
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 03 February 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.970457
This article is part of the Research TopicInhibitors of CDK family: New Perspective and Rationale for Drug Combination in Preclinical Model of Solid TumorsView all 10 articles
 Claudia Arndt1,2*†
Claudia Arndt1,2*† Antje Tunger3,4†
Antje Tunger3,4† Rebekka Wehner3,4,5
Rebekka Wehner3,4,5 Rebecca Rothe3,4
Rebecca Rothe3,4 Eleni Kourtellari4
Eleni Kourtellari4 Stephanie Luttosch4
Stephanie Luttosch4 Katharina Hannemann4
Katharina Hannemann4 Stefanie Koristka1
Stefanie Koristka1 Liliana R. Loureiro1
Liliana R. Loureiro1 Anja Feldmann1
Anja Feldmann1 Torsten Tonn5,6,7
Torsten Tonn5,6,7 Theresa Link3,5,8
Theresa Link3,5,8 Jan Dominik Kuhlmann3,5,8
Jan Dominik Kuhlmann3,5,8 Pauline Wimberger3,5,8
Pauline Wimberger3,5,8 Michael Philipp Bachmann1,3,5,9
Michael Philipp Bachmann1,3,5,9 Marc Schmitz3,4,5*
Marc Schmitz3,4,5*The cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor palbociclib is an emerging cancer therapeutic that just recently gained Food and Drug Administration approval for treatment of estrogen receptor (ER)-positive, human epidermal growth factor receptor (Her)2-negative breast cancer in combination with the ER degrader fulvestrant. However, CDK4/6 inhibitors are not cancer-specific and may affect also other proliferating cells. Given the importance of T cells in antitumor defense, we studied the influence of palbociclib/fulvestrant on human CD3+ T cells and novel emerging T cell-based cancer immunotherapies. Palbociclib considerably inhibited the proliferation of activated T cells by mediating G0/G1 cell cycle arrest. However, after stopping the drug supply this suppression was fully reversible. In light of combination approaches, we further investigated the effect of palbociclib/fulvestrant on T cell-based immunotherapies by using a CD3-PSCA bispecific antibody or universal chimeric antigen receptor (UniCAR) T cells. Thereby, we observed that palbociclib clearly impaired T cell expansion. This effect resulted in a lower total concentration of interferon-γ and tumor necrosis factor, while palbociclib did not inhibit the average cytokine release per cell. In addition, the cytotoxic potential of the redirected T cells was unaffected by palbociclib and fulvestrant. Overall, these novel findings may have implications for the design of treatment modalities combining CDK4/6 inhibition and T cell-based cancer immunotherapeutic strategies.
Approximately 80% of breast cancers are hormone receptor-positive (HR+) and therefore represent the largest subtype of this malignancy. Endocrine therapies targeting the estrogen receptor (ER) using aromatase inhibitors, such as letrozole, preventing ER signaling (Finn et al., 2015; Finn et al., 2016b; Goetz et al., 2017), selective ER degraders, like fulvestrant (Turner et al., 2015; Sledge et al., 2017; Turner et al., 2018), or selective ER modulators as tamoxifen (Tripathy et al., 2018b) substantially reduced tumor recurrence and improved overall survival (OS) (Abe et al., 2005). However, a significant proportion of patients suffers from relapse following single-agent treatment (Abe et al., 2005; Baselga et al., 2012). To overcome resistance to endocrine therapy new treatment options were developed, such as cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors, which significantly improved clinical outcomes for these patients (Finn et al., 2015; Turner et al., 2018). CDK4/6 are fundamental drivers of the cell cycle by regulating initiation and progression through the G1 phase and are therefore also key players in various malignancies (Yu et al., 2006; Choi et al., 2012). Common dysregulations of the CDK4/6-retinoblastoma protein (Rb) axis, like copy-number variation or overexpression as well as loss of negative regulators of the pathway, can lead to cancer formation (Sherr et al., 2016). Accordingly, CDKs have long been attractive targets for pharmacologic inhibition in tumor therapy (Adams et al., 2015; Sherr et al., 2016).
The cytostatic potential of single-agent CDK4/6 inhibitors has been shown in vitro, causing downregulation of transcription factor E2F target genes, loss of proliferation markers and cell cycle arrest in G1 (Fry et al., 2004). In particular, HR+ breast cancer is susceptible to CDK4/6 inhibitor therapy (Finn et al., 2009; O’Leary et al., 2016). Given the fact that activation of the cyclin D-CDK4/6 complex depends on mitogenic stimuli, synergistic combinations of CDK4/6 inhibitors with signal transduction inhibitors have been developed. In particular, the three orally available CDK4/6 inhibitors palbociclib (PD-0332991; Ibrance; Pfizer), ribociclib (LEE011; Kisqali; Novartis) and abemaciclib (LY2835219; Verzenio; Lilly) received approval by the Food and Drug Administration (FDA) for treatment of patients with ER+, human epidermal growth factor receptor 2-negative (HER2-) advanced or metastatic breast cancer in combination with an aromatase inhibitor or fulvestrant (Finn et al., 2016a; Cristofanilli et al., 2016; Hortobagyi et al., 2016). Various clinical trials within the framework of the PALOMA, MONALEESA, and MONARCH study families form the basis for the FDA approvals, showing improved progression-free survival (PFS) and OS for treatment with CDK4/6 inhibitors and endocrine therapy in breast cancer patients (Finn et al., 2016b; Cristofanilli et al., 2016; Tripathy et al., 2018a; Turner et al., 2018; Im et al., 2019; Johnston et al., 2020; Slamon et al., 2020; Sledge et al., 2020). Beyond ER+ breast cancer, promising activity of CDK4/6 inhibitors in mantle cell lymphoma (MCL), liposarcoma, melanoma, non-small cell lung cancer (NSCLC), glioblastoma, neuroblastoma and malignant rhabdoid tumors has been shown (Leonard et al., 2012; Dickson et al., 2016; Patnaik et al., 2016; Geoerger et al., 2017). Further studies are currently underway, e.g. the evaluation of ribociclib for treatment of prostate cancer (Scheinberg et al., 2020).
However, resistance to therapy frequently occurs in treated patients (Finn et al., 2016a; Finn et al., 2016b; Cristofanilli et al., 2016; Hortobagyi et al., 2016). For this reason, new therapeutic strategies are required to overcome the resistance to CDK4/6 inhibition. The combination with other strategies, such as immunotherapeutic approaches, may represent an interesting treatment modality. There is increasing evidence that CDKs not only regulate cell cycle progression in tumor cells, but also development, differentiation and activation of immune cells (Wells and Morawski, 2014; Ameratunga et al., 2019; Laphanuwat and Jirawatnotai, 2019). T cells play a major role in antitumor immune defense. Based on their antitumoral properties, such as production of proinflammatory cytokines and cytotoxic activity, T cells emerged as a promising tool for cancer immunotherapy. An attractive approach is the genetic modification of autologous T cells with chimeric antigen receptors (CARs) targeting tumor-associated antigens (TAAs). By this, T cells can be redirected against tumor cells in a major histocompatibility complex (MHC)-independent manner (Sadelain et al., 2013). Currently, there are several clinical trials of CAR T cells targeting HER-2 (NCT01935843, NCT01022138), cMet (NCT03060356) or mesothelin (NCT02580747) in breast cancer patients. In this study, we used the switchable UniCAR system (Bachmann, 2019), that is also currently under clinical investigation in a phase I clinical trial (NCT04230265). As an adaptor CAR system, UniCAR T cells recognize a small epitope not present on the cell surface. Thus, they are per se inactive and have to be combined with a tumor-reactive target module (TM) to induce tumor lysis, thereby separating the signaling and tumor-targeting function of CARs (Bachmann, 2019; Arndt et al., 2020a). Here, we are utilizing a well-established prostate stem cell antigen (PSCA)-specific TM to redirect UniCAR T cells against prostate cancer cells (Arndt et al., 2014b; 2014a). Alternative strategies to redirect T cells towards tumor cells are bispecific antibodies (bsAbs) that simultaneously target CD3 and a TAA. Due to the bsAb-mediated cross-linkage, T cells can be efficiently engaged for tumor cell killing independent of their TCR-specificity and costimulatory signals (Wolf et al., 2005; Offner et al., 2006). In a phase II study (NCT04224272), the combination of the HER-2-targeting bsAb ZW25 and palbociclib plus fulvestrant for HER2+/HR+ advanced breast cancer is under investigation. Carcinoembryonic antigen (NCT01730612) and PSCA (NCT03927573) are further antigens for targeting breast cancer cells with bsAbs. Here, we used the CD3-PSCA bsAb, which triggers an efficient T cell-mediated killing of PSCA+ tumor cells (Feldmann et al., 2012; Arndt et al., 2014b).
Based on these findings, the aim of the present study was to examine the impact of palbociclib and fulvestrant alone or in combination on CD3+ T cells and novel emerging T cell-based cancer immunotherapies. In this context, we explored the impact of these two therapeutic agents on proliferation, cytokine production and cytotoxic potential of PSCA-specific UniCAR T cells (Feldmann et al., 2017) and T cells redirected via CD3-PSCA bsAb (Feldmann et al., 2012).
All cell lines were maintained at 37°C in a humidified atmosphere with 5% CO2. Recombinant antibody producing 3T3 cell lines were cultured in DMEM complete media (Feldmann et al., 2011). The prostate cancer cell lines PC3-PSCA/PSMA Luc+ and LNCaP-PSCA Luc+ were generated and cultured as previously described (Feldmann et al., 2017).
Construction and cloning of PSCA TM and CD3-PSCA bsAb have been published elsewhere (Feldmann et al., 2012; Arndt et al., 2014b). Recombinant antibodies were produced by 3T3 cell lines (Feldmann et al., 2012; Arndt et al., 2014b). Antibody purification from cell culture supernatants was performed via Ni-NTA affinity chromatography (Feldmann et al., 2011). After dialysis of elution fractions against 1x PBS, proteins were characterized via SDS-PAGE and immunoblotting as published previously (Feldmann et al., 2011; Feldmann et al., 2012; Arndt et al., 2018).
The study was approved by the local institutional review board of the Faculty of Medicine of the TU Dresden (EK138042014). Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats of healthy donors via density gradient centrifugation. Untouched CD3+ T cells were isolated from freshly prepared PBMCs using immunomagnetic separation according to the manufacturer’s instructions (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The purity of the isolated cell population was > 90% as assessed by flow cytometric analysis. Isolated T cells were cultured in RPMI complete medium (Feldmann et al., 2011) supplemented with 50 U/ml interleukin (IL)-2 (Miltenyi Biotec GmbH).
Generation of UniCAR T cells was carried out as described recently (Feldmann et al., 2020). Briefly, T cells were stimulated with T Cell TransAct™ (Miltenyi Biotec GmbH) and genetically modified via lentiviral transduction (Cartellieri et al., 2014) using a multiplicity of infection of 1–2. During transduction and expansion, T cells were maintained in TexMACS™ medium (Miltenyi Biotec GmbH) supplemented with human IL-2, human IL-7 and human IL-15 (all Miltenyi Biotec GmbH). Experiments were conducted with unsorted UniCAR T cells that were kept in RPMI complete medium (Feldmann et al., 2011) without additional cytokines for 24 h. Based on the co-translated EGFP marker protein expression, the proportion of UniCAR+ T cells was assessed via flow cytometry prior to each experiment.
Analysis of surface molecules on CD3+ T cells was performed using the following monoclonal antibodies: APC-H7-conjugated anti-human CD3 (BD Biosciences, Heidelberg, Germany), anti-human CD4-VioBlue, anti-human CD3-FITC and anti-human CD8-APC (all Miltenyi Biotec GmbH). Immunofluorescence staining of cell surface molecules was performed using the relevant antibodies according to the provider’s instructions. After the staining procedure, cells were washed and evaluated by BD LSRFortessa™ flow cytometer or MACSQuant Analyzer 10 (Miltenyi Biotec GmbH). Before measurement, 7-AAD (BD Biosciences), DAPI (Miltenyi Biotec GmbH) or propidium iodide solution (Thermo Fisher Scientific, Waltham, Massachusetts, USA) was added for live/dead discrimination.
In order to distinguish effector and target cells, CD3+ T cells and freshly prepared or thawed UniCAR T cells were stained with cell proliferation dye eFluor™ 670 according to the manufacturer’s instructions (Thermo Fisher Scientific). Stained CD3+ T cells (2 × 105/well) were cultured in the presence of stimulating anti-CD3/CD28 beads (Thermo Fisher Scientific) as well as the presence or absence of palbociclib (1, 0.2 or 0.025 µM) and fulvestrant (0.1 or 0.025 µM) in different concentrations in round-bottomed 96-well plates. Palbociclib was added daily whereas fulvestrant was added only once to the corresponding wells, according to the clinical dosing schedule. Cells were harvested after 24, 48, 72, 96, 120, 144 or 168 h. After excluding doublets and distinguishing between live and dead cells, the percentage of eFluor670+-diminished T cells compared to the untreated control was analyzed and expressed as “% proliferation”. Additionally, number of T cells were determined over time by gating on single, living eFluor670+ cells. Samples were analyzed after DAPI staining by utilizing a MACSQuant VYB flow cytometer (Miltenyi Biotec GmbH). For analysis of reversibility, palbociclib was only added at the first day and cells were harvested after 48 or 120 h and stained with anti-CD3 antibody. Percentage of eFluor™ 670 diminished CD3+ T cells was monitored by a BD LSRFortessa™ flow cytometer.
eFluor™ 670 stained unstimulated or UniCAR-modified T cells were incubated with tumor cells at an effector-to-target cell (E:T) ratio of 5:1 in the presence or absence of 30 nM CD3-PSCA bsAb or PSCA TM in a 96 h co-cultivation assay. Palbociclib (1, 0.2 or 0.025 µM) was added after 0, 24, 48 and 72 h. Fulvestrant (0.1 or 0.025 µM) was added just once at the beginning of the experiment (0 h). Effector cell numbers were determined by flow cytometry after 96 h as previously published (Arndt et al., 2020b). In brief, 20 µl of each sample was transferred to a 96-well round bottom plate and mixed with DAPI solution prior to measurement with the MACSQuant VYB analyzer (Miltenyi Biotec GmbH). Samples were first gated for T cells using SSC-A/FSC-A parameters. Subsequently, doublets were excluded by FSC-H/FSC-A gating and live/dead cells were distinguished by DAPI. Gating on eFluor670+ or eFluor670+ EGFP+ cells identified T cells or UniCAR T cells and allowed determination of T cell number.
To analyze DNA replication in proliferating cells the Click-iT® Plus EdU Flow Cytometry Assay Kit was utilized according to the manufacturer’s instructions (Thermo Fisher Scientific). During DNA synthesis, the thymidine analog EdU (5-ethynyl-2′-deoxyuridine) was incorporated in the DNA and detected by flow cytometry after performing a copper catalyzed click reaction with the provided Alexa Fluor 488 dye. In brief, CD3+ T cells of four healthy donors (2 × 105/well), being prepared as described in Section 2.3, were stimulated with human T-cell activator CD3/CD28 Dynabeads™ (Thermo Fisher Scientific) and cultured in the presence or absence of palbociclib at different concentrations (0.025, 0.2 or 1 µM) in round-bottomed 96-well plates. Unstimulated, untreated as well as stimulated and DMSO treated cells served as controls. Palbociclib was added daily during the experimental period of 4 days. 18 h prior to cell harvesting, cells were incubated with 10 µM EdU. The cells were harvested after 96 h and stained with an APC-coupled anti-CD3 antibody (BD Biosciences). Following surface antibody staining, cells were fixed and permeabilized using Click-iT® fixative and 1x Click-iT® saponin-based reagent, respectively, for 15 min each. Further, Click-iT® Plus reaction cocktail was added and the samples were incubated, protected from light, for another 30 min at room temperature. After staining and washing procedure, samples were incubated with DAPI (0.4 ng/μl) to stain the cells for DNA content. Finally, flow cytometric analysis was performed using MACSQuant Analyzer 10 (Miltenyi Biotec GmbH) with the appropriate laser and filter settings. FlowLogic™ software (version 8.6; Inivai Technologies, Mentone Victoria, Australia) was used for data evaluation.
To evaluate the interferon (IFN)-γ and tumor necrosis factor (TNF) secretion of activated T cells, 5 × 104 effector cells (unstimulated T cells or UniCAR T cells) and 1 × 104 tumor cells were incubated in the presence or absence of 30 nM of recombinant antibody (CD3-PSCA bsAb or PSCA TM) in round-bottomed 96-well plates. Palbociclib (1, 0.2 or 0.025 µM) was added after 0, 24, 48 and 72 h. Fulvestrant (0.1 or 0.025 µM) was added just once at the beginning of the experiment (0 h). After 96 h, cell-free supernatants were collected. IFN-γ and TNF were quantified by ELISA according to the manufacturer’s instructions (BD Biosciences).
Luminescence-based killing assays were performed according to a previously published protocol (Mitwasi et al., 2017). Briefly, 5 × 104 effector cells (unstimulated T cells or UniCAR T cells) and 1 × 104 luciferase-expressing tumor cells were incubated with or without 30 nM of recombinant antibody (CD3-PSCA bsAb or PSCA TM). In addition, palbociclib (1, 0.2 or 0.025 µM) and/or fulvestrant (0.1 or 0.025 µM) were added to co-cultures. Alternatively, effector cells were first incubated with palbociclib (0.2 or 0.025 µM) and/or fulvestrant (0.1 or 0.025 µM) for 24 h. Thereafter, tumor cells and recombinant antibodies were added as described above. In addition, co-cultivation assays were repeatedly supplemented with palbociclib (1, 0.2 or 0.025 µM). Effector cells were prepared freshly or thawed 48 h prior to experiment. After 8 h of co-culture, 96-well white plates were centrifuged for 5 min at 360 × g. Subsequently, 100 μl supernatant was carefully removed and 50 μl ONE-Glo™ Luciferase reagent (Promega GmbH, Mannheim, Germany) added to each well. Following a 5 min incubation step, luminescence of each sample was measured using the Infinite® M200 pro microplate reader (Tecan Germany GmbH, Crailsheim, Germany). Specific tumor cell lysis was calculated as described elsewhere (Mitwasi et al., 2017).
Student’s t-test was performed to evaluate the significance of the results. To compare samples to a control sample One-way ANOVA with posthoc Dunnett multiple comparison test was performed using GraphPad Prism 7 software (GraphPad Prism Inc., La Jolla, CA, United States). Values of p ≤ 0.05 were considered as significant.
The expansion, cytokine secretion and cytotoxic activity of T cells play an important role in antitumor immunity. Due to the fact, that T cells rapidly proliferate after antigen-specific activation, we explored the effect of the CDK4/6 inhibitor on T cell proliferation. To investigate whether palbociclib and/or fulvestrant alter this function, CD3+ T cells were maintained in the presence or absence of palbociclib or fulvestrant alone or in combination. T cells were stimulated to proliferate by anti-CD3/CD28 beads. Proliferation was measured every 24 h for 7 days by flow cytometry on the basis of eFluor™ 670 dilution over time. As depicted in Figure 1A, T cells displayed a strong proliferation upon stimulation in comparison to unstimulated control starting after 48 h. Palbociclib significantly impaired this ability of the CD3+ T cells in a concentration-dependent manner. Notably, 0.025–1 µM palbociclib clearly reduced the proliferative capacity of CD3+ T cells. The reduced proliferation, as measured by the dilution of the proliferation dye eFluor670™, is also reflected in a reduced number of T cells (Supplementary Figure S1). In contrast, fulvestrant did not influence this functional property of T cells (Figure 1A; Supplementary Figure S1).
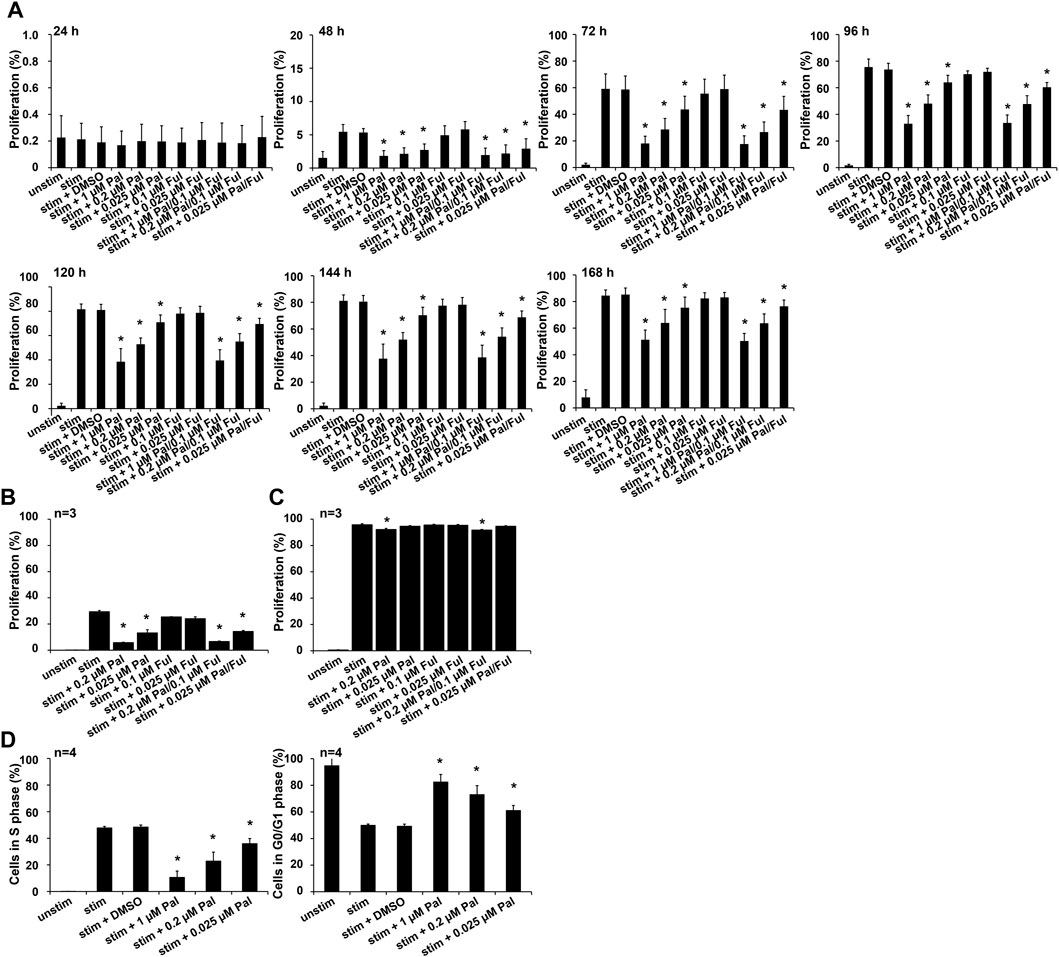
FIGURE 1. Influence of palbociclib and fulvestrant on T cell proliferation. (A) eFluor™ 670-stained T cells were stimulated by anti-CD3/CD28 beads and cultured in the presence or absence of palbociclib (daily addition), fulvestrant, or their combination at indicated concentrations for 24, 48, 72, 96, 120, 144, and 168 h. Cells were harvested and dilution of eFluor670™ dye was determined by flow cytometry. The results are depicted as the means ± SEM of four donors. (*p ≤ 0.05 compared to control sample “stim + DMSO”). (B,C) eFluor™ 670-stained T cells were stimulated by anti-CD3/CD28 beads and cultured in the presence or absence of palbociclib (single administration), fulvestrant, or their combination for (B) 48 h and (C) 120 h. Cells were harvested and dilution of proliferation dye eFluor670™ was determined by flow cytometry. The results are depicted as the means ± SEM of three donors. (*p ≤ 0.05 compared to control sample “stim”). (D) DNA replication in proliferating cells was analyzed by EdU flow cytometry assay. Therefore, T cells were stimulated by anti-CD3/CD28 beads and cultured in the presence of palbociclib (daily addition) at indicated concentrations for 96 h. 18 h prior to cell harvesting, cells were incubated with 10 µM EdU. Cells were harvested and percentage of EdU-positive cells was determined by flow cytometry. (*p ≤ 0.05 compared to control sample “stim + DMSO”).
We further investigated whether the inhibitory effect of palbociclib on T cell proliferation is reversible. Therefore, the CDK4/6 inhibitor was only added at the first day to stimulated CD3+ T cells instead of daily. Proliferation was analyzed after 48 h and 120 h. Although overall proliferation of stimulated T cells was less pronounced after 48 h, single-dosing of palbociclib alone or in combination with fulvestrant clearly impaired proliferation of CD3+ T cells (Figure 1B). However, after 120 h the inhibitory effect of palbociclib on T cell proliferation was almost abrogated (Figure 1C). These results indicate that the inhibitory effect of palbociclib on the proliferative capacity of T cells is reversible after stopping the drug application.
To study the underlying mechanisms of the palbociclib-mediated reduction in T cell proliferation, we performed an EdU assay with anti-CD3/CD28 bead-activated T cells treated with palbociclib (daily addition) for 96 h. We found a decreased percentage of T lymphocytes in S-phase of cell cycle when cells are exposed to 0.2 or 1 µM palbociclib (Figure 1D). In contrast, the percentage of T cells in G0/G1 phase increased under the same culture conditions. These findings indicate that the reduced proliferation of T cells is caused by a palbociclib-mediated G0/G1 cell cycle arrest.
In view of potential combination therapies, we explored the influence of palbociclib and fulvestrant on two PSCA-specific T cell-based immunotherapies. On the one hand, we selected the CD3-PSCA bsAb (Figure 2A) (Feldmann et al., 2012). As shown in previous studies, due to its dual specificity for CD3 and PSCA it can specifically cross-link T cells and PSCA-expressing tumor cells in a MHC- and TCR-independent manner that finally culminates in effective tumor cell elimination (Feldmann et al., 2012). On the other hand, we chose the switchable UniCAR T cell technology (Bachmann, 2019), which is composed of UniCAR-modified T cells and PSCA-specific TMs (Figure 2B) (Arndt et al., 2014a; Arndt et al., 2014b).
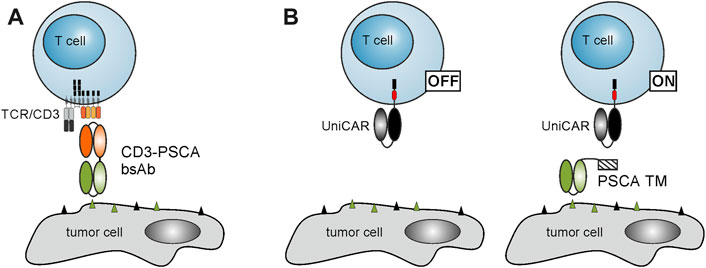
FIGURE 2. Schematic representation of T cell-based immunotherapies. (A) Due to its dual specificity for CD3 and PSCA, the CD3-PSCA bsAb is able to cross-link T cells and PSCA-expressing tumor cells. Subsequently, T cells are activated and kill the recognized target cell. (B) The UniCAR system is composed of UniCAR T cells and TAA-specific TMs. In the absence of TMs, UniCAR T cells are not activated. Upon addition of a PSCA-specific TM, UniCAR T cells can be cross-linked with PSCA-expressing tumor cells resulting in an efficient tumor cell lysis.
In order to assess the impact of palbociclib and fulvestrant on both T cell retargeting strategies, co-cultivation assays with two different prostate cancer cells lines were carried out. For this purpose, unstimulated T cells or UniCAR T cells in the presence or absence of the CD3-PSCA bsAb or the PSCA TM were used. Palbociclib and fulvestrant were added either alone or in combination at various concentrations.
For a sustained and efficient antitumor response mediated by T cell-based immunotherapies, proliferation and polyclonal expansion of effector T cell populations is required. Thus, first experiments aimed to investigate the influence of palbociclib and fulvestrant on the proliferative capacity of specifically activated bsAb-engaged T cells and UniCAR T cells. As shown in Figure 3, upon cross-linkage with PC3-PSCA/PSMA Luc+ (Figures 3A, B) or LNCaP-PSCA Luc+ cells (Figures 3C, D) via the CD3-PSCA bsAb, T cell numbers increased up to 5-fold compared to the negative controls without bsAb after 96 h. However, in the presence of 0.2 or 1 µM palbociclib the proliferation and expansion of bsAb-redirected T cells was efficiently inhibited (Figure 3). Under these conditions, numbers of T cells did not or only slightly increase and were in some cases almost equal to T cell counts detected in samples without the CD3-PSCA bsAb. Similar results were obtained when palbociclib was applied in combination with 0.1 μM fulvestrant. Although suppressive effects of palbociclib on T cell proliferation were less profound at a lower concentration of 0.025 μM, T cell expansion was still significantly reduced compared to samples lacking the small-molecule inhibitor. In contrast, the selective ER degrader fulvestrant did not alter bsAb-mediated T cell expansion.
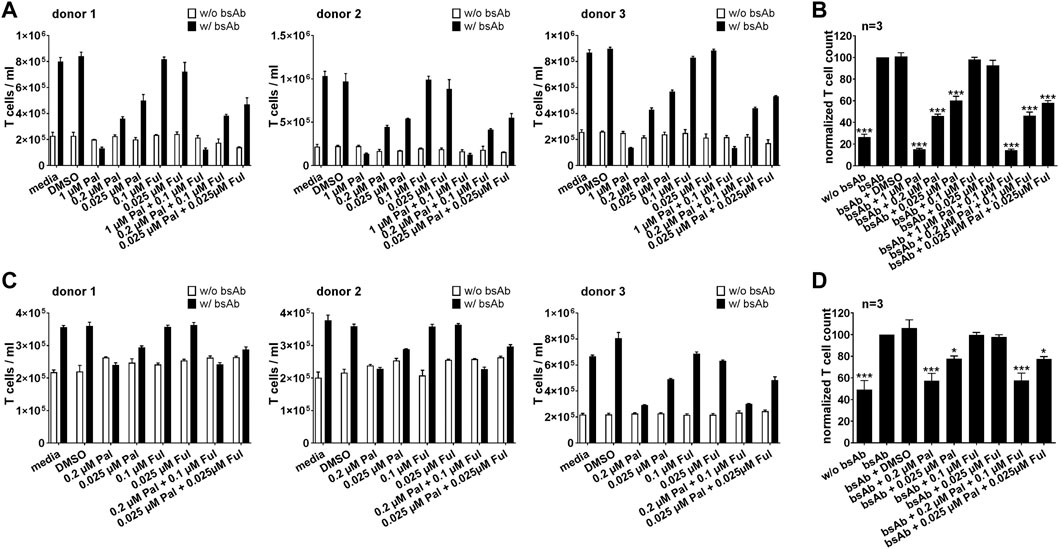
FIGURE 3. Effect of palbociclib and fulvestrant on bsAb-mediated T cell expansion. eFluor™ 670+ T cells and (A,B) PC3-PSCA/PSMA Luc+ or (C,D) LNCaP-PSCA Luc+ cells were incubated with or without CD3-PSCA bsAb. Palbociclib and/or fulvestrant were added at indicated concentrations. After 96 h, numbers of eFluor™ 670+ T cells were determined by flow cytometry using the MACSQuant® Analyzer. (A,C) Each diagram shows average T cell counts ± SEM of triplicates for one T cell donor. (B,D) Graphs summarize relative T cell counts ± SEM of three different T cell donors. T cell numbers in the presence of tumor cells and bsAb (“bsAb”) were equalized to 100%. (*p ≤ 0.05, ***p ≤ 0.001 compared to control sample “bsAb”; One-way ANOVA with posthoc Dunnett multiple comparison test).
These findings were not only limited to bsAb-redirected T cells, but could also be observed when palbociclib and fulvestrant were combined with the PSCA-specific UniCAR system. Expansion of redirected UniCAR T cells was significantly suppressed in the presence of 0.2 or 1 µM palbociclib alone or in combination with 0.1 μM fulvestrant (Figure 4). Fulvestrant alone exerted no inhibitory effects on the proliferative capacity of UniCAR T cells.
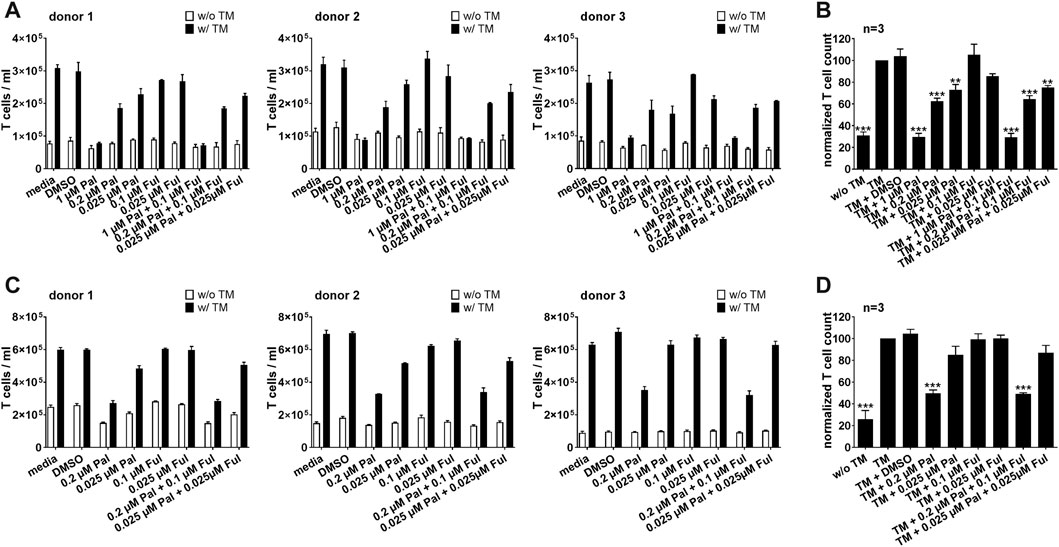
FIGURE 4. Effect of palbociclib and fulvestrant on TM-mediated UniCAR T cell expansion. eFluor™ 670+ UniCAR T cells and (A,B) PC3-PSCA/PSMA Luc+ or (C,D) LNCaP-PSCA Luc+ cells were incubated with or without 30 nM of PSCA TM. Palbociclib and/or fulvestrant were added at indicated concentrations. After 96 h, numbers of eFluor™ 670+ UniCAR T cells were determined by flow cytometry using the MACSQuant® Analyzer. (A,C) Each diagram shows average T cell counts ± SEM of triplicates for one T cell donor. (B,D) Graphs summarize relative UniCAR T cell counts ± SEM of three different T cell donors. UniCAR T cell numbers in the presence of tumor cells and TM (“TM”) were equalized to 100%. (***p ≤ 0.001 compared to control sample “TM”; One-way ANOVA with posthoc Dunnett multiple comparison test).
As the proinflammatory cytokines IFN-γ and TNF can increase the therapeutic efficacy of T cell-based immunotherapies, we investigated the impact of palbociclib and fulvestrant on the secretion of IFN-γ and TNF by UniCAR T cells and bsAb-redirected T cells. Therefore, cytokine assays were performed with LNCaP-PSCA Luc+ or PC3-PSCA/PSMA Luc+ cells. Upon cross-linkage with PC3-PSCA/PSMA Luc+ (Figures 5A, B) or LNCaP-PSCA Luc+ cells (Figures 5C, D) via the CD3-PSCA bsAb, a higher IFN-γ concentration in the supernatants compared to the control was detected. Palbociclib used at a concentration of 0.2 or 1 µM significantly reduced the IFN-γ concentration in the supernatants of bsAb-redirected T cells. A lower palbociclib concentration (0.025 µM) exerted only minor or no inhibitory effects. Likewise, in the presence of 1 µM palbociclib, significantly lower levels of TNF were detected in the co-culture supernatants after 96 h (Supplementary Figures S2A, B). Fulvestrant alone did not affect the bsAb-mediated IFN-γ or TNF secretion by T cells.
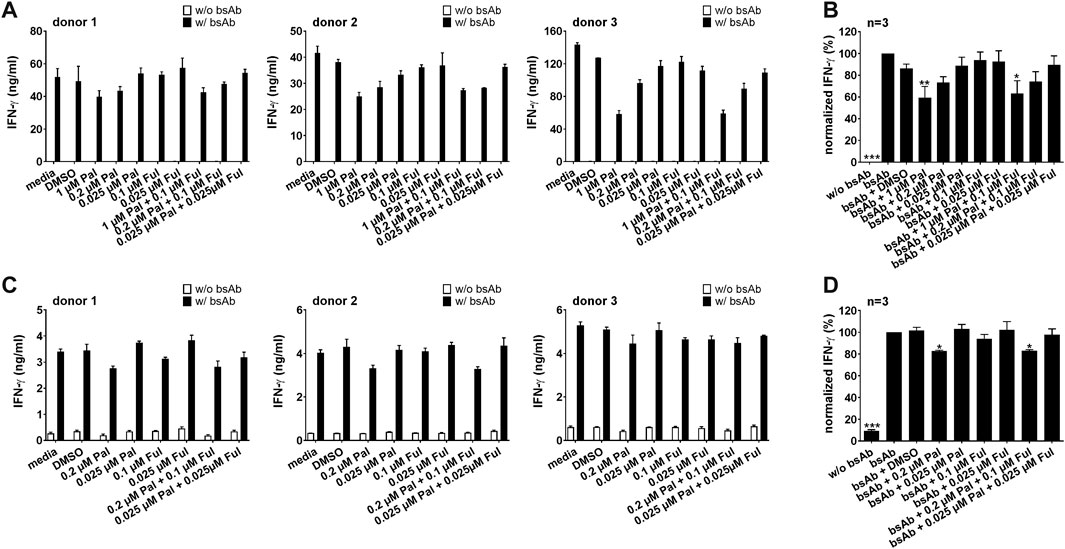
FIGURE 5. Effect of palbociclib and fulvestrant on IFN-γ release of bsAb-engaged T cells. T cells and (A,B) PC3-PSCA/PSMA Luc+ or (C,D) LNCaP-PSCA Luc+ cells were incubated with or without 30 nM of CD3-PSCA bsAb in the presence or absence of palbociclib and/or fulvestrant. After 96 h, IFN-γ concentrations in co-culture supernatants were analyzed by ELISA. (A,C) Each diagram shows average IFN-γ concentration ± SEM of triplicates for one T cell donor. (B,D) Graphs summarize relative IFN-γ release ± SEM of three different T cell donors. IFN-γ concentrations in the presence of T cells, tumor cells and bsAb (“bsAb”) were equalized to 100%. (*p ≤ 0.05, ***p ≤ 0.001 compared to control sample “bsAb”; One-way ANOVA with posthoc Dunnett multiple comparison test).
Similar results were observed when palbociclib and fulvestrant were combined with the PSCA-specific UniCAR system (Figure 6; Supplementary Figures S2C, D). Cross-linkage of PSCA-specific UniCAR T cells with prostate cancer cells resulted in a higher IFN-γ and TNF concentration in the supernatants compared to the control. In the presence of palbociclib, the concentration of IFN-γ and TNF in the supernatants of redirected UniCAR T cells was reduced in a concentration-dependent manner. While in the presence of 0.2 or 1 µM palbociclib IFN-γ and TNF were significantly reduced, a lower palbociclib concentration only considerably altered TNF release. In contrast, fulvestrant had no inhibitory effect on this functional characteristic.
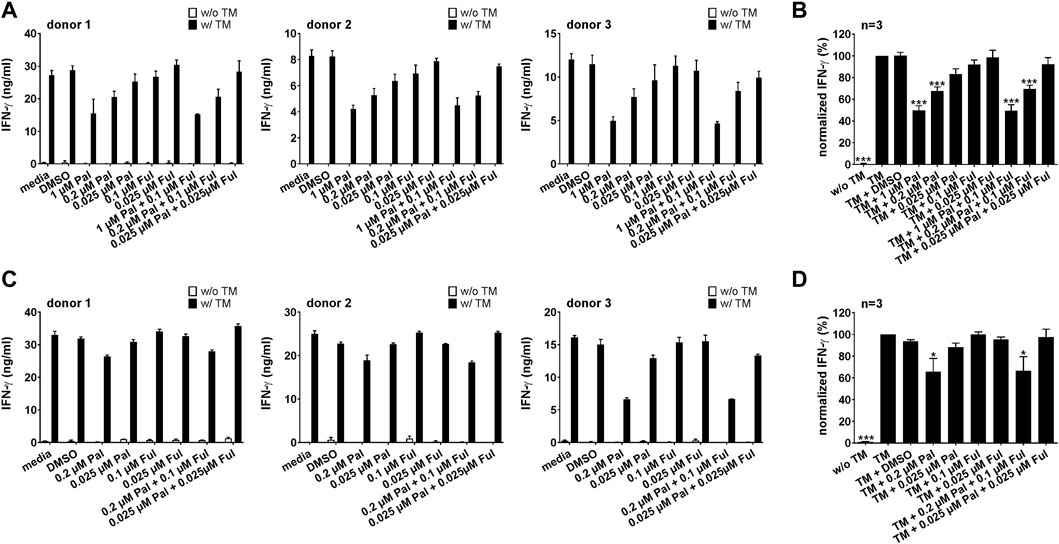
FIGURE 6. Effect of palbociclib and fulvestrant on IFN-γ release of TM-engaged UniCAR T cells. UniCAR T cells and (A,B) PC3-PSCA/PSMA Luc+ or (C,D) LNCaP-PSCA Luc+ cells were incubated with or without 30 nM of PSCA TM in the presence or absence of palbociclib and/or fulvestrant. After 96 h, IFN-γ concentrations in co-culture supernatants were analyzed by ELISA. (A,C) Each diagram shows average IFN-γ concentration ± SEM of triplicates for one T cell donor, respectively. (B,D) Graphs summarize relative IFN-γ release ± SEM of three different T cell donors. IFN-γ concentrations in the presence of UniCAR T cells, tumor cells and TM (“TM”) were equalized to 100%. (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 compared to control sample “TM”; One-way ANOVA with posthoc Dunnett multiple comparison test).
To explore whether the lower total concentrations of IFN-γ and TNF in the supernatants are associated with the palbociclib-mediated reduction of the T cell number, the amounts of IFN-γ and TNF detected in the supernatant after 96 h were calculated per T cell or UniCAR T cell. As summarized in Supplementary Figures S3, S4, in the presence of palbociclib average cytokine concentrations per cell did not significantly change or were even elevated compared to the settings without inhibitors, indicating that the lower concentrations of IFN-γ and TNF in the supernatants are associated with the palbociclib-induced decrease in T cell numbers.
For an effective combination therapy, it is important to ensure that palbociclib and fulvestrant do not impede antitumor cytotoxicity of T cell-based immunotherapies. Hence, the effect of palbociclib and fulvestrant on bsAb- or UniCAR T cell-mediated tumor cell killing was investigated by performing luminescence-based killing assays with LNCaP-PSCA Luc+ or PC3-PSCA/PSMA Luc+ cells. As shown in Figure 7 and Supplementary Figures S5A, 5B, the CD3-PSCA bsAb was able to specifically engage T cells for killing of PSCA+ tumor cells already after 8 h. Interestingly, palbociclib and/or fulvestrant did not modulate this functional property of T cells.
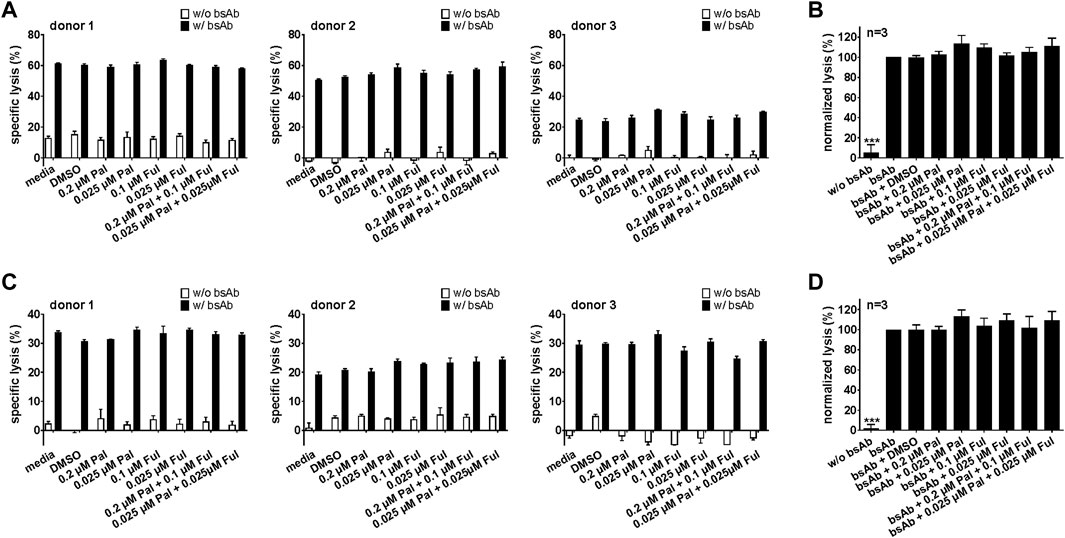
FIGURE 7. Effect of palbociclib and fulvestrant on bsAb-mediated tumor cell killing. T cells and (A,B) LNCaP-PSCA Luc+ or (C,D) PC3-PSCA/PSMA Luc+ cells were incubated with or without 30 nM of CD3-PSCA bsAb in the presence or absence of palbociclib and/or fulvestrant. After 8 h, tumor cell killing was calculated based on a luminescence-based killing assay. (A,C) Each diagram shows mean specific lysis ± SEM of triplicates for one T cell donor. (B,D) Graphs summarize relative tumor lysis ± SEM of three different T cell donors. Specific lysis in the presence of T cells and bsAb (“bsAb”) were equalized to 100%. (***p ≤ 0.001 compared to control sample “bsAb”; One-way ANOVA with posthoc Dunnett multiple comparison test).
Similar results were obtained for the PSCA-specific UniCAR system (Figure 8; Supplementary Figures S5C, D). Upon cross-linkage with prostate cancer cells via the PSCA TM, UniCAR T cells mediated efficient tumor cell lysis. Again, palbociclib and/or fulvestrant did not alter the cytotoxic activity of the UniCAR T cells.
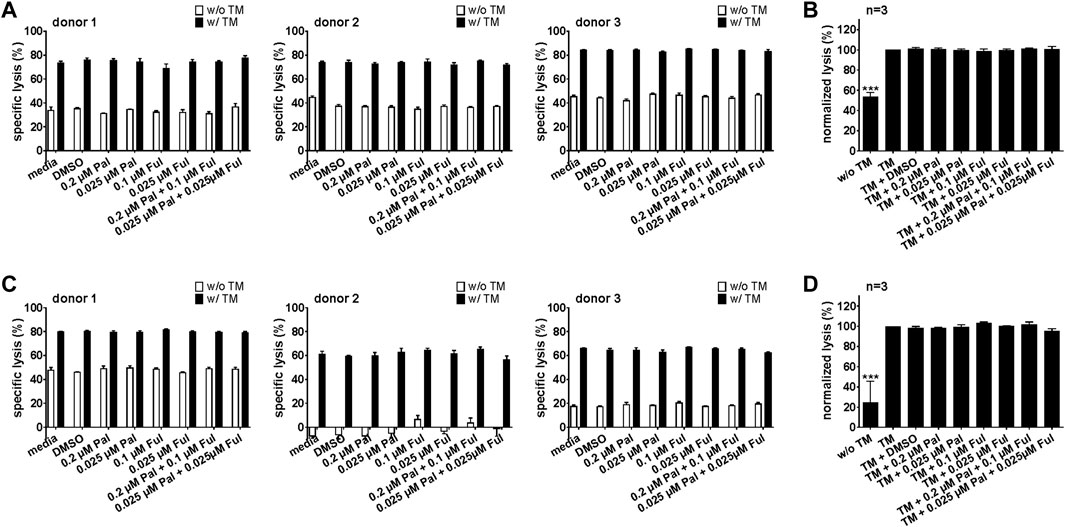
FIGURE 8. Effect of palbociclib and fulvestrant on UniCAR T cell-mediated tumor cell killing. UniCAR T cells and (A,B) LNCaP-PSCA Luc+ or (C,D) PC3-PSCA/PSMA Luc+ cells were incubated with or without 30 nM of PSCA TM in the presence or absence of palbociclib and/or fulvestrant. After 8 h, tumor cell killing was calculated based on a luminescence-based killing assay. (A,C) Each diagram shows mean specific lysis ± SEM of triplicates for one T cell donor. (B,D) Graphs summarize relative tumor lysis ± SEM of three different T cell donors. Specific lysis in the presence of UniCAR T cells and TM (“TM”) were equalized to 100%. (***p ≤ 0.001 compared to control sample “TM”; One-way ANOVA with posthoc Dunnett multiple comparison test).
In a next step, we also investigated whether pretreatment of T cells or UniCAR T cells with palbociclib and/or fulvestrant can influence their cytotoxic potential. For this purpose, T cells or UniCAR T cells were incubated with palbociclib and/or fulvestrant at 37°C. 24 h later, luminescence-based killing assays were performed in which drug-pretreated T cells or UniCAR T cells were co-cultured with PC3-PSCA/PSMA Luc+ cells and 30 nM CD3-PSCA bsAb or PSCA TM, respectively. Pre-incubation of T cells (Figures 9A,B) or UniCAR T cells (Figures 9C,D) with one or both drugs did neither influence tumor cell killing mediated by bsAb-redirected T cells nor by TM-engaged UniCAR T cells. These results provide evidence that palbociclib alone or in combination with fulvestrant retain the cytotoxic activity of bsAb-engaged T cells and UniCAR T cells.
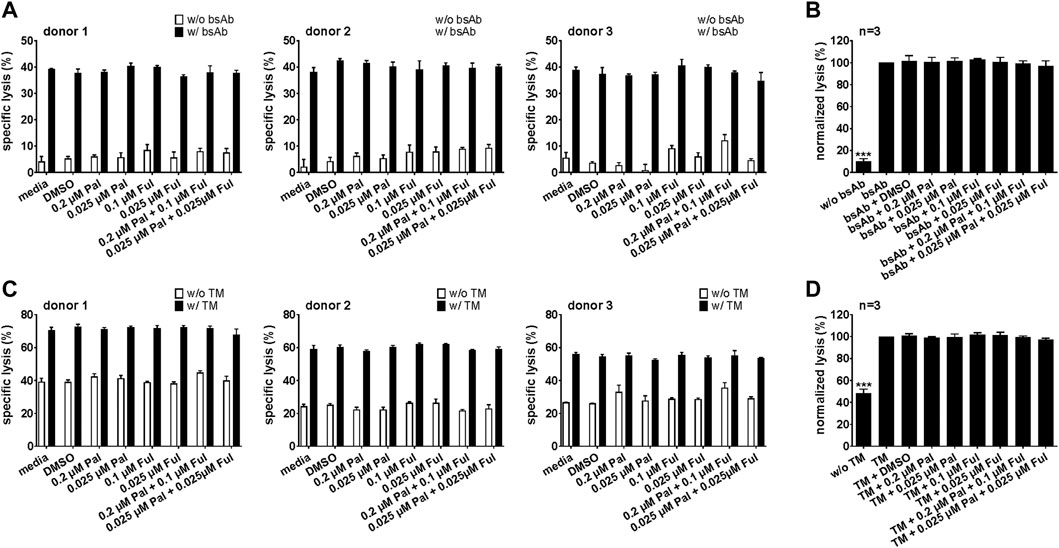
FIGURE 9. Cytotoxic capacity of palbociclib- and fulvestrant-pretreated bsAb-engaged T cells and UniCAR T cells. After pre-incubation of (A,B) T cells or (C,D) UniCAR T cells without or with palbociclib and/or fulvestrant for 24 h, luciferase-based killing assays were performed. Therefore, preincubated (A,B) T cells or (C,D) preincubated UniCAR T cells were cultured with PC3-PSCA/PSMA Luc+ cells in the presence or absence of 30 nM (A,B) CD3-PSCA bsAb or (C,D) PSCA TM. After 8 h, specific lysis was determined. (A,C) Each diagram shows mean specific lysis ± SEM of triplicates for one T cell donor. (B,D) Graphs summarize relative tumor lysis ± SEM of three different T cell donors. Specific lysis in the presence of (B) T cell and bsAb (“bsAb”) or (D) UniCAR T cells and TM (“TM”) were equalized to 100%. (***p ≤ 0.001 compared to control sample “bsAb” or “TM”; One-way ANOVA with posthoc Dunnett multiple comparison test).
CDK4/6 inhibitors in combination with endocrine therapy received FDA approval for treatment of patients with ER+, HER2- advanced or metastatic breast cancer (Finn et al., 2016a; Cristofanilli et al., 2016; Hortobagyi et al., 2016). This kind of therapy has shown clinical efficacy by prolonging PFS and OS (Finn et al., 2016a; Cristofanilli et al., 2016; Hortobagyi et al., 2016; Im et al., 2019; Johnston et al., 2020; Slamon et al., 2020; Sledge et al., 2020). However, a proportion of patients experienced disease progression (Finn et al., 2016a; Finn et al., 2016b; Cristofanilli et al., 2016; Hortobagyi et al., 2016). Potential explanations for therapy resistance to CDK4/6 inhibition in these patients are intrinsic or acquired resistance, including deregulations of the immune pathway, such as activation of inhibitory immune checkpoint pathways and suppression of immune stimulatory pathways in palbociclib-resistant cells (Pandey et al., 2019; Pandey et al., 2021; Pancholi et al., 2020). Therefore, identification of novel therapeutic strategies for treatment of patients resistant to CDK4/6 inhibition is urgently needed. In the last few decades, immunotherapy has become an important treatment modality. Since functional T cells are a crucial component of the tumor microenvironment for efficient tumor eradication, they emerged as key players of diverse immunotherapeutic strategies, including inhibition of immune checkpoint molecules and adoptive cellular therapy. With regard to therapeutic combination strategies, first success was achieved combining palbociclib with immune checkpoint inhibitors (CPI) in an ex vivo organotypic tumor spheroid culture system as well as in different mouse models (Deng et al., 2018; Zhang et al., 2018; Long et al., 2020). Furthermore, the comparison of patients with metastatic breast cancer treated with a combination of palbociclib, the CPI pembrolizumab and the aromatase inhibitor letrozole or with pembrolizumab and letrozole revealed that higher frequencies of blood-circulating effector memory T cells at baseline are potential predictive biomarkers of response to the combination of CDK4/6 inhibitors and CPI (Egelston et al., 2021). Additional attractive T cell-based strategies, such as bsAb (Wolf et al., 2005) and CAR T cell therapy (June et al., 2018; Arndt et al., 2020a), may also represent promising combinatorial partners for CDK4/6 inhibitors.
Emerging preclinical studies revealed diverse immunomodulatory effects of CDK4/6 inhibition, such as an enhanced immune infiltration into the tumor microenvironment, elevated antigen presentation and modulation of the cytokine milieu, supporting antitumor immune response (Ameratunga et al., 2019). Different murine models investigated the influence of CDK4/6 inhibition on tumor-infiltrating immune cells. In a breast cancer mouse model, a significant reduction of CD3+ tumor-infiltrating lymphocytes was observed under palbociclib treatment (Zhang et al., 2018). In contrast, Goel and colleagues reported significant increases of intratumoral CD3+ T cells in their transgenic mouse model of mammary carcinoma treated with abemaciclib or palbociclib (Goel et al., 2017). Another study also showed enhanced proportions of CD4+ and CD8+ T cells in lung tumors of genetically engineered mice after treatment with palbociclib or trilaciclib (Deng et al., 2018). However, this effect seems not to be based on increased proliferation, but on elevated homing of effector T cells to the tumor. These data are in line with observations by Schaer and colleagues, who demonstrated that abemaciclib increased the frequency of CD3+ T cell numbers within the tumor, but not the absolute numbers in a colon cancer mouse model (Schaer et al., 2018). By analyzing the functional status of the tumor-infiltrating T cells, Deng et al. (2018) observed an increased IFN-γ secretion by total splenocytes isolated from lung tumor-bearing mice, but not naïve mice, treated with trilaciclib in vivo. Moreover, Teo and colleagues have shown, that the CDK4/6 inhibitor ribociclib does not impair activation and cytotoxic potential of tumor-infiltrating CD8+ and CD4+ T cells in a triple-negative breast cancer mouse model (Teo et al., 2017).
Whereas all these findings rely on mouse models, little is known about the impact of palbociclib and fulvestrant on the functional properties of human immune cells. Heckler and colleagues investigated the impact of CDK4/6 inhibitors on activated CD8+ T cells of breast cancer patients and observed that CDK4/6 inhibition resulted in an increased frequency of memory CD8+ T cell precursors (Heckler et al., 2021). Based on the observation that higher frequencies of pre-existing effector memory T cells were detectable in the blood of responders to the combination of CDK4/6 inhibitors and CPI (Egelston et al., 2021), early treatment with CDK4/6 inhibitors to establish a memory CD8+ T cell pool followed by the CPI administration may represent a promising therapeutic strategy for cancer patients. Here, we examined the influence of palbociclib and fulvestrant on the proliferation and functional properties of bsAb-engaged T cells and UniCAR T cells in terms of a potential combinatorial approach of CDK4/6 inhibition and T cell-based immunotherapy. An important prerequisite for a durable and efficient antitumor T cell response is the proliferation and clonal expansion of the T cells. Therefore, we analyzed the impact of palbociclib and fulvestrant on the proliferative capacity of stimulated CD3+ T cells. We demonstrated that palbociclib clearly impairs the ability of T cells to proliferate upon anti-CD3/CD28 bead stimulation. This effect is based on a G0/G1 cell cycle arrest mediated by palbociclib. The palbociclib-mediated T cell inhibition was reversible after stopping the drug application. In contrast, fulvestrant did not influence this functional property. Furthermore, we analyzed the impact of palbociclib and fulvestrant on the proliferation of bsAb-engaged T cells and UniCAR T cells. Our studies were performed exemplarily with the CD3-PSCA bsAb (Feldmann et al., 2012) and the UniCAR technology using the PSCA TM (Arndt et al., 2014a; Arndt et al., 2014b; Bachmann, 2019). PSCA is a TAA upregulated in several major cancers, including prostate cancer, urinary bladder cancer, renal cell carcinoma, pancreatic cancer, ovarian mucinous tumor and NSCLC (Amara et al., 2001; Argani et al., 2001; Cao et al., 2005; Elsamman et al., 2006; Kawaguchi et al., 2010). Recently, PSCA expression in a subgroup of breast cancer patients was also reported (Link et al., 2017). Although T cell stimulation occurred via different methods, palbociclib but not fulvestrant prevented adequate proliferation and expansion of both bsAb-engaged T cells and redirected UniCAR T cells. Goel et al. also observed an inhibitory effect on the proliferation of CD4+ CD25- and CD8+ T cells derived from spleens and lymph nodes of wild-type mice after treatment with abemaciclib in vitro, concordant with our findings (Goel et al., 2017). Furthermore, ex vivo stimulation of splenocytes from lung tumor-bearing mice showed a reduced proliferation after anti-CD3/CD28 antibody stimulation and treatment with trilaciclib (Deng et al., 2018). In further experiments, we investigated the impact of palbociclib and/or fulvestrant on the cytokine production of T cells. The proinflammatory cytokine IFN-γ is an important player in the antitumoral immune response and may further enhance therapeutic effects of a T cell-based immunotherapy as it increases MHC class I expression and antigen presentation of tumor cells and further supports macrophages, cytotoxic T lymphocytes and natural killer cells in their antitumor response. TNF can also exhibit various antitumor effects, such as the induction of tumor cell apoptosis, and the recruitment and activation of tumor-reactive T cells. Palbociclib treatment of PSCA-specific UniCAR T cells and CD3-PSCA bsAb-engaged T cells co-cultured with prostate cancer cells resulted in lower total concentrations of IFN-γ and TNF in the supernatants. When investigating the underlying mechanism for this observation, we found that the lower concentrations of IFN-γ and TNF are linked to the palbociclib-mediated decrease in T cell numbers. For efficient tumor eradication, cytotoxic potential of T cells is a crucial parameter for immunotherapeutic strategies. For this reason, we further investigated the impact of palbociclib and fulvestrant on the UniCAR T cell- or bsAb-mediated cytotoxicity. The CD3-PSCA bsAb as well as the PSCA-specific UniCAR system have proven their capability to redirect T cells for efficient killing of PSCA+ tumor cells in vitro and in mouse models (Feldmann et al., 2012; Feldmann et al., 2017; Pishali Bejestani et al., 2017). We demonstrated under various conditions that neither palbociclib nor fulvestrant added either prior or during co-culture influence this functional property.
In summary, our data revealed that palbociclib reversibly impairs proliferation of activated CD3+ T cells and reduces expansion of UniCAR T cells and bsAb-engaged T cells. Furthermore, we observed reduced amounts of IFN-γ and TNF in the supernatants of palbociclib-treated PSCA-specific UniCAR T cells and CD3-PSCA bsAb-engaged T cells co-cultured with prostate cancer cells, which is caused by the palbociclib-mediated decrease in T cell numbers. The cytotoxic potential of PSCA-specific UniCAR T cells and CD3-PSCA bsAb-engaged T cells was not affected by palbociclib. Fulvestrant did not show impact on any of these functional properties of stimulated CD3+ T cells, UniCAR T cells and bsAb-engaged T cells. These results provide evidence that the CDK4/6 inhibitor palbociclib has not only an impact on the cell cycle of tumor cells but also on T cells, which are crucial players in an antitumor immune response. Hence, palbociclib-mediated alterations of T cell functionality should be taken into consideration for future therapeutic recommendations and potential combinatorial approaches with T cell-based immunotherapy. Thus, a palbociclib-free time period may be required for an efficient T cell-based immunotherapy.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The study was approved by the local institutional review board.
AT, CA, and MS contributed to conception and formal analysis. AT, CA, RR, EK, SL, KH, SK, and LL contributed to methodology and investigation. AT, CA, MB, and MS validated the study. AF, MB, and MS provided critical material and resources. AT, CA, and EK performed all experiments and curated data. AT and CA wrote the original draft of the manuscript. AF, RW, TT, TL, JK, PW, MB, and MS reviewed and edited the original draft of the manuscript. AT and CA visualized the data. MS supervised the project. AT, CA, and MS administered the project. All authors contributed to manuscript revision, read, and approved the submitted version.
This research was funded by the Federal Ministry of Education and Research (03ZU1111LA to AF and 03ZU1111LB to MB and MS).
CA is fellow of the Mildred Scheel Early Career Center Dresden P2 funded by the German Cancer Aid (Deutsche Krebshilfe). We thank Kim Weiße, Luisa Zimmermann, Christine Gräfe and Cathleen Lippitsch for excellent technical assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.970457/full#supplementary-material
Abe, O., Abe, R., Enomoto, K., Kikuchi, K., Koyama, H., Masuda, H., et al. (2005). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 365, 1687–1717. doi:10.1016/S0140-6736(05)66544-0
Adams, J. L., Smothers, J., Srinivasan, R., and Hoos, A. (2015). Big opportunities for small molecules in immuno-oncology. Nat. Rev. Drug Discov. 14, 603–622. doi:10.1038/nrd4596
Amara, N., Palapattu, G. S., Schrage, M., Gu, Z., Thomas, G. V., Dorey, F., et al. (2001). Prostate stem cell antigen is overexpressed in human transitional cell carcinoma. Cancer Res. 61, 4660–4665.
Ameratunga, M., Kipps, E., Okines, A. F. C., and Lopez, J. S. (2019). To cycle or fight—CDK4/6 inhibitors at the crossroads of anticancer immunity. Clin. Cancer Res. 25, 21–28. doi:10.1158/1078-0432.CCR-18-1999
Argani, P., Rosty, C., Reiter, R. E., Wilentz, R. E., Murugesan, S. R., Leach, S. D., et al. (2001). Discovery of new markers of cancer through serial analysis of gene expression: Prostate stem cell antigen is overexpressed in pancreatic adenocarcinoma. Cancer Res. 61, 4320–4324.
Arndt, C., Fasslrinner, F., Loureiro, L. R., Koristka, S., Feldmann, A., and Bachmann, M. (2020a). Adaptor car platforms—Next generation of T cell-based cancer immunotherapy. Cancers (Basel) 12, 1302. doi:10.3390/cancers12051302
Arndt, C., Feldmann, A., Koristka, S., Cartellieri, M., Dimmel, M., Ehninger, A., et al. (2014a). Simultaneous targeting of prostate stem cell antigen and prostate-specific membrane antigen improves the killing of prostate cancer cells using a novel modular T cell-retargeting system. Prostate 74, 1335–1346. doi:10.1002/pros.22850
Arndt, C., Feldmann, A., Töpfer, K., Koristka, S., Cartellieri, M., Temme, A., et al. (2014b). Redirection of CD4+ and CD8+ T lymphocytes via a novel antibody-based modular targeting system triggers efficient killing of PSCA+ prostate tumor cells. Prostate 74, 1347–1358. doi:10.1002/pros.22851
Arndt, C., Koristka, S., Feldmann, A., Bergmann, R., and Bachmann, M. (2018). Coomassie brilliant blue staining of polyacrylamide gels. Methods Mol. Biol. 1853, 27–30. doi:10.1007/978-1-4939-8745-0_4
Arndt, C., Loureiro, L. R., Feldmann, A., Jureczek, J., Bergmann, R., Máthé, D., et al. (2020b). UniCAR T cell immunotherapy enables efficient elimination of radioresistant cancer cells. Oncoimmunology 9, 1743036. doi:10.1080/2162402X.2020.1743036
Bachmann, M. (2019). The UniCAR system: A modular CAR T cell approach to improve the safety of CAR T cells. Immunol. Lett. 211, 13–22. doi:10.1016/j.imlet.2019.05.003
Baselga, J., Campone, M., Piccart, M., Burris, H. A., Rugo, H. S., Sahmoud, T., et al. (2012). Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N. Engl. J. Med. 366, 520–529. doi:10.1056/NEJMoa1109653
Cao, D., Ji, H., and Ronnett, B. M. (2005). Expression of mesothelin, fascin, and prostate stem cell antigen in primary ovarian mucinous tumors and their utility in differentiating primary ovarian mucinous tumors from metastatic pancreatic mucinous carcinomas in the ovary. Int. J. Gynecol. Pathol. 24, 67–72. doi:10.1097/01.pgp.0000139648.17750.35
Cartellieri, M., Koristka, S., Arndt, C., Feldmann, A., Stamova, S., Von Bonin, M., et al. (2014). A novel ex vivo isolation and expansion procedure for chimeric antigen receptor engrafted human T cells. PLoS One 9, e93745. doi:10.1371/journal.pone.0093745
Choi, Y. J., Li, X., Hydbring, P., Sanda, T., Stefano, J., Christie, A. L., et al. (2012). The requirement for cyclin D function in tumor maintenance. Cancer Cell 22, 438–451. doi:10.1016/j.ccr.2012.09.015
Cristofanilli, M., Turner, N. C., Bondarenko, I., Ro, J., Im, S.-A., Masuda, N., et al. (2016). Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet. Oncol. 17, 425–439. doi:10.1016/S1470-2045(15)00613-0
Deng, J., Wang, E. S., Jenkins, R. W., Li, S., Dries, R., Yates, K., et al. (2018). CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 8, 216–233. doi:10.1158/2159-8290.CD-17-0915
Dickson, M. A., Schwartz, G. K., Louise Keohan, M., D’Angelo, S. P., Gounder, M. M., Chi, P., et al. (2016). Progression-free survival among patients with well-differentiated or dedifferentiated liposarcoma treated with cdk4 inhibitor palbociclib a phase 2 clinical trial. JAMA Oncol. 2, 937–940. doi:10.1001/jamaoncol.2016.0264
Egelston, C., Guo, W., Yost, S., Lee, J. S., Rose, D., Avalos, C., et al. (2021). Pre-existing effector T-cell levels and augmented myeloid cell composition denote response to CDK4/6 inhibitor palbociclib and pembrolizumab in hormone receptor-positive metastatic breast cancer. J. Immunother. cancer 9, e002084. doi:10.1136/JITC-2020-002084
Elsamman, E. M., Fukumori, T., Tanimoto, S., Nakanishi, R., Takahashi, M., Toida, K., et al. (2006). The expression of prostate stem cell antigen in human clear cell renal cell carcinoma: A quantitative reverse transcriptase-polymerase chain reaction analysis. BJU Int. 98, 668–673. doi:10.1111/j.1464-410X.2006.06350.x
Feldmann, A., Arndt, C., Bergmann, R., Loff, S., Cartellieri, M., Bachmann, D., et al. (2017). Retargeting of T lymphocytes to PSCA- or PSMA positive prostate cancer cells using the novel modular chimeric antigen receptor platform technology “UniCAR. Oncotarget 8, 31368–31385. doi:10.18632/oncotarget.15572
Feldmann, A., Arndt, C., Töpfer, K., Stamova, S., Krone, F., Cartellieri, M., et al. (2012). Novel humanized and highly efficient bispecific antibodies mediate killing of prostate stem cell antigen-expressing tumor cells by CD8 + and CD4 + T cells. J. Immunol. 189, 3249–3259. doi:10.4049/jimmunol.1200341
Feldmann, A., Hoffmann, A., Bergmann, R., Koristka, S., Berndt, N., Arndt, C., et al. (2020). Versatile chimeric antigen receptor platform for controllable and combinatorial T cell therapy. Oncoimmunology 9, 1785608. doi:10.1080/2162402X.2020.1785608
Feldmann, A., Stamova, S., Bippes, C. C., Bartsch, H., Wehner, R., Schmitz, M., et al. (2011). Retargeting of T cells to prostate stem cell antigen expressing tumor cells: Comparison of different antibody formats. Prostate 71, 998–1011. doi:10.1002/pros.21315
Finn, R. S., Aleshin, A., and Slamon, D. J. (2016a). Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res. 18, 17. doi:10.1186/s13058-015-0661-5
Finn, R. S., Crown, J. P., Lang, I., Boer, K., Bondarenko, I. M., Kulyk, S. O., et al. (2015). The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet. Oncol. 16, 25–35. doi:10.1016/S1470-2045(14)71159-3
Finn, R. S., Dering, J., Conklin, D., Kalous, O., Cohen, D. J., Desai, A. J., et al. (2009). PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 11, R77. doi:10.1186/bcr2419
Finn, R. S., Martin, M., Rugo, H. S., Jones, S., Im, S.-A., Gelmon, K., et al. (2016b). PALMO-2: Palbociclib and Letrozole in Advanced Breast Cancer (first line hormone positive disease). N. Engl. J. Med. 375, 1925–1936. doi:10.1056/NEJMoa1607303
Fry, D. W., Harvey, P. J., Keller, P. R., Elliott, W. L., Meade, M. A., Trachet, E., et al. (2004). Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol. Cancer Ther. 3, 1427–1438. doi:10.1158/1535-7163.1427.3.11
Geoerger, B., Bourdeaut, F., DuBois, S. G., Fischer, M., Geller, J. I., Gottardo, N. G., et al. (2017). A phase I study of the CDK4/6 inhibitor ribociclib (LEE011) in pediatric patients with malignant rhabdoid tumors, neuroblastoma, and other solid tumors. Clin. Cancer Res. 23, 2433–2441. doi:10.1158/1078-0432.CCR-16-2898
Goel, S., Decristo, M. J., Watt, A. C., Brinjones, H., Sceneay, J., Li, B. B., et al. (2017). CDK4/6 inhibition triggers anti-tumour immunity. Nature 548, 471–475. doi:10.1038/nature23465
Goetz, M. P., Toi, M., Campone, M., Trédan, O., Bourayou, N., Sohn, J., et al. (2017). Monarch 3: Abemaciclib as initial therapy for advanced breast cancer. J. Clin. Oncol. 35, 3638–3646. doi:10.1200/JCO.2017.75.6155
Heckler, M., Ali, L. R., Clancy-Thompson, E., Qiang, L., Ventre, K. S., Lenehan, P., et al. (2021). Inhibition of CDK4/6 promotes CD8 T-cell memory formation. Cancer Discov. 11, 2564–2581. doi:10.1158/2159-8290.CD-20-1540
Hortobagyi, G. N., Stemmer, S. M., Burris, H. A., Yap, Y. S., Sonke, G. S., Paluch-Shimon, S., et al. (2016). Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N. Engl. J. Med. 375, 1738–1748. doi:10.1056/NEJMoa1609709
Im, S.-A., Lu, Y.-S., Bardia, A., Harbeck, N., Colleoni, M., Franke, F., et al. (2019). Overall survival with ribociclib plus endocrine therapy in breast cancer. N. Engl. J. Med. 381, 307–316. doi:10.1056/nejmoa1903765
Johnston, S. R. D., Harbeck, N., Hegg, R., Toi, M., Martin, M., Shao, Z. M., et al. (2020). Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE). J. Clin. Oncol. 38, 3987–3998. doi:10.1200/jco.20.02514
June, C. H., O’Connor, R. S., Kawalekar, O. U., Ghassemi, S., and Milone, M. C. (2018). CAR T cell immunotherapy for human cancer. Science 359, 1361–1365. doi:10.1126/science.aar6711
Kawaguchi, T., Sho, M., Tojo, T., Yamato, I., Nomi, T., Hotta, K., et al. (2010). Clinical significance of prostate stem cell antigen expression in non-small cell lung cancer. Jpn. J. Clin. Oncol. 40, 319–326. doi:10.1093/jjco/hyp181
Laphanuwat, P., and Jirawatnotai, S. (2019). Immunomodulatory roles of cell cycle regulators. Front. Cell Dev. Biol. 7, 23. doi:10.3389/fcell.2019.00023
Leonard, J. P., LaCasce, A. S., Smith, M. R., Noy, A., Chirieac, L. R., Rodig, S. J., et al. (2012). Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood 119, 4597–4607. doi:10.1182/blood-2011-10-388298
Link, T., Kuithan, F., Ehninger, A., Kuhlmann, J. D., Kramer, M., Werner, A., et al. (2017). Exploratory investigation of PSCA-protein expression in primary breast cancer patients reveals a link to HER2/neu overexpression. Oncotarget 8, 54592–54603. doi:10.18632/oncotarget.17523
Long, Q., Ma, A. H., Zhang, H., Cao, Z., Xia, R., Lin, T. Y., et al. (2020). Combination of cyclin-dependent kinase and immune checkpoint inhibitors for the treatment of bladder cancer. Cancer Immunol. Immunother. 69, 2305–2317. doi:10.1007/s00262-020-02609-5
Mitwasi, N., Feldmann, A., Bergmann, R., Berndt, N., Arndt, C., Koristka, S., et al. (2017). Development of novel target modules for retargeting of UniCAR T cells to GD2 positive tumor cells. Oncotarget 8, 108584–108603. doi:10.18632/oncotarget.21017
Offner, S., Hofmeister, R., Romaniuk, A., Kufer, P., and Baeuerle, P. A. (2006). Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol. Immunol. 43, 763–771. doi:10.1016/j.molimm.2005.03.007
O’Leary, B., Finn, R. S., and Turner, N. C. (2016). Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 13, 417–430. doi:10.1038/nrclinonc.2016.26
Pancholi, S., Ribas, R., Simigdala, N., Schuster, E., Nikitorowicz-Buniak, J., Ressa, A., et al. (2020). Tumour kinome re-wiring governs resistance to palbociclib in oestrogen receptor positive breast cancers, highlighting new therapeutic modalities. Oncogene 39, 4781–4797. doi:10.1038/s41388-020-1284-6
Pandey, K., An, H. J., Kim, S. K., Lee, S. A., Kim, S., Lim, S. M., et al. (2019). Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: A review. Int. J. Cancer 145, 1179–1188. doi:10.1002/ijc.32020
Pandey, K., Lee, E., Park, N., Hur, J., Cho, Y. B., Katuwal, N. B., et al. (2021). Deregulated immune pathway associated with palbociclib resistance in preclinical breast cancer models: Integrative genomics and transcriptomics. Genes (Basel). 12, 159. doi:10.3390/genes12020159
Patnaik, A., Rosen, L. S., Tolaney, S. M., Tolcher, A. W., Goldman, J. W., Gandhi, L., et al. (2016). Efficacy and safety of Abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non–small cell lung cancer, and other solid tumors. Cancer Discov. 6, 740–753. doi:10.1158/2159-8290.CD-16-0095
Pishali Bejestani, E., Cartellieri, M., Bergmann, R., Ehninger, A., Loff, S., Kramer, M., et al. (2017). Characterization of a switchable chimeric antigen receptor platform in a pre-clinical solid tumor model. Oncoimmunology 6, e1342909. doi:10.1080/2162402X.2017.1342909
Sadelain, M., Brentjens, R., and Rivière, I. (2013). The basic principles of chimeric antigen receptor design. Cancer Discov. 3, 388–398. doi:10.1158/2159-8290.CD-12-0548
Schaer, D. A., Beckmann, R. P., Dempsey, J. A., Huber, L., Forest, A., Amaladas, N., et al. (2018). The CDK4/6 inhibitor abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep. 22, 2978–2994. doi:10.1016/j.celrep.2018.02.053
Scheinberg, T., Kench, J., Stockler, M., Mahon, K. L., Sebastian, L., Stricker, P., et al. (2020). Pharmacodynamics effects of CDK4/6 inhibitor LEE011 (ribociclib) in high-risk, localised prostate cancer: A study protocol for a randomised controlled phase II trial (leep study: LEE011 in high-risk, localised prostate cancer). BMJ Open 10, e033667. doi:10.1136/bmjopen-2019-033667
Sherr, C. J., Beach, D., and Shapiro, G. I. (2016). Targeting CDK4 and CDK6: From discovery to therapy. Cancer Discov. 6, 353–367. doi:10.1158/2159-8290.CD-15-0894
Slamon, D. J., Neven, P., Chia, S., Fasching, P. A., De Laurentiis, M., Im, S.-A., et al. (2020). Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N. Engl. J. Med. 382, 514–524. doi:10.1056/nejmoa1911149
Sledge, G. W., Toi, M., Neven, P., Sohn, J., Inoue, K., Pivot, X., et al. (2017). Monarch 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2-advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 35, 2875–2884. doi:10.1200/JCO.2017.73.7585
Sledge, G. W., Toi, M., Neven, P., Sohn, J., Inoue, K., Pivot, X., et al. (2020). The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy - MONARCH 2: A randomized clinical trial. JAMA Oncol. 6, 116–124. doi:10.1001/jamaoncol.2019.4782
Teo, Z. L., Versaci, S., Dushyanthen, S., Caramia, F., Savas, P., Mintoff, C. P., et al. (2017). Combined CDK4/6 and PI3Kα inhibition is synergistic and immunogenic in triple-negative breast cancer. Cancer Res. 77, 6340–6352. doi:10.1158/0008-5472.CAN-17-2210
Tripathy, D., Im, S. A., Colleoni, M., Franke, F., Bardia, A., Harbeck, N., et al. (2018a). Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 19, 904–915. doi:10.1016/S1470-2045(18)30292-4
Tripathy, D., Sohn, J., Im, S.-A., Colleoni, M., Franke, F., Bardia, A., et al. (2018b). Abstract GS2-05: First-line ribociclib vs placebo with goserelin and tamoxifen or a non-steroidal aromatase inhibitor in premenopausal women with hormone receptor-positive, HER2-negative advanced breast cancer: Results from the randomized phase III MONALEESA-7 trial. Cancer Res. 78, GS2–05. GS2-05-GS2-05. doi:10.1158/1538-7445.sabcs17-gs2-05
Turner, N. C., Ro, J., André, F., Loi, S., Verma, S., Iwata, H., et al. (2015). Palbociclib in hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 373, 209–219. doi:10.1056/NEJMoa1505270
Turner, N. C., Slamon, D. J., Ro, J., Bondarenko, I., Im, S. A., Masuda, N., et al. (2018). Overall survival with palbociclib and fulvestrant in advanced breast cancer. N. Engl. J. Med. 379, 1926–1936. doi:10.1056/NEJMoa1810527
Wells, A. D., and Morawski, P. A. (2014). New roles for cyclin-dependent kinases in T cell biology: Linking cell division and differentiation. Nat. Rev. Immunol. 14, 261–270. doi:10.1038/nri3625
Wolf, E., Hofmeister, R., Kufer, P., Schlereth, B., and Baeuerle, P. A. (2005). BiTEs: Bispecific antibody constructs with unique anti-tumor activity. Drug Discov. Today 10, 1237–1244. doi:10.1016/S1359-6446(05)03554-3
Yu, Q., Sicinska, E., Geng, Y., Ahnström, M., Zagozdzon, A., Kong, Y., et al. (2006). Requirement for CDK4 kinase function in breast cancer. Cancer Cell 9, 23–32. doi:10.1016/j.ccr.2005.12.012
Keywords: cancer immunotherapy, CDK4/6, palbociclib, fulvestrant, bispecific antibody, CAR T cell, adoptive T cell therapy
Citation: Arndt C, Tunger A, Wehner R, Rothe R, Kourtellari E, Luttosch S, Hannemann K, Koristka S, Loureiro LR, Feldmann A, Tonn T, Link T, Kuhlmann JD, Wimberger P, Bachmann MP and Schmitz M (2023) Palbociclib impairs the proliferative capacity of activated T cells while retaining their cytotoxic efficacy. Front. Pharmacol. 14:970457. doi: 10.3389/fphar.2023.970457
Received: 16 June 2022; Accepted: 20 January 2023;
Published: 03 February 2023.
Edited by:
Andrea Cavazzoni, University of Parma, ItalyReviewed by:
Xavier Bisteau, Université libre de Bruxelles, BelgiumCopyright © 2023 Arndt, Tunger, Wehner, Rothe, Kourtellari, Luttosch, Hannemann, Koristka, Loureiro, Feldmann, Tonn, Link, Kuhlmann, Wimberger, Bachmann and Schmitz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudia Arndt, Yy5hcm5kQGh6ZHIuZGU=; Marc Schmitz, bWFyYy5zY2htaXR6QHR1LWRyZXNkZW4uZGU=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.