Comparison of the effectiveness and safety of perampanel and oxcarbazepine as monotherapy in children and adolescents with newly diagnosed focal epilepsy
- 1Department of Neurology, Wuhan Children’s Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Pediatric Rehabilitation, Hubei the Third People’s Hospital, Wuhan, China
A Corrigendum on
Comparison of the effectiveness and safety of perampanel and oxcarbazepine as monotherapy in children and adolescents with newly diagnosed focal epilepsy
by Yi J-Q, Huang S, Wu M-J, Ma J-H, Huang L-J, Liang S and Sun D (2023). Front. Pharmacol. 14:1189058. doi: 10.3389/fphar.2023.1189058
In the published article, there was an error in Figure 2 as published. The 3-month seizure freedom rate in OXC group should be 80.7% in the original figure. The 6-month seizure freedom rate in OXC group should be 43/55. The corrected Figure 2 and its caption appear below.
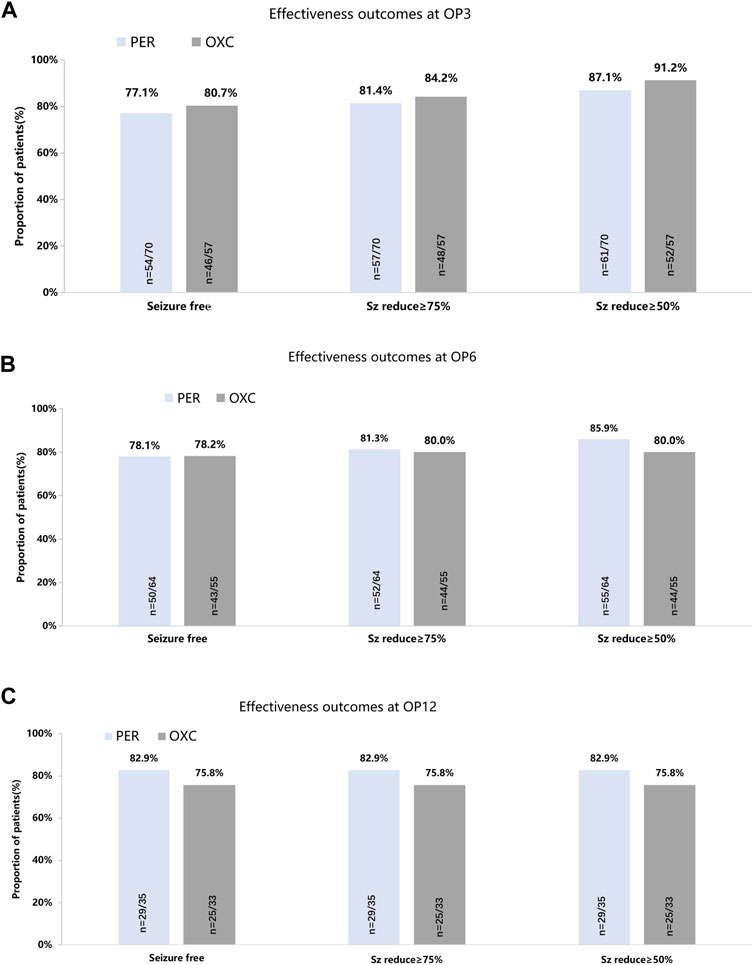
FIGURE 2. Seizure-response status and seizure-free status on PER/OXC monotherapy. (A) OP3; (B) OP6; and (C) OP12. Seizure free: seizure freedom; Sz reduce ≥75%: seizure reduction ≥75%; Sz reduce ≥50%: seizure reduction ≥50%.
In the published article, there was also an error in the text. The 6-month seizure freedom rate in OXC group should be 43/55.
A correction has been made to 3 Result, 3.2 Primary endpoint, paragraph 1. This sentence previously stated:
“In the PPS, 78.1% (50/64, 95% CI: 66.0%–87.5%) of children in the PER group and 78.2% (43/56) in the OXC group were seizure-free at 6 months.”
The corrected sentence appears below:
“In the PPS, 78.1% (50/64, 95% CI: 66.0%–87.5%) of children in the PER group and 78.2% (43/55) in the OXC group were seizure-free at 6 months.”
The authors apologize for these errors and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Keywords: perampanel, oxcarbazepine, newly diagnosed focal epilepsy, monotherapy, anti-seizure medications
Citation: Yi J-Q, Huang S, Wu M-J, Ma J-H, Huang L-J, Liang S and Sun D (2023) Corrigendum: Comparison of the effectiveness and safety of perampanel and oxcarbazepine as monotherapy in children and adolescents with newly diagnosed focal epilepsy. Front. Pharmacol. 14:1295784. doi: 10.3389/fphar.2023.1295784
Received: 17 September 2023; Accepted: 19 September 2023;
Published: 29 September 2023.
Edited and reviewed by:
Alfredo Vannacci, University of Florence, ItalyCopyright © 2023 Yi, Huang, Wu, Ma, Huang, Liang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Song Liang, c29uZzExOTYzQDE2My5jb20=; Dan Sun, YmxvdmVyaXZlckAxNjMuY29t
†These authors have contributed equally to this work
 Jia-Qin Yi
Jia-Qin Yi Sheng Huang1†
Sheng Huang1† Dan Sun
Dan Sun