- Department of Hematology, Institute of Hematology, West China Hospital Sichuan University, Chengdu, China
Background: For multiple myeloma (MM), the proportions of patients reaching the subsequent line of therapy (LOT) decline gradually and real-world data describing the attrition rates of LOT in Chinese MM were limited. Herein, we investigated the attrition rates by subsequent LOTs and their relevant risk factors in MM patients in China.
Methods: MM patients who had been hospitalized and received at least one LOT from January 2008 to August 2019 in West China Hospital Sichuan University were retrospectively recruited. Demographic and clinical characteristic data were obtained from the “HemaTank” Chinese Multiple Myeloma Database. The Cox proportional hazards regression model was applied to analyze the risk factors of frontline treatment attrition.
Results: A total of 1,255 newly diagnosed MM were enrolled, with 573 (45.7%) patients receiving only one LOT and 682 (54.3%) patients receiving more than one LOT. Thalidomide with dexamethasone/prednisone was the most common frontline treatment before 2017, while bortezomib-based regimens constituted the majority of frontline treatment in 2017 and beyond. The attrition rates from the first to the fifth LOT exhibited a gradual upward trend (45.7%, 48.7%, 58.9% and 62.5%, respectively). Meanwhile, 54.3%, 27.9%, 11.5%, and 4.3% of all the enrolled MM patients received a second, third, fourth and fifth LOT. MM who underwent autologous stem cell transplantation (ASCT) showed lower attrition rates across all LOTs (range 12%–56.8%) than MM without ASCT (range 49.1%–64.5%). The multivariate Cox regression model revealed that ISS stage III (HR 2.07, p < .001), elevated LDH (HR 1.47, p = .006), and comorbidities such as amyloidosis (HR 1.63, p = 0 .01), hepatic disease (HR 1.36, p = .022), pulmonary disease (HR 1.38, p = .022), and cardiac disease (HR 1.62, p = .004) were independent risk factors for MM patients attritted from the frontline treatment.
Conclusion: In this study, the attrition rates were generally high and increased gradually across all LOTs. Nearly half of MM patients received only one LOT, and higher tumor burden and more comorbidities may be associated with fewer subsequent LOTs. The high attrition rates highlight the importance of applying the most optimal frontline treatment regimen rather than salvaging subsequent LOTs.
Introduction
Multiple myeloma (MM) is the second most common hematologic malignancy, which is a biologically heterogeneous disease characterized by clonal evolution during disease progression and remains incurable currently (Morgan et al., 2012; Rajkumar, 2020; Yang et al., 2022). The proportions of MM patients reaching the subsequent line of therapy (LOT) decline gradually (Raab et al., 2016; Yong et al., 2016). Besides, the treatment response rate, response duration, overall survival (OS) and progression-free survival (PFS) decrease with each successive LOT in MM, while the incidence of toxicity increases (Yong et al., 2016; Cook et al., 2018; Hajek et al., 2018; Verelst et al., 2018). The response depth and duration of the frontline treatment of MM are associated with PFS and OS (Landgren and Iskander, 2017; Yan et al., 2019). Therefore, an optimal frontline treatment regimen rather than salvaging subsequent LOTs is critical for improving the outcomes of MM. A good understanding of the attrition rates of LOTs and risk factors of the frontline treatment attrition is essential to provide evidence for choosing the most optimal frontline therapy and clinical treatment sequence among the available standard of care in the real-world practice.
Attrition rates between LOTs vary in regions but are commonly high in MM (Fonseca et al., 2021). A real-world study across Europe demonstrated that the attrition rates of MM patients gradually increased from the first to fifth LOT (36.1%, 43.2%, 73.2%, and 72.8%, respectively) (Yong et al., 2016). Findings derived from 5704 MM patients in the United States (US) indicated that attrition rates are 50.4% between the first and second LOT and 54.5% between the second and third LOT (MacEwan et al., 2018). Meanwhile, Fonseca et al. observed that the attrition rates across all LOTs for non-transplant MM patients are higher than for transplant patients based on the data identified from three US patient-level databases (Fonseca et al., 2020). Over the past decades, the treatment pattern of MM in China has significantly changed with the availability of more novel agents (Huang et al., 2017; Cai et al., 2022). However, the treatment regimens during the same period are clinically distinct between China and European countries and the United States countries in real-world practice due to the later launch and medical insurance coverage of new agents in China (Qian et al., 2018; Fonseca et al., 2020; He et al., 2020; He et al., 2021; Atrash et al., 2022). Therefore, the attrition rates of LOTs may vary due to the different frontline treatment patterns in China. Besides, multiple factors may influence the selection of the frontline regimens for MM patients, such as clinical characteristics, performance status, comorbidities and economic burden, resulting in potential risk factors of treatment attrition.
To the best of our knowledge, there were no studies on attrition rates of distinct LOTs and their relevant risk factors in China. In this study, we aim to elucidate the attrition rates of separate LOTs and their associated risk factors in Chinese MM patients by collecting real-world data from the West China Hospital Sichuan University (WCHSCU). Findings gleaned from this work would provide valuable clues for MM enrollment in clinical trials and patient management in clinical practice.
Materials and methods
Study population
Newly diagnosed MM patients from January 2008 to August 2019 in WCHSCU were retrospectively enrolled in this study. The cutoff date of follow-up data was 31 October 2021. MM patients included in this study must meet the following criteria: 1) the diagnosis of MM was confirmed to the criteria of the International Myeloma Working Group (Rajkumar et al., 2014), 2) newly diagnosed MM patients who have been hospitalized in the department of hematology in WCHSCU, and 3) receiving at least one LOT. Exclusion criteria included patients who received only short-term glucocorticoid therapies and smoldering MM or monoclonal gammopathy of undetermined significance. This study was approved by the Ethics Committee of WCHSCU and conducted according to the Declaration of Helsinki.
Data extraction
Data were obtained from the “HemaTank” Chinese Multiple Myeloma Database (HCMMD). “HemaTank” was a project that aimed to construct databases that collect and analyze the real-world data of patients with multiple myeloma based on a standard dataset (Niu et al., 2022), and now it has been expanded to 19 tertiary referral hospitals in China. The baseline demographic features (e.g., age and gender), laboratory examination indexes (e.g., anemia, thrombocytopenia, leukocytopenia, renal insufficiency, hypercalcemia, immunoparesis, and ISS stage) and comorbidities (e.g., tuberculosis, hypertension, diabetes, amyloidosis, hepatic disease, pulmonary disease, and cardiac disease) were extracted from the database. Treatment regimens across all LOTs extracted from the database were manually reviewed. The common treatment regimens include thalidomide-dexamethasone/prednisone (TD/TP), bortezomib-dexamethasone (BD), bortezomib-cyclophosphamide-dexamethasone (BCD), bortezomib-thalidomide-dexamethasone (BTD), vincristine-adriamycin-dexamethasone-thalidomide (VADT), melphalan-prednisone-thalidomide (MPT), cyclophosphamide-dexamethasone/prednisone (CD/CP), and cyclophosphamide-thalidomide-dexamethasone (CTD).
Evaluation of attrition rates and associated risk factors
One LOT was defined as an initial administration of at least one kind of anti-tumor drug until an unplanned addition of a new drug or switching to a different drug or combination of new drugs due to any reason such as disease progression, lack of response, inadequate response, toxicity or other reasons (Rajkumar et al., 2015). In order to elucidate the proportion of MM patients dropped from each LOT, we calculated the attrition rates between distinct LOTs. Duration of treatment (defined as from the start time of treatment to the end of a specific LOT) was evaluated for each LOT. In this study, the attrition rate was defined as the ratio of MM patients who did not have a record of a subsequent LOT owing to death, loss to follow-up, or still stayed at the current LOT (i.e., no subsequent treatment during the study period). To understand the attrition rates in distinct clinical settings, we conducted the subgroup analysis by using the variables of age, gender, autologous stem cell transplantation (ASCT), treatment regimen and time. To elucidate the potential clinical and biological factors that influenced MM patients to receive more LOTs, we partitioned patients into 1 LOT (only treated with one LOT) and >1 LOT subgroups and examined the distinct distribution of the potential factors between two subgroups.
Statistical analysis
R software (version 4.1.2) was employed to achieve relevant analyses and plots. The continuous and categorical variables of the two LOT subgroups were assessed with Wilcoxon rank-sum test (Wilcoxon test) and Chi-square test, respectively. Exploration of factors associated with attrition rates was based on Cox proportional hazards regression model, and in the outcome binary variable, “1” indicates patient attrition, and “0” indicates non-attrition. Two-sided p values less than .05 were considered to be statistically significant.
Results
Baseline characteristics of MM
Finally, a total of 6,665 diagnosed MM patients were collected from the HCMMD of WCHSCU, including 3,175 hospitalized MM patients. Among them, 1,255 newly diagnosed MM patients meeting the inclusion criteria were enrolled in the study. The detailed demographic information, baseline clinical characteristics and comorbidities were presented in Table 1. The median age of all the enrolled MM patients was 62, ranging from 19 to 91. Only 117 (9.3%) patients received an ASCT treatment. Amongst, 682 (45.7%) patients were treated with only one LOT, and the residual patients (54.3%) received more than one LOTs. Their distinct distributions of baseline characteristics between subgroups were illustrated in Table 1. MM patients receiving more than one LOTs were significantly younger than patients with only one LOT (median age: 61 vs. 63 years, p = .001). A significant higher proportion of ISS stage III were observed in 1 LOT subgroup than >1 LOT subgroup (34.5% vs. 51.5%, p < .001). Besides, MM patients in the> 1 LOT subgroup harbored a significantly lower proportion of anemia, thrombocytopenia, renal insufficiency and comorbidities such as amyloidosis, hepatic diseases, pulmonary diseases and cardiac disorders (Table 1).
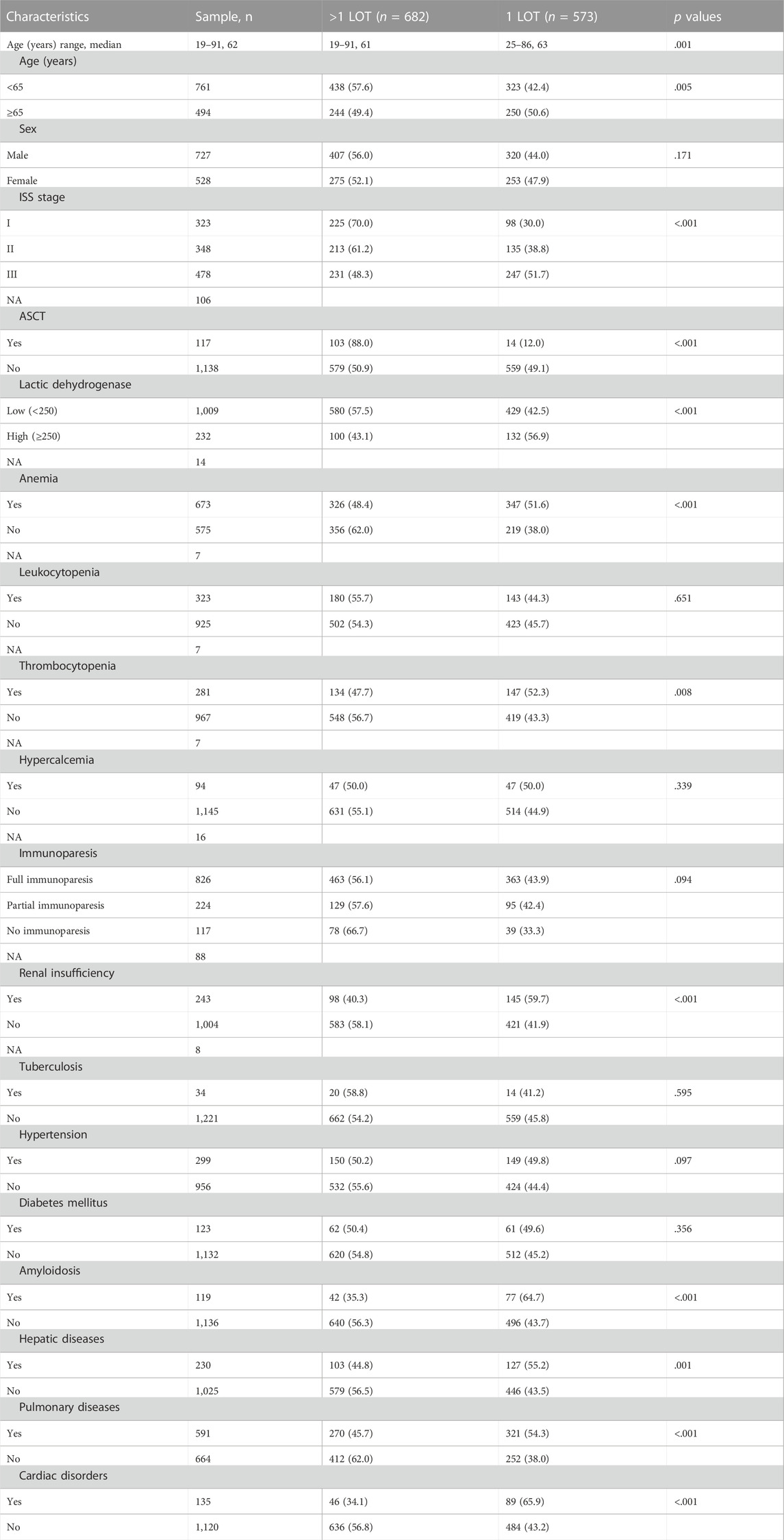
TABLE 1. Clinical features of included 1255 MM patients and their distinct distribution between patients with 1 LOT and >1 LOT.
Frontline treatment patterns in MM
Distinct chemotherapy agents are used for the frontline treatment of MM patients, and we therefore investigated the common therapy drugs and patterns in the MM first LOT. In overall patients, results indicated that TD (23.6%) was the most frequently used treatment pattern in Chinese MM frontline clinical settings (Figure 1A), followed by BD (15.0%), BCD (8.8%), VADT (8.3%), and BTD (7.6%). Considering that bortezomib was not included in National Reimbursement Drug List (NRDL) list until 2017, we also explored the evolution of treatment patterns for patients with MM before 2017 or 2017 and beyond. Subgroup analysis of frontline treatment demonstrated that the main treatment patterns before 2017 (N = 890) included TD (29.6%), VADT (11.6%), BD (10.6%), BTD (9.3%), and MPT (6.9%) (Figure 1B). Nevertheless, among MM patients in 2017 and beyond (N = 365), BD (25.7%) was the most commonly applied therapeutic regimen, followed by BCD (23.8%) and TD (8.8%) (Figure 1C). In addition, the choice of the frontline treatment patterns was usually influenced by the performance status and health conditions, so we performed the subgroup analyses of treatment regimens by age. Among patients <65 years old, the main treatment patterns were TD (21.0%), BD (14.3%), BCD (11.8%), VADT (10.9%), and BTD (6.8%), while TD (27.5%), BD (16.0%), BTD (8.7%), MPT (7.7%), and CD (5.5%) were frequently employed among patients ≥65 years old (Figures 1D, E).
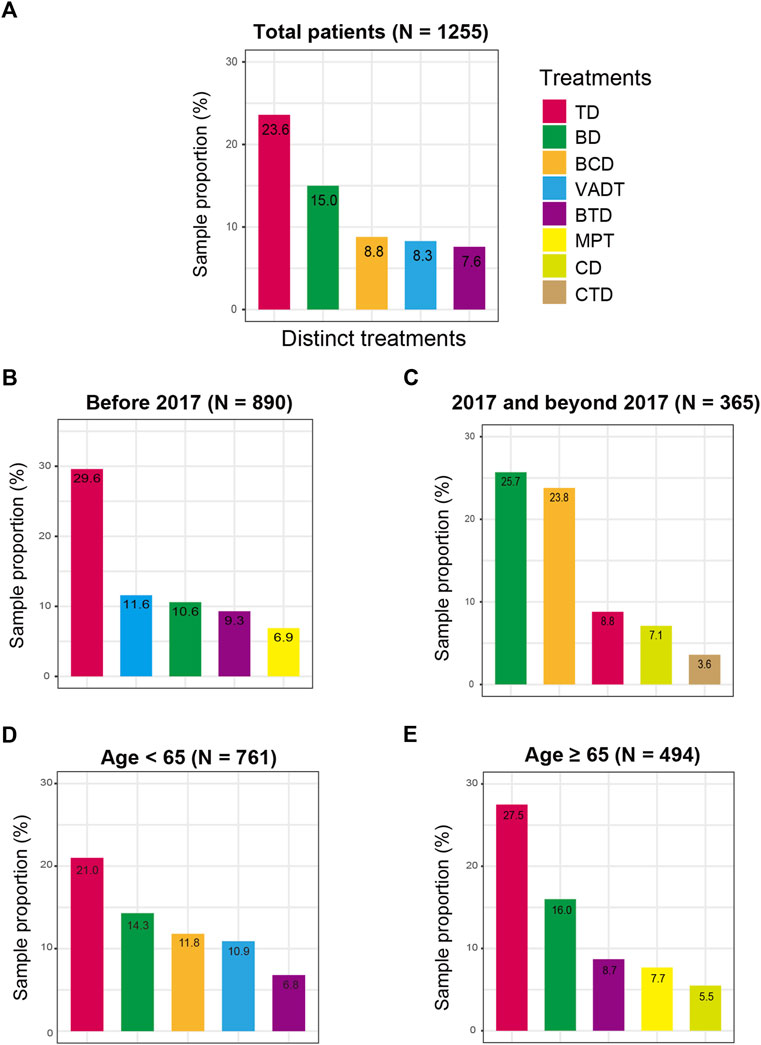
FIGURE 1. Frontline treatment patterns for MM patients in distinct clinical subgroups. (A) in the total MM patients; (B) before 2017; (C) after 2017; (D) in MM patients aged less than 65 years old; (E) in MM patients age 65 years or older.
Treatment duration between distinct LOTs
We calculated the treatment duration between distinct LOTs to understand the intervals between each LOT. Among the 1,255 patients, the median treatment duration for the 1st to 2nd LOT, 2nd to 3rd LOT, 3rd to 4th LOT and 4th to 5th LOT were 6.45, 6.57, 7.68, and 7.83 months respectively (Figure 2A). Further comparisons revealed a significant difference between the treatment duration of 1st to 2nd LOT and 3rd to 4th LOT (Wilcoxon rank-sum test p = .041; Figure 2A). We then investigated the distinct frontline treatment durations for the commonly used five treatment regimens (i.e., TD, BD, BCD, VADT, and BTD), which were significantly different (median time: 3.88, 6.05, 8.25, 7.83, and 6.93 months; Kruskal-Wallis H test p = .005; Figure 2B), with TD exhibiting the shortest treatment duration and BCD treatment showing the longest treatment duration (Wilcoxon rank-sum test p = .048; Figure 2B).
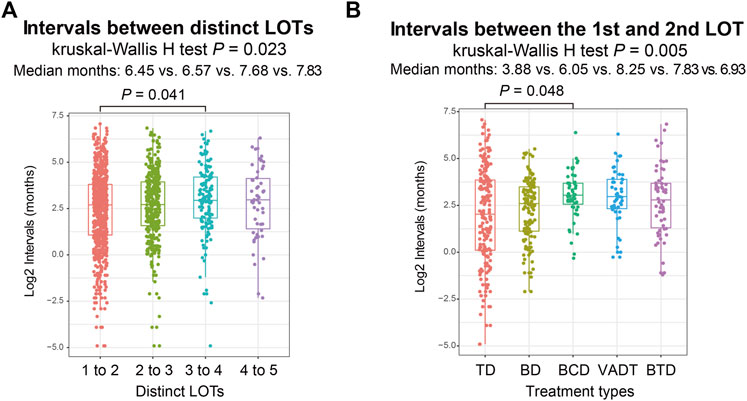
FIGURE 2. (A) Intervals between distinct LOTs in total MM patients. (B) Intervals from the first LOT to the second LOT among different frontline treatment regimens.
Attrition rates of subsequent LOTs
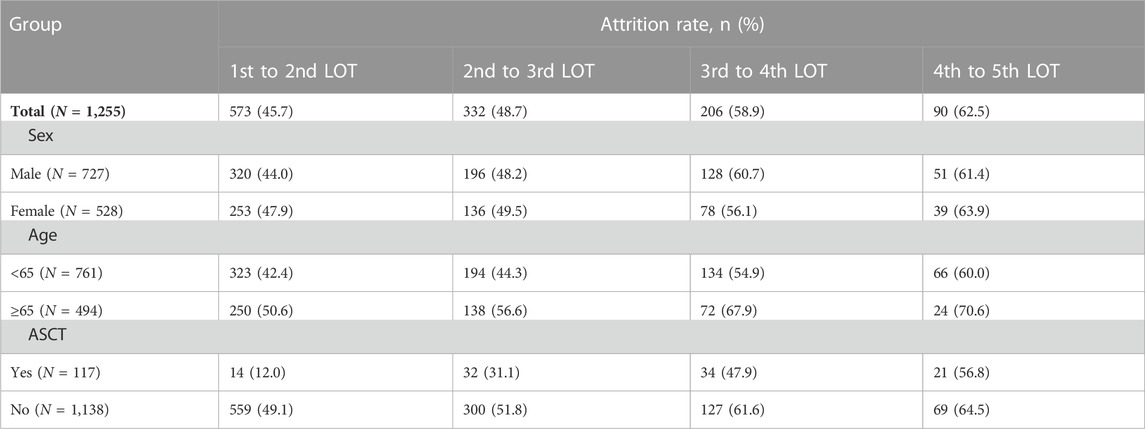
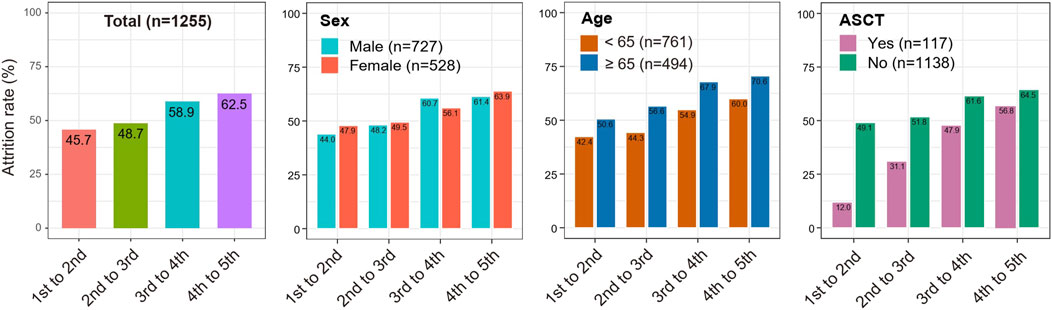
We calculated distinct attrition rates with the increased numbers of LOT. Results from the total MM patients revealed that the attrition rates from the first to the fifth LOT exhibited a gradual upward trend (45.7%, 48.7%, 58.9% and 62.5% for the 1st to 2nd LOT, 2nd to 3rd LOT, 3rd to 4th LOT and 4th to 5th LOT, respectively). (Table 2; Figure 3). As a result, 54.3%, 27.9%, 11.5%, and as few as 4.3% of all the MM patients enrolled in the study received a second, third, fourth and fifth LOT respectively (Figure 4).
Taking into account that clinical factors potentially influence MM attrition rate, we then performed stratification analyses based on the variables of gender, age, and ASCT. As shown in Figure 3, consistent trends of elevated attrition rates were observed in gender, age and ASCT subgroup analysis. Meanwhile, MM patients ≥65 years old showed relatively higher attrition rates than patients <65 years old in correspondingly LOTs (50.6% vs. 42.4%, 56.6% vs. 44.3%, 67.9% vs. 54.9% and 70.6% vs. 60.0% for the 1st to 2nd LOT, 2nd to 3rd LOT, 3rd to 4th LOT and 4th to 5th LOT, respectively). Similarly, MM who underwent ASCT showed higher attrition rates across all LOTs (range 12%–56.8%) than MM without ASCT (range 49.1%–64.5%). Notably, 13.7% of transplant MM patients received the fifth LOT, while only 3.3% of non-transplant MM patients entered the fifth LOT (Figure 4).
Risk factors associated with the treatment attrition
The incidence of MM patients reaching the second LOT during follow up in Supplementary Figure S1. To elucidate possible factors that are linked with subsequent MM LOTs, we collected multi-dimension indexes and compared their differences between 1 LOT (N = 573) and >1 LOT (N = 682) subgroups under univariate and multivariate Cox regression models (Table 3). Univariate analysis demonstrated that higher age, ISS stage III, full immunoparesis, anemia, thrombocytopenia, renal insufficiency, hypercalcemia, lactic dehydrogenase, hypertension, diabetes, amyloidosis, hepatic disease, pulmonary disease, and cardiac disease exhibited significant risk factors for MM patients attrited from the frontline therapy (all p < .05). Further multivariate Cox regression analysis revealed that ISS stage III, elevated lactic dehydrogenase, amyloidosis, hepatic disease, pulmonary disease, and cardiac disease remained still significant after adjusting for multiple confounding factors (all adjusted p < .05). Aa a result, patients in ISS III stage (HR: 2.07, 95% CI: 1.42–3.01, p < .001), with high lactic dehydrogenase (HR: 1.47, 95% CI: 1.11–1.94, p = .006), amyloidosis (HR: 1.63, 95% CI: 1.13–2.36, p = .01), hepatic disease (HR: 1.36, 95% CI: 1.05–1.77, p = .022), pulmonary disease (HR: 1.38, 95% CI: 1.08–1.76, p = .022), and cardiac disease (HR: 1.62, 95% CI: 1.16–2.26, p = .004) were more likely to be attrited (Table 3).

TABLE 3. Univaraite and multivaraite Cox regression models regarding factors associated with attrition rates.
Discussion
In this study, we identified the commonly applied frontline treatment pattern and assessed the attrition rates of distinct LOTs by collecting and analyzing the data of 1255 MM Chinese patients derived from the HCMMD. Notably, risk factors for the frontline treatment attrition were also explored. To the best of our knowledge, this is the first study to assess the attrition rates of LOTs for Chinese MM patients based on the real-world data. Besides, this study analyzed the frontline treatment patterns based on the largest sample size in China. Findings from this work would provide theoretical evidence for MM patients to receive distinct therapies at specific clinical stages.
This study demonstrated that MM frontline therapies in China are distinct from US and Western countries. The thalidomide-containing regimen is the most common frontline treatment in all the MM patients enrolled in the study, while Bortezomib-containing and lenalidomide regimens are the primary frontline treatment among MM patients from US and Western countries in the same period (Fonseca et al., 2020; He et al., 2021; Kumar et al., 2021; Atrash et al., 2022). It may be partly attributed to the economic factor and the availability of new agents. According to the MM dataset in this study, we also observed the obvious transitions of treatment patterns over time. A significant change in the treatment pattern of MM patients occurred around 2017, after which Bortezomib-based regimens became the main protocol. Although bortezomib was approved by China Food and Drug Administration in 2005, it was not included in the Chinese NRDL list until 2017. It can be seen that whether it can be reimbursed by medical insurance in China is a very critical factor influencing the treatment choices of physicians and patients in real-world practice. We also noticed that age might be a potential influence factor for MM patients to receive distinct frontline chemotherapies, and the treatment pattern of older MM was slightly different from other studies (Chan et al., 2019; Al Saleh et al., 2021; Cejalvo et al., 2021; Jimenez-Zepeda et al., 2021). TD, BD, and BTD were frequent treatment regimens in both age subgroups. Besides, BCD and VADT were also observed in the frontline treatment among patients <65 years old, but MPT and CD treatments were usually applied among patients ≥65 years old, which may be explained that oral administration of less intensive chemotherapy regimens is more convenient and safer for older adults.
This study demonstrated that the attrition rates were generally high in Chinese MM patients, almost consistent with previous studies in other countries (Raab et al., 2016; Yong et al., 2016; MacEwan et al., 2018; Antunes et al., 2019; Fonseca et al., 2020; Mohyuddin et al., 2021). The attrition rates fluctuated from 45.7% to 62.5% across all LOTs and exhibited a gradually increased tendency with the increase of LOT. Nevertheless, the increasing trend of attrition rates may be attributed to worse treatment efficacy, more comorbidities, death, or family economic burden with the subsequent LOT. As a result, 54.3%, 27.9%, 11.5%, and as few as 4.3% of all the MM patients enrolled in the study received a second, third, fourth and fifth LOT. Similarly, approximate 47%, 27%, 16%, and 9% of MM patients received a second, third, fourth and fifth LOT based on the data derived from three databases in the United States (up to 2018) (Fonseca et al., 2020), and 32%–16%, 14%–38%, 15%, and 1% of European MM patients received a second, third, fourth, and fifth LOT (Raab et al., 2016; Yong et al., 2016; Antunes et al., 2019). Therefore, when MM patients were diagnosed and received therapies at earlier clinical stages (e.g., first or second LOTs), more effective and rational drugs or treatment approaches should be conducted to obtain a more profound response and a favorable survival outcome.
This study also explored the attrition rates by subgroup analysis of age, gender and ASCT. In this study, 88% of transplant MM patients received a second LOT and 13.7% received a fifth LOT, while only 50.9% of non-transplant received a second LOT and 3.3% received a fifth LOT. Similar to the results of the study by Fonseca et al. (2020), the attrition rates across all LOTs were relatively lower in transplant MM patients than in non-transplant MM patients. Besides, this study revealed a distinct difference in the attrition rates among MM patients of different ages. The attrition rates from 1st LOT through 5th LOT among MM patients ≥65 years old were generally higher than MM patients <65 years old, which may be explained by the different baseline conditions of these two groups of MM patients and elderly MM patients are usually frail and presented with more comorbidities (Lee et al., 2021; Tang et al., 2022). The median treatment duration was approximately six to 8 months across all LOTs, similar to other real-world study (Fonseca et al., 2020).
In this study, we performed the subgroup analysis of the baseline characteristics and comorbidities between MM patients receiving only 1 LOT and >1 LOT. MM who did not enter the subsequent LOT were significantly older, presented higher levels of β2-microglobulin and LDH, and had higher incidences of anemia, thrombocytopenia, renal insufficiency and comorbidities such as amyloidosis, pulmonary diseases, hepatic diseases, and cardiac disorders. It may indicate that younger MM patients may have the ability to receive more successive treatment, and a relatively preferable clinical status and physical state may be the main reason for receiving more subsequent therapies. We also noticed a significantly decreased proportion of ISS stage III MM patients in the group with >1 LOT. Especially we also conducted the Cox proportional hazards model to assess the risk factors for treatment attrition of the frontline therapy of MM in this study. Based on the multivariate analysis, ISS stage III, high LDH, and higher incidence comorbidities such as pulmonary diseases, hepatic diseases, and cardiac disorders were independent risk factors for treatment attrition of the frontline therapy. Overall, factors of older age, high-risk stratification, higher tumor burden, more comorbidities, and worse performance status may be associated with fewer subsequent treatments, increased drug toxicities and resistances.
Several limitations may exist in this retrospective real-world study. Firstly, it should be noted that this study only enrolled hospitalized MM patients who had received at least one LOT but excluded patients only receiving short-term glucocorticoids, which underestimates the attrition rates due to excluding untreated MM patients. Therefore, the results should be interpreted in the context of inpatients who received anti-tumor therapy, not the entire MM population. Secondly, patients did not enter a subsequent LOT generally owing to three reasons (i.e., death, loss of follow-up, or staying at the current LOT), but the data did not include information on the disease status of patients who survived at the current LOT during the study period. Besides, since this real-world study was carried out in a tertiary referral hospital in Western China, MM patients who lost follow-up in this hospital may return to local hospitals for further therapies, resulting in overestimated attrition. Thirdly, the shift of LOTs may be due to disease progress or drug intolerance, which was not deeply discussed in this study.
Collectively, this study demonstrated the common frontline treatment patterns in Chinese MM patients and elucidated the attrition rates with distinct LOTs and potential risk factors linked with treatment attrition, which may provide more evidence for effective treatments at specific LOTs in MM.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of West China Hospital Sichuan University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
TN and WT concepted and designed the study. WT and JY perform the statistical analysis and drafted the manuscript. YNL, LZ, HL, JW, YIL, CZ, and YQ managed the patients and collected the data. YZ and TN revised the manuscript critically. All the authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was supported by Incubation Program for Clinical Trials (No. 19HXFH030), 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. ZYJC21007), and Natural Science Foundation of Sichuan Province (No. 2022NSFSC1299). The construction of the “HemaTank” Chinese Multiple Myeloma Database (HCMMD) was funded by China Primary Healthcare Foundation.
Acknowledgments
The authors would like to acknowledge Goodwill Hessian Health Technology Co., Ltd. for the database processing and Beijing DAO research technology Co., Ltd. for the statistic analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.979111/full#supplementary-material
References
Al Saleh, A. S., Visram, A., Parmar, H., Muchtar, E., Buadi, F. K., Dispenzieri, A., et al. (2021). Treatment and outcome of newly diagnosed multiple myeloma patients > 75 years old: A retrospective analysis. Leuk. Lymphoma 62 (12), 3011–3018. doi:10.1080/10428194.2021.1950708
Antunes, L., Rocha-Gonçalves, F., Chacim, S., Lefèvre, C., Pereira, M., Pereira, S., et al. (2019). Real-world treatment patterns, resource use and cost burden of multiple myeloma in Portugal. Eur. J. Cancer Care (Engl) 28 (4), e13026. doi:10.1111/ecc.13026
Atrash, S., Flahavan, E. M., Xu, T., Ma, E., Karve, S., Hong, W. J., et al. (2022). Treatment patterns and outcomes according to cytogenetic risk stratification in patients with multiple myeloma: A real-world analysis. Blood Cancer J. 12 (3), 46. doi:10.1038/s41408-022-00638-0
Cai, Z., Chang, Y., Chen, S., Chen, W., Du, J., Fu, C., et al. (2022). Guidelines for the diagnosis and management of multiple myeloma in China (2022 revision). Zhonghua Nei Ke Za Zhi 61 (5), 480–487. doi:10.3760/cma.j.cn112138-20220309-00165
Cejalvo, M. J., Bustamante, G., Gonzalez, E., Vazquez-Alvarez, J., Garcia, R., Ramirez-Payer, A., et al. (2021). Treatment patterns and outcomes in real-world transplant-ineligible patients newly diagnosed with multiple myeloma. Ann. Hematol. 100 (7), 1769–1778. doi:10.1007/s00277-021-04529-5
Chan, H., Chai, K., Shih, S., Lewsey, R., Chen, K., McDiarmid, B., et al. (2019). Frontline treatment of elderly non transplant-eligible multiple myeloma patients using CyBorD with or without thalidomide-based consolidation: A retrospective multi-centre analysis of real-world data. Br. J. Haematol. 187 (4), 470–477. doi:10.1111/bjh.16095
Cook, G., Zweegman, S., Mateos, M. V., Suzan, F., and Moreau, P. (2018). A question of class: Treatment options for patients with relapsed and/or refractory multiple myeloma. Crit. Rev. Oncol. Hematol. 121, 74–89. doi:10.1016/j.critrevonc.2017.11.016
Fonseca, R., Facon, T., Hashim, M., Nair, S., He, J., Ammann, E. M., et al. (2021). First-line use of daratumumab, lenalidomide, and dexamethasone confers survival benefit compared with second-line use of daratumumab-based regimens in transplant-ineligible patients with multiple myeloma: Analysis of different clinical scenarios. Blood 138 (1), 118. doi:10.1182/blood-2021-144914
Fonseca, R., Usmani, S. Z., Mehra, M., Slavcev, M., He, J., Cote, S., et al. (2020). Frontline treatment patterns and attrition rates by subsequent lines of therapy in patients with newly diagnosed multiple myeloma. BMC Cancer 20 (1), 1087. doi:10.1186/s12885-020-07503-y
Hajek, R., Jarkovsky, J., Maisnar, V., Pour, L., Spicka, I., Minarik, J., et al. (2018). Real-world outcomes of multiple myeloma: Retrospective analysis of the Czech registry of monoclonal gammopathies. Clin. Lymphoma Myeloma Leuk. 18 (6), e219–e240. doi:10.1016/j.clml.2018.04.003
He, J., He, D., Han, X., Zheng, G., Wei, G., Zhao, Y., et al. (2020). Bortezomib-based regimens for newly diagnosed multiple myeloma in China: A report of 12-year real-world data. Front. Pharmacol. 11, 561601. doi:10.3389/fphar.2020.561601
He, J., Schmerold, L., Van Rampelbergh, R., Qiu, L., Potluri, R., Dasgupta, A., et al. (2021). Treatment pattern and outcomes in newly diagnosed multiple myeloma patients who did not receive autologous stem cell transplantation: A real-world observational study: Treatment pattern and outcomes in patients with multiple myeloma. Adv. Ther. 38 (1), 640–659. doi:10.1007/s12325-020-01546-0
Huang, X., Lu, J., Chang, Y., Hou, J., Li, J., Chen, W., et al. (2017). The guidelines for the diagnosis and management of multiple myeloma in China (2017 revision). Zhonghua Nei Ke Za Zhi 56 (11), 866–870. doi:10.3760/cma.j.issn.0578-1426.2017.11.021
Jimenez-Zepeda, V. H., Venner, C., McCurdy, A., Masih-Khan, E., Atenafu, E. G., Sebag, M., et al. (2021). Real-world outcomes with bortezomib-containing regimens and lenalidomide plus dexamethasone for the treatment of transplant-ineligible multiple myeloma: A multi-institutional report from the Canadian myeloma research group database. Br. J. Haematol. 193 (3), 532–541. doi:10.1111/bjh.17350
Kumar, S., Williamson, M., Ogbu, U., Surinach, A., Arndorfer, S., and Hong, W. J. (2021). Front-line treatment patterns in multiple myeloma: An analysis of U.S.-based electronic health records from 2011 to 2019. Cancer Med. 10 (17), 5866–5877. doi:10.1002/cam4.4137
Landgren, O., and Iskander, K. (2017). Modern multiple myeloma therapy: Deep, sustained treatment response and good clinical outcomes. J. Intern Med. 281 (4), 365–382. doi:10.1111/joim.12590
Lee, H. S., Lee, J., Jo, J. C., Jung, S. H., Lee, J. J., Kim, D., et al. (2021). Development of a new clinical index to easily assess frailty of elderly patients with multiple myeloma in Asian population. Sci. Rep. 11 (1), 22907. doi:10.1038/s41598-021-02433-6
MacEwan, J. P., Batt, K., Yin, W., Peneva, D., Sison, S., Vine, S., et al. (2018). Economic burden of multiple myeloma among patients in successive lines of therapy in the United States. Leuk. Lymphoma 59 (4), 941–949. doi:10.1080/10428194.2017.1361035
Mohyuddin, G. R., Koehn, K., Abdallah, A. O., Goodman, A. M., and Prasad, V. (2021). Reporting of postprotocol therapies and attrition in multiple myeloma randomized clinical trials: A systematic review. JAMA Netw. Open 4 (4), e218084. doi:10.1001/jamanetworkopen.2021.8084
Morgan, G. J., Walker, B. A., and Davies, F. E. (2012). The genetic architecture of multiple myeloma. Nat. Rev. Cancer 12 (5), 335–348. doi:10.1038/nrc3257
Niu, T., Lu, J., Fu, C., and Sun, C. (2022). Chinese multiple myeloma standard dataset (version 2022). Peking: Peking Union Medical College Press.
Qian, X., Chen, H., Xia, J., Wang, J., Zhou, X., and Guo, H. (2018). Real-world clinical outcomes in elderly Chinese patients with multiple myeloma: A single-center experience. Med. Sci. Monit. 24, 5887–5893. doi:10.12659/MSM.907588
Raab, M. S., Cavo, M., Delforge, M., Driessen, C., Fink, L., Flinois, A., et al. (2016). Multiple myeloma: Practice patterns across Europe. Br. J. Haematol. 175 (1), 66–76. doi:10.1111/bjh.14193
Rajkumar, S. V., Dimopoulos, M. A., Palumbo, A., Blade, J., Merlini, G., Mateos, M. V., et al. (2014). International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 15 (12), e538–e548. doi:10.1016/s1470-2045(14)70442-5
Rajkumar, S. V. (2020). Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am. J. Hematol. 95 (5), 548–567. doi:10.1002/ajh.25791
Rajkumar, S. V., Richardson, P., and San Miguel, J. F. (2015). Guidelines for determination of the number of prior lines of therapy in multiple myeloma. Blood 126 (7), 921–922. doi:10.1182/blood-2015-05-647636
Tang, W., Li, Y., Zou, Z., Cui, J., Wang, F., Zheng, Y., et al. (2022). A stratified therapeutic model incorporated with studies on regulatory B cells for elderly patients with newly diagnosed multiple myeloma. Cancer Med. doi:10.1002/cam4.5228
Verelst, S. G. R., Blommestein, H. M., De Groot, S., Gonzalez-McQuire, S., DeCosta, L., de Raad, J. B., et al. (2018). Long-term outcomes in patients with multiple myeloma: A retrospective analysis of the Dutch population-based HAematological registry for observational studies (pharos). Hemasphere 2 (4), e45. doi:10.1097/HS9.0000000000000045
Yan, Y., Mao, X., Liu, J., Fan, H., Du, C., Li, Z., et al. (2019). The impact of response kinetics for multiple myeloma in the era of novel agents. Blood Adv. 3 (19), 2895–2904. doi:10.1182/bloodadvances.2019000432
Yang, P., Qu, Y., Wang, M., Chu, B., Chen, W., Zheng, Y., et al. (2022). Pathogenesis and treatment of multiple myeloma. MedComm 3 (2), e146. doi:10.1002/mco2.146
Keywords: multiple myeloma, attrition rate, line of therapy, frontline treatment, transplant
Citation: Tang W, Yang J, Li Y, Zhang L, Li H, Wang J, Liao Y, Zhang C, Qu Y, Zheng Y and Niu T (2023) Treatment attrition rates and relevant risk factors in multiple myeloma: A real-world study in China. Front. Pharmacol. 14:979111. doi: 10.3389/fphar.2023.979111
Received: 27 June 2022; Accepted: 03 January 2023;
Published: 12 January 2023.
Edited by:
Brian Godman, University of Strathclyde, United KingdomReviewed by:
Joycelyn Sim, Queen Mary Hospital, Hong Kong SAR, ChinaSimrit Parmar, University of Texas MD Anderson Cancer Center, United States
Copyright © 2023 Tang, Yang, Li, Zhang, Li, Wang, Liao, Zhang, Qu, Zheng and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Niu, bml1dGluZ0B3Y2hzY3UuY24=
†These authors have contributed equally to this work
 Wenjiao Tang
Wenjiao Tang Jinrong Yang†
Jinrong Yang† Yan Li
Yan Li Li Zhang
Li Zhang Yi Liao
Yi Liao Chunlan Zhang
Chunlan Zhang Ying Qu
Ying Qu Ting Niu
Ting Niu