- 1Department of Neurology, Peking University Aerospace School of Clinical Medicine (Aerospace Center Hospital), Beijing, China
- 2Department of Pediatrics, Peking University First Hospital, Beijing, China
- 3Department of Neurology, Peking University First Hospital, Beijing, China
Objectives: We compared and ranked the efficacy and tolerability of multiple prophylactic treatments for vestibular migraine (VM), including β-blockers, calcium channel blockers, antiseizure medications, and antidepressants such as tricyclics and serotonin–noradrenaline reuptake inhibitors.
Methods: PubMed, Web of Science, Embase, and Cochrane Center for Clinical Trials were systematically searched for relevant randomized clinical trials (RCTs) from March 2023 to May 2023. Studies on the efficacy and tolerability of prophylactic treatments for VM were included. Efficacy was measured using the average vertigo frequency per month and dizziness handicap inventory (DHI) improvement after 3–6 months of treatment. Tolerability was measured by the number of patients reporting at least one adverse event (AE). Network meta-analyses were performed according to a Bayesian framework and a random-effects model based on odds ratios or mean differences (MDs) and 95% confidence intervals (CIs). A sequence of ranking probability was calculated according to the surface under the cumulative ranking (SUCRA) curve. This network meta-analysis was previously registered with PROSPERO (CRD42023422258).
Results: Five RCTs comprising 334 patients were analyzed by synthesizing the published evidence. Considering the examined prophylactic therapies, there is significant evidence that valproate acid (VPA) is superior to placebo or abortive treatment alone (MD = −4.12, 95% CI = −8.09, −0.15) in reducing the frequency of vertigo. Flunarizine (MD = 20.00, 95% CI = 10.90, 29.10), valproate acid (MD = 18.88, 95% CI = 10.42, 27.34), and venlafaxine (MD = 11.48, 95% CI = 9.84, 13.12) were significantly more effective than placebo or abortive treatment in reducing DHI. VPA most strongly reduced the frequency of vertigo according to SUCRA, but it ranked third-to-last in tolerability. Flunarizine ranked best in DHI improvement but worst in tolerability. Metoprolol ranked worst for efficacy but best for tolerability.
Conclusion: VPA and flunarizine reduced the frequency of vertigo and improved DHI, but they had unfavorable tolerability. The effects of metoprolol on vertigo require further study. Given the low certainty and limited sample, additional head-to-head RCTs are warranted to further confirm efficacy.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/; Identifier CRD42023422258.
Introduction
Vestibular migraine (VM) is an underdiagnosed but increasingly recognized central condition that is characterized by recurrent vertigo attacks accompanied by migraine symptoms (Authors Anonymous, 2018). As the most common cause of episodic vertigo in both adults and children, VM affects up to 1.0%–2.7% of the population (Beh, 2022). Also previously known as “migrainous vertigo,” “migraine-associated vertigo,” “migraine-associated dizziness,” “migraine-anxiety-associated dizziness,” and “migraine-related vestibulopathy,” the term “vestibular migraine” has been accepted by the International Classification of Headaches as the unifying terminology that identifies both vestibular and migraine symptoms (Smyth et al., 2022).
The current consensus criteria for the diagnosis of VM were first published in 2012 by the International Bárány Society (Lempert et al., 2022). The current criteria are as follows: at least five episodes of vestibular symptoms (vertigo or dizziness) lasting between 5 min and 72 h, current or previous history of migraine with or without aura, one or more migraine features with at least 50% of the vestibular episodes, and not better explained by another diagnosis (Authors Anonymous, 2018).
The underlying pathophysiology of VM remains poorly understood, and most of the hypotheses are based on the established knowledge of migraine headaches. However, numerous theories have been formulated to explain the pathogenesis of VM, including cortical spreading depression, transmitter/vascular/inflammatory mechanisms, genetic predisposition, and functional brain changes (Furman et al., 2013). It has been suggested that brainstem vestibular nuclei and trigeminal nociceptive inputs are also involved in the pathogenesis of VM (Espinosa-Sanchez and Lopez-Escamez, 2015). Lastly, as some neurotransmitters (e.g., serotonin, noradrenaline, and dopamine) might be involved in the pathogenesis of VM, they could represent treatment options for this condition (Balaban, 2011).
Because current migraine treatment guidelines are based on migraines for which the ability of interventions to control vestibular symptoms was not assessed, there remains a clinical need for pragmatic management guidelines specific for VM using the available evidence.
Current pharmacological therapies can either be prophylactic to reduce the frequency and severity of future episodes or abortive to alleviate an acute attack. Because there are limited studies evaluating the efficacy of abortive treatments, this systematic review focuses on a comparison of prophylactic treatments.
There are various prophylactic treatment options addressing multiple aspects of VM, including β-blockers (such as propranolol and metoprolol), calcium channel blockers (such as flunarizine), antiseizure medications (such as topiramate and sodium valproate), antidepressants (such as venlafaxine), and emerging new drugs such as monoclonal antibodies against CGRP (such as erenumab, fremanezumab, and galcanezumab) (Smyth et al., 2022; Webster et al., 2023). As the mechanisms of action of these treatments are being studied and potential new targets are emerging, we can foresee a prospective future for the treatment of VM.
Because most previous studies comparing existing VM treatments on vestibular symptoms were observational, our systematic review was limited regarding the number of randomized clinical trials (RCTs) that compared these treatments. However, to the best of our knowledge, this is the first network meta-analysis (NMA) synthesizing established RCTs for VM and evaluating the efficacy and tolerability of multiple prophylactic treatments.
Methods
This NMA was previously registered with PROSPERO (CRD42023422258), and it adhered to the PRISMA statement for network meta-analyses (Supplementary Material) (Hutton et al., 2015).
Search strategy
Online databases including PubMed, Web of Science, Embase, and the Cochrane Center for Clinical Trials were systematically searched for relevant RCTs from 31 March 2023 to 1 May 2023. The search terms included contained VM (and previous names such as migraine-associated dizziness) and prophylactic treatments, including β-blockers, calcium channel blockers, antiseizure medications, vestibular rehabilitation, and antidepressants (the detailed search strings is presented in the Supplementary Material). The type of study was restricted to RCT. There was no limitation concerning year, publication, or language. Relevant reviews were retrieved, and their references were hand-searched.
Study inclusion
Studies were independently selected by two reviewers (H.C. and Y.W.) by screening titles and abstracts according to previously formulated criteria. Differences in opinion were discussed to obtain consensus. If consensus could not be achieved, arbitration was provided by X.Y.
Inclusion criteria
1) Patients: patients aged >18 with definite or probable VM could be included. Older studies containing diagnoses of “migrainous vertigo” or “migraine-associated vertigo” were also acceptable.
2) Interventions: prophylactic treatments for VM, including drugs, rehabilitation, and physical therapies, were considered eligible, regardless of whether they were combined with abortive treatments during vertigo episodes.
3) Outcomes: the efficacy outcome included either the average attack frequency per month after 3–6 months of treatment or symptomatic improvement as measured by questionnaires. The tolerability outcome included the number of patients reporting at least one adverse event (AE).
Data extraction and risk of bias evaluation
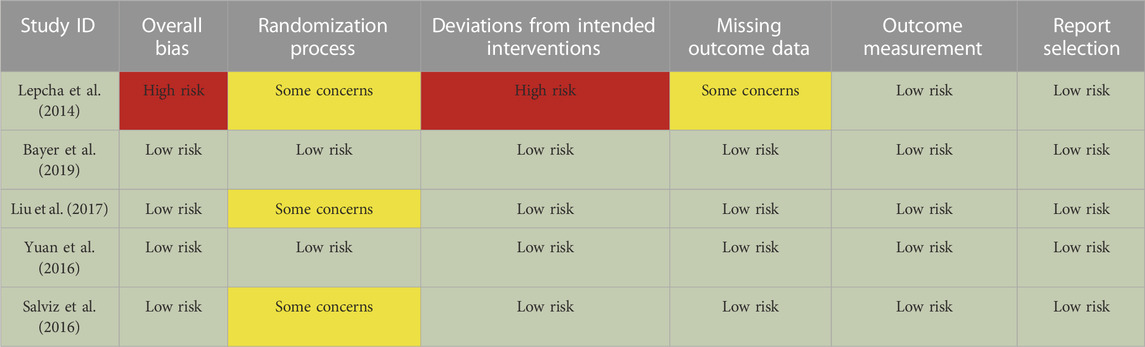
Data were extracted on previously designed spreadsheets and rechecked by two reviewers (H.C. and Y.W.). For one study described in two or more articles, all available data were obtained to provide a comprehensive outcome. To evaluate the risk of bias of the included RCTs, the Cochrane Collaboration Risk of Bias tool version 2 (RoB2) was adopted (Sterne et al., 2019).
Data synthesis
NMA was performed using R software (version 4.2.1, http://www.r-project.org). Specifically, the gemtc package used JAGS 4.3.0 based on a Bayesian framework, and the netmeta package was based on a frequentist framework (Turner et al., 2012; Cipriani et al., 2013). After extracting the outcomes as odds ratios (ORs) or mean differences (MDs) and 95% confidence intervals (CIs) for binary or continuous variables, respectively, we performed a random-effects meta-analysis. For included studies with more than one arm, each treatment arm was pooled to form a single node for the corresponding prophylactic treatment. Markov chain Monte Carlo methods were conducted for the prophylactic treatments to synthesize the results of direct and indirect comparisons (Lu and Ades, 2004). To quantify and evaluate heterogeneity, I2 was calculated, wherein I2 > 50% indicated high heterogeneity (Higgins et al., 2003). The surface under the cumulative ranking (SUCRA) curve of the ranking probability was calculated to evaluate the efficacy and tolerability of the investigated treatments. A higher SUCRA denotes superior tolerability, a longer latency between vertigo episodes, or improvement on the DHI.
Results
Description of included studies
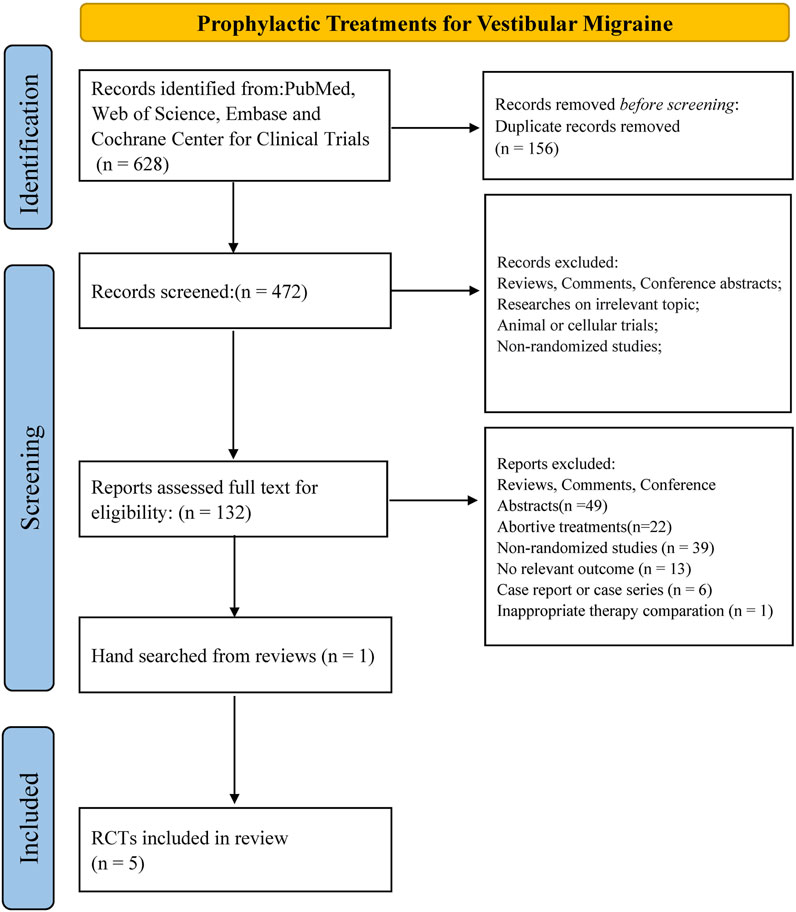
In total, 628 results were identified from PubMed, Web of Science, Embase, and the Cochrane Center for Clinical Trials. We removed 156 duplicates and assessed 472 abstracts. After checking 132 full-text articles for eligibility, we excluded 128 articles. One article was identified via hand-search of the references of a relevant review. In total, five RCTs including 334 patients were analyzed in this NMA (Lepcha et al., 2014; Salviz et al., 2016; Yuan et al., 2016; Liu et al., 2017; Bayer et al., 2019; Li et al., 2021) (Figure 1).
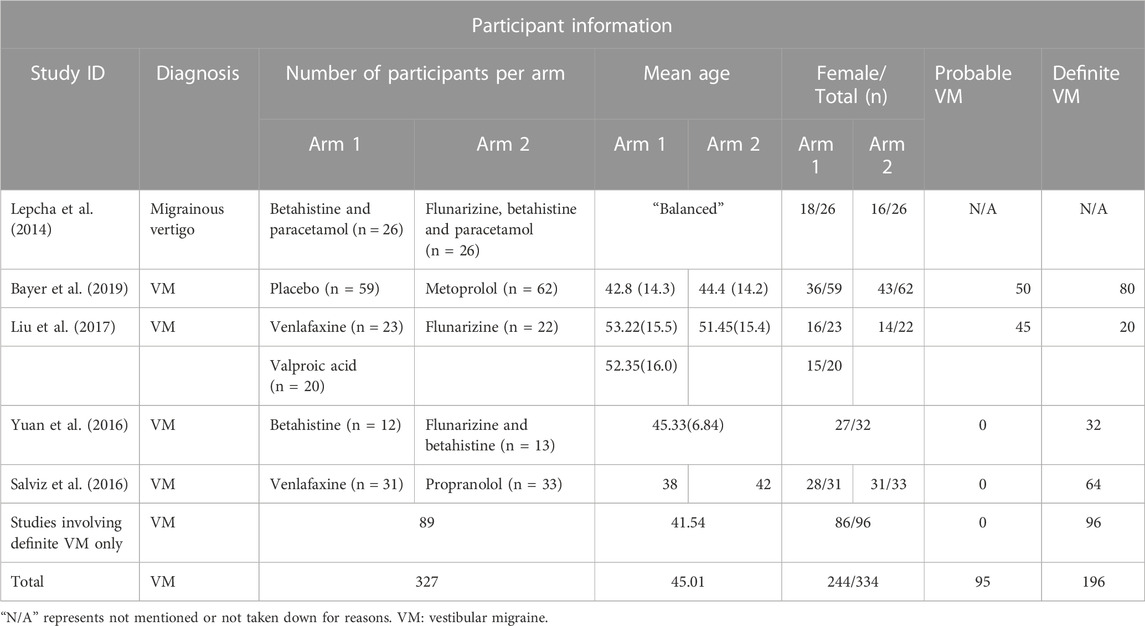
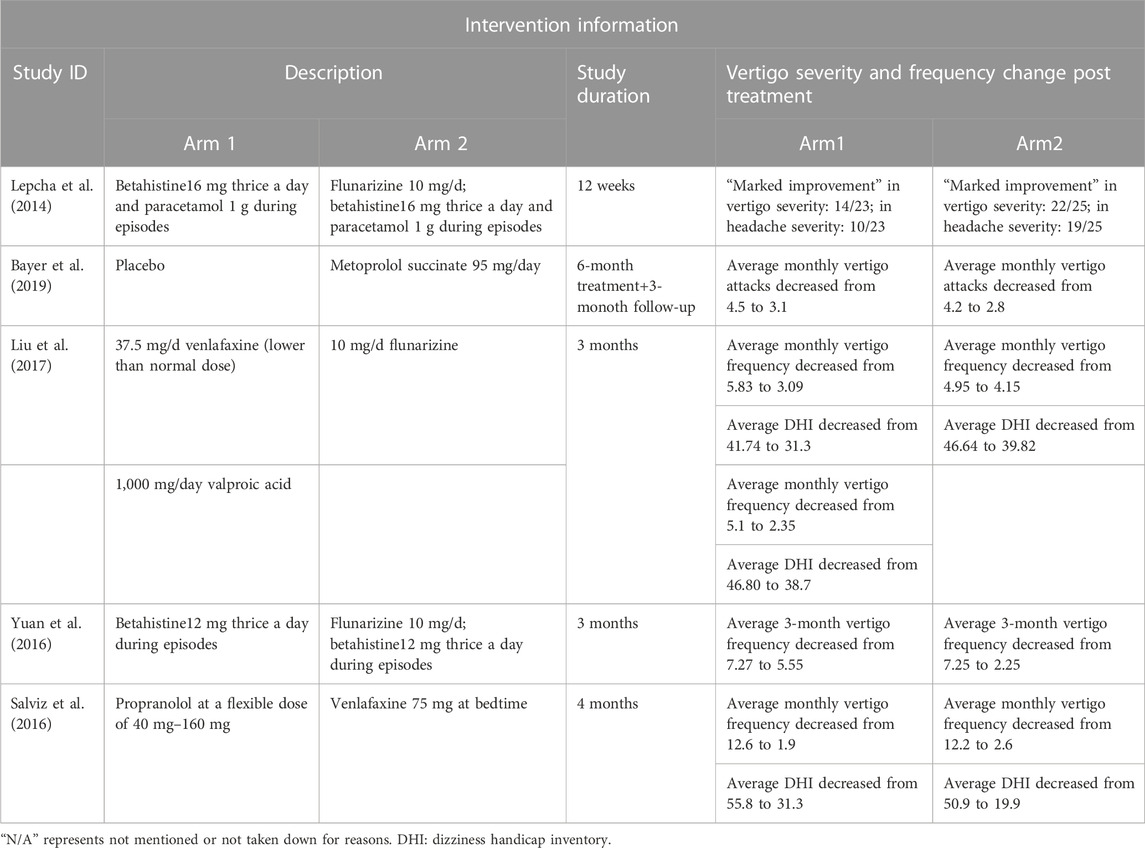
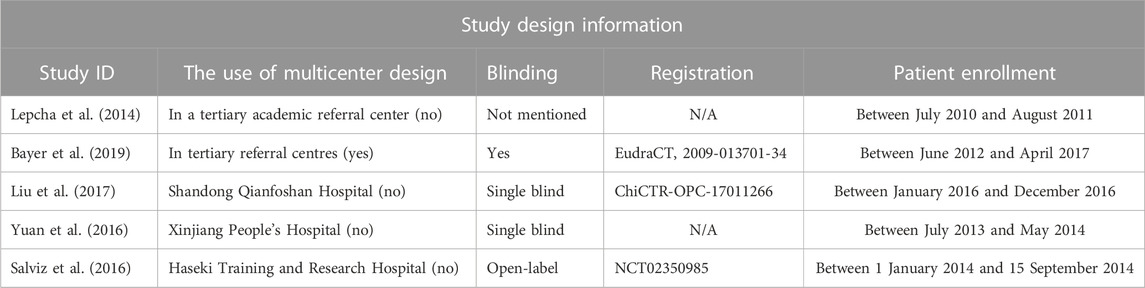
Baseline information and demographic characteristics are presented in Tables 1–3. The proportions of patients with definite and probable VM, age, gender, study design, dose, and the durations of treatments were obtained. All five RCTs assessed the efficacy and tolerability of prophylactic treatments (abortive treatment during episodes alone or placebo, three arms; flunarizine, three arms; venlafaxine, two arms; VPA, one arm; propranolol, one arm; metoprolol, one arm). The risk of bias was evaluated using the RoB2 tool (Table 4). Heterogeneity was low among the included RCTs, as the I2 in NMA analyses ranged between 4% and 12%.
Efficacy outcomes
Outcomes regarding the improvement of DHI and frequency of vertigo after 3–6 months of treatment were analyzed. The intention-to-treat population was used instead of the per-protocol population in the included studies. The network plots presented in Figures 2A–C summarize the comparisons, and thicker lines indicate more head-to-head studies.
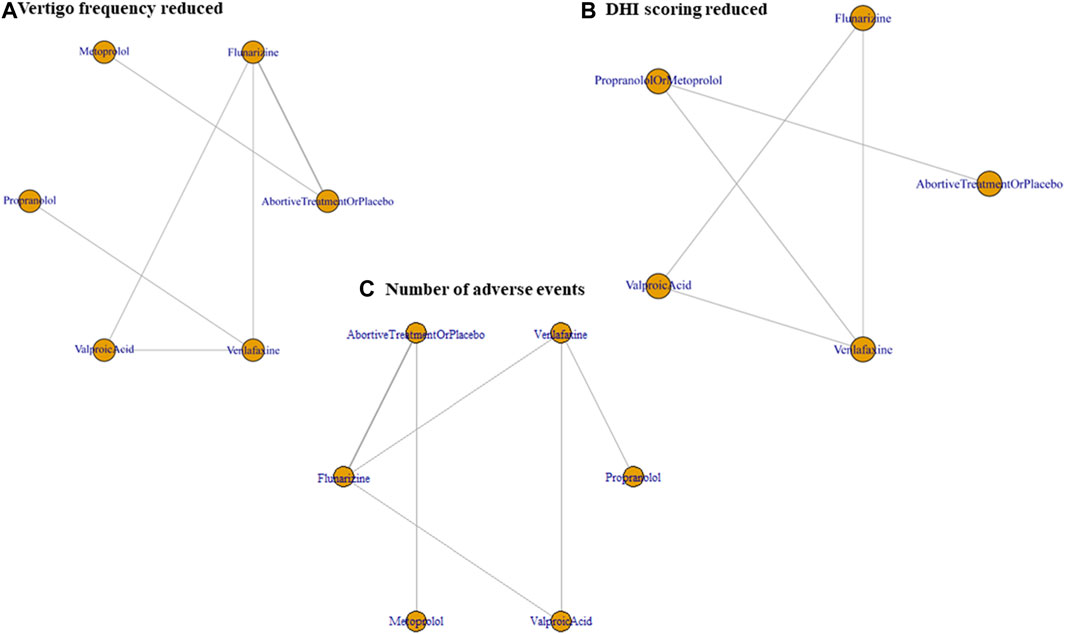
FIGURE 2. Network plot of prophylactic therapies comparing efficacy and tolerability. (A) Vertigo frequency reduction, (B) DHI improvement, and (C) the number of patients reporting any AE, all after 3–6 months of treatment. DHI, dizziness handicap inventory; AE, adverse event.
The frequentist method forest plot illustrated that VPA (MD = −4.12, 95% CI = −8.09, −0.15) significantly reduced the frequency of vertigo versus abortive therapy alone or placebo (Figure 3D). The Bayesian method failed to detect any significant difference because of the broad 95% CI (Figure 3A).
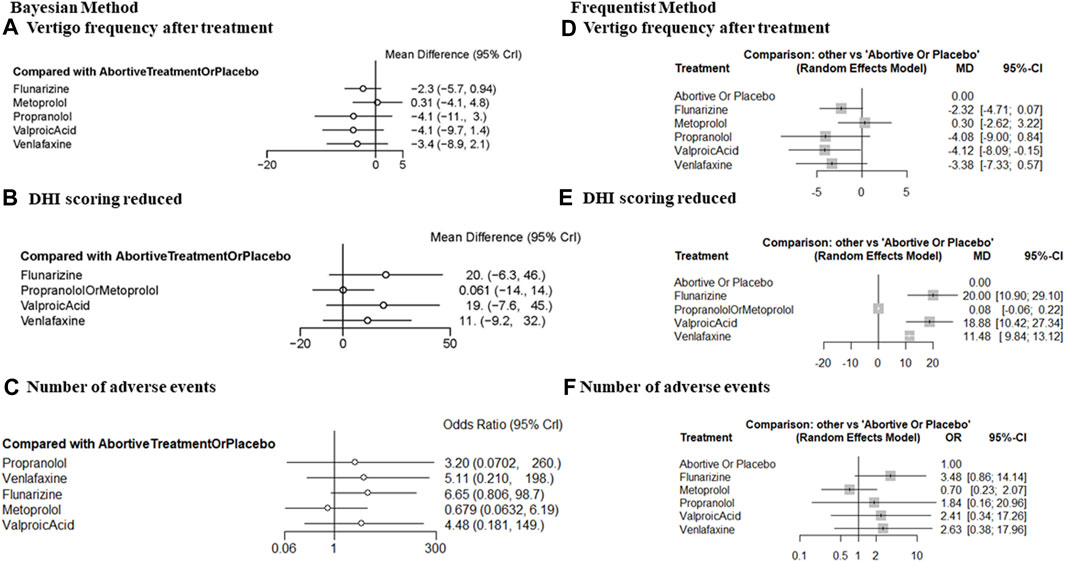
FIGURE 3. Forest plot comparing the efficacy and tolerability of prophylactic therapies with placebo or abortive treatment alone after 3–6 months of treatment. (A) The frequency of vertigo after treatment (Bayesian method), (B) DHI improvement (Bayesian method), (C) the number of patients reporting any AE, (Bayesian method); (D) the frequency of vertigo after treatment (frequentist method), (E) DHI improvement (frequentist method), and (F) the number of patients reporting any AE (frequentist method). DHI, dizziness handicap inventory; AE, adverse event.
Concerning DHI improvement after treatment, a significant effect of prophylactic therapy compared with abortive therapy alone or placebo was detected for flunarizine (MD = 20.00, 95% CI = 10.90, 29.10), valproate acid (MD = 18.88, 95% CI = 10.42, 27.34), and venlafaxine (MD = 11.48, 95% CI = 9.84, 13.12; Figure 3E). The β-blockers metoprolol and propranolol were pooled together to ensure the connection of the comparison network. For these β-blockers, there was no significant improvement (MD = 0.08, 95% CI = −0.06, 0.22). We interpret this finding with caution because the efficacy of metoprolol and propranolol could differ. Broad 95% CIs were observed in the Bayesian method because of the limited number of available RCTs and methodological limitations (Figure 3B).
According to SUCRA, we established a ranking of efficacy for reducing the frequency of vertigo as follows: VPA > propranolol > venlafaxine > flunarizine > placebo or abortive treatment alone > metoprolol (Figure 4A). Regarding DHI improvement, the drugs were ranked in the order flunarizine > VPA > venlafaxine > placebo or abortive treatment alone > metoprolol or propranolol (Figure 4B). Most of the evaluated prophylactic treatments (excluding β-blockers) performed better than placebo or abortive treatment alone, indicating generally favorable efficacy against VM.
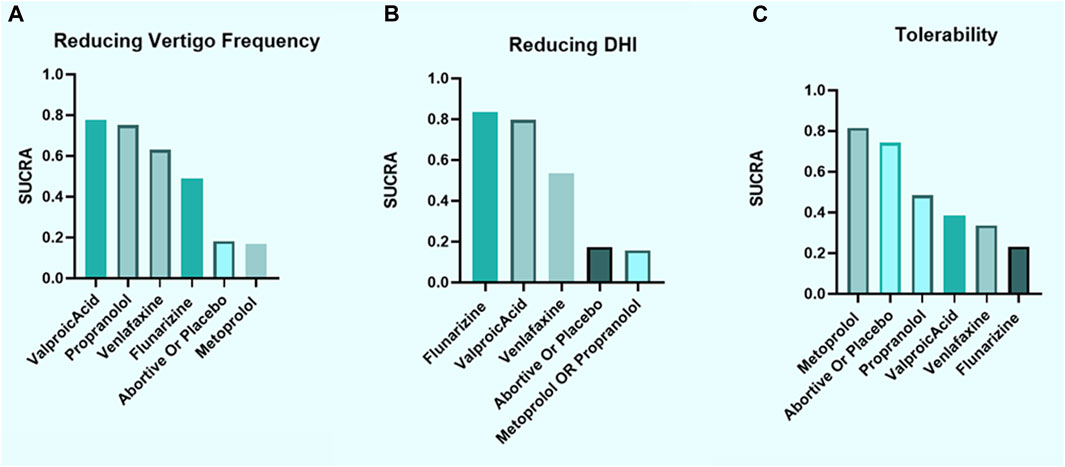
FIGURE 4. Ranking according to SUCRA for efficacy and tolerability. (A) Vertigo frequency reduction, (B) DHI improvement, and (C) tolerability considering the number of patients reporting any AE, all after 3–6 months of treatment. SUCRA, surface under the cumulative ranking curve; DHI, dizziness handicap inventory; AE, adverse event.
Although propranolol more effectively reduced the frequency of vertigo than metoprolol, there was no significant improvement in DHI with either treatment. However, given the low certainty of the results with the β-blockers because of the limited number of available RCTs, their efficacy in treating VM remains unclear. Therefore, further research with propranolol and metoprolol is warranted.
Although other data such as headache frequency, visual analog scales, vertigo symptom scales, and vestibular activities of daily living scales were also collected in our review, the limited number of RCTs that reported these outcomes prevented further analysis. Therefore, future studies addressing these outcomes are needed.
Tolerability outcomes
Tolerability outcomes were measured as the number of patients reporting any AEs. No significantly increased risk of AEs was observed. This conclusion was firm in both Bayesian and frequentist frameworks (Figures 3C, F).
According to SUCRA, the tolerability of the treatments was ranked as follows: metoprolol > placebo or abortive treatment alone > propranolol > VPA > venlafaxine > flunarizine (Figure 4C). Although only measuring the number of AEs regardless of their types could resulted in misinterpretation, β-blockers displayed favorable tolerability.
Discussion
In this network meta-analysis, we systematically summarized the comparative efficacy and tolerability of all available pharmacological interventions used as prophylactic treatments in patients with VM.
Most prophylactic therapies appeared to have good efficacy in reducing the frequency of vertigo and improving DHI. However, treatments with greater efficacy frequently had poor tolerability. VPA ranked first in reducing the frequency of vertigo but third-to-last in tolerability, whereas flunarizine ranked first in improving DHI but last in tolerability. Conversely, metoprolol, which ranked last in efficacy, ranked first in tolerability.
β-blockers have been commonly used for the prophylactic treatment of migraines (Smyth et al., 2022). Although the pathophysiology of migraines and the mechanisms of action of β-blockers in migraine prevention remain unclear, their high tolerability profile is encouraging for their clinical use. β-blockers might also provide some benefits concerning efficacy. Propranolol ranked second in reducing vertigo frequency, although it did not effectively improve DHI. This is in accordance with previous studies demonstrating that propranolol has high affinity for 5-hydroxytryptamine (serotonin or 5-HT) receptors, namely, 5HT2B and 5HT2C, which play a pivotal role in the pathophysiology of migraines. Propranolol also inhibits nitric oxide production by blocking inducible nitric oxide synthase, potentially suppressing the activation of the trigeminovascular complex (Fumagalli et al., 2020).
We advise caution in overinterpreting the inferior ranking of metoprolol regarding both the frequency of vertigo and DHI because the evidence was limited in the available RCTs. The only available RCT did not reach its target sample size because of early trial closure. Consequently, confirmatory analyses could not be performed. Furthermore, that trial had a relatively short duration, including a follow-up period of only 12 weeks. Therefore, additional studies with longer durations are necessary to obtain more comprehensive findings (Bayer et al., 2019). Together with propranolol, metoprolol is the only other β-blocker with the highest level of evidence and fewer side effects in major migraine guidelines. Pharmacologically, it is a moderate lipophilic β-1 selective antagonist, and unlike propranolol, it does not have affinity for 5-HT receptors (Fumagalli et al., 2020). In patients treated with β-blockers, the visual evoked potential amplitude tends to normalize, suggesting that β-blockers modulate cortical excitability and abnormal cortical information processing in migraines. Given the differences in the pharmacological mechanisms of metoprolol and propranolol, the pharmacological mechanisms of metoprolol might have less overlap with the possible pathogenesis of VM, which could explain why metoprolol is less effective than propranolol in the preventive treatment of VM in clinical practice.
VPA is an FDA-approved antiseizure medication for the preventative treatment of migraines (Khani et al., 2021). In our NMA, VPA ranked best in reducing the frequency of vertigo. This favorable efficacy might be associated with its mechanism. VPA increases GABA activity and inhibits NMDA-evoked neuroexcitatory signals, likely blocking cortical spreading depression during a migraine attack (Shnayder et al., 2023). VPA might also produce efficacy in patients with CACNA1A mutations by adjusting calcium channels (Espinosa-Sanchez and Lopez-Escamez, 2015; Hautakangas et al., 2022). However, tolerability must be considered when using VPA to treat VM based on its poor tolerability profile in this study. The AEs of VPA include asthenia/fatigue, dizziness/vertigo, nausea, tremor, and weight gain (Linde et al., 2013), as well as somnolence and dyspepsia (Liu et al., 2017).
Flunarizine is a non-selective calcium channel blocker that has been used to treat migraines since the 1980s (Louis, 1981). It has high lipid solubility, and it can cross the blood–brain barrier to antagonize histamine H1(Dyhrfjeld-Johnsen and Attali, 2019) and dopamine D2 receptors (Brücke et al., 1995). Our results illustrated that flunarizine has the best efficacy in improving DHI; however, it performed poorly in the vertigo frequency and tolerability outcomes. Flunarizine might increase various side effects such as extrapyramidal disturbances, somnolence, depression, weight gain, and drowsiness (Leone et al., 1991). In our study, the incidence of side effects of flunarizine in RCTs, including somnolence and weight gain (Lepcha et al., 2014), was as high as 24%. Some patients even withdrew from the study because of these side effects, and they were lost to follow-up (Li et al., 2021). In clinical applications, long-term administration of flunarizine should be carefully monitored.
Venlafaxine, which is a selective serotonin and noradrenaline reuptake inhibitor (Smyth et al., 2022), ranked third in reducing the frequency of vertigo, third in DHI improvement, and second-to-last in tolerability. The reported AEs of venlafaxine in the included RCTs were nausea, insomnia, palpitations, and somnolence (Liu et al., 2017). It should be noted that withdrawal syndrome can complicate the clinical use of venlafaxine (Smyth et al., 2022). Therefore, venlafaxine should be cautiously recommended for the preventive treatment of VM.
Together, prophylactic therapies exhibited good efficacy in reducing the frequency of vertigo and improving DHI. Respective strengths were observed among the drugs regarding the frequency of vertigo, DHI improvement, and tolerability.
Limitation
The limited number of RCTs restricted further analyses of important outcomes such as headache frequency, visual analog scales, vertigo symptom scales, and quality of life. Further, although we adopted a statistically suitable and appropriate NMA methodology, significance might have been underestimated. Although heterogeneity was low based on the I2 statistic, there could be incompatibilities in the baseline characteristics among the arms of the included RCTs. Both “probable” and “definite” VM were included in this review (although including only definite VM would largely strengthen the evidence, which was prevented in this review because of the limited number of studies that implemented this restriction). Hence, we strongly recommend that future researchers enroll only patients with definite VM. More RCTs are needed to clarify the efficacy and tolerability of prophylactic therapies for VM.
Conclusion
VPA and flunarizine appeared most effective in reducing the frequency of vertigo and improving DHI, but their tolerability was unfavorable. Conversely, metoprolol ranked last in efficacy for both the frequency of vertigo and DHI, but it ranked first in tolerability. This might emphasize the need for precision medicine in patients with different needs and symptoms. However, because of the limited number of available RCTs, additional RCTs comparing the efficacy and tolerability of prophylactic treatment for VM are warranted to confirm our findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
HC: Data curation, Formal Analysis, Investigation, Writing–original draft. YW: Data curation, Formal Analysis, Investigation, Writing–original draft. XL: Formal Analysis, Investigation, Resources, Writing–review and editing. KL: Formal Analysis, Resources, Supervision, Writing–review and editing. XY: Conceptualization, Supervision, Visualization, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1332973/full#supplementary-material
References
Authors Anonymous (2018). Headache classification committee of the international headache society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia 38 (1), 1–211. doi:10.1177/0333102417738202
Balaban, C. D. (2011). Migraine, vertigo and migrainous vertigo: links between vestibular and pain mechanisms. J. Vestib. Res. Equilib. Orientat. 21 (6), 315–321. doi:10.3233/VES-2011-0428
Bayer, O., Adrion, C., Al Tawil, A., Mansmann, U., and Strupp, M.PROVEMIG investigators (2019). Results and lessons learnt from a randomized controlled trial: prophylactic treatment of vestibular migraine with metoprolol (PROVEMIG). Trials 20 (1), 813. doi:10.1186/s13063-019-3903-5
Beh, S. C. (2022). Vestibular migraine. Curr. neurology Neurosci. Rep. 22 (10), 601–609. doi:10.1007/s11910-022-01222-6
Brücke, T., Wöber, C., Podreka, I., Wöber-Bingöl, C., Asenbaum, S., Aull, S., et al. (1995). D2 receptor blockade by flunarizine and cinnarizine explains extrapyramidal side effects. A SPECT study. J. Cereb. blood flow metabolism 15 (3), 513–518. doi:10.1038/jcbfm.1995.63
Cipriani, A., Higgins, J. P., Geddes, J. R., and Salanti, G. (2013). Conceptual and technical challenges in network meta-analysis. Ann. Intern. Med. 159 (2), 130–137. doi:10.7326/0003-4819-159-2-201307160-00008
Dyhrfjeld-Johnsen, J., and Attali, P. (2019). Management of peripheral vertigo with antihistamines: new options on the horizon. Br. J. Clin. Pharmacol. 85 (10), 2255–2263. doi:10.1111/bcp.14046
Espinosa-Sanchez, J. M., and Lopez-Escamez, J. A. (2015). New insights into pathophysiology of vestibular migraine. Front. neurology 6, 12. doi:10.3389/fneur.2015.00012
Fumagalli, C., Maurizi, N., Marchionni, N., and Fornasari, D. (2020). β-blockers: their new life from hypertension to cancer and migraine. Pharmacol. Res. 151, 104587. doi:10.1016/j.phrs.2019.104587
Furman, J. M., Marcus, D. A., and Balaban, C. D. (2013). Vestibular migraine: clinical aspects and pathophysiology. Neurology 12 (7), 706–715. doi:10.1016/S1474-4422(13)70107-8
Hautakangas, H., Winsvold, B. S., Ruotsalainen, S. E., Bjornsdottir, G., Harder, A. V. E., Kogelman, L. J. A., et al. (2022). Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nat. Genet. 54 (2), 152–160. doi:10.1038/s41588-021-00990-0
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ Clin. Res. ed.) 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 162 (11), 777–784. doi:10.7326/M14-2385
Khani, S., Hejazi, S. A., Yaghoubi, M., and Sharifipour, E. (2021). Comparative study of magnesium, sodium valproate, and concurrent magnesium-sodium valproate therapy in the prevention of migraine headaches: a randomized controlled double-blind trial. J. headache pain 22 (1), 21. doi:10.1186/s10194-021-01234-6
Lempert, T., Olesen, J., Furman, J., Waterston, J., Seemungal, B., Carey, J., et al. (2022). Vestibular migraine: diagnostic criteria1. J. Vestib. Res. Equilib. Orientat. 32 (1), 1–6. doi:10.3233/VES-201644
Leone, M., Grazzi, L., La Mantia, L., and Bussone, G. (1991). Flunarizine in migraine: a minireview. Headache 31 (6), 388–391. doi:10.1111/j.1526-4610.1991.hed3106388.x
Lepcha, A., Amalanathan, S., Augustine, A. M., Tyagi, A. K., and Balraj, A. (2014). Flunarizine in the prophylaxis of migrainous vertigo: a randomized controlled trial. Eur. archives oto-rhino-laryngology 271 (11), 2931–2936. doi:10.1007/s00405-013-2786-4
Li, G., Xu, J., Zhou, X., Wu, S., Yin, Y., Zhuang, J., et al. (2021). Lin chuang er bi yan hou tou jing. wai ke za zhi = J. Clin. otorhinolaryngology, head, neck Surg. 35 (9), 784–787. doi:10.13201/j.issn.2096-7993.2021.09.004
Linde, M., Mulleners, W. M., Chronicle, E. P., and McCrory, D. C. (2013). Valproate (valproic acid or sodium valproate or a combination of the two) for the prophylaxis of episodic migraine in adults. Cochrane database Syst. Rev. 2013 (6), CD010611. doi:10.1002/14651858.CD010611
Liu, F., Ma, T., Che, X., Wang, Q., and Yu, S. (2017). The efficacy of venlafaxine, flunarizine, and valproic acid in the prophylaxis of vestibular migraine. Front. neurology 8, 524. doi:10.3389/fneur.2017.00524
Louis, P. (1981). A double-blind placebo-controlled prophylactic study of flunarizine (Sibelium) in migraine. Headache 21 (6), 235–239. doi:10.1111/j.1526-4610.1981.hed2106235.x
Lu, G., and Ades, A. E. (2004). Combination of direct and indirect evidence in mixed treatment comparisons. Statistics Med. 23 (20), 3105–3124. doi:10.1002/sim.1875
Salviz, M., Yuce, T., Acar, H., Karatas, A., and Acikalin, R. M. (2016). Propranolol and venlafaxine for vestibular migraine prophylaxis: a randomized controlled trial. Laryngoscope 126 (1), 169–174. doi:10.1002/lary.25445
Shnayder, N. A., Grechkina, V. V., Khasanova, A. K., Bochanova, E. N., Dontceva, E. A., Petrova, M. M., et al. (2023). Therapeutic and toxic effects of valproic acid metabolites. Metabolites 13 (1), 134. doi:10.3390/metabo13010134
Smyth, D., Britton, Z., Murdin, L., Arshad, Q., and Kaski, D. (2022). Vestibular migraine treatment: a comprehensive practical review. Brain 145 (11), 3741–3754. doi:10.1093/brain/awac264
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ Clin. Res. ed.) 366, l4898. doi:10.1136/bmj.l4898
Turner, R. M., Davey, J., Clarke, M. J., Thompson, S. G., and Higgins, J. P. (2012). Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int. J. Epidemiol. 41 (3), 818–827. doi:10.1093/ije/dys041
Webster, K., Dor, A., Galbraith, K., Kassem, L. H., Harrington-Benton, N., Judd, O., et al. (2023). Pharmacological interventions for prophylaxis of vestibular migraine. Cochrane database Syst. Rev. 2023 (4), CD015187. doi:10.1002/14651858.CD015187.pub2
Keywords: vestibular migraine, prophylactic treatments, preventive treatment, valproate acid, flunarizine
Citation: Chu H, Wang Y, Ling X, Li K and Yang X (2023) Prophylactic treatments for vestibular migraine: a systematic review and network meta-analysis of randomized clinical trials. Front. Pharmacol. 14:1332973. doi: 10.3389/fphar.2023.1332973
Received: 04 November 2023; Accepted: 04 December 2023;
Published: 21 December 2023.
Edited by:
Francisco Lopez-Munoz, Camilo José Cela University, SpainReviewed by:
John Rothrock, Inova Health System, United StatesJiying Zhou, First Affiliated Hospital of Chongqing Medical University, China
Copyright © 2023 Chu, Wang, Ling, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Yang, eWFuZ3h1MjAxMUAxNjMuY29t
†These authors have contributed equally to this work
 Hongyuan Chu1,2†
Hongyuan Chu1,2† Xia Ling
Xia Ling Xu Yang
Xu Yang