- Department of Respiratory Medicine, The Second Hospital of Jilin University, Changchun, Jilin, China
Irisin, a myokine, is secreted by the movement of skeletal muscles. It plays an important role in metabolic homeostasis, insulin resistance, anti-inflammation, oxidative stress, and bone metabolism. Several studies have reported that irisin-related signaling pathways play a critical role in the treatment of various diseases, including obesity, cardiovascular disease, diabetes, and neurodegenerative disorders. Recently, the potential role of irisin in lung diseases, including chronic obstructive pulmonary disease, acute lung injury, lung cancer, and their associated complications, has received increasing attention. This article aims to explore the role of irisin in lung diseases, primarily focusing on the underlying molecular mechanisms, which may serve as a marker for the diagnosis as well as a potential target for the treatment of lung diseases, thus providing new strategies for their treatment.
Introduction
Globally, lung diseases, including chronic obstructive pulmonary disease (COPD), bronchial asthma, interstitial pneumonitis, obstructive sleep apnea hypoventilation syndrome (OSAHS), pulmonary embolism (PE), and lung cancer, have high morbidity and mortality rates, adding to the healthcare burden (GBD Chronic Respiratory Disease Collaborators, 2020). Effective measures to enhance the prevention and treatment of lung diseases are urgently required to improve the health of patients and to reduce the healthcare burden.
Irisin, a hormone-like substance, comprises of 112 amino acids. Fibronectin type III domain-containing protein 5 (FNDC5), an irisin precursor, is formed by the cleavage of irisin (Boström et al., 2012). Exercise improves cardiorespiratory fitness, and irisin is primarily secreted by skeletal muscles during exercise. Available literature suggests that soccer players have significantly higher serum irisin levels compared to healthy individuals, which may be attributed to exercise and skeletal muscle function (Gaudio et al., 2021). Irisin is essential for the maintenance of metabolic homeostasis, regulation of energy and heat production, promotion of white fat browning (Wang and Pan, 2016; Xiong et al., 2019; Chen et al., 2021; Aladag et al., 2023), attenuation of insulin resistance (IR) (Ye et al., 2019; Zheng et al., 2022), as well as regulation of bone metabolism (Buccoliero et al., 2021; Zhu et al., 2021) and neurological functions (Pignataro et al., 2021). A growing body of evidence suggests that irisin may have a protective role in lung diseases. Several studies have shown that it is aberrantly expressed in lung diseases and is involved in the pathogenesis of COPD, asthma, acute lung injury (ALI), pulmonary hypertension, lung cancer, and other lung diseases. It acts by reducing oxidative stress (Ho et al., 2018), improving endothelial cell function (Bi et al., 2020), resisting apoptosis, and inhibiting inflammatory factor production (Shao et al., 2017a; Ma et al., 2021). Moreover, irisin inhibits the migration and proliferation of cancer cells and is a potential target for the treatment of lung cancer (Shao et al., 2017b). It may be a potential biomarker and therapeutic target for lung diseases. In this article, we summarize the possible molecular mechanisms and functions of irisin in regulating lung diseases (Figure 1), contributing to a better understanding of the role of irisin in lung diseases, which could help to identify novel targets for the diagnosis or treatment of lung diseases as well as to develop promising interventional strategies for the treatment of these diseases.
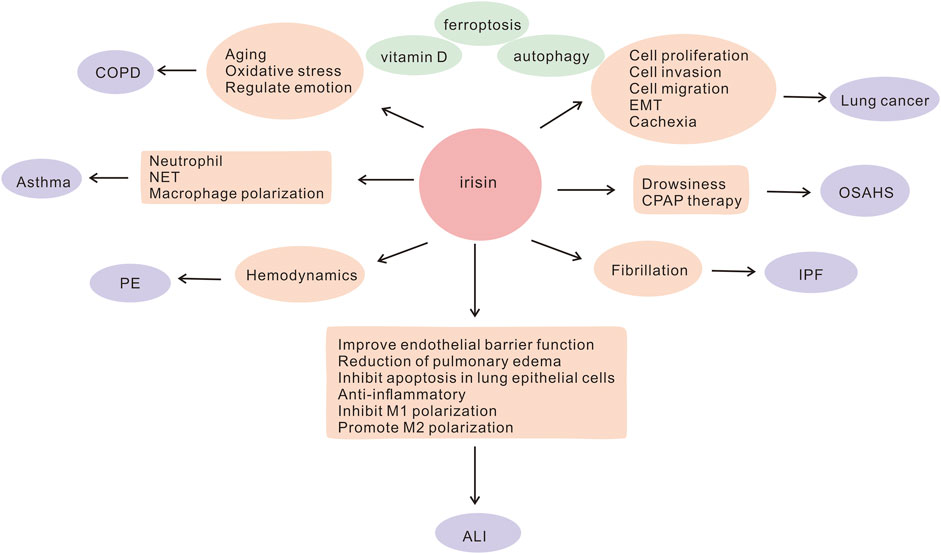
FIGURE 1. Involvement of irisin in the potential pathogenesis of lung diseases ALI, acute lung injury; COPD, chronic obstructive pulmonary disease; CPAP, continuous airway positive pressure; EMT, epithelial-mesenchymal transition; IPF, idiopathic pulmonary fibrosis; NET, neutrophil extracellular trap; OSAHS, obstructive sleep apnea hypoventilation syndrome; PE, pulmonary embolism.
Irisin and cellular pathways
Ferroptosis is an iron-dependent mode of cell death that plays a key role in the pathogenesis of lung disease (Li et al., 2022); therefore, targeting the associated pathways could be a novel strategy for the treatment of lung diseases, including idiopathic pulmonary fibrosis (IPF) (Guo et al., 2023a), ALI (Li et al., 2022), lung cancer (Zhao et al., 2023), COPD (Yoshida et al., 2019), and asthma (Li et al., 2023). Irisin appears to be an important regulator of ferroptosis. Serum irisin expression is reduced in patients with sepsis, with lower irisin levels indicating a higher sepsis severity (Wei et al., 2020). Irisin has an inhibitory effect on ferroptosis through different pathways and beneficial effects on sepsis-related organ damage, such as encephalopathy (Wang et al., 2022) and liver injury (Wei et al., 2020). It has effects on different causes of renal injury. For example, in in-vivo and in-vitro studies, Zhang et al. have demonstrated that irisin-associated activation of sirtuin 1 (SIRT1)/nuclear factor E2-related factor 2 (Nrf2) inhibits ferroptosis to attenuate renal injury in sepsis patients (Qiongyue et al., 2022). Contrarily, another study has revealed that irisin ameliorates renal injury in ischemia-reperfusion by promoting the ferroptosis protein glutathione peroxidase 4 (Zhang et al., 2021). Meanwhile, in pancreatic cancer, irisin increases ferroptosis and reactive oxygen species (ROS) accumulation as well as helps protect against pancreatic cancer progression (Yang and Leung, 2020). Therefore, irisin may be used to treat lung diseases by modulating ferroptosis-related pathways.
Macroautophagy (or autophagy for short) is the process by which cells remove damaged organelles and their own proteins to maintain cellular homeostasis. It is essential for lung health as well as disease, and it helps with bacterial clearance (Junkins et al., 2013). First, autophagy is an important part of lung development and morphogenesis, and its impairment may lead to bronchopulmonary dysplasia (Yeganeh et al., 2019). Autophagy is not only involved in lung development and maintenance of morphology, but it is also a key link in the development and treatment of lung diseases (Yeganeh et al., 2019; Zhang et al., 2022). It plays a crucial role in ALI (Zhang et al., 2018; Nosaka et al., 2020), asthma (Suzuki et al., 2022), IPF (Gui et al., 2015), lung cancer (Hou et al., 2020), and COPD (Bodas et al., 2019). There is an important link between irisin and autophagy. Irisin enhances the expression of the autophagy protein light chain 3-II in particulate matter less than 2.5 µm in diameter (PM2.5)-induced ALI and decreases the expression of p62 protein, which promotes autophagy and decreases the expression of proinflammatory factors such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, and IL-18 (Ma et al., 2023). In a diabetic cardiomyopathy model, irisin led to inhibition of autophagy in H9c2 cardiomyocytes and ameliorated IR through the phosphatidylinositol 3-kinase (PI3K)/AKT pathway (Song et al., 2021). In contrast, in C2C12 cells, irisin promoted autophagy and ameliorated IR through the p38–mitogen-activated protein kinase (MAPK)–peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC-1α) signaling pathway (Ye et al., 2019). These contradictory findings are likely due to the use of different cell lines and the complex pathogenesis involved. Irisin also plays a key role in bones and joints. It induces autophagy in bone marrow mesenchymal stem cells, promotes osteoblast differentiation, and plays a role in osteoporosis, as evidenced by the Wnt/β-catenin pathway (Chen et al., 2020). In addition, it has a protective role in degenerative disc disease. Exercise-associated irisin promotes autophagy in nucleus pulposus cells, inhibits cellular senescence and apoptosis, and helps to improve degenerative disc disease (Zhou et al., 2022). By ameliorating cardiac hypertrophy and inducing autophagy through the AMP-activated protein kinase (AMPK)–Unc-51-like autophagy-activating kinase 1 pathway as well as reducing cardiomyocyte apoptosis, irisin may be a novel target for the treatment of cardiac diseases (Li et al., 2018; Li R. et al., 2019). It has a paradoxical role in the regulation of autophagy, possibly due to different microenvironments and acting on different signaling pathways. Therefore, regulation of autophagy by irisin opens up ideas for treating lung diseases and may serve as a new approach to therapy.
Irisin not only plays an important role in regulating ferroptosis and autophagy in lung diseases, but it may be a predictive biomarker for lung diseases. Its aberrant expression in lung diseases is involved in the molecular pathogenesis of lung diseases as well as mood regulation, and it is closely linked to rehabilitative exercises, prognosis, and even treatment. However, further research is required to elucidate the potential role of irisin in lung diseases.
Irisin and lung diseases
The role of irisin is well established in metabolic, neurological, and cardiovascular diseases (Polyzos et al., 2018; Yu et al., 2019; Qi et al., 2022). Though its role in lung diseases is promising, it has been less studied and requires further exploration. In this article, we summarize the link between irisin and lung diseases as well as its mechanism of action in lung diseases (Table 1).
Irisin in ALI/acute respiratory distress syndrome (ARDS)
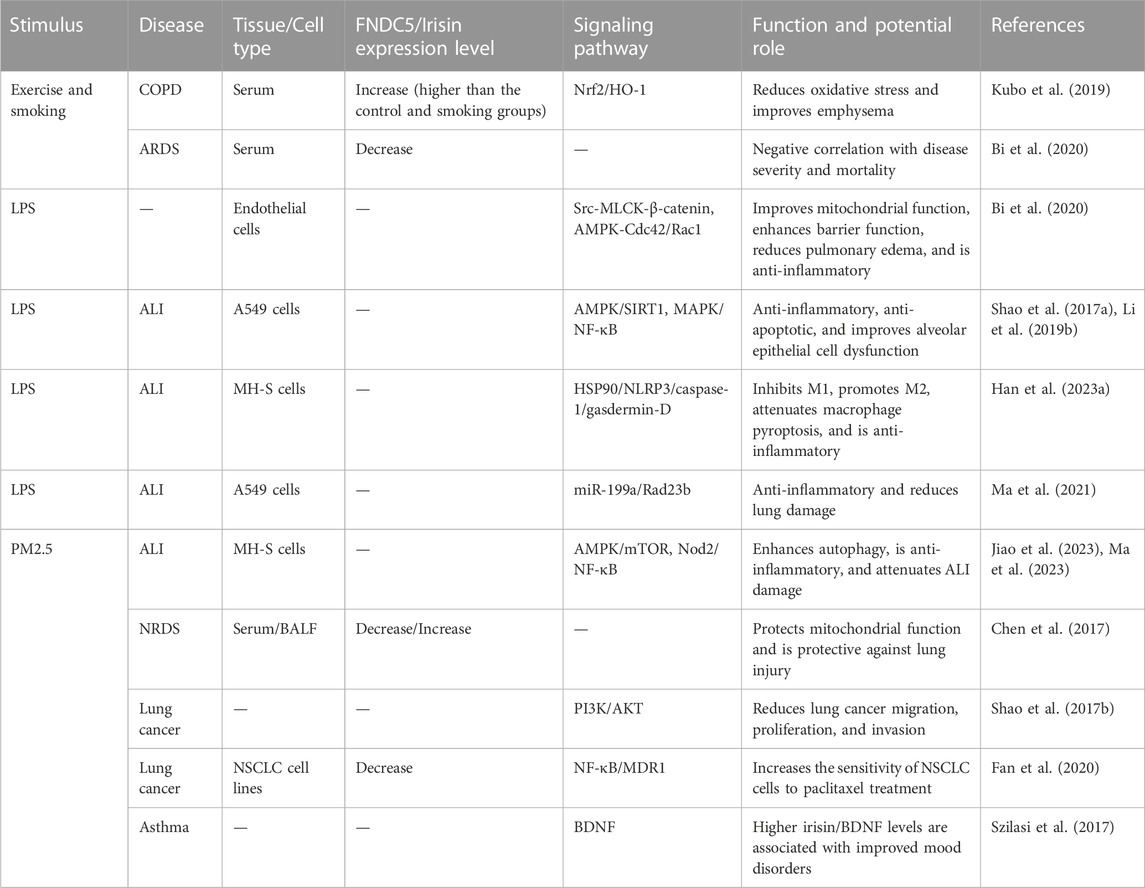
ALI/ARDS is a severe disease with a high mortality rate. It is caused by a variety of factors, including infection, trauma (Ware and Matthay, 2000), sepsis, and coronavirus disease 2019 infection (Roden et al., 2022; Sang et al., 2022). Its pathogenesis varies depending on the microenvironment of the disease. Recently, the role of irisin in ALI has received great attention. In patients with ARDS, the serum irisin level is less than that of healthy individuals; in addition, as the irisin level increases, the disease is milder and the prognosis is better (Bi et al., 2020). Similarly, the serum irisin levels are reported to be less in patients with neonatal respiratory distress syndrome than in healthy neonates, while its concentrations in bronchoalveolar lavage fluid are less than the serum levels. Moreover, exogenous administration of irisin prior to lung ischemia/reperfusion is reported to be protective (Chen et al., 2017). It is further suggested that irisin plays an anti-inflammatory role in the pathogenesis of ALI. Irisin improves endothelial barrier function and lung permeability as well as attenuates lung edema and inflammation in ALI, possibly through the AMPK/SIRT1 signaling pathway and inhibition of p-Src/myosin light-chain kinase/β-linker protein, activation of AMPK-Ras-related C3 botulinum toxin substrate 1/cell division control protein 42 homolog, and protection of mitochondrial function (Chen et al., 2017; Li et al., 2019; Bi et al., 2020). Similarly, the protective effect of irisin against ALI has been validated by Shao et al. in in-vivo and in-vitro ALI models. The results demonstrate that irisin suppresses MAPK and induces nuclear factor kappa B (NF-κB) pathways, and simultaneously inhibits apoptosis and inflammation in lung epithelial cells (Shao et al., 2017a). Irisin inhibits the heat shock protein 90/NOD-like receptor family pyrin domain containing 3/caspase-1/gasdermin D pathway, suppresses M1 polarization, promotes M2 polarization, and inhibits macrophage cell pyroptosis (Han et al., 2023). Another study has demonstrated that irisin can exert anti-inflammatory effects in lipopolysaccharide (LPS) and Nrf2-induced ALI by inhibiting miR-199a, thus upregulating downstream upregulated Rad23b (Ma et al., 2021). Irisin also has been shown to ameliorate inflammatory cell infiltration in PM2.5-induced ALI, and this is attributed to promotion of autophagy and inhibition of nucleotide-binding oligomerization domain-containing protein 2/NF-κB (Jiao et al., 2023; Ma et al., 2023). Thus, irisin exerts a protective effect against ALI through a variety of mechanisms and is a potential molecule for the treatment of ALI.
Irisin in COPD
COPD, a chronic airway obstructive heterogeneous disease, includes chronic bronchitis and emphysema. Its pathogenesis involves oxidative stress, cellular senescence, and inflammatory mechanisms (Barnes, 2021; Barnes, 2022). Its high morbidity and mortality rates have burdened the physical and mental health of patients as well as the healthcare system alike (López-Campos et al., 2016). Infection and smoking are among the most common risk factors for acute exacerbation of COPD. Traditional treatment can effectively control the disease and reduce the risk of acute exacerbation, but exacerbations are still unavoidable due to the heterogeneity of the disease. Multiple factors have been identified as COPD biomarkers, providing a new direction for individualized treatment. Irisin has been recognized as a promising biomarker for COPD. It has been shown that the serum irisin levels are lower in COPD patients than in healthy individuals (Ijiri et al., 2015), and these low levels are involved in the development of emphysema (Sugiyama et al., 2017). The serum levels are reduced in COPD patients who smoke (Kureya et al., 2016), and the levels are reported to be elevated in mice in the exercise and smoking group compared to mice in the smoking-only group. Exercise and irisin ameliorate cigarette-induced emphysema, and this effect is through the Nrf2/heme oxygenase 1 (HO-1) pathway (Kubo et al., 2019). Moreover, COPD mice have reduced skeletal muscle FNDC5/irisin expression, and this is due to the fact that irisin promotes skeletal muscle growth and exposure to cigarette smoke leads to skeletal muscle dysfunction (Zhang et al., 2022).
Aging and oxidative stress are important aspects of COPD pathogenesis, and irisin is closely related to both. Oxidative stress due to oxidative/antioxidative imbalance is a critical link in the pathogenesis of COPD. Many studies have demonstrated that irisin exhibits antioxidant activity that inhibits oxidative stress. For example, exogenous irisin administration has been shown to inhibit oxidative stress as well as ameliorate pancreatic inflammation and fibrosis in chronic pancreatitis (Ren et al., 2020); furthermore, it decreases nicotine-induced oxidative stress and endothelial cell dysfunction (Sarwar et al., 2022). In diabetes-induced cardiac microangiopathy, irisin increases antioxidant protein expression and reduces oxidative stress through activation of the ERK1/2/Nrf2/HO-1 pathway (Zhu et al., 2022). Moreover, exercise-induced secretion of irisin reduces oxidative stress and attenuates cigarette smoke-induced emphysema by activating the Nrf2/HO-1 pathway (Kubo et al., 2019). Nrf2/HO-1 has antioxidant activity and is closely related to irisin, which may attenuate oxidative stress in a variety of other diseases through a spectrum of mechanisms that may have beneficial effects in lung diseases. However, additional studies are required to explore these mechanisms.
Aging reduces skeletal muscle strength, impairs body function, and increases the chance of disease. COPD accelerates aging of the lungs, and exercise can slow this process. Serum irisin levels are significantly lower in the elderly than in young and middle-aged individuals (Chang et al., 2017). Similarly, the serum FNDC5 levels are reported to be reduced in aging mice, and exogenous irisin administration helps to ameliorate aging-associated impaired cardiac function, cardiac remodeling, and inflammation (Hu et al., 2022). Irisin helps to delay osteoblast senescence, and in a study involving patients undergoing total hip or knee arthroplasty, old age was associated with lower serum irisin levels (Colaianni et al., 2021). Moreover, irisin is positively correlated with vertebral and femoral bone density, and serum irisin levels have been found to be lower in osteoporotic patients (Colaianni et al., 2021). Exercise improves muscle mass and function, and resistance training increases blood expression of irisin and enhances muscle strength in the elderly (Kim et al., 2015). In COPD patients, the senescence suppressor gene klotho has been found to be expressed significantly less among smokers than nonsmokers, and the levels are positively correlated with irisin; thus, the relationship between senescence and irisin levels in COPD deserves to be investigated (Kureya et al., 2016). Irisin helps to ameliorate aging, and exercise training increases its expression, which may be potentially beneficial for COPD treatment.
Irisin in asthma
Asthma, a heterogeneous disease, involves chronic inflammation of the airways. Based on the proportion of inflammatory cells in the induced sputum, it is categorized as eosinophilic asthma, neutrophilic asthma, granulocyte-deficient asthma, and mixed-cell asthma. The pathogenesis of different types of asthma varies (Simpson et al., 2006).
Irisin and macrophages in asthma
Macrophages are important cells in the immune response, and exposure to allergenic stimuli in asthmatics leads to monocyte recruitment to promote an inflammatory response, whereas macrophages in the alveoli act as suppressors to maintain homeostasis (Zasłona et al., 2014). Irisin has a significant effect on macrophage polarization and regulates monocyte infiltration in different microenvironments. In cerebral ischemia, irisin has beneficial effects on neurons by inhibiting monocyte infiltration and microglia activation as well as decreasing the levels of the proinflammatory factors TNF-α and IL-6 (Li et al., 2017). Irisin regulates macrophage function, activity, and polarization. The macrophage activity is regulated by reducing ROS overproduction, thereby exerting anti-inflammatory effects (Mazur-Bialy, 2017). We speculate that irisin may likewise have a protective role in asthma by inhibiting monocyte infiltration in the lungs and promoting the activation of resident alveolar macrophages to exert an anti-inflammatory effect, which is worth investigating in the future.
M1-M2 macrophage polarization affects the asthma inflammatory subtype, with M1 and M2 polarization mainly involved in neutrophilic and eosinophilic asthma, respectively (Robbe et al., 2015). Depending on the microenvironment, irisin has a dual effect on macrophage polarization. In an LPS-induced mouse model of sepsis and in-vitro cell- and bone marrow-derived macrophages, irisin induces anti-inflammatory differentiation of M2 macrophages, an effect that has been shown to be mediated by the induction of the peroxisome proliferator-activated receptor gamma-related anti-inflammatory system and the Janus kinase 2-signal transducer and activator of transcription 6-dependent transcriptional activation of Nrf2-associated antioxidant genes (Tu et al., 2023). In addition, irisin-induced M2 polarization enhances osteogenesis in osteoblasts, an effect that may be related to AMPK activation (Ye et al., 2020). Moreover, aerobic exercise can effectively activate the FNDC5/irisin and PI3K/AKT signaling pathways, promote the polarization of M2 macrophages, and inhibit the inflammatory response of the liver after myocardial infarction (Wang et al., 2023). In mice, irisin administration following LPS stimulation leads to inhibition of M1 polarization and promotion of M2 polarization, thus reducing LPS-induced production and secretion of IL-1β, IL-18, and TNF-α, resulting in anti-inflammatory activity and reduced alveolar inflammatory cell infiltration (Han et al., 2023). However, the role of irisin in macrophage polarization in asthma inflammatory subtypes seems to be contradictory, possibly due to different experimental reagents and cells as well as the presence of multiple signaling pathways; the exact reason for this is unclear and requires further investigation.
Irisin and neutrophils in asthma
Neutrophilic asthma, a hormone-resistant asthma, is insensitive to hormone therapy, and a specific drug for its treatment is still being investigated. Irisin inhibits neutrophil infiltration and IL-1β expression at 24 h after cerebral hemorrhage, inhibits macrophage activation, increases M1 polarization to M2, and is neuroprotective (Wang et al., 2022b). Neutrophil extracellular traps (NETs) are a key component in asthma, and plasma NET biomarkers are reduced in asthmatics compared to healthy individuals and are negatively correlated with lung function (Varricchi et al., 2022). In an in-vitro model of neutrophilic inflammation, irisin reduced NET formation, inhibited pancreatitis inflammatory cell infiltration, and attenuated injury via the integrin αVβ5-P38/MAPK pathway (Han et al., 2023). Meanwhile, airway smooth muscle cells express αVβ5 integrin and activate transforming growth factor beta in vivo, thus promoting cellular hypertrophy in asthma models (Tatler et al., 2011). Nevertheless, the relationship between irisin and neutrophilic asthma remains to be elucidated.
Irisin and mental health
Irisin also plays an important role in regulating mood. Owing to physical discomfort, COPD patients often have abnormal moods, including anxiety and depression (Yohannes et al., 2022; Martínez-Gestoso et al., 2022). Irisin has a beneficial effect on improving mood. Serum irisin levels are lower in patients with poor moods, and an association has been reported to be related to brain-derived neurotrophic factor (BDNF) (Papp et al., 2017). Moreover, exercise leads to an increase in serum irisin levels; this effect is observed after 8 weeks of exercise training, and appropriate physical activity may help to improve COPD (Ijiri et al., 2015). The exercise-associated increase in irisin levels leads to an improved quality of life and prognosis in COPD patients (Greulich et al., 2014; Boeselt et al., 2017). Similar to its role in COPD, the irisin-BDNF signaling pathway contributes to attenuation of mood disorders, including anxiety and depression, in asthma patients (Szilasi et al., 2017).
Irisin in IPF
IPF is a type of interstitial lung disease with an unknown etiology and a poor prognosis (León-Román et al., 2022). There is an inextricable relationship between irisin and fibrosis. It has a protective effect in hepatic fibrosis, renal fibrosis, pancreatic fibrosis, and cardiac fibrosis, and it may be a potential target for therapy (Ren et al., 2020; Pan et al., 2021; Armandi et al., 2022; Yang et al., 2023). In a mouse model of carbon tetrachloride-induced hepatic fibrosis, irisin appears to play a key role and can alleviate endoplasmic reticulum stress and hepatic fibrosis through inhibition of protein kinase RNA-like endoplasmic reticulum kinase-mediated destabilization of heterogeneous nuclear ribonucleoprotein A1 (Liao et al., 2021). In addition, patients with the presence of the FNDC5 rs3480 A>G gene variant have a low prevalence of fibrosis in nonalcoholic fatty liver disease (NAFLD). In hepatic fibrosis, irisin is expressed at higher levels in both the serum and the hepatic tissue, and it has a profibrotic effect that is associated with hepatic stellate cell activation (Petta et al., 2017; Dong et al., 2020). In nonobese, nondiabetic, nonalcoholic patients with fatty liver disease, the role of irisin is reversed. The serum irisin levels are more pronounced in patients with more severe fibrosis, and correlation analyses have demonstrated a positive correlation between irisin and the representative fibrosis markers P type III collagen propeptide and type VI collagen cleavage product, which may participate in the pathogenesis of fibrosis (Armandi et al., 2022). In addition, the administration of nicotinamide ribose to mice with NAFLD has been shown to increase the plasma FNDC5/irisin levels as well as FNDC5 deubiquitination and deacetylation via sirtuin 2 for the treatment of NAFLD (Li et al., 2021). Irisin also reduces renal fibrosis. In-vivo and in-vitro studies on diabetic nephropathy have revealed that irisin attenuates epithelial-mesenchymal transition (EMT) and fibrosis as well as reduces renal injury by inhibiting the Smad/β-catenin pathway (Yang et al., 2023). Furthermore, irisin attenuates angiotensin II-induced and doxorubicin-induced cardiac fibrosis as well as cardiomyocyte hypertrophy (Chen et al., 2019; Pan et al., 2021). In conclusion, irisin is a promising target for the treatment of fibrotic diseases; however, its effect on fibrosis associated with interstitial lung disease remains to be investigated.
Irisin in lung cancer
Lung cancer is one of the cancers with a high mortality and morbidity. Cell proliferation, invasion, migration, angiogenesis, EMT, and drug resistance can all contribute to its progression. EMT can promote lung cancer invasion and metastasis (Tang et al., 2019; Wang et al., 2022), and it is associated with drug resistance in lung cancer (Cheng et al., 2020). Irisin expression levels vary in different types of cancers, and its role appears to be contradictory. The serum irisin levels were significantly lower in patients with colon, bladder, liver, and breast cancers than in healthy controls (Provatopoulou et al., 2015; Pazgan-Simon et al., 2020; Taken et al., 2022; Celik et al., 2023), whereas they were elevated in patients with kidney cancer (Altay et al., 2018). There are also differences regarding its effects. On the one hand, it is harmful to humans, with increased levels of irisin in liver cancer tissues without changes in the serum, and promotes cancer development (Shi et al., 2017). On the other hand, irisin is beneficial to humans and inhibits cell proliferation, migration, and invasion in ovarian, glioma, and pancreatic cancer cells (Zhang et al., 2019; Huang et al., 2020; Alizadeh Zarei et al., 2023). In pancreatic cancer, irisin is reported to inhibit EMT via the AMPK-mTOR pathway and to suppress tumor progression (Liu et al., 2018). In addition, irisin is highly expressed in breast cancer tissues and is associated with a longer survival and a good prognosis in patients (Cebulski et al., 2022). Irisin appears to be beneficial in lung cancer by reversing the activity of EMT through the PI3K/AKT pathway, which may be useful in controlling the proliferation, invasion, and migration of lung cancer and controlling cancer progression (Shao et al., 2017b). Importantly, FNDC5 expression is decreased in paclitaxel-resistant non-small-cell lung cancer, and exogenous irisin can increase the sensitivity of lung cancer to paclitaxel, thus contributing to treatment. This effect is mediated through the NF-κB/multidrug resistance protein 1 pathway (Fan et al., 2020). Moreover, cachexia, a muscular dystrophy, is a common manifestation of cancer patients and is associated with a poor prognosis. Irisin appears to be an important influencing factor for cachexia, with high levels contributing to a reduced likelihood of developing sarcopenia in metastasis-free colorectal cancer (Oflazoglu et al., 2022). In-vivo experiments demonstrate that irisin ameliorates sarcopenia in aged mice (Guo et al., 2023b). In summary, irisin is crucial in the development of cancer. Therefore, exogenous irisin administration is expected to be a therapeutic agent for targeting lung cancer.
Irisin in PE/idiopathic pulmonary hypertension
PE is an obstructive disease of the pulmonary arteries, and chronic PE can lead to pulmonary hypertension and right ventricular hypertrophy, a disease with a high morbidity rate. Irisin expression can be used to evaluate the hemodynamic changes in idiopathic pulmonary hypertension, and lower serum irisin levels are associated with higher mean pulmonary arterial pressures and a poorer prognosis (Sun et al., 2021). The serum irisin levels are significantly lower in patients with acute PE than in controls without PE, and they are negatively correlated with the PE severity index. Irisin detection is useful for the diagnosis of PE; however, its sensitivity and specificity are poorer than those of D-dimers (Gurger et al., 2021). Consistently, in a study of patients with acute PE, the group with a higher serum irisin level had a better prognosis, was hemodynamically more stable, and had a lower probability of deterioration (Sun et al., 2020). In summary, irisin can be used as a biomarker for the diagnosis of PE and has some significance in predicting the prognosis of patients.
Irisin in OSAHS
Irisin appears to play a crucial role in OSAHS, with irisin expression negatively correlating with OSAHS severity (Huang et al., 2020). Excessive daytime sleepiness symptoms are associated with high serum irisin and BDNF levels (More et al., 2019). Continuous positive airway pressure (CPAP) is an important technical tool for the treatment of OSAHS, and studies have shown that receiving short-term CPAP therapy increases irisin levels compared to subtherapeutic CPAP (Ng et al., 2017).
Irisin and treatment
Traditional therapeutic drugs
Glucocorticosteroids have anti-inflammatory and immunomodulatory properties. They are one of the main drugs used to treat lung diseases. However, they are associated with various adverse effects, including osteoporosis, muscle atrophy, high blood pressure, and high blood sugar levels, which need to be prevented. It has been reported that irisin has an ameliorative effect on dexamethasone-induced muscle atrophy (Chang and Kong, 2020). Moreover, in hepatocytes, glucocorticoid receptor enhances the transcription of the FNDC5 gene and the expression of irisin (Kim et al., 2017). Paradoxically, dexamethasone downregulates irisin expression (Mohammed et al., 2019). Meanwhile, N-acetylcysteine is an antioxidant that reduces ALI inflammation, asthmatic airway hyperresponsiveness, and emphysema formation as well as attenuates pulmonary fibrosis (Zhang et al., 2014; Breau et al., 2019; Lee et al., 2020; Zhao et al., 2022). It also has an important role in lung diseases and elevates irisin levels as well as attenuates oxidative stress and tissue damage in a diabetes model (Yalçın and Gürel, 2021). Furthermore, long-acting muscarinic receptor antagonists are bronchodilators that are one of the mainstays in the treatment of asthma and COPD. COPD treatment with long-acting muscarinic receptor antagonists can raise the serum irisin levels, and a positive correlation exists between the two, which can lead to an improved prognosis and have a beneficial effect on the patient (Mandal et al., 2018).
Irisin and vitamin D
Vitamin D is a potential medication for asthma and COPD, and it has been associated with asthmatic lung function, airway hyperresponsiveness, and sensitivity to glucocorticoids, with lower levels suggesting a worse prognosis (Sutherland et al., 2010). Vitamin D supplementation reduces asthma airway hyperresponsiveness and inflammation (Agrawal et al., 2013). Moreover, it decreases ALI and pulmonary emphysema-associated inflammation with beneficial effects (Shi et al., 2016; Hu et al., 2019). In type 2 diabetes, vitamin D supplementation increases SIRT1 and irisin expression as well as improves IR (Safarpour et al., 2020). Interestingly, the serum vitamin D and irisin levels are positively correlated in women with sarcopenia (Wang et al., 2022d). Vitamin D supplementation also increases the serum irisin levels in hyperparathyroidism, as demonstrated in skeletal muscle cells in-vitro, probably acting through the SIRT1 and PGC-1α pathways (Sanesi et al., 2023). Thus, vitamin D has a positive effect on irisin, increasing its levels and playing a critical role in various diseases.
Irisin and other potential therapies
In summary, ferroptosis and autophagy have important contributions in the pathogenesis of lung diseases, and alleviation of lung diseases by drugs that modulate ferroptosis-related pathways and autophagic processes is a potential therapeutic option. The close association between irisin and iron-dependent death as well as autophagy and targeting it opens up the opportunity of treating lung diseases and may serve as a new therapeutic approach worth developing.
Conclusion
In summary, irisin is closely associated with the diagnosis, treatment, and prognosis of lung diseases, making it an attractive target for the treatment of lung diseases. However, the mechanisms underlying the development and progression of various lung diseases are highly intricate and involve multiple influencing factors and signaling pathways. Exogenous administration of irisin may be a novel strategy for the treatment of lung diseases. Therefore, an in-depth study regarding the role of irisin in the development and progression of lung diseases will help to create new avenues for disease prevention and treatment. Moreover, additional studies are required to elucidate the mechanism of action of irisin in various lung diseases and its potential as a therapeutic target.
Author contributions
HD: Writing–original draft. XL: Writing–original draft. PG: Writing–review and editing. YH: Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the Natural Science Foundation of Jilin Province (YDZJ202201ZYTS052) and the National Science Foundation of China (82070037).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agrawal, T., Gupta, G. K., and Agrawal, D. K. (2013). Vitamin D supplementation reduces airway hyperresponsiveness and allergic airway inflammation in a murine model. Clin. Exp. allergy 43 (6), 672–683. doi:10.1111/cea.12102
Aladag, T., Mogulkoc, R., and Baltaci, A. K. (2023). Irisin and energy metabolism and the role of irisin on metabolic syndrome. Mini Rev. Med. Chem. 23, 1942–1958. doi:10.2174/1389557523666230411105506
Alizadeh Zarei, M., Seyed Hosseini, E., Haddad Kashani, H., Ahmad, E., and Nikzad, H. (2023). Effects of the exercise-inducible myokine irisin on proliferation and malignant properties of ovarian cancer cells through the HIF-1 α signaling pathway. Sci. Rep. 13 (1), 170. doi:10.1038/s41598-022-26700-2
Altay, D. U., Keha, E. E., Karagüzel, E., Menteşe, A., Yaman, S. O., and Alver, A. (2018). The diagnostic value of FNDC5/irisin in renal cell cancer. Int. Braz. J. Urol. 44 (4), 734–739. doi:10.1590/S1677-5538.IBJU.2017.0404
Armandi, A., Rosso, C., Nicolosi, A., Caviglia, G. P., Abate, M. L., Olivero, A., et al. (2022). Crosstalk between irisin levels, liver fibrogenesis and liver damage in non-obese, non-diabetic individuals with non-alcoholic fatty liver disease. J. Clin. Med. 11 (3), 635. doi:10.3390/jcm11030635
Barnes, P. J. (2021). Targeting cellular senescence as a new approach to chronic obstructive pulmonary disease therapy. Curr. Opin. Pharmacol. 56, 68–73. doi:10.1016/j.coph.2020.11.004
Barnes, P. J. (2022). Oxidative stress in chronic obstructive pulmonary disease. Antioxidants (Basel, Switz. 11 (5), 965. doi:10.3390/antiox11050965
Bi, J., Zhang, J., Ren, Y., Du, Z., Zhang, Y., Liu, C., et al. (2020). Exercise hormone irisin mitigates endothelial barrier dysfunction and microvascular leakage-related diseases. JCI insight 5 (13), e136277. doi:10.1172/jci.insight.136277
Bodas, M., Pehote, G., Silverberg, D., Gulbins, E., and Vij, N. (2019). Autophagy augmentation alleviates cigarette smoke-induced CFTR-dysfunction, ceramide-accumulation and COPD-emphysema pathogenesis. Free Radic. Biol. Med. 131, 81–97. doi:10.1016/j.freeradbiomed.2018.11.023
Boeselt, T., Nell, C., Lütteken, L., Kehr, K., Koepke, J., Apelt, S., et al. (2017). Benefits of high-intensity exercise training to patients with chronic obstructive pulmonary disease: a controlled study. Respir. Int. Rev. Thorac. Dis. 93 (5), 301–310. doi:10.1159/000464139
Boström, P., Wu, J., Jedrychowski, M. P., Korde, A., Ye, L., Lo, J. C., et al. (2012). A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481 (7382), 463–468. doi:10.1038/nature10777
Breau, M., Houssaini, A., Lipskaia, L., Abid, S., Born, E., Marcos, E., et al. (2019). The antioxidant N-acetylcysteine protects from lung emphysema but induces lung adenocarcinoma in mice. JCI insight 4 (19), e127647. doi:10.1172/jci.insight.127647
Buccoliero, C., Oranger, A., Colaianni, G., Pignataro, P., Zerlotin, R., Lovero, R., et al. (2021). The effect of Irisin on bone cells in vivo and in vitro. Biochem. Soc. Trans. 49 (1), 477–484. doi:10.1042/BST20200978
Cebulski, K., Nowińska, K., Jablońska, K., Romanowicz, H., Smolarz, B., Dzięgiel, P., et al. (2022). Expression of irisin/FNDC5 in breast cancer. Int. J. Mol. Sci. 23 (7), 3530. doi:10.3390/ijms23073530
Celik, Z., Baygutalp, N. K., Kilic, A. F., Tekin, S. B., Bakan, E., Gul, M. A., et al. (2023). Serum irisin levels in colorectal cancer patients. Eur. Rev. Med. Pharmacol. Sci. 27 (4), 1474–1479. doi:10.26355/eurrev_202302_31387
Chang, J. S., Kim, T. H., Nguyen, T. T., Park, K. S., Kim, N., and Kong, I. D. (2017). Circulating irisin levels as a predictive biomarker for sarcopenia: a cross-sectional community-based study. Geriatrics gerontology Int. 17 (11), 2266–2273. doi:10.1111/ggi.13030
Chang, J. S., and Kong, I. D. (2020). Irisin prevents dexamethasone-induced atrophy in C2C12 myotubes. Pflugers Archiv Eur. J. physiology 472 (4), 495–502. doi:10.1007/s00424-020-02367-4
Chen, K., Xu, Z., Liu, Y., Wang, Z., Li, Y., Xu, X., et al. (2017). Irisin protects mitochondria function during pulmonary ischemia/reperfusion injury. Sci. Transl. Med. 9 (418), eaao6298. doi:10.1126/scitranslmed.aao6298
Chen, R. R., Fan, X. H., Chen, G., Zeng, G. W., Xue, Y. G., Liu, X. T., et al. (2019). Irisin attenuates angiotensin II-induced cardiac fibrosis via Nrf2 mediated inhibition of ROS/TGFβ1/Smad2/3 signaling axis. Chemico-biological Interact. 302, 11–21. doi:10.1016/j.cbi.2019.01.031
Chen, X., Sun, K., Zhao, S., Geng, T., Fan, X., Sun, S., et al. (2020). Irisin promotes osteogenic differentiation of bone marrow mesenchymal stem cells by activating autophagy via the Wnt//β-catenin signal pathway. Cytokine 136, 155292. doi:10.1016/j.cyto.2020.155292
Chen, Y., Ding, J., Zhao, Y., Ju, S., Mao, H., and Peng, X. G. (2021). Irisin induces white adipose tissue browning in mice as assessed by magnetic resonance imaging. Exp. Biol. Med. (Maywood, NJ) 246 (14), 1597–1606. doi:10.1177/15353702211006049
Cheng, L., Gou, L., Wei, T., and Zhang, J. (2020). GBP1 promotes erlotinib resistance via PGK1-activated EMT signaling in non-small cell lung cancer. Int. J. Oncol. 57 (3), 858–870. doi:10.3892/ijo.2020.5086
Colaianni, G., Errede, M., Sanesi, L., Notarnicola, A., Celi, M., Zerlotin, R., et al. (2021). Irisin correlates positively with BMD in a cohort of older adult patients and downregulates the senescent marker p21 in osteoblasts. J. bone mineral Res. 36 (2), 305–314. doi:10.1002/jbmr.4192
Dong, H. N., Park, S. Y., Le, C. T., Choi, D. H., and Cho, E. H. (2020). Irisin regulates the functions of hepatic stellate cells. Endocrinol. metabolism (Seoul, Korea) 35 (3), 647–655. doi:10.3803/EnM.2020.658
Fan, G. H., Zhu, T. Y., and Huang, J. (2020). FNDC5 promotes paclitaxel sensitivity of non-small cell lung cancers via inhibiting MDR1. Cell. Signal. 72, 109665. doi:10.1016/j.cellsig.2020.109665
Gaudio, A., Rapisarda, R., Xourafa, A., Zanoli, L., Manfrè, V., Catalano, A., et al. (2021). Effects of competitive physical activity on serum irisin levels and bone turnover markers. J. Endocrinol. investigation 44 (10), 2235–2241. doi:10.1007/s40618-021-01529-0
GBD Chronic Respiratory Disease Collaborators (2020). Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 8 (6), 585–596. doi:10.1016/S2213-2600(20)30105-3
Greulich, T., Nell, C., Koepke, J., Fechtel, J., Franke, M., Schmeck, B., et al. (2014). Benefits of whole body vibration training in patients hospitalised for COPD exacerbations - a randomized clinical trial. BMC Pulm. Med. 14, 60. doi:10.1186/1471-2466-14-60
Gui, Y. S., Wang, L., Tian, X., Li, X., Ma, A., Zhou, W., et al. (2015). mTOR overactivation and compromised autophagy in the pathogenesis of pulmonary fibrosis. PloS one 10 (9), e0138625. doi:10.1371/journal.pone.0138625
Guo, M., Peng, T., Wu, C., Pan, X., and Huang, Z. (2023a). Engineering ferroptosis inhibitors as inhalable nanomedicines for the highly efficient treatment of idiopathic pulmonary fibrosis. Bioeng. (Basel, Switz. 10 (6), 727. doi:10.3390/bioengineering10060727
Guo, M., Yao, J., Li, J., Zhang, J., Wang, D., Zuo, H., et al. (2023b). Irisin ameliorates age-associated sarcopenia and metabolic dysfunction. J. cachexia, sarcopenia muscle 14 (1), 391–405. doi:10.1002/jcsm.13141
Gurger, M., Telo, S., Yilmaz, M., Atescelik, M., Gul, E., and Goktekin, M. C. (2021). Diagnostic and prognostic relevance of serum irisin level in patients with pulmonary embolism. Clin. Lab. 67 (3). doi:10.7754/Clin.Lab.2020.200608
Han, F., Ding, Z. F., Shi, X. L., Zhu, Q. T., Shen, Q. H., Xu, X. M., et al. (2023b). Irisin inhibits neutrophil extracellular traps formation and protects against acute pancreatitis in mice. Redox Biol. 64, 102787. doi:10.1016/j.redox.2023.102787
Han, Z., Ma, J., Han, Y., Yuan, G., Jiao, R., and Meng, A. (2023a). Irisin attenuates acute lung injury by suppressing the pyroptosis of alveolar macrophages. Int. J. Mol. Med. 51 (4), 32. doi:10.3892/ijmm.2023.5235
Ho, M. Y., Wen, M. S., Yeh, J. K., Hsieh, I. C., Chen, C. C., Hsieh, M. J., et al. (2018). Excessive irisin increases oxidative stress and apoptosis in murine heart. Biochem. biophysical Res. Commun. 503 (4), 2493–2498. doi:10.1016/j.bbrc.2018.07.005
Hou, H. H., Pan, H. J., Liao, W. Y., Lee, C. H., and Yu, C. J. (2020). Autophagy in fibroblasts induced by cigarette smoke extract promotes invasion in lung cancer cells. Int. J. cancer 147 (9), 2587–2596. doi:10.1002/ijc.33127
Hu, C., Zhang, X., Hu, M., Teng, T., Yuan, Y. P., Song, P., et al. (2022). Fibronectin type III domain-containing 5 improves aging-related cardiac dysfunction in mice. Aging Cell 21 (3), e13556. doi:10.1111/acel.13556
Hu, G., Dong, T., Wang, S., Jing, H., and Chen, J. (2019). Vitamin D(3)-vitamin D receptor axis suppresses pulmonary emphysema by maintaining alveolar macrophage homeostasis and function. EBioMedicine 45, 563–577. doi:10.1016/j.ebiom.2019.06.039
Huang, C. W., Chang, Y. H., Lee, H. H., Wu, J. Y., Huang, J. X., Chung, Y. H., et al. (2020a). Irisin, an exercise myokine, potently suppresses tumor proliferation, invasion, and growth in glioma. FASEB J. 34 (7), 9678–9693. doi:10.1096/fj.202000573RR
Huang, W., Liu, Y., Xu, H., Zhu, H., Guan, J., Yi, H., et al. (2020b). Association of the serum irisin level with obstructive sleep apnea: a body mass index- and physical activity-matched study. Endocr. J. 67 (6), 607–612. doi:10.1507/endocrj.EJ19-0590
Ijiri, N., Kanazawa, H., Asai, K., Watanabe, T., and Hirata, K. (2015). Irisin, a newly discovered myokine, is a novel biomarker associated with physical activity in patients with chronic obstructive pulmonary disease. Respirol. Carlt. Vic. 20 (4), 612–617. doi:10.1111/resp.12513
Jiao, R., Han, Z., Ma, J., Wu, S., Wang, Z., Zhou, G., et al. (2023). Irisin attenuates fine particulate matter induced acute lung injury by regulating Nod2/NF-κB signaling pathway. Immunobiology 228 (3), 152358. doi:10.1016/j.imbio.2023.152358
Junkins, R. D., Shen, A., Rosen, K., McCormick, C., and Lin, T. J. (2013). Autophagy enhances bacterial clearance during P. aeruginosa lung infection. PloS one 8 (8), e72263. doi:10.1371/journal.pone.0072263
Kim, H. J., So, B., Choi, M., Kang, D., and Song, W. (2015). Resistance exercise training increases the expression of irisin concomitant with improvement of muscle function in aging mice and humans. Exp. Gerontol. 70, 11–17. doi:10.1016/j.exger.2015.07.006
Kim, H. K., Jeong, Y. J., Song, I. S., Noh, Y. H., Seo, K. W., Kim, M., et al. (2017). Glucocorticoid receptor positively regulates transcription of FNDC5 in the liver. Sci. Rep. 7, 43296. doi:10.1038/srep43296
Kubo, H., Asai, K., Kojima, K., Sugitani, A., Kyomoto, Y., Okamoto, A., et al. (2019). Exercise ameliorates emphysema of cigarette smoke-induced COPD in mice through the exercise-irisin-nrf2 Axis. Int. J. chronic Obstr. Pulm. Dis. 14, 2507–2516. doi:10.2147/COPD.S226623
Kureya, Y., Kanazawa, H., Ijiri, N., Tochino, Y., Watanabe, T., Asai, K., et al. (2016). Down-regulation of soluble α-klotho is associated with reduction in serum irisin levels in chronic obstructive pulmonary disease. Lung 194 (3), 345–351. doi:10.1007/s00408-016-9870-7
Lee, P. H., Hong, J., and Jang, A. S. (2020). N-acetylcysteine decreases airway inflammation and responsiveness in asthma by modulating claudin 18 expression. Korean J. Intern. Med. 35 (5), 1229–1237. doi:10.3904/kjim.2019.105
León-Román, F., Valenzuela, C., and Molina-Molina, M. (2022). Idiopathic pulmonary fibrosis. Med. Clin. 159 (4), 189–194. doi:10.1016/j.medcli.2022.02.020
Li, D. J., Li, Y. H., Yuan, H. B., Qu, L. F., and Wang, P. (2017). The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism Clin. Exp. 68, 31–42. doi:10.1016/j.metabol.2016.12.003
Li, D. J., Sun, S. J., Fu, J. T., Ouyang, S. X., Zhao, Q. J., Su, L., et al. (2021). NAD(+)-boosting therapy alleviates nonalcoholic fatty liver disease via stimulating a novel exerkine Fndc5/irisin. Theranostics 11 (9), 4381–4402. doi:10.7150/thno.53652
Li, J., Deng, S. H., Li, J., Li, L., Zhang, F., Zou, Y., et al. (2022b). Obacunone alleviates ferroptosis during lipopolysaccharide-induced acute lung injury by upregulating Nrf2-dependent antioxidant responses. Cell. Mol. Biol. Lett. 27 (1), 29. doi:10.1186/s11658-022-00318-8
Li, R., Wang, X., Wu, S., Wu, Y., Chen, H., Xin, J., et al. (2019a). Irisin ameliorates angiotensin II-induced cardiomyocyte apoptosis through autophagy. J. Cell. physiology 234 (10), 17578–17588. doi:10.1002/jcp.28382
Li, R. L., Wu, S. S., Wu, Y., Wang, X. X., Chen, H. Y., Xin, J. J., et al. (2018). Irisin alleviates pressure overload-induced cardiac hypertrophy by inducing protective autophagy via mTOR-independent activation of the AMPK-ULK1 pathway. J. Mol. Cell. Cardiol. 121, 242–255. doi:10.1016/j.yjmcc.2018.07.250
Li, X., Jamal, M., Guo, P., Jin, Z., Zheng, F., Song, X., et al. (2019b). Irisin alleviates pulmonary epithelial barrier dysfunction in sepsis-induced acute lung injury via activation of AMPK/SIRT1 pathways. Biomed. Pharmacother. = Biomedecine Pharmacother. 118, 109363. doi:10.1016/j.biopha.2019.109363
Li, Y., Yan, B., Wu, Y., Peng, Q., Wei, Y., Chen, Y., et al. (2023). Ferroptosis participates in dibutyl phthalate-aggravated allergic asthma in ovalbumin-sensitized mice. Ecotoxicol. Environ. Saf. 256, 114848. doi:10.1016/j.ecoenv.2023.114848
Li, Y., Yang, Y., and Yang, Y. (2022a). Multifaceted roles of ferroptosis in lung diseases. Front. Mol. Biosci. 9, 919187. doi:10.3389/fmolb.2022.919187
Liao, X., Zhan, W., Li, R., Tian, T., Yu, L., and Yang, Q. (2021). Irisin ameliorates endoplasmic reticulum stress and liver fibrosis through inhibiting PERK-mediated destabilization of HNRNPA1 in hepatic stellate cells. Biol. Chem. 402 (6), 703–715. doi:10.1515/hsz-2020-0251
Liu, J., Song, N., Huang, Y., and Chen, Y. (2018). Irisin inhibits pancreatic cancer cell growth via the AMPK-mTOR pathway. Sci. Rep. 8 (1), 15247. doi:10.1038/s41598-018-33229-w
López-Campos, J. L., Tan, W., and Soriano, J. B. (2016). Global burden of COPD. Respirol. Carlt. Vic. 21 (1), 14–23. doi:10.1111/resp.12660
Ma, J., Han, Z., Jiao, R., Yuan, G., Ma, C., Yan, X., et al. (2023). Irisin ameliorates pm2.5-induced acute lung injury by regulation of autophagy through AMPK/mTOR pathway. J. Inflamm. Res. 16, 1045–1057. doi:10.2147/JIR.S390497
Ma, L. Y., Liu, J. M., Du, G. L., and Dang, X. B. (2021). Irisin attenuates lipopolysaccharide-induced acute lung injury by downregulating inflammatory cytokine expression through miR-199a-mediated Rad23b overexpression. Exp. Cell Res. 404 (2), 112593. doi:10.1016/j.yexcr.2021.112593
Mandal, J., Roth, M., Papakonstantinou, E., Sun, Q., Costa, L., Boeck, L., et al. (2018). Treatment with long acting muscarinic antagonists stimulates serum levels of irisin in patients with COPD. Pulm. Pharmacol. Ther. 48, 111–116. doi:10.1016/j.pupt.2017.10.011
Martínez-Gestoso, S., García-Sanz, M. T., Carreira, J. M., Salgado, F. J., Calvo-Álvarez, U., Doval-Oubiña, L., et al. (2022). Impact of anxiety and depression on the prognosis of copd exacerbations. BMC Pulm. Med. 22 (1), 169.
Mazur-Bialy, A. I. (2017). Irisin acts as a regulator of macrophages host defense. Life Sci. 176, 21–25. doi:10.1016/j.lfs.2017.03.011
Mohammed, M. A., Mahmoud, M. O., Awaad, A. S., Gamal, G. M., and Abdelfatah, D. (2019). Alpha lipoic acid protects against dexamethasone-induced metabolic abnormalities via APPL1 and PGC-1 α up regulation. Steroids 144, 1–7. doi:10.1016/j.steroids.2019.01.004
More, C. E., Papp, C., Harsanyi, S., Gesztelyi, R., Mikaczo, A., Tajti, G., et al. (2019). Altered irisin/BDNF axis parallels excessive daytime sleepiness in obstructive sleep apnea patients. Respir. Res. 20 (1), 67. doi:10.1186/s12931-019-1033-y
Ng, S. S., Liu, E. K., Ma, R. C., Chan, T. O., To, K. W., Chan, K. K., et al. (2017). Effects of CPAP therapy on visceral fat thickness, carotid intima-media thickness and adipokines in patients with obstructive sleep apnoea. Respirol. Carlt. Vic. 22 (4), 786–792. doi:10.1111/resp.12963
Nosaka, N., Martinon, D., Moreira, D., Crother, T. R., Arditi, M., and Shimada, K. (2020). Autophagy protects against developing increased lung permeability and hypoxemia by down regulating inflammasome activity and IL-1β in LPS plus mechanical ventilation-induced acute lung injury. Front. Immunol. 11, 207. doi:10.3389/fimmu.2020.00207
Oflazoglu, U., Caglar, S., Yılmaz, H. E., Önal, H. T., Varol, U., Salman, T., et al. (2022). The relationship between sarcopenia detected in newly diagnosed colorectal cancer patients and FGF21, irisin and CRP levels. Eur. Geriatr. Med. 13 (4), 795–803. doi:10.1007/s41999-022-00635-3
Pan, J. A., Zhang, H., Lin, H., Gao, L., Zhang, H. L., Zhang, J. F., et al. (2021). Irisin ameliorates doxorubicin-induced cardiac perivascular fibrosis through inhibiting endothelial-to-mesenchymal transition by regulating ROS accumulation and autophagy disorder in endothelial cells. Redox Biol. 46, 102120. doi:10.1016/j.redox.2021.102120
Papp, C., Pak, K., Erdei, T., Juhasz, B., Seres, I., Szentpéteri, A., et al. (2017). Alteration of the irisin-brain-derived neurotrophic factor axis contributes to disturbance of mood in COPD patients. Int. J. Chron. Obstruct. Pulmon. Dis. 12, 2023–2033.
Pazgan-Simon, M., Zuwala-Jagiello, J., Menzyk, T., Bator, M., Derra, A., Lekstan, A., et al. (2020). Serum betatrophin and irisin levels in hepatocellular carcinoma. J. physiology Pharmacol. 71 (1). doi:10.26402/jpp.2020.1.11
Petta, S., Valenti, L., Svegliati-Baroni, G., Ruscica, M., Pipitone, R. M., Dongiovanni, P., et al. (2017). Fibronectin type III domain-containing protein 5 rs3480 A>G polymorphism, irisin, and liver fibrosis in patients with nonalcoholic fatty liver disease. J. Clin. Endocrinol. metabolism 102 (8), 2660–2669. doi:10.1210/jc.2017-00056
Pignataro, P., Dicarlo, M., Zerlotin, R., Zecca, C., Dell'Abate, M. T., Buccoliero, C., et al. (2021). FNDC5/Irisin system in neuroinflammation and neurodegenerative diseases: update and novel perspective. Int. J. Mol. Sci. 22 (4), 1605. doi:10.3390/ijms22041605
Polyzos, S. A., Anastasilakis, A. D., Efstathiadou, Z. A., Makras, P., Perakakis, N., Kountouras, J., et al. (2018). Irisin in metabolic diseases. Endocrine 59 (2), 260–274. doi:10.1007/s12020-017-1476-1
Provatopoulou, X., Georgiou, G. P., Kalogera, E., Kalles, V., Matiatou, M. A., Papapanagiotou, I., et al. (2015). Serum irisin levels are lower in patients with breast cancer: association with disease diagnosis and tumor characteristics. BMC cancer 15, 898. doi:10.1186/s12885-015-1898-1
Qi, J. Y., Yang, L. K., Wang, X. S., Wang, M., Li, X. B., Feng, B., et al. (2022). Irisin: a promising treatment for neurodegenerative diseases. Neuroscience 498, 289–299. doi:10.1016/j.neuroscience.2022.07.018
Qiongyue, Z., Xin, Y., Meng, P., Sulin, M., Yanlin, W., Xinyi, L., et al. (2022). Post-treatment with irisin attenuates acute kidney injury in sepsis mice through anti-ferroptosis via the SIRT1/nrf2 pathway. Front. Pharmacol. 13, 857067. doi:10.3389/fphar.2022.857067
Ren, Y., Zhang, J., Wang, M., Bi, J., Wang, T., Qiu, M., et al. (2020). Identification of irisin as a therapeutic agent that inhibits oxidative stress and fibrosis in a murine model of chronic pancreatitis. Biomed. Pharmacother. = Biomedecine Pharmacother. 126, 110101. doi:10.1016/j.biopha.2020.110101
Robbe, P., Draijer, C., Borg, T. R., Luinge, M., Timens, W., Wouters, I. M., et al. (2015). Distinct macrophage phenotypes in allergic and nonallergic lung inflammation. Am. J. physiology Lung Cell. Mol. physiology 308 (4), L358–L367. doi:10.1152/ajplung.00341.2014
Roden, A. C., Boland, J. M., Johnson, T. F., Aubry, M. C., Lo, Y. C., Butt, Y. M., et al. (2022). Late complications of COVID-19. Archives pathology laboratory Med. 146 (7), 791–804. doi:10.5858/arpa.2021-0519-SA
Safarpour, P., Daneshi-Maskooni, M., Vafa, M., Nourbakhsh, M., Janani, L., Maddah, M., et al. (2020). Vitamin D supplementation improves SIRT1, Irisin, and glucose indices in overweight or obese type 2 diabetic patients: a double-blind randomized placebo-controlled clinical trial. BMC Fam. Pract. 21 (1), 26. doi:10.1186/s12875-020-1096-3
Sanesi, L., Dicarlo, M., Pignataro, P., Zerlotin, R., Pugliese, F., Columbu, C., et al. (2023). Vitamin D increases irisin serum levels and the expression of its precursor in skeletal muscle. Int. J. Mol. Sci. 24 (4), 4129. doi:10.3390/ijms24044129
Sang, A., Wang, Y., Wang, S., Wang, Q., Wang, X., Li, X., et al. (2022). Quercetin attenuates sepsis-induced acute lung injury via suppressing oxidative stress-mediated ER stress through activation of SIRT1/AMPK pathways. Cell. Signal. 96, 110363. doi:10.1016/j.cellsig.2022.110363
Sarwar, M., Ahsin, S., Saeed, G. N., and Ashraf, H. (2022). Role of r-irisin in nicotine-induced oxidative stress and endothelial dysfunction in BALB/c mice. J. Coll. Physicians Surgeons--Pakistan JCPSP 32 (9), 1175–1180. doi:10.29271/jcpsp.2022.09.1175
Shao, L., Li, H., Chen, J., Song, H., Zhang, Y., Wu, F., et al. (2017b). Irisin suppresses the migration, proliferation, and invasion of lung cancer cells via inhibition of epithelial-to-mesenchymal transition. Biochem. biophysical Res. Commun. 485 (3), 598–605. doi:10.1016/j.bbrc.2016.12.084
Shao, L., Meng, D., Yang, F., Song, H., and Tang, D. (2017a). Irisin-mediated protective effect on LPS-induced acute lung injury via suppressing inflammation and apoptosis of alveolar epithelial cells. Biochem. biophysical Res. Commun. 487 (2), 194–200. doi:10.1016/j.bbrc.2017.04.020
Shi, G., Tang, N., Qiu, J., Zhang, D., Huang, F., Cheng, Y., et al. (2017). Irisin stimulates cell proliferation and invasion by targeting the PI3K/AKT pathway in human hepatocellular carcinoma. Biochem. biophysical Res. Commun. 493 (1), 585–591. doi:10.1016/j.bbrc.2017.08.148
Shi, Y. Y., Liu, T. J., Fu, J. H., Xu, W., Wu, L. L., Hou, A. N., et al. (2016). Vitamin D/VDR signaling attenuates lipopolysaccharide-induced acute lung injury by maintaining the integrity of the pulmonary epithelial barrier. Mol. Med. Rep. 13 (2), 1186–1194. doi:10.3892/mmr.2015.4685
Simpson, J. L., Scott, R., Boyle, M. J., and Gibson, P. G. (2006). Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirol. Carlt. Vic. 11 (1), 54–61. doi:10.1111/j.1440-1843.2006.00784.x
Song, R., Zhao, X., Cao, R., Liang, Y., Zhang, D. Q., and Wang, R. (2021). Irisin improves insulin resistance by inhibiting autophagy through the PI3K/Akt pathway in H9c2 cells. Gene 769, 145209. doi:10.1016/j.gene.2020.145209
Sugiyama, Y., Asai, K., Yamada, K., Kureya, Y., Ijiri, N., Watanabe, T., et al. (2017). Decreased levels of irisin, a skeletal muscle cell-derived myokine, are related to emphysema associated with chronic obstructive pulmonary disease. Int. J. chronic Obstr. Pulm. Dis. 12, 765–772. doi:10.2147/COPD.S126233
Sun, N., Chen, Y., Fan, Y., Chang, J., Gao, X., Zhao, Y., et al. (2021). Plasma irisin levels are associated with hemodynamic and clinical outcome in idiopathic pulmonary arterial hypertension patients. Intern. Emerg. Med. 16 (3), 625–632. doi:10.1007/s11739-020-02467-0
Sun, N., Fan, Y., Chang, J., Chen, Y., Gao, X., Sun, H., et al. (2020). Plasma irisin level associated with hemodynamic parameters and predict clinical outcome in patients with acute pulmonary embolism. Respir. Med. 171, 106072. doi:10.1016/j.rmed.2020.106072
Sutherland, E. R., Goleva, E., Jackson, L. P., Stevens, A. D., and Leung, D. Y. (2010). Vitamin D levels, lung function, and steroid response in adult asthma. Am. J. Respir. Crit. care Med. 181 (7), 699–704. doi:10.1164/rccm.200911-1710OC
Suzuki, Y., Aono, Y., Akiyama, N., Horiike, Y., Naoi, H., Horiguchi, R., et al. (2022). Involvement of autophagy in exacerbation of eosinophilic airway inflammation in a murine model of obese asthma. Autophagy 18 (9), 2216–2228. doi:10.1080/15548627.2022.2025571
Szilasi, M. E., Pak, K., Kardos, L., Varga, V. E., Seres, I., Mikaczo, A., et al. (2017). The alteration of irisin-brain-derived neurotrophic factor Axis parallels severity of distress disorder in bronchial asthma patients. Front. Neurosci. 11, 653. doi:10.3389/fnins.2017.00653
Taken, K., Aslan, R., Eryilmaz, R., Alp, H. H., Huyut, Z., and Dönmez, M. (2022). Serum irisin is a novel biomarker for bladder cancer detection. Int. urology Nephrol. 54 (1), 55–61. doi:10.1007/s11255-021-03074-4
Tang, Z., Ding, Y., Shen, Q., Zhang, C., Li, J., Nazar, M., et al. (2019). KIAA1199 promotes invasion and migration in non-small-cell lung cancer (NSCLC) via PI3K-Akt mediated EMT. J. Mol. Med. (Berlin, Ger. 97 (1), 127–140. doi:10.1007/s00109-018-1721-y
Tatler, A. L., John, A. E., Jolly, L., Habgood, A., Porte, J., Brightling, C., et al. (2011). Integrin αvβ5-mediated TGF-β activation by airway smooth muscle cells in asthma. J. Immunol. 187 (11), 6094–6107. doi:10.4049/jimmunol.1003507
Tu, Y., Liu, J., Kong, D., Guo, X., Li, J., Long, Z., et al. (2023). Irisin drives macrophage anti-inflammatory differentiation via JAK2-STAT6-dependent activation of PPARγ and Nrf2 signaling. Free Radic. Biol. Med. 201, 98–110. doi:10.1016/j.freeradbiomed.2023.03.014
Varricchi, G., Modestino, L., Poto, R., Cristinziano, L., Gentile, L., Postiglione, L., et al. (2022). Neutrophil extracellular traps and neutrophil-derived mediators as possible biomarkers in bronchial asthma. Clin. Exp. Med. 22 (2), 285–300. doi:10.1007/s10238-021-00750-8
Wang, J., Zhu, Q., Wang, Y., Peng, J., Shao, L., and Li, X. (2022a). Irisin protects against sepsis-associated encephalopathy by suppressing ferroptosis via activation of the Nrf2/GPX4 signal axis. Free Radic. Biol. Med. 187, 171–184. doi:10.1016/j.freeradbiomed.2022.05.023
Wang, S., and Pan, J. (2016). Irisin ameliorates depressive-like behaviors in rats by regulating energy metabolism. Biochem. biophysical Res. Commun. 474 (1), 22–28. doi:10.1016/j.bbrc.2016.04.047
Wang, T., Yu, M., Li, H., Qin, S., Ren, W., Ma, Y., et al. (2023). FNDC5/Irisin inhibits the inflammatory response and mediates the aerobic exercise-induced improvement of liver injury after myocardial infarction. Int. J. Mol. Sci. 24 (4), 4159. doi:10.3390/ijms24044159
Wang, W., Xiao, L., Pan, D., and Hu, L. (2022c). ASF1B enhances migration and invasion of lung cancers cell via regulating the P53-mediated epithelial-mesenchymal transformation (EMT) signaling pathway. Neoplasma 69 (2), 361–369. doi:10.4149/neo_2021_210818N1181
Wang, Y., Gu, Y., Huang, J., Wu, H., Meng, G., Zhang, Q., et al. (2022d). Serum vitamin D status and circulating irisin levels in older adults with sarcopenia. Front. Nutr. 9, 1051870. doi:10.3389/fnut.2022.1051870
Wang, Y., Tian, M., Tan, J., Pei, X., Lu, C., Xin, Y., et al. (2022b). Irisin ameliorates neuroinflammation and neuronal apoptosis through integrin αVβ5/AMPK signaling pathway after intracerebral hemorrhage in mice. J. neuroinflammation 19 (1), 82. doi:10.1186/s12974-022-02438-6
Ware, L. B., and Matthay, M. A. (2000). The acute respiratory distress syndrome. N. Engl. J. Med. 342 (18), 1334–1349. doi:10.1056/NEJM200005043421806
Wei, S., Bi, J., Yang, L., Zhang, J., Wan, Y., Chen, X., et al. (2020). Serum irisin levels are decreased in patients with sepsis, and exogenous irisin suppresses ferroptosis in the liver of septic mice. Clin. Transl. Med. 10 (5), e173. doi:10.1002/ctm2.173
Xiong, Y., Wu, Z., Zhang, B., Wang, C., Mao, F., Liu, X., et al. (2019). Fndc5 loss-of-function attenuates exercise-induced browning of white adipose tissue in mice. FASEB J. 33 (5), 5876–5886. doi:10.1096/fj.201801754RR
Yalçın, A., and Gürel, A. (2021). Effects of N-acetylcysteine on kidney tissue, matrix metalloproteinase-2, irisin and oxidative stress in a diabetes mellitus model. Biotech. Histochem. 96 (8), 616–622. doi:10.1080/10520295.2021.1883738
Yang, B. C., and Leung, P. S. (2020). Irisin is a positive regulator for ferroptosis in pancreatic cancer. Mol. Ther. oncolytics 18, 457–466. doi:10.1016/j.omto.2020.08.002
Yang, Z., Wei, J., Wang, Y., Du, Y., Song, S., Li, J., et al. (2023). Irisin ameliorates renal tubulointerstitial fibrosis by regulating the smad4/β-catenin pathway in diabetic mice. Diabetes, metabolic syndrome Obes. targets Ther. 16, 1577–1593. doi:10.2147/DMSO.S407734
Ye, W., Wang, J., Lin, D., and Ding, Z. (2020). The immunomodulatory role of irisin on osteogenesis via AMPK-mediated macrophage polarization. Int. J. Biol. Macromol. 146, 25–35. doi:10.1016/j.ijbiomac.2019.12.028
Ye, X., Shen, Y., Ni, C., Ye, J., Xin, Y., Zhang, W., et al. (2019). Irisin reverses insulin resistance in C2C12 cells via the p38-MAPK-PGC-1α pathway. Peptides 119, 170120. doi:10.1016/j.peptides.2019.170120
Yeganeh, B., Lee, J., Ermini, L., Lok, I., Ackerley, C., and Post, M. (2019). Autophagy is required for lung development and morphogenesis. J. Clin. investigation 129 (7), 2904–2919. doi:10.1172/JCI127307
Yohannes, A. M., Murri, M. B., Hanania, N. A., Regan, E. A., Iyer, A., Bhatt, S. P., et al. (2022). Depressive and anxiety symptoms in patients with COPD: a network analysis. Respir. Med. 198: 106865.
Yoshida, M., Minagawa, S., Araya, J., Sakamoto, T., Hara, H., Tsubouchi, K., et al. (2019). Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nat. Commun. 10 (1), 3145. doi:10.1038/s41467-019-10991-7
Yu, Q., Kou, W., Xu, X., Zhou, S., Luan, P., Xu, X., et al. (2019). FNDC5/Irisin inhibits pathological cardiac hypertrophy. Clin. Sci. Lond. 133 (5), 611–627. doi:10.1042/CS20190016
Zasłona, Z., Przybranowski, S., Wilke, C., van Rooijen, N., Teitz-Tennenbaum, S., Osterholzer, J. J., et al. (2014). Resident alveolar macrophages suppress, whereas recruited monocytes promote, allergic lung inflammation in murine models of asthma. J. Immunol. 193 (8), 4245–4253. doi:10.4049/jimmunol.1400580
Zhang, D., Zhang, P., Li, L., Tang, N., Huang, F., Kong, X., et al. (2019). Irisin functions to inhibit malignant growth of human pancreatic cancer cells via downregulation of the PI3K/AKT signaling pathway. OncoTargets Ther. 12, 7243–7249. doi:10.2147/OTT.S214260
Zhang, D., Zhou, J., Ye, L. C., Li, J., Wu, Z., Li, Y., et al. (2018). Autophagy maintains the integrity of endothelial barrier in LPS-induced lung injury. J. Cell. physiology 233 (1), 688–698. doi:10.1002/jcp.25928
Zhang, J., Bi, J., Ren, Y., Du, Z., Li, T., Wang, T., et al. (2021). Involvement of GPX4 in irisin's protection against ischemia reperfusion-induced acute kidney injury. J. Cell. physiology 236 (2), 931–945. doi:10.1002/jcp.29903
Zhang, L., He, Y. L., Li, Q. Z., Hao, X. H., Zhang, Z. F., Yuan, J. X., et al. (2014). N-acetylcysteine alleviated silica-induced lung fibrosis in rats by down-regulation of ROS and mitochondrial apoptosis signaling. Toxicol. Mech. methods 24 (3), 212–219. doi:10.3109/15376516.2013.879974
Zhang, L., Li, C., Xiong, J., Chang, C., and Sun, Y. (2022b). Dysregulated myokines and signaling pathways in skeletal muscle dysfunction in a cigarette smoke-induced model of chronic obstructive pulmonary disease. Front. physiology 13, 929926. doi:10.3389/fphys.2022.929926
Zhang, Y., Zhang, J., and Fu, Z. (2022a). Role of autophagy in lung diseases and ageing. Eur. Respir. Rev. 31 (166), 220134. doi:10.1183/16000617.0134-2022
Zhao, G., Liang, J., Shan, G., Gu, J., Xu, F., Lu, C., et al. (2023). KLF11 regulates lung adenocarcinoma ferroptosis and chemosensitivity by suppressing GPX4. Commun. Biol. 6 (1), 570. doi:10.1038/s42003-023-04959-z
Zhao, H., Fu, L., Xiang, H. X., Xiang, Y., Li, M. D., Lv, B. B., et al. (2022). N-acetylcysteine alleviates pulmonary inflammatory response during benzo[a]pyrene-evoked acute lung injury. Environ. Sci. Pollut. Res. Int. 29 (3), 3474–3486. doi:10.1007/s11356-021-15914-y
Zheng, S., Chen, N., Kang, X., Hu, Y., and Shi, S. (2022). Irisin alleviates FFA induced β-cell insulin resistance and inflammatory response through activating PI3K/AKT/FOXO1 signaling pathway. Endocrine 75 (3), 740–751. doi:10.1007/s12020-021-02875-y
Zhou, W., Shi, Y., Wang, H., Chen, L., Yu, C., Zhang, X., et al. (2022). Exercise-induced FNDC5/irisin protects nucleus pulposus cells against senescence and apoptosis by activating autophagy. Exp. Mol. Med. 54 (7), 1038–1048. doi:10.1038/s12276-022-00811-2
Zhu, D., Zhang, X., Wang, F., Ye, Q., Yang, C., and Liu, D. (2022). Irisin rescues diabetic cardiac microvascular injury via ERK1/2/Nrf2/HO-1 mediated inhibition of oxidative stress. Diabetes Res. Clin. Pract. 183, 109170. doi:10.1016/j.diabres.2021.109170
Zhu, X., Li, X., Wang, X., Chen, T., Tao, F., Liu, C., et al. (2021). Irisin deficiency disturbs bone metabolism. J. Cell. physiology 236 (1), 664–676. doi:10.1002/jcp.29894
Glossary
Keywords: irisin, lung disease, biomarker, diagnostic, treatment
Citation: Dong H, Lv X, Gao P and Hao Y (2023) Potential role of irisin in lung diseases and advances in research. Front. Pharmacol. 14:1307651. doi: 10.3389/fphar.2023.1307651
Received: 05 October 2023; Accepted: 27 November 2023;
Published: 06 December 2023.
Edited by:
Cassiano Felippe Gonçalves-de-Albuquerque, Rio de Janeiro State Federal University, BrazilReviewed by:
Yong Wang, Nanjing University, ChinaJennifer Speth, University of Michigan, United States
Copyright © 2023 Dong, Lv, Gao and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Gao, Z2FvcGVuZzEyMzRAamx1LmVkdS5jbg==; Yuqiu Hao, aGFvLnl1LnFpLnVAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Hongna Dong†
Hongna Dong† Peng Gao
Peng Gao