
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 18 October 2023
Sec. Neuropharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1272969
This article is part of the Research TopicTherapies for Brain InjuryView all 7 articles
Traumatic brain injury (TBI) affects more than 2.5 million people in the U.S. each year and is the leading cause of death and disability in children and adults ages 1 to 44. Approximately 90% of TBI cases are classified as mild but may still lead to acute detrimental effects such as impaired cerebral blood flow (CBF) that result in prolonged impacts on brain function and quality of life in up to 15% of patients. We previously reported that nociceptin/orphanin FQ (N/OFQ) peptide (NOP) receptor antagonism reversed mild blast TBI-induced vestibulomotor deficits and prevented hypoxia. To explore mechanisms by which the NOP receptor-N/OFQ pathway modulates hypoxia and other TBI sequelae, the ability of the NOP antagonist, SB-612111 (SB), to reverse TBI-induced CBF and associated injury marker changes were tested in this study. Male Wistar rats randomly received sham craniotomy or craniotomy + TBI via controlled cortical impact. Injury severity was assessed after 1 h (modified neurological severity score (mNSS). Changes in CBF were assessed 2 h post-injury above the exposed cortex using laser speckle contrast imaging in response to the direct application of increasing concentrations of vehicle or SB (1, 10, and 100 µM) to the brain surface. TBI increased mNSS scores compared to baseline and confirmed mild TBI (mTBI) severity. CBF was significantly impaired on the ipsilateral side of the brain following mTBI, compared to contralateral side and to sham rats. SB dose-dependently improved CBF on the ipsilateral side after mTBI compared to SB effects on the respective ipsilateral side of sham rats but had no effect on contralateral CBF or in uninjured rats. N/OFQ levels increased in the cerebral spinal fluid (CSF) following mTBI, which correlated with the percent decrease in ipsilateral CBF. TBI also activated ERK and cofilin within 3 h post-TBI; ERK activation correlated with increased CSF N/OFQ. In conclusion, this study reveals a significant contribution of the N/OFQ-NOP receptor system to TBI-induced dysregulation of cerebral vasculature and suggests that the NOP receptor should be considered as a potential therapeutic target for TBI.
TBI is caused by an external force that causes alteration in brain physiology or pathology (Menon et al., 2010). TBI affects more than 50 million people each year (Maas et al., 2017), yet the FDA has not approved any therapeutic agents to treat TBI consequences (Vanderploeg et al., 2005; Dean and Sterr, 2013). The complicated pathophysiological consequences of TBI lead to significant disruption of delivery and increased consumption of oxygen in the brain which often results in cerebral ischemia (Algattas and Huang, 2013). According to the Mild TBI Committee of the American Congress of Rehabilitation Medicine (ACRM), mTBI severity is identified based on the Glasgow Coma Scale score of 13–15 and the severity and number of symptoms suffered by patients (Lefevre-Dognin et al., 2021). The primary tissue damage following TBI, and the subsequent events, impair the physiologic control of cerebral circulation and lead to cerebral vasospasms (Lewelt et al., 1980; Algattas and Huang, 2013), and disruption of the blood brain barrier (BBB) that contributes to vasogenic edema. Other TBI consequences that also contribute to impaired CBF include cerebral ischemia, cytotoxic edema, and increased intracranial pressure (ICP) (Lewelt et al., 1980; Algattas and Huang, 2013; Hinson et al., 2015). Both clinical and preclinical studies demonstrated that secondary pathophysiological consequences of TBI including anxiety, hyperthermia, and seizures may increase metabolic demand following brain injury (Awwad et al., 2015; Hinson et al., 2015; Stocchetti et al., 2015). Continued hemodynamic dysregulation following TBI may produce further apoptosis and necrosis in affected brain tissue. However, acute interventions to restore decreased CBF following TBI may protect brain tissue from further damage (Salehi et al., 2017).
The FDA approved the use of TBI blood biomarkers, glial fibrillary acidic protein (GFAP), and ubiquitin C-terminal hydrolase (UCH-L1), to evaluate the utility of imaging tests in adult mTBI patients (FDA, 2018). Other biochemical changes related to TBI pathology include upregulation of the axonal damage protein, neurofilament light chain (NF-L) (Liliang et al., 2010; Pandey et al., 2017; Kochanek et al., 2018; Iverson et al., 2022; Castaño-Leon et al., 2023), and upregulation and activation of the cytoskeleton-associated protein, Cofilin-1 (Campbell et al., 2012; Bahader et al., 2023) that is involved in actin filament dynamics and depolymerization.
Several animal models have been utilized experimentally to examine the biomechanical aspects of brain injury and to understand the detrimental, complex molecular cascades that are initiated by head trauma. The controlled cortical impact injury (CCI) is a mechanical model of TBI that uses a pneumatic or electromagnetic impact device to drive a rigid impactor onto the surgically exposed intact cortical dura. The CCI model produces morphologic and cerebrovascular injury responses that mirror aspects of human focal TBI. CCI impairs cerebral hemodynamic autoregulation relative to the severity of the impact and causes acute and prolonged reductions in CBF in the pericontusional cortex (Kroppenstedt et al., 2000; Liu et al., 2002; Zhang et al., 2002; Cherian and Robertson, 2003; Kroppenstedt et al., 2003; Cherian et al., 2004; Cherian et al., 2007; Liu et al., 2018).
The NOP receptor, the fourth member of the opioid receptor superfamily (Bunzow et al., 1994; Chen et al., 1994; Fukuda et al., 1994; Mollereau et al., 1994; Wang et al., 1994; Wick et al., 1994; Pan et al., 1995), and its endogenous neuropeptide, N/OFQ, are expressed in astrocytes, microglia, and neurons in the central and peripheral nervous and immune systems (Mollereau et al., 1996; Al Yacoub et al., 2022). Numerous studies demonstrated that N/OFQ levels in CSF (Armstead, 2000b; c) and brain tissue increase following injury (Witta et al., 2003; Awwad et al., 2018). These changes start early and last for a few days after the injury. Armstead’s group was the first to establish a link between N/OFQ levels upregulation and vasoconstriction of cortical cerebral arteries following cerebral ischemia, hypoxia, and TBI (Armstead, 2002; Al Yacoub et al., 2022). Administration of N/OFQ onto the exposed healthy cortex of newborn piglets induced pial artery dilation (Armstead, 1999), However, this process was reversed post-TBI. Topical N/OFQ to the injured cortex following TBI produced vasoconstriction (Armstead, 2000c), and pre-administration of a single dose of NOP receptor partial agonist attenuated the impaired cerebral vasoconstriction caused by TBI (Armstead, 2000c). We previously reported hypoxia in rat brains 8 days post-blast TBI, that was prevented by a single dose of the NOP antagonist, SB-612111 injected 30 min following blast (Awwad et al., 2018). Armstead’s work also indicated that several Mitogen-activated protein kinases (MAPKs) were involved in N/OFQ-induced vasoconstrictive actions following TBI. The studies herein investigated the effect of acute NOP receptor antagonist administration on CBF following mTBI in rats, and the effect of TBI on the N/OFQ-NOP receptor system and injury markers, cofilin-1, and MAPKs.
Male Wistar Han wildtype rats (N = 14) were purchased from Charles River Labs (Wilmington, MA) and allowed to acclimate for at least 7 days after arrival. Rats (200–300 g; 12–14 weeks of age) were housed in the animal facility under a 12-h light:12-h dark cycle (lights on at 0600) with free access to food and water. Experimental protocols were approved by the institutional animal care and use committee (IACUC) of the University of Oklahoma Health Sciences Center (OUHSC), and studies were conducted in compliance with animal welfare act (AWA) regulations, Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines 2.0 (Percie du Sert et al., 2020), and other federal statutes relating to animals and experiments involving animals. Rats were randomly assigned to receive either sham or mTBI surgery.
CCI was performed as previously described (Brody et al., 2007; Osier et al., 2015; Osier and Dixon, 2016) with modifications to enable CBF measurements on the uninjured contralateral side at the same time as the ipsilateral side as illustrated in Figure 1. Anesthetized rats (4% isoflurane with medical air induction; 2.5%–3% maintenance) underwent stereotaxic surgery with a midline incision, exposure of the skull using a retractor, and assignment of bregma as a reference using the stereotaxic manipulator (Stoelting Co., Wood Dale, IL). Control (sham) injury animals received a 9–11 mm craniotomy (Figure 1C) that spanned from the left parietal cortex to the right parietal cortex using a hand-held drill without impact, and durotomy to expose the brain cortex.
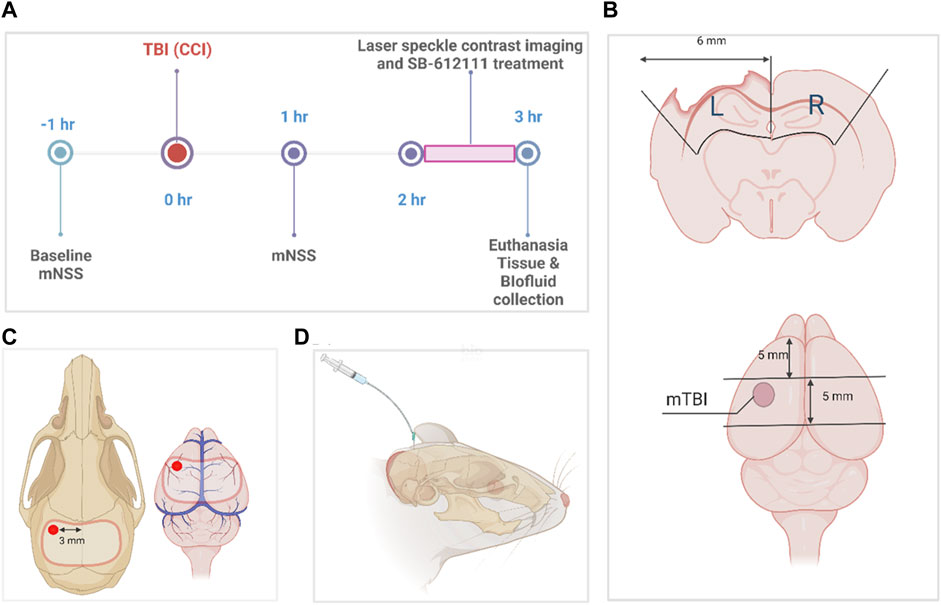
FIGURE 1. Experimental timeline and protocol diagrams. (A) indicates the times different data points and surgeries were performed. (B) illustrates tissue sample dissection of sham and mTBI brains for biochemical assays, while the size and location of the craniotomy performed for sham and mTBI surgeries and the location of the impact for mTBI is shown in (C). (D) illustrates CSF collection from the direct insertion of a 26-gauge needle into the cisterna magna (D). The dissection protocol in (B) was employed to combine tissue from parts of the somatosensory and motor cortex, corpus callosum, and hippocampus immediately below the area of impact (pericontusional area) and the comparable area in sham animals, to compare biochemical changes in tissues from the two groups. It observes anatomical borders of brain regions and specified dimensions to collect tissue from the ipsilateral and contralateral sides of the injury. This figure was created in BioRender.com.
TBI rats received a craniotomy followed by a mild controlled cortical impact (Figure 1C) with stereotaxic coordinates (1.8 mm posterior, 3.0 mm lateral to the left of the bregma) using the Impact One device (Leica Biosystems, IL) and the following actuator settings: Impactor flat tip diameter (2 mm), velocity (3 m/s), dwell time (100 m) and impact depth (4 mm). Because the animals needed to be transported to a different building to assess CBF, the bone flap was sealed in place with sterile bone wax, wounds were sutured with staples and tissue adhesive, and topical antibiotic ointment applied after each surgery. Rats remained under anesthesia sham or TBI impact until the craniotomy wound was sutured. Righting reflex time was recorded for each rat and defined as the time it took to stand on all 4 paws once anesthesia was discontinued. Body temperature and vital functions were monitored throughout surgery. Temperature was monitored using a rectal probe connected to a monitor and heating pad that adjusted temperature based on rat’s body temperature. Respiration was monitored by changes in breathing pattern (e.g., gasping, labored breathing) and/or cyanotic ears, tail, or feet. If present, isoflurane was reduced to increase oxygen delivery. Previous studies reported no changes in mean arterial blood pressure or arterial blood gasses at 30 min to 48 h following mild CCI in rats (Bryan et al., 1995; Smith and Hall, 1996; Kroppenstedt et al., 1999; Thomale et al., 2002a; Thomale et al., 2002b; Thomale et al., 2006; Muller et al., 2021).
The mNSS (Chen et al., 2001) was used to validate the severity of the injury as a measure of overall neurological function at baseline and at 1 h following surgery. The evaluation indices included a battery of motor (raising rat by the tail (0–3); walking on floor (0–3)), sensory (proprioceptive test (0–1); visual and tactile test (0–1)), Reflex: Pinna reflex (0–1); Corneal reflex (0–1); Startle reflex (0–1), resting movement (seizures, myoclonus, myodystony (0–1)), and beam balance (0–6) tests, where normal function received a value of 0. Neurological deficit severity was categorized based on cumulative score: Severe = 13–18, moderate = 6–12, mild = 1–6 (Chen et al., 2001). Rats lacking neurological deficits scored less than 1.
Laser Speckle Contrast Imaging (LSCI) technology was used to measure CBF in rats following TBI. Rats were placed in the isoflurane induction chamber for 5 min (isoflurane 4%), and anesthesia was then maintained with isoflurane (2%–3%) through the nose mask while the rats were in the stereotaxic frame. The temperature was controlled using a homeothermic controller. Once rats were in the stereotaxic frame and deep anesthesia was confirmed (no toe pinch or eye blinking reflexes), the incision was opened using a sterile blade on a scalpel. Bone wax and the skull bone flap from the craniotomy were removed to expose the cranial window for LSCI. The LSCI device (Perimed, Järfälla, Sweden) was positioned above the cranial window surface (the exposed dura mater). Drops of sterile saline were applied periodically over the exposed dura mater to keep it and cortical tissue moist during imaging. After initial CBF readings were obtained, the direct effect of vehicle (5% dimethylsulfoxide and 0.9% sodium chloride) on CBF was assessed before and after the topical application of sterile SB-612111 onto the exposed cortex with sterile pipettes for 5–10 min or until each measurement returned to baseline. To evaluate changes in CBF, an elliptic shape, with dimensions of ∼4 mm (height) and 2 mm (width), was drawn over each side of the brain in live images of each rat using the PIMsoft software (Perimed, Järfälla, Sweden). The inner borders of the shapes over each side were ∼1 mm from the midline between the two hemispheres. To identify reductions in CBF on the ipsilateral side after TBI or sham surgery, the relative percent change of CBF in the ipsilateral side to the contralateral side was generated by the software. To identify changes in CBF following the application of sequential doses of SB-612111, times of interest (TOI) in the CBF graph of each side were identified after each dose, and percent change relative to the baseline at each TOI after injury was generated and used for statistical analysis. Three rats were excluded: one died during anesthesia; excessive bleeding prevented CBF assessment for two others. The total number of rats with CBF assessments per group was 5 sham and 6 mTBI.
[(−)-cis-1-Methyl-7-[[4-(2,6-dichlorophenyl) piperidin-1-yl] methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol], SB-612111 (SB; Tocris Bioscience, Bristol, United Kingdom) was dissolved in sterile 5% dimethylsulfoxide and 0.9% sodium chloride vehicle to improve solubility and absorption of the drug. Three final concentrations (1 μM, 10 μM, and 100 µM) of the SB were applied sequentially to the exposed cortex following assessment with vehicle 2 h following CCI. SB concentrations were selected based on results from previous studies (Spagnolo et al., 2007; Liao et al., 2011; Vazquez-DeRose et al., 2013).
Rats were euthanized via intracardiac exsanguination under isoflurane anesthesia, wherein brain, CSF, and blood were collected. After whole blood cardiac exsanguination, whole blood was stored at room temperature for 30 min, the supernatant (serum) was collected after centrifugation at 5,000 × g, 4°C for 5 min (Shear et al., 2016) and flash frozen in 250 µL aliquots. CSF (∼100–200 µL) was collected from the direct insertion of a 26-gauge needle into the cisterna magna (Figure 1D). Brains were extracted and dissected using a matrix brain slicer (Zivic Instruments) to include separate 5 mm sections of ipsilateral (left) and contralateral (right) tissue (cortex, corpus callosum, and hippocampus) as illustrated in Figure 1B. Brain tissue was then homogenized and divided for radioimmunoassay, qPCR, and immunoblotting then were processed and stored in −80°C as described previously (Al Yacoub et al., 2023). We considered the general effect of topical SB on the dissected tissue to be negligible since it was present for only a short time and was washed away such that CBF returned to pre-SB treatment levels before rats were euthanized.
Peptide extraction to assess N/OFQ content from tissue homogenates, CSF and serum was performed in duplicate samples using an RIA kit (Phoenix Pharmaceuticals, Belmont, CA) as described in the manufacturer’s protocol. The concentration of soluble protein present in the brain tissue extract was determined by the BCA method. The total amount of N/OFQ immunoreactivity (IR) was calculated and expressed as pg/mL in CSF and serum samples, and as pg/mg for tissue samples. Samples were excluded if they fell outside of the range of the standard curve or if contaminated with blood.
Frozen tissue homogenates were thawed and treated with cell lysis buffer (50 mM Tris pH 7.5, 0.5 M NaCl, 50 mM NaF, 10 mM EDTA, 2 mM EGTA, 1% Triton X-100, 2 mM Na3VO4, 10 µM Na4P2O7, 250 µM PMSF) with freshly added protease and phosphatase inhibitor cocktail (Santa Cruz Biotechnology, Dallas, TX). The protein concentration in supernatants (14,000 x g at 4°C for 20 min) was measured using a BCA protein assay kit (Pierce™, ThermoFisher Scientific Inc.), then samples were solubilized in 4X sample loading buffer (LI-COR Biosciences, Lincoln, NE) and heated to 65°C for 20 min. Samples (20 µg of total protein) were resolved by Novex™ WedgeWell™ 8%–16% gradient Tris-Glycine gels (Thermo Fisher Scientific Inc.), transferred to nitrocellulose membranes and probed for the following proteins: GFAP (GPCA-GFAP, 1:4000; EnCor Biotechnology, Gainesville, FL), UCH-L1 (sc-271639, 1:200; Santa Cruz Biotechnology), NF-L (sc-20012, 1:200; Santa Cruz Biotechnology), and actin (A3853, 1:2000; Sigma-Aldrich). MAPK antibodies were purchased from Cell Signaling Technology, Beverly, MA, and were diluted as follows: ERK1/2 (4696S, 1:2000), phospho-ERK1/2 (4370S, 1:500), p-38 MAPK (9228S, 1:1000), SAPK/JNK (9252S, 1:1000). Blots were incubated in primary antibody overnight at 4°C and secondary antibody for 1 h at room temperature. IRDye® 800CW goat anti-rabbit (1:10000), IRDye® 680CW donkey anti-rabbit (1:10000), IRDye® 680CW donkey anti-mouse (1:10000), IRDye® 800CW donkey anti-mouse (1:10000), IRDye® 800CW donkey anti-goat (1:10000), IRDye® 680CW goat anti-mouse (1:10000), were purchased from LI-COR Biosciences (Lincoln, NE). Blots were processed, images captured, and band density analyzed using the Odyssey® CLx Infrared Imaging System (LI-COR Biosciences, Lincoln, NE). Band density was normalized to the loading control actin in the corresponding lane using Image Studio™ Lite image processing software Ver 5.2 (LI-COR Biosciences, Lincoln, NE). Quantification of the GFAP bands included the GFAP breakdown product bands.
TriPure reagent (Sigma-Aldrich, MO) was immediately added to brain tissue homogenate collected for mRNA extraction and stored at −80°C. cDNA was synthesized using Super-Script III Reverse Transcriptase (Sigma-Aldrich, MO). Real-time PCR was performed using PowerUp™ SYBR™ Green Master Mix (Applied Biosystems, Foster City, CA) and 125 nM forward and reverse primers of target genes (rat GAPDH Fwd: 5′-ACC CAG AAG ACT GTG GAT GG-3′, Rev: 5′-CAC ATT GGG GGT AGG AAC AC-3′; rat NOP Fwd: 5′-GTT CAA GGA CTG GGT GTT CAG CCA GGT AGT-3′; rat NOP Rev: 5′-TGC TGG CCG TGG TAC TGT CTC AGA ACT CTT-3′; rat preproN/OFQ Fwd: 5′-TGC ACC AGA ATG GTA ATG TG-3′, Rev: 5′-TAG CAA CAG GAT TGT GGT GA-3′, all from Sigma-Aldrich) in an ABI 7000 Sequence Detection System (Applied Biosystems, CA). GAPDH gene was used as an internal standard to which expression of other genes was normalized. Data were analyzed using the comparative Ct method and compared to control values from sham rats (Schmittgen and Livak, 2008).
Data Analysis and graph preparation were performed using GraphPad Prism 9.5.1 software (GraphPad Software, La Jolla, CA, United States). Data are expressed as mean ± SD unless indicated otherwise. Statistical comparisons were performed by two-way ANOVA with Tukey’s post-hoc analyses as automatically recommended by the software, or a two-tailed, unpaired student’s t-test as appropriate. Results were considered statistically significant if p < 0.05. All data were subjected to Shapiro-Wilk (N < 8) normality tests before analysis. Those groups that failed the normality test (p < 0.05) were subjected to an outlier test (ROUT; Q = 1%) as recommended, to determine if the outlier was responsible for the failed normality test. Pearson’s Correlation Analysis was performed with the following data aligned from each rat: tissue N/OFQ in the ipsilateral side of the brain and in CSF, differences in CBF in the ipsilateral side relative to contralateral after injury, and injury markers. Correlations were made with data from sham and mTBI groups.
CCI TBI produces mild severity injury with prolonged righting reflex time 1 h post-impact. To assess overall neurological function and to validate the severity of the impact produced, mNSS scores were determined before surgery (baseline) and at 1 hour following sham or TBI injury. No rats were excluded prior to TBI as all scored less than 1 (in the normal range). Two-way ANOVA analysis showed a significant interaction between injury and time [F (1, 20) = 58.58, p= <0.0001], the effect of injury [F (1, 20) = 58.58, p= <0.0001], and time [F (1, 20) = 63.13, p= <0.0001]. All rats that received mTBI yielded mNSS scores within the mild severity range (mNSS = 1–6) 1-h following injury (Figure 2A). TBI also prolonged righting reflex time compared to sham rats (p = 0.017; Figure 2B).
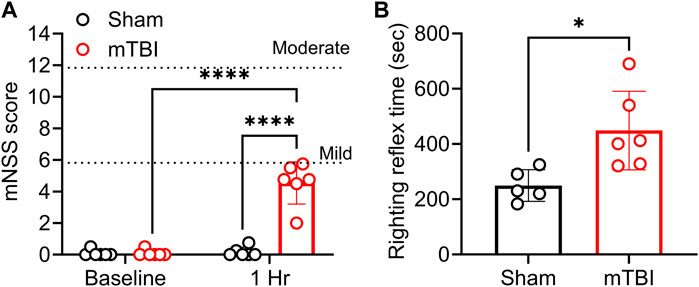
FIGURE 2. CCI produced mild TBI and prolonged the righting reflex time. TBI severity was determined by mNSS assessment; mNSS scores 1 h post-TBI and sham surgery are shown in (A); righting reflex time is found in (B). Both are presented as a scatter plot with mean ± SD (n = 5-6 per group). Dotted lines in panel (A) at 6 and 12 represent the upper limit of mild and moderate severity, respectively. Severe injury scores range from 13–18. Significant differences are represented as *p < 0.05 and ****p < 0.0001. Analysis of (A) was performed with two-way ANOVA with Tukey’s multiple comparisons tests, while results in (B) were analyzed using a student’s two-tailed unpaired t-test.
mTBI reduces CBF. Baseline CBF assessments could not be made due to instrumental limitations inherent to LSCI and the fact that the impactor and LSCI devices were located in two different buildings. Therefore, to assess the effect of mTBI on CBF, a reduction in CBF on the ipsilateral side was calculated in sham and CCI surgery animals relative to the same animal’s contralateral side using the PIMsoft software as described in the methods section. TBI reduced CBF to a greater extent 2 h post-surgery than rats that received sham surgery (p = 0.0032; Figure 3A). Representative CBF images of ipsilateral and contralateral sides of sham and CCI surgery rats are shown in Figure 3B. Areas of highest blood flow appear as bright red, while dark blue indicates the lowest levels of blood flow.
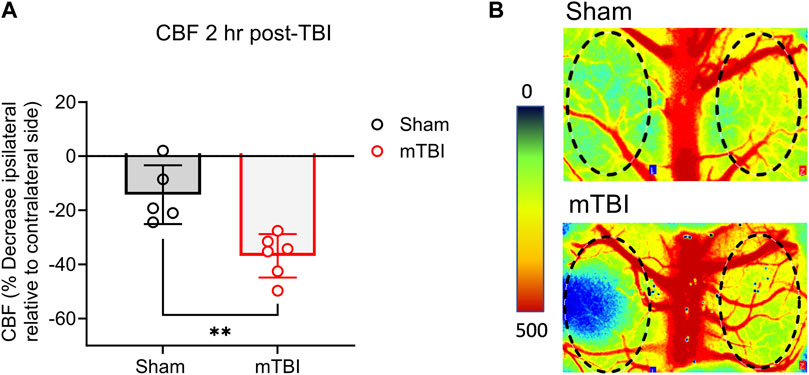
FIGURE 3. mTBI reduces CBF. (A) Values are presented as a scatter plot including mean ± SD (n = 5-6 per group). (B) Perfusion maps of the rat brain are visualized using LSCI and were pseudocolored using an arbitrary color map. Significant differences are represented as **p < 0.01 according to analysis with two-tailed, unpaired student’s t-test.
NOP receptor antagonist treatment improved CBF following mTBI but not after sham surgery. The vehicle and three concentrations of SB were applied topically, stepwise, to the dura once initial CBF measurements were completed. After each application, CBF was recorded until it stabilized (5–10 min) before the next application was made. CBF improved in the ipsilateral hemisphere following topical application of 10 µM (p = 0.0380) and 100 µM (p = 0.0011) SB to the exposed cortex of mTBI rats compared to vehicle application; 100 uM SB increased CBF significantly more than 1 uM SB (p = 0.0037; Figure 4A). CBF increased on the contralateral hemisphere in mTBI rats only with 100 µM SB compared to vehicle (p = 0.0146) and to 1 uM (p = 0.0154; Figure 4A). Two-way ANOVA analysis showed a significant effect of SB concentration on CBF [F (3, 28) = 12.31, p= <0.0001]. None of the SB concentrations altered CBF in either hemisphere following sham injury (Figure 4B). Representative images of CBF in sham and mTBI rats following surgery and each successive topical addition are shown in Figure 4C.
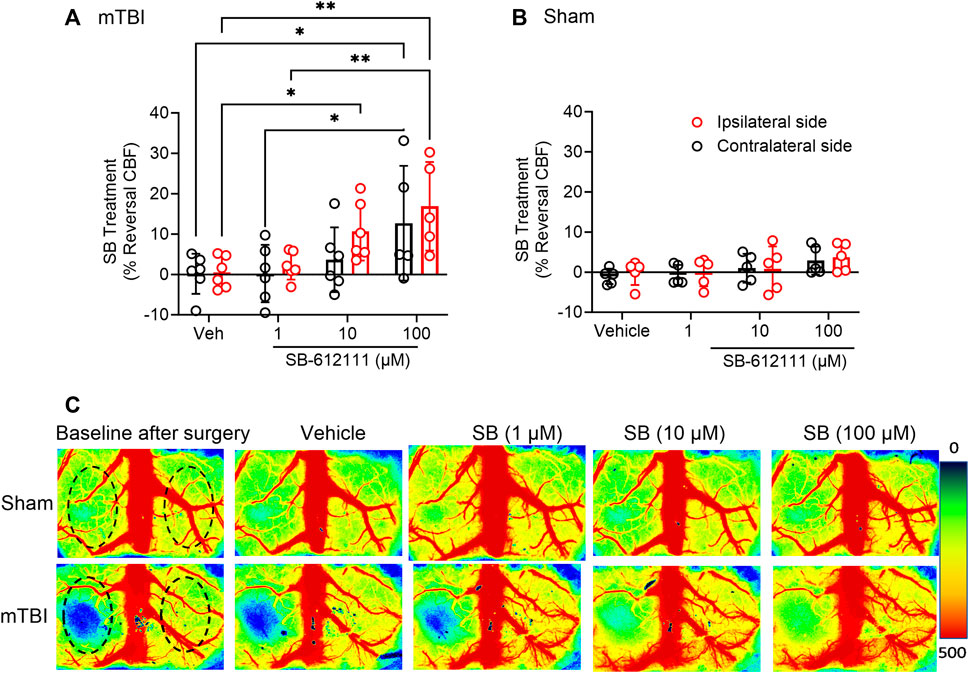
FIGURE 4. Topical application of SB-612111 improved CBF in the ipsilateral side following mTBI, but not sham surgery. SB improved CBF in the ipsilateral side following mTBI after addition of 10 µM and 100 μM, dropwise (A). Increased CBF on contralateral side in mTBI rats was noted only following 100 μM SB, compared to vehicle but not the contralateral side compared to vehicle. Vehicle and SB treatment has no effect on CBF following sham (B). (C) contains representative images of CBF in the cortex following sham (upper panels) and mTBI (lower panels) at after surgery and following treatment with vehicle, 1 μM, 10 μM, and 100 µM SB. Values are presented as mean ± SD. Significant differences are indicated as **p < 0.01; *p < 0.05 as determined by Repeated measures 2-way ANOVA with Tukey’s post-hoc test.
Increased N/OFQ levels in CSF following mTBI correlated with CBF decrease on the ipsilateral side. N/OFQ levels were measured in CSF, serum and tissue dissected from ipsilateral and contralateral hemispheres as illustrated in Figure 1B. Mild TBI increased N/OFQ levels 3 h post-TBI in CSF compared to sham (p = 0.0487; Figure 5A), but not in tissue (Figure 5B) or serum (Figure 5C). Figure 5D shows the results of the correlation analysis between percent change in CBF in the ipsilateral hemisphere relative to the contralateral hemisphere and levels of N/OFQ in CSF. N/OFQ levels in CSF negatively correlated with a percent decrease in CBF in the ipsilateral hemisphere relative to the contralateral hemisphere. No correlations between N/OFQ levels in ipsilateral tissue, or in serum and the decrease in CBF were found.
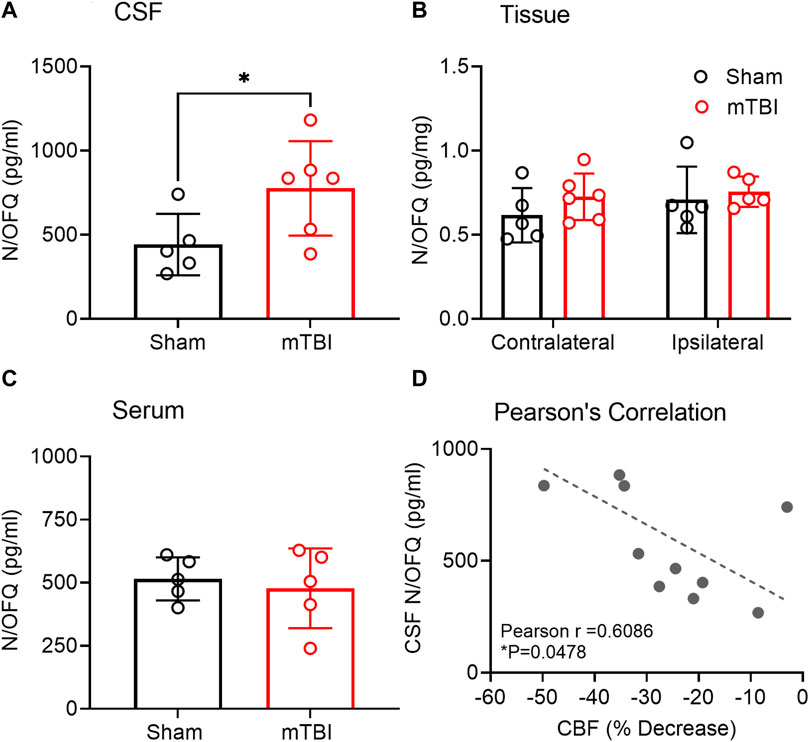
FIGURE 5. N/OFQ levels in CSF, serum, and tissue from contralateral and ipsilateral hemispheres collected 3 h post mTBI. Levels of N/OFQ were quantified using RIA in CSF (A) and serum (C) collected from rats following euthanasia 3 h post-TBI. Data were analyzed using a two-tailed unpaired t-test, and values are presented as mean ± SD (n = 5-6 per group). Differences from sham are represented as *p < 0.05). (B) indicates N/OFQ levels measured in ipsilateral and contralateral tissue collected 3 h post-TBI. Values are presented as mean ± SD (n = 5-6 per group). Two-way ANOVA with Tukey’s post-hoc test was employed to determine contributions of injury severity and side of the brain. (D) represents the results of a Pearson’s correlation analysis between N/OFQ levels in CSF and the % decrease in CBF on the ipsilateral side relative to the contralateral side, *p < 0.05. Two samples were excluded due to contamination with blood.
The effect of mTBI on N/OFQ peptide and NOP receptor mRNA was also examined. Ipsilateral hemisphere tissue mRNA was prepared and subjected to real-time PCR analysis as described above. No differences in mRNA levels between sham and mTBI rats were found (Figures 6A, B).
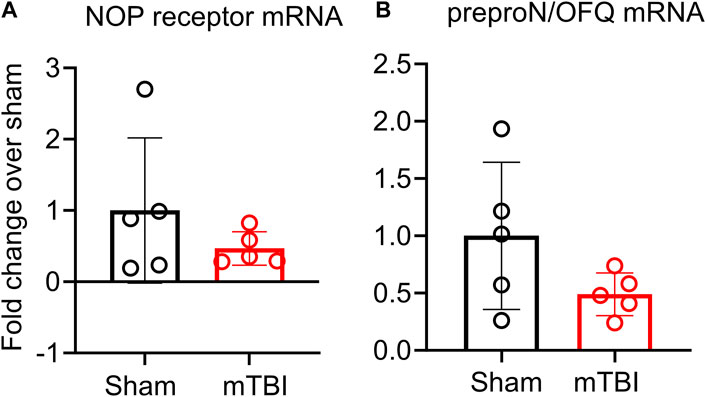
FIGURE 6. NOP receptor (A) and preproN/OFQ (B) mRNA expression in ipsilateral tissue is not altered 3 h post-mTBI. Messenger RNA was extracted from ipsilateral tissue for real-time PCR analysis as described in methods. Target gene expression in sham and mTBI-treated rat ipsilateral hemispheres were normalized to GAPDH gene expression and individual 2−ΔΔCT values from mTBI (n = 5) were normalized to the mean of individual 2−ΔΔCT values of the sham group (n = 6) to determine fold change in mRNA. Values are presented as mean ± SD and compared using a two-tailed unpaired student’s t-test.
mTBI increased ERK and cofilin-1 activation in ipsilateral brain tissue compared to sham. Phospho-ERK expression increased following mTBI in tissue from the ipsilateral hemisphere compared to ipsilateral tissue in sham (p = 0.0253; Figures 7A,B). Pearson correlation analysis between ipsilateral N/OFQ and phospho-ERK expression was performed and ipsilateral N/OFQ levels positively correlated with phospho-ERK expression (r = 0.8115, p = 0.0024). No differences between mTBI and sham groups in expression of total ERK, p38, and JNK in ipsilateral brain tissue 3 h post-mTBI were noted (Figures 7C–E respectively). Unlike ERK, which is activated by phosphorylation, cofilin-1 is activated by dephosphorylation (Meberg et al., 1998; Wang et al., 2005). Phospho-cofilin-1 expression decreased in ipsilateral TBI tissue compared to sham (p = 0.0270; Figures 8A,B), as determined by two-tailed, unpaired Student’s t-test, consistent with the presence of ischemia. No significant differences between mTBI and sham groups in expression of cofilin-1, UCH-L1, NF-L, and GFAP in ipsilateral brain tissue at 3 h post-mTBI were found (Figures 8C–F, respectively).
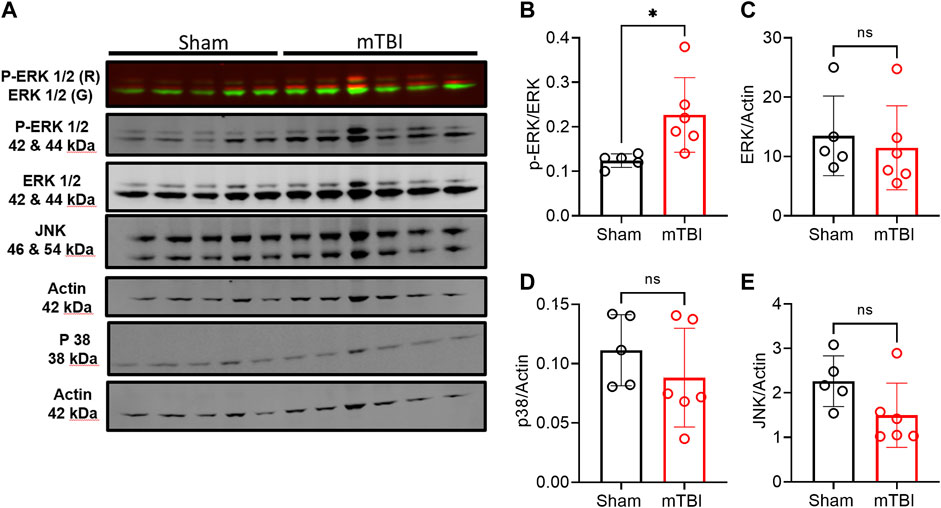
FIGURE 7. Expression of MAPKs in ipsilateral tissue from rat brains collected 3 h post-surgery. Representative immunoblots of MAPKs from brain tissue of 5–6 rats in each group at 3 h post-TBI are shown in (A). Expression of p-ERK (B), ERK (C), p38 (D), and JNK (E) were quantified by densitometric analysis of immunoblots normalized to actin loading control from the same lane except phospho-ERK was normalized to total ERK values. A two-tailed unpaired t-test was performed to assess difference from sham; significant differences are denoted by *p < 0.05. Values are presented as mean ± SD (n = 5-6 per group).

FIGURE 8. Expression of injury markers in ipsilateral tissue from rat brain hemispheres collected 3 h post-surgery. Representative immunoblots of TBI or ischemia-related markers from 5–6 rats in each group are shown in (A). (B, C) show expression of phospho-cofilin-1 (actin depolymerizing factor) and cofilin-1 (B, C, respectively). Phospho-cofilin-1 was normalized to total cofilin-1 values. UCHL-1 (neuronal injury marker) (D), NF-L [axonal injury marker; (E)], and GFAP [astrogliosis marker; (F)] were quantified by densitometric analysis of immunoblots and values were normalized to actin loading control from the same lane. A two-tailed unpaired t-test was performed to assess the difference between TBI, and sham, and significant differences are denoted by *p < 0.05. Values are presented as mean ± SD.
This study generated several important and novel findings to advance our understanding of the role of the N/OFQ-NOP receptor system in cerebrovascular dysregulation following focal mTBI with CCI. First, topical application of a NOP receptor antagonist onto the exposed cortex of rats within 1–2 h following mild CCI TBI improved CBF. Second, N/OFQ levels in CSF increased acutely (within 3 h of impact) following mTBI. Third, mTBI CCI increased activation of ERK MAPK and cofilin-1 within 3 h post-impact.
It is well established that CCI impairs cerebral hemodynamic autoregulation relative to the severity of the impact and causes acute and severe reductions in CBF in the pericontusional cortex (Kroppenstedt et al., 2000; Liu et al., 2002; Zhang et al., 2002; Cherian and Robertson, 2003; Kroppenstedt et al., 2003; Cherian et al., 2004; Cherian et al., 2007). The precise mechanism(s) underlying disruptions in the neurovascular unit and vasoconstriction that occur within hours following TBI remain poorly understood. However, previous findings hypothesized that vasospasm, including vasoconstriction of large and small cerebral vessels, is induced by increased blood pressure and subarachnoid hemorrhage after TBI (Izzy and Muehlschlegel, 2014), accompanied by increased transportation of endothelin receptors to the cellular membrane in the neurovascular unit (Kallakuri et al., 2007), and pericyte migration from the vascular wall (Dore-Duffy et al., 2000). Work by Armstead’s lab suggests that the N/OFQ-NOP receptor system may mediate this process by several potential mechanisms, including activation of ERK (Armstead, 2003; 2006). This is the first time that CCI-induced changes in CBF were shown to be correlated with N/OFQ levels and modulated by a NOP receptor antagonist. CCI causes acute reductions in CBF in pericontusional cortex (Kroppenstedt et al., 2000; Liu et al., 2002; Zhang et al., 2002; Cherian and Robertson, 2003; Kroppenstedt et al., 2003; Cherian et al., 2004; Cherian et al., 2007). A recent study in mice reported both acute and prolonged reductions (from 6 h to 21 days post-TBI) in cortical CBF following CCI using the laser speckle imaging approach (Liu et al., 2018). Our findings (Figure 3) support those previous reports of acute reduction in cortical CBF following CCI compared to sham. We were not able to measure cortical CBF baseline prior to sham or CCI because the LSCI apparatus was in a different building from where the surgery was performed. Therefore, the contralateral hemisphere was used as a baseline reference to evaluate the effect of mild impact or sham surgery on cortical CBF post-surgery as described in the methods section.
Based on previous findings from our group and others (Armstead, 2000c; Witta et al., 2003; Awwad et al., 2018), we hypothesized that mTBI would acutely increase N/OFQ levels in brain tissue and CSF. Our findings support the acute increase in N/OFQ levels in CSF shortly after TBI, as was demonstrated previously using the fluid percussion injury model of TBI (FPI) (Armstead, 2000c; Armstead, 2002) in piglets. However, the new findings demonstrate that N/OFQ levels in tissue are not yet upregulated at 3 h following mTBI CCI (Figure 5B), as reported for 8 days post-CCI TBI (Al Yacoub et al., 2023) 1 day post blast TBI (Awwad et al., 2018) or 6 hr–24 h following cortical stab injury (Witta et al., 2003). Similarly, this study found no increases in serum N/OFQ 3 h post-CCI (Figure 5C), as we’d previously reported for plasma 24 h post-blast injury (Awwad et al., 2018). This suggests that 3 h is too early to detect changes in N/OFQ levels in tissue or in blood. Collectively, this indicates that there is an acute release of N/OFQ into the CSF, but the process of replenishing peptide stores has not yet begun at this early time point. This is confirmed by our RT-PCR results in which no difference in N/OFQ mRNA tissue levels between sham and CCI TBI rats was evident (Figure 6B). Following stab TBI, N/OFQ mRNA was not elevated in pericontusional tissue until 24 h post-injury (Witta et al., 2003). However, the fact that N/OFQ levels in CSF correlated negatively with a percent decrease in CBF in the ipsilateral cortex relative to the contralateral cortex (Figure 5D) establishes an association between increased N/OFQ levels and mTBI-induced cerebrovascular disruption.
N/OFQ vasodilates and directly relaxes blood vessels under normal, non-injury conditions (Champion et al., 2002; Brookes et al., 2007; Simonsen et al., 2008). Systemic (Hashiba et al., 2003) and central (Burmeister and Kapusta, 2007) administration of N/OFQ reduces blood pressure and causes bradycardia. The indirect vasodilative effects of N/OFQ are mediated by histamine released from immune cells in the blood that occurs after NOP receptor activation in those cells (Brookes et al., 2007). Direct relaxation occurs by inhibiting pre-junctional adrenergic neurotransmission (Simonsen et al., 2008). The vasodilatory effect of N/OFQ is not nitric oxide or prostaglandin-dependent (Champion et al., 2002). Lambert’s group showed recently that sepsis increases NOP receptor mRNA expression in human vascular endothelial cells, but not in vascular smooth muscle cells in vitro (Bird et al., 2022). N/OFQ administration on cortical cerebral arteries elicited vasodilation that is protein kinase C (PKC), K(ATP), and k(Ca) activation dependent under normal conditions (Armstead, 1999). However, N/OFQ application caused vasoconstriction following ischemia and brain injury (Armstead, 2000a; Armstead, 2000b; Armstead, 2000c; Armstead, 2002). Administration of the NOP receptor putative antagonist, [F/G] NOC/OFQ (1–13), attenuated pial artery vasoconstriction and impaired CBF when applied topically to the cortex shortly after FPI TBI (Armstead, 2000c; Armstead, 2002). This peptide was later classified as a NOP receptor partial agonist, not an antagonist, based upon pharmacological studies conducted by several groups (McDonald et al., 2003; Kapusta et al., 2005; McDonald and Lambert, 2010; Asth et al., 2016). SB-612111 is a standard NOP receptor antagonist and one of the most potent and selective nonpeptide antagonists for the NOP receptor (Spagnolo et al., 2007). Our group previously demonstrated that a single treatment with SB injected shortly after mild blast TBI prevented the development of hypoxia in brain tissue of rats 8 days post-TBI (Awwad et al., 2018). One of the major goals of this study was to examine the acute effect of SB treatment on CBF (1–3 h) post-CCI TBI. We hypothesized that the acute upregulation of the N/OFQ-NOP receptor system contributes to CBF-induced deficits post-CCI TBI, and that a topical application of SB to the exposed cortex would attenuate the decrease in CBF following CCI TBI. Three different SB concentrations were applied to the exposed cortex, 1–100 μM, 2 h post-TBI. Two concentrations, 10 and 100 μM, improved CBF in ipsilateral tissue following TBI. No effects on sham rat CBF were found. The lack of effect of SB on CBF in sham was likely because CSF N/OFQ levels remained unchanged from baseline in those rats. Since we did not measure baseline CBF before surgery, it is not known if, or to what extent, CBF in the contralateral side was also affected by TBI. These findings improve our understanding of one of the mechanisms by which the N/OFQ-NOP receptor system contributes to TBI-induced deficits. However, further studies are needed to explore the effect of systemic administration of NOP receptor antagonists on cerebrovascular dysregulation post-TBI.
TBI also upregulates and activates MAPKs (Zeng et al., 2023). ERK and p38 MAPKs and protein kinase C (PKC) activation were involved in N/OFQ-mediated vasoconstrictive actions in the parietal cortex post-FPI TBI (Armstead, 2003; Philip and Armstead, 2003; Ross and Armstead, 2005). In this study, we evaluated changes in MAPKs and other injury markers in pericontusional tissue collected from sham and mTBI animals. We considered the general effect of topical SB on the dissected tissue to be negligible since it was present for only a short time and was washed away until previous CBF levels returned. This appears to be a fair assessment as increased phosphorylation of ERK was detected 3 h post-TBI in ipsilateral tissue compared to sham (Figure 7A). The levels of ERK phosphorylation correlated with tissue N/OFQ levels, which indicates an association between N/OFQ levels and activation of ERK. However, no changes in phosphorylation or total protein levels of p38 and JNK were detected at this early time point. Further studies are needed to link the timeline of vasoconstrictive actions and decreased CBF post-TBI with N/OFQ-NOP receptor system-mediated activation of MAPKs.
Acute elevations (2 h–5 days) in UCH-L1 (Liu et al., 2010; Osier et al., 2021) and NF-L (Korley et al., 2018; Karlsson et al., 2021) levels were previously detected in serum and CSF post-TBI. The earliest time at which changes in GFAP levels in injured brain tissue was detected is 3 days post-TBI (Wang et al., 2018; Niu et al., 2020), and our results are consistent with that. One of our aims in this study was to evaluate the effect of mTBI on injury markers in injured tissue at 3 h post-TBI. We found no changes in levels of UCH-L1, GFAP, or NF-L in injured tissue at this early time point (Figure 8) compared to sham. This is the first study to report on tissue levels of NF-L and UCH-L1 at 3 h post mild CCI TBI.
Cofilin-1 is a cytoskeleton-associated protein involved in actin filament dynamics and depolymerization (Pollard et al., 2000). TBI and cerebral ischemia increased cofilin expression and its de-phosphorylation in injured tissue, and it has been used as a marker of ischemia (Campbell et al., 2012; Bahader et al., 2023). Activation of cofilin by de-phosphorylation (e.g., reduced phosphorylation) leads to increased actin depolymerization and, as a result, dendritic remodeling and spine loss post-TBI (Campbell et al., 2012). Cofilin-1 activation is also involved in oxidative stress and microglial activation responses post-TBI (Bahader et al., 2023). Activated cofilin may be involved in BBB disruption by destabilizing tight junction adherent junction proteins connecting endothelia cells within the BBB (Toshima et al., 2001; Suurna et al., 2006; Nagumo et al., 2008; Shiobara et al., 2013), thus it may also become a useful marker of BBB integrity. Several signaling pathways are involved in cofilin-1 phosphorylation and dephosphorylation under physiological and pathophysiological conditions in the CNS (Namme et al., 2021). However, regulation of cofilin-1 expression and activation following TBI is not well studied. Our results supported findings from the few previous studies (Campbell et al., 2012; Bahader et al., 2023) that show an early activation of cofilin by dephosphorylation in ipsilateral tissue post-TBI. This activation of cofilin is an indication of the acute ischemic response in injured tissue resulting from tissue damage and decreased CBF post-TBI. No correlation was found between cofilin-1 or p-cofilin-1 expression in tissue, and N/OFQ levels in tissue or CSF. Further studies at later timepoints are needed to better understand if there is a relationship between N/OFQ and cofilin-1 activation, as well as the mechanism, in general, behind cofilin-1 activation and upregulation post-TBI.
The present study demonstrated the involvement of the N/OFQ-NOP receptor in decreased CBF 1–3 h post-mTBI. Mild TBI results in decreased CBF, ischemia, increased release of N/OFQ into the CSF, and activation of ERK MAPK as early as 3 h post-CCI TBI, while other injury markers and MAPKs were unchanged at this early time point. Together, our data suggest that acute blockade of the NOP receptor provides a protective effect against cerebrovascular dysregulation and potentially prevents the detrimental effects of decreased CBF post-mTBI.
We submit our manuscript as an original research article to Frontiers in Pharmacology as part of the Neuropharmacology Research Topic: Therapies for brain injury. Our manuscript falls under the theme of ‘Preclinical Pharmacology: animal models, validation of novel drug targets, the mechanism(s) of drug action, innovative methods for drug delivery to the brain’. Our study reveals a significant contribution of the Nociceptin/Orphanin FQ (N/OFQ)- Nociceptin/Orphanin FQ peptide (NOP) receptor system to TBI-induced dysregulation of cerebral vasculature and suggests that the NOP receptor should be considered as a potential therapeutic target for TBI. We utilized one of the 3 most widely used TBI models, controlled cortical impact (CCI), to produce mild TBI, and used the NOP receptor antagonist SB-612111 (SB) to dose-dependently reverse TBI-induced reductions in CBF. N/OFQ levels increased in the cerebral spinal fluid (CSF) acutely following mTBI, which correlated with the percent decrease in ipsilateral CBF. TBI also activated ERK and cofilin within 3 h post-TBI, and ERK activation positively correlated with increased CSF N/OFQ. These findings support the findings of a recent publication that demonstrate that recovery from CCI injury in NOP receptor −/− rats is more rapid and complete than in WT.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The animal study was approved by the Institutional animal care and use committee (IACUC) of the University of Oklahoma Health Sciences Center (OUHSC). The study was conducted in accordance with and institutional requirements.
OA: Conceptualization, Data curation, Formal Analysis, Methodology, Participation in project management, Visualization, Writing–review and editing, Investigation, Writing–original draft. ST: Methodology, Writing–review and editing, Supervision. YZ: Methodology, Writing–review and editing, Investigation. AC: Methodology, Writing–review and editing, Resources, Supervision. KS: Methodology, Resources, Supervision, Writing–review and editing, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Project administration, Visualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants to the American Heart Association (AHA CDA941290), the National Institute on Aging (K01AG073614, R03AG070479), Presbyterian Health Foundation, Cellular and Molecular GeroScience CoBRE (P20GM125528), Reynolds Foundation, the Oklahoma Nathan Shock Center (P30AG050911), NCI Cancer Center Support Grant (P30 CA225520), and the Oklahoma Tobacco Settlement Endowment Trust to ST, and the Presbyterian Health Foundation and Richard T. Anderson Endowment, OU College of Pharmacy to KS.
Parts of this work were presented as a poster presentation at the 39th Annual Symposium of the National Neurotrauma Society, including the AANS/CNS Joint Section on Neurotrauma and Critical Care on June 26–29, 2022, Atlanta, GA, United States (Al Yacoub et al., 2022). A draft of this manuscript was included as a chapter in a dissertation submitted by OA to the Graduate College of the University of Oklahoma Health Sciences Center in partial fulfillment of the requirements for a degree of Doctor of Philosophy in Pharmaceutical Sciences, June 2023 (Al Yacoub, 2023).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CBF, Cerebral blood flow; CSF, Cerebral spinal fluid; CCI, Controlled cortical impact; ERK, Extracellular signal regulated kinase; GFAP, Glial fibrillary acidic protein; MAPK, Mitogen-activated protein kinase; mNSS, Modified neurological severity score; mTBI, Mild traumatic brain injury; N/OFQ, Nociceptin/Orphanin FQ; NF-L, Neurofilament light chain; NOP, nociceptin/orphanin FQ (N/OFQ) peptide; PpN/OFQ, Prepronociceptin; SB, SB-612111; TBI, Traumatic brain injury; UCH-L1, Ubiquitin carboxy-terminal hydrolase—L1.
Al Yacoub, O. N., Awwad, H. O., and Standifer, K. M. (2023). Recovery from traumatic brain injury (TBI) is Nociceptin/Orphanin FQ peptide (NOP) receptor genotype-, sex-, and injury severity-dependent. J. Pharmacol. Exp. Ther. 2023. doi:10.1124/jpet.123.001664
Al Yacoub, O. N., Awwad, H. O., Zhang, Y., and Standifer, K. M. (2022a). Therapeutic potential of nociceptin/orphanin FQ peptide (NOP) receptor modulators for treatment of traumatic brain injury, traumatic stress, and their co-morbidities. Pharmacol. Ther. 231, 107982. doi:10.1016/j.pharmthera.2021.107982
Al Yacoub, O. N. (2023). The N/Ofq-Nop receptor system: a potential therapeutic target for traumatic brain injury. Oklahoma, USA: Doctor of Philosophy, University of Oklahoma Health Sciences Center.
Al Yacoub, O. T., Awwad, S., Csiszar, H., and Standifer, K. (2022b). “P143 nociceptin/orphanin fq peptide receptor antagonist rescues traumatic brain injury-induced impaired cerebral blood flow,” in The 39th Annual Symposium of the National Neurotrauma Society, Atlanta, Georgia, USA, June 26-29, 2022.
Algattas, H., and Huang, J. H. (2013). Traumatic Brain Injury pathophysiology and treatments: early, intermediate, and late phases post-injury. Int. J. Mol. Sci. 15 (1), 309–341. doi:10.3390/ijms15010309
Armstead, W. M. (2000a). Altered release of prostaglandins contributes to hypoxic/ischemic impairment of NOC/oFQ cerebrovasodilation. Brain Res. 859 (1), 104–112. doi:10.1016/s0006-8993(00)01949-1
Armstead, W. M. (2006). Differential activation of ERK, p38, and JNK MAPK by nociceptin/orphanin FQ in the potentiation of prostaglandin cerebrovasoconstriction after brain injury. Eur. J. Pharmacol. 529 (1-3), 129–135. doi:10.1016/j.ejphar.2005.08.059
Armstead, W. M. (1999). Nociceptin/orphanin FQ dilates pial arteries by K(ATP) and K(ca) channel activation. Brain Res. 835 (2), 315–323.
Armstead, W. M. (2003). PTK, ERK and p38 MAPK contribute to impaired NMDA-induced vasodilation after brain injury. Eur. J. Pharmacol. 474 (2-3), 249–254. doi:10.1016/s0014-2999(03)02012-0
Armstead, W. M. (2000b). Relationship between nociceptin/orphanin FQ and cerebral hemodynamics after hypoxia-ischemia in piglets. Am. J. Physiol. Heart Circ. Physiol. 278 (2), H477–H483. doi:10.1152/ajpheart.2000.278.2.H477
Armstead, W. M. (2000c). Role of nociceptin/orphanin FQ in age-dependent cerebral hemodynamic effects of brain injury. J. Neurotrauma 17 (9), 751–764. doi:10.1089/neu.2000.17.751
Armstead, W. M. (2002). Role of Nociceptin/Orphanin FQ in the physiologic and pathologic control of the cerebral circulation. Exp. Biol. Med. (Maywood) 227 (11), 957–968. doi:10.1177/153537020222701103
Asth, L., Ruzza, C., Malfacini, D., Medeiros, I., Guerrini, R., Zaveri, N. T., et al. (2016). Beta-arrestin 2 rather than G protein efficacy determines the anxiolytic-versus antidepressant-like effects of nociceptin/orphanin FQ receptor ligands. Neuropharmacology 105, 434–442. doi:10.1016/j.neuropharm.2016.02.003
Awwad, H. O., Durand, C. D., Gonzalez, L. P., Tompkins, P., Zhang, Y., Lerner, M. R., et al. (2018). Post-blast treatment with Nociceptin/Orphanin FQ peptide (NOP) receptor antagonist reduces brain injury-induced hypoxia and signaling proteins in vestibulomotor-related brain regions. Behav. Brain Res. 340, 183–194. doi:10.1016/j.bbr.2016.10.041
Awwad, H. O., Gonzalez, L. P., Tompkins, P., Lerner, M., Brackett, D. J., Awasthi, V., et al. (2015). Blast overpressure waves induce transient anxiety and regional changes in cerebral glucose metabolism and delayed hyperarousal in rats. Front. Neurol. 6, 132. doi:10.3389/fneur.2015.00132
Bahader, G. A., James, A. W., Almarghalani, D. A., and Shah, Z. A. (2023). Cofilin inhibitor protects against traumatic brain injury-induced oxidative stress and neuroinflammation. Biol. (Basel) 12 (4). doi:10.3390/biology12040630
Bird, M. F., Gallacher-Horley, B., McDonald, J., McVey, D. G., Al-Janabi, F., Guerrini, R., et al. (2022). In vitro sepsis induces Nociceptin/Orphanin FQ receptor (NOP) expression in primary human vascular endothelial but not smooth muscle cells. PLoS One 17 (9), e0274080. doi:10.1371/journal.pone.0274080
Brody, D. L., Mac Donald, C., Kessens, C. C., Yuede, C., Parsadanian, M., Spinner, M., et al. (2007). Electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury. J. Neurotrauma 24 (4), 657–673. doi:10.1089/neu.2006.0011
Brookes, Z. L., Stedman, E. N., Guerrini, R., Lawton, B. K., Calo, G., and Lambert, D. G. (2007). Proinflammatory and vasodilator effects of nociceptin/orphanin FQ in the rat mesenteric microcirculation are mediated by histamine. Am. J. Physiol. Heart Circ. Physiol. 293 (5), H2977–H2985. doi:10.1152/ajpheart.00448.2007
Bryan, R. M., Cherian, L., and Robertson, C. (1995). Regional cerebral blood flow after controlled cortical impact injury in rats. Anesth. Analg. 80 (4), 687–695. doi:10.1097/00000539-199504000-00007
Bunzow, J. R., Saez, C., Mortrud, M., Bouvier, C., Williams, J. T., Low, M., et al. (1994). Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett. 347 (2-3), 284–288.
Burmeister, M. A., and Kapusta, D. R. (2007). Centrally administered nociceptin/orphanin FQ (N/OFQ) evokes bradycardia, hypotension, and diuresis in mice via activation of central N/OFQ peptide receptors. J. Pharmacol. Exp. Ther. 322 (1), 324–331. doi:10.1124/jpet.107.120394
Campbell, J. N., Low, B., Kurz, J. E., Patel, S. S., Young, M. T., and Churn, S. B. (2012). Mechanisms of dendritic spine remodeling in a rat model of traumatic brain injury. J. Neurotrauma 29 (2), 218–234. doi:10.1089/neu.2011.1762
Castaño-Leon, A. M., Sánchez Carabias, C., Hilario, A., Ramos, A., Navarro-Main, B., Paredes, I., et al. (2023). Serum assessment of traumatic axonal injury: the correlation of GFAP, t-Tau, UCH-L1, and NfL levels with diffusion tensor imaging metrics and its prognosis utility. J. Neurosurg. 138 (2), 454–464. doi:10.3171/2022.5.Jns22638
Champion, H. C., Bivalacqua, T. J., Zadina, J. E., Kastin, A. J., Hyman, A. L., and Kadowitz, P. J. (2002). Role of nitric oxide in mediating vasodilator responses to opioid peptides in the rat. Clin. Exp. Pharmacol. Physiol. 29 (3), 229–232. doi:10.1046/j.1440-1681.2002.03634.x
Chen, J., Sanberg, P. R., Li, Y., Wang, L., Lu, M., Willing, A. E., et al. (2001). Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 32 (11), 2682–2688. doi:10.1161/hs1101.098367
Chen, Y., Fan, Y., Liu, J., Mestek, A., Tian, M., Kozak, C. A., et al. (1994). Molecular cloning, tissue distribution and chromosomal localization of a novel member of the opioid receptor gene family. FEBS Lett. 347 (2-3), 279–283.
Cherian, L., Goodman, J. C., and Robertson, C. (2007). Neuroprotection with erythropoietin administration following controlled cortical impact injury in rats. J. Pharmacol. Exp. Ther. 322 (2), 789–794. doi:10.1124/jpet.107.119628
Cherian, L., Hlatky, R., and Robertson, C. S. (2004). Comparison of tetrahydrobiopterin and L-arginine on cerebral blood flow after controlled cortical impact injury in rats. J. Neurotrauma 21 (9), 1196–1203. doi:10.1089/neu.2004.21.1196
Cherian, L., and Robertson, C. S. (2003). L-arginine and free radical scavengers increase cerebral blood flow and brain tissue nitric oxide concentrations after controlled cortical impact injury in rats. J. Neurotrauma 20 (1), 77–85. doi:10.1089/08977150360517209
Dean, P. J., and Sterr, A. (2013). Long-term effects of mild traumatic brain injury on cognitive performance. Front. Hum. Neurosci. 7, 30. doi:10.3389/fnhum.2013.00030
Dore-Duffy, P., Owen, C., Balabanov, R., Murphy, S., Beaumont, T., and Rafols, J. A. (2000). Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc. Res. 60 (1), 55–69. doi:10.1006/mvre.2000.2244
FDA (2018). FDA authorizes marketing of first blood test to aid in the evaluation of concussion in adults. Available at: https://www.fda.gov/news-events/press-announcements/fda-authorizes-marketing-first-blood-test-aid-evaluation-concussion-adults, US Food and Drug Administration (Accessed July 2, 2018).
Fukuda, K., Kato, S., Mori, K., Nishi, M., Takeshima, H., Iwabe, N., et al. (1994). cDNA cloning and regional distribution of a novel member of the opioid receptor family. FEBS Lett. 343 (1), 42–46.
Hashiba, E., Hirota, K., Kudo, T., Calo, G., Guerrini, R., and Matsuki, A. (2003). Effects of nociceptin/orphanin FQ receptor ligands on blood pressure, heart rate, and plasma catecholamine concentrations in Guinea pigs. Naunyn Schmiedeb. Arch. Pharmacol. 367 (4), 342–347. doi:10.1007/s00210-003-0704-9
Hinson, H. E., Rowell, S., and Schreiber, M. (2015). Clinical evidence of inflammation driving secondary brain injury: a systematic review. J. Trauma Acute Care Surg. 78 (1), 184–191. doi:10.1097/ta.0000000000000468
Iverson, G. L., Minkkinen, M., Karr, J. E., Berghem, K., Zetterberg, H., Blennow, K., et al. (2022). Examining four blood biomarkers for the detection of acute intracranial abnormalities following mild traumatic brain injury in older adults. Front. Neurol. 13, 960741. doi:10.3389/fneur.2022.960741
Izzy, S., and Muehlschlegel, S. (2014). Cerebral vasospasm after aneurysmal subarachnoid hemorrhage and traumatic brain injury. Curr. Treat. Options Neurol. 16 (1), 278. doi:10.1007/s11940-013-0278-x
Kallakuri, S., Kreipke, C. W., Rossi, N., Rafols, J. A., and Petrov, T. (2007). Spatial alterations in endothelin receptor expression are temporally associated with the altered microcirculation after brain trauma. Neurol. Res. 29 (4), 362–368. doi:10.1179/016164107x204675
Kapusta, D. R., Burmeister, M. A., Calo, G., Guerrini, R., Gottlieb, H. B., and Kenigs, V. A. (2005). Functional selectivity of nociceptin/orphanin FQ peptide receptor partial agonists on cardiovascular and renal function. J. Pharmacol. Exp. Ther. 314 (2), 643–651. doi:10.1124/jpet.104.082768
Karlsson, M., Yang, Z., Chawla, S., Delso, N., Pukenas, B., Elmér, E., et al. (2021). Evaluation of diffusion tensor imaging and fluid based biomarkers in a large animal trial of cyclosporine in focal traumatic brain injury. J. Neurotrauma 38 (13), 1870–1878. doi:10.1089/neu.2020.7317
Kochanek, P. M., Dixon, C. E., Mondello, S., Wang, K. K. K., Lafrenaye, A., Bramlett, H. M., et al. (2018). Multi-center pre-clinical consortia to enhance translation of Therapies and biomarkers for traumatic brain injury: operation brain trauma therapy and beyond. Front. Neurol. 9, 640. doi:10.3389/fneur.2018.00640
Korley, F. K., Nikolian, V. C., Williams, A. M., Dennahy, I. S., Weykamp, M., and Alam, H. B. (2018). Valproic acid treatment decreases serum glial fibrillary acidic protein and neurofilament light chain levels in swine subjected to traumatic brain injury. J. Neurotrauma 35 (10), 1185–1191. doi:10.1089/neu.2017.5581
Kroppenstedt, S. N., Kern, M., Thomale, U. W., Schneider, G. H., Lanksch, W. R., and Unterberg, A. W. (1999). Effect of cerebral perfusion pressure on contusion volume following impact injury. J. Neurosurg. 90 (3), 520–526. doi:10.3171/jns.1999.90.3.0520
Kroppenstedt, S. N., Stover, J. F., and Unterberg, A. W. (2000). Effects of dopamine on posttraumatic cerebral blood flow, brain edema, and cerebrospinal fluid glutamate and hypoxanthine concentrations. Crit. Care Med. 28 (12), 3792–3798. doi:10.1097/00003246-200012000-00004
Kroppenstedt, S. N., Thomale, U. W., Griebenow, M., Sakowitz, O. W., Schaser, K. D., Mayr, P. S., et al. (2003). Effects of early and late intravenous norepinephrine infusion on cerebral perfusion, microcirculation, brain-tissue oxygenation, and edema formation in brain-injured rats. Crit. Care Med. 31 (8), 2211–2221. doi:10.1097/01.Ccm.0000080482.06856.62
Lefevre-Dognin, C., Cogné, M., Perdrieau, V., Granger, A., Heslot, C., and Azouvi, P. (2021). Definition and epidemiology of mild traumatic brain injury. Neurochirurgie 67 (3), 218–221. doi:10.1016/j.neuchi.2020.02.002
Lewelt, W., Jenkins, L. W., and Miller, J. D. (1980). Autoregulation of cerebral blood flow after experimental fluid percussion injury of the brain. J. Neurosurg. 53 (4), 500–511. doi:10.3171/jns.1980.53.4.0500
Liao, Y. Y., Jiang, F., and Chiou, L. C. (2011). Quantitative study of the antagonistic effect of (-)-cis-1-Methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol (SB-612111) on nociceptin/orphanin FQ-mediated potassium channel activation in rat periaqueductal gray slices. Eur. J. Pharmacol. 657 (1-3), 84–88. doi:10.1016/j.ejphar.2011.01.057
Liliang, P. C., Liang, C. L., Weng, H. C., Lu, K., Wang, K. W., Chen, H. J., et al. (2010). Tau proteins in serum predict outcome after severe traumatic brain injury. J. Surg. Res. 160 (2), 302–307. doi:10.1016/j.jss.2008.12.022
Liu, H., Goodman, J. C., and Robertson, C. S. (2002). The effects of L-arginine on cerebral hemodynamics after controlled cortical impact injury in the mouse. J. Neurotrauma 19 (3), 327–334. doi:10.1089/089771502753594891
Liu, H., He, J., Zhang, Z., Liu, L., Huo, G., Sun, X., et al. (2018). Evolution of cerebral perfusion in the peri-contusional cortex in mice revealed by in vivo laser speckle imaging after traumatic brain injury. Brain Res. 1700, 118–125. doi:10.1016/j.brainres.2018.07.006
Liu, M. C., Akinyi, L., Scharf, D., Mo, J., Larner, S. F., Muller, U., et al. (2010). Ubiquitin C-terminal hydrolase-L1 as a biomarker for ischemic and traumatic brain injury in rats. Eur. J. Neurosci. 31 (4), 722–732. doi:10.1111/j.1460-9568.2010.07097.x
Maas, A. I. R., Menon, D. K., Adelson, P. D., Andelic, N., Bell, M. J., Belli, A., et al. (2017). Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 16 (12), 987–1048. doi:10.1016/s1474-4422(17)30371-x
McDonald, J., Barnes, T. A., Okawa, H., Williams, J., Calo, G., Rowbotham, D. J., et al. (2003). Partial agonist behaviour depends upon the level of nociceptin/orphanin FQ receptor expression: studies using the ecdysone-inducible mammalian expression system. Br. J. Pharmacol. 140 (1), 61–70. doi:10.1038/sj.bjp.0705401
McDonald, J., and Lambert, D. G. (2010). Binding of GTPgamma[35S] is regulated by GDP and receptor activation. Studies with the nociceptin/orphanin FQ receptor. Br. J. Pharmacol. 159 (6), 1286–1293. doi:10.1111/j.1476-5381.2009.00621.x
Meberg, P. J., Ono, S., Minamide, L. S., Takahashi, M., and Bamburg, J. R. (1998). Actin depolymerizing factor and cofilin phosphorylation dynamics: response to signals that regulate neurite extension. Cell Motil. Cytoskelet. 39 (2), 172–190. doi:10.1002/(sici)1097-0169(1998)39:2<172::Aid-cm8>3.0.Co;2-8
Menon, D. K., Schwab, K., Wright, D. W., and Maas, A. I. (2010). Position statement: definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 91 (11), 1637–1640. doi:10.1016/j.apmr.2010.05.017
Mollereau, C., Parmentier, M., Mailleux, P., Butour, J. L., Moisand, C., Chalon, P., et al. (1994). ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 341 (1), 33–38.
Mollereau, C., Simons, M. J., Soularue, P., Liners, F., Vassart, G., Meunier, J. C., et al. (1996). Structure, tissue distribution, and chromosomal localization of the prepronociceptin gene. Proc. Natl. Acad. Sci. U. S. A. 93 (16), 8666–8670. doi:10.1073/pnas.93.16.8666
Muller, C. R., Courelli, V., Lucas, A., Williams, A. T., Li, J. B., Dos Santos, F., et al. (2021). Resuscitation from hemorrhagic shock after traumatic brain injury with polymerized hemoglobin. Sci. Rep. 11 (1), 2509. doi:10.1038/s41598-021-81717-3
Nagumo, Y., Han, J., Bellila, A., Isoda, H., and Tanaka, T. (2008). Cofilin mediates tight-junction opening by redistributing actin and tight-junction proteins. Biochem. Biophys. Res. Commun. 377 (3), 921–925. doi:10.1016/j.bbrc.2008.10.071
Namme, J. N., Bepari, A. K., and Takebayashi, H. (2021). Cofilin signaling in the CNS physiology and neurodegeneration. Int. J. Mol. Sci. 22 (19). doi:10.3390/ijms221910727
Niu, F., Qian, K., Qi, H., Zhao, Y., Jiang, Y., and Sun, M. (2020). Antiapoptotic and anti-inflammatory effects of CPCGI in rats with traumatic brain injury. Neuropsychiatr. Dis. Treat. 16, 2975–2987. doi:10.2147/ndt.S281530
Osier, N. D., Bramlett, H. M., Shear, D. A., Mondello, S., Carlson, S. W., Dietrich, W. D., et al. (2021). Kollidon VA64 treatment in traumatic brain injury: operation brain trauma therapy. J. Neurotrauma 2021. doi:10.1089/neu.2021.0089
Osier, N. D., and Dixon, C. E. (2016). The controlled cortical impact model: applications, considerations for researchers, and future directions. Front. Neurol. 7, 134. doi:10.3389/fneur.2016.00134
Osier, N. D., Korpon, J. R., and Dixon, C. E. (2015). “Frontiers in neuroengineering controlled cortical impact model,” in Brain Neurotrauma: molecular, neuropsychological, and rehabilitation aspects. Editor F. H. Kobeissy (Boca Raton: CRC Press/Taylor & Francis).
Pan, Y. X., Cheng, J., Xu, J., Rossi, G., Jacobson, E., Ryan-Moro, J., et al. (1995). Cloning and functional characterization through antisense mapping of a kappa 3-related opioid receptor. Mol. Pharmacol. 47 (6), 1180–1188.
Pandey, S., Singh, K., Sharma, V., Pandey, D., Jha, R. P., Rai, S. K., et al. (2017). A prospective pilot study on serum cleaved tau protein as a neurological marker in severe traumatic brain injury. Br. J. Neurosurg. 31 (3), 356–363. doi:10.1080/02688697.2017.1297378
Percie du Sert, N., Hurst, V., Ahluwalia, A., Alam, S., Avey, M. T., Baker, M., et al. (2020). The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. Br. J. Pharmacol. 177 (16), 3617–3624. doi:10.1111/bph.15193
Philip, S., and Armstead, W. M. (2003). Differential role of PTK, ERK and p38 MAPK in superoxide impairment of NMDA cerebrovasodilation. Brain Res. 979 (1-2), 98–103. doi:10.1016/s0006-8993(03)02879-8
Pollard, T. D., Blanchoin, L., and Mullins, R. D. (2000). Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 29, 545–576. doi:10.1146/annurev.biophys.29.1.545
Ross, J., and Armstead, W. M. (2005). NOC/oFQ activates ERK and JNK but not p38 MAPK to impair prostaglandin cerebrovasodilation after brain injury. Brain Res. 1054 (1), 95–102. doi:10.1016/j.brainres.2005.06.065
Salehi, A., Zhang, J. H., and Obenaus, A. (2017). Response of the cerebral vasculature following traumatic brain injury. J. Cereb. blood flow metabolism 37 (7), 2320–2339. doi:10.1177/0271678X17701460
Schmittgen, T. D., and Livak, K. J. (2008). Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3 (6), 1101–1108. doi:10.1038/nprot.2008.73
Shear, D. A., Dixon, C. E., Bramlett, H. M., Mondello, S., Dietrich, W. D., Deng-Bryant, Y., et al. (2016). Nicotinamide treatment in traumatic brain injury: operation brain trauma therapy. J. Neurotrauma 33 (6), 523–537. doi:10.1089/neu.2015.4115
Shiobara, T., Usui, T., Han, J., Isoda, H., and Nagumo, Y. (2013). The reversible increase in tight junction permeability induced by capsaicin is mediated via cofilin-actin cytoskeletal dynamics and decreased level of occludin. PLoS One 8 (11), e79954. doi:10.1371/journal.pone.0079954
Simonsen, U., Laursen, B. E., and Petersen, J. S. (2008). ZP120 causes relaxation by pre-junctional inhibition of noradrenergic neurotransmission in rat mesenteric resistance arteries. Br. J. Pharmacol. 153 (6), 1185–1194. doi:10.1038/sj.bjp.0707688
Smith, S. L., and Hall, E. D. (1996). Mild pre- and posttraumatic hypothermia attenuates blood-brain barrier damage following controlled cortical impact injury in the rat. J. Neurotrauma 13 (1), 1–9. doi:10.1089/neu.1996.13.1
Spagnolo, B., Carrà, G., Fantin, M., Fischetti, C., Hebbes, C., McDonald, J., et al. (2007). Pharmacological characterization of the nociceptin/orphanin FQ receptor antagonist SB-612111 [(-)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol]: in vitro studies. J. Pharmacol. Exp. Ther. 321 (3), 961–967. doi:10.1124/jpet.106.116764
Stocchetti, N., Taccone, F. S., Citerio, G., Pepe, P. E., Le Roux, P. D., Oddo, M., et al. (2015). Neuroprotection in acute brain injury: an up-to-date review. Crit. Care 19 (1), 186. doi:10.1186/s13054-015-0887-8
Suurna, M. V., Ashworth, S. L., Hosford, M., Sandoval, R. M., Wean, S. E., Shah, B. M., et al. (2006). Cofilin mediates ATP depletion-induced endothelial cell actin alterations. Am. J. Physiol. Ren. Physiol. 290 (6), F1398–F1407. doi:10.1152/ajprenal.00194.2005
Thomale, U. W., Griebenow, M., Kroppenstedt, S. N., Unterberg, A. W., and Stover, J. F. (2006). The effect of N-acetylcysteine on posttraumatic changes after controlled cortical impact in rats. Intensive Care Med. 32 (1), 149–155. doi:10.1007/s00134-005-2845-4
Thomale, U. W., Kroppenstedt, S. N., Beyer, T. F., Schaser, K. D., Unterberg, A. W., and Stover, J. F. (2002a). Temporal profile of cortical perfusion and microcirculation after controlled cortical impact injury in rats. J. Neurotrauma 19 (4), 403–413. doi:10.1089/08977150252932361
Thomale, U. W., Schaser, K., Kroppenstedt, S. N., Unterberg, A. W., and Stover, J. F. (2002b). Cortical hypoperfusion precedes hyperperfusion following controlled cortical impact injury. Acta Neurochir. Suppl. 81, 229–231. doi:10.1007/978-3-7091-6738-0_59
Toshima, J., Toshima, J. Y., Amano, T., Yang, N., Narumiya, S., and Mizuno, K. (2001). Cofilin phosphorylation by protein kinase testicular protein kinase 1 and its role in integrin-mediated actin reorganization and focal adhesion formation. Mol. Biol. Cell 12 (4), 1131–1145. doi:10.1091/mbc.12.4.1131
Vanderploeg, R. D., Curtiss, G., and Belanger, H. G. (2005). Long-term neuropsychological outcomes following mild traumatic brain injury. J. Int. Neuropsychol. Soc. 11 (3), 228–236. doi:10.1017/s1355617705050289
Vazquez-DeRose, J., Stauber, G., Khroyan, T. V., Xie, X. S., Zaveri, N. T., and Toll, L. (2013). Retrodialysis of N/OFQ into the nucleus accumbens shell blocks cocaine-induced increases in extracellular dopamine and locomotor activity. Eur. J. Pharmacol. 699 (1-3), 200–206. doi:10.1016/j.ejphar.2012.11.050
Wang, J. B., Johnson, P. S., Imai, Y., Persico, A. M., Ozenberger, B. A., Eppler, C. M., et al. (1994). cDNA cloning of an orphan opiate receptor gene family member and its splice variant. FEBS Lett. 348 (1), 75–79.
Wang, M. L., Yu, M. M., Yang, D. X., Liu, Y. L., Wei, X. E., and Li, W. B. (2018). Longitudinal microstructural changes in traumatic brain injury in rats: a diffusional kurtosis imaging, histology, and behavior study. AJNR Am. J. Neuroradiol. 39 (9), 1650–1656. doi:10.3174/ajnr.A5737
Wang, Y., Shibasaki, F., and Mizuno, K. (2005). Calcium signal-induced cofilin dephosphorylation is mediated by Slingshot via calcineurin. J. Biol. Chem. 280 (13), 12683–12689. doi:10.1074/jbc.M411494200
Wick, M. J., Minnerath, S. R., Lin, X., Elde, R., Law, P. Y., and Loh, H. H. (1994). Isolation of a novel cDNA encoding a putative membrane receptor with high homology to the cloned mu, delta, and kappa opioid receptors. Brain Res. Mol. Brain Res. 27 (1), 37–44.
Witta, J., Buzas, B., and Cox, B. M. (2003). Traumatic brain injury induces nociceptin/orphanin FQ expression in neurons of the rat cerebral cortex. J. Neurotrauma 20 (6), 523–532. doi:10.1089/089771503767168456
Zeng, H., Zhao, S., Pang, Z., Wang, S., Cao, L., and Zhang, Y. (2023). Identification of key genes and pathways in the Hippocampus after traumatic brain injury: bioinformatics analysis and experimental validation. J. Integr. Neurosci. 22 (2), 44. doi:10.31083/j.jin2202044
Zhang, F., Sprague, S. M., Farrokhi, F., Henry, M. N., Son, M. G., and Vollmer, D. G. (2002). Reversal of attenuation of cerebrovascular reactivity to hypercapnia by a nitric oxide donor after controlled cortical impact in a rat model of traumatic brain injury. J. Neurosurg. 97 (4), 963–969. doi:10.3171/jns.2002.97.4.0963
Keywords: mild traumatic brain injury, Nociceptin/orphanin FQ (N/OFQ), cofilin-1, cerebral blood flow, Mitogen-activated protein kinases, controlled cortical impact, laser speckle contrast imaging, N/OFQ peptide receptor (NOP)
Citation: Al Yacoub ON, Tarantini S, Zhang Y, Csiszar A and Standifer KM (2023) The Nociceptin/Orphanin FQ peptide receptor antagonist, SB-612111, improves cerebral blood flow in a rat model of traumatic brain injury. Front. Pharmacol. 14:1272969. doi: 10.3389/fphar.2023.1272969
Received: 04 August 2023; Accepted: 05 October 2023;
Published: 18 October 2023.
Edited by:
Scott D. Moore, Duke University, United StatesReviewed by:
Douglas Scott DeWitt, University of Texas Medical Branch at Galveston, United StatesCopyright © 2023 Al Yacoub, Tarantini, Zhang, Csiszar and Standifer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kelly M. Standifer, a2VsbHktc3RhbmRpZmVyQG91aHNjLmVkdQ==
†Present address: Omar N. Al Yacoub, Department of Experimental and Clinical Pharmacology, College of Pharmacy, University of Minnesota, Minneapolis, MN, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.