
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Pharmacol. , 11 January 2024
Sec. Inflammation Pharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1354581
This article is a correction to:
Sorafenib increases cytochrome P450 lipid metabolites in patient with hepatocellular carcinoma
 Can G. Leineweber1,2,3
Can G. Leineweber1,2,3 Miriam Rabehl1,2
Miriam Rabehl1,2 Anne Pietzner1,2
Anne Pietzner1,2 Nadine Rohwer1,2,4
Nadine Rohwer1,2,4 Michael Rothe5
Michael Rothe5 Maciej Pech6
Maciej Pech6 Bruno Sangro7
Bruno Sangro7 Rohini Sharma8
Rohini Sharma8 Chris Verslype9
Chris Verslype9 Bristi Basu10
Bristi Basu10 Christian Sengel11
Christian Sengel11 Jens Ricke12
Jens Ricke12 Nils Helge Schebb13
Nils Helge Schebb13 Karsten-H. Weylandt1,2*†
Karsten-H. Weylandt1,2*† Julia Benckert14*†
Julia Benckert14*†A Corrigendum on
Sorafenib increases cytochrome P450 lipid metabolites in patient with hepatocellular carcinoma
by Leineweber CG, Rabehl M, Pietzner A, Rohwer N, Rothe M, Pech M, Sangro B, Sharma R, Verslype C, Basu B, Sengel C, Ricke J, Schebb NH, Weylandt K-H and Benckert J (2023). Front. Pharmacol. 14:1124214. doi: 10.3389/fphar.2023.1124214
In the published article, there was an error in Figures 2, 3, 5 as published. In an earlier version of the article, the asterisks in the figures mentioned above were not displayed as a visualization of the p-values. The corrected Figures and their unchanged captions appear below.
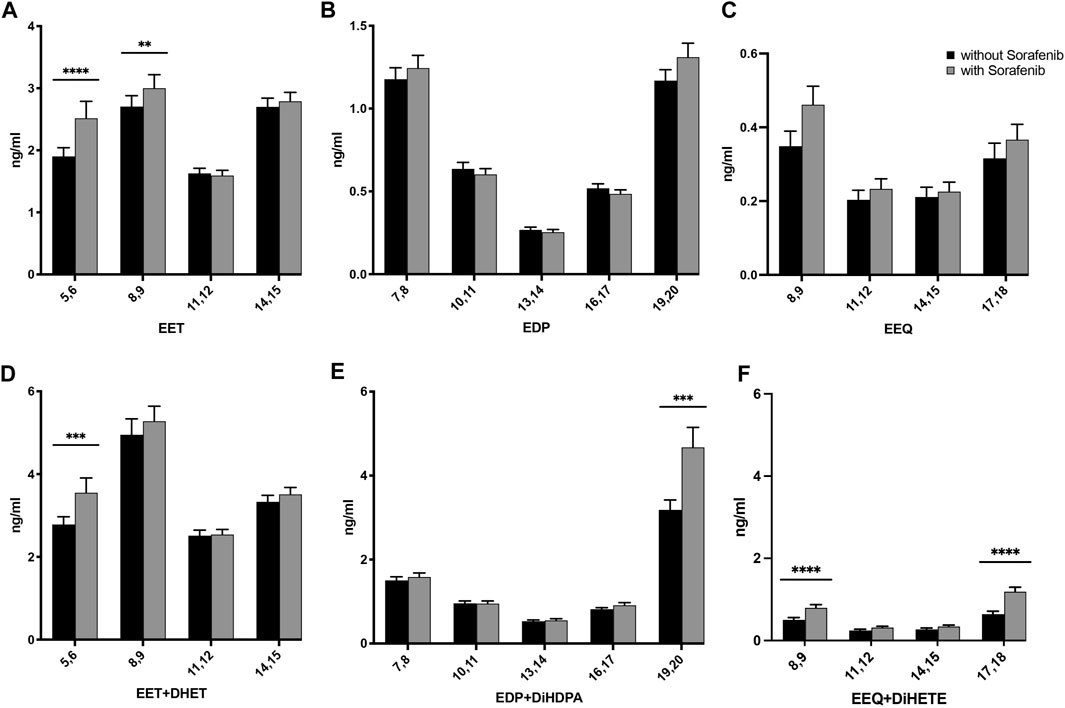
FIGURE 2. Effects on the concentrations of (A) AA-, (B) DHA-, and (C) EPA-derived epoxy-PUFA EETs, EDPs, and EEQs; and (D) AA-derived epoxy-PUFA plus dihydroxy-PUFA, (E) DHA-derived epoxy-PUFA plus dihydroxy-PUFA, and (F) EPA-derived epoxy-PUFA plus dihydroxy-PUFA in the plasma of n = 43 patients with hepatocellular carcinoma (HCC) without and undergoing sorafenib treatment (ng/mL ± standard error of the mean). Statistical differences were determined using the Wilcoxon signed-rank test (**p < 0.01; ***p < 0.001; ****p < 0.0001).
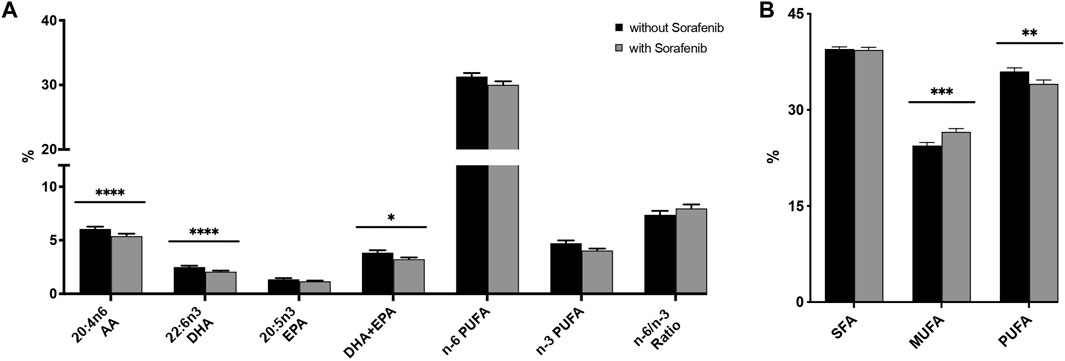
FIGURE 3. (A) Relative n-3 (docosahexaenoic acid, DHA; eicosapentaenoic acid, EPA) and n-6 (arachidonic acid, AA) PUFA levels in plasma from n = 43 patients with hepatocellular carcinoma (HCC) without and during sorafenib treatment individually, summarized and as a ratio. (B) Relative content of saturated fatty acids (SFA), monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA) in plasma from n = 43 patients with HCC without and undergoing sorafenib treatment. Statistical differences were determined using the Wilcoxon signed-rank test (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
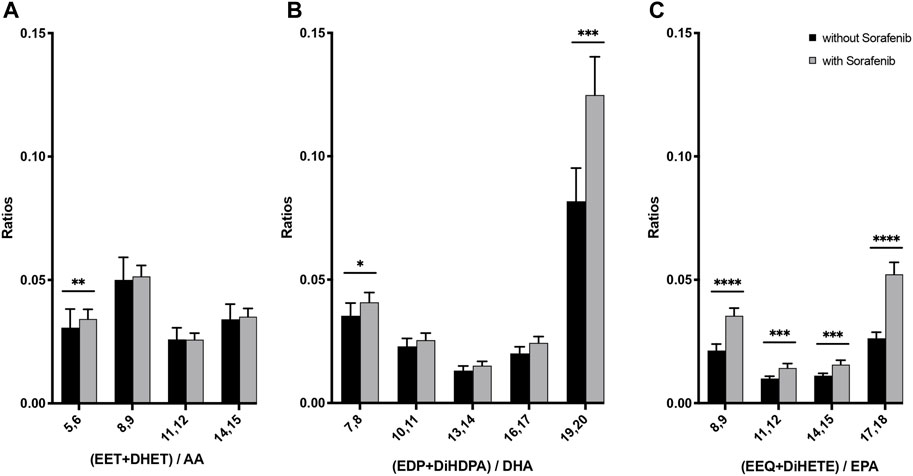
FIGURE 5. N-3 and n-6 PUFA-derived epoxides plus dihydroxy compounds as a marker for the presence of CYP metabolites in plasma from n = 43 patients with HCC without and undergoing sorafenib treatment. (A) Ratio of AA-derived products divided by AA plasma content, (B) ratio of DHA-derived products divided by DHA plasma content, (C) ratio of EPA-derived products divided by EPA plasma content (*p < 0.05, **p < 0.01; ***p < 0.001; ****p < 0.0001).
In the published article, there was an error in the Funding statement. The correct Funding statement appears below:
The author(s) declare financial support was received for publication of this article. Publication was funded by the Brandenburg Medical School (Medizinische Hochschule Brandenburg, MHB) publication fund supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) and by the Ministry of Science, Research and Culture of the State of Brandenburg.
In the published article, there was a typographical error. A correction has been made to the Introduction. The original sentence stated:
“These epoxymetabolites are then further metabolized via she into their biologically less active corresponding dihydroxy metabolites”
The corrected sentence appears below:
“These epoxymetabolites are then further metabolized via sEH into their biologically less active corresponding dihydroxy metabolites”
The authors apologize for these errors and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: hepatocellular carcinoma, cytochrome P450, sorafenib, EET, EDP, omega-3 fatty acids, oxylipins, lipidomics
Citation: Leineweber CG, Rabehl M, Pietzner A, Rohwer N, Rothe M, Pech M, Sangro B, Sharma R, Verslype C, Basu B, Sengel C, Ricke J, Schebb NH, Weylandt K-H and Benckert J (2024) Corrigendum: Sorafenib increases cytochrome P450 lipid metabolites in patient with hepatocellular carcinoma. Front. Pharmacol. 14:1354581. doi: 10.3389/fphar.2023.1354581
Received: 12 December 2023; Accepted: 13 December 2023;
Published: 11 January 2024.
Approved by:
Frontiers Editorial Office, Frontiers Media SA, SwitzerlandCopyright © 2024 Leineweber, Rabehl, Pietzner, Rohwer, Rothe, Pech, Sangro, Sharma, Verslype, Basu, Sengel, Ricke, Schebb, Weylandt and Benckert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karsten-H. Weylandt, a2Fyc3Rlbi53ZXlsYW5kdEBtaGItZm9udGFuZS5kZQ==; Julia Benckert, anVsaWEuYmVuY2tlcnRAY2hhcml0ZS5kZQ==
†These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.