- 1Guang'Anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Department of Emergency, Guang'anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 3Department of Dermatology, Guang'anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
Purpose: Antibiotic-resistant bacterial pneumonia poses a significant therapeutic challenge. In China, Chinese herbal compound (CHC) is commonly used to treat bacterial pneumonia. We aimed to evaluate the efficacy and safety of CHC and identify core herb combinations for the treatment of multidrug-resistant or extensively drug-resistant bacterial pneumonia.
Methods: Stata 16 and TSA 0.9.5.10 beta software were used for meta-analysis and trial sequential analysis (TSA), respectively. Exploring the sources of heterogeneity through meta-regression and subgroup analysis.
Results: Thirty-eight studies involving 2890 patients were included in the analyses. Meta-analysis indicated that CHC combined with antibiotics improved the response rate (RR = 1.24; 95% CI: 1.19–1.28; p < 0.0001) and microbiological eradication (RR = 1.41; 95% CI: 1.27–1.57; p < 0.0001), lowered the white blood cell count (MD = −2.09; 95% CI: −2.65 to −1.53; p < 0.0001), procalcitonin levels (MD = −0.49; 95% CI: −0.59 to −0.40; p < 0.0001), C-reactive protein levels (MD = −11.80; 95% CI: −15.22 to −8.39; p < 0.0001), Clinical Pulmonary Infection Scores (CPIS) (MD = −1.97; 95% CI: −2.68 to −1.26; p < 0.0001), and Acute Physiology and Chronic Health Evaluation (APACHE)-II score (MD = −4.08; 95% CI: −5.16 to −3.00; p < 0.0001), shortened the length of hospitalization (MD = −4.79; 95% CI: −6.18 to −3.40; p < 0.0001), and reduced the number of adverse events. TSA indicated that the response rate and microbiological eradication results were robust. Moreover, Scutellaria baicalensis Georgi, Fritillaria thunbergii Miq, Lonicera japonica Thunb, and Glycyrrhiza uralensis Fisch were identified as core CHC prescription herbs.
Conclusion: Compared with antibiotic treatment, CHC + antibiotic treatment was superior in improving response rate, microbiological eradication, inflammatory response, CPIS, and APACHE-II score and shortening the length of hospitalization. Association rule analysis identified four core herbs as promising candidates for treating antibiotic-resistant bacterial pneumonia. However, large-scale clinical studies are still required.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42023410587.
1 Introduction
Lower respiratory tract infections are the fourth leading cause of mortality worldwide, claiming 2.6 million lives in 2019 (World Health Organization, 2020). Although several organisms are implicated, bacteria remains the primary cause of pneumonia. The introduction of antibiotics considerably improved bacterial pneumonia treatment efficiency. However, antibiotic overuse and misuse in hospitals and the agricultural industry have contributed to the emergence of drug resistance (Chawla et al., 2022). In recent years (Zaman et al., 2017; Duval Raphaël et al., 2019; Salam et al., 2023), multidrug-resistant (MDR) bacteria, defined as bacteria with resistance to at least three different classes of antimicrobial agents, have become common, promoting selection pressure toward extensively drug-resistant(XDR) bacteria. One study reported that 28%–75% of patients with hospital-acquired pneumonia (HAP) carried MDR bacteria (Catia et al., 2019). These bacteria, most often Pseudomonas aeruginosa (P. aeruginosa)and methicillin-resistant Staphylococcus aureus (MRSA), are the main causes of early-onset and late-onset ventilator-associated pneumonia (VAP) (Torres et al., 2017). In addition to nosocomial pneumonia, recent studies have reported an increased risk of MDR and XDR bacteria spreading to the community (Van Duin and Paterson, 2020; NAIR and Niederman, 2021). In one study, the “PES” pathogens, including P. aeruginosa, extended-spectrum β-lactamase-positive Enterobacteriaceae, and MRSA, were responsible for 6% of hospitalized community-acquired pneumonia (CAP) cases and resulted in high mortality (Prina et al., 2015). Macaux et al. (Lou et al., 2018) reported that 35% of XDR bacteria carriers had not been hospitalized in the past 12 months. As the emergence of MDR and XDR bacteria reduces antibiotic efficacy, lower respiratory tract infections can not be controlled, leading to longer hospitalization durations, higher costs, and increased mortality (Venter, 2019; Yahav et al., 2021; Raycheva et al., 2022). The shortage of new antibiotics developed against MDR and XDR bacteria aggravates this problem (Terreni et al., 2021).
Currently, researchers have shifted their focus to developing new antibiotics from botanicals. Complex natural products with numerous molecular targets can reduce the incidence of resistance and thus exhibit considerable antibacterial activity against MDR bacteria (Filipa et al., 2020; Hafsa et al., 2022; Thangaiyan et al., 2022). Traditional Chinese medicine(TCM) is characterized by the use of botanicals, a rich source of bioactive phytocompounds. Many bioactive phytocompounds originating from TCM herbs, such as phenols, terpenoids, alkaloids, flavonoids, isothiocyanates, and indoles, hinder major drug-resistant factors, such as efflux pumps, enzyme activity, membrane permeability, and other virulence mechanisms, including quorum sensing (QS) and biofilm development (Gupta and Birdi, 2017; Su et al., 2020; Li et al., 2022). Chinese herbal compound (CHC) represents a prevalent therapeutic approach in TCM. Several randomized control trials (RCTs) have demonstrated the efficacy of CHC alone or in combination with antibiotics in treating MDR and XDR bacterial pneumonia, improving the eradication rate of antibiotic-resistant bacteria, reducing the inflammatory response and adverse effects, and shortening the duration of hospitalization. However, the therapeutic efficacy and safety of CHC or CHC combination therapy remains indeterminate due to the lack of comprehensive evaluation of these RCTs. Therefore, this study aimed to produce a novel meta-analysis and trial sequential analysis (TSA) to reliably estimate the clinical effects and safety of CHC for treating MDR and XDR bacterial pneumonia. Association rule mining using the Apriori algorithm was performed to identify core herbal combinations that could serve as potential therapeutic agents.
2 Methods
This study was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA) 2020 guidelines (Supplementary Material S1). The registration number of the study protocol is CRD42023410587 (PROSPERO: https://www.crd.york.ac.uk/prospero/).
2.1 Literature search strategy
RCTs that evaluated the efficacy and safety of CHC for MDR or XDR bacterial pneumonia were obtained from eight databases, including Pubmed, Embase, Cochrane Library, Web of Science (WOS), China National Knowledge Infrastructure (CNKI), Wanfang Data Knowledge Service Platform (Wanfang), VIP information resource integration service platform (VIP), and Chinese Biomedical Literature Database (CBM), and independently screened by two investigators (JS. and ZY.). The coverage dates ranged from the inception of each database (Pubmed, 1946; Embase, 1947; The Cochrane Library, 1995; WOS, 1900; CNKI, 1994; Wanfang, 1980; VIP, 1989; CBM, 2008) until 10 March 2023. Medical subject heading (MeSH) and free terms were combined for retrieval in this study, and the search terms were appropriately modified for the different databases. Keywords used for the literature search included “Drug-resistant bacterial pneumonia,” “Antibiotic-resistant bacterial pneumonia,” “Multidrug-resistant bacterial pneumonia,” “extensively drug-resistant bacterial pneumonia,” “Medicine, Chinese Traditional,” “Traditional Chinese Medicine,” “decoction,” “formula,” “randomized controlled trial,” and “RCT.” Complete retrieval methods are detailed in Supplementary Material S2.
2.2 Study selection
EndNote reference management software (version 20; Clarivate Analytics) was used for study selection. Two reviewers (DD. and YY.) separately screened the titles, abstracts, and full text for eligibility using the same criteria. Disagreements were resolved by a third reviewer (DY.).
2.2.1 Eligibility criteria
2.2.1.1 Type of studies
All RCTs, with or without blinding, were included in this study. No specified date or language restrictions were set.
2.2.1.2 Type of participants
Patients with any type of pneumonia (VAP, HAP, or CAP) caused by MDR or XDR bacteria were included without restrictions for sex, age, or race. Positive cultures from endotracheal aspirates or bronchoscopic sampling techniques were necessary for the diagnosis of antibiotic-resistant bacterial pneumonia. MDR bacteria were defined as bacteria unsusceptible to at least three different antimicrobial categories, and XDR bacteria were defined as bacteria unsusceptible to at least one agent in addition to two or fewer antimicrobial classes (Magiorakos et al., 2012). The pathogenetic diagnosis was based on at least two etiological examinations of cultures from lower respiratory tract secretions, including sputum, endotracheal aspirate, or bronchoalveolar lavage fluid. Furthermore, abnormal radiological findings indicating the presence of new or progressed pulmonary infiltrate(s) and clinical signs of infection, including the onset of fever (≥38°C), increased sputum production or change in sputum color to a more purulent state, peripheral leukocytosis, and decreased oxygenation or the requirement of oxygen supplementation therapy, were required (Kalil et al.; Olson and Davis, 2020).
2.2.1.3 Type of intervention
RCTs comparing CHC in the treatment group with antibiotic treatment in the control group, or comparing combined CHC + antibiotic treatment with antibiotic-only treatment, with or without a placebo, were included. All forms of oral administration or nasal feeding, such as through decoction, granules, or capsules, were included. No restrictions were placed on the dose or duration of CHC therapy.
2.2.1.4 Outcome measures
The primary outcome was set as response rate, calculated as (total number of patients—number of patients without efficacy)/total number of patients. Inefficacy was defined as unaltered or worsening signs and symptoms, such as pulmonary shadows visualized on X-ray or CT, pulmonary rales, fever, cough, and chest pain.
Secondary outcomes included: 1) microbiological response, defined as bacterial eradication determined by etiological examination of clinical samples collected from the site of infection showing no bacterial growth at the end of treatment; 2) inflammatory indicators, including absolute white blood cell (WBC) count, procalcitonin (PCT) levels, and C-reactive protein (CRP) levels; 3) scoring systems that assessed the severity of pneumonia, prognosis, and drug efficacy, including the Clinical Pulmonary Infection Scores (CPIS) and Acute Physiology and Chronic Health Evaluation (APACHE)-II score; 4) safety, evaluated by the incidence of adverse events (AEs) and adverse drug reactions (ADRs).
2.2.2 Exclusion criteria
Exclusion criteria included: 1) cohort studies, case reports, reviews, and conference abstracts; 2) studies that lacked primary outcomes, control groups, or full-text articles; 3) participants diagnosed without an etiological examination or a drug sensitivity test, or those diagnosed with an etiological examination indicating chlamydia, mycoplasma, or fungal infection; 4) interventions involving non-drug TCM therapy, such as acupuncture or massage.
2.3 Data extraction and quality assessment
A standardized data extraction form including the title, first author’s name, publication year, age, sample size, sex, interventions in the treatment and control groups, treatment duration, infection type, pathogen, setting, outcome measures, and reported AEs or ADRs was used. Five methodological quality domains were assessed using Rob 2.0 and included: 1) bias arising from the randomization process; 2) bias due to deviations from intended interventions; 3) bias due to missing outcome data; 4) bias in outcome measurements; 5) bias in the selection of the reported result. Each domain was categorized according to three levels, namely, “high risk of bias,” “low risk of bias,” or “some concerns”. Two reviewers (Y.G. and S.Z.) independently evaluated the methodological quality of each study, and disagreements were resolved by a third reviewer (JQ.).
2.4 Statistical analysis
Meta-analysis was performed using Stata 16.0. Binary outcomes were expressed in terms of relative risk (RR) and 95% confidence interval (CI). For continuous variables, the mean difference (MD) and 95% CI were used. When I2 was <50%, the fixed-model Mantel–Haenszel method was used to evaluate the pooled effect. When I2 was >50%, a random-effects model was used. A sensitivity analysis was performed to evaluate the stability of the research findings. To explore potential sources of heterogeneity, meta-regression and subgroup analyses were performed. Meta-regression was used when the number of included studies was greater than 10. Begg’s and Egger’s tests were used to assess publication bias, and trim-and-fill analyses were performed to confirm the results. To avoid type I and type II errors from the meta-analysis and to obtain more robust results (Pogue and Yusuf, 1998; Shah and Smith, 2019), TSA was conducted using TSA 0.9.5.10 beta software. The quality of evidence for all outcomes was evaluated using the GRADE system.
To investigate likely association rules of core herb combinations, we examined CHC constituent herb data. Preliminary information for data extraction was obtained by analyzing the frequency of each herb prior to association rule analysis. Apriori association rule analysis and plot generation were performed using the “arules” R-package (version 2023.03.0 + 386) to fit data and “arulesViz” to generate the plots (Merdun and Ozturk, 2005; Hahsler, 2017). To detect meaningful connections between variables in large databases, frequent hub item sets and association rules were mined using the Apriori algorithm. This method can identify relationships between projects and can be used in various medical studies designed to predict variable characteristics (Hsieh et al., 2020).
In the Apriori algorithm, the primary metrics used to measure associations include support, confidence, and lift. Support, defined as the proportion of transactions that contain the itemset in the dataset, measures the importance of the itemset and is expressed as P (A∩B). Confidence, which measures the probability of observing herb A in a transaction containing herb B, can be expressed as P(A∩B)/P(A) = P(B|A) but does not explain whether there is a meaningful correlation between the two transactions. Lift, which can be expressed as P (A with B)/P (A) P (B) = P(B|A)/P(B), can identify meaningful correlations between two transactions. Herb A and Herb B are unrelated when lift is close to 1, as they are probabilistically close to independent, whereas lift >1 indicates a strong relationship between Herb A and Herb B. To identify association rules, minimum support and confidence values were set at 20% and 80%, respectively, and core herb combinations demonstrating the most evident associations were identified.
3 Results
3.1 Study selection
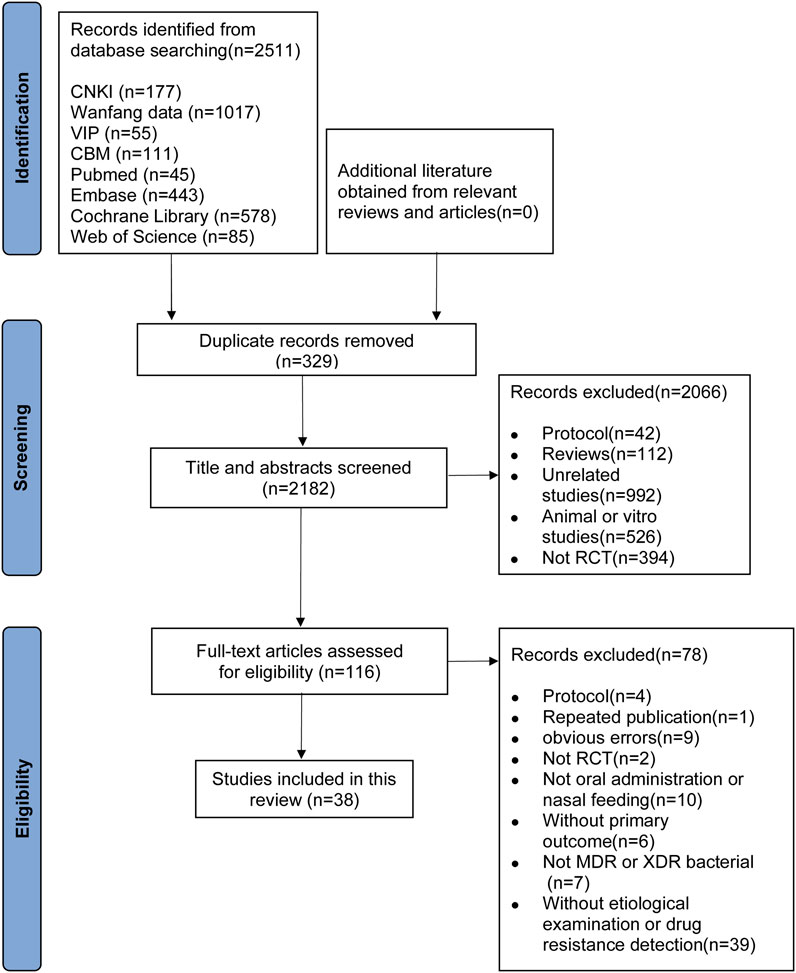
The initial search of the eight electronic databases yielded a total of 2511 articles. After removing duplicates, the remaining 2182 articles were screened for eligibility, and of the 116 eligible studies, 38 met the inclusion criteria (Han and Huang, 2006; Jin et al., 2015; Yang et al., 2015; Shujuan et al., 2015; Xue, 2015; Chengdu University of Traditional Chinese Medicine, 2016; Liu and Song, 2016; Liang et al., 2016; Zhu et al., 2016; Shi et al., 2017; Hu et al., 2017; Xie, 2017; Guo et al., 2017; Zhang and Liu, 2018; Xu et al., 2019; Huang et al., 2019a; Tan, 2019; Liang et al., 2019b; Liang et al., 2019a; Liu et al., 2019; Wang, 2019; Peng et al., 2019; Huang et al., 2019b; Yan et al., 2020; Beijing University of Chinese Medicine, 2020; Gao et al., 2021; Wang and Songtao, 2020; Zhong et al., 2020; Xiao et al., 2021; Clinical, 2021; Zhou and Luo, 2021; Li et al., 2022; Fu et al., 2022; Chen, 2022; Shandong University of Traditional Chinese Medicine, 2022; WANG, 2022; Yin et al., 2022) (Figure 1).
3.2 Study characteristics
Thirty-eight RCTs conducted in China between 2006 and 2022, involving 2890 patients, with 1431 patients in the intervention group and 1459 patients in the comparison group, were included. The median age of the patients was 59.55 years. Most of the studies included middle-aged or elderly adults, and only one study recruited toddlers. Table 1 summarizes the characteristics of the 38 included studies.
3.2.1 Setting
Of the 38 studies, 22 were conducted in intensive care units (ICUs); 6 were conducted in non-ICU settings, including neurology, rehabilitation medicine, respiratory, and integrated traditional Chinese and Western medicine wards, and 10 RCTs did not provide information on the setting.
3.2.2 Pathogens
The studies involved MDR/XDR P. aeruginosa, MDR/XDR Acinetobacter baumanii (A. baumannii), MRSA, ESBL-producing Escherichia coli, and mixed MDR/XDR bacteria. Nine RCTs did not provide information on pathogen type.
3.2.3 Treatment
Regarding treatment groups, only one study adopted CHC monotherapy, whereas the others used combined interventions. CHC therapies were prepared as decoctions or granules. Twenty-three RCTs included patients who received Chinese herbal compound prescriptions orally, while the remaining studies involved patients receiving these prescriptions via nasogastric feeding. All of these patients were from ICUs. Eighteen studies documented antibiotic treatment regimens based on sputum-culture drug-sensitivity test results or recommended guidelines, while the remaining 20 studies provided detailed documentation of the types and dosages of antibiotics administered in treatment. The antibiotics involved in these studies included carbapenems, β-lactam antibiotics, glycopeptides, aminoglycosides, oxazolidinones, and tetracyclines. The most frequently utilized antibiotic was a combination of imipenem and cilastatin sodium.
3.2.4 Outcome
All 38 studies included reported response rates. Regarding secondary outcomes, 55.26%, 47.37%, 60.53%, 47.37%, 28.95%, 18.42%, 15.79%, and 44.74% of the included RCTs reported microbiological response, WBC count, PCT levels, CRP levels, CPIS, APACHE-II score, length of hospitalization, and safety, respectively.
3.3 Risk of bias assessment
The Rob 2.0 tool was used to assess the quality of the included studies. The results of the risk of bias assessment are as follows: 1) Randomization process: All included studies declared randomization, but only 25 studies described the details of the randomization methods (by software or random number table). The allocation concealment approach was illustrated by only two studies. None of the studies exhibited baseline differences between the intervention and control groups, indicating poor randomization. Consequently, two studies were assessed as low-risk, and the rest had some concerns about the risk of bias. 2) Deviations from the intended interventions: As only one study adopted a single-blind method, and none of the studies used intention-to-treat (ITT) or modified-ITT analysis to evaluate differences between the intervention and control groups, the risk of bias associated with deviation from the intended interventions was rated as high for most studies. 3) Missing outcome data: Two studies reported missing outcome data due to dropouts. The rest of the included RCTs had no incomplete data and were marked as low-risk. 4) Measurement of the outcome: All studies were rated as having a low risk of bias, mostly because participants’ symptoms and laboratory results were used to assess response rates, which were rarely influenced by their knowledge. 5) Selection of the reported result: As none of the included studies had a registered protocol, adherence to pre-planned statistical analysis methods could not be assessed. Consequently, all included studies were assessed as having unclear risks. The overall risk of bias was high. The summary of methodological quality is displayed in Figure 2.
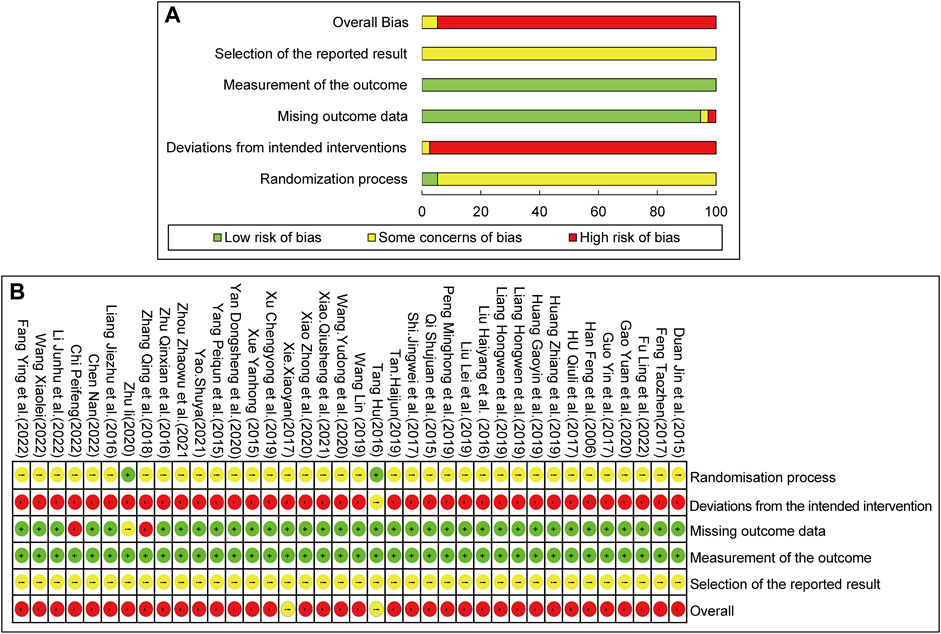
FIGURE 2. Risk of bias assessment for included studies: (A) Risk of bias graph. (B) Risk of bias summary.
3.4 Primary outcomes
3.4.1 Response rate
Thirty-eight studies involving 2890 patients compared CHC + antibiotic treatment with antibiotic-only treatment and reported the response rate for treating pneumonia caused by MDR or XDR bacteria. The heterogeneity test results revealed very low heterogeneity among studies (X2 = 38.33; p = 0.41; I2 = 3.47%), and therefore a fixed-effects model was used for the statistical analysis of the response rate. The meta-analysis revealed a significant response rate for CHC + antibiotic treatment (response rate = 1.24; 95% CI: 1.19–1.28; p < 0.0001). Subgroup analysis according to the average age, treatment duration, sample size, setting, infection type, and pathogen (Table 2, Supplementary Material S4) revealed statistically significant differences between CHC + antibiotic treatment and antibiotic-only treatment in the average age, treatment duration, sample size, and setting subgroups. In contrast, subgroup analysis of various infection types revealed no statistical difference for patients with CAP. Sensitivity analyses, in which single study was systematically excluded to determine whether one study had a significant impact on the results, indicated similar aggregated statistics and robust results (Figure 4, Supplementary Material S6).
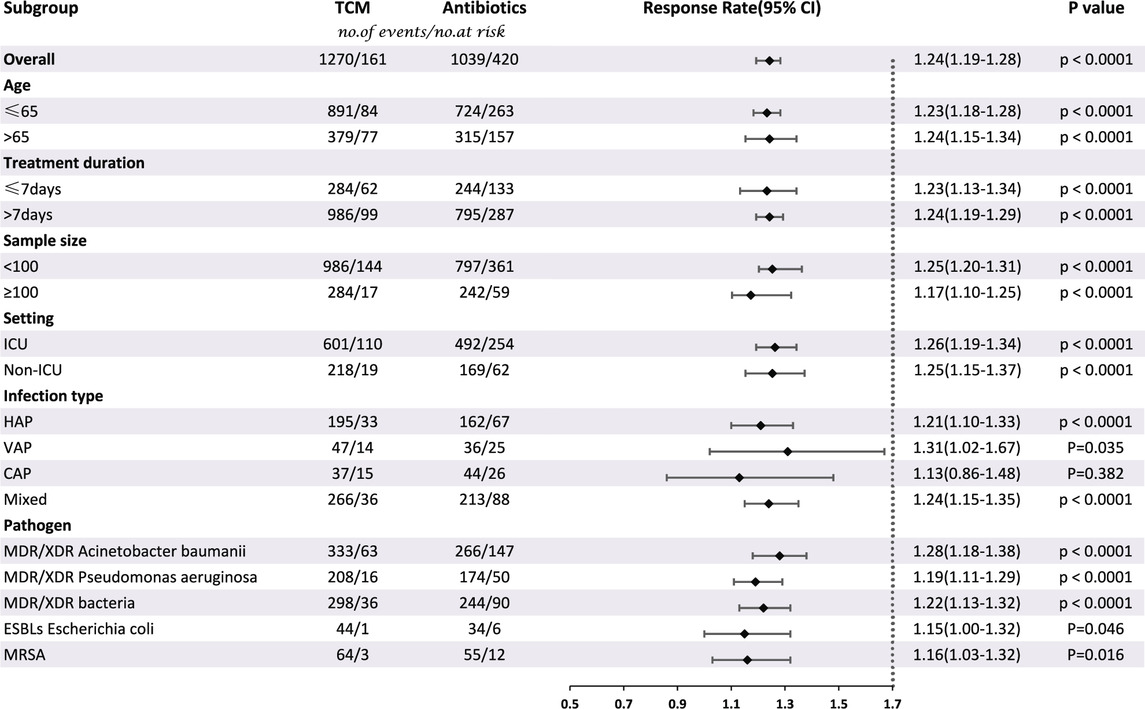
TABLE 2. Subgroup analysis of response rate according to average age, treatment duration, sample size, setting, infection type, and pathogen.
3.5 Secondary outcomes
3.5.1 Microbiological response
Twenty-one studies involving 1542 patients reported the efficacy of CHC + antibiotic versus antibiotic-only treatment for microbiological eradication. The heterogeneity test revealed low heterogeneity among studies (X2 = 23.97; p = 0.24; I2 = 16.55%); Therefore, the statistical analysis of the microbiological response was performed using a fixed-effects model. The results indicated that CHC + antibiotic treatment significantly eradicated bacteria compared with antibiotic-only treatment (RR = 1.41; 95% CI: 1.27–1.57; p < 0.0001). Subgroup analysis according to total sample size, setting, and infection type revealed no statistical improvement for the large sample size, non-ICU setting, and CAP subgroups. In contrast, among the other subgroups, CHC + antibiotic treatment exhibited statistically superior bactericidal effects when compared with antibiotic-only treatment (Table 3, Supplementary Material S4). MRSA eradication in the CHC + antibiotic group was lower than that in the antibiotic-only group, although the difference was not statistically significant. However, as the number of studies within the MRSA subgroup was limited, further investigation is required. Sensitivity analysis indicated that the pooled effect was robust (Figure 4, Supplementary Material S6).
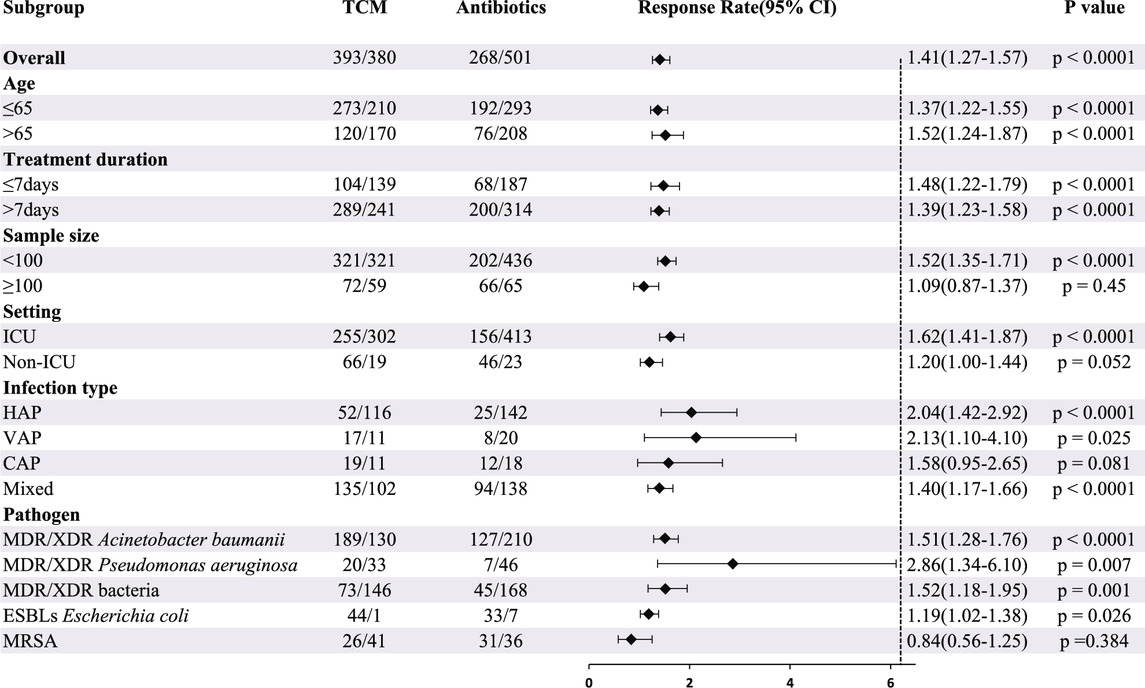
TABLE 3. Subgroup analysis of microbiological response according to average age, treatment duration, sample size, setting, infection type, and pathogen.
3.5.2 White blood cell count
Eighteen studies involving 1284 patients reported the WBC count for CHC + antibiotic treatment and antibiotic-only treatment groups. Heterogeneity tests revealed high heterogeneity among the studies (X2 = 261.52; p < 0.0001; I2 = 93.49%), and statistical analysis of this outcome was conducted using a random-effects model. The results indicated superior efficacy of CHC + antibiotics in reducing the WBC count compared with that of the control group (MD: −2.09; 95% CI: −2.65 to −1.53; p < 0.0001). Meta-regression indicated no significant difference in WBC count based on average age (p = 0.055, adjusted R2 = 26.77%), publication year (p = 0.603, adjusted R2 = −7.77%), sample size (p = 0.105, adjusted R2 = 10.52%), or treatment duration (p = 0.933, adjusted R2 = −10.40%) (Supplementary Material S5). Although study characteristics, such as setting, infection type, and pathogen, may have also contributed to heterogeneity, meta-regression analyses were not possible due to incomplete documentation of these baseline characteristics. Subgroup analysis revealed undiminished heterogeneity within each subgroup, indicating that the heterogeneity could not be explained by setting, infection type, or pathogen. Furthermore, subgroup analysis of various settings revealed no statistical difference between CHC + antibiotics and antibiotics only in the treatment of non-ICU patients (Supplementary Material S4). Sensitivity analysis indicated that the pooled effect was robust (Figure 4, Supplementary Material S6).
3.5.3 Procalcitonin levels
Twenty-three studies involving 1693 patients reported PCT levels for CHC + antibiotic and antibiotic-only groups. The heterogeneity test revealed high heterogeneity among the studies (X2 = 491.60; p < 0.0001; I2 = 95.52%), and statistical analysis of this outcome was conducted using a random-effects model. The results revealed a significantly lower PCT level in the CHC + antibiotic group than in the antibiotic-only group (MD = −0.49; 95% CI: −0.59 to −0.40; p < 0.0001). Meta-regression revealed no significant difference in PCT level based on average age (p = 0.92, adjusted R2 = −7.22%), publication year (p = 0.485, adjusted R2 = −4.17%), sample size (p = 0.080, adjusted R2 = 12.78%), or treatment duration (p = 0.831, adjusted R2 = −6.49%) (Supplementary Material S5). Subgroup analysis of various pathogens, settings, and infection types indicated that these factors were not the source of heterogeneity. Subgroup analysis of various infection types revealed that, although no statistical difference was seen between the CHC + antibiotic and antibiotic-only groups for patients with VAP, CHC + antibiotics significantly reduced the PCT level in patients with HAP, CAP, or a mixed infection type compared with antibiotics only (Supplementary Material S4). Sensitivity analysis indicated that the pooled effect was robust (Figure 4, Supplementary Material S6).
3.5.4 C-reactive protein levels
Eighteen studies involving 1282 patients reported the CRP levels for CHC + antibiotic and antibiotic-only groups. High heterogeneity among studies was detected (X2 = 1733.56; p < 0.0001; I2 = 99.02%), and statistical analysis of this outcome was therefore conducted using a random-effects model. The results revealed significantly lower CRP levels in the CHC + antibiotic group than those in the antibiotic-only group (MD = −11.80; 95% CI: −15.22 to −8.39; p < 0.0001). Meta-regression revealed no significant difference in CRP levels based on average age (p = 0.958, adjusted R2 = −8.57%), publication year (p = 0.062, adjusted R2 = 22.64%), sample size (p = 0.092, adjusted R2 = 14.66%), or treatment duration (p = 0.355, adjusted R2 = −0.24%) (Supplementary Material S5). Subgroup analysis indicated that the pathogen, setting, and infection type were not the source of heterogeneity. Subgroup analysis of various pathogens revealed that there was no statistically significant difference between CHC + antibiotic treatment and antibiotic treatment only in patients with pneumonia caused by MDR P. aeruginosa, whereas CHC + antibiotics had a superior outcome for treating pneumonia caused by mixed MDR/XDR bacteria and MDR/XDR A. baumannii than antibiotics alone (Supplementary Material S4). Sensitivity analysis indicated that the pooled effect was robust (Figure 4, Supplementary Material S6).
3.5.5 Clinical pulmonary infection scores
Eleven studies involving 898 patients reported CPISs for CHC + antibiotic and antibiotic-only groups. High heterogeneity (X2 = 332.03; p < 0.0001; I2 = 94.66%) was detected among the studies, and statistical analysis of this outcome was conducted using a random-effects model. The results indicated that CHC + antibiotics could significantly lower the CPIS more than antibiotics alone (MD = −1.97; 95% CI: −2.68 to −1.26; p < 0.0001). Meta-regression revealed no significant association between CPIS and average age (p = 0.608, adjusted R2 = −10.20%), publication year (p = 0.504, adjusted R2 = −7.70%), sample size (p = 0.240, adjusted R2 = 8.05%), or treatment duration (p = 0.399, adjusted R2 = −3.90%) (Supplementary Material S5). The source of heterogeneity could not be explained by the pathogen, setting, or infection type. Subgroup analysis based on the pathogen type revealed a superior outcome for CHC + antibiotics in the treatment of pneumonia caused by MDR P. aeruginosa but not for pneumonia caused by mixed MDR/XDR bacteria (Supplementary Material S4). Sensitivity analysis indicated that the pooled effect was robust (Figure 4, Supplementary Material S6).
3.5.6 APACHE-II score
Seven studies involving 424 patients reported APACHE-II scores for CHC + antibiotic and antibiotic-only treatment. High heterogeneity among the studies (X2 = 16.62; p = 0.011; I2 = 63.91%) was detected, and statistical analysis of this outcome was conducted using a random-effects model. The results indicated that the CHC + antibiotic group had lower APACHE-II scores than did the antibiotic-only group (MD = −4.08; 95% CI: −5.16 to −3.00; p < 0.0001). Subgroup analysis showed statistical differences in the average age, treatment duration, pathogen, and infection type subgroups. However, as the number of studies within each subgroup was limited, the impact of these characteristics on the APACHE-II score could not be assessed (Supplementary Material S4). Sensitivity analysis indicated that the pooled effect was robust (Figure 4, Supplementary Material S6).
3.5.7 Length of hospitalization
Six studies involving 394 patients reported the length of hospitalization for CHC + antibiotic and antibiotic-only groups. High heterogeneity among the studies (X2 = 27.26; p < 0.0001; I2 = 81.66%) was detected, and statistical analysis of this outcome was conducted using a random-effects model. The results revealed a shorter length of hospitalization for the CHC + antibiotic group than for the antibiotic-only group (MD = −4.79; 95% CI: −6.18 to −3.40; p < 0.0001). Although subgroup analyses by age, duration of treatment, pathogen, setting, and type of infection were conducted, the limited number of studies within each subgroup prevented an assessment of the impact of these characteristics on the length of hospitalization (Supplementary Material S4). Sensitivity analysis indicated that the pooled effect was robust (Figure 4, Supplementary Material S6).
3.5.8 Safety
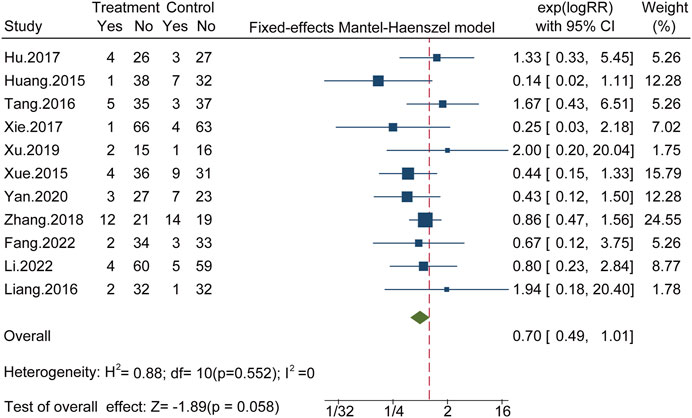
Eleven studies involving 859 patients reported AEs, with fewer AEs reported for the CHC + antibiotic group than for the antibiotic-only group. All 11 studies reported gastrointestinal tract symptoms, such as nausea, vomiting, and diarrhea. In one study, four cases of renal dysfunction were reported in the CHC + antibiotic group. Six studies claimed that, during the study, there were no AEs in the CHC + antibiotic group. AEs were not mentioned in the remaining 21 studies. No heterogeneity was detected among the studies (X2 = 8.79; p = 0.552; I2 = 0%), and statistical analysis of this outcome was conducted using a fixed-effects model. The results indicated fewer AEs in the CHC + antibiotic group than in the antibiotic-only group; However, the difference was not statistically significant (RR = 0.70; 95% CI: 0.49–1.01; p = 0.058) (Figure 3). Sensitivity analysis indicated that the pooled effect was robust (Figure 4, Supplementary Material S6).

FIGURE 4. Sensitivity analysis: CHC with antibiotics vs. Antibiotics: (A) Response rate, (B) Microbiological response, (C) WBC, (D) PCT, (E) CRP, (F) CPIS scores, (G) APACHE-II, (H) Length of hospitalization, (I) Adverse events.
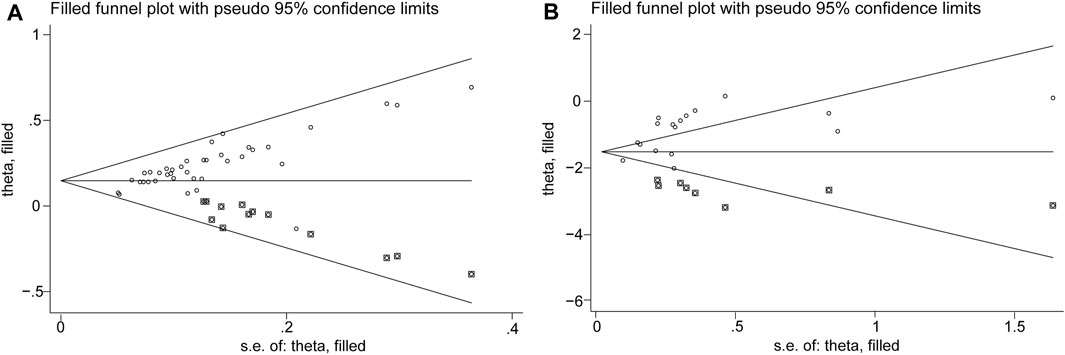
3.6 Publication bias
We assessed the impact of publication bias on response rate using Begg’s and Egger’s tests. The p-value for both Begg’s and Egger’s tests were <0.0001, suggesting publication bias. However, trim-and-fill analysis performed using the fixed-effects model did not reveal any publication bias. For the microbiological response, the p-values for Begg’s and Egger’s tests were 0.266 and 0.004, respectively, which were not indicative of publication bias. This was supported by trim-and-fill analysis, which also did not reveal any publication bias (Figure 5, Supplementary Material S7). According to Begg’s test, there was no evidence of publication bias for “WBC count,” “PCT level,” “CRP level,” “CIPS score,” “APACHE-II score,” “length of hospitalization,” and “safety.” However, the results of Egger’s test indicated a potential risk of publication bias for “WBC count,” “CRP level,” and “CIPS score.”
3.7 Quality of evidence according to outcome measures
The GRADE method was used to assess the quality of the evidence. When comparing CHC + antibiotics with antibiotics-only, the overall quality of evidence for each outcome was evaluated as moderate to very poor. The high risk of bias, inconsistency across studies, and imprecision of findings contributed to the low quality of evidence. Supplementary Material S8 provides a summary of the overall quality of evidence for each outcome.
3.8 Trial sequential analysis
The false positive and false negative findings in systematic reviews and meta-analyses can be controlled by TSA, which has therefore become an attractive statistical method. Therefore, we used TSA 0.9.5.10 beta software to ensure that the results of our meta-analyses were reliable and conclusive. The required information size in this study was estimated by α = 0.05 (two-sided) and β = 0.20 (power of 80%), based on the O'Brien–Fleming alpha-expenditure function. The RR reduction and event rates in both experimental and control groups were calculated from the mean of the event proportions.
3.8.1 Response rate
The TSA included 38 RCTs and was performed based on a controlled-event proportion of 71.21% and an RR reduction of −23%. The TSA identified the required information size (RIS) as 192. The cumulative Z-curve reached the RIS and crossed the RIS-adjusted boundary in support of CHC + antibiotic treatment, thus suggesting conclusive and robust results (Figure 6).
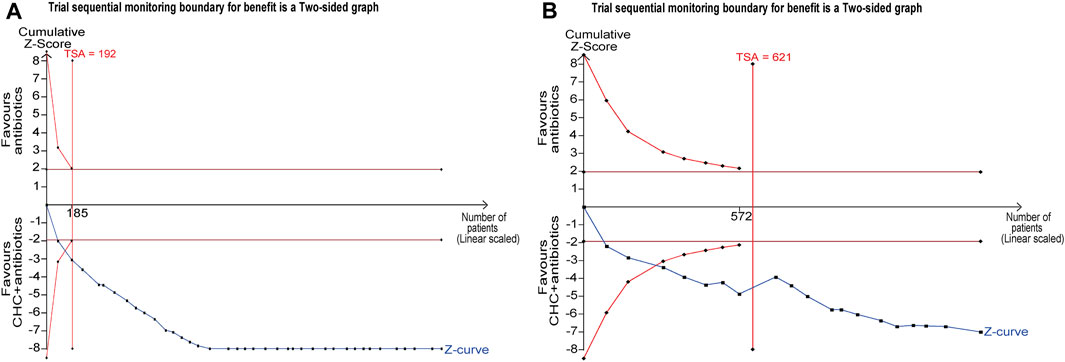
FIGURE 6. Trial sequential analysis (TSA) for response rate and microbiological response. (A) TSA of response rate with a control event proportion of 71.21%, type 1 error of 5%, power of 80%, and relative risk reduction of −23%. (B) TSA of microbiological response with a control event proportion of 34.40%, type 1 error of 5%, power of 80%, and relative risk reduction of −41%.
3.8.2 Microbiological response
The TSA included 21 RCTs and was performed based on a controlled-event proportion of 34.40% and an RR reduction of −41%. The RIS was 621. However, the number of patients did not reach the RIS, and the cumulative Z-curve crossed the conventional boundary line and RIS-adjusted boundary, indicating that CHC + antibiotic treatment was effective in eradicating MDR or XDR bacteria in patients with pneumonia (Figure 6).
3.9 Apriori algorithm-based association rule analysis
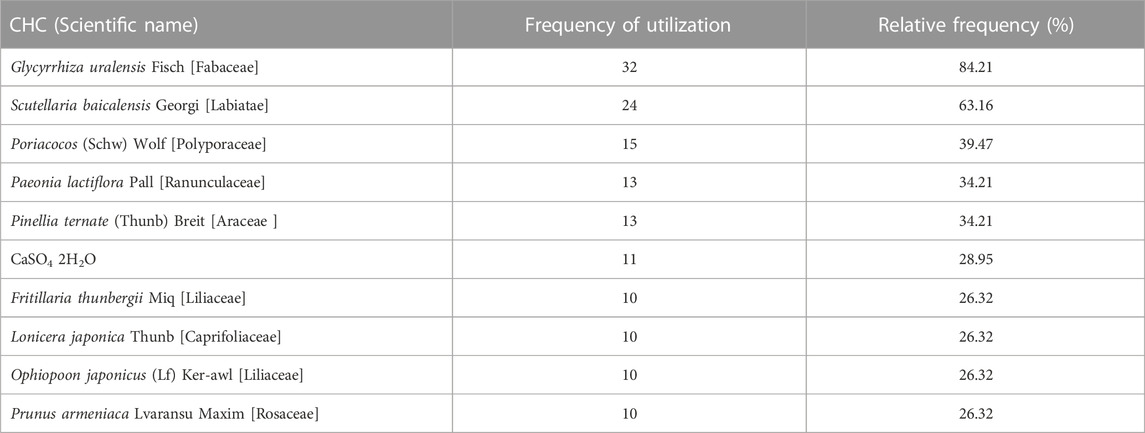
The association rule analysis included 110 herbs from 38 CHC prescriptions. CHC prescriptions are summarized in Table 4, and the distribution frequency of the herbs is summarized in Table 5. Eighteen association rules were detected when the minimum support and confidence values were set at 0.2 and 0.8, respectively. The lift was >1, which was indicative of effective association rules, with a higher degree of lift indicative of a stronger association. The scatter plot reflected the degree of lift, indicated by color depth, of each association rule, ranging from 0.950 to 1.425 (Table 6). The general distribution of the determined association rules was evaluated using a grouping matrix (Figure 7). The horizontal ordinate represented 10clusters, which were generated by 18 association rules. According to the scatter plot and grouping matrix diagram, four association rules of {Paeonia lactiflora Pall [Ranunculaceae]}=>{Scutellaria baicalensis Georgi [Labiatae]}, {Fritillaria thunbergii Miq [Liliaceae]}=>{S. baicalensis Georgi [Labiatae]}, {Lonicera japonica Thunb [Caprifoliaceae]}=>{S. baicalensis Georgi [Labiatae]}, and {F. thunbergii Miq [Liliaceae], Glycyrrhiza uralensis Fisch [Fabaceae]}=>{S. baicalensis Georgi [Labiatae]} were considered. The three rules with the highest lift were {F. thunbergii Miq [Liliaceae]}=>{S. baicalensis Georgi [Labiatae]}, {L. japonica Thunb [Caprifoliaceae]}=>{S. baicalensis Georgi [Labiatae]}, and {F. thunbergii Miq [Liliaceae], G. uralensis Fisch [Fabaceae]}=>{S. baicalensis Georgi [Labiatae]}, which also exhibited a confidence value exceeding 0.9. Therefore, S. baicalensis Georgi [Labiatae], F. thunbergii Miq [Liliaceae], L. japonica Thunb [Caprifoliaceae], and G. uralensis Fisch [Fabaceae] were identified as core CHC ingredients for the treatment of patients with pneumonia caused by MDR or XDR bacteria. A network graph presenting the relationship between these association rules is shown in Figure 8.
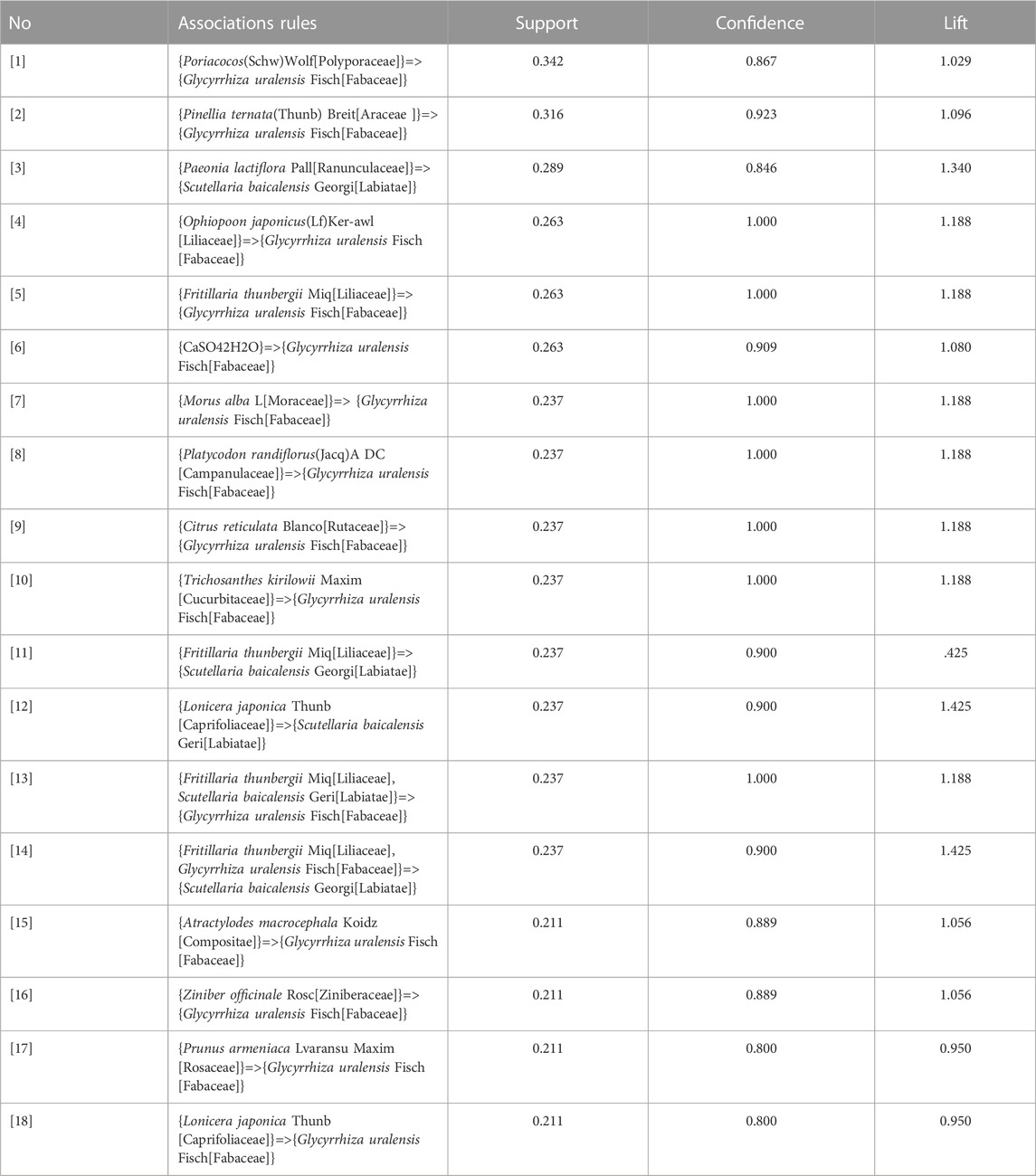
TABLE 6. Apriori algorithm-based association rules in the meta-analysis of CHC prescribed for pneumonia caused by MDR or XDR bacteria.
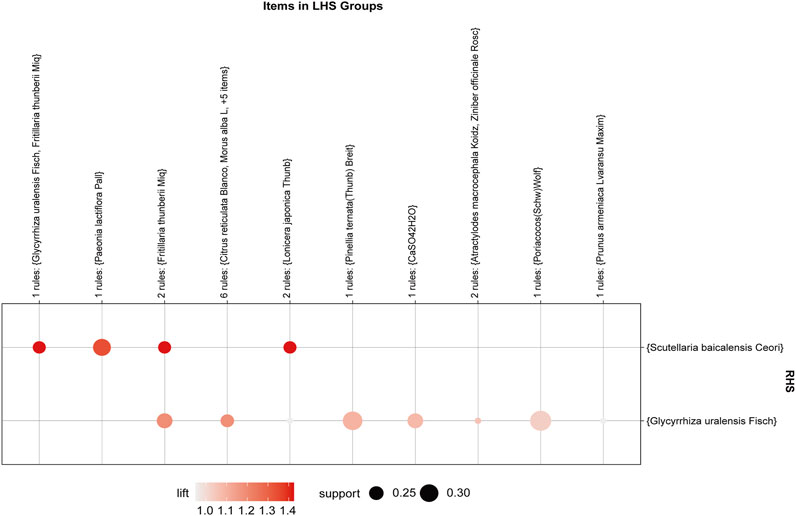
FIGURE 7. Grouping matrix of the association rules in the meta-analysis of Chinese herbs for pneumonia caused by MDR or XDR bacteria. The herb X and herb Y are called antecedent (left-hand side, LHS) and consequent (right-hand side, RHS) of the rules.
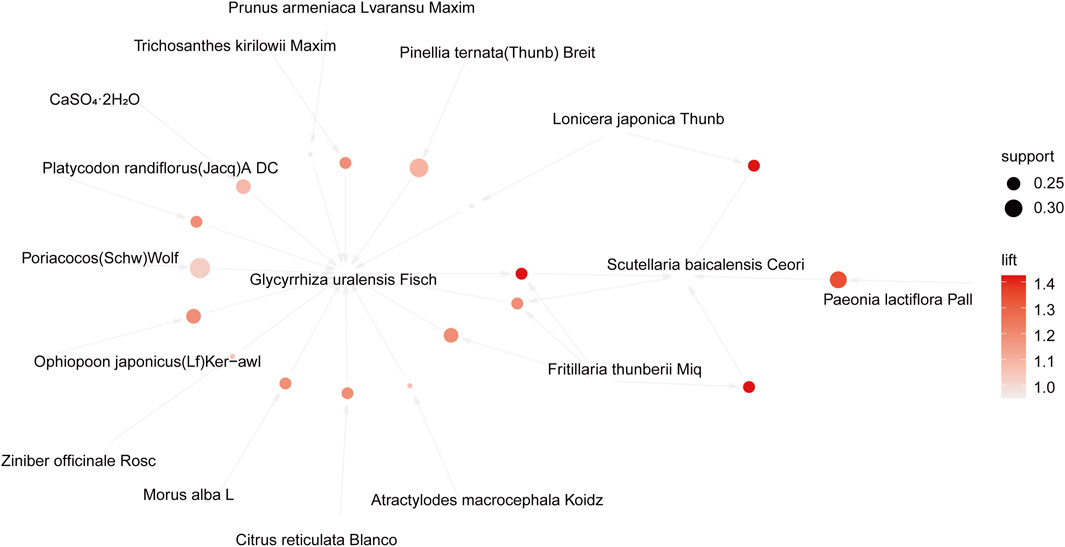
FIGURE 8. Network graph of the association rules in the meta-analysis of Chinese herbal compound prescribed for pneumonia caused by MDR or XDR bacteria.
4 Discussion
Traditional Chinese medicine(TCM) has been used to treat infectious diseases for thousands of years. The classic works of TCM, such as the “Inner Canon of Huangdi,” “Treatise on Febrile Disease,” and “Febrile Disease Differentiation,” record the rich theory behind treating infectious diseases and the effective methods of treatment. Recently, antibioticresistance has emerged as a global threat. The incidence of MDR and XDR bacteria increases annually, limiting the efficacy of antibiotic agents for the treatment of severe refractory infections (Ayukekbong et al., 2017). Accumulating evidence from clinical studies indicates that patients with pneumonia caused by MDR or XDR bacteria may achieve better clinical outcomes using CHC.
4.1 Summary of the main finding
In this study, 38 RCTs were analyzed, of which 37 compared CHC + antibiotics with the same antibiotics alone, and one study compared CHC alone with antibiotics. We found that the combination of CHC and antibiotics significantly increased the response rate. Sensitivity analysis demonstrated that the results were robust, and there was no evidence of publication bias as assessed by trim-and-fill analysis. TSA further explained that the clinical evidence of the efficacy rate of CHC combination therapy for pneumonia caused by MDR or XDR bacteria was determinate; thus, the analytic result is worth asserting. CHC + antibiotic combination treatment was more effective than antibiotics alone in improving the microbiological eradication rate. Subgroup analysis revealed no statistically significant difference in the CAP subgroups. TSA demonstrated that the results were conclusive and robust.
For inflammatory indicators, the combination treatment led to significant improvements in WBC, CRP, and PCT. We identified high heterogeneity in WBC, PCT, and CRP levels; however, the source of heterogeneity remains unclear. We speculated that large individual differences in WBC, PCT, and CRP levels may account for the high heterogeneity, leading to high variation in baseline levels, as well as the degree of improvement. In addition, inadequate study protocols, such as the lack of blinding and allocation concealment, may have contributed to heterogeneity.
In terms of score systems, we also found the CHC combination therapy group had lower CPIS and APACHE-II scores after treatment than the antibiotics-alone group. The CPIS and APACHE-II score were recommended to predict the clinical outcomes of HAP/VAP by recent guidelines (Torres et al., 2017; Martin-Loeches et al., 2018); Lower scores are associated with better outcomes (Zhou et al., 2015). According to our findings, combining CHC and antibiotics could eradicate MDR/XDR bacteria, reduce inflammatory indicator levels, and lower the CPIS and APACHE-II scores, resulting in better clinical outcomes for patients with pneumonia caused by MDR/XDR bacteria. Our results also indicated that patients receiving CHC combination therapy had a shorter length of hospitalization than those receiving antibiotic therapy alone.
4.2 Implications of mechanism research
Evidence from this study suggests that the concomitant use of CHC may be more effective than antibiotics alone in the treatment of pneumonia caused by MDR or XDR bacteria. During the antibiotic resistance crisis, many researchers shifted their attention to TCM and investigated the inhibitory effect of TCM herbs or bioactive phytocompounds on antibiotic resistance (Liu et al., 2020; Mohamed et al., 2020; Lan et al., 2021). As the main source of TCM herbs, natural products (NP) are characterized by more complex phytochemicals and spatial configurations than synthetic drugs (Koehn and Carter, 2005). Different extracts from the same herbs vary wildly, which may lead to distinct antimicrobial activity against various antibiotic-resistant bacteria. In one study, the high antimicrobial activity of L. ethanol extracts from Hypericum perforatum Linn against Lactobacillus plantarum (L. plantarum), Enterococcus faecalis (E. faecalis), Streptococcus mutans (S. mutans), and Streptococcus sobrinus (S. sobrinus)strains was observed. While S. sobrinus and L. plantarum were highly inhibited by their water extracts, a moderate antibacterial effect was observed in S. mutans and E. faecalis (Süntar et al., 2015). Antibiotic-resistance mechanisms of TCM herbs may involve the efflux pump system, enzyme activity, inhibition of biofilm (Tsai et al., 2018), drug target changes, permeability of the bacterial cell membrane (Zhao et al., 2019), and other drug-resistant mechanisms (Anand et al., 2020). Baicalin, a bioactive compound derived from S. baicalensis Georgi, could hinder the formation of P. aeruginosa biofilms by suppressing QS-related genes, including lasI, lasR, rhlI, rhlR, pqsR, and pqsA (Luo et al., 2017). Moreover, in vivo experiments demonstrated the bioactive anti-inflammatory effects of this compound in an acute pneumonia rat model induced by MDR P. aeruginosa (Lei et al., 2023). Other studie demonstrated that lonicerin inhibited biofilm formation and subsequently ameliorated P. aeruginosa infections in A549 cells by directly inhibiting AlgE activity without affecting bacterial viability or AlgE expression (Xu et al., 2019). Licorice extract was found to stimulate excessive intracellular reactive oxygen species production, inducing oxidative stress and generating a significant number of free radicals. This disrupted the cell wall membrane structure and functionality of Staphylococcus aureus (S. aureus). Additionally, post-treatment of S. aureus with licorice extract resulted in a notable reduction in the enzymatic activity of two critical cellular metabolic enzymes: succinate dehydrogenase and succinate dehydrogenase ATPase, thereby interfering with the organism’s energy metabolism system (Singh et al., 2015; Zhang et al., 2023). In one study, under the intervention of 1/4 MIC berberine and 1/8 MIC imipenem (Su and Wang, 2018), the MexXY-OprM efflux pump in P. aeruginosa was blocked due to the downregulation of MexZ, MexX, MexY, and the outer membrane protein OprM. Chelerythrine, isolated from the root of Toddalia asiatica (Linn) Lam, has been reported to damage the bacterial cell membrane and destroy channels across cell membranes, allowing protein leakage from the cell and suppressing protein biosynthesis. Therefore, it has a strong inhibitory effect on S. aureus and MRSA and on the beta-lactamases produced by these bacteria (He et al., 2018). Additionally, the synergistic effect of TCM herbs can enhance the sensitivity of antibiotic-resistant bacteria to antibiotics, leading to their use as sensitizers. For example, there is no significant difference in the antibacterial effect of pterostilbene and gentamycin alone. However, when combined, the two compounds completely inhibited bacterial growth and exhibited synergistic antibacterial effects (Lee et al., 2017). Research reported that the antimicrobial effect of mupirocin against strains of S. aureus and MRSA was enhanced when combined with piperine isolated from black pepper through the inhibition of ethidium bromide efflux (Mirza et al., 2011).
4.3 Limitations
Our study had some limitations. First, the randomization process of most studies was unclear, and blinding and allocation concealment was often lacking, which introduced a high risk of bias and lowered the methodological quality. Furthermore, none of these studies were registered; therefore, a study protocol was not obtained. Second, the sample sizes of all included studies were small, and therefore, the findings of these studies may not be generalizable. Third, some studies lacked comprehensive reports regarding research characteristics, such as pathogens, setting, and infection type, which limited the analyses of heterogeneity and exploration of the dominant population. Furthermore, the included studies lacked long-term outcome evaluation indicators for CHC + antibiotic treatment in drug-resistant bacterial pneumonia, such as potential relapse rates. Fourth, although we utilized meta-regression and subgroup analysis to investigate the sources of heterogeneity in this article, we were unable to identify them. Therefore, in future research, we aim to establish comprehensive protocols that yield more stable and reliable results. Fifth, all participants included in this study were exclusively sourced from China limiting the generalizability of the findings to other populations. We look forward to the emergence of future large-sample RCTs conducted in various regions to enhance the external validity and reliability of research findings.
5 Conclusion
Patients with pneumonia caused by MDR and XDR bacteria could benefit from CHC + antibiotic combination therapy, which improved response and microbiological eradication rates, reduced the inflammatory response, reduced the CPIS and APACHE-II score, and shortened the length of hospitalization. Scutellaria baicalensis Georgi [Labiatae], F. thunbergii Miq [Liliaceae], G. uralensis Fisch [Fabaceae], and L. japonica Thunb [Caprifoliaceae] were identified as core herbs for the treatment of antibiotic-resistant bacterial pneumonia.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
SZ: Visualization, Writing–original draft, Writing–review and editing. YG: Methodology, Writing–review and editing. JS: Investigation, Writing–review and editing. JQ: Project administration, Writing–review and editing. YY: Investigation, Writing–review and editing. DD: Investigation, Writing–review and editing. ZY: Investigation, Writing–review and editing. WQ: Supervision, Writing–review and editing. DY: Project administration, Writing–review and editing. XZ: Project administration, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and publication of this article. This work was funded by the Scientific and Technological Innovation Project of the China Academy of Chinese Medical Sciences (Ref CI 2021A02901). The funder did not participate in any process of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1282538/full#supplementary-material
References
Anand, U., Nandy, S., Mundhra, A., Das, N., Pandey, D. K., and Dey, A. (2020). A review on antimicrobial botanicals, phytochemicals and natural resistance modifying agents from Apocynaceae family: possible therapeutic approaches against multidrug resistance in pathogenic microorganisms. Drug Resist. Updat. Rev. Comment. Antimicrob. anticancer Chemother. 51, 100695. doi:10.1016/j.drup.2020.100695
Ayukekbong, J. A., Ntemgwa, M., and Atabe, A. N. (2017). The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob. Resist. Infect. Control 6 (1), 47. doi:10.1186/s13756-017-0208-x
Beijing University of Chinese Medicine (2020). Exploration on the treatment of hospital acquired pneumonia caused by drug-resistant bacteria with Xinjiada Yuansan. Beijing, China: Beijing University of Chinese Medicine.
Catia, C., Cristina, D., and Torres, A. (2019). An overview of guidelines for the management of hospital-acquired and ventilator-associated pneumonia caused by multidrug-resistant Gram-negative bacteria. Curr. Opin. Infect. Dis. 32, 656–662. doi:10.1097/QCO.0000000000000596
Chawla, M., Verma, J., Gupta, R., and Das, B. (2022). Antibiotic potentiators against multidrug-resistant bacteria: discovery, development, and clinical relevance. Front. Microbiol. 13, 887251. doi:10.3389/fmicb.2022.887251
Chen, N. (2022). Clinical study on fuzheng guben tang in the treatment of multidrug-resistance pneumonia after stroke. Mod. Med. Health Res. Electron. J. 6 (20), 93–96.
Chengdu University of Traditional Chinese Medicine (2016). The clinical research about treating the multidrug-resistence of bacterial pneumonia by the reinforce healthy qi and purge toxins. Sichuan Province, China: Chengdu University of Traditional Chinese Medicine.
Clinical, S. Y. Y. (2021). Study of xueduqing,A traditional Chinese medicine Compound,in the treatment of multidrug-resistant Pseudomonas aeruginosa pneumoniain elderly patients with phlegm-heat accumulation in lung. Hefei, China: Anhui University of Chinese Medicine.
Dachaihu, T. Z. F. (2017). Decoction in the treatment of encephalopathy after multiple resistance clinical observation on the Effect of drug bacteria infection on phlegm heat accumulation. Xianyang; Shaanxi University of Traditional Chinese Medicine.
Duval Raphaël, E., Marion, G., and Béatrice, D. (2019). Fight against antimicrobial resistance: we always need new antibacterials but for right bacteria. Molecules 24, 3152. doi:10.3390/molecules24173152
Filipa, B., Pinto, E., Anake, K., Pinto, M., and Sousa, E. (2020). Targeting antimicrobial drug resistance with marine natural products. Int. J. Antimicrob. Agents 56, 106005. doi:10.1016/j.ijantimicag.2020.106005
Fu, L., Wu, H., Yang, J., Wang, W., and Xia, F. (2022). Study on the intervention effect of qingfei shengmai decoction on multidrug-resistant acinetobacter baumannii infection in AECOPD. TRADITIONAL Chin. DRUG Res. Clin. Pharmacol. 33 (1), 120–125. doi:10.19378/j.issn.1003-9783.2022.01.017
Gao, Y., Liu, H., Mu, X., Qu, N., and Qin, Y. (2021). The clinical effects of fuzheng huazhuo decoction combined with western medicine in the treatment of senile drug-resistant bacterial pneumonia with deficiency of qi, phlegm and obstruction of lung. Chin. Pharmacoeconomics 28 (5), 104–107. doi:10.12010/j.issn.1673-5846.2020.01.026
Guo, Y., Su, J., Luo, B., Xu, H., Lin, Z., and Liang, J. (2017). Clinical observation of sepsis prescription combined with tigecycline and imipenem/cilastatin in the treatment of severe extensively drug-resistant Acinetobacter baumannii pneumonia. Chin. J. Clin. Ration. Drug Use 10 (8), 19–21. doi:10.15887/j.cnki.13-1389/r.2017.23.002
Gupta, P., and Birdi, T. (2017). Development of botanicals to combat antibiotic resistance. J. Ayurveda Integr. Med. 8 (4), 266–275. doi:10.1016/j.jaim.2017.05.004
Hafsa, Q., Haseeb Shah, A., Mudasir Ahmad, S., Alshehri, B., Almilaibary, A., and Ahmad Mir, M. (2022). Natural products and their semi-synthetic derivatives against antimicrobial-resistant human pathogenic bacteria and fungi. Saudi J. Biol. Sci. 29, 103376. doi:10.1016/j.sjbs.2022.103376
Hahsler, M. (2017). arulesViz: interactive visualization of association rules with R. R J. 9 (2), 163–175. doi:10.32614/rj-2017-047
Han, F., and Huang, J. (2006). Clinical observation on the treatment of ESBLs producing Escherichia coli pneumonia in children with integrated traditional Chinese and Western medicine. Hubei Tradit. Chin. Med. 28 (6), 9–10. doi:10.3969/j.issn.1000-0704.2006.06.004
He, N., Wang, P., Wang, P., Ma, C., and Kang, W. (2018). Antibacterial mechanism of chelerythrine isolated from root of Toddalia asiatica (Linn) Lam. BMC Complementary Altern. Med. 18 (1), 261. doi:10.1186/s12906-018-2317-3
Hsieh, P. C., Cheng, C. F., Wu, C. W., Tzeng, I. S., Kuo, C. Y., Hsu, P. S., et al. (2020). Combination of acupoints in treating patients with chronic obstructive pulmonary disease: an Apriori algorithm-based association rule analysis. Evidence-based complementary Altern. Med. 2020, 8165296. doi:10.1155/2020/8165296
Hu, Q., Cui, L., and Liu, C. (2017). Curative effect dbsernation of observation peitushengjin method combined treatment intervention of multi drug resistant bacteria of pulmonary infection. J. Basic Chin. Med. 23 (12), 1728–1730. doi:10.06325/0(2017)12-1728-03
Huang, G., Zhao, M., and Li, J. (2019a). Clinical efficacy of the Qingfei Tongluo decoction on pneumonia with multidrug-resistant bacteria after stroke. Clin. J. Chin. Med. 11 (25), 82–84. doi:10.3969/j.issn.1674-7860.2019.25.034
Huang, Z., Huang, X., and Gao, G. (2019b). Therapeutic effect of Xia Yu Huang Decoction on multidrug-resistant Pseudomonas aeruginosa infection in lower respiratory tract. Guide China Med. 17 (13), 161–162. doi:10.15912/j.cnki.gocm.2019.13.122
Jin, D., Zhou, W., and Tian, Y. (2015). Combined traditional Chinese and western medicine for the treatment of 50 cases of multidrug-resistant bacterial infection in the lower respiratory tract. Henan Tradit. Chin. Med. 35 (4), 882–883. doi:10.16367/j.issn.1003-5028.2015.04.0375
Kalil, A. C., Metersky, M. L., Klompas, M., Muscedere, J., Sweeney, D. A., Palmer, L. B., et al. (2016). Executive summary: management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American thoracic society. Clin. Infect. Dis. 63, 575–582. doi:10.1093/cid/ciw504
Koehn, F. E., and Carter, G. T. (2005). The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 4 (3), 206–220. doi:10.1038/nrd1657
Lan, J.-E., Li, X.-J., Zhu, X.-F., Sun, Z.-L., He, J.-M., Zloh, M., et al. (2021). Flavonoids from Artemisia rupestris and their synergistic antibacterial effects on drug-resistant Staphylococcus aureus. Nat. Prod. Res. 35 (11), 1881–1886. doi:10.1080/14786419.2019.1639182
Lee, W., Basri, D., and Ghazali, A. (2017). Bactericidal effect of pterostilbene alone and in combination with gentamicin against human pathogenic bacteria. Mol. (Basel, Switz. 22 (3), 463. doi:10.3390/molecules22030463
Lei, Li, Cui, H., Zhang, Y., Xie, W., Lin, Y., Guo, Y., et al. (2023). Baicalin ameliorates multidrug-resistant Pseudomonas aeruginosa induced pulmonary inflammation in rat via arginine biosynthesis. Biomed. Pharmacother. 162, 114660. doi:10.1016/j.biopha.2023.114660
Li, J., Feng, S., Liu, X., Jia, X., Qiao, F., Guo, J., et al. (2022). Effects of traditional Chinese medicine and its active ingredients on drug-resistant bacteria. Front. Pharmacol. 13, 837907. doi:10.3389/fphar.2022.837907
Li, J., Zhang, F., Liu, S., Zhang, H., Han, W., and Li, Y. (2022). Efficacy of modified Maxingyin decoction combined with tigecycline in the treatment of XDRAB ventilator-associated pneumonia and its effect on T lymphocyte subsets. China J. Mod. Med. 32 (13), 75–80. doi:10.3969/j.issn.1005-8982.2022.13.013
Liang, H., Tan, F., Liu, K., Cai, G., and Sun, B. (2019b). Effect of qingwen jiedu decoction on extensive drug-resistant Pseudomonas aeruginosa associated severe pneumonia. J. Emerg. Traditional Chin. Med. 28 (1), 44–46. doi:10.3969/j.issn.1004-745X.2019.01.012
Liang, J., Luo, S., Xian, Z., Tan, X., Lin, Z., and Ma, W. (2016). Two TCM decoctions in treatment of pulmonary patients with multi drug-resistant bacteria infection. Acta Chin. Med. 31 (216): 642–645. doi:10.16368/j.issn.1674-8999.2016.05.181
Liang, W., Liu, K., Cai, G., Sun, B., and Tan, F. (2019a). Effect of Qingwen Jiedu Decoction on ventilator-associated pneumonia of syndrome of phlegm-heat congesting in the lung caused by extensive drug-resistant acinetobacter baumannii. Mod. J. Integr. Traditional Chin. West. Med. 28 (1), 2509–2513. doi:10.3969/j.issn.1008-8849.2019.23.001
Liu, H., and Song, H. (2016). Modified xiaoqinglong decoction for the treatment of 30 cases of encephalopathy complicated with multidrug-resistant bacterial pneumonia. Henan Tradit. Chin. Med. 36 (5), 759–761. doi:10.16367/j.issn.1003-5028.2016.05.0324
Liu, L., Yan, D., and Zhang, Z. (2019). Hongteng Zijin decoction combined with antibiotics in the treatment of 45 cases of multidrug-resistant Acinetobacter baumannii pneumonia in ICU. Traditional Chin. Med. Res. 32 (4), 22–24. doi:10.3969/j.issn.1001-6910.2019.04.09
Liu, T., Luo, J., Bi, G., Du, Z., Kong, J., and Chen, Y. (2020). Antibacterial synergy between linezolid and baicalein against methicillin-resistant Staphylococcus aureus biofilm in vivo. Microb. Pathog. 147, 104411. doi:10.1016/j.micpath.2020.104411
Lou, M., Oulimata, N., Hugues, C., Pomares, T. B., Seytre, D., Bouchaud, O., et al. (2018). Extensively-drug-resistant bacteria carriers among overseas travellers: one-third had not been hospitalized previously. Int. J. Antimicrob. agents 52, 385–389. doi:10.1016/j.ijantimicag.2018.06.006
Luo, J., Dong, B., Wang, Ke, Cai, S., Liu, T., Cheng, X., et al. (2017). Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS One 12, e0176883. doi:10.1371/journal.pone.0176883
Magiorakos, A. P., Srinivasan, A., Carey, R. B., Carmeli, Y., Falagas, M. E., Giske, C. G., et al. (2012). Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18 (3), 268–281. doi:10.1111/j.1469-0691.2011.03570.x
Martin-Loeches, I., Rodriguez, A., and Torres, A. (2018). New guidelines for hospital-acquired pneumonia/ventilator-associated pneumonia: USA vs. Europe. Curr. Opin. Crit. care 24 (5), 347–352. doi:10.1097/MCC.0000000000000535
Merdun, H., and Ozturk, Y. (2005). Arules - a computational environment for mining association rules and frequent item sets. J. Stat. Softwa re 014 (15), 1–25. doi:10.1016/j.jspi.2004.04.017
Mirza, Z., Kumar, A., Kalia, N., Zargar, A., and Khan, I. A. (2011). Piperine as an inhibitor of the MdeA efflux pump of Staphylococcus aureus. J. Med. Microbiol. 60, 1472–1478. doi:10.1099/jmm.0.033167-0
Mohamed, E. H., Alghamdi, Y. S., Abdel-Hafez, S. M., Soliman, M. M., Alotaibi, S. H., Hassan, M. Y., et al. (2020). Susceptibility assessment of multidrug resistant bacteria to natural products. Dose-Response 18 (3), 1559325820936189. doi:10.1177/1559325820936189
Nair, G., and Niederman, M. S. (2021). Updates on community acquired pneumonia management in the ICU. J. Int. Encycl. Pharmacol. Ther. 217 (1), 107663. doi:10.1016/j.pharmthera.2020.107663
Olson, G., and Davis, A. M. (2020). Diagnosis and treatment of adults with community-acquired pneumonia. JAMA J. Am. Med. Assoc. 323 (9), 885–886. doi:10.1001/jama.2019.21118
Peng, M., Chen, X., Ruan, X., and Zhang, T. (2019). Observation on the therapeutic effect of Buzhong Yiqi decoction in the treatment of multidrug-resistant pneumonia after stroke. J. Pract. Traditional Chin. Med. 35 (12), 1457–1458.
Pogue, J., and Yusuf, S. (1998). Overcoming the limitations of current meta-analysis of randomised controlled trials. Lancet 351 (9095), 47–52. doi:10.1016/S0140-6736(97)08461-4
Prina, E., Ranzani, O., Polverino, E., Cillóniz, C., Ferrer, M., Fernandez, L., et al. (2015). Risk factors associated with potentially antibiotic-resistant pathogens in community-acquired pneumonia. Ann. Am. Thorac. Soc. 12 (2), 153–160. doi:10.1513/AnnalsATS.201407-305OC
Raycheva, R., Rangelova, V., and Kevorkyan, A. (2022). Cost analysis for patients with ventilator-associated pneumonia in the neonatal intensive care unit. Healthc. (Basel, Switz. 10 (6), 980. doi:10.3390/healthcare10060980
Salam, M. A., Al-Amin, M. Y., Salam, M. T., Pawar, J. S., Akhter, N., Rabaan, A. A., et al. (2023). Antimicrobial resistance: a growing serious threat for global public health. Healthc. (Basel) 11, 1946. doi:10.3390/healthcare11131946
Shah, A., and Smith, A. F. (2019). Trial sequential analysis: adding a new dimension to meta-analysis. Anaesthesia 74, 15–20. doi:10.1111/anae.14705
Shandong University of Traditional Chinese Medicine (2022). The clinical research of yiqi huoxue huatan decoction in cure drug-resistance acinetobacter baumann pneumonia. Jinan, China: Shandong University of Traditional Chinese Medicine.
Shi, J., Zhang, T., Zhou, H., Peng, M., and Lu, Y. (2017). The effect of modified Baihu decoction on the treatment of pneumonia caused by multidrug-resistant bacteria in stroke patients. Guangdong Med. J. 38 (z2), 148–150. doi:10.3969/j.issn.1001-9448.2017.z2.063
Shujuan, Q., Yunyan, Z., Xiaolan, L., Xiaomei, L., Li, L., and Jianwei, J. (2015). Clinical observation on tongfu xiefei formula in treating extensive drug-resistant acinetobacter baumannii pneumonia in ICU. J. North Pharm. 12 (3), 73.
Singh, V., Pal, A., and Darokar, M. P. (2015). A polyphenolic flavonoid glabridin: oxidative stress response in multidrug-resistant Staphylococcus aureus. Free Radic. Biol. Med. 87, 48–57. doi:10.1016/j.freeradbiomed.2015.06.016
Su, F., and Wang, J. (2018). Berberine inhibits the MexXY-OprM efflux pump to reverse imipenem resistance in a clinical carbapenem-resistant Pseudomonas aeruginosa isolate in a planktonic state. Exp. Ther. Med. 15 (1), 467–472. doi:10.3892/etm.2017.5431
Su, T., Qiu, Y., Hua, X., Ye, B., Luo, H., Liu, D., et al. (2020). Novel opportunity to reverse antibiotic resistance: to explore traditional Chinese medicine with potential activity against antibiotics-resistance bacteria. Front. Microbiol. 11, 610070. doi:10.3389/fmicb.2020.610070
Süntar, I., Oyardı, O., Akkol, E. K., and Ozçelik, B. (2015). Antimicrobial effect of the extracts from Hypericum perforatum against oral bacteria and biofilm formation. Pharm. Biol. 54 (6), 1065–1070. doi:10.3109/13880209.2015.1102948
Tan, H. (2019). Clinical observation on the treatment of poststroke pneumonia caused by multidrug-resistant bacteria (phlegm heat stasis in the lung) with self developed qingfei huatan formula. J. North Pharm. 16 (1), 57–58. doi:10.3969/j.issn.1672-8351.2019.01.044
Terreni, M., Taccani, M., and Pregnolato, M. (2021). New antibiotics for multidrug-resistant bacterial strains: latest research developments and future perspectives. . Mol. (Basel, Switz. 26 (9), 2671. doi:10.3390/molecules26092671
Thangaiyan, S., Packiavathy Issac, A. S. V., Bell, A. G. S., Carmona, A., Rashmi, V., Mariappan, S., et al. (2022). Tackling multiple-drug-resistant bacteria with conventional and complex phytochemicals. Front. Cell Infect. Microbiol. 12, 883839. doi:10.3389/fcimb.2022.883839
Torres, A., Niederman, M. S., Chastre, J., Ewig, S., Fernandez-Vandellos, P., Hanberger, H., et al. (2017). International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur. Respir. J. 50 (3), 1700582. doi:10.1183/13993003.00582-2017
Tsai, C., Lin, C., Hsu, C., Chang, C. M., Chang, I. W., Lin, L. W., et al. (2018). Using the Chinese herb Scutellaria barbata against extensively drug-resistant Acinetobacter baumannii infections: in vitro and in vivo studies. BMC complementary Altern. Med. 18 (1), 96. doi:10.1186/s12906-018-2151-7
Van Duin, D., and Paterson, D. (2020). Multidrug-resistant bacteria in the community: an update. Infect. Dis. Clin. N. Am. 34 (4), 709–722. doi:10.1016/j.idc.2020.08.002
Venter, H. (2019). Reversing resistance to counter antimicrobial resistance in the World Health Organisation's critical priority of most dangerous pathogens. Biosci. Rep. 39. doi:10.1042/BSR20180474
Wang, L. (2019). Clinical observation on the treatment of pulmonary infection caused by multidrug-resistant Pseudomonas aeruginosa after cerebral infarction by supplementing qi, invigorating spleen, clearing heat and resolving phlegm in traditional Chinese medicine. Mod. J. Integr. Traditional Chin. West. Med. 28 (1), 56–59. doi:10.3969/j.issn.1008-8849.2019.01
Wang, X. (2022). Modified dachaihu decoction in treating pulmonary multidrug-resistant bacteria infection after encephalopathy study on therapeutic value of phlegm-heat fu-organs syndrome, 7 (3), 45–48.
Wang, Y., and Songtao, R. (2020). Efficacy evaluation of sequential traditional Chinese medicine in adjuvant treatment of multidrug-resistant acinetobacter baumannii pneumonia in ICU. Liaoning J. Traditional Chin. Med. 47 (7), 87–90. doi:10.13192/j.issn.1000-1719.2020.07.024
World Health Organization (2020). The top 10 causes of death. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
Xiao, Q., Ma, M., Deng, M., and Zhang, Y. (2021). Clinical observation of xiaochaihu decoction in elderly patients with pulmonary extensive drug-resistant acinetobacter baumannii infection in ICU. J. Emerg. Traditional Chin. Med. 30 (5), 861–864. doi:10.3969/j.issn.1004-745X.2021.05.030
Xie, X. (2017). Clinical effect of TCM pneumonia mixture combined with linezolid on MRSA - induced pneumonia and the impact on serum inflammatory cytokines levels. Pract. J. Cardiac Cereb. Pneumal Vasc. Dis. 25 (7), 96–99. doi:10.3969/j.issn.1008-5971.2017.07.024
Xu, C., Xie, W., Wu, C., Yang, Y., Lu, X., Xiao, P., et al. (2019). Observation on the curative effect of minocycline and other combination drugs plus modified ephedra cimicifugae decoction on multidrug-resistant Acinetobacter baumannii pneumonia. Chin. J. New Clin. Med. 12 (10), 1079–1082. doi:10.3969/j.issn.1674-3806.2019.10.09
Xu, Z., Kun, Li, Pan, T., Liu, J., Li, B., Li, C., et al. (2019). Lonicerin, an anti-algE flavonoid against Pseudomonas aeruginosa virulence screened from Shuanghuanglian formula by molecule docking based strategy. J. Ethnopharmacol. 239, 111909. doi:10.1016/j.jep.2019.111909
Xue, Y. (2015). Clinical observation on the combination of traditional Chinese medicine pneumonia mixture and western medicine in the treatment of extensive drug-resistant acinetobacter baumannii pulmonary infection. J. Emerg. Traditional Chin. Med. 24 (6), 1076–1078. doi:10.3969/j.issn.1004-745X.2015.06.048
Yahav, D., Shepshelovich, D., and Tau, N. (2021). Cost analysis of new antibiotics to treat multidrug-resistant bacterial infections: mind the gap. Infect. Dis. Ther. 10 (1), 621–630. doi:10.1007/s40121-021-00412-y
Yan, D., Liu, L., Yang, Z., Wang, S., and Xu, M. (2020). Clinical observation on the combined treatment of red vine zijin decoction and western medicines for extensive drug-resistance of acinetobacter baumannii pulmonary infection. J. Basic Chin. Med. 26 (1), 76–77,84. doi:10.3969/j.issn.1006-3250.2020.01.026
Yang, P., Chen, C., Bi, X., and Liu, T. (2015). Clinical observation on the treatment of multidrug-resistant Pseudomonas aeruginosa pulmonary infection after cerebral infarction using the method of supplementing qi, strengthening spleen, and resolving phlegm. Chin. J. Integr. Med. Cardio-Cerebrovascular Dis. 13 (12), 1444–1445. doi:10.3969/j.issn.1672-1349.2015.12.034
Yin, F., Zhao, J., Chen, S., Liu, B., and Wang, H. (2022). Clinical observation on the treatment of drug-resistant bacterial Pneumonia with modified Dachaihu decoction and shengjiang powder. Chin. J. geriatric care 20 (5), 75–80. doi:10.3969/j.issn.1672-2671.2022.05.019
Zaman, S., Hussain, M., Nye, R., Mehta, V., Mamun, K. T., and Hossain, N. (2017). A review on antibiotic resistance: alarm bells are ringing. Cureus 9 (6), e1403. doi:10.7759/cureus.1403
Zhang, Q., and Liu, H. (2018). Efficacy and safety of decoction of glehnia and ophiopogon in the treatment of ventilator - associated pneumonia with multidrug resistance in the elderly. Int. J. Geriatrics 39 (2), 77–80,100. doi:10.3969/j.issn.1674-7593.2018.02.008
Zhang, S., Liang, H., and Sun, J. (2023). The antimicrobial activity of licorice extract against Staphylococcus aureus and its underlying mechanism of action. Food Ferment. industry, 1–10. doi:10.13995/j.cnki.11-1802/ts.036761
Zhao, L., Liu, H., Wang, L., Xu, Z. F., Tan, H. B., and Qiu, S. X. (2019). Rhodomyrtosone B, a membrane-targeting anti-MRSA natural acylgphloroglucinol from Rhodomyrtus tomentosa. J. Ethnopharmacol. 228, 50–57. doi:10.1016/j.jep.2018.09.011
Zhong, X., Cai, F., and Xu, Q. (2020). Clinical study on qingjin huatan tang in the treatment of pulmonary infection caused by multidrug -resistance Pseudomonas aeruginosa. J. Med. Forum 41 (6), 155–158.
Zhou, X., Ben, S., Chen, H., and Ni, S. S. (2015). A comparison of Apache II and CPIS scores for the prediction of 30-day mortality in patients with ventilator-associated pneumonia. Int. J. Infect. Dis. IJID official Publ. Int. Soc. Infect. Dis. 30, 144–147. doi:10.1016/j.ijid.2014.11.005
Zhou, Z., and Luo, X. (2021). Effect of tongyang xiezhuo prescription on cytokines, serum pulmonary surfactant protein, endothelial function and immunosuppression in elderly patients with encephalopathy complicated with pulmonary multidrug-resistant bacteria infection. Acta Chin. Med. 36 (5), 1078–1083. doi:10.16368/j.issn.1674-8999.2021.05.227
Keywords: Chinese herbal medicine, antimicrobial drug resistance, bacterial pneumonia, respiratory tract infection, meta-analysis, systematic review
Citation: Zhao S, Geng Y, Shi J, Qian J, Yang Y, Dai D, Yan Z, Qi W, Yu D and Zhao X (2023) Chinese herbal compound for multidrug-resistant or extensively drug-resistant bacterial pneumonia: a meta-analysis and trial sequential analysis with association rule mining to identify core herb combinations. Front. Pharmacol. 14:1282538. doi: 10.3389/fphar.2023.1282538
Received: 24 August 2023; Accepted: 26 October 2023;
Published: 20 December 2023.
Edited by:
Matar Seck, Cheikh Anta Diop University, SenegalReviewed by:
Chuan Wang, University of South China, ChinaVigyasa Singh, University of Arizona, United States
Copyright © 2023 Zhao, Geng, Shi, Qian, Yang, Dai, Yan, Qi, Yu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wensheng Qi, cWl3ZW5zaGVuZ2dhbUAxNjMuY29t; Daxing Yu, eGluZ3hpbmd5dTEyMUBzaW5hLmNvbQ==; Xin Zhao, ZWNobzAxMTFAc2luYS5jb20=
 Shuman Zhao1
Shuman Zhao1 Dan Dai
Dan Dai Daxing Yu
Daxing Yu