- 1Department of Clinical Pharmacy, College of Pharmacy, Umm AL-Qura University, Makkah, Saudi Arabia
- 2Department of Infection Prevention and Control Program, Alnoor Specialist Hospital Makkah, Makkah, Saudi Arabia
- 3Department of Pharmacy Practice, Faculty of Pharmacy, Bahauddin Zakariya University, Multan, Pakistan
- 4Department of Internal Medicine, Alnoor Specialist Hospital Makkah, Makkah, Saudi Arabia
- 5Department of Clinical Pharmacy, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Alkharj, Saudi Arabia
- 6Alnoor Specialist Hospital Makkah, Department of Infectious Diseases, Makkah, Saudi Arabia
- 7Pharmaceutical Care Department, Alnoor Specialist Hospital Makkah, Department of Infection Prevention and Control Program, Makkah, Saudi Arabia
- 8Department of Microbiology, Faculty of Medicine, Umm AL-Qura University, Makkah, Saudi Arabia
Background: Dose optimization of vancomycin plays a substantial role in drug pharmacokinetics because of the increased incidence of obesity worldwide. This systematic review was aimed to highlight the current dosing strategy of vancomycin among obese patients.
Methods: This systematic review was in concordance with Preferred Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. The literature search was carried out on various databases such as Scopus, PubMed/MEDLINE, ScienceDirect and EMBASE using Keywords and MeSH terms related to vancomycin dosing among obese patients. Google Scholar was also searched for additional articles. The English language articles published after January, 2000 were included in this study. The quality of the study was assessed using different assessment tools for cohort, and case reports.
Results: A total of 1,029 records were identified. After screening, 18 studies were included for the final review. Of total, twelve studies are retrospective and remaining six are case-control studies. A total of eight studies were conducted in pediatrics while remaining studies were conducted in adult population. Most of the studies reported the dosing interval every 6–8 h. Differences in target trough concentration exist with respect to target ranges. The administration of loading dose (20–25 mg/kg) followed by maintenance dose (15–25 mg/kg) of vancomycin is recommended in adult patients to target therapeutic outcomes. Moreover, a dose of 40–60 mg/kg/day appears appropriate for pediatric patients.
Conclusion: The initial dosing of vancomycin based on TBW could be better predictor of vancomycin trough concentration. However, the clinical significance is uncertain. Therefore, more studies are needed to evaluate the dosing strategy of vancomycin in overweight or obese patients.
Introduction
Obesity (defined as a body mass index [BMI] of 30 kg/m2 or higher) was once considered as a minor problem while adjusting doses of medication due to limited population of obese patients (Smit et al., 2020). But, now-a-days, obesity plays a substantial role in drug pharmacokinetics (PK) because of the increased prevalence of obesity around the globe. It has been well documented that due to pathophysiological changes related to obesity, such as impacted metabolic enzyme activity, increase in adipose tissues, renal dysfunction, increased cardiac output, and pharmacokinetic parameters of the drug, there is a significant impact on healthcare system, thus requiring a dosing optimization (Knibbe et al., 2015; Smit et al., 2018). In 2008, World Health Organization (WHO) reported that approximately 1.5 billion people aged above 20 years were obese of whom 200 million were males and about 300 million were females (Grace, 2012). According to WHO, approximately 60% of the global population i.e. 3.3 billion people will be classified as obese (1.1 billion people) or overweight (2.2 billion people) by the year of 2030 (Frühbeck et al., 2013).
When calculating medication dosages for obese patients, healthcare professionals need to take into account the patient’s body weight and body composition (Alessa et al., 2021; Haseeb et al., 2021; Haseeb et al., 2022a; Haseeb et al., 2022b). There are several types of weights that can be used in dose calculations for obese patients (Cies et al., 2012; Pai, 2021). Actual Body Weight (ABW) is the patient’s current weight in kilograms (kg). ABW is often used as the initial weight when calculating medication doses for obese patients. Ideal Body Weight is the weight that a person of the same height and gender would be if they had a “normal” body mass index (BMI) of 25 kg/m2. IBW is often used as a reference weight when calculating medication doses for obese patients. Adjusted Body Weight (AdjBW) is a weight that takes into account the patient’s actual weight and their ideal body weight. AdjBW is calculated using IBW and ABW. Total Body Weight (TBW) is a weight that takes into account the patient’s total body mass, including fat, muscle, and other tissues. TBW is often used when calculating medication doses for drugs that are distributed throughout the body. The choice of weight used in medication dose calculations for obese patients depends on the medication being administered, the patient’s overall health status, and other individual factors. It is important for healthcare professionals to use appropriate weights and dosages to ensure safe and effective medication therapy for obese patients (Bearden and Rodvold, 2000; Cies et al., 2012; Schwartz et al., 2016; Pai, 2021).
For decades, vancomycin, a first glycopeptide antibiotics was used to treat various infections caused by gram-positive bacteria, especially methicillin-resistant staphylococcus aureus (MRSA) (Mühlberg et al., 2020; Alghamdi et al., 2021). It is considered as a first-line therapy for the treatment of MRSA infections (Choo and Chambers, 2016). Despite of having numerous benefits of vancomycin, clinicians face different challenges regarding its dosing that achieves a maximum therapeutic concentration and area under curve (AUC) to minimum inhibitory concentration (MIC) ratio that is optimal for bactericidal activity, while reducing the side effects (Cusumano et al., 2020). The standard dose of vancomycin based on total body weight is 15–20 mg/kg that is to be administered every 8–12 h in a patient with normal renal function (Liu et al., 2011). Dosing and monitoring guidelines emphasize on achieving steady-state trough concentrations. Troughs should be at least 10 μg/mL, with targets of 15–20 μg/mL for microorganism with MIC ≥1 μg/mL and for patients with serious infectious diseases (Rybak et al., 2009). These guidelines recommend dosing strategy and monitoring of vancomycin treatment in obese patients in a manner similar to non-obese patients (Durand et al., 2018). Multisite studies have been documented the PK parameters of vancomycin in non-obese patients (Katip et al., 2016; Ma et al., 2020; Arjangpour et al., 2022). However, the data among obese patient is limited. Dose optimization of vancomycin in obese patients is becoming of greater importance in the light of public health data reporting increased prevalence of obesity around the globe. This systematic review highlights the current dosing strategy of vancomycin and its clinical outcomes in obese patients.
Materials and methods
Data sources and searches
A comprehensive systematic review was in concordance with Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines on December, 2021 to identify relevant articles (Moher, 2015). We performed an online search in databases such as Scopus, PubMed/MEDLINE, Science Direct, and EMBASE and the Cochrane Library Central Register of Controlled Trial databases with a time limit from January, 2000 to December, 2021. Two of the reviewers performed manual research in the reference lists of included studies. Moreover, the grey literature (e.g., Google Scholar) was also searched. The search terms included “pharmacokinetic parameters”, “dose optimization”, “vancomycin”, “dosing schedule”, “case-control”, “pediatrics”, “adults”, “overweight”, “body mass index” “obese patients”, “obesity”. Moreover, the corresponding Medical Subject Headings (MeSH) terms were also searched. The complete search strategy for grey literature research and databases is available in Supplementary Tables S1, S2.
Inclusion and exclusion criteria
The studies retrieved from grey literature and databases were merged and the duplicates were identified and removed using EndNote X9. The screening process was carried out in two steps. First, two of the reviewers independently screened the titles and abstracts and selected the relevant articles. Second, the selected titles and abstracts were then reviewed and validated by a third reviewer. The full-text articles were retrieved and screened by all authors for the final inclusion. The studies were included if they have met the inclusion and exclusion criteria. Studies were included if 1) they had reported about the dosing of vancomycin 2) assessed the therapeutic monitoring of vancomycin 3) published after January, 2000 4). The restriction of English language was imposed, while abstracts from conference proceedings were excluded from this study. The selected articles included was cohort studies, and case-control studies. This review also include studies in which normal-weight patients were compared with obese or overweight patients. Two reviewers (AS and ZS) independently screened the retrieved articles. In case of any disagreement, the final decision was made by third reviewer.
Data extraction
The standardized data collection form was utilized to extract data on the studies’ metadata (e.g., author names and year, design), patients’ characteristics (e.g., sample size, clinical condition of patient), drug administration (e.g., dosing schedule of drug), clinical outcomes and recommendation for future use. Three reviewers (SD, ZS and MG) retrieved aforementioned data from the articles. The disagreements were resolved by fourth reviewer.
Quality assessment
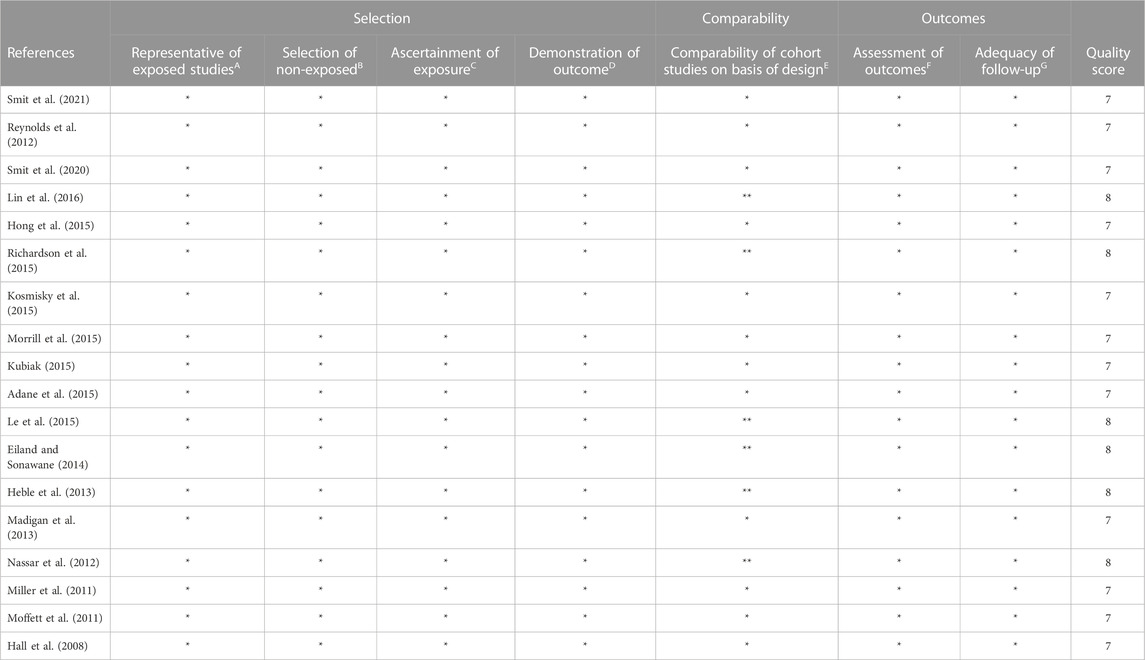
To critically appraise the selected articles, Newcastle-Ottawa scale (NOS) checklist were implemented for case-control and cohort studies (Wells, 2014). This scale categorizes the methodological quality of papers into three sub-scale i.e., selection, comparability and outcomes. The possible scores of NOS range from 0 to 8 for case-control and cohort studies. Any discrepancy while assessing the quality of studies was resolved by (ZS and WQ).
Results
Study characteristics
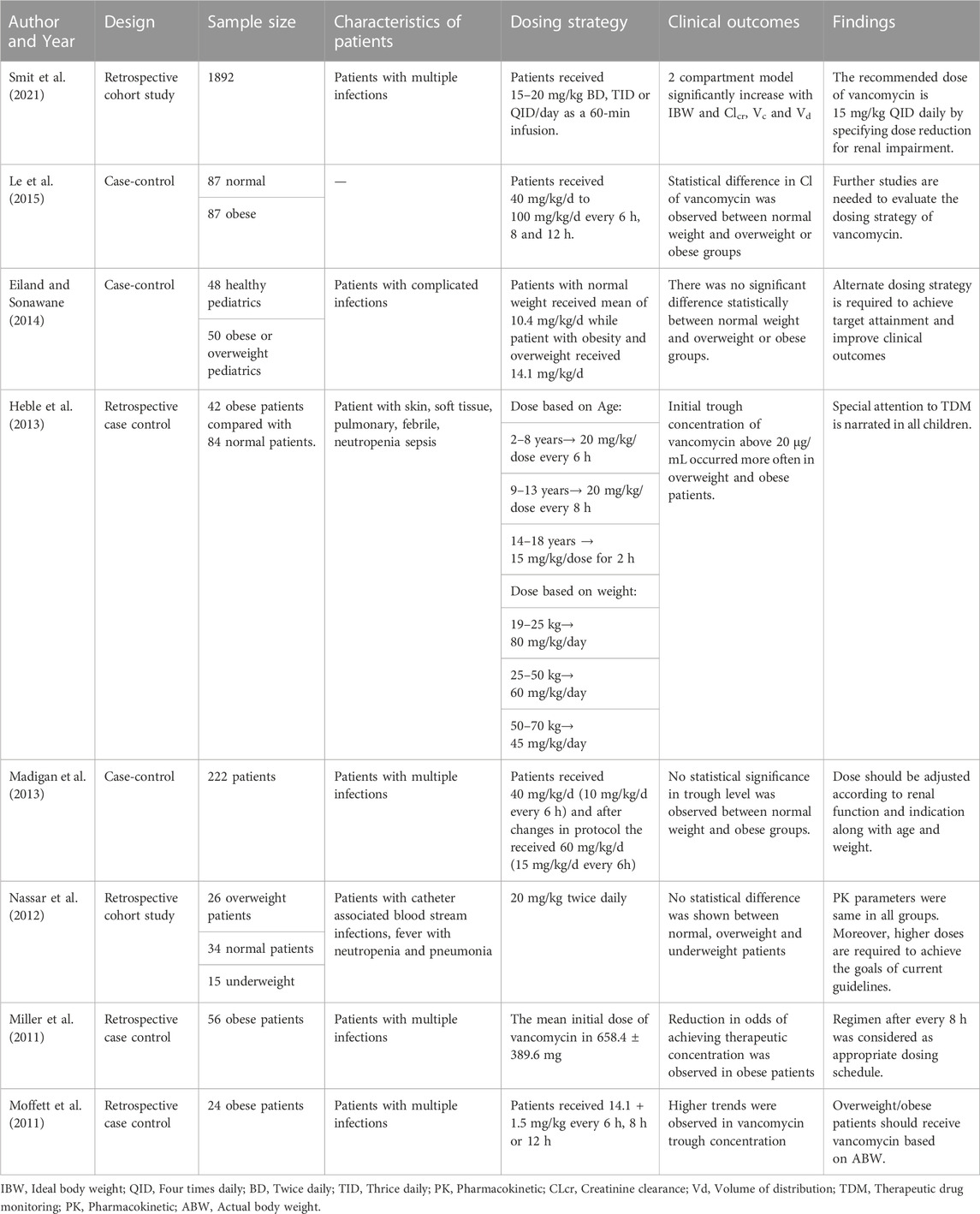
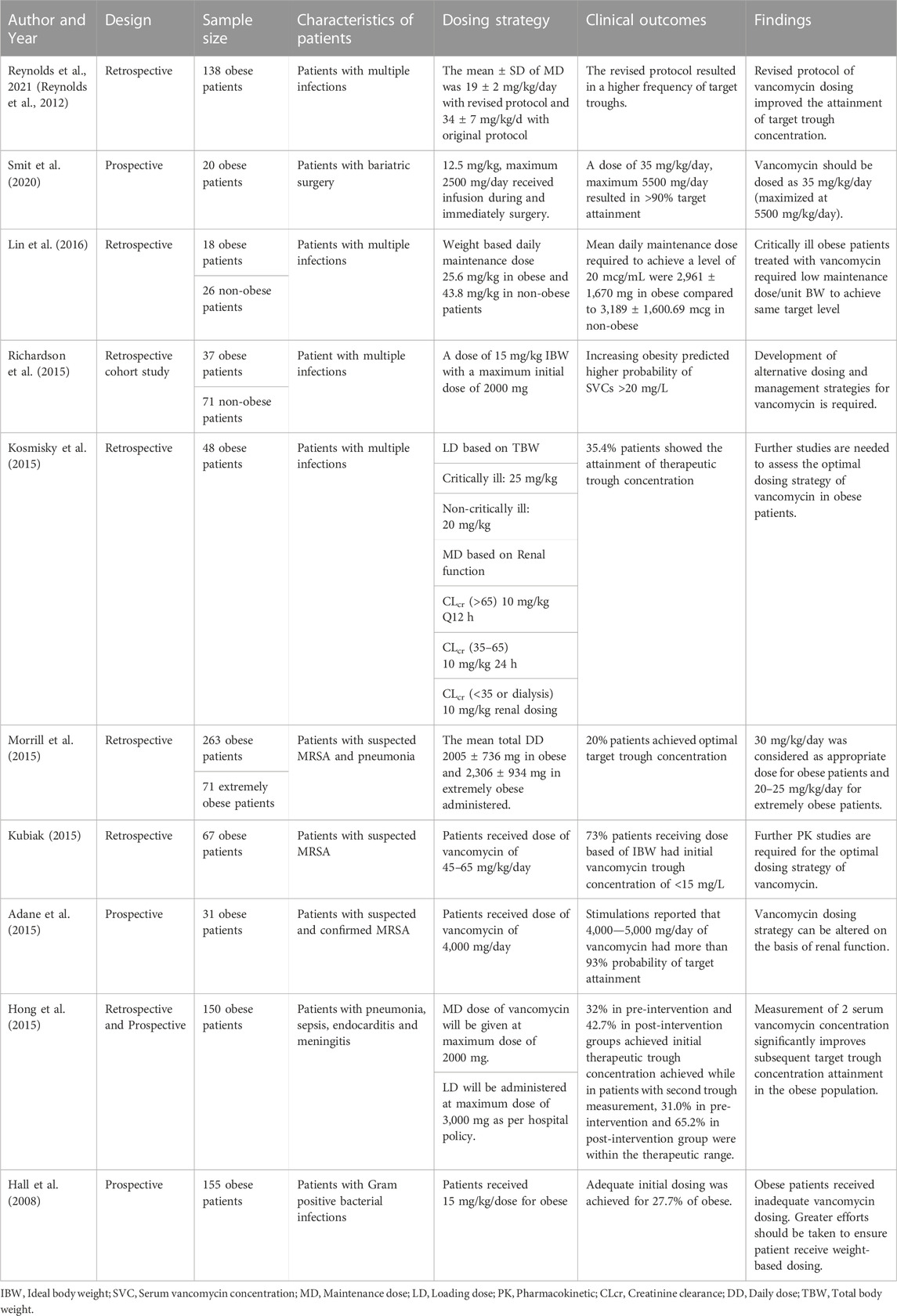
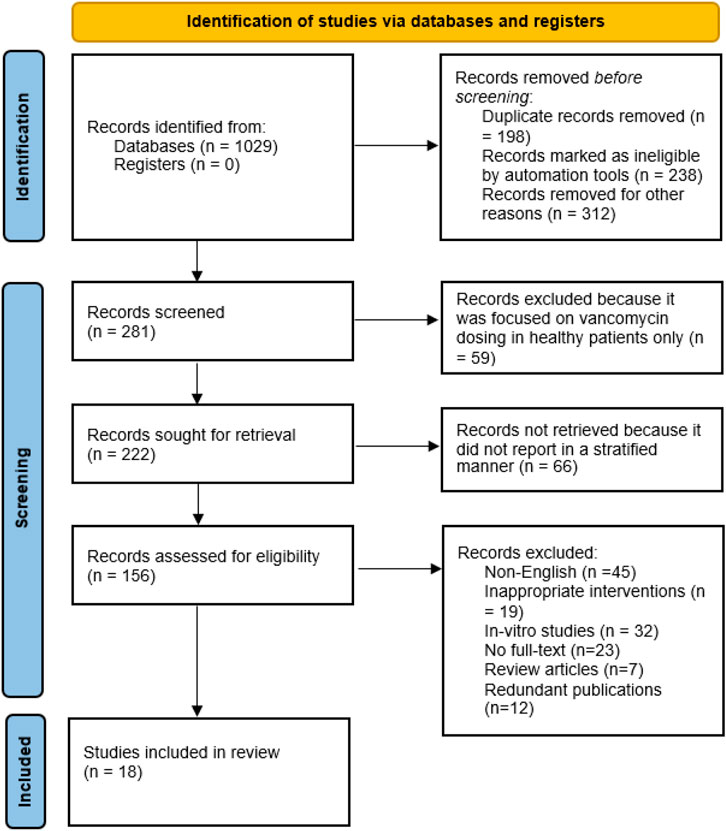
A total of 1,029 articles were searched using Scopus, PubMed/MEDLINE, Science Direct, and EMBASE and the Cochrane Library Central Register of Controlled Trial databases. Following the removal of duplicate studies, the abstracts of 281 studies were screened on the basis of exclusion and inclusion criteria. After the screening process, most of the articles were excluded due to following reasons: non-English (N = 45), in-vitro studies (N = 32), no full-text available (N = 23), inappropriate interventions (N = 19), literature reviews (N = 7) and redundant publications (N = 12). Based on inclusion criteria, eighteen studies published in English language were included in this systematic review (Hall et al., 2008; Miller et al., 2011; Moffett et al., 2011; Nassar et al., 2012; Reynolds et al., 2012; Heble et al., 2013; Madigan et al., 2013; Eiland and Sonawane, 2014; Adane et al., 2015; Hong et al., 2015; Kosmisky et al., 2015; Kubiak, 2015; Le et al., 2015; Morrill et al., 2015; Richardson et al., 2015; Lin et al., 2016; Smit et al., 2020; Smit et al., 2021). All required characteristics of studies were presented in Table 1 and Table 2. Of total, twelve studies are retrospective and remaining six are case-control studies. A total of eight studies were conducted in pediatrics while remaining studies were conducted in adult population. The PRISMA flow diagram reporting the procedure of selection of studies is shown in Figure 1.
Dosing strategy of vancomycin in pediatrics
In children, the recommended dose of vancomycin is 15–20 mg/kg/dose every 6 h. Majority of the studies reported the dosing interval every 6–8 h. However, in a Nassar et al., study, patients received dose of vancomycin twice daily (Nassar et al., 2012). A study reported that a dose of 20 mg/kg every 8 h was considered as an appropriate dosing schedule for obese pediatrics with multiple infections (Miller et al., 2011). However, another study suggested a dose of 15/mg/kg/dose twice daily by specifying dose reduction for renal dysfunction in patients with various multiple infections (Smit et al., 2021). Nassar and his colleagues reported that no statistical difference was shown among normal, overweight and obese patients with catheter-associated infections who received dose of 20 mg/kg/dose twice daily (Nassar et al., 2012). Moffett et al., reported that PK parameters were not altered in patients who received a dose of 14 mg/kg every 6 h or 8 h or 12 h (Moffett et al., 2011). However, in a pharmacokinetic model performed by Le and his colleagues documented lower clearance of vancomycin in overweight and obese patients using Bayesian estimation method (Le et al., 2015). Differences in target trough concentration exist with respect to target ranges 10–15 ug/mL, 10–20 ug/mL and 15–20 ug/mL in each included study.
Dosing strategy of vancomycin in adults
In adults, the recommended dose of vancomycin is 15–20 mg/kg/day every 8–12 h. Adane and his colleagues conducted a prospective study in which patients with suspected MRSA infections received dose of 4,000 mg/day and >90% probability target attainment was achieved using aforementioned dose (Adane et al., 2015). However, in retrospective study, authors recommended the dose of 45–65 mg/kg/dose on the basis of ideal body weight (IBW) in the patients with suspected MRSA infections (Kubiak, 2015). About 73% of patients had an initial trough concentration less than 15 mg/L (Kubiak, 2015). Similarly, another study reported that the dose of 15 mg/kg/dose given on the basis of IBW resulted in higher probability of serum vancomycin concentration (SVC) greater than 20 mg/L (Richardson et al., 2015). Another prospective study reported that the appropriate dose of vancomycin is 35 mg/kg/day in patients with bariatric surgery (Smit et al., 2020). Reynolds and his colleagues retrospectively compared the original protocol (a loading dose of 20–25 mg/kg, followed by the maintenance dose of 15 mg/kg every 8–12 h) with revised protocol (a loading dose of 20–25 mg/kg, followed by maintenance dose of 10 mg/kg/day (Reynolds et al., 2012). He concluded that the revised protocol improved the attainment of target trough concentration.
Quality assessment
The quality of the study was considered higher when scored ≥7, moderate when scored 5-6, and low when scored <5. A total of 6 studies were awarded 8 stars while the remaining studies were awarded 7 stars. The stars awarded for studies ranged from 7-8 and the average value was 7.7 (Table 3).
Discussion
However, literature exists for altered PK parameters of vancomycin in obese patients, the clinical practice with vancomycin dosing gives challenges to healthcare professionals. In this systematic review, we found that differences exist in the dosing strategy of vancomycin in pediatrics as well adult population with overweight and obesity. The evidence on PK studies in obese adults describes the correlation between the volume of distribution and total body clearance with total body weight (TBW) (Bearden and Rodvold, 2000; Leong et al., 2011; Alobaid et al., 2016). Instead of using adjusted or ideal body weight, the recommended dosing strategy for obese adult population is to utilize TBW to achieve therapeutic outcomes (Khare et al., 2020). The administration of loading dose (20–25 mg/kg) followed by maintenance dose (15–25 mg/kg) of vancomycin is recommended in adult patients to target therapeutic outcomes (Al-Kathiri et al., 2021). However, it remains unclear if changes in metabolism such as absorption, volume of distribution and clearance are different in pediatrics with overweight compared with pediatric with obesity because the majority of the articles studied these patients in one group.
According to IDSA guidelines, the recommended dose of vancomycin is 15 mg/kg/dose every 6 h in pediatrics and 15–20 mg/kg/dose every 8–12 h for the treatment of MRSA infections (Liu et al., 2011). The IDSA also suggested the AUC/MIC ratio of vancomycin should be ≥400, which corresponds to the target trough serum concentration of 15–20 μg/mL in adult population (Giuliano et al., 2010). A target trough concentration of 15–20 μg/mL was also recommended for pediatrics with serious infections such as endocarditis, bacteremia, pneumonia, meningitis, osteomyelitis and skin infections (Liu et al., 2011). These suggestions are based on limited data obtained from case-control studies, reports of expert committees and clinicians’ experience (Durand et al., 2018). We found that most of studies conducted in pediatrics did not follow the IDSA-recommended dosing strategy of 15 mg/kg/day every 6 h (Nassar et al., 2012; Madigan et al., 2013; Le et al., 2015). Therefore, further studies are required to examine the pediatric population with obesity and overweight taking higher doses of vancomycin. Several studies have been previously reported that the differences exist in trough concentration associated with target exposure due to age, weight or dosing frequency in obese as well as normal-weight patients. Therefore, healthcare practitioners should not adjust the dose based on trough concentration alone but preferably utilize the Bayesian estimation method to correlate the therapeutic drug monitoring (TDM) samples to identify exposure, as is also suggested in the modified TDM guidelines of vancomycin (Frymoyer et al., 2014; Janssen et al., 2016; Rybak et al., 2020).
Alternative dosing strategies of vancomycin have been introduced recently. Some studies suggested that a maintenance dose of 60 mg/kg/day according to TBW and the utilization of Bayesian estimation method of dosing showed the highest achievement of an AUC/MIC ratio of ≥400 in obese pediatrics with suspected or confirmed MRSA infections (Le et al., 2013; Le et al., 2014). A continuous dosing regimen of vancomycin resulted in an improved target attainment concentration when compared with the intermittent infusion of vancomycin in infants with sepsis (Gwee et al., 2019). Similarly, the adult population receiving the continuous infusion of vancomycin showed significantly lower incidence of nephrotoxicity than patient treated with intermittent infusion (Hao et al., 2016). Wesner and his colleagues introduced a nomogram on the basis of weight and creatinine clearance to calculate the dose of vancomycin for achieving serum vancomycin concentration (SVCs) level in obese population (Wesner, 2013). However, limited data were found on nephrotoxicity associated with higher vancomycin dosing. Higher tough serum concentration results in an increase in the risk of nephrotoxicity in overweight and obese children, although this concern needs further evaluation (Choi et al., 2017; Fiorito et al., 2018).
Further PK studies are required to understand the differences in current dosing protocols and to find out the clinical outcomes to evaluate the correlation between PK parameters and obesity in the pediatric and adult populations (Kyler et al., 2019). Additional and more extensive PK studies in which researchers examine AUC, Vd, and clearance of vancomycin that should be conducted in obese patients to optimize therapeutic dosing and minimize side effects. Following the different studies regarding an increase in MRSA resistance to vancomycin, increased serum trough levels are suggested for the treatment of various severe or invasive infections (Kullar et al., 2011; Leu et al., 2012; Elyasi et al., 2014; Almangour et al., 2017; Almangour et al., 2019). Therefore, future vancomycin PK studies should also be focused strictly on the obese population with severe infections such as endocarditis, bacteremia, MRSA infection, pneumonia, meningitis, osteomyelitis and skin infections. This would help clinicians in assessing the appropriate dosing strategy to achieve therapeutic outcomes.
This systematic review has set some limitations. Firstly, the data was extracted from small retrospective and prospective observational studies. Secondly, the sample size was generally small that may affect the outcomes. Thirdly, the majority of the studies reported dosing strategy from single-center that may reflect variability in vancomycin dosing protocol. Moreover, the clinical outcomes were not assessed as the main focus was on target trough concentration in majority of the studies, which lack prospective evidence to use this dosing protocols as a surrogate marker for clinical outcomes. The included studies did not assess the nephrotoxicity and other adverse events associated with higher doses of vancomycin in obese population.
Conclusion
The physiological changes in patients with obesity and overweight resulted in alteration of dosing protocol of vancomycin. Our study supports the practice of administering loading dose (20–25 mg/kg) followed by maintenance dose (15–25 mg/kg) of vancomycin in obese adults while in pediatrics, a dose of 40–60 mg/kg/day appears appropriate to achieve therapeutic outcomes. The initial dosing of vancomycin based on TBW could be better predictor of vancomycin trough concentration rather than adjusted or ABW. However, the clinical significance is uncertain. Therefore, there is an urgent need for better-defined dosing strategy in obese and overweight patients. Moreover, additional studies are needed to evaluate the alternate dosing strategy in overweight or obese patients and assess how obesity and overweight affects the PK parameter to increase the efficacy, reduced side effects and promote antibiotic cost savings.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The authors extend their appreciation to the Deanship for Research and Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number: IFP22UQU4290073DSR022.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.965284/full#supplementary-material
References
Adane, E. D., Herald, M., and Koura, F. (2015). Pharmacokinetics of vancomycin in extremely obese patients with suspected or confirmed S taphylococcus aureus infections. Pharmacotherapy 35 (2), 127–139. doi:10.1002/phar.1531
Alessa, M., Gramish, J., Almodaimegh, H., Khobrani, M. A., Hornsby, L., and Alhifany, A. A. (2021). Utilization of adjusted body weight for dosing unfractionated heparin in obese patients with venous thromboembolism: A retrospective matched cohort study. Trop. J. Pharm. Res. 20 (1), 191–195.
Alghamdi, M., Alotaibi, F., Ahmed, H., Alharbi, F., Bukhari, O., and Youssef, A.-R. (2021). Effect of medical education on the knowledge, attitude and compliance regarding infection control measures among dental students in Makkah. JUMS 7 (1), 14–17.
Almangour, T. A., Fletcher, V., Alessa, M., Alhifany, A. A., and Tabb, D. (2017). Multiple weekly dalbavancin dosing for the treatment of native vertebral osteomyelitis caused by methicillin-resistant Staphylococcus aureus: A case report. Am. J. Med. Case Rep. 18, 1315.
Almangour, T. A., Perry, G. K., Terriff, C. M., Alhifany, A. A., and Kaye, K. S. (2019). Dalbavancin for the management of gram-positive osteomyelitis: Effectiveness and potential utility. Diagn. Microbiol. Infect. Dis. 93 (3), 213–218.
Alobaid, A. S., Hites, M., Lipman, J., Taccone, F. S., and Roberts, J. A. (2016). Effect of obesity on the pharmacokinetics of antimicrobials in critically ill patients: A structured review. Int. J. Antimicrob. Agents 47 (4), 259–268. doi:10.1016/j.ijantimicag.2016.01.009
Al-Kathiri, W. H., Alharthi, A. S., Albeladi, F. H., AlDawsari, H. A., Alweqayyan, A. A., Alshahrani, M. S., et al. (2021). The impact of Vancomycin loading dose on the emergency department in Saudi Arabia: A multicenter cohort study. SJEMed 2 (2), 180.
Arjangpour, S., Sadeghi, K., Solduzian, M., and Mousavi, S. A. (2022). Vancomycin pharmacokinetic parameters in patients undergoing hematopoietic stem cell transplantation. J. Oncol. Pharm. Pract. 28 (1), 101–108. doi:10.1177/1078155220985317
Bearden, D. T., and Rodvold, K. A. (2000). Dosage adjustments for antibacterials in obese patients: Applying clinical pharmacokinetics. Clin. Pharmacokinet. 38, 415–426. doi:10.2165/00003088-200038050-00003
Choi, Y. C., Saw, S., Soliman, D., Bingham, A. L., Pontiggia, L., Hunter, K., et al. (2017). Intravenous vancomycin associated with the development of nephrotoxicity in patients with class III obesity. Ann. Pharmacother. 51 (11), 937–944. doi:10.1177/1060028017720946
Choo, E. J., and Chambers, H. F. (2016). Treatment of methicillin-resistant Staphylococcus aureus bacteremia. Infect. Chemother. 48 (4), 267–273. doi:10.3947/ic.2016.48.4.267
Cies, J. J., Chan, S., Hossain, J., Brenn, B. R., and Di Pentima, M. C. (2012). Influence of body mass index and antibiotic dose on the risk of surgical site infections in pediatric clean orthopedic surgery. Surg. Infect. 13 (6), 371–376. doi:10.1089/sur.2011.096
Cusumano, J. A., Klinker, K. P., Huttner, A., Luther, M. K., Roberts, J. A., and LaPlante, K. L. (2020). Towards precision medicine: Therapeutic drug monitoring–guided dosing of vancomycin and β-lactam antibiotics to maximize effectiveness and minimize toxicity. Am. J. Health. Syst. Pharm. 77 (14), 1104–1112. doi:10.1093/ajhp/zxaa128
Durand, C., Bylo, M., Howard, B., and Belliveau, P. (2018). Vancomycin dosing in obese patients: Special considerations and novel dosing strategies. Ann. Pharmacother. 52 (6), 580–590. doi:10.1177/1060028017750084
Eiland, L. S., and Sonawane, K. B. (2014). Vancomycin dosing in healthy-weight, overweight, and obese pediatric patients. J. Pediatr. Pharmacol. Ther. 19 (3), 182–188. doi:10.5863/1551-6776-19.3.182
Elyasi, S., Khalili, H., Dashti-Khavidaki, S., Emadi-Koochak, H., Mohammadpour, A., and Abdollahi, A. (2014). Elevated vancomycin trough concentration: Increased efficacy and/or toxicity? Iran. J. Pharm. Res IJPR 13 (4), 1241–1247.
Fiorito, T. M., Luther, M. K., Dennehy, P. H., LaPlante, K. L., and Matson, K. L. (2018). Nephrotoxicity with vancomycin in the pediatric population: A systematic review and meta-analysis. Pediatr. Infect. Dis. J. 37 (7), 654–661. doi:10.1097/INF.0000000000001882
Frühbeck, G., Toplak, H., Woodward, E., Yumuk, V., Maislos, M., Oppert, J. M., et al. (2013). Obesity: The gateway to ill health - an EASO position statement on a rising public health, clinical and scientific challenge in europe. Obes. Facts 6 (2), 117–120. doi:10.1159/000350627
Frymoyer, A., Hersh, A. L., El-Komy, M. H., Gaskari, S., Su, F., Drover, D. R., et al. (2014). Association between vancomycin trough concentration and area under the concentration-time curve in neonates. Antimicrob. Agents Chemother. 58 (11), 6454–6461. doi:10.1128/AAC.03620-14
Giuliano, C., Haase, K. K., and Hall, R. (2010). Use of vancomycin pharmacokinetic-pharmacodynamic properties in the treatment of MRSA infections. Expert Rev. anti-infective Ther. 8 (1), 95–106. doi:10.1586/eri.09.123
Grace, E. (2012). Altered vancomycin pharmacokinetics in obese and morbidly obese patients: What we have learned over the past 30 years. J. Antimicrob. Chemother. 67 (6), 1305–1310. doi:10.1093/jac/dks066
Gwee, A., Cranswick, N., McMullan, B., Perkins, E., Bolisetty, S., Gardiner, K., et al. (2019). Continuous versus intermittent vancomycin infusions in infants: A randomized controlled trial. Pediatrics 143 (2), e20182179. doi:10.1542/peds.2018-2179
Hall, R. G., Payne, K. D., Bain, A. M., Rahman, A. P., Nguyen, S. T., Eaton, S. A., et al. (2008). Multicenter evaluation of vancomycin dosing: Emphasis on obesity. Am. J. Med. 121 (6), 515–518. doi:10.1016/j.amjmed.2008.01.046
Hao, J.-J., Chen, H., and Zhou, J.-X. (2016). Continuous versus intermittent infusion of vancomycin in adult patients: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 47 (1), 28–35. doi:10.1016/j.ijantimicag.2015.10.019
Haseeb, A., Abourehab, M. A. S., Almalki, W. A., Almontashri, A. M., Bajawi, S. A., Aljoaid, A. M., et al. (2022). Trimethoprim-sulfamethoxazole (bactrim) dose optimization in pneumocystis jirovecii pneumonia (PCP) management: A systematic review. Int. J. Environ. Res. Public Health 19 (5), 2833. doi:10.3390/ijerph19052833
Haseeb, A., Alqurashi, M. K., Althaqafi, A. S., Alsharif, J. M., Faidah, H. S., Bushyah, M., et al. (2022). A systematic review on clinical safety and efficacy of vancomycin loading dose in critically ill patients. Antibiotics 11 (3), 409. doi:10.3390/antibiotics11030409
Haseeb, A., Faidah, H. S., Alghamdi, S., Alotaibi, A. F., Elrggal, M. E., Mahrous, A. J., et al. (2021). Dose optimization of colistin: A systematic review. Antibiotics 10 (12), 1454. doi:10.3390/antibiotics10121454
Heble, D. E., McPherson, C., Nelson, M. P., and Hunstad, D. A. (2013). Vancomycin trough concentrations in overweight or obese pediatric patients. Pharmacotherapy 33 (12), 1273–1277. doi:10.1002/phar.1321
Hong, J., Krop, L. C., Johns, T., and Pai, M. P. (2015). Individualized vancomycin dosing in obese patients: A two-sample measurement approach improves target attainment. Pharmacotherapy 35 (5), 455–463. doi:10.1002/phar.1588
Janssen, E. J., Valitalo, P. A. J., Allegaert, K., de Cock, R. F. W., Simons, S. H. P., Sherwin, C. M. T., et al. (2016). Towards rational dosing algorithms for vancomycin in neonates and infants based on population pharmacokinetic modeling. Antimicrob. Agents Chemother. 60 (2), 1013–1021. doi:10.1128/AAC.01968-15
Katip, W., Jaruratanasirikul, S., Pattharachayakul, S., Wongpoowarak, W., Jitsurong, A., and Lucksiri, A. (2016). The pharmacokinetics of vancomycin during the initial loading dose in patients with septic shock. Infect. Drug Resist. 9, 253–260. doi:10.2147/IDR.S121513
Khare, M., Azim, A., Kneese, G., Haag, M., Weinstein, K., Rhee, K. E., et al. (2020). Vancomycin dosing in children with overweight or obesity: A systematic review and meta-analysis. Hosp. Pediatr. 10 (4), 359–368. doi:10.1542/hpeds.2019-0287
Knibbe, C. A., Brill, M. J. E., van Rongen, A., Diepstraten, J., van der Graaf, P. H., and Danhof, M. (2015). Drug disposition in obesity: Toward evidence-based dosing. Annu. Rev. Pharmacol. Toxicol. 55, 149–167. doi:10.1146/annurev-pharmtox-010814-124354
Kosmisky, D. E., Griffiths, C. L., Templin, M. A., Norton, J., and Martin, K. E. (2015). Evaluation of a new vancomycin dosing protocol in morbidly obese patients. Hosp. Pharm. 50 (9), 789–797. doi:10.1310/hpj5009-789
Kubiak, D. W. (2015). “An evaluation of systemic vancomycin dosing in obese patients,” in Open forum infectious diseases (Oxford University Press).
Kullar, R., Leonard, S. N., Davis, S. L., Delgado, G., Pogue, J. M., Wahby, K. A., et al. (2011). Validation of the effectiveness of a vancomycin nomogram in achieving target trough concentrations of 15–20 mg/L suggested by the vancomycin consensus guidelines. Pharmacotherapy 31 (5), 441–448. doi:10.1592/phco.31.5.441
Kyler, K. E., Wagner, J., Hosey-Cojocari, C., Watt, K., and Shakhnovich, V. (2019). Drug dose selection in pediatric obesity: Available information for the most commonly prescribed drugs to children. Paediatr. drugs 21 (5), 357–369. doi:10.1007/s40272-019-00352-8
Le, J., Bradley, J. S., Murray, W., Romanowski, G. L., Tran, T. T., Nguyen, N., et al. (2013). Improved vancomycin dosing in children using area-under-the-curve exposure. Pediatr. Infect. Dis. J. 32 (4), e155–e163. doi:10.1097/INF.0b013e318286378e
Le, J., Capparelli, E. V., Wahid, U., Wu, Y. S. S., Romanowski, G. L., Tran, T. M., et al. (2015). Bayesian estimation of vancomycin pharmacokinetics in obese children: Matched case-control study. Clin. Ther. 37 (6), 1340–1351. doi:10.1016/j.clinthera.2015.05.006
Le, J., Ngu, B., Bradley, J. S., Murray, W., Nguyen, A., Nguyen, L., et al. (2014). Vancomycin monitoring in children using bayesian estimation. Ther. Drug Monit. 36 (4), 510–518. doi:10.1097/FTD.0000000000000039
Leong, J. V., Boro, M. S., and Winter, M. E. J. A. J. o. H.-S. P. (2011). Determining vancomycin clearance in an overweight and obese population. Am. J. Health. Syst. Pharm. 68 (7), 599–603. doi:10.2146/ajhp100410
Leu, W. J., Liu, Y. C., Wang, H. W., Chien, H. Y., Liu, H. P., and Lin, Y. M. (2012). Evaluation of a vancomycin dosing nomogram in achieving high target trough concentrations in Taiwanese patients. Int. J. Infect. Dis. 16 (11), e804–e810. doi:10.1016/j.ijid.2012.07.005
Lin, H., Yeh, D. D., and Levine, A. R. (2016). Daily vancomycin dose requirements as a continuous infusion in obese versus non-obese SICU patients. Crit. Care 20 (1), 205–207. doi:10.1186/s13054-016-1363-9
Liu, C., Bayer, A., Cosgrove, S. E., Daum, R. S., Fridkin, S. K., Gorwitz, R. J., et al. (2011). Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52 (3), e18–e55. doi:10.1093/cid/ciq146
Ma, K.-f., Liu, Y. X., Jiao, Z., Lv, J. H., Yang, P., Wu, J. Y., et al. (2020). Population pharmacokinetics of vancomycin in kidney transplant recipients: Model building and parameter optimization. Front. Pharmacol. 11 (1558), 563967. doi:10.3389/fphar.2020.563967
Madigan, T., Sieve, R. M., Graner, K. K., and Banerjee, R. (2013). The effect of age and weight on vancomycin serum trough concentrations in pediatric patients. Pharmacotherapy 33 (12), 1264–1272. doi:10.1002/phar.1331
Miller, M., Miller, J. L., Hagemann, T. M., Harrison, D., Chavez-Bueno, S., and Johnson, P. N. (2011). Vancomycin dosage in overweight and obese children. Am. J. Health Syst. Pharm. 68 (21), 2062–2068. doi:10.2146/ajhp110107
Moffett, B. S., Kim, S., and Edwards, M. S. (2011). Vancomycin dosing in obese pediatric patients. Clin. Pediatr. 50 (5), 442–446. doi:10.1177/0009922810393500
Moher, D. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. 4 (1), 1–9.
Morrill, H. J., Caffrey, A. R., Noh, E., and LaPlante, K. L. (2015). Vancomycin dosing considerations in a real-world cohort of obese and extremely obese patients. Pharmacotherapy 35 (9), 869–875. doi:10.1002/phar.1625
Mühlberg, E., Umstatter, F., Kleist, C., Domhan, C., Mier, W., and Uhl, P. (2020). Renaissance of vancomycin: Approaches for breaking antibiotic resistance in multidrug-resistant bacteria. Can. J. Microbiol. 66 (1), 11–16. doi:10.1139/cjm-2019-0309
Nassar, L., Hadad, S., Gefen, A., Shachor-Meyouhas, Y., Mashiach, T., Krivoy, N., et al. (2012). Prospective evaluation of the dosing regimen of vancomycin in children of different weight categories. Curr. Drug Saf. 7 (5), 375–381. doi:10.2174/157488612805076606
Pai, M. P. (2021). Antimicrobial dosing in specific populations and novel clinical methodologies: Obesity. Clin. Pharmacol. Ther. 109 (4), 942–951. doi:10.1002/cpt.2181
Reynolds, D. C., Waite, L. H., Alexander, D. P., and DeRyke, C. A. (2012). Performance of a vancomycin dosage regimen developed for obese patients. Am. J. Health. Syst. Pharm. 69 (11), 944–950. doi:10.2146/ajhp110324
Richardson, J., Scheetz, M., and O'Donnell, E. P. (2015). The association of elevated trough serum vancomycin concentrations with obesity. J. Infect. Chemother. 21 (7), 507–511. doi:10.1016/j.jiac.2015.03.007
Rybak, M. J., Le, J., Lodise, T. P., Levine, D. P., Bradley, J. S., Liu, C., et al. (2020). Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American society of health-system pharmacists, the infectious diseases society of America, the pediatric infectious diseases society, and the society of infectious diseases pharmacists. Am. J. Health Syst. Pharm. 71 (6), 1361–1364. doi:10.1093/cid/ciaa303
Rybak, M., Lomaestro, B., Rotschafer, J. C., Moellering, R., Craig, W., Billeter, M., et al. (2009). Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American society of health-system pharmacists, the infectious diseases society of America, and the society of infectious diseases pharmacists. Am. J. Health Syst. Pharm. 66 (1), 82–98. doi:10.2146/ajhp080434
Schwartz, B. S., Pollak, J., BaiLey-Davis, L., Hirsch, A. G., Cosgrove, S. E., Nau, C., et al. (2016). Antibiotic use and childhood body mass index trajectory. Int. J. Obes. 40 (4), 615–621. doi:10.1038/ijo.2015.218
Smit, C., De Hoogd, S., Bruggemann, R. J. M., and Knibbe, C. A. J. (2018). Obesity and drug pharmacology: A review of the influence of obesity on pharmacokinetic and pharmacodynamic parameters. Expert Opin. Drug Metab. Toxicol. 14 (3), 275–285. doi:10.1080/17425255.2018.1440287
Smit, C., Goulooze, S. C., Bruggemann, R. J. M., Sherwin, C. M., and Knibbe, C. A. J. (2021). Dosing recommendations for vancomycin in children and adolescents with varying levels of obesity and renal dysfunction: A population pharmacokinetic study in 1892 children aged 1–18 years. AAPS J. 23 (3), 53–10. doi:10.1208/s12248-021-00577-x
Smit, C., Wasmann, R. E., Goulooze, S. C., Wiezer, M. J., van Dongen, E. P. A., Mouton, J. W., et al. (2020). Population pharmacokinetics of vancomycin in obesity: Finding the optimal dose for (morbidly) obese individuals. Br. J. Clin. Pharmacol. 86 (2), 303–317. doi:10.1111/bcp.14144
Keywords: dose optimization, vancomycin, obese population, pediatrics, adults
Citation: Elrggal ME, Haseeb A, AlGethamy M, Ahsan U, Saleem Z, Althaqafi AS, Alshuail SS, Alsiddiqi ZA, Iqbal MS, Alzahrani AF, AlQarni A, Radwan RM, Qul AKS, Mahrous AJ, Alsharif JM, Alqurashi MK, Faidah HS and Aldurdunji M (2023) Dose optimization of vancomycin in obese patients: A systematic review. Front. Pharmacol. 14:965284. doi: 10.3389/fphar.2023.965284
Received: 10 June 2022; Accepted: 27 February 2023;
Published: 24 March 2023.
Edited by:
Pasquale Pagliano, University of Salerno, ItalyReviewed by:
Ali Saffaei, Ministry of Health and Medical Education, IranElmira Niknami, Shahid Beheshti University of Medical Sciences, Iran
Copyright © 2023 Elrggal, Haseeb, AlGethamy, Ahsan, Saleem, Althaqafi, Alshuail, Alsiddiqi, Iqbal, Alzahrani, AlQarni, Radwan, Qul, Mahrous, Alsharif, Alqurashi, Faidah and Aldurdunji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdul Haseeb, YW1oYXNlZWJAdXF1LmVkdS5zYQ==
 Mahmoud E. Elrggal
Mahmoud E. Elrggal Abdul Haseeb
Abdul Haseeb Manal AlGethamy2
Manal AlGethamy2 Umar Ahsan
Umar Ahsan Zikria Saleem
Zikria Saleem Sattam Saad Alshuail
Sattam Saad Alshuail Muhammad Shahid Iqbal
Muhammad Shahid Iqbal Ahmad Jamal Mahrous
Ahmad Jamal Mahrous Hani Saleh Faidah
Hani Saleh Faidah Mohammed Aldurdunji
Mohammed Aldurdunji