- 1School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 2Department of Anesthesiology, Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
Background: This study was conducted to evaluate the safety and efficacy of intravenous esketamine as an adjuvant for sedation or analgesia outside the operating room in adults and children.
Method: PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science, and Scopus were searched for potential randomized controlled studies randomized controlled trials comparing drug combinations of esketamine to any other single or combination drug regimens for sedation or analgesia outside the operating room.
Results: Twenty-five studies with a total of 3,455 participants were included in this review. The pooled results of adults showed that compared with drug regimens of the control group, intravenous esketamine combinations were significantly associated with decreased risk of oxygen desaturation (RR = 0.49, 95% CI = [0.34, 0.70]); hypotension (RR = 0.38, 95% CI = [0.31, 0.46]); bradycardia (RR = 0.23, 95% CI = [0.12, 0.43]); injection pain (RR = 0.37, 95% CI = [0.25, 0.53]); body movement (RR = 0.60, 95% CI = [0.41, 0.88]); and propofol consumption (SMD = −1.38, 95% CI = [−2.64, −0.11]), but an increased risk of psychiatric symptoms (RR = 3.10, 95% CI = [2.11, 4.54]) (RR = relative risk; CI = confidence intervals; SMD = standardized mean difference). Subgroup analysis showed that only the combination of esketamine and propofol significantly reduced the above incidence of respiratory and cardiovascular adverse events in adults. In addition, the pooled results of children showed that compared with drug regimens of the control group, esketamine and propofol co-administration significantly reduced the risk of hypotension (RR = 0.59, 95% CI = [0.37, 0.95]) but increased the risk of visual disturbance (RR = 6.62, 95% CI = [2.18, 20.13]) and dizziness (RR = 1.99, 95% CI = [1.17, 3,37]). Subgroup analysis indicated that esketamine>0.5 mg/kg significantly reduced the incidence of hypotension, but increased the risk of dizziness in children.
Conclusion: Intravenous use of esketamine, particularly in combination with propofol, may improve the safety and efficacy of sedation and analgesia outside the operating room, although the potential for psychiatric side effects warrants attention. Future research is recommended to investigate the role of esketamine with agents other than propofol.
1 Introduction
The need for sedation or analgesia outside the operating room is increasing because of improvements in medical technology (Pino, 2007). Various drugs such as propofol, benzodiazepines, opioids, ketamine, and dexmedetomidine, either alone or in combination, have been used (Jo and Kwak, 2019). Combination drugs help to optimize the desired effects while countering each other’s side effects (Hinkelbein et al., 2020). The common administration of opioid-propofol combination and other drug combinations have been shown to be more effective than single agents for sedation or analgesia (Boriosi et al., 2017; Hayes et al., 2021; Rathi et al., 2022; De Vries et al., 2023).
Esketamine, an antagonist of the N-methyl-D-aspartic acid (NMDA) receptor, is an s-enantiomer of ketamine. It produces approximately twice higher sedative activity but induces fewer side effects than ketamine (Peltoniemi et al., 2016). Esketamine and ketamine can deliver analgosedation action, but some drawbacks such as the induction of frequent vomiting and clustered psychiatric symptoms limit their single administration (Nakao et al., 2003). Low-dose esketamine as an adjuvant has been shown to have several benefits for sedation or anesthesia, including stabilizing hemodynamics and respiratory function, decreasing propofol requirements, or reducing postoperative pain sensitivity (Huang et al., 2023; Ren et al., 2023).
In this study, we conducted an updated systematic review and meta-analysis to include all drug combinations of intravenous esketamine for sedation or analgesia outside the operating room. We also aimed to compare the safety and efficacy of combination regimens of esketamine with other drug regimens of the control group in adults and children undergoing various types of surgical procedures outside the operating room.
2 Materials and methods
2.1 Literature search and selection criteria
This review was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2010). The PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science, and Scopus databases were searched for potential randomized controlled trials (RCTs) that assessed the effect of esketamine on sedation or analgesia in adults and children undergoing surgical procedures outside the operating room. The date parameters for the search were set from database inception to 19 March 2024. The search strategy was specific to each database and is available in Supplementary Material S1. Additional studies in the reference lists of selected articles were also screened for possible eligibility. Clinicaltrials.gov was also searched for possible ongoing studies. In our study, the PICOS criteria are as follows: Population (P): patients requiring sedation or analgesia in clinical settings outside the operating room; Intervention (I): intravenous administration of esketamine in combination with other sedative or analgesic agents; Comparator (C): other drug regimens without the adjunctive use of esketamine; Outcomes (O): the primary outcomes of interest were the safety of using esketamine for sedation or analgesia outside the operating room, particularly examining respiratory and cardiovascular adverse events; Study design (S): our study is based on RCTs.
The inclusion criteria were as follows: full-text available RCTs, intravenous esketamine in combination with other anesthetics, and studies with participants who had undergone sedation or analgesia outside the operating room. The exclusion criteria were as follows: failure to provide sufficient information or data, sedation performed in the ICU or emergency room, or studies with participants under general anesthesia with laryngeal insertion or tracheal intubation.
2.2 Data collection
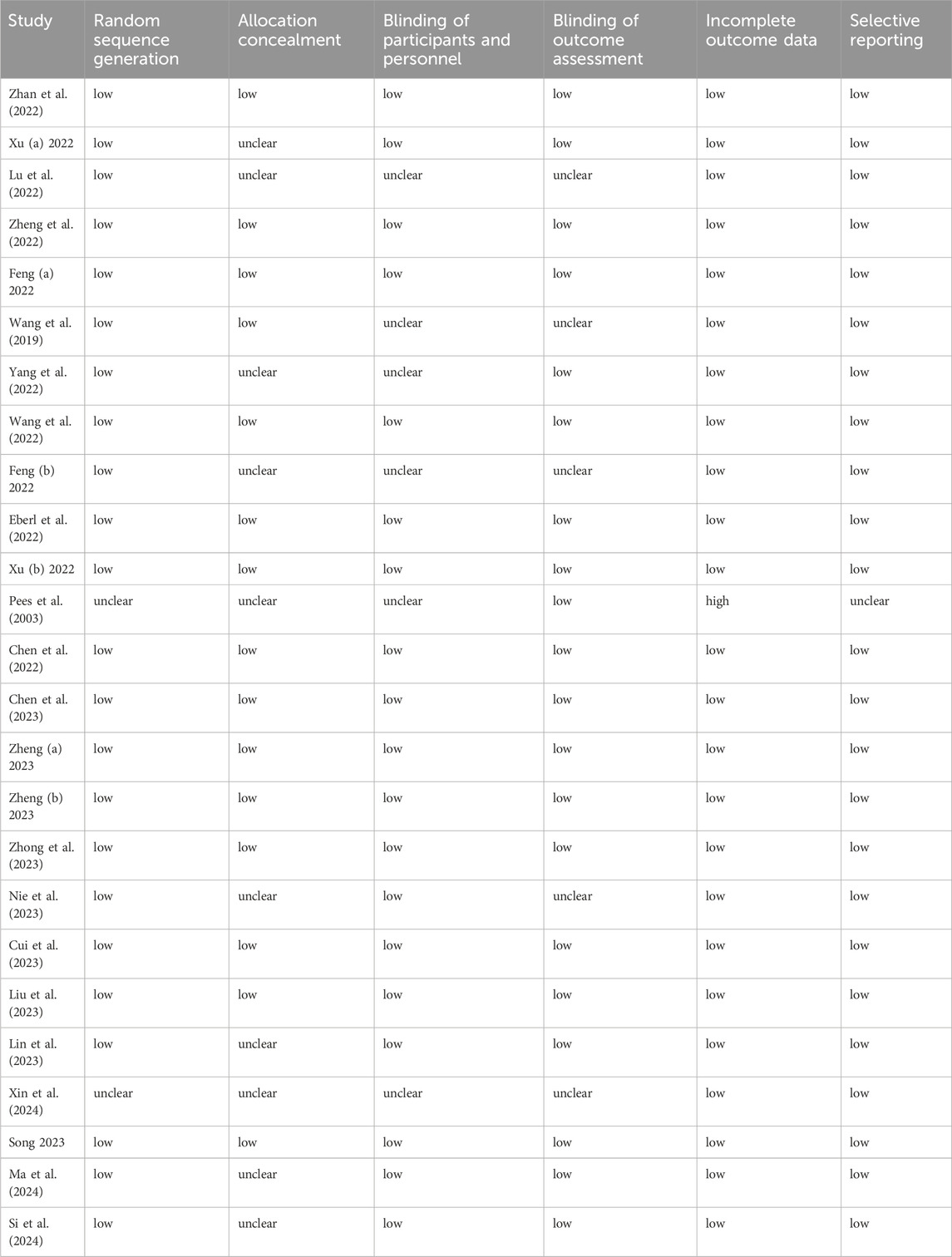
Two independent authors extracted data from the included studies based on a previously designed data extraction table. The following information was extracted: author, publication year, number of participants, age, intervention measures, type of surgery, and outcomes. The primary outcomes were respiratory and cardiovascular adverse events. The secondary outcomes were injection pain, inadequate sedation parameters (intraoperative cough and body movement), nausea and/or vomiting, propofol consumption, psychiatric symptoms, visual disturbance, dizziness, emergence delirium, awakening time, recovery time, and orientation recovery time. Where data were presented as values other than the mean and standard deviations, we attempted to contact the author to obtain raw data. If this was not possible, data were excluded from the meta-analysis. In the case of studies with more than one treatment group (including different doses of esketamine), we combined different doses as the esketamine≤0.5 mg/kg subgroup or esketamine>0.5 mg/kg subgroup (Higgins and Deeks, 2011). The risk of bias of the included studies was also independently assessed by two reviewers using the Cochrane risk of bias tool. Five parameters, namely, randomization sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting, from each included study were evaluated as low, unclear, or high risk of bias. The database searching, literature screening, data collection and risk of bias assessment were conducted independently by two authors, ZK and WM. Any discrepancies were resolved by discussion with the third author, PZ.
2.3 Statistical analysis
The relative risk (RR) with 95% confidence intervals (CI) was calculated for dichotomous data. The standardized mean difference (SMD) with 95% CI was used to express continuous variables owing to differences in measurement scales and methods of drug administration across included studies. Differences were considered statistically significant if p < 0.05, 95%CI of RR excluded 1, or 95% CI excluded 0 for the SMD. The I2 statistic was used to assess heterogeneity. If heterogeneity was significant (I2>50%), a random effects model was used; otherwise, a fixed effects model was used. Subgroup analysis was conducted according to the esketamine drug regimens (esketamine combined with propofol or other drugs) and esketamine dose (≤0.5 mg/kg or >0.5 mg/kg). We did not perform subgroup analysis if there were fewer than two trials in each subgroup. Additionally, sensitivity analysis was performed by omitting one study each time for primary outcomes to identify potential sources of heterogeneity. Publication bias was assessed by visual inspection of funnel plots. Statistical analyses were performed using Review Manager (RevMan) version 5.1 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011).
3 Results
3.1 Study search and characteristics
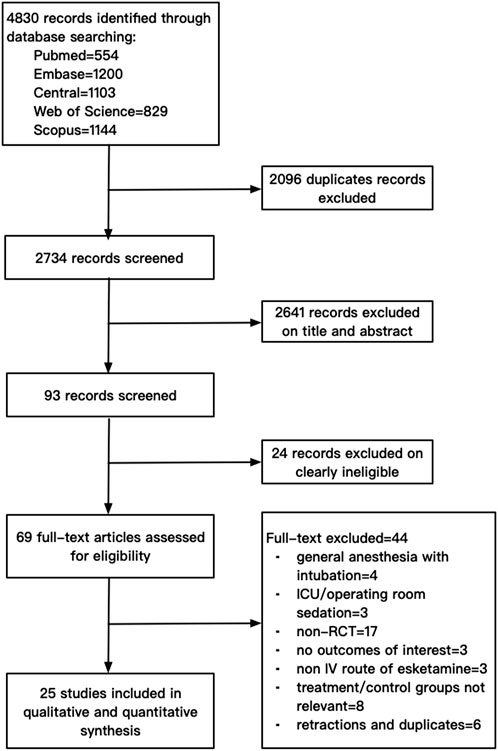
The electronic search yielded a total of 4,830 citations. After excluding 2096 duplicates, 2,734 studies were screened. After screening the titles and abstracts and assessing the full texts, 25 studies with a total of 3,455 participants were finally included in this review. A flowchart of the literature search for the included studies is shown in Figure 1. The data of 44 studies excluded by full-text screening are available in Supplementary Material S2.
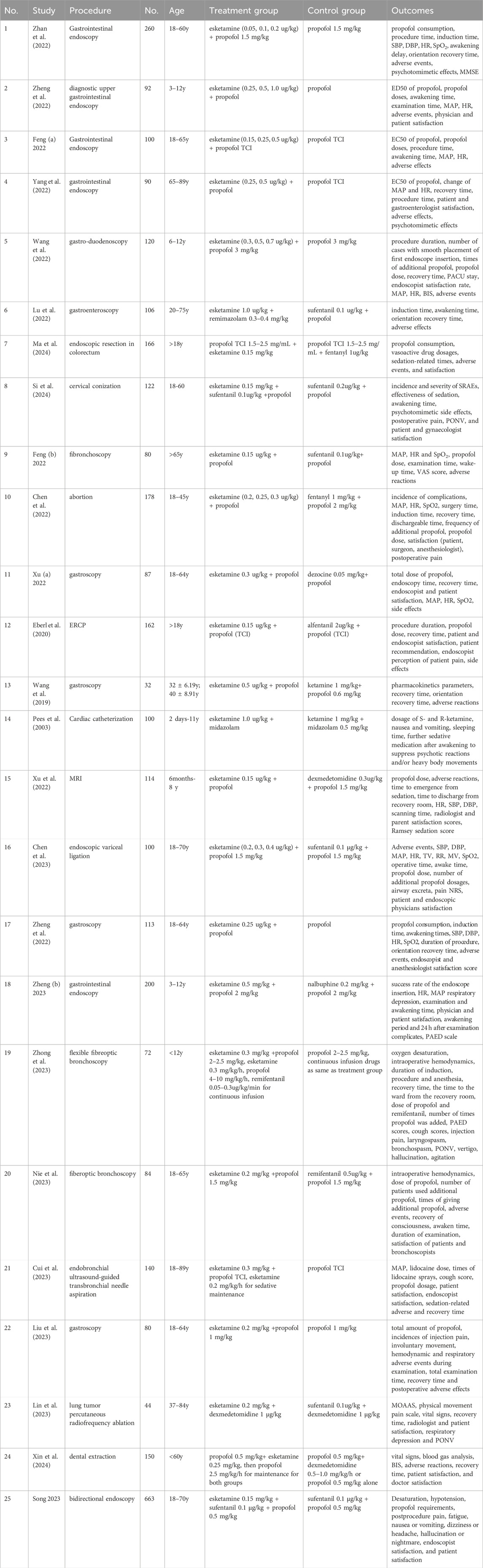
Six studies involved 698 pediatric participants (age range: 2 days to 12 years) (Pees et al., 2003; Wang et al., 2022; Xu (b) et al., 2022; Zheng (b) et al., 2023; Zheng et al., 2022; Zhong et al., 2023), and the 19 remaining studies involved 2,757 adult participants (18–89 years) (Chen et al., 2022; Chen et al., 2023; Cui et al., 2023; Eberl et al., 2020; Feng (a) et al., 2022; Feng (b) et al., 2022; Lin et al., 2023; Liu et al., 2023; Lu et al., 2022; Ma et al., 2024; Nie et al., 2023; Si et al., 2024; Song et al., 2023; Wang et al., 2019; Xin et al., 2024; Xu (a) et al., 2022; Yang et al., 2022; Zhan et al., 2022; Zheng (a) et al., 2023). Fifteen studies carried out diagnostic and therapeutic gastrointestinal endoscopy (Chen et al., 2023; Eberl et al., 2020; Feng (a) et al., 2022; Liu et al., 2023; Lu et al., 2022; Ma et al., 2024; Song et al., 2023; Wang et al., 2022; Wang et al., 2019; Xu (a) et al., 2022; Yang et al., 2022; Zhan et al., 2022; Zheng (a) et al., 2023; Zheng (b) et al., 2023; Zheng et al., 2022); four conducted fiberoptic bronchoscopy (Cui et al., 2023; Feng (b) et al., 2022; Nie et al., 2023; Zhong et al., 2023); and six studies involved abortions (Chen et al., 2022), cervical conization (Si et al., 2024), MRI (Xu (b) et al., 2022), cardiac catheterization (Pees et al., 2003), dental extraction (Xin et al., 2024), and percutaneous radiofrequency ablation of lung tumor (Lin et al., 2023), respectively. Twenty-two studies used a combination of esketamine and propofol (Chen et al., 2022; Chen et al., 2023; Cui et al., 2023; Eberl et al., 2020; Feng (a) et al., 2022; Feng (b) et al., 2022; Liu et al., 2023; Ma et al., 2024; Nie et al., 2023; Si et al., 2024; Song et al., 2023; Wang et al., 2022; Wang et al., 2019; Xin et al., 2024; Xu (a) et al., 2022; Xu (b) et al., 2022; Yang et al., 2022; Zhan et al., 2022; Zheng (a) et al., 2023; Zheng (b) et al., 2023; Zheng et al., 2022; Zhong et al., 2023), two studies used esketamine plus benzodiazepines (Pees et al., 2003; Lu et al., 2022), and one study used esketamine combined with dexmedetomidine (Lin et al., 2023). Eighteen studies involved one esketamine group with doses ranging from 0.15 to 1.0 μg/kg (Cui et al., 2023; Eberl et al., 2020; Feng (b) et al., 2022; Lin et al., 2023; Liu et al., 2023; Lu et al., 2022; Ma et al., 2024; Nie et al., 2023; Pees et al., 2003; Si et al., 2024; Song et al., 2023; Wang et al., 2019; Xin et al., 2024; Xu (a) et al., 2022; Xu (b) et al., 2022; Zheng (a) et al., 2023; Zheng (b) et al., 2023; Zhong et al., 2023), and seven studies involved more than one esketamine group with doses ranging from 0.05 to 1.0 μg/kg (Chen et al., 2022; Chen et al., 2023; Feng (a) et al., 2022; Wang et al., 2022; Yang et al., 2022; Zhan et al., 2022; Zheng et al., 2022). With respect to drug regimens in the control groups, 10 studies used propofol alone (Cui et al., 2023; Feng (a) et al., 2022; Liu et al., 2023; Nie et al., 2023; Wang et al., 2022; Yang et al., 2022; Zhan et al., 2022; Zheng (a) et al., 2023; Zheng (b) et al., 2023; Zheng et al., 2022); 13 studies used propofol in combination with opioids (Chen et al., 2022; Chen et al., 2023; Eberl et al., 2020; Feng (b) et al., 2022; Lu et al., 2022; Ma et al., 2024; Si et al., 2024; Song et al., 2023; Xu (a) et al., 2022; Zhong et al., 2023), ketamine (Wang et al., 2019), or dexmedetomidine (Xin et al., 2024; Xu (b) et al., 2022); one study used ketamine and midazolam (Pees et al., 2003); and one study used sufentanil in combination with dexmedetomidine (Lin et al., 2023). The characteristics of the included studies are shown in Table 1.
Fourteen studies had an overall low risk of bias, 10 studies had an unclear risk of bias, and one study had a high risk of bias. The sources of bias were primarily because of poor reporting of the randomization process and blinding, specifically a lack of description about allocation concealment. The risk of bias of the included studies is presented in Table 2.
3.2 Pooled results of adults
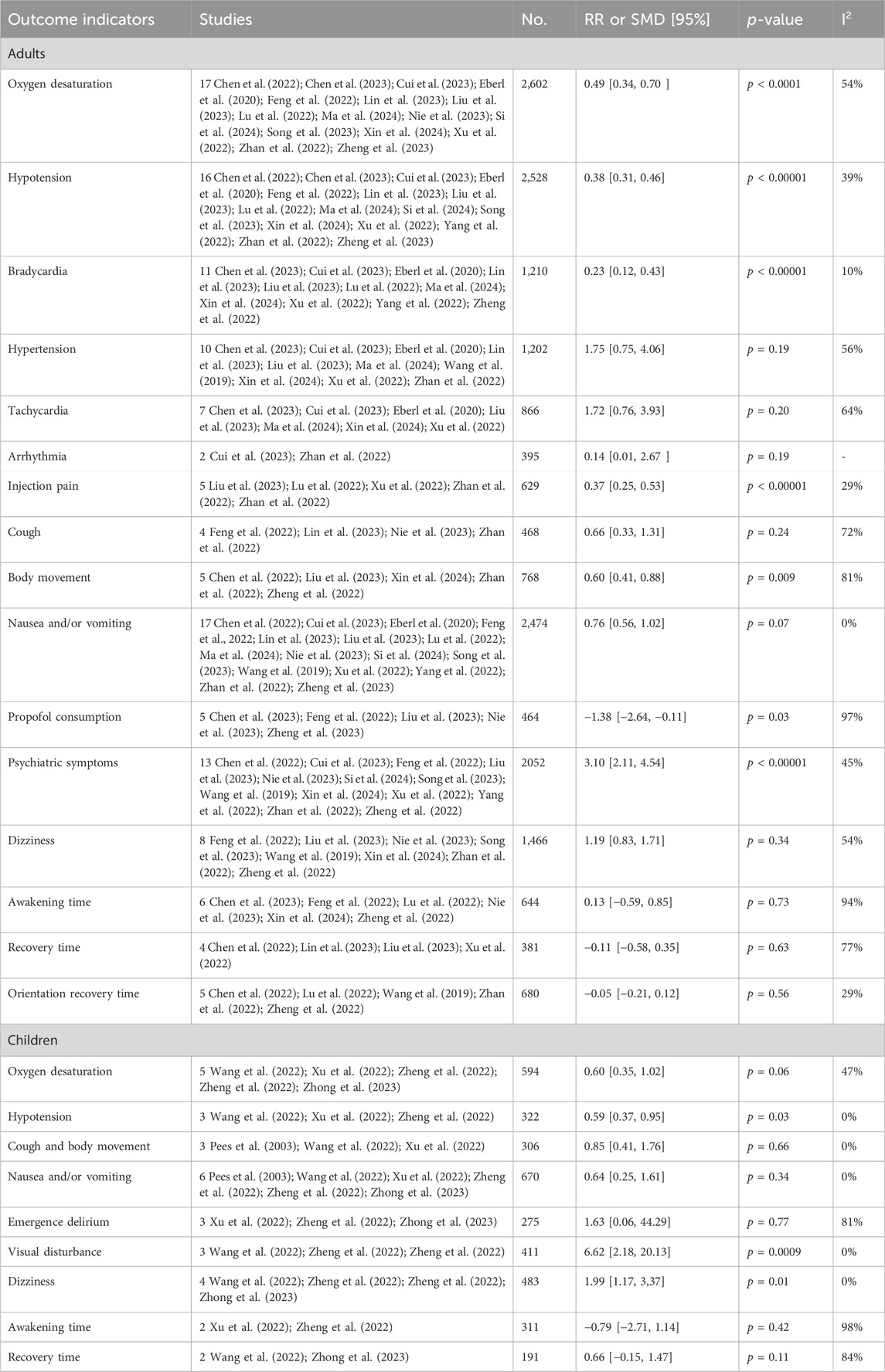
The pooled results in adults showed that compared with drug regimens of the control group, intravenous esketamine drug combinations significantly reduced the risk of oxygen desaturation (RR = 0.49, 95%CI = [0.34, 0.70], p < 0.0001); hypotension (RR = 0.38, 95%CI = [0.31, 0.46], p < 0.00001); bradycardia (RR = 0.23, 95%CI = [0.12, 0.43], p < 0.00001); injection pain (RR = 0.37, 95%CI = [0.25, 0.53], p < 0.00001); body movement (RR = 0.60, 95%CI = [0.41, 0.88], p = 0.009); and propofol consumption (SMD = −1.38, 95%CI = [−2.64, −0.11], p = 0.03) but increased the risk of psychiatric symptoms (RR = 3.10, 95%CI = [2.11, 4.54], p < 0.00001). No significant differences were found in hypertension (RR = 1.75, 95%CI = [0.75, 4.06], p = 0.19); tachycardia (RR = 1.72, 95%CI = [0.76, 3.93], p = 0.20); arrhythmia (RR = 0.14, 95%CI = [0.01, 2.67], p = 0.19); cough (RR = 0.66, 95%CI = [0.33, 1.31], p = 0.24); nausea and/or vomiting (RR = 0.76, 95%CI = [0.56, 1.02], p = 0.07); dizziness (RR = 1.19, 95%CI = [0.83, 1.71], p = 0.34); awakening time (SMD = 0.13, 95%CI = [−0.59, 0.85], p = 0.73); recovery time (SMD = −0.11, 95%CI = [−0.58, 0.35], p = 0.63); or orientation recovery time (SMD = −0.05, 95%CI = [−0.21, 0.12], p = 0.56) compared with drug regimens of the control group (Figures 2A–D; Table 3).
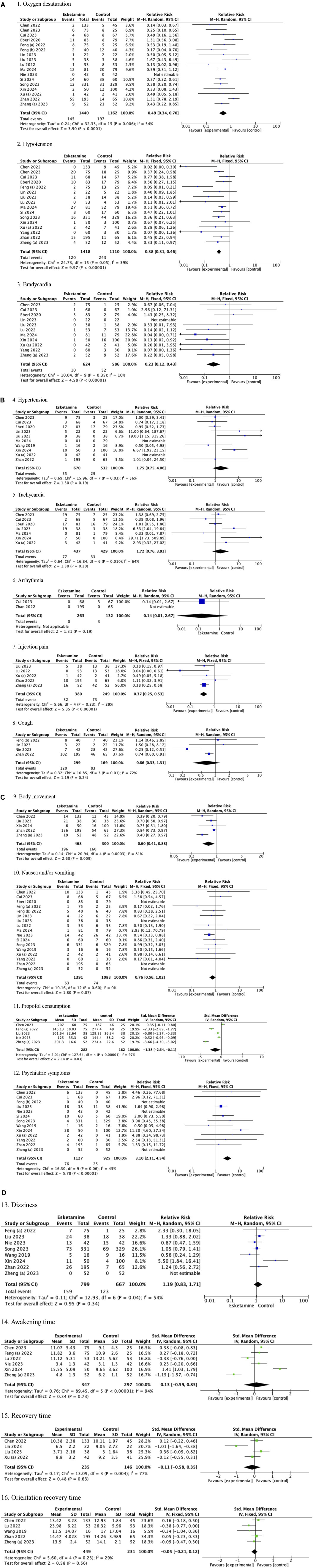
Figure 2. (A) Forest plots of adults (1. Oxygen desaturation, 2. Hypotension, and 3. Bradycardia). (B) Forest plots of adults (4. Hypertension, 5. Tachycardia, 6. Arrhythmia, 7. Injection pain, and 8. Cough). (C) Forest plots of adults (9. Body movement, 10. Nausea and/or vomiting, 11. Propofol consumption, and 12. Psychiatric symptoms). (D) Forest plots of adults (13. Dizziness, 14. Awakening time, 15. Recovery time, and 16. Orientation recovery time).
Subgroup analysis of esketamine regimens showed a significant reduction in the risk of oxygen desaturation (RR = 0.51, 95%CI = [0.35, 0.74], p = 0.0003); hypotension (RR = 0.38, 95%CI = [0.31, 0.46], p < 0.00001); and bradycardia (RR = 0.24, 95%CI = [0.12, 0.47], p < 0.0001) in the esketamine-propofol combination, and a marginally significant reduction (oxygen desaturation: RR = 0.23, 95%CI = [0.05, 1.06], p = 0.06, and test for subgroup differences p = 0.32; hypotension: RR = 0.26, 95%CI = [0.07, 1.00], p = 0.05, and test for subgroup differences p = 0.59; bradycardia: RR = 0.14, 95%CI = [0.02, 1.12], p = 0.06, and test for subgroup differences p = 0.64) in the esketamine-non-propofol combination in adults. No significant difference was found in nausea and/or vomiting in the subgroup analysis of esketamine regimens (Table 4). Given that only one study involved esketamine dose >0.5 ug/kg (Lu et al., 2022), we did not perform subgroup analysis of the esketamine dose in adults. Additionally, the pooled results of adults were not altered after eliminating this single esketamine>0.5 ug/kg study (data not shown).
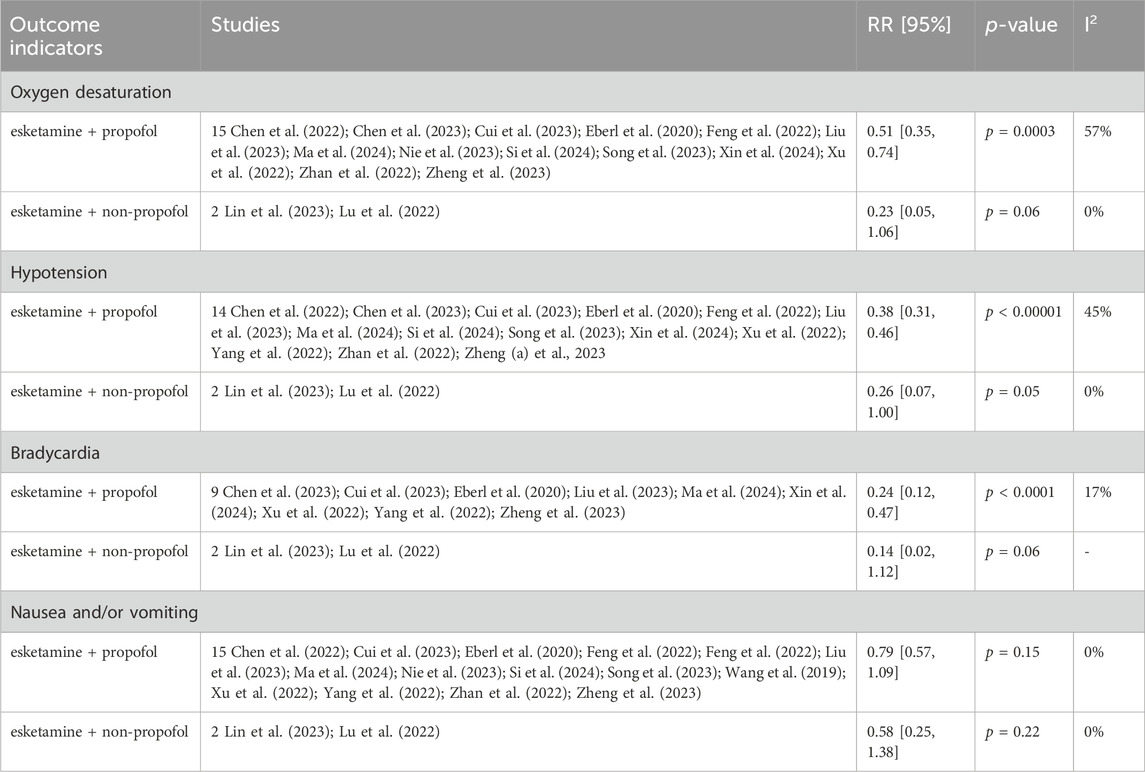
Table 4. Adults: esketamine drug regimens subgroup analysis (combination of esketamine with propofol versus other drugs).
3.3 Pooled results of children
The pooled results in children showed that compared with drug regimens of the control group, esketamine-propofol combination significantly reduced the risk of hypotension (RR = 0.59, 95%CI = [0.37, 0.95], p = 0.03) and increased the risk of visual disturbance (RR = 6.62, 95%CI = [2.18, 20.13], p = 0.0009) and dizziness (RR = 1.99, 95%CI = [1.17, 3,37], p = 0.01). No significant differences were found in oxygen desaturation (RR = 0.60, 95%CI = [0.35, 1.02], p = 0.06); cough and body movement (RR = 0.85, 95%CI = [0.41, 1.76], p = 0.66); nausea and/or vomiting (RR = 0.64, 95%CI = [0.25, 1.61], p = 0.34); emergence delirium (RR = 1.63, 95%CI = [0.06, 44.29], p = 0.77); awakening time (SMD = −0.79, 95%CI = [−2.71, 1.14], p = 0.42); and recovery time (SMD = 0.66, 95%CI = [−0.15, 1.47], p = 0.11) compared with drug regimens of the control group (Figures 3A,B; Table 3).
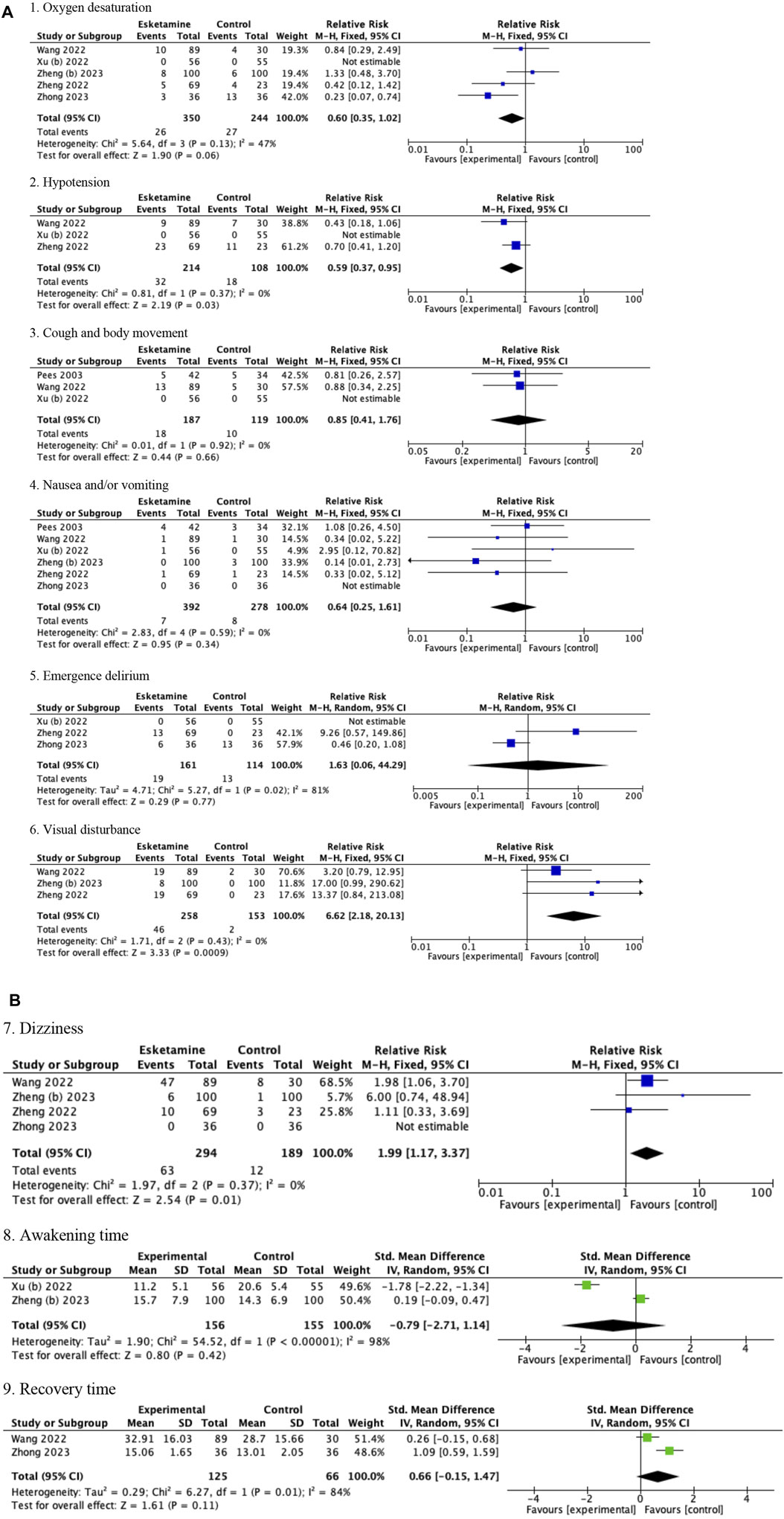
Figure 3. (A) Forest plots of children (1. Oxygen desaturation, 2. Hypotension, 3. Cough and body movement, 4. Nausea and/or vomiting, 5. Emergence delirium, and 6. Visual disturbance). (B) Forest plots of children (7. Dizziness, 8. Awakening time, and 9. Recovery time).
Because only one study used the esketamine-midazolam combination, which reported data on nausea and/or vomiting and body movement, we did not conduct a subgroup analysis of the esketamine regimens in children (Pees et al., 2003). After eliminating this single esketamine-midazolam study, the pooled results of children was not changed (data was not shown). Esketamine dose subgroup analysis showed a significant decrease in the risk of hypotension (RR = 0.33, 95%CI = [0.15, 0.75], p = 0.008) and dizziness (RR = 2.36, 95%CI = [1.34, 4.18], p = 0.003) in the esketamine>0.5 mg/kg subgroup, but a non-significant difference in the esketamine≤0.5 mg/kg subgroup (hypotension: RR = 0.73, 95%CI = [0.45, 1.18], p = 0.19, and test for subgroup differences p = 0.11; dizziness: RR = 1.72, 95%CI = [0.98, 3.02], p = 0.06, and test for subgroup differences p = 0.44). No significant difference was found in oxygen desaturation, nausea and/or vomiting, and visual disturbance in the esketamine dose subgroup analyses (Table 5).
3.4 Sensitivity analysis
We conducted a sensitivity analysis of the primary outcomes (cardiorespiratory adverse events) by eliminating one included study each time. There was no significant difference in the sensitivity analysis in adults. In children, the pooled results of oxygen desaturation were altered after eliminating the study by Zheng (b) 2023 (Zheng (b) et al., 2023), and that of hypotension was altered after eliminating the studies by Wang et al., 2022; Zheng et al. 2022; Wang et al., 2022; Zheng et al., 2022). Due to concerns regarding a high risk of bias, we performed a sensitivity analysis that excluded the study by Pees et al. (Pees et al., 2003). After this exclusion, the pooled results of cough and body movement, and nausea and/or vomiting in children remained consistent.
3.5 Publication bias analysis
We assessed publication bias for six indicators, each reported in no less than 10 studies, including oxygen desaturation, hypotension, bradycardia, hypertension, nausea and/or vomiting, and psychiatric symptoms, and the results of this analysis are shown in Supplementary Material S3. However, the symmetry of the four funnel plots for three of these indicators, such as oxygen desaturation, hypotension, hypertension and psychiatric symptoms, was less than ideal, suggesting a possible publication bias.
4 Discussion
In this study, we assessed the safety and efficacy of intravenous esketamine as an adjuvant for sedation or analgesia in adults and children undergoing surgical procedures outside the operating room. The unique pharmacodynamics of esketamine (such as dissociative anesthetic property, sympathetic excitatory activity, and local anesthetic action) and the synergistic effect of drug combinations were considered to account for the reduced risk of hypotension, bradycardia, oxygen desaturation, injection pain, and body movement and decreased propofol consumption (Li et al., 2022; Xu et al., 2022; Zhan et al., 2022; Zheng (a) et al., 2023).
Three recent systematic reviews and meta-analyses have investigated the effect of esketamine on sedation or non-intubated anesthesia (Chen H et al., 2023; Huang et al., 2023; Lian et al., 2023). Unlike our review, which aimed to include all combinations of intravenous esketamine, these studies focused specifically on the safety and efficacy of esketamine-propofol combination in comparison to other drug regimens. Our main findings were generally consistent with these three studies, except for one study by Huang et al., which included seven RCTs with 808 patients and reported no significant differences in respiratory depression and body movement with esketamine administration for procedural sedation, without providing a rationale for these finding (Huang et al., 2023). In addition, Chen H et al. included data only from adults, analyzed 14 RCTs and demonstrated that the esketamine-propofol combination provided more stable haemodynamic indices during induction of non-intubated anaesthesia (Chen H et al., 2023). Lian et al., pooling data from 18 RCTs involving 1962 patients, observed that the addition of esketamine to propofol significantly reduced recovery time compared with the saline group, but not compared with the opioid group. They further demonstrated that esketamine significantly lowered the required dose of propofol and the risk of overall complications when compared with both the saline and opioid groups (Lian et al., 2023).
In children, this study first demonstrated that esketamine adjunct to propofol sedation probably caused a lower risk of hypotension but a higher risk of visual disturbance and dizziness. Unlike in adults, the combined use of esketamine and propofol in children provided a limited benefit on the incidence of oxygen desaturation. This finding was consistent with that reported in two systematic reviews with ketamine-propofol combination, indicating only a reduced risk of hypotension and/or bradycardia but not oxygen desaturation in the pediatric population (Foo et al., 2020; Hayes et al., 2021). The possible explanation for these findings is the different respiratory physiology of children such as vulnerable airways, low functional residual capacity of the lungs, poor oxygen reserves, and high oxygen consumption and basal metabolic rate, which makes them more susceptible to respiratory depression from anesthetics (Zheng (b) et al., 2023; Zhong et al., 2023). All respiratory depression events were alleviated by airway management or mask oxygen delivery, and no serious complications occurred in children among the included studies (Wang et al., 2022; Xu (b) et al., 2022; Zheng (b) et al., 2023; Zheng et al., 2022; Zhong et al., 2023).
Another concern about esketamine usage is the psychic emergence reactions that manifest as vivid dreaming; extracorporeal experiences; and illusions with excitement, confusion, euphoria, and fear, and these are often accompanied by auditory and visual disturbances (Miller, 2006; Zhuang et al., 2009). They occur in the first hour of emergence and usually abate within one to several hours (Miller, 2006; Zhuang et al., 2009). Our results showed that the administration of esketamine to adults increased the incidence of psychiatric symptoms. These esketamine-related psychiatric symptoms did not require additional medical support in any of the included studies. Additionally, our study also observed similar awakening, recovery, and orientation recovery times in adults.
It is difficult for young children to express their experience and to self-report potential psychiatric symptoms (Xu (b) et al., 2022). Thus, adverse reactions such as visual disturbance (usually complaints of diplopia), dizziness, and emergence delirium were assessed instead in children. The incidence of visual disturbance and dizziness varied from 8% to 34.78% and 0%–73.3%, respectively, possibly depending on age, esketamine dose, and different surgical procedures among the included studies (Wang et al., 2022; Xu (b) et al., 2022; Zheng (b) et al., 2023; Zheng et al., 2022; Zhong et al., 2023). Furthermore, we found that visual disturbance was associated with esketamine regardless of its doses, while dizziness was associated with esketamine >0.5 mg/kg in this study. Those two postoperative complications were self-limited and did not require intervention among the included studies (Wang et al., 2022; Xu (b) et al., 2022; Zheng (b) et al., 2023; Zheng et al., 2022; Zhong et al., 2023). Although no significant difference was found, the incidence of emergence delirium (assessed using pediatric anesthesia emergence delirium [PAED] scores) in our study should be interpreted with caution. In contrast to previous studies, one meta-analysis that combined one pediatric study and two adults studies observed that esketamine-propofol combination was associated with an increased risk of emergence agitation (not based on PAED scores) (Huang et al., 2023). It is thought that visual disturbance or dizziness may influence the recovery quality of children (Wang et al., 2022; Zhong et al., 2023). However, no significant difference was found in awakening and recovery time in our review.
Combination with propofol is the most common drug regimen of esketamine for sedation or analgesia, and dexmedetomidine and remimazolam were also added to esketamine in adults in two included studies (Lu et al., 2022; Lin et al., 2023). Dexmedetomidine, a highly selective α2-adrenergic agonist, and remimazolam, a short-acting benzodiazepine, both have fewer cardiovascular and respiratory side effects than propofol (Kim and Fechner, 2022; Zhang et al., 2024). Interestingly, a subgroup analysis of esketamine regimens revealed a significant reduction in the risk of oxygen desaturation, hypotension and bradycardia only with the esketamine-propofol combination but not with the esketamine-dexmedetomidine and -remimazolam combinations. Clinical heterogeneity, sample size and limited number of studies may contribute to the discrepancies in the subgroup analysis of esketamine-based regimens. The results of the subgroup analyses should be interpreted with caution. Further research, particularly RCTs with large sample sizes, is warranted to clarify the impact of esketamine in combination with non-propofol agents on the safety and efficacy of sedation and analgesia.
Our study has some limitations: (i) We did not stratify the main analysis by different types of surgical procedures and drug regimens of the control group. This allowed us to broaden the applicability of the findings beyond a specific context. However, these factors may have caused heterogeneity among some analyses. Other factors such as the different definitions of some outcomes and strategies of esketamine and propofol administration may have also contributed to heterogeneity. (ii) Twenty-three of the 25 included studies (92%) were performed in China, which may have led to bias in the results. (iii) Some continuous variables (such as propofol consumption, awakening time, recovery time, and orientation recovery time) of the included studies were reported as medium or with different measurement units, making them unsuitable for direct data merging (Cui et al., 2023; Eberl et al., 2020; Feng (b) et al., 2022; Ma et al., 2024; Si et al., 2024; Song et al., 2023; Wang et al., 2022; Wang et al., 2019; Xu (a) et al., 2022; Xu (b) et al., 2022; Yang et al., 2022; Zhan et al., 2022; Zheng et al., 2022; Zhong et al., 2023). In our study, these data were excluded from the meta-analysis because we failed to obtain raw data from the authors. After qualitative analysis of these data, we found that esketamine groups was associated with lower propofol consumption in all 10 included studies that were ineligible for the meta-analysis (Eberl et al., 2020; Feng (b) et al., 2022; Ma et al., 2024; Song et al., 2023; Wang et al., 2022; Xu (a) et al., 2022; Xu (b) et al., 2022; Zhan et al., 2022; Zheng et al., 2022; Zhong et al., 2023). In addition, three of the seven “inappropriate” studies showed the significantly shorter awakening time and/or recovery time with esketamine administration (Wang et al., 2019; Eberl et al., 2020; Yang et al., 2022; Zheng et al., 2022; Cui et al., 2023; Ma et al., 2024; Si et al., 2024). (iv) Almost 90% of the included studies (22 out of 25) used drug regimens that comprised esketamine and propofol. We could perform the subgroup analysis of esketamine regimens for some outcomes in adults. Future studies are needed to explore the effect of different esketamine combinations on sedation or anesthesia. (v) Publication bias may exist for oxygen desaturation, hypotension, hypertension, and psychiatric symptoms by visual inspection of funnel plots, suggesting the need for cautious interpretation of results.
5 Conclusion
This study showed that intravenous esketamine combinations probably reduced the incidence of several cardiorespiratory depression events (hypotension, bradycardia, and oxygen desaturation); injection pain; body movement; and propofol requirement, but increased the risk of psychiatric symptoms in adults who have undergone different surgical procedures outside the operating room. However, subgroup analysis indicated that the incidence of hypotension, bradycardia, and oxygen desaturation in adults was significantly reduced only with the esketamine and propofol combination. In addition, this study also suggested that the combined use of esketamine and propofol may cause a lower risk of hypotension (esketamine>0.5 mg/kg), but a higher risk of visual disturbance and dizziness (esketamine>0.5 mg/kg) in children who have received sedation or analgesia outside the operating room. Although these short-term side effects usually do not require additional intervention, clinicians should be aware of the potential psychiatric symptoms of esketamine. Given the limited number of studies, future research is needed, particularly RCTs with substantial cohorts, to elucidate the effect of esketamine when combined with agents other than propofol on the safety and effectiveness of sedation and analgesia protocols.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
ZK: Data curation, Writing–original draft, Writing–review and editing. WM: Data curation, Writing–review and editing. YD: Data curation, Writing–review and editing. PZ: Data curation, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Science and Technology Plan Project of Sichuan Province, China [2022YFS0439], the Cadres Health research topic Sichuan Province, China [2022-217] and the youth talent fund project of Sichuan Provincial People’s Hospital [2021QN16].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1287761/full#supplementary-material
References
Boriosi, J. P., Eickhoff, J. C., Klein, K. B., and Hollman, G. A. (2017). A retrospective comparison of propofol alone to propofol in combination with dexmedetomidine for pediatric 3T MRI sedation. Paediatr. Anaesth. 27 (1), 52–59. doi:10.1111/pan.13041
Chen, H., Ding, X., Xiang, G., Xu, L., Liu, Q., Fu, Q., et al. (2023). Analysis of the efficacy of subclinical doses of esketamine in combination with propofol in non-intubated general anesthesia procedures - a systematic review and meta-analysis. Bmc. Anesthesiol. 23 (1), 245. doi:10.1186/s12871-023-02135-8
Chen, J., Zou, X., Hu, B., Yang, Y., Wang, F., Zhou, Q., et al. (2022). Effect of different doses of esketamine compared with fentanyl combined with propofol on hypotension in patients undergoing painless abortion surgery: a prospective, randomized, double-blind controlled clinical trial. Bmc. Anesthesiol. 22 (1), 305. doi:10.1186/s12871-022-01848-6
Chen, Y., Chen, J., Wang, Q., Lyu, H., Chen, X., Liu, R., et al. (2023). Safety and tolerability of esketamine in propofol based sedation for endoscopic variceal ligation with or without injection sclerotherapy: randomized controlled trial. Dig. Endosc. 35, 845–854. doi:10.1111/den.14539
Cui, S., Huang, P., Wei, Z., Guo, T., Zhang, A., and Huang, L. (2023). Esketamine combined with propofol tci versus propofol tci for deep sedation during endobronchial ultrasound-guided transbronchial needle aspiration: a prospective, randomized, and controlled trial. Int. J. Clin. Pract. 2023, 1155126. doi:10.1155/2023/1155126
De Vries, L. J., Veeger, N., Van Roon, E. N., and Lameijer, H. (2023). Low-dose ketamine or opioids combined with propofol for procedural sedation in the emergency department: a systematic review. Eur. J. Emerg. Med. 30 (4), 244–251. doi:10.1097/mej.0000000000001046
Eberl, S., Koers, L., van Hooft, J., de Jong, E., Hermanides, J., Hollmann, M. W., et al. (2020). The effectiveness of a low-dose esketamine versus an alfentanil adjunct to propofol sedation during endoscopic retrograde cholangiopancreatography: a randomised controlled multicentre trial. Eur. J. Anaesthesiol. 37 (5), 394–401. doi:10.1097/eja.0000000000001134
Feng, M., Shi, G., Cui, W., Zhang, N., Xie, Q., and Zhang, W. (2022a). The median effective concentration of propofol in combination with different doses of esketamine during gastrointestinal endoscopy in adults. Front. Pharmacol. 13, 1034236. doi:10.3389/fphar.2022.1034236
Feng, Y., Du, T., Wang, J., and Chen, Z. (2022b). Low dose of esketamine combined with propofol in painless fibronchoscopy in elderly patients. Med. Baltim. 101 (50), e31572. doi:10.1097/md.0000000000031572
Foo, T. Y., Mohd Noor, N., Yazid, M. B., Fauzi, M. H., Abdull Wahab, S. F., and Ahmad, M. Z. (2020). Ketamine-propofol (Ketofol) for procedural sedation and analgesia in children: a systematic review and meta-analysis. Bmc. Emerg. Med. 20 (1), 81. doi:10.1186/s12873-020-00373-4
Hayes, J. A., Aljuhani, T., De Oliveira, K., and Johnston, B. C. (2021). Safety and efficacy of the combination of propofol and ketamine for procedural sedation/anesthesia in the pediatric population: a systematic review and meta-analysis. Anesth. Analg. 132 (4), 979–992. doi:10.1213/ane.0000000000004967
Higgins, J. P., and Deeks, J. J. (2011). “Chapter 7: selecting studies and collecting data,” in Cochrane handbook for systematic reviews of interventions. Editors J. P. T. Higgins, and S. Green (The Cochrane Collaboration). Version 5.1.0 (updated March 2011).
Hinkelbein, J., Schmitz, J., Lamperti, M., and Fuchs-Buder, T. (2020). Procedural sedation outside the operating room. Curr. Opin. Anaesthesiol. 33 (4), 533–538. doi:10.1097/aco.0000000000000885
Huang, X., Lin, F., Chen, Q., and Hu, X. (2023). Safety and efficacy of the combination of esketamine and propofol in procedural sedation/analgesia: a systematic review and meta-analysis. Minerva. Anestesiol. 89, 680–689. doi:10.23736/s0375-9393.23.17100-8
Jo, Y. Y., and Kwak, H. J. (2019). Sedation strategies for procedures outside the operating room. Yonsei. Med. J. 60 (6), 491–499. doi:10.3349/ymj.2019.60.6.491
Kim, S. H., and Fechner, J. (2022). Remimazolam - current knowledge on a new intravenous benzodiazepine anesthetic agent. Korean J. Anesthesiol. 75 (4), 307–315. doi:10.4097/kja.22297
Li, C. Y., Chen, Z. Y., He, H. F., Wang, H. G., and Xu, L. M. (2022). A subclinical dose of esketamine pretreatment for propofol and rocuronium injection pain. Asian. J. Surg. 45 (12), 3038–3039. doi:10.1016/j.asjsur.2022.07.103
Lian, X., Lin, Y., Luo, T., Jing, Y., Yuan, H., and Guo, Y. (2023). Efficacy and safety of esketamine for sedation among patients undergoing gastrointestinal endoscopy: a systematic review and meta-analysis. Bmc. Anesthesiol. 23 (1), 204. doi:10.1186/s12871-023-02167-0
Lin, Z., Li, S., Zhou, Y., Lu, X., Yang, B., Yu, Z., et al. (2023). A comparative study of esketamine-dexmedetomidine and sufentanil-dexmedetomidine for sedation and analgesia in lung tumor percutaneous radiofrequency ablation (PRFA): a randomized double-blind clinical trial. Bmc. Anesthesiol. 23 (1), 304. doi:10.1186/s12871-023-02266-y
Liu, X., Xiao, Q., and Zhuang, S. (2023). Comparison of propofol-esketamine versus propofol for anesthesia in gastroscopy: a double-blind, randomized controlled clinical trial. Front. Med. (Lausanne). 10, 1184709. doi:10.3389/fmed.2023.1184709
Lu, C., Ren, J., Guo, X., and Qian, J. (2022). Effects of remimazolam combined with esketamine anesthesia on circulatory and respiratory function during painless gastroenteroscopy. Contrast. Media. Mol. Imaging. 2022, 1079099. doi:10.1155/2022/1079099
Ma, Y., Wang, J., Yang, Y., and Yao, M. (2024). Efficacy and safety of esketamine combined with propofol for curative endoscopic resection in colorectum: a prospective, randomized controlled trial. Bmc. Anesthesiol. 24 (1), 96. doi:10.1186/s12871-024-02475-z
Miller, R. D. (2006). Miller’s anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone, 171–183.
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Group, P. (2010). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8 (5), 336–341. doi:10.1016/j.ijsu.2010.02.007
Nakao, S., Nagata, A., Miyamoto, E., Masuzawa, M., Murayama, T., and Shingu, K. (2003). Inhibitory effect of propofol on ketamine-induced c-Fos expression in the rat posterior cingulate and retrosplenial cortices is mediated by GABAA receptor activation. Anaesthesiol. Scand. 47 (3), 284–290. doi:10.1034/j.1399-6576.2003.00040.x
Nie, J., Chen, W., Jia, Y., Zhang, Y., and Wang, H. (2023). Comparison of remifentanil and esketamine in combination with propofol for patient sedation during fiberoptic bronchoscopy. Bmc. Pulm. Med. 23 (1), 254. doi:10.1186/s12890-023-02517-1
Pees, C., Haas, N. A., Ewert, P., Berger, F., and Lange, P. E. (2003). Comparison of analgesic/sedative effect of racemic ketamine and S(+)-ketamine during cardiac catheterization in newborns and children. Pediatr. Cardiol. 24 (5), 424–429. doi:10.1007/s00246-002-0356-4
Peltoniemi, M. A., Hagelberg, N. M., Olkkola, K. T., and Saari, T. I. (2016). Ketamine: a review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin. Pharmacokinet. 55 (9), 1059–1077. doi:10.1007/s40262-016-0383-6
Pino, R. M. (2007). The nature of anesthesia and procedural sedation outside of the operating room. Curr. Opin. Anaesthesiol. 20 (4), 347–351. doi:10.1097/ACO.0b013e32827035c7
Rathi, G. V., Padawe, D., Takate, V., Dighe, K., Bansode, K. K., and Narwade, A. U. (2022). Comparative evaluation of ease of dental treatment and clinical efficiency of midazolam vs midazolam and ketamine combination for sedation in young uncooperative pediatric patients: a systematic review. Int. J. Clin. Pediatr. Dent. 15 (6), 680–686. doi:10.5005/jp-journals-10005-2456
Ren, Y. L., Yuan, J. J., Xing, F., Zhu, L. N., and Zhang, W. (2023). Effects of different doses of esketamine on pain sensitivity of patients undergoing thyroidectomy: a randomized controlled trial. Ther 12 (3), 739–750. doi:10.1007/s40122-023-00488-z
Si, J., Li, X., Wang, Y., Feng, N., and Cui, M. (2024). Effects of adding low-dose esketamine to sufentanil and propofol sedation during cervical conization: a single-centre, randomized controlled trial. Bmc. Anesthesiol. 24 (1), 15. doi:10.1186/s12871-023-02389-2
Song, N., Yang, Y., Zheng, Z., Shi, W. C., Tan, A. P., Shan, X. S., et al. (2023). Effect of esketamine added to propofol sedation on desaturation and hypotension in bidirectional endoscopy: a randomized clinical trial. JAMA. Netw. Open 6 (12), e2347886. doi:10.1001/jamanetworkopen.2023.47886
Wang, J., Hu, W., Zhao, X., Ren, W., Huang, X., and Zhang, B. (2022). Sedative effect and safety of different doses of S-ketamine in combination with propofol during gastro-duodenoscopy in school-aged children: a prospective, randomized study. Bmc. Anesthesiol. 22 (1), 346. doi:10.1186/s12871-022-01885-1
Wang, J., Huang, J., Yang, S., Cui, C., Ye, L., Wang, S. Y., et al. (2019). Pharmacokinetics and safety of esketamine in Chinese patients undergoing painless gastroscopy in comparison with ketamine: a randomized, open-label clinical study. Drug. Des. devel. Ther. 13, 4135–4144. doi:10.2147/dddt.s224553
Xin, Z., Wang, P., Wang, N., Li, B., Yu, T., Gong, K., et al. (2024). Comparison of three intravenous sedation techniques used for extracting mandibular third molars in dental patients. J. Hard Tissue Biol. 33 (1), 61–66. doi:10.2485/jhtb.33.61
Xu, C., Wei, X., Zhang, C., Huang, X., Lan, H., Xu, Y., et al. (2022). Esketamine prevents propofol-induced injection pain: randomized controlled trial. Front. Pharmacol. 13, 991559. doi:10.3389/fphar.2022.991559
Xu, S. X., Shan, X. S., Gao, J. M., Liu, H. X., Chen, W. R., Gao, S. S., et al. (2022). Effect of esketamine vs dexmedetomidine adjunct to propofol sedation for pediatric 3Tesla magnetic resonance imaging: a randomized, double-blind, controlled trial. Eur. J. Med. Res. 27 (1), 258. doi:10.1186/s40001-022-00890-x
Xu, Y., Zheng, Y., Tang, T., Chen, L., Zhang, Y., and Zhang, Z. (2022). The effectiveness of esketamine and propofol versus dezocine and propofol sedation during gastroscopy: a randomized controlled study. J. Clin. Pharm. Ther. 47 (9), 1402–1408. doi:10.1111/jcpt.13678
Yang, H., Zhao, Q., Chen, H. Y., Liu, W., Ding, T., Yang, B., et al. (2022). The median effective concentration of propofol with different doses of esketamine during gastrointestinal endoscopy in elderly patients: a randomized controlled trial. Br. J. Clin. Pharmacol. 88 (3), 1279–1287. doi:10.1111/bcp.15072
Zhan, Y., Liang, S., Yang, Z., Luo, Q., Li, S., Li, J., et al. (2022). Efficacy and safety of subanesthetic doses of esketamine combined with propofol in painless gastrointestinal endoscopy: a prospective, double-blind, randomized controlled trial. Bmc. Gastroenterol. 22 (1), 391. doi:10.1186/s12876-022-02467-8
Zhang, W., Wang, L., Zhu, N., Wu, W., and Liu, H. (2024). A prospective, randomized, single-blinded study comparing the efficacy and safety of dexmedetomidine and propofol for sedation during endoscopic retrograde cholangiopancreatography. BMC Anesthesiol. 24 (1), 191. doi:10.1186/s12871-024-02572-z
Zheng, L., Wang, Y., Ma, Q., Liang, W., Zhang, X., Ren, Z., et al. (2023a). Efficacy and safety of a subanesthetic dose of esketamine combined with propofol in patients with obesity undergoing painless gastroscopy: a prospective, double-blind, randomized controlled trial. Drug Des. Dev. Ther. 17, 1347–1356. doi:10.2147/DDDT.S408076
Zheng, X., Huang, J., Wei, S., Tao, Y., Shen, Y., Wang, Y., et al. (2023b). Efficacy and safety comparison of esketamine-propofol with nalbuphine-propofol for upper gastrointestinal endoscopy in children: a multi-center randomized controlled trial. Front. Pediatr. 11, 1126522. doi:10.3389/fped.2023.1126522
Zheng, X. S., Shen, Y., Yang, Y. Y., He, P., Wang, Y. T., Tao, Y. Y., et al. (2022). ED(50) and ED(95) of propofol combined with different doses of esketamine for children undergoing upper gastrointestinal endoscopy: a prospective dose-finding study using up-and-down sequential allocation method. J. Clin. Pharm. Ther. 47 (7), 1002–1009. doi:10.1111/jcpt.13635
Zhong, Y., Jiang, M., Wang, Y., Su, T., Lv, Y., Fan, Z., et al. (2023). Evaluating efficacy and safety of sub-anesthetic dose esketamine as an adjuvant to propofol/remifentanil analgosedation and spontaneous respiration for children flexible fibreoptic bronchoscopy: a prospective, double-blinded, randomized, and placebo-controlled clinical trial. Front. Pharmacol. 14, 1184663. doi:10.3389/fphar.2023.1184663
Keywords: esketamine, sedation, analgesia, outside of operating room, propofol
Citation: Kan Z, Min W, Dai Y and Zhang P (2024) Intravenous esketamine as an adjuvant for sedation/analgesia outside the operating room: a systematic review and meta-analysis. Front. Pharmacol. 15:1287761. doi: 10.3389/fphar.2024.1287761
Received: 02 September 2023; Accepted: 17 June 2024;
Published: 03 July 2024.
Edited by:
Somchai Amornyotin, Mahidol University, ThailandReviewed by:
Mojtaba Akbari, Isfahan University of Medical Sciences, IranYuanyuan Mao, Fifth Affiliated Hospital of Zhengzhou University, China
Stefano Turi, San Raffaele Hospital (IRCCS), Italy
Copyright © 2024 Kan, Min, Dai and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Zhang, YW5lcGVuZ3poYW5nQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Ziheng Kan1†
Ziheng Kan1† Yuee Dai
Yuee Dai Peng Zhang
Peng Zhang