- 1First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin, China
- 3State Key Laboratory of Component-Based Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, China
Introduction: Pogostemon cablin (PC) is used in traditional Chinese medicine and food, as it exerts pharmacological effects, such as immune-modulatory, antibacterial, antioxidant, antitumor, and antiviral. Currently, the pharmacokinetics (PK) studies of PC mainly focus on individual components. However, research on these individual components cannot reflect the actual PK characteristics of PC after administration. Therefore, the simultaneous determination of multiple components in rat plasma using UPLC-MS/MS was used for the pharmacokinetic study after oral administration of PC extract in this study, providing reference value for the clinical application of PC.
Methods: In the present study, a reliable and sensitive ultra-high performance liquid chromatography/tandem mass spectrometry (UPLC-MS/MS) method was developed and validated for the simultaneous determination of 15 prototype components (vanillic acid, vitexin, verbascoside, isoacteoside, hyperoside, cosmosiin, apigenin, β-rhamnocitrin, acacetin, ombuin, pogostone, pachypodol, vicenin-2, retusin, and diosmetin-7-O-β-D-glucopyranoside) in rat plasma after oral administration of the PC extract. Plasma samples were prepared via protein precipitation using acetonitrile, and icariin was used as the internal standard (IS).
Results: The intra-day and inter-day accuracies ranged from −12.0 to 14.3%, and the precision of the analytes was less than 11.3%. The extraction recovery rate of the analytes ranged from 70.6−104.5%, and the matrix effects ranged from 67.4−104.8%. Stability studies proved that the analytes were stable under the tested conditions, with a relative standard deviation lower than 14.1%.
Conclusion: The developed method can be applied to evaluate the PK of 15 prototype components in PC extracts of rats after oral administration using UPLC-MS/MS, providing valuable information for the development and clinical safe, effective, and rational use of PC.
1 Introduction
Medicinal plants are natural resources with crucial medicinal value, not only for the treatment of diseases but also for the enhancement of immunity and prevention of diseases. Therefore, an in-depth study of medicinal plants is of great significance (Zhu et al., 2022; Lai et al., 2023). Pogostemon cablin (Blanco) Benth., is a plant of the Lamiaceae family, commonly called patchouli, or “Guanghuoxiang.” Pogostemon cablin (PC), is extensively distributed in the tropical and subtropical areas of Asia, and widely cultivated in the Philippines, India, and southern and southwestern China (Su et al., 2017; Chen et al., 2020). As a traditional herbal medicine, PC was used as a stomach tonic to remove dampness, improve indigestion, and relieve vomiting and diarrhea. It was also used to treat the common cold, headache, nausea, fever, and so on (Zhang et al., 2019; Chinese Pharmacopoeia Committee, 2020; Li et al., 2023). There are a number of components in PC, such as monoterpenoids, triterpenoids, sesquiterpenoids, phytosterols, and flavonoids. Studies have suggested that PC has immune-modulatory, antibacterial, antioxidant, antitumor, and antiviral bioactivities due to its multi-component properties (Kiyohara et al., 2012; Kim et al., 2015; Liu et al., 2015; Junren et al., 2021). PC is one of the ingredients in the formulations of many famous traditional Chinese patent medicines, including Huoxiang Zhengqi Oral Liquid and Huodan Wan (Pills). It can be used to treat gastrointestinal disorders with Huoxiang Zhengqi Oral Liquid, and Huodan Wan can be used for cold rhinitis and nasal congestion caused by dampness-heat in clinical (Lan and Yuan, 2018; Zhao et al., 2019). In addition to the traditional use of herbal medicine, PC also has high edible value and health benefits due to its various nutrients, including amino acids, proteins, vitamins, and minerals (Zhang, 2004). The leaves or stems of fresh PC can be stir-fried, fried, dipped in sauce, cold mixed, pickled, cooked in soup or congee, and it is mainly used as side dishes and stews to taste because of its properties of fishiness-eliminating and flavor-enhancing. Huoxiang jam is considered a food with higher health value. Meanwhile, PC is combined with Mentha haplocalyx, Glycyrrhiza uralensis, Perilla frutescens, Ophiopogonis Radix, Chrysanthemum morifolium, and other raw materials, which can form a variety of medicinal teas with fitness, beauty, and disease treatment effects. Moreover, PC can be used as a fixing agent for various perfumes, and as a raw material for cosmetics and oral hygiene products (Huo et al., 2007; Zhang J. et al., 2016; Sandes et al., 2016).
Pharmacokinetics (PK) is the study of the absorption, distribution, metabolism, and excretion of drugs in vivo, which plays an important role in the determination of the drug’s kinetics and bioavailability. Notably, PK is very important for evaluating the absorption properties of different active components of traditional Chinese medicine (TCM). Moreover, the development of sensitive and reliable biological sample analysis technology to simultaneously determine multiple active ingredients in vivo is a hotspot in the PK research of TCM extracts, due to the complex composition and significant differences in the content (Cao et al., 2021). Therefore, an accurate and selective bioanalytical method is urgently required for the simultaneous determination of multiple biological components in plasma to understand the characteristics and diversity of the PK properties of PC.
In this study, a method for the simultaneous detection of 15 compounds (vanillic acid, vitexin, verbascoside, isoacteoside, hyperoside, cosmosiin, apigenin, β-rhamnocitrin, acacetin, ombuin, pogostone, pachypodol, diosmetin-7-O-β-D-glucopyranoside, vicenin-2, and retusin) in rat plasma was established and validated using ultra-high-performance liquid chromatography/tandem mass spectrometry (UPLC-MS/MS), which provided a reference for the clinical safe, effective and rational use of PC.
2 Materials and methods
2.1 Chemicals and reagents
Standard compound including vanillic acid, vitexin, verbascoside, isoacteoside, hyperoside, cosmosiin, apigenin, β-rhamnocitrin, acacetin, ombuin, pogostone, pachypodol, diosmetin-7-O-β-D-glucopyranoside, vicenin-2, and retusin (purity ≥98%) were purchased from Chengdu Desite Bio-Technology Co., Ltd. (Chengdu, China). Icariin [internal standard (IS)] was provided by Tianjin Yifang Zhongkang Pharmaceutical Technology Co., Ltd. (Tianjin, China). Fisher Scientific (Fair Lawn, NJ, United States) supplied the chromatographic grade methanol (ME) and acetonitrile (ACN). The chromatographic grade of formic acid (FA) was provided by ROE (St Louis, MO, United States). PC was purchased from Guangdong province (China).
2.2 Chromatography and mass spectrometry (MS)
The analyte was separated and detected through the utilization of a UPLC-MS/MS system, consisting primarily of the Agilent-1290 high-performance liquid chromatography system (Agilent, United States) and the Agilent-6470 triple quadrupole tandem mass spectrometer (Agilent, United States).
The components were separated using an ACQUITY UPLC®BEH C18 column (2.1 × 100 mm, 1.7 µm) maintained at a temperature of 25°C. 0.1% FA-water and ACN were used as mobile phases A and B. The gradient elution conditions were as follows: 0–4 min, 14%–53% B; 4–5 min, 53%–100% B; and 5–7 min, 100%–100% B. A flow rate of 0.3 mL/min and an injection volume of 5 μL were used.
Mass spectrometry analysis was conducted in the multiple reaction monitoring (MRM) mode with both positive and negative ionization (Zhu et al., 2022). The parameters for the mass spectrometer included drying gas (N2) temperature, gas flow rate, atomizer pressure, and capillary voltage of 320°C, 7 L/min, 35 psi, and 3500 V, respectively. The detailed MRM parameters can be found in Supplementary Table S1.
2.3 PC extract preparation
PC (0.5 kg) was accurately weighed and extracted with 10-fold ethanol (85%, v/v) under heated reflux conditions twice for 1.5 h. Next, the solutions for extraction underwent filtration and were subsequently combined. The ensuing mixture underwent concentration through evaporation under decreased pressure. Ultimately, the dried PC extract was stored in a dry environment for further analysis. The levels of vanillic acid, vitexin, verbascoside, isoacteoside, hyperoside, cosmosiin, apigenin, β-rhamnocitrin, acacetin, ombuin, pogostone, pachypodol, vicenin-2, retusin, and diosmetin-7-O-β-D-glucopyranoside in the PC extract are displayed in Supplementary Table S2.
2.4 Preparation of standard solutions, calibration standards, and quality control (QC) samples
Vanillic acid, vitexin, verbascoside, isoacteoside, hyperoside, cosmosiin, apigenin, β-rhamnocitrin, acacetin, ombuin, pogostone, pachypodol, vicenin-2, retusin, diosmetin-7-O-β-D-glucopyranoside, and icariin (IS) were individually weighed and mixed with ME to prepare standard stock solution (1 mg/mL). To obtain the subsequent working solutions, the standard stock solutions were diluted using ME.
To prepare the calibration solutions, 20 μL of a mixture of working solution and IS was added to blank rat plasma (100 μL), resulting in concentrations: 20, 50, 100, 200, 400, 1,000, 2000, 4,000, and 8,000 ng/mL for vanillic acid and vicenin-2; 0.5, 1.25, 2.5, 5, 10, 25, 50, 100, and 200 ng/mL for vitexin, cosmosiin, and ombuin; 0.3, 0.75, 1.5, 3, 6, 15, 30, 60, and 120 ng/mL for hyperoside, β-rhamnocitrin, acacetin, and diosmetin-7-O-β-D-glucopyranoside; 10, 25, 50, 100, 200, 500, 1,000, 2000, and 4,000 ng/mL for verbascoside; 2, 5, 10, 20, 40, 100, 200, 400, and 800 ng/mL for isoacteoside; 1.5, 3.75, 7.5, 15, 30, 75, 150, 300, and 600 ng/mL for apigenin; 1, 2.5, 5, 10, 20, 50, 100, 200, and 400 ng/mL for pachypodol; 0.2, 0.5, 1, 2, 4, 10, 20, 40, and 80 ng/mL for retusin; 125, 312.5, 635, 1,250, 2,500, 6,250, 12,500, 25,000, and 50,000 ng/mL for pogostone. QC samples at three concentrations (low, medium, and high) were obtained similarly. Prior to analysis, all solutions were stored in an environment of 4°C.
2.5 Preparation of plasma sample
The 100 μL plasma sample was combined with 20 μL of ME, 20 μL of IS (500 ng/mL), and 2.5 μL of FA, followed by vortexing for 1 min. This mixture underwent extraction with 600 μL ACN through vortexing at room temperature for 3 min. Subsequent to centrifugation at 14,000 rpm for a period of 10 min, the resulting supernatant was moved to another tube and dried under a stream of nitrogen. The dried residue was dissolved in 100 μL ME-ACN (50:50 v/v), followed by vortexing for 5 min, and subsequent centrifugation at 14,000 rpm for 10 min. Finally, 20 μL of the supernatant was used for analysis.
2.6 Method validation
2.6.1 Specificity
In order to assess specificity, chromatograms of rat plasma samples without any added analytes, plasma with added analytes, and post-dosing plasma samples from rats following oral ingestion of PC were analyzed.
2.6.2 Linearity and LLOQ
By graphing the correlation between the peak area ratios of each component to IS versus the concentration of those particular analytes to obtain calibration curves. The regression association was depicted utilizing a linear formula with a weighting factor of 1/x2. The determination of LLOQ relied on the baseline noise, ensuring a signal-to-noise ratio of around ten.
2.6.3 Precision and accuracy
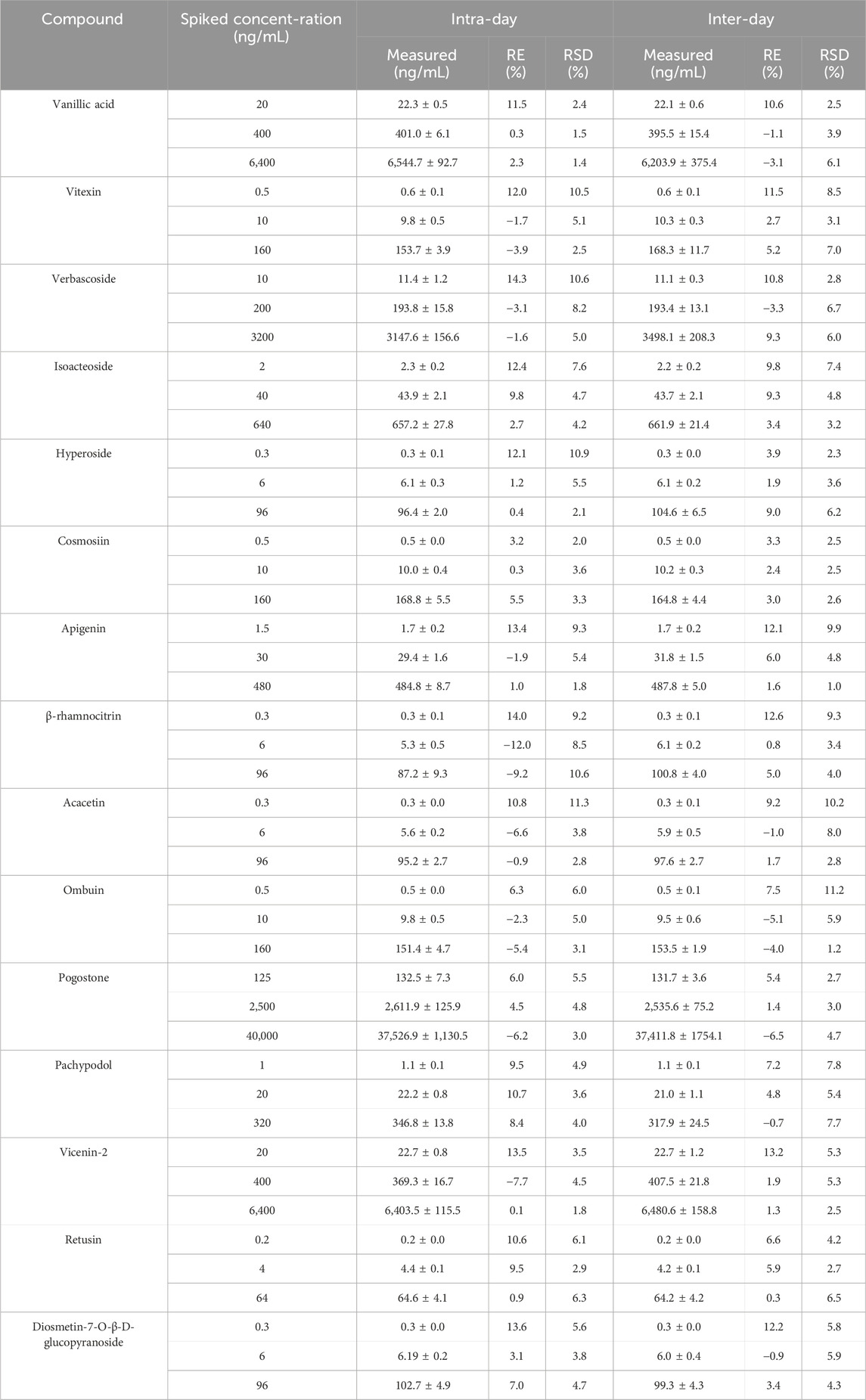
QC samples three concentrations (20, 400, and 6,400 ng/mL for vanillic acid and vicenin-2; 0.5, 10, and 160 ng/mL for vitexin, cosmosiin, and ombuin; 0.3, 6, and 96 ng/mL for hyperoside, β-rhamnocitrin, acacetin and diosmetin-7-O-β-D-glucopyranoside; 10, 200, and 3200 ng/mL for verbascoside; 2, 40, and 640 ng/mL for isoacteoside; 1.5, 30, and 480 ng/mL for apigenin; 1, 20, and 320 ng/mL for pachypodol; 0.2, 4, and 64 ng/mL for retusin; 125, 2,500, and 40,000 ng/mL for pogostone) were analyzed six repetitions over one or three consecutive days to assess the precision and accuracy. Relative error (RE) was used to determine accuracy, while the precisions were expressed as the relative standard deviation (RSD).
2.6.4 Extraction recovery and matrix effect
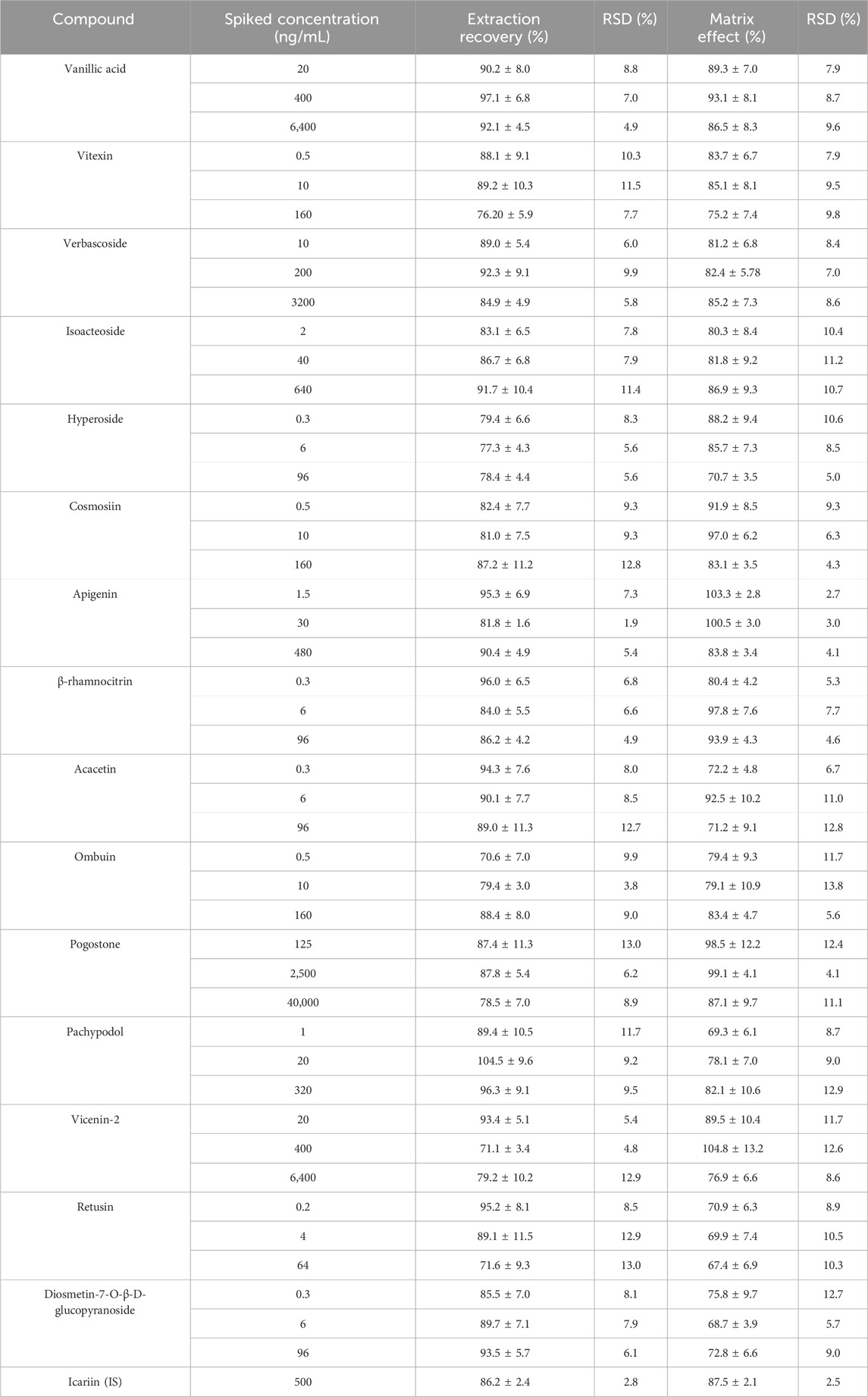
Analyzing the peak areas of QC samples and the peak areas of the post-extraction mixed samples to measure the extraction recovery rates at three concentrations. Matrix effects were determined by comparing the peak areas of QC samples in post-extracted mixed samples to those in standard solutions, calculating the ratio for evaluation.
2.6.5 Stability
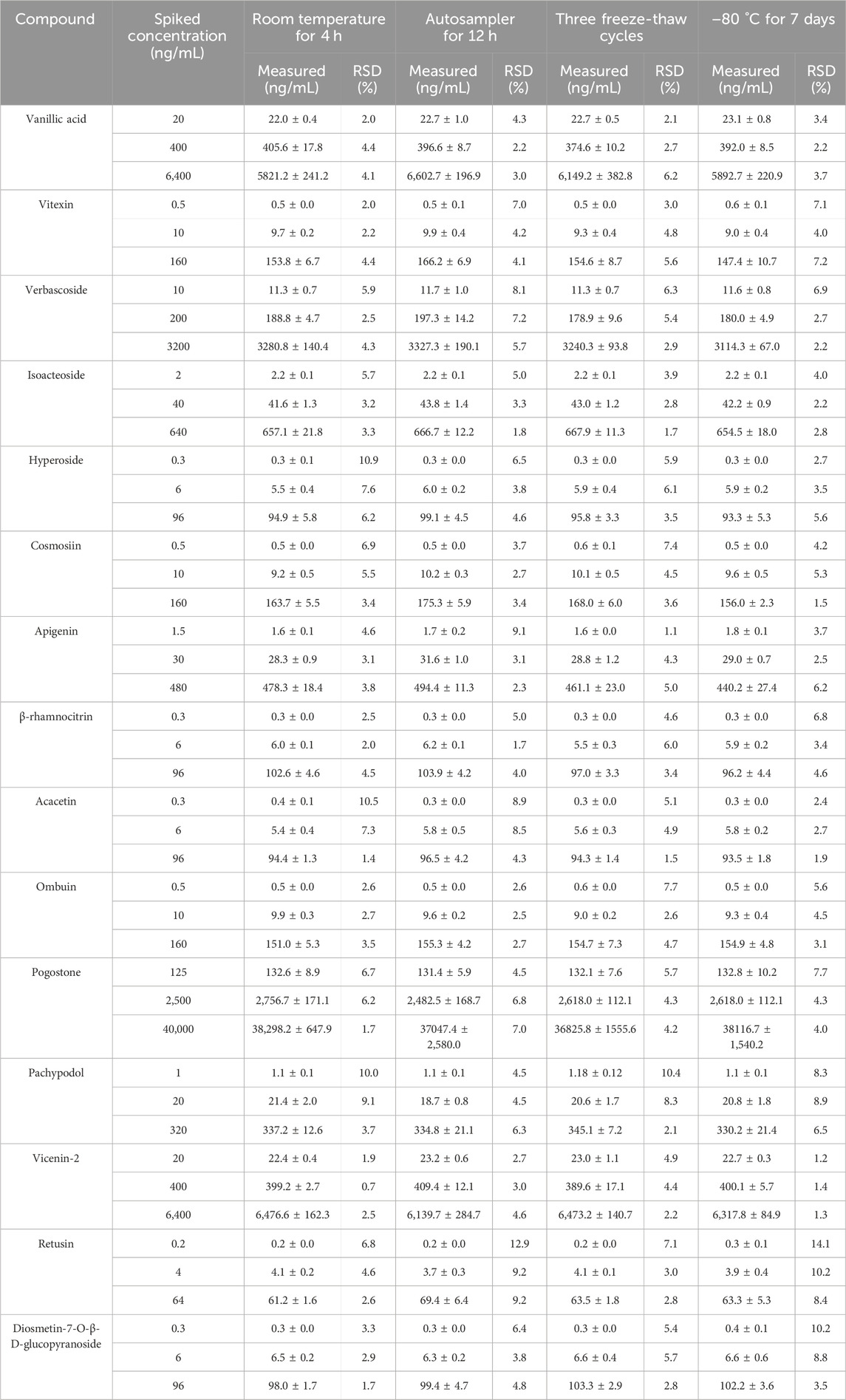
Plasma samples were retained for 12 h in an auto-sampler, exposed to room temperature for 4 h, undergoing three freeze-thaw cycles, and stored at −80°C for a period of 1 week to assess the stability of all compounds.
2.7 PK study
Six male rats (220 ± 10 g) were utilized in this experiment and were not restricted from drinking water but fasted for 12 h before the investigation. A concentration of 0.5 g/mL of PC extract was obtained by dissolving in 0.5% CMC-Na aqueous solution. The PC suspension of the 4.0 g/kg dose was given to rats by single oral administration. Samples of blood (300 μL) were obtained from the orbital venous plexus prior to and following oral dosing at 0, 0.03, 0.08, 0.25, 0.5, 0.75, 1, 2, 6, 10, 12, 24, 36, and 48 h. Subsequently, the plasma collected underwent centrifugation at a speed of 7000 rpm for a duration of 10 min. It was then moved to clean tubes and kept at a temperature of −80°C.
2.8 Data analysis
Data in this study was presented as the mean ± standard deviation (SD). The MassHunter Workstation software (version B.09.00, Agilent, United States) was utilized for determining the plasma levels of the 15 analytes. Additionally, PK parameters were analyzed using DAS 3.0 Software (Medical College of Wannan, China).
3 Result and discussion
3.1 Optimization of chromatography and MS
To improve the separation of the 15 compounds, an investigation was conducted on the impact of different columns, including ACQUITY UPLC BEH C18 (2.1 × 100 mm, 1.7 µm) and ACQUITY UPLC HSS T3 (2.1 × 100 mm, 1.8 µm), on the chromatographic peaks and retention times. The findings revealed that the ACQUITY UPLC BEH C18 (2.1 × 100 mm, 1.7 µm) column provided superior separation capability for the 15 compounds. Different mobile phases were also evaluated, such as 0.1% FA/water-ACN, 0.1% FA/water-ME, water-ME, and water-ACN, to optimize the separation of all compounds. The findings demonstrated that the 0.1% FA/water-ACN proved to be superior in improving the separation and peak profiles of the compounds. All 15 components and IS were eluted successfully within a 7-min, and with no observed interference peaks.
Optimizing the primary parameters of the MS was crucial in enhancing the response of compounds. In positive ion mode, the compounds retusin, diosmetin-7-O-β-D-glucopyranoside, and IS were examined, while others were assessed in negative ion mode.
3.2 Sample preparation
The study evaluated four different extraction methods to determine the most effective method for preparing plasma samples, including protein precipitation using ME or ACN, ethyl acetate liquid-liquid extraction, and extraction using a mixture of ME and ACN (v/v = 1:4). The findings indicated that the protein precipitation using ACN showed higher extraction recovery rates for the 15 analytes tested. Additionally, different volumes (400, 600, 800, and 1,000 μL) of precipitated solvent were used to assess the extraction recovery and matrix effect. The outcomes demonstrated that the extraction recovery and matrix effects of the ACN-protein precipitation using 600 μL satisfied the criteria for analyzing biological specimens, with no interference from endogenous compounds. Additionally, the impacts of various reconstitution solvents such as ME, ACN, 50% ME, and ME-ACN (v/v = 1:1, 1:4, and 4:1) were assessed. The results indicated that the redissolution effectiveness was optimal with a ME-ACN mixture of (v/v = 1:1).
3.3 Method validation
3.3.1 Specificity
The MRM chromatograms in Figure 1 displayed the rat plasma samples without any added analytes (A), plasma samples combined with the 15 compounds and IS (B), and post-dosing plasma samples from rats following oral ingestion of PC (C). The results suggested no noticeable interference peaks were noted at the retention time of the 15 compounds and IS.

Figure 1. The MRM chromatograms of 15 components and IS in rat plasma samples. (A) Plasma samples without any added analytes, (B) plasma samples combined with the 15 compounds and IS, (C) post-dosing plasma samples from rats following oral ingestion of PC. 1. Vanillic acid, 2. Vitexin, 3. Verbascoside, 4. Isoacteoside, 5. Hyperoside, 6. Cosmosiin, 7. Apigenin, 8. β-Rhamnocitrin, 9. Acacetin, 10. Ombuin, 11. Pogostone, 12. Pachypodol, 13. Vicenin-2, 14. Retusin 15. Diosmetin-7-O-β-D-glucopyranoside 16. Icariin (IS).
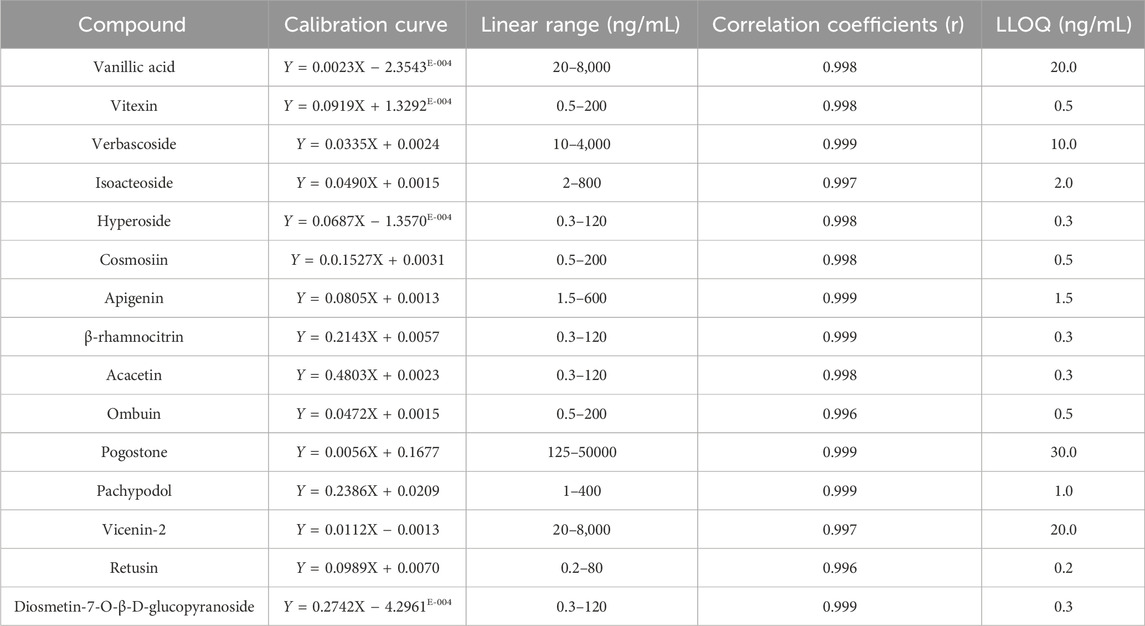
3.3.2 Linearity and LLOQ
The calibration curves, linear ranges, correlation coefficients, and LLOQ for 15 compounds are shown in Table 1. Within their respective concentration ranges, the calibration profiles of these 15 compounds exhibited excellent linearity (r > 0.996). The LLOQ of vanillic acid, vitexin, verbascoside, isoacteoside, hyperoside, cosmosiin, apigenin, β-rhamnocitrin, acacetin, ombuin, pogostone, pachypodol, diosmetin-7-O-β-D-glucopyranoside, vicenin-2, and retusin and were 20.0, 0.5, 10.0, 2.0, 0.3, 0.5, 1.5, 0.3, 0.3, 0.5, 30.0, 1.0, 0.3, 20.0, and 0.2 ng/mL, respectively.
3.3.3 Precision and accuracy
The precision and accuracy of QC samples at three concentrations are provided in Table 2. The RSD of intra- and inter-day were below 11.3%, the RE of intra-day ranged from −12.0%–14.3%, and the inter-day ranged from −6.5%–13.2%. These findings indicated that is accurate and precise to a satisfactory degree.
3.3.4 Extraction recovery and matrix effect
As listed in Table 3, the 15 analytes and the IS displayed extraction recovery and matrix effects within the ranges of 70.6%–104.5%, and 67.4%–104.8%, respectively. These findings revealed that the method’s extraction recovery and matrix effects were accurate and satisfactory.
3.3.5 Stability
As shown in Table 4, the RSD values for the stability of all analytes tested were less than 14.1%, indicating that they were adequately stable across different conditions. This further suggested that the established UPLC-MS/MS method could effectively measure the 15 components in rat plasma.
3.4 PK study
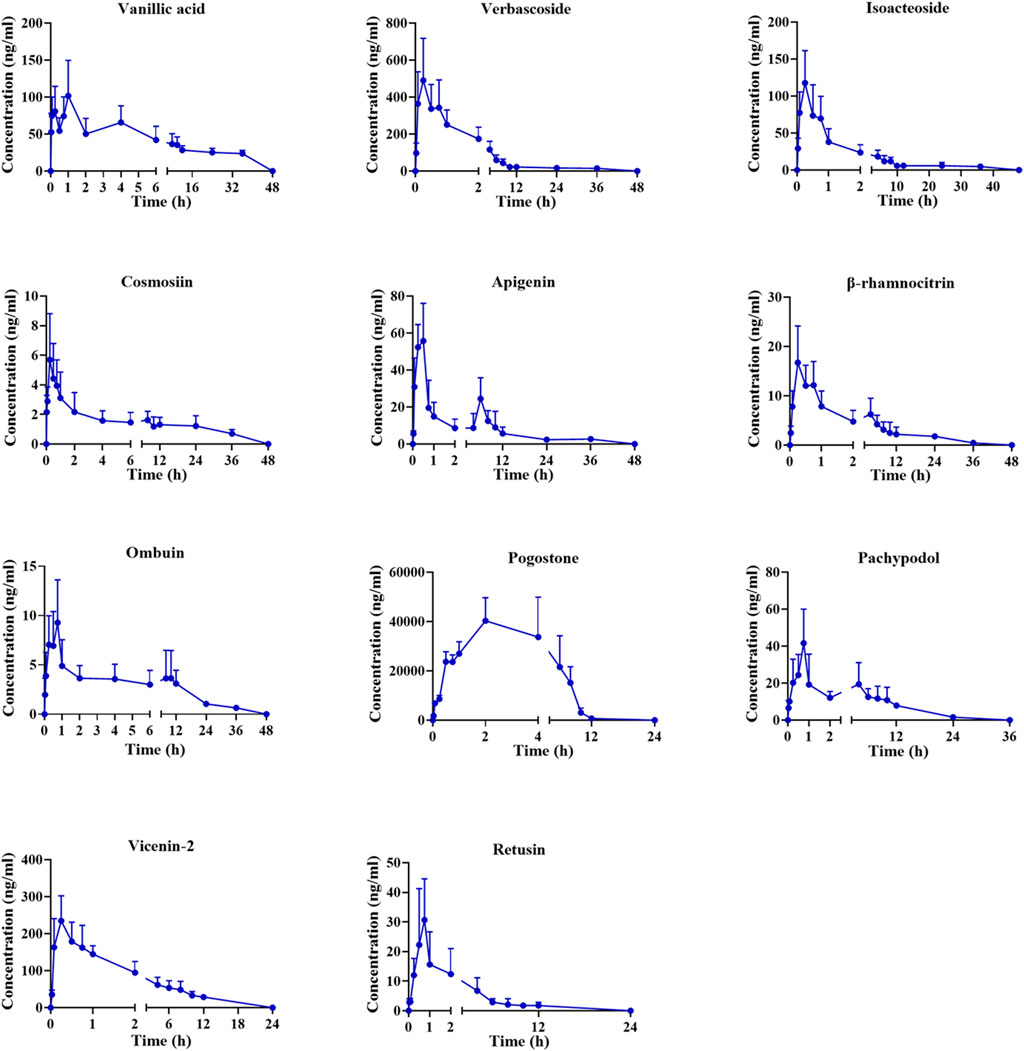
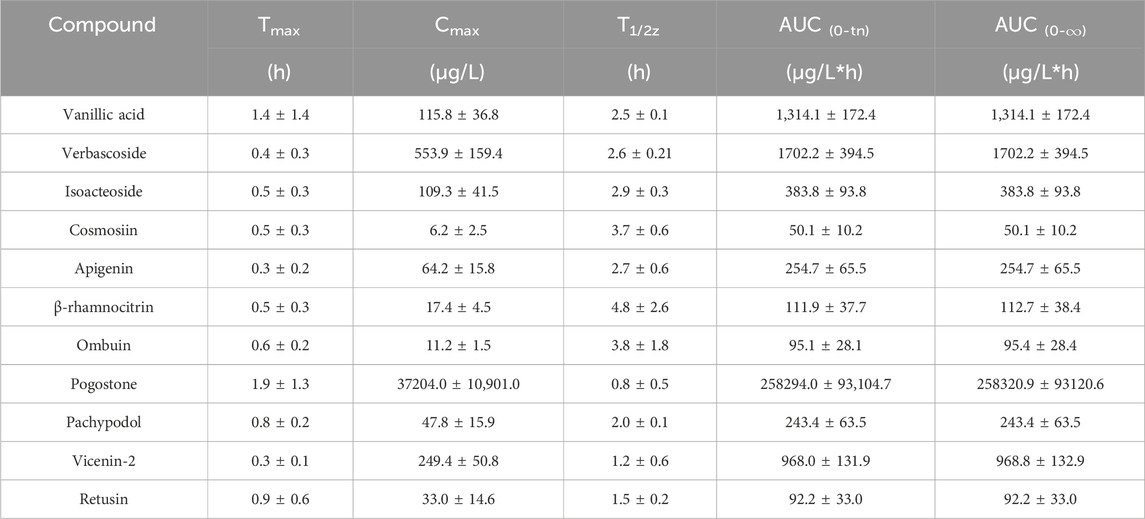
Following oral administration of the PC extract, 15 plasma constituents were analyzed using the validated method. Unfortunately, certain analytes such as vitexin, hyperoside, acacetin, and diosmetin-7-O-β-D-glucopyranoside were only detected in the initial plasma samples, leading to difficulties in obtaining a complete PK profile. As a result, these analytes were not included in our analysis. The mean plasma concentration-time curves for the remaining 11 analytes are displayed in Figure 2, with corresponding PK parameters detailed in Table 5.
The concentration maximum (Tmax) of vanillic acid, verbascoside, isoacteoside, cosmosiin, apigenin, β-rhamnocitrin, ombuin, pogostone, pachypodol, vicenin-2, and retusin were 1.4, 0.4, 0.5, 0.5, 0.3, 0.5, 0.6, 1.9, 0.8, 0.3 and 0.9 h, respectively. The Tmax of all components was within 2 h, indicating that the absorption of these compounds happened quickly. Pogostone was rapidly absorbed in rats after oral administration, potentially attributed to its low polarity profile and small molecule size (Liu et al., 2012; Chen et al., 2013). Verbascoside and isoacteoside could be rapidly absorbed in vivo, which might be related to their relatively large polarity, and previous research has shown comparable PK characteristics. (Zheng et al., 2015). The plasma half-life (T1/2) of pogostone was 0.8 ± 0.5 h, which was relatively short compared to other compounds. It is speculated that this might be due to its distribution and elimination rapidly in rats (Zheng et al., 2015). The area-under-the-curve (AUC) for pogostone significantly exceeded that of the other constituents, suggesting that pogostone exhibited a higher plasma exposure level, correlating with its abundant PC content. Additionally, the concentration maximum (Cmax) of pogostone was 37204.0 ± 10,901.0 ng/mL, coupled with its substantial exposure in vivo, suggesting that pogostone may be the mian active component in the PC extract (Li et al., 2021). Furthermore, the maximum concentration (Cmax) of pogostone was measured at 37204.0 ± 10,901.0 ng/mL.
The apigenin, β-rhamnocitrin, and pachypodol have a double-peak phenomenon in Figure 2, which is likely due to enterohepatic circulation (Wang et al., 2014). Additionally, the absorption of drugs in the gastrointestinal tract is a complex process that is influenced by many physical, chemical, and physiological factors. There are multiple absorption sites in different parts of the gastrointestinal tract, but due to the different permeability of the inner membrane of the cavity to drugs at different sites, the absorption rates and times differ following oral administration. Consequently, absorbed drugs overlap in the blood, creating a bimodal phenomenon (Zhou, 2003).
PK is an indispensable strategy for understanding the behavior in vivo after drug administration, which is of great significance in elucidating the mechanism of action, reducing toxic and side effects, optimizing the drug administration program, and guiding the clinical application of drugs (Jin et al., 2019; Liu et al., 2021). Currently, the PK studies of PC mainly focus on a few components, such as pogostone and verbascoside (Li et al., 2012; Chen et al., 2013; Huo et al., 2016). However, research on these individual components cannot reflect the actual PK characteristics of PC after administration. Therefore, in this study, the simultaneous determination of multiple components in rat plasma and PK study by UPLC-MS/MS, which is crucial in elucidating the pharmacological substance basis, mechanism of action, and optimal dosing regimen of PC, and may provide a reference for the clinical application of PC.
Notably, most of the current PK studies are in normal animals, and there are fewer in model animals. Numerous studies have shown that disease states may cause significant alterations in PK parameters (Chen et al., 2022; Liu et al., 2022; Xiong et al., 2023). Since the drugs are mainly used in the pathological state of the body, it is more meaningful to study the PK of TCM in pathological states (Luo et al., 2014). Moreover, PK and pharmacodynamics (PD) are two important interrelated and inseparable aspects in the field of pharmacological research of TCM. PK/PD modeling is extensively utilized in both preclinical and clinical drug research, which aids in gaining a comprehensive and precise understanding of drug effectiveness over time and plasma concentration. Additionally, it provides a valuable reference for optimizing clinical dosage, improving efficacy, and minimizing adverse effects. Therefore, it is vital to explore the ideas and methods of PK-PD modeling of TCM to elucidate the nature and laws (Zhang Z. et al., 2016; Zhu et al., 2023). Only normal rats were investigated in this study, while the PK of PC in model animals was lacking. In the future, The PK research of PC should focus on studies in disease states and develop PK-PD models to elucidate the pharmacodynamic basis and mechanism of action of PC.
4 Conclusion
In this study, a UPLC-MS/MS method was developed to simultaneously measure 15 components in rat plasma following the oral administration of PC extract. The method has the benefits of uncomplicated sample preparation and the simultaneous analysis of multiple components within a brief timeframe. Furthermore, the method is specific, stable, and reliable. Moreover, PK results are crucial in elucidating the pharmacodynamic material basis, mechanism of action, and optimal dosing regimen of PC, and may provide guidance for the future advancement and clinical application of PC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by The animal experiments were performed strictly in accordance with the Principles of Animal Care and Use approved by The Animal Ethic Review Committee of Tianjin University of Traditional Chinese Medicine (TCM-LAEC2023059). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YZ: Data curation, Formal Analysis, Investigation, Writing–original draft. ZL: Data curation, Formal Analysis, Writing–original draft. GY: Data curation, Formal Analysis, Writing–original draft. MG: Data curation, Formal Analysis, Methodology, Writing–original draft. XC: Data curation, Formal Analysis, Investigation, Writing–original draft. YC: Data curation, Formal Analysis, Investigation, Writing–original draft. HO: Data curation, Formal Analysis, Investigation, Writing–original draft. JH: Conceptualization, Funding acquisition, Resources, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by Tianjin Committee of Science and Technology, China (23ZYJDSS00010).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1293464/full#supplementary-material
Abbreviations
PC, P. cablin; TCM, traditional Chinese medicine; IS, internal standard; ME, methanol; ACN, acetonitrile; FA, formic acid; UPLC-MS/MS, ultra-high performance liquid chromatography/tandem mass spectrometry; MRM, multiple reaction monitoring; RE, relative error; RSD, relative standard deviation; LLOQ, lower limits of quantitation; QC, quality control; Tmax, concentration maximum; T1/2, plasma half-life; Cmax, concentration maximum; AUC, area-under-the-curve.
References
Cao, L., Zhan, S., Ji, X., Zheng, B., Ye, C., Chen, Z., et al. (2021). Research advance in multi-component pharmacokinetics of Chinese herbal extracts in recent five years. China J. Chin. Materia Medica 46, 3270–3287. doi:10.19540/j.cnki.cjcmm.20210310.601
Chen, H., Li, Y., Wu, X., Li, C., Li, Q., Qin, Z., et al. (2013). LC-MS/MS determination of pogostone in rat plasma and its application in pharmacokinetic studies. Biomed. Chromatogr. BMC 27, 1092–1099. doi:10.1002/bmc.2897
Chen, H., Luo, Y., Liu, J., Chen, J., Chen, Y., and Li, X. (2022). Comparative pharmacokinetic study of six lignans in normal and diabetic rats after oral administration of Saururus chinensis extract by LC-MS/MS. Biomed. Chromatogr. BMC 36, e5253. doi:10.1002/bmc.5253
Chen, Y., Luo, Q., Li, S., Li, C., Liao, S., Yang, X., et al. (2020). Antiviral activity against porcine epidemic diarrhea virus of Pogostemon cablin polysaccharide. J. Ethnopharmacol. 259, 113009. doi:10.1016/j.jep.2020.113009
Chinese Pharmacopoeia Committee (2020) Pharmacopoeia of the people’s Republic of China. Beijing: China Medical Science Press.
Huo, F., Sun, J., Shen, J., and Hu, Y. (2007). Study on the combination condiment for stewing fish by raw material of northeast Agastache rugosa. China Condiment, 60–62.
Huo, S., Li, J., Gao, L., Peng, X., Chen, X., Wen, Y., et al. (2016). Absorption, distribution and excretion of acteoside in rats. Chin. J. Hosp. Pharm. 36, 450–454. doi:10.13286/j.cnki.chinhosppharmacyj.2016.06.07
Jin, J., Xue, H., Sun, X., Zan, B., Li, Y., Wang, T., et al. (2019). Simultaneous determination of multiple compounds of Da-Huang-Xiao-Shi decoction in rat plasma by LC-MS/MS and its application in a pharmacokinetic study. J. Pharm. Biomed. analysis 174, 8–18. doi:10.1016/j.jpba.2019.05.050
Junren, C., Xie, X., Li, M., Xiong, Q., Li, G., Zhang, H., et al. (2021). Pharmacological activities and mechanisms of action of Pogostemon cablin Benth: a review. Chin. Med. 16, 5. doi:10.1186/s13020-020-00413-y
Kim, K. H., Beemelmanns, C., Clardy, J., and Cao, S. (2015). A new antibacterial octaketide and cytotoxic phenylethanoid glycosides from Pogostemon cablin (Blanco) Benth. Bioorg. Med. Chem. Lett. 25, 2834–2836. doi:10.1016/j.bmcl.2015.04.094
Kiyohara, H., Ichino, C., Kawamura, Y., Nagai, T., Sato, N., and Yamada, H. (2012). Patchouli alcohol: in vitro direct anti-influenza virus sesquiterpene in Pogostemon cablin Benth. J. Nat. Med. 66, 55–61. doi:10.1007/s11418-011-0550-x
Lai, K., Xiao, J., Wang, H., Qiu, J., Wan, X., Yan, B., et al. (2023). Mechanism and application prospects of microbial fertilizers in regulating quality formation of medicinal plants. China J. Chin. Materia Medica, 1–14. doi:10.19540/j.cnki.cjcmm.20230921.101
Lan, H., and Yuan, H. (2018). Treating cold rhinitis with huodan wan. Clin. J. Chin. Med. 10, 124–125.
Li, M., Chen, S., Wang, X., Li, L., Xu, Y., and Di, L. (2021). UPLC fingerprint of Pogostemonis Herbal and prediction of its potential quality markers based on network pharmacology. Chin. Traditional Herb. Drugs 52, 2665–2677.
Li, S., Feng, Y., Zhou, Y., Liao, C., Su, L., Liu, D., et al. (2023). Pogocablenes A-O, fifteen undescribed sesquiterpenoids with structural diversity from Pogostemon cablin. Phytochemistry 214, 113829. doi:10.1016/j.phytochem.2023.113829
Li, Y., Liang, H., Chen, H., Tan, L., Yi, Y., Qin, Z., et al. (2012). Anti-Candida albicans activity and pharmacokinetics of pogostone isolated from Pogostemonis Herba. Phytomedicine Int. J. phytotherapy Phytopharm. 20, 77–83. doi:10.1016/j.phymed.2012.08.008
Liu, F., Zeng, Y., Dai, P., Huang, K., Zhang, K., Tao, T., et al. (2022). Comparative pharmacokinetics of three bioactive diterpenoids of rabdosia serra extract in normal and con A-induced liver injury rats using UPLC-MS/MS. Front. Pharmacol. 13, 944949. doi:10.3389/fphar.2022.944949
Liu, J., Li, X., Peng, C., Lin, D., Wang, Y., Yang, Y., et al. (2015). 4-nor-β-Patchoulene sesquiterpenoids from the essential oil of Pogostemon cablin. Phytochem. Lett. 12, 27–30. doi:10.1016/j.phytol.2015.02.016
Liu, L., Liu, K., Wen, Y., Zhang, H., Lu, Y., and Yin, Z. (2012). Development of a fully automated on-line solid phase extraction and high-performance liquid chromatography with diode array detection method for the pharmacokinetic evaluation of bavachinin: a study on absolute bioavailability and dose proportionality. J. Chromatogr. B, Anal. Technol. Biomed. life Sci. 893, 21–28. doi:10.1016/j.jchromb.2012.02.020
Liu, X., Zhang, Y., Yang, X., Xu, W., Liu, L., Zhang, P., et al. (2021). Simultaneous determination of twenty-five compounds with anti-inflammatory activity in Spatholobi Caulis by using an optimized UFLC-MS/MS method: an application to pharmacokinetic study. J. Pharm. Biomed. analysis 204, 114267. doi:10.1016/j.jpba.2021.114267
Luo, T., Li, J., and Tong, R. (2014). Application status and prospect of the PK-PD model in Chinese medicine research. China J. Traditional Chin. Med. Pharm. 29, 332–335.
Sandes, S., Zucchi, M., Pinheiro, J., Bajay, M., Batista, C., Brito, F., et al. (2016). Molecular characterization of patchouli (Pogostemon spp) germplasm. Genet. Mol. Res. GMR 15 (1). doi:10.4238/gmr.15017458
Su, J., He, J., Su, Z., Zhou, L., Zeng, Y., Lai, X., et al. (2017). T cell inhibition by pogostone from Pogostemon cablin (Blanco) Benth: in vitro and in vivo immunosuppressive analysis. Mol. Med. Rep. 16, 4511–4520. doi:10.3892/mmr.2017.7147
Wang, Y., Yang, G., Guo, C., Pei, Q., Zhang, R., and Huang, L. (2014). Plasma double-peak phenomenon following oral administration. Chin. J. Clin. Pharmacol. Ther. 19, 341–345.
Xiong, S., Li, X., Chu, H., Deng, Z., Sun, L., Liu, J., et al. (2023). Comparative pharmacokinetics of four major compounds after oral administration of Mori Cortex total flavonoid extract in normal and diabetic rats. Front. Pharmacol. 14, 1148332. doi:10.3389/fphar.2023.1148332
Zhang, C., Liu, T., Yuan, X., Huang, H., Yao, G., Mo, X., et al. (2019). The plastid genome and its implications in barcoding specific-chemotypes of the medicinal herb Pogostemon cablin in China. PloS one 14, e0215512. doi:10.1371/journal.pone.0215512
Zhang, J., Wang, Y., Piao, C., Yu, H., Liu, J., Jiang, L., et al. (2016a). Application and prospect of agastache rugosa in food. Food Res. Dev. 37, 208–211.
Zhang, Q. (2004). Resources of Agastache Rugosa’s development and utilization. Ginseng Res., 10–12. doi:10.19403/j.cnki.1671-1521.2004.03.004
Zhang, Z., Qin, L., Peng, L., Zhang, Q., Wang, Q., Lu, Z., et al. (2016b). Pharmacokinetic-pharmacodynamic modeling to study the antipyretic effect of qingkailing injection on pyrexia model rats. Molecules 21, 317. doi:10.3390/molecules21030317
Zhao, M., Chen, Y., Wang, C., Xiao, W., Chen, S., Zhang, S., et al. (2019). Systems pharmacology dissection of multi-scale mechanisms of action of Huo-Xiang-Zheng-Qi formula for the treatment of gastrointestinal diseases. Front. Pharmacol. 9, 1448. doi:10.3389/fphar.2018.01448
Zheng, D., Chen, W., Ma, S., Shao, J., Wang, J., and Luo, Y. (2015). Simultaneous determination of three phenolic giycosides in Callicarpa nudiflora by UHPLC-MS methods and analysis of their pharmacokinetics in plasma of rats. Chin. Traditional Herb. Drugs 46, 3533–3538. doi:10.7501/j.issn.0253-2670.2015.23.015
Zhou, H. (2003). Pharmacokinetic strategies in deciphering atypical drug absorption profiles. J. Clin. Pharmacol. 43, 211–227. doi:10.1177/0091270002250613
Zhu, Y., Fan, Y., Cao, X., Wei, S., Zhang, M., Chang, Y., et al. (2023). Pharmacokinetic-pharmacodynamic (PK/PD) modeling to study the hepatoprotective effect of Perilla Folium on the acute hepatic injury rats. J. Ethnopharmacol. 313, 116589. doi:10.1016/j.jep.2023.116589
Keywords: Pogostemon cablin extract, prototype components, pharmacokinetic, rat plasma, UPLC-MS/MS
Citation: Zhu Y, Ouyang H, Lv Z, Yao G, Ge M, Cao X, Chang Y and He J (2024) Simultaneous determination of multiple components in rat plasma by UPLC-MS/MS for pharmacokinetic studies after oral administration of Pogostemon cablin extract. Front. Pharmacol. 15:1293464. doi: 10.3389/fphar.2024.1293464
Received: 13 September 2023; Accepted: 06 May 2024;
Published: 22 May 2024.
Edited by:
Ling Ye, Southern Medical University, ChinaReviewed by:
Xie-an Yu, Shenzhen Institute For Drug Control, ChinaBeatriz Garcia Mendes, Federal University of Santa Catarina, Brazil
Copyright © 2024 Zhu, Ouyang, Lv, Yao, Ge, Cao, Chang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun He, aGVqdW42NzNAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yameng Zhu
Yameng Zhu Huizi Ouyang1,2†
Huizi Ouyang1,2† Zhenguo Lv
Zhenguo Lv Yanxu Chang
Yanxu Chang Jun He
Jun He