
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 12 March 2024
Sec. Drugs Outcomes Research and Policies
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1304139
Background: Novel oral anticoagulants (NOACs) have been recommended by guidelines as the first-line drugs for preventing cardiogenic stroke. We aimed to provide an overview of the prescription trends and dosing appropriateness of NOACs in China.
Methods: We conducted a retrospective analysis of NOAC prescriptions using the Hospital Prescription Analysis Cooperation Project data from 2016 to 2022. Various patient features, such as gender, age, city, year, source, department visited, original diagnosis, dosing, cost, and insurance type, were collected and analyzed to examine the trends and dosing appropriateness of NOAC usage in ischemic stroke patients.
Results: 62,014 NOAC prescriptions were analyzed, including 16,602 for dabigatran, 45,253 for rivaroxaban, and 159 for apixaban. 85.14% of the patients were aged 65 or above, and tertiary hospitals accounted for 95.97% of NOAC prescriptions. NOAC prescriptions rose from 1828 in 2016 to 13,998 in 2021 but dropped to 13,166 in 2022. The percentage of annual prescriptions for NOACs among stroke patients has increased from 0.05% in 2016 to 0.37% in 2022. Total drug cost increased from ¥704541.18 in 2016 to ¥4128648.44 in 2021, then decreased to ¥1680109.14 in 2022. Prescriptions were divided into 48,321 appropriate and 11,262 inappropriate dosing groups, showing significant differences in medications, age, year, city type, hospital level, source, insurance type, and department visited (all p < 0.001). The median drug cost for inappropriate dosing was higher than for appropriate dosing (¥55.20 VS ¥83.80). The top comorbidities in ischemic stroke patients were atrial fibrillation (35.30%), hypertension (32.75%), and coronary heart disease (16.48%).
Conclusion: The application of NOACs in the Chinese population is increasing. Our findings highlight the frequent deviation from labeled dosing of NOACs in clinical practice. Continued efforts are necessary to promote the appropriate use of NOACs according to the standard dosage in the drug insert.
Ischemic stroke is a leading cause of neurological morbidity and mortality in neurological diseases worldwide (GBD, 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018). Recent epidemiological data reveals that in 2019, there were approximately 12.2 million incident cases of stroke and 101 million prevalent cases of stroke worldwide. Among these cases, ischemic stroke accounted for 62.4% of all incident strokes (GBD, 2019 Stroke Collaborators, 2021). Several factors can lead to the development of ischemic stroke, including arteriosclerosis affecting cerebral circulation, occlusion of small and medium-sized cerebral blood vessels, and embolism originating from the heart (cardiac embolism). Among the various causes of ischemic stroke, cardioembolic causes have been found to contribute to an increasing proportion of cases, and this trend is expected to continue in the coming decades (Kamel and Healey, 2017). Compared to non-cardiac stroke, atrial fibrillation (AF) related stroke exhibits a more unfavorable clinical outcome, with higher rates of recurrence, disability, and mortality (Yiin et al., 2019).
Anticoagulation therapy is considered to be the cornerstone of ischemic stroke prevention in non-valvular atrial fibrillation (NVAF) patients. Both vitamin K antagonists (VKAs) and novel oral anticoagulants (NOACs) have been proven effective in preventing stroke in patients with NVAF, ultimately prolonging life. NOACs have demonstrated superior efficacy and safety compared to VKAs(Ruff et al., 2014; Nazha et al., 2018; Tsai et al., 2020; Li et al., 2021), and they also have advantages such as rapid onset, short half-life, minimal drug interactions, and the absence of regular monitoring of the international normalized ratio (INR). They are extensively utilized for stroke prevention in AF patients, effectively reducing the formation of thrombosis and lowering the risk of recurrent stroke. Therefore, the current guidelines from Europe, North America, Japan, and China strongly advocate for NOACs as the first-line treatment for AF patients with a prior history of cardioembolic stroke, recommending lifelong anticoagulation therapy for secondary stroke prevention, unless contraindications are present (Kleindorfer et al., 2021; Steffel et al., 2021; Gladstone et al., 2022; Miyamoto et al., 2022; Liping et al., 2023). Furthermore, NOACs are particularly suitable for Asian patients, as they carry a significantly lower risk of bleeding and intracranial hemorrhage (ICH) while maintaining their effectiveness (Cho et al., 2019; Lee et al., 2020). NOACs have emerged as effective alternatives to warfarin for globally preventing cardiogenic stroke. Four specific NOACs, namely dabigatran, rivaroxaban, apixaban, and edoxaban, have obtained regulatory approval and gained guideline recommendations for secondary prevention of stroke in AF patients (Carnicelli et al., 2022). NOACs do not require dose adjustments based on clinical monitoring indicators, as the standard dose is clearly stated in the package insert and guidelines. However, it is common in clinical practice to prescribe NOACs at dosages that deviate from the recommended standard dosage in the drug insert. Nevertheless, there is currently limited understanding of the prescription patterns and dosing appropriateness of NOACs in Chinese patients with ischemic stroke in real-world settings. Therefore, this study aims to provide a comprehensive overview of the prescription trends and dosing appropriateness of NOACs for ischemic stroke in China.
This study was a retrospective analysis based on real-world prescription data. The data was obtained from the Hospital Prescription Analysis Cooperative Project (HPACP) database, which was conducted by the Chinese Pharmaceutical Association. The HPACP database included prescriptions from 120 medical institutions in 9 cities across China, including Beijing, Chengdu, Guangzhou, Harbin, Hangzhou, Shanghai, Shenyang, Tianjin, and Zhengzhou. A random sampling method was used to select 10 workday prescriptions for each quarter in the HPACP database, excluding national statutory holidays. Various patient features, such as gender, age, region, year, hospital level, source, department visited, original diagnosis, drug name, dosage, frequency, cost, and insurance type, were collected and analyzed to investigate the trends and dosing appropriateness of NOAC usage in ischemic stroke patients. The personal information of patients and clinicians was anonymized in the extracted prescriptions. The study received approval from the Ethics Committee of Beijing Tiantan Hospital, Capital Medical University.
The population we studied was patients who were using NOACs for anticoagulation therapy to prevent secondary stroke following an ischemic stroke. In the present study, prescriptions meeting the following criteria were included: 1) prescribed for patients diagnosed with ischemic stroke, with diagnostic keywords including stroke, cerebral apoplexy, ischemic cerebrovascular disease, cerebral infarction, transient ischemic attack, cerebral embolism, and cerebral thrombosis; 2) encompassing outpatient, emergency, and inpatient patients; 3) containing at least one of the following NOACs: Dabigatran, Rivaroxaban, Apixaban, Edoxaban; and 4) prescribed between the years 2016 and 2022. Prescriptions with missing age or sex information were excluded.
In this study, we conducted a descriptive analysis of the total number, proportion, cost, and trends of prescriptions. The median and interquartile range (IQR) were used to describe continuous variables that did not follow a normal distribution. Categorical variables were presented as frequency (%). The prescriptions were divided into two groups: appropriate dosing and inappropriate dosing, and the baseline characteristics of the two groups were analyzed using chi-square tests and nonparametric tests. p < 0.05 was considered statistically significant. All data were analyzed using Microsoft Excel and IBM SPSS Statistics (version 25; IBM Corporation, United States).
A total of 63,794 prescriptions for NOACs were initially extracted from the CPDHP database. Following refinement and screening processes, 62,014 prescriptions including dabigatran, Edoxaban and Apixaban, were selected for analysis in this study. While no prescriptions for edoxaban were identified among the extracted data (Figure 1).
The demographic features of the studied population are shown in Table 1. Most patients with ischemic stroke who used NOACs were 65 years or above, comprising 85.14% of the study population. Tertiary hospitals accounted for 95.70% of NOAC prescriptions, with 66.08% of these patients being from inpatient wards. Among the 9 cities included in the study, First-tier cities Beijing, Shanghai, and Guangzhou contributed to approximately half of the total prescriptions (Supplementary Table S1). 75.54% of patients using NOACs received full or partial reimbursement from Medicare.
Figure 2 illustrates the annual trends in the number/percentage of prescriptions and total drug cost for NOACs from 2016 to 2022. During this period, the total number of prescriptions for NOACs experienced consistent growth, rising from 1828 in 2016 to 13,998 in 2021, marking a substantial increase of 6.66-fold. However, in 2022, the number of prescriptions slightly decreased to 13,166, showing a decline of 5.94% compared to the previous year, which was mainly attributed to the decline in dabigatran prescriptions in 2022. Notably, the percentage of annual prescriptions for NOACs among stroke patients continued to grow, from 0.05% in 2016 to 0.37% in 2022, suggesting that stroke patients who require anticoagulant therapy are increasingly choosing to use NOACs. The annual number and percentage of prescriptions for rivaroxaban demonstrated a significant surge, increasing approximately 8 times from 2016 to 2022. In contrast, apixaban had relatively few prescriptions, with only sporadic prescriptions between 2016 and 2020. Nonetheless, it exhibited a clear growth trajectory in the past 2 years (Figure 2A; Supplementary Table S2).
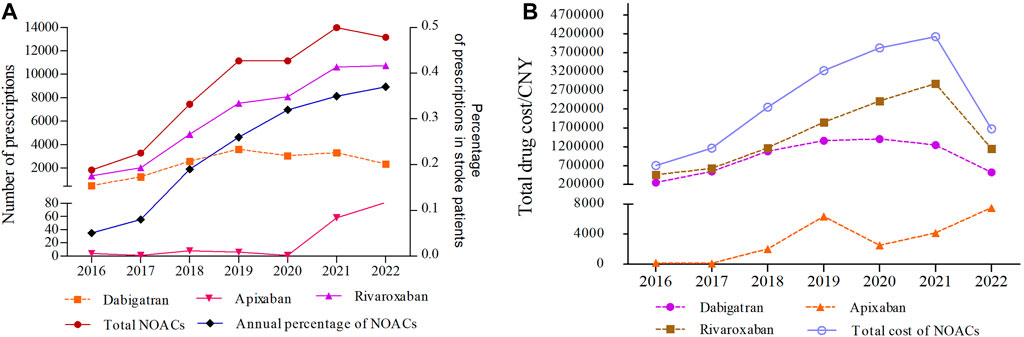
FIGURE 2. The annual trends in the number/percentage of prescriptions (A) and total drug cost for NOACs (B) from 2016 to 2022 (CNY, Chinese Yuan).
The annual trends in the total drug cost of NOACs from 2016 to 2022 are shown in Figure 2B. The total drug cost of NOACs experienced a substantial increase from ¥704541.18 in 2016 to ¥4128648.44 in 2021, rising by 4.86 times. However, in 2022, there was a rapid decline of 59.31% compared to the previous year. From 2016 to 2021, both dabigatran and rivaroxaban showed a consistent upward trajectory in terms of total drug cost, with dabigatran experiencing a 5.63-fold increase and rivaroxaban witnessing a 6.34-fold increase. These increments were in line with the corresponding rise in the number of prescriptions. However, a notable shift occurred in 2022, marked by a significant decline in the total drug cost (Figure 2B; Supplementary Tables S3, S4). The total drug costs for apixaban followed a similar pattern to the number of prescriptions, increasing steadily and continuing to rise from 2020 to 2022 (Figure 2B; Supplementary Table S5).
The prescriptions of NOACs were divided into appropriate dosing group and inappropriate dosing group according to the recommended standard dosage as stated in the drug insert. Taking into consideration the specific comorbidities and physical conditions of individual patients, we conducted a comprehensive assessment to determine the appropriate dosing range for NOACs. We defined the appropriate dosing range as those falling within the maximum and minimum dosages that align with the patient’s particular health conditions. Any prescriptions surpassing the maximum daily dosage or falling below the minimum effective dosage for the specific indications were classified as inappropriate dosing prescriptions. Conversely, prescriptions falling within the recommended dosing range specified in the drug insert were considered appropriate dosing prescriptions. More specifically, prescriptions were considered inappropriate when the daily dose of dabigatran exceeded 300 mg or fell below 150mg, rivaroxaban exceeded 30 mg or fell below 10 mg, and apixaban exceeded 20 mg or fell below 2.5 mg. Prescriptions with unclear dosages were excluded from the analysis, resulting in a total of 48,321 appropriate dosing prescriptions and 11,262 inappropriate ones.
The features of appropriate and inappropriate dosing prescriptions for NOACs are presented in Table 2. When comparing the baseline characteristics of the two groups, it was found that the median age was 78 years in the appropriate group and 80 years in the inappropriate group. In the inappropriate dosing group, more patients aged 65 years or above were prescribed NOACs than those aged 18–64 years (16.84% VS 2.06%), and more prescriptions were prescribed in other cities than in first-tier cities (10.44% VS 8.46%). Compared with the appropriate dosing group, the median drug cost of the inappropriate dosing group was higher (¥55.20 VS ¥83.80). There were significant differences between the two groups in terms of medications, age, year, city type, hospital level, patient source, insurance type, and department visited (all p˂0.001). However, there was no statistically significant difference in gender and total cost (p > 0.05).
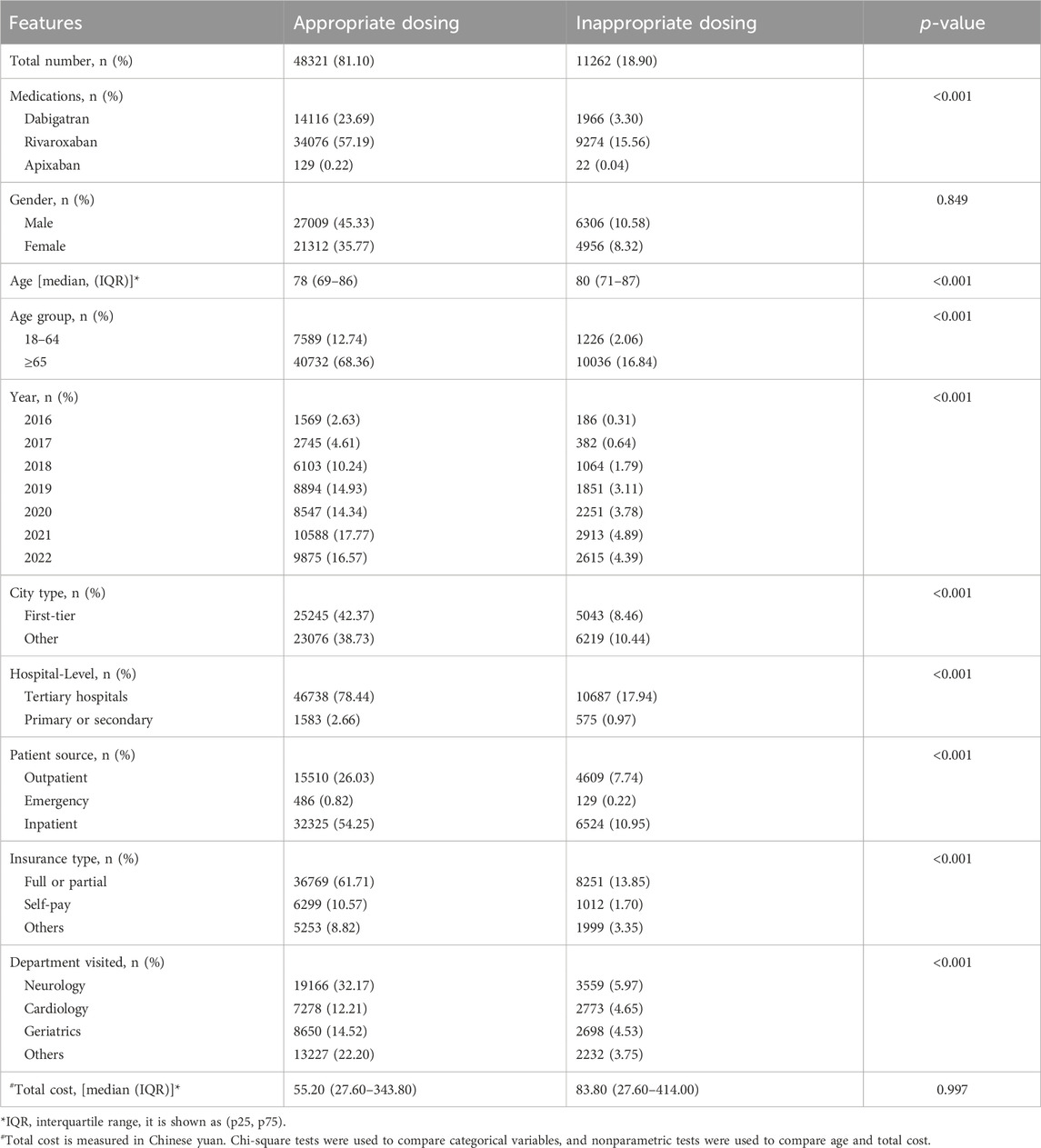
TABLE 2. Comparison of the features of patients with appropriate and inappropriate dosing prescriptions for NOACs.
The number of inappropriate dosing prescriptions increased in tandem with the total number of annual prescriptions, peaking in 2021 (Figure 3; Supplementary Table S6). Tertiary hospitals accounted for over 95% of these prescriptions, but their share has been gradually declining from 99.89% in 2016 to 94.69% in 2022. Conversely, prescriptions for NOACs in primary or secondary hospitals have remained relatively low, but they have shown consistent growth, rising from 2 in 2016 to 663 in 2022. Additionally, the number of appropriate dosing prescriptions in primary or secondary hospitals has also steadily increased alongside the total prescriptions (Supplementary Table S7).
From the perspective of the prescription trend in different cities, the number of appropriate dosing prescriptions in Chengdu and Hangzhou showed consistent and continuous growth, while the other 7 cities showed corresponding decreases from 2020 to 2022. The annual number of appropriate dosing prescriptions in Guangzhou has been ranked first among the 9 cities (Figure 4A; Supplementary Table S8). The number of inappropriate dosing prescriptions in Chengdu and Hangzhou had been increasing continuously, while the other 7 cities showed a significant decline in 2022 (Figure 4B; Supplementary Table S9).
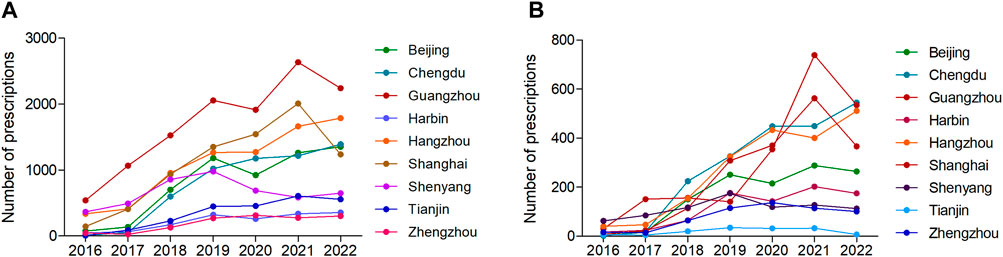
FIGURE 4. The number of (A) appropriate dosing prescriptions and (B) inappropriate dosing prescriptions in different cities from 2016 to 2022.
The annual trends of appropriate and inappropriate dosing prescriptions in different types of cities are presented in Figure 5. In the first-tier cities, the appropriate dosing and total prescriptions showed two declining inflection points in 2020 and 2022 respectively. In 2022, the appropriate dosing prescriptions and total prescriptions decreased by 26.70% and 19.97%, respectively. In other cities, the appropriate dosing and total prescriptions only decreased in 2020 and continued to increase from 2021 to 2022. For another, the number of inappropriate dosing prescriptions decreased in 2022 in the first-tier cities, while in other cities has been growing slowly from 2016 to 2022 (Figure 5; Supplementary Table S10).
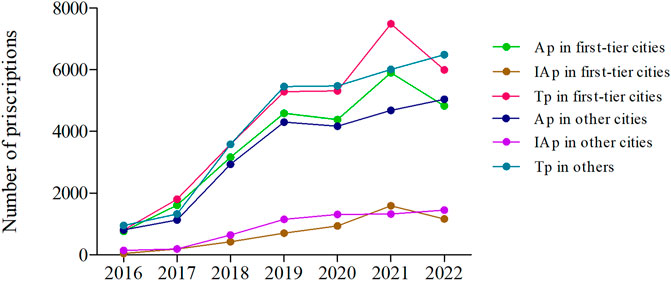
FIGURE 5. The annual trends of appropriate and inappropriate dosing prescriptions in different types of cities from 2016 to 2022. (Ap, appropriate dosing prescriptions; IAp, inappropriate dosing prescriptions; Tp, total prescriptions; First-tier cities include Beijing, Guangzhou, and Shanghai. Other cities include Chengdu, Harbin, Hangzhou, Shenyang, Tianjin, and Zhengzhou.)
To determine the prevalence of comorbidities in patients with ischemic stroke who received NOAC treatment, we conducted a comprehensive analysis based on the diagnostic information provided in the prescriptions. Among the patients who used NOACs for ischemic stroke, a significant majority (88.94%) had cerebral infarction. AF emerged as the most common comorbidity, accounting for 35.30% of cases, followed by hypertension (32.75%), coronary heart disease (16.48%), atherosclerosis (15.46%), diabetes mellitus (13.41%), cardiac insufficiency (10.08%), and hyperlipidemia (8.93%) (Table 3; Supplementary Figure S1).
This study investigated the utilization of NOACs in Chinese patients with ischemic stroke, using a large prescription database. The NOACs examined in the present study included dabigatran, rivaroxaban, and apixaban, while edoxaban was not detected. Considering the fluctuations in the number of stroke cases, we examined the percentage of NOAC prescriptions among stroke patients. Our analysis revealed a consistent annual rise in the proportion of NOAC prescriptions, indicating a growing preference among stroke patients in need of anticoagulant therapy for using NOACs. The growing preference for NOACs is attributed to their user-friendly nature, high effectiveness, safety profile, and expanding clinical evidence.
In terms of prescription frequency, rivaroxaban was the most prescribed, followed by dabigatran, with apixaban being the least. This discrepancy may be attributed to the time of NOACs covered under medical insurance and essential medicine. Rivaroxaban, a direct factor Xa inhibitor, was first approved for clinical use in China in 2009. It was subsequently included in the “National Reimbursement Drug List” (NRDL) that same year and was further incorporated into the “National Essential Drug List” (NEDL) in 2018 (National Health Commission of the People’s Republic of China, 2018). Rivaroxaban has thus garnered the most extensive safety experience among NOACs, which accounts for its highest number of prescriptions. Although both dabigatran and apixaban were approved for clinical use in 2013 and included in the NRDL in 2017 (Department of Human Resources and Social Security, 2017), dabigatran was additionally included in the NEDL in 2018 (National Health Commission of the People’s Republic of China, 2018), whereas apixaban was not. This disparity might explain the lower prescription proportion for apixaban. Edoxaban, approved for clinical use in late 2018, was included in the NRDL at the end of 2020 but has not yet been incorporated into the NEDL (Department of Human Resources and Social Security, 2020).
Our findings showed a steady increase in the frequency of NOAC use from 2016 to 2021 but decreased in 2022, which was mainly due to a decrease in the number of stroke patient visits in hospitals in 2022. Moreover, we observed a dramatic reduction in the total drug cost of NOACs in 2022, with a decline of 59.31% compared to 2021. This reduction can be attributed to the inclusion of dabigatran and rivaroxaban in the fifth batch of the National Drug Centralized Procurement List (NDCPL) in 2021, leading to a substantial reduction in drug prices. For instance, the price of the original drug rivaroxaban decreased by 49.95%, and the highest price of generic rivaroxaban decreased by 99.42%. Consequently, the implementation of the fifth batch of NDCPL in medical institutions nationwide in 2022 resulted in a quick and strong reduction in the total cost of NOACs.
The introduction of four NOACs with varying indications, doses, and criteria for dose reduction has made the process of determining the appropriate dosing more complex. This complexity poses a significant challenge in daily clinical practice and individualized treatment. The recommended dosing of NOACs for preventing stroke and systemic embolism in patients with AF in the Chinese and US guidelines is consistent with the label requirements (Steffel et al., 2018; Chinese Medical Association, 2021).
In the present study, we found that 18.90% of prescriptions for NOACs were either overdosing or underdosing, indicating that off-label dosing of NOACs in Chinese patients with ischemic stroke is common and needs more attention. Notably, a substantial proportion, approximately 40%, of AF patients receive NOAC dosing inconsistent with drug labeling in real-world clinical settings (Yu et al., 2020). These inconsistent prescribing patterns may be associated with poor safety and lack of efficacy benefits in patients with severe kidney disease. In patients with normal or mildly impaired renal function, these prescribing patterns may be associated with lower efficacy and no safety benefits (Yao et al., 2017). It is worth noting that overdosing on NOACs has been associated with worse clinical outcomes in Asian AF patients (Yu et al., 2020), suggesting that we should be more vigilant against overdosing use of NOACs in Chinese patients.
NOACs for the prevention of stroke in AF patients require dose adjustment based on certain clinical criteria (e.g., kidney function), but the off-label dosing of use is common. A recent study revealed that 12.6% of patients with NVAF did not adhere to the US Food and Drug Administration (FDA) label recommendations for NOAC administration, the incidence was higher in patients with worse renal function (31.9%) and was associated with less consistent long-term anticoagulation (Rymer et al., 2023). A Previous study showed that among the 1,473 patients with a renal indication for dose reduction, 43.0% were potentially overdosing, which was associated with a higher risk of major bleeding. Among 13,392 patients with no renal indication for dose reduction, 13.3% were potentially underdosing with NOACs, which was associated with a higher risk for stroke (Yao et al., 2017). Despite the potential benefits of lower doses of NOACs in patients with impaired renal function, those who receive less than the minimum recommended dosing still face an increased risk of thrombosis and poorer clinical outcomes. The AFIRE trial showed that underdose rivaroxaban (10 mg/day), compared with the standard dose (15 mg/day), was associated with a similar incidence of thrombotic events but a lower rate of bleeding events in AF patients with stable coronary artery disease (CAD) and preserved renal function (Arashi et al., 2022). A recent study revealed that utilization of lower-dose rather than standard-dose NOACs was associated with a higher incidence of death, stroke, and systemic embolism in patients with reduced kidney function (Harrington et al., 2023). These findings suggest that label adherence to NOAC dosing should be emphasized to achieve optimal clinical outcomes in Asian patients with AF.
Standard-dose anticoagulation is associated with a relatively good prevention of stroke and other embolism. One study showed that standard-dose apixaban (5 mg twice daily) was associated with significantly lower risks for stroke/systemic embolism and death than low-dose apixaban (2.5 mg twice daily) (Siontis et al., 2018). A network meta-analysis has shown that standard dose NOACs offer the most favorable risk-benefit profile compared to other oral anticoagulants. In Asian patients, standard-dose NOACs are a more attractive treatment option than underdosing NOACs due to their significant reduction in ischemic stroke without an increased risk of major bleeding (Cha et al., 2017). However, with safety concerns about increasing bleeding, off-label underdosing of NOACs is common in East Asian patients with AF (Cho et al., 2019; Chan et al., 2020). A study of oral anticoagulant dose adjustment in patients with acute ischemic stroke related to AF showed that one-third of patients with ischemic stroke used an inappropriate dosing regimen during oral Xa inhibitor therapy (26% underdose and 8% overdose), and underdosing was associated with lower functional plasma levels, higher clinical stroke severity, and worse functional outcome (Stoll et al., 2020). In another study involving Asian patients with NVAF, the risks of ischemic stroke, all-cause death, and composite clinical outcomes were higher in the off-label underdosing apixaban group than the on-label standard dose apixaban group, but the risks of major bleeding were comparable (Lee et al., 2021). These findings highlight the need for efforts to improve the quality and dosing of NOACs. We discovered a consistent increase in the number of inappropriate dosing prescriptions in Chengdu and Hangzhou from 2016 to 2022. This finding highlights the need for further investigation into the prescribing practices of physicians in these two cities. Identifying the factors influencing prescribed dosing, implementing standardized training and promotion, and advocating for the rational use of NOACs are essential steps to address this issue.
Our study also has some limitations. Firstly, this study only analyzed NOACs and did not include information on other anticoagulants, which limits our ability to investigate the overall changes in anticoagulation treatment for cardiogenic stroke. Secondly, the database did not provide information on treatment efficacy and adverse effects, preventing us from determining the effectiveness and safety of NOACs in patients. Thirdly, trends in the number and proportion of NOAC prescriptions have limitations in capturing the full picture of drug consumption. Lastly, this study mainly focused on samples from developed large cities in China and therefore may not accurately represent the situation in less developed areas and rural regions.
The application of NOACs in the Chinese population is increasing. The total cost of NOACs in ischemic stroke has been steadily increasing since 2016 but showed a sharp decline in 2022, demonstrating that the “drug price negotiation policy” in China have significantly contributed to the reduction in drug costs. Atrial fibrillation, hypertension, coronary heart disease, atherosclerosis, and diabetes were the most prevalent comorbidities among patients with ischemic stroke. In addition, our findings highlight the frequent deviation from labeled dosing of NOACs in clinical practice, which may result in poor efficacy in thromboprophylaxis and pose a high safety risk. Thus, it is crucial to improve adherence to the standard dosages of NOACs to enhance the effectiveness of stroke prevention. Continued efforts are necessary to promote the appropriate use of NOACs according to the standard dosage in the drug insert.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
MW: Data curation, Formal Analysis, Funding acquisition, Software, Writing–original draft, Conceptualization, Writing–review and editing. HJ: Methodology, Software, Validation, Visualization, Writing–original draft. KY: Data curation, Investigation, Software, Writing–review and editing. ZZ: Funding acquisition, Project administration, Supervision, Writing–review and editing, Methodology. BZ: Methodology, Software, Supervision, Validation, Writing–review and editing, Conceptualization.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1304139/full#supplementary-material
Arashi, H., Yamaguchi, J., Hagiwara, N., Yasuda, S., Kaikita, K., Akao, M., et al. (2022). Rivaroxaban underdose for atrial fibrillation with stable coronary disease: the AFIRE trial findings. Thromb. Haemost. 122 (9), 1584–1593. doi:10.1055/s-0042-1744543
Carnicelli, A. P., Hong, H., Connolly, S. J., Eikelboom, J., Giugliano, R. P., Morrow, D. A., et al. (2022). Direct oral anticoagulants versus warfarin in patients with atrial fibrillation: patient-level network meta-analyses of randomized clinical trials with interaction testing by age and sex. Circulation 145 (4), 242–255. doi:10.1161/CIRCULATIONAHA.121.056355
Cha, M. J., Choi, E. K., Han, K. D., Lee, S. R., Lim, W. H., Oh, S., et al. (2017). Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in asian patients with atrial fibrillation. Stroke 48 (11), 3040–3048. doi:10.1161/STROKEAHA.117.018773
Chan, Y. H., Chao, T. F., Chen, S. W., Lee, H. F., Yeh, Y. H., Huang, Y. C., et al. (2020). Off-label dosing of non-vitamin K antagonist oral anticoagulants and clinical outcomes in Asian patients with atrial fibrillation. Heart Rhythm 17 (12), 2102–2110. doi:10.1016/j.hrthm.2020.07.022
Chinese Medical Association (2021). Guideline for rational medication of atrial fibrillation in primary care. Chin. J. Gen. Pract. 20 (2), 166–174. doi:10.3760/cma.j.cn114798-20201210-01241
Cho, M. S., Yun, J. E., Park, J. J., Kim, Y. J., Lee, J., Kim, H., et al. (2019). Outcomes after use of standard- and low-dose non-vitamin K oral anticoagulants in asian patients with atrial fibrillation. Stroke 50 (1), 110–118. doi:10.1161/STROKEAHA.118.023093
Department of Human Resources and Social Security (2017). Notice of the Issuance of national basic medical insurance, work-related injury insurance and Maternity Insurance Drug List.
Department of Human Resources and Social Security (2020). Notice of the Issuance of national basic medical insurance, work-related injury insurance and Maternity Insurance Drug List.
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392 (10159), 1789–1858. doi:10.1016/S0140-6736(18)32279-7
GBD 2019 Stroke Collaborators (2021). Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 20 (10), 795–820. doi:10.1016/S1474-4422(21)00252-0
Gladstone, D. J., Lindsay, M. P., Douketis, J., Smith, E. E., Dowlatshahi, D., Wein, T., et al. (2022). Canadian stroke best practice recommendations: secondary prevention of stroke update 2020. Can. J. Neurol. Sci. 49 (3), 315–337. doi:10.1017/cjn.2021.127
Harrington, J., Carnicelli, A. P., Hua, K., Wallentin, L., Patel, M. R., Hohnloser, S. H., et al. (2023). Direct oral anticoagulants versus warfarin across the spectrum of kidney function: patient-level network meta-analyses from COMBINE AF. Circulation 147 (23), 1748–1757. doi:10.1161/CIRCULATIONAHA.122.062752
Kamel, H., and Healey, J. S. (2017). Cardioembolic stroke. Circ. Res. 120 (3), 514–526. doi:10.1161/CIRCRESAHA.116.308407
Kleindorfer, D. O., Towfighi, A., Chaturvedi, S., Cockroft, K. M., Gutierrez, J., Lombardi-Hill, D., et al. (2021). 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American heart association/American stroke association. Stroke 52 (7), e364–e467. doi:10.1161/STR.0000000000000375
Lee, S. R., Choi, E. K., Kwon, S., Jung, J. H., Han, K. D., Cha, M. J., et al. (2020). Oral anticoagulation in asian patients with atrial fibrillation and a history of intracranial hemorrhage. Stroke 51 (2), 416–423. doi:10.1161/STROKEAHA.119.028030
Lee, S. R., Choi, E. K., Park, S. H., Jung, J. H., Han, K. D., Oh, S., et al. (2021). Off-label underdosed apixaban use in Asian patients with non-valvular atrial fibrillation. Eur. Heart J. Cardiovasc. Pharmacother. 7 (5), 415–423. doi:10.1093/ehjcvp/pvab004
Li, H. J., Lin, S. Y., Lin, F. J., Hung, C. S., and Wang, C. C. (2021). Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in Asian patients with atrial fibrillation and valvular heart disease. Curr. Med. Res. Opin. 37 (4), 535–542. doi:10.1080/03007995.2021.1885365
Liping, L., Hongyu, Z., Wanying, D., Xiaochuan, H., Ximing, N., Huihui, L., et al. (2023). Guidelines for clinical management of cerebrovascular diseases (second edition) (Excerpt)——chapter four clinical management of ischaemic cerebrovascular diseases. Chin. J. Stroke 18 (8), 910–933. doi:10.3969/j.issn.1673-5765.2023.08.009
Miyamoto, S., Ogasawara, K., Kuroda, S., Itabashi, R., Toyoda, K., Itoh, Y., et al. (2022). Japan stroke society guideline 2021 for the treatment of stroke. Int. J. Stroke. 17 (9), 1039–1049. doi:10.1177/17474930221090347
National Health Commission of the People's Republic of China (2018). National essential medicines list (2018 edition).
Nazha, B., Pandya, B., Cohen, J., Zhang, M., Lopes, R. D., Garcia, D. A., et al. (2018). Periprocedural outcomes of direct oral anticoagulants versus warfarin in nonvalvular atrial fibrillation. Circulation 138 (14), 1402–1411. doi:10.1161/CIRCULATIONAHA.117.031457
Ruff, C. T., Giugliano, R. P., Braunwald, E., Hoffman, E. B., Deenadayalu, N., Ezekowitz, M. D., et al. (2014). Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 383 (9921), 955–962. doi:10.1016/S0140-6736(13)62343-0
Rymer, J. A., Chiswell, K., Young, L., Chiu, A., Liu, L., Webb, L., et al. (2023). Analysis of oral anticoagulant dosing and adherence to therapy among patients with nonvalvular atrial fibrillation. JAMA Netw. Open. 6 (6), e2317156. doi:10.1001/jamanetworkopen.2023.17156
Siontis, K. C., Zhang, X., Eckard, A., Bhave, N., Schaubel, D. E., He, K., et al. (2018). Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation 138 (15), 1519–1529. doi:10.1161/CIRCULATIONAHA.118.035418
Steffel, J., Collins, R., Antz, M., Cornu, P., Desteghe, L., Haeusler, K. G., et al. (2021). 2021 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace 23 (10), 1612–1676. doi:10.1093/europace/euab065
Steffel, J., Verhamme, P., Potpara, T. S., Albaladejo, P., Antz, M., Desteghe, L., et al. (2018). The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 39 (16), 1330–1393. doi:10.1093/eurheartj/ehy136
Stoll, S., Macha, K., Marsch, A., Gerner, S. T., Siedler, G., Frohlich, K., et al. (2020). Ischemic stroke and dose adjustment of oral Factor Xa inhibitors in patients with atrial fibrillation. J. Neurol. 267 (7), 2007–2012. doi:10.1007/s00415-020-09795-3
Tsai, C. T., Liao, J. N., Chiang, C. E., Lin, Y. J., Chang, S. L., Lo, L. W., et al. (2020). Association of ischemic stroke, major bleeding, and other adverse events with warfarin use vs non-vitamin K antagonist oral anticoagulant use in patients with atrial fibrillation with a history of intracranial hemorrhage. JAMA Netw. Open. 3 (6), e206424. doi:10.1001/jamanetworkopen.2020.6424
Yao, X., Shah, N. D., Sangaralingham, L. R., Gersh, B. J., and Noseworthy, P. A. (2017). Non-vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J. Am. Coll. Cardiol. 69 (23), 2779–2790. doi:10.1016/j.jacc.2017.03.600
Yiin, G., Li, L., Bejot, Y., and Rothwell, P. M. (2019). Time trends in atrial fibrillation-associated stroke and premorbid anticoagulation: population-based study and systematic review. Stroke 50 (1), 21–27. doi:10.1161/STROKEAHA.118.022249
Keywords: novel oral anticoagulants, NOACs, ischemic stroke, prescription trends, dosing appropriateness
Citation: Wu M, Jiang H, Yu K, Zhao Z and Zhu B (2024) The Prescription trends and dosing appropriateness analysis of novel oral anticoagulants in ischemic stroke patients: a retrospective study of 9 cities in China. Front. Pharmacol. 15:1304139. doi: 10.3389/fphar.2024.1304139
Received: 29 September 2023; Accepted: 28 February 2024;
Published: 12 March 2024.
Edited by:
Tin Wui Wong, Universiti Teknologi MARA Puncak Alam, MalaysiaReviewed by:
Janattul Ain Jamal, Universiti Teknologi MARA Puncak Alam, MalaysiaCopyright © 2024 Wu, Jiang, Yu, Zhao and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Zhu, emJ0Y21AMTYzLmNvbQ==; Zhigang Zhao, MTAyMnp6Z0BzaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.