
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 13 November 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1269935
This article is part of the Research Topic Exploring Immunotherapy Targets and Strategies for Cancer: A Multi-Omics Perspective View all 6 articles
 Yosuke Shibata1*
Yosuke Shibata1* Takeshi Kishida1*
Takeshi Kishida1* Taku Kouro2,3
Taku Kouro2,3 Feifei Wei2,3
Feifei Wei2,3 Yuka Igarashi2,3
Yuka Igarashi2,3 Hidetomo Himuro2,3
Hidetomo Himuro2,3 Takeaki Noguchi1
Takeaki Noguchi1 Mitsuyuki Koizumi1
Mitsuyuki Koizumi1 Takahisa Suzuki1
Takahisa Suzuki1 Kimito Osaka1
Kimito Osaka1 Yusuke Saigusa4
Yusuke Saigusa4 Tetsuro Sasada2,3*
Tetsuro Sasada2,3*Introduction: This study aimed to identify immune mediators, including cytokines, chemokines, and growth factors, in the plasma for predicting treatment efficacy and immune-related adverse events (irAEs) in advanced urothelial carcinoma (aUC) treated with immune checkpoint inhibitors (ICIs).
Methods: We enrolled 57 patients with aUC who were treated with the anti-programmed cell death protein 1 (PD-1) antibody pembrolizumab after the failure of platinum-based chemotherapy between February 2018 and December 2020. Plasma levels of 73 soluble immune mediators were measured before and 6 weeks after initiating pembrolizumab therapy. The association of estimated soluble immune mediators with clinical outcomes, including overall survival (OS), progression-free survival (PFS), anti-tumor responses, and irAEs, were statistically evaluated.
Results: In the multivariate analysis, levels of 18 factors at baseline and 12 factors during treatment were significantly associated with OS. Regarding PFS, baseline levels of 17 factors were significantly associated with PFS. Higher levels of interleukin (IL)-6, IL-8, soluble tumor necrosis factor receptor 1 (sTNF-R1), and IL-12 (p40), both at baseline and post-treatment, were significantly associated with worse OS. Conversely, low IL-6 and high TWEAK levels at baseline were associated with irAEs. Among identified factors, interferon (IFN) γ and IL-12 (p40) were repeatedly identified; high baseline levels of these factors were risk factors for worse OS and PFS, as well as progressive disease. Notably, using correlation and principal component analysis, factors significantly associated with clinical outcomes were broadly classified into three groups exhibiting similar expression patterns.
Discussion: Measuring plasma levels of soluble immune mediators, such as IL-6, IL-8, sTNF-R1, IFNγ, and IL-12 (p40), could be recommended for predicting prognosis and irAEs in ICI-treated patients with aUC.
Platinum-based chemotherapy is the most commonly used first-line therapy for advanced urothelial carcinoma (aUC). Following the failure of first-line regimens, pembrolizumab, a humanised monoclonal antibody targeting programmed death protein-1 (PD-1), was approved for second-line treatment as the first immune checkpoint inhibitor (ICI) based on the KEYNOTE-045 trial (Bellmunt et al., 2017). In 2021, maintenance therapy with avelumab was approved for patients who responded to platinum-based chemotherapy based on the findings of the JAVELIN Bladder 100 trial (Powles et al., 2020). Pembrolizumab, the most commonly used ICI for aUC, has shown a response rate of 21.1%, and 68% of patients with a good response to pembrolizumab were found to exhibit a long-term response exceeding 1 year (Bellmunt et al., 2017). Conversely, 60.9% of patients reportedly experienced immune-related adverse events (irAEs), 15% of which were ≥ grade 3 severe irAEs, thereby resulting in treatment discontinuation in 5.6% of patients (Bellmunt et al., 2017).
Currently, only a limited number of patients can receive third-line therapy following disease progression after second-line treatment with ICIs. The antibody-drug conjugates, enfortumab vedotin and sacituzumab govitecan, and the fibroblast growth factor receptor inhibitor, erdafitinib, have recently demonstrated efficacy as third-line treatments after ICI therapy (Tagawa et al., 2021; Yu et al., 2021; Siefker-Radtke et al., 2022). Thus, given that switching to subsequent treatment remains urgent in patients who fail to respond to pembrolizumab, biomarkers to predict the clinical effects before or at early time points of ICI treatment need to be developed.
In other cancers, such as lung cancer, the expression of programmed death ligand 1 (PD-L1) has been clinically employed as a biomarker for ICIs to predict their efficacy. However, in urothelial carcinoma (UC), the KEYNOTE-045 and JAVELIN Bladder 100 trials found no clinical significance for PD-L1 expression (Bellmunt et al., 2017; Powles et al., 2020). In the current study, we analyzed soluble immune mediators in the plasma of patients with aUC treated with pembrolizumab to determine their clinical roles in predicting ICI efficacy and irAEs.
Herein, we enrolled 57 patients initiated on the anti-PD-1 antibody pembrolizumab after platinum-based chemotherapy for aUC at Kanagawa Cancer Center between February 2018 and December 2020. Peripheral blood (heparin anticoagulated) was collected from these patients at pembrolizumab treatment initiation and 6 weeks after initiation to measure soluble immune mediators. All eligible patients received pembrolizumab (200 mg/body weight) every 3 weeks. The studies involving human participants were reviewed and approved by the Institutional Review Boards of Kanagawa Cancer Center. The patients/participants provided their written informed consent to participate in this study.
Before and after pembrolizumab administration, a bead-based multiplex assay (Bio-Plex 200 system; Bio-Rad Laboratories, Hercules, CA) was used to evaluate the plasma levels of soluble immune mediators, including cytokines, chemokines, and growth factors. As the plasma amounts were limited, we used two multiplex kits (Bio-Plex Pro™ Human Chemokine Panel 40-Plex kit and Human Inflammation Panel 1, 37-Plex kit; Bio-Rad Laboratories), which could measure many different immune mediators in a single well at the same time, in accordance with the manufacturer’s instructions. The kits contained the reagents to comprehensively measure 73 soluble immune mediators that may be important for antitumor immunity and inflammation: interleukin (IL)-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-11, IL-12 (p40), IL-12 (p70), IL-16, IL-19, IL-20, IL-22, IL-26, IL-27, IL-28A, IL-29, IL-32, IL-34, IL-35, interferon (IFN) α2, IFNβ, IFNγ, tumor necrosis factor (TNF) α, granulocyte-macrophage colony-stimulating factor, C-C motif chemokine ligand (CCL) 1, CCL2, CCL3, CCL7, CCL8, CCL11, CCL13, CCL15, CCL17, CCL19, CCL20, CCL21, CCL22, CCL23, CCL24, CCL25, CCL26, CCL27, C-X-C motif chemokine ligand (CXCL) 1, CXCL2, CXCL5, CXCL6, CXCL9, CXCL10, CXCL11, CXCL12, CXCL13, CXCL16, CX3CL1, macrophage migration inhibitory factor, sCD30, sCD163, chitinase 3-like-1, gp130, IL-6Rα, soluble TNF (sTNF)-R1, sTNF-R2, APRIL, BAFF, LIGHT, pentraxin-3, TSLP, TWEAK, osteocalcin, osteopontin, matrix metalloproteinase (MMP)-1, MMP-2, and MMP-3.
In brief, patients’ plasma stored at −80°C was thawed at 4°C overnight and was diluted four times with sample diluent buffer. The diluted plasma (50 μL) was incubated with antibody-coupled beads in the filter plate (Multiscreen HTS BV, 1.2 μm, Merck Millipore, Burlington, MA) for 1 h at room temperature on plate mixer. Then, the plate was set on the vacuum manifold and beads were washed with 100 μL of wash buffer three times and incubated with biotinylated detection antibody cocktail for 30 min at room temperature on the plate mixer. The beads were then washed three times with wash buffer and incubated with streptavidin-phycoerythrin for 10 min at room temperature on a plate shaker. After three washes, beads were resuspended in 150 μL of assay buffer and subjected to analysis on the Bio-Plex 200 system. The fluorescence intensity values obtained were plotted against standard curves generated from standard cocktail measurements to calculate the concentrations of soluble immune mediators. All assays were performed on the same day using reagent kits and standards manufactured from the same lot.
Tumor response was assessed according to the Response Evaluation Criteria in Solid Tumours, version 1.1. Adverse events were evaluated according to the Common Terminology Criteria for Adverse Events, version 5.0. Two analyses were performed: the levels of soluble immune mediators before treatment (baseline analysis) and potential alterations after 6 weeks of treatment (change analysis). Overall survival (OS) was calculated from the start date of pembrolizumab therapy to the date of death or last follow-up. Patients who were alive were censored at the date of the last contact. Progression-free survival (PFS) was calculated from the start date of pembrolizumab therapy to the date of progression, death, or last follow-up. OS and PFS were evaluated using Cox proportional hazard regression models and log-rank tests. Logistic regression analysis was used to estimate the odds ratios for progressive disease (PD) and irAEs. In the baseline analysis with Cox proportional hazard regression models, continuous measurements of baseline soluble immune mediator levels were approximately log-normally distributed and analysed as log-10 transformed. In the baseline analysis with the log-rank test, patients were classified into “high” and “low” groups by setting the median as the cut-off value. To analyze the altered levels, patients were divided into two groups: those with decreased levels and those with unchanged or increased levels after pembrolizumab administration.
For multivariate analysis of OS, the following clinical factors known to be significantly associated with OS were included in the model: Eastern Cooperative Oncology Group performance status (ECOG PS) and liver metastases (Yanagisawa et al., 2022). For multivariate analysis of PFS, PD, and irAE, variables were selected using the forward stepwise method using Bayesian information criterion from the following clinical factors: age, sex, ECOG PS, liver metastasis, lung metastasis, bone metastasis, lymph node metastasis only, radical surgical history, pathological grade, neutrophil-to-lymphocyte ratio, platelet count, hemoglobin, albumin, and irAE history. Each soluble immune mediator was used in multivariate analysis. All these clinical factors could have been better included in the multivariate model; however, the small number of events limited the number of clinical factors that could be included in the model. The mediators were characterized using principal component analysis. The correlations between each mediator were evaluated using Spearman’s rank correlation coefficient analysis. All p-values were two-sided, with a significance level set at 0.05. Statistical analyses were performed using EZR (version 3.5.2; https://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html) and R (version 4.1.3; https://www.r-project.org/). The network was visualised using the Gephi software (Ver 0.9.1; https://gephi.org/).
Table 1 presents the patient’s background. The median follow-up was 7.2 months (range: 0.2–34.8), and the median age at pembrolizumab initiation was 73 years (range: 44–85). Primary cancer sites were bladder in 23 (40.4%), ureter in 18 (31.6%), and renal pelvis in 16 patients (28.1%). Histologic types included UC in 50 patients (87.7%), squamous cell variant in 5 patients (8.8%), and sarcomatoid variant or micropapillary variant in 1 patient (1.8%) each. Seventeen patients (29.8%) exhibited only lymph node metastasis. Of the 39 patients with visceral metastases, 12 (21.1%), 21 (36.8%), and 14 (24.6%) presented liver, lung, and bone metastases, respectively.
All patients were treated with pembrolizumab after one or more platinum-based chemotherapy regimens. Two patients received ICI treatment prior to pembrolizumab. One received durvalumab plus platinum-based chemotherapy, and the other received avelumab as maintenance therapy after platinum-based chemotherapy. At the time of analysis, 7 patients continued pembrolizumab; however, 48 patients discontinued pembrolizumab owing to treatment failure. In addition, two patients, one with grade 3 hypothyroidism and grade 2 myositis and another with grade 3 arthritis, discontinued pembrolizumab because of irAEs. The median OS and PFS were 12.6 [95% confidence interval (CI) 5.1–17.3] and 3.0 (95% CI 1.9–5.9) months, respectively. The objective response rate of pembrolizumab was 40.4% (23/57), while 4 (7.0%) patients exhibited complete response (CR), 19 (33.3%) had a partial response (PR), 4 (7.0%) experienced stable disease (SD), and 30 (52.6%) showed PD. Notably, two patients who discontinued pembrolizumab owing to irAEs achieved CR and PR.
ECOG PS and liver metastasis, which are frequently associated with OS, were selected and included in the multivariate analysis models; each soluble immune mediator was included in this model (Table 2). Considering the multivariate analysis, 18 mediators [IL-6, IL-8, IL-12 (p40), IFNγ, CCL1, CCL3, CCL7, CCL20, CXCL1, CXCL6, CXCL13, sCD30, sCD163, IL-6Rα, sTNF-R1, sTNF-R2, LIGHT, MMP-1] in the baseline analysis and 12 mediators [IL-6, IL-8, IL-12 (p40), IL-16, IL-22, IL-27, IL-28A, CCL21, CCL22, CXCL16, sTNF-R1, osteocalcin] in the change analysis were significantly associated with OS (Table 2).
Notably, IL-6, IL-8, IL-12 (p40), and sTNF-R1 were significantly associated with OS both at baseline and during treatment (baseline/change; p = 0.002/0.020, p = 0.022/<0.001, p = 0.038/0.022, and p = 0.001/0.035, respectively). High baseline levels of these factors and increased levels after treatment were significantly associated with worse OS. Subgroups stratified by these mediators were subjected to Kaplan-Meier analysis of OS (Figure 1). High baseline levels and increased levels after treatment of IL-6, IL-8, and sTNF-R1, but not those of IL-12 (p40), were significantly associated with worse OS (baseline/change; p < 0.001/0.017, p = 0.016/<0.001, p = 0.002/0.020, and p = 0.077/0.181, respectively; by log-rank analysis).
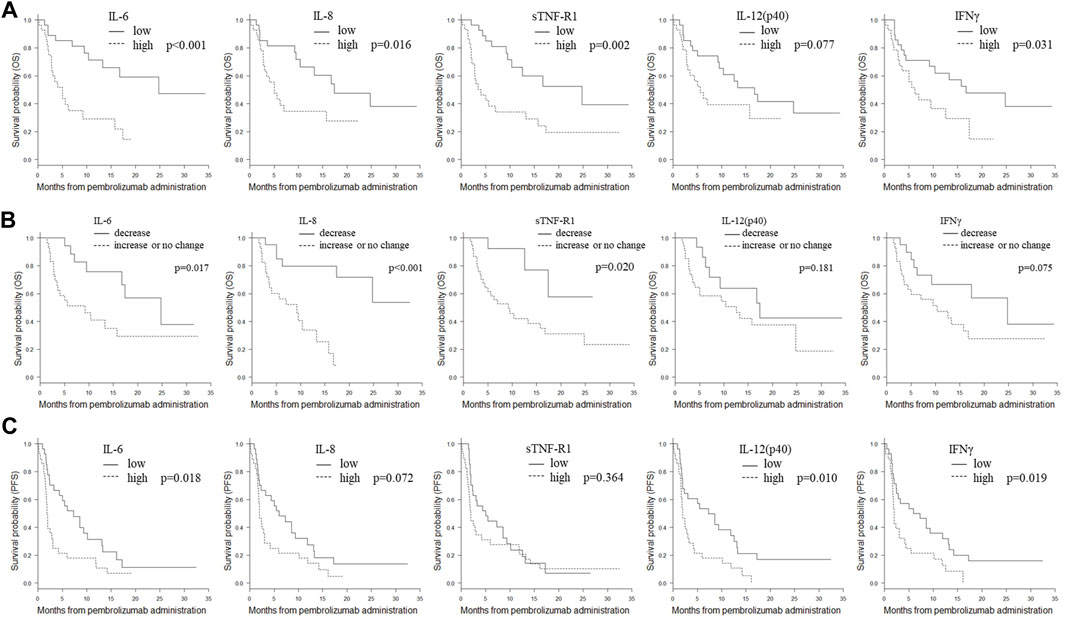
FIGURE 1. Kaplan-Meier plots of OS or PFS in the subgroups stratified by IL-6, IL-8, sTNFR-1, IL-12 (p40), and IFNγ. (A) Kaplan-Meier plots of OS in subgroups stratified by baseline plasma levels of IL-6, IL-8, sTNFR-1, IL-12 (p40), and IFNγ. The cut-off values between the high and low groups were the median values. p-values (log-rank test) are shown. (B) Kaplan-Meier plots of OS in the subgroups stratified by changes in plasma IL-6, IL-8, sTNFR-1, IL-12 (p40), and IFNγ after treatment. Patients were divided into two groups: those with decreased levels and those with unchanged or increased levels after treatment. p-values (log-rank test) are shown. (C) Kaplan-Meier plots of PFS in subgroups stratified by plasma levels of IL-6, IL-8, sTNFR-1, IL-12 (p40), and IFNγ after treatment. The cut-off values between the high and low groups were the median values. p-values (log-rank test) are shown. Abbreviations: IL, interleukin; IFNγ, interferon γ; OS, overall survival; PFS, progression-free survival; sTNF-R1, soluble tumor necrosis factor receptor 1.
For PFS analysis, liver metastasis and platelet count were selected using the stepwise method and adapted to the multivariate model, with each mediator forced into this model (Table 3). Multivariate analysis revealed that 17 mediators [IL-2, IL-12 (p40), IL-29, IL-32, IL-35, IFNα2, IFNγ, CCL8, CCL15, CCL23, CXCL5, CXCL11, CXCL16, sCD30, LIGHT, TSLP, and MMP-2] in the baseline analysis were significantly associated with PFS.
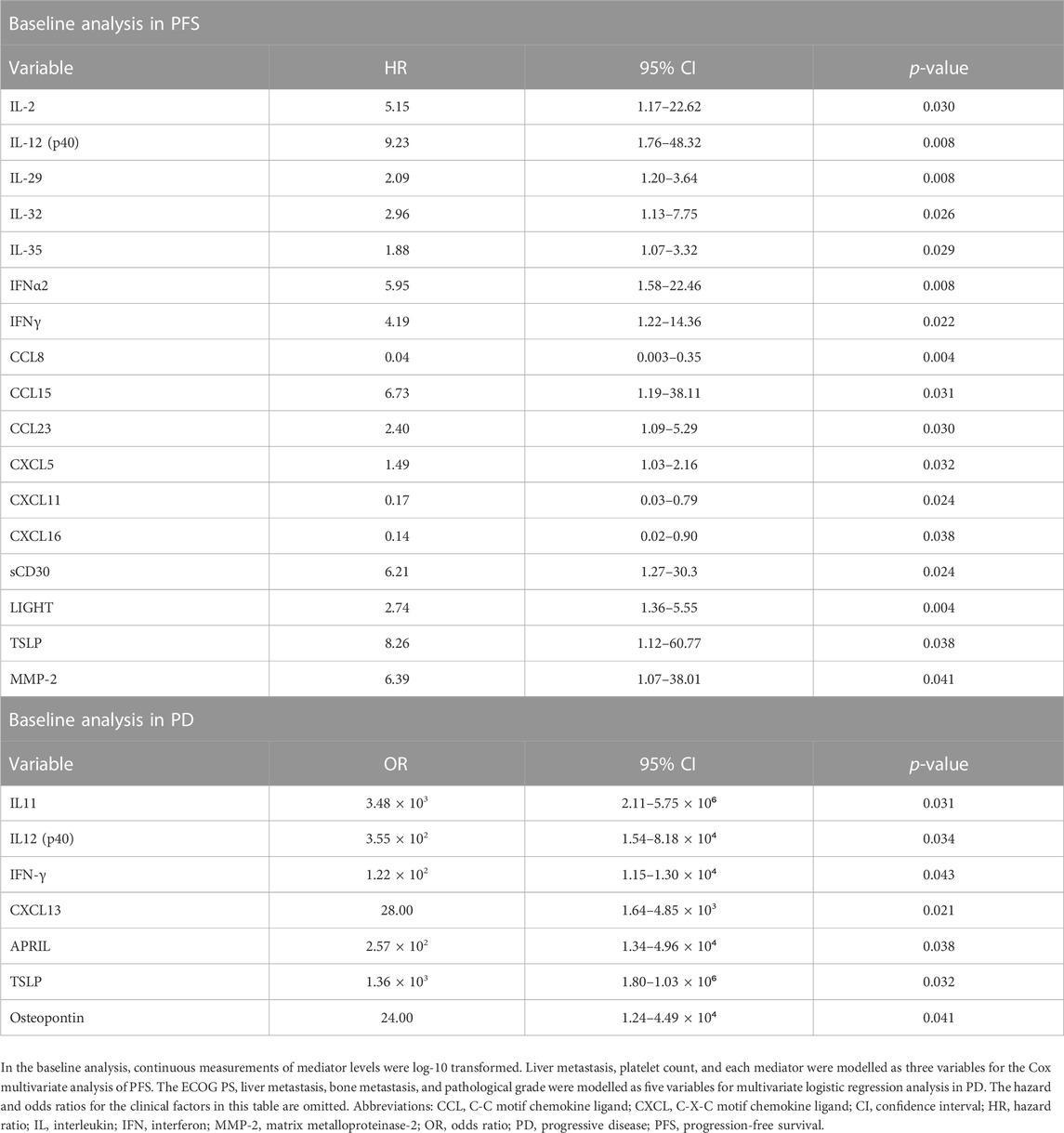
TABLE 3. Cox multivariate analysis of PFS and multivariate logistic regression analysis of progressive disease.
For PD analysis, ECOG PS, liver metastasis, bone metastasis, and pathological grade were selected using the stepwise method and adapted to the multivariate model; each mediator was forced into this model (Table 3). In multivariate analysis, seven mediators [IL-11, IL-12 (p40), IFNγ, CXCL13, APRIL, TSLP, and osteopontin] in the baseline analysis were significantly associated with PD.
Notably, high baseline levels of IL-12 (p40) and IFNγ, which showed a significant association with PD, were also significantly associated with worse PFS and OS (PD/PFS/OS; p = 0.034, 0.008, 0.038, and p = 0.043, 0.022, and 0.022, respectively). Kaplan-Meier analysis of OS and PFS for IL-12 (p40) and IFNγ was performed (Figure 1). In the baseline analysis, low expression levels of IL-12 (p40) and IFNγ were significantly associated with prolonged PFS (p = 0.010 and p = 0.018, respectively). In the baseline analysis, low IFNγ expression was significantly associated with prolonged OS (p = 0.031); however, no association was detected in the change analysis (p = 0.075).
IrAEs were documented in 32 patients (56.1%), with ≥ grade 3 noted in 12 patients (21.1%); however, there were no deaths attributed to adverse events (Table 4). For multivariate analysis, liver metastasis was selected using a stepwise method and included in the model. When each of the mediators at baseline was forced into the model, low IL-6 (OR 0.182, 95% CI 0.03–0.88, p = 0.034) and high TWEAK (OR 350, 95% CI 3.62–33900, p = 0.012) levels at baseline were associated with irAEs.
Using principal component analysis, we characterised identified mediators significantly associated with clinical outcomes, including OS, PFS, and irAEs. As shown in Figure 2A, these mediators were broadly classified into three groups. The first group contained five mediators, including IL-22, IL-27, IL-29, IL-35, and LIGHT, whereas the second group contained 11 factors, including IL-16, CCL1, CCL8, CCL22, CXCL1, CXCL5, CXCL11, IL-6Rα, TWEAK, osteocalcin, and MMP-2. The third group comprised 26 factors: IL-2, IL-6, IL-8, IL-11, IL-12 (p40), IL-28A, IL-32, IFNα2, IFNγ, CCL3, CCL7, CCL15, CCL20, CCL21, CCL23, CXCL6, CXCL13, CXCL16, sCD30, sCD163, sTNF-R1, sTNF-R2, APRIL, TSLP, osteopontin, and MMP-1. Notably, most mediators listed in the third group were highly correlated with each other (r > 0.5, p < 0.05, Spearman’s rank correlation coefficient analysis), as shown in Figure 2B.

FIGURE 2. Classification of the plasma immune mediators. (A) The plasma immune mediators significantly associated with clinical outcomes, including OS, PFS, PD, and irAEs, were classified into three groups by principal component analysis. (B) The correlation among the plasma immune mediators significantly associated with clinical outcomes was examined using Spearman’s rank correlation coefficient analysis. Strong correlations (r > 0.5, p < 0.05; 118 pairs) were visualised using the weighted network, with the breadth of the line proportional to the correlation coefficient (from 0.50 to 0.84).
In patients with aUC who fail to respond to ICI as second-line therapy, the OS was found to be 3.1 months, and only one-third of these patients can proceed to third-line therapy after ICI treatment failure (Gómez de Liaño Lista et al., 2020) mainly because of the rapid aUC progression and/or physical exhaustion caused by preceding treatments. Accordingly, several patients with aUC without indication of third-line treatment are compelled to undergo the best supportive care (BSC). Notably, the OS of patients who received third-line therapy after ICI treatment was 8.3 months, whereas that of patients with BSC was markedly brief, limited to approximately 1.5 months (Gómez de Liaño Lista et al., 2020). In addition, enfortumabu vedotin, acituzumab govitecan, and erdafitinib were recently approved as a promising therapy after ICI failure for aUC (Tagawa et al., 2021; Yu et al., 2021; Siefker-Radtke et al., 2022). Therefore, the development of valuable biomarkers for predicting the effects of ICI and the occurrence of irAEs before or during the early stages of treatment is urgently needed.
The Food and Drug Administration has approved PD-L1 expression, microsatellite instability/mismatch repair deficiency, and tumor mutational burden as predictive biomarkers of ICI (Twomey and Zhang, 2021). Although PD-L1 expression might be a predictive biomarker for ICI based on the findings of some previous trials on UC, the methods and results of PD-L1 assessment in each trial were different and inconsistent across trials. Integrated biomarkers that combine multiple factors have been shown to afford better results than a single Food and Drug Administration-approved predictive biomarker (Wang et al., 2021). Based on these results, a multifaceted approach may be necessary to precisely predict ICI efficacy. Although limited UC studies are available, comprehensive measurement of soluble immune mediators in plasma may be a promising strategy (Lim and Yoon, 2021).
In the present study, multivariate models demonstrated that baseline levels or post-treatment changes of several different immune mediators in plasma were significantly associated with OS and/or PFS in patients with aUC receiving pembrolizumab therapy. The principal component analysis demonstrated that these mediators, which were significantly associated with clinical outcomes, could be broadly classified into three groups. Interestingly, mediators that correlated with OS were mainly classified in the third group, whereas those correlated with PFS were more frequent in the second group. In addition, several examined factors were highly correlated with each other, although the precise mechanisms responsible for such a correlation remain unknown. Given that plasma immune mediators with distinct functions displayed similar expression patterns, it can be suggested that multiple factors, rather than a single factor, might affect the immunological tumor microenvironment concurrently and could be associated with clinical outcomes in patients treated with ICI. In-depth investigations assessing the functions and interactions of these mediators in ICI treatment would potentially reveal a more accurate prediction of ICI efficacy and aid in developing new treatment approaches.
It should be noted that both high baseline levels and elevated post-treatment levels of IL-6, IL-8, and sTNFR-1 were significantly associated with worse OS. IL-6 is involved in immunosuppression via various mechanisms. For example, IL-6-mediated STAT3 activation promotes the production of immunosuppressive molecules, such as vascular endothelial growth factor and arginase, and induces immunosuppressive cells, such as myeloid-derived suppressor cells (MDSCs) (Zhou et al., 2017), which reportedly serve as biomarkers of OS in patients with melanoma treated with ICI (Meyer et al., 2014). In addition, IL-6 limits anti-tumor T-cell responses by promoting the differentiation of M2 macrophages or acting on dendritic cells to inhibit the expression of MHC-II, CD80/86, and IL-12 and promote IL-10 production (Zhou et al., 2017). Furthermore, IL-6 activates MMP and forms desmoplastic tumor stroma (Dechow et al., 2004), which acts as a barrier to avoid recognition by the immune system and subsequent T-cell infiltration (Salmon and Donnadieu, 2012). Although higher IL-6 levels are reportedly associated with worse OS and PFS in patients with melanoma and non-small-cell lung carcinoma undergoing ICI (Keegan et al., 2020; Laino et al., 2020), reports on patients with UC are lacking. IL-8, a proinflammatory chemokine, inhibits anti-tumor immune responses by inducing MDSCs in the tumor microenvironment and expanding the desmoplastic tumor stroma, similar to IL-6 (Alfaro et al., 2016). In addition, IL-8 promotes an angiogenic response that leads to cancer metastasis and proliferation (David et al., 2016). Similar to our findings, higher plasma IL-8 levels have been associated with lower efficacy of anti-PD-L1 antibody (Yuen et al., 2020) in patients with UC. sTNFR-1 competes with the transmembrane form by binding to circulating TNFα, thereby inhibiting its action. Although TNFα is a pleiotropic cytokine with contradictory roles in oncoimmunology (Mercogliano et al., 2021), sTNFR-1-mediated inhibition of TNFα might suppress the anti-tumor activity of ICI in patients with UC. In addition, sTNFR-1 can reportedly increase IL-6 mRNA expression and stimulate IL-6 release (Wang and Bjorling, 2011), which may clarify the high correlation between plasma sTNFR-1 and IL-6 levels in the present study.
Herein, high baseline IL-12 (p40) and IFNγ levels were significantly associated with PD and worse OS and PFS, suggesting their importance as predictive markers. IFNγ is a well-known cytokine with anti-tumor immune effects, including activation and proliferation of tumor-infiltrating lymphocytes (Ikeda et al., 2002). Increased IFNγ levels after ICI treatment were strongly associated with better patient response and survival in non-small cell lung cancer (Boutsikou et al., 2018). In contrast, IFNγ has pro-tumor immune effects and plays a dual role in the tumor microenvironment. IFNγ promotes the expression of immunosuppressive molecules, such as indoleamine-2,3-dioxygenase 1 (IDO1), PD-L1, PD-L2, inducible nitric oxide synthase, Fas, and Fas ligand (Gocher et al., 2022). Furthermore, prolonged exposure to IFNγ signalling, associated with tumor growth, has been shown to promote these immunosuppressive molecules, resulting in resistance to ICI therapy (Benci et al., 2016). In the present study, the negative correlation between high IFNγ expression and OS and ICI response may be attributed to the pro-tumor immune effects of the IFNγ pathway. IL-12 (p40) is a cytokine and subunit of heterodimer IL-23. Both IL-12 (p40) and IL-23 are pro-tumor cytokines. IL-12 (p40) helps cancer cells escape cell death by suppressing the IFN-γ pathway and inhibiting cytotoxic T-cell infiltration (Kundu et al., 2017). IL-23 promotes IL-17 production and, like IL-6, activates STAT3 signalling. IL-17 promotes tumor angiogenesis and induces immunosuppressive molecules, such as tumor-associated macrophages and MDSCs, in the tumor microenvironment (Mirlekar and Pylayeva-Gupta, 2021). In renal cell carcinoma, IL-23 blockade augments the therapeutic effect of the anti-PD-1 antibody (Fu et al., 2019). The present study suggests the importance of the IFNγ pathway in ICI therapy, and further studies could identify potential strategies to enhance ICI treatment by inhibiting the pro-tumor effects of IFNγ and inducing its anti-tumor effects.
Changes in the post-treatment levels of IL-16, IL-22, IL-27, IL-28A, CCL21, CCL22, CXCL16, and osteocalcin were significantly associated with OS, similar to those of IL-6, IL-8, IL-12 (p40), and sTNF-R1. Elevated post-treatment levels of IL-27, CCL21, CCL22, and osteocalcin were associated with a favorable OS. These mediators have been reported to promote antitumor immune activity in the tumor microenvironment, and multiple studies have demonstrated their association with ICI therapy. For example, IL-27 directly stimulates CD8+ T cell differentiation and enhances antitumor cytotoxic T lymphocyte responses (Liu et al., 2013). An increase in IL-27 expression has been reported with anti-PD-L1 antibody treatment, leading to the suppression of tumor growth (Wenthe et al., 2022). CCL21 promotes the infiltration of T cells and dendritic cells into tumors, thereby enhancing immune activity (Sharma et al., 2020), and the synergistic tumor-suppressive effects of the combination of CCL21 and ICI in lung cancer and pancreatic cancer mouse models (Salehi-Rad et al., 2017; Chen et al., 2021). High expression levels of CCL22 and CCL17 are positively correlated with CD4+ T cell infiltration and a favorable prognosis with ICI treatment in patients with head and neck carcinoma (Zhou et al., 2022). Osteocalcin promotes T cell proliferation and IFNγ production, inhibiting tumor cell growth (Hayashi et al., 2017), and high expression levels of osteocalcin were associated with favorable OS in patients with head and neck carcinoma treated with the anti-PD-L1 antibody durvalumab (Arends et al., 2021). In contrast, elevated posttreatment levels of IL-16, IL-22, IL-28A, and CXCL16 were associated with poor OS. IL-22 has been identified as a poor prognostic factor in bladder cancer (Zeng et al., 2020). In addition, IL-22 has been shown to promote PD-L1 expression through the activation of STAT3 (Wolk et al., 2010; Chen et al., 2019), and the anti-PD-L1 antibody nivolumab has been reported to be effective in bladder tumors with a high density of intratumoral IL22-producing cells (Zeng et al., 2020). However, in this study, elevated post-treatment levels of IL-22 were associated with poor OS in the change analysis, suggesting that the function and immune evasion mechanisms of IL-22 differ between locations (intratumoral vs. peripheral), although further research is required. Furthermore, since no reports linking IL-16, IL-28A, and CXCL16 to ICIs have been found, further studies are needed.
Herein, low IL-6 and high TWEAK levels at baseline were significantly associated with irAEs. The mechanisms of irAEs remain poorly elucidated. However, irAEs have been associated with high plasma levels of proinflammatory cytokines in patients with melanoma patients (Lim et al., 2019). For example, high levels of IL-6, a typical inflammatory cytokine, are considered a risk factor for irAE, and tocilizumab, an anti-IL-6 receptor monoclonal antibody, can reportedly prevent irAEs in patients with melanoma undergoing ICI therapy (Dimitriou et al., 2021). However, the current study revealed that high IL-6 levels were negatively associated with irAEs. Given that there was an incubation period before the onset of irAEs and patients with UC exhibiting high IL6 levels had a markedly poor prognosis, disease progression may have occurred rapidly before the development of irAE. TWEAK signalling has been reported to activate inflammation and promote tumor invasion and growth (Winkles, 2008). Although it is speculated that TWEAK-mediated inflammation may be associated with the development of irAEs, no available reports have demonstrated an association between TWEAK levels and irAEs.
This study comprehensively and quantitatively surveyed soluble immune mediators in the plasma of aUC patients undergoing ICI treatment, and demonstrated associations of various immune mediators with immunotherapy response, and irAEs. These results indicate that interactions between multiple mediators may affect the tumor’s immunological microenvironment. However, this study had several limitations. First, the sample size was relatively small, which restricted the number of clinical factors included in the multivariate analysis and might limit the generalizability of the findings. Second, the uneven distribution of primary cancer sites and histologic types as well as the lack of standardized chemotherapy regimens prior to pembrolizumab treatment could potentially introduce bias. Third, the median follow-up duration was relatively short, mainly because of the rapid progression of aUC. Fourth, although many immune mediators have been shown to be associated with clinical outcomes, the clinical significance and predictive value of each mediator remain to be addressed in detail. Therefore, further large-scale studies with longer follow-up periods are warranted to provide a more comprehensive understanding of the patient outcomes. If further studies could establish immune mediators as predictive biomarkers of ICI response and irAEs, measurement of soluble immune mediators in plasma will be a promising strategy to guide treatment decisions and provide meaningful prognostic information, given the ease of monitoring over time and the reduced invasiveness that blood sampling offers.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of Kanagawa Cancer Center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
YoS: Data curation, Formal Analysis, Methodology, Writing–original draft. TKi: Supervision, Writing–review and editing. TKo: Investigation, Writing–review and editing. FW: Investigation, Writing–review and editing. YI: Investigation, Writing–review and editing. HH: Investigation, Writing–review and editing. TN: Investigation, Writing–review and editing. MK: Investigation, Writing–review and editing. TaS: Investigation, Writing–review and editing. KO: Investigation, Writing–review and editing. YuS: Formal Analysis, Methodology, Writing–review and editing. TeS: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Supervision, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by AMED (Grant Number JP19ae0101076) and Kanagawa Cancer Center Hospital-Research Institute Joint Study (Grant Number 2020-14).
We thank Kahori Shouji, Megumi Abe, Akiko Orikasa, and Makoto Wakatsuki (Kanagawa Cancer Center Research Institute) for sample handling and data acquisition. We thank Editage (www.editage.com) for English language editing.
TeS received personal fees from Bristol Myers Squibb and Chugai Pharmaceutical Co., and grants from BrightPath Biotherapeutics Co., and Taiho Pharmaceutical Co.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
aUC, advanced urothelial carcinoma; CCL, C-C motif chemokine ligand; CR, complete response; CXCL, C-X-C motif chemokine ligand; ECOG PS, Eastern Cooperative Oncology Group performance status; IFN, interferon; IL, interleukin; irAE, immune-related adverse event; MDSCs, myeloid-derived suppressor cells; MIF, macrophage migration inhibitory factor; MMP, matrix metalloproteinase; OS, overall survival; PD, progressive disease; PD-1, programmed cell death protein 1; PD-L1, programmed death ligand 1; PFS, progression-free survival; PR, partial response; SD, stable disease; sTNF-R, soluble tumor necrosis factor receptor; TNF, tumor necrosis factor.
Alfaro, C., Teijeira, A., Oñate, C., Pérez, G., Sanmamed, M. F., Andueza, M. P., et al. (2016). Tumor-produced interleukin-8 attracts human myeloid-derived suppressor cells and elicits extrusion of neutrophil extracellular traps (NETs). Clin. Cancer Res. 22 (15), 3924–3936. doi:10.1158/1078-0432.Ccr-15-2463
Arends, R., Guo, X., Baverel, P. G., González-García, I., Xie, J., Morsli, N., et al. (2021). Association of circulating protein biomarkers with clinical outcomes of durvalumab in head and neck squamous cell carcinoma. Oncoimmunology 10 (1), 1898104. doi:10.1080/2162402x.2021.1898104
Bellmunt, J., de Wit, R., Vaughn, D. J., Fradet, Y., Lee, J. L., Fong, L., et al. (2017). Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 376 (11), 1015–1026. doi:10.1056/NEJMoa1613683
Benci, J. L., Xu, B., Qiu, Y., Wu, T. J., Dada, H., Twyman-Saint Victor, C., et al. (2016). Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell 167 (6), 1540–1554. doi:10.1016/j.cell.2016.11.022
Boutsikou, E., Domvri, K., Hardavella, G., Tsiouda, D., Zarogoulidis, K., and Kontakiotis, T. (2018). Tumour necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: a pragmatic approach in clinical practice. Ther. Adv. Med. Oncol. 10, 1758835918768238. doi:10.1177/1758835918768238
Chen, Q., Yin, H., Pu, N., Zhang, J., Zhao, G., Lou, W., et al. (2021). Chemokine C-C motif ligand 21 synergized with programmed death-ligand 1 blockade restrains tumor growth. Cancer Sci. 112 (11), 4457–4469. doi:10.1111/cas.15110
Chen, S., Crabill, G. A., Pritchard, T. S., McMiller, T. L., Wei, P., Pardoll, D. M., et al. (2019). Mechanisms regulating PD-L1 expression on tumor and immune cells. J. Immunother. Cancer 7 (1), 305. doi:10.1186/s40425-019-0770-2
David, J. M., Dominguez, C., Hamilton, D. H., and Palena, C. (2016). The IL-8/IL-8R Axis: a double agent in tumor immune resistance. Vaccines (Basel) 4 (3), 22. doi:10.3390/vaccines4030022
Dechow, T. N., Pedranzini, L., Leitch, A., Leslie, K., Gerald, W. L., Linkov, I., et al. (2004). Requirement of matrix metalloproteinase-9 for the transformation of human mammary epithelial cells by Stat3-C. Proc. Natl. Acad. Sci. U. S. A. 101 (29), 10602–10607. doi:10.1073/pnas.0404100101
Dimitriou, F., Hogan, S., Menzies, A. M., Dummer, R., and Long, G. V. (2021). Interleukin-6 blockade for prophylaxis and management of immune-related adverse events in cancer immunotherapy. Eur. J. Cancer 157, 214–224. doi:10.1016/j.ejca.2021.08.031
Fu, Q., Xu, L., Wang, Y., Jiang, Q., Liu, Z., Zhang, J., et al. (2019). Tumor-associated macrophage-derived interleukin-23 interlinks kidney cancer glutamine addiction with immune evasion. Eur. Urol. 75 (5), 752–763. doi:10.1016/j.eururo.2018.09.030
Gocher, A. M., Workman, C. J., and Vignali, D. A. A. (2022). Interferon-γ: teammate or opponent in the tumour microenvironment? Nat. Rev. Immunol. 22 (3), 158–172. doi:10.1038/s41577-021-00566-3
Gómez de Liaño Lista, A., van Dijk, N., de Velasco Oria de Rueda, G., Necchi, A., Lavaud, P., Morales-Barrera, R., et al. (2020). Clinical outcome after progressing to frontline and second-line Anti-PD-1/PD-L1 in advanced urothelial cancer. Eur. Urol. 77 (2), 269–276. doi:10.1016/j.eururo.2019.10.004
Hayashi, Y., Kawakubo-Yasukochi, T., Mizokami, A., Hazekawa, M., Yakura, T., Naito, M., et al. (2017). Uncarboxylated osteocalcin induces antitumor immunity against mouse melanoma cell growth. J. Cancer 8 (13), 2478–2486. doi:10.7150/jca.18648
Ikeda, H., Old, L. J., and Schreiber, R. D. (2002). The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 13 (2), 95–109. doi:10.1016/s1359-6101(01)00038-7
Keegan, A., Ricciuti, B., Garden, P., Cohen, L., Nishihara, R., Adeni, A., et al. (2020). Plasma IL-6 changes correlate to PD-1 inhibitor responses in NSCLC. J. Immunother. Cancer 8 (2), e000678. doi:10.1136/jitc-2020-000678
Kundu, M., Roy, A., and Pahan, K. (2017). Selective neutralization of IL-12 p40 monomer induces death in prostate cancer cells via IL-12-IFN-γ. Proc. Natl. Acad. Sci. U. S. A. 114 (43), 11482–11487. doi:10.1073/pnas.1705536114
Laino, A. S., Woods, D., Vassallo, M., Qian, X., Tang, H., Wind-Rotolo, M., et al. (2020). Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J. Immunother. Cancer 8 (1), e000842. doi:10.1136/jitc-2020-000842
Lim, J. U., and Yoon, H. K. (2021). Potential predictive value of change in inflammatory cytokines levels subsequent to initiation of immune checkpoint inhibitor in patients with advanced non-small cell lung cancer. Cytokine 138, 155363. doi:10.1016/j.cyto.2020.155363
Lim, S. Y., Lee, J. H., Gide, T. N., Menzies, A. M., Guminski, A., Carlino, M. S., et al. (2019). Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1-based immunotherapy. Clin. Cancer Res. 25 (5), 1557–1563. doi:10.1158/1078-0432.Ccr-18-2795
Liu, Z., Liu, J. Q., Talebian, F., Wu, L. C., Li, S., and Bai, X. F. (2013). IL-27 enhances the survival of tumor antigen-specific CD8+ T cells and programs them into IL-10-producing, memory precursor-like effector cells. Eur. J. Immunol. 43 (2), 468–479. doi:10.1002/eji.201242930
Mercogliano, M. F., Bruni, S., Mauro, F., Elizalde, P. V., and Schillaci, R. (2021). Harnessing tumor necrosis factor alpha to achieve effective cancer immunotherapy. Cancers (Basel) 13 (3), 564. doi:10.3390/cancers13030564
Meyer, C., Cagnon, L., Costa-Nunes, C. M., Baumgaertner, P., Montandon, N., Leyvraz, L., et al. (2014). Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol. Immunother. 63 (3), 247–257. doi:10.1007/s00262-013-1508-5
Mirlekar, B., and Pylayeva-Gupta, Y. (2021). IL-12 family cytokines in cancer and immunotherapy. Cancers (Basel) 13 (2), 167. doi:10.3390/cancers13020167
Powles, T., Park, S. H., Voog, E., Caserta, C., Valderrama, B. P., Gurney, H., et al. (2020). Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 383 (13), 1218–1230. doi:10.1056/NEJMoa2002788
Salehi-Rad, R., Walser, T., So, S., Park, S., Sharma, L., Jay, S. D., et al. (2017). CCL21 combined with PD-1 blockade cooperatively inhibits tumor growth in KRAS murine model of NSCLC. J. Thorac. Oncol. 12, S1537. doi:10.1016/j.jtho.2017.06.026
Salmon, H., and Donnadieu, E. (2012). Within tumors, interactions between T cells and tumor cells are impeded by the extracellular matrix. Oncoimmunology 1 (6), 992–994. doi:10.4161/onci.20239
Sharma, S., Kadam, P., and Dubinett, S. (2020). CCL21 programs immune activity in tumor microenvironment. Adv. Exp. Med. Biol. 1231, 67–78. doi:10.1007/978-3-030-36667-4_7
Siefker-Radtke, A. O., Necchi, A., Park, S. H., García-Donas, J., Huddart, R. A., Burgess, E. F., et al. (2022). Efficacy and safety of erdafitinib in patients with locally advanced or metastatic urothelial carcinoma: long-term follow-up of a phase 2 study. Lancet Oncol. 23 (2), 248–258. doi:10.1016/s1470-2045(21)00660-4
Tagawa, S. T., Balar, A. V., Petrylak, D. P., Kalebasty, A. R., Loriot, Y., Fléchon, A., et al. (2021). TROPHY-U-01: a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J. Clin. Oncol. 39 (22), 2474–2485. doi:10.1200/jco.20.03489
Twomey, J. D., and Zhang, B. (2021). Cancer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. Aaps J. 23 (2), 39. doi:10.1208/s12248-021-00574-0
Wang, Y., Tong, Z., Zhang, W., Zhang, W., Buzdin, A., Mu, X., et al. (2021). FDA-approved and emerging next generation predictive biomarkers for immune checkpoint inhibitors in cancer patients. Front. Oncol. 11, 683419. doi:10.3389/fonc.2021.683419
Wang, Z. Y., and Bjorling, D. E. (2011). Tumor necrosis factor-α induces expression and release of interleukin-6 by human urothelial cells. Inflamm. Res. 60 (6), 525–532. doi:10.1007/s00011-010-0298-x
Wenthe, J., Naseri, S., Hellström, A. C., Moreno, R., Ullenhag, G., Alemany, R., et al. (2022). Immune priming using DC- and T cell-targeting gene therapy sensitizes both treated and distant B16 tumors to checkpoint inhibition. Mol. Ther. Oncolytics 24, 429–442. doi:10.1016/j.omto.2022.01.003
Winkles, J. A. (2008). The TWEAK-Fn14 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nat. Rev. Drug Discov. 7 (5), 411–425. doi:10.1038/nrd2488
Wolk, K., Witte, E., Witte, K., Warszawska, K., and Sabat, R. (2010). Biology of interleukin-22. Semin. Immunopathol. 32 (1), 17–31. doi:10.1007/s00281-009-0188-x
Yanagisawa, T., Mori, K., Katayama, S., Mostafaei, H., Quhal, F., Laukhtina, E., et al. (2022). Pretreatment clinical and hematologic prognostic factors of metastatic urothelial carcinoma treated with pembrolizumab: a systematic review and meta-analysis. Int. J. Clin. Oncol. 27 (1), 59–71. doi:10.1007/s10147-021-02061-0
Yu, E. Y., Petrylak, D. P., O'Donnell, P. H., Lee, J. L., van der Heijden, M. S., Loriot, Y., et al. (2021). Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 22 (6), 872–882. doi:10.1016/s1470-2045(21)00094-2
Yuen, K. C., Liu, L. F., Gupta, V., Madireddi, S., Keerthivasan, S., Li, C., et al. (2020). High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat. Med. 26 (5), 693–698. doi:10.1038/s41591-020-0860-1
Zeng, H., Liu, Z., Wang, Z., Zhou, Q., Qi, Y., Chen, Y., et al. (2020). Intratumoral IL22-producing cells define immunoevasive subtype muscle-invasive bladder cancer with poor prognosis and superior nivolumab responses. Int. J. Cancer 146 (2), 542–552. doi:10.1002/ijc.32715
Zhou, J., Qu, Z., Sun, F., Han, L., Li, L., Yan, S., et al. (2017). Myeloid STAT3 promotes lung tumorigenesis by transforming tumor immunosurveillance into tumor-promoting inflammation. Cancer Immunol. Res. 5 (3), 257–268. doi:10.1158/2326-6066.Cir-16-0073
Zhou, W., Zhang, X., Feng, Y., Zhang, Y., and Liu, Z. (2022). The CC ligand chemokine family members CCL17/CCL22 predict the survival and response to immune checkpoint blockade therapy of patients with head and neck squamous cell carcinoma. Curr. Probl. Cancer 46 (6), 100896. doi:10.1016/j.currproblcancer.2022.100896
Keywords: immune checkpoint inhibitors, anti-PD-1 antibody, pembrolizumab, cytokine, chemokine, immune-related adverse event
Citation: Shibata Y, Kishida T, Kouro T, Wei F, Igarashi Y, Himuro H, Noguchi T, Koizumi M, Suzuki T, Osaka K, Saigusa Y and Sasada T (2023) Immune mediators as predictive biomarkers for anti-PD-1 antibody therapy in urothelial carcinoma. Front. Pharmacol. 14:1269935. doi: 10.3389/fphar.2023.1269935
Received: 31 July 2023; Accepted: 30 October 2023;
Published: 13 November 2023.
Edited by:
Vladimir Brusic, The University of Nottingham Ningbo, ChinaReviewed by:
Jifa Zhang, Sutro Biopharma, Inc., United StatesCopyright © 2023 Shibata, Kishida, Kouro, Wei, Igarashi, Himuro, Noguchi, Koizumi, Suzuki, Osaka, Saigusa and Sasada. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yosuke Shibata, eS5zaGliYS4zMjlAZ21haWwuY29t; Tetsuro Sasada, dHNhc2FkYUBrY2NoLmpw; Takeshi Kishida, a2lzaGlkYXRAa2NjaC5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.