- 1Department of Neurology, Kazan State Medical University, Kazan, Russia
- 2Department of Neurology, Interregional Clinical Diagnostic Center, Kazan, Russia
Background: There has been intensive research into enhancing the effects of reperfusion therapy to mitigate hemorrhagic transformation (HT) in stroke patients. Using neuroprotective agents alongside intravenous thrombolysis (IVT) appears a promising approach. Cerebrolysin is one of the candidates since it consists of neuropeptides mimicking the action of neurotrophic factors on brain protection and repair.
Objectives: We looked at treatment effects of Cerebrolysin as an early add-on to IVT in stroke patients with varying HT risk.
Methods: It was post hoc analysis of the CEREHETIS trial (ISRCTN87656744). Patients with middle cerebral artery infarction (n = 238) were selected from the intention-to-treat population. To stratify participants according to their HT risk, the DRAGON, SEDAN and HTI scores were computed for each eligible subject using on-admission data. The study endpoints were any and symptomatic HT, and functional outcome measured with the modified Rankin Scale (mRS) on day 90. Favorable functional outcome (FFO) was defined as an mRS ≤2. The performance of each stratification tool was estimated with regression approaches. Heterogeneous treatment effect analysis was conducted using techniques of meta-analysis and the matching-smoothing method.
Results: The HTI score outperformed other tools in terms of HT risk stratification. Heterogeneity of Cerebrolysin treatment effects was moderate (I2, 35.8%–56.7%; H2, 1.56–2.31) and mild (I2, 10.9%; H2, 1.12) for symptomatic and any HT, respectively. A significant positive impact of Cerebrolysin on HT and functional outcome was observed in the moderate (HTI = 1) and high (HTI ≥2) HT risk patients, but it was neutral in those with the low (HTI = 0) risk. In particular, there was a steady decline in the rate of symptomatic (HTI = 0 vs. HTI = 4: by 4.3%, p = 0.077 vs. 21.1%, p < 0.001) and any HT (HTI = 0 vs. HTI = 4: by 1.2%, p = 0.737 vs. 32.7%, p < 0.001). Likewise, an mRS score reduction (HTI = 0 vs. HTI = 4: by 1.8%, p = 0.903 vs. 126%, p < 0.001) with a reciprocal increase of the fraction of FFO patients (HTI = 0 vs. HTI = 4: by 1.2% p = 0.757 vs. 35.5%, p < 0.001) was found.
Conclusion: Clinically meaningful heterogeneity of Cerebrolysin treatment effects on HT and functional outcome was established in stroke patients. The beneficial effects were significant in those whose estimated on-admission HT risk was either moderate or high.
Introduction
Over the past decade, there has been intensive research into enhancing the effects of reperfusion therapy by mitigating its adverse consequences such as reperfusion injury and hemorrhagic transformation (HT), and promoting functional recovery in stroke patients (Saver, 2011). The most straightforward and promising approach appears to be the use of neuroprotective agents with multimodal pleiotropic properties along with intravenous thrombolysis (IVT) (Otsu et al., 2020). Cerebrolysin, a porcine brain derivate, is one of the candidates since it consists of low-molecular weight neuropeptides and free amino acids, which mimic the action of endogenous neurotrophic factors on protection and repair of the central nervous system (Masliah and Díez-Tejedor, 2012).
Since early 1970-s, Cerebrolysin has been widely used in treatment of acute ischemic stroke, traumatic brain injury and cognitive impairment in over 50 European, Middle East, Latin American and Asian countries but not in the United States, United Kingdom and Australia (Cui et al., 2019; Staszewski et al., 2022; Jarosz et al., 2023; Ziganshina et al., 2023). A plethora of clinical trials and meta-analyses have suggested Cerebrolysin enhances early post-stroke recovery, improves neurological deficit after stroke, and bears an excellent tolerability and safety profile (Lang et al., 2013; Guekht et al., 2017; Bornstein et al., 2018). Therefore, it has been included in the guideline on pharmacological support in motor rehabilitation after acute ischemic stroke issued by the European Academy of Neurology and the European Federation of Neurorehabilitation Societies (Beghi et al., 2021).
In our original study published in March 2023 (Khasanova and Kalinin, 2023), we looked at the effects of Cerebrolysin with IVT versus IVT alone in stroke patients. The rationale behind the trial came from two aspects. First, recombinant tissue plasminogen activator (rtPA) increases the HT rate by degrading the blood-brain barrier (BBB) integrity and promoting neuroinflammation and excitotoxicity (Wang et al., 2004; Kelly et al., 2006; Guan et al., 2019). On the other hand, Cerebrolysin ameliorates rtPA adverse effects and, therefore, can potentially protect from IVT-related HT (Veinbergs et al., 2000; Teng et al., 2021; Guan et al., 2019). Besides bearing neuroprotective properties directed to mitigate the reperfusion injury, Cerebrolysin also attenuates BBB permeability (Zhang et al., 2010; Teng et al., 2021), which plays an essential role in HT pathophysiology (Spronk et al., 2021).
Thus, Cerebrolysin as an early add-on to IVT turned out to be beneficial for stroke patients: the treatment was safe, and reduced the symptomatic HT rate and early neurological deficit. Furthermore, our findings were coherent with the results of a similar trial on patients with severe stroke and futile recanalization after IVT (Poljakovic et al., 2021).
However, subjects differ not only in their background characteristics, but also in how they respond to a particular treatment or intervention (Xie et al., 2012). Accordingly, it is reasonable to assume that patients’ response to the Cerebrolysin treatment would vary with respect to their HT risk stratification on admission, and hence, discovering a group of patients benefiting most by the Cerebrolysin treatment could be of paramount importance for decision making in acute stroke settings. Therefore, we conducted post hoc analysis of the CEREHETIS trial (Khasanova and Kalinin, 2023) to look at treatment effects of Cerebrolysin as an early add-on to the reperfusion therapy in stroke patients with varying HT risk.
Methods
CEREHETIS was a prospective, randomized, open-label, active control, multicenter, parallel-group phase IIIb pilot study (trial registration number: ISRCTN87656744). The trial protocol, patient inclusion/exclusion criteria and original results have been published previously (Khasanova and Kalinin, 2023).
Each eligible patient was randomly assigned into either the Cerebrolysin or control group. Both arms received a standard dose of 0.9 mg/kg alteplase (Actilyse®, Boehringer Ingelheim GmbH, Germany), rtPA, administered intravenously within 4.5 h after symptom onset (maximal dosage 90 mg, 10% of the drug given in bolus and the rest in 60 min via intravenous infusion). Patients in the intervention group were additionally given 30 mL of Cerebrolysin® (EVER Pharma GmbH, Austria) diluted in 100 mL of normal saline and infused intravenously through a separate line over 20 min. The Cerebrolysin treatment was initiated simultaneously with IVT and continued once daily for 14 consecutive days.
The study primary endpoints were the rate of any and symptomatic HT verified on a follow-up computed tomography scan from day 0 to day 14. Symptomatic HT was defined according to the ECASS III trial: any apparently extravascular blood in the brain or within the cranium that was associated with clinical deterioration, as defined by an increase of 4 points or more in the score on the National Institutes of Health Stroke Scale (NIHSS), or that led to death and that was identified as the predominant cause of the neurologic deterioration (Hacke et al., 2008).
The secondary endpoint was functional outcome measured with the modified Rankin Scale (mRS) on day 90. Favorable functional outcome (FFO) was defined as an mRS score of ≤2 on day 90.
Study measurements
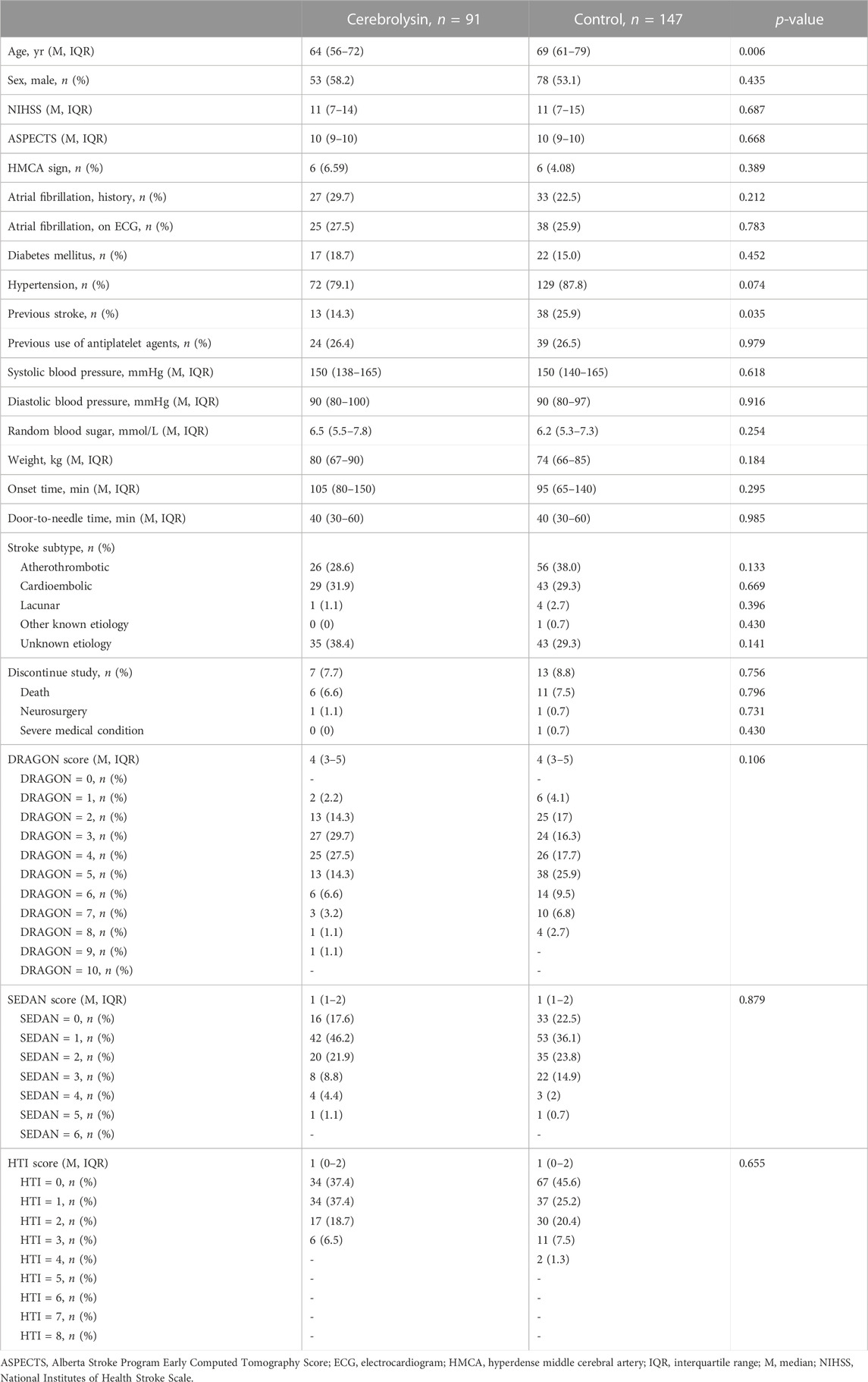
For the current study, patients with middle cerebral artery (MCA) infarction were selected from the intention-to-treat population of the CEREHETIS trial. To stratify participants according to their HT risk, several prediction tools were used: the DRAGON, SEDAN and HTI scores were computed for each eligible patient using on-admission data. By specification of each scale, a point increment corresponded to an increase in the HT probability.
Systematic reviews and meta-analyses (Whiteley et al., 2012; Sun et al., 2023) have established a number of on-admission predictors of IVT-related HT in stroke patients such as atrial fibrillation, age, serum glucose level, NIHSS, hyperdense MCA (HMCA) sign, Alberta Stroke Program Early Computed Tomography Score (ASPECTS), and onset-to-treatment time. Notwithstanding a variety of existing HT risk assessment models (Kalinin et al., 2017), our choice was restricted by several factors: an appreciation of those predictors and infarct vascular territory by each tool, effortless calculation taking into account the available dataset, external validation, and feasible use in clinical practice and trials (Fu et al., 2022).
The DRAGON score (HMCA sign/ASPECTS <10: both = 2, either = 1, none = 0; pre-stroke mRS >1: yes = 1; age: ≥80 years = 2, 65–79 years = 1, <65 years = 0; glucose: >8 mmol/L = 1; onset-to-treatment time: >90 min = 1; NIHSS: >15 = 3, 10–15 = 2, 5–9 = 1, 0–4 = 0; score range 0–10) has been designed and validated to predict functional outcome in stroke patients after IVT (Strbian et al., 2012a). However, it can also be used to assess the symptomatic HT risk since factors predicting poor functional outcome and HT are very similar (Whiteley et al., 2012).
The SEDAN score (glucose: 8.1–12.0 mmol/L = 1, >12.0 mmol/L = 2; ASPECTS <10: yes = 1; HMCA sign: yes = 1; age: >75 years = 1; NIHSS: ≥10 = 1; score range 0–6) is another well-established and externally validated tool to estimate the probability of IVT-related symptomatic HT (Strbian et al., 2012b).
The HTI score (ASPECTS: 10–7 = 0, 6–5 = 1, 4–3 = 2, 2–0 = 3; NIHSS: 0–11 = 0, 12–17 = 1, 18–23 = 2, >23 = 3; HMCA sign: yes = 1; atrial fibrillation on ECG: yes = 1; score range 0–8) has been developed and externally validated to predict any HT in MCA stroke patients regardless of IVT (Kalinin et al., 2017; Andrade et al., 2022).
Statistical analysis
Statistical analysis was performed with Stata/MP v.14.2 and 17.0 (StataCorp LLC, United States). The descriptive statistics included median (M) with the interquartile range (IQR) for non-normally distributed continuous data and percentage for categorical data. The groups were compared with the Mann–Whitney U test and Pearson’s χ2-test for continuous and categorical variables, respectively.
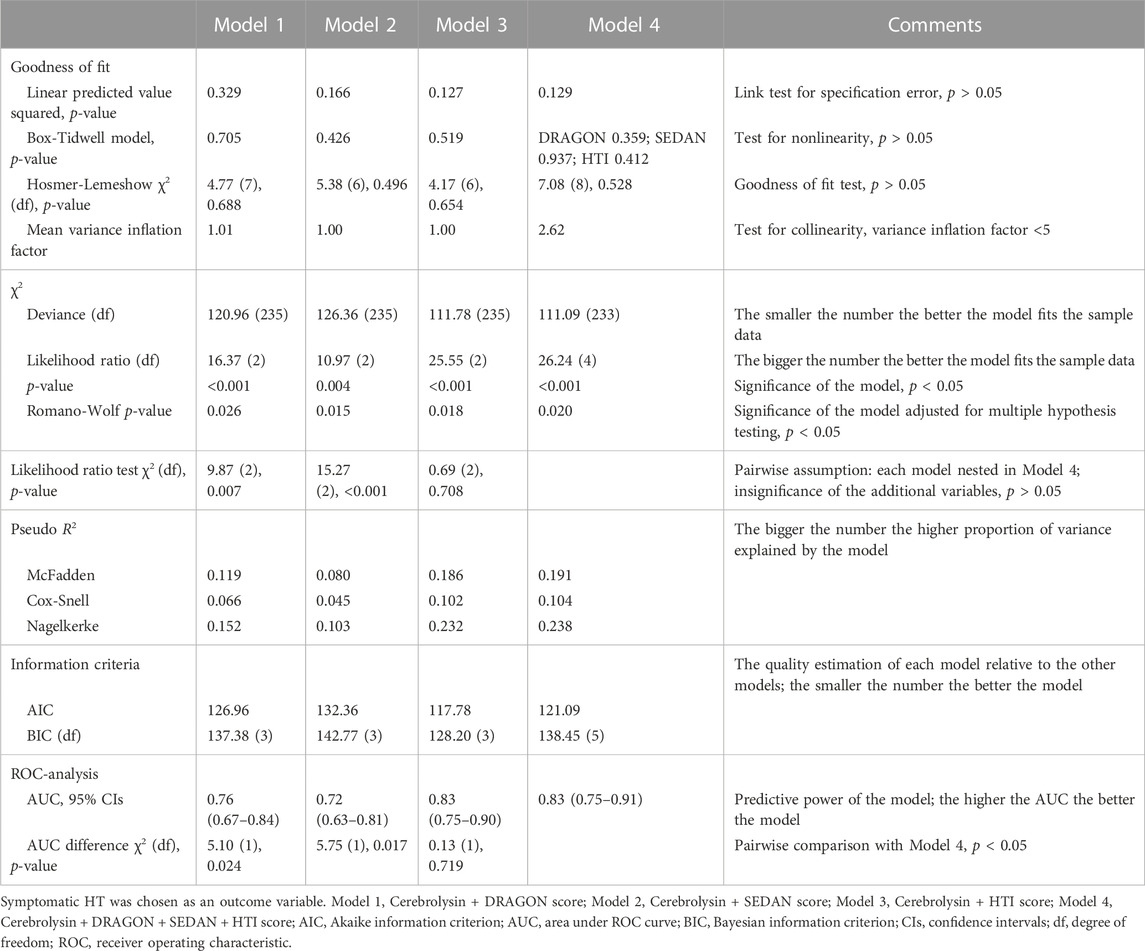
Four models–Cerebrolysin treatment adjusted for the DRAGON, SEDAN, HTI score, and Cerebrolysin treatment confounded by all three scores combined–were fitted with binary logistic regression. HT (symptomatic and any) and FFO were successively defined as a dependent variable. However, only symptomatic HT was specified as a dependent variable for the assessment of postestimation statistics since its odds ratio (OR) was significantly affected by the Cerebrolysin treatment (Khasanova and Kalinin, 2023). The performance of each stratification tool was evaluated in an array of postestimation tests. Based on the results of the assessment, the best fitted model was selected for further analysis.
In order to explore a range of Cerebrolysin treatment responses in patients with varying HT risk, the chosen model was fitted with binary logistic regression followed by the assessment of conditional marginal effects. Likewise, average treatment effects (ATE), average treatment effects on the treated (ATT) and average treatment effects on the untreated (ATC) were estimated using propensity score kernel matching. In addition to the matched treatment effects, naive ATE (unconditional mean differences; without regression adjustment) (NATE) and potential outcome averages were reported (Jann, 2017).
Moreover, generalized ordered logistic regression with the proportional and partial proportional odds assumption (Williams, 2006) was applied to highlight association between the mRS score and the selected model followed by the assessment of conditional marginal effects. The parallel (proportional) odds assumption was checked with the Brant test.
Heterogeneity of Cerebrolysin treatment effects was evaluated using in-built Stata commands for meta-analysis (StataCorp, 2021). Patients were divided into several subgroups according to their HT risk score assigned by the selected model. Each subgroup was declared as a “study”. For a comparison of two-sample binary outcomes, Cerebrolysin treatment effect sizes of the “studies” measured with the risk difference were estimated in the fixed-effects Mantel–Haenszel model. Likewise, Hedges’ g, the standardized mean difference, was used in the fixed-effects inverse-variance model to compare two-sample continuous outcomes. The random-effects restricted maximum likelihood model was applied for both types of the outcomes with estimation of the risk difference or Hedges’ g, respectively. Heterogeneity statistics and a forest plot were analyzed in the output of each model. The presence of small-study effects was checked with the Egger test.
Finally, advanced heterogeneous treatment effect (HTE) analysis was performed using the matching-smoothing method (Xie et al., 2012). Cerebrolysin and the selected model were specified as a treatment and independent variable, respectively. Symptomatic and any HT, mRS, and FFO were successively defined as a dependent variable.
Following the propensity score estimation for each subject using a logit model, kernel matching was performed on the logarithm of the OR. As a result, plots of a local polynomial smooth of the treatment effect against the propensity score were generated using a local polynomial fit of degree 1 (local-linear smoothing), and the 95% confidence intervals (CIs) were estimated. The Epanechnikov kernel function was used for matching as well as for calculation of the weighted local polynomial estimate. The bandwidth was not specified, and a rule-of-thumb bandwidth estimator was calculated and used.
Furthermore, each score of the selected model was matched with the corresponding propensity score to estimate their treatment effects, and the two-tailed p-values were computed using the z-score of the 95% CIs. The HTEs included estimates from both the treated and the untreated patients.
Whenever possible, bootstrapping was performed with 1,000 samples and computing normal-based CIs to reduce sampling bias, overfitting and prediction errors. Files of Stata code (Supplementary Data Sheet S3) and raw data (Supplementary Data Sheets S1, S2) can be found in the Supplementary Material.
Results
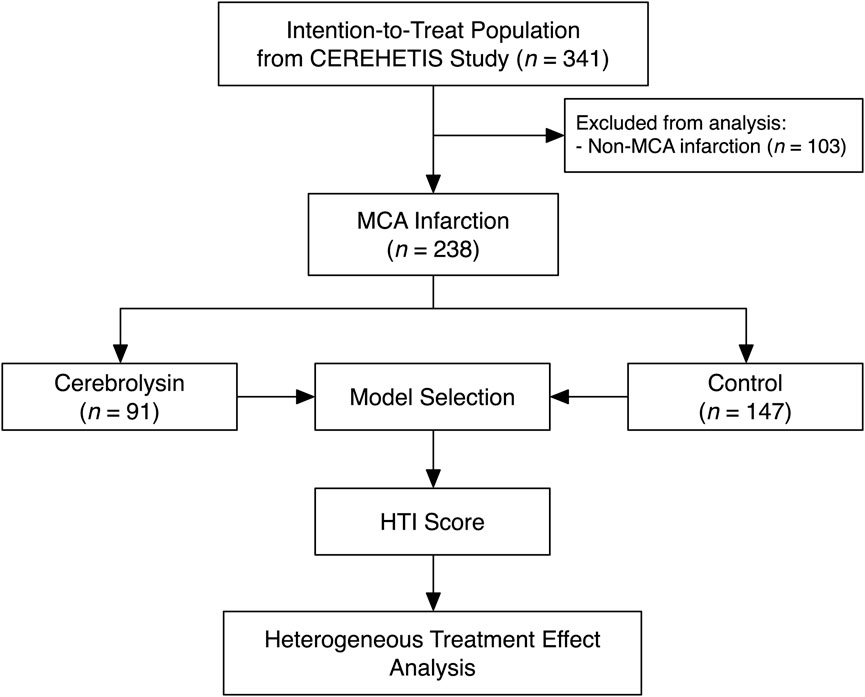
Of 341 participants comprising the intention-to-treat population of the CEREHETIS trial, 238 MCA stroke patients were selected for analysis (Figure 1).
At baseline, patients in the Cerebrolysin arm were slightly younger and, as a result, had fewer cases of previous stroke (Table 1). That was of no surprise, since the covariate disbalance had already been observed in the original cohort (Khasanova and Kalinin, 2023).
Although the score distribution did not extend over the full scale for the DRAGON (0–10), SEDAN (0–6) and HTI (0–8) score (Table 1) due to the study exclusion criteria (e.g., patients with neither minor (NIHSS <4) nor severe (NIHSS >25) stroke nor high hemorrhagic risk were eligible for IVT), each tool was able to predict HT and FFO and confounded the Cerebrolysin treatment (Figures 2A–C). However, only the HTI score became significant in the combined model (Figure 2D) and its performance was superior to the competitors in the postestimation tests (Table 2), hence it was selected for further analysis.
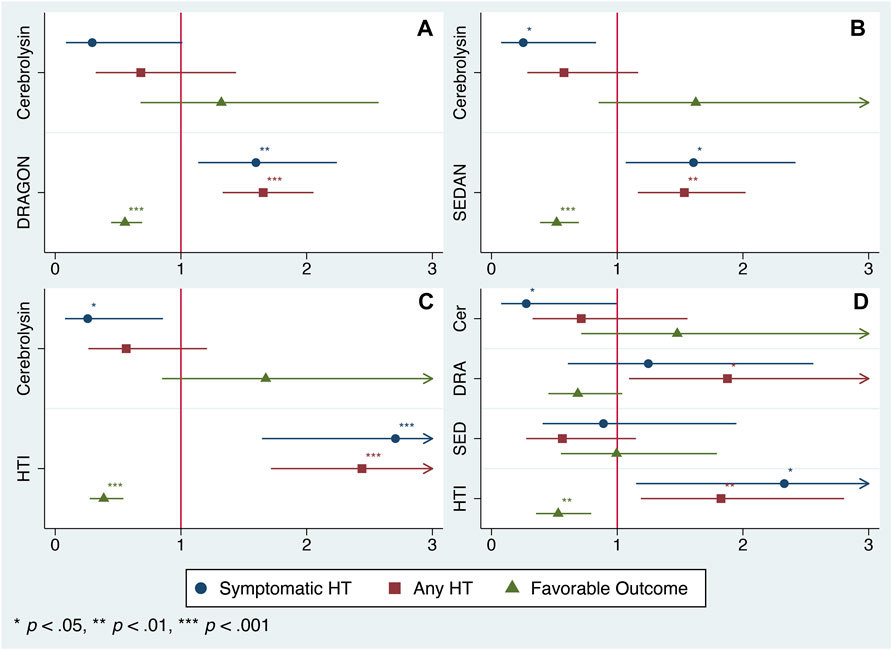
FIGURE 2. Model selection. Odds ratio (OR) of the Cerebrolysin treatment adjusted for selected scores with normal-based 95% CIs (n = 238). (A). Model 1: OR adjusted for the DRAGON score. (B). Model 2: OR adjusted for the SEDAN score. (C). Model 3: OR adjusted for the HTI score. (D). Model 4: OR adjusted for the DRAGON, SEDAN and HTI score combined.
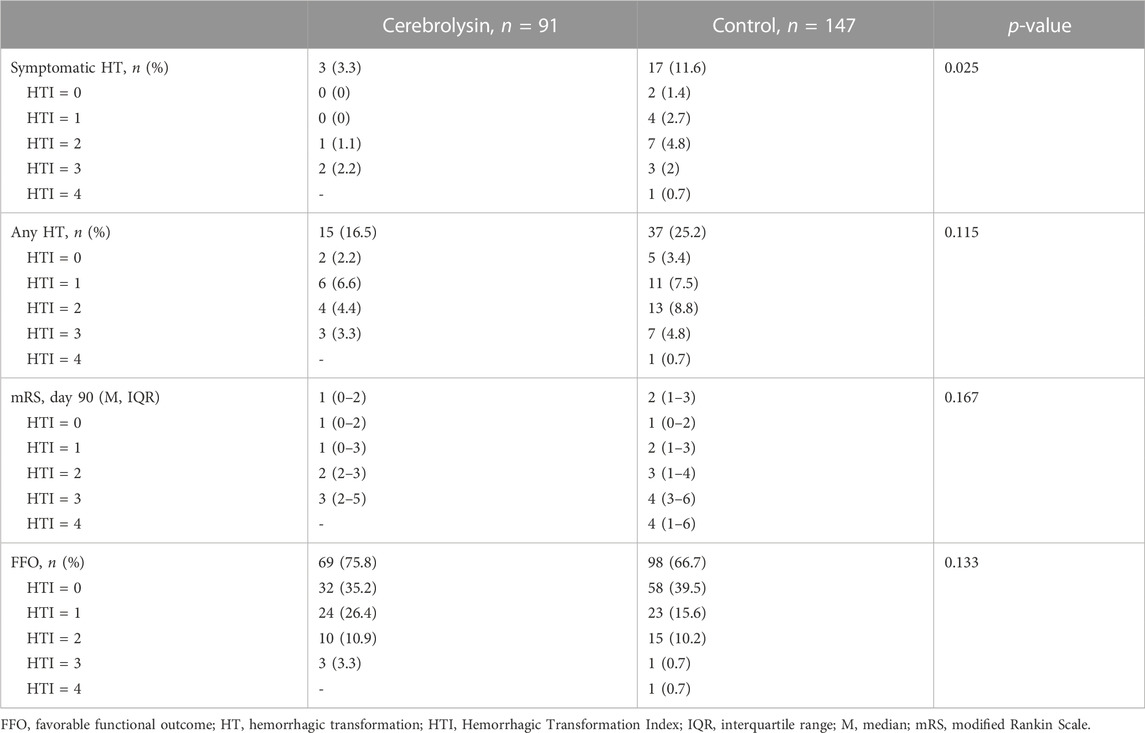
The summarized output of the employed statistical techniques–two-way contingency tables, logistic regression and propensity score matching followed by the treatment effect assessment–was consistent with our original results (Khasanova and Kalinin, 2023): the Cerebrolysin treatment significantly reduced the symptomatic HT rate and outlined a tendency to alleviate any HT and poor functional outcome (Table 3; Figures 2C, 3A, C).
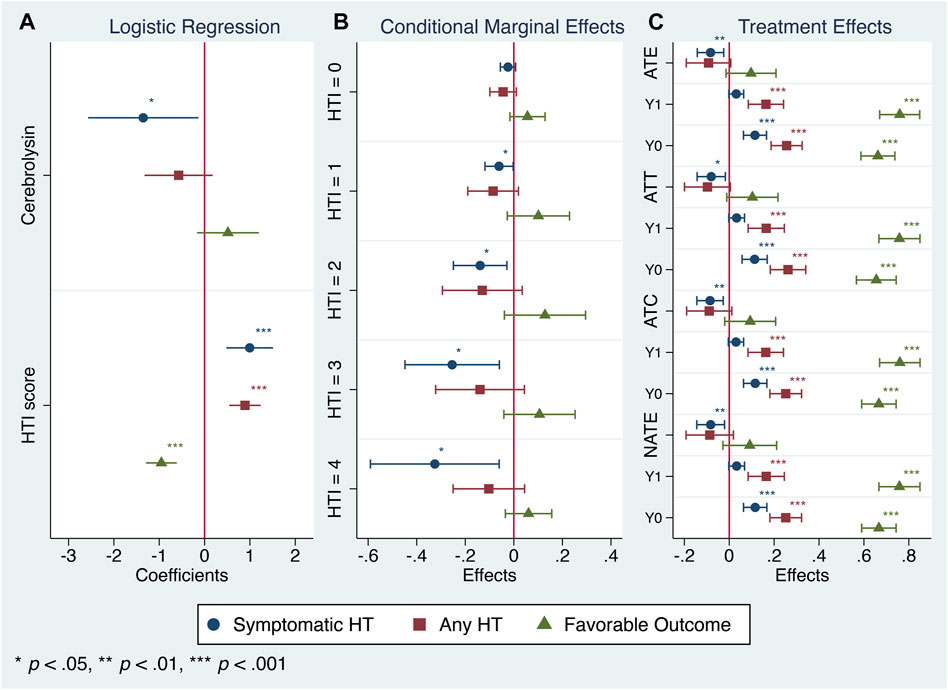
FIGURE 3. Cerebrolysin treatment responses in patients with varying HTI scores (n = 238). (A). Logistic regression analysis. Coefficients with normal-based 95% CIs are reported. (B). Conditional marginal effects of the Cerebrolysin group on probability of symptomatic HT, any HT and favorable functional outcome by the HTI score with 95% CIs. (C). Treatment effects of Cerebrolysin adjusted for the HTI score with normal-based 95% CIs. Average treatment effects (ATE), average treatment effects on the treated (ATT), average treatment effects on the untreated (ATC), naive average treatment effects (NATE) and potential outcome averages (Y0–the outcome that would be obtained if a patient does not get the treatment, Y1–the outcome that would be obtained if a patient gets the treatment) are reported.
Since CEREHETIS was a prospective randomized trial with almost equal covariate distribution between the treated and the untreated (Table 1), the estimated treatment effects (ATE, ATT, ATC, NATE) and their corresponding potential outcome averages did not differ much from one another (Figure 3C), and the HTI score had no influence on the treatment assignment (propensity score matching: HTI coefficient = 0.006; 95% CI: −0.258, 0.270; p = 0.962).
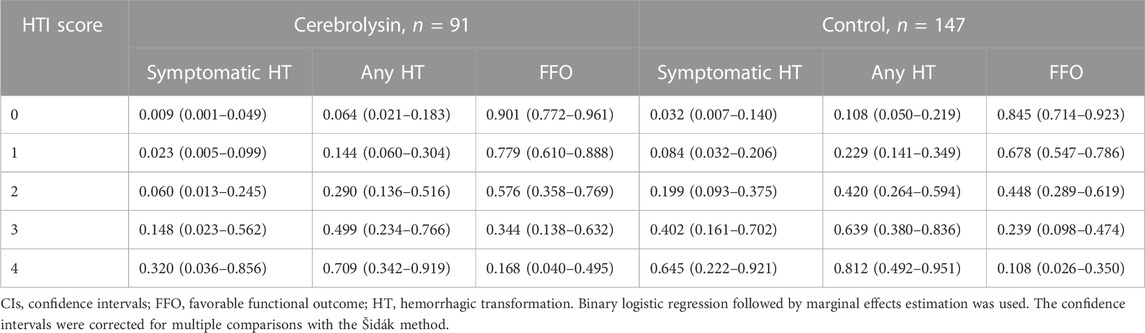
Based on the HT predicted probability adjusted for the HTI score (Table 4), HT risk can be graded for the sake of simplicity as low (HTI = 0), moderate (HTI = 1) and high (HTI ≥2).
In that respect, the assessment of conditional marginal effects of the Cerebrolysin treatment on probability of symptomatic HT by the HTI score revealed a clear pattern–the impact was neutral in the low HT risk patients but became prominent in those with the moderate and high risk. Accordingly, the Cerebrolysin treatment decreased the symptomatic HT rate by 2.4% (p > 0.05) in patients with HTI = 0, which further dropped step-wise to 32.5% (p < 0.05) at HTI = 4 (Figure 3B).
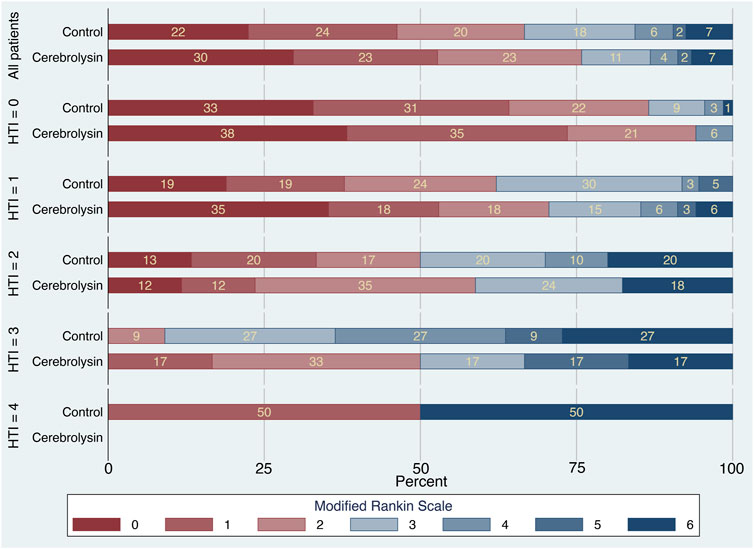
The mRS stacked bar plot arranged by the HTI score demonstrated two positive trends in functional outcome of the Cerebrolysin group. First, at HTI = 0 and 1, there was a decrease in the percentage of patients with mRS = 3 on day 90 (HTI = 0: 0% in the Cerebrolysin arm vs. 9% in the control; HTI = 1: 15% vs. 30%, respectively). Second, the overall percentage of patients with FFO was higher in the Cerebrolysin group. Interestingly, it was more prominent at HTI = 3 (50% vs. 9%, respectively) (Figure 4).
The Brant test turned out to be insignificant (χ2 (10) = 4.31, p = 0.932), hence the parallel regression assumption was not violated and generalized ordered logistic regression with proportional as well as partial proportional odds was justified for analysis. Both approaches defined the HTI score as a significant predictor of functional outcome (Figure 5).
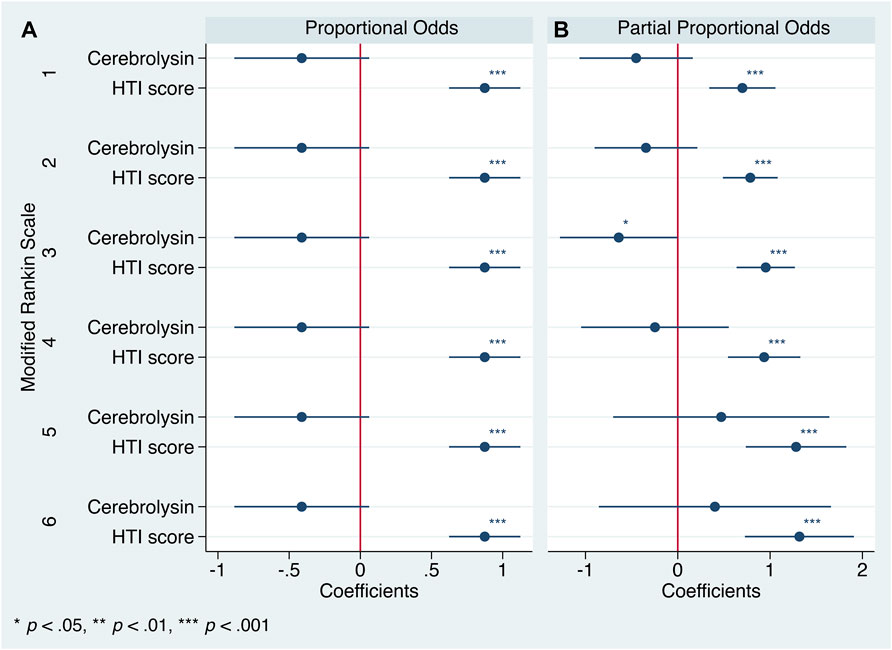
FIGURE 5. Generalized ordered logistic regression analysis: coefficients with 95% CIs (n = 238). mRS = 0 is the base category. (A). Proportional odds assumption. (B). Partial proportional odds assumption.
After applying the partial proportional odds assumption, the aforementioned first trend did achieve the statistical significance but was concealed by the use of a less flexible technique, the parallel one. However, both procedures failed to provide robust estimates of the second trend (Figures 5, 6).
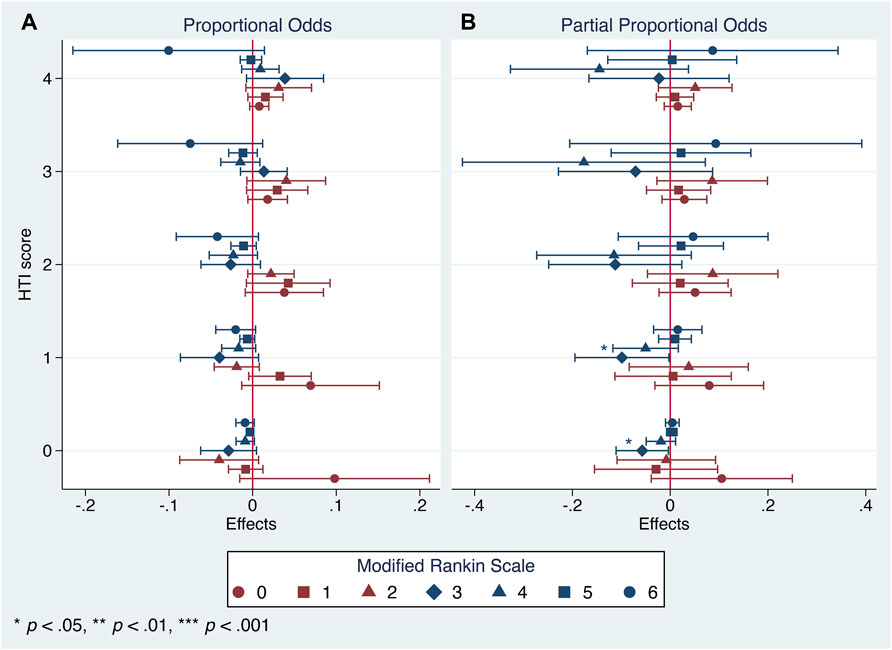
FIGURE 6. Generalized ordered logistic regression analysis: conditional marginal effects of the Cerebrolysin group on probability of varying mRS scores on day 90 by the HTI score with 95% CIs (n = 238). (A). Proportional odds assumption. (B). Partial proportional odds assumption.
Although the applied statistical approaches did establish some clinical variability in Cerebrolysin treatment effects among patients with varying HT risk, their heterogeneity was confirmed using methods of meta-analysis. The subgroup of patients with HTI = 4 was omitted during the procedure due to missing values in the Cerebrolysin arm (Table 3).
The overall and subgroup direction and size of Cerebrolysin treatment effects on HT and functional outcome did not differ much between the general population (the random-effects model) and studied cohort (the fixed-effects model) and were coherent with the results of the previous tests. By the way, no small-study effects, the phenomenon that smaller subgroups (“studies”) showed different treatment effects than large ones, were found in any model of interest (Egger test, p > 0.05). Heterogeneity of Cerebrolysin treatment effects turned out to be moderate (I2, 35.8%–56.7%; H2, 1.56–2.31) and mild (I2, 10.9%; H2, 1.12) for symptomatic and any HT, respectively. However, the approach was unrevealing for functional outcome (Figures 7, 8).
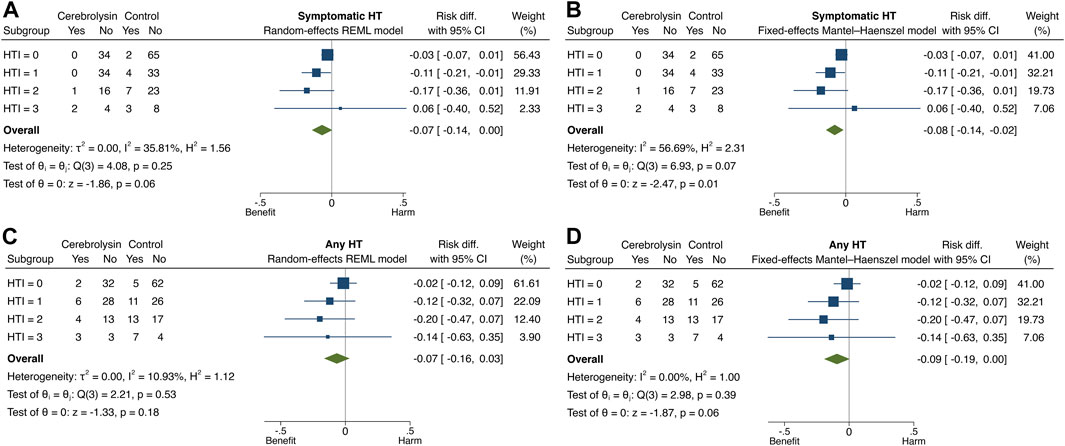
FIGURE 7. Forest plot and heterogeneity statistics of Cerebrolysin treatment effects on HT (n = 236). (A). Symptomatic HT, random-effects restricted maximum likelihood (REML) model. (B). Symptomatic HT, fixed-effects Mantel–Haenszel model. (C). Any HT, random-effects REML model. (D). Any HT, fixed-effects Mantel–Haenszel model.
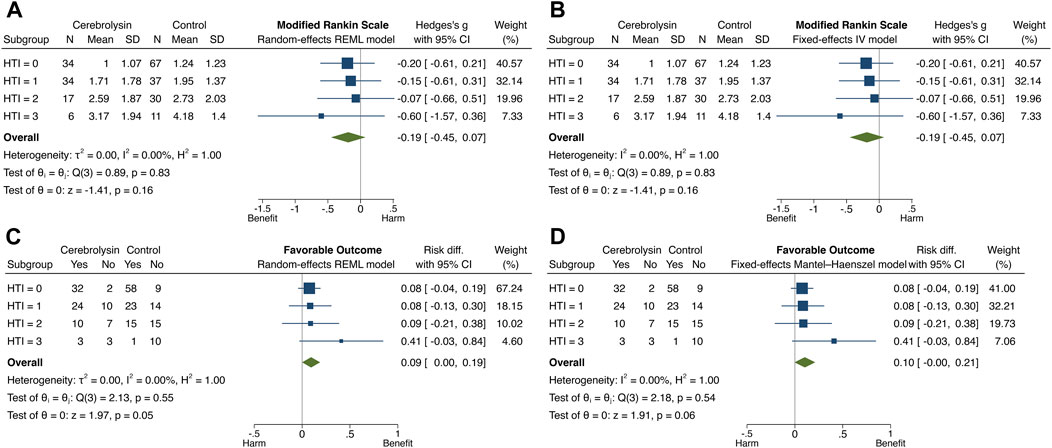
FIGURE 8. Forest plot and heterogeneity statistics of Cerebrolysin treatment effects on functional outcome (n = 236). (A). Modified Rankin Scale (mRS), random-effects restricted maximum likelihood (REML) model. (B). mRS, fixed-effects inverse-variance (IV) model. (C). Favorable functional outcome (FFO), random-effects REML model. (D). FFO, fixed-effects Mantel–Haenszel model.
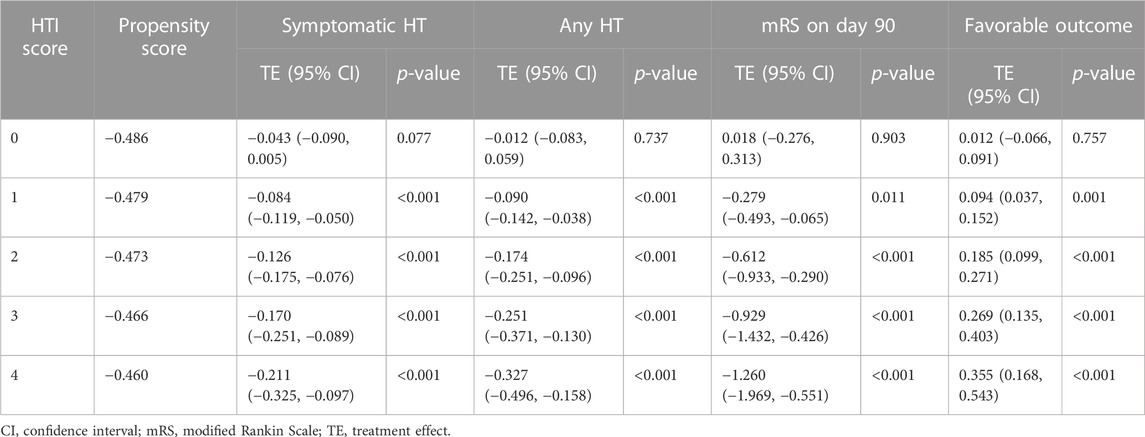
Nevertheless, HTE analysis by means of the matching-smoothing method demonstrated a significant positive impact of the Cerebrolysin treatment on HT and functional outcome: it was neutral when the risk of HT and poor functional outcome was low (HTI = 0) but gradually became evident in the moderate (HTI = 1) and high (HTI ≥2) HT risk patients. Largely, Cerebrolysin HTEs appeared in a linear-slope manner (Figure 9).
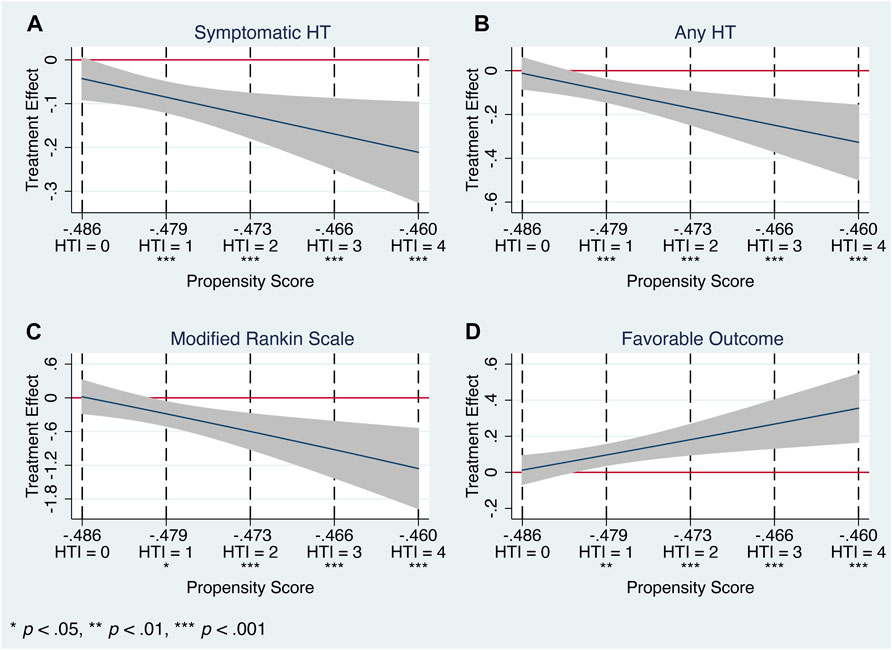
FIGURE 9. Heterogeneous treatment effects of Cerebrolysin with 95% CIs using the matching-smoothing method (n = 238). A local polynomial fit of degree 1 (local-linear smoothing) is used. Each HTI score is matched to the corresponding propensity score with a dashed vertical line. (A). Symptomatic HT. (B). Any HT. (C). Modified Rankin Scale on day 90. (D). Favorable functional outcome.
In particular, there was a steady decline in the rate of symptomatic (HTI = 0 vs. HTI = 4: by 4.3%, p = 0.077 vs. 21.1%, p < 0.001, respectively) and any HT (HTI = 0 vs. HTI = 4: by 1.2%, p = 0.737 vs. 32.7%, p < 0.001, respectively). Likewise, Cerebrolysin treatment resulted in an overall reduction of the mRS score, which was significantly greater with increasing HTI scores (HTI = 0 vs. HTI = 4: by 1.8%, p = 0.903 vs. 126%, p < 0.001, respectively). Reciprocally, a growing fraction of patients with FFO (HTI = 0 vs. HTI = 4: by 1.2% p = 0.757 vs. 35.5%, p < 0.001, respectively) was observed with climbing HTI scores (Table 5; Figure 9).
Thus, eligible for IVT patients with estimated on-admission HT risk of the moderate or high grade turned out to be the most responsive to the Cerebrolysin treatment.
Discussion
Using sophisticated statistical analysis, our post hoc study established clinically meaningful heterogeneity of Cerebrolysin treatment effects with respect to HT mitigation and functional outcome improvement among patients with varying HT risk. More importantly, the positive impact of the Cerebrolysin treatment grew steadily along with increasing HT risk. In general, the study results were coherent with the original report (Khasanova and Kalinin, 2023) and discovered a group of patients benefiting most by the proposed treatment.
Treatment effect refers to the causal effect of a treatment or intervention on an outcome of interest based on the counterfactuals (e.g., difference in outcomes with/without using the drug). HTE analysis focuses on assessing varying treatment effects for individuals or subgroups in a population (Gong et al., 2021).
Estimation of HTEs plays an essential role in randomized clinical trials, in particular, to identify patients who benefit most by the intervention for developing personalized treatment (Imai and Ratkovic, 2013; Ling et al., 2023). Meanwhile, traditional statistical approaches like descriptive statistics and regression analysis have a strong tendency to focus on the significance of the estimated overall ATE rather than systematically evaluate the variation in treatment effects across subgroups. As a result, there has been the standard practice to report a number of single estimates representing the treatment efficacy (Imai and Strauss, 2011).
HTEs are usually examined with a predictive model for individual outcomes followed by the exploration of interactions between treatment allocation and important patients’ baseline characteristics. In contrast, our HTE analysis was in favor of the two-stage approach (Watson and Holmes, 2020) since it allowed us to avoid overfitting and transformations of the outcome measurements.
Moreover, assessment of HTEs could especially be useful in settings in which univariate subgroup analyses are unrevealing (Raghavan et al., 2022). For instance, heterogeneity of treatment effects alone may be considered as a sufficient explanation for negative results in studies of some neuroprotective agents because even in the largest trials sample size is inadequate to detect effect size equivalent to those with IVT (Samsa and Matchar, 2001; Muir, 2002). Indeed, various regression approaches failed to provide robust estimates of the functional outcome improvement in our study, whereas HTE analysis did unambiguously confirm those patterns.
However, HTE analysis has not been widely used despite the fact that treatment effects are rarely perfectly homogeneous over the population. Perhaps, part of the reason is the complexity of its methods (Gong et al., 2021). Indeed, only few studies addressing HTEs in stroke patients have been published by now (Kent et al., 2001; Kent et al., 2021; Zhou et al., 2022; Ngufor et al., 2023; Zhu et al., 2023), but none of them related to neuroprotection. Therefore, we have striven to adhere meticulously to the proposed guidelines (Gabler et al., 2009) to report the results of our HTE analysis.
A number of tools have been suggested to predict HT in stroke patients (Kalinin et al., 2017). Although many of them are reliable and composed of the same set of predictors with some variations, only the HTI score takes into account a vascular territory of the infarcted brain lesions. This could be a reason why the HTI score outperformed the DRAGON and SEDAN scores in HT risk stratification in MCA stroke patients.
There are numerous well-established differences between stroke in the posterior and anterior circulation (Libman et al., 2001; Sarikaya et al., 2011; Merwick and Werring, 2014), which could be a source of additional heterogeneity of treatment effects. Moreover, some HT risk assessment tools turned out to be of little value in patients with posterior circulation stroke (Sung et al., 2013). Therefore, only MCA stroke patients were included in the study because we considered them to a certain extent as a homogeneous cohort from the anatomical point of view.
Permeability–surface area product (PS) is a known perfusion imaging marker of BBB permeability and an independent HT predictor. The HTI score is able to predict not only the HT probability but also brain perfusion data, in particular, PS values. The more PS rises in the infarct core following stroke, the higher the HTI score, and hence, the higher the HT risk (Kalinin et al., 2019).
Moreover, rtPA increases the HT rate by degrading the BBB integrity and promoting neuroinflammation and excitotoxicity (Wang et al., 2004; Kelly et al., 2006; Guan et al., 2019). On the other hand, Cerebrolysin ameliorates rtPA adverse effects (Veinbergs et al., 2000; Teng et al., 2021; Guan et al., 2019), and our original report has confirmed neuroprotective and BBB stabilizing features of Cerebrolysin in clinical settings: an improvement in the diffusion-tensor imaging metrics and PS was observed in the infarcted lesions (Khasanova and Kalinin, 2023). Therefore, benefiting most by the Cerebrolysin treatment in HT-risky circumstances could be explained by more efficient salvage of the ischemic brain tissue as well as more prominent attenuation of BBB permeability and mitigation of rtPA adverse effects with the Cerebrolysin use.
Based on high-quality evidence, current clinical guidelines strongly recommend IVT for patients with acute ischemic stroke of <4.5 h duration (Berge et al., 2021). Since the earliest publications of systematic reviews and meta-analyses on IVT-related HT, a paradigm that no reliable method can provide the individualization of treatment according to predicted HT risk has dominated (Whiteley et al., 2012), which practically makes all available prediction tools useless. As a result, prior HT risk assessment is not even required to start IVT in stroke patients. In that respect, our research is one of the first steps to shift that paradigm.
Several clinical studies have investigated the concomitant use of various agents, which reduce the HT rate and exert multimodal effects, alongside IVT. However, phase III clinical trials are required to confirm the observed positive results (Otsu et al., 2020). The ESCAPE-NA1 trial, a large-scale study of the neuroprotective agent nerinetide, failed to demonstrate an improvement in functional outcome due to a possible drug-drug interaction with alteplase (Hill et al., 2020). In that respect, Cerebrolysin could be an alternative to nerinetide and other candidates given its ability to mitigate rtPA-related adverse effects, well-established safety profile and long-lasting use in clinical practice worldwide.
Clinical practitioners frequently encounter so called the risk-treatment paradox, in which patients at the highest risk (and with the greatest potential to gain from the treatment) are treated less often than those at lower risk and with less potential to benefit (Spertus et al., 2015). In that respect, our study is another step towards integrating individualized risk stratification within routine clinical practice to disregard this misconception, to remind clinicians of each patient’s potential benefits from the treatment and to ensure more patient-orientated, evidence-based care with favorable outcomes.
The strength of the study emerged from stratification of stroke patients according to their HT risk followed by a complex statistical approach to analyze heterogeneity of Cerebrolysin treatment effects.
Nevertheless, there are a few limitations in our study. First, an anatomical restriction: only MCA stroke patients were selected for analysis. Heterogeneity of treatment effects in subjects with vertebrobasilar infarction can significantly affect the outcome of interest and requires a separate subanalysis.
Second, an incomplete range of the HTI score: due to study exclusion criteria, the population with an HTI score of ≤4 was only analyzed. Obviously, patients with an HTI score beyond this limit would fall into a category of extremely high risk of HT and would have a large infarct core and severe stroke. In those circumstances, IVT might be considered in some selected cases based on the results of advanced brain imaging (core/perfusion mismatch) and other contraindications (Berge et al., 2021). In that respect, a clinical trial on patients with severe stroke and futile recanalization after IVT has suggested Cerebrolysin as an add-on to IVT is safe and reduces the HT and mortality rate (Poljakovic et al., 2021). Moreover, recent clinical trials on mechanical endovascular thrombectomy (EVT) in patients with a large infarct core (Yoshimura et al., 2022; Huo et al., 2023; Sarraj et al., 2023) have shown a significant improvement in functional outcome compared with the standard medical care alone despite an increased HT rate in the intervention group. Thus, it is fairly reasonable to assume the Cerebrolysin treatment combined with reperfusion therapy could result in a reduction of the HT rate and further enhancement of functional outcome in those patients.
At last, post hoc analyses inherently suffer from a well-known statistical problem: the subgroups are formed after the trial is conducted, and such analyses bear a risk of finding statistically significant results when no true relationship exists (Imai and Strauss, 2011). In that respect, there are a number of challenges related to researcher bias in secondary data analysis such as prior knowledge of data, non-hypothesis-driven research, inappropriateness and lack of flexibility in data analysis (Baldwin et al., 2022). Therefore, a variety of approaches were applied to address this issue (Table 6). Yet, further research with prespecified HT risk subgroups is required.
Recommendations for clinical practice
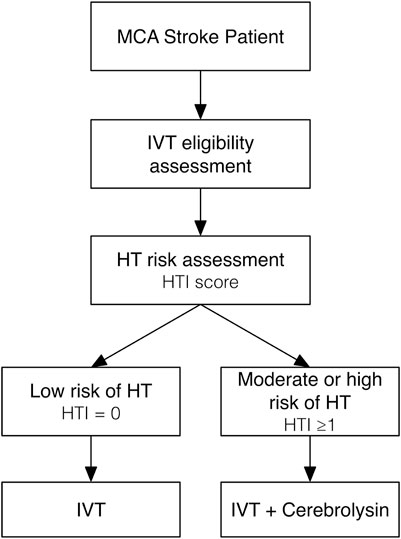
Based on the results of our original research and post hoc analysis, recommendations for clinical practice can be proposed as follows (Figure 10):
1. All patients with clinical and imaging data of acute MCA ischemic stroke of <4.5 h duration should be checked against eligibility criteria for IVT.
2. The HT risk assessment with prediction tools like the HTI score should be performed before starting IVT.
3. For IVT-eligible patients with the low (HTI = 0) risk of HT, IVT alone may be considered.
4. For IVT-eligible patients with the moderate (HTI = 1) or high (HTI ≥2) risk of HT, IVT with a concurrent add-on of a neuroprotective agent like Cerebrolysin (intravenous infusion of 30 mL in 100 mL of normal saline via separate line over 20 min) is strongly recommended to prevent early HT.
5. Following IVT, treatment with a neuroprotective agent like Cerebrolysin (the same dose once daily) might be continued for as long as 14 consecutive days to prevent delayed HT and to further enhance functional recovery.
Future research directions
Nowadays mechanical EVT has become the standard reperfusion therapy for acute ischemic stroke due to large vessel occlusion in the anterior circulation (Powers et al., 2019). Besides early-window (<6 h) reperfusion, EVT is being extensively studied in other clinical settings: prior IVT bridging, late-window (6–24 h) and very late-window (>24 h) time frame, a large infarct core, and subsequent futile recanalization (Manning et al., 2018; Lin et al., 2022; Kobeissi et al., 2023; Sattari et al., 2023; Shen et al., 2023). However, reperfusion injury and BBB disruption leading to HT and poor outcome inevitably occur in some patients. Future research on Cerebrolysin along with EVT and prior HT risk assessment is required in those clinical scenarios. As the first step, an ongoing clinical trial, the efficacy of Cerebrolysin treatment as an add-on therapy to mechanical thrombectomy in patients with acute ischemic stroke due to large vessel occlusion, has already set the rate of symptomatic HT as a secondary endpoint (Staszewski et al., 2022).
In posterior circulation stroke, the HT risk after IVT is half that of anterior one, with similar functional outcomes and higher risk of death, acknowledging limitations of the NIHSS for stroke severity or infarct size adjustment (Sarikaya et al., 2011; Keselman et al., 2020). On the other hand, EVT increases the HT rate but significantly decreases the risk of 90-day mortality (Dong et al., 2022; Xu et al., 2023). Therefore, more research is required to assess the HT risk and the use of Cerebrolysin alongside reperfusion therapy in those clinical settings.
Finally, COVID-19 has had an adverse impact on acute ischemic stroke patients who undergo reperfusion therapy, leading to an elevated risk of HT, higher mortality and lower likelihood of FFO (Stuckart et al., 2023). Hence, further studies can highlight an impact of Cerebrolysin combined with reperfusion therapy in COVID-19 patients.
Conclusion
Clinically meaningful HTEs of Cerebrolysin as an early add-on to reperfusion therapy were established in patients with varying HT risk. In terms of FFO achievement and HT rate reduction after IVT, the Cerebrolysin treatment appeared to be beneficial in those whose estimated on-admission HT risk was either moderate or high. The evidence is sufficient to guide different treatment recommendations in one or more subgroups but warrants future research with prespecified hypotheses.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The requirement of ethical approval was waived by the Local Ethics Committee of the Interregional Clinical Diagnostic Center, Kazan, Russia for the studies involving humans because the current study represented post hoc analysis of the original research, and it did not require a separate ethical approval. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board also waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the current study represented post hoc analysis of the original research, and it did not require to obtain separate written informed consent.
Author contributions
MK: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing–original draft, Writing–review and editing. DK: Conceptualization, Formal Analysis, Methodology, Supervision, Validation, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1288718/full#supplementary-material
Abbreviations
ASPECTS, Alberta Stroke Program Early Computed Tomography Score; ATC, average treatment effects on the untreated; ATE, average treatment effects; ATT, average treatment effects on the treated; BBB, blood-brain barrier; CI, confidence interval; DRAGON, (hyper)Dense cerebral artery sign/early infarct signs on admission computed tomography scan, prestroke modified RAnkin scale, Glucose level at baseline, Onset-to-treatment time, baseline NIHSS; EVT, endovascular thrombectomy; FFO, favorable functional outcome; HMCA, hyperdense middle cerebral artery; HT, hemorrhagic transformation; HTE, heterogeneous treatment effect; HTI, Hemorrhagic Transformation Index; IQR, interquartile range; IVT, intravenous thrombolysis; M, median; MCA, middle cerebral artery; mRS, modified Rankin Scale; NATE, naive average treatment effects; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; PS, permeability–surface area product; rtPA, recombinant tissue plasminogen activator; SEDAN, baseline blood Sugar, Early infarct signs, (hyper)Dense cerebral artery sign, Age, NIHSS on admission.
References
Andrade, J. B. C., Mohr, J. P., Ahmad, M., Lima, F. O., Barros, L. C. M., and Silva, G. S. (2022). Accuracy of predictive scores of hemorrhagic transformation in patients with acute ischemic stroke. Arq. Neuropsiquiatr. 80 (5), 455–461. doi:10.1590/0004-282X-ANP-2021-0091
Baldwin, J. R., Pingault, J. B., Schoeler, T., Sallis, H. M., and Munafò, M. R. (2022). Protecting against researcher bias in secondary data analysis: challenges and potential solutions. Eur. J. Epidemiol. 37 (1), 1–10. doi:10.1007/s10654-021-00839-0
Beghi, E., Binder, H., Birle, C., Bornstein, N., Diserens, K., Groppa, S., et al. (2021). European Academy of Neurology and European Federation of Neurorehabilitation Societies guideline on pharmacological support in early motor rehabilitation after acute ischaemic stroke. Eur. J. Neurol. 28 (9), 2831–2845. doi:10.1111/ene.14936
Berge, E., Whiteley, W., Audebert, H., De Marchis, G. M., Fonseca, A. C., Padiglioni, C., et al. (2021). European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur. Stroke J. 6 (1), I–LXII. doi:10.1177/2396987321989865
Bornstein, N. M., Guekht, A., Vester, J., Heiss, W. D., Gusev, E., Hömberg, V., et al. (2018). Safety and efficacy of Cerebrolysin in early post-stroke recovery: a meta-analysis of nine randomized clinical trials. Neurol. Sci. 39 (4), 629–640. doi:10.1007/s10072-017-3214-0
Cui, S., Chen, N., Yang, M., Guo, J., Zhou, M., Zhu, C., et al. (2019). Cerebrolysin for vascular dementia. Cochrane Database Syst. Rev. 2019 (11), CD008900. doi:10.1002/14651858.CD008900.pub3
Dong, S., Li, Y., Guo, J., Luo, Y., Fang, J., Tang, L., et al. (2022). Endovascular treatment combined with standard medical treatment improves outcomes of posterior circulation stroke: a systematic review and meta-analysis. Front. Neurol. 13, 694418. doi:10.3389/fneur.2022.694418
Fu, C. H., Chen, C. H., Lin, C. H., Lee, C. W., Lee, M., Tang, S. C., et al. (2022). Comparison of risk scores in predicting symptomatic intracerebral hemorrhage after endovascular thrombectomy. J. Formos. Med. Assoc. 121 (7), 1257–1265. doi:10.1016/j.jfma.2021.09.005
Gabler, N. B., Duan, N., Liao, D., Elmore, J. G., Ganiats, T. G., and Kravitz, R. L. (2009). Dealing with heterogeneity of treatment effects: is the literature up to the challenge? Trials 10, 43. doi:10.1186/1745-6215-10-43
Gong, X., Hu, M., Basu, M., and Zhao, L. (2021). Heterogeneous treatment effect analysis based on machine-learning methodology. CPT Pharmacometrics Syst. Pharmacol. 10 (11), 1433–1443. doi:10.1002/psp4.12715
Guan, X., Wang, Y., Kai, G., Zhao, S., Huang, T., Li, Y., et al. (2019). Cerebrolysin ameliorates focal cerebral ischemia injury through neuroinflammatory inhibition via CREB/PGC-1α pathway. Front. Pharmacol. 10, 1245. doi:10.3389/fphar.2019.01245
Guekht, A., Vester, J., Heiss, W. D., Gusev, E., Hoemberg, V., Rahlfs, V. W., et al. (2017). Safety and efficacy of Cerebrolysin in motor function recovery after stroke: a meta-analysis of the CARS trials. Neurol. Sci. 38 (10), 1761–1769. doi:10.1007/s10072-017-3037-z
Hacke, W., Kaste, M., Bluhmki, E., Brozman, M., Dávalos, A., Guidetti, D., et al. (2008). Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 359 (13), 1317–1329. doi:10.1056/NEJMoa0804656
Hill, M. D., Goyal, M., Menon, B. K., Nogueira, R. G., McTaggart, R. A., Demchuk, A. M., et al. (2020). Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet 395 (10227), 878–887. doi:10.1016/S0140-6736(20)30258-0
Huo, X., Ma, G., Tong, X., Zhang, X., Pan, Y., Nguyen, T. N., et al. (2023). Trial of endovascular therapy for acute ischemic stroke with large infarct. N. Engl. J. Med. 388 (14), 1272–1283. doi:10.1056/NEJMoa2213379
Imai, K., and Ratkovic, M. (2013). Estimating treatment effect heterogeneity in randomized program evaluation. Ann. Appl. Stat. 7 (1), 443–470. doi:10.1214/12-aoas593
Imai, K., and Strauss, A. (2011). Estimation of heterogeneous treatment effects from randomized experiments, with application to the optimal planning of the get-out-the-vote campaign. Polit. Anal. 19 (1), 1–19. doi:10.1093/pan/mpq035
Jann, B. (2017). Statistical software components S458346. Chestnut Hill, Mass: Boston College Department of Economics. revised 19 Sep 2020. Available at https://ideas.repec.org/c/boc/bocode/s458346.html. kmatch: stata module for multivariate-distance and propensity-score matching, including entropy balancing, inverse probability weighting, (coarsened) exact matching, and regression adjustment.
Jarosz, K., Kojder, K., Andrzejewska, A., Solek-Pastuszka, J., and Jurczak, A. (2023). Cerebrolysin in patients with TBI: systematic review and meta-analysis. Brain Sci. 13 (3), 507. doi:10.3390/brainsci13030507
Kalinin, M. N., Khasanova, D. R., and Ibatullin, M. M. (2017). The hemorrhagic transformation index score: a prediction tool in middle cerebral artery ischemic stroke. BMC Neurol. 17, 177. doi:10.1186/s12883-017-0958-3
Kalinin, M. N., Khasanova, D. R., and Ibatullin, M. M. (2019). Kompleksnaia otsenka perfuzionnykh dannykh golovnogo mozga u bol’nykh s ostrym ishemicheskim insul’tom dlia prediktsii gemorragicheskoĭ transformatsii [A comprehensive assessment of brain perfusion data in patients with acute ischemic stroke for prediction of hemorrhagic transformation]. Zh Nevrol Psikhiatr Im S S Korsakova 119 (3. Vyp. 2), 24–36. Russian. doi:10.17116/jnevro201911903224
Kelly, M. A., Shuaib, A., and Todd, K. G. (2006). Matrix metalloproteinase activation and blood-brain barrier breakdown following thrombolysis. Exp. Neurol. 200, 38–49. doi:10.1016/j.expneurol.2006.01.032
Kent, D. M., Saver, J. L., Kasner, S. E., Nelson, J., Carroll, J. D., Chatellier, G., et al. (2021). Heterogeneity of treatment effects in an analysis of pooled individual patient data from randomized trials of device closure of patent foramen ovale after stroke. JAMA 326 (22), 2277–2286. doi:10.1001/jama.2021.20956
Kent, T. A., Soukup, V. M., and Fabian, R. H. (2001). Heterogeneity affecting outcome from acute stroke therapy: making reperfusion worse. Stroke 32 (10), 2318–2327. doi:10.1161/hs1001.096588
Keselman, B., Gdovinová, Z., Jatuzis, D., Melo, T. P. E., Vilionskis, A., Cavallo, R., et al. (2020). Safety and outcomes of intravenous thrombolysis in posterior versus anterior circulation stroke: results from the safe implementation of treatments in stroke registry and meta-analysis. Stroke 51 (3), 876–882. doi:10.1161/STROKEAHA.119.027071
Khasanova, D. R., and Kalinin, M. N. (2023). Cerebrolysin as an early add-on to reperfusion therapy: risk of hemorrhagic transformation after ischemic stroke (CEREHETIS), a prospective, randomized, multicenter pilot study. BMC Neurol. 23, 121. doi:10.1186/s12883-023-03159-w
Kobeissi, H., Ghozy, S., Adusumilli, G., Kadirvel, R., Brinjikji, W., Rabinstein, A. A., et al. (2023). Endovascular therapy for stroke presenting beyond 24 hours: a systematic review and meta-analysis. JAMA Netw. Open 6 (5), e2311768. doi:10.1001/jamanetworkopen.2023.11768
Lang, W., Stadler, C. H., Poljakovic, Z., and Fleet, D. (2013). A prospective, randomized, placebo-controlled, double-blind trial about safety and efficacy of combined treatment with alteplase (rt-PA) and Cerebrolysin in acute ischaemic hemispheric stroke. Int. J. Stroke 8 (2), 95–104. doi:10.1111/j.1747-4949.2012.00901.x
Libman, R. B., Kwiatkowski, T. G., Hansen, M. D., Clarke, W. R., Woolson, R. F., and Adams, H. P. (2001). Differences between anterior and posterior circulation stroke in TOAST. Cerebrovasc. Dis. 11 (4), 311–316. doi:10.1159/000047659
Lin, C. H., Saver, J. L., Ovbiagele, B., Huang, W. Y., and Lee, M. (2022). Endovascular thrombectomy without versus with intravenous thrombolysis in acute ischemic stroke: a non-inferiority meta-analysis of randomized clinical trials. J. Neurointerv Surg. 14 (3), 227–232. doi:10.1136/neurintsurg-2021-017667
Ling, Y., Upadhyaya, P., Chen, L., Jiang, X., and Kim, Y. (2023). Emulate randomized clinical trials using heterogeneous treatment effect estimation for personalized treatments: methodology review and benchmark. J. Biomed. Inf. 137, 104256. doi:10.1016/j.jbi.2022.104256
Manning, N. W., Warne, C. D., and Meyers, P. M. (2018). Reperfusion and clinical outcomes in acute ischemic stroke: systematic review and meta-analysis of the stent-retriever-based, early window endovascular stroke trials. Front. Neurol. 9, 301. doi:10.3389/fneur.2018.00301
Masliah, E., and Díez-Tejedor, E. (2012). The pharmacology of neurotrophic treatment with Cerebrolysin: brain protection and repair to counteract pathologies of acute and chronic neurological disorders. Drugs Today (Barc) 48 (Suppl. l), 3–24. doi:10.1358/dot.2012.48(Suppl.A).1739716
Merwick, Á., and Werring, D. (2014). Posterior circulation ischaemic stroke. BMJ 348; g3175. Erratum in: BMJ. 2014;348:g3773. doi:10.1136/bmj.g3175
Muir, K. W. (2002). Heterogeneity of stroke pathophysiology and neuroprotective clinical trial design. Stroke 33 (6), 1545–1550. doi:10.1161/01.str.0000018684.86293.ab
Ngufor, C., Yao, X., Inselman, J. W., Ross, J. S., Dhruva, S. S., Graham, D. J., et al. (2023). Identifying treatment heterogeneity in atrial fibrillation using a novel causal machine learning method. Am. Heart J. 260, 124–140. doi:10.1016/j.ahj.2023.02.015
Otsu, Y., Namekawa, M., Toriyabe, M., Ninomiya, I., Hatakeyama, M., Uemura, M., et al. (2020). Strategies to prevent hemorrhagic transformation after reperfusion therapies for acute ischemic stroke: a literature review. J. Neurol. Sci. 419, 117217. doi:10.1016/j.jns.2020.117217
Poljakovic, Z., Supe, S., Ljevak, J., Starcevic, K., Peric, I., Blazevic, N., et al. (2021). Efficacy and safety of Cerebrolysin after futile recanalisation therapy in patients with severe stroke. Clin. Neurol. Neurosurg. 207, 106767. doi:10.1016/j.clineuro.2021.106767
Powers, W. J., Rabinstein, A. A., Ackerson, T., Adeoye, O. M., Bambakidis, N. C., Becker, K., et al. (2019). Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke 50 (12), e344–e418. Erratum in: Stroke. 2019;50(12):e440-e441. doi:10.1161/STR.0000000000000211
Raghavan, S., Josey, K., Bahn, G., Reda, D., Basu, S., Berkowitz, S. A., et al. (2022). Generalizability of heterogeneous treatment effects based on causal forests applied to two randomized clinical trials of intensive glycemic control. Ann. Epidemiol. 65, 101–108. doi:10.1016/j.annepidem.2021.07.003
Samsa, G. P., and Matchar, D. B. (2001). Have randomized controlled trials of neuroprotective drugs been underpowered? An illustration of three statistical principles. Stroke 32 (3), 669–674. doi:10.1161/01.str.32.3.669
Sarikaya, H., Arnold, M., Engelter, S. T., Lyrer, P. A., Mattle, H. P., Georgiadis, D., et al. (2011). Outcomes of intravenous thrombolysis in posterior versus anterior circulation stroke. Stroke 42 (9), 2498–2502. doi:10.1161/STROKEAHA.110.607614
Sarraj, A., Hassan, A. E., Abraham, M. G., Ortega-Gutierrez, S., Kasner, S. E., Hussain, M. S., et al. (2023). Trial of endovascular thrombectomy for large ischemic strokes. N. Engl. J. Med. 388 (14), 1259–1271. doi:10.1056/NEJMoa2214403
Sattari, S. A., Antar, A., Sattari, A. R., Feghali, J., Hung, A., Lee, R. P., et al. (2023). Endovascular thrombectomy versus endovascular thrombectomy preceded by intravenous thrombolysis: a systematic review and meta-analysis. World Neurosurg. 177, 39–58. doi:10.1016/j.wneu.2023.05.033
Saver, J. L. (2011). Improving reperfusion therapy for acute ischaemic stroke. J. Thromb. Haemost. 9 (Suppl. 1), 333–343. doi:10.1111/j.1538-7836.2011.04371.x
Shen, H., Killingsworth, M. C., and Bhaskar, S. M. M. (2023). Comprehensive meta-analysis of futile recanalization in acute ischemic stroke patients undergoing endovascular thrombectomy: prevalence, factors, and clinical outcomes. Life (Basel) 13 (10), 1965. doi:10.3390/life13101965
Spertus, J. A., Decker, C., Gialde, E., Jones, P. G., McNulty, E. J., Bach, R., et al. (2015). Precision medicine to improve use of bleeding avoidance strategies and reduce bleeding in patients undergoing percutaneous coronary intervention: prospective cohort study before and after implementation of personalized bleeding risks. BMJ 350, h1302. doi:10.1136/bmj.h1302
Spronk, E., Sykes, G., Falcione, S., Munsterman, D., Joy, T., Kamtchum-Tatuene, J., et al. (2021). Hemorrhagic transformation in ischemic stroke and the role of inflammation. Front. Neurol. 12, 661955. doi:10.3389/fneur.2021.661955
Staszewski, J., Stȩpień, A., Piusińska-Macoch, R., Dȩbiec, A., Gniadek-Olejniczak, K., Frankowska, E., et al. (2022). Efficacy of cerebrolysin treatment as an add-on therapy to mechanical thrombectomy in patients with acute ischemic stroke due to large vessel occlusion: study protocol for a prospective, open label, single-center study with 12 Months of follow-up. Front. Neurol. 13, 910697. doi:10.3389/fneur.2022.910697
StataCorp (2021). Stata: release 17. Statistical software. College Station, TX: StataCorp LLC.Stata meta-analysis reference manual
Strbian, D., Engelter, S., Michel, P., Meretoja, A., Sekoranja, L., Ahlhelm, F. J., et al. (2012b). Symptomatic intracranial hemorrhage after stroke thrombolysis: the SEDAN score. Ann. Neurol. 71 (5), 634–641. doi:10.1002/ana.23546
Strbian, D., Meretoja, A., Ahlhelm, F. J., Pitkäniemi, J., Lyrer, P., Kaste, M., et al. (2012a). Predicting outcome of IV thrombolysis-treated ischemic stroke patients: the DRAGON score. Neurology 78 (6), 427–432. doi:10.1212/WNL.0b013e318245d2a9
Stuckart, I., Kabsha, A., Siepmann, T., Barlinn, K., and Barlinn, J. (2023). Intravenous thrombolysis and endovascular therapy for acute ischemic stroke in COVID-19: a systematic review and meta-analysis. Front. Neurol. 14, 1239953. doi:10.3389/fneur.2023.1239953
Sun, J., Lam, C., Christie, L., Blair, C., Li, X., Werdiger, F., et al. (2023). Risk factors of hemorrhagic transformation in acute ischaemic stroke: a systematic review and meta-analysis. Front. Neurol. 14, 1079205. doi:10.3389/fneur.2023.1079205
Sung, S. F., Chen, C. H., Chen, Y. W., Tseng, M. C., Shen, H. C., and Lin, H. J. (2013). Predicting symptomatic intracerebral hemorrhage after intravenous thrombolysis: stroke territory as a potential pitfall. J. Neurol. Sci. 335 (1-2), 96–100. doi:10.1016/j.jns.2013.08.036
Teng, H., Li, C., Zhang, Y., Lu, M., Chopp, M., Zhang, Z. G., et al. (2021). Therapeutic effect of Cerebrolysin on reducing impaired cerebral endothelial cell permeability. Neuroreport 32 (5), 359–366. doi:10.1097/WNR.0000000000001598
Veinbergs, I., Mante, M., Mallory, M., and Masliah, E. (2000). Neurotrophic effects of Cerebrolysin in animal models of excitotoxicity. J. Neural Transm. Suppl. 59, 273–280. doi:10.1007/978-3-7091-6781-6_29
Wang, X., Tsuji, K., Lee, S. R., Ning, M., Furie, K. L., Buchan, A. M., et al. (2004). Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke 35 (11 Suppl. 1), 2726–2730. doi:10.1161/01.STR.0000143219.16695.af
Watson, J. A., and Holmes, C. C. Graphing and reporting heterogeneous treatment effects through reference classes. Trials. 2020;21:386. Erratum in: Trials. 2020;21:865, doi:10.1186/s13063-020-04306-1
Whiteley, W. N., Slot, K. B., Fernandes, P., Sandercock, P., and Wardlaw, J. (2012). Risk factors for intracranial hemorrhage in acute ischemic stroke patients treated with recombinant tissue plasminogen activator: a systematic review and meta-analysis of 55 studies. Stroke 43 (11), 2904–2909. doi:10.1161/STROKEAHA.112.665331
Williams, R. (2006). Generalized ordered logit/partial proportional odds models for ordinal dependent variables. Stata J. 6 (1), 58–82. doi:10.1177/1536867x0600600104
Xie, Y., Brand, J. E., and Jann, B. (2012). Estimating heterogeneous treatment effects with observational data. Sociol. Methodol. 42 (1), 314–347. doi:10.1177/0081175012452652
Xu, J., Chen, X., Chen, S., Cao, W., Zhao, H., Ni, W., et al. (2023). Endovascular treatment for basilar artery occlusion: a meta-analysis. Stroke Vasc. Neurol. 8 (1), 1–3. doi:10.1136/svn-2022-001740
Yoshimura, S., Sakai, N., Yamagami, H., Uchida, K., Beppu, M., Toyoda, K., et al. (2022). Endovascular therapy for acute stroke with a large ischemic region. N. Engl. J. Med. 386 (14), 1303–1313. doi:10.1056/NEJMoa2118191
Zhang, C., Chopp, M., Cui, Y., Wang, L., Zhang, R., Zhang, L., et al. (2010). Cerebrolysin enhances neurogenesis in the ischemic brain and improves functional outcome after stroke. J. Neurosci. Res. 88 (15), 3275–3281. doi:10.1002/jnr.22495
Zhou, Y., Xing, P., Li, Z., Zhang, X., Zhang, L., Zhang, Y., et al. (2022). Effect of occlusion site on the safety and efficacy of intravenous alteplase before endovascular thrombectomy: a prespecified subgroup analysis of DIRECT-MT. Stroke 53 (1), 7–16. doi:10.1161/STROKEAHA.121.035267
Zhu, E., Chen, Z., Ai, P., Wang, J., Zhu, M., Xu, Z., et al. (2023). Analyzing and predicting the risk of death in stroke patients using machine learning. Front. Neurol. 14, 1096153. doi:10.3389/fneur.2023.1096153
Keywords: Cerebrolysin, hemorrhagic transformation, functional outcome, stroke, reperfusion therapy, alteplase, heterogeneous treatment effect
Citation: Kalinin MN and Khasanova DR (2024) Heterogeneous treatment effects of Cerebrolysin as an early add-on to reperfusion therapy: post hoc analysis of the CEREHETIS trial. Front. Pharmacol. 14:1288718. doi: 10.3389/fphar.2023.1288718
Received: 11 September 2023; Accepted: 11 December 2023;
Published: 05 January 2024.
Edited by:
Rui Tao, Florida Atlantic University, United StatesReviewed by:
Stefan Strilciuc, University of Medicine and Pharmacy Iuliu Hatieganu, RomaniaAngela Ruban, Tel Aviv University, Israel
Copyright © 2024 Kalinin and Khasanova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mikhail N. Kalinin, bmluaWxha0BnbWFpbC5jb20=
†ORCID: Mikhail N. Kalinin, orcid.org/0000-0002-3664-6888; Dina R. Khasanova, orcid.org/0000-0002-8825-2346
 Mikhail N. Kalinin
Mikhail N. Kalinin Dina R. Khasanova
Dina R. Khasanova