- 1Beijing University of Chinese Medicine, Beijing, China
- 2Institute of Metabolic Diseases, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
Diabetes mellitus has become a major public health issue globally, putting an enormous burden on global health systems and people. Among all diseased groups, a considerable part of patients are elderly, while their clinical features, pathogenic processes, and medication regimens are different from patients of other ages. Despite the availability of multiple therapies and techniques, there are still numerous elderly diabetes patients suffering from poor blood glucose control, severe complications, and drug adverse effects, which negatively affect the quality of life in their golden years. Traditional Chinese Medicine (TCM) has been widely used in the treatment of diabetes for several decades, and its relevant clinical practice has confirmed that it has a satisfactory effect on alleviating clinical symptoms and mitigating the progression of complications. Chinese herbal medicine and its active components were used widely with obvious clinical advantages by multiple targets and signaling pathways. However, due to the particular features of elderly diabetes, few studies were conducted to explore Traditional Chinese Medicine intervention on elderly diabetic patients. This study reviews the research on clinical features, pathogenic processes, treatment principles, and TCM treatments, hoping to provide fresh perspectives on the prevention and management strategies for elderly diabetes.
1 Introduction
Diabetes mellitus (DM) is characterized by an absolute or relative lack of insulin creation as well as insulin resistance (IR) in target tissues, which leads to hyperglycemia and glucose intolerance (Ma et al., 2022). It can be classified into four types: type 1 diabetes (T1DM), type 2 diabetes (T2DM), gestational diabetes, and diabetes driven by islet disease or medicines (Fan et al., 2022; Yang and Yang, 2022). With an expanding lifespan and conversion in diet structure, the number of patients with diabetes-related high-risk factors increases, which contributes to the increased prevalence of diabetes, especially among elderly people (LeRoith et al., 2019). Elderly diabetes refers to the patients above the age of sixty, including patients diagnosed with diabetes before the age of sixty and patients diagnosed with diabetes after the age of sixty (Chinese Elderly Type 2 Diabetes Prevention and Treatment of Clinical Guidelines Writing Group et al., 2022). According to prior studies, around 28.8% of Chinese individuals aged 60–69 suffered from diabetes. This figure jumped to 31.8% among those aged over 70, which was significantly higher than young and middle-aged populations (Li et al., 2020). However, the present knowledge, diagnosis, treatment, and self-management capacity of elderly diabetes patients is inadequate, which causes the blood glucose level out of control (Chinese Elderly Type 2 Diabetes Prevention and Treatment of Clinical Guidelines Writing Group et al., 2022). Many people were first diagnosed with diabetes because of serious complications such as ischemic cardiovascular and cerebrovascular disease. This not only greatly affects patients' quality of life, but also creates an enormous burden on society and the medical system.
Current treatments for diabetes include insulin secretagogues, biguanides, insulin sensitizers, alpha-glucosidase inhibitors, incretin mimetics, amylin antagonists, and sodium-glucose co-transporter-2 (SGLT2) inhibitors, glucagon-like peptide-1 (GLP-1) receptors agonists (Padhi et al., 2020). Though these drugs can enhance insulin sensitivity, promote insulin secretion, and improve metabolic disorders, there are still a large number of patients suffering from poor blood glucose control, serious complications and drug side effects. Previous studies have shown that Traditional Chinese Medicine (TCM) was extremely effective in stabilizing blood glucose fluctuations, preventing the occurrence of brittle diabetes, slowing the development of complications, and enhancing patients’ life quality (Tian et al., 2019). Therefore, attention has steadily been drawn to comprehensive regimens with integrative medicine for diabetes.
Pathogenic pathways peculiar to elderly diabetes include gut microbiota disturbance, cell senescence, mitochondrial failure, oxidative stress, and alterations in epigenetic expression. Also, many Chinese herbs (such as Puerariae Lobatae, Radix Scutellaria baicalensis Georgi, Salvia miltiorrhiza) play a role in elderly diabetes through the aforementioned mechanisms (Xiao et al., 2020; Wang et al., 2022c; Yingrui et al., 2022; Niu et al., 2023). Furthermore, different Chinese medicine formulations may be chosen for symptomatic therapy based on the stage of diabetes to fully exert the advantage of TCM. Here, we reviewed the characteristics, pathogenesis and related TCM treatments of elderly diabetes, and analyzed the research status and potential clinical situations.
2 Clinical features in elderly diabetes
2.1 Invisible typical symptoms
The typical symptoms of DM are excessive intake of water and food, urination, and weight loss, which are invisible in elderly populations. These symptoms can be important hints for diagnosis in young and middle-aged people, but in elderly people they are masked or manifested with other atypical symptoms due to a variety of factors such as reduced body functions due to natural aging, diseases, medications, and other factors.
It should be noted that polyuria due to DM is an increase in the volume of urine. Many diseases of the elderly, such as prostate enlargement in men and urinary tract infections in women, leads to an increase in the frequency of urination, which can be distinguished from polyuria due to DM. In addition to DM, excessive thirst and intake of water are also common in the elderly (El Osta et al., 2014; Islas-Granillo et al., 2017). Previous studies have shown that the majority of older adults experience hyposalivation or dry mouth, which may be associated with a decrease in the number of teeth in the mouth, aging, the female gender, and other diseases (hypertension, cardiovascular disease, neurologic disorders, and psychological disorders) (van der Putten et al., 2003; Flink et al., 2008; Abdullah, 2015; Ohara et al., 2016). Therefore, diseases with the same characteristics as diabetes tend to mask those symptoms of the elderly, which also shows the limitations of diabetes diagnosis relying on symptoms and physical examination screening.
In elderly groups, the syndromes of diabetes may manifest itself in other forms. Diminished pressure receptor-mediated regulation of thirst in that weakens physical function of Aging body, induces higher thirst osmolality set point (Koch and Fulop, 2017). The symptoms of excessive thirst and drinking could be replaced by fatigue and cognitive deficits (Hoen et al., 2021). Diabetes causes glycation of blood fibrinogen and decreased production of fibrinolytic enzymes, which directly affects fibrinolysis and leads to hypercoagulable state (Ajjan et al., 2013). Excessively coagulated blood increases the risk of stroke, acute coronary syndrome, or intermittent claudication in elderly. Thus, all of these atypical forms of morbidity may act as the initial symptoms of elderly diabetes (Mordarska and Godziejewska-Zawada, 2017).
2.2 High risk of hypoglycemia
Hypoglycemia is more common in the elderly diabetic populations. A cohort study based on 987 elderly diabetic patients showed that about one-third of the elderly patients have hypoglycemia, within 3.3% of cases were severe (Bordier et al., 2015). As well known, hypoglycemia in the elderly is closely related to anti-diabetes regimens like sulfonylureas and insulin (Ling et al., 2021).
Decreased autonomic function due to aging results in a reduced response intensity to hypoglycemic symptoms in elderly diabetic patients. n healthy individuals, aging also allows attenuation of blood glucose recovery and reductions of counter-regulatory responses. For example, emerging warning signals associated with hypoglycemia (such as sweating, shivering, or hunger) generated by stimulation of adrenergic system, may not be present in the elderly (Mordarska and Godziejewska-Zawada, 2017). In addition, negative feedback regulation to glucagon secretion often becomes inadequate and limited.
2.3 Increased incidence of complications and coexisting disorders
In the natural course of diabetes, the incidence of complications increases with the duration of diabetes, making elderly adults at high-risk for microvascular (retinopathy, nephropathy, neuropathy) and macrovascular (coronary heart disease, stroke, peripheral arterial disease) complications of DM (Gregg et al., 2002). It has been shown that the most common cardiovascular complications in elderly patients with diabetes are coronary heart disease, followed by lower extremity vascular disease, cerebrovascular disease, and heart failure (Sinclair et al., 2015; Bauduceau et al., 2018). Also, gradual loss of skeletal muscle mass, cognitive impairment, depression, urinary incontinence, falls, fractures, and other geriatric syndromes are often comorbid or concomitant. Elderly patients with T2DM have accelerated loss of lean leg mass, muscle strength and functional capacity compared to the normoglycemic group (Leenders et al., 2013). Plasma dipeptidyl peptidase-4 activity was shown to be independently associated with mild cognitive impairment in elderly T2DM (Zheng et al., 2016). Indian survey shows that nearly one-fifth of elderly diabetes patients are suffering from depression, urban living and financial support can prevent the occurrence of depression (Sodhi et al., 2023). Diabetes duration, neuropathy and albuminuria are risk factors for urinary incontinence in elderly women with diabetes and are particularly associated with severe incontinence (Vischer et al., 2009). Increased risk of hip fracture is seen primarily in patients treated with insulin, while T2DM patients treated with any glucose control medication are consistently at increased risk of non-skeletal fall injuries (Wallander et al., 2017).
Compared to those clinical features of young and middle-aged adults, the problems faced by elderly population, include the characteristics of invisible typical symptoms, high risk of hypoglycemia, and increased incidence of complications and coexisting disorders (macrovascular and microvascular lesions, sarcopenia, cognitive dysfunction, depression, urinary incontinence, falls, and fracture). For these reasons, screening, and prevention of progression from prediabetes to diabetes are particularly important. According to the guideline (LeRoith et al., 2019), fasting glucose and/or HbA1c screening is recommended every 2 years for elderly population without diabetes, lifestyle interventions for those with pre-diabetes, and 2-h oral glucose tolerance test for those with established diabetes. Elderly diabetes patients should also undergo fingertip glucose testing as part of their daily testing regimen and regular cognitive screening.
3 Pathogenesis of elderly diabetes
3.1 Gut microbiota disturbance
Pathogenesis of elderly diabetes (including Gut microbiota disturbance, Cellular senescence, Oxidative stress and mitochondrial dysfunction, Immune and inflammatory, and Epigenetics) has been summarized. Relevant details are indicated at Figure 1. A huge number of microorganisms such as bacteria, archaea, fungi, viruses, and phages habitats in gastrointestinal tract (Wang J. et al., 2023). The gut microbiota prototype forms prenatally, undergo rapid establishment during the neonatal and infancy stages, evolves with growth and becomes virtually stable in adulthood (Zhuang et al., 2019). Changes in the structure and quantity of gut microbiota, which serve as pathogenic mechanisms in chronic noncommunicable illnesses (Illiano et al., 2020), may have an impact on corresponding activities such as food digestion (Paone and Cani, 2020), energy metabolism (Li M. et al., 2022), immunological and genetic modulation (Wiertsema et al., 2021; Xu et al., 2023), as well as the integrity of the intestinal barrier (Adolph et al., 2019; Paone and Cani, 2020).
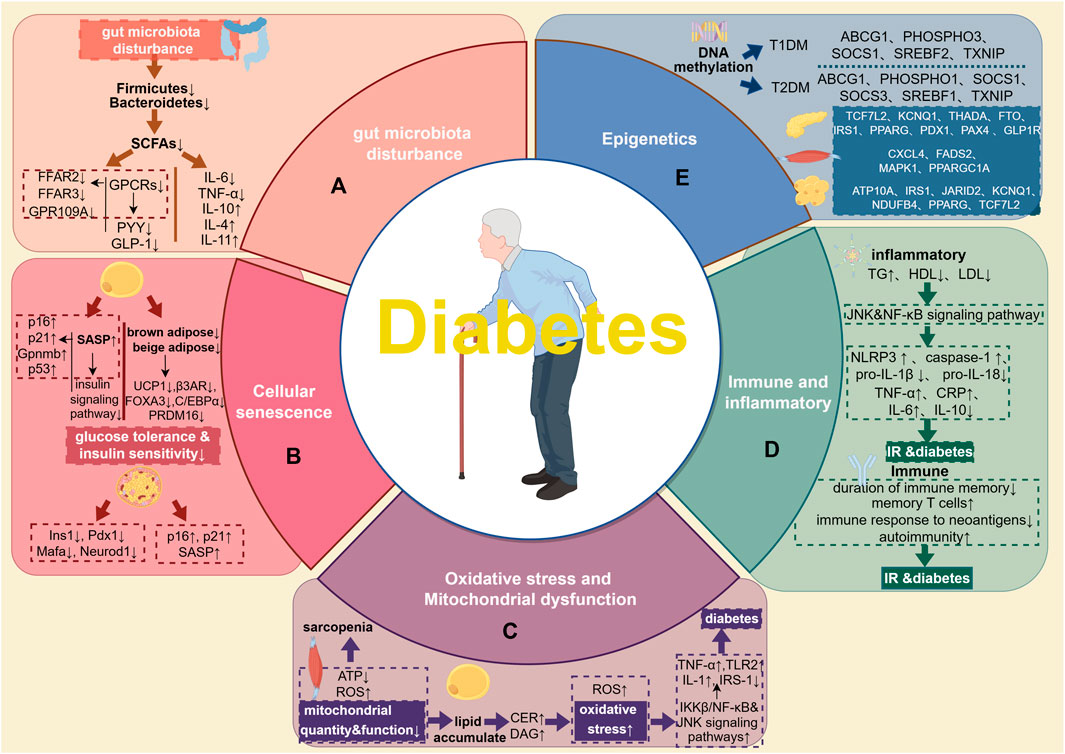
FIGURE 1. Pathogenesis of elderly diabetes. (A), gut microbiota. The lack of Firmicutes and Bacteroidetes in the elderly may result in a decrease of SCAFs, and then put the intestines and pancreatic beta cells in a state of immune disorder and inflammation, leading to disorders of blood sugar homeostasis and metabolism. (B), cellular senescence. Aging adipocytes disrupt the insulin signaling pathway by secreting SASP and regulating the expression of aging-related genes. At the same time, reduced volume of brown and beige adipose tissue and significantly reduced expression of genes related to thermogenesis and differentiation both jointly aggravate IR. In senescent islet cells, the expression of Ins1, Pdx1, Mafa, and Neurod1 decreases, and the expression of aging-related markers p16, p21, and SASP increases. (C), oxidative stress, and mitochondrial dysfunction. Changes in skeletal muscle mitochondrial function, reduced ATP synthesis, and increased ROS production may be the causes of skeletal sarcopenia in the elderly with diabetes. Impaired mitochondrial oxidative function in skeletal muscle leads to excessive lipid deposition, increased CER and DAG, and further exacerbates oxidative stress, inflammation and diabetes. (D), Immune and inflammatory. Elderly patients with diabetes are often accompanied by disorders of lipid metabolism, which activates related inflammatory pathways such as JNK and NF-κB, which in turn leads to the activation of pro-inflammatory factors such as NLRP3, caspase-1, TNF-a, CRP, IL-6 secretion and pro-IL-1β, pro-IL-18 cleavage increased. At the same time, the expression of anti-inflammatory factors such as IL-10 is reduced. In terms of immunity, the main manifestations are the shortened duration of immune memory, accumulation of memory T cells, the lack of immune response to neoantigens, and a higher tendency to autoimmunity, further induced the occurrence of IR and diabetes. (E), Epigenetics. Epigenetic research on diabetes mostly focuses on DNA methylation. Methylation of ABCG1, P1, SHOSPHO3, SREBF1 and TXNIP is associated with a higher risk of T2DM; methylation of ABCG1, PHOSPHO3, SOCS1, SREBF2 and TXNIP is associated with T1DM. Different tissues have different methylation sites. In the pancreatic tissue of T2DM patients, the methylation sites are TCF7L2, KCNQ1, THADA, FTO, IRS1, PPARG, PDX1, PAX4 and GLP1R; in the skeletal muscle tissue, the methylation sites are CXCL4, FADS2, MAPK1, PPARGC1A and other sites; in adipose tissue, the methylation sites are ATP10A, IRS1, JARID2, KCNQ1, NDUFB4, PPARG and TCF7L2.
In 1907, Elie Metchnikoff postulated that one of the factors contributing to the decline in physical health was the gut microbiota and its metabolites (Cavaillon and Legout, 2016). In recent years, there has been a growing awareness of the correlation between gut microbiota and aging process. It had shown that gut microbiota was extremely different between the elderly and young population, manifested by a decrease in species diversity and probiotics, an increase in individual differences and harmful bacteria (Duncan and Flint, 2013; Mangiola et al., 2018). After comparing the gut microbiota between the elderly and young population in South Korea, Seung Yun Lee et al. found that Firmicutes, Bacteroidetes, Cyanobacteria, Fusobacteria, and Proteobacteria were more common in young people, while the abundance of Negativicutes was higher in the elderly (Lee et al., 2021). Furthermore, Tianyi Li et al. (Li et al., 2019) and Yongcheng Ni et al. (Ni et al., 2018) discovered that Firmicutes abundance was positively correlated with blood glucose level and sensitivity to insulin treatment in elderly T2DM patients, while the abundance of anaerobic bacteria (such as Bacteroidetes) was negatively correlated. Firmicutes are the major butyrate-producing bacteria in the human body, while Bacteroidetes are mainly acetate and propionate producers (Tsai et al., 2021). Previous research has revealed that short chain fatty acids, (SCFAs) including acetate, propionate (Chambers et al., 2015a; Wu et al., 2022), and butyrate (Gao et al., 2009; Chang et al., 2014; Mollica et al., 2017; Zhang et al., 2017; 2019; Li Z. et al., 2018; Stachowska et al., 2021) may target G protein-coupled receptors (GPCRs) such as FFAR2、FFAR3、GPR109A on the intestine, pancreatic islets, and immune cells, which may affect the metabolic function (Mayorga-Ramos et al., 2022). It has been demonstrated that SCFAs activate intestinal GPCRs to control the release of glucagon-like peptide 1 (GLP-1) and peptide YY (PYY) (Coppola et al., 2021), which in turn influences insulin secretion and the hypothalamic perception of energy intake. Furthermore, in pancreatic β-cell mitochondria, butyrate can suppress the expression of fission genes (DRP1, FIS1) and fusion genes (MFN1, MFN2, OPA1).
This can not only decrease the production of pro-inflammatory factors (such as IL-6 and TNF-α), but also increase the secretion of anti-inflammatory factors (such as IL-10, IL-4, and IL-11) (Mollica et al., 2017; Xiong et al., 2019; Noureldein et al., 2020; Prause et al., 2021). Hence, the lack of Firmicutes and Bacteroidetes in the elderly may result in a decrease of SCAFs, and then put the intestines and pancreatic beta cells in a state of immune disorder and inflammation, leading to disorders of blood sugar homeostasis and metabolism.
Besides SCFAs, Shin Yoshimoto et al. investigated metabolic products of gut microbiota in young and elderly groups found that choline, trimethylamine (TMA), N-8-acetylspermidine, 2-hydroxy-4-methylvaleric acid, and 5-methylcytosine were more abundant in elderly individuals (Yoshimoto et al., 2021). These metabolites have been proven to be high-risk factors for geriatric diseases such as metabolic syndrome (Chen et al., 2019; Zhou et al., 2021), cardiovascular disease (Nayak et al., 2020; Li et al., 2021), and tumors (Yin et al., 2022).
In elderly individuals, the administration of Bifidobacterium has been observed to regulate intestinal barrier function, exert anti-inflammatory and antioxidant effects, and result in an increased abundance of this organism in stool samples. These effects have been found to be associated with a reduction in fasting blood glucose levels and improvement in insulin resistance among patients diagnosed with T2DM (Schiffrin et al., 2007; Ouwehand et al., 2008). The utilization of B subtilis natto DG101 in conjunction with suitable medications has demonstrated promising outcomes in the management of T2DM (Cardinali et al., 2020). Additionally, the potential cognitive benefits of probiotics can be attributed to their ability to reduce visceral fat and mitigate inflammation (Azuma et al., 2023).
3.2 Cellular senescence
Cellular senescence is a complicated yet ubiquitous process which is essential for biological embryonic development, tissue remodeling, and wound healing (Huang et al., 2022b). Cellular senescence is often distinguished by a reduction in the capacity for cell division, stoppage of the cell cycle, and the secretion of senescence-associated secretory phenotypes (SASP), which include cytokines, chemokines, growth factors, and proteases (Basisty et al., 2020). These chemicals can be delivered extracellularly via small vesicles, resulting in alterations to intercellular communication and the exertion of regulatory influences on adjacent cells, tissues, and even faraway tissues (Özcan et al., 2016; Terlecki-Zaniewicz et al., 2018). Nevertheless, the onset of age-related diseases is inextricably tied to the process of excessive and protracted senescence (Palmer et al., 2019; Yousefzadeh et al., 2021). Clinical studies revealed that increasing age is one of the risk factors for elderly diabetes, which is closely related to the aging of tissues and organs (Markle-Reid et al., 2018; Kumar et al., 2023).
Studies verified that the cellular senescence of diabetes target organs (such as the adipose tissue and pancreas) might accelerate the occurrence and progression of diabetes. Furthermore, obesity, lipid metabolism problems, and hyperglycemia also exacerbated this process (Narasimhan et al., 2021). Adipose tissue is crucial for storing energy, maintaining body temperature, safeguarding internal organs, and play a role in wound healing, immune as well as endocrine regulation. (Zwick et al., 2018). Nevertheless, the composition and functionality of adipose tissue undergo substantial alterations with aging or serious metabolic stress. These changes include the redistribution of body adipose, an increase in chronic aseptic inflammation, a decline in the function of adipose progenitor cells, heightened lipotoxicity of nearby tissues caused by ectopic fat deposition, diminished secretion and sensitivity of hormones derived from adipose tissue (Palmer and Kirkland, 2016). By secreting SASP and recruiting immune cells, ageing adipocytes may aggravate inflammatory responses and disrupt the insulin signaling pathway, which aggravate IR and raise the risk of T2DM and metabolic syndrome (Khosla et al., 2020). A comparison of adipose tissue between young and elderly people showed that the volume of brown and beige adipose tissue around shoulder blades, blood vessels, and kidneys was smaller in the elderly. Furthermore, the expression of adipose tissue thermogenesis and differentiation related genes (UCP1, β3AR, FOXA3, C/EBPα and PRDM16) significantly decreased (Ma et al., 2014; Wang et al., 2019; Ikeda and Yamada, 2020; Ou et al., 2022). This may cause the fibrosis of fat precursor cells and reduced the differentiation of beige cells in the elderly, causing more fat to be stored under the skin and around internal organs, inducing and enhancing the risk of metabolic diseases. Aside from functional alterations, adipocytes from elderly people and mice also show increased expression of aging-related genes such as p16, p21, Gpnmb, and p53. Activation and overexpression of p53 can lead to DNA damage, downregulation of insulin signaling protein transcription, inflammatory response, and macrophage infiltration, which have been confirmed to be associated with reduced lipogenesis, IR, and the occurrence of diabetes (Vergoni et al., 2016; Lahalle et al., 2021). However, the mechanism of other aging molecules such as p16 and p21 induced IR and diabetes is still unclear.
Additionally, it has confirmed that the aging of pancreatic beta cells is also associated with elderly diabetes. Age-related alterations in pancreatic beta cell function include decreased insulin production, decreased beta cell mass, and delayed cell proliferation. These processes might be potential roads that lead to elderly diabetes (Aguayo-Mazzucato, 2020). Cristina Aguayo-Mazzucato et al. compared the pancreatic islets of young and elderly mice and recognized that senescent islet cells accumulated obviously in the elderly mice, companied the downregulation of Ins1, Pdx1, Mafa, Neurod1, while aging markers (p16, p21), SASP (Ccl2, Il1a, Il6, Tnf) expression increased (Aguayo-Mazzucato et al., 2019; Aguayo-Mazzucato, 2020). An analogous pattern can be observed in human beings, where the prevalence of senescent islet β cells will progressively escalate because of IR, obesity, and diabetes.
Intervention strategies in the cell senescence process may be able to ameliorate metabolic problems and the progression of diabetes in the elderly (Tchkonia et al., 2021). Suda et al. and Wang et al. have confirmed that reduced expression of senescent cells and senescence markers in adipose tissue can improve glucose tolerance and insulin sensitivity (Suda et al., 2021). Currently, commonly used anti-aging combination include Dasatinib plus Quercetin (D + Q). This solution can target senescent adipocyte progenitor cells, reducing adipose tissue inflammation, increasing adipose tissue and peripheral insulin sensitivity, as well as promoting adipocyte progenitor cell development, ultimately treating diabetes in the elderly (Hickson et al., 2019; Wang et al., 2022a).
3.3 Oxidative stress and mitochondrial dysfunction
Mitochondria are double-membraned organelles that play a pivotal role in ATP synthesis and energy metabolism. Fatty acids and glucose both contribute to energy metabolism via glycolysis and the tricarboxylic acid cycle. The majority of high-energy electrons are transmitted downstream after combining with the reduced coenzyme (NADH or FADH2) of the respiratory chain in the mitochondria. Thereafter, they combine with oxygen to produce water and ATP (Nolfi-Donegan et al., 2020; Prasun, 2020). However, some electrons are not transferred through the respiratory chain but react directly with oxygen to produce superoxide radicals and hydrogen peroxide (Addabbo et al., 2009). This in turn damages cell membranes, proteins, enzymes, and DNA, leading to cell death. When mitochondrial dysfunction occurs, excessive accumulation of ROS prevents superoxide dismutase and reducing glutathione peroxidase in mitochondria from exerting their antioxidant effects. This process is called oxidative stress (Nolfi-Donegan et al., 2020).
Mitochondrial function weakens with age, owing to lower antioxidant capacity, decreased ATP synthesis, mitochondrial DNA (mtDNA) damage, mitochondrial protein oxidation, decreased electron transport chain efficiency, and worse quality control during mitophagy (Chistiakov et al., 2014). Previous studies have confirmed that mitochondrial dysfunction and oxidative stress in target organs (e.g., skeletal muscle, adipose tissue) are related to elderly diabetes. Sarcopenia, in addition to typical microvascular and macrovascular disorders, has emerged as the third significant complication among older diabetes patients. It is a significant role in patients’ poor quality of life and impairment. Changes in skeletal muscle mitochondrial function, ATP reduced synthesis and increased ROS generation may be possible causes of sarcopenia in elderly diabetes (Izzo et al., 2021). Paul Coen et colleagues discovered that the quantity and function of mitochondria in skeletal muscle were significantly diminished in the elderly when compared to young persons, and that the number of mitochondria in skeletal muscle was positively connected with insulin sensitivity (Coen et al., 2013). Pelletier et al. discovered that the oxidative phosphorylation and oxidation capacities of elderly muscle were diminished, which inhibited oxidative breakdown of lipids in muscles then increased lipid accumulate (St-Jean-Pelletier et al., 2017). Lipid accumulation raises ceramide (CER) and diacylglycerol (DAG) levels, which lower the function of mitochondrial oxidative phosphorylation and disrupting the electron transport chain (Chaurasia and Summers, 2021). As a consequence, substantial volumes of ROS were produced. ROS production activates downstream targets such as the IKKβ/NF-κB and JNK signaling pathways, increases inflammatory factor secretion (TNF-α, TLR2, IL-1), decreases IRS-1 activity, and affects insulin signaling (Michot et al., 2013). Increased levels of inflammation diminish IRS-1 expression and disrupt insulin signaling, resulting in elderly diabetes. Through comparing adipose tissue from young and elderly people, Laura Pelletier et al. found that mitochondrial dysfunction, oxidative stress, and adipocyte dysfunction were more obvious in elderly adipose tissue which accompanied by impaired lipogenesis and insulin sensitivity (L et al., 2021).
3.4 Immune and inflammatory
There are two types of immune systems: innate immunity and adaptive immunity. The complement system and various types of white blood cells (natural killer cells, mast cells, eosinophils, basophils, phagocytic cells, macrophages, neutrophils, and dendritic cells) comprise the innate immune system, which can produce cytokines and recruit immune cells to the site of infection or inflammation, then activate the adaptive immune process via antigen presentation (Daryabor et al., 2020). Adaptive immune cells include B cells and T cells, which mainly participate in humoral immunity and cellular immunity processes (SantaCruz-Calvo et al., 2022). A great number of research over the last 2 decades have demonstrated that immunometabolism is a key mechanism for controlling adaptive and innate immunity (Makowski et al., 2020). That is, metabolic processes can influence immune cell activity, and immunological and inflammatory responses may be the root causes of metabolic diseases.
However, as age increases, the immune system of the elderly will show a senescence state, which is manifested by a shortened duration of immune memory, accumulation of memory T cells, a lack of immune response to neoantigens, a higher tendency to autoimmunity, and a persistent low-grade inflammatory state throughout the body (Franceschi et al., 2018; Bulut et al., 2020; Moskalev et al., 2020). Inflammation can be caused by a variety of factors, including heightened levels of proinflammatory cytokines in the blood flow, disrupted lipid metabolism, alterations in the composition of gut microbiota, compromised immune system functioning, and the presence of meta-inflammation (Singh and Newman, 2011).
Previous studies have confirmed that the level of cellular inflammatory factors such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and C-reactive protein (CRP) was increased in elderly patients with diabetes, and anti-inflammatory factors such as IL-10 were reduced (Gorska-Ciebiada et al., 2015; Saukkonen et al., 2018; Yang et al., 2018; Leite et al., 2021). These inflammatory factors have been confirmed to have a negative effect on insulin production, secretion and insulin signaling pathway (Shoelson et al., 2006; Akbari and Hassan-Zadeh, 2018), which may induce programmed cell death of pancreatic β cells (Maedler et al., 2009), and aggravate the risk of IR and diabetes. (Wang et al., 2013).
Furthermore, elderly patients with diabetes are often accompanied by abnormalities in lipid metabolism, mainly manifested by high triglycerides, low high-density lipoprotein cholesterol, and elevated small-density low-density lipoprotein cholesterol. (Gyawali et al., 2018). These pro-inflammatory lipids result in the activation of inflammatory signaling pathways, including JNK and NF-κB (Osborn and Olefsky, 2012; Tall and Yvan-Charvet, 2015). Activation of inflammatory signaling pathways lead to increased metabolic inflammatory stress (Hotamisligil, 2017) and promote the activation of NLRP3 (Masters et al., 2010; Wen et al., 2011; Wen et al., 2013) and caspase-1 as well as the cleavage of pro-IL-1β and pro-IL-18 (Wen et al., 2012), thereby aggravating systemic chronic inflammation and inducing the occurrence of IR (Masters et al., 2010).
Anti-inflammation and regulating immune cell function are ways to alleviate insulin resistance and pancreatic β-cell dysfunction. Commonly used drugs include metformin, GLP-1 receptor agonists (GLP-1 RAs) and dipeptidyl peptidase 4 (DPP4) inhibitors (Jo and Fang, 2021). In addition, IL-1R antagonists can reduce islet inflammation caused by hyperglycemia, thereby improving islet β-cell dysfunction, alleviating insulin resistance and blood sugar homeostasis. However, the clinical efficacy of the above-mentioned drugs for elderly diabetes patients remains to be seen due to a lack of clinical research.
3.5 Epigenetics
Epigenetics is the study of heritable changes in the genetic information of gene-related characteristics that do not involve in modifying the DNA sequence, such as DNA methylation, histone modifications, and non-coding RNA (Allis and Jenuwein, 2016; Ling and Rönn, 2019). Furthermore, several enzymes are involved in post-transcriptional protein modification, such as acetylation, methylation, phosphorylation, and ubiquitination. However, most clinical studies have shown that there is a close relationship between chromatin formation, histone modifications, DNA methylation and gene activity, which have differential epigenetic changes expressed in different target tissues. So, it is difficult to determine which epigenetic phenomenon appears first.
The direct effects of aging on metabolic regulation exacerbate the underlying pathophysiological processes in elderly patients with diabetes. Aging effects interact with diabetes to accelerate the progression of many common diabetes complications (LeRoith et al., 2019). This is often associated with varying degrees of underlying IR, excess obesity, beta cell dysfunction, and sarcopenia, and increase the complexity of diabetes management in this age group (Bellary et al., 2021). Most of the current epigenetics research in diabetes are focused on DNA methylation. Previous studies have shown that the methylation patterns of diabetic patients are significantly different from those of healthy population, and there are also obvious differences in the methylation sites of different target tissues in patients (Singh et al., 2020). John Chambers et al. followed 25,372 Indian Asians and Europeans and found that methylation of ABCG1, PHOSPHO1, SOCS3, SREBF1 and TXNIP in the blood was associated with a higher risk of future T2DM; in type 1 diabetes Methylation of ABCG1, PHOSPHO3, SOCS1, SREBF2 and TXNIP sites was found (Chambers et al., 2015b). But when it comes to specific tissues, their methylation sites are different. When comparing pancreatic tissue from healthy people and T2D patients, multiple studies have found that TCF7L2, KCNQ1, THADA, FTO, IRS1, PPARG, PDX1, PAX4, and GLP1R sites are methylated, which may be related to the methylation of pancreatic islet β after transcriptional inactivation. Decreased cell secretory function and activation of pro-inflammatory pathways (Dayeh et al., 2016; Suárez et al., 2023). In skeletal muscle, CXCL4, FADS2, MAPK1, PPARGC1A and other sites; in adipose tissue, it shows methylation of ATP10A, IRS1, JARID2, KCNQ1, NDUFB4, PPARG, and TCF7L2.
4 Treatment principles of elderly diabetes
With the decline of pancreatic beta cell function and the increase of IR, the prevalence of diabetes is gradually increasing in the elderly (Motta et al., 2008a; Tekin and Zimmerman, 2020). Common comorbidities in elderly patients with diabetes include chronic kidney disease, cognitive impairment, chronic airway disease, and infection (Fagot-Campagna et al., 2005; Lin et al., 2016). Multiple medications may have adverse effects (Noale et al., 2016), so appropriate management of comorbidities should be included in the guidelines for elderly diabetes patients (Caughey et al., 2010). The challenge of managing elderly diabetes patients is that it is highly heterogeneous (Bennett, 2015), which requires personal assessment of treatment and care options (Munshi et al., 2020), as well as comprehensive education of patients (Tekin and Zimmerman, 2020).
4.1 Western medicine treatment principles and clinical intervention
The target level of blood glucose and the use of anti-diabetes medications should be comprehensively evaluated according to clinical status, risk of hypoglycemia, and complications of diabetes (Scheen et al., 2014). Failure of oral glycemic control therapy is often observed in elderly patients, so insulin is often chosen as the preferred medication (Rajpal et al., 2021). However, the dangers associated with hypoglycemia cannot be ignored. Regimens using DPP-4 inhibitors alone or in combination with basal insulin have been shown to be safe and effective and may be an alternative to basal injection regimens in elderly patients (Umpierrez and Pasquel, 2017). Reducing the frequency and severity of hypoglycemia is the key to achieving better compliance in elderly patients with diabetes (Motta et al., 2008b). In addition, the participation of family members is the basis for the good treatment effect of elderly diabetic patients (Baig et al., 2015). Besides, aiming to maintain or improve general health, the management goals of elderly patients with diabetes also include the assessment and treatment of atherosclerosis and microvascular disease (Adu-Sarkodie, 2017).
Specific clinical guidelines for T2DM in elderly have been published in Europe and the United States, but they do not specifically address advanced chronic kidney disease in elderly people with diabetes. Elderly patients with diabetes are different from younger patients, mainly due to their frailty and shorter life expectancy, requiring different treatment strategies to be tailored (Abaterusso et al., 2008). Studies have found that in the treatment of elderly diabetic nephropathy, excessive reduction of blood pressure to the current target is unsafe (Williams, 2013). The selection of some specific medications could improve the cure rate, reduce blood glucose, and improve kidney function for diabetic nephropathy in elderly (Cao and Chen, 2021).
Diabetic peripheral neuropathy (DPN) is the main form of neuropathy and a leading cause of disability. Small fiber neuropathy (SFN) can develop in older people with pre-diabetes, prior to large fiber damage (Akbar et al., 2023). The symptoms of DPN consist primarily of spontaneous, intractable pain that is diffuse and persistent and can last for weeks to months. Clinical treatment focuses on alleviating clinical symptoms, improving blood glucose control and cardiovascular risk factors (Yang et al., 2022). Pain in the elderly need arouse attention from clinicians and patients (Marchesi et al., 2024). In pain management, anticonvulsants such as pregabalin and gabapentin are the first-line treatment of choice, followed by amitriptyline, duloxetine, and venlafaxine (Cernea and Raz, 2021). Opioids and related medications are recommended for short-term use during acute exacerbations of pain (Kozma et al., 2012; Feldman et al., 2019; Marchesi et al., 2024). These interventions play an important role in diminishing DPN’s symptoms and complications. For patients who do not respond to monotherapy, combination therapy may be beneficial (Rafiullah and Siddiqui, 2022). Basic interventions also include nutritional recommendations (mecobalamin, etc.) and functional exercise (Didangelos et al., 2020; 2021; Seyedizadeh et al., 2020; Shen et al., 2023b; Marchesi et al., 2024). However, diet and exercise are often neglected in the treatment of elderly patients with diabetes. Nutritional recommendations and exercise resistance training based on elderly subjects to increase muscle mass have good value in reducing diabetes parameters (Constans and Lecomte, 2007).
4.2 TCM treatment principles
The comprehensive and holistic management with TCM for diabetes patients is gradually being favored by modern healthcare systems (Xiao and Luo, 2018). The combination of TCM characteristics and western medicine to prevent and treat diabetes becomes a new attempt (Wang et al., 2020b). More and more studies have emphasized that the bioactive components of TCM participate in those mechanism mentioned above (Nie et al., 2019; Ai et al., 2020; Tang et al., 2021; Huang et al., 2022c). TCM emphasizes Yin and Yang balance and a holistic approach. Chinese herbal medicine bidirectional regulation of body metabolism, to maintain the balance of the body’s internal environment. TCM individual treatment focuses on syndrome differentiation, multi-level and multi-target treatment, and patients with diabetes can significantly relieve symptoms under the treatment of TCM theory (Tong et al., 2012).
Clinical TCM classifies diabetes as “Xiaoke” and “Pidan”. Some scholars put forward the idea of “state-target” differentiation and treatment (Zhang et al., 2021a), and believe that the stage identification and treatment of elderly diabetes are different from the general diabetes population. According to the different stages of clinical manifestations and pathological changes, syndrome differentiation and treatment can alleviate the symptoms and physique of patients (Wang et al., 2020b). The pathological characteristics of elderly patients with diabetes are gradually becoming weak, accompanied by insufficient digestive ability. Due to long-term nutritional metabolism deficit, often just at the beginning of the disease will see the phenomenon of physical weakness, so elderly diabetes is easy to enter the stage of deficiency, damage, often combined with a variety of complications. TCM believes that most elderly patients are sick for too long and lead to physical weakness, with the gradual loss of Yang physical characteristics, so strong Yang is often the treatment principle of elderly diseases.
TCM believes that most elderly patients have a long course of disease, which leads to physical weakness and gradual imbalance of Qi, Blood, Yin, and Yang. Therefore, the use of TCM intervention strategies, focusing on improving immunity, improving circulation, and reducing inflammation may becoming the key to the treatment of elderly diabetes.
5 TCM treatments of elderly diabetes
Over the past few years, numerous investigations have been carried out to find evidence-based anti-diabetes TCM formulas but few for elderly diabetes. We conducted a literature review on PubMed and CNKI updated until November 2023, for eligible studies on the Traditional Medicine accepted for use in clinical settings by the elderly population with diabetes or diabetic complications. And we searched keywords and Medical Subject Headings (MeSH) terms pertinent to the intervention of interest, such as “elderly diabetes”, and “Traditional Medicine”. All involved TCM interventions for elderly diabetes were summarized (Table 1).
Though there were insufficient direct clinical proofs on TCM for diabetes and related complications in elderly patients, it is beyond question that TCM addresses the health of the population. Moreover, TCM have provide patient-centered treatment strategies for blood glucose problem that can partly replace western medicine. A meta-analysis reported that TCM could significantly improve glucose control and clinical indices in patients with diabetes and effectively delay the progression of diabetes (Tian et al., 2019). As early as 2015, the first RCT on TCM formula for diabetes and mechanism exploration with gut microbiota was conducted, providing powerful clinical proofs on this issue (Xu et al., 2015). A traditional Chinese herbal formula, Gegen Qinlian Decoction, can exert similar diabetes-control effects with metformin in a dose-dependent manner (Tong et al., 2011; Xu et al., 2020; Tian et al., 2021).
Also, studies reported in Chinese concentrated on senile disease (such as DKD) and other common symptoms or signs in elderly diabetes like constipation and diabetic gastroparesis. It is obvious that the danger and particularity of elderly diabetes have attracted more attention in recent years, and we’ve also just seen the size of these settlements balloon. Various TCM interventions could solve a majority of problems for elderly diabetes. And different complications suit for different TCM interventions. For example, acupuncture plays a crucial role on diabetic peripheral neuropathy while TCM exercise therapy (such as Taichi) helps weight loss and muscle function recovery (Jiang et al., 2020; Huang. et al., 2022a; Shen et al., 2023b; Li et al., 2023).
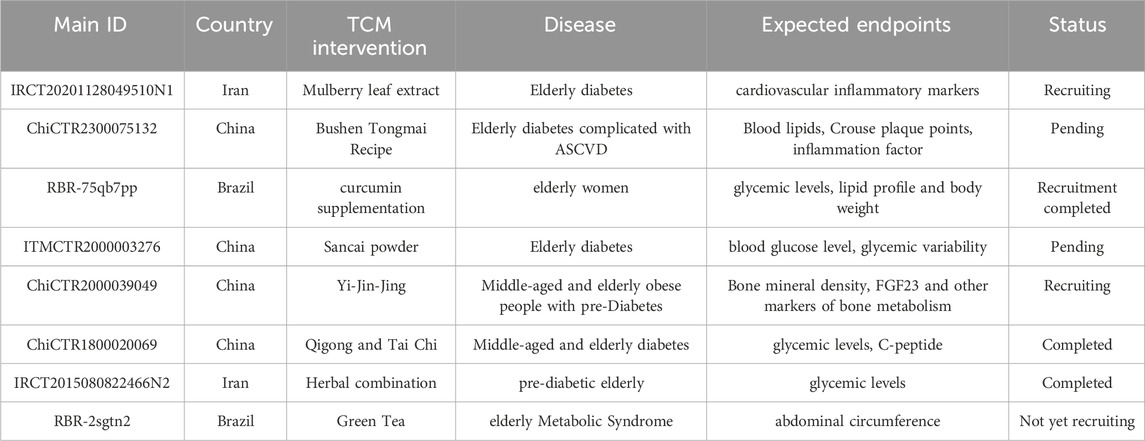
Clinical trials on TCM intervention in elderly diabetes was also retrieved from WHO International Clinical Trials Registry Platform (https://www.who.int/clinical-trials-registry-platform), ClinicalTrials.gov. (https://clinicaltrials.gov/). and Chinese Clinical Trial Registry (https://www.chictr.org.cn/). We noticed that there was an increasing trend in trials on TCM intervention in elderly diabetes (Table 2). Such TCM treatment of elderly diabetes research is currently in progress.
Furthermore, we have reviewed the common mechanism of TCM reported for aging and diabetes, or direct mechanism for elderly diabetes mentioned above. So far, those mechanism could be well implemented by distinctive TCM non-pharmacological approaches (Figure 2) and herbal treatment (Table 3). TCM had the effect of modulating gut microbiota and improving glucose metabolisms in T2DM patients and pre-clinical experiments (Zheng et al., 2021b). Numerous studies reviewed the efficacy and mechanisms of Chinese herbal medicine on DKD, DR, and DPN, which have been reported to be critical for diabetes in the elderly (Lu et al., 2019; Ai et al., 2020; Chen et al., 2022b; Liu et al., 2022). Whlie TCM could give full play to the advantages of multiple targets and intervene in many important mechanisms of elderly diabetes. Take Gegen Qinlian Decoction (GQD) as an example, its prescirption may ameliorate T2DM with hyperlipidemia via enriching beneficial gut microbiota (such as Blautia and Faecalibacterium spp) and decline harmful gut microbiota which closely related to the occurrence and development of T2DM (Tong et al., 2018). Also, the exosomal miRNA expression profile and signaling pathways related to T2DM was changed obviously following GQD treatment to provide a potential strategy for elderly diabetes. For elderly DKD, Dihong formula, Qi-Kui granules, and treatment based on syndrome differentiation were reported to exert the effect of oxidative stress inhibition and kidney protection.
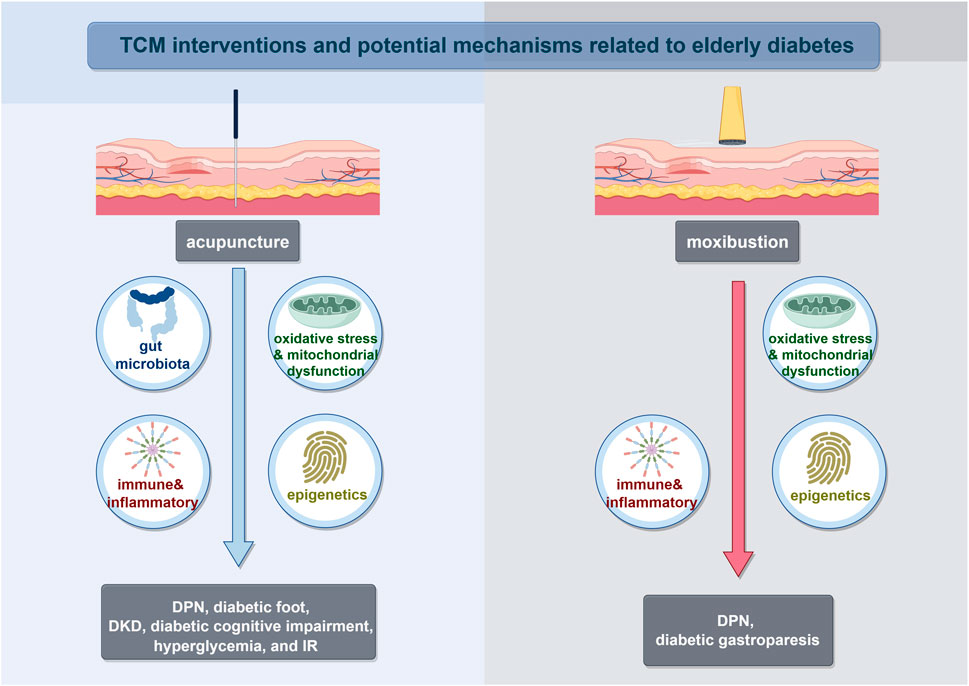
FIGURE 2. TCM interventions and potential mechanisms related to elderly diabetes. Abbreviations: Diabetic peripheral neuropathy (DPN); Diabetic kidney disease (DKD); Insulin resistance (IR).
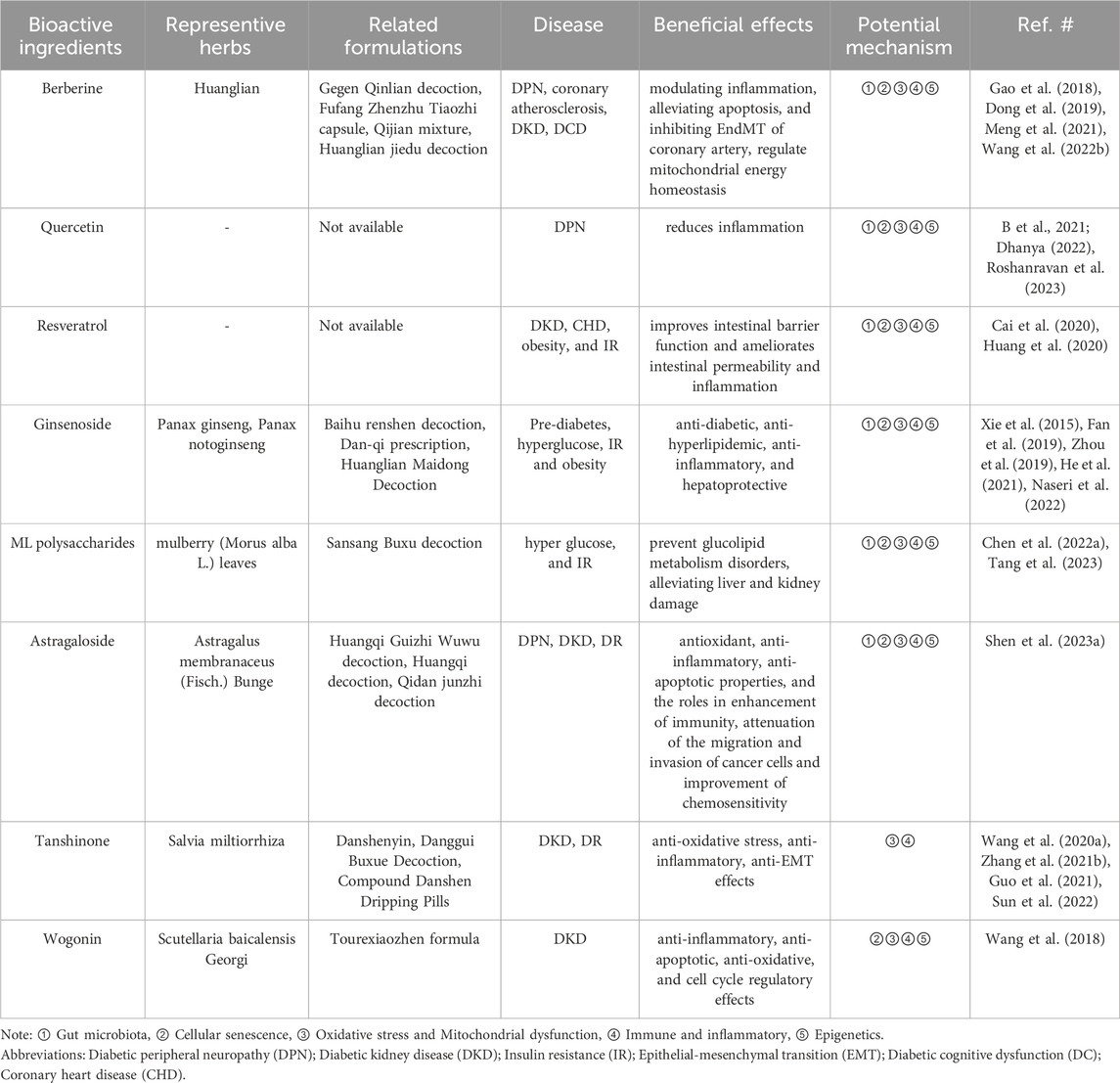
TABLE 3. Representative examples of major anti-diabetic effects of TCM ingredients, herbs and formulations, as well as potential mechanisms related to elderly diabetes.
There were also various natural products (including oleuropein, cyclocarya paliurus polysaccharides, hyperoside, and so on) that do not used in traditional clinical practice, while existed proofs have reported excellent potentials for elderly diabetes considering consistent curative mechanism (Yao et al., 2020; Zheng et al., 2021a; Liu et al., 2021). Quercetin, an important natural flavonoid, not only regulate gut microbiota disorders in diabetes animal models, but also inhibiting oxidative stress and inflammatory responses to restore mitochondrial dysfunction (Yi et al., 2021; Cui et al., 2022). Moreover, there are numerous natural products from TCM used for various diabetes related complications, including DR, DPN and so on. Furthermore, we categorized and analyzed their main bioactive ingredients (Table 3). This review partly covers the key works on the effects and underlying mechanisms of TCM, herbal ingredients and synergistic effects of constituent compatibility in treating elderly diabetes, providing additional ideas to address this threat.
6 Discussion and prospects
Diabetes is a systemic chronic metabolic disease caused by a combination of genetic, nutritional, environmental, and other factors. With the improvement of living circumstances and the aging of population, diabetes is affecting an increasing number of senior individuals. As a result, early and thorough interventions have substantial therapeutic implications for delaying diabetes development, preserving target organs, preventing complications, and increasing patients' quality of life. Even though a range of medicines is being utilized to treat diabetes, current diabetes management in the elderly is still inadequate.
Diabetic patients in China frequently undergo TCM treatment in addition to conventional care, and the results are frequently superior to conventional treatment alone. Multiple studies have demonstrated that TCM can relieve clinical symptoms and postpone the development of diabetes by regulating exosome secretion and epigenetic expression, as well as enhancing gut microbiota and eliminating oxidative stress. However, due to the unique diagnostic methods of TCM and the complexity of TCM components, as well as the fact that the pathogenesis of diabetes in the elderly differs from that of other age groups, there is still a lack of large-scale, multi-center, randomized, and controlled clinical trials of TCM in the treatment of elderly diabetes. Furthermore, clinical studies have yet to validate several results, although clinical trials have proven improvements in clinical symptoms, the fundamental mechanisms remain unknown. To overcome these limitations, we should conduct additional analyses and searches for key compounds and targets of TCM in the treatment of elderly diabetes. Also, there is an urgent need to clarify the drug dose-response relationship and ensure the reliability of the results through experimental verification in the future. Besides, a high-quality TCM clinical research protocol should be established to facilitate the conduction of large-scale, multi-center, controlled trials, so that to provide stronger evidence for TCM treatment of diabetes in the elderly.
Author contributions
XY: Writing–original draft. CX: Writing–original draft. KC: Writing–original draft. DG: Writing–original draft. HW: Project administration, Writing–review and editing. CT: Project administration, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by the National Natural Science Foundation of China (82305192), Scientific Research Start-up Funds for New Faculty at Beijing University of Chinese Medicine (2023-JYB-900202-004), and scientific research Funds for HuTian visiting professors at Beijing University of Chinese Medicine (2023-XJ-KYQD-006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1339744/full#supplementary-material
References
Abaterusso, C., Lupo, A., Ortalda, V., De Biase, V., Pani, A., Muggeo, M., et al. (2008). Treating elderly people with diabetes and stages 3 and 4 chronic kidney disease. Clin. J. Am. Soc. Nephrol. 3, 1185–1194. doi:10.2215/CJN.00410108
Abdullah, M. J. (2015). Prevalence of xerostomia in patients attending Shorish dental speciality in Sulaimani city. J. Clin. Exp. Dent. 7, e45–e53. doi:10.4317/jced.51867
Addabbo, F., Montagnani, M., and Goligorsky, M. S. (2009). Mitochondria and reactive oxygen species. Hypertension 53, 885–892. doi:10.1161/HYPERTENSIONAHA.109.130054
Adolph, T. E., Mayr, L., Grabherr, F., Schwärzler, J., and Tilg, H. (2019). Pancreas-microbiota cross talk in health and disease. Annu. Rev. Nutr. 39, 249–266. doi:10.1146/annurev-nutr-082018-124306
Adu-Sarkodie, N. Y. (2017). Clinical management of diabetes mellitus in the older adult patient. Curr. Diabetes Rev. 13, 225–238. doi:10.2174/1573399812666161206151706
Aguayo-Mazzucato, C. (2020). Functional changes in beta cells during ageing and senescence. Diabetologia 63, 2022–2029. doi:10.1007/s00125-020-05185-6
Aguayo-Mazzucato, C., Andle, J., Lee, T. B., Midha, A., Talemal, L., Chipashvili, V., et al. (2019). Acceleration of β cell aging determines diabetes and senolysis improves disease outcomes. Cell Metab. 30, 129–142. doi:10.1016/j.cmet.2019.05.006
Ai, X., Yu, P., Hou, Y., Song, X., Luo, J., Li, N., et al. (2020). A review of traditional Chinese medicine on treatment of diabetic retinopathy and involved mechanisms. Biomed. Pharmacother. 132, 110852. doi:10.1016/j.biopha.2020.110852
Ajjan, R. A., Gamlen, T., Standeven, K. F., Mughal, S., Hess, K., Smith, K. A., et al. (2013). Diabetes is associated with posttranslational modifications in plasminogen resulting in reduced plasmin generation and enzyme-specific activity. Blood 122, 134–142. doi:10.1182/blood-2013-04-494641
Akbar, M., Wandy, A., Soraya, G. V., Goysal, Y., Lotisna, M., and Basri, M. I. (2023). Sudomotor dysfunction in diabetic peripheral neuropathy (DPN) and its testing modalities: a literature review. Heliyon 9, e18184. doi:10.1016/j.heliyon.2023.e18184
Akbari, M., and Hassan-Zadeh, V. (2018). IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology 26, 685–698. doi:10.1007/s10787-018-0458-0
Allis, C. D., and Jenuwein, T. (2016). The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 17, 487–500. doi:10.1038/nrg.2016.59
Azuma, N., Mawatari, T., Saito, Y., Tsukamoto, M., Sampei, M., and Iwama, Y. (2023). Effect of continuous ingestion of bifidobacteria and dietary fiber on improvement in cognitive function: a randomized, double-blind, placebo-controlled trial. Nutrients 15, 4175. doi:10.3390/nu15194175
Baig, A. A., Benitez, A., Quinn, M. T., and Burnet, D. L. (2015). Family interventions to improve diabetes outcomes for adults. Ann. N. Y. Acad. Sci. 1353, 89–112. doi:10.1111/nyas.12844
Basisty, N., Kale, A., Jeon, O. H., Kuehnemann, C., Payne, T., Rao, C., et al. (2020). A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 18, e3000599. doi:10.1371/journal.pbio.3000599
Bauduceau, B., Le Floch, J.-P., Halimi, S., Verny, C., Doucet, J., and Intergroup, S. F. D./SFGG (2018). Cardiovascular complications over 5 Years and their association with survival in the GERODIAB cohort of elderly French patients with type 2 diabetes. Diabetes Care 41, 156–162. doi:10.2337/dc17-1437
Bellary, S., Kyrou, I., Brown, J. E., and Bailey, C. J. (2021). Type 2 diabetes mellitus in older adults: clinical considerations and management. Nat. Rev. Endocrinol. 17, 534–548. doi:10.1038/s41574-021-00512-2
Bennett, K. (2015). Diabetes in older people. Clin. Med. (Lond) 15, 465–467. doi:10.7861/clinmedicine.15-5-465
Bordier, L., Buysschaert, M., Bauduceau, B., Doucet, J., Verny, C., Lassmann Vague, V., et al. (2015). Predicting factors of hypoglycaemia in elderly type 2 diabetes patients: contributions of the GERODIAB study. Diabetes Metab. 41, 301–303. doi:10.1016/j.diabet.2015.03.001
Bulut, O., Kilic, G., Domínguez-Andrés, J., and Netea, M. G. (2020). Overcoming immune dysfunction in the elderly: trained immunity as a novel approach. Int. Immunol. 32, 741–753. doi:10.1093/intimm/dxaa052
Cai, T.-T., Ye, X.-L., Li, R.-R., Chen, H., Wang, Y.-Y., Yong, H.-J., et al. (2020). Resveratrol modulates the gut microbiota and inflammation to protect against diabetic nephropathy in mice. Front. Pharmacol. 11, 1249. doi:10.3389/fphar.2020.01249
Cao, X., and Chen, P. (2021). The effects of alprostadil combined with α-lipoic acid in the treatment of senile diabetic nephropathy. Am. J. Transl. Res. 13, 10823–10829.
Cardinali, N., Bauman, C., Rodriguez Ayala, F., and Grau, R. (2020). Two cases of type 2 diabetes mellitus successfully treated with probiotics. Clin. Case Rep. 8, 3120–3125. doi:10.1002/ccr3.3354
Caughey, G. E., Roughead, E. E., Vitry, A. I., McDermott, R. A., Shakib, S., and Gilbert, A. L. (2010). Comorbidity in the elderly with diabetes: identification of areas of potential treatment conflicts. Diabetes Res. Clin. Pract. 87, 385–393. doi:10.1016/j.diabres.2009.10.019
Cavaillon, J.-M., and Legout, S. (2016). Centenary of the death of Elie Metchnikoff: a visionary and an outstanding team leader. Microbes Infect. 18, 577–594. doi:10.1016/j.micinf.2016.05.008
Cernea, S., and Raz, I. (2021). Management of diabetic neuropathy. Metabolism 123, 154867. doi:10.1016/j.metabol.2021.154867
Chambers, E. S., Viardot, A., Psichas, A., Morrison, D. J., Murphy, K. G., Zac-Varghese, S. E. K., et al. (2015a). Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 64, 1744–1754. doi:10.1136/gutjnl-2014-307913
Chambers, J. C., Loh, M., Lehne, B., Drong, A., Kriebel, J., Motta, V., et al. (2015b). Epigenome-wide association of DNA methylation markers in peripheral blood from Indian Asians and Europeans with incident type 2 diabetes: a nested case-control study. Lancet Diabetes Endocrinol. 3, 526–534. doi:10.1016/S2213-8587(15)00127-8
Chang, F., Song, R., Du, Y., Hu, Z., and Li, J. (2021). Clinical observation on Yupingfeng powder in treating hyperhidrosis of senile type 2 diabetes mellitus. Guangming J. Chin. Med. 36, 1265–1268.
Chang, P. V., Hao, L., Offermanns, S., and Medzhitov, R. (2014). The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. U. S. A. 111, 2247–2252. doi:10.1073/pnas.1322269111
Chaurasia, B., and Summers, S. A. (2021). Ceramides in metabolism: key lipotoxic players. Annu. Rev. Physiol. 83, 303–330. doi:10.1146/annurev-physiol-031620-093815
Chen, C.-P., Chan, K.-C., Ho, H.-H., Huang, H.-P., Hsu, L.-S., and Wang, C.-J. (2022a). Mulberry polyphenol extracts attenuated senescence through inhibition of Ras/ERK via promoting Ras degradation in VSMC. Int. J. Med. Sci. 19, 89–97. doi:10.7150/ijms.64763
Chen, D.-Q., Wu, J., and Li, P. (2022b). Therapeutic mechanism and clinical application of Chinese herbal medicine against diabetic kidney disease. Front. Pharmacol. 13, 1055296. doi:10.3389/fphar.2022.1055296
Chen, S., Henderson, A., Petriello, M. C., Romano, K. A., Gearing, M., Miao, J., et al. (2019). Trimethylamine N-oxide binds and activates PERK to promote metabolic dysfunction. Cell Metab. 30, 1141–1151. doi:10.1016/j.cmet.2019.08.021
Chiao, Y.-W., Chen, Y.-J., Kuo, Y.-H., and Lu, C.-Y. (2018). Traditional Chinese medical care and incidence of stroke in elderly patients treated with antidiabetic medications. Int. J. Environ. Res. Public Health 15, 1267. doi:10.3390/ijerph15061267
Chinese Elderly Type 2 Diabetes Prevention and Treatment of Clinical Guidelines Writing Group, Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Society, Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Health Care Society, Geriatric Professional Committee of Beijing Medical Award Foundation, National Clinical Medical Research Center for Geriatric Diseases PLA General Hospital (2022). Clinical guidelines for prevention and treatment of type 2 diabetes mellitus in the elderly in China (2022 edition). Zhonghua Nei Ke Za Zhi 61, 12–50. doi:10.3760/cma.j.cn112138-20211027-00751
Chistiakov, D. A., Sobenin, I. A., Revin, V. V., Orekhov, A. N., and Bobryshev, Y. V. (2014). Mitochondrial aging and age-related dysfunction of mitochondria. Biomed. Res. Int. 2014, 238463. doi:10.1155/2014/238463
Coen, P. M., Jubrias, S. A., Distefano, G., Amati, F., Mackey, D. C., Glynn, N. W., et al. (2013). Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 68, 447–455. doi:10.1093/gerona/gls196
Constans, T., and Lecomte, P. (2007). Non pharmacological treatments in elderly diabetics. Diabetes Metab. 33 (1), S79–S86. doi:10.1016/s1262-3636(07)80060-7
Coppola, S., Avagliano, C., Calignano, A., and Berni Canani, R. (2021). The protective role of butyrate against obesity and obesity-related diseases. Molecules 26, 682. doi:10.3390/molecules26030682
Cui, Z., Zhao, X., Amevor, F. K., Du, X., Wang, Y., Li, D., et al. (2022). Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front. Immunol. 13, 943321. doi:10.3389/fimmu.2022.943321
Daryabor, G., Atashzar, M. R., Kabelitz, D., Meri, S., and Kalantar, K. (2020). The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Front. Immunol. 11, 1582. doi:10.3389/fimmu.2020.01582
Dayeh, T., Tuomi, T., Almgren, P., Perfilyev, A., Jansson, P.-A., de Mello, V. D., et al. (2016). DNA methylation of loci within ABCG1 and PHOSPHO1 in blood DNA is associated with future type 2 diabetes risk. Epigenetics 11, 482–488. doi:10.1080/15592294.2016.1178418
Dhanya, R. (2022). Quercetin for managing type 2 diabetes and its complications, an insight into multitarget therapy. Biomed. Pharmacother. 146, 112560. doi:10.1016/j.biopha.2021.112560
Didangelos, T., Karlafti, E., Kotzakioulafi, E., Kontoninas, Z., Margaritidis, C., Giannoulaki, P., et al. (2020). Efficacy and safety of the combination of superoxide dismutase, alpha lipoic acid, vitamin B12, and carnitine for 12 Months in patients with diabetic neuropathy. Nutrients 12, 3254. doi:10.3390/nu12113254
Didangelos, T., Karlafti, E., Kotzakioulafi, E., Margariti, E., Giannoulaki, P., Batanis, G., et al. (2021). Vitamin B12 supplementation in diabetic neuropathy: a 1-year, randomized, double-blind, placebo-controlled trial. Nutrients 13, 395. doi:10.3390/nu13020395
Dong, J., Zuo, Z., Yan, W., Liu, W., Zheng, Q., and Liu, X. (2019). Berberine ameliorates diabetic neuropathic pain in a rat model: involvement of oxidative stress, inflammation, and μ-opioid receptors. Naunyn Schmiedeb. Arch. Pharmacol. 392, 1141–1149. doi:10.1007/s00210-019-01659-6
Duncan, S. H., and Flint, H. J. (2013). Probiotics and prebiotics and health in ageing populations. Maturitas 75, 44–50. doi:10.1016/j.maturitas.2013.02.004
El Osta, N., Hennequin, M., Tubert-Jeannin, S., Abboud Naaman, N. B., El Osta, L., and Geahchan, N. (2014). The pertinence of oral health indicators in nutritional studies in the elderly. Clin. Nutr. 33, 316–321. doi:10.1016/j.clnu.2013.05.012
Fagot-Campagna, A., Bourdel-Marchasson, I., and Simon, D. (2005). Burden of diabetes in an aging population: prevalence, incidence, mortality, characteristics and quality of care. Diabetes Metab. 31, 5S35–5S52. Spec No 2. doi:10.1016/s1262-3636(05)73650-8
Fan, W., Pang, H., Xie, Z., Huang, G., and Zhou, Z. (2022). Circular RNAs in diabetes mellitus and its complications. Front. Endocrinol. (Lausanne) 13, 885650. doi:10.3389/fendo.2022.885650
Fan, X., Zhang, C., Niu, S., Fan, B., Gu, D., Jiang, K., et al. (2019). Ginsenoside Rg1 attenuates hepatic insulin resistance induced by high-fat and high-sugar by inhibiting inflammation. Eur. J. Pharmacol. 854, 247–255. doi:10.1016/j.ejphar.2019.04.027
Fang, H.-S., Liu, C.-C., Jia, C.-H., Chen, Y.-H., and Li, C.-Y. (2015). Risk of developing coronary artery disease in patients with type 2 diabetes receiving traditional Chinese medicine therapy. J. Altern. Complement. Med. 21, 604–609. doi:10.1089/acm.2014.0079
Feldman, E. L., Callaghan, B. C., Pop-Busui, R., Zochodne, D. W., Wright, D. E., Bennett, D. L., et al. (2019). Diabetic neuropathy. Nat. Rev. Dis. Prim. 5, 42. doi:10.1038/s41572-019-0097-9
Feng, C., and Tong, X. (2009). 24 cases of elderly patients with diabetes mellitus and constipation treated with Jichuan decoction. Jilin J. Chin. Med. 29, 129. doi:10.13463/j.cnki.jlzyy.2009.02.016
Flink, H., Bergdahl, M., Tegelberg, A., Rosenblad, A., and Lagerlöf, F. (2008). Prevalence of hyposalivation in relation to general health, body mass index and remaining teeth in different age groups of adults. Community Dent. Oral Epidemiol. 36, 523–531. doi:10.1111/j.1600-0528.2008.00432.x
Franceschi, C., Garagnani, P., Parini, P., Giuliani, C., and Santoro, A. (2018). Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14, 576–590. doi:10.1038/s41574-018-0059-4
Gao, K., Yang, R., Zhang, J., Wang, Z., Jia, C., Zhang, F., et al. (2018). Effects of Qijian mixture on type 2 diabetes assessed by metabonomics, gut microbiota and network pharmacology. Pharmacol. Res. 130, 93–109. doi:10.1016/j.phrs.2018.01.011
Gao, Z., Yin, J., Zhang, J., Ward, R. E., Martin, R. J., Lefevre, M., et al. (2009). Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58, 1509–1517. doi:10.2337/db08-1637
Gong, J. (2013). Clinical observation of 48 cases of senile diabetic nephropathy treated by TCM syndrome differentiation. Guid. J. Traditional Chin. Med. Pharm. 19, 51–52. doi:10.13862/j.cnki.cn43-1446/r.2013.09.032
Gorska-Ciebiada, M., Saryusz-Wolska, M., Borkowska, A., Ciebiada, M., and Loba, J. (2015). Serum levels of inflammatory markers in depressed elderly patients with diabetes and mild cognitive impairment. PLoS One 10, e0120433. doi:10.1371/journal.pone.0120433
Gregg, E. W., Engelgau, M. M., and Narayan, V. (2002). Complications of diabetes in elderly people. BMJ 325, 916–917. doi:10.1136/bmj.325.7370.916
Guo, Y., Sun, J., Zhang, R., Yang, P., Zhang, S., and Wu, Z. (2021). Salvia miltiorrhiza improves type 2 diabetes: a protocol for systematic review and meta-analysis. Med. Baltim. 100, e23843. doi:10.1097/MD.0000000000023843
Gyawali, P., Martin, S. A., Heilbronn, L. K., Vincent, A. D., Taylor, A. W., Adams, R. J. T., et al. (2018). The role of sex hormone-binding globulin (SHBG), testosterone, and other sex steroids, on the development of type 2 diabetes in a cohort of community-dwelling middle-aged to elderly men. Acta Diabetol. 55, 861–872. doi:10.1007/s00592-018-1163-6
He, Q., Zhang, T., Jin, B., Wu, Y., Wu, J., Gao, P., et al. (2021). Exploring the regulatory mechanism of modified huanglian Maidong decoction on type 2 diabetes mellitus biological network based on systematic pharmacology. Evid. Based Complement. Altern. Med. 2021, 1768720. doi:10.1155/2021/1768720
Hickson, L. J., Langhi Prata, L. G. P., Bobart, S. A., Evans, T. K., Giorgadze, N., Hashmi, S. K., et al. (2019). Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 47, 446–456. doi:10.1016/j.ebiom.2019.08.069
Hoen, L., Pfeffer, D., Zapf, R., Raabe, A., Hildebrand, J., Kraft, J., et al. (2021). Association of drug application and hydration status in elderly patients. Nutrients 13, 1929. doi:10.3390/nu13061929
Hotamisligil, G. S. (2017). Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185. doi:10.1038/nature21363
Hsu, P.-C., Tsai, Y.-T., Lai, J.-N., Wu, C.-T., Lin, S.-K., and Huang, C.-Y. (2014). Integrating traditional Chinese medicine healthcare into diabetes care by reducing the risk of developing kidney failure among type 2 diabetic patients: a population-based case control study. J. Ethnopharmacol. 156, 358–364. doi:10.1016/j.jep.2014.08.029
Huang, C.-Y., Mayer, P. K., Wu, M.-Y., Liu, D.-H., Wu, P.-C., and Yen, H.-R. (2022a). The effect of Tai Chi in elderly individuals with sarcopenia and frailty: a systematic review and meta-analysis of randomized controlled trials. Ageing Res. Rev. 82, 101747. doi:10.1016/j.arr.2022.101747
Huang, D.-D., Shi, G., Jiang, Y., Yao, C., and Zhu, C. (2020). A review on the potential of Resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed. Pharmacother. 125, 109767. doi:10.1016/j.biopha.2019.109767
Huang, W., Hickson, L. J., Eirin, A., Kirkland, J. L., and Lerman, L. O. (2022b). Cellular senescence: the good, the bad and the unknown. Nat. Rev. Nephrol. 18, 611–627. doi:10.1038/s41581-022-00601-z
Huang, Y., Lu, J., Zhao, Q., Chen, J., Dong, W., Lin, M., et al. (2022c). Potential therapeutic mechanism of traditional Chinese medicine on diabetes in rodents: a review from an NMR-based metabolomics perspective. Molecules 27, 5109. doi:10.3390/molecules27165109
Ikeda, K., and Yamada, T. (2020). UCP1 dependent and independent thermogenesis in Brown and beige adipocytes. Front. Endocrinol. (Lausanne) 11, 498. doi:10.3389/fendo.2020.00498
Illiano, P., Brambilla, R., and Parolini, C. (2020). The mutual interplay of gut microbiota, diet and human disease. FEBS J. 287, 833–855. doi:10.1111/febs.15217
Islas-Granillo, H., Borges-Yáñez, A., Fernández-Barrera, M. Á., Ávila-Burgos, L., Patiño-Marín, N., Márquez-Corona, M. de L., et al. (2017). Relationship of hyposalivation and xerostomia in Mexican elderly with socioeconomic, sociodemographic and dental factors. Sci. Rep. 7, 40686. doi:10.1038/srep40686
Izzo, A., Massimino, E., Riccardi, G., and Della Pepa, G. (2021). A narrative review on sarcopenia in type 2 diabetes mellitus: prevalence and associated factors. Nutrients 13, 183. doi:10.3390/nu13010183
Jiang, H. L., Jia, P., Fan, Y. H., Li, M. D., Cao, C. C., Li, Y., et al. (2020). Manual acupuncture or combination with vitamin B to treat diabetic peripheral neuropathy: a systematic review and meta-analysis of randomized controlled trials. Biomed. Res. Int. 2020, 4809125. doi:10.1155/2020/4809125
Jo, S., and Fang, S. (2021). Therapeutic strategies for diabetes: immune modulation in pancreatic β cells. Front. Endocrinol. (Lausanne) 12, 716692. doi:10.3389/fendo.2021.716692
Khosla, S., Farr, J. N., Tchkonia, T., and Kirkland, J. L. (2020). The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 16, 263–275. doi:10.1038/s41574-020-0335-y
Ko, C. H., Yi, S., Ozaki, R., Cochrane, H., Chung, H., Lau, W., et al. (2014). Healing effect of a two-herb recipe (NF3) on foot ulcers in Chinese patients with diabetes: a randomized double-blind placebo-controlled study. J. Diabetes 6, 323–334. doi:10.1111/1753-0407.12117
Koch, C. A., and Fulop, T. (2017). Clinical aspects of changes in water and sodium homeostasis in the elderly. Rev. Endocr. Metab. Disord. 18, 49–66. doi:10.1007/s11154-017-9420-5
Kozma, C. M., Benson, C., Slaton, T. L., Kim, M. S., and Vorsanger, G. J. (2012). Opioids before and after initiation of pregabalin in patients with diabetic peripheral neuropathy. Curr. Med. Res. Opin. 28, 1485–1496. doi:10.1185/03007995.2012.713338
Kumar, P., Liu, C., Suliburk, J., Hsu, J. W., Muthupillai, R., Jahoor, F., et al. (2023). Supplementing Glycine and N-acetylcysteine (GlyNAC) in older adults improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, physical function, and aging hallmarks: a randomized clinical trial. J. Gerontol. A Biol. Sci. Med. Sci. 78, 75–89. doi:10.1093/gerona/glac135
Lahalle, A., Lacroix, M., De Blasio, C., Cissé, M. Y., Linares, L. K., and Le Cam, L. (2021). The p53 pathway and metabolism: the tree that hides the forest. Cancers (Basel) 13, 133. doi:10.3390/cancers13010133
Lee, S. Y., Lee, D. Y., Kang, H. J., Kang, J. H., Cho, M. G., Jang, H. W., et al. (2021). Differences in the gut microbiota between young and elderly persons in Korea. Nutr. Res. 87, 31–40. doi:10.1016/j.nutres.2020.12.013
Leenders, M., Verdijk, L. B., van der Hoeven, L., Adam, J. J., van Kranenburg, J., Nilwik, R., et al. (2013). Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J. Am. Med. Dir. Assoc. 14, 585–592. doi:10.1016/j.jamda.2013.02.006
Leite, M. M., Dutra, M. T., da Costa, M. V. G., Funghetto, S. S., Silva, A. de O., de Lima, L. R., et al. (2021). Comparative evaluation of inflammatory parameters and substitute insulin resistance indices in elderly women with and without type 2 diabetes mellitus. Exp. Gerontol. 150, 111389. doi:10.1016/j.exger.2021.111389
LeRoith, D., Biessels, G. J., Braithwaite, S. S., Casanueva, F. F., Draznin, B., Halter, J. B., et al. (2019). Treatment of diabetes in older adults: an endocrine society* clinical practice guideline. J. Clin. Endocrinol. Metab. 104, 1520–1574. doi:10.1210/jc.2019-00198
Li, C. (2022). Clinical observation on Danhuang MINGMU decoction in the treatment of senile diabetic retinopathy. J. Chengde Med. Univ. 39, 215–218. doi:10.15921/j.cnki.cyxb.2022.03.006
Li, M., Wang, S., Li, Y., Zhao, M., Kuang, J., Liang, D., et al. (2022). Gut microbiota-bile acid crosstalk contributes to the rebound weight gain after calorie restriction in mice. Nat. Commun. 13, 2060. doi:10.1038/s41467-022-29589-7
Li, T., Li, H., Li, W., Chen, S., Feng, T., Jiao, W., et al. (2019). Interleukin-37 sensitize the elderly type 2 diabetic patients to insulin therapy through suppressing the gut microbiota dysbiosis. Mol. Immunol. 112, 322–329. doi:10.1016/j.molimm.2019.06.008
Li, T., Miao, Y., Wang, Y., and Cui, L. (2018). Yunnan journal of traditional Chinese medicine and materia medica. Yunnan J. Traditional Chin. Med. Materia Medica 39, 104–105. doi:10.16254/j.cnki.53-1120/r.2018.04.045
Li, X., Liu, Y., Jing, Z., Fan, B., Pan, W., Mao, S., et al. (2023). Effects of acupuncture therapy in diabetic neuropathic pain: a systematic review and meta-analysis. Complement. Ther. Med. 78, 102992. doi:10.1016/j.ctim.2023.102992
Li, X., Su, C., Jiang, Z., Yang, Y., Zhang, Y., Yang, M., et al. (2021). Berberine attenuates choline-induced atherosclerosis by inhibiting trimethylamine and trimethylamine-N-oxide production via manipulating the gut microbiome. NPJ Biofilms Microbiomes 7, 36. doi:10.1038/s41522-021-00205-8
Li., Y., Chen, Q., Liao, W., Lin, S., Xie, Z., and Tang, Q. (2022). Effect analysis of TCM health management in elderly patients with type 2 diabetes. J. Anhui Med. Coll. 21, 39–41.
Li, Y., Teng, D., Shi, X., Qin, G., Qin, Y., Quan, H., et al. (2020). Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ 369, m997. doi:10.1136/bmj.m997
Li, Z., Yi, C.-X., Katiraei, S., Kooijman, S., Zhou, E., Chung, C. K., et al. (2018). Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 67, 1269–1279. doi:10.1136/gutjnl-2017-314050
Lin, W., Chen, C., Guan, H., Du, X., and Li, J. (2016). Hospitalization of elderly diabetic patients: characteristics, reasons for admission, and gender differences. BMC Geriatr. 16, 160. doi:10.1186/s12877-016-0333-z
Lin, Zi, He, W., Zhang, Fu, Du, S., and Lin, X. (2023). 43 cases of senile type 2 diabetes mellitus with yin deficiency and blood stasis were treated with di Hong Fang. Fujian J. Traditional Chin. Med. 54, 62–64. doi:10.13260/j.cnki.jfjtcm.2023.04021
Ling, C., and Rönn, T. (2019). Epigenetics in human obesity and type 2 diabetes. Cell Metab. 29, 1028–1044. doi:10.1016/j.cmet.2019.03.009
Ling, S., Zaccardi, F., Lawson, C., Seidu, S. I., Davies, M. J., and Khunti, K. (2021). Glucose control, sulfonylureas, and insulin treatment in elderly people with type 2 diabetes and risk of severe hypoglycemia and death: an observational study. Diabetes Care 44, 915–924. doi:10.2337/dc20-0876
Liu, J., Zhang, Y., Sheng, H., Liang, C., Liu, H., Moran Guerrero, J. A., et al. (2021). Hyperoside suppresses renal inflammation by regulating macrophage polarization in mice with type 2 diabetes mellitus. Front. Immunol. 12, 733808. doi:10.3389/fimmu.2021.733808
Liu, X.-J., Hu, X.-K., Yang, H., Gui, L.-M., Cai, Z.-X., Qi, M.-S., et al. (2022). A review of traditional Chinese medicine on treatment of diabetic nephropathy and the involved mechanisms. Am. J. Chin. Med. 50, 1739–1779. doi:10.1142/S0192415X22500744
Lu, Z., Zhong, Y., Liu, W., Xiang, L., and Deng, Y. (2019). The efficacy and mechanism of Chinese herbal medicine on diabetic kidney disease. J. Diabetes Res. 2019, 2697672. doi:10.1155/2019/2697672
Ma, C.-X., Ma, X.-N., Guan, C.-H., Li, Y.-D., Mauricio, D., and Fu, S.-B. (2022). Cardiovascular disease in type 2 diabetes mellitus: progress toward personalized management. Cardiovasc Diabetol. 21, 74. doi:10.1186/s12933-022-01516-6
Ma, J., and Xu, X. (2017). Tongluo Xifeng Decoction in treating senile diabetic combined with acute stroke. Jilin J. Traditional Chin. Med. 37, 133–136. doi:10.13463/j.cnki.jlzyy.2017.02.008
Ma, X., Xu, L., Gavrilova, O., and Mueller, E. (2014). Role of forkhead box protein A3 in age-associated metabolic decline. Proc. Natl. Acad. Sci. U. S. A. 111, 14289–14294. doi:10.1073/pnas.1407640111
Maedler, K., Dharmadhikari, G., Schumann, D. M., and Størling, J. (2009). Interleukin-1 beta targeted therapy for type 2 diabetes. Expert Opin. Biol. Ther. 9, 1177–1188. doi:10.1517/14712590903136688
Makowski, L., Chaib, M., and Rathmell, J. C. (2020). Immunometabolism: from basic mechanisms to translation. Immunol. Rev. 295, 5–14. doi:10.1111/imr.12858
Mangiola, F., Nicoletti, A., Gasbarrini, A., and Ponziani, F. R. (2018). Gut microbiota and aging. Eur. Rev. Med. Pharmacol. Sci. 22, 7404–7413. doi:10.26355/eurrev_201811_16280
Marchesi, N., Fahmideh, F., Pascale, A., Allegri, M., and Govoni, S. (2024). Neuropathic pain in aged people: an unresolved issue open to novel drug approaches, focusing on painful diabetic neuropathy. Curr. Neuropharmacol. 22, 53–64. doi:10.2174/1570159X21666230807103642
Markle-Reid, M., Ploeg, J., Fraser, K. D., Fisher, K. A., Bartholomew, A., Griffith, L. E., et al. (2018). Community program improves quality of life and self-management in older adults with diabetes mellitus and comorbidity. J. Am. Geriatr. Soc. 66, 263–273. doi:10.1111/jgs.15173
Masters, S. L., Dunne, A., Subramanian, S. L., Hull, R. L., Tannahill, G. M., Sharp, F. A., et al. (2010). Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 11, 897–904. doi:10.1038/ni.1935
Mayorga-Ramos, A., Barba-Ostria, C., Simancas-Racines, D., and Guamán, L. P. (2022). Protective role of butyrate in obesity and diabetes: new insights. Front. Nutr. 9, 1067647. doi:10.3389/fnut.2022.1067647
Meng, J., Zhu, Y., Ma, H., Wang, X., and Zhao, Q. (2021). The role of traditional Chinese medicine in the treatment of cognitive dysfunction in type 2 diabetes. J. Ethnopharmacol. 280, 114464. doi:10.1016/j.jep.2021.114464
Meyer-Hamme, G., Friedemann, T., Greten, J., Gerloff, C., and Schroeder, S. (2021). Electrophysiologically verified effects of acupuncture on diabetic peripheral neuropathy in type 2 diabetes: the randomized, partially double-blinded, controlled ACUDIN trial. J. Diabetes 13, 469–481. doi:10.1111/1753-0407.13130
Michot, C., Mamoune, A., Vamecq, J., Viou, M. T., Hsieh, L.-S., Testet, E., et al. (2013). Combination of lipid metabolism alterations and their sensitivity to inflammatory cytokines in human lipin-1-deficient myoblasts. Biochim. Biophys. Acta 1832, 2103–2114. doi:10.1016/j.bbadis.2013.07.021
Mollica, M. P., Mattace Raso, G., Cavaliere, G., Trinchese, G., De Filippo, C., Aceto, S., et al. (2017). Butyrate regulates liver mitochondrial function, efficiency, and dynamics in insulin-resistant obese mice. Diabetes 66, 1405–1418. doi:10.2337/db16-0924
Mordarska, K., and Godziejewska-Zawada, M. (2017). Diabetes in the elderly. pm 2, 38–43. doi:10.5114/pm.2017.68589
Moskalev, A., Stambler, I., and Caruso, C. (2020). Innate and adaptive immunity in aging and longevity: the foundation of resilience. Aging Dis. 11, 1363–1373. doi:10.14336/AD.2020.0603
Motta, M., Bennati, E., Capri, M., Ferlito, L., and Malaguarnera, M. (2008a). Diabetes mellitus in the extreme longevity. Exp. Gerontol. 43, 102–105. doi:10.1016/j.exger.2007.06.012
Motta, M., Bennati, E., Ferlito, L., Passamonte, M., Cardillo, E., and Malaguarnera, M. (2008b). A review on the actual trends of insulin treatment in elderly with diabetes. Arch. Gerontol. Geriatr. 47, 151–161. doi:10.1016/j.archger.2007.07.009
Munshi, M. N., Meneilly, G. S., Rodríguez-Mañas, L., Close, K. L., Conlin, P. R., Cukierman-Yaffe, T., et al. (2020). Diabetes in ageing: pathways for developing the evidence base for clinical guidance. Lancet Diabetes Endocrinol. 8, 855–867. doi:10.1016/S2213-8587(20)30230-8
Narasimhan, A., Flores, R. R., Robbins, P. D., and Niedernhofer, L. J. (2021). Role of cellular senescence in type II diabetes. Endocrinology 162, bqab136. doi:10.1210/endocr/bqab136
Naseri, K., Saadati, S., Sadeghi, A., Asbaghi, O., Ghaemi, F., Zafarani, F., et al. (2022). The efficacy of ginseng (panax) on human prediabetes and type 2 diabetes mellitus: a systematic review and meta-analysis. Nutrients 14, 2401. doi:10.3390/nu14122401
Nayak, A., Liu, C., Mehta, A., Ko, Y.-A., Tahhan, A. S., Dhindsa, D. S., et al. (2020). N8-Acetylspermidine: a polyamine biomarker in ischemic cardiomyopathy with reduced ejection fraction. J. Am. Heart Assoc. 9, e016055. doi:10.1161/JAHA.120.016055
Ni, Y., Mu, C., He, X., Zheng, K., Guo, H., and Zhu, W. (2018). Characteristics of gut microbiota and its response to a Chinese Herbal Formula in elder patients with metabolic syndrome. Drug Discov. Ther. 12, 161–169. doi:10.5582/ddt.2018.01036
Nie, Q., Chen, H., Hu, J., Fan, S., and Nie, S. (2019). Dietary compounds and traditional Chinese medicine ameliorate type 2 diabetes by modulating gut microbiota. Crit. Rev. Food Sci. Nutr. 59, 848–863. doi:10.1080/10408398.2018.1536646
Niu, W., Miao, J., Li, X., Guo, Q., Zhang, N., Deng, Z., et al. (2023). Combined systematic pharmacology and urine metabonomics to study the therapeutic mechanism of type 2 diabetic treated with the herbal pair of Salvia miltiorrhiza Bunge and Pueraria Montana var. lobata (Willd.) Sanjappa and Pradeep. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1217, 123627. doi:10.1016/j.jchromb.2023.123627
Noale, M., Veronese, N., Cavallo Perin, P., Pilotto, A., Tiengo, A., Crepaldi, G., et al. (2016). Polypharmacy in elderly patients with type 2 diabetes receiving oral antidiabetic treatment. Acta Diabetol. 53, 323–330. doi:10.1007/s00592-015-0790-4
Nolfi-Donegan, D., Braganza, A., and Shiva, S. (2020). Mitochondrial electron transport chain: oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 37, 101674. doi:10.1016/j.redox.2020.101674
Noureldein, M. H., Bitar, S., Youssef, N., Azar, S., and Eid, A. A. (2020). Butyrate modulates diabetes-linked gut dysbiosis: epigenetic and mechanistic modifications. J. Mol. Endocrinol. 64, 29–42. doi:10.1530/JME-19-0132
Ohara, Y., Hirano, H., Yoshida, H., Obuchi, S., Ihara, K., Fujiwara, Y., et al. (2016). Prevalence and factors associated with xerostomia and hyposalivation among community-dwelling older people in Japan. Gerodontology 33, 20–27. doi:10.1111/ger.12101
Osborn, O., and Olefsky, J. M. (2012). The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 18, 363–374. doi:10.1038/nm.2627
Ou, M.-Y., Zhang, H., Tan, P.-C., Zhou, S.-B., and Li, Q.-F. (2022). Adipose tissue aging: mechanisms and therapeutic implications. Cell Death Dis. 13, 300. doi:10.1038/s41419-022-04752-6
Ouwehand, A. C., Bergsma, N., Parhiala, R., Lahtinen, S., Gueimonde, M., Finne-Soveri, H., et al. (2008). Bifidobacterium microbiota and parameters of immune function in elderly subjects. FEMS Immunol. Med. Microbiol. 53, 18–25. doi:10.1111/j.1574-695X.2008.00392.x
Özcan, S., Alessio, N., Acar, M. B., Mert, E., Omerli, F., Peluso, G., et al. (2016). Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging (Albany NY) 8, 1316–1329. doi:10.18632/aging.100971
Padhi, S., Nayak, A. K., and Behera, A. (2020). Type II diabetes mellitus: a review on recent drug based therapeutics. Biomed. Pharmacother. 131, 110708. doi:10.1016/j.biopha.2020.110708
Palmer, A. K., Gustafson, B., Kirkland, J. L., and Smith, U. (2019). Cellular senescence: at the nexus between ageing and diabetes. Diabetologia 62, 1835–1841. doi:10.1007/s00125-019-4934-x
Palmer, A. K., and Kirkland, J. L. (2016). Aging and adipose tissue: potential interventions for diabetes and regenerative medicine. Exp. Gerontol. 86, 97–105. doi:10.1016/j.exger.2016.02.013
Paone, P., and Cani, P. D. (2020). Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut 69, 2232–2243. doi:10.1136/gutjnl-2020-322260
Prasun, P. (2020). Mitochondrial dysfunction in metabolic syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165838. doi:10.1016/j.bbadis.2020.165838
Prause, M., Pedersen, S. S., Tsonkova, V., Qiao, M., and Billestrup, N. (2021). Butyrate protects pancreatic beta cells from cytokine-induced dysfunction. Int. J. Mol. Sci. 22, 10427. doi:10.3390/ijms221910427
Rafiullah, M., and Siddiqui, K. (2022). Pharmacological treatment of diabetic peripheral neuropathy: an update. CNS Neurol. Disord. Drug Targets 21, 884–900. doi:10.2174/1871527320666210303111939
Rajpal, A., Sayyed Kassem, L., and Aron, D. C. (2021). Management of diabetes in elderly patients during the COVID-19 pandemic: current and future perspectives. Expert Rev. Endocrinol. Metab. 16, 181–189. doi:10.1080/17446651.2021.1927708
Roshanravan, N., Askari, S. F., Fazelian, S., Ayati, M. H., and Namazi, N. (2023). The roles of quercetin in diabetes mellitus and related metabolic disorders; special focus on the modulation of gut microbiota: a comprehensive review. Crit. Rev. Food Sci. Nutr. 63, 2990–3003. doi:10.1080/10408398.2021.1983765
SantaCruz-Calvo, S., Bharath, L., Pugh, G., SantaCruz-Calvo, L., Lenin, R. R., Lutshumba, J., et al. (2022). Adaptive immune cells shape obesity-associated type 2 diabetes mellitus and less prominent comorbidities. Nat. Rev. Endocrinol. 18, 23–42. doi:10.1038/s41574-021-00575-1
Saukkonen, T., Mutt, S. J., Jokelainen, J., Saukkonen, A.-M., Raza, G. S., Karhu, T., et al. (2018). Adipokines and inflammatory markers in elderly subjects with high risk of type 2 diabetes and cardiovascular disease. Sci. Rep. 8, 12816. doi:10.1038/s41598-018-31144-8
Scheen, A. J., Paquot, N., and Bauduceau, B. (2014). Diabetes mellitus in the elderly: from the epidemiological challenge to a personalized approach. Rev. Med. Liege 69, 323–328.
Schiffrin, E. J., Thomas, D. R., Kumar, V. B., Brown, C., Hager, C., Van’t Hof, M. A., et al. (2007). Systemic inflammatory markers in older persons: the effect of oral nutritional supplementation with prebiotics. J. Nutr. Health Aging 11, 475–479.
Seyedizadeh, S. H., Cheragh-Birjandi, S., and Hamedi Nia, M. R. (2020). The effects of combined exercise training (Resistance-Aerobic) on serum kinesin and physical function in type 2 diabetes patients with diabetic peripheral neuropathy (randomized controlled trials). J. Diabetes Res. 2020, 6978128. doi:10.1155/2020/6978128
Shen, Q., Fang, J., Guo, H., Su, X., Zhu, B., Yao, X., et al. (2023a). Astragaloside IV attenuates podocyte apoptosis through ameliorating mitochondrial dysfunction by up-regulated Nrf2-ARE/TFAM signaling in diabetic kidney disease. Free Radic. Biol. Med. 203, 45–57. doi:10.1016/j.freeradbiomed.2023.03.022
Shen, Y., Shi, Q., Nong, K., Li, S., Yue, J., Huang, J., et al. (2023b). Exercise for sarcopenia in older people: a systematic review and network meta-analysis. J. Cachexia Sarcopenia Muscle 14, 1199–1211. doi:10.1002/jcsm.13225
Shoelson, S. E., Lee, J., and Goldfine, A. B. (2006). Inflammation and insulin resistance. J. Clin. Invest 116, 1793–1801. doi:10.1172/JCI29069
Sinclair, A., Dunning, T., and Rodriguez-Mañas, L. (2015). Diabetes in older people: new insights and remaining challenges. Lancet Diabetes Endocrinol. 3, 275–285. doi:10.1016/S2213-8587(14)70176-7
Singh, R., Chandel, S., Dey, D., Ghosh, A., Roy, S., Ravichandiran, V., et al. (2020). Epigenetic modification and therapeutic targets of diabetes mellitus. Biosci. Rep. 40, BSR20202160. doi:10.1042/BSR20202160
Singh, T., and Newman, A. B. (2011). Inflammatory markers in population studies of aging. Ageing Res. Rev. 10, 319–329. doi:10.1016/j.arr.2010.11.002
Sodhi, B., Malik, M., Agarwal, P., and Basu, S. (2023). The prevalence and predictors of depression and disability in older adults and elderly patients with Diabetes in India: cross-sectional analysis from the Longitudinal Study on Ageing. Diabetes Metab. Syndr. 17, 102765. doi:10.1016/j.dsx.2023.102765
Stachowska, E., Wiśniewska, M., Dzieżyc, A., and Bohatyrewicz, A. (2021). Could the use of butyric acid have a positive effect on microbiota and treatment of type 2 diabetes? Eur. Rev. Med. Pharmacol. Sci. 25, 4570–4578. doi:10.26355/eurrev_202107_26250
St-Jean-Pelletier, F., Pion, C. H., Leduc-Gaudet, J.-P., Sgarioto, N., Zovilé, I., Barbat-Artigas, S., et al. (2017). The impact of ageing, physical activity, and pre-frailty on skeletal muscle phenotype, mitochondrial content, and intramyocellular lipids in men. J. Cachexia Sarcopenia Muscle 8, 213–228. doi:10.1002/jcsm.12139
Suárez, R., Chapela, S. P., Álvarez-Córdova, L., Bautista-Valarezo, E., Sarmiento-Andrade, Y., Verde, L., et al. (2023). Epigenetics in obesity and diabetes mellitus: new insights. Nutrients 15, 811. doi:10.3390/nu15040811
Suda, M., Shimizu, I., Katsuumi, G., Yoshida, Y., Hayashi, Y., Ikegami, R., et al. (2021). Senolytic vaccination improves normal and pathological age-related phenotypes and increases lifespan in progeroid mice. Nat. Aging 1, 1117–1126. doi:10.1038/s43587-021-00151-2
Sun, L., Yang, Z., Zhao, W., Chen, Q., Bai, H., Wang, S., et al. (2022). Integrated lipidomics, transcriptomics and network pharmacology analysis to reveal the mechanisms of Danggui Buxue Decoction in the treatment of diabetic nephropathy in type 2 diabetes mellitus. J. Ethnopharmacol. 283, 114699. doi:10.1016/j.jep.2021.114699
Tall, A. R., and Yvan-Charvet, L. (2015). Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 15, 104–116. doi:10.1038/nri3793
Tang, C., Bao, T., Zhang, Q., Qi, H., Huang, Y., Zhang, B., et al. (2023). Clinical potential and mechanistic insights of mulberry (Morus alba L.) leaves in managing type 2 diabetes mellitus: focusing on gut microbiota, inflammation, and metabolism. J. Ethnopharmacol. 306, 116143. doi:10.1016/j.jep.2023.116143
Tang, G., Li, S., Zhang, C., Chen, H., Wang, N., and Feng, Y. (2021). Clinical efficacies, underlying mechanisms and molecular targets of Chinese medicines for diabetic nephropathy treatment and management. Acta Pharm. Sin. B 11, 2749–2767. doi:10.1016/j.apsb.2020.12.020
Tchkonia, T., Palmer, A. K., and Kirkland, J. L. (2021). New horizons: novel approaches to enhance healthspan through targeting cellular senescence and related aging mechanisms. J. Clin. Endocrinol. Metab. 106, e1481–e1487. doi:10.1210/clinem/dgaa728
Tekin, Z., and Zimmerman, R. S. (2020). Noninsulin diabetes therapies in older adults. Clin. Geriatr. Med. 36, 385–394. doi:10.1016/j.cger.2020.03.001
Terlecki-Zaniewicz, L., Lämmermann, I., Latreille, J., Bobbili, M. R., Pils, V., Schosserer, M., et al. (2018). Small extracellular vesicles and their miRNA cargo are anti-apoptotic members of the senescence-associated secretory phenotype. Aging (Albany NY) 10, 1103–1132. doi:10.18632/aging.101452
Tian, J., Bai, B., Gao, Z., Yang, Y., Wu, H., Wang, X., et al. (2021). Alleviation effects of GQD, a traditional Chinese medicine formula, on diabetes rats linked to modulation of the gut microbiome. Front. Cell Infect. Microbiol. 11, 740236. doi:10.3389/fcimb.2021.740236
Tian, J., Jin, D., Bao, Q., Ding, Q., Zhang, H., Gao, Z., et al. (2019). Evidence and potential mechanisms of traditional Chinese medicine for the treatment of type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes. Metab. 21, 1801–1816. doi:10.1111/dom.13760
Tian, J., Lian, F., Yang, L., and Tong, X. (2018). Evaluation of the Chinese herbal medicine Jinlida in type 2 diabetes patients based on stratification: results of subgroup analysis from a 12-week trial. J. Diabetes 10, 112–120. doi:10.1111/1753-0407.12559
Tong, X., Xu, J., Lian, F., Yu, X., Zhao, Y., Xu, L., et al. (2018). Structural alteration of gut microbiota during the amelioration of human type 2 diabetes with hyperlipidemia by metformin and a traditional Chinese herbal formula: a multicenter, randomized, open label clinical trial. mBio 9, 023922–e2417. doi:10.1128/mBio.02392-17
Tong, X.-L., Dong, L., Chen, L., and Zhen, Z. (2012). Treatment of diabetes using traditional Chinese medicine: past, present and future. Am. J. Chin. Med. 40, 877–886. doi:10.1142/S0192415X12500656
Tong, X.-L., Zhao, L.-H., Lian, F.-M., Zhou, Q., Xia, L., Zhang, J.-C., et al. (2011). Clinical observations on the dose-effect relationship of gegen qin lian decoction on 54 out-patients with type 2 diabetes. J. Tradit. Chin. Med. 31, 56–59. doi:10.1016/s0254-6272(11)60013-7
Tsai, F.-J., Ho, T.-J., Cheng, C.-F., Liu, X., Tsang, H., Lin, T.-H., et al. (2017). Effect of Chinese herbal medicine on stroke patients with type 2 diabetes. J. Ethnopharmacol. 200, 31–44. doi:10.1016/j.jep.2017.02.024
Tsai, H.-J., Tsai, W.-C., Hung, W.-C., Hung, W.-W., Chang, C.-C., Dai, C.-Y., et al. (2021). Gut microbiota and subclinical cardiovascular disease in patients with type 2 diabetes mellitus. Nutrients 13, 2679. doi:10.3390/nu13082679
Tsai, Y.-Y., Chen, K.-J., Yang, Y.-H., and Lin, Y.-H. (2020). Use of traditional Chinese medicine may delay the need for insulin treatment in patients with type 2 diabetes: a population-based cohort study. J. Altern. Complement. Med. 26, 628–635. doi:10.1089/acm.2019.0375
Umpierrez, G. E., and Pasquel, F. J. (2017). Management of inpatient hyperglycemia and diabetes in older adults. Diabetes Care 40, 509–517. doi:10.2337/dc16-0989
Uno, T., Ohsawa, I., Tokudome, M., and Sato, Y. (2005). Effects of Goshajinkigan on insulin resistance in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 69, 129–135. doi:10.1016/j.diabres.2004.11.017
van der Putten, G. J., Brand, H. S., Bots, C. P., and van Nieuw Amerongen, A. (2003). Prevalence of xerostomia and hyposalivation in the nursing home and the relation with number of prescribed medication. Tijdschr. Gerontol. Geriatr. 34, 30–36.
Vergoni, B., Cornejo, P.-J., Gilleron, J., Djedaini, M., Ceppo, F., Jacquel, A., et al. (2016). DNA damage and the activation of the p53 pathway mediate alterations in metabolic and secretory functions of adipocytes. Diabetes 65, 3062–3074. doi:10.2337/db16-0014
Vischer, U.-M., Bauduceau, B., Bourdel-Marchasson, I., Blickle, J.-F., Constans, T., Fagot-Campagna, A., et al. (2009). A call to incorporate the prevention and treatment of geriatric disorders in the management of diabetes in the elderly. Diabetes Metab. 35, 168–177. doi:10.1016/j.diabet.2009.02.003
Wallander, M., Axelsson, K. F., Nilsson, A. G., Lundh, D., and Lorentzon, M. (2017). Type 2 diabetes and risk of hip fractures and non-skeletal fall injuries in the elderly: a study from the fractures and fall injuries in the elderly cohort (frailco). J. Bone Min. Res. 32, 449–460. doi:10.1002/jbmr.3002
Wang, E., Wang, L., Ding, R., Zhai, M., Ge, R., Zhou, P., et al. (2020a). Astragaloside IV acts through multi-scale mechanisms to effectively reduce diabetic nephropathy. Pharmacol. Res. 157, 104831. doi:10.1016/j.phrs.2020.104831
Wang, J., Ma, Q., Li, Y., Li, P., Wang, M., Wang, T., et al. (2020b). Research progress on Traditional Chinese Medicine syndromes of diabetes mellitus. Biomed. Pharmacother. 121, 109565. doi:10.1016/j.biopha.2019.109565
Wang, J., Zhu, N., Su, X., Gao, Y., and Yang, R. (2023). Gut-microbiota-derived metabolites maintain gut and systemic immune homeostasis. Cells 12, 793. doi:10.3390/cells12050793
Wang, L., Feng, F., Zhou, J., Yuan, R., Zha, M., Pu, Q., et al. (2023). Short-term effect of Qi-Kui granules combined with dulaglutide in the treatment of clinical stage of diabetic kidney disease in the elderly. Pract. Geriatr. 37, 348–351.
Wang, L., Wang, B., Gasek, N. S., Zhou, Y., Cohn, R. L., Martin, D. E., et al. (2022a). Targeting p21Cip1 highly expressing cells in adipose tissue alleviates insulin resistance in obesity. Cell Metab. 34, 75–89.e8. doi:10.1016/j.cmet.2021.11.002
Wang, L., Zhang, D., Zhan, W., Zeng, Z., Yin, J., Wang, K., et al. (2022b). Chinese medicine Fufang Zhenzhu Tiaozhi capsule ameliorates coronary atherosclerosis in diabetes mellitus-related coronary heart disease minipigs. Biomed. Pharmacother. 156, 113831. doi:10.1016/j.biopha.2022.113831
Wang, M., Wang, Z., Zhou, J., Sun, W., Wang, Y., Han, M., et al. (2018). Effects of traditional Chinese herbal medicine in patients with diabetic kidney disease: study protocol for a randomized controlled trial. Trials 19, 389. doi:10.1186/s13063-018-2749-6
Wang, W., Ishibashi, J., Trefely, S., Shao, M., Cowan, A. J., Sakers, A., et al. (2019). A PRDM16-driven metabolic signal from adipocytes regulates precursor cell fate. Cell Metab. 30, 174–189. doi:10.1016/j.cmet.2019.05.005
Wang, X., Bao, W., Liu, J., Ouyang, Y.-Y., Wang, D., Rong, S., et al. (2013). Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 36, 166–175. doi:10.2337/dc12-0702
Wang, Y. (2020). Clinical analysis of supplemented Shenqi Dihuang Decoction in treating senile diabetes mellitus in community. Cardiovasc. Dis. Electron. J. Integr. Traditional Chin. ;Western Med. 8, 161. doi:10.16282/j.cnki.cn11-9336/r.2020.12.145
Wang, Z., Du, H., Peng, W., Yang, S., Feng, Y., Ouyang, H., et al. (2022c). Efficacy and mechanism of pueraria lobata and pueraria thomsonii polysaccharides in the treatment of type 2 diabetes. Nutrients 14, 3926. doi:10.3390/nu14193926
Wen, H., Gris, D., Lei, Y., Jha, S., Zhang, L., Huang, M. T.-H., et al. (2011). Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 12, 408–415. doi:10.1038/ni.2022
Wen, H., Miao, E. A., and Ting, J. P.-Y. (2013). Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity 39, 432–441. doi:10.1016/j.immuni.2013.08.037
Wen, H., Ting, J. P.-Y., and O’Neill, L. A. J. (2012). A role for the NLRP3 inflammasome in metabolic diseases--did Warburg miss inflammation? Nat. Immunol. 13, 352–357. doi:10.1038/ni.2228
Weng, S.-W., Chang, C.-C., Chen, T.-L., Yeh, C.-C., Hu, C.-J., Lane, H.-L., et al. (2021). Risk of diabetes in stroke patients who used Bu Yang Huan Wu Tang: a nationwide propensity-score matched study. Phytomedicine 80, 153376. doi:10.1016/j.phymed.2020.153376
Wiertsema, S. P., van Bergenhenegouwen, J., Garssen, J., and Knippels, L. M. J. (2021). The interplay between the gut microbiome and the immune system in the context of infectious diseases throughout life and the role of nutrition in optimizing treatment strategies. Nutrients 13, 886. doi:10.3390/nu13030886
Williams, M. E. (2013). Diabetic kidney disease in elderly individuals. Med. Clin. North Am. 97, 75–89. doi:10.1016/j.mcna.2012.10.011
Wu, C.-T., Tsai, Y.-T., Lin, J.-G., Fu, S.-L., and Lai, J.-N. (2018). Chinese herbal products and the reduction of risk of breast cancer among females with type 2 diabetes in Taiwan: a case-control study. Med. Baltim. 97, e11600. doi:10.1097/MD.0000000000011600
Wu, F., Xiao, Y., and Xie, L. (2021). Study on clinical effect of traditional Chinese medicine intervention on elderly patients with diabetes of phlegm and dampness constitution. Chin. J. Mod. Drug Appl. 15, 197–199. doi:10.14164/j.cnki.cn11-5581/r.2021.16.077
Wu, Q., Dong, J., Bai, X., Jiang, Y., Li, J., Fan, S., et al. (2022). Propionate ameliorates diabetes-induced neurological dysfunction through regulating the PI3K/Akt/eNOS signaling pathway. Eur. J. Pharmacol. 925, 174974. doi:10.1016/j.ejphar.2022.174974
Xiao, E., and Luo, L. (2018). Alternative therapies for diabetes: a comparison of western and traditional Chinese medicine (TCM) approaches. Curr. Diabetes Rev. 14, 487–496. doi:10.2174/1573399813666170519103230
Xiao, S., Liu, C., Chen, M., Zou, J., Zhang, Z., Cui, X., et al. (2020). Scutellariae radix and coptidis rhizoma ameliorate glycolipid metabolism of type 2 diabetic rats by modulating gut microbiota and its metabolites. Appl. Microbiol. Biotechnol. 104, 303–317. doi:10.1007/s00253-019-10174-w
Xie, Z., Loi Truong, T., Zhang, P., Xu, F., Xu, X., and Li, P. (2015). Dan-Qi prescription ameliorates insulin resistance through overall corrective regulation of glucose and fat metabolism. J. Ethnopharmacol. 172, 70–79. doi:10.1016/j.jep.2015.05.041
Xiong, W., Ma, Z., An, D., Liu, Z., Cai, W., Bai, Y., et al. (2019). Mitofusin 2 participates in mitophagy and mitochondrial fusion against angiotensin II-induced cardiomyocyte injury. Front. Physiol. 10, 411. doi:10.3389/fphys.2019.00411
Xu, H. (2019). Effects of sini decoction on glucose metabolism, lipid metabolism and glucose transporter 4 in elderly patients with type 2 diabetes mellitus. Electron. J. Clin. Med. Literature 6, 1–2+4. doi:10.16281/j.cnki.jocml.2019.64.001
Xu, J., Lian, F., Zhao, L., Zhao, Y., Chen, X., Zhang, X., et al. (2015). Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J. 9, 552–562. doi:10.1038/ismej.2014.177
Xu, S., Li, X., Zhang, S., Qi, C., Zhang, Z., Ma, R., et al. (2023). Oxidative stress gene expression, DNA methylation, and gut microbiota interaction trigger Crohn’s disease: a multi-omics Mendelian randomization study. BMC Med. 21, 179. doi:10.1186/s12916-023-02878-8
Xu, X., Gao, Z., Yang, F., Yang, Y., Chen, L., Han, L., et al. (2020). Antidiabetic effects of gegen qinlian decoction via the gut microbiota are attributable to its key ingredient berberine. Genomics Proteomics Bioinforma. 18, 721–736. doi:10.1016/j.gpb.2019.09.007
Yang, K., Wang, Y., Li, Y.-W., Chen, Y.-G., Xing, N., Lin, H.-B., et al. (2022). Progress in the treatment of diabetic peripheral neuropathy. Biomed. Pharmacother. 148, 112717. doi:10.1016/j.biopha.2022.112717
Yang, W., Li, Y., Wang, J.-Y., Han, R., and Wang, L. (2018). Circulating levels of adipose tissue-derived inflammatory factors in elderly diabetes patients with carotid atherosclerosis: a retrospective study. Cardiovasc Diabetol. 17, 75. doi:10.1186/s12933-018-0723-y
Yang, X.-D., and Yang, Y.-Y. (2022). Ferroptosis as a novel therapeutic target for diabetes and its complications. Front. Endocrinol. (Lausanne) 13, 853822. doi:10.3389/fendo.2022.853822
Yao, Y., Yan, L., Chen, H., Wu, N., Wang, W., and Wang, D. (2020). Cyclocarya paliurus polysaccharides alleviate type 2 diabetic symptoms by modulating gut microbiota and short-chain fatty acids. Phytomedicine 77, 153268. doi:10.1016/j.phymed.2020.153268
Yi, H., Peng, H., Wu, X., Xu, X., Kuang, T., Zhang, J., et al. (2021). The therapeutic effects and mechanisms of quercetin on metabolic diseases: pharmacological data and clinical evidence. Oxid. Med. Cell Longev. 2021, 6678662. doi:10.1155/2021/6678662
Yin, H., Huang, Z., Niu, S., Ming, L., Jiang, H., Gu, L., et al. (2022). 5-Methylcytosine (m5C) modification in peripheral blood immune cells is a novel non-invasive biomarker for colorectal cancer diagnosis. Front. Immunol. 13, 967921. doi:10.3389/fimmu.2022.967921
Yingrui, W., Zheng, L., Guoyan, L., and Hongjie, W. (2022). Research progress of active ingredients of Scutellaria baicalensis in the treatment of type 2 diabetes and its complications. Biomed. Pharmacother. 148, 112690. doi:10.1016/j.biopha.2022.112690
Yoshimoto, S., Mitsuyama, E., Yoshida, K., Odamaki, T., and Xiao, J.-Z. (2021). Enriched metabolites that potentially promote age-associated diseases in subjects with an elderly-type gut microbiota. Gut Microbes 13, 1–11. doi:10.1080/19490976.2020.1865705
Yousefzadeh, M. J., Flores, R. R., Zhu, Y., Schmiechen, Z. C., Brooks, R. W., Trussoni, C. E., et al. (2021). An aged immune system drives senescence and ageing of solid organs. Nature 594, 100–105. doi:10.1038/s41586-021-03547-7
Zhang, B., Huang, Y., Jin, Z., Zhao, L., and Wang, L. (2021a). Professor Tong Xiaolin’s experience in the treatment of senile diabetes with the syndrome of qi deficiency and yang debility. Jilin J. Chin. Med. 41, 865–867. doi:10.13463/j.cnki.jlzyy.2021.07.007
Zhang, L., Du, J., Yano, N., Wang, H., Zhao, Y. T., Dubielecka, P. M., et al. (2017). Sodium butyrate protects -against high fat diet-induced cardiac dysfunction and metabolic disorders in type II diabetic mice. J. Cell Biochem. 118, 2395–2408. doi:10.1002/jcb.25902
Zhang, L., Han, L., Wang, X., Wei, Y., Zheng, J., Zhao, L., et al. (2021b). Exploring the mechanisms underlying the therapeutic effect of Salvia miltiorrhiza in diabetic nephropathy using network pharmacology and molecular docking. Biosci. Rep. 41, BSR20203520. doi:10.1042/BSR20203520
Zhang, W.-Q., Zhao, T.-T., Gui, D.-K., Gao, C.-L., Gu, J.-L., Gan, W.-J., et al. (2019). Sodium butyrate improves liver glycogen metabolism in type 2 diabetes mellitus. J. Agric. Food Chem. 67, 7694–7705. doi:10.1021/acs.jafc.9b02083
Zhang, Z.-L., Ji, X.-Q., Zhao, S.-H., Zhang, J.-J., Kang, T., and Yang, X.-J. (2008). Randomized controlled study on effects of the needling method for regulating spleen-stomach on coronary heart disease complicated by type 2 diabetes mellitus complicated. Zhongguo Zhen Jiu 28, 629–633.
Zheng, S., Huang, K., and Tong, T. (2021a). Efficacy and mechanisms of oleuropein in mitigating diabetes and diabetes complications. J. Agric. Food Chem. 69, 6145–6155. doi:10.1021/acs.jafc.1c01404
Zheng, T., Qin, L., Chen, B., Hu, X., Zhang, X., Liu, Y., et al. (2016). Association of plasma DPP4 activity with mild cognitive impairment in elderly patients with type 2 diabetes: results from the GDMD study in China. Diabetes Care 39, 1594–1601. doi:10.2337/dc16-0316
Zheng, Y., Ding, Q., Wei, Y., Gou, X., Tian, J., Li, M., et al. (2021b). Effect of traditional Chinese medicine on gut microbiota in adults with type 2 diabetes: a systematic review and meta-analysis. Phytomedicine 88, 153455. doi:10.1016/j.phymed.2020.153455
Zhong, L., Shi, C., Hou, Q., Yang, R., Li, M., and Fu, X. (2022). Promotive effects of four herbal medicine ARCC on wound healing in mice and human. Health Sci. Rep. 5, e494. doi:10.1002/hsr2.494
Zhong, W. (2018). Analysis of the effect of Zhenwu decoction plus and minus in the treatment of type 2 diabetic retinopathy in the elderly. J. China Prescr. Drug 16, 115–116.
Zhou, L., Li, X., Li, S., Wen, X., Peng, Y., and Zhao, L. (2021). Relationship between dietary choline intake and diabetes mellitus in the national health and nutrition examination survey 2007-2010. J. Diabetes 13, 554–561. doi:10.1111/1753-0407.13143
Zhou, P., Xie, W., He, S., Sun, Y., Meng, X., Sun, G., et al. (2019). Ginsenoside Rb1 as an anti-diabetic agent and its underlying mechanism analysis. Cells 8, 204. doi:10.3390/cells8030204
Zhuang, L., Chen, H., Zhang, S., Zhuang, J., Li, Q., and Feng, Z. (2019). Intestinal microbiota in early life and its implications on childhood health. Genomics Proteomics Bioinforma. 17, 13–25. doi:10.1016/j.gpb.2018.10.002
Zhuang, W. (2019). Effects of sini decoction on glucose metabolism, lipid metabolism and glucose transporter 4 in elderly patients with type 2 diabetes mellitus. Electron. J. Clin. Med. Literature 6, 23. doi:10.16281/j.cnki.jocml.2019.61.017
Keywords: elderly diabetes, traditional Chinese medicine, clinical manifestation, pathogenic mechanism, treatment
Citation: Yang X, Xue C, Chen K, Gao D, Wang H and Tang C (2024) Characteristics of elderly diabetes patients: focus on clinical manifestation, pathogenic mechanism, and the role of traditional Chinese medicine. Front. Pharmacol. 14:1339744. doi: 10.3389/fphar.2023.1339744
Received: 16 November 2023; Accepted: 28 December 2023;
Published: 11 January 2024.
Edited by:
Guanhu Yang, Ohio University, United StatesReviewed by:
Nicoletta Marchesi, University of Pavia, ItalyYing Zou, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, China
Copyright © 2024 Yang, Xue, Chen, Gao, Wang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng Tang, dGFuZ2NoZW5nMDcxOUBidWNtLmVkdS5jbg==; Han Wang, d2FuZ2hhbjQzMTNAYnVjbS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Xiaofei Yang
Xiaofei Yang Chongxiang Xue
Chongxiang Xue Keyu Chen
Keyu Chen Dongyang Gao2
Dongyang Gao2 Han Wang
Han Wang Cheng Tang
Cheng Tang