- 1Institute of Liver Diseases, Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Shanghai Key Laboratory of Traditional Chinese Clinical Medicine, Shuguang Hospital, Shanghai, China
- 3Institute of Interdisciplinary Science, Shanghai University of Traditional Chinese Medicine, Shanghai, China
Background and aims: The serum metabolites changes in patients with hepatitis B virus (HBV)-related cirrhosis as progression. Peroxisome proliferator-activated receptor gamma (PPARγ) is closely related to lipid metabolism in cirrhotic liver. However, the relationship between fatty acids and the expression of hepatic PPARγ during cirrhosis regression remains unknown. In this study, we explored the serum metabolic characteristics and expression of PPARγ in patients with histological response to treatment with entecavir.
Methods: Sixty patients with HBV-related cirrhosis were selected as the training cohort with thirty patients each in the regression (R) group and non-regression (NR) group based on their pathological changes after 48-week treatment with entecavir. Another 72 patients with HBV-related cirrhosis and treated with entecavir were collected as the validation cohort. All of the serum samples were tested using ultra-performance liquid chromatography coupled to tandem mass spectrometry. Data were processed through principal component analysis and orthogonal partial least square discriminant analysis. Hepatic PPARγ expression was observed using immunohistochemistry. The relationship between serum fatty acids and PPARγ was calculated using Pearson’s or Spearman’s correlation analysis.
Results: A total of 189 metabolites were identified and 13 differential metabolites were screened. Compared to the non-regression group, the serum level of fatty acids was higher in the R group. At baseline, the expression of PPARγ in hepatic stellate cells was positively correlated with adrenic acid (r2 = 0.451, p = 0.046). The expression of PPARγ in both groups increased after treatment, and the expression of PPARγ in the R group was restored in HSCs much more than that in the NR group (p = 0.042). The adrenic acid and arachidonic acid (AA) in the R group also upgraded more than the NR group after treatment (p = 0.037 and 0.014).
Conclusion: Baseline serum differential metabolites, especially fatty acids, were identified in patients with HBV-related cirrhosis patients who achieved cirrhosis regression. Upregulation of adrenic acid and arachidonic acid in serum and re-expression of PPARγ in HSCs may play a crucial role in liver fibrosis improvement.
Introduction
Liver cirrhosis, one of the most threatening diseases worldwide, may develop into hepatocellular carcinoma or other decompensation events. Viral infection, especially hepatitis B virus (HBV) infection, and alcohol abuse are common pathogenic factors in China and Asia (GBD, 2017 Cirrhosis Collaborators, 2020). Cirrhosis has been shown to be reversed when pathogenic factors are removed (Chang et al., 2010; D'Ambrosio et al., 2012). However, not all patients benefit from antiviral treatment, and there remain 42%–73% of patients with chronic hepatitis B (CHB) with fibrosis or cirrhosis who fail to obtain satisfactory therapeutic effects in terms of histological changes following 48–78 weeks of entecavir treatment (Schiff et al., 2008; Chang et al., 2010; Sun et al., 2017; Sun et al., 2018; Wu et al., 2018).
A previous study revealed that metabolic pathways related to carbohydrates, amino acids, and lipids changed were altered under conditions of liver fibrosis or cirrhosis among humans, mice, and rats (Chang and Yang, 2019). Another study reported that several metabilites such as plasma lactate, tyrosine, methionine and phosphatidylcholines may predict survival in patients with decompensated cirrhosis (McPhail et al., 2016). In HBV-related cirrhosis, serum bile acids are closely associated with pathological progression (Wang et al., 2016). Metabolomics analysis or certain serum metabolites can distinguish and predict liver cirrhosis and decompensated cirrhosis (Wang et al., 2012). Thus, the progression of liver fibrosis is closely related to metabolomics, while there are fewer reports on the changes in metabolomics during fibrosis regression.
The peroxisome proliferator-activated receptor (PPAR) family regulates downstream targeted pathways, such as TGF-β, MAPKs, and NF-κB p65 (Li J. et al., 2021), and it is involved in the pathological process of liver fibrosis. A previous study showed that intrahepatic expression of PPARγ was significantly reduced in patients with liver fibrosis and cirrhosis. Many studies have focused on the role of PPARγ in hepatic stellate cell (HSC) activation (Bae et al., 2010). In hepatic steatosis, PPARγ is also related to lipid metabolism in hepatocytes (Wang et al., 2020) and has significant anti-inflammatory properties (Chinetti et al., 2003). Indeed, He et al. demonstrated that PPARγ activation was necessary for docosahexaenoic acid (DHA) to reduce liver fibrosis (He et al., 2019). However, the expression of PPARγ in the liver tissues of patients with HBV-related cirrhosis remains unknown, especially during the regression of cirrhosis. Meanwhile, the relationship between PPARγ and serum metabolites, especially fatty acids, involved in the histological response of HBV cirrhosis requires further exploration.
Regarding patients with HBV-related cirrhosis who are treated by entecavir, it remains unclear why some people show a histological response, while others do not, and the mechanism of action. In this study, we conducted metabolomics and multiplex immunofluorescence staining to unravel the relationship between serum metabolites and PPARγ expression in patients who histologically responded to treatment.
Methods
Clinical studies
Study participants
Patients were recruited from the cohort between September 2014 to October 2018 in 20 hospitals from 12 provinces of China (Li ZX. et al., 2021) (Clinical Trial. gov: NCT 02241590). All of the patients participated in the biobank initiative. At each research visit, serum samples were collected and immediately stored at −80°C. Liver biopsy was performed twice before and after 48-week entecavir treatment at a dose of 0.5 mg once daily. This study was approved by the ethics committee of Shuguang Hospital (approval No. 2014–331-27–01) and other participating hospitals. All of the participants provided informed consent for the study.
Definition of fibrosis regression
In this study, the efficacy criteria for entecavir on liver histology were assessed through the change in liver fibrosis stage from before (pre-) to after (post-) therapy using the Ishak scoring system (Ishak et al., 1995). According to the pathological histological results of the liver biopsy before and after treatment, a decrease in the Ishak score of at least 1 point was considered to indicate fibrosis regression or non-regression. Three experienced pathologists, who were blinded to the clinical information of the liver biopsy sample, reached a consensus on the Ishak fibrosis score on each biopsy following discussion.
Inclusion criteria
The key inclusion criteria were as follows: 1) aged 18–60 years; 2) serologically proven active CHB infection based on documented history and detectable levels of HBV DNA >20 IU/mL; 3) Ishak fibrosis score of 5 or 6 before treatment; and 4) completed a 48-week treatment course, followed by liver biopsy.
Exclusion criteria
Patients were excluded if they had a history of hepatic decompensation, other chronic liver disease within treatment, or poor compliance to antiviral treatment. Patients with no liver biopsy available for evaluation before and after treatment were also excluded.
Blood sample preparation and clinical parameter analysis
Overnight fasting venous blood samples were obtained using the conventional method before initiating antiviral therapy. Samples were centrifuged at 3,000 g for 15 min at room temperature. Serum was aliquoted and stored at −80°C for metabolomics analysis. The following clinical parameters were measured by a conventional method: platelet count (PLT), white blood cells (WBC), hemoglobin (HGB), blood glucose, aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), albumin, total bilirubin (TBIL), triglyceride (TG), total cholesterol (TC), low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, creatinine (Cr), prothrombin time (PT), alpha fetoprotein (AFP), hepatitis B e antigen (HBeAg), and HBV DNA levels.
UPLC-MS (ultra-performance liquid chromatography coupled to tandem mass spectrometry) analysis
Chemicals and reagents
All of the standards for the targeted metabolites were obtained from Sigma-Aldrich (St. Louis, MO, United States), Steraloids Inc. (Newport, RI, United States), and TRC Chemicals (Toronto, ON, Canada). All of the standards were accurately weighed and prepared in water, methanol, sodium hydroxide solution, or hydrochloric acid solution to obtain individual stock solutions at a concentration of 5.0 mg/mL. The appropriate amount of each stock solution was mixed to create stock calibration solutions.
Formic acid was of Optima grade and was obtained from Sigma-Aldrich (St. Louis, MO, United States). Methanol (Optima LC-MS), acetonitrile (Optima LC-MS), and isopropanol (Optima LC-MS) were purchased from Thermo Fisher Scientific (FairLawn, NJ, United States). Ultrapure water was produced using a Mill-Q Reference system equipped with a LC-MS Pak filter (Millipore, Billerica, MA, United States).
Experimental procedure
The method was optimized according to a previously reported protocol (Lan et al., 2016). Serum samples were thawed in an ice bath to diminish sample degradation. The serum samples were processed according to the following steps: 20 μL of serum was added to a 96-well plate, which was then transferred to an Eppendorf epMotion Workstation (Eppendorf Inc., Hamburg, Germany); 120 μL of ice cold methanol and partial internal standards were automatically added to each sample and vortexed vigorously for 5 min; the plate was centrifuged at 4,000 g for 30 min (Allegra X-15R, Beckman Coulter, Inc., Indianapolis, IN, United States) and then returned to the workstation; 30 μL of supernatant was transferred to a clean 96-well plate, and 20 μL of freshly prepared derivative reagent was added to each well; the plate was sealed and derivatization was performed at 30°C for 60 min; after derivatization, 330 μL of ice-cold 50% methanol solution was added to dilute the sample; the plate was stored at 20°C for 20 min, followed by centrifugation at 4,000 g at 4°C for 30 min; 135 μL of supernatant was transferred to a new 96-well plate with 10 μL of internal standards in each well; serial dilutions of derivatized stock standards were added to the left wells; and finally, the plate was sealed for LC-MS analysis.
An UPLC-MS system (ACQUITY UPLC-Xevo TQ-S, Waters Corp., Milford, MA, United States) was used to quantify all targeted metabolites. The optimized instrument settings are described in the Supplementary Material.
Multiplex immunohistochemistry staining
Multiplex immunohistochemistry staining (mIHC) was performed to analyze the expression of various factors in liver tissue. Deparaffinized and hydrated tissue sections were boiled in sodium citrate buffer (pH 6.0) for antigen retrieval. mIHC was performed using a four-color immunohistochemistry staining kit (PANOVUE, 10079100020) according to the manufacturer’s protocol. Next, α-SMA antibody (1:400 dilution, #19245, CST) was incubated overnight and washed with TBS. Subsequently, 30 μL of proportionally diluted tyramide signal amplification fluorescent solution (1:100) was incubated at room temperature for 20 min. Then, the PPARγ antibody (1:300 dilution, ab59256, Abcam) was also processed following the same conditions and steps. The stained slides were scanned and quantified using the HALO Highplex FL (Indica Labs; Albuquerque, NM) analysis module of Halo software (Erber et al., 2021).
Statistical analysis
The raw data files generated by UPLC-MS/MS were processed using MassLynx software (v4.1, Waters, Milford, MA, USA) to perform peak integration, calibration, and quantitation for each metabolite. The powerful package R Studio was used for statistical analyses. Statistical algorithms were adapted from the widely used statistical analysis software packages in R studio (http://cran.r-project.org/). Multidimensional statistics were based on the orthogonal partial least square discriminant analysis (OPLS-DA) model. The overall contribution of each variable to the model was described by VIP (variable import in the project), and the threshold was usually set to VIP >1. Univariate analysis was based on p-value obtained from the t-test or Mann–Whitney U test, and the fold change was used to filter variables before drawing a volcano plot.
We selected differentially expressed metabolites based on the criterion as absolute values of log2FC > 0 and p < 0.05.
Continuous variables are expressed as the mean ± standard deviation and as median (Q1, Q3) in cases where the data were abnormally distributed. Only observed values were used in the data analyses. Normally distributed data were analyzed by t-test, while those that were non-normally distributed were analyzed by non-parametric test using SAS 9.4. p-values <0.05 were considered to be statistically significant.
Results
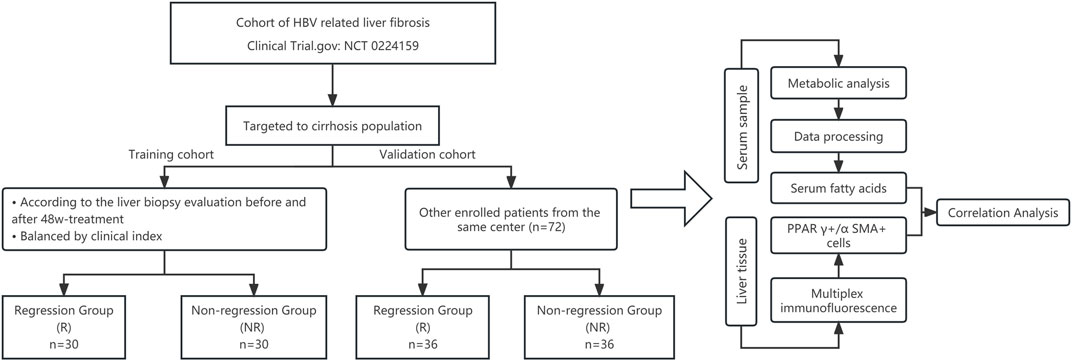
The flowchart of this study is illustrated in Figure 1.
Characterization of the study population
Considering the effects of sex, age, body mass index, and level of HBV-DNA on antiviral efficacy and metabolism (Sun et al., 2020), we balanced these factors to select cases for serum metabolomics tests. According to the pathological histological results of the liver biopsy, 60 patients were divided into regression and non-regression groups. The histology of the liver biopsies, as assessed by the Ishak scoring system, including HE and Sirius red staining, is shown in Supplementary Figure S1. The demographic and clinical characteristics of the two groups are detailed in Table 1. The two groups showed no significant differences in demographic and clinical characteristics before antiviral treatment (p > 0.05).
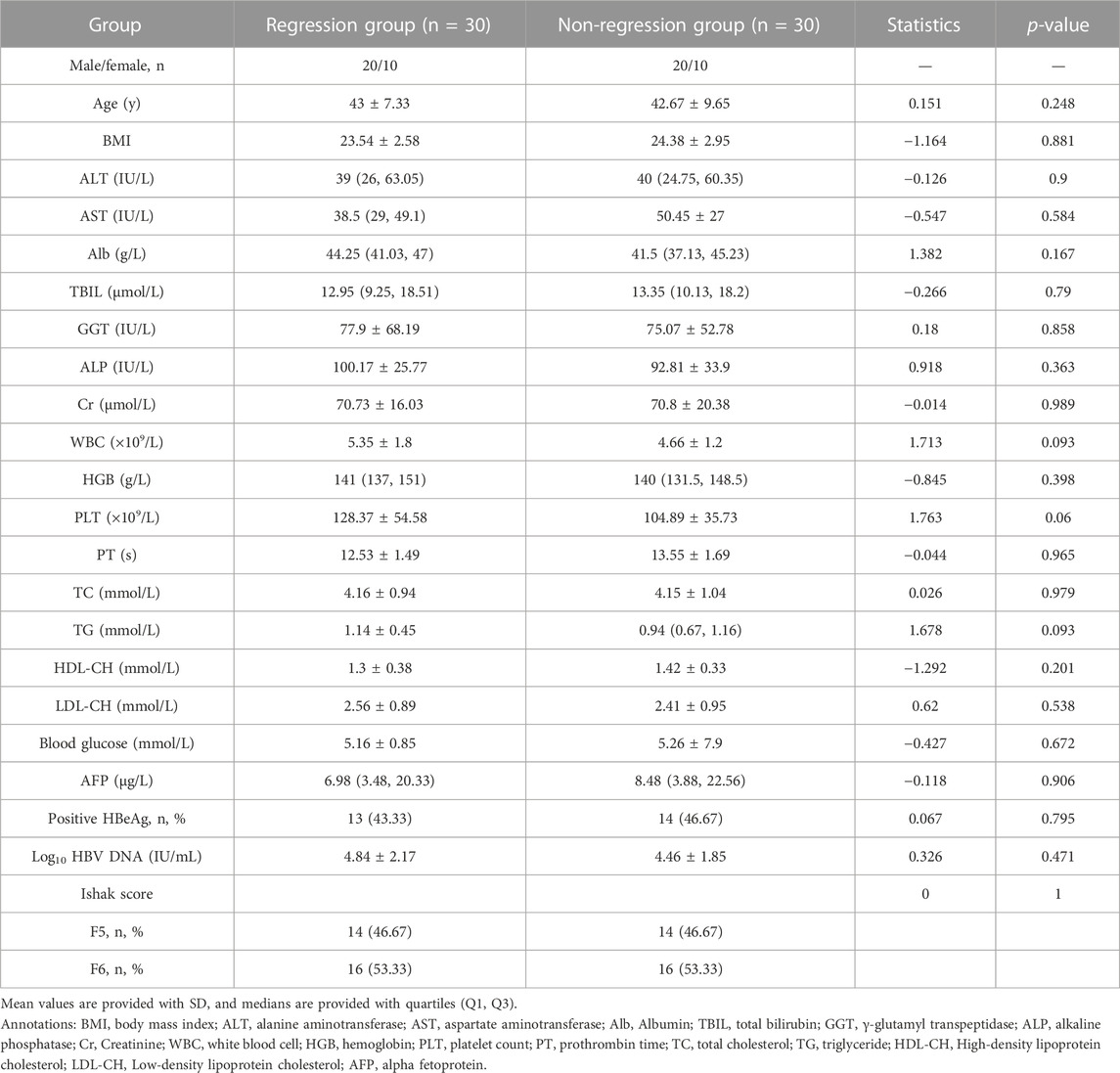
TABLE 1. Demographic and clinical characteristics of patients with liver fibrosis before antiviral therapy.
Serum metabolomics analysis and discovery of differential metabolites
In this study, we applied a quantitative targeted metabolomics profile. Quality control (QC) samples were applied to ensure the stability of the test. The principal component analysis (PCA) scores with QC samples are shown in Supplementary Figure S2A. QC samples gathering near the center of the score matrix projection showed that the sample analysis process was stable. Samples 2, 11, and 49 were far from the center and were identified as outliers and not included in the following analysis. A total of 189 metabolites were identified. Fatty acids (23.28%), amino acids (21.69%), organic acids (14.81%), carbohydrates (8.47%), and carnitine (7.41%) were the top five metabolites found in the two groups (Supplementary Figure S3A).
We applied multidimensional and single-dimensional statistical methods to screen for differential metabolites. In multidimensional statistics analysis, the distribution of regression and non-regression populations in the PCA score map showed no significant difference between each sample except for the outlier, as mentioned before (Supplementary Figure S3B). The PCA scores showed that the detected metabolites in the two groups were relatively similar in composition. Further OPLS-DA analysis showed that the metabolites of the two groups were partly distinguished. Moreover, 59 metabolites were selected when considering the contribution of VIP and correlation coefficients (the screening condition was defined as VIP >1, Supplementary Figure S3C).
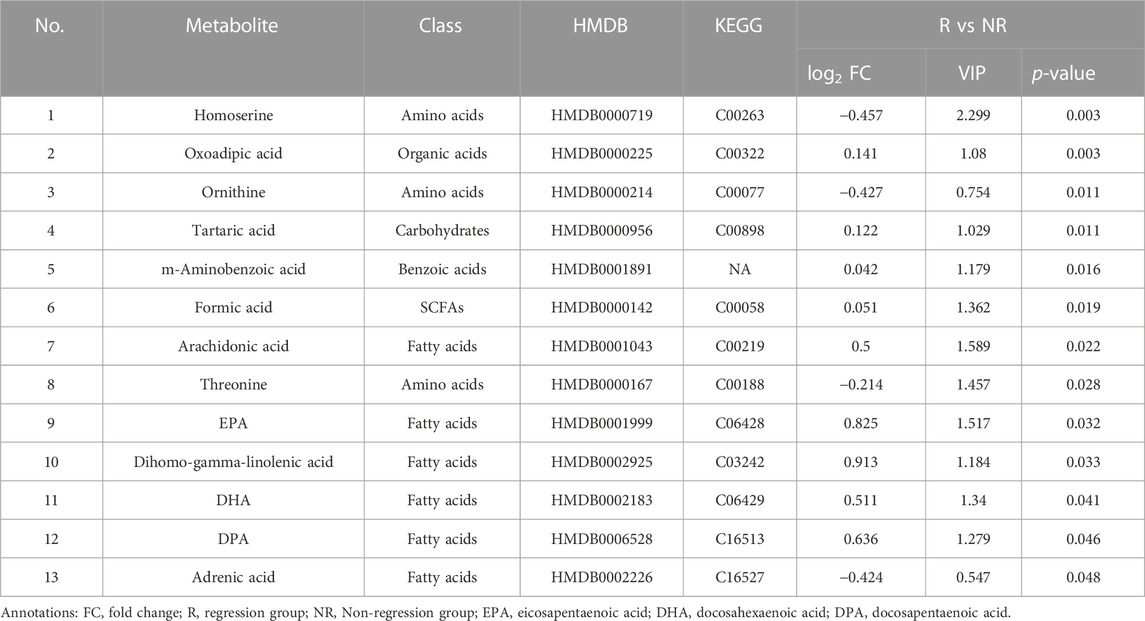
We selected differential metabolites from univariate analysis according to the following criterion: p < 0.05 and the absolute value of log2FC (fold change) > 0. The volcano plot revealed 13 metabolites that were significantly different between the two populations (Figure 2).
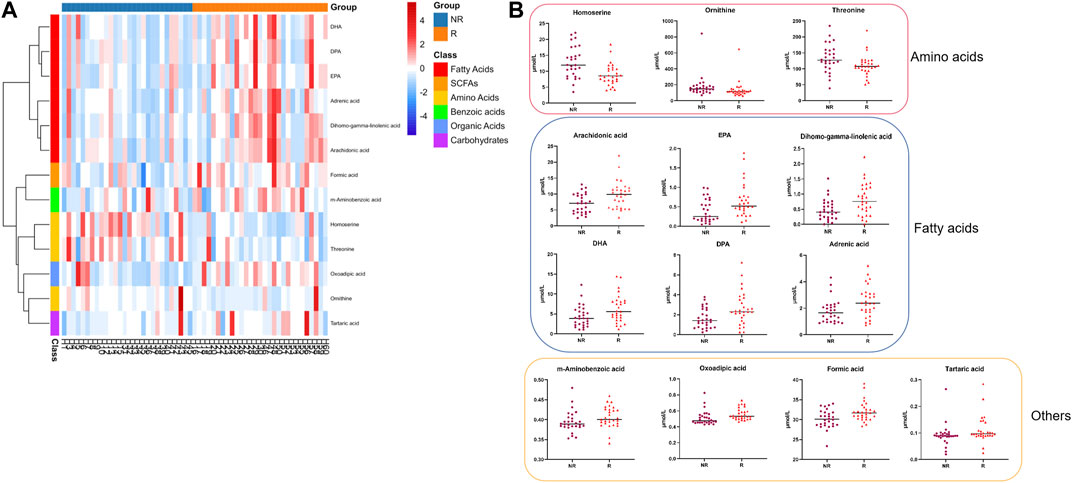
FIGURE 2. Differential serum metabolite expression between groups at baseline. (A). Heatmap of differential metabolites. (B). Serum level of 13 differential metabolites between the R and NR groups. R: Regression, NR: Non-regression.
Compared to the metabolic levels of the patients in the NR group, the levels of homoserine, ornithine, and threonine were lower in the patients in the R group. In contrast, the other 10 metabolites, including arachidonic acid and adrenic acid, were increased in the patients in the R group (Table 2).
Identification and validation of serum fatty acids at baseline
The differential metabolites mainly belonged to fatty acids and amino acids, as shown in Figure 2. The serum levels of fatty acids, including adrenic acid, arachidonic acid, dihomo-gamma-linolenic acid, eicosapentaenoic acid (EPA), docosahexaenoic acid (DPA), and DHA, were higher in patients in the R group (all p < 0.05).
To validate these fatty acids, the serums of other enrolled patients with HBV-related cirrhosis from the same centers were selected for metabolomics analysis. As shown in Table 3, there were no significant differences in sex, age, BMI, ALT, AST, ALB, TBIL, Cr, PT, WBC, HGB, PLT, AFP, and Ishak scores (p > 0.05), suggesting that the demographic and clinical data are comparable between the two groups. Meanwhile, the results in Figure 3 suggest that serum levels of fatty acids, including arachidonic acid, dihomo-gamma-linolenic acid, EPA, DPA, and DHA, were also higher in the patients in the R group (all p < 0.05).
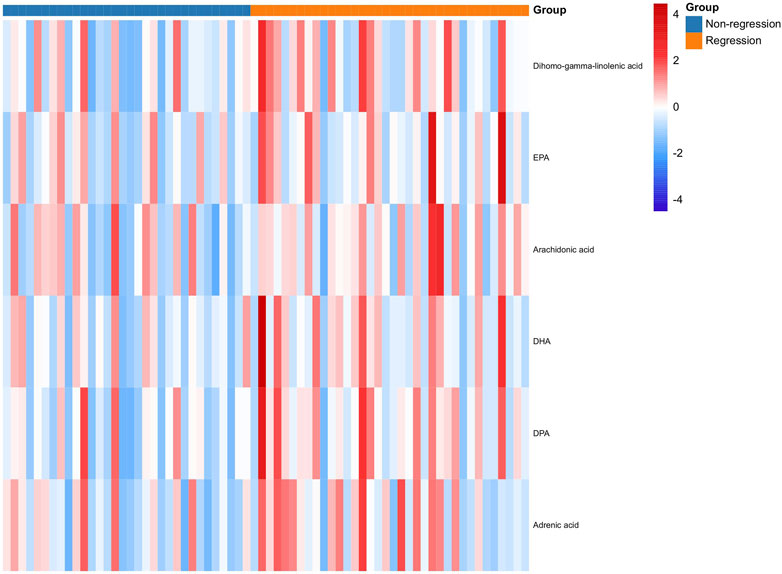
FIGURE 3. Expression of fatty acids between the R and NR groups in the validation cohort at baseline. R: Regression, NR: Non-regression.
Correlations between serum fatty acids and expression of PPARγ in the liver
PPARγ is mainly expressed in hepatocyte, HSCs, Kupffer cells, and liver sinusoidal endothelial cells (Boyer-Diaz et al., 2021). The general expression of PPAR in healthy liver was higher than that in the fibrotic or cirrhotic liver, especially fatty liver. We also observed that PPARγ was predominantly expressed in hepatocytes (Figure 4A). Previous studies have focused on the relationship between PPARγ loss and HSC activation (Liu et al., 2020). Interestingly, the serum level of adrenic acid was positively correlated with PPARγ+ HSCs in the cirrhotic liver (r2 = 0.451, p = 0.046).
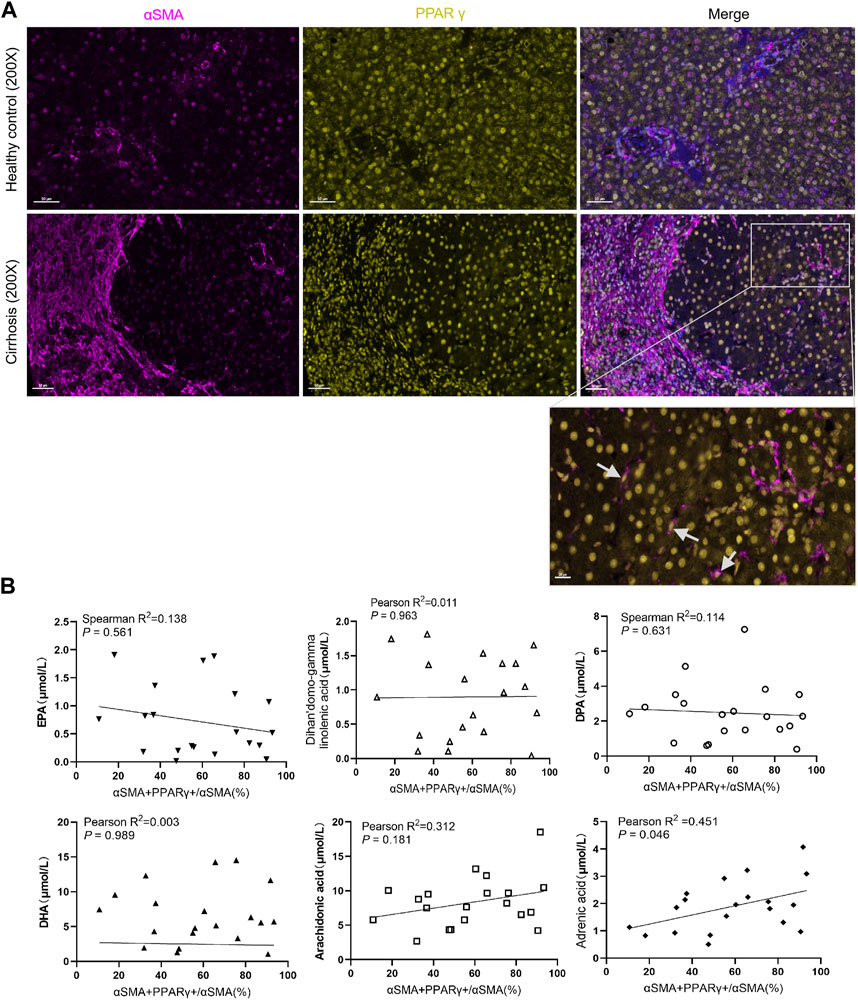
FIGURE 4. Expression of PPARγ in the liver and its relationship with serum fatty acids. (A). Expression of PPARγ is downregulated in patients with chronic hepatitis B with cirrhosis. (B). Correlation analysis between the proportion of PPARγ+αSMA+/αSMA + cells in the liver and the serum fatty acid level. The level of adrenic acid was positively correlated with the expression of PPARγ+ HSC.
Changes in serum fatty acid metabolism and PPARγ expression after treatment
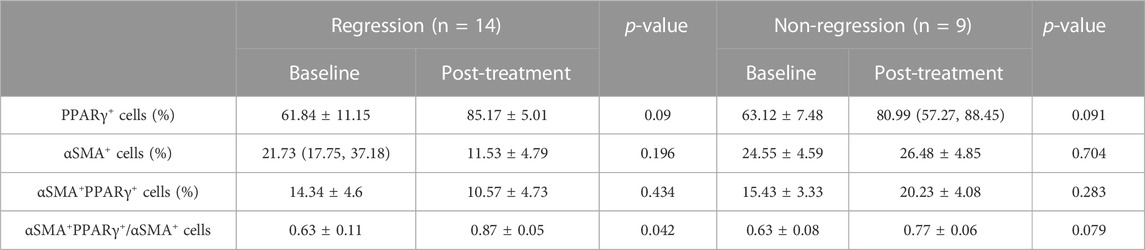
After 48 weeks of antiviral treatment, PPARγ expression was restored in hepatocytes and HSCs in the regression group. Some PPARγ+ HSCs were seen at the collagen fiber deposits (Figure 5A). The changes in these six fatty acids stratified by liver fibrosis changes (fibrosis regression and non-regression) are shown in Figure 5B. Longitudinal change values of these six fatty acids were evaluated as predictors of fibrosis improvement at week 48, especially the change in adrenic acid and arachidonic acid (p = 0.037 and 0.014). Meanwhile, as shown in Table 4, the ratio of PPARγ+ in αSMA+ cells was elevated after treatment (0.63 ± 0.11 vs 0.87 ± 0.05, p = 0.042).
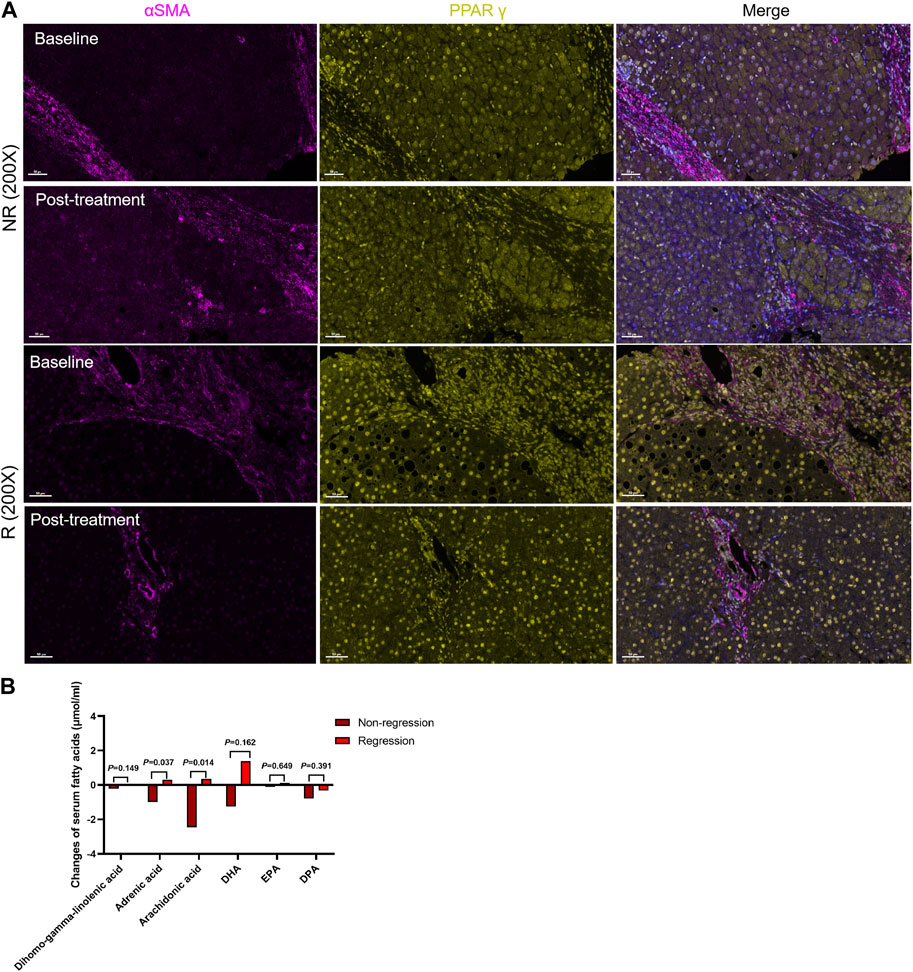
FIGURE 5. Changes in serum fatty acid metabolism and PPARγ expression after treatment. (A). The R group expressed more PPARγ in hepatic stellate cells, while the proportion of PPARγ+ HSCs increased in the regression group following treatment. (B). Changes in the levels of six serum fatty acids between the two groups.
Dicussion
HBV-related liver fibrosis can regress or be reversed by antiviral therapy, such as entecavir or tenofovir. In previous studies, the regression rate following 78-week treatment ranged from 27% to 44% (Sun et al., 2017; Sun et al., 2018; Wu et al., 2018). However, it is still unclear whether the effect of antiviral therapy is affected by initial factors. It has been reported that metabolites can predict the pathological progression of CHB or the survival of decompensated liver cirrhosis (McPhail et al., 2016; Wang et al., 2016). Nevertheless, the relationship between baseline metabolites and the population with a histological response has not yet been reported.
Serum metabolomics is a method for diagnosing and treating diseases by detecting metabolites, intracellular substances, and microbial metabolites in the blood. In medicine, serum metabolomics play an important role in prevention, treatment, and prediction (Zhang et al., 2018; Ottka et al., 2021; Sun et al., 2021).
In this study, we found differences in serum metabolism between the regression and no-regression groups before antiviral treatment, which may influence the efficacy of treatment. Differential serum metabolites were mainly classified as fatty acids and amino acids and mainly included arachidonic acid, adrenic acid, EPA, and DHA. Fatty acids such as arachidonic acid paly a potential causal associations with the risk of NAFLD and cirrhosis (Chen et al., 2023). EPA is also involved in multiple pathways regulating hepatic inflammation and fibrosis (Fraser et al., 2022). The levels of EPA, arachidonic acid and other fatty acids also decreased in patients with HBV-related cirrhosis (Arain et al., 2017).
The level of fatty acid changes as liver fibrosis improves. Four eicosanoids, including adrenic acid, are significantly associated with the liver fibrosis stage at baseline in NAFLD (Caussy et al., 2020). Meanwhile, serum adrenic acid level was positively related to the expression of PPARγ+ αSMA+ in the cirrhotic liver. PPARγ is one of the marker of quiescent HSCs. Some studies have suggested that fatty acid metabolism affects the expression of PPARγ through regulating HSC activation (Liu et al., 2021; Ning et al., 2022). As is well known, upgrading eicosanoids, such as EPA or DHA, can inhibit HSC activation through re-expression of PPARγ (He et al., 2017). In this study, we observed that the expression of PPARγ+ αSMA+ in the livers of patients in the R group was higher and positively correlated with the serum level of adrenic acid at baseline.
Study showed that after 24-week treatment, changes in plasma eicosanoids, such as adrenic acid, arachidonic acid, DHA, and EPA, are associated with in liver fibrosis improvement in NAFLD (Caussy et al., 2020). Our findings showed a similar trend in HBV-related cirrhosis compared to nonalcoholic steatohepatitis. After 48 weeks of antiviral treatment, the serum levels of fatty acids, especially adrenic acid and AA, upgraded much higher in the R group, as the expression of PPARγ was increased in HSCs. This finding indicates that upregulation of adrenic acid and AA and PPARγ expressed in HSCs may play a crucial role in liver fibrosis improvement.
Our research has a few limitations that warrant discussion. First, we only included patients with HBV-related cirrhosis (Ishak F5-6). Therefore, our findings may be not applicable to patients with cirrhosis related to other factors. Second, the samples were from multicenter randomized double-blind trials in China. Although they covered a wide area, due to differences in living and eating habits, the findings remain to be verified by extending the study overseas. Finally, as this is an observational research, we need advanced assays to explore the potential mechanisms of metabolites and pathways; thus, a prospective study with larger samples and in vitro and in vivo experiments is required to verify the hypothesis.
Conclusion
For HBV-related cirrhosis receiving entecavir treatment, patients who histologically respond to therapy had higher serum levels of several fatty acids at baseline. The expression of PPARγ was positively correlated with the serum level of adrenic acid. After treatment, a change in serum adrenic acid and arachidonic acid level were associated with fibrosis improvement, as the restoration of PPARγ in HSCs may be related to these two fatty acids. These findings provide potential therapeutic targets and pharmacological mechanisms of populations that respond to treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the IRB of Shuguang Hospital Affiliated with Shanghai University of Traditional Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
H-NF: Formal Analysis, Writing–original draft. Z-MZ: Conceptualization, Writing–review and editing. KH: Data curation and Methodology. X-NW: Writing–review and editing. Y-KD: Validation, Writing–review and editing. C-HL: Conceptualization, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Shanghai Key Specialty of Traditional Chinese Clinical Medicine (shslczdzk01201), the China Postdoctoral Science Foundation (2022M722162) and National Natural Science Foundation of China (No. 82305100).
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1329266/full#supplementary-material
References
Arain, S. Q., Talpur, F. N., Channa, N. A., Ali, M. S., and Afridi, H. I. (2017). Serum lipid profile as a marker of liver impairment in hepatitis B Cirrhosis patients. Lipids Health Dis. 16 (1), 51. doi:10.1186/s12944-017-0437-2
Bae, M. A., Rhee, S. D., Jung, W. H., Ahn, J. H., Song, B. J., and Cheon, H. G. (2010). Selective inhibition of activated stellate cells and protection from carbon tetrachloride-induced liver injury in rats by a new PPARgamma agonist KR62776. Arch. Pharm. Res. 33 (3), 433–442. doi:10.1007/s12272-010-0313-3
Boyer-Diaz, Z., Aristu-Zabalza, P., Andrés-Rozas, M., Robert, C., Ortega-Ribera, M., Fernández-Iglesias, A., et al. (2021). Pan-PPAR agonist lanifibranor improves portal hypertension and hepatic fibrosis in experimental advanced chronic liver disease. J. Hepatol. 74 (5), 1188–1199. doi:10.1016/j.jhep.2020.11.045
Caussy, C., Chuang, J. C., Billin, A., Hu, T., Wang, Y., Subramanian, G. M., et al. (2020). Plasma eicosanoids as noninvasive biomarkers of liver fibrosis in patients with nonalcoholic steatohepatitis. Ther. Adv. Gastroenterol. 13, 1756284820923904. doi:10.1177/1756284820923904
Chang, M. L., and Yang, S. S. (2019). Metabolic signature of hepatic fibrosis: from individual pathways to systems biology. Cells 8 (11), 1423. doi:10.3390/cells8111423
Chang, T. T., Liaw, Y. F., Wu, S. S., Schiff, E., Han, K. H., Lai, C. L., et al. (2010). Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 52 (3), 886–893. doi:10.1002/hep.23785
Chen, J., Ruan, X., Sun, Y., Li, X., Yuan, S., and Larsson, S. C. (2023). Plasma phospholipid arachidonic acid in relation to non-alcoholic fatty liver disease: mendelian randomization study. Nutrition 106, 111910. doi:10.1016/j.nut.2022.111910
Chinetti, G., Fruchart, J. C., and Staels, B. (2003). Peroxisome proliferator-activated receptors: new targets for the pharmacological modulation of macrophage gene expression and function. Curr. Opin. Lipidol. 14 (5), 459–468. doi:10.1097/00041433-200310000-00006
D'Ambrosio, R., Aghemo, A., Rumi, M. G., Ronchi, G., Donato, M. F., Paradis, V., et al. (2012). A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology 56 (2), 532–543. doi:10.1002/hep.25606
Erber, R., Spoerl, S., Mamilos, A., Krupar, R., Hartmann, A., Ruebner, M., et al. (2021). Impact of spatially heterogeneous trop-2 expression on prognosis in oral squamous cell carcinoma. Int. J. Mol. Sci. 23 (1), 87. doi:10.3390/ijms23010087
Fraser, D. A., Wang, X., Lund, J., Nikolić, N., Iruarrizaga-Lejarreta, M., Skjaeret, T., et al. (2022). A structurally engineered fatty acid, icosabutate, suppresses liver inflammation and fibrosis in NASH. J. Hepatol. 76 (4), 800–811. doi:10.1016/j.jhep.2021.12.004
GBD 2017 Cirrhosis Collaborators (2020). The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 5 (3), 245–266. doi:10.1016/S2468-1253(19)30349-8
He, J., Bai, K., Hong, B., Zhang, F., and Zheng, S. (2017). Docosahexaenoic acid attenuates carbon tetrachloride-induced hepatic fibrosis in rats. Int. Immunopharmacol. 53, 56–62. doi:10.1016/j.intimp.2017.09.013
He, J., Hong, B., Bian, M., Jin, H., Chen, J., Shao, J., et al. (2019). Docosahexaenoic acid inhibits hepatic stellate cell activation to attenuate liver fibrosis in a PPARγ-dependent manner. Int. Immunopharmacol. 75, 105816. doi:10.1016/j.intimp.2019.105816
Ishak, K., Baptista, A., Bianchi, L., Callea, F., De Groote, J., Gudat, F., et al. (1995). Histological grading and staging of chronic hepatitis. J. Hepatol. 22 (6), 696–699. doi:10.1016/0168-8278(95)80226-6
Lan, K., Su, M., Xie, G., Ferslew, B. C., Brouwer, K. L., Rajani, C., et al. (2016). Key role for the 12-hydroxy group in the negative ion fragmentation of unconjugated C24 bile acids. Anal. Chem. 88 (14), 7041–7048. doi:10.1021/acs.analchem.6b00573
Li, J., Guo, C., and Wu, J. (2021a). The agonists of peroxisome proliferator-activated receptor-γ for liver fibrosis. Drug Des. Devel Ther. 15, 2619–2628. doi:10.2147/DDDT.S310163
Li, Z. X., Zhao, Z. M., Liu, P., Zheng, Q. S., and Liu, C. H. (2021b). Treatment of HBV cirrhosis with fuzheng huayu tablet and entecavir: design of a randomized, double-blind, parallel and multicenter clinical trial. Chin. J. Integr. Med. 27 (7), 509–513. doi:10.1007/s11655-020-3257-6
Liu, S., Zhang, H., Li, Y., Zhang, Y., Bian, Y., Zeng, Y., et al. (2021). S100A4 enhances protumor macrophage polarization by control of PPAR-γ-dependent induction of fatty acid oxidation. J. Immunother. Cancer 9 (6), e002548. doi:10.1136/jitc-2021-002548
Liu, X., Xu, J., Rosenthal, S., Zhang, L. J., McCubbin, R., Meshgin, N., et al. (2020). Identification of lineage-specific transcription factors that prevent activation of hepatic stellate cells and promote fibrosis resolution. Gastroenterology 158 (6), 1728–1744. doi:10.1053/j.gastro.2020.01.027
McPhail, M. J. W., Shawcross, D. L., Lewis, M. R., Coltart, I., Want, E. J., Antoniades, C. G., et al. (2016). Multivariate metabotyping of plasma predicts survival in patients with decompensated cirrhosis. J. Hepatol. 64 (5), 1058–1067. doi:10.1016/j.jhep.2016.01.003
Ning, Z., Guo, X., Liu, X., Lu, C., Wang, A., Wang, X., et al. (2022). USP22 regulates lipidome accumulation by stabilizing PPARγ in hepatocellular carcinoma. Nat. Commun. 13 (1), 2187. doi:10.1038/s41467-022-29846-9
Ottka, C., Vapalahti, K., Puurunen, J., Vahtera, L., and Lohi, H. (2021). A novel canine nuclear magnetic resonance spectroscopy-based metabolomics platform: validation and sample handling. Vet. Clin. Pathol. 50 (3), 410–426. doi:10.1111/vcp.12954
Schiff, E., Simsek, H., Lee, W. M., Chao, Y. C., Sette, H., Janssen, H. L., et al. (2008). Efficacy and safety of entecavir in patients with chronic hepatitis B and advanced hepatic fibrosis or cirrhosis. Am. J. Gastroenterol. 103 (11), 2776–2783. doi:10.1111/j.1572-0241.2008.02086.x
Sun, R., Zhao, H., Huang, S., Zhang, R., Lu, Z., Li, S., et al. (2021). Prediction of liver weight recovery by an integrated metabolomics and machine learning approach after 2/3 partial hepatectomy. Front. Pharmacol. 12, 760474. doi:10.3389/fphar.2021.760474
Sun, Y., Wu, X., Zhou, J., Meng, T., Wang, B., Chen, S., et al. (2020). Persistent low level of hepatitis B virus promotes fibrosis progression during therapy. Clin. Gastroenterol. Hepatol. 18 (11), 2582–2591. doi:10.1016/j.cgh.2020.03.001
Sun, Y., Zhou, J., Wang, L., Wu, X., Chen, Y., Piao, H., et al. (2017). New classification of liver biopsy assessment for fibrosis in chronic hepatitis B patients before and after treatment. Hepatology 65 (5), 1438–1450. doi:10.1002/hep.29009
Sun, Y., Zhou, J., Wu, X., Chen, Y., Piao, H., Lu, L., et al. (2018). Quantitative assessment of liver fibrosis (qFibrosis) reveals precise outcomes in Ishak "stable" patients on anti-HBV therapy. Sci. Rep. 8 (1), 2989. doi:10.1038/s41598-018-21179-2
Wang, B., Chen, D., Chen, Y., Hu, Z., Cao, M., Xie, Q., et al. (2012). Metabonomic profiles discriminate hepatocellular carcinoma from liver cirrhosis by ultraperformance liquid chromatography-mass spectrometry. J. Proteome Res. 11 (2), 1217–1227. doi:10.1021/pr2009252
Wang, X., Xie, G., Zhao, A., Zheng, X., Huang, F., Wang, Y., et al. (2016). Serum bile acids are associated with pathological progression of hepatitis B-induced cirrhosis. J. Proteome Res. 15 (4), 1126–1134. doi:10.1021/acs.jproteome.5b00217
Wang, Y., Nakajima, T., Gonzalez, F. J., and Tanaka, N. (2020). PPARs as metabolic regulators in the liver: lessons from liver-specific PPAR-null mice. Int. J. Mol. Sci. 21 (6), 2061. doi:10.3390/ijms21062061
Wu, S. D., Liu, L. L., Cheng, J. L., Liu, Y., Cheng, L. S., Wang, S. Q., et al. (2018). Longitudinal monitoring of liver fibrosis status by transient elastography in chronic hepatitis B patients during long-term entecavir treatment. Clin. Exp. Med. 18 (3), 433–443. doi:10.1007/s10238-018-0501-x
Keywords: metabolomics, PPAR γ, HBV-related cirrhosis, regression, fatty acids
Citation: Fan H-N, Zhao Z-M, Huang K, Wang X-N, Dai Y-K and Liu C-H (2023) Serum metabolomics characteristics and fatty-acid-related mechanism of cirrhosis with histological response in chronic hepatitis B. Front. Pharmacol. 14:1329266. doi: 10.3389/fphar.2023.1329266
Received: 28 October 2023; Accepted: 06 December 2023;
Published: 21 December 2023.
Edited by:
Zhengsheng Zou, Fifth Medical Center of the PLA General Hospital, ChinaReviewed by:
Jinsheng Guo, Fudan University, ChinaTian Lan, Guangdong Pharmaceutical University, China
Copyright © 2023 Fan, Zhao, Huang, Wang, Dai and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun-Kai Dai, eXVuLWthaWRhaUBAaG90bWFpbC5jb20=; Cheng-Hai Liu, Y2hlbmdoYWlsaXVAaG90bWFpbC5jb20=
†These authors share first authorship
 Hai-Na Fan
Hai-Na Fan Zhi-Min Zhao
Zhi-Min Zhao Kai Huang
Kai Huang Xiao-Ning Wang
Xiao-Ning Wang Yun-Kai Dai
Yun-Kai Dai Cheng-Hai Liu1,2*
Cheng-Hai Liu1,2*