- 1Department of Cardiology, Toranomon Hospital, Tokyo, Japan
- 2National Cerebral and Cardiovascular Center, Suita, Japan
- 3Division of Public Health, Center for Community Medicine, Jichi Medical University, Shimotsuke, Japan
- 4National Hospital Organization, Yonago Medical Center, Yonago, Japan
- 5StaGen Co., Ltd., Tokyo, Japan
Objectives: This study investigates the impact of xanthine oxidase inhibitors (XOI) on mortality in patients with cardiovascular diseases. XOI withdrawal has been reported to increased mortality risk due to rapid adenosine triphosphate (ATP) deficiency. This study aims to determine whether XOI treatment reduces mortality and whether XOI withdrawal increases mortality.
Methods: This is a real-world database study using the Japanese Registry of All Cardiac and Vascular Diseases (J-ROAD). We analyzed 1,648,891 hospitalized patients aged 20–90 with acute coronary syndrome or heart failure. In the first study, mortality rates were compared between patients without urate-lowering agents (n = 1,292,486) and those with XOI agents (n = 315,388, excluding 41,017 on other urate-lowering agents). In the second study, mortality rates were compared between the XOI continuous medication group (n = 226,261) and the XOI withdrawal group (n = 89,127).
Results: After multiple adjustments, XOI treatment group showed significantly lower mortality compared with that without any urate-lowering agent (odds ratio (OR), 0.576, 95% confidence interval (CI), 0.567–0.587, p < .001). In the sub-analysis, the group with allopurinol (OR, 0.578; 95% CI, 0.557–0.600), febuxostat (OR, 0.610; 95% CI, 0.599–0.622), and topiroxostat (HR, 0.545; 95% CI, 0.473–0.628) showed lower OR of mortality compared with that without any urate-lowering agent. XOI withdrawal group led to significantly higher death rates compared to XOI continuous group (19.8% vs. 0.03%; p < .001).
Conclusion: XOI treatment for patients with cardiovascular diseases is associated with reduced mortality. Conversely, XOI withdrawal is linked to elevated mortality risk. This emphasizes the importance of both prescribing and discontinuing XOI carefully to optimize patient outcomes.
Introduction
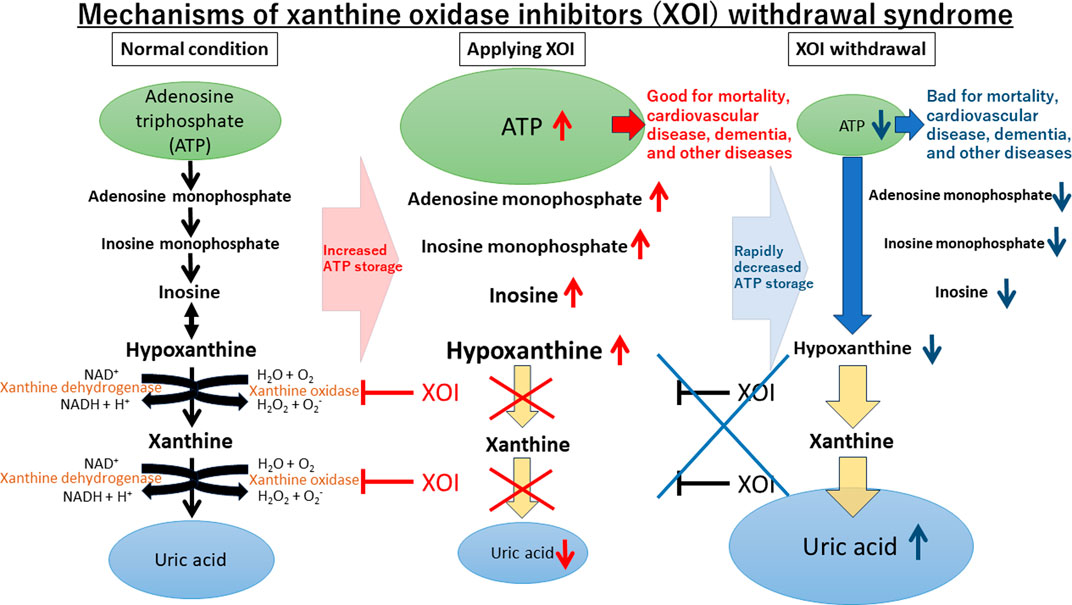
Uric acid, the end product of adenosine triphosphate (ATP) metabolism in humans, is influenced by xanthine oxidase (XO). XO inhibitors (XOI) suppress the production of uric acid and potentially store ATP (Kuwabara et al., 2023). XOI discontinuation has shown a XOI withdrawal syndrome with ATP depletion and increased mortality (Johnson et al., 2019; Ghang et al., 2020). Cardiovascular Safety of Febuxostat and Allopurinol in Patients with Gout and Cardiovascular Morbidities (CARES) trial showed that febuxostat use is associated with increased cardiovascular-related death compared to allopurinol (White et al., 2018). However, the CARES showed that nearly 85% of deaths occurred while subjects were off of therapy (Bubb, 2019). The CARES sub-analysis found increased major adverse cardiovascular events (MACE) and cardiovascular death verse events were increased in the initial stage after discontinuation of febuxostat or allopurinol (Ghang et al., 2022). The FDA showed black-box warnings for febuxostat (Abeles and Pillinger, 2019), yet the febuxostat versus allopurinol streamlined trial (FAST), which had a low dropout rate, found no group differences in cardiovascular outcomes or death between the two groups (Mackenzie et al., 2020). These results suggest that the primary causes of MACE or death are associated with the withdrawal of XOI regardless of the type of XOI used. We hypothesize that the main cause of death is from XOI withdrawal by removing beneficial effects of XOI like reducing uric acid, reducing reactive oxygen species (ROS) and inflammation, or storing ATP (Feig et al., 2008; Johnson et al., 2019).
This study tests our hypothesis that oral XOI administration improves mortality, but discontinuation leads to excess deaths. This study analyzes inpatient data of acute coronary syndrome (ACS) or heart failure patients, a high-risk population, to compare mortality with and without XOI and XOI continuation and discontinuation. This investigation aims to shed light on the potential benefits of XOI administration and the risks associated with discontinuation in this vulnerable population.
Materials and methods
Study design and study subjects
This study retrospectively analyzed the Japanese Registry of All Cardiac and Vascular Diseases (J-ROAD) database, which is a nationwide registry collected by the Japanese Circulation Society (JCS). The database consists of all participating (associated) training hospitals in the JCS (Yasuda et al., 2016; Yasuda et al., 2018). The main diagnoses or comorbidities of each patient were coded using the International Classification of Disease and Related Health Problems 10th revision (ICD-10) codes. As no information specifying individuals was included, the requirement for informed consent was waived. This study complied with the principles of the Declaration of Helsinki regarding investigations in human subjects and was approved by the Toranomon Hospital Institutional Research Ethics Review Board (Approved number 2208).
We collected and analyzed the J-ROAD data from April 2014 to March 2020. The study consists of two studies. As the first study, we compared all-cause mortality in inpatient with ACS or heart failure at admission between with XOI and without any urate-lowering medication. In the sub-analysis of the first study, we checked each XOI (allopurinol, febuxostat, and topiroxostat) affects mortality. As the second study, we compared rate of death between XOI continuous group and withdrawal group.
Patient involvement
No patients were involved in setting the research question or outcome measures, nor were they involved in the design and implementation of the study. There are no plans to involve patients in dissemination.
Inclusion and exclusion criteria
The J-ROAD database had 9,825,635 inpatients data from April 2014 to March 2020. We included inpatients aged between 20 and 90 years with ACS or heart failure at admission.
which ICD-10 code was I200, I21, I22, and I50. We showed the flow diagram of the study (Figures 1, 2).
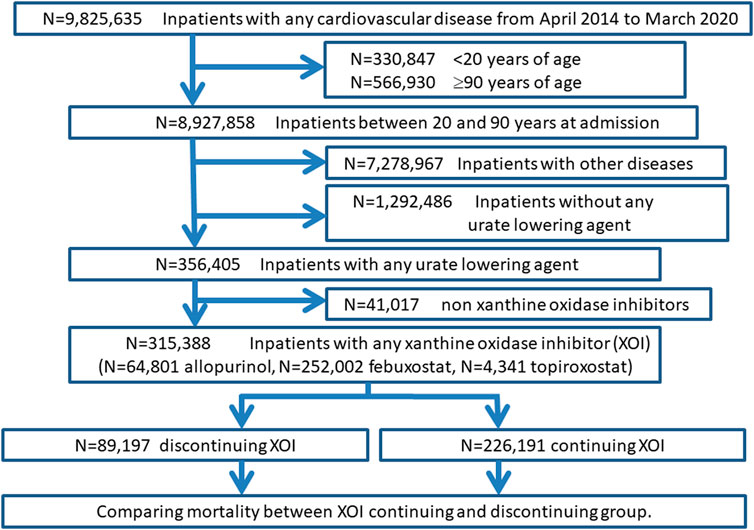
FIGURE 2. Flow diagram of the second study: xanthine oxidase inhibitors discontinuation and mortality.
Statistical analysis
The statistically significant level was set at probability (p) < 0.05 (two sided). Data are expressed as mean ± standard deviation or as percent frequency unless otherwise specified. Comparisons between two groups were performed with student t-tests for normally distributed variables, and χ2 analyses for categorical data.
In the first study, we compared all-cause mortality between patients with XOI and without any urate-lowering agents. To analyze the factors associated with mortality, a multilevel mixed-effect logistic regression analysis using institution as a random intercept was performed. The factors associated with mortality were evaluated both by crude models (non-adjusted) and by adjusted multivariable models with age, sex, smoking, hypertension, diabetes mellitus, dyslipidemia, chronic kidney disease, cancer, and XOI.
In the second study, we checked mortality by four group categories: 1) those who were on XOI at admission and continued XOI at discharge, 2) those who were on XOI at admission and discontinued XOI at discharge, 3) those who were not on XOI at admission (XOI was given during hospitalization) and continued XOI at discharge, and 4) those were not on XOI at admission (XOI was given during hospitalization) and discontinued XOI at discharge. We compared continuous XOI group including 1) and 3) and withdrawal group including 2) and 4) by χ2 analyses and calculate odds ratio.
Statistical analyses were performed using the SPSS Statistics software version 25 for Windows (IBM SPSS Statistics; IBM, New York, USA) and the Stata version 14.2 for Windows (StataCorp, College Station, TX, United States).
Ethical considerations
We adhered to the principles of the Declaration of Helsinki. Informed consent was obtained from all subjects by a comprehensive agreement method provided by St. Luke’s International Hospital. All data were collected and compiled in a protected computer database. Individual data were anonymous without identifiable personal information. St. Luke’s International Hospital Ethics Committee approved the protocol for this study.
Results
Demographics of this study subjects
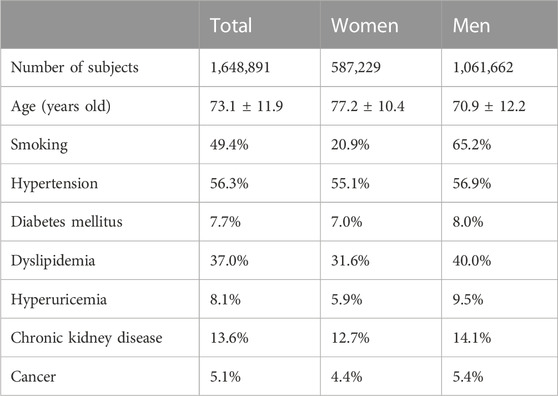
Of 9,825,635 inpatients data in J-ROAD from April 2014 to March 2020, we included 8,927,858 inpatients out of excluding 330,847 inpatients younger than 20 years and 566,930 inpatients older than 90 years. Of those, we included 1,648,891 inpatients with ACS or heart failure. The backgrounds of the study subjects were shown in Table 1. We divided the inpatients into with urate-lowering agent (N = 356,405) and without any urate-lowering agent (N = 1,292,486). Finally, we compared mortality between 1,292,486 without urate-lowering agent and 315,388 with XOI agent after excluding 41,017 subjects with urate-lowering agent other than XOI.
Xanthine oxidase inhibitors treatment and mortality
The number of patients with each XOI prescription was following: 64,801 allopurinol, 252,002 febuxostat, and 4,341 topiroxostat. The mortality of the whole patients of this study was 7.6%. The mortality of the study inpatient with allopurinol, febuxostat, and topiroxostat were 5.0%, 5.6%, and 4.8%, respectively, which were significantly lower than that without any urate-lowering agent.
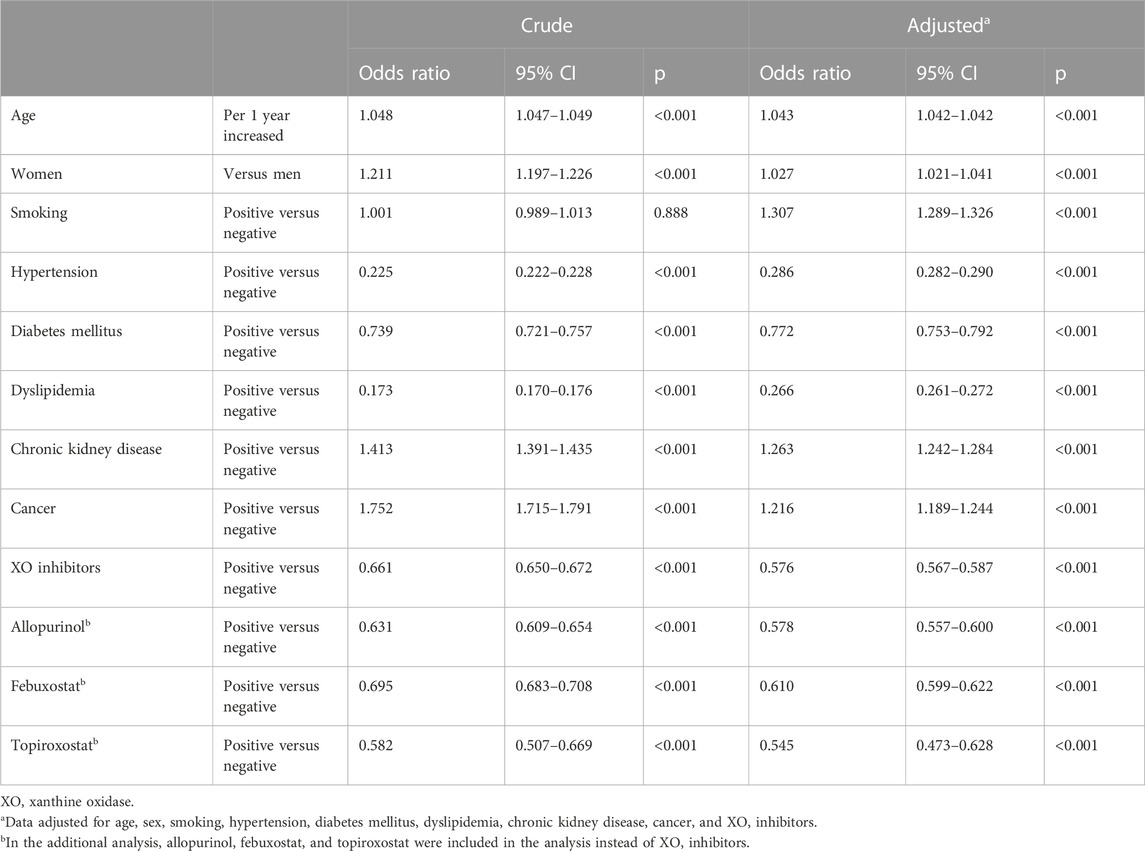
After multivariable adjustments with age, sex, smoking, hypertension, diabetes mellitus, dyslipidemia, chronic kidney disease, and cancer, the group with XOI showed significantly lower odds ratio (OR) of mortality compared with that without any urate-lowering agent (OR, 0.576; 95% CI, 0.567–0.587; p < .001). In the sub-analysis, the group with allopurinol (OR, 0.578; 95% CI, 0.557–0.600; p < .001), febuxostat (OR, 0.610; 95% CI, 0.599–0.622; p < .001), and topiroxostat (HR, 0.545; 95% CI, 0.473–0.628; p < .001) showed lower OR of mortality compared with that without any urate-lowering agent (Table 2).
Xanthine oxidase withdrawal syndrome
Of 356,405 inpatients with any urate-lowering agent, 315,388 were on XOI (41,017 were on other urate-lowering agent). The XOI adherents were divided into the following four groups, and the number of deaths and mortality rates for each were identified as follows;
(1) those who were on XOI at admission and continued XOI at discharge: 219,020 patients, of which 59 deaths (0.03%), (2) those who were on XOI at admission and discontinued XOI at discharge: 89,035 patients, of which 17,663 deaths (19.84%), (3) those who were not on XOI at admission (XOI was given during hospitalization) and continued XOI at discharge: 7,241 patients, of which 11 deaths (0.15%), (4) those were not on XOI at admission (XOI was given during hospitalization) and discontinued XOI at discharge: 92 patients, of which 11 deaths (11.96%).
We compared rate of death between continuous medication group including (1) and (3) and withdrawal group including (2) and (4). The rate of death in XOI withdrawal group was 620 times higher than XOI continuous group (19.8% versus 0.03%; p < .001).
Discussion
This study aimed to assess the prognostic impact of oral administration and discontinuation of XOI in patients hospitalized for ACS or heart failure. The findings revealed that hospitalized patients receiving XOI associated a 42% lower mortality rate compared to those without any urate-lowering agent. This trend was consistent across individual XOIs—allopurinol, febuxostat, and topiroxostat. The results suggest that XOI could reduce mortality regardless of the type of XOI used. Conversely, discontinuation of XOI was associated with a staggering 620-fold increase in mortality when compared to the group that continued XOI therapy. These results substantiate our hypothesis that XOI has intrinsic beneficial effects, while withdrawal negates these effects (Johnson et al., 2019). These results are compatible with some previous studies including the analyses of Medicare data or the United Kingdom Clinical Research Practice Datalink which indicated beneficial effects of allopurinol including reduced mortality in long-term studies (MacIsaac et al., 2016; Singh et al., 2017).
The suggested mechanisms underlying XOI’s cardiovascular benefits encompass the mitigation of negative effects from ROS or hyperuricemia, as well as the positive effects of ATP augmentation (Feig et al., 2008; Kuwabara et al., 2018). The production of uric acid via XO generates ROS like superoxide or hydroxyl radical, which can be harmful to various cells and tissues. Therefore, inhibiting its generation with XOI could alleviate the consequences of ROS production. While uric acid possesses antioxidant properties in serum, it acts as a prooxidant molecule intracellularly. It activates a specific catalytic subunit of nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase, NOX4 (Lanaspa et al., 2012). In the endothelium, uric acid reduces the availability of nitric oxide, a vasodilator, leading to vascular endothelial damage and dysfunction, which contributes to the development of cardiovascular diseases (Kuwabara et al., 2018). While the ROS mechanisms require longer durations to manifest, Noman et al. demonstrated the prompt benefits of allopurinol over a mere 6-week period in stable chronic angina patients (Noman et al., 2010). This implies that XOI’s positive impact becomes evident rapidly, and withdrawal promptly reverses these effects. ATP directly affects many organs including heart and its effects are rapid. Upon XOI cessation, the avoided deaths reappear by the lack of ATP particularly impacting the heart, leading to increased death in at-risk individuals. The notion of a “XOI withdrawal syndrome” is similar to withdrawal effects observed with cardiovascular risk-reducing medications like beta-blockers (Prins et al., 2015), a cornerstone in treatment for heart failure with reduced ejection fraction (McDonagh et al., 2021; Tsutsui et al., 2021; Heidenreich et al., 2022).
Although this study assessed all-cause mortality, detailed cause-specific mortality data were constrained by data limitations. Notably, dementia is a major contributor to poor mortality (Wolfson et al., 2001; Mitchell, 2015). Recent studies indicated a potential association between gout or hyperuricemia and dementia, suggesting higher uric acid levels was associate with lower prevalence of dementia (Scheepers et al., 2019; Min et al., 2021). Conversely, a meta-analysis unveiled a negative relationship between allopurinol exposure and dementia risk (Lai et al., 2022), implying allopurinol’s protective effects. To delve into this, we undertook additional analyses to investigate XOI’s potential link to dementia. The results demonstrated that the XOI group exhibited a significantly lower OR of dementia compared to the group without any urate-lowering agent, even after accounting for various factors (OR, 0.893; 95% CI, 0.874–0.912; p < 0.001) (Table 3). In the subgroup analysis, allopurinol and febuxostat demonstrated a similar trend, whereas topiroxostat did not attain statistical significance. Our results align with recent studies indicating that allopurinol and febuxostat were associated with a reduced risk of developing Alzheimer’s disease or dementia (Singh and Cleveland, 2018; Fang et al., 2022). These findings lend support to our hypothesis suggesting that XOI might potentially contribute to dementia reduction through ATP storage (Figure 3).
Allopurinol versus usual care in UK patients with ischaemic heart disease (ALL-HEART) study showed that treatment with allopurinol did not affect the risk of cardiovascular outcomes (Mackenzie et al., 2022). In the ALL-HEART study, the serum uric acid level in the allopurinol group was 0.34 mmol/L (5.7 mg/dL) at baseline and decreased to 0.18 mmol/L (3.0 mg/dL) after treatment. However, this study did not specifically assess the effects of hyperuricemia treatment due to its design. Additionally, the study experienced a high dropout rate, with 57.4% of participants in the allopurinol group withdrawing from the randomized treatment. The CARES trials showed that all-cause mortality and cardiovascular death was higher in febuxostat group compared with allopurinol group, (White et al., 2018), but a post hoc analysis revealed most deaths in the drug discontinuation group (Johnson et al., 2019). Given the results of our study, it raises the possibility that there were more cardiovascular events in the group that withdrew allopurinol and fewer cardiovascular events in the group that continued allopurinol in the ALL-HEART study. Further analyses comparing the outcomes between the groups that continued and those that withdrew from the treatment are necessary. We hypothesize that in the ALL-HEART study, the group that continued medication may have experienced a reduction in events, while the discontinuation group saw an increase, leading to an overall non-significant difference in outcomes.
Our study has several limitations. First, we could not assess uric acid levels and a history of gouts from this database. Recently, a study showed that gout flares are associated with a transient increase in cardiovascular events especially within 120 days after gout flares (Cipolletta et al., 2022). The rate of gout in patients with XOI should be higher than those without any urate-lowering agent, and these groups were higher risk of cardiovascular disease. However, the better prognosis in the XOI group is a noteworthy result. Second, some of the deaths in the XOI discontinuation group were due to the fact that other medications were also discontinued, which indicates it is impossible to determine a direct causal relationship between XOI withdrawal and death. In addition, it is influenced by the fact that some patients tend to stop taking their medications prior to death. Therefore, we may overestimate the effects of XOI withdrawal syndrome. However, there are similar reports about XOI withdrawal syndrome, (Bubb, 2019; Johnson et al., 2019; Ghang et al., 2020; Ghang et al., 2022), and we should take care of discontinuing XOI. Third, we did not assess the impact of other medications, including those for ACS and heart failure. These medications can influence mortality, representing a limitation of this study. However, XOI has minimal confounding effects with other medicines, and we believe that our results remain reasonably acceptable. Fourth, our study has shown favorable outcomes in patients with hypertension, diabetes, and dyslipidemia, which may appear unreasonable. However, considering that the study specifically focused on patients hospitalized for cardiovascular diseases, these positive prognostic results could be attributed to the proper treatment provided to these patients. Fifth, due to the nature of this being a retrospective database study, we have been unable to obtain detailed information on the reasons for the initiation or discontinuation of medications and specific information on cardiovascular deaths. Previous studies have shown that XOI is often prescribed for asymptomatic hyperuricemia rather than for gout in Japan (Hakoda and Kasagi, 2019). Therefore, we assume that in this study, XOI was frequently used for treating asymptomatic hyperuricemia. Although detailed information on cardiovascular deaths would have been more informative, the available data on all-cause mortality still provide valuable insights into the XOI withdrawal syndrome observed in this study. Sixth, this study included patients admitted with ACS or heart failure, recognizing that many cases were complex and involved multiple conditions. Although a sub-analysis for each disease would be valuable, the complexity and overlapping nature of these diseases often make isolated analysis difficult in this database. Further research focused on individual diseases is needed. Finally, it is important to acknowledge that our study is limited in its scope, as it exclusively evaluated Japanese population. To assess generalizability, similar studies should be conducted with other populations. In Japan, insurance covers urate-lowering drugs for hyperuricemia, and the concept of treatment for hyperuricemia may differ from that in other countries.
In conclusion, our study found that inpatients with ACS or heart failure receiving XOI treatment exhibited favorable mortality outcomes. On the other hand, XOI withdrawal was associated with a significantly higher risk of death, indicating the presence of XOI withdrawal syndrome. These findings emphasize the importance of not only prescribing XOI medication but also carefully considering the withdrawal process to optimize patient outcomes.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The data underlying this article were provided by the Japanese Circulation Society by permission. Data will be shared on request to the corresponding author with permission of the Japanese Circulation Society. Requests to access these datasets should be directed to J-ROAD Secretariat, ZHBjLWpyb2FkQG1sLm5jdmMuZ28uanA=.
Ethics statement
The studies involving humans were approved by the Toranomon Hospital Institutional Research Ethics Review Board (Approved number 2208). The studies were conducted in accordance with the local legislation and institutional requirements. The requirement for written informed consent from participants or their legal guardians/next of kin was waived, as this study utilizes an anonymized database.
Author contributions
MK: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. MN: Data curation, Formal Analysis, Methodology, Software, Supervision, Validation, Writing–review and editing. YS: Data curation, Methodology, Software, Writing–review and editing. YI: Data curation, Methodology, Software, Writing–review and editing. RA: Investigation, Project administration, Supervision, Writing–review and editing. TK: Investigation, Supervision, Writing–review and editing. IH: Investigation, Methodology, Project administration, Supervision, Writing–review and editing. NK: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. JSPS KAKENHI Grant (20K17168 and 23K07493), the research grant by Toranomon Hospital, and the research grant by Gout and uric acid foundation of Japan supported the study.
Conflict of interest
Author NK works as the Chairman of StaGen Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abeles, A. M., and Pillinger, M. H. (2019). Febuxostat and the black box blues. ACR Open Rheumatol. 1, 343–344. doi:10.1002/acr2.11047
Bubb, M. R. (2019). Excess Deaths Upon Cessation of Xanthine Oxidase Inhibitor Treatment-Data From the Cardiovascular Safety of Febuxostat and Allopurinol in Patients With Gout and Cardiovascular Morbidities Trial: comment on the Article by Choi et al. Arthritis Rheumatol. 71, 1391–1392. doi:10.1002/art.40914
Cipolletta, E., Tata, L. J., Nakafero, G., Avery, A. J., Mamas, M. A., and Abhishek, A. (2022). Association between gout flare and subsequent cardiovascular events among patients with gout. JAMA 328, 440–450. doi:10.1001/jama.2022.11390
Fang, J., Zhang, P., Wang, Q., Chiang, C. W., Zhou, Y., Hou, Y., et al. (2022). Artificial intelligence framework identifies candidate targets for drug repurposing in Alzheimer's disease. Alzheimers Res. Ther. 14, 7. doi:10.1186/s13195-021-00951-z
Feig, D. I., Kang, D. H., and Johnson, R. J. (2008). Uric acid and cardiovascular risk. N. Engl. J. Med. 359, 1811–1821. doi:10.1056/NEJMra0800885
Ghang, B., Ahn, S. M., Kim, J., Kim, Y. G., Lee, C. K., and Yoo, B. (2020). Discontinuing febuxostat might cause more deaths than continuing febuxostat: the untold story from the CARES trial. Rheumatol. Oxf. 59, 1439–1440. doi:10.1093/rheumatology/kez552
Ghang, B. Z., Lee, J. S., Choi, J., Kim, J., and Yoo, B. (2022). Increased risk of cardiovascular events and death in the initial phase after discontinuation of febuxostat or allopurinol: another story of the CARES trial. RMD Open 8, e001944. doi:10.1136/rmdopen-2021-001944
Hakoda, M., and Kasagi, F. (2019). Increasing trend of asymptomatic hyperuricemia under treatment with urate-lowering drugs in Japan. Mod. Rheumatol. 29, 880–884. doi:10.1080/14397595.2018.1519149
Heidenreich, P. A., Bozkurt, B., Aguilar, D., Allen, L. A., Byun, J. J., Colvin, M. M., et al. (2022). 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of cardiology/American heart association joint committee on clinical Practice guidelines. Circulation 145, e895–e1032. doi:10.1161/CIR.0000000000001063
Johnson, T. A., Kamatani, N., and Kuwabara, M. (2019). Xanthine Oxidase Inhibitor Withdrawal Syndrome? Comment on the Article by Choi et al. Arthritis Rheumatol. 71, 1966–1967. doi:10.1002/art.41066
Kuwabara, M., Kanbay, M., and Hisatome, I. (2018). Uric acid and hypertension because of arterial stiffness. Hypertension 72, 582–584. doi:10.1161/HYPERTENSIONAHA.118.11496
Kuwabara, M., Kodama, T., Ae, R., Kanbay, M., Andres-Hernando, A., Borghi, C., et al. (2023). Update in uric acid, hypertension, and cardiovascular diseases. Hypertens. Res. 46, 1714–1726. doi:10.1038/s41440-023-01273-3
Lai, S. W., Hwang, B. F., Kuo, Y. H., Liu, C. S., and Liao, K. F. (2022). Allopurinol use and the risk of dementia: a meta-analysis of case-control studies. Med. Baltim. 101, e29827. doi:10.1097/MD.0000000000029827
Lanaspa, M. A., Sanchez-Lozada, L. G., Choi, Y. J., Cicerchi, C., Kanbay, M., Roncal-Jimenez, C. A., et al. (2012). Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 287, 40732–40744. doi:10.1074/jbc.M112.399899
Macisaac, R. L., Salatzki, J., Higgins, P., Walters, M. R., Padmanabhan, S., Dominiczak, A. F., et al. (2016). Allopurinol and cardiovascular outcomes in adults with hypertension. Hypertension 67, 535–540. doi:10.1161/HYPERTENSIONAHA.115.06344
Mackenzie, I. S., Ford, I., Nuki, G., Hallas, J., Hawkey, C. J., Webster, J., et al. (2020). Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): a multicentre, prospective, randomised, open-label, non-inferiority trial. Lancet 396, 1745–1757. doi:10.1016/S0140-6736(20)32234-0
Mackenzie, I. S., Hawkey, C. J., Ford, I., Greenlaw, N., Pigazzani, F., Rogers, A., et al. (2022). Allopurinol versus usual care in UK patients with ischaemic heart disease (ALL-HEART): a multicentre, prospective, randomised, open-label, blinded-endpoint trial. Lancet 400, 1195–1205. doi:10.1016/S0140-6736(22)01657-9
Mcdonagh, T. A., Metra, M., Adamo, M., Gardner, R. S., Baumbach, A., Bohm, M., et al. (2021). 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 42, 3599–3726. doi:10.1093/eurheartj/ehab368
Min, K. H., Kang, S. O., Oh, S. J., Han, J. M., and Lee, K. E. (2021). Association between gout and dementia in the elderly: a nationwide population-based cohort study. Am. J. Geriatr. Psychiatry 29, 1177–1185. doi:10.1016/j.jagp.2021.01.016
Mitchell, S. L. (2015). CLINICAL PRACTICE. Advanced dementia. N. Engl. J. Med. 372, 2533–2540. doi:10.1056/NEJMcp1412652
Noman, A., Ang, D. S., Ogston, S., Lang, C. C., and Struthers, A. D. (2010). Effect of high-dose allopurinol on exercise in patients with chronic stable angina: a randomised, placebo controlled crossover trial. Lancet 375, 2161–2167. doi:10.1016/S0140-6736(10)60391-1
Prins, K. W., Neill, J. M., Tyler, J. O., Eckman, P. M., and Duval, S. (2015). Effects of beta-blocker withdrawal in acute decompensated heart failure: a systematic review and meta-analysis. JACC Heart Fail 3, 647–653. doi:10.1016/j.jchf.2015.03.008
Scheepers, L., Jacobsson, L. T. H., Kern, S., Johansson, L., Dehlin, M., and Skoog, I. (2019). Urate and risk of Alzheimer's disease and vascular dementia: a population-based study. Alzheimers Dement. 15, 754–763. doi:10.1016/j.jalz.2019.01.014
Singh, J. A., and Cleveland, J. D. (2018). Comparative effectiveness of allopurinol versus febuxostat for preventing incident dementia in older adults: a propensity-matched analysis. Arthritis Res. Ther. 20, 167. doi:10.1186/s13075-018-1663-3
Singh, J. A., Ramachandaran, R., Yu, S., and Curtis, J. R. (2017). Allopurinol use and the risk of acute cardiovascular events in patients with gout and diabetes. BMC Cardiovasc Disord. 17, 76. doi:10.1186/s12872-017-0513-6
Tsutsui, H., Ide, T., Ito, H., Kihara, Y., Kinugawa, K., Kinugawa, S., et al. (2021). JCS/JHFS 2021 guideline focused update on diagnosis and treatment of acute and chronic heart failure. Circ. J. 85, 2252–2291. doi:10.1253/circj.CJ-21-0431
White, W. B., Saag, K. G., Becker, M. A., Borer, J. S., Gorelick, P. B., Whelton, A., et al. (2018). Cardiovascular safety of febuxostat or allopurinol in patients with gout. N. Engl. J. Med. 378, 1200–1210. doi:10.1056/NEJMoa1710895
Wolfson, C., Wolfson, D. B., Asgharian, M., M'lan, C. E., Ostbye, T., Rockwood, K., et al. (2001). A reevaluation of the duration of survival after the onset of dementia. N. Engl. J. Med. 344, 1111–1116. doi:10.1056/NEJM200104123441501
Yasuda, S., Miyamoto, Y., and Ogawa, H. (2018). Current status of cardiovascular medicine in the aging society of Japan. Circulation 138, 965–967. doi:10.1161/CIRCULATIONAHA.118.035858
Keywords: xanthine oxidase, xanthine oxidase inhibitors, mortality, withdrawal syndrome, epidemiology, uric acid, hyperuricemia
Citation: Kuwabara M, Nakai M, Sumita Y, Iwanaga Y, Ae R, Kodama T, Hisatome I and Kamatani N (2024) Xanthine oxidase inhibitors treatment or discontinuation effects on mortality: evidence of xanthine oxidase inhibitors withdrawal syndrome. Front. Pharmacol. 14:1289386. doi: 10.3389/fphar.2023.1289386
Received: 05 September 2023; Accepted: 11 December 2023;
Published: 08 January 2024.
Edited by:
Tanveer Ahmed Khan, National Institute of Health, PakistanReviewed by:
Milos Stojiljkovic, University of Banja Luka, Bosnia and HerzegovinaNeha Kapoor, Suresh Gyan Vihar University, India
Copyright © 2024 Kuwabara, Nakai, Sumita, Iwanaga, Ae, Kodama, Hisatome and Kamatani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masanari Kuwabara, a3V3YW1hc2E3MjhAZ21haWwuY29t
 Masanari Kuwabara
Masanari Kuwabara Michikazu Nakai
Michikazu Nakai Yoko Sumita2
Yoko Sumita2 Naoyuki Kamatani
Naoyuki Kamatani