- 1Science, Evidence and Analytics Directorate, National Institute for Health and Care Excellence, Manchester, United Kingdom
- 2Norwich Medical School, University of East Anglia, Norwich, United Kingdom
Objectives: As the initial crisis of the COVID-19 pandemic recedes, healthcare decision makers are likely to want to make rational evidence-guided choices between the many interventions now available. We sought to update a systematic review to provide an up-to-date summary of the cost-effectiveness evidence regarding tests for SARS-CoV-2 and treatments for COVID-19.
Methods: Key databases, including MEDLINE, EconLit and Embase, were searched on 3 July 2023, 2 years on from the first iteration of this review in July 2021. We also examined health technology assessment (HTA) reports and the citations of included studies and reviews. Peer-reviewed studies reporting full health economic evaluations of tests or treatments in English were included. Studies were quality assessed using an established checklist, and those with very serious limitations were excluded. Data from included studies were extracted into predefined tables.
Results: The database search identified 8,287 unique records, of which 54 full texts were reviewed, 28 proceeded for quality assessment, and 15 were included. Three further studies were included through HTA sources and citation checking. Of the 18 studies ultimately included, 17 evaluated treatments including corticosteroids, antivirals and immunotherapies. In most studies, the comparator was standard care. Two studies in lower-income settings evaluated the cost effectiveness of rapid antigen tests and critical care provision. There were 17 modelling analyses and 1 trial-based evaluation.
Conclusion: A large number of economic evaluations of interventions for COVID-19 have been published since July 2021. Their findings can help decision makers to prioritise between competing interventions, such as the repurposed antivirals and immunotherapies now available to treat COVID-19. However, some evidence gaps remain present, including head-to-head analyses, disease-specific utility values, and consideration of different disease variants.
Systematic Review Registration: [https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021272219], identifier [PROSPERO 2021 CRD42021272219].
1 Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its associated disease (COVID-19) pandemic placed healthcare systems and wider economies under massive strain in 2020 and 2021. Decisions about diagnostic tests and treatments for the disease were made rapidly, forgoing traditional, rigorous health technology assessments (HTAs) that healthcare interventions are subjected to in many countries. Now that the early pandemic crisis has passed, HTA organisations will increasingly view COVID-19 as being equivalent to any other condition, and seek to understand the cost effectiveness of tests and treatments for it. Such evidence can support reimbursement decisions and the efficient allocation of scarce healthcare resources.
In July 2021, the first iteration of a systematic literature review to identify economic evaluations of tests and treatments for COVID-19 was conducted (Elvidge et al., 2022). Its objective was to identify up-to-date cost-effectiveness estimates for COVID-19 tests and treatments, and the methodological approaches, limitations and uncertainties present in published economic evaluations. Since then, the pandemic context, evidence base, and disease have evolved considerably. The present study reports a timely two-year update of the review, to provide a contemporary understanding of the cost-effectiveness evidence for COVID-19 tests and treatments.
This study has been supported by Next-Generation Health Technology Assessment (HTx), which is a Horizon 2020 project supported by the European Union, lasting for 5 years from January 2019. Its main aim is to create a framework for the next-generation of HTA to support patient-centred, societally oriented, real-time decision making on access to and reimbursement for health technologies throughout Europe.
2 Materials and methods
We performed an update of a previously published systematic literature review to identify full economic evaluations of diagnostics (e.g., tests) for SARS-CoV-2 and treatments (e.g., pharmaceuticals) for COVID-19 (Elvidge et al., 2021; Elvidge et al., 2022). The date range spanned the previous search date, 12th July 2021, to 3rd July 2023. The search strategy was consistent with the original search, including citation checking of included studies and efforts to identify relevant grey literature. Studies were included if they were full economic evaluations, comparing both the costs and health outcomes of 2 or more alternative tests for SARS-CoV-2 or treatments for COVID-19.
Every identified title and abstract was screened against the selection criteria by 2 reviewers (JE and NN/GH). For studies that were identified as potentially relevant, full-text articles were sought and assessed against the selection criteria by both reviewers. Studies that met the selection criteria were quality assessed by both reviewers, using the NICE economic evaluation quality checklist (National Institute for Health and Care Excellence NICE, 2012). Those judged to have very serious limitations were excluded. For each included study, data extraction was conducted by 1 reviewer using prespecified tables consistent with the original review. Extracted data for each study were checked and validated by another reviewer. At all stages, discrepancies between the reviewers were resolved through discussion or, if needed, adjudication by a senior reviewer (DD). Key study characteristics are presented in Tables 1, 2, and findings in Table 3. Due to extensive heterogeneity between studies, results were synthesised narratively (Shields and Elvidge, 2020).
3 Results
3.1 Included studies
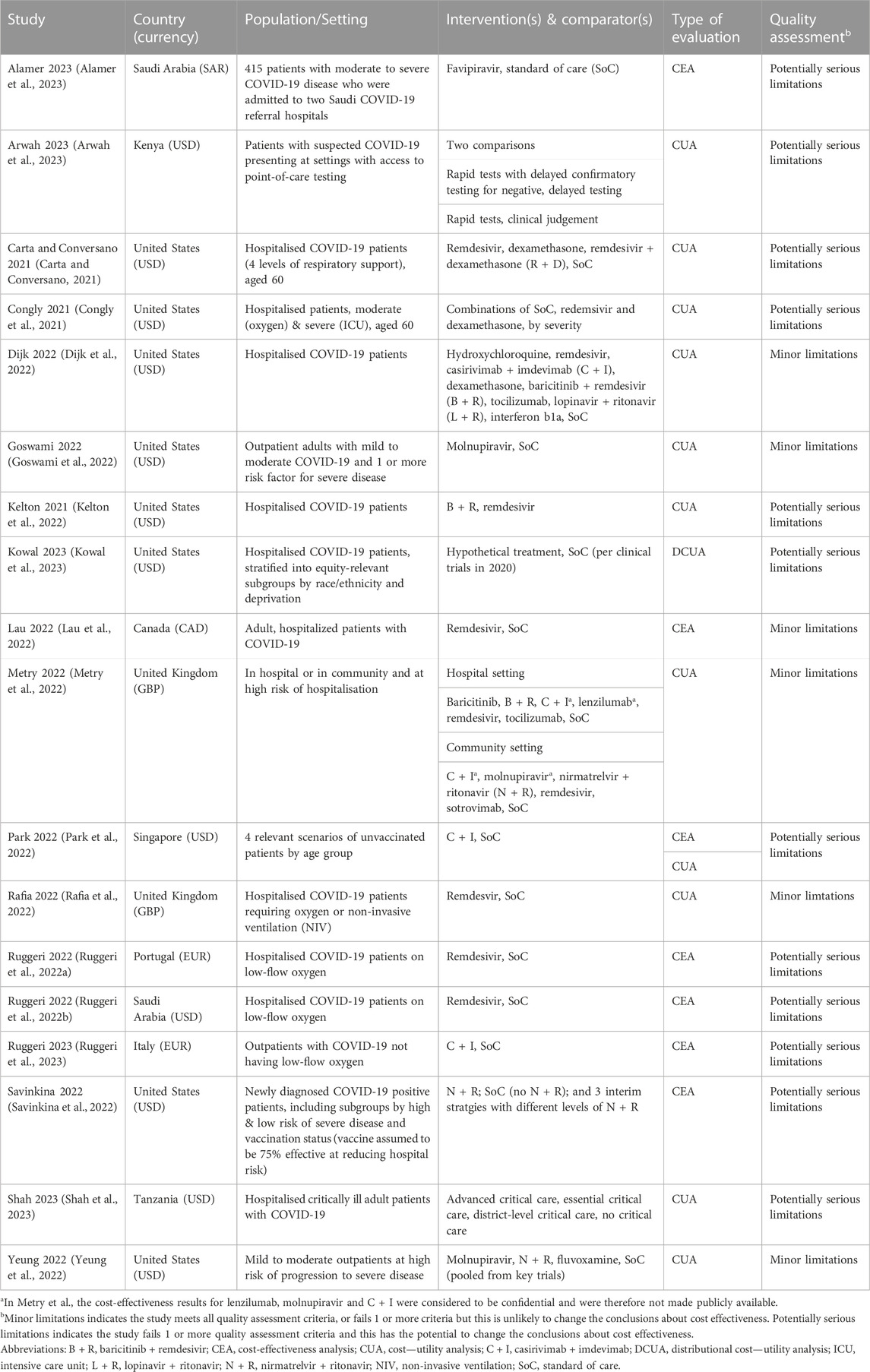
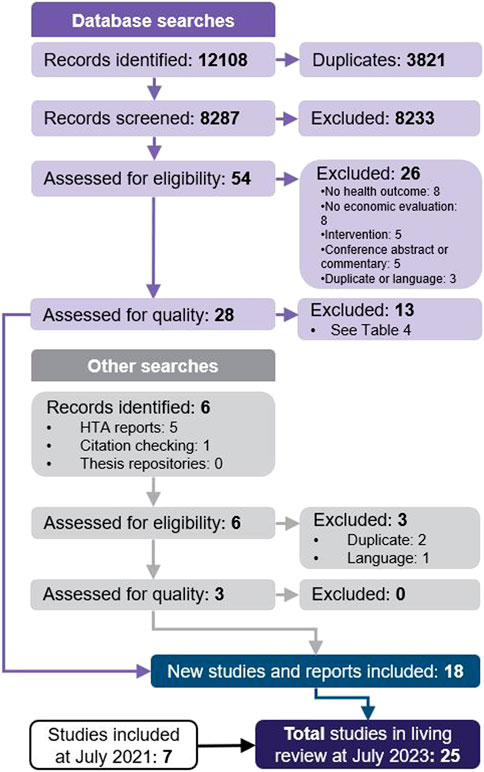
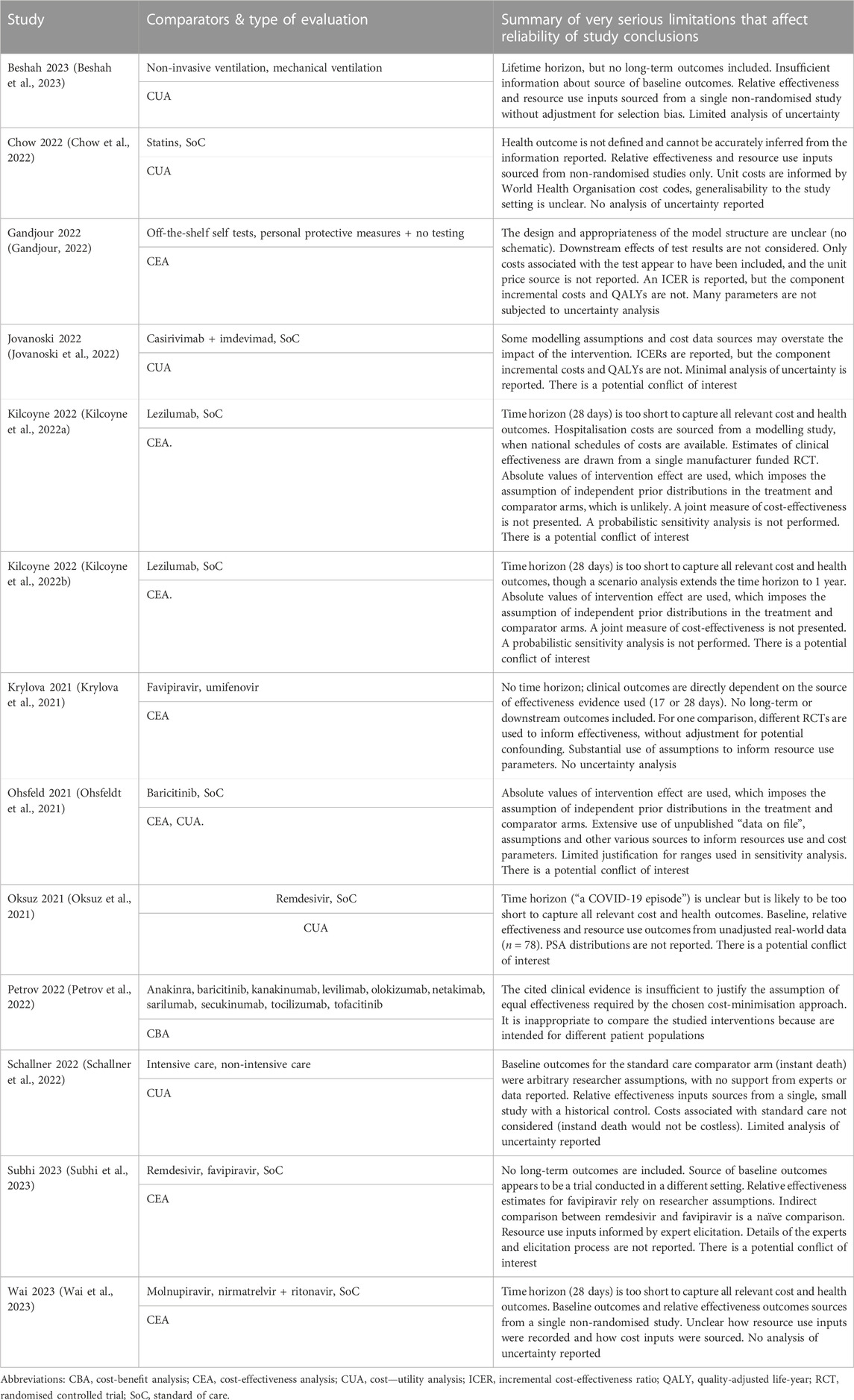
Search strategies and results per database are provided in Supplementary Material. A total of 8,287 unique records were identified for initial screening of titles and abstracts (Figure 1). Of those, 8,233 were excluded, most commonly because they did not report a primary economic evaluation. Therefore, 54 studies proceeded to full-text review, with 28 meeting the inclusion criteria. Six studies were also identified through searches of grey literature: 1 through citation checking, which met our inclusion criteria, and 5 HTA reports, of which 2 met our criteria. Two reported on the same HTA and were considered to be duplicates, and 1 was not available in English. A total of 31 studies proceeded to quality assessment, of which 13 were excluded due to the presence of very serious limitations (Table 4). Finally, 18 studies of acceptable quality were included in this two-year update (Carta and Conversano, 2021; Congly et al., 2021; Ruggeri et al., 2022a; Ruggeri et al., 2022b; Dijk et al., 2022; Goswami et al., 2022; Kelton et al., 2022; Lau et al., 2022; Metry et al., 2022; Park et al., 2022; Rafia et al., 2022; Savinkina et al., 2022; Yeung et al., 2022; Alamer et al., 2023; Arwah et al., 2023; Kowal et al., 2023; Ruggeri et al., 2023; Shah et al., 2023).
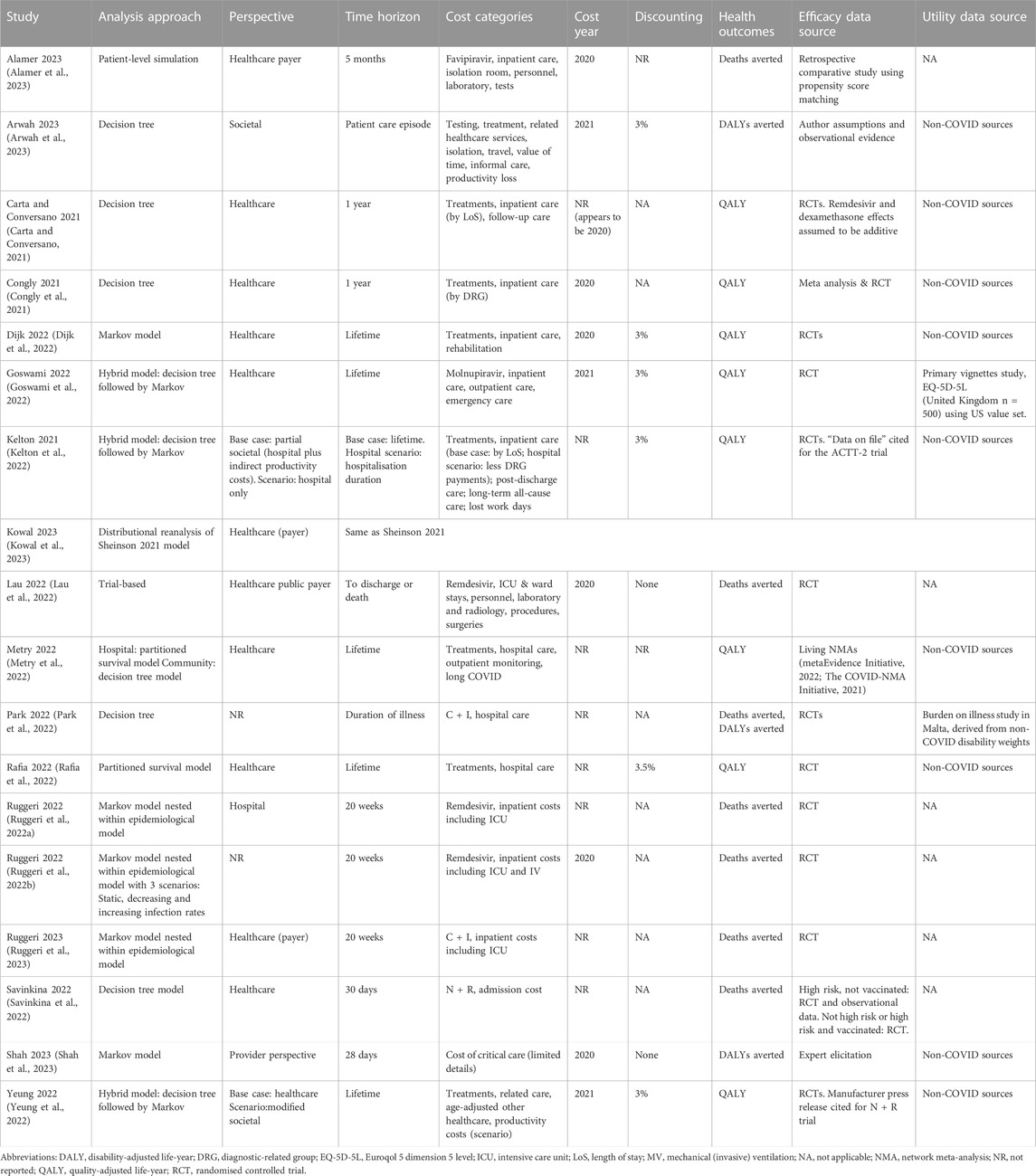
Included studies evaluated interventions in community or outpatient settings (5/18) (Goswami et al., 2022; Park et al., 2022; Savinkina et al., 2022; Yeung et al., 2022; Ruggeri et al., 2023), where patients are at risk of admission to hospital, or an inpatient hospital setting (11/18) (Carta and Conversano, 2021; Congly et al., 2021; Ruggeri et al., 2022a; Ruggeri et al., 2022b; Dijk et al., 2022; Kelton et al., 2022; Lau et al., 2022; Rafia et al., 2022; Alamer et al., 2023; Kowal et al., 2023; Shah et al., 2023); one study included both settings (1/18) (Metry et al., 2022). One study evaluated point-of-care tests in an unspecified health facility (1/18) (Arwah et al., 2023). Of studies based in inpatient hospital settings, some were aimed at specific populations and places within the care pathway, namely moderate disease with non-invasive ventilation (3/12) (Ruggeri et al., 2022a; Ruggeri et al., 2022b; Rafia et al., 2022) or critical care (1/12) (Shah et al., 2023), though most had mixed or unspecified populations (8/12) (Carta and Conversano, 2021; Congly et al., 2021; Dijk et al., 2022; Kelton et al., 2022; Lau et al., 2022; Metry et al., 2022; Alamer et al., 2023; Kowal et al., 2023). Most studies (12/18) (Carta and Conversano, 2021; Congly et al., 2021; Dijk et al., 2022; Goswami et al., 2022; Lau et al., 2022; Metry et al., 2022; Rafia et al., 2022; Savinkina et al., 2022; Yeung et al., 2022; Alamer et al., 2023; Kowal et al., 2023; Ruggeri et al., 2023) took a healthcare system or payer perspective in their base-case analyses, while 2/18 took a provider (e.g., hospital) perspective (Ruggeri et al., 2022a; Shah et al., 2023), 2/18 took a partial societal perspective (Kelton et al., 2022; Arwah et al., 2023), and 2/18 did not explicitly report a perspective (Ruggeri et al., 2022b; Park et al., 2022). Multiple studies were conducted in the United States (8/18) (Carta and Conversano, 2021; Congly et al., 2021; Dijk et al., 2022; Goswami et al., 2022; Kelton et al., 2022; Savinkina et al., 2022; Yeung et al., 2022; Kowal et al., 2023), Saudi Arabia (2/18) (Ruggeri et al., 2022b; Alamer et al., 2023) and the United Kingdom (2/18) (Metry et al., 2022; Rafia et al., 2022), while single studies were conducted in each of Canada (Lau et al., 2022), Italy (Ruggeri et al., 2023), Kenya (Arwah et al., 2023), Portugal (Ruggeri et al., 2022a), Singapore (Park et al., 2022) and Tanzania (Shah et al., 2023). Most studies reported costs in US dollars (12/18), with 4/18 converting to US dollars from the local currency (Ruggeri et al., 2022b; Park et al., 2022; Arwah et al., 2023; Shah et al., 2023), while 6/18 reported costs in the local non-US currency (Ruggeri et al., 2022a; Lau et al., 2022; Metry et al., 2022; Rafia et al., 2022; Alamer et al., 2023; Ruggeri et al., 2023).
Included studies evaluated one or more of the following pharmacological treatments for COVID-19, usually in addition to standard care: remdesivir (9/18) (Carta and Conversano, 2021; Congly et al., 2021; Ruggeri et al., 2022a; Ruggeri et al., 2022b; Dijk et al., 2022; Kelton et al., 2022; Lau et al., 2022; Metry et al., 2022; Rafia et al., 2022), casirivimab + imdevimab (3/18) (Dijk et al., 2022; Park et al., 2022; Ruggeri et al., 2023), baricitinib + remdesivir (3/18) (Dijk et al., 2022; Kelton et al., 2022; Metry et al., 2022), dexamethasone (3/18) (Carta and Conversano, 2021; Congly et al., 2021; Dijk et al., 2022), nirmatrelivir + ritonivir (3/18) (Metry et al., 2022; Savinkina et al., 2022; Yeung et al., 2022), molnupiravir (2/18) (Goswami et al., 2022; Yeung et al., 2022), and tocilizumab (2/18) (Dijk et al., 2022; Metry et al., 2022). The following medicines were evaluated by single studies: baricitinib (Metry et al., 2022), favipiravir (Alamer et al., 2023), fluvoxamine (Yeung et al., 2022), hydroxychloroquine (Dijk et al., 2022), interferon beta-1a (Dijk et al., 2022), lopinavir + ritonivir (Dijk et al., 2022), remdesivir + dexamethasone (Carta and Conversano, 2021), sotrovimab (Metry et al., 2022). One study (1/18) evaluated lenzilumab alongside other treatments (lenzilumab, molnupiravir and casirivimab + imdevimab) but did not publish cost-effectiveness results for these other treatments due to confidentiality (Metry et al., 2022). One study (1/18) evaluated a hypothetical pharmacological treatment for COVID-19, with an efficacy profile derived from the ACTT-1 (remdesivir) and RECOVERY (dexamethasone) trials, and a price of $2,500 per course (Kowal et al., 2023). In all cases, standard care without the pharmacological intervention of interest was a comparator. One study (1/18) evaluated a test for SARS-CoV-2 (Arwah et al., 2023), and one (1/18) evaluated the cost effectiveness of different levels of critical care for the treatment of severe COVID-19 in a lower-middle income country setting (Shah et al., 2023). The comparators were not treating COVID-19 in critical care services; treating COVID-19 with basic critical care in district hospitals, reflecting standard care; essential critical care, defined as treating people with severe and critical disease with advanced care such as supplemental oxygen; and advanced critical care, where people with critical disease are treated with life-sustaining therapies such as mechanical ventilation.
Cost—utility analyses (CUAs) were reported by 12/18 studies, quantifying costs and a preference-based measure of health (Carta and Conversano, 2021; Congly et al., 2021; Dijk et al., 2022; Goswami et al., 2022; Kelton et al., 2022; Metry et al., 2022; Park et al., 2022; Yeung et al., 2022; Arwah et al., 2023; Kowal et al., 2023; Shah et al., 2023). In most cases (9/12), quality-adjusted life years (QALYs) were used (Carta and Conversano, 2021; Congly et al., 2021; Dijk et al., 2022; Goswami et al., 2022; Kelton et al., 2022; Metry et al., 2022; Rafia et al., 2022; Yeung et al., 2022; Kowal et al., 2023); the rest (3/12) used disability-adjusted life years (DALYs) (Park et al., 2022; Arwah et al., 2023; Shah et al., 2023). One of the QALY-based analyses was a distributional CUA (Kowal et al., 2023), and was a re-analysis of a study that was included in the initial review (Sheinson et al., 2021). Cost-effectiveness analyses (CEAs) were reported by 7/18 studies (Ruggeri et al., 2022a; Ruggeri et al., 2022b; Lau et al., 2022; Park et al., 2022; Savinkina et al., 2022; Alamer et al., 2023; Ruggeri et al., 2023). All CEA studies used deaths averted as their non-preference-based measure of health. One study (1/18) was a CEA conducted alongside a clinical trial (Lau et al., 2022). All other studies (17/18) reported model-based analyses, comprising decision trees (6/17) (Carta and Conversano, 2021; Congly et al., 2021; Metry et al., 2022; Park et al., 2022; Savinkina et al., 2022; Arwah et al., 2023); Markov models (6/17) (Ruggeri et al., 2022a; Ruggeri et al., 2022b; Dijk et al., 2022; Kowal et al., 2023; Ruggeri et al., 2023; Shah et al., 2023), some of which were nested within a disease epidemiology model (3/17) (Ruggeri et al., 2022a; Ruggeri et al., 2022b; Ruggeri et al., 2023); “hybrid” models, with a decision tree to model acute disease followed by a long-term Markov component (3/17) (Goswami et al., 2022; Kelton et al., 2022; Yeung et al., 2022); partitioned survival models (2/17) (Metry et al., 2022; Rafia et al., 2022); and a patient-level simulation (1/17) (Alamer et al., 2023). A potential financial conflict of interest in favour the intervention under evaluation was reported by 6/18 included studies (Ruggeri et al., 2022b; Goswami et al., 2022; Kelton et al., 2022; Lau et al., 2022; Ruggeri et al., 2023).
3.2 Cost effectiveness
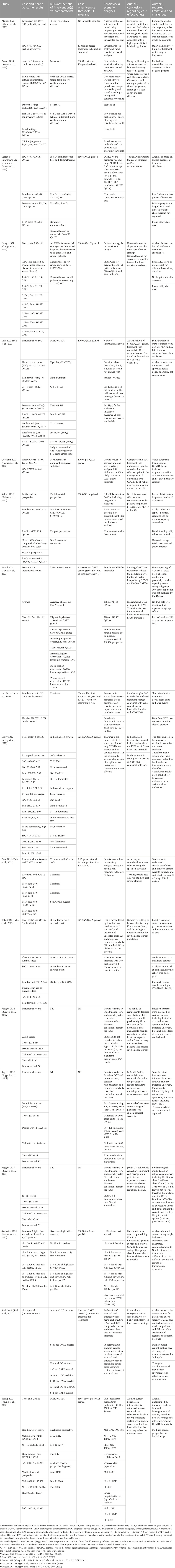
3.2.1 Treatments: inpatient hospital setting
For evaluations based in inpatient hospital populations, with or without supplemental oxygen, 8/12 studies were CUAs that specified one or more willingness-to-pay thresholds to determine cost effectiveness of evaluated interventions. Dexamethasone was found to be cost effective compared with standard care (Carta and Conversano, 2021; Congly et al., 2021; Dijk et al., 2022); this conclusion was robust to sensitivity analyses, and a value of information analysis indicated there would be no value in further research (Dijk et al., 2022). Remdesivir was also generally cost effective versus standard care (Carta and Conversano, 2021; Congly et al., 2021; Dijk et al., 2022; Rafia et al., 2022), though 1 study noted that this result was highly sensitive to whether it confers a survival benefit or not (Rafia et al., 2022). If it does, the reported ICER was around $17,000 per QALY gained, rising to over $1 million per QALY gained if it does not reduce the risk of death. Its mortality hazard ratio should be at least 0.92 for it to be cost effective. Further, 1 study found that using dexamethasone for all hospitalised patients dominated any strategy that involved remdesivir (Congly et al., 2021). Baricitinib in addition to remdesivir was found to be cost effective versus standard care in 2 studies (Dijk et al., 2022; Metry et al., 2022), and this was robust to sensitivity analyses. In a US study (Kelton et al., 2022), it had an ICER of around $22,000 per QALY gained compared with using remdesivir alone, in a partial societal analysis, and was dominant from a hospital perspective. However, this was in conflict with a fully incremental analysis from a United Kingdom healthcare perspective (Metry et al., 2022), that suggested barcitinib monotherapy was the most cost effective treatment for hospitalised patients, with ICERs of around $7,500–19,000 per QALY gained depending on the patient’s need for oxygen. The conflicting results may be explained by healthcare resource cost differences between the US and United Kingdom, or the studies’ different sources for relative effectiveness data; one (Kelton et al., 2022) made use of data on file from a single trial (ACTT-2), while the other (Metry et al., 2022) used outputs from published “living” network meta analyses (metaEvidence Initiative, 2022; The COVID-NMA Initiative, 2021). In a study that compared treatments with standard care but not with each other (Dijk et al., 2022), casirivimab + imdevimab and tocilizumab were estimated to have ICERs of around $13,000 and $40,000 per QALY gained, respectively, while hydroxychloroquine, interferon beta-1a and lopinavir + ritonivir were found to be cost saving but detrimental to health outcomes (QALYs). The resulting southwest-qaudrant ICERs were around $46,000, $5,500 and $15,000, respectively, which, at the specified threshold of $100,000 per QALY gained, imply the cost savings would not be sufficient to offset the health outcomes foregone. Value of information analysis identified that further research to examine disinvestment in hydroxychloroquine may be worthwhile, though it is not widely used.
One QALY-based evaluation of inpatient pharmacological treatment (Kowal et al., 2023) was a distributional re-analysis of a study previously included in this review (Sheinson et al., 2021). Treatment was found to be more cost effective in more deprived populations, with an ICER of $28,000 per QALY gained in the most deprived group and $29,800 in the least deprived group. Including the existing inequitable distribution of opportunity costs in the US health system, population-level net health benefits would remain positive up to a treatment cost of $60,100 per patient.
The one other CUA in the inpatient setting (Shah et al., 2023) evaluated using different levels of critical care to treat people with COVID-19, and used DALYs averted as the health outcome. At a specified conservative threshold in Tanzania of $101 per DALY averted, using essential critical care (e.g., supplemental oxygen) for people with COVID-19 was cost effective compared with no critical care, with an ICER of $37 per DALY averted, and district-level critical care ($14 per DALY averted). The equivalent ICERs for using advanced critical care (e.g., mechanical ventilation critical disease) were above the threshold, at $186 and $144, respectively.
There were 4 CEAs evaluating treatments in the inpatient setting, with all using deaths averted as the health outcome. Two studies used the same economic model with country-specific input data, and found remdesivir to reduce deaths and costs compared with standard care in Portugal [7 deaths averted and $1.2 m saved per 1,000 cases (Ruggeri et al., 2022a)] and Saudi Arabia [6.7, $980,000 (Ruggeri et al., 2022b)]. One trial-based analysis reached similar results in the setting of Canada (Lau et al., 2022); it was dominant in 58% of probabilistic sensitivity analysis (PSA) simulations, and the ICER was below $100,000 for death averted in 82%. Favipiravir was evaluated by 1 study (Alamer et al., 2023), and was also found to reduce deaths and costs in Saudi Arabia, with a saving of $4,500 per death averted.
3.2.2 Treatments: outpatient and community setting
For patients treated in the outpatient and community setting, at risk of progressing to severe disease requiring inpatient or critical care, 4 studies compared active treatments with standard care only. Among them, casirivimab + imdevimab was found to have an ICER of $800 per DALY averted in 1 study (Park et al., 2022)–below the specified $74,000 per DALY threshold in Singapore–and to reduce deaths and save costs in Italy in another study (Ruggeri et al., 2023). The conclusions of both studies were robust to sensitivity analyses undertaken; in the latter case, casirivimab + imdevimab was dominant in over 90% of simulations. One study estimated that molnupiravir would dominate standard care, generating incremental QALYs and reducing costs (Goswami et al., 2022).
In the only identified study that explicitly compared different strategies for using a treatment in subgroups defined by vaccination status (Savinkina et al., 2022), base-case results suggested that nirmatrelivir + ritonivir would dominate standard care for unvaccinated high-risk groups. However, that result was sensitive to the relative effect estimate, with a plausible lower-bound effect leading to an ICER of over $300,000 per death averted. Nirmatrelivir + ritonivir would be less cost effective if used in vaccinated and low-risk groups, with the ICER rising to $5 m per death averted if used for all patients. In a US HTA analysis (Yeung et al., 2022), it had an ICER of $21–26,000 per QALY gained versus standard care, depending on the perspective chosen, and in a United Kingdom fully incremental analysis conducted for HTA (Metry et al., 2022), the ICER was around $8,500 per QALY gained (remdesivir and sotrovimab were dominated). The US HTA study also found fluvoxamine would be cost effective compared with standard care relative to typical US thresholds (ICER: $8–20,000 per QALY gained), while molnupiravir had an ICER of $61,000 per QALY gained from a healthcare perspective, and $43,000 per QALY gained from a partial societal perspective that captured productivity costs. Therefore, the perspective would be a relevant factor for healthcare decision makers using the specified conservative threshold of $50,000 per QALY. In a scenario focusing on an unvaccinated patient population, in whom the effects of COVID-19 may be more severe, the US HTA (Yeung et al., 2022) found that nirmatrelivir + ritonivir, fluvoxamine and molnpiravir would be more cost effective versus standard care (with healthcare perspective ICERs of $15,000, $4,000 and $48,000 per QALY gained, respectively).
3.2.3 Diagnostic tests
The single study that evaluated a diagnostic test (Arwah et al., 2023) found that rapid antigen tests, plus a delayed nucleic acid amplifying test (NAAT) used in a confirmatory way, had an ICER of $965 per DALY averted compared with typical standard care in Kenya: delayed NAAT alone. This would be cost effective by a close margin relative to the specified local threshold of $1,003 per DALY; in PSA, the probability of the ICER being below the threshold was 53%. The ICER was sensitive to the underlying disease prevalence and the accuracy of rapid and confirmatory tests. In a scenario where NAAT is not available, rapid testing was estimated to dominate a “no testing” strategy that relies on clinical judgement.
4 Discussion
4.1 Principal findings
This updated systematic review indicates that pharmacological treatments that have been repurposed for to treat COVID-19 in recent years have the potential to be cost effective. In particular, the use of the low-cost corticosteroid dexamethasone—which has become routine practice to treat severe COVID-19 in an inpatient setting—appears to be clearly cost effective. Remdesivir and baricitinib, potentially in combination, appear to be promising candidates to treat severe disease, too. Limited cost-effectiveness evidence in the inpatient setting for casirivimab + imdevimab, tocilizumab, hydroxychloroquine, interferon beta-1a and lopinavir + ritonivir suggests the latter 3 treatments may produce worse health outcomes than standard care without a commensurate cost saving to be considered by decision makers.
In the outpatient and community setting, there is some evidence that casirivimab + imdevimab, fluvoxamine and molnupiravir may be cost effective over standard care. Results from 3 studies indicate that nirmatrelivir + ritonivir may be a cost effective treatment in the community setting among patients at high risk of hospitalisation (such as unvaccinated people), though the one fully incremental analysis among them does not include casirivimab + imdevimab, fluvoxamine or molnupiravir in the published results. The 2 studies that evaluated non-pharmacological interventions were both in lower-income settings. They suggested that rapid antigen tests may be cost effective where there is slow existing testing infrastructure, and certainly where there is none; and using the most advanced forms of critical care to treat COVID-19 might be difficult to justify, on cost-effectiveness grounds, in a resource-limited setting.
Compared with the first iteration of this review (Elvidge et al., 2022) conducted in July 2021, this update has identified economic evaluations of a much larger set of interventions for COVID-19. Previously, the evidence base was limited to evaluations of monotherapy and combination therapy use of dexamethasone and remdesivir, which were early candidate interventions for the treatment of COVID-19 in hospital. While we have identified additional cost-effectiveness evidence regarding these treatments, other pharmacological interventions have received marketing authorisation to treat the disease since 2021, and it is logical that healthcare decisions makers will be interested in understanding which of them offer value for money. Here, we have identified such evidence for antiviral therapies (casirivimab + imdevimab, favipiravir, lopinavir + ritonivir, molnupiravir, nirmatrelivir + ritonivir), immunotherapies (baricitinib, sotrovimab, tocilizumab) and various other repurposed medicines (fluvoxamine, hydroxychloroquine, interferon beta-1a).
We identified 1 study evaluating the cost effectiveness of a test for SARS-CoV-2, representing 6% of our included studies (1/18) compared with 29% in the initial review (2/7). It is likely that this reflects the changing pandemic context over time, such that comparing alternative testing strategies is no longer a prominent concern, relative to assessing the value of the growing number of available treatment options. The study evaluating rapid antigen tests was one of 2 included studies evaluating non-pharmacological interventions; the other estimated the value of using scarce critical care resources to treat people with COVID-19. Notably, both studies were conducted in lower-income settings (Kenya and Tanzania, respectively). This suggests testing strategies and the allocation of scarce hospital resources for COVID-19 remain a prominent concern for healthcare decision makers in settings where the most effective and newly licensed pharmacological interventions are less likely to be available.
The context around COVID-19 has changed substantially since the first iteration of this review. However, it does not appear that economic evaluations have adapted their methods to reflect these changes. This is likely to limit their scope to inform decision-making. First, vaccination programmes have been successful across the world and the vast majority of people, particularly in high and middle income countries, have now received at least one dose of a vaccine (Mathieu et al., 2020). Despite this, there appears to be limited consideration within economic evaluations of the impact of vaccination on disease severity and implications for cost effectiveness. Some identified studies did report scenario analyses in unvaccinated populations who are more likely to experience severe symptoms, and one (Savinkina et al., 2022) study from the US explicitly compared alternative strategies for using nirmatrelivir + ritonivir in different patient subgroups defined by their vaccination status and risk of hospitalisation. This approach is likely required to properly define who would benefit from treatments, but has not become widespread. Second, there are several reasons that parameters derived from studies completed at different stages of the pandemic may not be generalisable to the present day. These include pressures and constraints on hospital care during acute phases of the pandemic, differing approaches to standard care and changing disease variants. One study (Yeung et al., 2022) reported a scenario analysis in the context of a hypothethical variant with lower baseline risk of hospitalisation, though this did not consider differential treatment effectiveness for different variants. Differential efficacy between variants has been observed in practice, and means cost-effectiveness evaluations may need to increasingly examine value for money in specific subpopulations (Coronavirus COVID-19 Update, 2022). While some studies did note that the changing composition of COVID-19 over time may limit the generalisability of their cost-effectiveness conclusions (Metry et al., 2022; Park et al., 2022; Ruggeri et al., 2023), this appears to be an underconsidered issue. Third, there are now treatments that are established as best-practice due to the emergence of results from large-scale platform trials. Indeed, within this review dexamethasone is highlighted as a low-cost option that is effective for patients with respiratory support. However, there have been limited attempts to compare established treatments with each other. There is a need for more fully incremental cost-effectiveness analyses that compare alternative options simultaneously, rather than indirectly through how they compare with standard care. This may require measures of relative effectiveness to be derived from network meta analyses, such as used Metry et al. (2022), rather than data from local sites or individual trials.
In terms of the analytical methods used, most included studies were model-based analyses, which is consistent with the first iteration of this review. The modelling methods used remained similar. Time horizons varied from short term to lifetime; decision tree, Markov, and hybrid model structures were prominent; the utility weights used by CUAs were often generalised from non-COVID sources; and the known long-terms effects of disease (“long COVID”) were generally not captured. However, it is notable that some models may be overrepresented in the evidence base, due to repeated adaptations or reanalyses of the same model. In particular, remdesivir was evaluated in settings of Portugal (Ruggeri et al., 2022a) and Saudia Arabia (Ruggeri et al., 2022b) using a common model with country-specific inputs; casirivimab + imdevimab was evaluated in Italy (Ruggeri et al., 2023) using essentially the same model; and an existing CUA of a hypothethical treatment was reanalysed through a distributional lens (Kowal et al., 2023).
4.2 Strengths and limitations
This update to our “living” systematic review is methodologically consistent with the first publication (Elvidge et al., 2022), and so the same issues concerning the search strategy and generalisability of findings apply here. Our review aimed to provide a comprehensive account of available evidence and as such, a large number of unique records were identified by the database search (8,287); this is due to the known sensitivity of search terms used to identify economic evaluations (Hubbard et al., 2022). This increases the sensitivity of the search, reducing the likelihood of missing relevant studies, but it also means future updates will continue to require a labour-intensive screening process. In addition, like before, we chose to exclude studies that met our selection criteria but were judged to have very serious limitations that may materially affect the conclusions about cost effectiveness. This follows the process used in NICE clinical guideline development (National Institute for Health and Care Excellence NICE, 2012), to avoid conflating results across studies of varying quality, and was predefined in our study protocol (Elvidge et al., 2021). In this update, it led to the exclusion of 13 potentially relevant studies (Table 4). While 2 reviewers conducted this quality assessment, discussing and resolving any disagreements, we recognise that this is necessarily subjective; other reviewers may have reached different quality assessment decisions, or simply included all studies that met the selection criteria. There may also be included studies which did not meet the bar for exclusion, but which have analytical flaws or are based on parameters that are biased or do not reflect best available evidence and this may impact their findings. Further, we excluded public health interventions (e.g., lockdowns, face masks) and vaccinations from our review. The economic value of such interventions may still be of interest to some healthcare decision makers, for example where vaccine coverage is relatively low. Finally, our review is, like any, at risk of publication bias. Several of the included studies exhibit a potential conflict of interest. We cannot know whether those analyses would have reached publication if they had demonstrated negative conclusions about the intervention, nor how many such analyses exist and were not published.
4.3 Implications for future research
After 3 years’ worth of cost-effectiveness research for COVID-19 interventions, there are some new and some persistent evidence gaps that would benefit from further thought. Head-to-head economic evaluations of interventions are in the minority of the identified studies. There are now several treatments available in both the pre-hospital and hospital settings, and fully incremental analyses that compare options simultaneously may be valuable for decision making. The cost effectiveness of tests and treatments may be influenced by what happens later in the clinical pathway, and so a whole-disease model that reports fully incremental results would be a valuable resource. In the context of a continuously evolving disease, and with variable standard care and vaccination uptake in different settings, a whole-disease model could allow for rapid re-analyses in light of new evidence or changes in the prevailing conditions. In general, researchers should routinely reflect on the potential implications of vaccination status and disease variants for the generalisability of their conclusions. Further, these authors recommend that researchers routinely conduct detailed sensitivity analyses examining potential cost effectiveness under a wide range of baseline outcomes and relative effectiveness. Such analyses may help to ‘future-proof’ their studies to ensure they remain useful under different prevailing coditions. There remains a need for robust evidence about the health-related quality of life impact of COVID-19 in the short and long term, to support the conduct of CUAs. Finally, several identified studies were CEAs that used deaths averted as their health outcome with relatively short time horizons. This may be reasonable in some circumstances, and particularly if one intervention appears both more effective and have lower costs. However, in general, decision makers should be aware that where a treatment has an effect on survival, short-term analyses will not fully capture all relevant differences in outcomes between it the comparator.
5 Conclusion
This updated review of economic evaluations of tests and treatments for COVID-19, covering the period from July 2021 and July 2023, provides a contemporary summary of the cost-effectiveness evidence. Compared with the first iteration of the review (up to July 2021), we have identified 18 additional studies of acceptable quality that healthcare decision makers, such as HTA and payer organisations, may consider to inform their COVID-related decision making. In particular, the evidence may support prioritisation between the numerous antiviral therapies and immunotherapies. Conclusions about some treatments, such as dexamethasone (cost effective) and hydroxychloroquine (not cost effective), support current standard practice. An evidence gap remains for a whole-disease model that can support holistic decision making about multiple tests and treatments at linked decision points in a fully incremental way.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
JE: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Supervision, Writing–original draft, Writing–review and editing. GH: Formal Analysis, Writing–original draft, Writing–review and editing. NN: Formal Analysis, Writing–original draft. DN: Conceptualization, Data curation, Validation, Writing–review and editing. DD: Formal Analysis, Methodology, Supervision, Writing–review and editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. The HTx project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement Nº 825162. This dissemination reflects only the author’s view and the European Commission is not responsible for any use that may be made of the information it contains.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1291164/full#supplementary-material
References
Alamer, A., Almutairi, A. R., Halloush, S., Al-jedai, A., Alrashed, A., AlFaifi, M., et al. (2023). Cost-effectiveness of Favipiravir in moderately to severely ill COVID-19 patients in the real-world setting of Saudi arabian pandemic referral hospitals. Saudi Pharm. J. 31 (4), 510–516. doi:10.1016/j.jsps.2023.02.003
Arwah, B., Mbugua, S., Ngure, J., Makau, M., Mwaura, P., Kamau, D., et al. (2023). Cost & cost-effectiveness of implementing SD biosensor antigen detecting SARs-CoV-2 rapid diagnostic tests in Kenya. Preprint. Health Econ. doi:10.1101/2023.01.05.23284225
Beshah, S. A., Zeru, A., Tadele, W., Defar, A., Getachew, T., and Fekadu Assebe, L. (2023). A cost-effectiveness analysis of COVID-19 critical care interventions in Addis Ababa, Ethiopia: a modeling study. Cost Eff. Resour. Allocation 21 (1), 40. doi:10.1186/s12962-023-00446-8
Carta, A., and Conversano, C. (2021). Cost utility analysis of Remdesivir and Dexamethasone treatment for hospitalised COVID-19 patients - a hypothetical study. BMC Health Serv. Res. 21 (1), 986. doi:10.1186/s12913-021-06998-w
Chow, R., Simone, C. B., Prsic, E. H., and Shin, H. J. (2022). Cost-effectiveness analysis of statins for the treatment of hospitalized COVID-19 patients. Ann. Palliat. Med. 11 (7), 2285–2290. doi:10.21037/apm-21-2797
Congly, S. E., Varughese, R. A., Brown, C. E., Clement, F. M., and Saxinger, L. (2021). Treatment of moderate to severe respiratory COVID-19: a cost-utility analysis. Sci. Rep. 11 (1), 17787. doi:10.1038/s41598-021-97259-7
Coronavirus (COVID-19) Update (2022). Coronavirus (COVID-19) update: FDA limits use of certain monoclonal antibodies to treat COVID-19 due to the omicron variant [press release].
Dijk, S. W., Krijkamp, E. M., Kunst, N., Gross, C. P., Wong, J. B., and Hunink, M. G. M. (2022). Emerging therapies for COVID-19: the value of information from more clinical trials. Value Health 25 (8), 1268–1280. doi:10.1016/j.jval.2022.03.016
Elvidge, J., Summerfield, A., Nicholls, D., and Dawoud, D. (2021). Diagnosis and treatment of COVID-19: a systematic review of economic evaluations. PROSPERO review protocol: CRD42021272219.
Elvidge, J., Summerfield, A., Nicholls, D., and Dawoud, D. (2022). Diagnostics and treatments of COVID-19: a living systematic review of economic evaluations. Value Health 25 (5), 773–784. doi:10.1016/j.jval.2022.01.001
Exchange rates (indicator) (2023). Exchange rates (indicator). Available from: https://data.oecd.org/conversion/exchange-rates.htm (Accessed September 08, 2023).
Gandjour, A. (2022). Benefits, risks, and cost-effectiveness of COVID-19 self-tests from a consumer’s perspective. BMC Health Serv. Res. 22 (1), 47. doi:10.1186/s12913-021-07277-4
Goswami, H., Alsumali, A., Jiang, Y., Schindler, M., Duke, E. R., Cohen, J., et al. (2022). Cost-effectiveness analysis of molnupiravir versus best supportive care for the treatment of outpatient COVID-19 in adults in the US. PharmacoEconomics 40 (7), 699–714. doi:10.1007/s40273-022-01168-0
Hubbard, W., Walsh, N., Hudson, T., Heath, A., Dietz, J., and Rogers, G. (2022). Development and validation of paired MEDLINE and Embase search filters for cost-utility studies. BMC Med. Res. Methodol. 22 (1), 310. doi:10.1186/s12874-022-01796-2
Jovanoski, N., Kuznik, A., Becker, U., Hussein, M., and Briggs, A. (2022). Cost-effectiveness of casirivimab/imdevimab in patients with COVID-19 in the ambulatory setting. J. Manag. Care & Specialty Pharm. 28 (5), 555–565. doi:10.18553/jmcp.2022.21469
Kelton, K., Klein, T., Murphy, D., Belger, M., Hille, E., McCollam, P. L., et al. (2022). Cost-effectiveness of combination of baricitinib and remdesivir in hospitalized patients with COVID-19 in the United States: a modelling study. Adv. Ther. 39 (1), 562–582. doi:10.1007/s12325-021-01982-6
Kilcoyne, A., Jordan, E., Thomas, K., Pepper, A. N., Zhou, A., Chappell, D., et al. (2022a). Clinical and economic benefits of lenzilumab plus standard of care compared with standard of care alone for the treatment of hospitalized patients with coronavirus disease 19 (COVID-19) from the perspective of national health service england. Clin. Outcomes Res. 14, 231–247. doi:10.2147/CEOR.S360741
Kilcoyne, A., Jordan, E., Zhou, A., Thomas, K., Pepper, A. N., Chappell, D., et al. (2022b). Clinical and economic benefits of lenzilumab plus standard of care compared with standard of care alone for the treatment of hospitalized patients with COVID-19 in the United States from the hospital perspective. J. Med. Econ. 25 (1), 160–171. doi:10.1080/13696998.2022.2030148
Kowal, S., Ng, C. D., Schuldt, R., Sheinson, D., and Cookson, R. (2023). The impact of funding inpatient treatments for COVID-19 on health equity in the United States: a distributional cost-effectiveness analysis. Value Health 26 (2), 216–225. doi:10.1016/j.jval.2022.08.010
Krylova, O., Krasheninnikov, A., Mamontova, E., Tananakina, G., and Belyakova, D. (2021). Pharmacoeconomic analysis of treatment regimens for coronavirus infection coronavirus disease-19. Open Access Macedonian J. Med. Sci. 9 (E), 1182–1189. doi:10.3889/oamjms.2021.7015
Lau, V. I., Fowler, R., Pinto, R., Tremblay, A., Borgia, S., Carrier, F. M., et al. (2022). Cost-effectiveness of remdesivir plus usual care versus usual care alone for hospitalized patients with COVID-19: an economic evaluation as part of the Canadian Treatments for COVID-19 (CATCO) randomized clinical trial. CMAJ Open 10 (3), E807–E817. doi:10.9778/cmajo.20220077
Mathieu, E., Ritchie, H., Guirao-Rodés, L., Appel, C., Giattino, C., Hassell, J., et al. (2020). Coronavirus pandemic (COVID-19).
metaEvidence Initiative (2022). Living meta-analysis and evidence synthesis of therapies for COVID19.
Metry, A., Pandor, A., Ren, S., Shippam, A., Clowes, M., Dark, P., et al. (2022). Therapeutics for people with COVID-19 [ID4038]. Assessment report, 2022.
National Institute for Health and Care Excellence (NICE) (2012). The guidelines manual—appendix G: methodology checklist: economic evaluations (Section 2: study limitations).
Ohsfeldt, R., Kelton, K., Klein, T., Belger, M., Mc Collam, P. L., Spiro, T., et al. (2021). Cost-effectiveness of baricitinib compared with standard of care: a modeling study in hospitalized patients with COVID-19 in the United States. Clin. Ther. 43 (11), 1877–1893.e4. doi:10.1016/j.clinthera.2021.09.016
Oksuz, E., Malhan, S., Gonen, M. S., Kutlubay, Z., Keskindemirci, Y., Jarrett, J., et al. (2021). Cost-effectiveness analysis of remdesivir treatment in COVID-19 patients requiring low-flow oxygen therapy: payer perspective in Turkey. Adv. Ther. 38 (9), 4935–4948. doi:10.1007/s12325-021-01874-9
Park, M., Tan, K. B., Vasoo, S., Dickens, B. L., Lye, D., and Cook, A. R. (2022). Estimated health outcomes and costs associated with use of monoclonal antibodies for prevention or mitigation of SARS-CoV-2 infections. JAMA Netw. Open 5 (4), e225750. doi:10.1001/jamanetworkopen.2022.5750
Petrov, V. I., Ryazanova, N. Y., Ponomareva, A. V., Shatalova, O. V., and Levina, Y. V. (2022). CLINICAL AND ECONOMIC ANALYSIS OF GENETICALLY ENGINEERED BIOLOGICS CONSUMPTION BY PATIENTS WITH COVID-19. Pharm. Pharmacol. 10 (2), 198–206. doi:10.19163/2307-9266-2022-10-2-198-206
Rafia, R., Martyn-St James, M., Harnan, S., Metry, A., Hamilton, J., and Wailoo, A. (2022). A cost-effectiveness analysis of remdesivir for the treatment of hospitalized patients with COVID-19 in england and wales. Value Health 25 (5), 761–769. doi:10.1016/j.jval.2021.12.015
Ruggeri, M., Signorini, A., and Caravaggio, S. (2023). Casirivimab and imdevimab: costeffectiveness analysis of the treatment based on monoclonal antibodies on outpatients with Covid-19. PLOS ONE 18 (2), e0279022. doi:10.1371/journal.pone.0279022
Ruggeri, M., Signorini, A., Caravaggio, S., Alraddadi, B., Alali, A., Jarrett, J., et al. (2022b). Modeling the potential impact of remdesivir treatment for hospitalized patients with COVID-19 in Saudi Arabia on healthcare resource use and direct hospital costs: a hypothetical study. Clin. Drug Investig. 42, 669–678. doi:10.1007/s40261-022-01177-z
Ruggeri, M., Signorini, A., Caravaggio, S., Rua, J., Luís, N., Braz, S., et al. (2022a). Estimation model for healthcare costs and intensive care units access for covid-19 patients and evaluation of the effects of remdesivir in the Portuguese context: hypothetical study. Clin. Drug Investig. 42 (4), 345–354. doi:10.1007/s40261-022-01128-8
Savinkina, A., Paltiel, A. D., Ross, J. S., and Gonsalves, G. (2022). Population-level strategies for nirmatrelvir/ritonavir prescribing—a cost-effectiveness analysis. Open Forum Infect. Dis. 9 (12), ofac637. doi:10.1093/ofid/ofac637
Schallner, N., Lieberum, J., Kalbhenn, J., Bürkle, H., and Daumann, F. (2022). Intensive care unit resources and patient-centred outcomes in severe COVID -19: a prospective single-centre economic evaluation. Anaesthesia 77 (12), 1336–1345. doi:10.1111/anae.15844
Shah, H. A., Baker, T., Schell, C. O., Kuwawenaruwa, A., Awadh, K., Khalid, K., et al. (2023). Cost effectiveness of strategies for caring for critically ill patients with COVID-19 in Tanzania. PharmacoEconomics - Open 7 (4), 537–552. doi:10.1007/s41669-023-00418-x
Sheinson, D., Dang, J., Shah, A., Meng, Y., Elsea, D., and Kowal, S. (2021). A cost-effectiveness framework for COVID-19 treatments for hospitalized patients in the United States. Adv. Ther. 38 (4), 1811–1831. doi:10.1007/s12325-021-01654-5
Shields, G., and Elvidge, J. (2020). Challenges in synthesising cost-effectiveness estimates. Syst. Rev. 9 (1), 289. doi:10.1186/s13643-020-01536-x
Subhi, A., Shamy, A. M. E., Hussein, S. A. M., Jarrett, J., Kozma, S., Harfouche, C., et al. (2023). Use of anti-viral therapies in hospitalised COVID-19 patients in the United Arab Emirates: a cost-effectiveness and health-care resource use analysis. BMC Health Serv. Res. 23 (1), 383. doi:10.1186/s12913-023-09376-w
Wai, A. K.-C., Chan, C. Y., Cheung, A. W.-L., Wang, K., Chan, S. C.-L., Lee, T. T.-L., et al. (2023). Association of Molnupiravir and Nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19. Lancet Regional Health - West. Pac. 30, 100602. doi:10.1016/j.lanwpc.2022.100602
Keywords: cost-effectiveness, COVID-19, diagnostics, economic evaluation, health technology assessment, pharmacological, living review, cost-utility analysis
Citation: Elvidge J, Hopkin G, Narayanan N, Nicholls D and Dawoud D (2023) Diagnostics and treatments of COVID-19: two-year update to a living systematic review of economic evaluations. Front. Pharmacol. 14:1291164. doi: 10.3389/fphar.2023.1291164
Received: 08 September 2023; Accepted: 30 October 2023;
Published: 16 November 2023.
Edited by:
Robert L. Lins, Independent Researcher, Antwerp, BelgiumCopyright © 2023 Elvidge, Hopkin, Narayanan, Nicholls and Dawoud. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jamie Elvidge, amFtaWUuZWx2aWRnZUBuaWNlLm9yZy51aw==
 Jamie Elvidge
Jamie Elvidge Gareth Hopkin1
Gareth Hopkin1 Nithin Narayanan
Nithin Narayanan David Nicholls
David Nicholls Dalia Dawoud
Dalia Dawoud