- 1School of Pharmacy, Institute of Pharmacokinetics, Anhui University of Chinese Medicine, Hefei, Anhui, China
- 2Center for Xin’an Medicine and Modernization of Traditional Chinese Medicine of IHM, Grand Health Research Institute of Hefei Comprehensive National Science Center, Anhui University of Chinese Medicine, Hefei, China
- 3Anhui Education Department (AUCM), Engineering Technology Research Center of Modernized Pharmaceutics, Hefei, Anhui, China
- 4Anhui Province Key Laboratory of Pharmaceutical Preparation Technology and Application, Anhui University of Chinese Medicine, Hefei, Anhui, China
- 5Anhui Genuine Chinese Medicinal Materials Quality Improvement Collaborative Innovation Center, Hefei, Anhui, China
- 6Anhui Academy of Chinese Medicine, Anhui University of Chinese Medicine, Hefei, China
- 7Hefei Food and Drug Inspection Center, Hefei, Anhui, China
Background: Kai-Xin-San (KXS) is one of the classic famous traditional Chinese medicine prescriptions for amnesia, which has been applied for thousands of years. Modern pharmacological research has found that KXS has significant therapeutic efficacy on nervous system diseases, which is related to its antioxidant activity. However, the antioxidant material basis and quality markers (Q-makers) of KXS have not been studied. Objective: The objective of this study is to explore the Q-makers of antioxidant activity of KXS based on spectrum-effect relationship.
Methods: Specifically, the metabolites in KXS extracts were identified by UPLC-Q-Exactive Orbitrap MS/MS. The fingerprint profile of KXS extracts were established by high-performance liquid chromatography (HPLC) and seven common peaks were identified. Meanwhile, 2, 2-diphenyl-1-picrylhydrazyl (DPPH) test was used to evaluate the free radical scavenging ability of KXS. The spectrum-effect relationship between its HPLC fingerprint and DPPH free radical scavenging activity was preliminarily examined by the Pearson correlation analysis, grey relation analysis (GRA), and orthogonal partial least squares discrimination analysis (OPLS-DA). Further, the antioxidant effect of KXS and its Q-makers were validated through human neuroblastoma (SH-SY5Y) cells experiment.
Results: The results showed that 103 metabolites were identified from KXS, and the similarity values between HPLC fingerprint of twelve batches of KXS were greater than 0.900. At the same time, the results of Pearson correlation analysis showed that the peaks 8, 1, 14, 17, 18, 24, 16, 21, 15, 13, 6, 5, and 3 from KXS were positively correlated with the scavenging activity values of DPPH. Combined with the results of GRA and OPLS-DA, peaks 1, 3, 5 (Sibiricose A6), 6, 13 (Ginsenoside Rg1), 15, and 24 in the fingerprints were screen out as the potential Q-makers of KXS for antioxidant effect. Besides, the results of CCK-8 assay showed that KXS and its Q-makers remarkably reduced the oxidative damage of SH-SY5Y cells caused by H2O2. However, the antioxidant activity of KXS was decreased significantly after Q-makers were knocked out.
Conclusion: In conclusion, the metabolites in KXS were successfully identified by UPLC-Q-Exactive Orbitrap MS/MS, and the Q-makers of KXS for antioxidant effect was analyzed based on the spectrum-effect relationship. These results are beneficial to clarify the antioxidant material basis of KXS and provide the quality control standards for new KXS products development.
1 Introduction
In recent years, more and more traditional Chinese medicine (TCM) prescriptions have been unearthed, some of which are known as classic famous prescriptions because of their wide application, precise efficacy, and obvious characteristics (Li et al., 2015). Kai-Xin-San (KXS) is one of the classic famous prescriptions for amnesia, recorded by Sun Simiao in Bei Ji Qian Jin Yao Fang during the Tang dynasty (652 AD) (Wang et al., 2019a; Fu et al., 2020). KXS is composed of Polygalae Radix (Polygala tenuifolia Willd.), Ginseng Radix et Rhizoma (Panax ginseng C. A. Mey.), Acori Tatarinowii Rhizoma (Acorus tatarinowii Schott), and Poria cocos (Poria cocos (Schw.) Wolf) (Qiong et al., 2016). As a tranquilizing medicine, Polygalae Radix has the effect of calming the mind, improving intelligence, and regulating the heart and kidney (Jiang et al., 2021). It was widely applied in treating Alzheimer’s disease (AD), depression, epilepsy, and other central nervous disorders (Zhao et al., 2020). Meanwhile, Ginseng Radix et Rhizoma is a great tonic medicine for benefiting Qi and invigorating vitality, with pharmacological effects which include improving learning and memory, stimulating the central nervous system, anti-tumor effects, and strengthening the immune system (Shi et al., 2013; Jiang et al., 2019; Yu et al., 2020). Additionally, studies have reported that Acori Tatarinowii Rhizoma has various pharmacological effects, including antioxidant and anti-depression properties, protecting nerve cells, alleviation of learning and memory impairment, and anti-myocardial ischemia (Zhang et al., 2015; Wen et al., 2023). In addition to regulating gut microbiota, Poria cocos has anti-inflammatory and antioxidant pharmacological activities in treating diseases (Fang et al., 2021; Xu et al., 2022). The compatible combination of these four drugs makes KXS have the advantages and characteristics in treating neurological diseases (Cao et al., 2018).
So far, many pharmacological studies have shown that KXS significantly improves depression and AD through anti-oxidation and anti-inflammation, and by inhibiting apoptosis (Guo et al., 2019; Hu et al., 2020a; Jiao et al., 2022). The metabolites of KXS are the real material basis for its efficacy (Yi et al., 2020). Among the metabolites in KXS, many of them have antioxidant activity (Xiao et al., 2020; Zhong et al., 2020; Lyu et al., 2021; Balakrishnan et al., 2022; He et al., 2022; Tang et al., 2022). For example, 3,6′-disinapoylsucrose, a metabolite of Polygalae Radix, has a protective effect against Aβ1-42-induced pathological damages, which may be associated with the reduction of Aβ deposition and anti-oxidation (Tang et al., 2022). Likewise, Ginsenoside Rg1, β-asarone, and pachymic acid possess significant antioxidant effects (Zhong et al., 2020; Balakrishnan et al., 2022; He et al., 2022). As is known, oxidative stress is one of the pathogenic mechanisms of AD (Zhao et al., 2020), as well as an important factor in the pathogenesis of depression and anxiety (Xiao et al., 2020; Zhong et al., 2020; Lyu et al., 2021; Balakrishnan et al., 2022; He et al., 2022; Tang et al., 2022). It is necessary to study the antioxidant activity of KXS and its material basis with a key marker. However, the overall spectrum–effect relationship between the antioxidant efficacy and the metabolites of KXS is still unclear.
The quality control of TCM is always a challenge due to the complexity of the metabolites and the ambiguity of the pharmacodynamic material basis. Fortunately, the quality marker (Q-marker) of TCM has fundamentally improved the idea and model of the quality assessment from the transmission and traceability, specificity, effectiveness, testability, and compound formula compatibility of metabolites (Ren et al., 2020). Proposed by Academician Liu, the Q-marker refers to the metabolites inherent in TCM or formed in the process of processing and preparation, which reflects the safety and effectiveness of TCM (Zhang et al., 2021a). As the intrinsic metabolites in botanical drugs and Chinese medicine products, Q-markers are crucial for establishing TCM quality assessment standards and traceability. In recent years, there have been several technical means to investigate the Q-markers of TCM (Xie et al., 2019; Zhang et al., 2021a). Combining the advantages of ultra-performance liquid chromatography (UPLC) and high-resolution mass spectrometry (MS), UPLC-MS/MS can produce secondary mass spectral information (including precursor and fragment ions) for aiding structural inference with good selectivity, sensitivity, mass accuracy, and exclusive detection (Yang et al., 2021). Among them, UPLC-Q-Exactive Orbitrap MS/MS conducts qualitative and quantitative analyses of complex botanical or biological sample metabolites using high selectivity of the quadrupole to parent ions and high resolution of precise mass numbers of the orbital ion trap (Orbitrap) with a low matrix effect (Wang et al., 2019b).
In recent years, the fingerprint profile established by high-performance liquid chromatography (HPLC) has been comprehensively, qualitatively, and quantitatively developed for the identification and quality evaluation of the complex multi-component TCM system (Fan et al., 2020). HPLC fingerprints can effectively separate the diverse metabolites in TCM and screen its characteristic metabolites based on the spectrum–effect relationship (Chen et al., 2020; Fan et al., 2020). As a stable free radical, 2,2-diphenyl-1-picrylhydrazyl (DPPH) is commonly used to measure the free radical scavenging activity of antioxidants and find the high antioxidant metabolites in TCM (Yu et al., 2021).
After the identification of the metabolites in KXS, the spectrum–effect relationship can be further determined based on HPLC fingerprinting and the DPPH scavenging activity of KXS, which is helpful to screen Q-markers for the antioxidant activity of KXS. Therefore, the metabolites in KXS were first identified by UPLC-Q-Exactive Orbitrap MS/MS in this study. Then, the HPLC fingerprint of KXS was established under gradient elution conditions with 12 batches of raw material, and their free radical scavenging activity was assessed by the DPPH test. The spectrum–antioxidant effect relationship of KXS was analyzed by the Pearson correlation analysis, gray relational analysis (GRA), and orthogonal partial least squares discriminant analysis (OPLS-DA). Finally, the Q-markers for the antioxidant effect of KXS were screened by the spectrum–effect relationship analysis and in vitro validated by the CCK-8 method. This study provided a solid research foundation for screening KXS antioxidant metabolites and establishing the quality evaluation standards for the future development of KXS products.
2 Materials and methods
2.1 Materials
Polygalae Radix, Ginseng Radix et Rhizoma, Poria cocos, and Acori Tatarinowii Rhizoma were obtained from Anhui Jingdao Co., Ltd. (Anhui, China), which were cultivated in their original authentic producing origins (Supplementary Table S1). These species were identified by Professor Can Peng in the Anhui University of Chinese Medicine, according to the 2020 edition of the Chinese Pharmacopoeia.
The reference substances of Sibiricose A5 (batch number: PS000944; purity: 99.41%) and Sibiricose A6 (batch number: PS000945; purity: 96.62%) were acquired from Chengdu Push Bio-Technology Co., Ltd. (Chengdu, China). β-Asarone (batch number: MUST-21082510; purity: 99.76%) and α-asarone (batch number: MUST-21092810; purity: 99.94%) were bought from Chengdu Man Site Co., Ltd. (Chengdu, China). Polygalaxanthone III (batch number: 111850-202006; purity: 95.30%), 3′,6-disinapoylsucrose (batch number: 111848-202006; purity: 96.50%), and Ginsenoside Rg1 (batch number: 110703-202034; purity: 94.00%) were obtained from National Institutes for Food and Drug Control (Beijing, China). DPPH was purchased from Shanghai Yuanye Co., Ltd. (Shanghai, China), and the H2O2 solution was obtained from Sigma (Shanghai, China). Mass spectrometric acetonitrile was purchased from Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China). HPLC-grade methanol and acetonitrile were obtained from Thermo Fisher Scientific (Massachusetts, United States).
2.2 Qualitative analysis of metabolites in KXS
2.2.1 Preparation of the sample solution
In this study, 12 combinations were selected by the random number table method from 81 permutations of Polygalae Radix, Ginseng Radix et Rhizoma, Poria cocos, and Acori Tatarinowii Rhizoma with different producing origins (Zhou et al., 2022). Then, Polygalae Radix, Ginseng Radix et Rhizoma, Poria cocos, and Acori Tatarinowii Rhizoma were separately crushed, sieved, and mixed with a weight ratio of 1: 1: 2: 1 to obtain KXS (Zhang and Zeng, 2020). The KXS extract was obtained by adding 2 g of KXS into 25 mL of 75% methanol and extracting by ultrasound for 30 min (Shang et al., 2023). The extract was filtered through a 0.22-μm-pore-size membrane before testing.
2.2.2 UPLC-Q-Exactive Orbitrap MS/MS analysis
The chromatographic and mass spectrometry conditions were performed in the Orbitrap Exploris 120 high-resolution mass spectrometer (Thermo Scientific, Bremen, Germany) using an ACQUITY UPLC BEH C18 column (1.7 μm, 2.1 × 100 mm, Waters). The mobile phase consisted of acetonitrile (A) and 0.1% formic acid solution (B) at a flow rate of 0.2 mL/min. The gradient protocol was as follows (A: B, v/v): 0–9 min, 7%–14% A; 9–13 min, 14%–16% A; 13–19 min, 16%–19% A; 19–23 min, 19%–23% A; 23–32 min, 23%–36% A; 32–37 min, 36%–38% A; and 37–45 min, 38%–46% A. The column temperature was 30°C, and the volume of sample injection was 2 μL.
The ESI ion source temperature was 120°C, and the scanning range of MS was m/z 50–1,500 Da. The capillary voltage was 3.5 kV in the positive ion collection mode and −2.5 kV in the negative ion collection mode. The cone voltage was 50 V, the ion source temperature was 110°C, the cone gas flow was 50 L/h, the atomization gas (N2) flow was 600 L/h, and the solvent removal temperature was 350°C. Leucine enkephalin was used as the calibration solution with an accurate mass number.
2.3 HPLC fingerprint method
2.3.1 Chromatographic conditions
HPLC analysis was performed using the Thermo Fisher Ultimate 3000 high-performance liquid chromatograph with a UV detector and chromatographic column of Thermo-AcclaimTM120-C18 (250*4.6 mm, 5 μm). The mobile phase consisted of acetonitrile (A) and 0.1% aqueous phosphoric acid solution (B). The HPLC elution conditions were optimized as follows (A: B, v/v): 0–10 min, 95%–85% B; 10–20 min, 85%–84% B; 20–31 min, 84%–82% B; 31–33 min, 82%–77% B; 33–52 min, 77%–60% B; 52–70 min, 60%–48% B; 70–76 min, 48%–27% B; 76–93 min, 27%–20% B; 93–100 min, 27%–5% B; and 100–102 min, 5% B. The detection wavelength of the UV detector was set at 230 nm, and the column temperature was maintained at 30°C. The volume of sample injection was 10 μL, and the flow rate was 1.0 mL·min−1.
2.3.2 Preparation of the standard solution
The seven reference standards (Sibiricose A5, Sibiricose A6, Polygalaxanthone III, 3′,6-disinapoylsucrose, Ginsenoside Rg1, β-asarone, and α-asarone) were precisely weighed and dissolved in 75% methanol. Subsequently, the final concentrations of Sibiricose A5, Sibiricose A6, Polygalaxanthone III, 3′,6-disinapoylsucrose, Ginsenoside Rg1, β-asarone, and α-asarone were 0.50, 0.50, 0.12, 0.20, 0.87, 0.09, and 0.10 mg·mL−1, respectively, obtained by diluting the aforementioned solution.
2.3.3 Validation of the methodology
For the specificity of the method, the blank solvent (75% methanol) was injected into the HPLC system for determination, according to the chromatographic conditions given in “2.3.1”. Method precision was evaluated by six successive injections of the KXS sample solution (Sample S1). Similarly, six sample solutions were prepared according to “2.3.2”, and the repeatability was estimated by the method in “2.3.1”. The stability of the KXS sample solution was analyzed within 1 day (0, 2, 4, 8, 12, and 24 h) (Hu et al., 2020b).
2.3.4 Similarity evaluation of HPLC fingerprints and peak identification
The obtained fingerprints (S1–S12) were imported into the Similarity Evaluation System for Chromatographic Fingerprint of TCM (Version 2004A, Beijing, China) and analyzed. With the aid of the similarity evaluation system for the TCM chromatographic fingerprint, the HPLC fingerprints of KXS were matched automatically (Goodarzi et al., 2013). The reference fingerprint was generated using the median method, and the similarity values between the reference fingerprint and the chromatogram of 12 batches of KXS were calculated.
Seven standard solutions and the mixed standard solution were injected into the HPLC system, respectively. According to the retention time (RT) of seven reference substances, the chromatographic peaks of the seven metabolites in the KXS extracts were identified.
2.4 DPPH free radical scavenging activity test
The experiment was performed according to the procedure described by Xiao et al. (2022). Briefly, 2 mL of the DPPH free radical solution (0.04 g/L in 75% methanol) and 2 mL of the blank solvent were mixed in the test tube, and allowed to react in the dark for 30 min. Finally, the absorbance value of the sample was measured using a UV spectrophotometer (λ = 517 nm) and recorded as A1. Similarly, different concentrations of the sample solution (6.25–200 μg/mL) were added into the DPPH free radical solution, respectively. After placing in the dark for 30 min, the absorbance of the samples was measured at λ = 517 nm as A2. At the same time, different concentrations of the sample solution and blank solvent were also mixed, and absorbance was measured as A3. The following equation was applied to compute the free radical scavenging rate (K).
In this experiment, the median scavenging concentration (SC50), that is, the drug concentration with a DPPH free radical scavenging rate of 50%, was calculated by non-linear regression analysis.
2.5 Spectrum–effect relationship analysis
The spectrum–effect relationship between the HPLC fingerprints and the DPPH free radical scavenging activity of KXS extracts was examined by the Pearson correlation analysis, GRA, and OPLS-DA. In the Pearson correlation model, the areas of 25 common peaks in the HPLC fingerprints were set as one variable, and the SC50 values of KXS scavenging DPPH free radicals were used as another variable to analyze the correlation coefficients. Meanwhile, the DPPH free radical scavenging effect of KXS was used as the reference sequence for GRA, and the common characteristic peaks of the fingerprint profile (X1–X25) were listed as the comparative sequence. The contribution of each common peak to the efficacy was determined by comparing the gray correlation between the comparative sequence and the reference sequence. Similarly, OPLS-DA was also analyzed by SPSSAU (SPSS Inc., United States) and SIMCA (Umetrics Inc., Sweden).
2.6 Preparation of the sample and Q-marker-knockout samples of KXS
Under the HPLC conditions for separating KXS, the Q-marker sample and the sequential knockout samples of KXS were collected separately, according to the spectrum–effect relationship and their RT (Liu et al., 2021). The eluents were rotationally evaporated to remove the mobile phase, and the Q-marker sample, the target knockout metabolites, and the negative samples were obtained.
2.7 In vitro cell experiment
Here, human neuroblastoma (SH-SY5Y) cells were provided by the China Center for Type Culture Collection (Shanghai, China) and grown in complete DMEM at 37°C with 5% CO2. Then, the SH-SY5Y cells were seeded in 96-well plates at a concentration of 104 cells/well and treated with 200 μmol/L H2O2 for 24 h to cause oxidative damage (Gai et al., 2019). To validate the antioxidant activity of KXS and its Q-markers, the Q-markers and sequential knockout samples of KXS were evenly dispersed in complete DMEM with 200 μg/mL (concentration of KXS) and added to the H2O2-induced SH-SY5Y cells for 24 h incubation. Following the treatment period, a solution containing WST-1 was added and incubated for another 1 h. The spectrophotometric absorbance was measured at 450 nm, and the cell viability was calculated using the following equation:
2.8 Statistical analysis
The metabolite identification was analyzed by Xcalibur 2.1 and Compound Discoverer 3.0 software applications. The dose–response curves were analyzed with the probit model by SPSS statistics software (SPSS 23.0, SPSS Inc., United States) (Ma et al., 2018). SPSSAU (SPSS Inc., United States) and SIMCA (Umetrics Inc., Swedish) were used for the Pearson correlation analysis, GRA, and OPLS-DA analysis (Liu et al., 2019). The data from the cell experiment were analyzed using GraphPad Prism 9.0 software. Student’s t-test and one-way analysis of variance (ANOVA) were applied for statistical analysis.
3 Results
3.1 Phytochemical analysis of KXS
The total ion chromatograms (TICs) of the KXS extract acquired by UPLC-Q-Exactive Orbitrap MS/MS in the positive and negative ion modes are shown in Figure 1, and the identified metabolites are listed in Supplementary Table S2. In total, we identified 103 metabolites in KXS by comparison with the database and related literature studies (Wang et al., 2019b; He et al., 2022). Among them, Sibiricose A5, Sibiricose A6, Polygalaxanthone III, and 3′,6-disinapoylsucrose from Polygalae Radix, Ginsenoside Rg1 from Ginseng Radix et Rhizoma, and β-asarone and α-asarone from Acori Tatarinowii Rhizoma were reported to have antioxidant activity (Kumar et al., 2012; Shi et al., 2015; Liu et al., 2018a; Balakrishnan et al., 2022). Here, these successfully identified antioxidant metabolites can be further used for spectrum–effect relationship analysis.
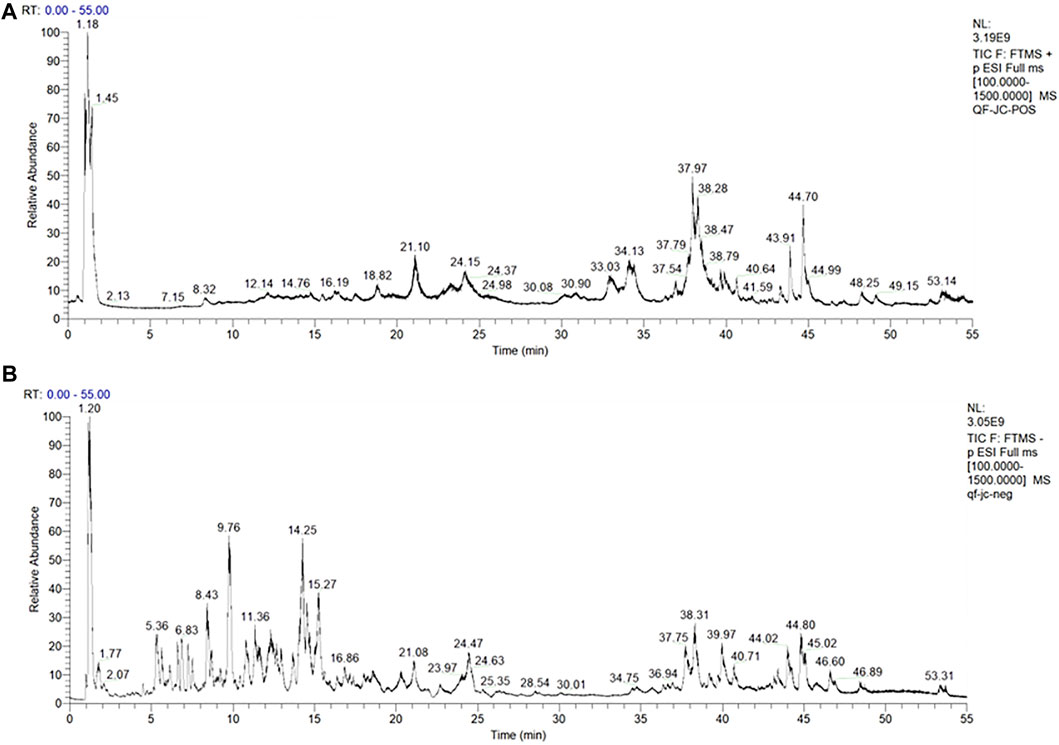
FIGURE 1. TIC of KXS extracts acquired by UPLC-Q-Exactive Orbitrap MS/MS in the positive (A) and negative (B) ion modes.
3.2 HPLC method validation
The results of specificity showed that the blank solvent did not interfere with the determination, namely, the method had good specificity. The relative standard deviation (RSD) of RT and the average peak area (APA) of common peaks were calculated. The RSD of method precision, reproducibility, and storage stability of sample solutions within 24 h appeared less than 3.00%. All test results demonstrated that this chromatographic method was reliable in the KXS fingerprint analysis (Zhang et al., 2021b).
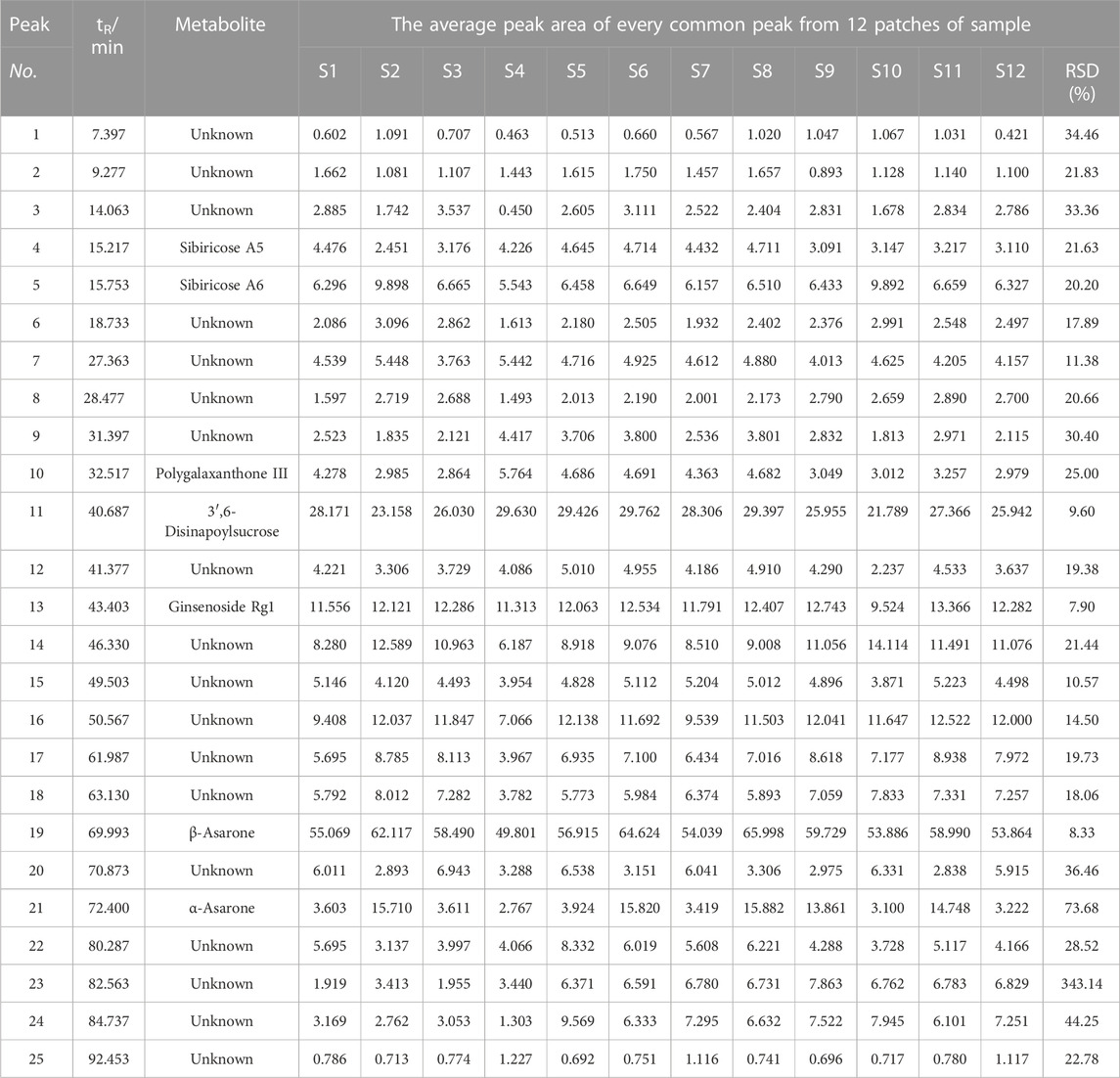
The characteristic HPLC fingerprints of 12 batches of KXS extracts are shown in Figure 2A, while the developed reference atlas was displayed in Figure 2B. As common peaks, 25 peaks with good stability were chosen. Based on the RT of seven reference standards, the common peaks of 4, 5, 10, 11, 13, 19, and 21 were identified as Sibiricose A5, Sibiricose A6, Polygalaxanthone III, 3′,6-disinapoylsucrose, Ginsenoside Rg1, β-asarone, and α-asarone, respectively. APA of the 25 common peaks of 12 batches of KXS extracts and the RSD value are shown in Table 1. These results revealed the contents of 25 metabolites represented by common peaks varied greatly in the S1–S12 KXS extracts.
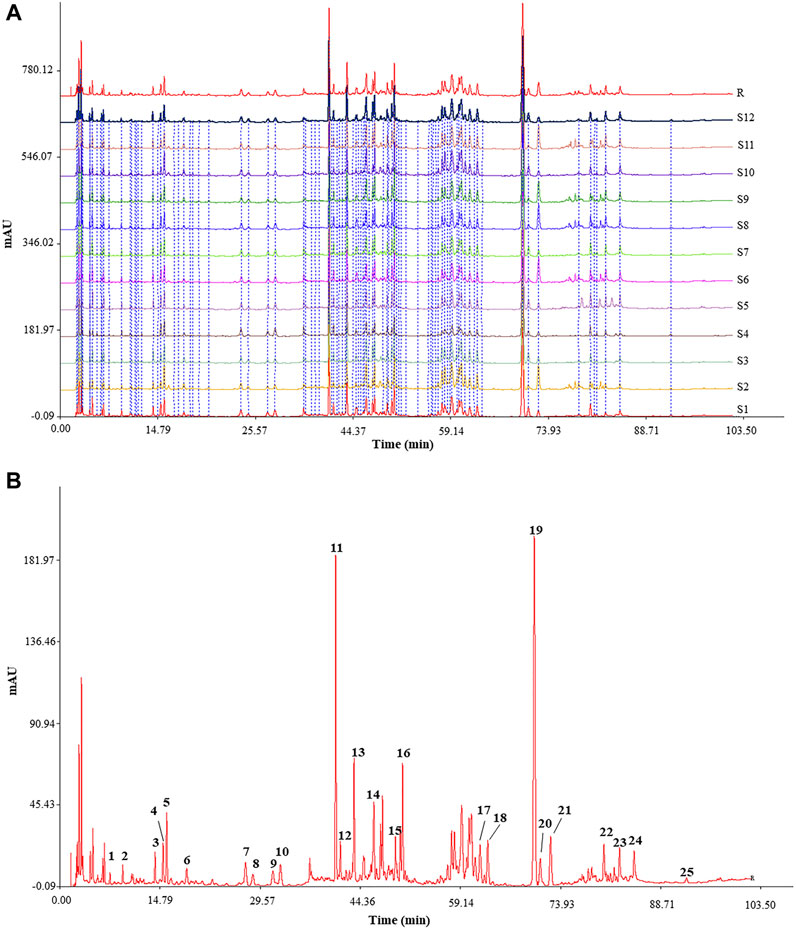
FIGURE 2. Typical HPLC fingerprints of 12 batches of KXS extracts (A) and reference chromatogram generated from KXS extracts. (B) Peak 4: Sibiricose A5; Peak 5: Sibiricose A6; Peak 10: Polygalaxanthone III; Peak 11: 3′,6-disinapoylsucrose; Peak 13: Ginsenoside Rg1; Peak 19: β-asarone; Peak 21: α-asarone.
3.3 Similarity analysis of HPLC fingerprints
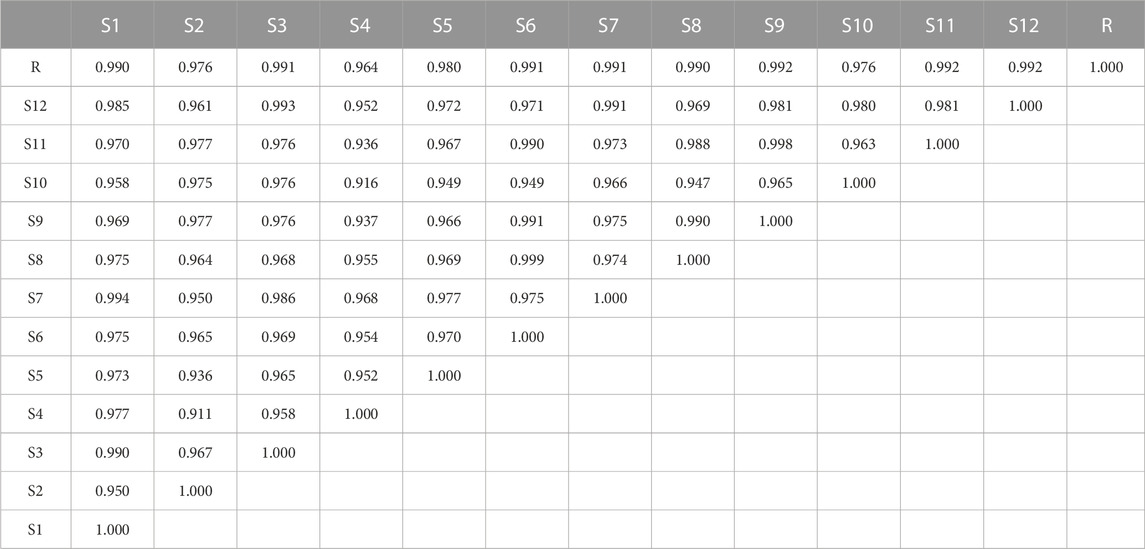
To evaluate the similarity of HPLC fingerprints, 12 batches of KXS sample solutions were analyzed by comparing each sample fingerprint with the reference fingerprint (R) (Wang et al., 2021). As shown in Table 2, the similarity values between each HPLC fingerprint of KXS were in the range of 0.911–0.999. These similarity values were greater than 0.900, meaning that the quality of KXS was relatively consistent and stable.
3.4 Results of DPPH free radical scavenging activity
The dose–response curves and their SC50 values of the DPPH free radical scavenging activity are listed in Table 3. It can be seen that these SC50 values of 12 batches of KXS extracts fluctuated within the range of 3.69–37.32 μg·mL−1. Meanwhile, the concentration–response curves demonstrated that the DPPH free radical inhibitory capacity of the KXS extracts (at a concentration of 6.25–200 μg/mL) possessed a good concentration–effect relationship (Bettencourt et al., 2019). Although KXS extracts can effectively scavenge DPPH free radicals, their antioxidant activities need further pharmacological relevant study.
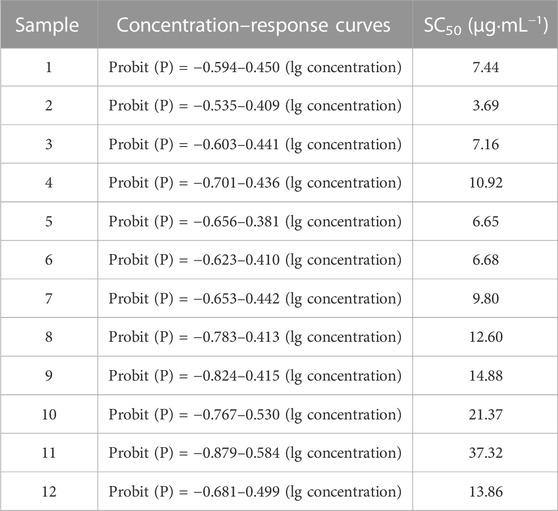
TABLE 3. Concentration–effect curves and SC50 values of 12 batches of KXS extracts in scavenging DPPH free radicals.
3.5 Results of Pearson correlation analysis
The results of Pearson correlation analysis are shown in Figure 3A, where red and blue represent positive and negative correlations, respectively. It can be seen that peaks 8, 1, 14, 17, 18, 24, 16, 21, 15, 13, 6, 5, and 3 in KXS were positively correlated with the DPPH free radical scavenging capacity, suggesting that these metabolites were the main bioactive metabolites for the DPPH free radical scavenging capacity of KXS.
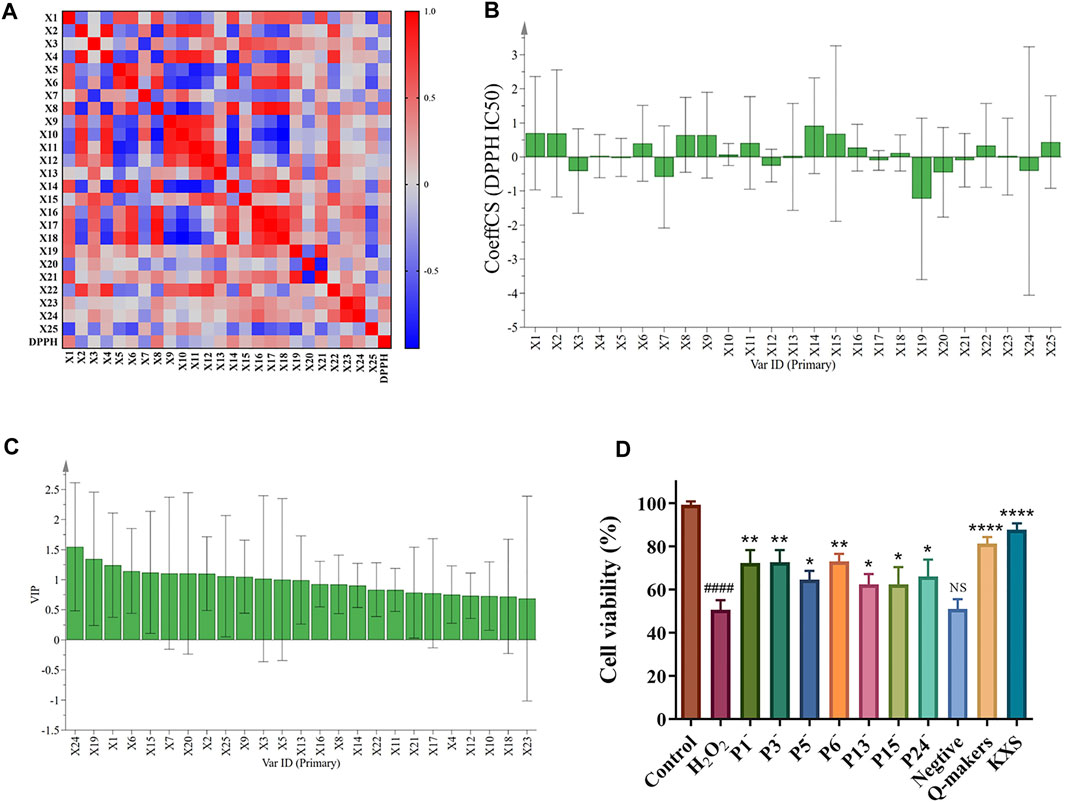
FIGURE 3. Results of Pearson correlation analysis (A), standardized regression coefficient of scavenging activity ratio of 25 common peaks (B), the VIP contribution of 25 common peaks to the antioxidant activity of KXS extracts (C), and the cell viability of H2O2-induced SH-SY5Y cells after treating with the different knock-outed KXS samples (D). (P1-/ P3-/ P5-/ P6-/ P13-/ P15-/ P24-: the samples knocked out peak 1, 3, 5, 6, 13, 15, 24, respectively; negative: the sample knocked out all 1, 3, 5, 6, 13, 15, and 24 peaks; Q-makers: the sample containing peak 1, 3, 5, 6, 13, 15, and 24). Significantly different compared to the control group: ####p < 0.0001; compared to the H2O2 group: ****p < 0.0001, **p < 0.01, *p < 0.05; NS: no significant difference.
3.6 Results of GRA
The correlation results of GRA analysis are listed in Table 4. Subsequently, the ranking results of GRA were as follows: X14 > X8 > X1 > X5 (Sibiricose A6) > X24 > X17 > X18 > X6 > X16 > X13 (Ginsenoside Rg1) > X19 (β-asarone) > X23 > X15 > X11 (3’, 6-disinapoylsucrose) > X7 > X12 > X20 > X25 > X22 > X9 >X3 > X4 (Sibiricose A5) > X21 (α-asarone) > X2> X10 (Polygalaxanthone III). The importance of common peaks increases with their rank, indicating that this metabolite has a greater contribution to scavenging capacity (Nijat et al., 2021). Furthermore, all relational grades are above 0.6, revealing that the DPPH free radical scavenging capacity is the result of the joint action of multiple metabolites in the KXS formula.
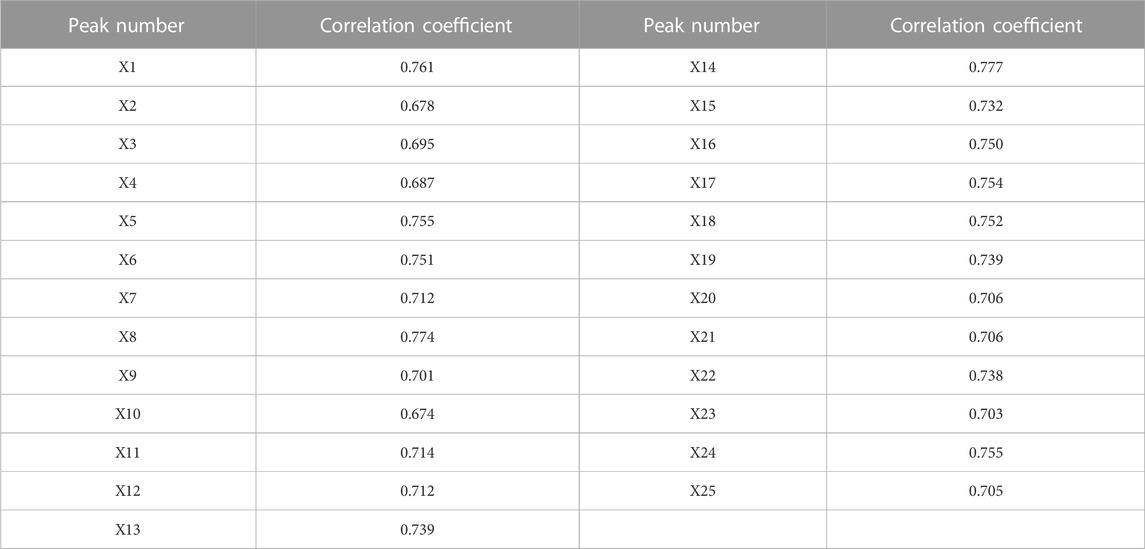
TABLE 4. Correlation results between common peaks of the HPLC fingerprint and DPPH free radical scavenging effect of KXS extracts.
3.7 Results of OPLS-DA
The normalized regression coefficients of the DPPH free radical scavenging capacity of 25 common peaks in KXS extracts are plotted in Figure 3B. The regression equation for the KXS scavenging DPPH free radical capacity is as follows:
Y = 1.395 + 0.697*X1 + 0.693*X2 − 0.414*X3 + 0.022*X4 − 0.012*X5 + 0.399*X6 − 0.587*X7 + 0.649*X8 + 0.642*X9 + 0.070*X10 + 0.411*X11 − 0.254*X12 + 0.001*X13 + 0.917*X14 + 0.686*X15 + 0.273*X16 − 0.102*X17 + 0.116*X18 − 1.230*X19 − 0.452*X20 − 0.097*X21 + 0.338*X22 + 0.008*X23 − 0.410*X24 + 0.437*X25.
Variable influence on projection (VIP) explains the contribution of the independent variable to the dependent variable. The larger the VIP value, the greater the contribution of the independent variable to the dependent variable. Additionally, VIP> 1 indicates a significant contribution to the dependent variable (Xia et al., 2021). As shown in Figure 3C, the scavenging rate of DPPH free radicals was used as the efficacy index for VIP; VIP of 24, 19, 1, 6, 15, 7, 20, 2, 25, 9, 3, 5, and 13 peaks had significant contributions to the antioxidant effect. According to the contribution degree, the antioxidant capacities of KXS extracts were ranked as follows: X24 > X19 (β-asarone) > X1 > X6 > X15 > X7 > X20 > X2 > X25 > X9 > X3 > X5 (Sibiricose A6) > X13 (Ginsenoside Rg1) > X16 > X8 > X14 > X22 > X11 (3′,6-disinapoylsucrose) > X21 (α-asarone) > X17 > X4 (Sibiricose A5) > X12 > X10 (Polygalaxanthone III) > X18 > X23. By cross-enriching the peaks screened by Pearson correlation analysis, GRA, and OPLS-DA, peaks 1, 3, 5 (Sibiricose A6), 6, 13 (Ginsenoside Rg1), 15, and 24 were consequently obtained, which can be considered the Q-markers of KXS for the DPPH free radical scavenging capacity.
3.8 Results of the antioxidant activity test
To further validate the relationship between the screened Q-markers and antioxidant activity, the CCK-8 assay was applied to measure the viability of SH-SY5Y cells before and after KXS treatment. The results in Figure 3D demonstrated that 200 μmol/L of H2O2 significantly damaged the SH-SY5Y cells after 24 h treatment (p<0.0001). The cell viability was increased after treating with the samples that knocked out peaks 1, 3, 5, 6, 13, 15, and 24, respectively (p < 0.01, p < 0.05). Interestingly, KXS and its Q-markers remarkably reduced the oxidative damage caused by H2O2 and increased the damaged cell survival (p < 0.0001). However, the antioxidant activity of the samples was reduced after these Q-markers were knocked out, respectively. Notably, when all Q-markers were knocked out, there was no significant difference in cell viability in the negative group compared to the H2O2 group. These results indicated that the Q-markers of KXS screened in this study were the main active substances for the antioxidant activity of KXS.
4 Discussion
Recently, the quality evaluation standard of TCM has become the most concerned issue with the widespread application of TCM and the improvement of people’s awareness of drug safety (Ren et al., 2020). To address the common quality control problems in TCM, Academician Liu (Liu et al., 2018b) proposed the concept of Q-marker, which brings a new juncture for the research of quality standards of TCM. However, Q-marker screening for the antioxidant effect of KXS has not been conducted so far. Therefore, the novelties of this study include screening the Q-markers of KXS for antioxidant activity by the spectrum–effect relationship based on the identification of the metabolites in KXS by UPLC-Q-Exactive Orbitrap MS/MS and validating the efficacy of Q-markers at the cellular level. First, we applied UPLC-Q-Exactive Orbitrap MS/MS and identified 103 metabolites in KXS. Subsequently, we established the HPLC fingerprints of 12 batches of KXS from different origins and evaluated their similarity. The results showed that the similarity values between each sample were greater than 0.900, which meant that the quality of KXS was relatively consistent and stable.
It is well known that the ability of scavenging DPPH free radicals is positively correlated with the antioxidant activity of drugs (Chen and Huang, 2019). The quality of Chinese botanical drugs is affected by different factors such as the growing environment, geographical location, cultivation technology, and processing (Liu et al., 2018c; Zhao et al., 2019). In this study, even though all of the KXS contained α-asarone, its content varied from batch to batch. As a result, the SC50 values of 12 batches of KXS were in the range of 3.69–37.32 μg/mL, indicating that KXS had a strong free radical scavenging ability with concentration dependence, and the antioxidant activity of KXS was correlated with the content of metabolites (Lee et al., 2016; Li et al., 2022). Meanwhile, these results objectively reflect the necessity of establishing a stable quality control system for scientific development of TCM. This is exactly the reason why we screen the Q-markers of KXS to more comprehensively control its quality and ensure its efficacy in the future.
The Pearson correlation analysis and GRA are multi-factor statistical analysis that reflects the correlation degree of elements between two systems (Murali et al., 2022). The results in this study showed that the correlations between the peak areas of the common peaks in KXS and their DPPH free radical scavenging activity were all greater than 0.6, indicating that multiple metabolites in KXS were associated with free radical scavenging activity. In particular, the peaks of 8, 1, 14, 17, 18, 24, 16, 21, 15, 13, 6, 5, and 3 from KXS were positively correlated with the DPPH scavenging activity. However, the Pearson correlation analysis and GRA mainly reflect the association between elements in the system and lack the overall description. Fortunately, the OPLS-DA method can compensate for this deficiency (Genisheva et al., 2018). In the current study, OPLS-DA was also applied to analyze the DPPH radical scavenging activity of 12 batches of KXS. The OPLS-DA results revealed that VIP of peaks 24, 19, 1, 6, 15, 7, 20, 2, 25, 9, 3, 5, and 13 was higher than 1, suggesting that these metabolites had significant contributions to its antioxidant effect (Gao et al., 2019; Wang et al., 2020). Combining with the aforementioned results of three analyses and taking the common intersection of the screened peaks, the Q-markers of KXS for the DPPH free radical scavenging activity were obtained as peaks 1, 3, 5 (Sibiricose A6), 6, 13 (Ginsenoside Rg1), 15, and 24. To further validate the Q-markers of KXS for antioxidant activity, the protective effect of the Q-marker sample and the Q-marker-knockout samples on H2O2-induced SH-SY5Y cells was detected by the CCK-8 method (Choi et al., 2023). Our results showed that KXS and its Q-markers remarkably reduced the SH-SY5Y cell damage caused by H2O2. However, the antioxidant activity of Q-markers of completely or partially knocked out samples was significantly reduced compared to KXS. These results indicated that the Q-markers of KXS screened in this study were the main bioactive metabolites for the antioxidant activity of KXS.
Because of the extensive sources and complex metabolites, it is difficult to determine the pharmacodynamic material basis of TCM (Liang et al., 2022). Comfortingly, the spectrum–effect relationship has become an important tool for exploring the relationship between the pharmacological effects and the material basis of TCM (Rao et al., 2022). It organically connects the fingerprint (active metabolites) and pharmacological data on TCM, and systematically reveals the relationship between them through a reasonable spectrum–effect model and analytical method (Wang et al., 2019c). In our research, the Pearson correlation analysis, GRA, and OPLS-DA were innovatively combined to investigate the spectrum–effect relationship between the HPLC fingerprint and the antioxidant activity of KXS extracts. The results revealed that the antioxidant activity of KXS was a comprehensive representation of various metabolites. Our analysis suggests that peaks 1, 3, 5, 6, 13, 15, and 24 are the potential antioxidant Q-markers of KXS, among which peaks 5 and 13 are Sibiricose A6 and Ginsenoside Rg1, respectively. Certainly, the metabolites of KXS are complex, and other metabolites needed to be further identified in the future.
5 Conclusion
In summary, 103 metabolites were identified from KXS by UPLC-MS/MS. The typical HPLC fingerprints of 12 batches of KXS extracts were established in this study, and the similarity values were in the range of 0.911–0.999. Meanwhile, the radical scavenging activity of 12 batches of KXS was determined by the DPPH method. Their SC50 values ranged from 3.69 to 37.32 μg/mL, with a definite concentration–effect relationship. Combined with the Pearson correlation analysis, GRA, and OPLS-DA, the potential antioxidant Q-markers in KXS were screened, which are peaks 1, 3, 5 (Sibiricose A6), 6, 13 (Ginsenoside Rg1), 15, and 24 in its HPLC fingerprints. Finally, the antioxidant activity of Q-markers of KXS was validated in H2O2-induced SH-SY5Y cells. Here, the identification of KXS metabolites and its spectrum–antioxidant effect relationship provide a pharmacodynamic material basis for its antioxidant efficacy, as well as a scientific reference for its Q-markers and the quality control standards.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in MetaboLights Database MTBLS8806.
Author contributions
XS: writing–original draft. XY: writing–review and editing. DL: data curation, software, and writing–review and editing. LZ: software, formal analysis, investigation, and writing–review and editing. SQ: data curation and writing–review and editing. JL: methodology, supervision, and writing–original draft. WT: formal analysis, project administration, and writing–original draft. CP: resources, validation, and writing–review and editing. JW: data curation, funding acquisition, and writing–review and editing. XC: conceptualization, methodology, and writing–review and editing. HW: methodology, project administration, and writing–review and editing. CZ: funding acquisition, visualization, and writing–review and editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Project of National Natural Science Foundation of China (51303006), the Anhui Province Key Research and Development Program Project (202203a07020031), the Provincial Natural Science Foundation of Anhui Province (KJ2021ZD0065, KJ 2019A0314, KJ2018ZD031, and 1408085MH196), and the Innovation and Entrepreneurship Training Program for College Students (202210369022). Grand Health Research Institute of Hefei Comprehensive National Science Center, Center for Xin’an Medicine and Modernization of Traditional Chinese Medicine of IHM ‘Jie Bang Gua Shuai’ Project (2023CXMMTCM014).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1270836/full#supplementary-material
Abbreviations
AUCM Anhui Education Department, AD Alzheimer’s disease, APA Average peak area, DPPH 2,2-Diphenyl-1-picrylhydrazyl, GRA Gray relational analysis, HPLC High-performance liquid chromatography, KXS Kai-Xin-San, OPLS-DA Orthogonal partial least squares discriminant analysis, Q-marker Quality marker, RSD Relative standard deviation, RT Retention time, TCM Traditional Chinese medicine, VIP Variable influence on projection.
References
Balakrishnan, R., Cho, D., Kim, I., Seol, S., and Choi, D. (2022). Molecular mechanisms and therapeutic potential of α- and β-Asarone in the treatment of neurological disorders. Antioxidants (Basel) 11 (2), 281. doi:10.3390/antiox11020281
Bettencourt, A. P., Castro, M., Silva, J. P., Fernandes, F., Coutinho, O. P., Sousa, M. J., et al. (2019). Phenolic imidazole derivatives with dual antioxidant/antifungal activity: synthesis and structure-activity relationship. Med. Chem. 15, 341–351. doi:10.2174/1573406414666181005143431
Cao, C., Xiao, J., Liu, M., Ge, Z., Huang, R., Qi, M., et al. (2018). Active components, derived from Kai-xin-san, a herbal formula, increase the expressions of neurotrophic factor NGF and BDNF on mouse astrocyte primary cultures via cAMP-dependent signaling pathway. J. Ethnopharmacol. 224, 554–562. doi:10.1016/j.jep.2018.06.007
Chen, F., and Huang, G. (2019). Antioxidant activity of polysaccharides from different sources of ginseng. Int. J. Biol. Macromol. 125, 906–908. doi:10.1016/j.ijbiomac.2018.12.134
Chen, Y., Pan, G., Xu, W., Sun, Q., Wang, B., Zhang, Y., et al. (2020). Spectrum-effect relationship study between HPLC fingerprints and antioxidant activity of Sabia parviflora. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1140, 121970. doi:10.1016/j.jchromb.2020.121970
Choi, J., Choi, S. Y., Hong, Y., Han, Y. E., Oh, S. J., Lee, B., et al. (2023). The central administration of vitisin a, extracted from Vitis vinifera, improves cognitive function and related signaling pathways in a scopolamine-induced dementia model. Biomed. Pharmacother. 163, 114812. doi:10.1016/j.biopha.2023.114812
Fan, Q., Yang, R., Yang, F., Xia, P., and Zhao, L. (2020). Spectrum-effect relationship between HPLC fingerprints and antioxidant activity of Angelica sinensis. Biomed. Chromatogr. 34, e4707. doi:10.1002/bmc.4707
Fang, C., Paul, C., Day, C., Chang, R., Kuo, C., Ho, T., et al. (2021). Poria cocos (Fuling) targets TGFβ/Smad7 associated collagen accumulation and enhances Nrf2-antioxidant mechanism to exert anti-skin aging effects in human dermal fibroblasts. Environ. Toxicol. 36 (5), 729–736. doi:10.1002/tox.23075
Fu, H., Xu, Z., Zhang, X. L., and Zheng, G. Q. (2020). Kaixinsan, a well-known Chinese herbal prescription, for Alzheimer's disease and depression: a preclinical systematic review. Front. Neurosci. 13, 1421. doi:10.3389/fnins.2019.01421
Gai, C., Feng, W. D., Qiang, T. Y., Ma, H. J., Chai, Y., Zhang, S. J., et al. (2019). Da-Bu-Yin-Wan and Qian-Zheng-San ameliorate mitochondrial dynamics in the Parkinson’s disease cell model induced by MPP. Front. Pharmacol. 10, 372. doi:10.3389/fphar.2019.00372
Gao, S., Chen, H., and Zhou, X. (2019). Study on the spectrum-effect relationship of the xanthine oxidase inhibitory activity of Ligustrum lucidum. J. Sep. Sci. 42, 3281–3292. doi:10.1002/jssc.201900531
Genisheva, Z., Quintelas, C., Mesquita, D. P., Ferreira, E. C., Oliveira, J. M., and Amaral, A. L. (2018). New PLS analysis approach to wine volatile compounds characterization by near infrared spectroscopy (NIR). Food Chem. 246, 172–178. doi:10.1016/j.foodchem.2017.11.015
Goodarzi, M., Russell, P. J., and Heyden, Y. V. (2013). Similarity analyses of chromatographic herbal fingerprints: a review. Anal. Chim. Acta 804, 16–28. doi:10.1016/j.aca.2013.09.017
Guo, S., Wang, J., Xu, H., Rong, W., Gao, C., Yuan, Z., et al. (2019). Classic prescription, Kai-Xin-San, ameliorates Alzheimer's Disease as an effective multitarget treatment: from neurotransmitter to protein signaling pathway. Oxid. Med. Cell Longev. 2019, 9096409. doi:10.1155/2019/9096409
He, Y., Zhong, J. H., Wei, X. D., Huang, C. Y., Peng, P. L., Yao, J., et al. (2022). Pachymic acid ameliorates pulmonary hypertension by regulating Nrf2-Keap1-ARE pathway. Curr. Med. Sci. 42 (1), 56–67. doi:10.1007/s11596-021-2414-2
Hu, X., Wang, D., Pang, Y., Wu, Z., Huan, H., Chen, Z., et al. (2020b). Development of chromatographic fingerprint for quality analysis of diploid and tetraploid Lonicera japonica. J. Tradit. Chin. Med. 40 (1), 73–82.
Hu, Y., Liu, X., Zhang, T., Chen, C., Dong, X., Can, Y., et al. (2020a). Behavioral and biochemical effects of KXS on postmyocardial infarction depression. Front. Pharmacol. 11, 561817. doi:10.3389/fphar.2020.561817
Jiang, N., Wei, S., Zhang, Y., He, W., Pei, H., Huang, H., et al. (2021). Protective effects and mechanism of Radix Polygalae against neurological diseases as well as effective substance. Front. Psychiatry 12, 688703. doi:10.3389/fpsyt.2021.688703
Jiang, Y. X., Chen, Y., Yang, Y., Chen, X. X., and Zhang, D. D. (2019). Screening five Qi-Tonifying herbs on M2 phenotype macrophages. Evid. Based Complement. Altern. Med. 2019, 9549315. doi:10.1155/2019/9549315
Jiao, Y. N., Zhang, J. S., Qiao, W. J., Tian, S. Y., Wang, Y. B., Wang, C. Y., et al. (2022). Kai-Xin-San inhibits tau pathology and neuronal apoptosis in aged SAMP8 mice. Mol. Neurobiol. 59 (5), 3294–3309. doi:10.1007/s12035-021-02626-0
Kumar, H., Kim, B. W., Song, S. Y., Kim, J. S., Kim, I. S., Kwon, Y. S., et al. (2012). Cognitive enhancing effects of alpha asarone in amnesic mice by influencing cholinergic and antioxidant defense mechanisms. Biosci. Biotechnol. Biochem. 76 (8), 1518–1522. doi:10.1271/bbb.120247
Lee, J. W., Mo, E. J., Choi, J. E., Jo, Y. H., Jang, H., Jeong, J. Y., et al. (2016). Effect of Korean Red Ginseng extraction conditions on antioxidant activity, extraction yield, and ginsenoside Rg1 and phenolic content: optimization using response surface methodology. J. Ginseng Res. 40, 229–236. doi:10.1016/j.jgr.2015.08.001
Li, J. H., Cao, X. P., Wei, J. J., Song, L., Liao, F. J., Zheng, G. Q., et al. (2015). Chuanxiong chadiao powder, a famous Chinese herbal prescription, for headache: a systematic review and meta-analysis. Complement. Ther. Med. 23 (4), 577–590. doi:10.1016/j.ctim.2015.06.012
Li, X., Wang, Y. B., Wang, C. C., Jing, R., Mu, L. H., Liu, P., et al. (2022). Antidepressant mechanism of Kaixinsan and its active compounds based on upregulation of antioxidant thioredoxin. Evid. Based Complement. Altern. Med. 2022, 7302442. doi:10.1155/2022/7302442
Liang, C., Yao, Y., Ding, H., Li, X., Li, Y., and Cai, T. (2022). Rapid classification and identification of chemical components of Astragali radix by UPLC-Q-TOF-MS. Phytochem. Anal. 33, 943–960. doi:10.1002/pca.3150
Liu, C., Ma, C., Lu, J., Cui, L., Li, M., Huang, T., et al. (2021). A rapid method and mechanism to identify the active compounds in Malus micromalus Makino fruit with spectrum-effect relationship, components knock-out and molecular docking technology. Food Chem. Toxicol. 150, 112086. doi:10.1016/j.fct.2021.112086
Liu, C. X., Liu, L., and Guo, D. A. (2018b). Quality marker of TCMs: concept and applications. Phytomedicine 44, 85–86. doi:10.1016/j.phymed.2018.05.015
Liu, H., Wang, J., Liu, M., Zhao, H., Yaqoob, S., Zheng, M., et al. (2018a). Antiobesity effects of ginsenoside Rg1 on 3T3-L1 preadipocytes and high fat diet-induced obese mice mediated by AMPK. Nutrients 10 (7), 830. doi:10.3390/nu10070830
Liu, H., Zhu, S., Liu, Q., and Zhang, Y. (2019). Spectrum-effect relationship study between HPLC fingerprints and antioxidant of honeysuckle extract. Biomed. Chromatogr. 33, e4583. doi:10.1002/bmc.4583
Liu, W., Wang, D., Hou, X., Yang, Y., Xue, X., Jia, Q., et al. (2018c). Effects of growing location on the contents of main active components and antioxidant activity of Dasiphora fruticosa (L.) Rydb. by chemometric methods. Chem. Biodivers. 15 (7), e1800114. doi:10.1002/cbdv.201800114
Lyu, W., Ouyang, M., Ma, X., Han, T., Pi, D., and Qiu, S. (2021). Kai-Xin-San attenuates doxorubicin-induced cognitive impairment by reducing inflammation, oxidative stress, and neural degeneration in 4T1 breast cancer mice. Evid. Based Complement. Altern. Med. 2021, 5521739. doi:10.1155/2021/5521739
Ma, Y. L., Zhu, D. Y., Thakur, K., Wang, C. H., Wang, H., Ren, Y. F., et al. (2018). Antioxidant and antibacterial evaluation of polysaccharides sequentially extracted from onion (Allium cepa L.). Int. J. Biol. Macromol. 111, 92–101. doi:10.1016/j.ijbiomac.2017.12.154
Murali, B., Vijaya Ramnath, B. M., Rajamani, D., Nasr, E. A., Astarita, A., and Mohamed, H. (2022). Experimental investigations on dry sliding wear behavior of kevlar and natural fiber-reinforced hybrid composites through an RSM-GRA hybrid approach. Mater. (Basel) 15 (3), 749. doi:10.3390/ma15030749
Nijat, D., Lu, C. F., Lu, J. J., Abdulla, R., Hasan, A., Aidarhan, N., et al. (2021). Spectrum-effect relationship between UPLC fingerprints and antidiabetic and antioxidant activities of Rosa rugosa. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1179, 122843. doi:10.1016/j.jchromb.2021.122843
Qiong, W., Yong-Liang, Z., Ying-Hui, L., Shan-Guang, C., Jiang-Hui, G., Yi-Xi, C., et al. (2016). The memory enhancement effect of Kai Xin San on cognitive deficit induced by simulated weightlessness in rats. J. Ethnopharmacol. 187, 9–16. doi:10.1016/j.jep.2016.03.070
Rao, S. W., Duan, Y. Y., Pang, H. Q., Xu, S. H., Hu, S. Q., Cheng, K. G., et al. (2022). Spectrum-effect relationship analysis of bioactive compounds in zanthoxylum nitidum (Roxb.) DC. by ultra-high performance liquid chromatography mass spectrometry coupled with comprehensive filtering approaches. Front. Pharmacol. 13, 794277. doi:10.3389/fphar.2022.794277
Ren, J. L., Zhang, A. H., Kong, L., Han, Y., Yan, G. L., Sun, H., et al. (2020). Analytical strategies for the discovery and validation of quality-markers of traditional Chinese medicine. Phytomedicine 67, 153165. doi:10.1016/j.phymed.2019.153165
Shang, B., Zhao, Z., Zeng, Q., Su, J., Xu, B., Liu, Y., et al. (2023). Research on key quality attributes of substance benchmark of the famous classical formula Kai-Xin-San. China J. Chin. materia medica 48 (02), 382–389. doi:10.19540/j.cnki.cjcmm.20220520.301
Shi, J., Tian, J., Zhang, X., Zeng, C., Wei, M., Wang, P., et al. (2013). A combination extract of renshen (panax ginseng), yinyanghuo (herba epimedii brevicornus), yuanzhi (radix palygalae) and jianghuang (rhizoma curcumae longae) decreases glycogen synthase kinase 3beta expression in brain cortex of APPV7171 transgenic mice. J. Tradit. Chin. Med. 33 (2), 211–217. doi:10.1016/s0254-6272(13)60127-2
Shi, Q., Chen, J., Zhou, Q., Lei, H., Luan, L., Liu, X., et al. (2015). Indirect identification of antioxidants in Polygalae Radix through their reaction with 2,2-diphenyl-1-picrylhydrazyl and subsequent HPLC-ESI-Q-TOF-MS/MS. Talanta 144, 830–835. doi:10.1016/j.talanta.2015.07.032
Tang, X., Zhao, Y., Liu, Y., Liu, Y., Liu, Y., Niu, F., et al. (2022). 3,6'-disinapoyl sucrose attenuates Aβ1-42-induced neurotoxicity in Caenorhabditis elegans by enhancing antioxidation and regulating autophagy. J. Cell Mol. Med. 26 (4), 1024–1033. doi:10.1111/jcmm.17153
Wang, H., Tan, J., Shang, X., Zheng, X., Liu, X., Wang, J., et al. (2019b). Porous organic cage incorporated monoliths for solid-phase extraction coupled with liquid chromatography-mass spectrometry for identification of ecdysteroids from Chenopodium quinoa Willd. J. Chromatogr. A 1583, 55–62. doi:10.1016/j.chroma.2018.11.019
Wang, J., Fan, L., Hu, M., Ma, F., and Qi, J. (2020). Spectrum-effect relationship between fingerprints and hemopoietic effects of small molecular fraction of Polygoni Multiflori radix praeparata. Biomed. Chromatogr. 34, e4821. doi:10.1002/bmc.4821
Wang, Q., Yu, X., Sun, L., Tian, R., He, H., Wang, S., et al. (2021). Fingerprint analysis of phenolic acid extract of Salvia miltiorrhiza by digital reference standard analyzer with one or two reference standards. Chin. Med. 16 (1), 8. doi:10.1186/s13020-020-00408-9
Wang, X. J., Zhang, A. H., Kong, L., Yu, J. B., Gao, H. L., Liu, Z. D., et al. (2019a). Rapid discovery of quality-markers from Kaixin San using chinmedomics analysis approach. Phytomedicine 54, 371–381. doi:10.1016/j.phymed.2017.12.014
Wang, Y. L., Zhang, Q., Yin, S. J., Cai, L., Yang, Y. X., Liu, W. J., et al. (2019c). Screening of blood-activating active components from Danshen-Honghua herbal pair by spectrum-effect relationship analysis. Phytomedicine 54, 149–158. doi:10.1016/j.phymed.2018.09.176
Wen, J., Yang, Y., and Hao, J. (2023). Acori Tatarinowii Rhizoma: a comprehensive review of its chemical composition, pharmacology, pharmacokinetics and toxicity. Front. Pharmacol. 14, 1090526. doi:10.3389/fphar.2023.1090526
Xia, W., Liu, Q., Zhou, H., Hua, S., Dong, L., Han, X., et al. (2021). Study on the Spectrum-effect relationship of the traditional effect of saponins in glycyrrhiza uralensis fisch. Int. J. Anal. Chem. 2021, 6617033. doi:10.1155/2021/6617033
Xiao, L., Li, H., Tian, J., Jin, N., Zhang, J., Yang, F., et al. (2020). The traditional formula Kai-Xin-San alleviates polyglutamine-mediated neurotoxicity by modulating proteostasis network in caenorhabditis elegans. Rejuvenation Res. 23 (3), 207–216. doi:10.1089/rej.2018.2149
Xiao, Y., Shan, X., Wang, H., Hong, B., Ge, Z., Ma, J., et al. (2022). Spectrum-effect relationship between HPLC fingerprint and antioxidant of “San-Bai Decoction” extracts. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1208, 123380. doi:10.1016/j.jchromb.2022.123380
Xie, G., Xu, Q., Li, R., Shi, L., Han, Y., Zhu, Y., et al. (2019). Chemical profiles and quality evaluation of Buddleja officinalis flowers by HPLC-DAD and HPLC-Q-TOF-MS/MS. J. Pharm. Biomed. Anal. 164, 283–295. doi:10.1016/j.jpba.2018.10.030
Xu, T., Zhang, H., Wang, S., Xiang, Z., Kong, H., Xue, Q., et al. (2022). A review on the advances in the extraction methods and structure elucidation of Poria cocos polysaccharide and its pharmacological activities and drug carrier applications. Int. J. Biol. Macromol. 217, 536–551. doi:10.1016/j.ijbiomac.2022.07.070
Yang, H., Luo, X. J., Zeng, Y. H., and Mai, B. X. (2021). Determination of tetrabromobisphenol-A/S and their eight derivatives in abiotic (soil/dust) samples using ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 1647, 462152. doi:10.1016/j.chroma.2021.462152
Yi, P., Zhang, Z., Huang, S., Huang, J., Peng, W., and Yang, J. (2020). Integrated meta-analysis, network pharmacology, and molecular docking to investigate the efficacy and potential pharmacological mechanism of Kai-Xin-San on Alzheimer's disease. Pharm. Biol. 58 (1), 932–943. doi:10.1080/13880209.2020.1817103
Yu, L., Zhang, Y., Zhao, X., He, Y., Wan, H., Wan, H., et al. (2021). Spectrum-effect relationship between HPLC fingerprints and antioxidant activity of Yangyin Tongnao prescription. J. Anal. Methods Chem. 2021, 6650366. doi:10.1155/2021/6650366
Yu, S., Wang, S., Huang, S., Wang, W., Wei, Z., Ding, Y., et al. (2020). Radix et Rhizoma Ginseng chemoprevents both initiation and promotion of cutaneous carcinoma by enhancing cell-mediated immunity and maintaining redox homeostasis. J. Ginseng Res. 44 (4), 580–592. doi:10.1016/j.jgr.2019.05.004
Zhang, L., and Zeng, F. (2020). Textual research on the dosage of Kai xin san in qian Jin Yao Fang. J. Beijing Univ. Traditional Chin. Med. 43 (08), 641–644. doi:10.3969/j.issn.1006-2157.2020.08.005
Zhang, W., Song, D., Xu, D., Wang, T., Chen, L., and Duan, J. (2015). Characterization of polysaccharides with antioxidant and immunological activities from Rhizoma Acori Tatarinowii. Carbohydr. Polym. 133, 154–162. doi:10.1016/j.carbpol.2015.07.018
Zhang, Y., Wu, M., Xi, J., Pan, C., Xu, Z., Xia, W., et al. (2021b). Multiple-fingerprint analysis of Poria cocos polysaccharide by HPLC combined with chemometrics methods. J. Pharm. Biomed. Anal. 198, 114012. doi:10.1016/j.jpba.2021.114012
Zhang, Y., Yan, G., Song, M., Bian, X., Xu, T., Zhang, Y., et al. (2021a). Identification and quantification of markers in azedarach fructus and toosendan fructus. J. Pharm. Biomed. Anal. 202, 114173. doi:10.1016/j.jpba.2021.114173
Zhao, M. M., Wang, K. R., Gu, R., and Zhong, S. H. (2019). A comparative study on shared-use medicines in Tibetan and Chinese medicine. J. Ethnobiol. Ethnomed 15 (1), 43. doi:10.1186/s13002-019-0320-5
Zhao, X., Cui, Y., Wu, P., Zhao, P., Zhou, Q., Zhang, Z., et al. (2020). Polygalae Radix: a review of its traditional uses, phytochemistry, pharmacology, toxicology, and pharmacokinetics. Fitoterapia 147, 104759. doi:10.1016/j.fitote.2020.104759
Zhong, S., Wang, L., Gu, R., Zhang, W., Lan, R., and Qin, X. (2020). Ginsenoside Rg1 ameliorates the cognitive deficits in D-galactose and AlCl3-induced aging mice by restoring FGF2-Akt and BDNF-TrkB signaling axis to inhibit apoptosis. Int. J. Med. Sci. 17 (8), 1048–1055. doi:10.7150/ijms.43979
Keywords: Kai-Xin-San, Q-marker, HPLC fingerprint, spectrum–effect relationship, antioxidant activity
Citation: Shan X, Yang X, Li D, Zhou L, Qin S, Li J, Tao W, Peng C, Wei J, Chu X, Wang H and Zhang C (2023) Research on the quality markers of antioxidant activity of Kai-Xin-San based on the spectrum–effect relationship. Front. Pharmacol. 14:1270836. doi: 10.3389/fphar.2023.1270836
Received: 03 August 2023; Accepted: 30 October 2023;
Published: 27 December 2023.
Edited by:
Xingjiang Xiong, China Academy of Chinese Medical Sciences, ChinaReviewed by:
Chunyu Li, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaHonghui Shen, Beijing Chaoyang Integrative Medicine Emergency Medical Center, China
Copyright © 2023 Shan, Yang, Li, Zhou, Qin, Li, Tao, Peng, Wei, Chu, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoqin Chu, Y2h1eHE0MjBAMTYzLmNvbQ==; Haixuan Wang, MTE0MzczMjM4QHFxLmNvbQ==; Caiyun Zhang, Y3l6aGFuZzZAdXN0Yy5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Xiaoxiao Shan1,2,3,4,5,6†
Xiaoxiao Shan1,2,3,4,5,6† Can Peng
Can Peng Xiaoqin Chu
Xiaoqin Chu Caiyun Zhang
Caiyun Zhang