
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 24 October 2023
Sec. Ethnopharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1273407
This article is part of the Research Topic Mechanism of Traditional Medicine on Intestinal Mucosal barrier in Ulcerative colitis View all 5 articles
Damage to the intestinal mucosal barrier play an important role in the pathogenesis of ulcerative colitis (UC). Discovering the key regulators and repairing the disturbed barrier are crucial for preventing and treating UC. Traditional Chinese medicine (TCM) has been proved to be effective on treating UC and has exhibited its role in repairing the intestinal mucosal barrier. We summarized the evidence of TCM against UC by protecting and repairing the physical barrier, chemical barrier, immune barrier, and biological barrier. Mechanisms of increasing intestinal epithelial cells, tight junction proteins, and mucins, promoting intestinal stem cell proliferation, restoring the abundance of the intestinal microbiota, and modulating the innate and adaptive immunity in gut, were all involved in. Some upstream proteins and signaling pathways have been elucidated. Based on the existing problems, we suggested future studies paying attention to patients’ samples and animal models of UC and TCM syndromes, conducting rescue experiments, exploring more upstream regulators, and adopting new technical methods. We hope this review can provide a theoretical basis and novel ideas for clarifying the mechanisms of TCM against UC via repairing the intestinal mucosal barrier.
Ulcerative colitis (UC) is a chronic inflammatory bowel disease, characterized by inflammatory ulceration of the colon and rectum. Patients with UC are troubled with persistent or recurrent diarrhea, bloody stools, and abdominal pain. In recent decades, the incidence of UC has been increasing worldwide, attracting more and more attention (Cosnes et al., 2011; Molodecky et al., 2012; Ananthakrishnan, 2015). Unfortunately, UC still cannot be cured. The aim of treatment is to induce and maintain remission, and to prevent disability, colectomy, and colorectal cancer in the long term. Based on the assessment of disease severity and extent, medications including 5-aminosalicylic acid (5-ASA) drugs, corticosteroids, immune-suppressants, and biological therapy are recommended for the treatment of UC (Harbord et al., 2017; Rubin et al., 2019). Although the early use of biological therapy, including infliximab, adalimumab, and golimumab, improves the efficacy, most medications are of various limitations, and a considerable proportion of patients still fail to achieve or maintain remission after treatment (Ungaro et al., 2017). There is an urgent need to find new therapeutic targets and new therapies for UC.
The intestinal mucosal barrier plays an important role in maintaining intestinal homeostasis and takes part in the pathogenesis of UC (Turner, 2009). It is composed of physical barrier, chemical barrier, immune barrier, and biological barrier. The physical barrier is the mainstay and prevents harmful substances from entering the intestinal mucosa, while the chemical barrier is a supplement to the physical barrier. The immune barrier prevents the damage of pathogenic antigens to the gut via the innate and adaptive immunity, and the biological barrier provides colonization resistance and helps nutrition absorption. The integrity and interaction of each barrier ensure the normal function of the intestinal mucosal barrier and the intestinal homeostasis. Early on in the pathogenesis of UC, damage to the intestinal mucosal barrier and a defective immune response to commensal bacteria might be the main mechanisms (Kobayashi et al., 2020; An et al., 2022). Mucosal injury allows the gut microbiota to trigger a sustained and uninhibited inflammatory response. Some studies also demonstrated that the intestinal barrier dysfunction could precede the clinical diagnosis of inflammatory bowel disease (IBD) by years (Torres et al., 2020; Turpin et al., 2020), which meant that discovering the key regulators and repairing the disturbed intestinal mucosal barrier would be crucial for preventing and treating UC. However, there is still a lack of medications targeting this mechanism (Ma et al., 2019; Alsoud et al., 2021; Hanzel et al., 2022).
Based on syndrome differentiation, traditional Chinese medicine (TCM) has been widely used in the treatment of digestive diseases for thousands of years. Accumulating evidences have shown that TCM has a definite therapeutic effect on UC. A multicenter, double-blind trial by Naganuma et al. found that compared with placebo, 8 weeks of Strobilanthes cusia (Nees) Kuntze [Acanthaceae; indigo naturalis] was effective in inducing a clinical response in Japanese patients with active UC (Mayo scores of 6 or more) (Naganuma et al., 2018). Shen et al. conducted a multicenter, randomized, controlled, double-blind study and demonstrated that Qing-Chang-Hua-Shi granules, a Chinese herbal formula, combined with continued 5-ASA 4 g/d therapy for 12 weeks led to a higher rates of clinical remission in moderately active UC patients (Shen et al., 2021). Another randomized controlled study showed that compared with mesalazine, Fufangkushen colon-coated capsule was similarly effective and safe in the treatment of active UC (Gong et al., 2012). Hu et al. conducted a meta-analysis and found that TCM combined with probiotics could alleviate clinical symptoms, inhibit intestinal inflammatory response, and reduce the disease recurrence with less adverse events (Hu et al., 2022). A large number of experiments in vivo and vitro also proved the efficacy of TCM formulas and metabolites on UC (Zheng et al., 2022a; Liu et al., 2022). While TCM is effective on UC, the potential mechanisms are still partially unknown. Studies have been conducted to unveil the underlying mechanisms of TCM against UC, and repairing the intestinal mucosal barrier has been reported to play an important role (Zheng et al., 2022a; Liu et al., 2022). Our team has been engaged in researches on TCM for UC for a long time, and has also found that TCM can exert therapeutic effects via repairing the intestinal physical barrier and regulating the intestinal microbiota (Mao et al., 2017; Kou et al., 2020; Sun et al., 2020; Wang et al., 2022a).
Therefore, we wrote the present review to summarize the evidence of TCM treating UC by protecting and repairing the mucosal barrier, hoping bringing new thinking to future studies.
The intestinal physical barrier mainly consists of intestinal epithelial cells, tight junctions, and extracellular mucus (Odenwald and Turner, 2017). The intestinal epithelial cells, including goblet cells, Paneth cells, and M cells, play a central role in the intestinal physical barrier (Allaire et al., 2018). These cells can be continually renewed by pluripotent intestinal epithelial stem cells residing in the base of crypts, and some of them, including enteroendocrine cells, goblet cells and Paneth cells, are specialized for secreting mucus, AMPs, and secreted immunoglobulin A (SIgA) (Peterson and Artis, 2014). The intestinal epithelial cells can also sense and respond to microbial stimuli, and participate in the coordination of appropriate immune responses. Tight junctions, including transmembrane proteins like claudin, occludin, zonula occludens 1 (ZO-1), and cingulin, connect adjacent intestinal epithelial cells, forming the physical barrier between the apical and the basolateral plasma membrane domains (Groschwitz and Hogan, 2009). Tight junctions also contribute to the establishment of cell polarity (Cereijido et al., 1998). Mucin is secreted by goblet cells, and plays an important role in isolating intestinal mucosa from pathogenic microbes. Studies have shown that injured epithelial cells, mucus abnormalities, and disordered tight junctions all have a close association with active UC (Van der Sluis et al., 2006; Chelakkot et al., 2018).
Gegen Qinlian decoction, composed of Pueraria montana var. lobata [Fabaceae; kudzuvine root], Scutellaria baicalensis Georgi [Lamiaceae; scutellariae baicalensis radix], and Coptis chinensis Franch. [Ranunculaceae; coptidis rhizoma], was firstly recorded in “Treatise on Febrile Diseases”.
By means of clearing dampness and heat, it has been widely used in the treatment of diarrhea in China. Wang et al. conducted an experiment in vivo and showed that receiving Gegen Qinlian decoction for 6 days could alleviate dextran sulfate sodium (DSS) induced colitis in C57BL/6 mice. It also improved mucus thickness and tight junction proteins ZO-1, occludin and Claudin1, which could be reversed by administration of aryl hydrocarbon receptor (AhR) antagonists. Further experiments showed that the AhR-mediated effect of Gegen Qinlian decoction on repairing the intestinal physical barrier might be induced by regulating gut microbiota-related tryptophan metabolism and restoring the generation of indole derivatives (Wang et al., 2023). Another study in vivo showed that 10 days of Jiawei Gegen Qinlian decoction and the main metabolites, including puerarin, baicalein, berberine, and glycyrrhiic acid, could alleviate DSS-induced colitis in Sprague-Dawley (SD) rats and Kunming mice. The activities of Dao and D-lactate in serum were significantly decreased, and the levels of ZO-1, occludin and claudin1 in colon tissue were increased, which meant that the therapeutic effect was associated with the reduced intestinal permeability (Li et al., 2021a). Zhao et al. found that Gegen Qinlian decoction could repair the intestinal physical barrier in both acute and chronic colitis caused by DSS, which was related to the increased level of MUC2 mRNA, reduced goblet cell differentiation, and promoted intestinal stem cell proliferation. A bidirectional regulatory effect of Gegen Qinlian decoction on the Notch signal transduction might account for the above results and was verified by experiments in vitro and in TLR4 knockout mice (Zhao et al., 2020).
Huangqin decoction, composed of Scutellaria baicalensis Georgi [Lamiaceae; scutellariae baicalensis radix], Paeonia lactiflora Pall. [Paeoniaceae; paeoniae radix alba], Glycyrrhiza glabra L. [Fabaceae; glycyrrhizae radix et rhizoma], and Ziziphus jujuba Mill. [Rhamnaceae; jujubae fructus], was also proved to have a therapeutic effect on UC. In “Treatise on Febrile Diseases”, Huangqin decoction was recorded with clearing heat and stopping dysentery. Similar to Gegen Qinlian decoction, Huangqin decoction could alleviate DSS-induced colitis and upregulate tight junction proteins, such as claudin-1 and ZO-1 (Zheng et al., 2022b). Li et al. (2021b) found that 7 days of Huangqin decoction could alleviate DSS-induced colitis and protect the epithelial barrier integrity by inhibiting the apoptosis of epithelial cells and regulating ESR1 and PTGS2, but the study did not conduct further validation experiments. Mo et al. used DSS + high-fat diet + hot and humid environment to simulate UC with a dampness-heat syndrome in mice. Results showed that compared with Salazosulfapyridine, receiving Huangqin decoction for 7days relieved colitis and downregulated IFN-γ/JAK/ETS signaling pathway related proteins, which reduced the excessive apoptosis of the intestinal epithelial cells. No rescue experiment was conducted likewise (Mo et al., 2022).
Shaoyao decoction, originated from Huangqin decoction, is composed of Paeonia lactiflora Pall. [Paeoniaceae; paeoniae radix alba], Rheum palmatum L. [Polygonaceae; rhei radix et rhizoma], Angelica sinensis (Oliv.) Diels [Apiaceae; angelicae sinensis radix], Coptis chinensis Franch. [Ranunculaceae; coptidis rhizoma], Areca catechu L. [Arecaceae; arecae semen tostum], Dolomiaea costus (Falc.) Kasana & A.K.Pandey [Asteraceae; aucklandiae radix], Glycyrrhiza glabra L. [Fabaceae; glycyrrhizae radix et rhizoma], Scutellaria baicalensis Georgi [Lamiaceae; scutellariae baicalensis radix], and Neolitsea cassia (L.) Kosterm. [Lauraceae; cinnamomi cortex]. Receiving Shaoyao decoction for 7 days could alleviate DSS-induced colitis in Kunming mice. It was also shown to have a regulatory effect on the MKP1/NF-κB/NLRP3, which could upregulate the expression of mucin and occludin. MKP1 inhibitor could reverse the effect of Shaoyao decoction (Wei et al., 2021).
Similar mechanisms were observed in experiments on Wumei Wan, which also came from “Treatise on Febrile Diseases”. Wumei Wan consists of Prunus mume (Siebold) Siebold & Zucc. [Rosaceae; mume fructus], Coptis chinensis Franch. [Ranunculaceae; coptidis rhizoma], Phellodendron amurense Rupr. [Rutaceae; phellodendri amurensis cortex], Asarum heterotropoides F.Schmidt [Aristolochiaceae; asari radix et rhizoma], Neolitsea cassia (L.) Kosterm. [Lauraceae; cinnamomi cortex], Angelica sinensis (Oliv.) Diels [Apiaceae; angelicae sinensis radix], Zanthoxylum bungeanum Maxim. [Rutaceae; zanthoxyli pericarpium], Cyperus rotundus L. [Cyperaceae; cyperi rhizoma], Zingiber officinale Roscoe [Zingiberaceae; zingiberis rhizoma praeparatum], and Panax ginseng C.A.Mey. [Araliaceae; ginseng radix et rhizoma], and is suitable for patients with a TCM syndrome of upper heat and lower cold. Yan et al. (2022a) showed that receiving Wumei Wan for 7 days could alleviate DSS-induced acute colitis in mice, and increase the number of goblet cells and the secretion of mucus. Their another experiment showed that 15 days of Wumei Wan could relieve DSS-induced chronic colitis in mice, inhibit the apoptosis of epithelial cells, and promotes cell proliferation. Mucin 2 was also significantly upregulated. The effect of Wumei Wan on repairing the mucosal barrier might be related to the regulation of Hippo/Yes-associated protein (YAP) signaling, but there was a lack of a rescue verification (Yan et al., 2022b).
Xianglian Pill, a Chinese patent medicine, is composed of Coptis chinensis Franch. [Ranunculaceae; coptidis rhizoma] and Dolomiaea costus (Falc.) Kasana & A.K.Pandey [Asteraceae; aucklandiae radix]. It was shown to be active on alleviating DSS-induced colitis and increasing the expression of Claudin-1 and ZO-1 in the colon, which might be induced by enhancing the autophagy via blocking the activation of PI3K/Akt/mTOR signaling pathway. Adding the autophagy inhibitor 3-MA could reverse the effect of Xianglian Pill (Wang et al., 2021).
Shenling Baizhu San, a commonly used formula for diarrhea, consists of 10 Chinese herbs. Studies showed the efficacy of Shenling Baizhu San on UC (Chen et al., 2022). Treatment for 2 weeks could alleviate DSS-induced colitis and increase the expression of tight junction proteins (Liu et al., 2018).
Tongxieyaofang, firstly recorded in the Danxi’s Mastery of Medicine, is composed of Atractylodes macrocephala Koidz. [Asteraceae; atractylodis macrocephalae rhizoma], Paeonia lactiflora Pall. [Paeoniaceae; paeoniae radix alba], Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk. [Apiaceae; saposhnikoviae radix] (Fangfeng), Citrus × aurantium f. deliciosa (Ten.) M.Hiroe [Rutaceae; citri reticulatae pericarpium viride] (Chenpi). A Chinese clinical study on forty patients with UC showed that treatment of Tongxieyaofang could increase the expression of protective factors of the intestinal mucosa barrier (Yu et al., 2020).
Qingchang Wenzhong decoction, an effective herbal prescription for UC, consisted with Coptis chinensis Franch. [Ranunculaceae; coptidis rhizoma], Zingiber officinale Roscoe [Zingiberaceae; zingiberis rhizoma praeparatum], Sophora flavescens Aiton [Fabaceae; sophorae flavescentis radix], Strobilanthes cusia (Nees) Kuntze [Acanthaceae; indigo naturalis], Sanguisorba officinalis L. [Rosaceae; sanguisorbae radix], Dolomiaea costus (Falc.) Kasana & A.K.Pandey [Asteraceae; aucklandiae radix], Salvia miltiorrhiza Bunge [Lamiaceae; Salviae miltiorrhizae radix et rhizoma], and Glycyrrhiza glabra L. [Fabaceae; glycyrrhizae radix et rhizoma], and was shown to be effective in improving the intestinal permeability through upregulating the expressions of tight junction proteins and numbers of goblet cells. It could also promote the intestinal stem cells-mediated epithelial regeneration, which might be related to the activation of Wnt/β-catenin signals (Sun et al., 2021).
Strobilanthes cusia (Nees) Kuntze [Acanthaceae; indigo naturalis] appeared for the first time in a book in Tang Dynasty. It could clear dampness and heat based on TCM theory. In experiments on Strobilanthes cusia (Nees) Kuntze [Acanthaceae; indigo naturalis], treatment for 1 week could alleviate DSS-induced colitis in mice and increase the expression of E-cadherin, occludin, ZO-1, and MUC2. The mechanism of reinforced intestinal physical barrier might be associated with the effects of anti-inflammation and intestinal microbiota regulation (Yang et al., 2021).
Patrinia villosa (Thunb.) Dufr. [Caprifoliaceae; patriniae herba], firstly recorded in “ShenNongBenCaoJing”, is usually used for appendicitis, enteritis and gynecological inflammation. After the treatment of water extract of Patrinia villosa (Thunb.) Dufr. [Caprifoliaceae; patriniae herba] for 2 weeks, TNBS-induced colitis was alleviated in mice. The mucous epithelium and the goblet cells were significantly increased. The effect might be related to the anti-inflammatory effect of Patrinia villosa (Thunb.) Dufr. [Caprifoliaceae; patriniae herba] via impacting bile acid metabolism and inhibiting NF-κB signaling pathways. No rescue experiment was conducted to verify the results (Wang et al., 2022b).
Piper wallichii (Miq.) Hand.-Mazz. [Piperaceae; piper wallichii] is a Chinese herbal medicine, with the effect of dispelling wind, dredging collaterals, and promoting blood circulation. It has been used for intestinal diseases in Asia for a long time. A study showed that receiving the ethanol extract of Piper wallichii (Miq.) Hand.-Mazz. [Piperaceae; piper wallichii] for 12 days could alleviate DSS-induced colitis in mice, compared with tofacitinib. It could also repair the physical barrier by upregulating the expression of occludin, increasing cell proliferation, and inhibiting cell apoptosis. Further results suggested that the effect might be induced by inhibiting the TLR4/NF-κB/COX-2 signal pathway, while no rescue experiment was conducted (Zhao et al., 2023).
Aloe vera (L.) Burm.f. [Asphodelaceae; aloe], a Chinese herbal medicine, is usually used to treat gastrointestinal diseases such as constipation and colitis. Shi et al. found that receiving aloe vera for 10 days mitigated DSS-induced colitis and enhanced mucin expression, which correlated with decreased inflammation in gut. However, they did not further explore the upstream mechanisms underlying the correlation (Shi et al., 2021).
Another botanical drug, Astragalus mongholicus Bunge [Fabaceae; astragali radix praeparata cum melle], with the effect of tonifying Qi, was found to be effective on alleviating DSS-induced colitis, restoring the epithelial structure and mucous membrane architecture (Li et al., 2022a).
Metabolites are the basis of the efficacy of TCM formulas, and researches on metabolites attract much attention. Berberine, an alkaloid extracted from some botanical drugs, has been studied for the treatment of UC. Zhu et al. showed that compared with sulphasalazin, administration of berberine hydrochloride for 6weeks could alleviate DSS-induced colitis in Wistar rats. It also significantly increased the protein expression levels of tight junctions, including occludin, claudin-1, ZO-1 and VCAM-1, which might be the results of blocking IL-6/STAT3/NF-κB signaling pathway (Zhu et al., 2019). Li et al. conducted experiments in vivo and vitro, demonstrating that berberine could upregulate the expression level of tight junction proteins and maintain a normal intestinal permeability (Li et al., 2020a; Li et al., 2020b). Wu et al. confirmed Berberine’ effects on DSS-induced colitis in cats. 7 days of Berberine could upregulate the expression of tight junction ZO-1 and occludin in colon tissue, which might be associated with the regulation of gut microbiota. No rescue experiment was conducted (Li et al., 2022b).
Indigo and indirubin are the main metabolites of Strobilanthes cusia (Nees) Kuntze [Acanthaceae; indigo naturalis]. Xie et al. (2023) showed that they could relieve DSS-induced colitis in Balb/C mice, and increase the expression of MUC2 and tight junction proteins.
Ginger polysaccharides, major metabolites of Zingiber officinale Roscoe [Zingiberaceae; rhizoma zingiberis recens], were also reported to be helpful in repairing the intestinal physical barrier indicated by the increased expression of occludin-1 and ZO-1 (Hao et al., 2022).
Aloe A and B are the main metabolites of Aloe vera (L.) Burm.f. [Asphodelaceae; aloe]. Shi et al. conducted experiments in vitro and found that receiving aloe A and B upregulated both intracellular and extracellular MUC2 expressions in LPS-stimulated LS174T cells (Shi et al., 2021).
Pulsatilla chinensis (Bunge) Regel [Ranunculaceae; pulsatillae radix], a Chinese herb for clearing heat and detoxification, is usually used for dysentery. Pulsatilla chinensis saponins are the main metabolites in Pulsatilla chinensis (Bunge) Regel [Ranunculaceae; pulsatillae radix], and are reported with anti-tumor, anti-inflammatory, anti-virus, and other pharmacological efficacies. Liu et al. found that receiving ethanolic extracts of Pulsatilla chinensis saponins for 9 days could attenuate DSS-induced colitis, increase the number of goblet cells, and reduce the injury of epithelial cells (Liu et al., 2021a).
Houttuynia cordata Thunb. [Saururaceae; houttuyniae herba] is a botanical drug in China, known for clearing heat and detoxification. Houttuynia cordata polysaccharides are abundant in Houttuynia cordata Thunb. [Saururaceae; houttuyniae herba]. Cen et al. reported that compared with Sulfasalazine, houttuynia cordata polysaccharides could relieve DSS-induced colitis in vivo, and increase the number of goblet cells and the expression of ZO-1 and MUC2. Experiments in vitro also confirmed that they could reduce the apoptosis of intestinal epithelial cells (Cen et al., 2022).
A large number of studies have explored and confirmed the effect of TCM on repairing the intestinal physical barrier, manifesting as increasing the number of colonic epithelial cells and the expression of tight junction proteins, reducing goblet cell differentiation, and promoting intestinal stem cell proliferation and mucus secretion, which is significantly related to the decreased colonic inflammation. The broad prospect of TCM against UC targeting the physical barrier can be viewed by these studies. Furthermore, some studies have explored the upstream mechanisms, which includes the regulation of signaling pathways, namely, Notch, IFN-γ/JAK/ETS, PI3K/Akt/mTOR, NF-κB, Wnt/β-catenin, and Hippo/YAP pathway, alterations of intestinal microbiota-related metabolisms, and modulation of target proteins, like AhR, ESR1, and PTGS2. Only 4 studies conducted rescue experiments to verify the effect mediated by the upstream targets (Zhao et al., 2020; Wang et al., 2021; Wei et al., 2021; Wang et al., 2023).
The intestinal chemical barrier is a supplement to the intestinal physical barrier, including bile acids, digestive enzymes, lysozyme, AMPs, and mucins, etc. Few associated researches focused on repairing the intestinal chemical barrier. Mucins, which was mentioned in the part of the intestinal physical barrier, are the primary constituent of the mucous layer lining the gut. Mucins separate the intestinal microbiota and the intestinal epithelium, and participate in cell signaling, adhesion, growth, and immune modulation (Boltin et al., 2013). The metabolism of bile acids is another point at issue. Bile acids play an important part in maintaining the chemical barrier and regulating immune system with the gut microbiota involved, and the balance of bile acid composition was found to be disrupted in UC patients (Li et al., 2021c). Digestive enzymes and lysozymes have a bactericidal and bacteriolytic effect on microbes, while AMPs are an integral part of the innate immune system, which will be described in the part of the intestinal immune barrier (Kang et al., 2017; An et al., 2022). Colonic epithelial cells of UC patients displayed a significant increase of AMPs and lysozyme (Muniz et al., 2012).
Related researches are few. Li et al. found that Jiawei Gegen Qinlian decoction, and the main metabolites, including puerarin, baicalein, berberine, and glycyrrhiic acid, might have an effect on bile acids metabolism by a gut microbiota-dependent manner (Li et al., 2021a). Wang et al. showed the regulatory effect of Patrinia villosa (Thunb.) Dufr. [Caprifoliaceae; patriniae herba] on the bile acid metabolism via the metabolic analysis of serum and liver sample (Wang et al., 2022b). The specific mechanism of TCM on bile acids metabolism are still partially unknown.
The intestinal biological barrier generally refers to the intestinal microbiota which comprises trillions of microorganisms. The intestinal microbiota is known as an essential “organ” of hosts, and takes part in the digestion and absorption of nutrients, promotes intestinal cell growth, prevents the colonization of pathogens, and maintains the normal immune function of the gut by releasing antimicrobial substances and improving resistance to harmful bacteria (Hu et al., 2021; An et al., 2022; Parizadeh and Arrieta, 2023). The exact composition of the intestinal microbiota is still unclear, and it is host-specific. Studies have shown that Firmicutes and Bacteroides make up 90% of the gut microbiota, and other phyla include Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia (Arumugam et al., 2011). The main genera under the Firmicutes phylum are Bacillus, Lactobacillus, Enterococcus, Clostridium, and Ruminococcus, while the predominant genera in Bacteroidota are Bacteroides and Prevotella (Aggarwal et al., 2013). The composition of the intestinal microbiota also evolves continuously throughout the life and is susceptible to exogenous and endogenous factors (Marchesi et al., 2016). In UC patients, studies showed a reduction in the diversity of the intestinal microbiota, with decreased proportions of Firmicutes and increases in Proteobacteria (Machiels et al., 2014; Imhann et al., 2018; Nishino et al., 2018). In specific microbes, the proportion of beneficial bacteria such as the Roseburia spp and lactobacillus decreased, and the number of harmful bacteria such as Escherichia coli, Bacteroides fragilis, and Helicobacter increased (Jess et al., 2011; Tuci et al., 2011).
In addition, the intestinal microbiota produces short chain fatty acids (SCFAs), bile acids, and tryptophan, which are important in maintaining the intestinal immune system and the integrity of the intestinal barrier (Macfarlane and Macfarlane, 2003; Marchesi et al., 2016). Metabolic disorders caused by gut microbial imbalance can also affect the integrity of the intestinal barrier, which in turn aggravates UC.
There is also an interplay between the intestinal microbiota and intestinal epithelial cells, mucus barrier, and immune cells, which contributes to maintain the homeostasis of the intestinal microenvironment. The disturbed interaction might induce the development of UC (Fang et al., 2021).
Wang et al. found that Gegen Qinlian decoction restored tryptophan metabolism and regulated tryptophan metabolism-related gut microbiota. At the phylum level, Gegen Qinlian decoction could reduce Proteobacteria and increase the abundance of Firmicutes and Bacteroidetes. At the order level, the relative abundance of Enterobacteriales decreased after treatment, whereas the relative abundance of Bacteroidales and Clostridiales increased, which were considered to be the main bacterial communities metabolizing tryptophan to produce indole derivatives (Wang et al., 2023).
Zheng et al. demonstrated that Huangqin decoction could restore the abundance of the intestinal microbiota, increase the abundance of Firmicutes, and decrease the abundance of Bacteroidetes. At the genus level, Lactobacillus and Bacteroidetes significantly increased, while Triclospira and Raptoidetes significantly decreased (Zheng et al., 2022b).
Rhubarb Peony decoction, composed of Rheum palmatum L. [Polygonaceae; rhei radix et rhizoma], Paeonia × suffruticosa Andrews [Paeoniaceae; moutan cortex], (Danpi), Prunus persica (L.) Batsch [Rosaceae; persicae semen] (Taoren), Benincasa hispida (Thunb.) Cogn. [Cucurbitaceae; benincasae semen], and Natrii sulfas, was used for intestinal carbuncle in TCM. It was also reported to have an efficacy on colitis induced by DSS. Luo et al. (2019) showed that 2 weeks of Rhubarb Peony decoction promoted the growth of butyric acid-producing bacteria, namely, Butyricicoccus pullicaecorum, and regulated the producing of SCFA.
A prospective cohort study on Shenling Baizhu San showed that the formula combined with mesalamine could improve the diversity and abundance of the intestinal microbiota, increase the percentages of Bacteroides, Blautia, Bifidobacterium and Lactobacillus in patients with active UC, which are the major sources of tryptophan metabolites (Jiao et al., 2022).
Baitouweng decoction, composed of Pulsatilla chinensis (Bunge) Regel [Ranunculaceae; pulsatillae radix], Fraxinus chinensis subsp. rhynchophylla (Hance) A.E.Murray [Oleaceae; fraxini cortex], Coptis chinensis Franch. [Ranunculaceae; coptidis rhizoma], and Phellodendron amurense Rupr. [Rutaceae; phellodendri amurensis cortex], was firstly recorded in “Treatise on Febrile Diseases”. It is commonly used to treat dysentery or diarrhea caused by a dampness-heat syndrome. Studies found that administration of Baitouweng decoction for 7–10 days could attenuate DSS-induced colitis, increase the abundance of Firmicutes, Proteobacteria, Actinobacteria, Tenericutes, and decrease the abundance of Bacteroidetes (Hua et al., 2021; Xuan-Qing et al., 2021).
Sanhuangshu’ai decoction, firstly recorded in “Life-Saving Book of Classified Syndromes”, comprises of Coptis chinensis Franch. [Ranunculaceae; coptidis rhizoma], Scutellaria baicalensis Georgi [Lamiaceae; scutellariae baicalensis radix], Phellodendron amurense Rupr. [Rutaceae; phellodendri amurensis cortex], Artemisia annua L. [Asteraceae; artemisiae annuae herba]. It was shown that, after the treatment of Sanhuangshu’ai decoction for 7 days, DSS-induced colitis was alleviated, and the decrease of Lactobacillus and population abundance of intestinal flora caused by DSS was prevented (Wu et al., 2020).
Some experienced decoctions have also been shown to alleviate UC in clinical practice and experiments. Qingchang Wengzhong decoction was reported to have an effect on UC via a gut microbiota-dependent manner. Sun et al. showed that it could enrich the relative abundance of Lactobacillus, reduce pathogenic species, such as Bacteroides and Streptococcus, and enhance tryptophan metabolism. Rescue experiments were conducted and there were evidences that the effect of Qingchang Wenzhong decoction on regulating the gut microbiota could be transferred by fecal microbiota transplantation, and antibiotics could neutralize the beneficial effect (Sun et al., 2021).
Kuijieyuan decoction is a clinically validated TCM formula, used for alleviating symptoms associated with UC. It is composed of Astragalus mongholicus Bunge [Fabaceae; astragali radix praeparata cum melle], Scleromitrion diffusum (Willd.) R.J.Wang [Rubiaceae; oldenlandiae diffusae herba], Cirsium arvense var. arvense [Asteraceae; cirsii herba], Pulsatilla chinensis (Bunge) Regel [Ranunculaceae; pulsatillae radix], Prunella vulgaris L. [Lamiaceae; prunellae spica], Coptis chinensis Franch. [Ranunculaceae; coptidis rhizoma], Reynoutria japonica Houtt. [Polygonaceae; polygoni cuspidati rhizoma et radix], Atractylodes lancea (Thunb.) DC. [Asteraceae; atractylodis rhizome], and Glycyrrhiza glabra L. [Fabaceae; glycyrrhizae radix et rhizoma]. Liu et al. (2020) found that Kuijieyuan decoction increased the proportion of Alloprevotella, Treponema, Prevotellaceae, and Prevotella, and reduced the proportion of Escherichia, Shigella, and Desulfovibrio in colon.
Guchang Zhixie Wan is another clinically validated TCM formula produced by Shanxi institute of traditional Chinese medicine pharmaceutical factory. It consists of Prunus mume (Siebold) Siebold & Zucc. [Rosaceae; mume fructus], Zingiber officinale Roscoe [Zingiberaceae; zingiberis rhizoma praeparatum], Coptis chinensis Franch. [Ranunculaceae; coptidis rhizoma], Papaver somniferum L. [Papaveraceae; papaveris pericarpium], Dolomiaea costus (Falc.) Kasana & A.K.Pandey [Asteraceae; aucklandiae radix], and Corydalis yanhusuo (Y.H.Chou & Chun C.Hsu) W.T.Wang ex Z.Y.Su & C.Y.Wu [Papaveraceae; corydalis rhizoma]. It was evidenced that it was effective on decreasing the relative abundance of Turicibacter and increasing the relative abundance of Ruminococcaceae_UCG-005 (Wang et al., 2020).
Yang et al. (2021) found that Strobilanthes cusia (Nees) Kuntze [Acanthaceae; indigo naturalis] could increase the abundance of Lactobacillus and decrease the abundance of Streptococcus and Desulfovibrio. Zingiber officinale Roscoe [Zingiberaceae; rhizoma zingiberis recens] and Panax ginseng C.A.Mey. [Araliaceae; ginseng radix et rhizoma] were also reported to increase the beneficial bacteria such as Muribaculaceae Norank, Lachnospiraceae, and Akkermansia, and reduce harmful bacteria such as Bacteroides, Parabacteroides and Desulfovibrio. They could modulate the composition and diversity of the intestinal microbiota to attenuate inflammatory responses (Wan et al., 2022). Li et al. observed that Astragalus mongholicus Bunge [Fabaceae; astragali radix praeparata cum melle] decreased the relative abundance of Allobaculum, Shigella and Oscillospirillum, which might be pathogenic bacteria. It significantly increased the relative abundance of Akkermansia, which was positively correlated with anti-inflammatory cytokines IL-10 and IgA (Zhu et al., 2019). Prunus humilis Bunge [Rosaceae; pruni semen] could increase the abundance of beneficial bacteria, including Parasutterella, Bacteroides, Roseburia and Blautia (Ran et al., 2022).
Li et al. (2022b) found that berberine caused an increase in the proportion of Lactobacillus, Prevoteaceae, Bifidobacteria, and Verrucomimicrobia, and a decrease in the proportion of Bacteroides and Proteobacteria in DSS-induced UC mice. Wu et al. (2022) also showed an increase in the relative abundance of Firmicutes and Lactobacillus, and a decrease in Proteobacteria after treatment of berberine. Cen et al. (2022) found that houttuynia cordata polysaccharides increased the number of Firmicutes and Bacteroides in the intestinal microbiota, and decreased the number of Proteobacteria. Ginger polysaccharides could significantly reduce the abundance of Proteobacteria, improve the balance of Firmicutes/Bacteroidetes ratio, and increase the abundance of Lactobacillus and decreasing Bacteroides (Hao et al., 2022). Pulsatilla chinensis Saponins was effective on increasing the diversity of the gut microbiota, especially the beneficial bacteria like norank_F_Muribaculaceae and norank_F_norank_O_Clostridia_UCG-014 (Liu et al., 2021a). Chlorogenic acid, a phenolic acid extracted from Lonicera japonica Thunb. [Caprifoliaceae; lonicerae japonicae flos], might restore the diversity of gut microbiota, reduce the abundance of Firmicutes and Bacteroidetes, and markedly increase the proportion of Akkermansia (Zhang et al., 2017). Combination therapy with indigo and indirubin was demonstrated to be effective on increasing the amounts of beneficial bacteria, like norank_f_Muribaculaceae and Lactobacillus (Xie et al., 2023).
Targeting gut microbiota is a hot topic in researches on UC, as well as TCM. Extensive studies provided evidences that TCM could alleviate UC via restoring the abundance of the gut microbiota, enriching the relative abundance of beneficial bacteria, reducing pathogenic species, and regulating the metabolism of bile acids, tryptophan, and butyric acid. Most studies were conducted without rescue experiments, which played a critical role in confirming these mechanisms. By transplanting the fecal microbiota of animals treated by TCM and using antibiotic therapy, the role of the gut microbiota in the effect of TCM can be verified from positive and negative perspectives. In addition, 16S rRNA sequencing has been employed in most studies to analyze the intestinal microbiota, which has a limitation of similarity within 16S for genus- and species-level differentiation (Church et al., 2020). The application of macrogenomics sequencing will contribute to screening the specific bacterial strains induced by TCM.
The intestinal immune barrier is mainly composed of gut-associated lymphoid tissue (GALT), SIgA, and antibacterial substances such as mucins and AMPs. It is not only responsible for recognizing and eliminating pathogens, food antigens, and detrimental luminal substances, but also provides tolerance to commensal microbiota (Ren et al., 2019). GALT comprises Peyer’s lymph nodes, mesenteric lymph nodes, isolated lymphoid follicles, and scattered lymphocytes, and contains dendritic cells, T and B lymphocytes, plasma cells, innate lymphoid cells, macrophages, and neutrophils (Mörbe et al., 2021). GALT participates in recognizing and presenting antigens, and activating T and B lymphocytes to establish effective adaptive immune response (Luongo et al., 2009). SIgA, released by B cells, resides primarily on intestinal mucus layer. The main role of SIgA is to coat bacteria to form antigen-antibody complexes to neutralize the toxins produced by bacteria. AMPs play an important role in the innate immune system, which contain pathogens locally, exert an anti-inflammatory effect, and recruit immune cells. In UC patients, the dysfunction of the intestinal immune barrier contributes to the direct interactions between pathogens and the mucosal immune system, causing abnormal immune responses.
Macrophages are key regulators of intestinal microenvironment homeostasis and can be polarized to different phenotypes in response to different signals, which mainly includes the classically activated macrophages (M1 macrophages) secreting pro-inflammatory cytokines and the alternatively activated macrophages (M2 macrophages) secreting anti-inflammatory cytokines. A large number of studies showed that TCM formulas, such as Gegen Qinlian decoction, Wumei Wan, Shenling Baizhu San, Tongxieyaofang and Qingchang Wenzhong decoction, etc., could inhibit macrophage polarization towards M1 direction and promote M2 polarization. Meanwhile, they could increase the secretion of anti-inflammatory cytokines like IL-10, and decrease pro-inflammatory cytokines like IL-6, IL-1 β, and TNF- α. (Liu et al., 2021b; Yan et al., 2022a; Yan et al., 2022b; Yan et al., 2022c; Lu et al., 2022; Yu et al., 2022). The regulatory effect of Wumei Wan on macrophage was reported to be related to the regulation of p38MAPK, NF-κB and STAT6 signaling pathways (Yan et al., 2022a; Yan et al., 2022b; Yan et al., 2022c). Qingchang Wenzhong decoction might modulate M1 macrophage polarization via JAK2/STAT3 signaling pathway. Rescue experiments have not been conducted to verify the upstream mechanisms (Lu et al., 2022).
Helper T cells (Th), including Th1, Th2, Th9, and Th17, and regulatory T cells (Treg) play an important part in the UC’s pathogenesis via regulating the adaptive immune response. There is evidence that Th1 and Th17 can promote the development of UC, while cytokines produced by Th2 and Treg cells can inhibit the above pro-inflammation effects (Shi et al., 2011; Dominguez-Villar and Hafler, 2018; Lloyd and Snelgrove, 2018). Targeting the skewed axis of Th1/Th2 and Th17/Treg cells attracted many studies on TCM. Shenling Baizhu San, Baitouweng decoction, Rhubarb Peony decoction, Yiyi Fuzi Baijiang decoction, Kuijieling decoction and some botanical drugs, including Astragalus mongholicus Bunge [Fabaceae; astragali radix praeparata cum melle] and Piper wallichii (Miq.) Hand.-Mazz. [Piperaceae; piper wallichii], were shown to have an effect on the adaptive immune system by regulating the balance of Th17/Treg (Li et al., 2017; Yu et al., 2018; Luo et al., 2019; Miao et al., 2021; Li et al., 2022a; Qi et al., 2022; Liu et al., 2023; Xiao et al., 2023). The effect of Rhubarb Peony decoction might be related to promoting the growth of butyric acid-producing bacteria, while Yiyi Fuzi Baijiang decoction’s effect might be related to bile acid metabolites, especially 3-oxoLCA and isoalloLCA (Luo et al., 2019; Liu et al., 2023). Kuijieling might regulate the Treg/Th17 cell balance via the RA/RARα signaling pathway (Xiao et al., 2023). Astragalus mongholicus Bunge [Fabaceae; astragali radix praeparata cum melle] also showed an immunomodulatory effect on regulating the balance of Th1/Th2 cells (Zhu et al., 2019).
Wen et al. found that the group 3 innate lymphoid cells (ILC3s) and MHC II expression in mesenteric lymph nodes of UC mice were strongly suppressed and its inhibitory effect on T follicular helper cells (Tfh cells) was reduced, which led to the proliferation of Tfh cells and induced the class switching of IgA + B cells by increasing IL-4 levels, and hypersecretion of IgA. The expression of IgA levels in colon further aggravated colon mucosa. Alcoholic extracts of Yujin Powder could ameliorate UC through upregulating the proportion of ILC3s and expression of MHC II, enhancing the inhibition of ILC3s on Tfh cells, further regulating IgA response and IgA targeted colonic mucosal flora (Wen et al., 2022). Ran et al. also demonstrated that Prunus humilis Bunge [Rosaceae; pruni semen] improved the expression of SIgA related genes and further regulated intestinal mucosal immune function. There seems to be controversy about the role of TCM formulas in regulating IgA level in colon (Ran et al., 2022). While the experimental subjects were all induced by DSS, the types of mice and the different durations of experiments might be the reasons for the different results. Further researches need to be conducted to illustrate the changes of IgA level.
Studies showed that berberine could alleviate DSS-induced UC by increasing the expression of SIgA, reducing the infiltration of neutrophils, dendritic cells, macrophages and NKTs, regulating the intestinal Treg/Th17 balance (Yan et al., 2012; Li et al., 2020a; Li et al., 2020b). Houttuynia cordata polysaccharides were reported to have an effect on suppressing the infiltration of macrophages and restoring the dysfunction of Th17/Treg cells (Cen et al., 2022).
Baicalin, a flavonoid isolated from Scutellaria baicalensis Georgi [Lamiaceae; scutellariae baicalensis radix], was reported to be able to decrease the proliferation of CD4+ CD29+ T cells, lead to a significant lower ratio of RORC/FOXP3 and indirectly regulate the balance of Th17/Treg differentiation in vitro (Yu et al., 2014).
Chlorogenic acid, might inhibit the NF- κB signaling pathway, leading to the suppression of the infiltration of macrophages, neutrophils, and CD3+ T cells in colon (Zatorski et al., 2015; Zhang et al., 2017).
Indirubin and isatin are metabolites of Strobilanthes cusia (Nees) Kuntze [Acanthaceae; indigo naturalis]. It was shown that indirubin alone, or combined with isatin, could significantly inhibit DSS-induced CD4 + T cell infiltration in mouse colon, and promote the generation of Foxp3 - expressing regulatory T cells (Gao et al., 2016; Gao et al., 2018). Xie et al. (2023) showed that combination of indigo and indirubin could reduce the populations of neutrophils, macrophages, and dendritic cells in the lamina propria, and significantly increase the relative quantity of CD335+CD11b+ NK cells.
Given UC is induced by the imbalance of immune homeostasis, a lot of studies focus on the impact of TCM on the intestinal immune barrier. Relevant mechanisms cover the regulation of macrophage polarization, balancing Th1/Th2 and Th17/Treg, modulating the secretion of IgA, and reducing the infiltration of neutrophils, macrophages, and dendritic cells. In some researches analyzing the proportion of immune cells, the flow cytometry analysis was replaced by detecting the related inflammatory cytokines levels or immunohistochemical analysis, which was ineligible. Besides, rescue experiments were absent from most studies. Adoptive transfer experiments on targeted immune cells, overexpressing and silencing targeted proteins should be applied to verify the logical relationship in future studies on related mechanisms. The regulatory effects of TCM on inflammatory factors is not within the scope of this review.
TCM, including TCM formulas and metbolites, is effective in treating UC, which has been confirmed by clinical trials and experiments. TCM usually acts on multi-targets and multi-pathways, and there is no exception in the treatment of UC. Considering the important role of the intestinal mucosal barrier in the pathogenesis of UC, our review summarized the mechanisms of TCM against UC by repairing the intestinal barrier, as shown in Tables 1, 2.
There is compelling evidence that TCM formulas and metabolites exert an effect on repairing the intestinal physical barrier by increasing colonic epithelial cells, tight junction proteins, and mucus secretion, and promoting intestinal stem cell proliferation. The biological barrier was repaired via restoring the abundance of the intestinal microbiota, enriching beneficial bacteria, reducing pathogenic species, and regulating microbial metabolites. Firmicutes, Bacteroidetes, Actinobacteria, Tenericutes, Bacteroidales, Clostridiales, Lactobacillus, Bacteroides, Blautia, Bifidobacterium, Alloprevotella, Treponema, Prevotella, Ruminococcaceae_UCG-005, Muribaculaceae Norank, Lachnospiraceae, Akkermansia, Parasutterella, Roseburia, Prevoteaceae, Bifidobacteria, norank_F_Muribaculaceae, and norank_F_norank_O_Clostridia_UCG-014 might be beneficial microorganisms, and Proteobacteria, Bacteroidetes, Enterobacteriales, Triclospira, Raptoidetes, Streptococcus, Escherichia, Desulfovibrio, Turicibacter, Parabacteroides, Allobaculum, Shigella, and Oscillospirillum were reported as potential harmful microorganisms. The immune barrier was restored due to the regulation of macrophage polarization, Th1/Th2 and Th17/Treg cells balance, the secretion of IgA, and reducing the infiltration of pro-inflammatory cells. Studies targeting the intestinal chemical barrier are few. Upstream mechanisms were explored, and the regulation of signal pathways, including Notch, IFN-γ/JAK/ETS, PI3K/Akt/mTOR, NF-κB, Wnt/β-catenin, Hippo/YAP, p38MAPK, STAT6, JAK2/STAT3, and RA/RARα pathways, alterations of intestinal microbiota-related metabolisms, and modulation of target proteins, like AhR, ESR1, and PTGS2, were involved. Detailed information was drawn in Figure 1.
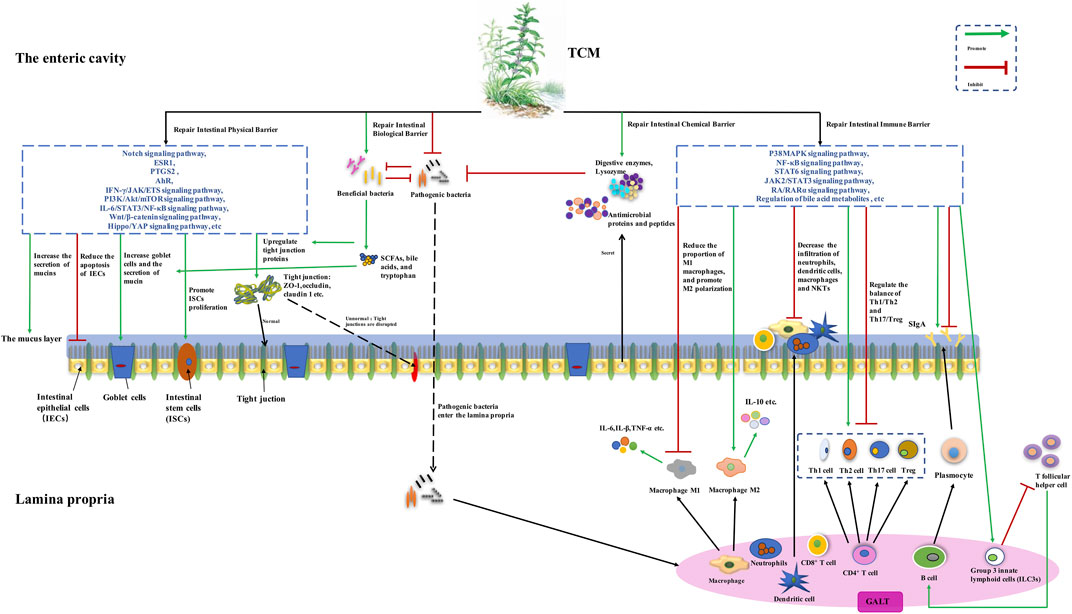
FIGURE 1. The potential mechanisms of traditional Chinese medicine (TCM) for ulcerative colitis (UC) based on repairing the intestinal mucosal barrier. TCM may increase the number of colonic goblet cells and the expression of tight junction proteins, reduce goblet cell differentiation, and promote intestinal stem cell proliferation and mucus secretion, via modulating signal pathways, including Notch, IFN-γ/JAK/ETS, PI3K/Akt/mTOR, NF-κB, Wnt/β-catenin, Hippo/YAP, AhR, ESR1, and PTGS2. The abundance of the intestinal microbiota is restored, with the relative abundance of beneficial bacteria being enriched and pathogenic species being reduced. The metabolisms of bile acids, tryptophan, and butyric acid are regulated. Macrophage polarization, balancing Th1/Th2 and Th17/Treg, changing the secretion of IgA, and reducing the infiltration of neutrophils, macrophages, and dendritic cells, were also involved in repairing the intestinal immune barrier, and modulating p38MAPK, NF-κB, STAT6, JAK2/STAT3, and RA/RARα pathways, and regulation of bile acid metabolites were found to be the upstream mechanisms.
However, attention should be paid to some problems. Firstly, most studies of TCM adopted DSS or TNBS induced colitis models. On one hand, studies on UC patients are few. On the other hand, since the treatment of TCM is based on the syndrome differentiation, new animal models simultaneously imitating diseases and syndromes deserve exploring. Secondly, there is a lack of rescue experiments in most researches, which results in incredibility and flaws in study results. For example, the impact of TCM on the gut microbiota can be verified through fecal bacteria transplantation and antibiotic therapy. There is also a scarcity of overexpression or silencing experiments of targeted proteins and signaling pathways. Meanwhile, the underlying upstream mechanisms require more elucidation. Last but not least, new technical methods, like multi omics and single-cell sequencing, will contribute to the discovery of new mechanisms.
In conclusion, we have summarized evidences in TCM formulas and metabolites, which can ameliorate experimental colitis by repairing the intestinal mucosal barrier. Increasing intestinal epithelial cells, tight junction proteins, and mucins, promoting intestinal stem cell proliferation, upregulating the abundance of the intestinal microbiota, especially beneficial bacteria, and modulating the innate and adaptive immunity in gut, were all involved in. Some targeted proteins and signaling pathways have been found to be the upstream mechanisms. Considering potential problems, suggestions including using patients’ samples and animal models of UC and TCM syndromes, conducting rescue experiments, exploring upstream mechanisms, and application of new technical methods have been put forward. Our review will provide a theoretical basis and novel ideas for future studies on clarifying the mechanisms of TCM against UC via repairing the intestinal mucosal barrier.
YZ: Data curation, Formal Analysis, Investigation, Writing–original draft. JM: Data curation, Formal Analysis, Investigation, Writing–original draft. TM: Formal Analysis, Writing–original draft, Methodology. QH: Data curation, Formal Analysis, Investigation, Writing–original draft. PZ: Conceptualization, Funding acquisition, Supervision, Writing–review and editing. LS: Writing–review and editing, Funding acquisition.
This work was supported by grants from the National Natural Science Foundation of China (no. 82004113 and 82104810), and Young Elite Scientists Sponsorship Program by CAST (2021-QNRC02-B06).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aggarwal, N., Kitano, S., Puah, G. R. Y., Kittelmann, S., Hwang, I. Y., and Chang, M. W. (2013). Microbiome and human health: current understanding, engineering, and enabling technologies. Chem. Rev. 123, 31–72. doi:10.1021/acs.chemrev.2c00431
Allaire, J. M., Crowley, S. M., Law, H. T., Chang, S. Y., Ko, H. J., and Vallance, B. A. (2018). The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. 39, 677–696. doi:10.1016/j.it.2018.04.002
Alsoud, D., Verstockt, B., Fiocchi, C., and Vermeire, S. (2021). Breaking the therapeutic ceiling in drug development in ulcerative colitis. Lancet Gastroenterol. Hepatol. 6, 589–595. doi:10.1016/S2468-1253(21)00065-0
An, J., Liu, Y., Wang, Y., Fan, R., Hu, X., Zhang, F., et al. (2022). The role of intestinal mucosal barrier in autoimmune disease: a potential target. Front. Immunol. 13, 871713. doi:10.3389/fimmu.2022.871713
Ananthakrishnan, A. N. (2015). Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 12, 205–217. doi:10.1038/nrgastro.2015.34
Arumugam, M., Raes, J., Pelletier, E., Le Paslier, D., Yamada, T., Mende, D. R., et al. (2011). Enterotypes of the human gut microbiome. Nature 473, 174–180. doi:10.1038/nature09944
Boltin, D., Perets, T. T., Vilkin, A., and Niv, Y. (2013). Mucin function in inflammatory bowel disease: an update. J. Clin. Gastroenterol. 47, 106–111. doi:10.1097/MCG.0b013e3182688e73
Cen, L., Yi, T., Hao, Y., Shi, C., Shi, X., Lu, Y., et al. (2022). Houttuynia cordata polysaccharides alleviate ulcerative colitis by restoring intestinal homeostasis. Chin. J. Nat. Med. 20, 914–924. doi:10.1016/S1875-5364(22)60220-6
Cereijido, M., Valdés, J., Shoshani, L., and Contreras, R. G. (1998). Role of tight junctions in establishing and maintaining cell polarity. Annu. Rev. Physiol. 60, 161–177. doi:10.1146/annurev.physiol.60.1.161
Chelakkot, C., Ghim, J., and Ryu, S. H. (2018). Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 50, 103. doi:10.1038/s12276-018-0126-x
Chen, J., Shen, B., and Jiang, Z. (2022). Traditional Chinese medicine prescription Shenling BaiZhu powder to treat ulcerative colitis: clinical evidence and potential mechanisms. Front. Pharmacol. 13, 978558. doi:10.3389/fphar.2022.978558
Church, D. L., Cerutti, L., Gürtler, A., Griener, T., Zelazny, A., and Emler, S. (2020). Performance and application of 16S rRNA gene cycle sequencing for routine identification of bacteria in the clinical microbiology laboratory. Clin. Microbiol. Rev. 33, 000533–e119. doi:10.1128/CMR.00053-19
Cosnes, J., Gower-Rousseau, C., Seksik, P., and Cortot, A. (2011). Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 140, 1785–1794. doi:10.1053/j.gastro.2011.01.055
Dominguez-Villar, M., and Hafler, D. A. (2018). Regulatory T cells in autoimmune disease. Nat. Immunol. 19, 665–673. doi:10.1038/s41590-018-0120-4
Fang, J., Wang, H., Zhou, Y., Zhang, H., Zhou, H., and Zhang, X. (2021). Slimy partners: the mucus barrier and gut microbiome in ulcerative colitis. Exp. Mol. Med. 53, 772–787. doi:10.1038/s12276-021-00617-8
Gao, W., Guo, Y., Wang, C., Lin, Y., Yu, L., Sheng, T., et al. (2016). Indirubin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice through the inhibition of inflammation and the induction of Foxp3-expressing regulatory T cells. Acta histochem. 118, 606–614. doi:10.1016/j.acthis.2016.06.004
Gao, W., Zhang, L., Wang, X., Yu, L., Wang, C., and Gong, Y. (2018). The combination of indirubin and isatin attenuates dextran sodium sulfate induced ulcerative colitis in mice. Biochem. Cell. Biol. 96, 636–645. doi:10.1139/bcb-2018-0041
Gong, Y., Zha, Q., Li, L., Liu, Y., Yang, B., Liu, L., et al. (2012). Efficacy and safety of Fufangkushen colon-coated capsule in the treatment of ulcerative colitis compared with mesalazine: a double-blinded and randomized study. J. Ethnopharmacol. 141, 592–598. doi:10.1016/j.jep.2011.08.057
Groschwitz, K. R., and Hogan, S. P. (2009). Intestinal barrier function: molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 124, 3–20. quiz 21-2. doi:10.1016/j.jaci.2009.05.038
Hanzel, J., Hulshoff, M. S., Grootjans, J., and D'Haens, G. (2022). Emerging therapies for ulcerative colitis. Expert Rev. Clin. Immunol. 18, 513–524. doi:10.1080/1744666X.2022.2069562
Hao, W., Chen, Z., Yuan, Q., Ma, M., Gao, C., Zhou, Y., et al. (2022). Ginger polysaccharides relieve ulcerative colitis via maintaining intestinal barrier integrity and gut microbiota modulation. Int. J. Biol. Macromol. 219, 730–739. doi:10.1016/j.ijbiomac.2022.08.032
Harbord, M., Eliakim, R., Bettenworth, D., Karmiris, K., Katsanos, K., Kopylov, U., et al. (2017). Third european evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current Management. J. Crohns Colitis 11, 769–784. doi:10.1093/ecco-jcc/jjx009
Hu, Y., Ye, Z., Wu, M., She, Y., Li, L., Xu, Y., et al. (2021). The communication between intestinal microbiota and ulcerative colitis: an exploration of pathogenesis, animal models, and potential therapeutic strategies. Front. Med. (Lausanne) 8, 766126. doi:10.3389/fmed.2021.766126
Hu, Y., Ye, Z., She, Y., Li, L., Wu, M., Qin, K., et al. (2022). Efficacy and safety of probiotics combined with traditional Chinese medicine for ulcerative colitis: a systematic review and meta-analysis. Front. Pharmacol. 13, 844961. doi:10.3389/fphar.2022.844961
Hua, Y. L., Jia, Y. Q., Zhang, X. S., Yuan, Z. W., Ji, P., Hu, J. J., et al. (2021). Baitouweng Tang ameliorates DSS-induced ulcerative colitis through the regulation of the gut microbiota and bile acids via pathways involving FXR and TGR5. Biomed. Pharmacother. 137, 111320. doi:10.1016/j.biopha.2021.111320
Imhann, F., Vich Vila, A., Bonder, M. J., Fu, J., Gevers, D., Visschedijk, M. C., et al. (2018). Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut 67, 108–119. doi:10.1136/gutjnl-2016-312135
Jess, T., Simonsen, J., Nielsen, N. M., Jørgensen, K. T., Bager, P., Ethelberg, S., et al. (2011). Enteric salmonella or campylobacter infections and the risk of inflammatory bowel disease. Gut 60, 318–324. doi:10.1136/gut.2010.223396
Jiao, C., Zhang, Q., Yang, M., Ma, J., Zhao, X., Tang, N., et al. (2022). Shenling Baizhu San ameliorates ulcerative colitis by regulating the gut microbiota and its tryptophan metabolites: a complementary medicine to mesalamine. J. Ethnopharmacol. 291, 115145. doi:10.1016/j.jep.2022.115145
Kang, H. K., Kim, C., Seo, C. H., and Park, Y. (2017). The therapeutic applications of antimicrobial peptides (AMPs): a patent review. J. Microbiol. 55, 1–12. doi:10.1007/s12275-017-6452-1
Kobayashi, T., Siegmund, B., Le Berre, C., Wei, S. C., Ferrante, M., Shen, B., et al. (2020). Ulcerative colitis. Nat. Rev. Dis. Prim. 6, 74. doi:10.1038/s41572-020-0205-x
Kou, F. S., Shi, L., Li, J. X., Wang, Z. B., Shi, R., Mao, T. Y., et al. (2020). Clinical evaluation of traditional Chinese medicine on mild active ulcerative colitis: a multi-center, randomized, double-blind, controlled trial. Med. Baltim. 99, e21903. doi:10.1097/MD.0000000000021903
Li, Y., Liu, Y., Yan, S. G., Zhao, J., Tang, M. F., and Sun, H. (2017). Effect of the classical three prescriptions for regulating intestinal function on cytokines as IL-17, IL-23, IL-6, IL-10 and TNF-α in colonic of ulcerative colitis rat. Mod. J. Integr. Tradit. Chin. West. Med. 26, 920–924. doi:10.3969/j.issn.1008-8849.2017.09.003
Li, H., Fan, C., Lu, H., Feng, C., He, P., Yang, X., et al. (2020a). Protective role of berberine on ulcerative colitis through modulating enteric glial cells-intestinal epithelial cells-immune cells interactions. Acta Pharm. Sin. B 10, 447–461. doi:10.1016/j.apsb.2019.08.006
Li, H., Feng, C., Fan, C., Yang, Y., Yang, X., Lu, H., et al. (2020b). Intervention of oncostatin M-driven mucosal inflammation by berberine exerts therapeutic property in chronic ulcerative colitis. Cell. Death Dis. 11, 271. doi:10.1038/s41419-020-2470-8
Li, Q., Cui, Y., Xu, B., Wang, Y., Lv, F., Li, Z., et al. (2021a). Main active components of Jiawei Gegen Qinlian decoction protects against ulcerative colitis under different dietary environments in a gut microbiota-dependent manner. Pharmacol. Res. 170, 105694. doi:10.1016/j.phrs.2021.105694
Li, M. Y., Li, M. X., Xu, N., Li, Z. H., Zhang, Y. M., Gan, Y. X., et al. (2021b). Effects of Huangqin decoction on ulcerative colitis by targeting estrogen receptor alpha and ameliorating endothelial dysfunction based on system pharmacology. J. Ethnopharmacol. 271, 113886. doi:10.1016/j.jep.2021.113886
Li, N., Zhan, S., Tian, Z., Liu, C., Xie, Z., Zhang, S., et al. (2021c). Alterations in bile acid metabolism associated with inflammatory bowel disease. Inflamm. bowel Dis. 27, 1525–1540. doi:10.1093/ibd/izaa342
Li, J., Ma, Y., Li, X., Wang, Y., Huo, Z., Lin, Y., et al. (2022a). Fermented astragalus and its metabolites regulate inflammatory status and gut microbiota to repair intestinal barrier damage in dextran sulfate sodium-induced ulcerative colitis. Front. Nutr. 9, 1035912. doi:10.3389/fnut.2022.1035912
Li, X., Xu, S., Zhang, Y., Li, K., Gao, X. J., and Guo, M. Y. (2022b). Berberine depresses inflammation and adjusts smooth muscle to ameliorate ulcerative colitis of cats by regulating gut microbiota. Microbiol. Spectr. 10, e0320722. doi:10.1128/spectrum.03207-22
Liu, C., Shi, J., and Huang, J. (2018). Effects of Shenling Baizhu San on the tight junction and MLCK/MLC pathway in mice with ulcerative colitis. J. Chin. Med. Mat. 41 (09), 2180–2184. doi:10.13863/j.issn1001-4454.2018.09.036
Liu, B., Piao, X., Niu, W., Zhang, Q., Ma, C., Wu, T., et al. (2020). Kuijieyuan decoction improved intestinal barrier injury of ulcerative colitis by affecting TLR4-Dependent PI3K/AKT/NF-κB oxidative and inflammatory signaling and gut microbiota. Front. Pharmacol. 11, 1036. doi:10.3389/fphar.2020.01036
Liu, Y., Zhou, M., Yang, M., Jin, C., Song, Y., Chen, J., et al. (2021a). Pulsatilla chinensis saponins ameliorate inflammation and DSS-Induced ulcerative colitis in rats by regulating the composition and diversity of intestinal flora. Front. Cell. Infect. Microbiol. 11, 728929. doi:10.3389/fcimb.2021.728929
Liu, X., Fan, Y., Du, L., Mei, Z., and Fu, Y. (2021b). In silico and in vivo studies on the mechanisms of Chinese medicine formula (Gegen Qinlian decoction) in the treatment of ulcerative colitis. Front. Pharmacol. 12, 665102. doi:10.3389/fphar.2021.665102
Liu, Y., Li, B. G., Su, Y. H., Zhao, R. X., Song, P., Li, H., et al. (2022). Potential activity of traditional Chinese medicine against ulcerative colitis: a review. J. Ethnopharmacol. 10, 115084. doi:10.1016/j.jep.2022.115084
Liu, M., Wang, Z., Liu, X., Xiao, H., Liu, Y., Wang, J., et al. (2023). Therapeutic effect of Yiyi Fuzi Baijiang formula on TNBS-induced ulcerative colitis via metabolism and Th17/Treg cell balance. J. Ethnopharmacol. 309, 116301. doi:10.1016/j.jep.2023.116301
Lloyd, C. M., and Snelgrove, R. J. (2018). Type 2 immunity: expanding our view. Sci. Immunol. 3, eaat1604. doi:10.1126/sciimmunol.aat1604
Lu, Q., Li, J., Ding, P., Mao, T., Shi, L., Sun, Z., et al. (2022). Qingchang Wenzhong decoction alleviates DSS-Induced inflammatory bowel disease by inhibiting M1 macrophage polarization in vitro and in vivo. Biomed. Res. Int. 2022, 9427076. doi:10.1155/2022/9427076
Luo, S., Wen, R., Wang, Q., Zhao, Z., Nong, F., Fu, Y., et al. (2019). Rhubarb Peony decoction ameliorates ulcerative colitis in mice by regulating gut microbiota to restoring Th17/Treg balance. J. Ethnopharmacol. 231, 39–49. doi:10.1016/j.jep.2018.08.033
Luongo, D., D'Arienzo, R., Bergamo, P., Maurano, F., and Rossi, M. (2009). Immunomodulation of gut-associated lymphoid tissue: current perspectives. Int. Rev. Immunol. 28, 446–464. doi:10.3109/08830180903236486
Ma, C., Battat, R., Dulai, P. S., Parker, C. E., Sandborn, W. J., Feagan, B. G., et al. (2019). Innovations in oral therapies for inflammatory bowel disease. Drugs 79, 1321–1335. doi:10.1007/s40265-019-01169-y
Macfarlane, S., and Macfarlane, G. T. (2003). Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 62, 67–72. doi:10.1079/PNS2002207
Machiels, K., Joossens, M., Sabino, J., De Preter, V., Arijs, I., Eeckhaut, V., et al. (2014). A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63, 1275–1283. doi:10.1136/gutjnl-2013-304833
Mao, T., Li, J., Liu, L., Zhao, W., Liu, Y., Gao, K., et al. (2017). Qingchang Wenzhong decoction attenuates DSS-induced colitis in rats by reducing inflammation and improving intestinal barrier function via upregulating the MSP/RON signalling pathway. Evid. Based Complement. Altern. Med. 2017, 4846876. doi:10.1155/2017/4846876
Marchesi, J. R., Adams, D. H., Fava, F., Hermes, G. D., Hirschfield, G. M., Hold, G., et al. (2016). The gut microbiota and host health: a new clinical frontier. Gut 65, 330–339. doi:10.1136/gutjnl-2015-309990
Miao, Z., Chen, L., Feng, H., Gu, M., Yan, J., Xu, Y., et al. (2021). Baitouweng decoction ameliorates ulcerative colitis in mice partially attributed to regulating Th17/Treg balance and restoring intestinal epithelial barrier. Front. Pharmacol. 11, 531117. doi:10.3389/fphar.2020.531117
Mo, X., Tang, K., Deng, L., Zhou, X., Li, X., Zhang, Y., et al. (2022). Prevention of ulcerative colitis by Huangqin decoction: reducing the intestinal epithelial cell apoptosis rate through the IFN-γ/JAK/ETS signalling pathway. Pharm. Biol. 60, 1116–1125. doi:10.1080/13880209.2022.2070220
Molodecky, N. A., Soon, I. S., Rabi, D. M., Ghali, W. A., Ferris, M., Chernoff, G., et al. (2012). Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54. doi:10.1053/j.gastro.2011.10.001
Mörbe, U. M., Jørgensen, P. B., Fenton, T. M., von Burg, N., Riis, L. B., Spencer, J., et al. (2021). Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 14, 793–802. doi:10.1038/s41385-021-00389-4
Muniz, L. R., Knosp, C., and Yeretssian, G. (2012). Intestinal antimicrobial peptides during homeostasis, infection, and disease. Front. Immunol. 3, 310. doi:10.3389/fimmu.2012.00310
Naganuma, M., Sugimoto, S., Mitsuyama, K., Kobayashi, T., Yoshimura, N., Ohi, H., et al. (2018). Efficacy of indigo naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology 154, 935–947. doi:10.1053/j.gastro.2017.11.024
Nishino, K., Nishida, A., Inoue, R., Kawada, Y., Ohno, M., Sakai, S., et al. (2018). Analysis of endoscopic brush samples identified mucosa-associated dysbiosis in inflammatory bowel disease. J. Gastroenterol. 53, 95–106. doi:10.1007/s00535-017-1384-4
Odenwald, M. A., and Turner, J. R. (2017). The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 14, 9–21. doi:10.1038/nrgastro.2016.169
Parizadeh, M., and Arrieta, M. C. (2023). The global human gut microbiome: genes, lifestyles, and diet. Trends Mol. Med. 27, 789–801. doi:10.1016/j.molmed.2023.07.002
Peterson, L. W., and Artis, D. (2014). Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 14, 141–153. doi:10.1038/nri3608
Qi, Y. F., Niu, M. L., and Zhang, L. J. (2022). Effect of Shenling Baizhu Powder on Th17/Treg immune balance in rats with ulcerative colitis. China’s Naturop. 30, 88–92. doi:10.19621/j.cnki.11-3555/r.2022.0631
Ran, B., Guo, C. E., Zhang, Y., Han, C., Cao, T., Huang, H., et al. (2022). Preventive effect of Chinese dwarf cherry [cerasus humilis (bge.) sok.] fermentation juice on dextran sulfate sodium-induced ulcerative colitis rats through the regulation of IgA and the intestinal immune barrier. Food Funct. 13, 5766–5781. doi:10.1039/d1fo04218a
Ren, Z., Guo, C., Yu, S., Zhu, L., Wang, Y., Hu, H., et al. (2019). Progress in mycotoxins affecting intestinal mucosal barrier function. Int. J. Mol. Sci. 20, 2777. doi:10.3390/ijms20112777
Rubin, D. T., Ananthakrishnan, A. N., Siegel, C. A., Sauer, B. G., and Long, M. D. (2019). ACG clinical guideline: ulcerative colitis in adults. Am. J. Gastroenterol. 114, 384–413. doi:10.14309/ajg.0000000000000152
Shen, H., Zhang, S., Zhao, W., Ren, S., Ke, X., Gu, Q., et al. (2021). Randomised clinical trial: efficacy and safety of Qing-Chang-Hua-Shi granules in a multicenter, randomized, and double-blind clinical trial of patients with moderately active ulcerative colitis. Biomed. Pharmacother. 139, 111580. doi:10.1016/j.biopha.2021.111580
Shi, X. Z., Winston, J. H., and Sarna, S. K. (2011). Differential immune and genetic responses in rat models of Crohn's colitis and ulcerative colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G41–G51. doi:10.1152/ajpgi.00358.2010
Shi, G., Jiang, H., Feng, J., Zheng, X., Zhang, D., Jiang, C., et al. (2021). Aloe vera mitigates dextran sulfate sodium-induced rat ulcerative colitis by potentiating colon mucus barrier. J. Ethnopharmacol. 279, 114108. doi:10.1016/j.jep.2021.114108
Sun, Z., Li, J., Dai, Y., Wang, W., Shi, R., Wang, Z., et al. (2020). Indigo naturalis alleviates dextran sulfate sodium-induced colitis in rats via altering gut microbiota. Front. Microbiol. 11, 731. doi:10.3389/fmicb.2020.00731
Sun, Z., Li, J., Wang, W., Liu, Y., Liu, J., Jiang, H., et al. (2021). Qingchang Wenzhong decoction accelerates intestinal mucosal healing through modulation of dysregulated gut microbiome, Intestinal Barrier and Immune Responses in Mice. Front. Pharmacol. 12, 738152. doi:10.3389/fphar.2021.738152
Torres, J., Petralia, F., Sato, T., Wang, P., Telesco, S. E., Choung, R. S., et al. (2020). Serum biomarkers identify patients who will develop inflammatory bowel diseases up to 5 years before diagnosis. Gastroenterology 159, 96–104. doi:10.1053/j.gastro.2020.03.007
Tuci, A., Tonon, F., Castellani, L., Sartini, A., Roda, G., Marocchi, M., et al. (2011). Fecal detection of mycobacterium avium paratuberculosis using the IS900 DNA sequence in crohn's disease and ulcerative colitis patients and healthy subjects. Dig. Dis. Sci. 56, 2957–2962. doi:10.1007/s10620-011-1699-6
Turner, J. R. (2009). Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809. doi:10.1038/nri2653
Turpin, W., Lee, S. H., Raygoza Garay, J. A., Madsen, K. L., Meddings, J. B., Bedrani, L., et al. (2020). Increased intestinal permeability is associated with later development of crohn's disease. Gastroenterology 159, 2092–2100.e5. doi:10.1053/j.gastro.2020.08.005
Ungaro, R., Mehandru, S., Allen, P. B., Peyrin-Biroulet, L., and Colombel, J. F. (2017). Ulcerative colitis. Lancet 389, 1756–1770. doi:10.1016/S0140-6736(16)32126-2
Van der Sluis, M., De Koning, B. A., De Bruijn, A. C., Velcich, A., Meijerink, J. P., Van Goudoever, J. B., et al. (2006). Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131, 117–129. doi:10.1053/j.gastro.2006.04.020
Wan, Y., Yang, L., Li, H., Ren, H., Zhu, K., Dong, Z., et al. (2022). Zingiber officinale and Panax ginseng ameliorate ulcerative colitis in mice via modulating gut microbiota and its metabolites. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1203, 123313. doi:10.1016/j.jchromb.2022.123313
Wang, Z., Liang, Y., Yu, J., Zhang, D., Ren, L., Zhang, Z., et al. (2020). Guchang Zhixie Wan protects mice against dextran sulfate sodium-induced colitis through modulating the gut microbiota in colon. J. Ethnopharmacol. 5, 112991. doi:10.1016/j.jep.2020.112991
Wang, B., Gong, Z., Zhan, J., Yang, L., Zhou, Q., and Yuan, X. (2021). Xianglian Pill suppresses inflammation and protects intestinal epithelial barrier by promoting autophagy in DSS induced ulcerative colitis mice. Front. Pharmacol. 11, 594847. doi:10.3389/fphar.2020.594847
Wang, J. L., Han, X., Li, J. X., Shi, R., Liu, L. L., Wang, K., et al. (2022a). Differential analysis of intestinal microbiota and metabolites in mice with dextran sulfate sodium-induced colitis. World J. Gastroenterol. 28, 6109–6130. doi:10.3748/wjg.v28.i43.6109
Wang, J., Wang, X., Ma, X., Xu, B., Chen, L., Chen, C., et al. (2022b). Therapeutic effect of patrinia villosa on TNBS-induced ulcerative colitis via metabolism, vitamin D receptor and NF-κB signaling pathways. J. Ethnopharmacol. 288, 114989. doi:10.1016/j.jep.2022.114989
Wang, X., Huang, S., Zhang, M., Su, Y., Pan, Z., Liang, J., et al. (2023). Gegen Qinlian decoction activates AhR/IL-22 to repair intestinal barrier by modulating gut microbiota-related tryptophan metabolism in ulcerative colitis mice. J. Ethnopharmacol. 302, 115919. doi:10.1016/j.jep.2022.115919
Wei, Y. Y., Fan, Y. M., Ga, Y., Zhang, Y. N., Han, J. C., and Hao, Z. H. (2021). Shaoyao decoction attenuates DSS-induced ulcerative colitis, macrophage and NLRP3 inflammasome activation through the MKP1/NF-κB pathway. Phytomedicine 92, 153743. doi:10.1016/j.phymed.2021.153743
Wen, Y., Zhang, W., Yang, R., Jiang, L., Zhang, X., Wang, B., et al. (2022). Regulation of Yujin Powder alcoholic extracts on ILC3s-TD IgA-colonic mucosal flora axis of DSS-induced ulcerative colitis. Front. Microbiol. 13, 1039884. doi:10.3389/fmicb.2022.1039884
Wu, Z. C., Zhao, Z. L., Deng, J. P., Huang, J. T., Wang, Y. F., and Wang, Z. P. (2020). Sanhuang Shu'ai decoction alleviates DSS-induced ulcerative colitis via regulation of gut microbiota, inflammatory mediators and cytokines. Biomed. Pharmacother. 125, 109934. doi:10.1016/j.biopha.2020.109934
Wu, C., Zheng, T., Chen, H., Zou, P., Zhang, M., Wang, J., et al. (2022). Effect and mechanism of pharmaceutical excipients on berberine to alleviate ulcerative colitis via regulating gut microbiota. Molecules 27, 5997. doi:10.3390/molecules27185997
Xiao, S., Yan, Y., Shao, M., Zhou, X., Niu, Z., Wu, Y., et al. (2023). Kuijieling decoction regulates the Treg/Th17 cell balance in ulcerative colitis through the RA/RARα signaling pathway. J. Ethnopharmacol. 318, 116909. doi:10.1016/j.jep.2023.116909
Xie, J., Tian, S., Liu, J., Huang, S., Yang, M., Yang, X., et al. (2023). Combination therapy with indigo and indirubin for ulcerative colitis via reinforcing intestinal barrier function. Oxid. Med. Cell. Longev. 2023, 2894695. doi:10.1155/2023/2894695
Xuan-Qing, CHEN., Xiang-Yu, L. V., and Shi-Jia, L. I. U. (2021). Baitouweng decoction alleviates dextran sulfate sodium-induced ulcerative colitis by regulating intestinal microbiota and the IL-6/STAT3 signaling pathway. J. Ethnopharmacol. 265, 113357. doi:10.1016/j.jep.2020.113357
Yan, F., Wang, L., Shi, Y., Cao, H., Liu, L., Washington, M. K., et al. (2012). Berberine promotes recovery of colitis and inhibits inflammatory responses in colonic macrophages and epithelial cells in DSS-treated mice. Am. J. Physiol. Gastrointest. Liver Physiol. 1302, G504–G514. doi:10.1152/ajpgi.00312.2011
Yan, S., Wei, H., Jia, R., Zhen, M., Bao, S., Wang, W., et al. (2022a). Wumei Wan ameliorates murine ulcerative colitis by regulating macrophage polarization. Front. Pharmacol. 13, 859167. doi:10.3389/fphar.2022.859167
Yan, S., Wang, P., Wei, H., Jia, R., Zhen, M., Li, Q., et al. (2022b). Treatment of ulcerative colitis with Wumei Wan by inhibiting intestinal inflammatory response and repairing damaged intestinal mucosa. Phytomedicine 105, 154362. doi:10.1016/j.phymed.2022.154362
Yan, S., Wang, P., Wei, H., Jia, R., Zhen, M., Li, Q., et al. (2022c). Treatment of ulcerative colitis with Wu-Mei-Wan by inhibiting intestinal inflammatory response and repairing damaged intestinal mucosa. Phytomedicine 105, 154362. doi:10.1016/j.phymed.2022.154362
Yang, Q. Y., Ma, L. L., Zhang, C., Lin, J. Z., Han, L., He, Y. N., et al. (2021). Exploring the mechanism of indigo naturalis in the treatment of ulcerative colitis based on TLR4/MyD88/NF-κB signaling pathway and gut microbiota. Front. Pharmacol. 12, 674416. doi:10.3389/fphar.2021.674416
Yu, F. Y., Huang, S. G., Zhang, H. Y., Ye, H., Chi, H. G., Zou, Y., et al. (2014). Effects of baicalin in CD4 + CD29 + T cell subsets of ulcerative colitis patients. World J. Gastroenterol. 20, 15299–15309. doi:10.3748/wjg.v20.i41.15299
Yu, H. Q., Jia, Y. X., Cheng, Y. X., Ma, W., Duan, Y. Q., Ming, H. X., et al. (2018). Effect of Shenling Baizhu Powder on expression of IL-6, IL-10 and C-FOS gene in colon tissue of UC rats with spleen deficiency and dampness. Lishizhen Med. meteria medica Res. 29, 1049–1052. doi:10.3969/j.issn.1008-0805.2018.05.008
Yu, Y., Wang, Q. H., Chen, Y. J., Li, C., and Zhang, M. Y. (2020). Regulatory effect of Tongxie Yaofang on immune function and intestinal mucosal barrier in patients with ulcerative colitis. Chin. J. Integr. Chin. West. Med. Dig. 28, 858–862.
Yu, W., Wang, G., Lu, C., Liu, C., Jiang, L., Jiang, Z., et al. (2022). Pharmacological mechanism of Shenling Baizhu formula against experimental colitis. Phytomedicine 98, 153961. doi:10.1016/j.phymed.2022.153961
Zatorski, H., Sałaga, M., Zielińska, M., Piechota-Polańczyk, A., Owczarek, K., Kordek, R., et al. (2015). Experimental colitis in mice is attenuated by topical administration of chlorogenic acid. Naunyn Schmiedeb. Arch. Pharmacol. 388, 643–651. doi:10.1007/s00210-015-1110-9
Zhang, Z., Wu, X., Cao, S., Cromie, M., Shen, Y., Feng, Y., et al. (2017). Chlorogenic acid ameliorates experimental colitis by promoting growth of akkermansia in mice. Nutrients 9, 677. doi:10.3390/nu9070677
Zhao, Y., Luan, H., Gao, H., Wu, X., Zhang, Y., and Li, R. (2020). Gegen Qinlian decoction maintains colonic mucosal homeostasis in acute/chronic ulcerative colitis via bidirectionally modulating dysregulated Notch signaling. Phytomedicine 68, 153182. doi:10.1016/j.phymed.2020.153182
Zhao, J., Wu, R., Wei, P., Ma, Z., Pei, H., Hu, J., et al. (2023). Ethanol extract of piper wallichii ameliorates DSS-induced ulcerative colitis in mice: involvement of TLR4/NF-κB/COX-2 signaling pathway. J. Ethnopharmacol. 308, 116293. doi:10.1016/j.jep.2023.116293
Zheng, S., Xue, T., Wang, B., Guo, H., and Liu, Q. (2022a). Chinese medicine in the treatment of ulcerative colitis: the mechanisms of signaling pathway regulations. Am. J. Chin. Med. 50, 1781–1798. doi:10.1142/S0192415X22500756
Zheng, Y., Liang, C., Li, Z., Chen, J., Chen, Z., Jiang, Y., et al. (2022b). Study on the mechanism of Huangqin Decoction on rats with ulcerative colitis of damp-heat type base on mtDNA, TLR4, p-PI3K, p-Akt protein expression and microbiota. J. Ethnopharmacol. 295, 115356. doi:10.1016/j.jep.2022.115356
Keywords: intestinal mucosal barrier, mechanism, pathogenesis, traditional Chinese medicine, ulcerative colitis
Citation: Zong Y, Meng J, Mao T, Han Q, Zhang P and Shi L (2023) Repairing the intestinal mucosal barrier of traditional Chinese medicine for ulcerative colitis: a review. Front. Pharmacol. 14:1273407. doi: 10.3389/fphar.2023.1273407
Received: 06 August 2023; Accepted: 10 October 2023;
Published: 24 October 2023.
Edited by:
Guang Chen, The University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Shi Xue Dai, Guangdong Provincial People’s Hospital, ChinaCopyright © 2023 Zong, Meng, Mao, Han, Zhang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Zhang, enA4OTEyMjNAMTI2LmNvbQ==; Lei Shi, YjAxMzUwQGJ1Y20uZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.