
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 23 October 2023
Sec. Experimental Pharmacology and Drug Discovery
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1291235
This article is part of the Research Topic Reviews in Experimental Pharmacology and Drug Discovery 2023: Pharmacological management of non-communicable diseases View all 7 articles
Background: Ondansetron is a selective antagonist of the serotonin 5-HT3 receptor that is commonly used to treat morning sickness. It is estimated that 70%–80% of pregnant women suffer from morning sickness, a condition characterized by nausea and vomiting. However, it is still controversial regarding its safety during pregnancy, and continued research will be necessary to fully understand the risks and benefits associated with its use. Therefore, we aimed to identify and provide details of the efficacy and safety of ondansetron in clinical trials.
Methods: A search was conducted of the ClinicalTrials.gov database on 13 April 2023, using the search term “ondansetron and pregnancy.” Inclusion and exclusion criteria were defined to identify relevant clinical trials. The inclusion criteria encompassed clinical trials related to pregnancy that utilized ondansetron as a treatment, while other clinical trials were excluded from consideration. All data extractions such as study title, study status, study type, intervention details, and outcome were collected.
Results: A total of 18 clinical trials were identified, of which only 6 focused on studying the effects of ondansetron. Their respective study titles, statuses, conditions, interventions, outcome measures, and enrollment sizes have been written in detail. The information collected from these trials will contribute to our understanding of the potential benefits and risks of ondansetron in the context of pregnancy and its complications.
Conclusion: Ondansetron has been shown to be an effective treatment for nausea and vomiting, including pregnancy-related morning sickness. Further research is needed to better understand the potential risks and benefits associated with its use in pregnant women.
Systematic Review Registration: ClinicalTrials.gov, identifier
Ondansetron is a selective serotonin receptor antagonist that prevents nausea and vomiting associated with chemotherapy, radiotherapy, and surgery and is commonly used to treat morning sickness (Wolf, 2000). It is estimated that 70%–80% of pregnant women suffer from morning sickness, a condition characterized by nausea and vomiting (Lee and Saha, 2011). Conditions such as malnutrition and dehydration can cause risks to the health of both the fetus and the mother (Maltepe, 2014). The hormonal changes that occur during pregnancy, such as elevated levels of chorionic gonadotropin (hCG), may result in increased levels of serotonin in the body, which, in turn, might be involved in causing maternal nausea and vomiting during pregnancy (Cengiz et al., 2015; Thibeault et al., 2019). Ondansetron works in the brain by selectively binding to specific serotonin (5-HT3) receptors (Simino et al., 2016). These are located on the terminals of the vagus nerve, which innervates the gastrointestinal tract (Griddine and Bush, 2022). Therefore, the serotonin receptors are blocked, inhibiting serotonin release. Serotonin, also known as 5-hydroxytryptamine (5-HT), is a neurotransmitter that regulates nausea and vomiting (Griddine and Bush, 2022). By preventing serotonin from binding to its receptors, ondansetron reduces nausea and vomiting (Yokoi et al., 2017). Even though the FDA initially approved ondansetron only for treating chemotherapy- and surgery-related nausea and vomiting (Hesketh et al., 2017), off-label prescribing of the drug for morning sickness has occurred in some cases (Griddine and Bush, 2022).
Ondansetron is, however, still controversial regarding its safety during pregnancy, and continued research is necessary to fully understand the risks and benefits associated with its use (Parker et al., 2018). Several reports have used different studies to determine ondansetron’s effectiveness in treating morning sickness in pregnant women (Colvin et al., 2013; Kennedy, 2016). The results of these studies have been promising, showing that ondansetron is an effective treatment for morning sickness. It is well tolerated, with few side effects, and can be taken in pill or injection form (Slattery et al., 2022). Even though ondansetron appears to be effective, safety concerns have been raised regarding its use during pregnancy (Michie and Hodson, 2020). Studies have found that it may increase the risk of cardiac arrhythmias and congenital disorders if prescribed in the first trimester of pregnancy (Kaplan et al., 2019). Some theories have been proposed that long-term exposure to ondansetron and continuous inhibition of serotonin can affect some physiological processes, including fetal development. However, the overall risk appears to be low, and other studies have reported conflicting results (Danielsson et al., 2014; Freedman et al., 2014).
It is important to remember that despite ondansetron’s apparent effectiveness at reducing nausea and vomiting caused by morning sickness during pregnancy, its safety is still under debate. For this reason, the severity of a patient’s symptoms and alternative treatment options should be considered before prescribing ondansetron during pregnancy (Ernst, 2019; Solihah et al., 2023). Regular monitoring of the patient’s condition is recommended to ensure the safety of the mother and baby. Finally, healthcare providers should provide support to the patient throughout the duration of the treatment. Using clinical trial results from ClinicalTrials.gov, this systematic review examines the efficacy and safety of ondansetron for morning sickness during pregnancy.
A comprehensive investigation was carried out on ClinicalTrials.gov on 13 April 2023, to identify all pertinent studies about the utilization of ondansetron (commonly known as Zofran) as a therapeutic intervention for ailments or conditions associated with pregnancy. The search was conducted by inputting the term “pregnancy and ondansetron” into the search engine of the website to yield relevant results.
The process of identifying pertinent clinical trials relied on the specific criteria outlined in the research area of interest. The inclusion criteria encompassed clinical trials related to pregnancy that utilized ondansetron as a treatment, while other clinical trials were excluded from consideration.
Data such as study title, study status, study type, intervention details, and outcome.
As of 13 April 2023, a comprehensive search on ClinicalTrials.gov revealed 18 registered clinical trials specifically related to pregnancy and its complications. These trials focused on ondansetron as a potential treatment and were carefully identified and documented for further analysis. Among them, only 6 trials were dedicated to studying the effects of ondansetron, as highlighted in Table 1, thus aligning with our research objectives and meeting our study inclusion criteria. It is noteworthy that all 6 studies have been reported as completed clinical trials. Their respective study titles, statuses, conditions, interventions, outcome measures, and enrollment sizes have been detailed in Table 1.
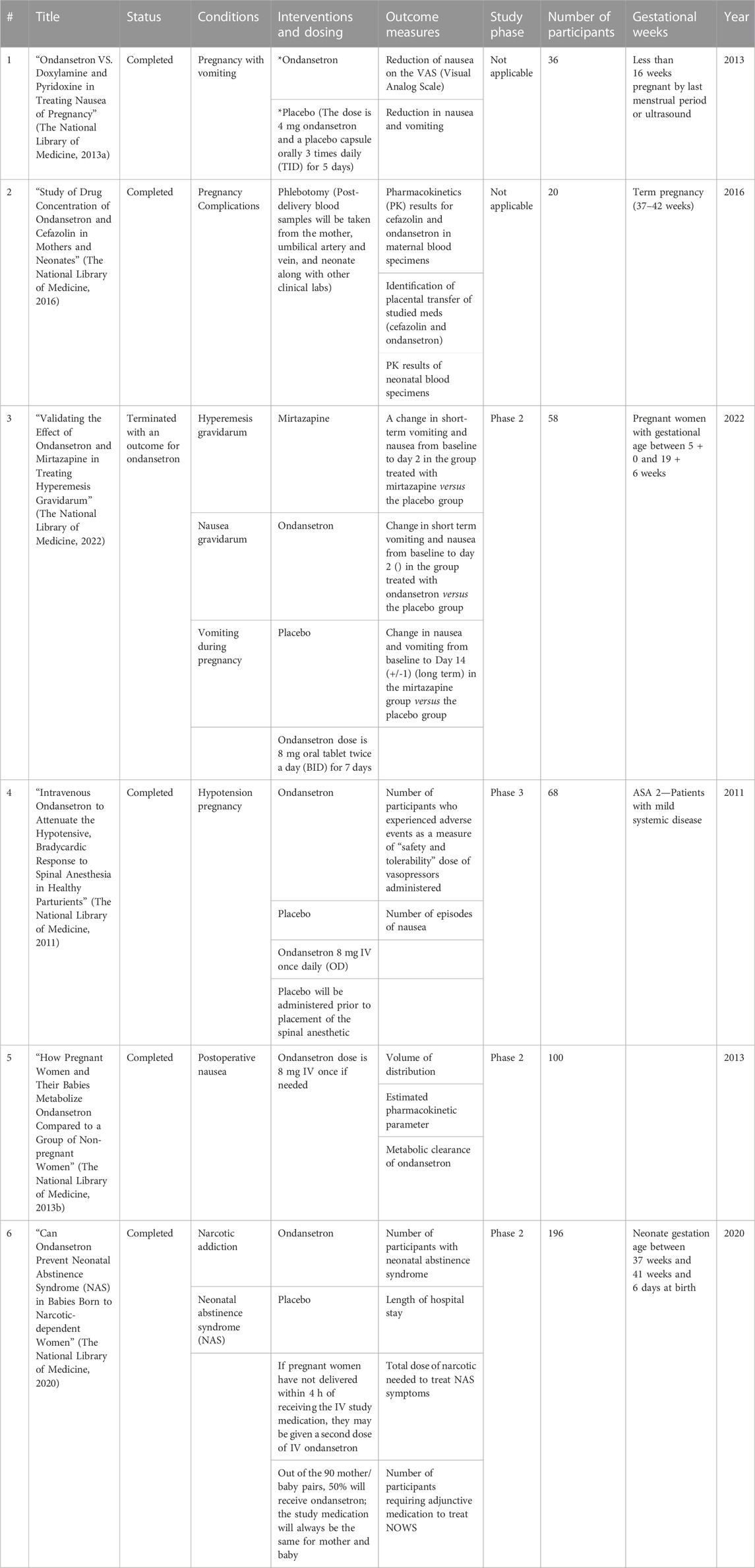
TABLE 1. Data from https://clinicaltrials.gov, updated on 13 April 2023.
A total of 508 adult female participants from a search of ClinicalTrials.gov were included in the systematic review.
Along with ondansetron, some studies have used mirtazapine and metoclopramide for different purposes.
In recent years, ondansetron has received increasing attention for its efficacy and safety in treating morning sickness in pregnancy (Kennedy, 2016). Approximately 80% of pregnant women experience morning sickness, or nausea and vomiting, during pregnancy, particularly during the first trimester (Koren, 2014). Such symptoms often negatively impact their quality of life (Clark et al., 2013). There has been significant interest in ondansetron as several studies over the past few years have shown it to be an effective treatment for morning sickness (Quinlan and Hill, 2003; Kennedy, 2016; Fejzo et al., 2019). Various studies have shown that ondansetron works by blocking serotonin receptors in the brain, alleviating nausea and vomiting symptoms in pregnant women (Heckroth et al., 2021). However, despite the evidence suggesting its efficacy, there is still some concern about its use during pregnancy and it is important to conduct further research to better understand the potential risks and benefits associated with its use in pregnant women (Carstairs, 2016; Kaplan et al., 2019).
Ondansetron’s pharmacokinetics are characterized by rapid absorption, extensive distribution, and hepatic metabolism (Simpson and Hicks, 1996). The drug exhibits swift absorption following oral administration, resulting in peak plasma concentrations manifesting within a span of 1–2 h. Oral ondansetron bioavailability is approximately 60% due to first-pass metabolism in the liver. The drug is extensively distributed throughout the body, with a volume of distribution (Vd) of approximately 140 L. Ondansetron is highly protein-bound, with more than 70% of the drug bound to plasma proteins. The drug is metabolized in the liver by several cytochrome P450 enzymes, primarily CYP3A4 and CYP2D6, and eliminated mainly via feces (Roila and Del Favero, 1995; Christofaki and Papaioannou, 2014).
Ondansetron’s pharmacodynamics are dose-dependent and exhibit a ceiling effect. The drug has a half-life of approximately 4 h and a duration of action of 8–12 h (Lozano, 2013). Ondansetron’s therapeutic dose range is 4–8 mg, and higher doses do not provide additional benefits (Meiri et al., 2007). The drug is generally well-tolerated; the most common adverse effects are headaches, constipation, and diarrhea. Ondansetron may also prolong the QT interval and increase the risk of cardiac arrhythmias (Charbit et al., 2005; Freedman et al., 2014).
Ondansetron has been shown to be an effective treatment for nausea and vomiting, including pregnancy-related morning sickness. The drug works by blocking serotonin receptors in the brain, reducing vomiting reflex activation. Despite evidence suggesting its efficacy, there are still concerns about its use during pregnancy, particularly during the first trimester. This is due to potential risks to fetal development, including congenital malformations. While some studies have reported a statistically significant association between ondansetron exposure and an increased risk of cardiac malformations, conflicting evidence has also been reported. Further research is warranted to assess the potential risks and benefits associated with the use of ondansetron in pregnant women to ensure that it can be used safely with minimal risk to both the mother and unborn baby.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
AA: Data collection, Investigation, Methodology, Software, Writing–original draft, Writing–review and editing.
The author declare that no financial support was received for the research, authorship, and/or publication of this article.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Carstairs, S. D. (2016). Ondansetron use in pregnancy and birth defects: a systematic review. Obstetrics Gynecol. 127 (5), 878–883. doi:10.1097/AOG.0000000000001388
Cengiz, H., Dagdeviren, H., Caypinar, S. S., Kanawati, A., Yildiz, S., and Ekin, M. (2015). Plasma serotonin levels are elevated in pregnant women with hyperemesis gravidarum. Archives Gynecol. obstetrics 291, 1271–1276. doi:10.1007/s00404-014-3572-2
Charbit, B., Albaladejo, P., Funck-Brentano, C., Legrand, M., Samain, E., and Marty, J. (2005). Prolongation of QTc interval after postoperative nausea and vomiting treatment by droperidol or ondansetron. J. Am. Soc. Anesthesiol. 102 (6), 1094–1100. doi:10.1097/00000542-200506000-00006
Christofaki, M., and Papaioannou, A. (2014). Ondansetron: a review of pharmacokinetics and clinical experience in postoperative nausea and vomiting. Expert Opin. Drug Metabolism Toxicol. 10 (3), 437–444. doi:10.1517/17425255.2014.882317
Clark, S., Hughes, B., and McDonald, S. S. (2013). The impact of nausea and vomiting of pregnancy on quality of life: report of a national consumer survey and recommendations for improving care. Obstetrical Gynecol. Surv. 68 (9), S1–S10. doi:10.1097/ogx.0b013e3182a8784d
Colvin, L., Gill, A. W., Slack-Smith, L., Stanley, F. J., and Bower, C. (2013). Off-label use of ondansetron in pregnancy in Western Australia. BioMed Res. Int. 2013, 909860. doi:10.1155/2013/909860
Danielsson, B., Wikner, B. N., and Källén, B. (2014). Use of ondansetron during pregnancy and congenital malformations in the infant. Reprod. Toxicol. 50, 134–137. doi:10.1016/j.reprotox.2014.10.017
Ernst, E. (2019). “Complementary/alternative medicine during pregnancy,” in Therapeutics in pregnancy and lactation (England, UK: Routledge), 207–213.
Fejzo, M. S., Trovik, J., Grooten, I. J., Sridharan, K., Roseboom, T. J., Vikanes, Å., et al. (2019). Nausea and vomiting of pregnancy and hyperemesis gravidarum. Nat. Rev. Dis. Prim. 5 (1), 62. doi:10.1038/s41572-019-0110-3
Freedman, S. B., Uleryk, E., Rumantir, M., and Finkelstein, Y. (2014). Ondansetron and the risk of cardiac arrhythmias: a systematic review and postmarketing analysis. Ann. Emerg. Med. 64 (1), 19–25. doi:10.1016/j.annemergmed.2013.10.026
Griddine, A., and Bush, J. S. (2022). “Ondansetron,” in StatPearls (Petersburg, Florida: StatPearls Publishing).
Heckroth, M., Luckett, R. T., Moser, C., Parajuli, D., and Abell, T. L. (2021). Nausea and vomiting in 2021: a comprehensive update. J. Clin. gastroenterology 55 (4), 279–299. doi:10.1097/MCG.0000000000001485
Hesketh, P. J., Kris, M. G., Basch, E., Bohlke, K., Barbour, S. Y., Clark-Snow, R. A., et al. (2017). Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 35 (28), 3240–3261. doi:10.1200/JCO.2017.74.4789
Kaplan, Y. C., Richardson, J. L., Keskin-Arslan, E., Erol-Coskun, H., and Kennedy, D. (2019). Use of ondansetron during pregnancy and the risk of major congenital malformations: a systematic review and meta-analysis. Reprod. Toxicol. 86, 1–13. doi:10.1016/j.reprotox.2019.03.001
Kennedy, D. (2016). Ondansetron and pregnancy: understanding the data. Obstet. Med. 9 (1), 28–33. doi:10.1177/1753495X15621154
Koren, G. (2014). Treating morning sickness in the United States: changes in prescribing are needed. Am. J. Obstetrics Gynecol. 211 (6), 602–606. doi:10.1016/j.ajog.2014.08.017
Lee, N. M., and Saha, S. (2011). Nausea and vomiting of pregnancy. Gastroenterol. Clin. 40 (2), 309–334. doi:10.1016/j.gtc.2011.03.009
Lozano, R. (2013). Mirtazapine and ondansetron: a dual pharmacodynamic and pharmacokinetic interaction. Afr. J. Psychiatry 16 (1), 56.
Maltepe, C. (2014). Surviving morning sickness successfully: from patient’s perception to rational management. J. Popul. Ther. Clin. Pharmacol. 21 (3), e555–e564.
Meiri, E., Jhangiani, H., Vredenburgh, J. J., Barbato, L. M., Carter, F. J., Yang, H. M., et al. (2007). Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr. Med. Res. Opin. 23 (3), 533–543. doi:10.1185/030079907x167525
Michie, L. A., and Hodson, K. K. (2020). Ondansetron for nausea and vomiting in pregnancy: re-evaluating the teratogenic risk. London, England: SAGE Publications Sage UK, 3–4.
Parker, S. E., Van Bennekom, C., Anderka, M., and Mitchell, A. A.National Birth Defects Prevention Study (2018). Ondansetron for treatment of nausea and vomiting of pregnancy and the risk of specific birth defects. Obstetrics Gynecol. 132 (2), 385–394. doi:10.1097/AOG.0000000000002679
Quinlan, J. D., and Hill, D. A. (2003). Nausea and vomiting of pregnancy. Am. Fam. physician 68 (1), 121–128.
Roila, F., and Del Favero, A. (1995). Ondansetron clinical pharmacokinetics. Clin. Pharmacokinet. 29 (2), 95–109. doi:10.2165/00003088-199529020-00004
Simino, G. P. R., Marra, L. P., Andrade, E. I. G. d., Acúrcio, F. d. A., Reis, I. A., De Araújo, V. E., et al. (2016). Efficacy, safety and effectiveness of ondansetron compared to other serotonin-3 receptor antagonists (5-HT3RAs) used to control chemotherapy-induced nausea and vomiting: systematic review and meta-analysis. Expert Rev. Clin. Pharmacol. 9 (9), 1183–1194. doi:10.1080/17512433.2016.1190271
Simpson, K. H., and Hicks, F. M. (1996). Clinical pharmacokinetics of ondansetron. A review. J. Pharm. Pharmacol. 48 (8), 774–781. doi:10.1111/j.2042-7158.1996.tb03973.x
Slattery, J., Quinten, C., Candore, G., Pinheiro, L., Flynn, R., Kurz, X., et al. (2022). Ondansetron use in nausea and vomiting during pregnancy: a descriptive analysis of prescription patterns and patient characteristics in UK general practice. Br. J. Clin. Pharmacol. 88 (10), 4526–4539. doi:10.1111/bcp.15370
Solihah, R., Purwati, A. E., Sandriani, S., and Mira, N. (2023). Literature Review: herbs to prevent nausea and vomiting in pregnant women. Nurul Ilmi J. Health Sci. Midwifery 1 (1), 37–44. doi:10.52221/nuri.v1i1.209
The National Library of Medicine (2016). NCT01357369. Available at: https://clinicaltrials.gov/study/NCT01357369.
The National Library of Medicine (2011). NCT01414777. Available at: https://clinicaltrials.gov/study/NCT01414777.
The National Library of Medicine (2013a). NCT01668069. Available at: https://clinicaltrials.gov/study/NCT01668069.
The National Library of Medicine (2013b). NCT01801475. Available at: https://clinicaltrials.gov/study/NCT01801475.
The National Library of Medicine (2020). NCT01965704. Available at: https://clinicaltrials.gov/study/NCT01965704.
The National Library of Medicine (2022). NCT03785691. Available at: https://clinicaltrials.gov/study/NCT03785691.
Thibeault, A.-A. H., Sanderson, J. T., and Vaillancourt, C. (2019). Serotonin-estrogen interactions: what can we learn from pregnancy? Biochimie 161, 88–108. doi:10.1016/j.biochi.2019.03.023
Wolf, H. (2000). Preclinical and clinical pharmacology of the 5-HT3 receptor antagonists. Scand. J. Rheumatology 29 (113), 37–45. doi:10.1080/030097400446625
Keywords: ondansetron, pregnancy, clinical trials, serotonin, vomiting, morning sickness
Citation: Ashour AM (2023) Efficacy and safety of ondansetron for morning sickness in pregnancy: a systematic review of clinical trials. Front. Pharmacol. 14:1291235. doi: 10.3389/fphar.2023.1291235
Received: 08 September 2023; Accepted: 11 October 2023;
Published: 23 October 2023.
Edited by:
Hanan Farouk Aly, National Research Centre, EgyptReviewed by:
Khalid Ibrahim, University of Zakho, IraqCopyright © 2023 Ashour. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed M. Ashour, YW1hc2hvdXJAdXF1LmVkdS5zYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.