- 1Department of Dermatology, Peking University Binhai Hospital, Tianjin, China
- 2Hematology and Oncology, Peking University Binhai Hospital, Tianjin, China
- 3Central Laboratory, Peking University Binhai Hospital, Tianjin, China
- 4Department of Epidemiology and Biostatistics, School of Public Health, Peking University Health Science Centre, Beijing, China
Background: Multiple immune checkpoint inhibitors (ICIs) and targeted therapies have been widely used as adjuvant treatments for high-risk resected melanoma, with unclear comparative efficacy and safety.
Methods: PubMed, Embase, the Cochrane Library, and ClinicalTrials.gov were searched from database inception until 6 June 2023. We included RCTs that assess adjuvant ICIs or targeted therapies in high-risk resected melanoma. Frequentist random-effect network meta-analyses (NMA) were performed. The primary outcome was recurrence-free survival (RFS).
Results: Eleven trials including 10,712 patients and comparing 10 treatments (nivolumab [Nivo], ipilimumab 3 mg/kg [Ipi3], Ipi10, pembrolizumab [Pemb], vemurafenib [Vemu], bevacizumab [Beva], Nivo + Ipi1, Nivo + Ipi3, dabrafenib plus trametinib [Dab + Tram], and placebo/observation [Pla/Obs]) were included. NMA showed that all treatments showed RFS benefit over placebo/observation except Ipi3 (hazard ratio [HR], 0.78; 95% CI, 0.58–1.05). Combination therapy of Nivo + Ipi3 was the most effective treatment, which significantly improved RFS compared with other treatments. NMA also showed that all treatments were associated with an increased risk of grade 3-5 adverse events over placebo/observation except Nivo (HR, 1.25; 95% CI, 0.87–1.80). NMA suggested that Nivo and Pemb were the two safest treatments except for placebo/observation. Although three combination therapies ranked as the top three in terms of RFS, they did not show significant overall survival benefits compared to monotherapies including Pemb, Nivo, Ipi3, and Ipi10.
Conclusion: In this NMA, adjuvant Nivo and Pemb are the preferred options in patients with resected melanoma considering the benefits and harms. Combination therapy of Nivo + Ipi3 may be a promising strategy, but more evidence from phase 3 trials is needed.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=438667, PROSPERO (CRD42023438667).
1 Introduction
Melanoma is a major public health problem and is the leading cause of skin cancer death worldwide, and has steadily increased in recent years (Stege et al., 2021; Siegel et al., 2023). It is estimated that 97,610 new cases of melanoma and 7,990 deaths will occur in 2023 in the United States (Siegel et al., 2023). Surgical excision is the preferred treatment for early-stage melanoma, yet 40%–60% of patients with high-risk melanoma or nodal involvement will eventually experience loco-regional relapse or tumor progression (Stege et al., 2021). Immune Checkpoint inhibitors (ICIs) and targeted therapy were approved for the treatment of melanoma in the United States and Europe. Several therapies have been shown to improve outcomes of patients with unresectable metastatic melanoma when used as the initial treatment, including ipilimumab, nivolumab, pembrolizumab, and combinations of BRAF plus MEK inhibitors (dabrafenib plus trametinib) in randomized control trials (RCTs). (Hodi et al., 2010; Long et al., 2014; Robert et al., 2015; Hodi et al., 2016; Larkin et al., 2019). In recent years, these treatments have been employed in the adjuvant setting to prevent the recurrence of melanoma following surgical excision (Long et al., 2017; Eggermont et al., 2018a). BRIM8 was a phase 3 trial that compared adjuvant vemurafenib to placebo, which demonstrated a potential improvement in disease-free survival (DFS) and well-tolerated treatment for patients with resected BRAF mutation-positive melanoma (Maio et al., 2018) Bevacizumab, a monoclonal antibody that targets VEGF, has shown improvement in DFS, but not in overall survival (OS) for patients with high-risk resected melanoma (Corrie et al., 2018). However, the optimal treatment regimen for high-risk resected melanoma remains unclear. Thus, we systematically reviewed evidence from RCTs to evaluate the efficacy and safety of different ICIs and targeted therapies for resected melanoma.
2 Materials and methods
2.1 Data sources and searches
We searched for literature published on PubMed, Embase, Cochrane Library, and grey literature from the ClinicalTrials.gov website up to date 6 June 2023. The search strategies were developed and piloted by the review team for bibliographic databases and clinical trial registries using medical subject headings or Emtree and text words for “adjuvant”, “resect”, melanoma”, and “randomized controlled trials” (see Supplementary Material). We conducted this meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, (Moher et al., 2009), and the protocol was registered on PROSPERO (CRD42023438667).
2.2 Eligibility criteria
We included RCTs (phase 2/3) that assessed adjuvant ICIs or targeted therapies in high-risk (American Joint Committee on Cancer stage IIB-IV) resected melanoma. Patients diagnosed with stage IIB and IIC melanoma, even in the absence of identified lymph node involvement, face a higher risk of recurrence and melanoma-specific death compared to those with stage IIIA disease and a similar risk to those with stage IIIB disease. (Yushak et al., 2019). Our search was restricted to papers published in English. We excluded conference abstracts, ongoing trials, and studies that had insufficient data to analyze. Two reviewers independently screened the titles and abstracts of all identified records. We retrieved full-length records of those deemed eligible and screened these again to confirm inclusion. Disagreements were resolved by discussion, or with the help of a third reviewer when consensus could not be reached.
2.3 Outcomes
The primary outcome was RFS. Secondary outcomes were distant metastasis-free survival (DMFS), overall survival (OS), and grade 3-5 adverse events (AEs). In some studies, only disease-free survival or relapse-free survival was reported, which were considered equivalent to recurrence-free survival.
2.4 Data extraction and quality assessment
Two reviewers independently extracted data on the study characteristics, patient characteristics, interventions, comparisons, and outcomes from each study using a standardized, piloted form. Hazard ratios (HRs) with 95% Cis were extracted for survival data if available. When studies presented outcomes for various follow-up periods, we extracted data for the longest follow-up period. Both ipilimumab at 3 mg/kg and 10 mg/kg were approved therapies for melanoma, we separated them as two treatment regimens. Since the combination therapy of nivolumab plus ipilimumab 1 mg/kg once every 6 weeks probably has different outcomes compared to nivolumab plus ipilimumab 3 mg/kg once every 3 weeks, (Weber et al., 2023), the two combination therapies were analysed separately. The risk of bias of RCTs was assessed by the Cochrane Collaboration’s tool (RoB 2), (Sterne et al., 2019), which includes the following domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result.
3 Results
3.1 Characteristics of included studies
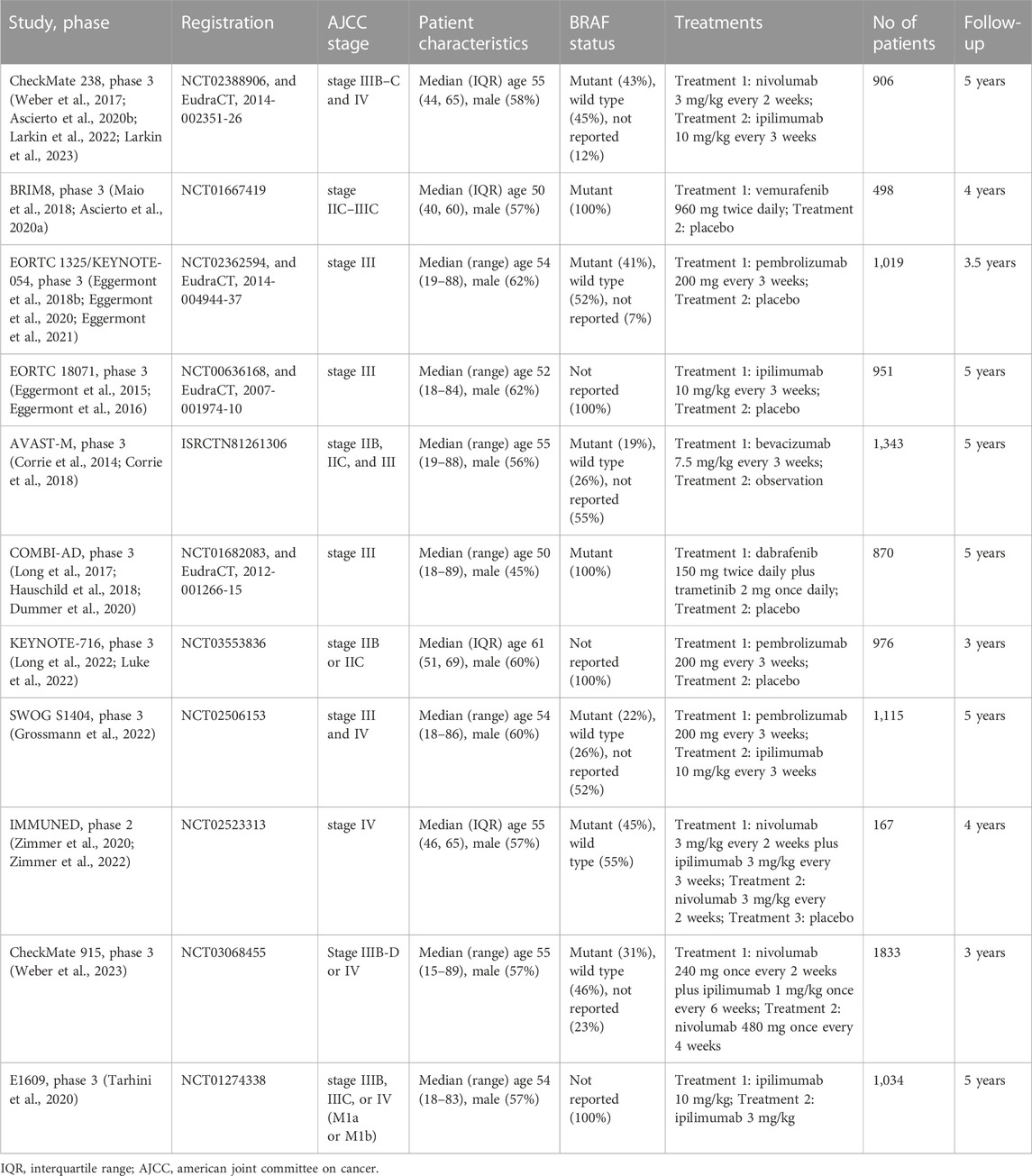
This systematic literature search initially identified 6,707 records, after excluding duplicates and irrelevant papers, 130 papers were evaluated in full text for eligibility (Supplementary Figure S1). Finally, 23 research papers originating from 11 RCTs enrolling 10,712 participants were included in this NMA. (Corrie et al., 2014; Eggermont et al., 2015; Eggermont et al., 2016; Long et al., 2017; Weber et al., 2017; Eggermont et al., 2018b; Corrie et al., 2018; Hauschild et al., 2018; Maio et al., 2018; Ascierto et al., 2020a; Ascierto et al., 2020b; Dummer et al., 2020; Eggermont et al., 2020; Tarhini et al., 2020; Zimmer et al., 2020; Eggermont et al., 2021; Grossmann et al., 2022; Larkin et al., 2022; Long et al., 2022; Luke et al., 2022; Zimmer et al., 2022; Larkin et al., 2023; Weber et al., 2023). Ten treatment strategies were analyzed, including nivolumab [Nivo], ipilimumab 3 mg/kg [Ipi3], Ipi10, pembrolizumab [Pemb], vemurafenib [Vemu], bevacizumab [Beva], Nivo + Ipi1, Nivo + Ipi3, dabrafenib plus trametinib [Dab + Tram], and placebo/observation [Pla/Obs]. The follow-up periods ranged from 3 to 5 years. Only one RCT was a phase 2 trial, (Zimmer et al., 2020), and the rest of included RCTs were phase 3 trials. One trial had observation as a control group compared with Beva. (Corrie et al., 2014). Five two-arm trials had placebo as a control group, with one compared with Vemu, (Maio et al., 2018), two compared with Pemb, (Eggermont et al., 2018b; Luke et al., 2022), one compared with Ipi10,28 and one compared with Dab + Tram. (Long et al., 2022). Three trials had Ipi10 as the control group compared with Nivo, Pemb, and Ipi3, respectively (Weber et al., 2017; Tarhini et al., 2020; Grossmann et al., 2022). One three-arm trial compared Nivo + Ipi3 with Nivo and placebo was included. (Zimmer et al., 2020). The remaining trial compared combination therapy of Nivo + Ipi1 with Nivo. (Weber et al., 2023). All trials were previously registered and provided registration numbers. Participants’ median age and proportion of men at baseline ranged from 50 to 61 years and from 45% to 62%, respectively. Characteristics of individual studies are summarised in Table 1.
3.2 Risk of bias
A qualitative assessment was performed by assessing various indicators for each individual trial using RoB 2. Eight of 11 trials were classified as low risk of bias, two trials had some concerns, and one trial was classified as high risk of bias owing to deviations from intended interventions. Three trials were open-label and the remaining 8 were double-blind. Risk of bias assessments in individual studies, including reasons, are listed in the characteristics of included studies in Supplementary Table S1.
3.3 Primary outcome
3.3.1 Recurrence-free survival
Eleven trials (10 treatments) including 10,712 participants reported RFS (Figure 1). In the NMA, we found that all treatments showed benefit over Pla/Obs except Ipi3 (HR, 0.78; 95% CI, 0.58–1.05) (Figure 2A). Treatment ranking probabilities suggested that Nivo + Ipi3 had the highest probability (P-score 0.997) of being the best treatment, which significantly improved RFS compared with other treatments. Compared with Ipi10, RFS benefit was demonstrated for Nivo + Ipi3 (HR, 0.31; 95% CI, 0.17–0.57), Dab + Tram (HR, 0.66; 95% CI, 0.53–0.83), Nivo + Ipi1 (HR, 0.67; 95% CI, 0.53–0.85), Nivo (HR, 0.73; 95% CI, 0.61–0.86), and Pemb (HR, 0.78; 95% CI, 0.66–0.92). There was no significant difference in RFS between Dab + Tram (P-score 0.791), Nivo + Ipi1 (P-score 0.784), Nivo (P-score 0.661), Pemb (P-score 0.569), and Vemu (P-score 0.479). There was no heterogeneity among treatments (I2 = 0%). We did not find substantial evidence of inconsistency between direct and indirect evidence.

FIGURE 1. Network plots of treatment comparisons. RFS, recurrence-free survival; OS, overall survival; DMFS, distant metastasis-free survival; Nivo+Ipi3, nivolumab plus ipilimumab 3 mg/kg; Dab+Tram, dabrafenib plus trametinib; Nivo+Ipi1, nivolumab plus ipilimumab 1 mg/kg; Nivo, nivolumab; Pemb, pembrolizumab; Vemu, vemurafenib; Ipi10, ipilimumab 10 mg/kg; Ipi3, ipilimumab 3 mg/kg; Beva, bevacizumab; Pla/Obs, placebo/observation.
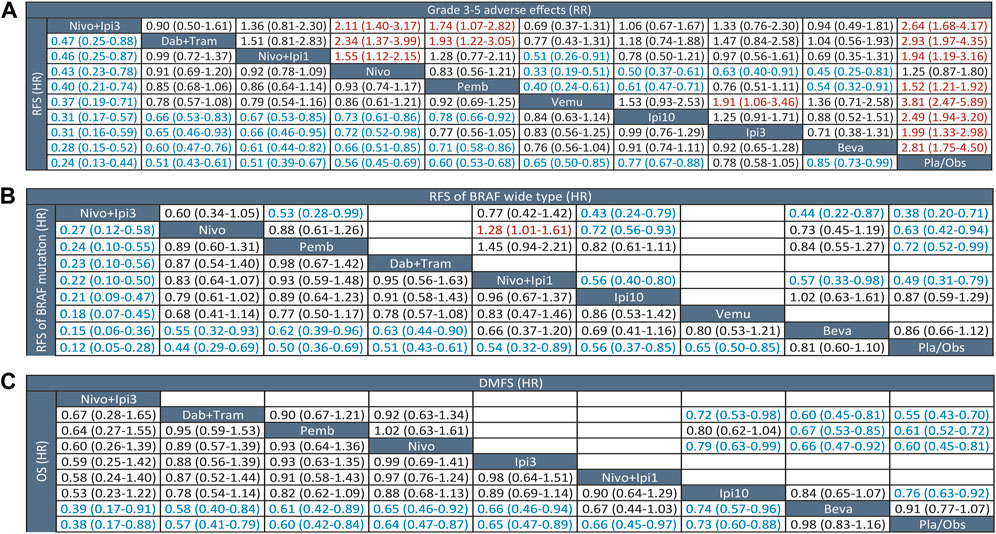
FIGURE 2. League tables of outcome analyses. (A) RFS (lower left) and grade 3-5 adverse effects (upper right). (B) RFS of BRAF mutation (lower left) and wide type (upper right). (C) OS (lower left) and DMFS (lower left). RFS, recurrence-free survival; OS, overall survival; DMFS, distant metastasis-free survival; Nivo+Ipi3, nivolumab plus ipilimumab 3 mg/kg; Dab+Tram, dabrafenib plus trametinib; Nivo+Ipi1, nivolumab plus ipilimumab 1 mg/kg; Nivo, nivolumab; Pemb, pembrolizumab; Vemu, vemurafenib; Ipi10, ipilimumab 10 mg/kg; Ipi3, ipilimumab 3 mg/kg; Beva, bevacizumab; Pla/Obs, placebo/observation.
3.3.2 Subgroup analysis
We performed a prespecified subgroup analysis for BRAF status. Eight trials (9 treatments) were included for patients with BRAF mutations, and NMA showed that all treatments showed benefit over Pla/Obs except Beva (HR, 0.81; 95% CI, 0.60–1.10) (Figure 2B). Nivo + Ipi3 was also the best treatment for mutant BRAF, which significantly improved RFS compared with other treatments. Only six trials (7 treatments) were included for patients with BRAF-wide type, and NMA showed that all treatments showed benefit over Pla/Obs except Beva and Ipi3 (Figure 2B).
3.4 Secondary outcomes
3.4.1 Distant metastasis-free survival
Only six trials (6 treatments) reported data on DMFS (Figure 1). All treatments showed DMFS as beneficial over Pla/Obs except Beva (HR, 0.91; 95% CI, 0.77–1.07) (Figure 2C). NMA showed that Dab + Tram (P-score 0.882) ranked first for DMFS, followed by Nivo (P-score 0.767), Pemb (P-score 0.731), Ipi10 (P-score 0.401), Beva (P-score 0.192), and Pla/Obs (P-score 0.026), and there was no significant difference between the top 3 treatments. There was no heterogeneity among treatments (I2 = 0%) and no significant inconsistency between direct and indirect evidence.
3.4.2 Overall survival
Eight trials (9 treatments) were included for OS (Figure 1). The results of NMA showed that all treatments showed OS benefit over Pla/Obs except Beva (Figure 2C). NMA also showed that Nivo + Ipi3 (P-score 0.900) ranked the best treatment, followed by Dab + Tram (P-score 0.722), Pemb (P-score 0.681), Nivo (P-score 0.592), Ipi3 (P-score 0.573), Nivo + Ipi1 (P-score 0.534), Ipi10 (P-score 0.357), Beva (P-score 0.085), and Pla/Obs (P-score 0.056), but there were no significant differences between the first 7 treatments. No heterogeneity (I2 = 0%) and significant inconsistency was found.
3.4.3 Grade 3-5 adverse events
Eleven trials (10 treatments) including 10,712 participants reported data of grade 3–5 AEs (Figure 1). In the NMA, we found that all treatments were associated with an increased risk of grade 3–5 AEs over Pla/Obs except Nivo (RR, 1.25; 95% CI, 0.87–1.80) (Figure 2A). Treatment ranking probabilities suggested that Nivo (P-score 0.880) and Pemb (P-score 0.763) were the two safest treatments except Pla/Obs. There was no significant difference in AEs between Nivo + Ipi1 (P-score 0.577), Ipi3 (P-score 0.565), Ipi10 (P-score 0.356), Nivo + Ipi3 (P-score 0.307), Beva (P-score 0.270), and Dab + Tram (P-score 0.229). In addition, Vemu (P-score 0.064) ranked the worst treatment. There was moderate heterogeneity among treatments (I2 = 63.2%), but no significant inconsistency between direct and indirect evidence was found.
4 Discussion
This systematic review and NMA included 11 RCTs (10 treatments) involving 10,712 patients and provided an overview of efficacy and safety outcomes of different treatment strategies in patients with high-risk resected melanoma. We found that the combination therapy of Nivo + Ipi3 ranks highest in terms of RFS and OS, and demonstrates superior RFS compared with other treatments. In addition, Nivo is found to have the lowest risk of grade 3–5 AEs and shows significant benefit compared to other treatment strategies except for Pemb. Meanwhile, both Nivo and Pemb are only inferior to Nivo + Ipi3 in terms of RFS. Although combination therapies including Nivo + Ipi3, Dab + Tram, and Nivo + Ipi1 rank as the top three treatments in terms of RFS, they do not show a significant difference in OS compared to monotherapies including Pemb, Nivo, Ipi3, and Ipi10. Furthermore, Vemu has been found to be the least tolerable treatment with the highest risk of grade 3–5 AEs, and Beva ranks last in terms of RFS, DMFS, and OS.
This is the latest and comprehensive NMA to investigate the efficacy and safety of ICIs and targeted therapy in adjuvant treatment of high-risk resected melanoma depending on evidence from RCTs. This review has several strengths. Firstly, the NMA followed the PRISMA guidelines and had a protocol registered in PROSPERO. We conducted a comprehensive search of multiple databases and included all available RCTs of ICIs and targeted therapy for patients with resected melanoma. Secondly, the risk of bias for included trials was assessed using a valid methodological tool. Thirdly, we have performed a comprehensive analysis including three efficacy outcomes (RFS, DMFS, and OS), subgroups of BRAF status (mutation or wide type), and safety outcome (grade 3–5 AEs) to facilitate optimal evidence-informed decision-making for people and clinicians regarding adjunct treatment strategies for resected melanoma.
One NMA of six trials published in Chinese had previously examined ICIs and targeted therapy for resected melanoma, (Zhang et al., 2022), and its results on RFS were consistent with our review. However, our study included a greater number of trials and treatment strategies and provided more comprehensive insights into DMFS, OS, and safety. Another NMA comparing adjuvant Nivo to other treatments in adults with resected melanoma concluded that Nivo provides an effective treatment option with a promising risk-benefit profile. (Toor et al., 2021). However, the literature search for the previous review was conducted in 2019, and some of the latest RCTs involving ICIs and targeted therapy were not included in the assessment. Furthermore, Ba and colleagues analyzed the efficacy and tolerability of adjuvant therapy for resected high-risk stage III-IV cutaneous melanoma, and the results suggested that Nivo and Pemb seem to be preferable adjuvant therapies. (Ba et al., 2023). However, it should be noted that most of the studies included in the analysis did not involve current ICIs or targeted therapy, and many of the trials were open-label and conducted in the early stages of research.
Although we compared grade 3–5 AEs between different therapies, the most common adverse events were different in these therapies. For example, fatigue and maculopapular rash were the most common events among patients treated with pembrolizumab, while fatigue and diarrhea were most common among patients treated with ipilimumab in the SWOG S1404 trial. In the COMBI-AD trial, comprising dabrafenib plus trametinib, the most common events were pyrexia, fatigue, and nausea.
Although we compared grade 3–5 AEs among different therapies, the most common events varied across these treatments. For instance, among patients treated with pembrolizumab, fatigue, and maculopapular rash were the most common events, whereas fatigue and diarrhea were more prevalent among patients treated with ipilimumab in the SWOG S1404 trial. (Grossmann et al., 2022). In the COMBI-AD trial, the dabrafenib plus trametinib group reported pyrexia, fatigue, and nausea as the most common events. (Long et al., 2017).
In the IMMUNED trial, combination therapy of Nivo + Ipi demonstrated superiority in terms of RFS compared to Nivo monotherapy. (Zimmer et al., 2020; Zimmer et al., 2022). However, in the CheckMate 915 trial, (Weber et al., 2023), this superiority was not observed, which may be due to the lower and less frequent dosage of Ipi used (83% lower exposure). Of note, the IMMUNED trial was the only phase 2 trial included, with only 167 patients enrolled, which is much smaller than other trials. Therefore, future large-scale phase 3 trials of Nivo + Ipi3 in resected melanoma are needed to further investigate its efficacy and safety.
4.1 Limitations
This study has several limitations. First, the number of enrolled trials is insufficient, and most of the evidence was derived from indirect comparisons due to the nature of the network analysis. Second, some trials did not report data on DMFS, OS, and BRAF status, which limited our comprehensive analysis of these outcomes due to missing data. Third, moderate heterogeneity was present among the included trials for grade 3–5 AEs. Finally, our analysis was based on study-level data rather than individual patient data, which limited the power of our analysis. Despite these limitations, we believe that our study provides the most comprehensive and up-to-date analysis of ICIs and targeted therapy in the adjuvant treatment of resected melanoma.
5 Conclusion
This systematic review and NMA identified 11 RCTs with 10,712 patients with high-risk resected melanoma. This study found that Nivo and Pemb are the preferred options in patients with resected melanoma considering the benefits and harms. Combination therapy of Nivo + Ipi3 showed the most significant improvement in RFS, but the risk of AEs was higher than Nivo and Pemb, highlighting the need for additional evidence from phase 3 trials. Additionally, although combination therapy showed a favorable improvement in RFS compared to monotherapy, this improvement did not translate into an OS benefit based on current evidence. Furthermore, Vemu is not recommended due to its poor tolerability, and Beva is not preferred due to its lower efficacy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
BZ conceived and designed the study. FS and YY undertook the literature review and extracted the data. BZ coded the statistical analysis, figures, and appendix. FS and YY interpreted the data and wrote the first draft of the manuscript. All authors reviewed and revised subsequent drafts and approved the final version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1284240/full#supplementary-material
References
Ascierto, P. A., Del Vecchio, M., Mandala, M., Gogas, H., Arance, A. M., Dalle, S., et al. (2020b). Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 21 (11), 1465–1477. doi:10.1016/S1470-2045(20)30494-0
Ascierto, P. A., Lewis, K. D., Di Giacomo, A. M., Demidov, L., Mandalà, M., Bondarenko, I., et al. (2020a). Prognostic impact of baseline tumour immune infiltrate on disease-free survival in patients with completely resected, BRAF(v600) mutation-positive melanoma receiving adjuvant vemurafenib. Ann. Oncol. 31 (1), 153–159. doi:10.1016/j.annonc.2019.10.002
Ba, H., Zhu, F., Zhang, X., Mei, Z., and Zhu, Y. (2023). Comparison of efficacy and tolerability of adjuvant therapy for resected high-risk stage III-IV cutaneous melanoma: a systemic review and Bayesian network meta-analysis. Ther. Adv. Med. Oncol. 15, 17588359221148918. doi:10.1177/17588359221148918
Corrie, P. G., Marshall, A., Dunn, J. A., Middleton, M. R., Nathan, P. D., Gore, M., et al. (2014). Adjuvant bevacizumab in patients with melanoma at high risk of recurrence (AVAST-M): preplanned interim results from a multicentre, open-label, randomised controlled phase 3 study. Lancet Oncol. 15 (6), 620–630. doi:10.1016/S1470-2045(14)70110-X
Corrie, P. G., Marshall, A., Nathan, P. D., Lorigan, P., Gore, M., Tahir, S., et al. (2018). Adjuvant bevacizumab for melanoma patients at high risk of recurrence: survival analysis of the AVAST-M trial. Ann. Oncol. 29 (8), 1843–1852. doi:10.1093/annonc/mdy229
Dummer, R., Hauschild, A., Santinami, M., Atkinson, V., Mandalà, M., Kirkwood, J. M., et al. (2020). Five-Year analysis of adjuvant dabrafenib plus trametinib in stage III melanoma. N. Engl. J. Med. 383 (12), 1139–1148. doi:10.1056/NEJMoa2005493
Eggermont, A. M., Chiarion-Sileni, V., Grob, J. J., Dummer, R., Wolchok, J. D., Schmidt, H., et al. (2015). Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 16 (5), 522–530. doi:10.1016/S1470-2045(15)70122-1
Eggermont, A. M. M., Blank, C. U., Mandala, M., Long, G. V., Atkinson, V., Dalle, S., et al. (2018b). Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N. Engl. J. Med. 378 (19), 1789–1801. doi:10.1056/NEJMoa1802357
Eggermont, A. M. M., Blank, C. U., Mandala, M., Long, G. V., Atkinson, V. G., Dalle, S., et al. (2020). Longer follow-up confirms recurrence-free survival benefit of adjuvant pembrolizumab in high-risk stage III melanoma: updated results from the EORTC 1325-MG/KEYNOTE-054 trial. J. Clin. Oncol. 38 (33), 3925–3936. doi:10.1200/JCO.20.02110
Eggermont, A. M. M., Blank, C. U., Mandala, M., Long, G. V., Atkinson, V. G., Dalle, S., et al. (2021). Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 22 (5), 643–654. doi:10.1016/S1470-2045(21)00065-6
Eggermont, A. M. M., Chiarion-Sileni, V., Grob, J. J., Dummer, R., Wolchok, J. D., Schmidt, H., et al. (2016). Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N. Engl. J. Med. 375 (19), 1845–1855. doi:10.1056/NEJMoa1611299
Eggermont, A. M. M., Robert, C., and Ribas, A. (2018a). The new era of adjuvant therapies for melanoma. Nat. Rev. Clin. Oncol. 15 (9), 535–536. doi:10.1038/s41571-018-0048-5
Grossmann, K. F., Othus, M., Patel, S. P., Tarhini, A. A., Sondak, V. K., Knopp, M. V., et al. (2022). Adjuvant pembrolizumab versus IFNα2b or ipilimumab in resected high-risk melanoma. Cancer Discov. 12 (3), 644–653. doi:10.1158/2159-8290.CD-21-1141
Hauschild, A., Dummer, R., Schadendorf, D., Santinami, M., Atkinson, V., Mandalà, M., et al. (2018). Longer follow-up confirms relapse-free survival benefit with adjuvant dabrafenib plus trametinib in patients with resected BRAF V600-mutant stage III melanoma. J. Clin. Oncol. 36 (35), 3441–3449. doi:10.1200/JCO.18.01219
Hodi, F. S., Chesney, J., Pavlick, A. C., Robert, C., Grossmann, K. F., McDermott, D. F., et al. (2016). Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 17 (11), 1558–1568. doi:10.1016/S1470-2045(16)30366-7
Hodi, F. S., O'Day, S. J., McDermott, D. F., Weber, R. W., Sosman, J. A., Haanen, J. B., et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363 (8), 711–723. doi:10.1056/NEJMoa1003466
Larkin, J., Chiarion-Sileni, V., Gonzalez, R., Grob, J. J., Rutkowski, P., Lao, C. D., et al. (2019). Five-Year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 381 (16), 1535–1546. doi:10.1056/NEJMoa1910836
Larkin, J., Del Vecchio, M., Mandala, M., Gogas, H., Arance Fernandez, A. M., Dalle, S., et al. (2023). Adjuvant nivolumab versus ipilimumab in resected stage III/IV melanoma: 5-year efficacy and biomarker results from CheckMate 238. Clin. cancer Res. official J. Am. Assoc. Cancer Res. 29, 3352–3361. doi:10.1158/1078-0432.CCR-22-3145
Larkin, J., Weber, J., Del Vecchio, M., Gogas, H., Arance, A. M., Dalle, S., et al. (2022). Adjuvant nivolumab versus ipilimumab (CheckMate 238 trial): reassessment of 4-year efficacy outcomes in patients with stage III melanoma per AJCC-8 staging criteria. Eur. J. Cancer 173, 285–296. doi:10.1016/j.ejca.2022.06.041
Long, G. V., Hauschild, A., Santinami, M., Atkinson, V., Mandalà, M., Chiarion-Sileni, V., et al. (2017). Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N. Engl. J. Med. 377 (19), 1813–1823. doi:10.1056/NEJMoa1708539
Long, G. V., Luke, J. J., Khattak, M. A., de la Cruz Merino, L., Del Vecchio, M., Rutkowski, P., et al. (2022). Pembrolizumab versus placebo as adjuvant therapy in resected stage IIB or IIC melanoma (KEYNOTE-716): distant metastasis-free survival results of a multicentre double-blind, randomised, phase 3 trial. Lancet Oncol. 23 (11), 1378–1388. doi:10.1016/S1470-2045(22)00559-9
Long, G. V., Stroyakovskiy, D., Gogas, H., Levchenko, E., de Braud, F., Larkin, J., et al. (2014). Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N. Engl. J. Med. 371 (20), 1877–1888. doi:10.1056/NEJMoa1406037
Luke, J. J., Rutkowski, P., Queirolo, P., Del Vecchio, M., Mackiewicz, J., Chiarion-Sileni, V., et al. (2022). Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet 399 (10336), 1718–1729. doi:10.1016/S0140-6736(22)00562-1
Maio, M., Lewis, K., Demidov, L., Mandalà, M., Bondarenko, I., Ascierto, P. A., et al. (2018). Adjuvant vemurafenib in resected, BRAF(V600) mutation-positive melanoma (BRIM8): a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 19 (4), 510–520. doi:10.1016/S1470-2045(18)30106-2
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G.PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ Clin. Res. ed) 339, b2535. doi:10.1136/bmj.b2535
Robert, C., Schachter, J., Long, G. V., Arance, A., Grob, J. J., Mortier, L., et al. (2015). Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 372 (26), 2521–2532. doi:10.1056/NEJMoa1503093
Siegel, R. L., Miller, K. D., Wagle, N. S., and Jemal, A. (2023). Cancer statistics, 2023. CA Cancer J. Clin. 73 (1), 17–48. doi:10.3322/caac.21763
Stege, H., Haist, M., Nikfarjam, U., Schultheis, M., Heinz, J., Pemler, S., et al. (2021). The status of adjuvant and neoadjuvant melanoma therapy, new developments and upcoming challenges. Target. Oncol. 16 (5), 537–552. doi:10.1007/s11523-021-00840-3
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj 366, l4898. doi:10.1136/bmj.l4898
Tarhini, A. A., Lee, S. J., Hodi, F. S., Rao, U. N. M., Cohen, G. I., Hamid, O., et al. (2020). Phase III study of adjuvant ipilimumab (3 or 10 mg/kg) versus high-dose interferon alfa-2b for resected high-risk melanoma: north American intergroup E1609. J. Clin. Oncol. 38 (6), 567–575. doi:10.1200/JCO.19.01381
Toor, K., Middleton, M. R., Chan, K., Amadi, A., Moshyk, A., and Kotapati, S. (2021). Comparative efficacy and safety of adjuvant nivolumab versus other treatments in adults with resected melanoma: a systematic literature review and network meta-analysis. BMC Cancer 21 (1), 3. doi:10.1186/s12885-020-07538-1
Weber, J., Mandala, M., Del Vecchio, M., Gogas, H. J., Arance, A. M., Cowey, C. L., et al. (2017). Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N. Engl. J. Med. 377 (19), 1824–1835. doi:10.1056/NEJMoa1709030
Weber, J. S., Schadendorf, D., Del Vecchio, M., Larkin, J., Atkinson, V., Schenker, M., et al. (2023). Adjuvant therapy of nivolumab combined with ipilimumab versus nivolumab alone in patients with resected stage IIIB-D or stage IV melanoma (CheckMate 915). J. Clin. Oncol. 41 (3), 517–527. doi:10.1200/JCO.22.00533
Yushak, M., Mehnert, J., Luke, J., and Poklepovic, A. (2019). Approaches to high-risk resected stage II and III melanoma. Am. Soc. Clin. Oncol. Educ. book Am. Soc. Clin. Oncol. Annu. Meet. 39, e207–e211. doi:10.1200/EDBK_239283
Zhang, N., Li, S., and Li, J. (2022). Efficacy of immune checkpoint inhibitors and targeted therapy in adjuvant treatment of resectable melanoma: a network meta-analysis. Chin. Journa Dermatology 55 (7), 603–609. doi:10.35541/cjd.20200767
Zimmer, L., Livingstone, E., Hassel, J. C., Fluck, M., Eigentler, T., Loquai, C., et al. (2020). Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 395 (10236), 1558–1568. doi:10.1016/S0140-6736(20)30417-7
Zimmer, L., Livingstone, E., Hassel, J. C., Fluck, M., Eigentler, T. K., Loquai, C., et al. (2022). Adjuvant nivolumab plus ipilimumab or nivolumab alone versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): final results of a randomised, double-blind, phase 2 trial. Lancet 400 (10358), 1117–1129. doi:10.1016/S0140-6736(22)01654-3
Keywords: melanoma, immune checkpoint inhibitors, network meta-analysis, adjuvant treatment, efficacy
Citation: Sheng F, Yan Y and Zeng B (2023) Efficacy and safety of immune checkpoint inhibitors and targeted therapies in resected melanoma: a systematic review and network meta-analysis. Front. Pharmacol. 14:1284240. doi: 10.3389/fphar.2023.1284240
Received: 28 August 2023; Accepted: 24 October 2023;
Published: 07 November 2023.
Edited by:
Zhiyu Zhang, Fourth Affiliated Hospital of China Medical University, ChinaReviewed by:
Ainara Soria Rivas, Ramón y Cajal University Hospital, SpainPennacchioli Elisabetta, European Institute of Oncology (IEO), Italy
Copyright © 2023 Sheng, Yan and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baoqi Zeng, emVuZ2Jhb3FpQDEyNi5jb20=
†These authors have contributed equally to this work
 Feng Sheng1†
Feng Sheng1† Baoqi Zeng
Baoqi Zeng