- 1Department of Pharmacology, Faculty of Pharmacy, The University of Lahore, Lahore Campus, Lahore, Pakistan
- 2Department of Pharmacology, Institute of Pharmacy, Faculty of Pharmaceutical and Allied Health Sciences, Lahore College for Women University, Lahore, Pakistan
- 3Department of Pharmacology, University of Health Sciences, Lahore, Pakistan
- 4Institute of Pharmaceutical Sciences, University of Veterinary and Animal Sciences, Lahore, Pakistan
- 5Department of Chemistry and Biochemistry, Faculty of Medicine and Pharmacy, Ibn Zohr University, Laayoune, Morocco
- 6Department of Food Science, Faculty of Agricultural and Food Sciences, Laval University, Quebec, QC, Canada
- 7Department of Pharmaceutics, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
- 8Department of Scientific Translation, Faculty of Translation, University of Bahri, Khartoum, Sudan
Background: Lawsone (2-hydroxy-1,4-naphthoquinone) is naturally present in Lawsonia Inermis and flowers of Eicchornia crassipes. This study assessed the anti-arthritic potential of Lawsone, using FCA-induced Sprague-Dawley rats.
Methods: Arthritic progress was analyzed through a macroscopic scoring scale, measurement of paw edema, and histopathological changes. Effects of Lawsone on mRNA expression levels of inflammatory markers were examined using the reverse transcription PCR technique. ELISA technique was used to evaluate the PGE2 levels. Moreover, levels of biochemical and hematological parameters were also analyzed.
Results: The research elucidated that Lawsone showed an inhibitory potential towards arthritic progress and ameliorated the paw edema. The histopathological analysis also validated the inhibition in arthritic development. Treatment with Lawosne reduced the expression levels of inflammatory markers in rats i.e., VEGF, TNF-α, MMP-2, MMP-3, NF-κB, IL-1β, and IL-6. PGE2 levels (all p < 0.001) were also found reduced in treatment groups. Lab investigations showed improved results of hematological and hepatic parameters in the treated groups as compared to the positive control. This study found no hepatotoxic or nephrotoxic effects of Lawsone in the test doses.
Conclusion: Lawsone possesses an anti-arthritic property which could be ascribed to its immunomodulatory and anti-inflammatory effects.
1 Introduction
RA is defined as an autoimmune multisystem disease, having primary symptoms like pain, inflammation of synovial membrane & peripheral joints, deterioration of articular tissues, morning stiffness, and constrained joint mobility (Scherer, Haupl, and Burmester, 2020).
It involves multiple systems and has a complex pathology (Kaur and Sultana, 2012). RA involves both immune cells, i.e., B-cells, T-cells, macrophages, and soluble inflammatory mediators of our defense system (Yap et al., 2018). RA development is the consequence of the imbalance between the expressed values of pro-inflammatory and anti-inflammatory cytokines, resulting in the proliferation of cells in the synovial layer and infiltration of cytokines, chemokines, growth factors, and hormones in the joints RA (Bugatti et al., 2014). RA can also result in severe disabilities, hence compromising the quality of life in both physical and mental spheres (Matcham et al., 2014).
In the case of RA, the average mortality ratio varies from 1.28 to 2.89. The overall data from the population of developed countries shows the RA occurrence rate to be 0.5%–1% (Uroos et al., 2017; Li and Zhang, 2020). As per World Health Organization (WHO) report, RA causes 0.8% of total global years of life lost due to disability (YLD). According to the Global Burden of Disease (GBD) 2017 report, the prevalence of RA is higher in developed countries, such as North America, Western Europe, and the Caribbean, followed by India and South America (Finckh et al., 2022). The risk of RA seems to be greater in women than men and also enhances with age (Shabbir et al., 2014). The prominent risk factors for RA are female gender, age, tobacco use, silica exposure, and obesity (Yap et al., 2018).
Inflammatory and edematous conditions like RA are usually treated by corticosteroids, non-steroidal anti-inflammatory drugs (NSAIDs), disease-modifying anti-rheumatic drugs (DMARDs), opiates and anti-TNFs. NSAIDs, like piroxicam, are preferred to be the “first-line” therapeutic drugs for the treatment of RA, as they restrict the upregulation of IL-6, IL-1, TNF-α, and pro-inflammatory prostaglandins by blocking the cyclooxygenase pathway of the arachidonic acid cascade. DMARDs, e.g., methotrexate, are immune-modulators that regulate a disturbed immune system (Uroos et al., 2017). American College of Rheumatology guidelines for the treatment of RA (2021) strongly recommends the use of methotrexate as monotherapy with moderate-to-high RA disease activity for DMARD naïve patients. These recommendations are over the use of sulfasalazine or hydroxychloroquine. The guidelines also acknowledge that short term glucocorticoids are often important to reduce the symptoms of RA before the beginning of onset of action of DMARDs. However, the duration of glucocorticoid therapy should be as short as possible and the lowest effective dose should be used. Along with this acknowledgment, the guidelines conditionally recommend the use of conventional synthetic DMARDs without short term glucocorticoids (Fraenkel et al., 2021).
DMARDs cause mild alopecia, rash, nausea, loss of appetite, raised rheumatoid nodules, oral ulcers and other neurological complications (Guo et al., 2018). Anti-TNFs drugs inhibit the expression of TNF and block the inflammatory response. But they cause headache, abdominal pain, vomiting, diarrhea, itching, bruising, bleeding, rash, cellulitis, and lower respiratory tract complications. Corticosteroids such as prednisone hormones, effectively reduce synovitis. However, long-term usage of corticosteroids also includes increase risk of osteoporosis, diabetes mellitus, peptic ulcer, gastrointestinal bleeding, cataracts, and infections. Short-term opiates, such as fentanyl and oxycodone, help in pain management in RA but the long-term ones are linked with side effects such as respiratory depression, dependence, and tolerance (Mobashar et al., 2020a).
All of this demands the development of a novel but safe anti-arthritic regimen (Uroos et al., 2017). There have been discovered many plant-based products that showed significant effects in the reduction of chronic joint inflammation, such as rheumatoid arthritis (Bang et al., 2009; Mubashir et al., 2014).
Lawsone (Lawsonia, 2-hydroxy-1,4 napthaquinone), has the orange-red colour artifact and is naturally present in Lawsonia Inermis and flowers of Eicchornia crassipes (Kapadia et al., 2013). It has anti-inflammatory, hepatoprotective, anti-bacterial, anti-fungal, molluscicidal, anti-parasitic, and antiplatelet and anti-cancer activities (Cape, Bowman, and Kramer, 2006; Adeli-Sardou et al., 2019). Its wound-healing activity was reported by using ethanolic extract of Lawsone which depicted a visible wound-healing effect in incision and excision models of rat. Lawsone increased the pentobarbitone-induced sleeping time in an anti-inflammatory model of rats. It is also used in folk medicine for treatment of inflammatory diseases (Badoni Semwal et al., 2014). The in vitro anti-inflammatory potential of the Lawsone complexes depicted the modulation in the activity of NF-κB and TNF-α with results similar to prednisolone (Vanco et al., 2017). It also proved to be effective the in amelioration of interleukins levels, resulting in the reduction of inflammation and edema (Biradar and Veeresh, 2013). A study has previously reported cytotoxic effects of lawsone which are probably mediated through the release of O2−, H2O2, and OH−. The study indicated that Lawsone is non-mutagenic (Sauriasari et al., 2007). This research assessed the anti-arthritic potential of Lawsone, using FCA-induced Sprague-Dawley rats.
2 Methodology
2.1 Test animals
Sprague-Dawley rats of both sexes, with a weight range of 150–250 g, were subjected to investigate the anti-arthritic property of Lawsone. Sprague-Dawley rats were obtained from the University of Veterinary & Animal Sciences, Lahore. Rats were fed with a standard pellet diet and water ad libitum. They were kept under 12 h dark/light cycles and average conditions of humidity (60%–70%) and temperature of (28°C ± 2°C) at the University of Veterinary & Animal Sciences, Lahore. They were familiarized with the surroundings 1 week earlier than the trial (Kyei, Koffuor, and Boampong, 2012).
2.2 Assessment of anti-arthritic effect
30 Sprague-Dawley rats were dispersed into 5 groups. Except for the negative control (group 1), arthritis was induced in the rest of the 4 groups by injecting FCA (0.1 mL) into the sub-plantar area, starting from day 0. Group 1 and arthritic control, i.e., group 2 were fed with normal saline (NS) per oral (Akhtar and Arham Shabbir, 2019), from day 8–22. The reference group (group 3) was dosed with piroxicam (10 mg/kg b.w., i.p.) (Shabbir et al., 2014). Group 4 was given Lawsone (100 mg/kg b.w., p.o.) and group 5 was given Lawsone (200 mg/kg b.w., p.o.) from day 8–22. The anti-inflammatory doses of Lawsone were selected from the literature review. Previously Lawsone has shown anti-inflammatory and anti-oxidant activity in in vivo rat models of inflammation at the doses of 100 and 200 mg/kg b.w. (Biradar and Veeresh, 2013). Lawsone was obtained from Alfa Aesar (LOT: 10192582).
2.3 Measurement of the arthritic score and paw edema
Morphological features of arthritis were analyzed macroscopically for all the rats on the 8th, 13th, 18th, and 23rd days (Akhtar and Arham Shabbir, 2019). A score of 0 was used for the normal paw. Scores 1 to 2 were for mild to moderate inflammation and erythema of digits. Score 3 was given to severe inflammation and erythema of digits. Score 4 was given to significant deformity and incapability of movement of limbs. The paw volume was also recorded on the same days using a digital vernier caliper.
2.4 Histopathological evaluation
The ankle joints of all groups, injected with FCA, were separated and fixed with 10% formalin, and afterward decalcified by a decalcifying agent (10% formic acid with 10% formalin). Then, samples were fixed with paraffin and stained by the H&E method, after cutting into slices of 5 µm thickness. Arthritic parameters such as bone eruption, formation of pannus, and intrusion of inflammatory cells were analyzed. Results were predicted through a scale having 0, 1, 2, 3, and 4 ranges for normal, mild, moderate, and gross variations respectively.
2.5 Analysis of mRNA expression levels of pro-inflammatory cytokines: TNF-α, IL-1β, IL-6, NF-κB, MMP-2, MMP-3, and VEGF
Blood samples of rats were collected and RNA is extracted using the Total RNA Isolation (TRIzol) method. A Nanodrop spectrophotometer (major science mini-300) was used to quantify the total RNA extracted. Then, cDNA was generated by following the RevertAid First Strand cDNA synthesis kit’s protocol (Thermo Scientific LOT 00960732). GAPDH was taken as a housekeeping gene. Primers of IL-1β, MMP-2, and MMP-3 were designed manually. Sequences of other primers, i.e., IL-6, TNF-α, NF-κB, and VEGF were followed from previously published papers, as exhibited in Table 1. cDNA (1 µL) was centrifuged with forward-reverse primer mix (1 µL), nuclease-free water, and PCR Master Mix (5 µL). The thermal cycler was operated for 35 cycles of the following 3 phases: denaturation (95°C for 10 s), annealing (according to temperatures displayed in Table 4) for 20 s, and subsequently extension (72°C for 30 s) cycles.
2.6 Determination PGE2 levels
Rat Prostaglandin E2 ELISA kit (Bioassay Technology Laboratory Cat No. E-0504Ra) was used to determine serum levels of PGE2. Results were obtained by measuring Optical Density (OD) using ELISA reader (BIORAD, PR 4100) at 450 nm wavelength.
2.7 Assessment of hematological parameters
An automated hemocytometer was used to calculate the hemoglobin (Hb) content as well as WBCs, RBCs, and platelet counts.
2.8 Biochemical parameters
Serum samples were processed to calculate the values of AST, ALT, urea and creatinine, through an automated chemistry analyzer.
2.9 Statistical analysis
Data were given as Mean ± SD, using one-way analysis of variance (ANOVA) and post hoc Tukey’s test, to arbitrate the significant difference among groups. Here, p < 0.05 was reflected as statistically significant.
3 Results
3.1 Lawsone repressed arthritic progress
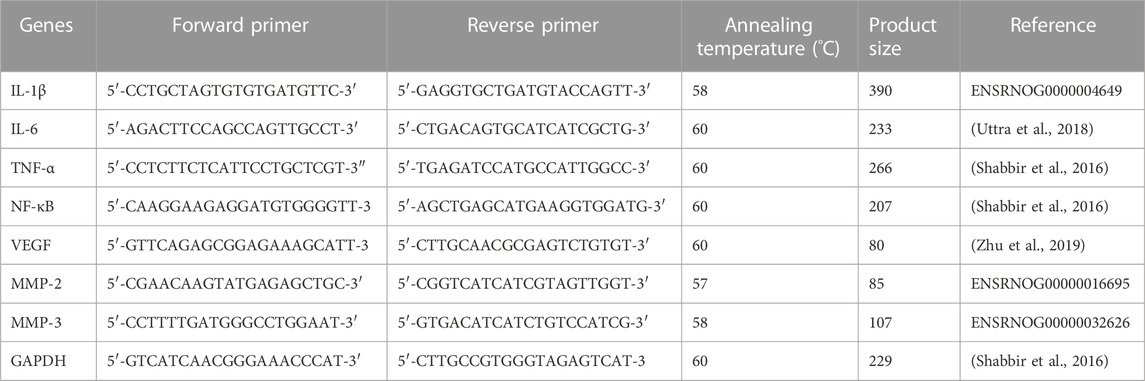
Arthritic development, manifesting swelling and erythema, was observed macroscopically after sub-planter induction of FCA. Treatment with Lawsone was initiated from day 8th till the 23rd day. The arthritic control group indicated a significant (p < 0.001) escalation in arthritic development than the vehicle control group since the 8th day. Lawsone treated groups displayed significant diminution in arthritis in contrast to group 2 on the 13th, 18th, and 23rd day respectively. On the 18th and 23rd days, macroscopic observation depicted a significant decrease in arthritis-related swelling and erythema in groups treated with Lawsone as shown in Figure 1.
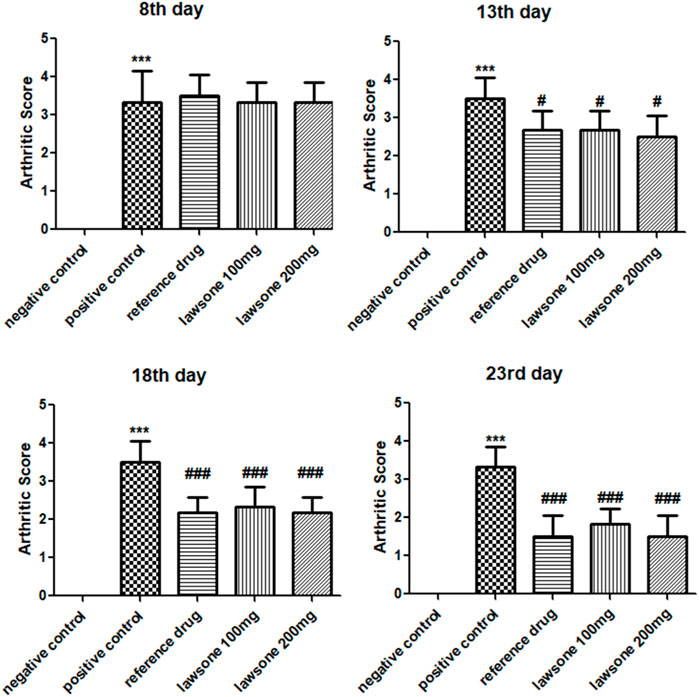
FIGURE 1. showing arthritic score in all groups after arthritic induction by FCA on 8th, 13th, 18th & 23rd day. Data is calculated as mean ± SD (n = 06). ***p < 0.001 in comparison to vehicle control group and #p < 0.05; ##p < 0.01; ###p < 0.001 in correlation to arthritic control group. Values of negative control group are zero, therefore are non-visible.
3.2 Lawsone decreased paw edema
After FCA induction, paw edema was measured in all groups of Sprague Dawley rats using a digital vernier caliper on the 8th, 13th, and 23rd days. Paw volumes observed on the 8th day exhibited a significant rise in paw edema than the vehicle control group. On the other hand, Lawsone treated groups displayed a significant decline in paw edema on the 13th day than the positive control group. On, the 23rd day, all treated groups exhibited significantly reduced levels of paw edema (p < 0.001) in contrast to the positive control group as presented in Figure 2.
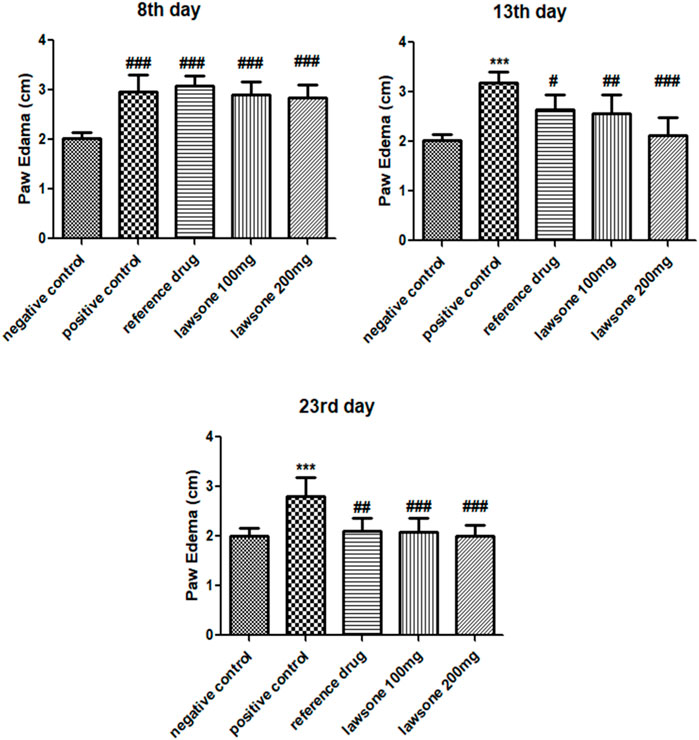
FIGURE 2. On 8th day, treatment groups were compared with negative control group, while on other days, they were compared with positive control. Data is calculated as mean ± SD (n = 06). ***p < 0.001 in correlation to vehicle control group and #p < 0.05; ##p < 0.01; ###p < 0.001 in correlation to arthritic control group.
3.3 Lawsone modulated histopathological parameters
Histopathological evaluation showed a significant reduction in arthritic score in the arthritic control group than the vehicle control group. All treated groups demonstrated attenuation of arthritic score at day 23 as shown in Figure 3 and Figure 4.
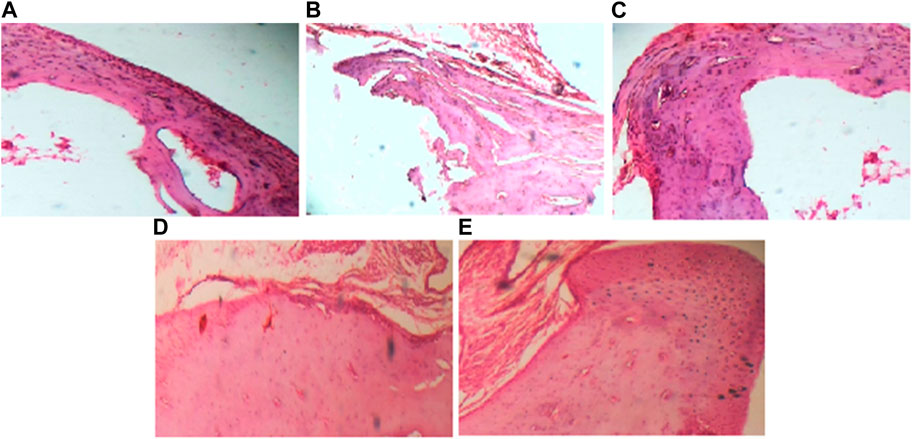
FIGURE 3. (40x) Histopathological evaluation of Sprague Dawley rats after FCA induction and treatment. (A) depicts negative control group; (B) depicts positive control group; (C) depicts reference drug group; (D) depicts group treated with Lawsone 100 mg; (E) depicts group treated with Lawsone 200 mg.
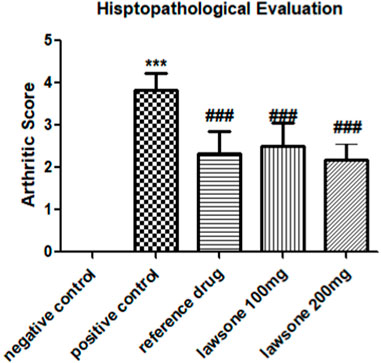
FIGURE 4. Histopathological evaluation of Sprague Dawley rats after FCA induction and treatment. Data is calculated as mean ± SD (n = 06). ***p < 0.001 as compared to vehicle control group and ###p < 0.001 in contrast to arthritic control group.
3.4 Lawsone declined the mRNA expression values of TNF-α, IL-1β, IL-6 & NF-κB
Blood samples treated with Lawsone were collected and processed. Significant downregulation of pro-inflammatory cytokines and matrix metalloproteinase enzymes was found, as shown in Figure 5. Arthritic control showed significant upregulation of TNF-α, IL-1β, and IL-6 in comparison to the vehicle control group. Expression levels of all these markers were significantly suppressed by the phytochemical as compared to the arthritic control group.
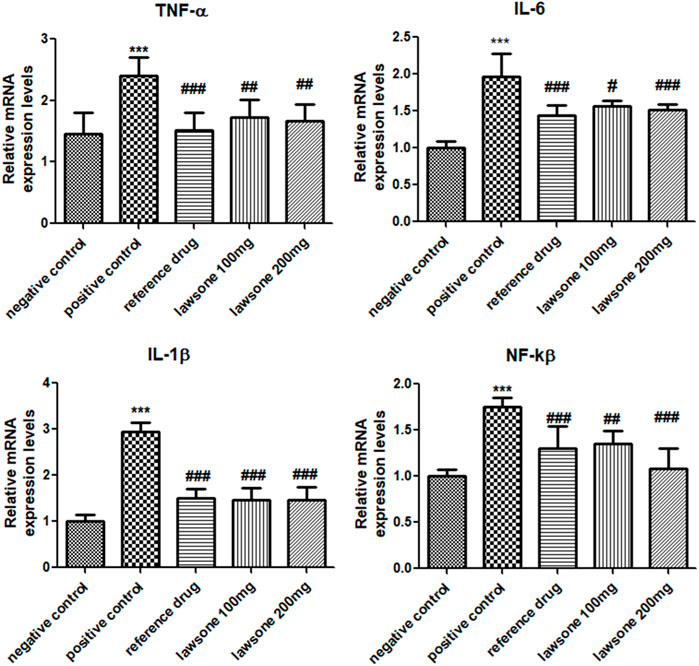
FIGURE 5. Lawsone reduced the mRNA expression values of TNF-α, IL-1β, IL-6 and NF-κB. Values are stated as mean ± SD (n = 06). ***p < 0.001 in contrast to vehicle control group and #p < 0.05; ###p < 0.001 in correlation to arthritic control group.
The positive control group displayed a significant (p < 0.001) escalation of NF-κB than the vehicle control group. Significantly (p < 0.001) reduced expressed values of NF-κB were found in Lawsone-treated groups than the arthritic control group.
3.5 Lawsone attenuated MMP-2, MMP-3 and VEGF expression levels
Significant (p < 0.001) upregulation of MMP-3, MMP-2 & VEGF in the arthritic control group was observed as opposed to the vehicle control group. However, suppression of MMP-3, MMP-2, and VEGF levels was found statistically significant after treatment with Lawsone than the positive control group as shown in Figure 6.
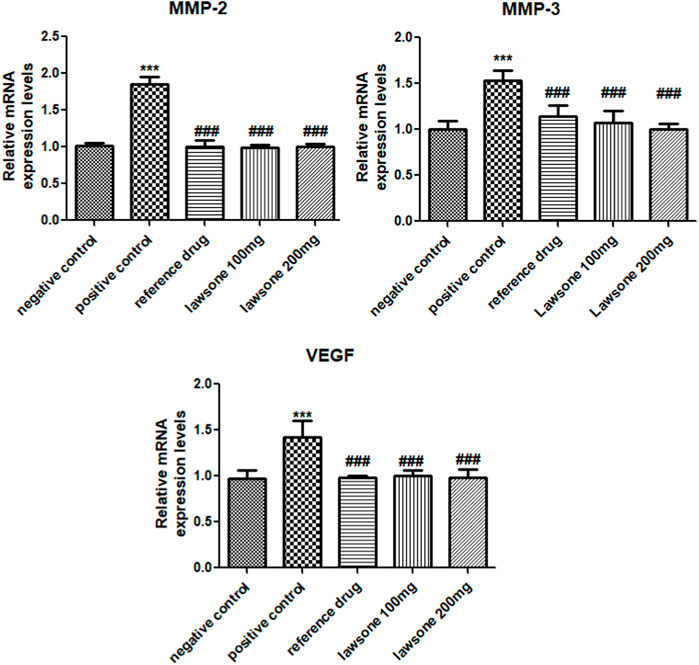
FIGURE 6. Lawsone declined the mRNA expression values of MMP-2, MMP-3 & VEGF. Values are calculated as mean ± SD (n = 06). ***p < 0.001 in contrast to vehicle control group and #p < 0.05; ##p < 0.001; ###p < 0.001 in contrast to arthritic control group.
3.6 Lawsone significantly reduced PGE2 levels
A substantial (p < 0.001) raise of PGE2 was depicted in the positive control group as compared to the negative control group. However, when groups treated with Lawsone showed a significant (p < 0.001) decline of PGE2 levels than the arthritic control group as shown in Table 2.
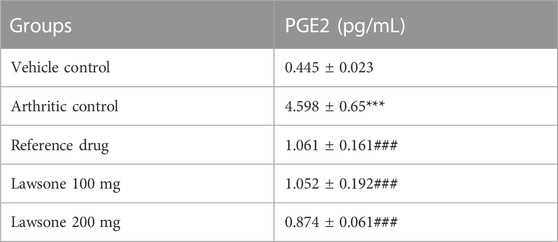
TABLE 2. Lawsone significantly declined PGE2 Levels as compared to arthritic control group. Data is stated as mean ± SD (n = 06). ***p < 0.001 in contrast to vehicle control and ###p < 0.001 as compared to arthritic control group.
3.7 Lawsone modulated hematological markers
A significant decline (p < 0.001) in RBC and Hb content was detected in the arthritic control group than in the vehicle control group. Lawsone treated groups showed an increase in RBC and Hb content to the positive control group. The data showed non-significant difference among all groups for TLC count.
However, the arthritic control group presented a significant elevation (p < 0.001) in PLT count than the vehicle control group. Lawsone treated groups showed lessened values (p < 0.05) of PLT count than the arthritic control group. Modulation in hematological markers is shown in Table 3.
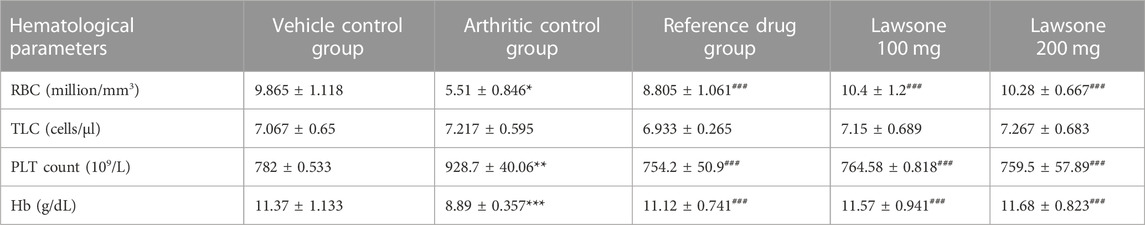
TABLE 3. Lawsone modulated Hematological Markers: Data is stated as mean ± SD (n = 06). **p < 0.01; ***p < 0.001 in contrast to negative control group and #p < 0.05; ##p < 0.01; ###p < 0.001 in correlation to positive control group.
3.8 Lawsone improved markers of liver function test
Significantly increased ALT (p < 0.001) and AST (p < 0.01) values were found in the arthritic control group than the vehicle control group. Hepatic parameters were found significantly improved in treated groups (Table 4).

TABLE 4. Lawsone improved markers of liver function test and showed no nephrotoxic effect: Values are stated as mean ± SD (n = 06). **p < 0.01; ***p < 0.001 in contrast to vehicle control and #p < 0.05; ##p < 0.01 in contrast to arthritic control group.
3.9 No nephrotoxic effect of lawsone
The values of urea and creatinine, in all groups, were not statistically significantly different as compared to each other. This depicts that Lawsone is safe in terms of renal parameters (Table 4).
4 Discussion
In our current research, the anti-arthritic effect of Lawsone was analyzed, by using an FCA-induced rat arthritic model (Mobashar et al., 2022). FCA was chosen to induce arthritis in the Sprague-Dawley rat model because the clinical and pathological changes induced by FCA are considered similar to those that appear in human RA, which includes increased paw-edema volumes and thickening of the soft-tissue at the site of injection (Patil et al., 2011). It is the autoimmune disorder of connective tissues, especially the joints, cartilage, and bones, causing their disability and other systemic disorders, influenced by both genetic (MHC genes) and environmental factors (smoking, diet, obesity, infections, and microbiota (Croia et al., 2019). The major cause of RA development is the imbalance between the expression levels of pro-inflammatory and anti-inflammatory cytokines. Upregulation of pro-inflammatory cytokines or downregulation of anti-inflammatory cytokines results in the development of RA (Mobashar et al., 2020b). Characteristic features of RA involve the conversion of the synovial membrane into pannus, destroying the surrounding cartilage and bone with the presence of activated macrophages and synovial fibroblasts that form matrix metalloproteinase (Gautam, Singh, and Nainwani, 2013). Common complications in RA involve neurological disorders, tendon rupture, and joint damage (Yap et al., 2018). Its symptoms also involve fever, fatigue, and weight loss (Mobashar et al., 2020a). Chronic RA can also affect some major organs like the lung, heart, skin, eyes, kidneys, and digestive system (Radu and Bungau, 2021; Maity and Wairkar, 2022). Arthritis can cause severe disability, thus compromising health, and may even cause premature death (Murugananthan et al., 2013; Choudhary et al., 2014).
Lawsone has been shown to possess anti-inflammatory and anti-oxidant properties. These properties were accredited with ameliorative effect of lawsone in acute pancreatitis (Biradar and Veeresh, 2013). Lawsone is also known to inhibit paw edema in rat model by suppressing the inflammatory markers like, TNF-α and NF-ĸB (Vanco et al., 2017). In a recent study, we found Lawsone as the major active ingredient of Eichhornia crassipes and validated the plant for anti-arthritic property. It was suggested to evaluate the phytochemical for its potential against arthritis in future (Sattar et al., 2023). Therefore, we designed the current study to evaluate the anti-arthritic property of Lawsone using similar inflammatory markers and protocols as mentioned in our previous publication. Current research showed that the Lawsone downregulated the inflammatory markers, both in vitro and in vivo, and inhibited the development of RA in the treated groups. The anti-arthritic potential was evident by attenuation of histopathological parameters, arthritic score, paw edema, and hematological markers.
Cytokines play a crucial role in triggering the auto-immune system, leading to severe synovitis and the destruction of articular tissues (Alunno et al., 2017; Helli et al., 2019). The inhibitory effect of Lawsone on the expression of pro-inflammatory markers (TNF-α, IL-1β, IL-6, NF-ĸB, and VEGF) and matrix metalloproteinase enzymes (MMP-2 and MMP-3) was determined in current study.
Increased values of TNF-α result in inflammation and articular damage by stimulating the expression of cytokines and encouraging the immune cells such as, B-lymphocytes, T-lymphocytes, and NK cells, to proliferate and differentiate (Farrugia and Baron, 2016). It also acts synergistically with IL-1 in the degradation of proteoglycans, resulting in joint damage (Schiff, 2000). Inhibition of TNF-α results in the downregulation of the expression of other inflammatory cytokines (Wiens et al., 2010). IL-1β is responsible for bone erosion and pannus formation in RA, by binding to IL-1 receptor, mostly expressed in cartilage pannus (Harrison et al., 2008). It can stimulate pro-inflammatory cytokines in the cell lines significantly, especially the transcription and translation of MMPs, IL-6, and TNF-α (Chen et al., 2019).
IL-6 exerts its pleiotropic activity after binding to its receptor and triggering events via JAK-mediated events in target cells. It targets the plasmablasts and causes the proliferation of autoantibodies. Moreover, IL-6 actively promotes the maturation of T follicular helper cells, which then release IL-21, which is also a factor for the differentiation of B cells. Overall dysregulation of IL-6 is an important factor that leads to a decrease in inflammation.
NF-κB is found in inactive form in cytoplasm, where an inhibitory protein IкB is attached with it. Various cytokines such as, TNF-α, IL-1β, and chemokines phosphorylate IкB resulting in activaton of NF-κB (Roman-Blas and Jimenez, 2016). Activated NF-ĸB is notorious for mediating synovial inflammatory processes by proliferation of fibroblast-like synoviocytes, triggering transcription and expression of pro-inflammatory cytokines, causing the production of osteoclasts which leads to bone erosion, unwanted apoptosis, initiation of Th1 activation, and upregulation of synovial cells (Mobashar et al., 2020b; Ding et al., 2023).
VEGF is responsible for synovial inflammation, hyperplasia, and angiogenesis in the affected joints. It is produced by rheumatoid synoviocytes and upregulated by pro-inflammatory cytokines, such as TNF-α, IL-1, and IL-8, and hypoxia. It initiates a self-perpetuating cycle of the inflammatory response by attaching to Flt-1 receptors on macrophages, leading to synovial hyperplasia through various signaling intermediates such as, MAPK, Phosphoinositide 3-kinase (PI3K), protein kinase B (AKT), phospholipase C-γ (PlC-γ) and small GTPases (Yoo et al., 2008). Elevated serum levels of VEGF are detected in RA patients more than healthy controls or patients with osteoarthritis, thus VEGF is now taken as one of the first indicators in the investigation of RA (Malemud, 2007).
MMPs are also notorious for the bone remodeling, differentiation, recruitment and migration of osteoblasts and osteocytes, and solubilization of osteoid. TNF-α, IL-6, and IL-1β activate MMP genes in RA synovial fibroblasts through the binding of several different transcription factors (Araki and Mimura, 2017).
It is well known that cytokines, e.g., IL-1 and TNF-α, and cytokine inducers increase the levels of PGE2 during immune response (Davidson et al., 2001). PGE2 is a lipid-mediator and is responsible for bone erosion, destruction of articular cartilage, vasodilation, extravasation of fluid, and acute pain (Fattahi and Mirshafiey, 2012) in RA. It evokes cAMP/PKA signaling pathway through four different prostanoid E (EP) receptors on synovium, chondrocytes, liver, and monocytic phagocytes (McCoy, Wicks, and Audoly, 2002).
In current study, treatment with Lawsone downregulated the relative mRNA expressions levels of inflammatory markers, which are mostly known to be amplified in immunomodulatory diseases (Seibl et al., 2003; Huang et al., 2007; Kim et al., 2007; Gheorghe et al., 2009). The downregulation of these pro-inflammatory markers by Lawsone indicated that the amelioration of RA could be ascribed to the phytochemical’s potential immunomodulatory and anti-inflammatory properties (Izhar et al., 2021).
The most important function in the activation of proinflammatory cytokines and prognosis of RA is played by the Janus kinase/signal transducers and activators of transcription (JAK/STAT) signal transduction pathway. Normally, JAK/STAT negative regulators and active STAT protein inhibitors restrict cytokine activation. However, both regulators are malfunctioned in RA, resulting in constant positive signaling of JAK/STAT pathway and consequently increased expression levels of MMPs (Malemud, 2023). MMPs degrade the collagen matrix and promotes the inavasion of synovial panuus into the articular cartilage through various cell signaling pathways, including MAPK, NF-κB, and AMP-activated protein kinase (AMPK) pathwyas (Araki and Mimura, 2017). It is well known that imbalance between Th1 and Th2 cells is a key factor in the pathogenesis of RA, where Th1 mediated cell variants predominantly differentiate the others. This predominance is achieved by p38 MAPK pathway and inhitbion of p38 activity can prevent the differentiation of Th1 cells (Ding et al., 2023). The anti-inflammatory effect of Lawsone could possibly be mediated by mentioned pathways, however, further studies are required to evaluate the effects of Lawsone on signaling pathways involved in pathogenesis of RA.
In the current study, lab investigations showed reduced values of RBC count and Hb content in the positive control group, which indicates the anemic condition of rats (Song et al., 2013). It may be due to the deficiency in the generation of cells as a result of declined bone marrow functioning and/or reduced iron storage in the reticuloendothelial system. Anemia in RA is mainly due to the alteration in the production and functioning of hepcidin and ferroportin, due to pro-inflammatory cytokines, especially 1L-6. It can lead to other diseases as well such as arteriosclerosis (Atwa et al., 2022). Moreover, the rise in PLT counts in the positive control group indicates the immunomodulatory as a defense response to attacking pathogens (Naz et al., 2020). Treatment with Lawsone normalized the levels of evaluated hematological parameters (RBC, PLT, and Hb content), Likewise, evaluation of hepatic and renal markers also proved the safety of Lawsone in terms of hepatotoxicity or nephrotoxicity.
4 Conclusion
The current research proved that Lawsone possesses significant anti-inflammatory and anti-arthritic properties against FCA-induced RA in animal models. Treatment with Lawsone reduced arthritic progress and paw edema along with histopathological parameters. Diminution of RA may be attributed to the decline in the expression levels of inflammatory parameters like TNF- α, IL-1β, IL-6, NF-ĸB, MMP-2, MMP-3, and VEGF by the phytochemical. Lawsone also reduced the levels of PGE2 and results were found comparable to piroxicam in amelioration of RA. Current study presented the data on mRNA expression levels of inflammatory markers. For further validation of the data, it is suggested to analyse protein levels of tested inflammatory markers in future studies using Western Blot technique.
Data availability statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statement
The animal study was approved by The current research is approved by the Institutional Research Ethics Committee, University of Lahore under IREC-2019-90. Notably, all animal experimentation was conducted in accordance with applicable laws, regulations, and guidelines, prioritizing animal welfare and minimizing any potential harm. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SS: Conceptualization, Data curation, Investigation, Writing–original draft. AS: Conceptualization, Methodology, Supervision, Writing–review and editing. MS: Formal analysis, Validation, Resources. TA: Formal analysis, Data curation, Visualization. SMA: Resources, Investigation, Validation. MB: Project administration, Resources, Writing–review and editing. H-AN: Funding acquisition, Visulalization, Software. YABJ: Funding acquisition, validation, Software. MD: Investigation, Resources, Project administration. AM: Validation, software, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research work was partially supported by Higher Education Commission Indigenous 5000 fellowship program (Phase 2, Batch IV) awarded to Ms. Sara Sattar. The authors would like to extend their sincere appreciation to the Researchers Supporting Project, King Saud University, Riyadh, Saudi Arabia for funding this work through the project number (RSP 2023R457).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
RA, Rheumatoid Arthritis; FCA, Freund’s Complete Adjuvant; H&E, Hematoxylin and Eosin; PGE2, Prostaglandin E2; ELISA, Enzyme-linked Immunosorbent Assay; ALT, Alanine Transaminase; AST, Aspartate Aminotransferase; Hb, Hemoglobin; CBC, Complete Blood Count; VEGF, Vascular Endothelial Growth Factor; TNF-α, Tumor Necrosis Factor- α; MMP-2 and MMP-3, matrix metalloproteinase; NF-κB, Nuclear Factor-Kappa B; IL-1β and IL-6, Interleukins.
References
Adeli-Sardou, M., Yaghoobi, M. M., Torkzadeh-Mahani, M., and Dodel, M. (2019). Controlled release of lawsone from polycaprolactone/gelatin electrospun nano fibers for skin tissue regeneration. Int. J. Biol. Macromol. 124, 478–491. doi:10.1016/j.ijbiomac.2018.11.237
Akhtar, G., and Arham Shabbir, (2019). Urginea indica attenuated rheumatoid arthritis and inflammatory paw edema in diverse animal models of acute and chronic inflammation. J. Ethnopharmacol. 238, 111864. doi:10.1016/j.jep.2019.111864
Alunno, A., Carubbi, F., Giacomelli, R., and Gerli, R. (2017). Cytokines in the pathogenesis of rheumatoid arthritis: new players and therapeutic targets. BMC Rheumatol. 1, 3. doi:10.1186/s41927-017-0001-8
Araki, Y., and Mimura, T. (2017). Matrix Metalloproteinase Gene Activation Resulting from Disordred Epigenetic Mechanisms in Rheumatoid Arthritis. Int. J. Mol. Sci. 18 (5), 905. doi:10.3390/ijms18050905
Atwa, E. T., Omar, H. M., Amin, A., and Hammad, M. (2022). Red cell distribution width and mean platelet volume in rheumatoid arthritis patients: Its association with disease activity. Reumatol. Clin. Engl. Ed. 18 (7), 399–405. doi:10.1016/j.reumae.2021.04.011
Badoni Semwal, R., Semwal, D. K., Combrinck, S., Cartwright-Jones, C., and Viljoen, A. (2014). Lawsonia inermis L. (henna): ethnobotanical, phytochemical and pharmacological aspects. J. Ethnopharmacol. 155 (1), 80–103. doi:10.1016/j.jep.2014.05.042
Bang, J. S., Oh, D. H., Choi, H. M., Sur, B. J., Lim, S. J., Kim, J. Y., et al. (2009). Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1beta-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res. Ther. 11 (2), R49. doi:10.1186/ar2662
Biradar, S., and Veeresh, B. (2013). Protective effect of lawsone on L-Arginine induced acute pancreatitis in rats. Indian J. Exp. Biol. 51 (3), 256–261.
Bugatti, S., Vitolo, B., Caporali, R., Montecucco, C., and Manzo, A. (2014). B cells in rheumatoid arthritis: From pathogenic players to disease biomarkers. Biomed. Res. Int. 2014, 681678. doi:10.1155/2014/681678
Cape, J. L., Bowman, M. K., and Kramer, D. M. (2006). Computation of the redox and protonation properties of quinones: towards the prediction of redox cycling natural products. Phytochemistry 67 (16), 1781–1788. doi:10.1016/j.phytochem.2006.06.015
Chen, J., Wu, W., Zhang, M., and Chen, C. (2019). Taraxasterol suppresses inflammation in IL-1β-induced rheumatoid arthritis fibroblast-like synoviocytes and rheumatoid arthritis progression in mice. Int. Immunopharmacol. 70, 274–283. doi:10.1016/j.intimp.2019.02.029
Choudhary, M., Kumar, V., Gupta, P. K., and Singh, S. (2014). Anti-arthritic activity of Barleria prionitis Linn. leaves in acute and chronic models in Sprague Dawley rats. Bull. Fac. Pharm. Cairo Univ. 52 (2), 199–209. doi:10.1016/j.bfopcu.2014.07.002
Croia, C., Bursi, R., Sutera, D., Petrelli, F., Alunno, A., and Puxeddu, I. (2019). One year in review 2019: pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 37 (3), 347–357.
Davidson, J., Abul, H. T., Milton, A. S., and Rotondo, D. (2001). Cytokines and cytokine inducers stimulate prostaglandin E2 entry into the brain. Pflugers Arch. 442 (4), 526–533. doi:10.1007/s004240100572
Ding, Q., Hu, W., Wang, R., Yang, Q., Zhu, M., Li, M., et al. (2023). Signaling pathways in rheumatoid arthritis: implications for targeted therapy. Signal Transduct. Target Ther. 8 (1), 68. doi:10.1038/s41392-023-01331-9
Farrugia, M., and Baron, B. (2016). The role of TNF-α in rheumatoid arthritis: a focus on regulatory T cells. J. Clin. Transl. Res. 2 (3), 84–90.
Fattahi, M. J., and Mirshafiey, A. (2012). Prostaglandins and rheumatoid arthritis. Arthritis 2012, 239310. doi:10.1155/2012/239310
Finckh, A., Gilbert, B., Hodkinson, B., Bae, S. C., Thomas, R., Deane, K. D., et al. (2022). Global epidemiology of rheumatoid arthritis. Nat. Rev. Rheumatol. 18 (10), 591–602. doi:10.1038/s41584-022-00827-y
Fraenkel, L., Bathon, J. M., England, B. R., St Clair, E. W., Arayssi, T., Carandang, K., et al. (2021). 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. Hob. 73 (7), 924–939. doi:10.1002/acr.24596
Gautam, R. K., Singh, D., and Nainwani, R. (2013). Medicinal plants having anti-arthritic potential: A review. Int. J. Pharm. Sci. Rev. Res. 19 (1), 96–102.
Gheorghe, K. R., Korotkova, M., Catrina, A. I., Backman, L., Af Klint, E., Claesson, H.-E., et al. (2009). Expression of 5-lipoxygenase and 15-lipoxygenase in rheumatoid arthritis synovium and effects of intraarticular glucocorticoids. Arthritis Res. Ther. 11 (3), R83–R11. doi:10.1186/ar2717
Guo, Q., Wang, Y., Xu, D., Nossent, J., Pavlos, N. J., and Xu, J. (2018). Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 6, 15. doi:10.1038/s41413-018-0016-9
Harrison, P., Pointon, J. J., Chapman, K., Roddam, A., and Wordsworth, B. P. (2008). Interleukin-1 promoter region polymorphism role in rheumatoid arthritis: a meta-analysis of IL-1B-511A/G variant reveals association with rheumatoid arthritis. Rheumatol. Oxf. 47 (12), 1768–1770. doi:10.1093/rheumatology/ken374
Helli, B., Shahi, M. M., Mowla, K., Jalali, M. T., and Haghighian, H. K. (2019). A randomized, triple-blind, placebo-controlled clinical trial, evaluating the sesamin supplement effects on proteolytic enzymes, inflammatory markers, and clinical indices in women with rheumatoid arthritis. Phytother. Res. 33 (9), 2421–2428. doi:10.1002/ptr.6433
Huang, Q. Q., Ma, Y., Adebayo, A., and Pope, R. M. (2007). Increased macrophage activation mediated through toll-like receptors in rheumatoid arthritis. Arthritis & Rheumatism Official J. Am. Coll. Rheumatology 56 (7), 2192–2201. doi:10.1002/art.22707
Izhar, H., Shabbir, A., Shahzad, M., Mobashar, A., and Ahmed, S. S. (2021). Phyllanthus reticulatus Prevents Ethanol-Induced Gastric Ulcer via Downregulation of IL-8 and TNF-α Levels. Evid. Based Complement. Altern. Med. 2021, 1734752. doi:10.1155/2021/1734752
Kapadia, G. J., Rao, G. S., Sridhar, R., Ichiishi, E., Takasaki, M., Suzuki, N., et al. (2013). Chemoprevention of skin cancer: effect of Lawsonia inermis L. (Henna) leaf powder and its pigment artifact, lawsone in the Epstein- Barr virus early antigen activation assay and in two-stage mouse skin carcinogenesis models. Anticancer Agents Med. Chem. 13 (10), 1500–1507. doi:10.2174/18715206113139990096
Kaur, G., and Sultana, S. (2012). Evaluation of antiarthritic activity of isoeugenol in adjuvant induced arthritis in murine model. Food Chem. Toxicol. 50 (8), 2689–2695. doi:10.1016/j.fct.2012.05.016
Kim, H.-R., Cho, M.-L., Kim, K.-W., Juhn, J.-Y., Hwang, S.-Y., Yoon, C.-H., et al. (2007). Up-regulation of IL-23p19 expression in rheumatoid arthritis synovial fibroblasts by IL-17 through PI3-kinase-NF-kappaB- and p38 MAPK-dependent signalling pathways. Rheumatology 46 (1), 57–64. doi:10.1093/rheumatology/kel159
Kyei, S., Koffuor, G. A., and Boampong, J. N. (2012). Antiarthritic effect of aqueous and ethanolic leaf extracts of Pistia stratiotes in adjuvant-induced arthritis in Sprague-Dawley rats. J. Exp. Pharmacol. 4, 41–51. doi:10.2147/JEP.S29792
Li, X. Z., and Zhang, S. N. (2020). Herbal compounds for rheumatoid arthritis: Literatures review and cheminformatics prediction. Phytother. Res. 34 (1), 51–66. doi:10.1002/ptr.6509
Maity, S., and Wairkar, S. (2022). Dietary polyphenols for management of rheumatoid arthritis: Pharmacotherapy and novel delivery systems. Phytother. Res. 36 (6), 2324–2341. doi:10.1002/ptr.7444
Malemud, C. J. (2007). Growth hormone, VEGF and FGF: involvement in rheumatoid arthritis. Clin. Chim. Acta 375 (1-2), 10–19. doi:10.1016/j.cca.2006.06.033
Malemud, C. J. (2023). The role of the JAK/STAT signal pathway in rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 10 (5-6), 117–127. doi:10.1177/1759720X18776224
Matcham, F., Scott, I. C., Rayner, L., Hotopf, M., Kingsley, G. H., Norton, S., et al. (2014). The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin. Arthritis Rheum. 44 (2), 123–130. doi:10.1016/j.semarthrit.2014.05.001
McCoy, J. M., Wicks, J. R., and Audoly, L. P. (2002). The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J. Clin. Invest. 110 (5), 651–658. doi:10.1172/JCI15528
Mobashar, A., Shabbir, A., Shahzad, M., and ul Hassan, S. (2020a). Evaluation of Immunomodulatory and Antiarthritic Potential of Trigonella gharuensis Extracts. Evid. Based Complement. Altern. Med. 2020, 8836080. doi:10.1155/2020/8836080
Mobashar, A., Shabbir, A., Shahzad, M., and Gobe, G. (2022). Preclinical Rodent Models of Arthritis and Acute Inflammation Indicate Immunomodulatory and Anti-Inflammatory Properties of Juglans regia Extracts. Evid. Based Complement. Altern. Med. 2022, 1695701. doi:10.1155/2022/1695701
Mobashar, A., Shabbir, A., Shahzad, M., and Hassan, S.Ul. (2020b). Evaluation of Immunomodulatory and Antiarthritic Potential of Trigonella gharuensis Extracts. Evid. Based Complement. Altern. Med. 2020, 8836080. doi:10.1155/2020/8836080
Mubashir, K., Ganai, B. A., Ghazanfar, K., and Akbar, S. (2014). Evaluation of Antiarthritic Potential of Methanolic Extract of Gentiana kurroo Royle. Arthritis 2014, 810615. doi:10.1155/2014/810615
Murugananthan, G., Kumar, G. S., Chethan, P. S., and Mohan, S. (2013). Anti-Arthritic and Anti-Inflammatory Constituents from Medicinal Plants. J. Appl. Pharm. Sci. 3.
Naz, R., Ahmed, Z., Shahzad, M., Shabbir, A., and Kamal, F. (2020). Amelioration of Rheumatoid Arthritis by Anacardium occidentale via Inhibition of Collagenase and Lysosomal Enzymes. Evid. Based Complement. Altern. Med. 2020, 8869484. doi:10.1155/2020/8869484
Patil, K. R., Patil, C. R., Jadhav, R. B., Mahajan, V. K., Patil, P. R., and Gaikwad, P. S. (2011). Anti-Arthritic Activity of Bartogenic Acid Isolated from Fruits of Barringtonia racemosa Roxb. (Lecythidaceae). Evid. Based Complement. Altern. Med. 2011, 785245. doi:10.1093/ecam/nep148
Radu, A. F., and Bungau, S. G. (2021). Management of Rheumatoid Arthritis: An Overview. Cells 10 (11), 2857. doi:10.3390/cells10112857
Roman-Blas, J. A., and Jimenez, S. A. (2016). NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthr. Cartil. 14 (9), 839–848. doi:10.1016/j.joca.2006.04.008
Seibl, R., Birchler, T., Loeliger, S., Hossle, J. P., E Gay, R., Saurenmann, T., et al. (2003). Expression and regulation of Toll-like receptor 2 in rheumatoid arthritis synovium. Am. J. pathology 162 (4), 1221–1227. doi:10.1016/S0002-9440(10)63918-1
Sattar, S., Shabbir, A., Shahzad, M., Akhtar, T., Ahmad, A., Alnasser, S. M., et al. (2023). Eichhornia crassipes Ameliorated Rheumatoid Arthritis by Modulating Inflammatory Cytokines and Metalloproteinase Enzymes in a Rat Model. Medicina 59 (9), 1594. doi:10.3390/medicina59091594
Sauriasari, R., Wang, D. H., Takemura, Y., Tsutsui, K., Masuoka, N., Sano, K., et al. (2007). Cytotoxicity of lawsone and cytoprotective activity of antioxidants in catalase mutant Escherichia coli. Toxicology 235 (1-2), 103–111. doi:10.1016/j.tox.2007.03.019
Scherer, H. U., Haupl, T., and Burmester, G. R. (2020). The etiology of rheumatoid arthritis. J. Autoimmun. 110, 102400. doi:10.1016/j.jaut.2019.102400
Schiff, M. H. (2000). Role of interleukin 1 and interleukin 1 receptor antagonist in the mediation of rheumatoid arthritis. Ann. Rheum. Dis. 59 (1), i103–i108. doi:10.1136/ard.59.suppl_1.i103
Shabbir, A., Shahzad, M., Ali, A., and Zia-ur-Rehman, M. (2014). Anti-arthritic activity of N'-[(2,4-dihydroxyphenyl)methylidene]-2-(3,4-dimethyl-5,5-dioxidopyrazolo[4,3-c] [1,2]benzothiazin-1(4H)-yl)acetohydrazide. Eur. J. Pharmacol. 738, 263–272. doi:10.1016/j.ejphar.2014.05.045
Shabbir, A., Shahzad, M., Ali, A., and Zia-ur-Rehman, M. (2016). Discovery of New Benzothiazine Derivative as Modulator of Pro- and Anti-inflammatory Cytokines in Rheumatoid Arthritis. Inflammation 39 (6), 1918–1929. doi:10.1007/s10753-016-0427-y
Song, S. N., Iwahashi, M., Tomosugi, N., Uno, K., Yamana, J., Yamana, S., et al. (2013). Comparative evaluation of the effects of treatment with tocilizumab and TNF-alpha inhibitors on serum hepcidin, anemia response and disease activity in rheumatoid arthritis patients. Arthritis Res. Ther. 15 (5), R141. doi:10.1186/ar4323
Uroos, M., Abbas, Z., Sattar, S., Umer, N., Shabbir, A., Rehman Shafiq Ur, , et al. (2017). Nyctanthes arbor-tristis Ameliorated FCA-Induced Experimental Arthritis: A Comparative Study among Different Extracts. Evid. Based Complement. Altern. Med. 2017, 4634853. doi:10.1155/2017/4634853
Uttra, A. M., Alamgeer, M. S., Shabbir, A., and Jahan, S. (2018). Ephedra gerardiana aqueous ethanolic extract and fractions attenuate Freund Complete Adjuvant induced arthritis in Sprague Dawley rats by downregulating PGE2, COX2, IL-1β, IL-6, TNF-α, NF-kB and upregulating IL-4 and IL-10. J. Ethnopharmacol. 224, 482–496. doi:10.1016/j.jep.2018.06.018
Vanco, J., Travnicek, Z., Hosek, J., and Suchy, P. (2017). In vitro and in vivo anti-inflammatory active copper(II)-lawsone complexes. PLoS One 12 (7), e0181822. doi:10.1371/journal.pone.0181822
Wiens, A., Venson, R., Correr, C. J., Otuki, M. F., and Pontarolo, R. (2010). Meta-analysis of the efficacy and safety of adalimumab, etanercept, and infliximab for the treatment of rheumatoid arthritis. Pharmacotherapy 30 (4), 339–353. doi:10.1592/phco.30.4.339
Yap, H. Y., Tee, S. Z., Wong, M. M., Chow, S. K., Peh, S. C., and Teow, S. Y. (2018). Pathogenic Role of Immune Cells in Rheumatoid Arthritis: Implications in Clinical Treatment and Biomarker Development. Cells 7 (10), 161. doi:10.3390/cells7100161
Yoo, S., Kwok, S., and Kim, an W. (2008). Proinflammatory Role of Vascular Endothelial Growth Factor in the Pathogenesis of Rheumatoid Arthritis: Prospects for Therapeutic Intervention. Mediat. Inflamm. 2008, 129873. doi:10.1155/2008/129873
Keywords: arthritis, inflammation, cytokines, rheumatism, phytochemical
Citation: Sattar S, Shabbir A, Shahzad M, Akhtar T, Anjum SM, Bourhia M, Nafidi H-A, Bin Jardan YA, Dauelbait M and Mobashar A (2023) Evaluation of anti-inflammatory and immunomodulatory potential of Lawsone (2-hydroxy-1,4-naphthoquinone) using pre-clinical rodent model of rheumatoid arthritis. Front. Pharmacol. 14:1279215. doi: 10.3389/fphar.2023.1279215
Received: 17 August 2023; Accepted: 29 September 2023;
Published: 13 October 2023.
Edited by:
Xianyu Li, China Academy of Chinese Medical Sciences, ChinaReviewed by:
Ali Sharif, Lahore College for Women University, PakistanLiufeng Mao, Guangdong Pharmaceutical University, China
Copyright © 2023 Sattar, Shabbir, Shahzad, Akhtar, Anjum, Bourhia, Nafidi, Bin Jardan, Dauelbait and Mobashar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arham Shabbir, YXJoYW0uc2hhYmJpckBsY3d1LmVkdS5waw==; Musaab Dauelbait, bXVzYWFiZWxuYWltQGdtYWlsLmNvbQ==
 Sara Sattar1
Sara Sattar1 Arham Shabbir
Arham Shabbir Mohammed Bourhia
Mohammed Bourhia Yousef A. Bin Jardan
Yousef A. Bin Jardan Musaab Dauelbait
Musaab Dauelbait