
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 15 January 2024
Sec. Pharmacology of Anti-Cancer Drugs
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1269922
This article is part of the Research Topic The Biomarkers, Mechanism, and Therapeutic Strategies of Cancer Immunotherapy Resistance View all 14 articles
Background: The clinical selection of three CDK4/6 inhibitors presents a challenging issue, owing to the absence of distinct clinical case characteristics, biomarkers, and their comparable clinical benefits in progression-free survival and overall survival To inform clinical treatment decisions, we conducted a comprehensive evaluation of the adverse events associated with CDK4/6 inhibitors in combination with endocrine therapy for hazard ratio+/HER2-breast cancer.
Methods: We searched Cochrane, PubMed, Embase, and Web of Science databases from their inception until 1 August 2022. The results were summarized narratively, and we assessed the methodological quality, reporting quality, and evidence quality of AEs by AMSTAR-2, PRISMA, and GRADE.
Results: Our analysis included 24 meta-analyses systematic reviews that evaluated the quality of AEs in 13 cases of early breast cancer (EBC) and 158 cases of advanced breast cancer The addition of CDK4/6 inhibitors was found to significantly increase AEs of any grade and AEs of grade 3 or higher in early breast cancer, along with a significant increase in the risk of treatment discontinuation. In advanced breast cancer, high and moderate-quality evidence indicated that CDK4/6 inhibitors significantly increased AEs across all grades, including grade 3/4 AEs, leucopenia, grade 3/4 leucopenia, neutropenia, grade 3/4 neutropenia, anemia, grade 3/4 anemia, nausea, grade 3/4 constipation, fatigue, pyrexia, venous thromboembolism abdominal pain, and cough. However, they did not significantly elevate the incidence of grade 3/4 diarrhea. Subgroup analysis revealed that palbociclib primarily increased hematologic toxicity, particularly grade 3/4 neutropenia, anemia, and thrombocytopenia. Ribociclib was mainly associated with grade 3/4 neutropenia, prolonged QT interval, and alopecia. Abemaciclib was closely linked with diarrhea and elevated blood creatinine levels.
Conclusion: The AEs associated with CDK4/6 inhibitors vary, necessitating individualized and precise clinical selection for optimal management. This approach should be based on the patient’s medical history and the distinct characteristics of different CDK4/6 inhibitors to improve the patient’s quality of life.
Systematic Review Registration: [https://systematicreview.gov/], identifier [CRD42022350167]
One of the primary factors contributing to the recurrence and metastasis of hormone receptor-positive (HR+)/HER2-negative (HER2-) early breast cancer (EBC) and advanced breast cancer (ABC) is resistance to endocrine therapy (ET) (Jeselsohn et al., 2015). Due to significant advancements in molecular biology, molecular targeted therapy for breast cancer has become increasingly popular. Notably, CDK4/6 inhibitors represent a major breakthrough in overcoming ET resistance and reducing the recurrence and metastasis of breast cancer. Numerous global, multicenter, clinical randomized controlled trials (RCTs) (PALOMA, MONARCH, MONALEESA, PALLAS, PENELOPE-B, etc.) conducted from 2014 to the present have investigated the efficacy of CDK4/6 inhibitors in combination with ET for HR+/HER2- EBC and ABC(Romero, 2017; Slamon et al., 2018; Tripathy et al., 2018; Hortobagyi et al., 2019; Loibl et al., 2021; Mayer et al., 2021). These combination therapies have significantly improved clinical prognosis. Consequently the Food and Drug Administration (FDA) approved three CDK4/6 inhibitors, palbociclib, ribociclib, and abemaciclib, for use as first and second-line treatments for HR+/HER2-metastatic breast cancer (MBC), based on these promising clinical trials (Mullard, 2017; Burstein et al., 2021). Additionally, abemaciclib received FDA approval for concurrent use with ET adjuvant treatment in patients with EBC who are HR+/HER2-, Ki-67 ≥ 20%, lymph node positivity, and at high risk of recurrence (Royce et al., 2022).
The safety profile of CDK4/6 inhibitors has garnered considerable attention. A thorough assessment of the adverse events (AEs) associated with CDK4/6 inhibitors can enhance clinical decision-making, monitoring, and management, thereby improving patient compliance and quality of life. Numerous systematic reviews/meta-analyses (SRs/MAs) have evaluated the safety of CDK4/6 inhibitors in combination with ET, focusing on diverse aspects such as bone marrow, gastrointestinal, skin AEs, and deep vein thrombosis (DVT) (Shohdy et al., 2017; Kassem et al., 2018; Thein et al., 2020; Silvestri et al., 2021). Nevertheless, limitations in study design, methodology, and procedures have resulted in varied evidence strengths, offering limited guidance for clinical practice. Based on this, this study is the first to summarize the AEs of CDK4/6 inhibitors combined with ET from SRs/MAs included in RCTs and to provide a comprehensive assessment using methodological quality, report quality and quality of evidence, with the aim of providing a basis for selection and reliable evidence for the clinical use of CDK4/6 inhibitors.
An umbrella review of the AEs of CDK4/6 inhibitors in the treatment of breast cancer patients was conducted according to the Joanna Briggs Institute (JBI) Manual for Evidence Synthesis. The protocol has been previously registered and published in The International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42022350167).
A comprehensive literature search was conducted in the Cochrane, PubMed, Embase, and Web of Science databases from their inception until August 1st. The objective was to gather literature pertaining to the use of CDK4/6 inhibitors in combination with ET for breast cancer. Both subject terms and free terms were employed in each database. Search phrases included “breast cancer," “Cyclin-Dependent Kinases 4 and 6 Inhibitors,” “Systematic review,” “Meta-analysis,” and the searches were limited to English (Supplementary Data S1).
Inclusion criteria were based on the PICOS (participant, intervention, comparison, outcome, and study type) framework (P) Participants were breast cancer patients of any race, age, or disease stage (I/C) the trial group received CDK4/6 inhibitors combined with ET, while the control group received ET alone or with placebo (O) outcomes measured included AEs, with data such as risk difference (RD), relative risk (RR), odds ratio (OR), hazard ratio (HR) (S) Study types were meta-analyses (MAs) and systematic reviews (SRs) comprising exclusively RCTs.
Exclusion criteria were: (a) duplicate publications; (b) articles that did not report the necessary data; (c) systematic review reevaluation plans, conference abstracts, etc.
Two reviewers (WY and XDB) independently conducted the screening and data extraction process. Initially, duplicate titles were removed, followed by screening of titles and abstracts, and then full-text evaluation based on the inclusion and exclusion criteria. Data extracted included first author, publication year, country, study population, sample size, interventions/control measures, outcome measures, and quality assessment methods. Any disagreements were resolved through the consensus of a third reviewer (CHH).
Following the Joanna Briggs Institute guidelines for Umbrella Reviews, we conducted a descriptive analysis of AEs for CDK4/6 inhibitors in combination with ET for breast cancer, without reanalyzing data from RCTs or MAs/SRs (Aromataris et al., 2015). We summarized indicators for CDK4/6 inhibitors, including RD, RR, OR, HR, 95% confidence interval (95% CI), and p-value. I2 was utilized to assess study heterogeneity, with statistical significance set at a p-value <0.05.
Two independent evaluators (WY and XDB) performed assessments using the Assessment of Multiple Systematic Reviews 2 (AMSTAR-2) scale, PRISMA statement, and the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) instrument and resolved disagreements by consensus of a third-party reviewer (Supplementary Data S2). First, the AMSTAR-2 scale, a systematic evaluative methodological quality assessment tool, was used to assess the study’s methodological quality (Shea et al., 2009; Shea et al., 2017). The AMSTAR-2 scale, a tool for assessing methodological quality, includes 16 items scored as “yes,” “no,” or “partially yes,” based on the criteria fulfillment. The PRISMA 2020 checklist was employed for assessing reporting quality, comprising 27 items (42 sub-item levels) (Hutton et al., 2015). Each item was scored as 1 for complete reporting, 0.5 for partial, and 0 for non-reporting, with a total possible score of 42. Scores of 33–42 indicated relatively complete reporting, 25–32 indicated some deficiencies, and scores below 25 signified serious information deficiencies (Page et al., 2021). The GRADE tool evaluated evidence quality for each outcome by examining risk of bias, inconsistency, indirectness, precision, and publication bias, categorizing outcome indicators as high, moderate, low, or very low based on downgrades (Schunemann et al., 2020; Zeng et al., 2021).
From four database searches, 425 pieces of literature were initially retrieved. After removing duplicates, screening titles and abstracts, and evaluating full texts, 24 studies met the inclusion criteria. The literature screening process and results are depicted (Figure 1). Publications spanned from 2017 to 2021, with study numbers ranging from 3 to 9 and sample sizes from 1,352 to 12,647. Regarding the included population, two studies focused on HR+/HER2- EBC(Agostinetto et al., 2021; Gao et al., 2021), with the remainder addressing HR+/HER2- ABC. In terms of therapeutic interventions, one study (Ramos-Esquivel et al., 2020) compared CDK4/6 inhibitor combined with fulvestrant versus fulvestrant alone, two studies (Ramos-Esquivel et al., 2018; Shimoi et al., 2020) compared CDK4/6 inhibitor with AI versus AI alone, and the rest involved CDK4/6 inhibitor combined with ET versus ET treatment. For study outcomes, 11 studies reported pooled AE outcomes, such as all-grade AEs, grade 3/4 AEs, and grades 3–5 AEs (Deng et al., 2018; Martel et al., 2018; Messina et al., 2018; Ramos-Esquivel et al., 2018; Ramos-Esquivel et al., 2020; Shimoi et al., 2020; Zheng et al., 2020; Agostinetto et al., 2021; Gao et al., 2021; Li et al., 2021; Tian et al., 2021). The other studies reported specific AE outcomes without pooled AE data (Table 1).
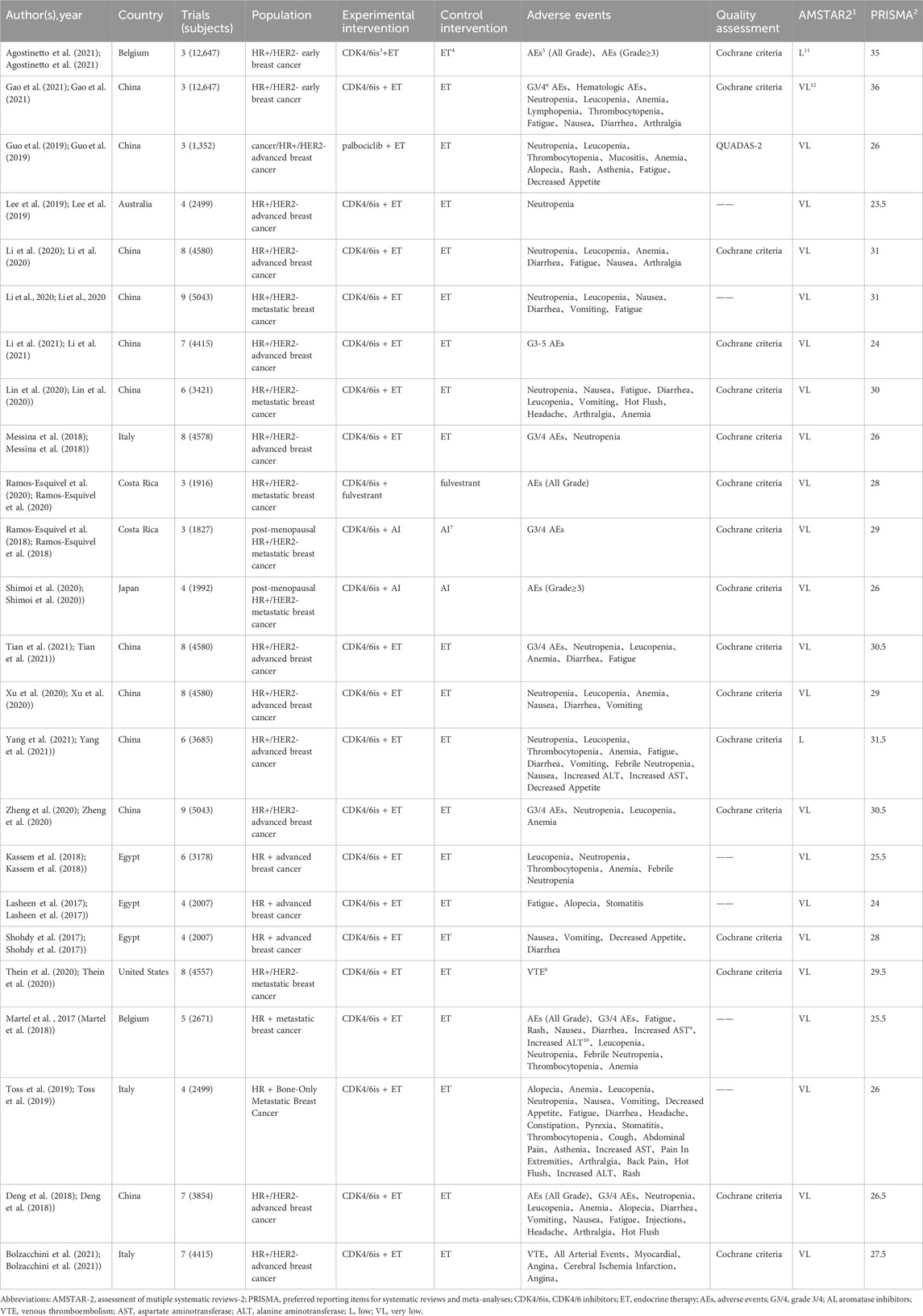
TABLE 1. Main characteristics of systematic reviews and meta-analyses included in the umbrella review.
AMSTAR-2, an extensive critical appraisal tool, facilitates rapid and reproducible assessments of systematic reviews of randomized controlled trials (RCTs) for interventions, thereby identifying high-quality evaluations for decision-makers (Shea et al., 2017). We utilized the AMSTAR-2 scale to appraise the methodological quality of the included studies. Two studies were evaluated as low quality (Agostinetto et al., 2021; Yang et al., 2021), while the remainder were deemed very low quality. For specific AMSTAR-2 criteria, entries 1, 3, and 11 achieved 100% compliance, indicating all included PICO, specified the types of literature included, and employed appropriate statistical methods for combined result analysis. Conversely, entries 7, 8, 10, and 14 had compliance rates of 0%, 13%, 8%, and 17%, respectively, highlighting significant gaps in justifying the inclusion of study types, listing and explaining excluded literature, detailing study characteristics, reporting funding sources, and elucidating result heterogeneity (Table 1; Figure 2, and Supplementary Data S3).
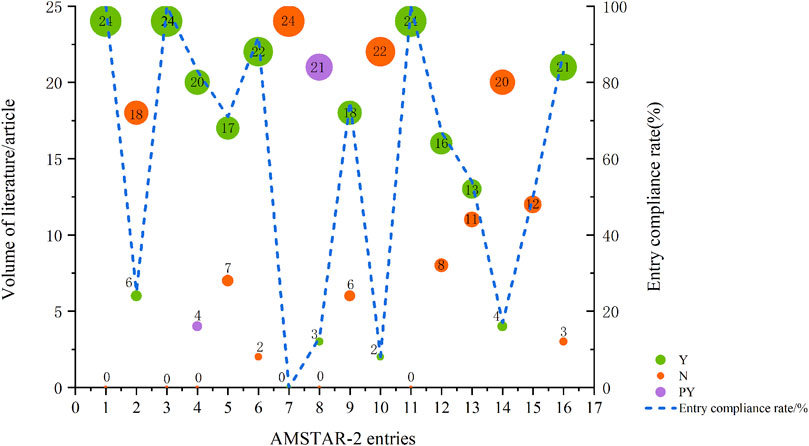
FIGURE 2. Results of the AMSTAR-2 assessment. Abbreviations: Y, Yes; N, No; PY, Partial Yes; each entry is Y for full compliance, PY for partial compliance, and N for non-compliance; entry compliance rate = (number of documents complying with this entry/total documents) * 100%.
PRISMA 2020 serves as a reporting guideline for systematic reviews of health intervention studies, regardless of study design. Its comprehensive reporting allows readers to evaluate the methodological soundness and credibility of study results (Page et al., 2021). We applied the PRISMA checklist to assess the reporting quality of the 24 included papers, which scored between 23.5 and 36. Two reports, scoring from 33 to 42, were relatively complete (Agostinetto et al., 2021; Gao et al., 2021). Nineteen reports, scoring from 25 to 32, exhibited some deficiencies. Three reports (Lasheen et al., 2017; Lee et al., 2019; Li et al., 2021) scoring less than 25 had serious deficiencies. Common omissions included item 7 (detailing the search strategy for databases), 13f (conducting sensitivity analysis in synthesis methods), 15 (assessing certainty), 16b (explaining literature exclusion reasons in results), 20d (providing sensitivity analysis in synthesis results), 22 (providing evidence certainty in results), 23c (discussing any limitations in the review process), and 24abc (providing registration and protocol-related information) (Figure 3; Table 1 and Supplementary Data S4).
Patients treated with CDK4/6 inhibitors, compared to ET alone, experienced a higher incidence of AEs. The main AE categories were distributed across nine areas (n = number of MAs): summary AEs (n = 14), hematological AEs (n = 15), gastrointestinal AEs (n = 13), systemic AEs (n = 11), liver AEs (n = 2), circulatory AEs s (n = 2), skin AEs (n = 5), pain-related AEs (n = 5) and other types AEs (n = 2). Notably, hematological and gastrointestinal AEs were more prevalent, with frequent reports of leucopenia, neutropenia, thrombocytopenia, anemia, diarrhea, nausea, and vomiting. Among systemic AEs, reports of fatigue were substantial (Figure 4).
A total of 2 MAs for EBC investigated the AEs of CDK4/6 inhibitors in combination with ET, focusing on summary AEs, hematologic AEs, gastrointestinal AEs, systemic AEs, and other AEs (Agostinetto et al., 2021; Gao et al., 2021). Adjuvant CDK4/6 inhibitors were notably associated with an increased overall incidence of all-grade AEs and grade 3 AEs, although the results showed considerable heterogeneity and the evidence quality was extremely low, based on data pooled from two studies. Gao HF’s research highlighted a focus on hematologic and systemic AEs, especially grade 3/4 leucopenia, neutropenia, lymphopenia, thrombocytopenia, and fatigue. However, the incidence of other AEs did not significantly differ between combination therapy and ET alone (Gao et al., 2021). Gao HF also conducted a subgroup analysis of CDK4/6 inhibitors, finding no significant differences in grade 3/4 AEs between abemaciclib and palbociclib. Agostinetto E et al. reported that adding a CDK4/6 inhibitor to ET significantly increased the likelihood of early treatment cessation (OR = 22.11, 95%CI: 9.45–51.69, p < 0.001) (Agostinetto et al., 2021) (Figure 5, Supplementary Data S5).
Twenty-two MAs reported on the AEs of CDK4/6 inhibitor combined with ET in patients with ABC, pooling the most comprehensive data and high-quality evidence for each AE (Figure 6, Supplementary Data S5). Nine studies were examined for summary AEs (Deng et al., 2018; Messina et al., 2018; Ramos-Esquivel et al., 2018; Shimoi et al., 2020; Zheng et al., 2020; Li et al., 2021; Tian et al., 2021). The results showed that the combination of CDK4/6 inhibitor and ET significantly increased the incidence of all-grade AEs and grade 3 or higher AEs in postmenopausal, bone metastasis-only, or ABC patients. Despite the overall benefit of CDK4/6 inhibitors combined with ET for AEs, Ramos-Esquivel A et al. reported more favorable outcomes for CDK4/6 inhibitors combined with fulvestrant, indicating that while the number of serious AEs was higher in the combination group, the advantage ratio for any serious AE was not statistically significant (OR = 1.51.95% CI: 0.74–3.08, p = 0.26), and the evidence quality was low (Ramos-Esquivel et al., 2020).
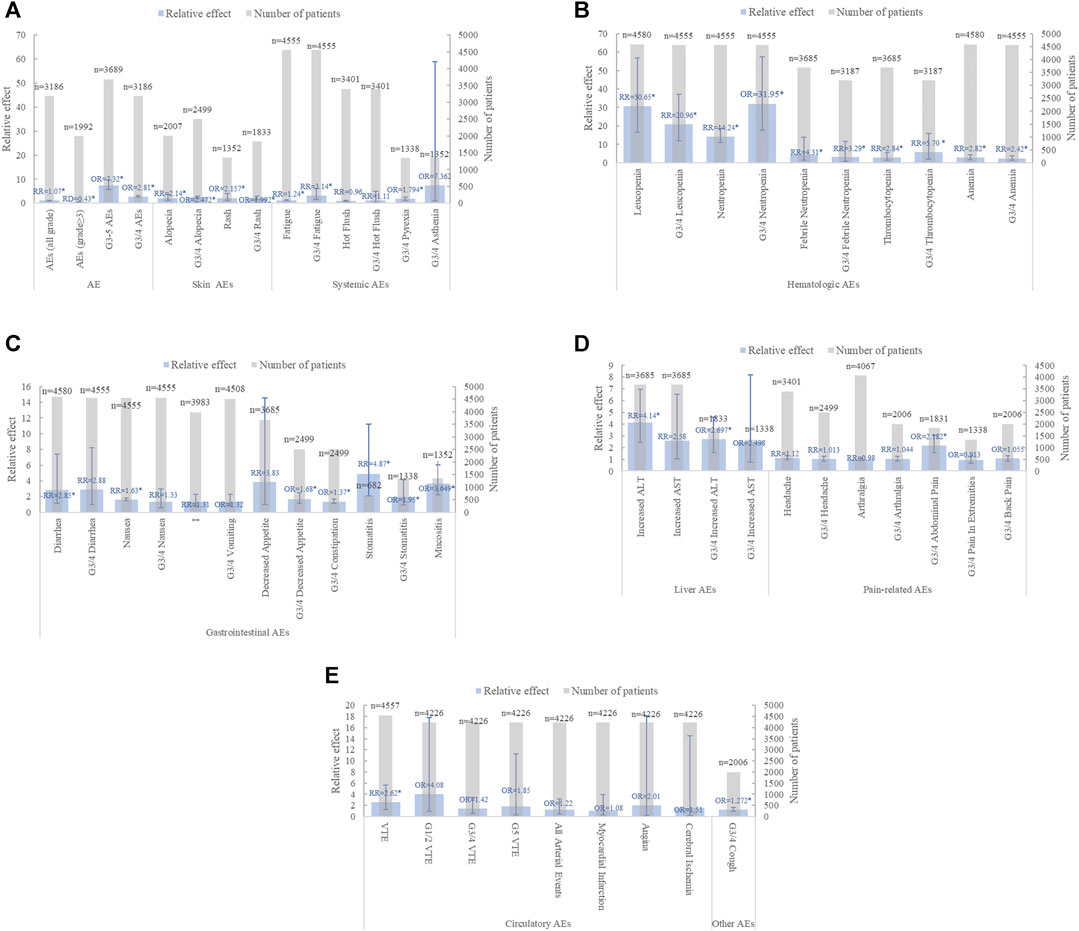
FIGURE 6. Summary chart of adverse events in advanced breast cancer (A) summary AE、skin AEs and systemic AEs (B) hematological AEs (C) gastrointestinal AEs (D) Liver AEs and pain-related AEs (E) Circulatory AEs and other types AEs (n = 2).
In the category of any-grade AEs, the incidence of hematologic and gastrointestinal AEs significantly increased when CDK4/6 inhibitors were combined with ET. Notably, significant increases were observed in Neutropenia (n = 9), Leukopenia (n = 8), Anemia (n = 8), Thrombocytopenia (n = 3), Febrile Neutropenia (n = 1), Diarrhea (n = 6), Fatigue (n = 6), Alopecia (n = 3), and VTE (n = 2). However, the association between nausea, vomiting, and decreased appetite in the gastrointestinal tract with combination therapy showed mixed results. Li JM(Li et al., 2020), Xu ZH (Xu et al., 2020), and Yang L (Yang et al., 2021) found no significant difference in nausea and vomiting between the two treatment groups. In contrast, six and three additional studies, respectively, concluded that CDK4/6 inhibitors significantly increased the incidence of nausea and vomiting. Furthermore, Shohdy KS (Shohdy et al., 2017) analyzed 1,350 patients from three clinical trials and observed a significant increase in decreased appetite with combination therapy, while Yang L analyzed 3,685 breast cancer patients from six clinical trials and reported no significant difference between the groups (Yang et al., 2021).
In the category of grade 3/4 AEs, hematologic, gastrointestinal, and systemic AEs were prevalent in the CDK4/6 inhibitor group combined with ET. Among hematologic AEs, grade 3/4 neutropenia (n = 8), leukopenia (n = 7), anemia (n = 6), and thrombocytopenia (n = 3) were significantly more common in the combination treatment than in the ET group alone. Notably, GUO LH (Guo et al., 2019) was more controversial regarding the grade 3/4 Fatigue outcome in systemic AEs. Guo LH (Guo et al., 2019) reported no significant risk of grade 3/4 fatigue in the combination therapy group, while six other studies indicated a higher risk (Lasheen et al., 2017; Martel et al., 2018; Toss et al., 2019; Li et al., 2020; Lin et al., 2020; Tian et al., 2021). Interestingly, diarrhea, which showed a significant increase in any-grade AEs, did not significantly differ in grade 3/4 AEs (Shohdy et al., 2017; Toss et al., 2019; Li et al., 2020; Lin et al., 2020; Tian et al., 2021).
Seven studies conducted a subgroup analysis of AEs by CDK4/6 inhibitor type (Lasheen et al., 2017; Kassem et al., 2018; Ramos-Esquivel et al., 2018; Li et al., 2020; Lin et al., 2020; Ramos-Esquivel et al., 2020; Tian et al., 2021) Palbociclib exhibited increased hematologic toxicity, particularly in grade 3/4 neutropenia (n = 2), anemia (n = 1), and thrombocytopenia, while ribociclib was more associated with grade 3/4 neutropenia (n = 2), prolonged QT interval (n = 2), and alopecia (n = 1). Abemaciclib was closely linked to diarrhea (n = 4) and elevated blood creatinine (n = 1).
The likelihood of drug toxicity necessitating therapeutic dose reduction and cessation is a crucial aspect of drug safety assessment. In a meta-analysis by Kassem L, the rate of dose reduction in the CDK4/6 inhibitor group varied from 31.6% to 53.9%, and discontinuation rates due to toxicity ranged from 2.6% to 19.6% (Kassem et al., 2018). Most dose-limiting toxicities were hematologic AEs, although clarity is lacking on whether AEs like fatigue, stomatitis (Lasheen et al., 2017), and gastrointestinal toxicity (Shohdy et al., 2017) can be managed through dose adjustment or discontinuation.
GRADE provides a framework for authors of systematic reviews and health technology assessments to rate the certainty of their evidence (Zeng et al., 2021). This study summarized 13 AE outcomes for two EBCs and 158 AE outcomes for 22 ABC studies. The evidence quality for EBC was predominantly low (4 items, about 30.77%) and very low (9 items, about 69.23%), as assessed using GRADE. The highest level of evidence in ABC was for the venous thromboembolism (VTE) outcome (Thein et al., 2020), Moderate quality evidence was found in five studies (Deng et al., 2018), Tian Q (Tian et al., 2021), Xu ZH (Xu et al., 2020), Li J (Li et al., 2020), Toss A (Toss et al., 2019) addressing various AEs including all-grade AEs, grade 3/4 AEs, leukopenia, grade 3/4 leukopenia, neutropenia, grade 3/4 neutropenia, anemia, grade 3/4 anemia, grade 3/4 diarrhea, and nausea, among others (20 items, about 12.66%). The remaining evidence was categorized as low quality (70 items, about 44.30%) and very low quality (67 items, about 42.40%). Overall, the reduced quality of evidence was largely attributed to inconsistency, risk of bias, and publication bias (Supplementary Data S5).
MA and SR have emerged as crucial supports for clinical decision-making, representing the highest level of evidence in the hierarchy of evidence-based medicine. Conducting effective quality evaluations is essential for the efficient utilization of MA and SR (Gelardi et al., 2021). The meticulous summarization and quality assessment of the clinical AEs of CDK4/6 inhibitors in combination with ET in this study aimed to enhance clinical decision-making, monitoring, and management, thereby improving patient compliance and clinical outcomes.
Our review identified 24 MAs and SRs evaluating AEs in 13 EBC cases and 158 ABC cases. Our findings indicated that AEs associated with CDK4/6 inhibitors spanned nine disease areas, including summary AEs, hematologic AEs, gastrointestinal AEs, systemic AEs, liver AEs, circulatory AEs, skin AEs, pain-related AEs, and other AEs. In HR+/HER2- EBC patients, CDK4/6 inhibitor addition significantly correlated with all-grade AEs and grade 3/4 AEs, and notably increased early treatment discontinuation risk. Subgroup analysis showed that EBC AEs were independent of whether palbociclib or abemaciclib was used. For HR+/HER2- ABC patients, combined therapy significantly elevated AEs across all grades, including grade 3/4 leukopenia, neutropenia, anemia, diarrhea, nausea, constipation, fatigue, pyrexia, VTE, abdominal pain, and cough. However, their safety profile was considered acceptable, with evidence quality mostly high to medium. Subgroup analysis of CDK4/6 inhibitor types in ABC revealed that palbociclib was associated with greater hematologic toxicity, especially grade 3/4 neutropenia, anemia, and thrombocytopenia, while ribociclib was linked mainly to grade 3/4 neutropenia, QT interval prolongation, and alopecia. Abemaciclib is closely associated with diarrhea and elevated blood creatinine.
With the increasing use of CDK4/6 inhibitors combined with ET in first and second-line clinical settings, anticipating AEs' risk, timely diagnosis, and management are pivotal in enhancing patients' quality of life (Martel et al., 2018). Despite a high incidence of hematologic, gastrointestinal, and systemic AEs in both EBC and ABC, most were reversible and manageable. Neutropenia was the most common hematologic toxicity, particularly with palbociclib and ribociclib, but it led to febrile neutropenia or infection at much lower rates than chemotherapy. The mechanism of CDK4/6 inhibitors involves reversible blocking of neutrophil precursor cycles, inhibiting proliferation, which is reversible upon discontinuation and tends to lessen over time (Kassem et al., 2018). Thus, clinical applications require monitoring of complete blood counts and timely intervention through discontinuation, dose adjustment, or symptomatic treatment based on individual safety and tolerability (Thill and Schmidt, 2018). Gastrointestinal AEs, such as diarrhea and nausea, especially with abemaciclib, did not significantly increase serious gastrointestinal risk. This may be attributed to abemaciclib’s CDK9 inhibitory effect (Marra and Curigliano, 2019). As diarrhea is typically short-lived and of low severity, it can be managed through dose adjustment or antidiarrheal medications like loperamide. Fatigue, significantly increased in any grade and grade 3/4 AEs, poses a challenge in systemic AEs due to its vague and multidimensional nature, making identification of contributing factors and mitigation measures difficult (Lasheen et al., 2017).
Significantly, the findings related to nausea and vomiting associated with CDK4/6 inhibitors were inconsistent across different AE categories. This inconsistency may stem from variations in the types of CDK4/6 inhibitors used in clinical trials, their modes of administration, differences in study subjects, and the range of clinical trials included in the SRs. Consequently, further research is necessary to reach definitive conclusions. Additionally, we noted substantial heterogeneity in some outcomes. Fundamental aspects of the included studies, such as study population, design, intervention/control measures (dose), follow-up activities, analysis procedures, and outcome indicators, were not adequately detailed, and limited raw data might have contributed to this heterogeneity.
The clinical selection of the three CDK4/6 inhibitors presents a challenging issue, primarily due to the absence of distinct clinical case characteristics, predictive biomarkers, and comparable clinical benefits in PFS and OS(Agostinetto et al., 2021; Gao et al., 2021; Munzone et al., 2021; Falato et al., 2023). A subgroup analysis focusing on CDK4/6 inhibitor types in ABC aimed to address this issue. Palbociclib was associated with increased neutropenia, anemia, and thrombocytopenia, while ribociclib was linked mainly to neutropenia, prolonged QT interval, and alopecia. Abemaciclib was strongly correlated with diarrhea and elevated blood creatinine. This suggests that patients with ABC who have hematological disorders or tendencies may be better suited for abemaciclib, while those with gastrointestinal disorders and renal dysfunction or tendencies might be more appropriate for ribociclib or palbociclib. Furthermore, patients with cardiac disorders or tendencies might be more suitable for ribociclib or abemaciclib. This could be a significant opportunity to improve patient quality of life, advance clinical decision-making accuracy, and achieve personalized precision medicine.
To our knowledge, this is the first to use an umbrella review of studies to summarize CDK4/6 inhibitors combined with ET for HR+/HER2-breast cancer AEs. This approach mitigates the bias inherent in individual MAs/SRs and enhances the accuracy of study outcomes. Second, we included MAs/SRs that used only RCT study types and excluded literature that used single-arm studies, and non-randomized prospective, retrospective, and observational studies as study types, which further reduced the interference of subjective factors and improved the scientific validity of the findings. In Additionally, this review employed the recently published PRISMA 2020 guidelines for reporting quality assessment (Sohrabi et al., 2021), a significant update over the PRISMA 2009 guidelines typically referenced in most MAs/SRs (Shea et al., 2009). The PRISMA 2020 guidelines offer improvements in areas like data items, data synthesis methods, study outcome selection, and results synthesis. These updated guidelines facilitate the generation of high-quality evidence that can support clinical decision-making and practice (Page et al., 2021).
This review, however, is not without limitations. Firstly, despite employing the latest versions of AMSTAR-2, PRISMA 2020, and GRADE for quality assessment of the selected literature, it is important to note that all three scales are inherently subjective, which could introduce bias to our findings. To mitigate this, two researchers independently conducted the assessments, with any differences resolved through consensus by a third-party reviewer (CHH), thereby aiming to maximize the accuracy of our evaluation. Moreover, the MAs/SRs included in this study were based on global multicenter RCTs, potentially leading to publication bias and a diminished quality of evidence due to the limited number of RCTs.
In patients with HR+/HER2- EBC, the addition of CDK4/6 inhibitors significantly increased the incidence of all-grade AEs and grade 3/4 AEs compared to ET alone, along with a notable rise in the risk of early treatment discontinuation. In patients with HR+/HER2- ABC, palbociclib was associated with increased hematologic toxicity, primarily grade 3/4 neutropenia, anemia, and thrombocytopenia, while ribociclib was linked mainly to grade 3/4 neutropenia, QT interval prolongation, and alopecia. Abemaciclib was closely related to diarrhea and elevated blood creatinine. Despite these findings, the safety of these inhibitors was deemed acceptable, and the overall quality of evidence was mostly moderate. The comprehensive summary and evaluation of these AEs will aid in the selection of tailored and precise treatments based on the history of breast cancer patients. Furthermore, it will assist clinicians in effectively anticipating, diagnosing, and managing AEs associated with CDK4/6 inhibitors, ultimately enhancing patients' quality of life.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
DP: Visualization, Writing–original draft. YW: Methodology, Writing–original draft. DX: Methodology, Writing–original draft. GS: Data curation, Writing–review and editing. HC: Methodology, Project administration, Writing–review and editing. DF: Formal Analysis, Writing–review and editing. MZ: Formal Analysis, Writing–review and editing. JL: Writing–review and editing, Writing–original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by National Natural Science Foundation of China (No:82374452), the Academic Leaders of the 2022 High-Level Talent Training Program in Chinese Medicine (No [2022]148), and the Emerging Tumor Supportive Therapy Topic (No. cphcf-2022-191).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1269922/full#supplementary-material
Agostinetto, E., Vian, L., Caparica, R., Bruzzone, M., Ceppi, M., Lambertini, M., et al. (2021). CDK4/6 inhibitors as adjuvant treatment for hormone receptor-positive, HER2-negative early breast cancer: a systematic review and meta-analysis. ESMO Open 6 (2), 100091. doi:10.1016/j.esmoop.2021.100091
Aromataris, E., Fernandez, R., Godfrey, C. M., Holly, C., Khalil, H., and Tungpunkom, P. (2015). Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int. J. Evid. Based Healthc. 13 (3), 132–140. doi:10.1097/XEB.0000000000000055
Bolzacchini, E., Pomero, F., Fazio, M., Civitelli, C., Fabro, G., Pellegrino, D., et al. (2021). Risk of venous and arterial thromboembolic events in women with advanced breast cancer treated with CDK 4/6 inhibitors: a systematic review and meta-analysis. Thromb. Res. 208, 190–197. doi:10.1016/j.thromres.2021.11.009
Burstein, H. J., Somerfield, M. R., Barton, D. L., Dorris, A., Fallowfield, L. J., Jain, D., et al. (2021). Endocrine treatment and targeted therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: ASCO guideline update. J. Clin. Oncol. 39 (35), 3959–3977. doi:10.1200/JCO.21.01392
Deng, Y., Ma, G., Li, W., Wang, T., Zhao, Y., and Wu, Q. (2018). CDK4/6 inhibitors in combination with hormone therapy for hr+/her2− advanced breast cancer: a systematic review and meta-analysis of randomized controlled trials. Clin. Breast Cancer 18 (5), e943–e953. doi:10.1016/j.clbc.2018.04.017
Falato, C., Schettini, F., Pascual, T., Braso-Maristany, F., and Prat, A. (2023). Clinical implications of the intrinsic molecular subtypes in hormone receptor-positive and HER2-negative metastatic breast cancer. Cancer Treat. Rev. 112, 102496. doi:10.1016/j.ctrv.2022.102496
Gao, H., Lin, Y., Zhu, T., Ji, F., Zhang, L., Yang, C., et al. (2021). Adjuvant CDK4/6 inhibitors combined with endocrine therapy in HR-positive, HER2-negative early breast cancer: a meta-analysis of randomized clinical trials. Breast 59, 165–175. doi:10.1016/j.breast.2021.07.002
Gelardi, F., Kirienko, M., and Sollini, M. (2021). Climbing the steps of the evidence-based medicine pyramid: highlights from Annals of Nuclear Medicine 2019. Eur. J. Nucl. Med. Mol. Imaging 48 (5), 1293–1301. doi:10.1007/s00259-020-05073-6
Guo, L., Hu, Y., Chen, X., Li, Q., Wei, B., and Ma, X. (2019). Safety and efficacy profile of cyclin-dependent kinases 4/6 inhibitor palbociclib in cancer therapy: a meta-analysis of clinical trials. Cancer Med. 8 (4), 1389–1400. doi:10.1002/cam4.1970
Hortobagyi, G. N., Stemmer, S. M., Burris, H. A., Yap, Y. S., Sonke, G. S., Paluch-Shimon, S., et al. (2019). Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 30 (11), 1842. doi:10.1093/annonc/mdz215
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 162 (11), 777–784. doi:10.7326/M14-2385
Jeselsohn, R., Buchwalter, G., De Angelis, C., Brown, M., and Schiff, R. (2015). ESR1 mutations-a mechanism for acquired endocrine resistance in breast cancer. Nat. Rev. Clin. Oncol. 12 (10), 573–583. doi:10.1038/nrclinonc.2015.117
Kassem, L., Shohdy, K. S., Lasheen, S., Abdel-Rahman, O., and Bachelot, T. (2018). Hematological adverse effects in breast cancer patients treated with cyclin-dependent kinase 4 and 6 inhibitors: a systematic review and meta-analysis. Breast Cancer-Tokyo 25 (1), 17–27. doi:10.1007/s12282-017-0818-4
Lasheen, S., Shohdy, K. S., Kassem, L., and Abdel-Rahman, O. (2017). Fatigue, alopecia and stomatitis among patients with breast cancer receiving cyclin-dependent kinase 4 and 6 inhibitors: a systematic review and meta-analysis. Expert Rev. anticanc. 17 (9), 851–856. doi:10.1080/14737140.2017.1355242
Lee, K. W. C., Lord, S., Finn, R. S., Lim, E., Martin, A., Loi, S., et al. (2019). The impact of ethnicity on efficacy and toxicity of cyclin D kinase 4/6 inhibitors in advanced breast cancer: a meta-analysis. Breast Cancer Res. Tr. 174 (1), 271–278. doi:10.1007/s10549-018-5054-x
Li, J., Fu, F., Yu, L., Huang, M., Lin, Y., Mei, Q., et al. (2020). Cyclin-dependent kinase 4 and 6 inhibitors in hormone receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer: a meta-analysis of randomized clinical trials. Breast Cancer Res. Tr. 180 (1), 21–32. doi:10.1007/s10549-020-05528-2
Li, J., Huo, X., Zhao, F., Ren, D., Ahmad, R., Yuan, X., et al. (2020). Association of cyclin-dependent kinases 4 and 6 inhibitors with survival in patients with hormone receptor-positive metastatic breast cancer: a systematic review and meta-analysis. JAMA Netw. Open 3 (10), e2020312. doi:10.1001/jamanetworkopen.2020.20312
Li, Y., Li, L., Du, Q., Li, Y., Yang, H., and Li, Q. (2021). Efficacy and safety of CDK4/6 inhibitors combined with endocrine therapy in hr+/HER-2- abc patients: a systematic review and meta-analysis. Cancer Invest. 39 (5), 369–378. doi:10.1080/07357907.2021.1910705
Lin, M., Chen, Y., Jin, Y., Hu, X., and Zhang, J. (2020). Comparative overall survival of CDK4/6 inhibitors plus endocrine therapy vs. Endocrine therapy alone for hormone receptor-positive, HER2-negative metastatic breast cancer. J. Cancer. 11 (24), 7127–7136. doi:10.7150/jca.48944
Loibl, S., Marme, F., Martin, M., Untch, M., Bonnefoi, H., Kim, S. B., et al. (2021). Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer-the penelope-B trial. J. Clin. Oncol. 39 (14), 1518–1530. doi:10.1200/JCO.20.03639
Marra, A., and Curigliano, G. (2019). Are all cyclin-dependent kinases 4/6 inhibitors created equal? NPJ Breast Cancer 5, 27. doi:10.1038/s41523-019-0121-y
Martel, S., Bruzzone, M., Ceppi, M., Maurer, C., Ponde, N. F., Ferreira, A. R., et al. (2018). Risk of adverse events with the addition of targeted agents to endocrine therapy in patients with hormone receptor-positive metastatic breast cancer: a systematic review and meta-analysis. Cancer Treat. Rev. 62, 123–132. doi:10.1016/j.ctrv.2017.09.009
Mayer, E. L., Dueck, A. C., Martin, M., Rubovszky, G., Burstein, H. J., Bellet-Ezquerra, M., et al. (2021). Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 22 (2), 212–222. doi:10.1016/S1470-2045(20)30642-2
Messina, C., Cattrini, C., Buzzatti, G., Cerbone, L., Zanardi, E., Messina, M., et al. (2018). CDK4/6 inhibitors in advanced hormone receptor-positive/HER2-negative breast cancer: a systematic review and meta-analysis of randomized trials. Breast Cancer Res. Tr. 172 (1), 9–21. doi:10.1007/s10549-018-4901-0
Mullard, A. (2017). FDA approves Novartis's CDK4/6 inhibitor. Nat. Rev. Drug Discov. 16 (4), 229. doi:10.1038/nrd.2017.62
Munzone, E., Pagan, E., Bagnardi, V., Montagna, E., Cancello, G., Dellapasqua, S., et al. (2021). Systematic review and meta-analysis of post-progression outcomes in ER+/HER2− metastatic breast cancer after CDK4/6 inhibitors within randomized clinical trials. ESMO Open 6 (6), 100332. doi:10.1016/j.esmoop.2021.100332
Page, M. J., Mckenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Ramos-Esquivel, A., Hernández-Romero, G., and Landaverde, D. U. (2020). Cyclin-dependent kinase 4/6 inhibitors in combination with fulvestrant for previously treated metastatic hormone receptor-positive breast cancer patients: a systematic review and meta-analysis of randomized clinical trials. Cancer Treat. Res. Commun. 23, 100175. doi:10.1016/j.ctarc.2020.100175
Ramos-Esquivel, A., Hernández-Steller, H., Savard, M., and Landaverde, D. U. (2018). Cyclin-dependent kinase 4/6 inhibitors as first-line treatment for post-menopausal metastatic hormone receptor-positive breast cancer patients: a systematic review and meta-analysis of phase III randomized clinical trials. Breast Cancer-Tokyo 25 (4), 479–488. doi:10.1007/s12282-018-0848-6
Romero, D. (2017). Breast cancer: PALOMA-2 - hope beyond the threshold. Nat. Rev. Clin. Oncol. 14 (1), 1. doi:10.1038/nrclinonc.2016.202
Royce, M., Osgood, C., Mulkey, F., Bloomquist, E., Pierce, W. F., Roy, A., et al. (2022). FDA approval summary: abemaciclib with endocrine therapy for high-risk early breast cancer. J. Clin. Oncol. 40 (11), 1155–1162. doi:10.1200/JCO.21.02742
Schunemann, H. J., Mustafa, R. A., Brozek, J., Steingart, K. R., Leeflang, M., Murad, M. H., et al. (2020). GRADE guidelines: 21 part 2. Test accuracy: inconsistency, imprecision, publication bias, and other domains for rating the certainty of evidence and presenting it in evidence profiles and summary of findings tables. J. Clin. Epidemiol. 122, 142–152. doi:10.1016/j.jclinepi.2019.12.021
Shea, B. J., Hamel, C., Wells, G. A., Bouter, L. M., Kristjansson, E., Grimshaw, J., et al. (2009). AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J. Clin. Epidemiol. 62 (10), 1013–1020. doi:10.1016/j.jclinepi.2008.10.009
Shea, B. J., Reeves, B. C., Wells, G., Thuku, M., Hamel, C., Moran, J., et al. (2017). AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358, j4008. doi:10.1136/bmj.j4008
Shimoi, T., Sagara, Y., Hara, F., Toyama, T., and Iwata, H. (2020). First-line endocrine therapy for postmenopausal patients with hormone receptor-positive, HER2-negative metastatic breast cancer: a systematic review and meta-analysis. Breast Cancer-Tokyo 27 (3), 340–346. doi:10.1007/s12282-020-01054-7
Shohdy, K. S., Lasheen, S., Kassem, L., and Abdel-Rahman, O. (2017). Gastrointestinal adverse effects of cyclin-dependent kinase 4 and 6 inhibitors in breast cancer patients: a systematic review and meta-analysis. Ther. Adv. Drug Saf. 8 (11), 337–347. doi:10.1177/2042098617722516
Silvestri, M., Cristaudo, A., Morrone, A., Messina, C., Bennardo, L., Nistico, S. P., et al. (2021). Emerging skin toxicities in patients with breast cancer treated with new cyclin-dependent kinase 4/6 inhibitors: a systematic review. Drug Saf. 44 (7), 725–732. doi:10.1007/s40264-021-01071-1
Slamon, D. J., Neven, P., Chia, S., Fasching, P. A., De Laurentiis, M., Im, S. A., et al. (2018). Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J. Clin. Oncol. 36 (24), 2465–2472. doi:10.1200/JCO.2018.78.9909
Sohrabi, C., Franchi, T., Mathew, G., Kerwan, A., Nicola, M., Griffin, M., et al. (2021). PRISMA 2020 statement: what's new and the importance of reporting guidelines. Int. J. Surg. 88, 105918. doi:10.1016/j.ijsu.2021.105918
Thein, K. Z., Htut, T. W., Ball, S., Swarup, S., Sultan, A., and Oo, T. H. (2020). Venous thromboembolism risk in patients with hormone receptor-positive HER2-negative metastatic breast cancer treated with combined CDK 4/6 inhibitors plus endocrine therapy versus endocrine therapy alone: a systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res. Tr. 183 (2), 479–487. doi:10.1007/s10549-020-05783-3
Thill, M., and Schmidt, M. (2018). Management of adverse events during cyclin-dependent kinase 4/6 (CDK4/6) inhibitor-based treatment in breast cancer. Ther. Adv. Med. Oncol. 10, 1758835918793326. doi:10.1177/1758835918793326
Tian, Q., Gao, H., Zhou, Y., and Yang, J. (2021). Overall survival and progression-free survival with cyclin-dependent kinase 4/6 inhibitors plus endocrine therapy in breast cancer: an updated meta-analysis of randomized controlled trials. Eur. Rev. Med. Pharmacol. Sci. 25 (23), 7252–7267. doi:10.26355/eurrev_202112_27418
Toss, A., Venturelli, M., Sperduti, I., Molinaro, E., Isca, C., Barbieri, E., et al. (2019). First-line treatment for endocrine-sensitive bone-only metastatic breast cancer: systematic review and meta-analysis. Clin. Breast Cancer 19 (6), e701–e716. doi:10.1016/j.clbc.2019.06.011
Tripathy, D., Im, S. A., Colleoni, M., Franke, F., Bardia, A., Harbeck, N., et al. (2018). Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 19 (7), 904–915. doi:10.1016/S1470-2045(18)30292-4
Xu, Z., Zhang, H., Wei, D., Xie, L., and Xu, C. (2020). Cyclin-dependent kinase 4/6 inhibitor in combination with endocrine therapy versus endocrine therapy only for advanced breast cancer: a systematic review and meta-analysis. Transl. Cancer Res. 9 (2), 657–668. doi:10.21037/tcr.2019.11.46
Yang, L., Xue, J., Yang, Z., Wang, M., Yang, P., Dong, Y., et al. (2021). Side effects of CDK4/6 inhibitors in the treatment of HR+/HER2− advanced breast cancer: a systematic review and meta-analysis of randomized controlled trials. Ann. Palliat. Med. 10 (5), 5590–5599. doi:10.21037/apm-21-1096
Zeng, L., Brignardello-Petersen, R., Hultcrantz, M., Siemieniuk, R., Santesso, N., Traversy, G., et al. (2021). GRADE guidelines 32: GRADE offers guidance on choosing targets of GRADE certainty of evidence ratings. J. Clin. Epidemiol. 137, 163–175. doi:10.1016/j.jclinepi.2021.03.026
Zheng, J., Wu, J., Wang, C., Zhuang, S., Chen, J., and Ye, F. (2020). Combination cyclin-dependent kinase 4/6 inhibitors and endocrine therapy versus endocrine monotherapy for hormonal receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: a systematic review and meta-analysis. PLoS One 15 (6), e0233571. doi:10.1371/journal.pone.0233571
Keywords: CDK4/6 inhibitor, adverse event, umbrella review, randomized controlled trial, endocrine therapy, HR+/ HER2-breast cancer
Citation: Pu D, Wu Y, Xu D, Shi G, Chen H, Feng D, Zhang M and Li J (2024) The adverse events of CDK4/6 inhibitors for HR+/ HER2- breast cancer: an umbrella review of meta-analyses of randomized controlled trials. Front. Pharmacol. 15:1269922. doi: 10.3389/fphar.2024.1269922
Received: 31 July 2023; Accepted: 03 January 2024;
Published: 15 January 2024.
Edited by:
Xiaoxin Ren, Regeneron Pharmaceuticals, Inc., United StatesReviewed by:
Neng Wang, Guangzhou University of Chinese Medicine, ChinaCopyright © 2024 Pu, Wu, Xu, Shi, Chen, Feng, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingwei Li, NzEwMDAzOTVAc2R1dGNtLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.