- 1Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children, Sichuan University, Chengdu, China
Objectives: There is no curative treatment for childhood obesity. We aim to synthesize published Randomized Controlled Trials (RCTs) evidence on the efficacy of exenatide in obese children and adolescents.
Methods: We conducted a comprehensive search and analysis of relevant studies in popular databases such as PubMed, Web of Science, Embase, and Cochrane Library. Our focus was on RCTs that examined the effectiveness of exenatide for treating obesity in children. We primarily assessed changes in body weight, body mass index (BMI), fasting plasma glucose (FPG), or HbA1c levels. Additionally, we considered any adverse events reported during the treatment period, with particular attention to hypoglycemia. To evaluate the quality of RCTs included in our study, we employed the Cochrane bias assessment tool.
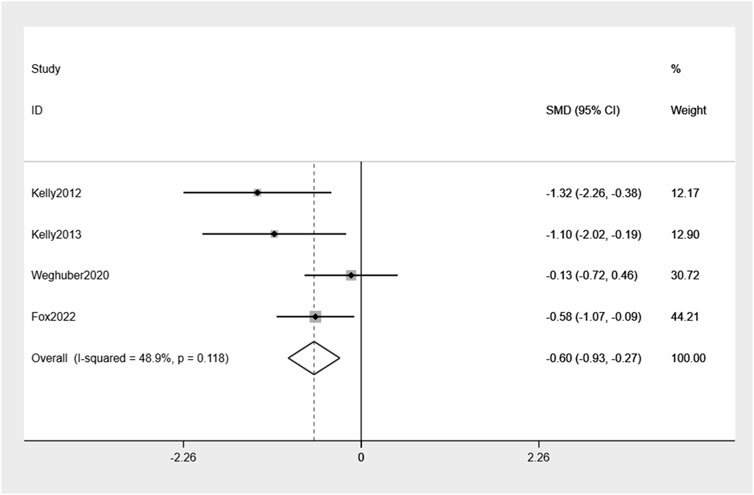
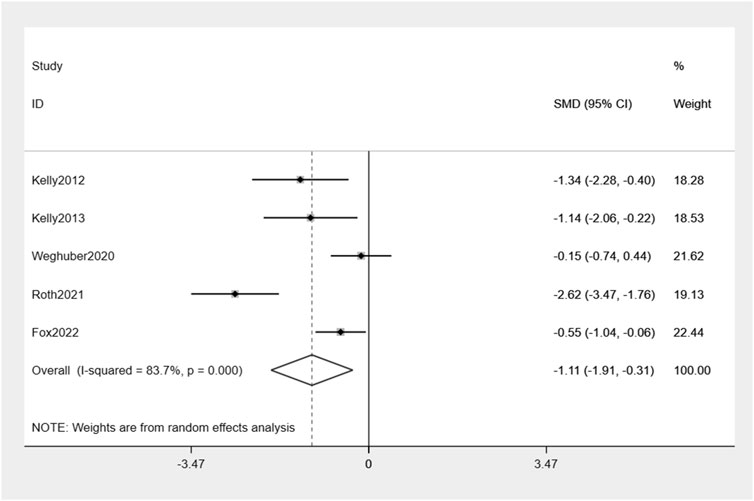
Results: Five studies met the inclusion criteria. A group of 100 children were assigned to receive treatment with exenatide. Compared with controls, exenatide therapy reduced body weight and BMI by −0.6% (95% CI −0.93, −0.27), −1.11% (95% CI −1.91, −0.31), respectively. Undesirable consequences encompass gastrointestinal symptoms, with the majority of instances being characterized by mild severity.
Conclusion: Exenatide demonstrates efficacy in the treatment of pediatric and adolescent obesity.
Systematic Review Registration: PROSPERO https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=413706
1 Introduction
In the last 5 decades or so, the prevalence of obesity has increased worldwide, according to the World Health Organization (WHO), over 650 million adults are obese, three times more than the rate in 1975, and another 1.3 billion people are overweight worldwide (Carreira et al., 2022). The COVID-19 pandemic made the situation more serious, with more than 50% of individuals with obesity reporting increased weight during the lockdown (Sideli et al., 2021). Basically, the rate of increase in childhood obesity has been higher than that in adults and it is expected that there will be 108 million obese young people worldwide (Afshin et al., 2017). Excessive body weight frequently leads to long-term health complications. Children can develop diabetes mellitus (Suárez et al., 2023), cardiovascular disease (Singh et al., 2013), many cancers (Lauby-Secretan et al., 2016; Afshin et al., 2017), and depression (Oz and Kıvrak, 2023). The health problem of obesity severely affects the quality of life and presents a more serious health threat. Hence, it is crucial to ensure timely and consistent weight reduction during childhood.
The lack of weight-loss treatments goes against the idea of worldwide health equality. The initial approach to addressing childhood obesity entails making changes to one’s lifestyle. Which means children need help to choose better foods and find ways to exercise more. Indeed, long-term weight loss maintenance through lifestyle changes is elusive for most obese children. This option can be further advanced to drug treatment, and among the most hopeful categories of medications is the agonist for glucagon-like peptide-1 receptor agonist (GLP-1RA), which reduces appetite through activation of GLP-1 receptors in the hypothalamus and slows down the rate at which a person’s stomach empties, promotes satiety, and reduces food intake (Bojanowska, 2005; Alhadeff and Grill, 2014; Burcelin and Gourdy, 2017). Besides weight loss, GLP-1 RA has been shown to improve glucose control by the glucose-dependent increased in postprandial insulin secretion, and inhibition of glucagon secretion improves many obesity-associated risk factors and complications, such as impaired glucose tolerance, reduced insulin sensitivity, elevated blood pressure, impaired vascular function, and increased inflammatory response (Koska et al., 2010; Okerson et al., 2010; Kelly et al., 2013).
Exenatide, a member of the GLP-1RA class, was initially developed and subsequently granted approval for managing Type 2 diabetes (T2DM) in adults and have also been shown to reduce weight in adults with obesity (Vilsbøll et al., 2012). The Core DURATION trial series was conducted from 2008 to 2013, the duration of exenatide treatment ranges from 24 to 30 weeks, and the average weight loss of patients during treatment ranged from 2.0 to 3.7 kg (Drucker et al., 2008; Bergenstal et al., 2010; Diamant et al., 2010; Blevins et al., 2011; Russell-Jones et al., 2012; Buse et al., 2013). Multiple meta-analyses of RCTs in adults with T2DM and/or obesity have shown benefits of exenatide for glycemic control and weight loss (Vilsbøll et al., 2012; Su et al., 2016; Monami et al., 2017; Waldrop et al., 2018). The use of liraglutide, a GLP-1 agonist, has been approved by the FDA for treating children who have T2DM(Bacha, 2019). Additional evidence is required to support the utilization of exenatide in pediatric patients.
This study aims to consolidate the findings from various randomized controlled trials (RCTs) and conduct a meta-analysis to evaluate the effectiveness and safety of exenatide in managing obesity among children.
2 Materials and methods
The review of existing literature was conducted in accordance with the guidelines provided by PRISMA, which are designed for systematic reviews and meta-analyses (Moher et al., 2009).
2.1 Eligibility criteria
We devised a framework called “PICOS” (Patient, Intervention, Comparison, Outcome and Study design) to establish the criteria for eligibility: 1. Population: Pediatric patients (≤18 years) with a diagnosis of obesity as defined by the study authors; 2. Intervention: exenatide as the intervention group. Medications that affect blood sugar or other parameters of metabolic syndrome should not be taken within 3 months prior to screening; 3. Comparison: control group receiving either placebo or standard treatment. Both the intervention and control groups take exenatide as an adjunct to diet and exercise; 4. Outcome: clinical trials meeting the eligibility criteria were required to document results for a minimum of one among the subsequent endpoints: HbA1c, FDP, body weight or BMI change; 5. Study design: RCTs. The exclusion criteria encompassed studies that were not randomized controlled trials (non-RCTs), as well as systematic reviews, meta-analyses, narrative reviews, editorials, abstracts, reports, and case series.
2.2 Information sources and search strategy
PubMed, Web of Science, Embase, and Cochrane Library were searched up until 6 March 2023, with no cut-off criteria for publication date, using the following search terms: (“Exenatide”) AND (“Pediatric Obesity”) AND (“Children”) AND (“Adolescent”). Keywords and Mesh/Emtree thesauri were applied to establish search strategies for each database. Two different investigators (C.B. and Z.X.Y.) independently evaluated the search results. A search strategy example is depicted in Supplementary Figure S1.
2.3 Study selection
After exclusion of duplicates, two reviewers (C.B. and Z.C.) independently screened the title and abstract. In the process of title and abstract screening, we carefully chose articles that seemed to fulfill the eligibility criteria. In case of any discrepancies between the two reviewers, they were resolved through discussion or referred to the corresponding author (L.X.H.) for final judgment.
2.4 Data extraction and quality assessment
The provided RCTs were analyzed to obtain the subsequent details: primary author, year of publication, number of participants, demographic characteristics of the study population, and geographical location, blood glucose (i.e., HbA1c in percent and FPG in mg/dL) and weight measures (i.e., weight in kilogram, BMI in kg/m2) with corresponding standard deviations (SDs) at baseline and follow-up, change-from-baseline blood glucose and weight outcomes with corresponding SDs, difference in change-from-baseline blood glucose and weight outcomes with corresponding confidence intervals (CIs) or p values, target drug dose and follow-up duration, the adverse events, and incidence of hypoglycemic episodes. Two reviewers (Z.X.Y. and X.D.Q.) extracted data from each trials independently. Missing SDs at follow-up were imputed by reported SDs at baseline, or vice versa (Cumpston et al., 2019). The risk of bias assessment for the included RCTs was conducted by two reviewers (C.B. and Z.X.Y.) utilizing the Cochrane Collaboration tool (Sterne et al., 2019). Disputes were settled through deliberation or assessed by the corresponding author (L.X.H.).
2.5 Meta-analysis
The effects of exenatide on difference Among change-from-baseline body weight, BMI, HbA1c, FPG outcomes were summarized by employing (i) statistical models with random effects and weighted variances to account for variations in individual study findings; (ii) statistical models with fixed effects and weighted variances assuming consistent effect estimates across studies. The findings of meta-analyses were presented in the form of mean differences (M.D.) for continuous outcomes and relative risks (R.R.) for dichotomous outcomes. The Hartung-Knapp technique was utilized to update the 95% confidence intervals (C.I.s) for the effects. The inconsistency index (I2) was used to assess heterogeneity between studies, with studies having an I2 value greater than 50% considered to have significant heterogeneity. Funnel plots and Egger’s regression tests were employed to examine asymmetry in the effect estimates at the study level. Sensitivity analyses using leave-one-out methods were conducted to test the robustness of the meta-analysis models. Subgroup analysis was performed to explore potential sources of heterogeneity. Statistical analyses were carried out using STATA software from Stata-Corp LLC located in College Station, TX, United States of America.
3 Results
3.1 Summary of study characteristics
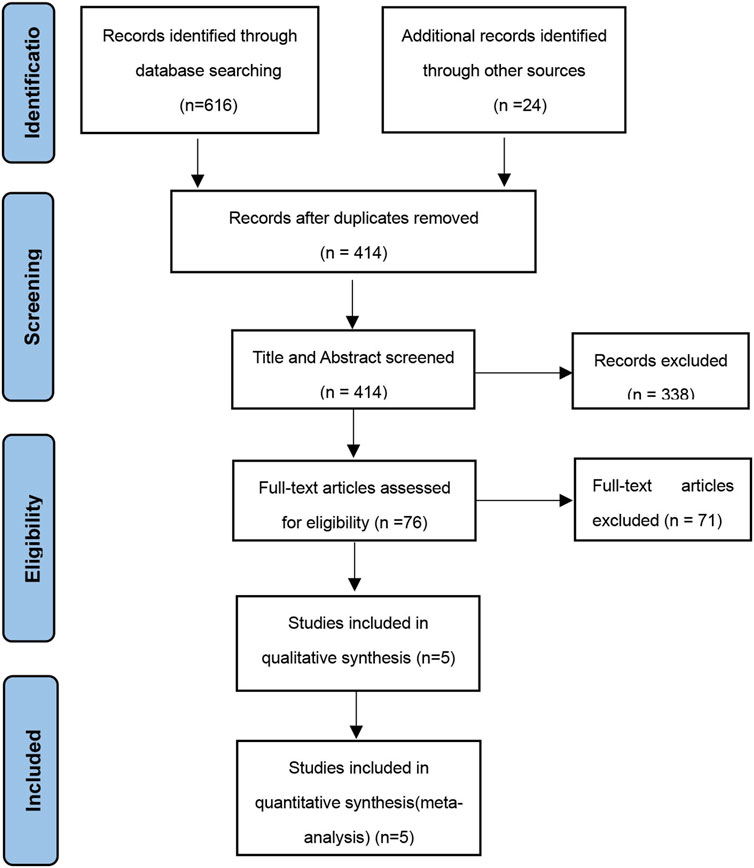
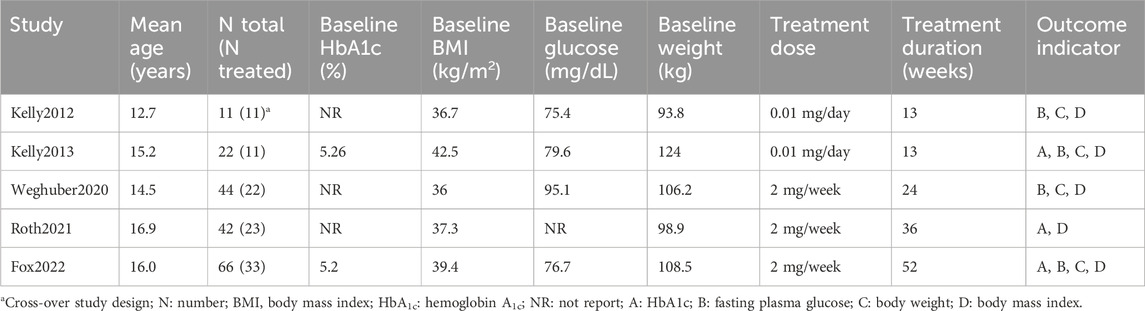
The study selection process is visually represented in Figure 1 through a flow diagram. Five RCTs met the criteria for inclusion in the systematic review (Kelly et al., 2012; Kelly et al., 2013; Weghuber et al., 2020; Roth et al., 2021; Fox et al., 2022). The key features of these studies are outlined in Table 1. Only one study was conducted in Europe (Weghuber et al., 2020), and the others in the United States. All the studies were published from 2012 to 2022. Mean ages of children ranged from 12.7 to 16.9 years old, treatment durations from 11 to 52 weeks, and study sizes from 11 to 66 children. Across all studies, 100 children were allocated to exenatide therapy. Treatment dose of five mcg, twice per day in two studies (EX10BID) (Kelly et al., 2012; Kelly et al., 2013), 2 mg once-weekly in three studies (EX2QW) (Weghuber et al., 2020; Roth et al., 2021; Fox et al., 2022). The participants in one study were patients with intracranial tumors after hypothalamic injury or children with hypothalamic obesity (Weghuber et al., 2020). One clinical trial utilized an open-label, crossover design (Kelly et al., 2012), while the remaining studies followed a double-blind, randomized design with placebo control. We expect positive changes in the health status of pediatric obese patients treated with exenatide in these anticipate observing a decrease in the occurrence of unfavorable incidents across five conducted studies, providing efficacy and safety results.
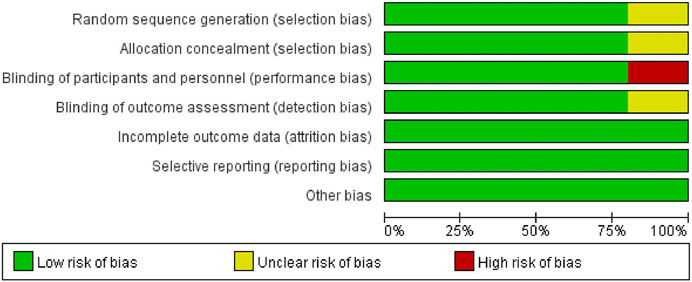
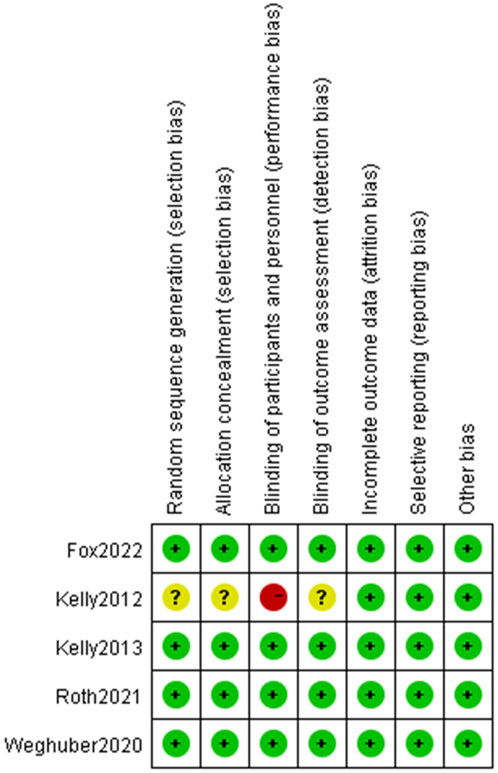
The assessment of potential bias in the studies included can be observed in Figure 2 and Figure 3. The studies conducted by Kelly (Kelly et al., 2012) was rated as poor quality due to open-label, crossover designing. While it was presumed that the remaining studies possessed a high level of quality.
3.2 Effect of exenatide on primary outcomes
The examination of the four randomized controlled trials indicated a slight reduction in body weight, amounting to −0.60% (95% CI: −0.93, −0.27), compared to placebo (Figure 4). Mild heterogeneity between the studies was observed (I2 = 48.9%).
The analysis combining the results of all five studies revealed a significant decrease in BMI by −1.11% (95% CI: -1.91, −0.31) compared to placebo (Figure 5). There was moderate heterogeneity in effect estimates among the studies (I2 = 83.7%).
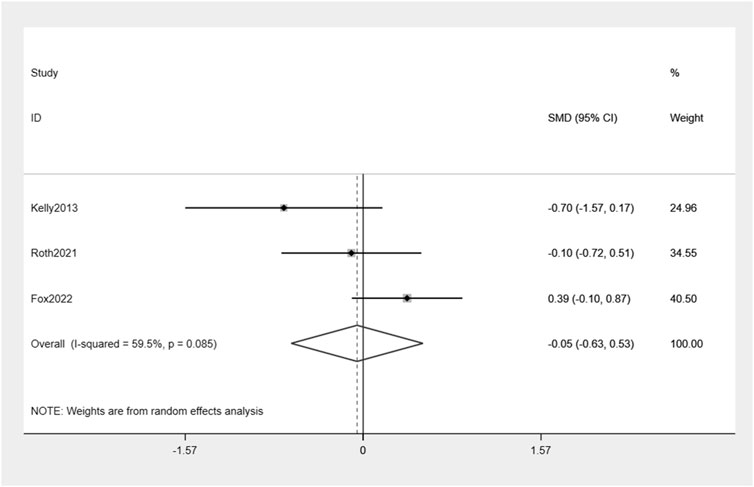
Exenatide treatment did not result in a significant reduction in HbA1c levels compared to placebo (−0.05%, 95% CI: -0.63, 0.53) (Figure 6). Moderate heterogeneity between the studies was observed (I2 = 59.5%).
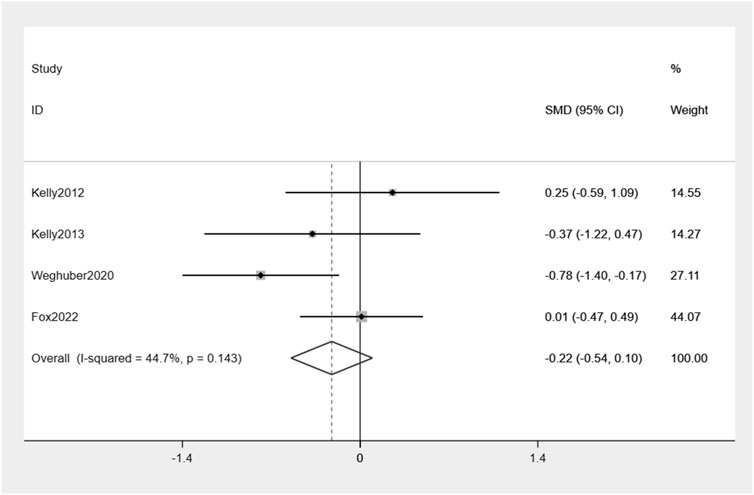
Similarly, exenatide exhibits no significant reduction in FPG when compared to the placebo group: −0.22% (95% CI: −0.54, 0.10) (Figure 7). There was a moderate level of variability in effect estimates across the studies (I2 = 44.7%).
3.3 Sensitivity analyses
Leave-one-out analyses are depicted in Supplementary Figures S2, S3. For BMI and HbA1c, exclusion of any single arm did not significantly alter heterogeneity among studies.
3.4 Subgroup analysis of BMI
We used BMI for subgroup analysis among the participants based on baseline body weight, baseline BMI, obesity type and treatment does.
The results showed that participants with baseline body weight<100 kg (34 children) were associated with a greater decrease in percentage of BMI change by −1.99% (95% CI: -3.24, −0.74) compared to participants with baseline body weight>100 kg (67 children): -0.52% (95% CI: −0.99, −0.06) (Supplementary Figure S4).
Participants with baseline BMI>38 kg/m2 (45 children)were associated with a slight decrease in percentage of BMI change by −0.71% (95% CI: -1.23, −0.20), while there was no significant reduction in BMI among participants with baseline BMI<38 kg/m2 (56 children): -1.34% (95% CI: -2.86, 0.17) (Supplementary Figure S5).
Children with hypothalamic obesity (23 children) were associated with a greater decrease in percentage of BMI change by −2.62% (95% CI: -3.47, −1.76). While an overall reduction of BMI in obesity children without hypothalamic (78 children) injure by −0.68% (95% CI: -1.17, −0.19) (Supplementary Figure S6).
Participants receiving treatment doses of EX10BID (23 children) were associated with a greater decrease in the percentage of BMI change by −1.24% (95% CI: -1.89, −0.58). While participants who used with EX2QW (78 children) did not show a significant decrease in BMI: -1.06% (95% CI: -2.28, 0.16) (Supplementary Figure S7).
3.5 Subgroup analysis of body weight
We used body weight for subgroup analysis among the participants based on baseline body weight, baseline BMI and treatment does.
The results showed that participants with baseline body weight<100 kg (44 children) were associated with a greater decrease in percentage of body weight change by −1.32% (95% CI: -2.26, −0.38) compared to participants with baseline body weight>100 kg (34 children) by −0.50% (95% CI: −0.85, −0.15) (Supplementary Figure S8).
Participants with baseline BMI>38 kg/m2 (45 children) were associated with a greater decrease in percentage of body weight change by −0.70% (95% CI: -1.13, −0.26), while participants with baseline BMI<38 kg/m2 (33 children) does not decrease in body weight: -0.47% (95% CI: -0.97, 0.04) (Supplementary Figure S9).
Participants receiving treatment doses of EX10BID (23 children) showed a greater decrease in the percentage of body weight change by −1.21% (95% CI: -1.86, −0.55). While participants who used with EX2QW (55 children) exhibited a smaller decrease in body weight by −0.39% (95% CI: -0.77, −0.02) (Supplementary Figure S10).
3.6 Reported adverse events
All five studies provided data on the adverse events associated with exenatide therapy. Gastrointestinal symptoms, such as nausea, vomiting, and diarrhea, were identified as the most prevalent side effects. The majority of these instances were classified as mild in intensity. Notably, there was no statistically significant disparity observed in the occurrence rate between individuals receiving exenatide treatment and those administered a placebo. Only one serious adverse event in the exenatide treatment about a participant was hospitalized for chemical dependency treatment after a drug (not related to the study) and alcohol overdose (Fox et al., 2022). And none of the five studies documented any instances of hypoglycemia.
4 Discussion
In this systematic review and meta-analysis, the evidence from five RCTs was combined to analyze the efficacy and safety of exenatide in obese children Compared with previous systematic reviews (Su et al., 2016; Tsapas et al., 2021; Zhang et al., 2021; Guo et al., 2022), this research examined the effects of exenatide on children who are overweight or obese but do not have diabetes.
Currently, few drugs are approved for childhood obesity in children, and the evidence for treatment options is inadequate (Cornejo-Estrada et al., 2023b). GLP-1 agonists have been authorized for managing adult T2DM and obesity, while liraglutide has obtained approval for treating children with T2DM and obesity. Despite the structural similarity between exenatide and liraglutide, variations in their pharmacokinetic and pharmacodynamic properties lead to distinct effects on reducing hyperglycemia and weight. Liraglutide, a GLP-1 receptor agonist, exhibits a remarkable similarity of over 97% in its amino acid sequence to human GLP-1. It possesses an extended half-life of approximately 13 h. Conversely, exenatide has a shorter half-life compared to liraglutide, being only half as long, and demonstrates only 53% homology in its amino acid sequence with human GLP-1 (Lund et al., 2014). Exenatide and liraglutide have been demonstrated to be effective in numerous clinical studies for patients with T2DM(Bergenstal et al., 2010; Hall et al., 2013). In a head-to-head trial, patients who received liraglutide demonstrated greater weight loss (3.9 vs. 2.7 kg) compared to individuals treated with exenatide (Buse et al., 2013). However, exenatide and liraglutide have been demonstrated to be similar effective in promoting weight loss in numerous studies (Scott et al., 2013; McAdam-Marx et al., 2016; Saunders et al., 2016; Fadini et al., 2019). The current research extensively examined the use of exenatide in overweight children, offering a comprehensive analysis. The results demonstrate that treatment with exenatide is associated with a reduction in body weight and BMI among pediatric patients. These findings are consistent with those of Gou H et al., whose study also showed that liraglutide use was associated with lower body weight and BMI in pediatric patients (Gou et al., 2023). The results of another meta-analysis confirmed that there was no clinically significant difference in weight loss and BMI between liraglutide use and pediatric patients. However, it should be noted that only three studies were included in the systematic review, all of which were conducted in the United States (Cornejo-Estrada et al., 2023a). In a post hoc analysis of the randomized, placebo-controlled, SCALE Teens trial investigating predictive characteristics of liraglutide versus placebo for achieving a reduction in BMI of ≥5% and ≥10% after 56 weeks (Bensignor et al., 2023), it was found that liraglutide was more effective than placebo in reducing BMI by ≥ 5% and ≥10%. In the randomized, double-blind trial involving adolescents with obesity, the use of liraglutide in combination with lifestyle therapy resulted in a significantly greater reduction in BMI and body weight compared to placebo combined with lifestyle therapy. (Kelly et al., 2020).
In relation to effectiveness, there was no observed decrease in HbA1c and FDP levels among obese children treated with exenatide. Overall, exenatide reduced body weight by −0.6% and reduced BMI by −1.11%; however, higher mean baseline values of BMI corresponded to larger treatment effects on BMI, but lower mean baseline values of body weight corresponded to larger treatment effects on body weight change. Furthermore, the effect of both body weight loss was much larger in children treaded with EX10BID than EX2QW (−1.21% vs. −0.39%), and the similar effect on BMI (−1.24% vs. −1.06%). Interestingly, effects of exenatide on BMI were much more obvious among children with hypothalamic obesity than children without hypothalamic obesity (-2.62% vs. −0.68%).
In relation to the aspect of safety, children exhibited good tolerance towards exenatide therapy. Only few adverse events reported across the RCTs. And no instances of hypoglycemia have been documented. The prevalent adverse effects observed were gastrointestinal symptoms, particularly nausea, vomiting, abdominal discomfort, and diarrhea. These manifestations frequently resolved spontaneously without any intervention. These findings align with previous research conducted on adult populations (Vilsbøll et al., 2012; Eng et al., 2014; Su et al., 2016; Afshin et al., 2017). A small number of headaches and injection site reactions were also reported, but there was no significant difference between the two groups, in keeping with studies in adult patients (Zaccardi et al., 2016).
Our research findings indicate that a greater percentage of participants achieved weight loss and reduction in BMI with EX10BID compared to EX2QW, aligning with the outcomes observed in previous meta-analyses (Wang et al., 2019). Therefore, the findings indicated a possible correlation between drug dosage and weight loss. It was unexpected that there was no significant decrease in HbA1c levels among obese children. This might reflect the lower pharmacokinetic effect of EX2QW than EX10BID, as only one study with EX10BID was included in HbA1c analysis. A similar pattern was seen in FDP change. Furthermore, one study with EX2QW described that adjusted estimated treatment difference in percent change in HbA1c was 0 (95% CI: −0.1 to 0.1), and in FPG was 0.5 (95% CI: −0.26–3.6), perhaps this is due to the fact that this study focues on eight-loss maintenance in adolescents with severe obesity design, high baseline BMI (39.4 ± 4.9 kg/m2) and low baseline FDP (78.5 ± 10.9 mg/dL) were the characteristics of participants in the exenatide-treated group enrolled in this study (Fox et al., 2022). In adults, weight loss may be greater in those with obesity-only (−2.85 and −4.47 kg) than in those with T2DM (−2.49 and −1.4 to −1.6 kg) (Chadda et al., 2021). EX2QW, the extended-release version of exenatide, has demonstrated efficacy and safety in individuals diagnosed with T2DM, offering enhanced convenience and improved patient adherence (Guo, 2016), resulting in a comparable reduction in body weight as that observed with EX10BID (Brunton and Davidson, 2016). In our investigation, EX10BID exhibited a notable advantageous impact on the management of body weight and alterations in BMI. This finding aligns with the meta-analysis conducted by Na Su (Su et al., 2016), both EX20BID and EX10BID were found to have a significant positive impact on weight loss in comparison to the placebo. However, this meta-analysis did not find sufficient evidence to support the use of once-weekly exenatide for non-diabetic individuals. Different clinical trials have targeted different patients, and more RCTs are needed in future to validate different doses of exenatide in non-diabetic obese and diabetic patients.
To our current knowledge, this research marks the first-ever meta-analysis performed to evaluate the efficacy and safety of exenatide in children diagnosed with obesity.
It is important to acknowledge certain constraints associated with this meta-analysis. First, differences in study design, baseline obesity severity and treatment dose may contribute to heterogeneity in the meta-analysis outcomes. Furthermore, the inclusion of a mere five RCTs was constrained by the small size of the samples. Finally, a cost analysis was not addressed by this systematic review and meta-analysis because there was insufficient data.
In conclusion, our comprehensive analysis revealed notable advantages of exenatide in obese children. Nevertheless, the existing literature was constrained by potential bias and a limited number of participants. More RCTs are needed in the future to confirm the efficacy and safety of this drug in treating pediatric obesity.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
BC: Writing–original draft, Writing–review and editing. ZZ: Writing–original draft, Writing–review and editing. XZ: Writing–original draft, Writing–review and editing. DX: Writing–original draft, Writing–review and editing. XL: Writing–original draft, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Science Foundation of China (82071353).
Acknowledgments
We extend our gratitude to the researchers and study participants for their valuable contributions. Additionally, we express our sincere appreciation to your esteemed journal for providing insightful feedback on our manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1290184/full#supplementary-material
References
Afshin, A., Forouzanfar, M., Reitsma, M., Sur, P., Estep, K., Lee, A., et al. (2017). Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 377 (1), 13–27. doi:10.1056/NEJMoa1614362
Alhadeff, A., and Grill, H. (2014). Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307 (4), R465–R470. doi:10.1152/ajpregu.00179.2014
Bacha, F. (2019). FDA approval of GLP-1 receptor agonist (liraglutide) for use in children. Lancet Child. Adolesc. Health 3 (9), 595–597. doi:10.1016/s2352-4642(19)30236-6
Bensignor, M., Bramante, C., Bomberg, E., Fox, C., Hale, P., Kelly, A., et al. (2023). Evaluating potential predictors of weight loss response to liraglutide in adolescents with obesity: a post hoc analysis of the randomized, placebo-controlled SCALE Teens trial. Pediatr. Obes. 18 (9), e13061. doi:10.1111/ijpo.13061
Bergenstal, R., Wysham, C., Macconell, L., Malloy, J., Walsh, B., Yan, P., et al. (2010). Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet London, Engl. 376 (9739), 431–439. doi:10.1016/s0140-6736(10)60590-9
Blevins, T., Pullman, J., Malloy, J., Yan, P., Taylor, K., Schulteis, C., et al. (2011). DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J. Clin. Endocrinol. metabolism 96 (5), 1301–1310. doi:10.1210/jc.2010-2081
Bojanowska, E. (2005). Physiology and pathophysiology of glucagon-like peptide-1 (GLP-1): the role of GLP-1 in the pathogenesis of diabetes mellitus, obesity, and stress. Obes. stress 11 (8), RA271–278.
Brunton, S., and Davidson, J. (2016). Exenatide once weekly: a review of Pharmacology and treatment considerations in type 2 diabetes. Clin. Ther. 38 (3), 582–594. doi:10.1016/j.clinthera.2016.01.014
Burcelin, R., and Gourdy, P. (2017). Harnessing glucagon-like peptide-1 receptor agonists for the pharmacological treatment of overweight and obesity. Obes. Rev. 18 (1), 86–98. doi:10.1111/obr.12465
Buse, J., Nauck, M., Forst, T., Sheu, W., Shenouda, S., Heilmann, C., et al. (2013). Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet London, Engl. 381 (9861), 117–124. doi:10.1016/s0140-6736(12)61267-7
Carreira, D., Robison, J., Robison, S., and Fitch, A. (2022). Obesity treatment in orthopaedic surgery. J. Am. Acad. Orthop. Surg. 30 (24), e1563–e1570. doi:10.5435/jaaos-d-21-01083
Chadda, K., Cheng, T., and Ong, K. (2021). GLP-1 agonists for obesity and type 2 diabetes in children: systematic review and meta-analysis. J. Am. Acad. Orthop. Surg. 22 (6), e13177. doi:10.1111/obr.13177
Cornejo-Estrada, A., Nieto-Rodríguez, C., León-Figueroa, D., Moreno-Ramos, E., Cabanillas-Ramirez, C., and Barboza, J. (2023a). Efficacy of liraglutide in obesity in children and adolescents: systematic review and meta-analysis of randomized controlled trials. Child. Basel, Switz. 10 (2), 208. doi:10.3390/children10020208
Cornejo-Estrada, A., Nieto-Rodríguez, C., León-Figueroa, D., Moreno-Ramos, E., Cabanillas-Ramirez, C., and Barboza, J. J. C. (2023b). Efficacy of liraglutide in obesity in children and adolescents: systematic review and meta-analysis of randomized controlled trials. Child. (Basel) 10 (2), 208. doi:10.3390/children10020208
Cumpston, M., Li, T., Page, M., Chandler, J., Welch, V., Higgins, J., et al. (2019). Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 10, ED000142. doi:10.1002/14651858.Ed000142
Diamant, M., Van Gaal, L., Stranks, S., Northrup, J., Cao, D., Taylor, K., et al. (2010). Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet London, Engl. 375 (9733), 2234–2243. doi:10.1016/s0140-6736(10)60406-0
Drucker, D., Buse, J., Taylor, K., Kendall, D., Trautmann, M., Zhuang, D., et al. (2008). Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet London, Engl. 372 (9645), 1240–1250. doi:10.1016/s0140-6736(08)61206-4
Eng, C., Kramer, C., Zinman, B., and Retnakaran, R. J. L. (2014). Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet 384 (9961), 2228–2234. doi:10.1016/s0140-6736(14)61335-0
Fadini, G., Bonora, B., Lapolla, A., Fattor, B., Morpurgo, P., Simioni, N., et al. (2019). Comparative effectiveness of exenatide once-weekly versus liraglutide in routine clinical practice: a retrospective multicentre study and meta-analysis of observational studies. Diabetes, Obes. metabolism 21 (5), 1255–1260. doi:10.1111/dom.13623
Fox, C., Clark, J., Rudser, K., Ryder, J., Gross, A., Nathan, B., et al. (2022). Exenatide for weight-loss maintenance in adolescents with severe obesity: a randomized, placebo-controlled trial. Obes. (Silver Spring) 30 (5), 1105–1115. doi:10.1002/oby.23395
Gou, H., Zhai, Y., and Guo, J. (2023). Efficacy and safety of liraglutide for weight management in children and adolescents: a systematic review and meta-analysis of randomized controlled trials. Eur. J. Pediatr. 182 (11), 5095–5108. doi:10.1007/s00431-023-05186-8
Guo, X. (2016). The value of short- and long-acting glucagon-like peptide-1 agonists in the management of type 2 diabetes mellitus: experience with exenatide. Curr. Med. Res. Opin. 32 (1), 61–76. doi:10.1185/03007995.2015.1103214
Guo, X., Zhou, Z., Lyu, X., Xu, H., Zhu, H., Pan, H., et al. (2022). The antiobesity effect and safety of GLP-1 receptor agonist in overweight/obese patients without diabetes: a systematic review and meta-analysis. Diabetes A Syst. Rev. Meta-Analysis 54 (7), 458–471. doi:10.1055/a-1844-1176
Hall, G., McMahon, A., Dain, M., Wang, E., and Home, P. (2013). Primary-care observational database study of the efficacy of GLP-1 receptor agonists and insulin in the UK. Diabet. Med. a J. Br. Diabet. Assoc. 30 (6), 681–686. doi:10.1111/dme.12137
Kelly, A., Auerbach, P., Barrientos-Perez, M., Gies, I., Hale, P., Marcus, C., et al. (2020). A randomized, controlled trial of liraglutide for adolescents with obesity. N. Engl. J. Med. 382 (22), 2117–2128. doi:10.1056/NEJMoa1916038
Kelly, A., Metzig, A., Rudser, K., Fitch, A., Fox, C., Nathan, B., et al. (2012). Exenatide as a weight-loss therapy in extreme pediatric obesity: a randomized, controlled pilot study. Control. pilot study 20 (2), 364–370. doi:10.1038/oby.2011.337
Kelly, A., Rudser, K., Nathan, B., Fox, C., Metzig, A., Coombes, B., et al. (2013). The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial. Clin. trial 167 (4), 355–360. doi:10.1001/jamapediatrics.2013.1045
Koska, J., Schwartz, E., Mullin, M., Schwenke, D., and Reaven, P. J. D. c. (2010). Improvement of postprandial endothelial function after a single dose of exenatide in individuals with impaired glucose tolerance and recent-onset type 2 diabetes. Diabetes Care 33 (5), 1028–1030. doi:10.2337/dc09-1961
Lauby-Secretan, B., Scoccianti, C., Loomis, D., Grosse, Y., Bianchini, F., Straif, K., et al. (2016). Body fatness and cancer--viewpoint of the IARC working group. N. Engl. J. Med. 375 (8), 794–798. doi:10.1056/NEJMsr1606602
Lund, A., Knop, F., and Vilsbøll, T. (2014). Glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes: differences and similarities. Eur. J. Intern Med. 25 (5), 407–414. doi:10.1016/j.ejim.2014.03.005
McAdam-Marx, C., Nguyen, H., Schauerhamer, M., Singhal, M., Unni, S., Ye, X., et al. (2016). Glycemic control and weight outcomes for exenatide once weekly versus liraglutide in patients with type 2 diabetes: a 1-year retrospective cohort analysis. Clin. Ther. 38 (12), 2642–2651. doi:10.1016/j.clinthera.2016.11.003
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G.PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6 (7), e1000097. doi:10.1371/journal.pmed.1000097
Monami, M., Dicembrini, I., Nreu, B., Andreozzi, F., Sesti, G., and Mannucci, E. (2017). Predictors of response to glucagon-like peptide-1 receptor agonists: a meta-analysis and systematic review of randomized controlled trials. Acta Diabetol. 54 (12), 1101–1114. doi:10.1007/s00592-017-1054-2
Okerson, T., Yan, P., Stonehouse, A., and Brodows, R. (2010). Effects of exenatide on systolic blood pressure in subjects with type 2 diabetes. Am. J. Hypertens. 23 (3), 334–339. doi:10.1038/ajh.2009.245
Oz, B., and Kıvrak, A. (2023). Evaluation of depression, anxiety symptoms, emotion regulation difficulties, and self-esteem in children and adolescents with obesity. Arch. Pediatr. 30(4):226–231. doi:10.1016/j.arcped.2023.02.003
Roth, C., Perez, F., Whitlock, K., Elfers, C., Yanovski, J., Shoemaker, A., et al. (2021). A phase 3 randomized clinical trial using a once-weekly glucagon-like peptide-1 receptor agonist in adolescents and young adults with hypothalamic obesity. Diabetes Obes. Metab. 23 (2), 363–373. doi:10.1111/dom.14224
Russell-Jones, D., Cuddihy, R., Hanefeld, M., Kumar, A., González, J., Chan, M., et al. (2012). Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): a 26-week double-blind study. Diabetes care 35 (2), 252–258. doi:10.2337/dc11-1107
Saunders, W., Nguyen, H., and Kalsekar, I. (2016). Real-world glycemic outcomes in patients with type 2 diabetes initiating exenatide once weekly and liraglutide once daily: a retrospective cohort study. Diabetes, metabolic syndrome Obes. targets Ther. 9, 217–223. doi:10.2147/dmso.S103972
Scott, D., Boye, K., Timlin, L., Clark, J., and Best, J. (2013). A network meta-analysis to compare glycaemic control in patients with type 2 diabetes treated with exenatide once weekly or liraglutide once daily in comparison with insulin glargine, exenatide twice daily or placebo. Diabetes, Obes. metabolism 15 (3), 213–223. doi:10.1111/dom.12007
Sideli, L., Lo Coco, G., Bonfanti, R., Borsarini, B., Fortunato, L., Sechi, C., et al. (2021). Effects of COVID-19 lockdown on eating disorders and obesity: a systematic review and meta-analysis. Eur. Eat. Disord. Rev. 29 (6), 826–841. doi:10.1002/erv.2861
Singh, G., Danaei, G., Farzadfar, F., Stevens, G., Woodward, M., Wormser, D., et al. (2013). The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS One 8 (7), e65174. doi:10.1371/journal.pone.0065174
Sterne, J., Savović, J., Page, M., Elbers, R., Blencowe, N., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Su, N., Li, Y., Xu, T., Li, L., Kwong, J., Du, H., et al. (2016). Exenatide in obese or overweight patients without diabetes: a systematic review and meta-analyses of randomized controlled trials. Int. J. Cardiol. 219, 293–300. doi:10.1016/j.ijcard.2016.06.028
Suárez, R., Chapela, S., Álvarez-Córdova, L., Bautista-Valarezo, E., Sarmiento-Andrade, Y., Verde, L., et al. (2023). Epigenetics in obesity and diabetes mellitus: new insights. New Insights 15 (4), 811. doi:10.3390/nu15040811
Tsapas, A., Karagiannis, T., Kakotrichi, P., Avgerinos, I., Mantsiou, C., Tousinas, G., et al. (2021). Comparative efficacy of glucose-lowering medications on body weight and blood pressure in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Obes. Metab. 23 (9), 2116–2124. doi:10.1111/dom.14451
Vilsbøll, T., Christensen, M., Junker, A., Knop, F., and Gluud, L. J. B. (2012). Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 344, d7771. doi:10.1136/bmj.d7771
Waldrop, G., Zhong, J., Peters, M., Goud, A., Chen, Y., Davis, S., et al. (2018). Incretin-based therapy in type 2 diabetes: an evidence based systematic review and meta-analysis. J. Diabetes Complicat. 32 (1), 113–122. doi:10.1016/j.jdiacomp.2016.08.018
Wang, J., Jin, X., An, P., Yu, S., and Mu, Y. (2019). The effects of exenatide once weekly (exqw) and exenatide twice a day (exbid) on beta-cell function in type 2 diabetes: a systematic review and network meta-analysis. J. Diabetes Res. 2019, 8083417. doi:10.1155/2019/8083417
Weghuber, D., Forslund, A., Ahlström, H., Alderborn, A., Bergström, K., Brunner, S., et al. (2020). A 6-month randomized, double-blind, placebo-controlled trial of weekly exenatide in adolescents with obesity. Pediatr. Obes. 15 (7), e12624. doi:10.1111/ijpo.12624
Zaccardi, F., Htike, Z., Webb, D., Khunti, K., and Davies, M. (2016). Benefits and harms of once-weekly glucagon-like peptide-1 receptor agonist treatments: a systematic review and network meta-analysis. A Syst. Rev. Netw. Meta-analysis 164 (2), 102–113. doi:10.7326/m15-1432
Keywords: exenatide, obesity, efficacy, child, meta-analysis
Citation: Chen B, Zou Z, Zhang X, Xiao D and Li X (2024) Exenatide for obesity in children and adolescents: Systematic review and meta-analysis. Front. Pharmacol. 15:1290184. doi: 10.3389/fphar.2024.1290184
Received: 07 September 2023; Accepted: 11 March 2024;
Published: 03 April 2024.
Edited by:
Meixian Zhang, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, ChinaReviewed by:
Silvia V. Conde, New University of Lisbon, PortugalDazhi Fan, Foshan Women and Children Hospital, China
Katarzyna Wiglusz, Wroclaw Medical University, Poland
Copyright © 2024 Chen, Zou, Zhang, Xiao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xihong Li, bGl4aWhvbmdoeGV5QDE2My5jb20=
 Bin Chen
Bin Chen Zhuan Zou
Zhuan Zou Xiaoyan Zhang1,2
Xiaoyan Zhang1,2 Dongqiong Xiao
Dongqiong Xiao Xihong Li
Xihong Li