- 1Mental Health Integrated Care Community, Minneapolis Veterans Administration Health Care System, Minneapolis, MN, United States
- 2School of Neuroscience, Virginia Tech, Blacksburg, VA, United States
- 3Department of Psychiatry and Behavioral Medicine, Virginia Tech Carillion School of Medicine, Roanoke, VA, United States
- 4Clinical Research Center for Mental Disorders, Chinese-German Institute of Mental Health, Shanghai Pudong New Area Mental Health Center, Tongji University, Shanghai, China
- 5Department of Psychiatry, Shanghai East Hospital, School of Medicine, Tongji University, Shanghai, China
- 6Department of Statistics and Center for Biostatistics and Health Data Science, Virginia Tech, Blacksburg, VA, United States
Objective: This study sought to investigate the relationship between antibiotic exposure and subsequent risk of psychiatric disorders.
Methods: This retrospective cohort study used a national database of 69 million patients from 54 large healthcare organizations. We identified a cohort of 20,214 (42.5% male; 57.9 ± 15.1 years old [mean ± SD]) adults without prior neuropsychiatric diagnoses who received antibiotics during hospitalization. Matched controls included 41,555 (39.6% male; 57.3 ± 15.5 years old) hospitalized adults without antibiotic exposure. The two cohorts were balanced for potential confounders, including demographics and variables with potential to affect: the microbiome, mental health, medical comorbidity, and overall health status. Data were stratified by age and by sex, and outcome measures were assessed starting 6 months after hospital discharge.
Results: Antibiotic exposure was consistently associated with a significant decrease in the risk of novel mood disorders and anxiety and stressor-related disorders in: men (mood (OR 0.84, 95% CI 0.77, 0.91), anxiety (OR 0.88, 95% CI 0.82, 0.95), women (mood (OR 0.94, 95% CI 0.89,1.00), anxiety (OR 0.93, 95% CI 0.88, 0.98), those who are 26–49 years old (mood (OR 0.87, 95% CI 0.80, 0.94), anxiety (OR 0.90, 95% CI 0.84, 0.97)), and in those ≥50 years old (mood (OR 0.91, 95% CI 0.86, 0.97), anxiety (OR 0.92, 95% CI 0.87, 0.97). Risk of intentional harm and suicidality was decreased in men (OR 0.73, 95% CI 0.55, 0.98) and in those ≥50 years old (OR 0.67, 95% CI 0.49, 0.92). Risk of psychotic disorders was also decreased in subjects ≥50 years old (OR 0.83, 95 CI: 0.69, 0.99).
Conclusion: Use of antibiotics in the inpatient setting is associated with protective effects against multiple psychiatric outcomes in an age- and sex-dependent manner.
Introduction
Mental illness affects one in five adults in the United States and is one of the leading causes of disability worldwide (Smith, 2014; Bose et al., 2018). Our understanding of the myriad factors that contribute to the etiology of psychiatric disorders remains limited. Antibiotic medications treat bacterial disease by destroying select bacteria throughout the body, which can disrupt the gut microbiome and inflammatory signaling throughout the body (Bercik and Collins, 2014; Zareifopoulos and Panayiotakopoulos, 2017; Palleja et al., 2018). Such alterations may alter the risk of the emergence of a variety of psychiatric conditions (Yuan et al., 2019; Borkent et al., 2022).
Emerging evidence has linked bacterial infections and antibiotic exposure with both susceptibility and with resilience to mental health disorders (Tome and Filipe, 2011; Lambricht et al., 2017; Kridin and Ludwig, 2023). For example, isoniazid, an antibiotic used to treat tuberculosis, inhibits monoamine oxidase activity, thus increasing monoamine levels and in this way producing antidepressant effects (Butler et al., 2019). Other examples include minocycline, which can be an effective adjunct treatment for major depressive disorder (Miyaoka et al., 2012; Zazula et al., 2021). Studies in rodents have documented that presence of specific bacterial species decrease depressive-like behaviors (Lowry et al., 2007; Siebler et al., 2018), while antibiotic administration during adolescence leads to increased anxiety-like behaviors (Lach et al., 2020). In humans, antibiotic exposure during early development can alter neurocognitive function (Slykerman et al., 2019), and increase risk of psychiatric disorders, including ADHD (Rees, 2014; Aversa et al., 2021). Similarly, a retrospective study using a large database of electronic health records from the UK found increased risk of depression and anxiety in adults after antibiotic exposure (Lurie et al., 2015). However, more recent large-scale analyses found no adverse neuropsychiatric outcomes due to antibiotic exposure (Wilcox et al., 2020), and a protective effect for opioid use disorder (Freedman et al., 2022). Neuropsychiatric effects of antibiotic exposure likely depend on antibiotic class, timing of antibiotic administration, and co-administration of other medications (Freedman et al., 2022; Clegg et al., 2023; Kridin and Ludwig, 2023).
To help define effects of antibiotic exposure on mental health we conducted a retrospective cohort study using a large national database from TriNetX (https://www.trinetx.com/). This database contains millions of de-identified, anonymized patient electronic medical records, which we used to determine the risk for major classes of psychiatric diagnoses following antibiotic treatment. Diagnostic classes included: mood disorders, anxiety and stressor-related disorders, intentional self-harm and suicidality, and psychotic disorders. Covariates included twenty-six variables, including demographic variables, comorbid conditions (e.g. pain, inflammation, obesity, etc.), CNS medications and others. Our results indicate that antibiotic administration during hospitalization decreases the risk of all four of the psychiatric outcomes that we examined in a sex- and age-dependent manner.
Methods
This is an observational retrospective study using weighted data. We utilized data from TriNetX (https://www.trinetx.com/), a global health research network providing access to statistics on electronic medical records (diagnoses, procedures, medications, laboratory values, genomic information) from approximately 69 million patients in 54 large healthcare organizations. TriNetX received a waiver from Western Institutional Review Board (Puyallup, WA) since only aggregated counts, statistical summaries of de-identified information, but no protected health information is received, and no study-specific activities are performed in retrospective analyses. We also received an Institutional Review Board (IRB) waiver locally from the Carilion Clinic IRB.
Setting
Patient cohorts were identified and analyzed in the TriNetX database in November 2020. All health care organizations available in the database at that time were used in this study. Cases were admitted to the hospital and treated with one or more antibiotics between 2013–2015. Control subjects were hospital inpatients during this same timeframe but did not receive antibiotic treatment. Six months after hospital discharge, we looked for the presence of electronic medical record codes related to mental illness diagnoses. The time gap between the antibiotic exposure window and outcome measures was included in the study design to reduce protopathic bias (Faillie J. L., 2015).
Participants
Patients were males and females between the ages of 18–89 years old at the start of the Index Event (hospital admission). All patients in the electronic patient records database were included if they were: a) 18 years of age or older; b) admitted inpatients during 2013–2015; c) not receiving antibiotics on admission (or within 6 months prior to admission); d) had no previous record of neuropsychiatric disorders; e) never had documented CRP measures greater than 3 mg/L; and f) had at least one post-discharge follow-up visit recorded in the database at least 6 months after discharge.
Exclusion Criteria. Patients in the electronic records database were excluded from our analyses if they were: a) under 18 years of age or older than 89 years of age; b) not admitted inpatients during 2013–2015; c) receiving antibiotics on admission (or within 6 months prior to admission); d) had previous record of neuropsychiatric disorders; e) had documented CRP measures greater than 3 mg/L at any time; or f) did not have at least one post-discharge follow-up visit recorded in the database at least 6 months after discharge.
Bias
To minimize bias, we followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline (see Supplemental Table S8 for STROBE checklist) (von Elm et al., 2007a; von Elm et al., 2007b; von Elm et al., 2007c; von Elm et al., 2007d). Furthermore, to account for protopathic bias, outcomes were not assessed until 6 months after hospital discharge (Faillie J.-L., 2015). This was done to minimize the association of the outcome with the initial patient complaint during the start of the Index Event. Referral bias was accounted for by sampling across multiple health care organizations. To help account for Berkson bias, where patients with more than one disease are more likely to be hospitalized thus increasing the potential for overestimation in the cases, cohorts were balanced on factors influencing contact with healthcare services (Sutton-Tyrrell, 1991).
Variables
Outcome measures were assessed 6 months to 5 years after discharge. Primary outcome measures included: 1) anxiety and stress-related disorders, 2) mood disorders, 3) psychotic disorders, and 4) intentional self-harm and suicidality. Supplemental Table S1 provides a list of diagnostic codes for each of these outcomes. The predictor of interest is an antibiotic prescription occurring during inpatient hospitalization. For a full listing of electronic health record codes used to define antibiotic prescription see Supplemental Table S2. Two stratifying variables were considered: sex (male and female) and age group (Age: 18 to 25, Age: 26 to 49, Age: 50 or older). Twenty-six variables were used for weighting variables, including five demographic variables–sex, age at hospitalization, race, ethnicity, and length of hospitalization (Supplemental Table S3), and twenty-one diagnostic and medication variables listed in Supplemental Table S4. In models considering the effect of treatment group by sex, age was included as a continuous weighting variable; in models considering treatment effects by age group, sex was included as a weighting variable. For a full description of the diagnostic variables, including TriNetX codes and the codes that were grouped together, see Supplemental Tables S5–S7. Data for Antibiotics After Discharge were collected from 2 weeks after discharge and up to 3 months after discharge. All other characteristics were taken from the beginning of the electronic health record up until 2 weeks post-discharge.
Study size
The downloaded dataset contained electronic health records from 502,444 adult patients with an index medical encounter between 2013–2015, excluding those with any neuropsychiatric diagnoses prior to the index encounter. The downloaded dataset was further restricted to: a) exclude those having CRP measures >3 mg/L; b) include only index encounters coded as an inpatient hospitalization; and c) include only those with a subsequent medical encounter recorded at least 6 months post-discharge.
Statistical methods
Preliminary Analyses. Derived data fields were calculated for age at hospital admission, length of hospitalization, and antibiotics prescribed post-discharge. In the event of missing data, the underlying mechanism of missingness was evaluated prior to implementing methodology to minimize bias from missing data for matching variables. Age at hospital admission could not be calculated for 2.93% of the sample due to missing year of birth. Age was imputed from the mean in these cases. Sex was missing for one individual and was imputed from the mode. For 2% of the sample, hospital discharge was coded before hospital admission. For these cases, length of hospitalization was set to zero.
Entropy Balancing. Entropy balancing uses an algorithm to find a single multiplier for each observation such that all covariates are balanced (Hainmueller, 2012). Entropy balancing was applied to each subset of the data by stratifying variables: female patients, male patients, patients aged 18 to 25, patients aged 26 to 49, and those 50 and older. Patient characteristics included in entropy balancing are shown in Table 1 and Table 2. Demographic patient characteristics included: age at admission, gender, race, and ethnicity. Clinical patient characteristics included medical diagnoses and medications.
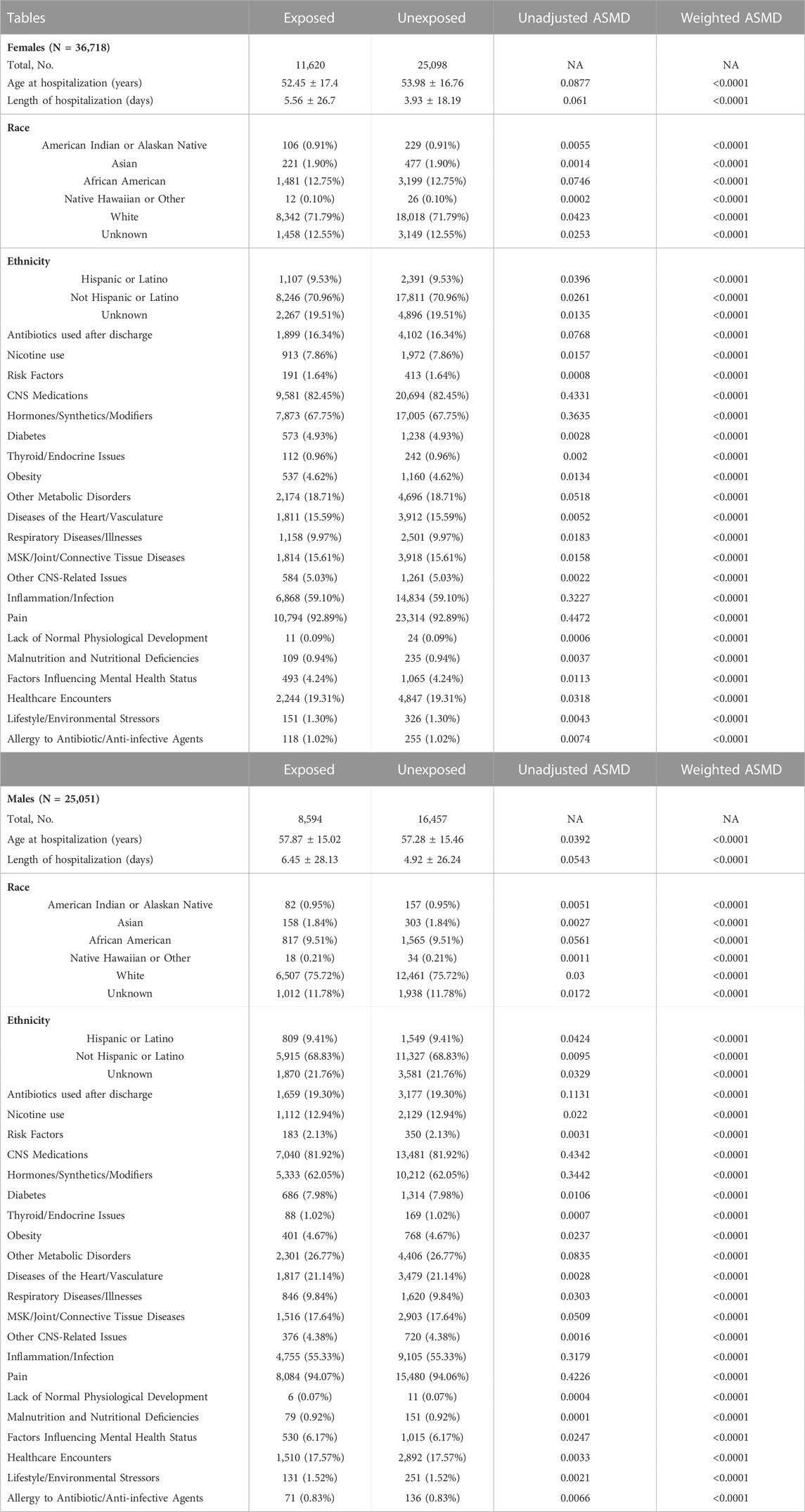
TABLE 1. Demographics and covariates used in the model for the data stratified by sex. Age at hospitalization and length of hospitalization are shown as mean ± SD. Length of hospitalization is shown in days for informational purposes. For the purposes of entropy balancing and creation of weighted data subsets it was converted to years. Total number of subjects (and their proportion of total cohort) is shown for each category. Absolute standardized mean differences (ASMD) are presented before and after propensity score matching. Abbreviations: NA–not applicable, MSK–musculoskeletal, CNS–central nervous system.
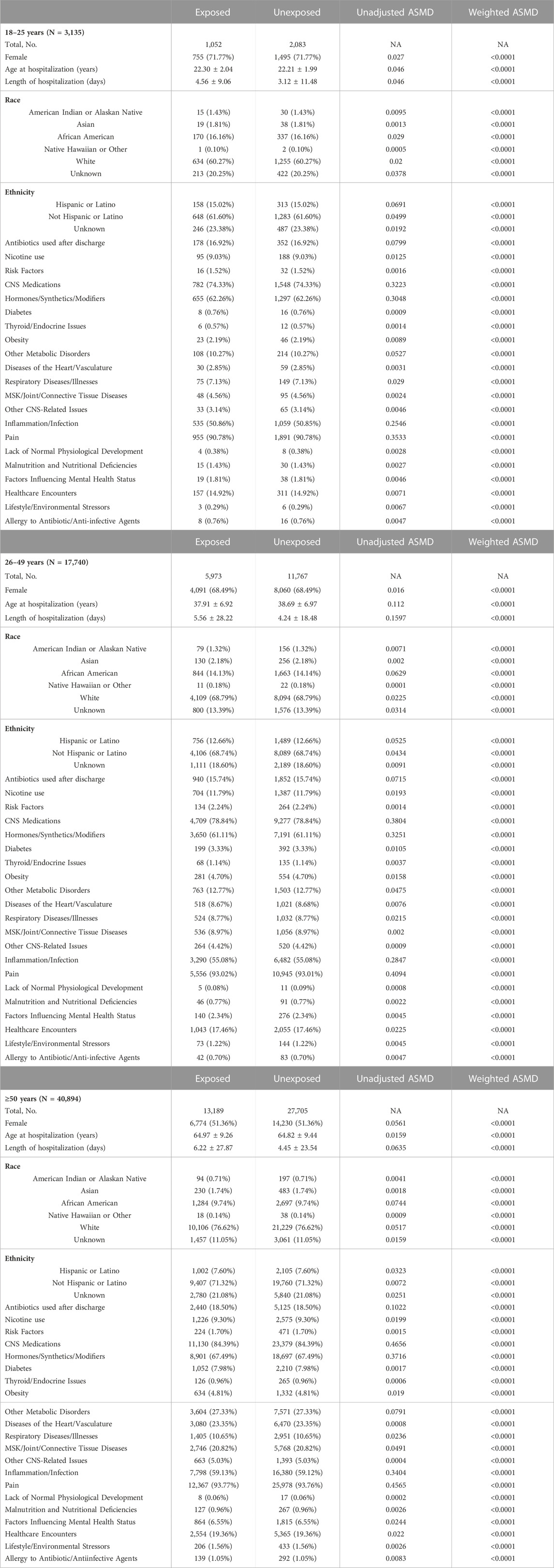
TABLE 2. Demographics and covariates used in the model for the data stratified by age. Age at hospitalization and length of hospitalization are shown as mean ± SD. Length of hospitalization is shown in days for informational purposes. For the purposes of entropy balancing and creation of weighted data subsets it was converted to years. Total number of subjects (and their proportion of total cohort) is shown for each category. Absolute standardized mean differences (ASMD) are presented before and after propensity score matching. Abbreviations: NA–not applicable, MSK–musculoskeletal, CNS–central nervous system.
Absolute standardized mean differences (ASMDs) for all matching variables were calculated for the original data subset and the weighted data subset. ASMDs were computed after weighting by taking the mean for each variable among the treatment group and the mean for each variable among the control group. These values were subtracted and standardized by dividing the difference by the standard deviation before any adjustments. The goal of weighting was to make adjustments such that the sample mimics a randomized trial. Small ASMDs (typically ≤0.1) indicate that the weighting method has balanced the groups to resemble a randomized trial (Rubin, 2001). After weighting ASMDs for all of the covariates fell below the 0.1 benchmark (Table 1 and Table 2).
Primary Analyses. Separate weighted analyses were generated for each sex and age group using the entropy balancing algorithm. Univariate logistic regression models were used to estimate parameters for each weighted sex and age effect. The resultant parameter estimates from the separate entropy balanced analyses, along with their associated standard errors, were used to compare estimated exposure effects across groups using Wald test statistics.
Results
Participants
Dataset of included participants consisted of 61,769 individuals. This cohort was first stratified by age and then re-analyzed after stratification by age.
Total sample size of female patients was 36,718–11,620 antibiotic-treated and 25,098 control (Table 1). The mean age at hospitalization in the treatment group was 52.45 ± 17.40 years (mean ± SD) and 53.98 ± 16.76 years in the control group. Total sample size of male patients was 25,051–8,594 treated with antibiotics and 16,457 control (Table 1). The mean age at hospitalization for the antibiotic treatment group was 57.87 ± 15.02 years and 57.28 ± 15.46 years in the control group. The groups were well balanced with an ASMD <0.0001 after weighting. All other variables given in Table 1 can be assessed in a similar manner.
Patient characteristics for the three age groups used for entropy balancing are summarized in Table 2. The total sample size of patients in the 18–25-year-old bracket was 3,135–1,052 antibiotic-treated and 2,083 control. In this age group, the mean age at hospitalization was 22.3 ± 2.04 years and 22.21 ± 1.99 years in the treatment group and in the control group, respectively. The total sample size of patients in the 26–49-year-old bracket was 17,740–5,973 antibiotic-treated and 11,767 control. The mean age at hospitalization was 37.91 ± 6.92 and 38.69 ± 6.97 in the treatment group and in the control group, respectively. The total sample size of patients in the 50 and older bracket was 40,894–13,189 treated and 27,705 control. The mean age at hospitalization in the treatment group was 64.97 ± 9.26 and 64.82 ± 9.44 in the control group.
Outcomes: Treatment group effects stratified by sex
Among women, we found significantly reduced risk of mood disorders (OR 0.94, 95% CI 0.89–1.00; p = 0.040) and anxiety and stress-related disorders (OR 0.93, 95% CI 0.88–0.98; p = 0.006; Table 3 and Figure 1). Among men, antibiotic use was associated with significantly reduced risk of: mood disorders (OR 0.84, 95% CI 0.77–0.91; p < 0.0001), anxiety and stress-related disorders (OR 0.88, 95% CI 0.82–0.95; p = 0.0006), and intentional self-harm and suicidality (OR 0.73, 95% CI 0.55–0.98; p = 0.040; Table 3 and Figure 1).
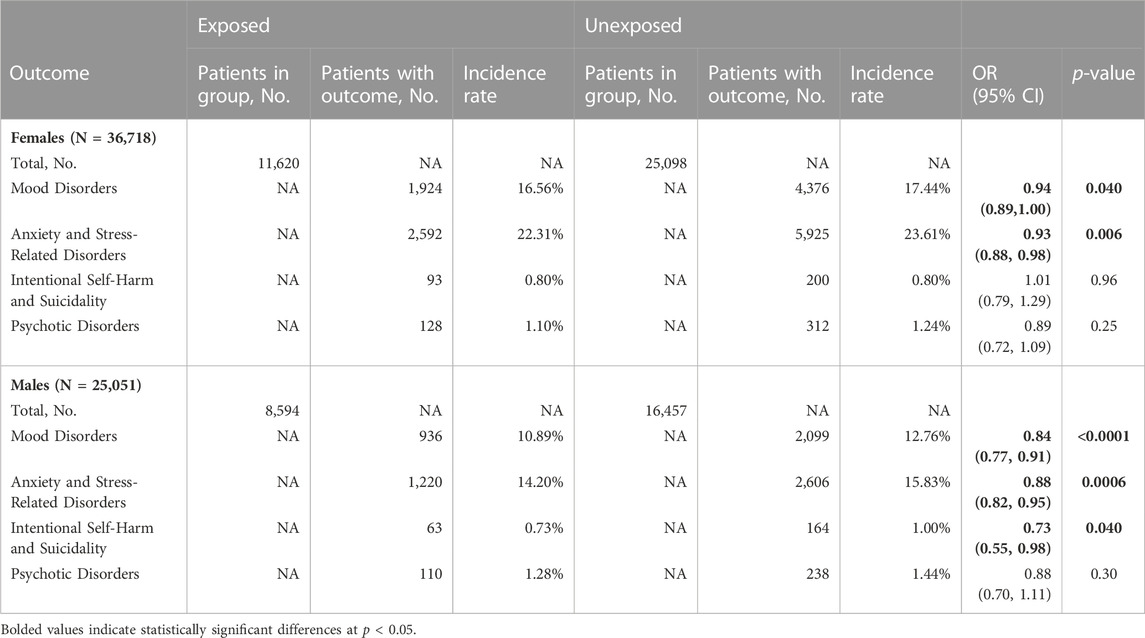
TABLE 3. Impact of antibiotic administration on psychiatric outcomes in female and male cohorts. NA–not applicable, OR–odds ratio.

FIGURE 1. Odds ratios of for the emergence psychiatric conditions following inpatient antibiotic exposure. The data were analyzed after stratification by sex. Statistical analyses revealed a significant decrease in the risk of mood disorders and anxiety and stress-related disorders in men and women. We also observed a decrease in the risk of intentional self-harm/suicidality in males. For additional details see Table 3. * -- p < 0.05, ** -- p < 0.001, *** -- p < 0.0001.
Outcomes: Treatment group effects stratified by age
We did not detect a significant relationship between antibiotic use and any of the outcome measures in the 18–25 years group. In the 26–49 years group we observed significant reduction in the risk of mood disorders (OR 0.87, 95% CI 0.80–0.94; p = 0.0009) and in that of anxiety and stress-related disorders (OR 0.90, 95% CI 0.84–0.97; p = 0.0072; Table 4 and in Figure 2). In the ≥50 years group we detected significant reductions in all four outcome variables: mood disorders (OR 0.91, 95% CI 0.86–0.97; p = 0.0031), anxiety and stress-related disorders (OR 0.92, 95% CI 0.87–0.97; p = 0.0018), intentional self-harm and suicidality (OR 0.67, 95% CI 0.49–0.92; p = 0.014), and psychotic disorders (OR 0.83, 95% CI 0.69–0.99; p = 0.040; Table 4 and in Figure 2).
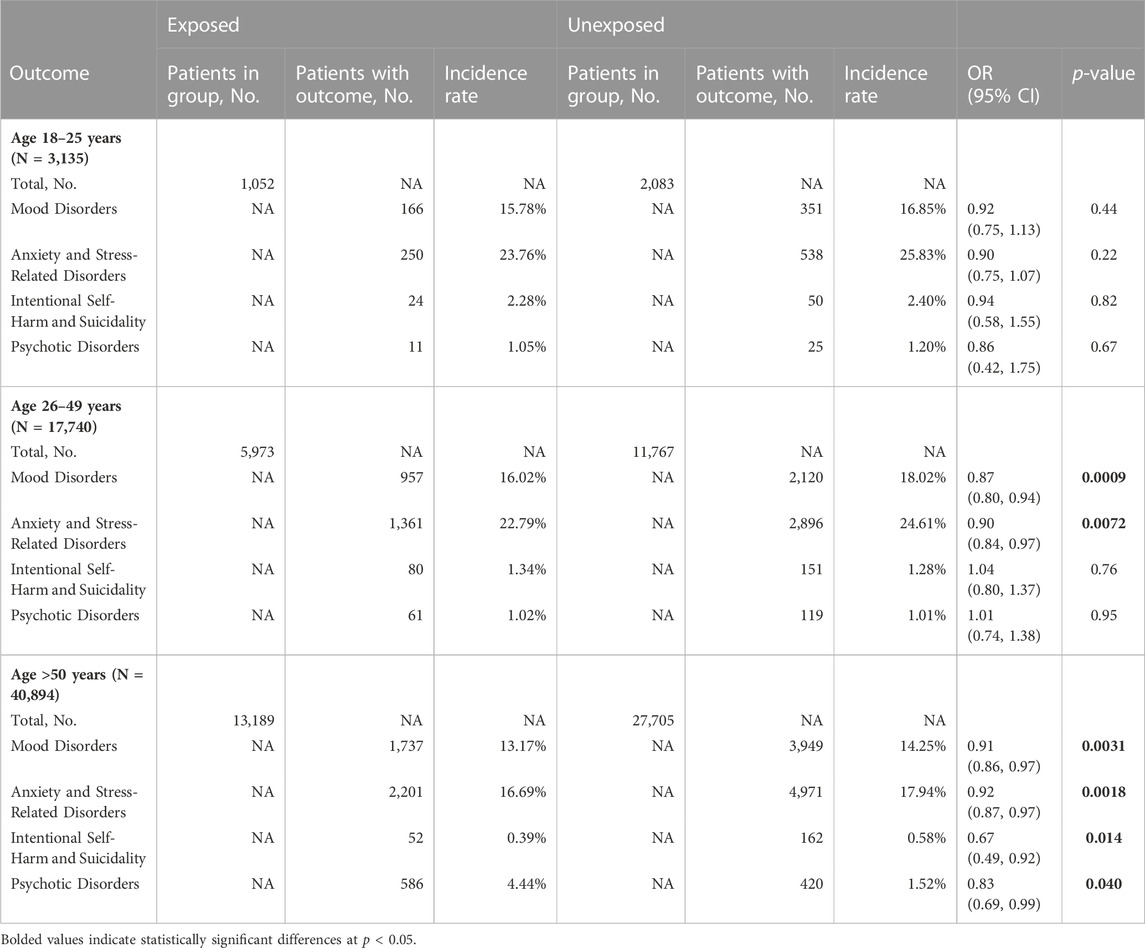
TABLE 4. Impact of antibiotic administration in cohorts stratified by age. NA–not applicable, OR–odds ratio.
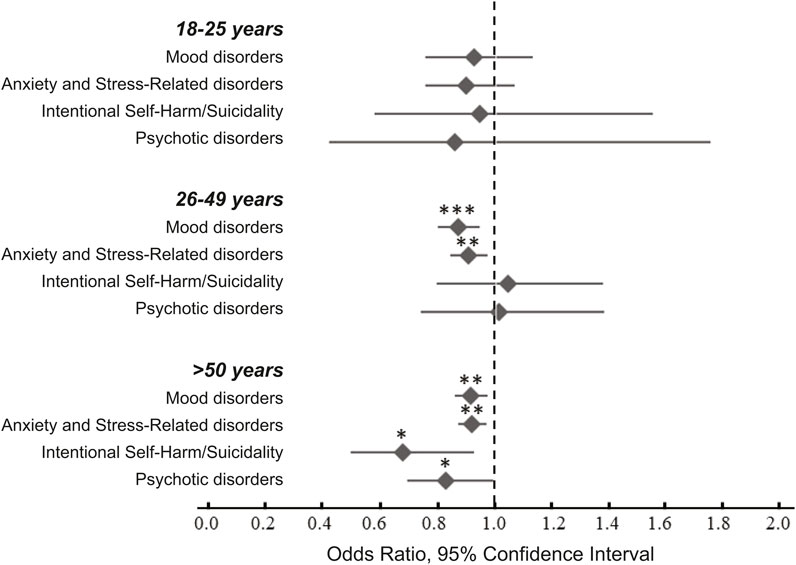
FIGURE 2. Odds ratios of for the emergence psychiatric conditions following antibiotic exposure during hospitalization. The data were analyzed after stratification by age. Statistical analyses revealed significant decreased risk of mood disorders and anxiety and stress-related disorders in the 26–49 years and in the ≥50 years age groups. There was also a significant decrease in the risk of intentional harm/suicidality and psychotic disorders in the ≥50 years group. For additional details see Table 4. * -- p < 0.05, ** -- p < 0.001, *** -- p < 0.0001.
Discussion
In the current study we utilized a large multi-site database to generate large well-balanced cohorts stratified by sex and by age. Our analyses indicate that antibiotic administration in the inpatient setting is associated with protective effects on psychiatric outcomes. Our most consistent observation was that antibiotic administration was associated with lower risk of mood disorders and anxiety and stress-related disorders. This was true in females, males, and in individuals 26–49 years old and in those ≥50 years old. Similarly, risk of self-harm and suicidality was lower in males and in individuals ≥50 years old. Subjects in this older age group were also at lower risk of psychosis, likely due to the improvement of delirium after treatment of infection (Rummans et al., 1995).
One of the strengths of our study is that we had access to a large multisite database that contained nearly 70 million patient records. Thus, were able to carefully balance cohorts and to control for a large number confounds, including as race, sex, age, medical comorbidities, and co-administration of CNS medications. We also utilized STROBE criteria to minimize bias in our study as well as to increase reliability (von Elm et al., 2007b; von Elm et al., 2007c; von Elm et al., 2007d). It is well established that minimization of bias is critically important in a variety of clinical studies, including retrospective cohort studies such as this one (von Elm et al., 2007b; von Elm et al., 2007c; von Elm et al., 2007d) as well as in meta-analyses and reviews (Morgan et al., 2018a; Morgan et al., 2019).
Previous reports found positive associations between antibiotic use and anxiety (Lurie et al., 2015), depression (Lurie et al., 2015), mania (Walrave et al., 2016; Yolken et al., 2016; Lambricht et al., 2017; Puri et al., 2021), suicidal ideation (LaSalvia et al., 2010; Kaur et al., 2016), and psychosis (Farrington et al., 1995; Moorthy et al., 2008; Michalak et al., 2017). However, much of this evidence is based on case reports, studies with limited numbers of subjects and without appropriate control for confounding variables.
Large-scale that explored the link between antibiotic exposure and psychiatric outcomes provide a mixed picture. Lurie, et al. reported an increase in the risk of depression and anxiety, but not of psychosis (Lurie et al., 2015), while Wilcox, et al. did not find any adverse effects as a consequence of antibiotic exposure (Wilcox et al., 2020). Others have reported a decrease in the risk of opioid use disorder (OUD) when opioids are co-prescribed with antibiotics (Freedman et al., 2022), while administration of antibiotics alone can increase risk of OUD (Clegg et al., 2023). Our observations extend these prior observations and indicate that antibiotic administration in hospitalized patients can decrease the risk of novel psychiatric disorders in a sex- and age-dependent manner. A significant strength of our approach is the use of entropy balancing to account for group differences in several factors that may alter risk for neuropsychiatric disorders. We controlled for multiple confounding variables, such as inflammation, CNS medications, pain, medical co-morbidities (e.g. obesity, cardiovascular disease, and diabetes), and others. We only considered patients who were administered antibiotics while in hospital, suggesting that type of setting in which these medications are prescribed may be an important determinant of their effects on mental health.
Our study is consistent with prior reports of protective effects of antibiotics in mental health. While traditionally considered to worsen depression and suicidality, a more recent analysis suggests that isotretinoin protects against several neuropsychiatric outcomes (Kridin and Ludwig, 2023). A similar study found no associated protective or harmful effect of isotretinoin on neuropsychiatric outcomes (Paljarvi et al., 2022). Minocycline adjunctive therapy may be beneficial in schizophrenia, with documented improvement in cognitive (Levkovitz et al., 2010; Zhang et al., 2019), positive (Zhang et al., 2019), and negative (Levkovitz et al., 2010; Liu et al., 2014; Palleja et al., 2018) symptoms and reduction of inflammatory cytokines IL-1β and IL-6 (Palleja et al., 2018; Zhang et al., 2019). Minocycline treatment may also have protective effects on depressive disorders (Cai et al., 2020; Husain et al., 2020). Taken together with our observations, these results suggest that antibiotic administration is protective against a range of neuropsychiatric outcomes.
Timing of antibiotic exposure along with classes of medications used may be critical factors in associated risk of mental illness. Children exposed to antibiotics in the first 3 years of life have increased risk for mood and anxiety disorders, depending on antibiotic class (Delara et al., 2020). Specifically, postnatal exposure to tetracyclines, aminoglycosides, quinolones, or sulfonamides was associated with increased risk of mood and anxiety disorders by the time study participants were adolescents (Delara et al., 2020). Conversely, exposure to macrolides, lincosamides, or streptogramins were associated with reduced risk of mood and anxiety disorders (Delara et al., 2020). Our study focused exclusively on late adolescent and adult exposure (18 + years of age at hospital admission). In general, younger adults (18–50 years) are at a greater risk for psychiatric disorders when compared to older populations (Bose et al., 2018). While antibiotic exposure had no effect on risk of novel psychiatric disorders in the youngest age group (18–25 years) in this study, we observed reduced risk of mood disorders and anxiety and stress-related disorders in the 26–49-year-old group, and decreased risk of mood disorders, anxiety and stress-related disorders, self-harm/suicidality, and psychotic disorders in the 50 years and older age group. Thus, protective effects of antibiotic exposure in hospitalized individuals may be age dependent.
Limitations
There are several limitations to the current study due to the reliance on electronic healthcare records. We were unable to fully account for differences in socioeconomic status and environmental confounding factors between groups by using real-time EMR data (Morgan et al., 2018a; Morgan et al., 2018b; Morgan et al., 2019). We attempted to address this by balancing cohorts based on “Factors influencing contact with Health services (Z00-Z99)”. These codes include various items such as: body mass index (Z68), persons with potential health hazards related to socioeconomic and psychosocial circumstances (Z55-Z65), and a variety of reasons people may encounter health care services. Some of these shortcomings were mitigated by our use of the STROBE guideline and checklist to minimize bias and improve reliability (von Elm et al., 2007c; von Elm et al., 2007d).
This study was restricted to an inpatient population thus limiting the overall generalizability of results to the population at large. However, our study provides evidence that inpatient antibiotic administration may be beneficial to the individual’s long-term mental health. Prior studies that used electronic healthcare records may not have adequately balanced cohorts, or may not have covaried for all confounding variables, potentially resulting in erroneous conclusions.
Additionally, we were limited with regards to the antibiotic classes, duration of treatment, indications for antibiotic prescribing, and the impact of stratifying by these variables on psychiatric outcomes. Due to the nature of our de-identified real-time data, TriNetX relies on prescription claims to compile medication treatment information. Consequently, detailed information such as the duration of treatment and specific indications is not available within our dataset. We did not distinguish between different classes of antibiotics, and included patients that were treated with any class of these medications. Specific medications and antibiotic classes are listed in Supplemental Table S2. Future studies will be required to determine the impact of specific classes of antibiotics on psychiatric outcomes.
Conclusions
We found that antibiotic treatment during hospitalization is associated with decreased subsequent risk of several classes of psychiatric disorders, which depended on the patients’ sex and age. Our findings suggest that antibiotic treatment in the hospital setting may provide protection against future psychiatric disorders. While antibiotic administration may be protective in the hospital setting, outpatient and/or off-label use of certain classes of antibiotics may predispose individuals for mood or anxiety disorders (Kaur et al., 2016). Future prospective studies should be able to parse out these differences.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.trinetx.com/. The raw data will be made available upon request.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
IK: Conceptualization, Data curation, Investigation, Methodology, Resources, Supervision, Visualization, Writing–original draft, Writing–review and editing. MG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing–original draft, Writing–review and editing. YL: Formal Analysis, Methodology, Software, Visualization, Writing–review and editing. JW: Conceptualization, Formal Analysis, Investigation, Methodology, Software, Writing–review and editing. AH: Conceptualization, Investigation, Methodology, Project administration, Software, Writing–review and editing. AK: Resources, Software, Supervision, Writing–review and editing. SC: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. SC received funding from National Institute of Mental Health (NIH), Grant Number R01MH105447.
Acknowledgments
We would like to thank Martha Tenzer and the Health Analytics Research team at Carillion Clinic, Roanoke, VA for their help with using the TriNetX database. We are grateful to Benjamin Zeitlin and Alicia Lozano for their help with statistical analyses. The authors were granted access to TriNetX data for research purposes and no constraints on the analyses or on the publication of results.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1290052/full#supplementary-material
References
Aversa, Z., Atkinson, E. J., Schafer, M. J., Theiler, R. N., Rocca, W. A., Blaser, M. J., et al. (2021). Association of infant antibiotic exposure with childhood health outcomes. Mayo Clin. Proc. 96 (1), 66–77. doi:10.1016/j.mayocp.2020.07.019
Bercik, P., and Collins, S. M. (2014). The effects of inflammation, infection and antibiotics on the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 817, 279–289. doi:10.1007/978-1-4939-0897-4_13
Borkent, J., Ioannou, M., Laman, J. D., Haarman, B. C. M., and Sommer, I. E. C. (2022). Role of the gut microbiome in three major psychiatric disorders. Psychol. Med. 52 (7), 1222–1242. doi:10.1017/S0033291722000897
Bose, J., et al. (2018). Key substance use and mental health indicators in the United States: results from the 2017 national survey on drug use and health (HHS publication No. SMA 18-5068, NSDUH series H-53). Rockville, MD, USA: Substance Abuse and Mental Health Services Administration.
Butler, M. I., Sandhu, K., Cryan, J. F., and Dinan, T. G. (2019). From isoniazid to psychobiotics: the gut microbiome as a new antidepressant target. Br. J. Hosp. Med. (Lond) 80 (3), 139–145. doi:10.12968/hmed.2019.80.3.139
Cai, D.-B., Zheng, W., Zhang, Q. E., Ng, C. H., Ungvari, G. S., Huang, X., et al. (2020). Minocycline for depressive symptoms: a meta-analysis of randomized, double-blinded, placebo-controlled trials. Psychiatr. Q. 91 (2), 451–461. doi:10.1007/s11126-019-09707-3
Clegg, T. J., Kawmi, N., and Graziane, N. M. (2023). Different classes of antibiotics have varying effects on the risk of developing opioid use disorder: a national database study. J. Subst. Use 28 (1), 101–111. doi:10.1080/14659891.2021.2010140
Delara, M., McMillan, D. E., Nickel, N. C., Jong, G. W., Seitz, D. P., and Mignone, J. (2020). Early life exposure to antibiotics and the risk of mood and anxiety disorders in children and adolescents: a population-based cohort study. J. Psychiatr. Res., doi:10.1016/j.jpsychires.2020.11.003
Faillie, J. L. (2015a). Indication bias or protopathic bias? Br. J. Clin. Pharmacol. 80 (4), 779–780. doi:10.1111/bcp.12705
Faillie, J.-L. (2015b). Indication bias or protopathic bias? Br. J. Clin. Pharmacol. 80, 779–780. doi:10.1111/bcp.12705
Farrington, J., Stoudemire, A., and Tierney, J. (1995). The role of ciprofloxacin in a patient with delirium due to multiple etiologies. Gen. Hosp. Psychiatry 17 (1), 47–53. doi:10.1016/0163-8343(94)00065-l
Freedman, Z. G., Kane, J. A., King, T. S., and Graziane, N. M. (2022). The effect of prescribing antibiotics with opioids on the development of opioid use disorder: a national database study. J. Addict. Dis. 40 (1), 62–70. doi:10.1080/10550887.2021.1926889
Hainmueller, J. (2012). Entropy balancing for causal effects: a multivariate reweighting method to produce balanced samples in observational studies. Polit. Anal. 20 (1), 25–46. doi:10.1093/pan/mpr025
Husain, M. I., Chaudhry, I. B., Khoso, A. B., Hodsoll, J., Ansari, M. A., et al. (2020). Minocycline and celecoxib as adjunctive treatments for bipolar depression: a multicentre, factorial design randomised controlled trial. Lancet Psychiatry 7 (6), 515–527. doi:10.1016/S2215-0366(20)30138-3
Kaur, K., Fayad, R., Saxena, A., Frizzell, N., Chanda, A., Das, S., et al. (2016). Fluoroquinolone-related neuropsychiatric and mitochondrial toxicity: a collaborative investigation by scientists and members of a social network. J. community Support. Oncol. 14 (2), 54–65. doi:10.12788/jcso.0167
Kridin, K., and Ludwig, R. J. (2023). Isotretinoin and the risk of psychiatric disturbances: a global study shedding new light on a debatable story. J. Am. Acad. Dermatol 88 (2), 388–394. doi:10.1016/j.jaad.2022.10.031
Lach, G., Fülling, C., Bastiaanssen, T. F. S., Fouhy, F., Donovan, A. N. O., Ventura-Silva, A. P., et al. (2020). Enduring neurobehavioral effects induced by microbiota depletion during the adolescent period. Transl. Psychiatry 10 (1), 382. doi:10.1038/s41398-020-01073-0
Lambricht, S., Van Oudenhove, L., and Sienaert, P. (2017). Antibiotics and mania: a systematic review. J. Affect Disord. 219, 149–156. doi:10.1016/j.jad.2017.05.029
LaSalvia, E. A., Domek, G. J., and Gitlin, D. F. (2010). Fluoroquinolone-induced suicidal ideation. General Hosp. psychiatry 32 (1), 108–110. doi:10.1016/j.genhosppsych.2009.03.002
Levkovitz, Y., Mendlovich, S., Riwkes, S., Braw, Y., Levkovitch-Verbin, H., Gal, G., et al. (2010). A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J. Clin. Psychiatry 71 (2), 138–149. doi:10.4088/JCP.08m04666yel
Liu, F., Guo, X., Wu, R., Ou, J., Zheng, Y., Zhang, B., et al. (2014). Minocycline supplementation for treatment of negative symptoms in early-phase schizophrenia: a double blind, randomized, controlled trial. Schizophr. Res. 153 (1-3), 169–176. doi:10.1016/j.schres.2014.01.011
Lowry, C. A., Hollis, J. H., de Vries, A., Pan, B., Brunet, L. R., Hunt, J. R. F., et al. (2007). Identification of an immune-responsive mesolimbocortical serotonergic system: potential role in regulation of emotional behavior. Neuroscience 146 (2), 756–772. doi:10.1016/j.neuroscience.2007.01.067
Lurie, I., Yang, Y. X., Haynes, K., Mamtani, R., and Boursi, B. (2015). Antibiotic exposure and the risk for depression, anxiety, or psychosis: a nested case-control study. J. Clin. psychiatry 76 (11), 1522–1528. doi:10.4088/JCP.15m09961
Michalak, K., Sobolewska-Włodarczyk, A., Włodarczyk, M., Sobolewska, J., Woźniak, P., and Sobolewski, B. (2017). Treatment of the fluoroquinolone-associated disability: the pathobiochemical implications. Oxidative Med. Cell. Longev. 2017, 8023935. doi:10.1155/2017/8023935
Miyaoka, T., Wake, R., Furuya, M., Liaury, K., Ieda, M., Kawakami, K., et al. (2012). Minocycline as adjunctive therapy for patients with unipolar psychotic depression: an open-label study. Prog. Neuropsychopharmacol. Biol. Psychiatry 37 (2), 222–226. doi:10.1016/j.pnpbp.2012.02.002
Moorthy, N., Raghavendra, N., and Venkatarathnamma, P. N. (2008). Levofloxacin-induced acute psychosis. Indian J. Psychiatry 50 (1), 57–58. doi:10.4103/0019-5545.39762
Morgan, R. L., Thayer, K. A., Santesso, N., Holloway, A. C., Blain, R., Eftim, S. E., et al. (2018a). Evaluation of the risk of bias in non-randomized studies of interventions (ROBINS-I) and the ‘target’ experiment’ concept in studies of exposures: rationale and preliminary instrument development. Environ. Int. 120, 382–387. doi:10.1016/j.envint.2018.08.018
Morgan, R. L., Thayer, K. A., Santesso, N., Holloway, A. C., Blain, R., Eftim, S. E., et al. (2019). A risk of bias instrument for non-randomized studies of exposures: a users’ guide to its application in the context of GRADE. Environ. Int. 122, 168–184. doi:10.1016/j.envint.2018.11.004
Morgan, R. L., Whaley, P., Thayer, K. A., and Schünemann, H. J. (2018b). Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ. Int. 121 (Pt 1), 1027–1031. doi:10.1016/j.envint.2018.07.015
Paljarvi, T., McPherson, T., Luciano, S., Herttua, K., and Fazel, S. (2022). Isotretinoin and adverse neuropsychiatric outcomes: retrospective cohort study using routine data. Br. J. Dermatol 187 (1), 64–72. doi:10.1111/bjd.21049
Palleja, A., Mikkelsen, K. H., Forslund, S. K., Kashani, A., Allin, K. H., Nielsen, T., et al. (2018). Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 3 (11), 1255–1265. doi:10.1038/s41564-018-0257-9
Puri, P., Parnami, P., Chitkara, A., Athwal, P. S. S., and Khetrapal, S. (2021). Antibiomania: a rare case of metronidazole-induced mania. Cureus 13 (1), e12414. doi:10.7759/cureus.12414
Rees, J. C. (2014). Obsessive-compulsive disorder and gut microbiota dysregulation. Med. hypotheses 82 (2), 163–166. doi:10.1016/j.mehy.2013.11.026
Rubin, D. B. (2001). Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv. Outcomes Res. Methodol. 2, 169–188. doi:10.1023/a:1020363010465
Rummans, T. A., Evans, J. M., Krahn, L. E., and Fleming, K. C. (1995). Delirium in elderly patients: evaluation and management. Mayo Clin. Proc. 70 (10), 989–998. doi:10.4065/70.10.989
Siebler, P. H., Heinze, J. D., Kienzle, D. M., Hale, M. W., Lukkes, J. L., Donner, N. C., et al. (2018). Acute administration of the nonpathogenic, saprophytic bacterium, Mycobacterium vaccae, induces activation of serotonergic neurons in the dorsal raphe nucleus and antidepressant-like behavior in association with mild hypothermia. Cell Mol. Neurobiol. 38 (1), 289–304. doi:10.1007/s10571-017-0564-3
Slykerman, R. F., Coomarasamy, C., Wickens, K., Thompson, J. M. D., Stanley, T. V., Barthow, C., et al. (2019). Exposure to antibiotics in the first 24 months of life and neurocognitive outcomes at 11 years of age. Psychopharmacology 236 (5), 1573–1582. doi:10.1007/s00213-019-05216-0
Sutton-Tyrrell, K. (1991). Assessing bias in case-control studies. Proper selection of cases and controls. Stroke 22 (7), 938–942. doi:10.1161/01.str.22.7.938
Tome, A. M., and Filipe, A. (2011). Quinolones: review of psychiatric and neurological adverse reactions. Drug Saf. 34 (6), 465–488. doi:10.2165/11587280-000000000-00000
von Elm, E., Altman, D. G., Egger, M., Pocock, S. J., Gøtzsche, P. C., Vandenbroucke, J. P., et al. (2007a). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370 (9596), 1453–1457. doi:10.1016/S0140-6736(07)61602-X
von Elm, E., Altman, D. G., Egger, M., Pocock, S. J., Gøtzsche, P. C., Vandenbroucke, J. P., et al. (2007b). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology 18 (6), 800–804. doi:10.1097/EDE.0b013e3181577654
von Elm, E., Altman, D. G., Egger, M., Pocock, S. J., Gøtzsche, P. C., Vandenbroucke, J. P., et al. (2007c). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 4 (10), e296. doi:10.1371/journal.pmed.0040296
von Elm, E., Altman, D. G., Egger, M., Pocock, S. J., Gøtzsche, P. C., Vandenbroucke, J. P., et al. (2007d). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann. Intern Med. 147 (8), 573–577. doi:10.7326/0003-4819-147-8-200710160-00010
Walrave, T. R. W. M., Mohammad, S., and Ploeger, R. R. (2016). Mania induced by antibiotic therapy. Tijdschr. Psychiatr. 58 (8), 603–606.
Wilcox, M. A., Villasis-Keever, A., Sena, A. G., Knoll, C., and Fife, D. (2020). Evaluation of disability in patients exposed to fluoroquinolones. BMC Pharmacol. Toxicol. 21 (1), 40. doi:10.1186/s40360-020-00415-4
Yolken, R., Adamos, M., Katsafanas, E., Khushalani, S., Origoni, A., Savage, C., et al. (2016). Individuals hospitalized with acute mania have increased exposure to antimicrobial medications. Bipolar Disord. 18 (5), 404–409. doi:10.1111/bdi.12416
Yuan, N., Chen, Y., Xia, Y., Dai, J., and Liu, C. (2019). Inflammation-related biomarkers in major psychiatric disorders: a cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl. Psychiatry 9 (1), 233. doi:10.1038/s41398-019-0570-y
Zareifopoulos, N., and Panayiotakopoulos, G. (2017). Neuropsychiatric effects of antimicrobial agents. Clin. drug Investig. 37 (5), 423–437. doi:10.1007/s40261-017-0498-z
Zazula, R., Husain, M. I., Mohebbi, M., Walker, A. J., Chaudhry, I. B., Khoso, A. B., et al. (2021). Minocycline as adjunctive treatment for major depressive disorder: pooled data from two randomized controlled trials. Aust. N. Z. J. Psychiatry 55 (8), 784–798. doi:10.1177/0004867420965697
Keywords: risk, antibiotic, psychosis, retrospective, suicidality, mood disorders, anxiety, sex
Citation: Kerman IA, Glover ME, Lin Y, West JL, Hanlon AL, Kablinger AS and Clinton SM (2024) Antibiotic exposure is associated with decreased risk of psychiatric disorders. Front. Pharmacol. 14:1290052. doi: 10.3389/fphar.2023.1290052
Received: 06 September 2023; Accepted: 01 December 2023;
Published: 08 January 2024.
Edited by:
Magdalena Sowa-Kucma, College of Medical Sciences, University of Rzeszow, PolandReviewed by:
Rafal Roman Jaeschke, Department of Psychiatry, Faculty of Medicine, Jagiellonian University Medical College, PolandPaul E. Alele, Mbarara University of Science and Technology, Uganda
Copyright © 2024 Kerman, Glover, Lin, West, Hanlon, Kablinger and Clinton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ilan A. Kerman, aWxhbmtlcm1hbkB2dC5lZHU=
†These authors contributed equally to this work
 Ilan A. Kerman
Ilan A. Kerman Matthew E. Glover2†
Matthew E. Glover2† Yezhe Lin
Yezhe Lin Jennifer L. West
Jennifer L. West Anita S. Kablinger
Anita S. Kablinger Sarah M. Clinton
Sarah M. Clinton