
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Pharmacol. , 16 January 2024
Sec. Pharmacoepidemiology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1249531
 Swetha Ramanathan1
Swetha Ramanathan1 Charlesnika T. Evans2,3
Charlesnika T. Evans2,3 Ronald C. Hershow1
Ronald C. Hershow1 Gregory S. Calip4
Gregory S. Calip4 Susan Rowan5
Susan Rowan5 Colin Hubbard6
Colin Hubbard6 Katie J. Suda7,8*
Katie J. Suda7,8*Background: Antibiotics prescribed as infection prophylaxis prior to dental procedures have the potential for serious adverse drug events (ADEs). However, the extent to which guideline concordance and different dental settings are associated with ADEs from antibiotic prophylaxis is unknown.
Aim: The purpose was to assess guideline concordance and antibiotic-associated ADEs and whether it differs by VA and non-VA settings.
Methods: Retrospective cohort study of antibiotic prophylaxis prescribed to adults with cardiac conditions or prosthetic joints from 2015 to 2017. Multivariable logistic regression models were fit to assess the impact of ADEs, guideline concordance and dental setting. An interaction term of concordance and dental setting evaluated whether the relationship between ADEs and concordance differed by setting.
Results: From 2015 to 2017, 61,124 patients with antibiotic prophylaxis were identified with 62 (0.1%) having an ADE. Of those with guideline concordance, 18 (0.09%) had an ADE while 44 (0.1%) of those with a discordant antibiotic had an ADE (unadjusted OR: 0.84, 95% CI: 0.49–1.45). Adjusted analyses showed that guideline concordance was not associated with ADEs (OR: 0.78, 95% CI: 0.25–2.46), and this relationship did not differ by dental setting (Wald χ^2 p-value for interaction = 0.601).
Conclusion: Antibiotic-associated ADEs did not differ by setting or guideline concordance.
A concern with dental antibiotic prophylaxis is the potential for adverse drug events (ADEs) [e.g., Clostridioides difficile infection (CDI)] (Becker, 2014; Ouanounou et al., 2020). Knowledge of patient medical history is vital to help prevent ADEs, however, most health information in dental clinics is self-reported by the patient and not through medical clinician notes in the electronic health record (EHR) (Jones et al., 2017). The Department of Veterans Affairs is the largest integrated healthcare system in the US and incorporates an integrated EHR. This EHR integration is rare in private sector dentistry (Rudman et al., 2010). However, data is scarce whether access to an integrated EHR can prevent antibiotic-associated ADEs by facilitating dentists’ access to risk factors for ADEs contained in the medical record. In addition, little is known if prescribing antibiotic prophylaxis consistent with guidelines mitigates the risk of ADEs. The most recent guidelines on the use of antibiotic prophylaxis to prevent infective endocarditis (IE) and prosthetic joint infection (PJI) were released in 2007 and 2013, respectively (Rethman et al., 2013). Current guidelines from the American Heart Association (AHA) recommend use of antibiotic prophylaxis to prevent IE in patients with specific cardiac conditions undergoing invasive dental procedures (Wilson et al., 2007). Guidelines from the American Academy of Orthopedic Surgeons (AAOS) and the American Dental Association (ADA) do not recommend the routine use of antibiotics for prevention of PJI among those with prosthetic joints (Rethman et al., 2013).
A systematic review and meta-analysis by Cahill et al. (2017) showed that antibiotic prophylaxis is effective in reducing the incidence of bacteremia. However, this may not translate to incidence of IE. Observational studies suggest that antibiotic prophylaxis is not protective with the exception of patients with cardiac conditions at high risk of an adverse outcome if infective endocarditis does occur (Thornhill et al., 2023b; 2022b). Regardless, Cahill and others have concluded that the evidence base for use of antibiotic prophylaxis for IE is limited and has significant limitations (Cahill et al., 2017; Suda et al., 2023). Furthermore, despite recommendations and evidence that suggest that antibiotic prophylaxis for PJIs is not necessary (Rethman et al., 2013; Thornhill et al., 2023a; 2022a), dentists continue to prescribe antibiotics for patients with prosthetic joints. A survey sent to dentists showed that though 95% followed the AHA guidelines for IE, guidelines were not followed in patients with prosthetic joint replacements (Spittle et al., 2017). The survey identified that 72% of dentists prescribed antibiotics within the first 2 years after joint replacement (the highest risk period for the development of PJI) and 58% continue to prescribe beyond 2 years. These findings are particularly important as antibiotic prophylaxis is not without risk. Thornhill et al. (2015) identified that even short antibiotic treatments associated with IE prophylaxis resulted in adverse drug reactions, including C. difficile infection (CDI). Therefore, the goal of this study was to assess an association between guideline concordance and antibiotic-associated ADEs and evaluate whether it differs by VA (with an integrated EHR) and non-VA dental settings. As the VA has access to medical data, we hypothesize that VA patients will have less ADEs associated with antibiotic prophylaxis compared to non-VA patients.
This was a retrospective cohort study of Veterans and non-Veterans with dental antibiotic prescriptions/visits from 1 January 2015, through 31 December 2017. The cohort included adults (18+) with a cardiac condition or prosthetic joint, identified using ICD-9/10 codes. Veteran data was obtained from the Corporate Data Warehouse. Non-Veteran data was obtained from the IBM Health Marketscan Commercial Claims/Encounters, Medicare Supplemental, Coordination Benefits and IBM Health Dental Claims. Data extraction and analysis were conducted between 2019 and 2022. This study received approval from the Edward Hines, Jr. Institutional Review Board and the University of Illinois at Chicago Institutional Review Board.
The main independent variable was guideline concordance defined as a prescription for an antibiotic with a days’ supply of ≤3 days for a patient with a cardiac condition (prosthetic values/material, history of infective endocarditis, cardiac transplant, congenital heart disease) undergoing a dental procedure warranting antibiotic prophylaxis (e.g., gingival manipulation or perforation of the oral mucosa) according to the AHA guidelines (Wilson et al., 2007). Antibiotics prescribed to patients with prosthetic joints was defined as guideline discordant according to AAOS/ADA guidelines (Rethman et al., 2013). The primary outcome variable (“ADEs”) was a composite indicator variable for the occurrence of anaphylaxis and antibiotic-related allergic reactions (within 14 days of antibiotic receipt), or CDI (within 30 days of antibiotic receipt), identified using ICD-9 and ICD-10 codes (Gross et al., 2019b).
Additional covariates included dental setting (VA/Non-VA), age (18–34, 35–44, 45–54, 55–64, and ≥65 years), gender (Male/Female), US geographic region (North, Midwest, South, West), location (Urban/Rural), and antibiotic agent [amoxicillin, clindamycin, cephalexin, azithromycin, penicillin, doxycycline, other (e.g., fluroquinolones)]. Prescriptions for amoxicillin/clavulanate were grouped with amoxicillin. Antibiotics were analyzed as dichotomous variables (Yes vs. No). Dental procedures were identified using the American Dental Association’s Code on Dental procedures and Nomenclature (CDT) codes and grouped into categories of service and analyzed as a dichotomous variable (Received vs. Did not receive procedure). Comorbid conditions were assessed using the composite Charlson Comorbidity index and the individual clinical conditions that are elements of the index, identified using ICD-9/10 codes.
The frequency distribution of ADEs was examined using Student’s t-test and Pearson Chi-square tests. Multivariable logistic regression was conducted with covariates selected based on statistical significance (p < 0.05) and epidemiological consideration. The final model was selected using backwards selection, using the Akaike Information Criterion (AIC). Additionally, a term modeling the potential interaction of guideline concordance and dental setting was included in the multivariable model and the Wald chi-square test was used to evaluate if the relationship between downstream outcomes and concordant prescribing differed by setting. All analyses were conducted with STATA software version 14.2 (StataCorp, College Station, TX).
From 2015 to 2017, 61,124 patients with antibiotic prophylaxis prescriptions were identified with 20,014 (32.7%) defined as guideline concordant and 62 (0.1%) having an ADE (61.3% of ADEs reported in non-VA settings and 38.7% of ADEs reported in VA settings). There were 42 people with an allergic reaction, 1 with anaphylaxis, and 19 with CDI. Of those with guideline concordance, 18 (0.09%) had an ADE while 44 (0.1%) of those with guideline discordance had an ADE (unadjusted OR: 0.84, 95% CI: 0.49–1.45). Comorbidities increased the odds of an ADE while those with diagnostic or preventive procedures had decreased odds of ADEs (Table 1).

TABLE 1. Distribution of characteristics by adverse drug events among those with cardiac conditions or prosthetic joints receiving a dental antibiotic prescription.
The multivariable regression model found that guideline concordance was associated with a lowered odds of ADEs (OR: 0.78, 95% CI: 0.25–2.46), though this finding was not statistically significant (Table 2). Patients receiving care from a VA dentist were more likely to experience ADEs (OR = 2.91, 95% CI 1.37–6.15). Furthermore, there was no evidence that the relationship between guideline concordance and ADEs was modified by dental setting in multivariable models (Wald
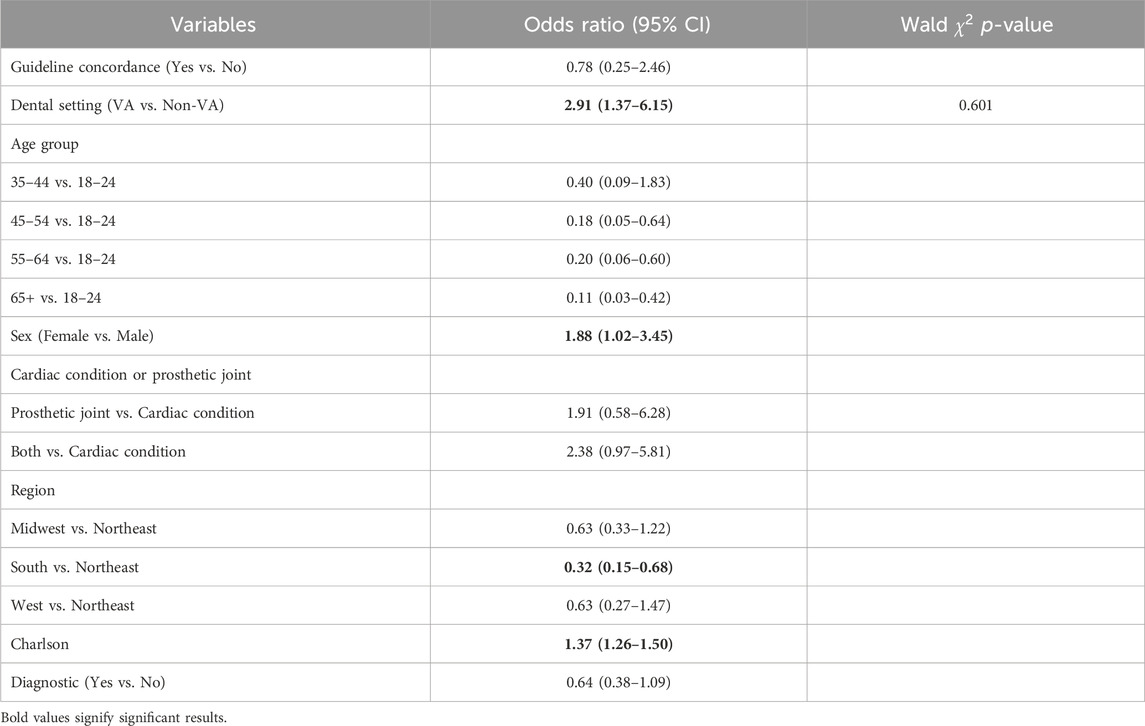
TABLE 2. Multivariable model for adverse drug events among those with cardiac conditions or prosthetic joints receiving a dental antibiotic prescription.
This is the first study to compare ADEs related to guideline concordance of antibiotic prophylaxis by VA and non-VA dental settings. There was no association between guideline concordance and ADEs, indicating that any use of antibiotic prophylaxis regardless of appropriateness is associated with risk. There was also no difference in the occurrence of ADEs by dental setting. Thus, our hypothesis, that VA dentists’ access to a nationally integrated EHR may facilitate the identification of risk factors for antibiotic-associated ADEs (e.g., history of CDI) was rejected. Potential explanation for this finding may be that VA and non-VA patients differed in characteristics. While we attempted to adjust for age, co-morbidities (through the Charlson score), and dental procedures, differences between populations may remain. For example, past exposure to antibiotics or history of ADEs was not assessed.
While the overall incidence was low, serious antibiotic-associated ADEs, such as CDI and antibiotic allergies, can be life-threatening. Gross et al. (2019b) identified 1.4% of a cohort with commercial insurance receiving unnecessary antibiotic prophylaxis had an ADE which is larger than our findings. However, Gross included ED visits and used a 4-year study period (vs. 3 years herein) (Gross et al., 2019b). A prospective study found that 1.5% of patients developed an adverse reaction with 0.9% reporting rash and 0.5% reporting diarrhea after receipt of a single dose of a prophylactic antibiotic (Sandrowski et al., 2020). Prior work in VA found antibiotic prophylaxis resulted in 690 (0.9%) CDI cases within 90-days of surgery (Branch-Elliman et al., 2019). A subsequent analysis of VA dental patients found that 0.05% had a positive diagnostic test for C. difficile within 30 days of antibiotic prophylaxis (Wilson et al., 2022). Additionally, a retrospective cohort study in Canada found 1.5% of patients who received a pre-operative antibiotic developed CDI (Carignan et al., 2008). Thus, previous studies reveal a range of estimates of the incidence of ADEs, with variation likely deriving from study methodologies (i.e., ICD-9 codes vs. prospective data collection).
To prevent ADEs after dental antibiotic prophylaxis, it is also important to understand why VA patients had a higher odds of ADEs. One reason may be because access to the medical chart allows for better recording of ADEs and/or population differences. A secondary analysis of the National Health Interview Survey of Veterans and those in the general population found that VA patient populations have poorer health, more medical conditions, and higher healthcare resource use compared to the general population (Agha et al., 2000). Assessing data from this study, the VA population was older but the non-VA cohort was slightly sicker. The final model controlled for both age and Charlson within this study. However, the Charlson index does not consider every comorbidity and it is possible that some comorbidities not captured in this model may be driving this result. It is also possible that, despite controlling for age, behavioral or other aspects related to age not accounted for could be influencing this result. For example, older patients may be more likely to follow through with health concerns such as rashes or diarrhea. Alternatively, unique characteristics related to medical setting or VA population may be the reason for this finding. These considerations are discussed in depth below.
Another reason for the differences in ADEs by dental setting is that Veterans may have increased access to care compared to the general population. Eligibility for dental care in the VA is different than other VA benefits (VA dental care, 2020). Those that receive dental care have a set of eligibility criteria in which some individuals have access to any dental care while others have limited access VA dental care (2020). Those that receive any dental care are those with a service-connected dental disability/condition or were a former prisoner of war. Coverage for dental care is very different from those in the non-VA population who can purchase or enroll into commercial dental insurance. Thereby, these VA dental patients may require more frequent and/or complex dental care (i.e., more invasive oral surgeries) than the general population resulting in increased contact with the healthcare system. Therefore, VA patients may be more likely to follow through with concerns such as allergies or CDI than those in the non-VA sector.
Apart from differing dental eligibilities and compensation at VA facilities, VA patients may also have easier access to care. VA patients typically have lower wait times, waiting on average 20 min less for primary care physicians compared to non-VA patients (Penn et al., 2019). Other work published on VA care suggests that VA patients also receive longer appointment times with their physicians (Shulkin, 2016). A combination of lower wait times and increased time with their physician, may motivate more VA patients to seek care with a primary or other clinician types thereby diagnosing more adverse drug events as compared to private sector individuals. Having reliable access to care and lower wait times may make Veterans more likely to follow up with such concerns with healthcare providers.
Current guidelines for antibiotic prophylaxis have been updated, over time, to account for unnecessary overprescribing and have reduced the number of people recommended to receive prophylaxis for IEs and PJIs. Recent guidelines advocate for prophylaxis in select patients with cardiac conditions and recommend no antibiotic prophylaxis for those with prosthetic joints (Wilson et al., 2007; Rethman et al., 2013). Guideline revisions along with antibiotic stewardship may have led to improved prescribing. Previous data have shown that during the time the guidelines were updated in 2007, dental antibiotic prescribing decreased by 38.1% for tetracyclines, 29.9% for cephalosporins, and 13.5% for penicillins with an overall decrease in proportion of prescriptions of 0.7% (from 10.7% in 2005 to 10% in 2010) (Suda et al., 2016). However, more recent data showed that dental prescribing rates remain unchanged between 2012 through 2019, during the AAOS/ADA guideline revisions (Ramanathan et al., 2023). Using administrative claims data, unnecessary antibiotic prophylaxis slightly decreased in a commercially insured population (Hubbard et al., 2022), but increased in the Veteran population (Suda et al., 2022). These results suggest that guideline changes alone may not impact prescribing and antibiotic stewardship should be incorporated into dental settings. While limited, studies have shown that the implementation of antibiotic stewardship strategies can be effective in dental clinics (Gross et al., 2019a; Goff et al., 2022). Consistent with recommendations by the Centers for Disease Control and Prevention, antibiotic stewardship should be a strategy employed in VA and non-VA dental settings as part of a daily clinical practice (The Core Elements of Outpatient Antibiotic Stewardship, 2023).
In order to conduct rigorous medication safety assessments, future research should validate methods to determine antibiotic-associated ADEs. While surveillance datasets (e.g., Medwatch) can be used to identify ADEs, these datasets cannot be linked to other claims or EHR datasets. For example, it is currently difficult to identify an association of unnecessary prescribing and occurrence of an ADE due to limited variables available for analysis in surveillance datasets. Obtaining granular data is essential to determining prevalence and risk factors for ADEs, variables which can be obtained from large claims and EHR databases.
This study is not without limitations. First, this study could not account for all differences between VA and non-VA settings. Second, we were not able identify the prescribing provider in non-VA data, though analogous approaches were used to identify each cohort and used methods applied in prior work. Ramanathan et al. (2023) Third, these analyses focus on a specific population in VA and non-VA settings, and the results may not be generalizable. Fourth, there could be misclassification as algorithms were used to determine guideline concordance, including undercoding of ICD-9/10s to identify ADEs. Fifth, this study did not account for prior antibiotic exposure, history of ADEs, or indication for prophylaxis which may have influenced the findings. Sixth, the small prevalence of the outcome can result in biased regression estimates, future studies should verify the validity of these findings. Finally, the study could not identify patients who did not seek care for an ADE. However, this study used two of the largest and most comprehensive data sources for dental research. A strength of this study is the first to evaluate downstream adverse events from dental antibiotic prophylaxis in two different dental settings.
The results of this study provide the foundation for understanding the relationship between dental setting, guideline concordance, and downstream ADEs. The results suggest there is no relationship between guideline concordance of antibiotic prophylaxis and downstream ADEs. Furthermore, this relationship did not differ by VA and non-VA dental settings.
The data analyzed in this study is subject to the following licenses/restrictions: We are committed to collaborating and sharing these data to maximize their value to improve veterans and others’ health and healthcare, to the greatest degree consistent with current Veterans Health Administration regulations and policy. We can provide access to the programming code used to identify the sample and conduct analyses and will conduct additional analyses as requested. We cannot provide a link to the database directly as it would compromise patients’ anonymity, and permissions from VA are needed to obtain the data. The Marketscan dataset used for this study is proprietary and unable to be shared. Interested individuals may contact the corresponding author (KS) for information. Requests to access these datasets should be directed to KS, a3N1ZGFAcGl0dC5lZHU=.
The studies involving humans were approved by the University of Illinois at Chicago and the Hines VA. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
SR contributed to conceptualization, methodology, investigation, formal analysis, and wrote the original draft. CE, RH, GC, and SR, contributed to conceptualization, methodology, and manuscript review and editing. CH contributed to conceptualization, methodology, investigation, and manuscript review and editing. KS contributed to conceptualization, methodology, manuscript review and editing, and funding acquisition. All authors contributed to the article and approved the submitted version.
This study was supported by AHRQ grant R01 HS25177 and VHA HSR&D Grant No. HX002452.
We acknowledge the contributions of Drs. Awadalla and Mehta for their guidance on this analysis. The views expressed in this manuscript are those of the author and do not reflect the position or policy of the Agency for Healthcare Research and Quality, the Department of Veterans Affairs, or the U.S. government.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Agha, Z., Lofgren, R., VanRuiswyk, J., and Layde, P. (2000). Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch. Intern Med. 160, 3252–3257. doi:10.1001/archinte.160.21.3252
Becker, D. E. (2014). Adverse drug reactions in dental practice. Anesth. Prog. 61, 26–33. doi:10.2344/0003-3006-61.1.26
Branch-Elliman, W., O’Brien, W., Strymish, J., Itani, K., Wyatt, C., Gupta, K., et al. (2019). Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg. 154, 590–598. doi:10.1001/jamasurg.2019.0569
Cahill, T. J., Harrison, J. L., Jewell, P., Onakpoya, I., Chambers, J. B., Dayer, M., et al. (2017). Antibiotic prophylaxis for infective endocarditis: a systematic review and meta-analysis. Heart 103, 937–944. doi:10.1136/heartjnl-2015-309102
Carignan, A., Allard, C., Pépin, J., Cossette, B., Nault, V., and Valiquette, L. (2008). Risk of Clostridium difficile infection after perioperative antibacterial prophylaxis before and during an outbreak of infection due to a hypervirulent strain. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 46, 1838–1843. doi:10.1086/588291
Goff, D. A., Mangino, J. E., Trolli, E., Scheetz, R., and Goff, D. (2022). Private practice dentists improve antibiotic use after dental antibiotic stewardship education from infectious diseases experts. Open Forum Infect. Dis. 9, ofac361. doi:10.1093/ofid/ofac361
Gross, A. E., Hanna, D., Rowan, R., Susan, A., Bleasdale, S. C., and Suda, K. J. (2019a). Successful implementation of an antibiotic stewardship program in an academic dental practice. Open Forum Infect. Dis. 6, ofz067. doi:10.1093/ofid/ofz067
Gross, A. E., Suda, K. J., Zhou, J., Calip, G. S., Rowan, S. A., Hershow, R. C., et al. (2019b). Serious antibiotic-related adverse effects following unnecessary dental prophylaxis in the United States. Infect. Control Hosp. Epidemiol. 42, 110–112. doi:10.1017/ice.2020.1261
Hubbard, C. C., Evans, C. T., Calip, G. S., Zhou, J., Rowan, S. A., and Suda, K. J. (2022). Appropriateness of antibiotic prophylaxis before dental procedures, 2016-2018. Am. J. Prev. Med. 62, 943–948. doi:10.1016/j.amepre.2021.11.004
Jones, J., Snyder, J., Gesko, D., and Helgeson, M. (2017). Integrated medical-dental delivery systems: models in a changing environment and their implications for dental education. J. Dent. Educ. 81, eS21–eS29. doi:10.21815/JDE.017.029
Ouanounou, A., Ng, K., and Chaban, P. (2020). Adverse drug reactions in dentistry. Int. Dent. J. 70, 79–84. doi:10.1111/idj.12540
Penn, M., Bhatnagar, S., Kuy, S., Lieberman, S., Elnahal, S., Clancy, C., et al. (2019). Comparison of wait times for new patients between the private sector and United States department of veterans Affairs medical centers. JAMA Netw. Open 2, e187096. doi:10.1001/jamanetworkopen.2018.7096
Ramanathan, S., Evans, C. T., Hershow, R. C., Calip, G. S., Rowan, S., Hubbard, C., et al. (2023). Comparison of guideline concordant antibiotic prophylaxis in Veterans Affairs and non-Veterans Affairs dental settings among those with cardiac conditions or prosthetic joints. BMC Infect. Dis. 23, 427. doi:10.1186/s12879-023-08400-y
Ramanathan, S., Yan, C. H., Hubbard, C., Calip, G. S., Sharp, L. K., Evans, C. T., et al. (2023). Changes in antibiotic prescribing by dentists in the United States, 2012-2019. Infect. Control Hosp. Epidemiol. 1–6. doi:10.1017/ice.2023.151
Rethman, M. P., Watters, W., Buck, H., Abt, E., Anderson, P. A., Carroll, K. C., et al. (2013). The American Academy of Orthopaedic Surgeons and the American Dental Association clinical practice guideline on the prevention of orthopaedic implant infection in patients undergoing dental procedures. J. Bone Jt. Surg. Am. 95, 745–747. doi:10.2106/00004623-201304170-00011
Rudman, W. J., Hart-Hester, S., Jones, W. A., Caputo, N., and Madison, M. (2010). Integrating medical and dental records: a new frontier in health information management. J. AHIMA 81, 36–39.
Sandrowski, K., Edelman, D., Rivlin, M., Jones, C., Wang, M., Gallant, G., et al. (2020). A prospective evaluation of adverse reactions to single-dose intravenous antibiotic prophylaxis during outpatient hand surgery. Hand N. Y. N. 15, 41–44. doi:10.1177/1558944718787264
Spittle, L. S., Muzzin, K. B., Campbell, P. R., DeWald, J. P., and Rivera-Hidalgo, F. (2017). Current prescribing practices for antibiotic prophylaxis: a survey of dental practitioners. J. Contemp. Dent. Pract. 18, 559–566. doi:10.5005/jp-journals-10024-2084
Suda, K. J., Costello, B. J., Sutherland, S. E., and Kalenderian, E. (2023). RE: maxillofacial Surgeons beware: some AHA “moderate risk” patients develop endocarditis after exodontia. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 81, 261. doi:10.1016/j.joms.2022.12.011
Suda, K. J., Fitzpatrick, M. A., Gibson, G., Jurasic, M. M., Poggensee, L., Echevarria, K., et al. (2022). Antibiotic prophylaxis prescriptions prior to dental visits in the Veterans’ Health Administration (VHA), 2015-2019. Infect. Control Hosp. Epidemiol. 43, 1565–1574. doi:10.1017/ice.2021.521
Suda, K. J., Roberts, R. M., Hunkler, R. J., and Taylor, T. H. (2016). Antibiotic prescriptions in the community by type of provider in the United States, 2005-2010. J. Am. Pharm. Assoc. 56, 621–626. doi:10.1016/j.japh.2016.08.015
The Core Elements of Outpatient Antibiotic Stewardship (2023). The Core elements of outpatient antibiotic stewardship.
Thornhill, M. H., Crum, A., Rex, S., Stone, T., Campbell, R., Bradburn, M., et al. (2022a). Analysis of prosthetic joint infections following invasive dental procedures in england. JAMA Netw. Open 5, e2142987. doi:10.1001/jamanetworkopen.2021.42987
Thornhill, M. H., Dayer, M. J., Prendergast, B., Baddour, L. M., Jones, S., and Lockhart, P. B. (2015). Incidence and nature of adverse reactions to antibiotics used as endocarditis prophylaxis. J. Antimicrob. Chemother. 70, 2382–2388. doi:10.1093/jac/dkv115
Thornhill, M. H., Gibson, T. B., Pack, C., Rosario, B. L., Bloemers, S., Lockhart, P. B., et al. (2023a). Quantifying the risk of prosthetic joint infections after invasive dental procedures and the effect of antibiotic prophylaxis. J. Am. Dent. Assoc. 154, 43–52.e12. doi:10.1016/j.adaj.2022.10.001
Thornhill, M. H., Gibson, T. B., Yoon, F., Dayer, M. J., Prendergast, B. D., Lockhart, P. B., et al. (2022b). Antibiotic prophylaxis against infective endocarditis before invasive dental procedures. J. Am. Coll. Cardiol. 80, 1029–1041. doi:10.1016/j.jacc.2022.06.030
Thornhill, M. H., Gibson, T. B., Yoon, F., Dayer, M. J., Prendergast, B. D., Lockhart, P. B., et al. (2023b). Endocarditis, invasive dental procedures, and antibiotic prophylaxis efficacy in US Medicaid patients. Oral Dis. doi:10.1111/odi.14585
VA dental care [WWW Document], 2020. Veterans aff. Available at: https://www.va.gov/health-care/about-va-health-benefits/dental-care/ (Accessed 8.17.2021).
Wilson, G. M., Evans, C. T., Fitzpatrick, M. A., Poggensee, L., Gibson, G., Jurasic, M. M., et al. (2022). Clostridioides difficile infection following dental antibiotic prescriptions in a cohort of US veterans. Infect. Control Hosp. Epidemiol. 44, 494–496. doi:10.1017/ice.2021.516
Wilson, W., Taubert, K. A., Gewitz, M., Lockhart, P. B., Baddour, L. M., Levison, M., et al. (2007). Prevention of infective endocarditis: guidelines from the American heart association: a guideline from the American heart association rheumatic fever, endocarditis, and kawasaki disease committee, council on cardiovascular disease in the young, and the council on clinical cardiology, council on cardiovascular surgery and anesthesia, and the quality of care and outcomes research interdisciplinary working group. Circulation 116, 1736–1754. doi:10.1161/CIRCULATIONAHA.106.183095
Keywords: antibiotic, dentist, adverse drug event, medication safety, prescribing patterns
Citation: Ramanathan S, Evans CT, Hershow RC, Calip GS, Rowan S, Hubbard C and Suda KJ (2024) Guideline concordance and antibiotic-associated adverse events between Veterans administration and non-Veterans administration dental settings: a retrospective cohort study. Front. Pharmacol. 15:1249531. doi: 10.3389/fphar.2024.1249531
Received: 28 June 2023; Accepted: 04 January 2024;
Published: 16 January 2024.
Edited by:
Mohammed Salahudeen, University of Tasmania, AustraliaCopyright © 2024 Ramanathan, Evans, Hershow, Calip, Rowan, Hubbard and Suda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katie J. Suda, a3N1ZGFAcGl0dC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.