
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 12 January 2024
Sec. Ethnopharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1268000
Background: The complexity of Chinese medicine treatment for Alzheimer’s disease (AD) utilizing a multi-herb therapy makes the evidence in current studies insufficient. Herb pairs are the most fundamental form of multi-herb formulae. Among the Chinese herbal formulas for AD treatment, Polygala tenuifolia (PT) and Acorus tatarinowii (AT) appeared as the most commonly used herbal pairs in combination.
Objective: The aim of this study is to evaluate the clinical efficacy and safety of the combination of PT and AT in the treatment of AD.
Methods: We systematically searched and screened randomized controlled trials of pairing PT and AT for the treatment of AD patients in eight databases with a search deadline of June 26, 2023. Authors, year of publication, title, and basic information such as subject characteristics (age, sex, and race), course of disease, control interventions, dose, and treatment duration were extracted from the screened studies. Primary outcomes assessed included mini-mental state examination (MMSE), activities of daily living (ADL), and AD assessment scale-cognitive subscale (ADAS-cog), while secondary outcomes included efficiency and adverse events. The quality of the included studies was assessed using the Cochrane risk of bias tool. The mean difference with 95% confidence intervals (MD [95% CI]) and risk ratio (RR) was selected as the effect size, and the data were analyzed and evaluated using RevMan 5.4 and Stata 16.
Results: A total of sixteen eligible and relevant studies involving 1103 AD participants were included. The combination of PT and AT plus conventional drugs was superior to single conventional drugs in MMSE [MD = 2.57, 95%CI: (1.44, 3.69); p < 0.00001; I2 = 86%], ADL [MD = −3.19, 95%CI: (−4.29, −2.09); p < 0.00001; I2 = 0%], and ADAS-cog scores [MD = −2.09, 95%CI: (−3.07, −1.10); p < 0.0001; I2 = 0%]. The combination of PT and AT plus conventional drugs had a significantly more favorable benefit in clinical effectiveness [RR = 1.27, 95%CI: (1.12, 1.44); p = 0.0002; I2 = 0%]. Adverse events were not increased with the combination of PT and AT plus conventional drugs compared to conventional drugs [RR = 0.65, 95%CI: (0.35, 1.19); p = 0.16; I2 = 0%]. The experimental group treated with the combination of PT and AT alone for AD was comparable in MMSE, ADL, and ADAS-cog scores compared with the control group treated with single conventional drugs.
Conclusion: Compared to single conventional drugs, the combination of PT and AT may be used as an alternative therapy to improve global cognition and functioning in AD, and the combination of PT and AT as adjunctive therapy appears to produce a better therapeutic response to AD in terms of efficacy without increasing the risk of adverse events. However, the very low to low quality of available evidence limits confidence in the findings.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42023444156.
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by memory loss, insidious episodes of cognitive deficits, and multiple dysfunctions such as aphasia, apraxia, agnosia, or executive decline, either individually or in combination (Dafre and Wasnik, 2023). The increasing aging of the global population and the increasing prevalence of AD have transformed AD into a major global medical, social, and economic dilemma (Passeri et al., 2022a). Currently, 50 million individuals are living with dementia globally, and this figure is projected to escalate to 152 million by 2050 (Scheltens et al., 2021).
Existing approved and marketed therapeutic strategies for AD include drugs such as donepezil, galantamine, carbapenems, and memantine, which belong to two families of anticholinesterase inhibitors and antiglutamatergic drugs (Balázs et al., 2021; Doroszkiewicz and Mroczko, 2022). These medications are used to limit disease progression, stabilize cognitive function, and attempt to improve the quality of life for patients with AD (Se Thoe et al., 2021; Srivastava et al., 2021). Nonetheless, current treatment options are only able to improve the clinical symptoms of AD patients for a limited period, and curative treatment is still not available (Plascencia-Villa and Perry, 2021). Studies have shown that none of the medications currently approved by the Food and Drug Administration (FDA) for AD treatment have positive effects on neuronal damage and brain atrophy or even progressive deterioration of cognition (Vaz and Silvestre, 2020). It is also worth noting that the latest monoclonal antibody approved by the FDA as an immunotherapy, aducanumab, has shown very limited improvement in cognitive function in AD, and its effectiveness remains highly controversial to date (Syed, 2020). Therefore, the search for new and effective treatments is a necessity.
Over thousands of years, the advantages of the diagnostic and therapeutic experience accumulated by Chinese medicine are extremely significant and have demonstrated an important role in the multi-target and multi-pathway treatment of AD (Deng et al., 2022; Ding et al., 2022; Arora et al., 2023). Polygala tenuifolia (PT) is a well-known traditional Chinese medicine, which has neuroprotective properties for treating amnesia and improving mental ability, with mechanisms of action involving anti-inflammatory, autophagic, anti-apoptotic, and anti-oxidative stress (Wang et al., 2021; Zhang et al., 2023). Acorus calamus (AT) is a traditional natural medicinal plant in the empirical system of Chinese medicine, and modern pharmacological studies have provided evidence of the pharmacological effects of its bioactive constituents, which can significantly improve brain diseases and neurological disorders such as Alzheimer’s disease (Zhang et al., 2022; Xu et al., 2023; Wang et al., 2023). The beneficial effects of the combination of PT and AT in ameliorating cognitive symptoms of AD through mechanisms such as the inhibition of inflammatory factors and modulation of intestinal flora have been demonstrated in preclinical studies (Xiong et al., 2022a; Jiao et al., 2022). Kaixin San (KXS), recorded in Sun Simiao’s “Prescriptions for Emergencies” in the Tang Dynasty, was used to treat dementia which has significant efficacy (Bo et al., 2022). In addition, the combination of PT and AT is frequently used in all medications and is a core prescription for the treatment of dementia (Yi et al., 2018).
Modern pharmacological studies have shown that the active constituents of PT and AT and their therapeutic mechanisms are complex. Polygala saponins are the major bioactive constituents of PT and have therapeutic potential in various neurological disorders. PTBP-1-3, a heteropolysaccharide isolated from PT, has an extremely potent inhibitory effect on neuroinflammation and may be one of the bioactive components in PT to improve cognitive function. It has been shown that tenuifolin, isolated from PT roots, has a significant improvement in cognitive function, preventing apoptosis, loss of mitochondrial membrane potential, and activation of caspases-3 in SH-SY5Y cells. It was found that PT may inhibit the accumulation of hyperphosphorylated tau through the 26S proteasome pathway to improve tau binding to microtubules, thereby improving cognitive performance. SCP-oil is the main active component of AT. SCP-oil promotes neuroprotection by reducing the activation of NLRP3 inflammatory vesicles through the inhibition of the NF-κB signaling pathway. ATP50-3, an active component of crude polysaccharide from AT, exerts anti-neuroinflammatory and neuroprotective effects by modulating the TLR4-mediated MyD88/NF-κB and PI3K/Akt signaling pathways.
In recent decades, TCM research has predominantly concentrated on cultivating potential candidates from Chinese medicinal herbs, with insufficient attention given to the judicious application of these traditional herbs. While multi-herb therapy stands as a pivotal characteristic of TCM, the contemporary endeavor to modernize this conventional wisdom encounters formidable challenges owing to its staggering complexity (Wang et al., 2012). Herb pairs, constituting the fundamental and simplest form of multi-herb formulations, serve as a central representative of Chinese herbal compatibility. TCM has multiple targets, and the efficacy and value of the combination of PT and AT, as a herb pair with unique advantages of TCM, have not yet been evaluated for its practical clinical application in the treatment of AD. We carried out a comprehensive systematic review and meta-analysis to explore the clinical efficacy and safety of combining PT and AT in the treatment of AD. This is the first systematic review and meta-analysis aimed to evaluate the global cognition and functioning effect and safety of the combination of PT and AT used alone or as adjunctive therapies in randomized controlled trials (RCTs) for the treatment of AD.
This study was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines and was registered in the International Prospective Register of Systematic Reviews (Number CRD42023444156).
Inclusion criteria: Types of studies: RCTs were included irrespective of blinding and language. Participants: Patients with AD, no restrictions on case origin, age, sex, or disease duration. Clear diagnostic criteria are proposed; Western medical diagnostic criteria meet one of the following diagnostic criteria: a) NIA-AA, b) NINCDS-ADRDA, c) DSM-Ⅴ, d) IWG-2, and e) ICD-10. A clear TCM syndrome type is proposed; TCM diagnostic criteria meet one of the following diagnostic criteria: a) Guidance principle of clinical study on new drugs of traditional Chinese medicine and b) Criteria for diagnosis, diagnostic typing, and efficacy assessment of senile dementia. Types of interventions: The intervention in the experimental group was any form of formula that included all types of PT and AT used in combination, either alone or in combination with conventional AD treatment with drugs. There were no restrictions on dosage, frequency of use, or formulations. Treatment duration was ≥ 12 weeks. Comparison: Interventions in the control group included no treatment, placebo, or conventional drugs for AD with the unrestricted mode of administration and dosage.
Exclusion criteria: Duplicate studies including reviews, guidelines, letters, conference abstracts, commentaries, case reports, and animal and cellular experiments; medical history of neurological, psychiatric, or other systemic disorders that may have an impact on cognitive function (e.g., depression, stroke, vascular dementia (VaD), and mild cognitive impairment); interventions combined with other TCM treatments such as acupuncture, moxibustion, auricular acupuncture, and physiotherapy; lack of quantitative data on outcome indicators and research with incomplete or unavailable data; and failure to provide study data for mean and SD or SE or CI.
Two authors (JZT and MQW) conducted independent searches of PubMed, Embase, Cochrane, the Web of Science, China National Knowledge Infrastructure (CNKI), the Wanfang Database, SINOMED, and the China Science and Technology Journal Database to screen and collect relevant studies published up to June 26, 2023.
The terms and search strategy were as follows: “(Alzheimer disease OR Alzheimer* OR dementia OR AD OR cogniti*) AND (Polygala OR Yuanzhi OR Yuan Zhi OR Polygala tenuifolia OR Polygala sibirica OR Polygala senega OR Seneca snakeroot OR Milkwort OR Polygala root OR Polygalae Radix OR Radix Polygalae) AND (Acorus OR Shichangpu OR Shi Chang Pu OR Acori Tatarinowii Rhizoma OR Acorus gramineus OR Acorus tatarinowii Schott OR Acorus tatarinowii OR Acorus calamus).” A manual search for all retrieved potentially relevant articles and references lists was carried out to find additional available articles. The complete search strategy used is described in Supplemental Material S2.
Two authors (JZT and MQW) independently assessed eligibility for inclusion by screening titles, abstracts, and full texts according to specified inclusion and exclusion criteria. Any disagreements were discussed to be resolved. In cases where consensus could not be reached, a third author (JS) was consulted. All reasons for exclusion were recorded.
Two authors (JNN and TL) independently extracted information using a pre-specified form of information extraction. Information to be derived from the studies obtained from the screening includes the lead authors of the paper, the year of publication, participants, age, sex, ethnicity, AD diagnostic criteria, inclusion criteria, specific interventions, treatment duration, outcome indicators, and occurrence of adverse events.
The primary outcomes assessed in this investigation were mini-mental state examination (MMSE), AD assessment scale-cognitive subscale (ADAS-cog), and activities of daily living (ADL).
The secondary outcomes assessed in this investigation were clinical treatment efficiency, TCM symptom score, and the incidence of adverse events.
Two authors (JNN and TL) independently assessed the risk of bias in eligible studies. The Cochrane risk of bias tool was used to assess randomization, allocation concealment, blinding of the intervention, completeness of outcome information, selective reporting, and other sources of bias, such as missed visits or dropout bias. Each item was categorized into three levels, “high risk,” “low risk,” and “unknown risk.” Items that were unclear in the study were further checked by contacting the corresponding author. Again, any disagreements were discussed with a third author (JS). When at least 10 studies were included in the quantitative analysis synthesis, funnel plots were constructed to assess publication bias among studies.
We chose to use Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) as a criterion for assessing the overall quality of evidence for the outcome indicators of the included trials, which was synthesized in terms of five dimensions: risk of bias, imprecision, inconsistency, indirectness, and publication bias (Schünemann et al., 2019). It was conducted independently by two authors (JZT and YCZ), with disagreements being resolved by a third author (JS).
We contacted the researchers or authors to obtain unavailable original outcome data when needed. When we did not receive a response, we used only the available data for analysis.
All meta-analyses were conducted statistically using RevMan 5.4 and Stata (version 16.0) software. We performed statistical analysis by calculating the MD and the corresponding 95% CI for continuous variable data as effect sizes and risk ratios for dichotomous variable outcomes.
For statistical heterogeneity, we used the I2 for testing and assessment. We decided to use a random-effects model for the analysis; or else, when statistical heterogeneity was not significant (p > 0.05, I2 ≤ 50%), we chose to use a fixed-effects model for the analysis. For continuous outcomes and dichotomous outcomes, we performed meta-analysis using the Mantel–Haenszel method and inverse variance method, respectively.
Possible sources of significant heterogeneity were assessed by subgroup analysis and further explored for factors with the greatest influence on high heterogeneity. We performed subgroup analyses of treatment measures and the duration of intervention. If sufficient data were available, the subgroup analysis would be carried out to explore any other effect that might explain any heterogeneity.
The presence of publication bias was assessed by the observation of the symmetry of the funnel plots, followed by Egger’s test for further statistics and validation (Sterne et al., 2011). Publication bias was assessed using a visual analysis of the funnel plots and Egger’s test when there were at least seven RCTs included in a meta-analysis.
Sensitivity analysis was used to examine if significant differences existed in individual studies or outliers that would markedly affect the robustness of overall study outcomes. Statistically significant differences were defined by a p-value of < 0.05, which applied to all test results in this study.
Following an initial comprehensive search, 2195 eligible articles were identified. Subsequently, 158 duplicates were removed, and 2564 articles were excluded after a meticulous review of titles and abstracts. Further detailed reading of the full text content of 193 articles led to the final inclusion of 16 articles. The study retrieval process is illustrated in Figure 1.
A total of 16 RCTs were eventually included in this meta-analysis, which involved 1103 AD participants (Table 1). Among all included studies, six trials compared the combination of PT and AT alone with drugs; five of the trials used donepezil, and one used piracetam for the control intervention drug. Ten trials compared the combination of PT and AT plus drugs with drugs, with nine trials using donepezil and one trial using memantine in combination with oxiracetam as the control intervention drug. Eight studies reported the inclusion of subjects with mild-to-moderate AD, and the remaining studies failed to report. Nine studies had a 3-month treatment duration, one study had a 4-month treatment duration, and six studies had a 6-month treatment duration (Table 2). Specific basic information on the herbal prescription ingredients reported in all included trials is provided in Supplemental Material S1.
A total of 16 studies were included for the risk of bias assessment. In the detailed assessment, 11 studies were assessed to be at low risk during randomization, and the remaining five studies were assessed to be at unknown risk because they were described as “randomized” without specifying the exact method of randomization. Regarding blinding of participants and personnel, all studies were assessed as high risk. One study suggested that the blinding method should be single-blind, and the allocation of the remaining studies was unclear in terms of concealment of allocation, the blinding method, and trial implementation. All studies were considered to have a low risk of bias in the domain of selective reporting. As for the incomplete outcome data, nine studies were assessed as having an unknown risk because they were not explicitly reported, and five studies were low risk. All studies did not explicitly mention detection bias and other bias and were assessed as having an unknown risk. The results of the risk of bias evaluation are shown in Figure 2 and Supplemental Material S3.
Sixteen studies were analyzed using MMSE as an evaluation metric, totaling 1103 subjects. The effects of the combination of PT and AT on AD were not significantly different in terms of MMSE when compared to conventional drug therapy [MD = 0.33, 95%CI: (−0.64, 1.31); p = 0.5; I2 = 42%]. We found a significant reduction in heterogeneity (42% vs. 8%) after excluding Chen’s study, which may be because piracetam was chosen as the intervention drug for the control group in this trial, whereas all the other studies used donepezil at the same dose and dosage. The combination of PT and AT plus control was more effective in improving MMSE compared to control [MD = 2.57, 95%CI: (1.44, 3.69); p < 0.00001; I2 = 86%] (Table 3; Supplemental Material S4, Supplementary Figure S1).
The results of the subgroup analyses indicated that treatment duration was not a source of significant heterogeneity. Compared with the control group, there was no significant difference in the effect of the combination of PT and AT on MMSE at a treatment duration of 3 months (MD = −0.28, 95%CI: [−0.98, 0.43]; p = 0.44; I2 = 0%), whereas the combination of PT and AT plus drugs was superior to the use of drugs alone (MD = 2.55, 95%CI: [0.96, 4.14]; p = 0.002; I2 = 92%). At a treatment duration of 6 months, the combination of PT and AT still appeared to show less effect on MMSE than the control group (MD = 1.72, 95%CI: [0.01, 3.42]; p = 0.05; I2 = 29%), while the combination of PT and AT plus drugs remained superior to the use of drugs alone (MD = 2.27, 95%CI: [0.81, 3.74]; p = 0.002; I2 = 49%) (Table 4; Supplemental Material S4, Supplementary Figures S5, 8).
A meta-analysis of seven randomized controlled trials including 502 patients showed no statistically significant differences in the effects of the combination of PT and AT on ADAS-cog compared to conventional drug in the control group (MD = −0.66, 95%CI: [−1.69, −0.37]; p = 0.21; I2 = 0%). The combination of PT and AT plus conventional drugs was more effective in improving ADAS-cog compared to Western drugs (MD = −2.09, 95%CI: [−3.07, −1.10]; p < 0.0001; I2 = 0%) (Table 3; Supplemental Material S4, Supplementary Figure S3).
Subgroup analyses of treatment duration showed that at 3 months of treatment, the combination of PT and AT was not as effective as conventional drug therapy for ADAS-cog [MD = −0.80, 95%CI: (−2.17, 0.58); p = 0.26; I2 = 0%]. At 6 months of treatment, the combination of PT and AT plus drugs was superior for ADAS-cog [MD = −1.98, 95%CI: (−3.12, −0.84); p = 0.0007; I2 = 0%] (Table 4; Supplemental Material S4, Supplementary Figures S7, 10).
Eight studies, involving 584 participants, used ADL scores as an evaluation metric. The results of meta-analysis using a fixed-effects model showed no statistically significant difference in the effect of the combination of PT and AT on ADL compared with conventional drug therapy [MD = −0.83, 95%CI: (−1.73, 0.08); p = 0.07; I2 = 0%]. The combination of PT and AT plus drugs showed significant beneficial effects on ADL scores compared to drugs [MD = −3.19, 95%CI: (−4.29, −2.09); p < 0.00001; I2 = 0%] (Table 3; Supplemental Material S4, Supplementary Figure S2).
The results of the subgroup analyses showed no significant difference in the therapeutic effect of the combination of PT and AT on ADL in AD patients compared with drugs at a treatment duration of 3 months [MD = −0.56, 95%CI: (−1.59, 0.46); p = 0.28; I2 = 0%]. At a treatment duration of 3 months, there was no significant difference in the benefits of the combination of PT and AT plus conventional drug therapy on ADL in AD patients compared with conventional drug therapy [MD = −2.38, 95%CI: (−5.34, 0.59); p = 0.12; I2 = 0%]. At a treatment duration of 6 months, the combination of PT and AT plus control treatment had a significant potentiating effect on ADLs in AD patients [MD = −3.14, 95%CI: (−4.36, −1.92); p < 0.00001; I2 = 0%] (Table 4; Supplemental Material S4, Supplementary Figures S6, 9).
Nine studies involving 601 subjects referred to the TCM symptom score, and the results of the random-effects model showed that the combination of PT and AT had a superior effect on the TCM symptom score [MD = −3.08, 95%CI: (−4.51, −1.64); p < 0.0001; I2 = 0%] compared to the control group treated with conventional drugs, while the combination of PT and AT plus conventional drugs also showed more beneficial effects [MD = −4.32, 95%CI: (−5.89, −2.75); p < 0.00001; I2 = 80%] (Table 3; Supplemental Material S4, Supplementary Figure S4).
Subgroup analyses of treatment duration showed that the combination of PT and AT plus conventional drugs in the control group improved the TCM symptom score significantly better than the control group, both at 3 months [MD = −4.38, 95%CI: (−6.16, −2.60); p < 0.00001; I2 = 71%] and 6 months [MD = −4.03, 95%CI: (−7.36, −0.69); p = 0.02; I2 = 91%] of treatment (Table 4, Supplemental Material S4, Supplementary Figure S11.
Only one study characterized the clinical effectiveness of the combination of PT and AT versus conventional drugs; therefore, meta-analysis could not be performed. Five studies reported the clinical effectiveness of the combination of PT and AT plus conventional drugs, involving a total of 401 subjects. The results showed that the combination of PT and AT plus conventional drugs had a significantly more favorable benefit in terms of clinical effectiveness [RR = 1.27, 95%CI: (1.12, 1.44); p = 0.0002; I2 = 0%] (Supplemental Material S4, Supplementary Figure S14).
Eight randomized controlled trials containing 604 subjects reported adverse events. Symptoms reported for non-serious adverse events included dizziness and nausea, and symptoms resolved on their own without intervention. No trials reported serious adverse events. The combination of PT and AT showed lower rates of adverse events compared to conventional drug therapy (8.1% vs. 25.8%), while the difference between the combination of PT and AT plus conventional drug therapy was not significant compared to conventional drug therapy (8.3% vs. 13%). Fixed-effects model analysis showed that the adverse event rates for the combination of PT and AT were significantly lower than those for conventional drugs therapy [RR = −0.33, 95%CI: (0.19, 0.60); p = 0.0003; I2 = 0%] (Supplemental Material S4, Supplementary Figure S12). Adverse events were not increased with the combination of PT and AT plus conventional drugs compared to conventional drugs [RR = 0.65, 95%CI: (0.35, 1.19); p = 0.16; I2 = 0%] (Supplemental Material S4, Supplementary Figure S13).
Sensitivity analyses showed that the quality of the included trials did not have an impact on the stability of the results, which remained stable and reliable (Supplemental Material S5, Supplementary Tables S3, 5). Due to the available data, we only assessed the publication bias of the combination of PT and AT on MMSE in AD patients (Zhang et al., 2022). Analysis results of the publication bias of the combination of PT and AT on MMSE by Egger’s test (p = 0.119) suggested no publication bias present, and the results of funnel plot assay were consistent with this (Figure 3, Supplemental Material S5; Supplementary Table S4). Visual inspection of the funnel plots showed some asymmetry (Figure 4), and when we assessed the combination of PT and AT plus control on MMSE with Egger’s test (p = 0.066), no publication bias was observed (Supplemental Material S5, Supplementary Table S6).
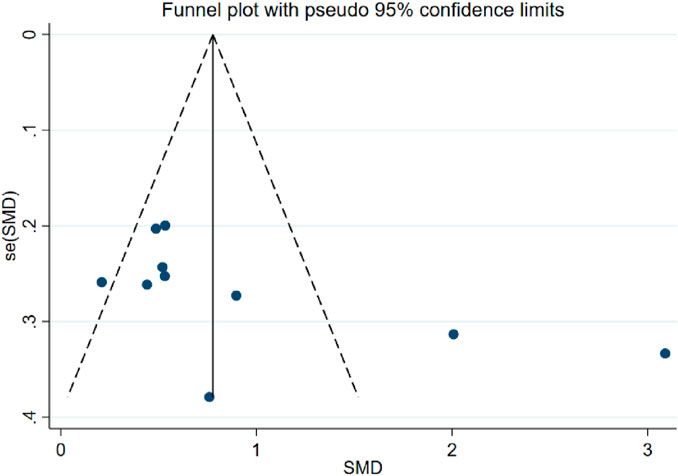
FIGURE 4. Forest plot of MMSE when compared with the combination of PT and AT plus control with control.
Very low-quality evidence suggested that the effects of the combination of PT and AT alone on MMSE, ADLs, and ADAS-cog in patients with AD are comparable to those of conventional drug therapy. Low-quality evidence suggested that the combination of PT and AT has a better effect on TCM symptom scores in AD patients than conventional drugs. Very low-quality evidence showed that the effects of the combination of PT and AT plus conventional drugs in MMSE and TCM symptom scores were comparable to conventional drug treatments. Evidence of low to moderate quality demonstrates that the combination of PT and AT plus conventional drugs is superior to conventional drugs on MMSE, ADL, and ADAS-cog in AD patients (Figure 5). Results of subgroup analyses based on the treatment duration of AD revealed that the quality of evidence for the outcomes of MMSE, ADL, ADAS-cog, and TCM was low or very low. Full details of the evidence summary are in Supplemental Material S6.
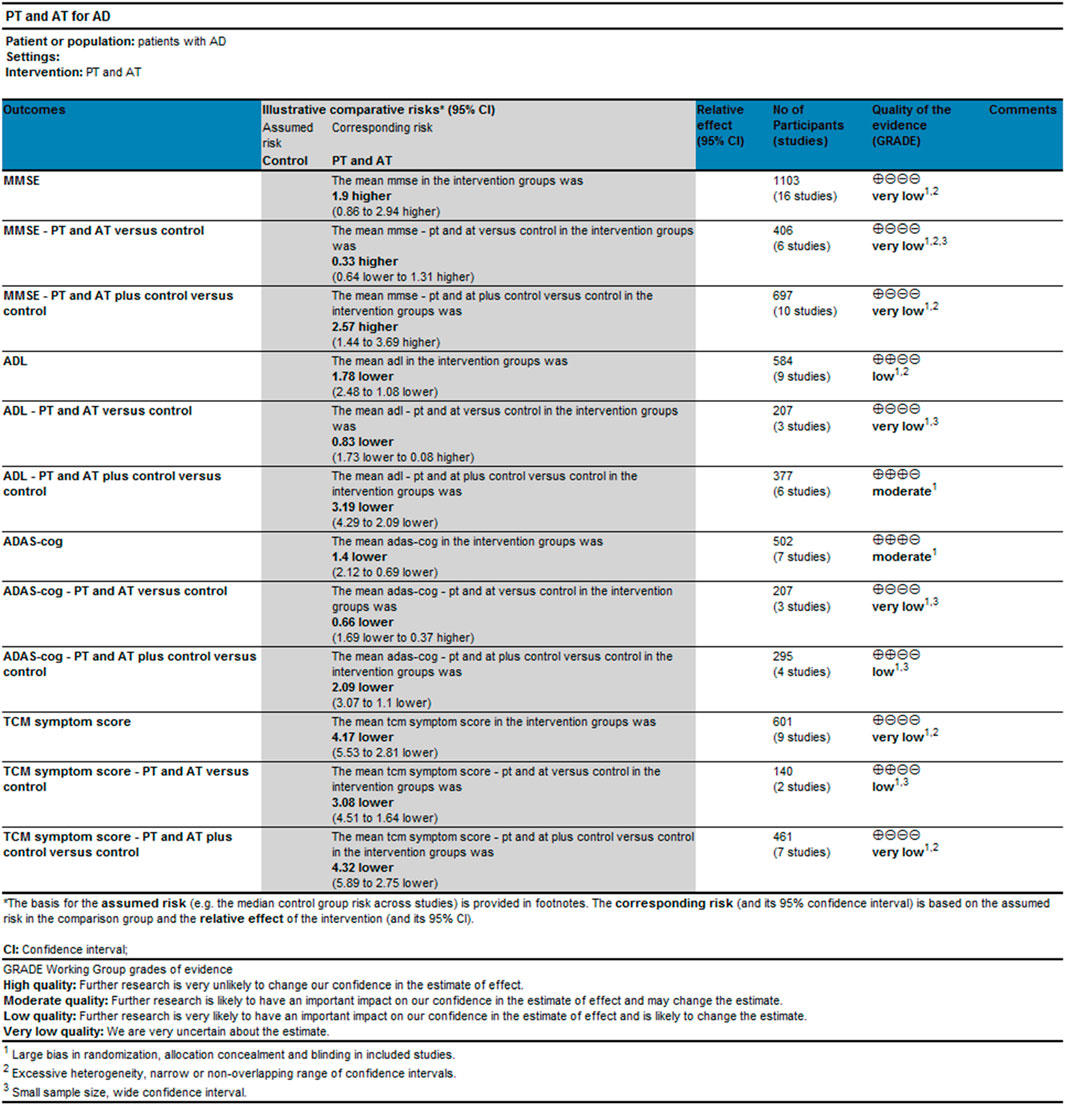
FIGURE 5. Results of evidence quality evaluation of GRADE for the combination of PT and AT discussion.
The pathomechanism of AD is complex, and numerous studies have been conducted but still have not led to drugs and targets that can effectively delay the progression of the disease (Ferrari and Sorbi, 2021; Passeri et al., 2022b). TCM formulas with PT as the principal drug have been repeatedly shown to improve cognitive symptoms in AD by consistently regulating phosphorylated tau (Qu et al., 2021; Li et al., 2023). AT has also been implicated in the therapeutic potential of AD by modulating the BDNF/ERK/CREB signaling pathway and the synapse-associated protein PSD-95 (Ning et al., 2021). The results of modern network pharmacology and experimental studies show that the combination of PT and AT has multi-component characteristics and advantages and can exert inhibitory effects on AD through multiple pathways, such as down-regulating the expression levels of TLR4 and NF-κB and related inflammatory factor targets (e.g., TNF-α, IL-1β, and IL-6) (Su et al., 2022; Wu et al., 2022; Cao et al., 2023).
TCM plays an important role in the clinical treatment of AD due to its unique advantages in combined medication (Tan et al., 2022; Xu et al., 2023). The combination of PT and AT is high-frequency drug pairs in TCM formulas for the treatment of AD. Polygala tenuifolia has the effects of tranquilizing the mind and promoting intelligence, coordinating the heart and the kidney, dispelling phlegm, and opening the orifices. Acorus tatarinowii is ranked first among the herbaceous medicines in “Shen Nong’s herbal classic,” with the effects of inducing resuscitation, calming the heart tranquilizing the mind, and removing dampness to restore normal functioning of the stomach. We found that out of the 16 RCTs included in this study, 11 were related to “kidney deficiency” in TCM syndrome differentiation, 5 were related to “spleen deficiency,” 8 were related to “phlegm turbidity,” and 4 were related to “blood stasis.” Therefore, we believe that the treatment of AD with the combination of PT and AT conforms to the evolution of TCM syndromes during the progression of AD disease, demonstrating a certain syndrome correlation effect.
This study evaluated and analyzed the effects of the combination of PT and AT on AD and included 16 RCTs involving 1103 participants. In this review, six trials compared the combination of PT and AT with conventional drugs (five donepezil and one piracetam). Ten RCTs compared the combination of PT and AT plus conventional drugs with conventional drugs (nine donepezil and one memantine + oxiracetam). Meanwhile, AD patients included in this study were mainly mild-to-moderate AD. For the clinical treatment of mild-to-moderate AD, donepezil is still the conventional drug mainly used. The analysis results indicated that the use of the combination of PT and AT alone did not have a significant effect on MMSE, ADL, and ADAS-cog in AD patients. Although the combination of PT and AT alone has shown a trend toward better outcomes with longer interventions, their clinical relevance remains limited. However, the combination of PT and AT plus conventional drugs showed more beneficial effects on MMSE, ADL, and ADAS-cog in patients with AD than drugs alone. In addition, we noted significant heterogeneity in the effects of both the combination of PT and AT and the combination of PT and AT plus conventional drugs on MMSE in patients with AD. By subgroup analysis of treatment duration, we were still unable to determine the source of heterogeneity. Based on the availability of included studies and the adequacy of data, we are currently unable to analyze to determine whether the factors affecting heterogeneity are related to other factors such as the dosage form, dosage, and subjectivity of TCM syndrome differentiation. In terms of safety reports, the combination of PT and AT had fewer adverse events than conventional drugs, while the combination of PT and AT plus conventional drugs was comparable to conventional drugs. In addition, the results of the subgroup analyses suggested that the effects of the combination of PT and AT plus conventional drugs on MMSE, ADL, and ADAS-cog were significant at 6 months of treatment duration, but given the clinical peculiarities of the AD disease course, increasing or adjusting the dosage over time and evolution is necessary, which may also increase the potential for secondary adverse reactions. Moreover, “syndrome” is of great importance in the process of TCM treatment, and it constantly changes with the passage of time and the evolution of the disease process (Li et al., 2021; Lu et al., 2021). Therefore, further prospective randomized controlled trials with large samples and follow-up are needed to assess the long-term efficacy of TCM treatments to avoid the limitation that conventional drug interventions are only effective in the short term.
We judged the overall quality of evidence for global cognition and functioning to be very low when the combination of PT and AT plus conventional drugs was compared with conventional drugs. The overall quality of evidence for global cognition and functioning was very low to moderate when the combination of PT and AT plus conventional drugs was compared with conventional drugs.
In the results of the meta-analyses evaluating the two treatment regimens separately, the direction of the effect sizes associated with the improvement of global cognition and functioning was consistent. In terms of the improvement in global cognition and functioning from the analyses of the included studies, we obtained positive results. The combination of PT and AT can be used as alternative therapies to conventional drugs for the treatment of AD, and the efficacy of the combination of PT and AT plus control is superior. However, the low and very low quality of available evidence limits confidence in the findings.
In our included studies, the maximum treatment duration was 6 months. The efficacy of both the combination of PT and AT and the combination of PT and AT plus control was better at 6 months than at 3 months of treatment duration. Our findings suggest that the combination of PT and AT demonstrated comparable effects to conventional drugs in terms of improvement of AD, with fewer adverse events, and showed a strong trend toward positive effects and marginal significance at 6 months of continuous treatment. With the longer duration of AD, a 6-month treatment duration does not appear to be the optimal period for the combination of PT and AT to be used to cure the disease; however, it still needs to be supported by more evidence, and the results need to be interpreted with caution. In the future, prospective trials may be needed to examine when a ceiling effect occurs in treatment interventions involving the combination of PT and AT.
We also tried to explore whether the therapeutic effect of the combination of PT and AT on AD is related to the amount or dosage form of the herbs, but the data from the existing studies were not sufficient to support us in finding a suitable methodology to analyze it. The bioavailability of the combination of PT and AT alone or in combination with conventional drugs may require more studies to conclude.
If the composition of TCM prescriptions in each study is strictly considered, it may be impossible to quantitatively summarize data from almost all TCM clinical studies. The present study focused on a meta-analysis of the macroscopic assessment of AD with the combination of PT and AT combined with conventional drugs compared with the combination of PT and AT alone. The resulting heterogeneity has been a methodological challenge for meta-analysis in TCM clinical studies (Dong et al., 2017).
In addition, it has been reported in some studies that TCM may have potential toxicity in clinical applications (Teo et al., 2016; Pan et al., 2020). Studies have shown that no toxic reactions were observed with PT and AT in relevant cell and animal experiments (Chen and Jia, 2020; Xiong et al., 2022b; Chen et al., 2022). Our findings suggested that the combination of PT and AT has fewer adverse effects in AD treatment and is safer for clinical application. It is also worth mentioning that the combination of PT and AT plus control showed a trend of fewer adverse reactions compared to when conventional drugs were applied. With the prolongation of the disease duration and treatment cycle of AD, the increase in the dosage of conventional drugs is inevitable (Cummings et al., 2019), and the combination of PT and AT may be beneficial in reducing the side effects of conventional drugs. More rigorous clinical trials are needed to explore the optimal dosage and timing of the combination of PT and AT use for benefit during different stages of AD.
To our knowledge, this is the first systematic review and meta-analysis to assess the effect of the combination of PT and AT on AD. We followed strictly the Cochrane methodology and conducted systematic and comprehensive searches in different databases. We compared different interventions and treatment durations separately based on the specificity of the AD disease itself and TCM treatment and focused on TCM syndrome differentiation during AD treatment. In addition, we carefully screened the data for each indicator in the combined analyses of the included studies, and we excluded three studies that used different ADL scoring criteria that resulted in opposite clinically significant outcomes to ensure homogeneity of findings.
This study still had several limitations. Due to the multi-component and multi-target nature of TCM treatments, we were unable to clarify the dose relationship between the intervention doses of the combination of PT and AT and changes in outcomes. The combination of PT and AT may not always be used as a principal drug in TCM formulas, and we are unable to analyze whether other active ingredients that improve outcomes in AD patients are also included in the formulas so that TCM treatments may show better improvement. The limitations of risks of bias that existed in the original study may have reduced the quality of the evidence, but we aimed to explore the effects of the combination of PT and AT in patients with AD, and therefore, this study is still of importance. In addition, the treatment duration in this study was a maximum of 6 months, and more RCTs with longer treatment durations are needed in the future to determine how effective the combination of PT and AT is for long-term intervention in AD. Given these publication biases that cannot be completely excluded, we should be cautious in interpreting the results. Sample sizes should be increased and longer treatment durations should be available in future RCTs to improve test efficacy. The design should be further refined in future RCTs, with more rigorous inclusion and exclusion criteria for interventions and reporting of more explicit quality control of prescriptions and chemical analyses studies of drugs.
Compared to single conventional drugs, the combination of PT and AT may be used as an alternative therapy to improve global cognition and functioning in AD, and the combination of PT and AT as an adjunctive therapy appears to produce a better therapeutic response to AD in terms of efficacy without increasing the risk of adverse events. However, the very low to low quality of available evidence limits confidence in the findings. More prospective studies with tightly controlled conditions are needed to provide confirmatory evidence.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
YZ: writing–original draft and writing–review and editing. JT: writing–review and editing. JN: writing–review and editing. MW: writing–original draft and writing–review and editing. TL: writing–original draft and writing–review and editing. JS: writing–review and editing.
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Fundamental Research Funds for the Central Universities (No. 2019-JYB-TD-007), the National Key Research and Development Program of China (2018YFC1704100), the National Natural Science Foundation of China (No. 82074362), and Qihuang Scholar Foundation (China).
The authors would like to thank all the members from their research group who made helpful comments.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1268000/full#supplementary-material
Arora, A., Behl, T., Sehgal, A., Singh, S., Sharma, N., Abdellatif, A. A. H., et al. (2023). Elucidating the promising role of traditional Chinese medicine in neuroprotection against oxidative stress encompassing Alzheimer's disease. Environ. Sci. Pollut. Res. Int. 30 (14), 39546–39557. doi:10.1007/s11356-023-25800-4
Balázs, N., Bereczki, D., and Kovács, T. (2021). Cholinesterase inhibitors and memantine for the treatment of Alzheimer and non-Alzheimer dementias. Ideggyogyaszati Szle. 74 (11-12), 379–387. doi:10.18071/isz.74.0379
Bo, M., Zhang, H., Xu, J., Zhao, H., Jia, X., Wang, G., et al. (2022). Systematic review of Kaixinsan in treating depression: efficacy and pharmacological mechanisms. Front. Behav. Neurosci. 16, 1061877. doi:10.3389/fnbeh.2022.1061877
Cao, Y., Li, M., Gu, L., Zhao, X., Zhou, A., Miao, Y., et al. (2023). Chinese traditional formula Kaixin San suppressed ferroptosis of hippocampal neurons and cardiomyocytes in mice with paradoxical sleep deprivation. J. Ethnopharmacol. 304, 116034. doi:10.1016/j.jep.2022.116034
Chen, S., and Jia, J. (2020). Tenuifolin attenuates amyloid-β42-induced neuroinflammation in microglia through the NF-κB signaling pathway. J. Alzheimers Dis. 76 (1), 195–205. doi:10.3233/JAD-200077
Chen, Z., Yang, Y., Han, Y., and Wang, X. (2022). Neuroprotective effects and mechanisms of senegenin, an effective compound originated from the roots of polygala tenuifolia. Front. Pharmacol. 13, 937333. doi:10.3389/fphar.2022.937333
Chen, Y., and Yuan, Y. (2008). Clinical study on the treatment of kidney deficiency and Marrow reduction syndrome in Alzheimer's disease with. Huonao Fang. (32), 81–82.
Cummings, J. L., Tong, G., and Ballard, C. (2019). Treatment combinations for Alzheimer's disease: current and future pharmacotherapy options. J. Alzheimers Dis. 67 (3), 779–794. doi:10.3233/JAD-180766
Dafre, R., and Wasnik, P. (2023). Current diagnostic and treatment methods of Alzheimer's disease: a narrative review. Cureus 15 (9), e45649. doi:10.7759/cureus.45649
Deng, C., Chen, H., Meng, Z., and Meng, S. (2022). Roles of traditional Chinese medicine regulating neuroendocrinology on AD treatment. Front. Endocrinol. 13, 955618. doi:10.3389/fendo.2022.955618
Ding, M. R., Qu, Y. J., Hu, B., and An, H. M. (2022). Signal pathways in the treatment of Alzheimer's disease with traditional Chinese medicine. Biomed. Pharmacother. = Biomedecine Pharmacother. 152, 113208. doi:10.1016/j.biopha.2022.113208
Dong, Q., Guo, Q. H., Luo, B. Y., and Xu, Y. (2017). Expert consensus on the management of post-stroke cognitive impairment. Chin. J. Stroke. 12 (6), 519–531.
Doroszkiewicz, J., and Mroczko, B. (2022). New possibilities in the therapeutic approach to Alzheimer's disease. Int. J. Mol. Sci. 23 (16), 8902. doi:10.3390/ijms23168902
Ferrari, C., and Sorbi, S. (2021). The complexity of Alzheimer's disease: an evolving puzzle. Physiol. Rev. 101 (3), 1047–1081. doi:10.1152/physrev.00015.2020
Gu, Y. L. (2019). Efficacy of jiawei Diankuang mengxing decoction for Alzheimer's disease patients with phlegm-stasis syndrome [D]. Hunan University of Traditional Chinese Medicine, (Chinese).
Guan, X. J. (2022). The clinical observation on alzheimer’s disease of spleen deficiency and Sputum resistance type with modified Kaixin san [D]. Shandong University of Traditional Chinese Medicine, (Chinese).
Jiao, Y. N., Zhang, J. S., Qiao, W. J., Tian, S. Y., Wang, Y. B., Wang, C. Y., et al. (2022). Kai-xin-san inhibits tau pathology and neuronal apoptosis in aged SAMP8 mice. Mol. Neurobiol. 59 (5), 3294–3309. doi:10.1007/s12035-021-02626-0
Kelaimujiang, G. (2019). The clinical research on Alzheimer's disease of kidney essence deficiency treated with Yizhi chidai recipe [D]. Xinjiang Medical University, (Chinese).
Li, B., Li, J., Hao, Y., Xie, P., Yue, S., Wang, S., et al. (2023). Yuanzhi Powder inhibits tau pathology in SAMP8 mice: mechanism research of a traditional Chinese formula against Alzheimer's disease. J. Ethnopharmacol. 311, 116393. doi:10.1016/j.jep.2023.116393
Li, S., Wu, Z., and Le, W. (2021). Traditional Chinese medicine for dementia. Alzheimer's dementia J. Alzheimer's Assoc. 17 (6), 1066–1071. doi:10.1002/alz.12258
Li, X. W. (2017). A clinical study on mild and moderate alzheimer’s disease of kidney deficiency and Marrow depletion syndrome treated with modified Shuyu Pill. Hubei University of Traditional Chinese Medicine, (Chinese).
Liang, J. F., Qin, C., and Yang, B. Clinical observation on bushenyizhi granule combined with western medicine in treatment of alzheimer’s disease. 2010;8(01):39–41.
Lin, D. X., and Chen, Z. (2018). A clinical study on treating AD with Kaixin San plus donepezil hydrochloride tablets. Clin. J. Chin. Med. 10 (23), 73–75.
Lin, Y. Q. (2017). Efficacy of phlegm-resolving orifice-opening decoction for Alzheimer's disease patients with phlegm-turbidity obstructing orifices pattern. Fujian University of Traditional Chinese Medicine, (Chinese).
Lu, M., Zhou, Y. H., Li, X. N., Sun, H. D., Guo, J. D., Wu, B. W., et al. (2021). Research on regularity of traditional Chinese medicine in treatment of Alzheimer's disease based on data mining. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J. Chin. materia medica 46 (6), 1558–1563. doi:10.19540/j.cnki.cjcmm.20200611.502
Ning, F., Chen, L., Chen, L., Liu, X., Zhu, Y., Hu, J., et al. (2021). Combination of Polygoni Multiflori radix Praeparata and acori tatarinowii rhizoma alleviates learning and memory impairment in scopolamine-treated mice by regulating synaptic-related proteins. Front. Pharmacol. 12, 679573. doi:10.3389/fphar.2021.679573
Pan, X., Zhou, J., Chen, Y., Xie, X., Rao, C., Liang, J., et al. (2020). Classification, hepatotoxic mechanisms, and targets of the risk ingredients in traditional Chinese medicine-induced liver injury. Toxicol. Lett. 323, 48–56. doi:10.1016/j.toxlet.2020.01.026
Passeri, E., Elkhoury, K., Morsink, M., Broersen, K., Linder, M., Tamayol, A., et al. (2022b). Alzheimer's disease: treatment strategies and their limitations. Int. J. Mol. Sci. 23 (22), 13954. doi:10.3390/ijms232213954
Passeri, E., Elkhoury, K., Morsink, M., Broersen, K., Linder, M., Tamayol, A., et al. (2022a). Alzheimer's disease: treatment strategies and their limitations. Int. J. Mol. Sci. 23 (22), 13954. doi:10.3390/ijms232213954
Peng, X. M. (2014). Clinical observation of Kaixin Jiannao granule in treating alzheimer disease of spleen and kidney deficiency, Sputum turbid blocking aperture syndrome [D]. Hunan University of Traditional Chinese Medicine, (Chinese).
Plascencia-Villa, G., and Perry, G. (2021). Preventive and therapeutic strategies in Alzheimer's disease: focus on oxidative stress, redox metals, and ferroptosis. Antioxidants redox Signal. 34 (8), 591–610. doi:10.1089/ars.2020.8134
Qu, S., Liu, M., Cao, C., Wei, C., Meng, X. E., Lou, Q., et al. (2021). Chinese medicine formula kai-xin-san ameliorates neuronal inflammation of CUMS-induced depression-like mice and reduces the expressions of inflammatory factors via inhibiting TLR4/IKK/NF-κB pathways on BV2 cells. Front. Pharmacol. 12, 626949. doi:10.3389/fphar.2021.626949
Scheltens, P., De Strooper, B., Kivipelto, M., Holstege, H., Chételat, G., Teunissen, C. E., et al. (2021). Alzheimer's disease. Lancet 397 (10284), 1577–1590. doi:10.1016/S0140-6736(20)32205-4
Schünemann, H. J., Higgins, J. P., Vist, G. E., Glasziou, P., Akl, E. A., Skoetz, N., et al. (2019). Completing ‘Summary of findings’ tables and grading the certainty of the evidence, 375–402.
Se Thoe, E., Fauzi, A., Tang, Y. Q., Chamyuang, S., and Chia, A. Y. Y. (2021). A review on advances of treatment modalities for Alzheimer's disease. Life Sci. 276, 119129. doi:10.1016/j.lfs.2021.119129
Srivastava, S., Ahmad, R., and Khare, S. K. (2021). Alzheimer's disease and its treatment by different approaches: a review. Eur. J. Med. Chem. 216, 113320. doi:10.1016/j.ejmech.2021.113320
Sterne, J. A., Sutton, A. J., Ioannidis, J. P., Terrin, N., Jones, D. R., Lau, J., et al. (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ Clin. Res. ed) 343, d4002. doi:10.1136/bmj.d4002
Su, S. J., Chen, Y., Yang, H. Y., Liu, H. N., Han, L., Wang, H., et al. (2022). Exploration on mechanism of Polygalae Radix and Acori Tatarinowii Rhizoma in treating Alzheimer's disease based on network pharmacology and experimental verification. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J. Chin. materia medica 47 (12), 3348–3360. doi:10.19540/j.cnki.cjcmm.20211216.707
Syed, Y. Y. (2020). Sodium oligomannate: first approval. Drugs 80 (4), 441–444. doi:10.1007/s40265-020-01268-1
Tan, W., Qi, L., Hu, X., and Tan, Z. (2022). Research progress in traditional Chinese medicine in the treatment of Alzheimer's disease and related dementias. Front. Pharmacol. 13, 921794. doi:10.3389/fphar.2022.921794
Teo, D. C., Ng, P. S., Tan, S. H., Lim, A. T., Toh, D. S., Chan, S. Y., et al. (2016). Drug-induced liver injury associated with Complementary and Alternative Medicine: a review of adverse event reports in an Asian community from 2009 to 2014. BMC Complement. Altern. Med. 16, 192. doi:10.1186/s12906-016-1168-z
Vaz, M., and Silvestre, S. (2020). Alzheimer's disease: recent treatment strategies. Eur. J. Pharmacol. 887, 173554. doi:10.1016/j.ejphar.2020.173554
Wang, M., Tang, H. P., Wang, S., Hu, W. J., Li, J. Y., Yu, A. Q., et al. (2023). Acorus tatarinowii schott: a review of its botany, traditional uses, phytochemistry, and pharmacology. Mol. (Basel, Switz. 28 (11), 4525. doi:10.3390/molecules28114525
Wang, S., Hu, Y., Tan, W., Wu, X., Chen, R., Cao, J., et al. (2012). Compatibility art of traditional Chinese medicine: from the perspective of herb pairs. J. Ethnopharmacol. 143 (2), 412–423. doi:10.1016/j.jep.2012.07.033
Wang, S. L., Yang, H., Hui, Z., Zhang, J. H., Tong, Z. X., Xu, C. C., et al. (2022). Clinical study of Yizhi Qixin decoction combined with donepezil in the treatment of Alzheimer's disease. Int. J. Traditional Chin. Med. 20 (13), 2480–2483.
Wang, X. F., Xiao, H. H., Wu, Y. T., Kong, L., Chen, J. C., Yang, J. X., et al. (2021). Active constituent of Polygala tenuifolia attenuates cognitive deficits by rescuing hippocampal neurogenesis in APP/PS1 transgenic mice. BMC complementary Med. Ther. 21 (1), 267. doi:10.1186/s12906-021-03437-5
Wang, P. (2021). Clinical observation on the supplementary treatment of senile dementia with bushen. Huoxue Huatan Tang 37 (04), 609–610.
Wu, Q., Li, X., Jiang, X. W., Yao, D., Zhou, L. J., Xu, Z. H., et al. (2022). Yuan-Zhi decoction in the treatment of Alzheimer's disease: an integrated approach based on chemical profiling, network pharmacology, molecular docking and experimental evaluation. Front. Pharmacol. 13, 893244. doi:10.3389/fphar.2022.893244
Xiong, W., Zhao, X., Xu, Q., Wei, G., Zhang, L., Fan, Y., et al. (2022a). Qisheng Wan formula ameliorates cognitive impairment of Alzheimer's disease rat via inflammation inhibition and intestinal microbiota regulation. J. Ethnopharmacol. 282, 114598. doi:10.1016/j.jep.2021.114598
Xiong, W., Zhao, X., Xu, Q., Wei, G., Zhang, L., Fan, Y., et al. (2022b). Qisheng Wan formula ameliorates cognitive impairment of Alzheimer's disease rat via inflammation inhibition and intestinal microbiota regulation. J. Ethnopharmacol., 282, 114598.
Xu, Y. M., Lu, F. M., Xu, H. C., Zhang, J., Hei, S. Y., Qiu, Y. H., et al. (2023b). Kai-xin-san improves cognitive impairment via wnt/β-catenin and IRE1/XBP1s signalings in APP/PS1 mice. Rejuvenation Res. 26 (3), 105–115. doi:10.1089/rej.2022.0063
Xu, Z., Zhou, X., Hong, X., Wang, S., Wei, J., Huang, J., et al. (2023a). Essential oil of Acorus tatarinowii Schott inhibits neuroinflammation by suppressing NLRP3 inflammasome activation in 3 × Tg-AD transgenic mice. Phytomedicine Int. J. phytotherapy Phytopharm. 112, 154695. doi:10.1016/j.phymed.2023.154695
Yang, X. C., Li, N., Xu, S. F., Xu, L. P., Lou, D. F., and Wang, J. Effacacy observation on tiaoxin prescription in treatment of mild alzheimer disease. 2018;33(10):2020–2024.
Yang, F., Yang, X. P., Xu, Y., Qiu, X. M., Chen, W. Y., and Li, W. H. Clinical observation of Huanshaodan in the treatment of mild to moderate alzheimer’s disease of spleen and kidney deficiency type. 2020;42(04):538–542.
Yang, L., Mao, Y. R., Han, G. X., Cheng, P. J., Xiao, Y. Z., and Miao, Y. Y. Clinical study on jieyu Yizhi tang for alzheimer disease of liver depression and spleen deficiency type. 2022;54(08):77–80.
Yi, Y. Q., Fang, R., Ge, J. W., Cheng, S. W., Wang, G. Z., and Liu, L. (2018). Analysis on medication rules for treatment of dementia by ancient physicians based on data mining methods. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J. Chin. materia medica 43 (16), 3376–3381. doi:10.19540/j.cnki.cjcmm.20180419.004
Zhang, L., Yong, Y. Y., Deng, L., Wang, J., Law, B. Y., Hu, M. L., et al. (2023). Therapeutic potential of Polygala saponins in neurological diseases. Phytomedicine Int. J. phytotherapy Phytopharm. 108, 154483. doi:10.1016/j.phymed.2022.154483
Zhang, Z., Xu, J., Ma, S., Lin, N., Hou, M., Wei, M., et al. (2022). Integration of network pharmacology and molecular docking Technology reveals the mechanism of the therapeutic effect of xixin decoction on Alzheimer's disease. Comb. Chem. high throughput Screen. 25 (10), 1785–1804. doi:10.2174/1386207325666220523151119
Zhang, L., Wang, Y. Y., Zhou, J. B., Ke, B., Yang, Y. B., Qin, J., et al. Effects and mechanisms of Dihuang Yinzi Decoction on the treatment of Alzheimer’s disease patients. 2018;33(11):4948–4952.
Keywords: Acorus tatarinowii, Alzheimer’s disease, clinical application, herb combinations, Polygala tenuifolia, traditional Chinese medicine, randomized controlled trials
Citation: Zhang Y, Tian J, Ni J, Wei M, Li T and Shi J (2024) Polygala tenuifolia and Acorus tatarinowii in the treatment of Alzheimer’s disease: a systematic review and meta-analysis. Front. Pharmacol. 14:1268000. doi: 10.3389/fphar.2023.1268000
Received: 27 July 2023; Accepted: 11 December 2023;
Published: 12 January 2024.
Edited by:
Valentina Echeverria Moran, United States Department of Veterans Affairs, United StatesReviewed by:
Manish Kumar, Chitkara University, IndiaCopyright © 2024 Zhang, Tian, Ni, Wei, Li and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Shi, c2hpamluZzg3QGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.