- 1Department of Health Services Administration, China Medical University, Taichung, Taiwan
- 2Department of Pharmacology, Chung Shan Medical University, Taichung, Taiwan
- 3Department of Pharmacy, Chung Shan Medical University Hospital, Taichung, Taiwan
- 4School of Medicine, Chung Shan Medical University, Taichung, Taiwan
Background: Recent studies have demonstrated that patients with type 2 diabetes mellitus (T2DM) who receive metformin have a decreased risk of developing age-related macular degeneration (AMD). However, other studies have also suggested that metformin may increase the risk of AMD development. Therefore, this study investigated the association between treatment with metformin and the risk of AMD in patients with T2DM by using Taiwan’ National Health Insurance Research Database.
Methods: Patients who received a diagnosis of new-onset T2DM between 2002 and 2013 were enrolled in this study. The patients were divided into patients treated and not treated with metformin to evaluate the risk of AMD after 5 years of follow-up. The logistic regression was used to estimate the risk of AMD associated with the intensity of treatment with metformin.
Result: A total of 7 517 patients (103.16 patients per 10,000 people) developed AMD in 5 years after DM diagnosis. After adjusting for the relevant variables, patients with T2DM treated with <5 defined daily dose (DDD)/month of metformin had a lower risk of AMD (odds ratios [OR]: 0.93; 95% confidence interval [CI]: 0.88 0.99). Patients treated with >25 DDD/month of metformin had a higher risk of AMD (OR: 1.39; 95% CI: 1.08-1.78).
Conclusion: Metformin use may be associated with a risk of AMD among patients with T2DM in a dose-dependent association manner, with the greater benefit at lower DDD/month. However, higher DDD/month exhibited an increased risk of AMD.
Introduction
Age-related macular degeneration (AMD) is the major cause of central irreversible blindness or visual loss among patients aged >50 years in developed countries (Chakravarthy et al., 2010). AMD is typically classified into early and late forms. Patients with early AMD are usually asymptomatic, whereas patients in the late stage of AMD may develop severe progressive vision loss. AMD can be categorized into the 2 following clinical types: nonexudative (dry) and exudative (wet) AMD (Fernandes et al., 2022). Incidence rates of AMD lesions increase substantially with age (Mitchell et al., 2002).
The pathogenesis of AMD is complicated and can be associated with several risk factors, including aging, ocular disorders, systemic diseases, cigarette smoking, diet, body mass index, genetic susceptibility, and environmental conditions (Lim et al., 2012; Ersoy et al., 2014). Studies have investigated whether type 2 diabetes mellitus (T2DM) play a role in AMD development and progression. Several studies have found a positive correlation between T2DM and AMD (Nitsch et al., 2008; Topouzis et al., 2009; Chen et al., 2014; He et al., 2018), whereas some other studies expressed no such effect (Fraser-Bell et al., 2008; Xu et al., 2009). In addition, an inverse association was observed in the Age-Related Eye Disease Study (Clemons et al., 2006).
Several retrospective clinical studies demonstrated that metformin may have a potential role in AMD development (Chen et al., 2019; Lee et al., 2019; Blitzer et al., 2021), while active treatment with metformin is associated an increased risk of dry AMD (Eton et al., 2022). In addition, a meta-analysis study show that metformin is not protective against AMD development (Romdhoniyyah et al., 2021). A study reported that treatment with metformin of low-to-moderate doses is associated with a lower risk of AMD, while higher doses of metformin use did not have reduced risk of AMD development (Blitzer et al., 2021). Conflicting data on the association between metformin exposure dosage and the risk of AMD development. Therefore, we conducted a large-scale nationwide study to determine the association between treatment with metformin and the risk of AMD in patients with T2DM by using data from the National Health Insurance Research Database (NHIRD).
Material and method
Data source
This study used the Longitudinal Health Insurance Database (LHID) from 2001 to 2018 as the study database provided by the Health and Welfare Data Science Center (HWDC) of the Ministry of Health and Welfare in Taiwan. The LHID encompasses data pertaining to every individual who is registered within Taiwan’s National Health Insurance (NHI) program. The NHI contains health insurance claims data for 99% of Taiwan’s 23 million residents. Disease diagnoses were coded according to the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) and ICD, 10th Revision, Clinical Modification (ICD-10-CM). The NHIRD can be used to obtain real-world evidence to support clinical decisions and healthcare policy-making (Chang et al., 2017; Hsieh et al., 2019; Lai et al., 2020). Therefore, we used data from the LHID to analyze the dose-response association of metformin use and risk of AMD among T2DM patients in Taiwan.
Ethics approval
This study was exempted from informed consent because the personal identification data were encrypted and transformed in the LHID. This study protocol was approved by the Central Regional Research Ethics Committee of China Medical University, Taiwan (No. CRREC-109-011).
Study participants
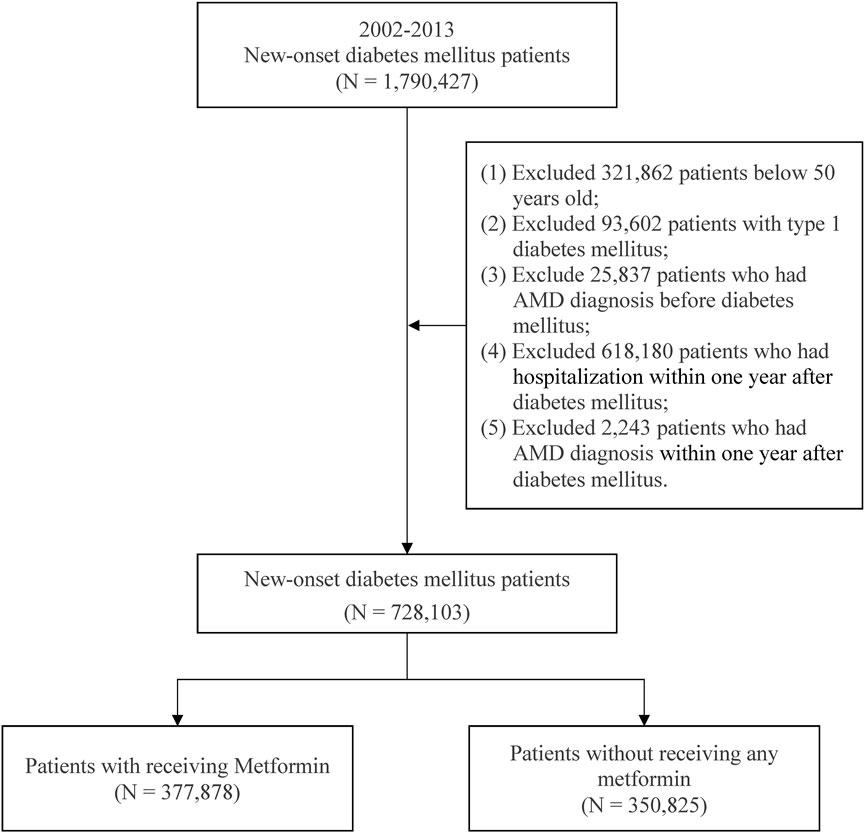
This study enrolled patients with new-onset diabetes mellitus (DM) aged ≥50 years from 2002 to 2013. DM (ICD-9-CM: 250) was indicated by the presence of 3 outpatient diagnoses. Metformin of the present study was defined according to the Anatomical Therapeutic Chemical (ATC) code A10BA02. The study participant exclusion criteria contained (Chakravarthy et al., 2010) type 1 DM patients, (Fernandes et al., 2022), patients having a diagnosis of AMD before DM, (Mitchell et al., 2002), patients having a diagnosis of AMD in the first year after DM, and (Lim et al., 2012) patients hospitalized within 1 year after DM diagnosis. After selection (Figure 1). There were a total of 728 703 patients with new-onset DM were included in the study. Patients treated with and without metformin were 377 878 patients and 350 825 patients, respectively.
Study design
This study was a cross-sectional study and used the defined daily dose (DDD) for assessing metformin intake. The DDD is characterized by the World Health Organization as the anticipated average daily maintenance dose for adults. However, the DDD does not necessarily reflect the recommended or prescribed daily dose (Grimmsmann and Himmel, 2011). The DDD of metformin is 2 g (Wellington, 2005), and the observation period prior to treatment with metformin in the present study was 1 year after DM. Based on the study design from previous studies (Chang et al., 2018; Huang et al., 2022a; Huang et al., 2022b), we categorized patients according to the average monthly DDD (expressed as DDD/month) into 5 ranges: 0, <5, 5 15, 15–25, and >25, respectively.
All patients were observed for a 5-year period to investigate the risk of incident AMD. The definition of incident AMD (ICD-9-CM: 362.50-362.52, 362.57; ICD-10-CM: H35.31-H35.32, H35.36) was indicated by 3 or more outpatient visits within 1 year. Control variables were sex, age, income level, urbanization, diabetes complications severity (DCSI), and AMD-related comorbidities. The DCSI was used to assess the severity of diabetes (Young et al., 2008; Chang et al., 2012). The comorbidities were hyperlipidemia (ICD-9-CM: 272.0-272.4), hyperuricemia (ICD-9-CM: 790.6), cerebrovascular disease (CVD; ICD-9-CM: 430-438), obesity (ICD-9-CM: 278.00), alcoholism (ICD-9-CM: 303), nonalcoholic fatty liver disease (NAFLD; ICD-9-CM: 571.8), rheumatoid arthritis (RA; ICD-9-CM: 714), hypothyroidism (ICD-9-CM: 244.9), hepatitis B virus (HBV; ICD-9-CM: 070.33), hepatitis C virus (HCV; ICD-9-CM: 070.54), sleep disturbance (ICD-9-CM: 780), systematic lupus erythematosus (SLE; ICD-9-CM: 710.0), chronic kidney disease (CKD; ICD-9-CM: 585), migraine (ICD-9-CM: 346.90), and hyperthyroidism (ICD-9-CM: 242.9).
Statistical analysis
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, United States). The chi-square test was used to evaluate differences between patients treated with and without metformin. Multiple logistic regression was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for AMD risk after adjustment for sex, age, income level, urbanization, diabetes severity, and comorbidities. All statistical results with p < .05 were regarded as statistically significant.
Results
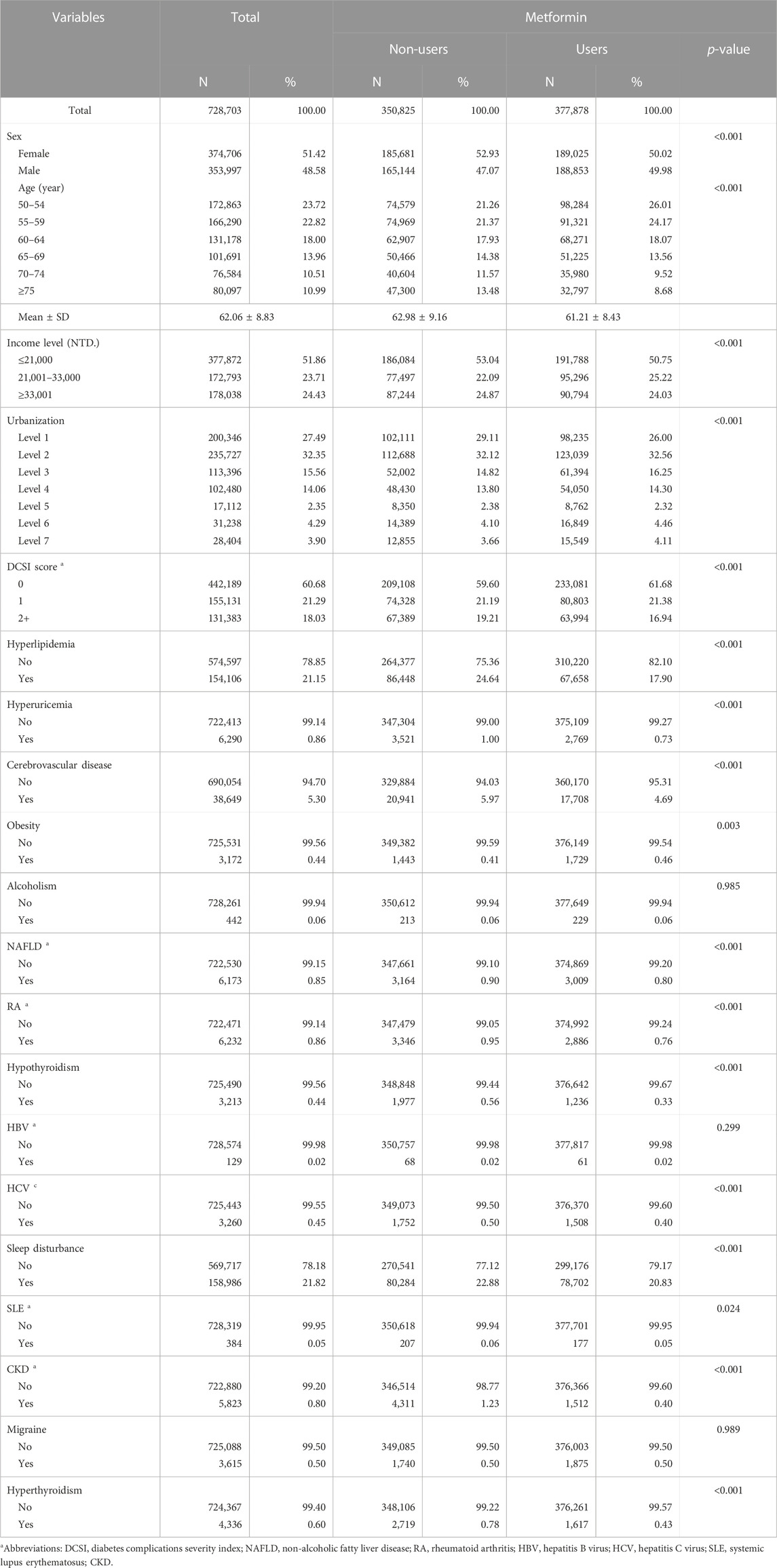
Table 1 presents the baseline characteristics of all patients. The average age of all patients was 62.06 ± 8.83 years, and 51.42% of all patients were women. Regarding age groups, 23.72% were 50–54 years old, 22.82% were 55–59 years old, 18.00% were 60–64 years old, 13.96% were 65–69 years old, 10.51% were 70–74 years old, and 10.99% were above 75 years old. In patients treated with metformin, the average age was 61.21 ± 8.43 years.
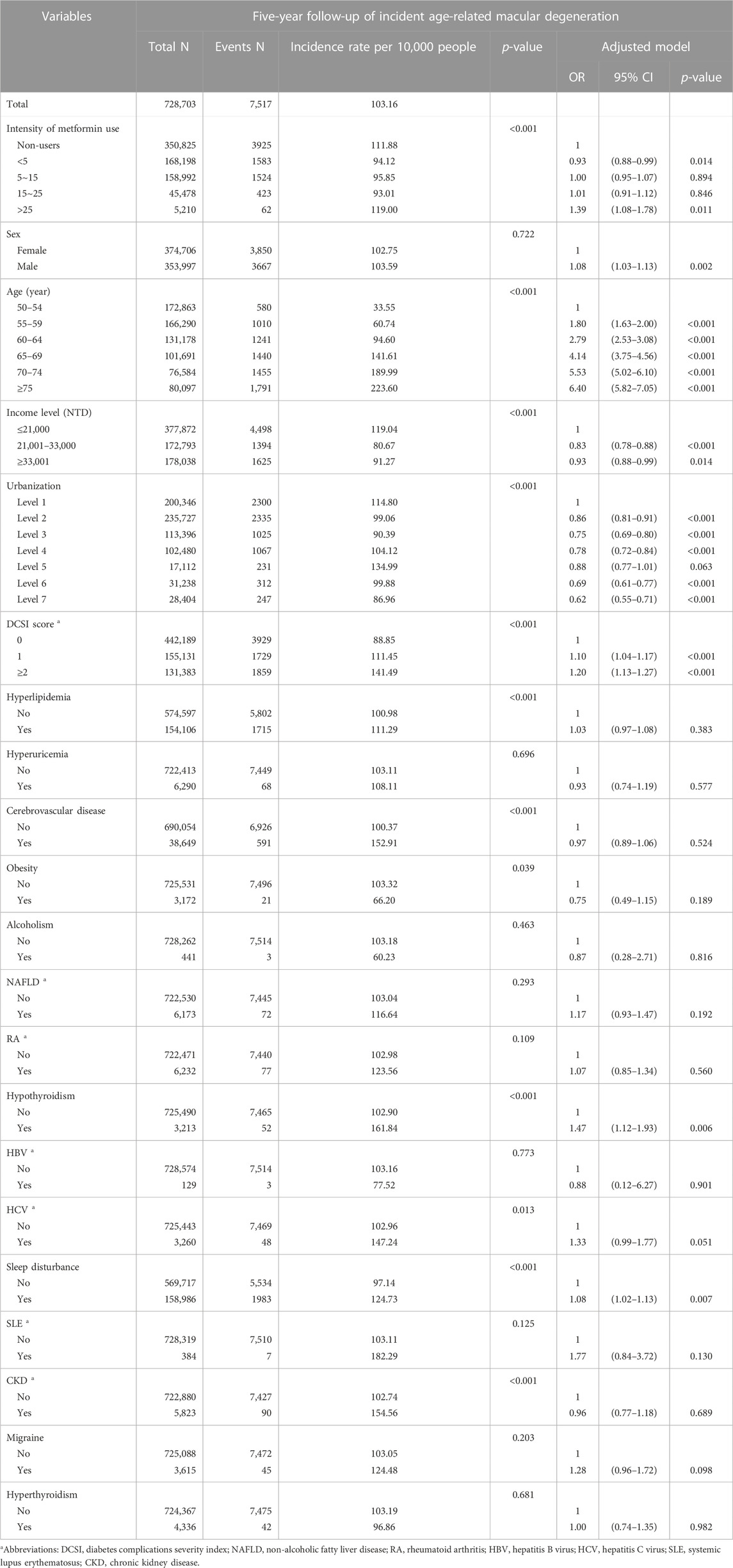
Table 2 presents the incidence rate per 10,000 people of AMD and the risk of AMD after 5 years of follow-up. Patients not treated with metformin were 350 825 and the incidence rate of AMD was 111.88 patients per 10,000 people; patients treated with metformin <5 DDD/month were 168 198 and the incidence rate of AMD was 94.12 patients per 10,000 people; patients treated with metformin 5–15 DDD/month were 158 992 and the incidence rate of AMD was 95.85 patients per 10,000 people; patients treated with metformin 15–25 DDD/month were 45 478 and the incidence rate of AMD was 93.01 patients per 10,000 people; patients treated with metformin >25 DDD/month were 5210 and the incidence rate of AMD was 119.00 patients per 10,000 people.
After adjusting for the relevant variables containing sex, age, income level, urbanization, DCSI, and AMD-related comorbidities, we determined that patients with DM treated with metformin at <5, 5–15, 15–25, and >25 DDD/month for AMD had ORs of 0.93 (95% CI: 0.88–0.99), 1.00 (95% CI: 0.95-1.07), 1.01 (95% CI: 0.91-1.12), and 1.39 (95% CI: 1.08-1.78), respectively. Patients aged ≥75 years had an OR of 6.40 (95% CI: 5.82-7.05) compared to patients aged 50–54 years. Patients with a DCSI score of 2 had a higher risk of AMD (OR: 1.20, 95% CI: 1.13-1.27). Moreover, Patients with comorbid hypothyroidism (OR: 1.47, 95% CI: 1.12-1.93), sleep disturbance (OR: 1.08, 95% CI: 1.02-1.13) had a higher risk of AMD at 5-year follow-up. However, patients with comorbid hyperlipidemia, hyperuricemia, CVD, obesity, alcoholism, NAFLD, RA, HBV, HCV, SLE, CKD, migraine, or hyperthyroidism did not exhibit a notable risk of AMD.
Discussion
This study found that treatment with metformin may be associated with the risk of AMD among patients with T2DM in a dose-response relationship manner. The results suggest that the intensity of treatment with metformin <5 DDD/month is associated with a lower risk of AMD at 5 years after initial DM diagnosis. However, patients with T2DM treated with >25 DDD/month of metformin experienced higher risks of AMD at 5 years. In addition, we found that among patients T2DM treated with metformin, older patients and patients with a higher DCSI score had a higher risk of AMD. Furthermore, patients with T2DM with comorbid sleep disturbance and hypothyroidism had a higher risk of AMD.
DM may play a significant role in the progression and development of AMD. Previous studies have demonstrated a positive correlation between DM and AMD (Nitsch et al., 2008; Topouzis et al., 2009; Choi et al., 2011; Chen et al., 2014; Khotcharrat et al., 2015; Vassilev et al., 2015; He et al., 2018). Several pathophysiological mechanisms may be associated with DM and AMD. Oxidative stress and chronic inflammation may explain the correlation between DM and the risk of AMD. Oxidative stress causes outer blood–retinal barrier degeneration that contributes to AMD progression (Jung et al., 2022), and oxidative stress is a risk factor for the development of insulin resistance through insulin signal disruption (Houstis et al., 2006; Newsholme et al., 2019).
Metformin achieves its antioxidative and anti-inflammatory effects through the activation of AMP-activated protein kinase (AMPK) (Lee et al., 2013; Zhao et al., 2020) and reduction in reactive oxygen species (Hou et al., 2010). Recent studies have demonstrated that AMPK plays a major role in the regulation of systemic glucose homeostasis and metabolic stress. AMPK is a conserved energy sensor and master regulator of glucose metabolism, which restores cellular energy balance during metabolic stress (Garcia and Shaw, 2017) and might be involved in AMD pathogenesis (Brown et al., 2019a). Metformin inhibited oxidative stress on human retinal pigment epithelium (RPE) cells by stimulating the AMPK signaling pathway in a mouse model of AMD (Xu et al., 2018). Antioxidant and anti-inflammatory effects of metformin can protect the RPE cells against the lesions of early AMD (Jiang et al., 2022).
Our findings demonstrated that patients with T2DM treated with <5 DDD/month of metformin had a lower risk of AMD at 5 years after initial DM diagnosis. Animal studies and physiology studies have suggested that metformin may play a beneficial role in the prophylaxis of AMD (Amin et al., 2022). Several studies suggested that metformin may have a role in AMD development and progression (Romdhoniyyah et al., 2021; Chen et al., 2019; Blitzer et al., 2021). A large-scale study reported the protective outcomes of metformin use in the development of AMD, with a 42% reduction (Brown et al., 2019b). A systematic review and meta-analysis study found that treatment with metformin is not associated with a significant lower risk of AMD (Romdhoniyyah et al., 2021). Another large case-control study reported that treatment with metformin is associated with a lower risk of AMD, with the lowest ORs associated with low-to-moderate doses (Blitzer et al., 2021). This study suggests that metformin use more than 2 years in patients aged 55 years and older is correlated with 5%–10% decreased odds ratio of AMD development.
Our findings revealed that patients treated with >25 DDD/month of metformin exhibited a higher risk of AMD after 5 years of follow-up. A case–control study observed no significant associations between AMD risk and cumulative duration or exposure of treatment with metformin (Lee et al., 2019). Another study with a small sample size found a conflicting relationship between metformin exposure and dry AMD, with the findings based on assessment of metformin cumulative dosage and the intensity of the treatment with metformin (Eton et al., 2022). A study based on medical claims from a large US insurer also indicated that conflicting associations between metformin exposure and development of dry AMD. Cumulative metformin dosage model showed a significant association between the risk of dry AMD with cumulative dosage, with the lowest dosage quartile associated with a decreased risk of dry AMD and the highest dosage associated with an increased risk (Eton et al., 2022). Active treatment with metformin is associated with an increased risk of dry AMD, whereas prior treatment with metformin is associated with decreased risk (Eton et al., 2022). Our findings are similar to a large nationwide case-control study revealed that the use of metformin may protect against AMD development in a dose-dependent manner (Blitzer et al., 2021). This research found that metformin may be useful as a preventive treatment for AMD with strongest at low to moderate doses, while higher dose did not have reduced risk of AMD development. This study reported that doses of greater than 1080 g of metformin use more than 2 years did not have decreased risk of AMD development, while was particularly for low to moderate doses of metformin revealed the greatest potential benefit (Blitzer et al., 2021). The greatest reduction in AMD risk was observed at metformin doses of 271–600 g over 2 years with an OR of 0.91, and doses of 1–270 g and 600–1080 g over 2 years were also correlated with decreased OR, 0.93 and 0.95, respectively (Blitzer et al., 2021).
Vitamin B12 deficiency may play a role in AMD development in patients with T2DM receiving long-term treatment with metformin. Treatment with metformin can induce vitamin B12 malabsorption by increasing bacterial overgrowth, altering gut bacterial flora in the enteric canal, and binding to the vitamin B12 intrinsic factor (Zhang et al., 2016). Malabsorption contributes to a decreased serum vitamin B12 plasma level. Current evidence suggests that metformin impairs vitamin B12 status in a dose-dependent and duration-dependent association manner (Infante et al., 2021). A meta-analysis suggest a negative association between metformin use and vitamin B12 plasma levels in T2DM patients (Chapman et al., 2016), and higher cumulative exposure and longer duration of metformin treatment were associated with an increased risk of vitamin B12 deficiency (Khattar et al., 2016; Huang et al., 2022a; Huang et al., 2022b; Huang et al., 2023). Patients received metformin with therapy duration ≥ 5 years and a metformin dose of ≥ 1500 mg/day for a duration of at least 6 month was associated vitamin B12 deficiency, especially the highest risk has been found in patients with a daily metformin dose of ≥ 2000 mg (Infante et al., 2021). T2DM patients received metformin dosage of >2,000 mg/day increased the risk of vitamin B12 deficiency 22 times (Ko et al., 2014). However, the underlying mechanism accounting for metformin-induced vitamin B12 deficiency in patients with long-term and high-dose of metformin use remains unclear. Nevertheless, the proposed underlying mechanisms due to the alteration in small intestine motility, resulting in small intestinal bacterial overgrowth and subsequent B12 deficiency or by directly decreasing vitamin B12 absorption (Ting et al., 2006; Damiao et al., 2016); malabsorption leads to a decreased serum vitamin B12 level. Vitamin B12 and homocysteine may play a role in reducing the risk of AMD. Vitamin B12 deficiencies, folate, or elevated serum homocysteine levels were used as predictors of a high risk of AMD (Gopinath et al., 2013). Vitamin B12 is essential for the conversion of homocysteine to methionine in the methionine cycle (Allen, 2012). Vitamin B12 deficiency can impair the remethylation of homocysteine; moreover, metformin-induced vitamin B12 deficiency is potentially associated with hyperhomocysteinemia (Russo et al., 2011). An animal study found that excess homocysteine levels on the structure and function of retinal pigment epithelial that contribute to the development of AMD-like features (Ibrahim et al., 2016). Human study have reported that plasma homocysteine level was elevated in patients with AMD and highlighted a strong correlation between hyperhomocysteinemia and the development of AMD (Huang et al., 2015). A cross-sectional study found that increased total serum homocysteine and low vitamin B12 concentrations were independently associated with a higher risk of AMD (Rochtchina et al., 2007). The beneficial effects of vitamin B12 and folate on the risk of AMD are partly mediated by lowering the concentration of serum homocysteine (Rochtchina et al., 2007). Although treatment with metformin can decrease the risk of AMD (Brown et al., 2019a; Xu et al., 2018; Jiang et al., 2022), when long-term and high-dose or high cumulative dosage of metformin use were associated with biochemical B12 deficiency and hyperhomocysteinemia (Russo et al., 2011), may offset the protection effect of metformin and could lead to enhance the risk of AMD (Rochtchina et al., 2007). Routine assessment of vitamin B12 levels in individuals treated with metformin should be considered (Aroda et al., 2016; Al-Hamdi et al., 2020). Due to the clinical benefits of metformin use, its associated side effects such as metformin-induced vitamin B12 deficiency is often overlooked in T2DM patients. However, the diagnosis of metformin-induced vitamin B12 deficiency may be difficult (Al-Hamdi et al., 2020). The underlying mechanisms of metformin cumulative dosage and the risk of AMD remain unclear. Thus, further prospective clinical trials are warranted to investigate the protective effect of metformin on AMD, especially regarding duration and dosage of therapy.
Our findings showed that T2DM patients treated with metformin, older patients, and having a higher DCSI score linked to an increased risk of AMD. Previous studies have identified several risk factors for AMD, including aging, ocular disorders, systemic diseases, smoking, diet, genetic susceptibility, and environmental risk factors (Lim et al., 2012), with aging being the strongest risk factor (Aldebert et al., 2018). In the general population, vitamin B12 plasma levels decline with age, and thus, the prevalence of vitamin B12 deficiency increases with age. Age is a strong risk factor for the development of AMD, and individuals aged <50 years have a lower risk of AMD (Jiang et al., 2022) compared with older adults, who also have a higher risk of vitamin B12 deficiency (Gonzalez-Gross et al., 2007). The DCSI is a useful tool for adjusting for baseline severity of disease and predicting mortality and the risk of hospitalization among patients with DM (Young et al., 2008). Our study showed that patients with T2DM treated with metformin with higher DCSI scores had an increased risk of AMD. Thus, DCSI may be used as an indicator for assessing the risk of AMD development.
Our study results demonstrated that patients with T2DM treated with metformin and with comorbid sleep disturbance and hypothyroidism had a higher risk of AMD. A Taiwan population-based study indicated that insomnia is an independent indicator of an increased risk of AMD (Tsai et al., 2020). Thyroid disease is associated with an increased risk of AMD (Xu et al., 2021).
This study adopted a population-based design and used data from the NHIRD. Because we included the entire Taiwanese population in this study, the sample size is large and sufficient for reducing selection bias and providing high-quality data. Second, the characteristics of the database provide sufficient statistical power for investigating the association between treatment with metformin and the risk of AMD among patients with T2DM. Finally, the intensity of treatment with metformin (DDD/month) was <5, 5–15, 15–25, >25 for determining the relationship between patients with T2DM and the risk of AMD.
This population-based cohort study has several limitations. First, information regarding family histories of AMD among patients with T2DM was unavailable. Second, patients’ personal data and their lifestyle habits, such as body mass index, cigarette smoking habits, alcohol consumption, dietary habits, and physical activity (factors that are associated with AMD risk), were unavailable. Due to the limitations of the Taiwan National Health Insurance inpatient medical claims system, the information on the medication dosage during hospitalization was lacking from the NHIRD. Therefore, the use of metformin during hospitalization was not included in the present study, which may result in an underestimation of metformin’s DDD in our study. Third, the diagnoses of AMD and other comorbidities were coded in accordance with the ICD-9-CM and ICD-10-CM. Nonetheless, the NHI Bureau of Taiwan randomly reviews the charts and interviews patients to assess the accuracy of the diagnoses, which improves the accuracy and validity of the NHIRD. Fourth, Information regarding biochemical parameters (e.g., fasting glucose, HbA1C, urine protein) is unavailable in the database but may affect developing AMD factors. The severity of DM and the disease duration of DM may also affect developing AMD. Therefore, the present study enrolled the new-onset DM patients as the study subjects and used the DCSI to adjust the severity of DM to reduce the bias. This study was a nationwide population-based study. Thus, the study results have accuracy and representativeness. Finally, this study is a type of epidemiology observational study that analyzes data from a nationwide database. The study result can only provide evidence to demonstrate that metformin is related to incident AMD. It is essential to obtain more information from other databases or questionnaires to conduct a prospective study or randomized controlled trial to analyze the cause-effect relation in future research.
Conclusion
In conclusion, this study provides evidence that treatment with metformin may be associated with the risk of AMD among patients with T2DM in a dose-dependent association manner. Patients treated with <5 DDD/month of metformin had a decreased risk of AMD at 5 years. However, >25 DDD/month exhibited an increased risk of AMD.
Data availability statement
The database used to support the findings of this study was provided by the Health and Welfare Data Science Center, Ministry of Health and Welfare (HWDC, MOHW) under license and so cannot be made freely available. Requests for access to these data should be made to HWDC (https://dep.mohw.gov.tw/dos/cp-5119-59201-113.html).
Ethics statement
The studies involving humans were approved by Central Regional Research Ethics Committee of China Medical University, Taiwan. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the database is anonymous to protect their privacy. The requirement for informed consent was waived.
Author contributions
K-HH: Conceptualization, Formal Analysis, Funding acquisition, Writing–original draft, Writing–review and editing. Y-LC: Conceptualization, Writing–original draft, Writing–review and editing. CL: Conceptualization, Writing–original draft, Writing–review and editing. S-YG: Conceptualization, Writing–original draft, Writing–review and editing. T-HT: Conceptualization, Formal Analysis, Writing–original draft, Writing–review and editing. N-JC: Conceptualization, Writing–original draft, Writing–review and editing. C-YL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Chung Shan Medical University Hospital, Taiwan (CSH-2023-C-003), Chung Shan Medical University, Taiwan (CSMU-INT-112-02), China Medical University Taiwan (CMU110-MF-120 and CMU111-MF-111), and the Ministry of Science and Technology Taiwan (MOST 109-2410-H-039-004-MY2).
Acknowledgments
We are grateful to China Medical University, Taiwan, Chung Shan Medical University, Taiwan, and Chung Shan Medical University Hospital, Taiwan for providing administrative, technical, and funding.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aldebert, G., Faillie, J. L., Hillaire-Buys, D., Mura, T., Carriere, I., Delcourt, C., et al. (2018). Association of anticholinergic drug use with risk for late age-related macular degeneration. JAMA Ophthalmol. 136 (7), 770–778. doi:10.1001/jamaophthalmol.2018.1719
Al-Hamdi, A., Al-Gahhafi, M., Al-Roshdi, S., Jaju, S., Al-Mamari, A., and Al Mahrezi, A. M. (2020). Vitamin B12 deficiency in diabetic patients on metformin therapy: a cross-sectional study from Oman. Sultan Qaboos Univ. Med. J. 20 (1), e90–e94. doi:10.18295/squmj.2020.20.01.013
Amin, S. V., Khanna, S., Parvar, S. P., Shaw, L. T., Dao, D., Hariprasad, S. M., et al. (2022). Metformin and retinal diseases in preclinical and clinical studies: insights and review of literature. Exp. Biol. Med. (Maywood). 247 (4), 317–329. doi:10.1177/15353702211069986
Aroda, V. R., Edelstein, S. L., Goldberg, R. B., Knowler, W. C., Marcovina, S. M., Orchard, T. J., et al. (2016). Long-term metformin use and vitamin B12 deficiency in the diabetes prevention program outcomes study. J. Clin. Endocrinol. Metab. 101 (4), 1754–1761. doi:10.1210/jc.2015-3754
Blitzer, A. L., Ham, S. A., Colby, K. A., and Skondra, D. (2021). Association of metformin use with age-related macular degeneration: a case-control study. JAMA Ophthalmol. 139 (3), 302–309. doi:10.1001/jamaophthalmol.2020.6331
Brown, E. E., Ball, J. D., Chen, Z., Khurshid, G. S., Prosperi, M., and Ash, J. D. (2019b). The common antidiabetic drug metformin reduces odds of developing age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 60 (5), 1470–1477. doi:10.1167/iovs.18-26422
Brown, E. E., Lewin, A. S., and Ash, J. D. (2019a). AMPK may play an important role in the retinal metabolic ecosystem. Adv. Exp. Med. Biol. 1185, 477–481. doi:10.1007/978-3-030-27378-1_78
Chakravarthy, U., Wong, T. Y., Fletcher, A., Piault, E., Evans, C., Zlateva, G., et al. (2010). Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 10, 31. doi:10.1186/1471-2415-10-31
Chang, H. Y., Weiner, J. P., Richards, T. M., Bleich, S. N., and Segal, J. B. (2012). Validating the adapted diabetes complications severity index in claims data. Am. J. Manag. Care 18 (11), 721–726.
Chang, S. H., Chou, I. J., Yeh, Y. H., Chiou, M. J., Wen, M. S., Kuo, C. T., et al. (2017). Association between use of non-vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA 318 (13), 1250–1259. doi:10.1001/jama.2017.13883
Chang, Y. T., Tsai, H. L., Kung, Y. T., Yeh, Y. S., Huang, C. W., Ma, C. J., et al. (2018). Dose-dependent relationship between metformin and colorectal cancer occurrence among patients with type 2 diabetes-A nationwide cohort study. Transl. Oncol. 11 (2), 535–541. doi:10.1016/j.tranon.2018.02.012
Chapman, L. E., Darling, A. L., and Brown, J. E. (2016). Association between metformin and vitamin B(12) deficiency in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab. 42 (5), 316–327. doi:10.1016/j.diabet.2016.03.008
Chen, X., Rong, S. S., Xu, Q., Tang, F. Y., Liu, Y., Gu, H., et al. (2014). Diabetes mellitus and risk of age-related macular degeneration: a systematic review and meta-analysis. PLoS One 9 (9), e108196. doi:10.1371/journal.pone.0108196
Chen, Y. Y., Shen, Y. C., Lai, Y. J., Wang, C. Y., Lin, K. H., Feng, S. C., et al. (2019). Association between metformin and a lower risk of age-related macular degeneration in patients with type 2 diabetes. J. Ophthalmol. 2019, 1649156. doi:10.1155/2019/1649156
Choi, J. K., Lym, Y. L., Moon, J. W., Shin, H. J., and Cho, B. (2011). Diabetes mellitus and early age-related macular degeneration. Arch. Ophthalmol. 129 (2), 196–199. doi:10.1001/archophthalmol.2010.355
Clemons, T. E., Rankin, M. W., and McBee, W. L.Age-Related Eye Disease Study Research Group (2006). Cognitive impairment in the age-related Eye disease study: AREDS report no. 16. Arch. Ophthalmol. 124 (4), 537–543. doi:10.1001/archopht.124.4.537
Damiao, C. P., Rodrigues, A. O., Pinheiro, M. F., Cruz, R. A. F., Cardoso, G. P., Taboada, G. F., et al. (2016). Prevalence of vitamin B12 deficiency in type 2 diabetic patients using metformin: a cross-sectional study. Sao Paulo Med. J. 134 (6), 473–479. doi:10.1590/1516-3180.2015.01382111
Ersoy, L., Ristau, T., Hahn, M., Karlstetter, M., Langmann, T., Droge, K., et al. (2014). Genetic and environmental risk factors for age-related macular degeneration in persons 90 years and older. Invest. Ophthalmol. Vis. Sci. 55 (3), 1842–1847. doi:10.1167/iovs.13-13420
Eton, E. A., Wubben, T. J., Besirli, C. G., Hua, P., McGeehan, B., and VanderBeek, B. L. (2022). Association of metformin and development of dry age-related macular degeneration in a U.S. insurance claims database. Eur. J. Ophthalmol. 32 (1), 417–423. doi:10.1177/1120672121997288
Fernandes, A. R., Zielinska, A., Sanchez-Lopez, E., Dos Santos, T., Garcia, M. L., Silva, A. M., et al. (2022). Exudative versus nonexudative age-related macular degeneration: physiopathology and treatment options. Int. J. Mol. Sci. 23 (5), 2592. doi:10.3390/ijms23052592
Fraser-Bell, S., Wu, J., Klein, R., Azen, S. P., Hooper, C., Foong, A. W., et al. (2008). Cardiovascular risk factors and age-related macular degeneration: the Los Angeles Latino Eye Study. Am. J. Ophthalmol. 145 (2), 308–316. doi:10.1016/j.ajo.2007.10.007
Garcia, D., and Shaw, R. J. (2017). AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol. Cell 66 (6), 789–800. doi:10.1016/j.molcel.2017.05.032
Gonzalez-Gross, M., Sola, R., Albers, U., Barrios, L., Alder, M., Castillo, M. J., et al. (2007). B-vitamins and homocysteine in Spanish institutionalized elderly. Int. J. Vitam. Nutr. Res. 77 (1), 22–33. doi:10.1024/0300-9831.77.1.22
Gopinath, B., Flood, V. M., Rochtchina, E., Wang, J. J., and Homocysteine, M. P. (2013). Homocysteine, folate, vitamin B-12, and 10-y incidence of age-related macular degeneration. Am. J. Clin. Nutr. 98 (1), 129–135. doi:10.3945/ajcn.112.057091
Grimmsmann, T., and Himmel, W. (2011). Discrepancies between prescribed and defined daily doses: a matter of patients or drug classes? Eur. J. Clin. Pharmacol. 67 (8), 847–854. doi:10.1007/s00228-011-1014-7
He, M. S., Chang, F. L., Lin, H. Z., Wu, J. L., Hsieh, T. C., and Lee, Y. C. (2018). The association between diabetes and age-related macular degeneration among the elderly in taiwan. Diabetes Care 41 (10), 2202–2211. doi:10.2337/dc18-0707
Hou, X., Song, J., Li, X. N., Zhang, L., Wang, X., Chen, L., et al. (2010). Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem. Biophys. Res. Commun. 396 (2), 199–205. doi:10.1016/j.bbrc.2010.04.017
Houstis, N., Rosen, E. D., and Lander, E. S. (2006). Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440 (7086), 944–948. doi:10.1038/nature04634
Hsieh, C. Y., Su, C. C., Shao, S. C., Sung, S. F., Lin, S. J., Kao Yang, Y. H., et al. (2019). Taiwan's national health insurance research database: past and future. Clin. Epidemiol. 11, 349–358. doi:10.2147/CLEP.S196293
Huang, K. H., Chang, Y. L., Gau, S. Y., Tsai, T. H., and Lee, C. Y. (2022a). Dose-response association of metformin with Parkinson's disease odds in type 2 diabetes mellitus. Pharmaceutics 14 (5), 946. doi:10.3390/pharmaceutics14050946
Huang, K. H., Lee, C. H., Cheng, Y. D., Gau, S. Y., Tsai, T. H., Chung, N. J., et al. (2022b). Correlation between long-term use of metformin and incidence of NAFLD among patients with type 2 diabetes mellitus: a real-world cohort study. Front. Endocrinol. (Lausanne) 13, 1027484. doi:10.3389/fendo.2022.1027484
Huang, K. H., Tsai, Y. F., Lee, C. B., Gau, S. Y., Tsai, T. H., Chung, N. J., et al. (2023). The correlation between metformin use and incident dementia in patients with new-onset diabetes mellitus: a population-based study. J. Pers. Med. 13 (5), 738. doi:10.3390/jpm13050738
Huang, P., Wang, F., Sah, B. K., Jiang, J., Ni, Z., Wang, J., et al. (2015). Homocysteine and the risk of age-related macular degeneration: a systematic review and meta-analysis. Sci. Rep. 5, 10585. doi:10.1038/srep10585
Ibrahim, A. S., Mander, S., Hussein, K. A., Elsherbiny, N. M., Smith, S. B., Al-Shabrawey, M., et al. (2016). Hyperhomocysteinemia disrupts retinal pigment epithelial structure and function with features of age-related macular degeneration. Oncotarget 7 (8), 8532–8545. doi:10.18632/oncotarget.7384
Infante, M., Leoni, M., Caprio, M., and Fabbri, A. (2021). Long-term metformin therapy and vitamin B12 deficiency: an association to bear in mind. World J. Diabetes 12 (7), 916–931. doi:10.4239/wjd.v12.i7.916
Jiang, J., Chen, Y., Zhang, H., Yuan, W., Zhao, T., Wang, N., et al. (2022). Association between metformin use and the risk of age-related macular degeneration in patients with type 2 diabetes: a retrospective study. BMJ Open 12 (4), e054420. doi:10.1136/bmjopen-2021-054420
Jung, W., Yoon, J. M., Han, K., Kim, B., Hwang, S., Lim, D. H., et al. (2022). Association between age-related macular degeneration and the risk of diabetes mellitus: a nationwide cohort study. Biomedicines 10 (10), 2435. doi:10.3390/biomedicines10102435
Khattar, D., Khaliq, F., Vaney, N., and Madhu, S. V. (2016). Is metformin-induced vitamin B12 deficiency responsible for cognitive decline in type 2 diabetes? Indian J. Psychol. Med. 38 (4), 285–290. doi:10.4103/0253-7176.185952
Khotcharrat, R., Patikulsila, D., Hanutsaha, P., Khiaocham, U., Ratanapakorn, T., Sutheerawatananonda, M., et al. (2015). Epidemiology of age-related macular degeneration among the elderly population in Thailand. J. Med. Assoc. Thai 98 (8), 790–797.
Ko, S. H., Ko, S. H., Ahn, Y. B., Song, K. H., Han, K. D., Park, Y. M., et al. (2014). Association of vitamin B12 deficiency and metformin use in patients with type 2 diabetes. J. Korean Med. Sci. 29 (7), 965–972. doi:10.3346/jkms.2014.29.7.965
Lai, S. W., Liao, K. F., Lin, C. L., Lin, C. C., and Lin, C. H. (2020). Longitudinal data of multimorbidity and polypharmacy in older adults in Taiwan from 2000 to 2013. Biomed. (Taipei) 10 (2), 1–4. doi:10.37796/2211-8039.1013
Lee, H., Jeon, H. L., Park, S. J., and Shin, J. Y. (2019). Effect of statins, metformin, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers on age-related macular degeneration. Yonsei Med. J. 60 (7), 679–686. doi:10.3349/ymj.2019.60.7.679
Lee, J. H., Kim, J. H., Kim, J. S., Chang, J. W., Kim, S. B., Park, J. S., et al. (2013). AMP-activated protein kinase inhibits TGF-beta-angiotensin II-aldosterone-high glucose-and albumin-induced epithelial-mesenchymal transition. Am. J. Physiol. Ren. Physiol. 304 (6), F686–F697. doi:10.1152/ajprenal.00148.2012
Lim, L. S., Mitchell, P., Seddon, J. M., Holz, F. G., and Wong, T. Y. (2012). Age-related macular degeneration. Lancet 379 (9827), 1728–1738. doi:10.1016/S0140-6736(12)60282-7
Mitchell, P., Wang, J. J., Foran, S., and Smith, W. (2002). Five-year incidence of age-related maculopathy lesions: the blue mountains Eye study. Ophthalmology 109 (6), 1092–1097. doi:10.1016/s0161-6420(02)01055-2
Newsholme, P., Keane, K. N., Carlessi, R., and Cruzat, V. (2019). Oxidative stress pathways in pancreatic beta-cells and insulin-sensitive cells and tissues: importance to cell metabolism, function, and dysfunction. Am. J. Physiol. Cell Physiol. 317 (3), C420-C433–C33. doi:10.1152/ajpcell.00141.2019
Nitsch, D., Douglas, I., Smeeth, L., and Fletcher, A. (2008). Age-related macular degeneration and complement activation-related diseases: a population-based case-control study. Ophthalmology 115 (11), 1904–1910. doi:10.1016/j.ophtha.2008.06.035
Rochtchina, E., Wang, J. J., Flood, V. M., and Mitchell, P. (2007). Elevated serum homocysteine, low serum vitamin B12, folate, and age-related macular degeneration: the Blue Mountains Eye Study. Am. J. Ophthalmol. 143 (2), 344–346. doi:10.1016/j.ajo.2006.08.032
Romdhoniyyah, D. F., Harding, S. P., Cheyne, C. P., and Beare, N. A. V. (2021). Metformin, A potential role in age-related macular degeneration: a systematic review and meta-analysis. Ophthalmol. Ther. 10 (2), 245–260. doi:10.1007/s40123-021-00344-3
Russo, G. T., Di Benedetto, A., Magazzu, D., Giandalia, A., Giorda, C. B., Ientile, R., et al. (2011). Mild hyperhomocysteinemia, C677T polymorphism on methylenetetrahydrofolate reductase gene and the risk of macroangiopathy in type 2 diabetes: a prospective study. Acta Diabetol. 48 (2), 95–101. doi:10.1007/s00592-009-0169-5
Ting, R. Z., Szeto, C. C., Chan, M. H., Ma, K. K., and Chow, K. M. (2006). Risk factors of vitamin B(12) deficiency in patients receiving metformin. Arch. Intern Med. 166 (18), 1975–1979. doi:10.1001/archinte.166.18.1975
Topouzis, F., Anastasopoulos, E., Augood, C., Bentham, G. C., Chakravarthy, U., de Jong, P. T., et al. (2009). Association of diabetes with age-related macular degeneration in the EUREYE study. Br. J. Ophthalmol. 93 (8), 1037–1041. doi:10.1136/bjo.2008.146316
Tsai, D. C., Chen, H. C., Leu, H. B., Chen, S. J., Hsu, N. W., Huang, C. C., et al. (2020). The association between clinically diagnosed insomnia and age-related macular degeneration: a population-based cohort study. Acta Ophthalmol. 98 (2), e238–e244. doi:10.1111/aos.14238
Vassilev, Z. P., Ruigomez, A., Soriano-Gabarro, M., and Garcia Rodriguez, L. A. (2015). Diabetes, cardiovascular morbidity, and risk of age-related macular degeneration in a primary care population. Invest. Ophthalmol. Vis. Sci. 56 (3), 1585–1592. doi:10.1167/iovs.14-16271
Wellington, K. (2005). Rosiglitazone/metformin. Drugs 65 (11), 1581–1592. ; discussion 93-4. doi:10.2165/00003495-200565110-00013
Xu, L., Kong, L., Wang, J., and Ash, J. D. (2018). Stimulation of AMPK prevents degeneration of photoreceptors and the retinal pigment epithelium. Proc. Natl. Acad. Sci. U. S. A. 115 (41), 10475–10480. doi:10.1073/pnas.1802724115
Xu, L., Xie, X. W., Wang, Y. X., and Jonas, J. B. (2009). Ocular and systemic factors associated with diabetes mellitus in the adult population in rural and urban China. The Beijing Eye Study. Eye (Lond). 23 (3), 676–682. doi:10.1038/sj.eye.6703104
Xu, Z., Zhang, M., Zhang, Q., Xu, T., and Tao, L. (2021). Thyroid disease is associated with higher age-related macular degeneration risk: results from a meta-analysis of epidemiologic studies. Ophthalmic Res. 64 (5), 696–703. doi:10.1159/000515273
Young, B. A., Lin, E., Von Korff, M., Simon, G., Ciechanowski, P., Ludman, E. J., et al. (2008). Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am. J. Manag. Care 14 (1), 15–23.
Zhang, Q., Li, S., Li, L., Li, Q., Ren, K., Sun, X., et al. (2016). Metformin treatment and homocysteine: a systematic review and meta-analysis of randomized controlled trials. Nutrients 8 (12), 798. doi:10.3390/nu8120798
Zhao, X., Liu, L., Jiang, Y., Silva, M., Zhen, X., and Zheng, W. (2020). Protective effect of metformin against hydrogen peroxide-induced oxidative damage in human retinal pigment epithelial (RPE) cells by enhancing autophagy through activation of AMPK pathway. Oxid. Med. Cell Longev. 2020, 2524174. doi:10.1155/2020/2524174
Keywords: age-related macular degeneration, metformin, type 2 diabetes mellitus, Pharmacoepidemiology, real world evidence (RWE)
Citation: Huang K-H, Chang Y-L, Lee CB, Gau S-Y, Tsai T-H, Chung N-J and Lee C-Y (2023) Dose-response association of metformin use and risk of age-related macular degeneration among patients with type 2 diabetes mellitus: a population-based study. Front. Pharmacol. 14:1275095. doi: 10.3389/fphar.2023.1275095
Received: 09 August 2023; Accepted: 10 November 2023;
Published: 22 November 2023.
Edited by:
Eugene Van Puijenbroek, Netherlands Pharmacovigilance Centre Lareb, NetherlandsReviewed by:
Zullies Ikawati, Gadjah Mada University, IndonesiaMichael Lloyd Christensen, University of Tennessee Health Science Center (UTHSC), United States
Rizaldy Taslim Pinzon, Duta Wacana Christian University, Indonesia
Copyright © 2023 Huang, Chang, Lee, Gau, Tsai, Chung and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chien-Ying Lee, Y3NoZDAxNUBjc211LmVkdS50dw==
†These authors have contributed equally to this work and share first authorship
 Kuang-Hua Huang1†
Kuang-Hua Huang1† Chiachi Bonnie Lee
Chiachi Bonnie Lee Shuo-Yan Gau
Shuo-Yan Gau Chien-Ying Lee
Chien-Ying Lee