- 1Department of Cardiology, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 2National Integrated Traditional and Western Medicine Center for Cardiovascular Disease, China-Japan Friendship Hospital, Beijing, China
- 3Centre for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
- 4Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 5Institute of Cardiovascular Diseases, Beijing University of Chinese Medicine, Beijing, China
Background: Chinese patent medicines (CMPs) have curative effectiveness in preventing coronary restenosis. However, the relative efficacy between different CPMs has not been sufficiently investigated.
Methods: Randomized clinical trials were searched from electronic databases including PubMed, Web of Science, Cochrane Library, Embase, CNKI, VIP, WanFang, SinoMed, Chinese Clinical Trial Registry, and ClinicalTrials.gov. Bayesian network meta-analysis was performed to analyze CPMs’ efficacy in preventing angiographic restenosis, recurrence angina, acute myocardial infarction, and target lesion revascularization after percutaneous coronary intervention.
Results: This network meta-analysis included 47 trials with 5,077 patients evaluating 11 interventions. Regarding angiographic restenosis, the efficacy of CPMs (except Xuezhikang capsule) combined with standard treatment (Std) was superior to Std alone, and Guanxin Shutong capsule plus Std reduced the risk of angiographic restenosis by 76% (relative risk 0.24, 95% confidence interval 0.11–0.45, and very low to moderate certainty of evidence), most likely the best intervention. Fufang Danshen dripping pill combined with Std showed superiority over other interventions for relieving recurrence angina, which can reduce the risk by 83% (RR 0.17, 95% CI 0.04–0.51, very low to moderate certainty of evidence) compared to Std alone. In acute myocardial infarction after percutaneous coronary intervention, compared with Std alone, Danhong injection plus Std displayed a significant effect (RR 0.11, 95% CI 0.00–0.69, very low to moderate certainty of evidence) and was the best treatment probably. Chuanxiongqin tablet plus Std was the most effective treatment for reducing target lesion revascularization by 90% (RR 0.10, 95% CI 0.00–0.60, very low to moderate certainty of evidence) compared with Std alone.
Conclusion: The results indicated that CPMs combined with Std reduced the risk of coronary restenosis after percutaneous coronary intervention. However, the results should be interpreted cautiously due to significant data limitations.
1 Introduction
Percutaneous coronary intervention (PCI) improves myocardial perfusion by dredging a narrow or even occluded coronary artery lumen through the cardiac catheterization technique (Grines et al., 2016). However, due to the complex pathological mechanism of thrombosis, intimal hyperplasia, and inflammatory response, patients undergoing PCI have a risk of coronary restenosis (Jukema et al., 2012; Melnik et al., 2022). Importantly, PCI for restenosis is associated with a higher risk of major adverse cardiac events than PCI for de novo lesions (Giustino et al., 2022). Although standard treatment (Std) recommended by the guideline (Lawton et al., 2022), including aspirin, clopidogrel, and statin, has been shown to reduce restenosis risk, in-stent restenosis still occurs at a rate of 5% in patients with PCI (Moussa et al., 2020). Given that millions of people undergo PCI treatment annually worldwide (Tsao et al., 2022; Chinese Cardiovascular Health and Disease report writing group, 2023), restenosis can be considered a significant public health problem.
As an adjunct drug, traditional Chinese medicine (TCM) combined with Std has a specific curative effectiveness in preventing restenosis (Wu et al., 2019). Among TCM, many clinical trials on Chinese patent medicines (CPMs) in preventing restenosis have been conducted due to the advantages of standardized dosage and composition, stable and controllable quality, and convenient taking (Xu et al., 2000; Li et al., 2004; Mao et al., 2015; Zhou et al., 2021). However, the relative efficacy between different CPMs has not been sufficiently investigated.
Network meta-analysis (NMA), a novel meta-analysis strategy, can integrate direct and indirect evidence. It allows comparisons across multiple treatments simultaneously even if they were not directly compared previously. Furthermore, a Bayesian approach to NMA provides the probability estimates that enable clinicians to select the optimal treatment option (Sutton and Abrams, 2001). The objective of this study was to evaluate the comparative effectiveness of CPMs in preventing coronary restenosis after PCI using a Bayesian NMA approach.
2 Materials and methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the extension statement for network meta-analysis (PRISMA-NMA) to report the current results (Hutton et al., 2015; Page et al., 2021).
2.1 Standard evaluation of CMPs
To make this study reproducible, we reported CMPs according to the requirements of the ConPhyMP consensus (Heinrich et al., 2020). Accurate scientific nomenclature for botanical drugs referred to Rivera’s suggestion (Rivera et al., 2014) and was validated taxonomically in the databases of “Medicinal Plant Names Services” (https://mpns.science.kew.org/mpns-portal/). In addition, we referred to the Chinese Pharmacopoeia 2020 regarding the names of non-botanical drugs. The relevant information about CMPs referred to the original study, the Chinese Pharmacopoeia 2020, and the National Medical Products Administration. The details are shown in Supplementary Tables S1, S2.
2.2 Inclusion criteria
We determined the literature to be included according to the PICOS principle: 1) patients: who accepted the treatment of PCI. 2) Intervention: the treatment group received Std for coronary heart disease (including aspirin, clopidogrel, statin, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and β-blocker) and additional CPMs. 3) Comparison: the control group received a Std treatment for coronary heart disease, with or without a placebo. 4) Outcome: the angiographic restenosis rate, defined as target vessel lumen stenosis ≥50% according to the angiographic results. 5) Study design: randomized controlled trial (RCT).
2.3 Exclusion criteria
We excluded duplicate literature, research lacking important data, articles in which the full text was not found, published papers using the same data, and trials followed for less than 4 weeks.
2.4 Sources and search strategy of the literature
We searched PubMed, Web of Science, Cochrane Library, Embase, CNKI, VIP, WanFang, and SinoMed from these electronic databases’ inception to 13 April 2023. In addition, two clinical trial registration platforms, namely, Chinese Clinical Trial Registry (https://www.chictr.org.cn/) and ClinicalTrials.gov (https://clinicaltrials.gov/), were also searched. A detailed search strategy for English databases can be seen in Supplementary Texts S1–S4.
2.5 Literature screening and data extraction
All studies were screened and data were extracted independently by two researchers. First, we used EndNote X9 software to remove duplicates. Then, two researchers read the title and abstract to finish the preliminary screening and finally read the full text to decide whether a study was included appropriately. In case of disagreement, the third researcher helped resolve the problem.
Data extraction still adopted the double entry and cross-check method. We tried to contact the authors to acquire missing information when we encountered incomplete data. Data extraction included title, author, disease, sample size, age, sex, intervention measures, course of treatment, outcome indicators, and follow-up time.
2.6 Bias risk and GRADE certainty assessment
The Cochrane Risk of Bias 2 tool (Cochrane Collaboration, London, United Kingdom) (Sterne et al., 2019) was used to assess the risk of bias of the included studies by two researchers. The tool assesses bias across six distinct domains: randomization process, deviations from intended interventions, missingness in outcome data, measurement of the outcome, selection of reported results, and overall bias. The judgment of each domain includes low risk, some concerns, and high risk. We used the GRADE approach for the entire network to provide the framework for rating the certainty of the evidence of each paired comparison as high, moderate, low, or very low (Puhan et al., 2014; Brignardello-Petersen et al., 2020).
2.7 Outcomes
The primary outcome was the angiographic restenosis rate defined as target vessel lumen stenosis ≥50% according to the angiographic results. Furthermore, we focused on the secondary outcomes, including recurrence angina, acute myocardial infarction (AMI) after PCI, and target lesion revascularization (TLR), defined as clinical restenosis.
2.8 Statistical analysis
NMA estimating the treatment effectiveness was conducted using Bayesian Markov chain Monte Carlo algorithms. The outcomes in this study were all categorical variables, and the statistical analysis results were expressed as relative risk (RR) with 95% confidence intervals (CIs). To account for clinical and methodological heterogeneity among studies when comparing treatment effectiveness, random-effects models were selected. The heterogeneity of the entire network was estimated using a global I2 statistic.
Models were calculated by generating 50,000 sample iterations with an initial burn-in period of 20,000 iterations (thin = 1). Four chains with different initial values were run simultaneously to assess convergence using the trace and density and Brooks–Gelman–Rubin diagnostic plots. The potential scale reduction factor value of the Brooks–Gelman–Rubin diagnostics close to 1 indicates approximate convergence. The surface under the cumulative ranking (SUCRA) curve value was calculated based on the evaluation of rank probabilities. No consistency evaluation was performed because all data were from indirect treatment comparisons and no head-to-head RCTs.
We performed subgroup network meta-analysis for angiographic restenosis according to the number of patients included in the original study (≥100 or <100). Sensitivity analysis was carried out by excluding studies with a high-risk bias. All statistical analyses of the NMA were conducted using the “gemtc” package in R v4.0.2 software (R Project; www.r-project.org). In addition, plots of network diagrams visualizing network geometry and node connectivity and funnel plots examining publication bias were created using Stata/SE 15.1.
3 Results
3.1 Study selection
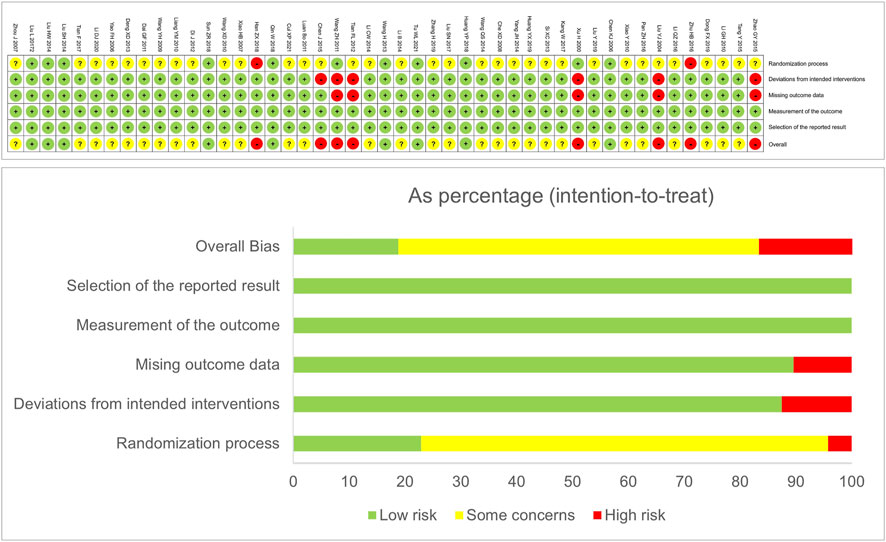
A total of 6,058 articles were found through the electronic database search, including 253 from PubMed, 89 from the Cochrane Library, 190 from the Web of Science, 462 from Embase, 864 from CNKI, 1,524 from WanFang, 1,397 from VIP, and 1,279 from CBMdisc. Relevant trials were not found from Chinese Clinical Trial Registry and ClinicalTrials.gov. After combining these articles, 2,505 duplicates were removed. After removing duplicates, 3,553 articles were screened for the title and abstract, of which 3,373 were excluded. Afterward, 180 relevant articles were screened for eligibility by reading the full text. Finally, 47 articles (Supplementary Text S5) that met the inclusion criteria were included in our Bayesian NMA. The details of the literature screening process are shown in Figure 1.
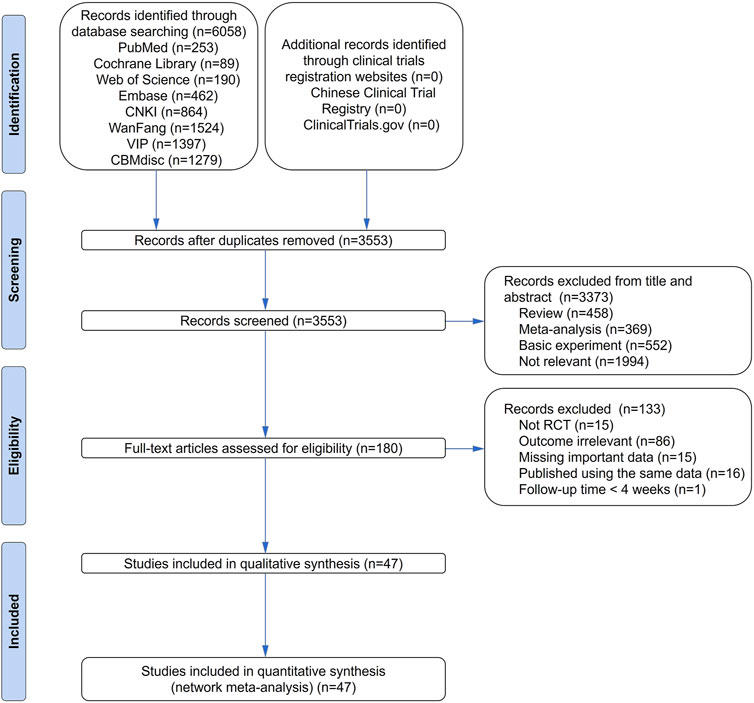
FIGURE 1. Summary of trial identification and selection. RCT, randomized controlled trial; TCM, traditional Chinese medicine.
3.2 Study characteristics
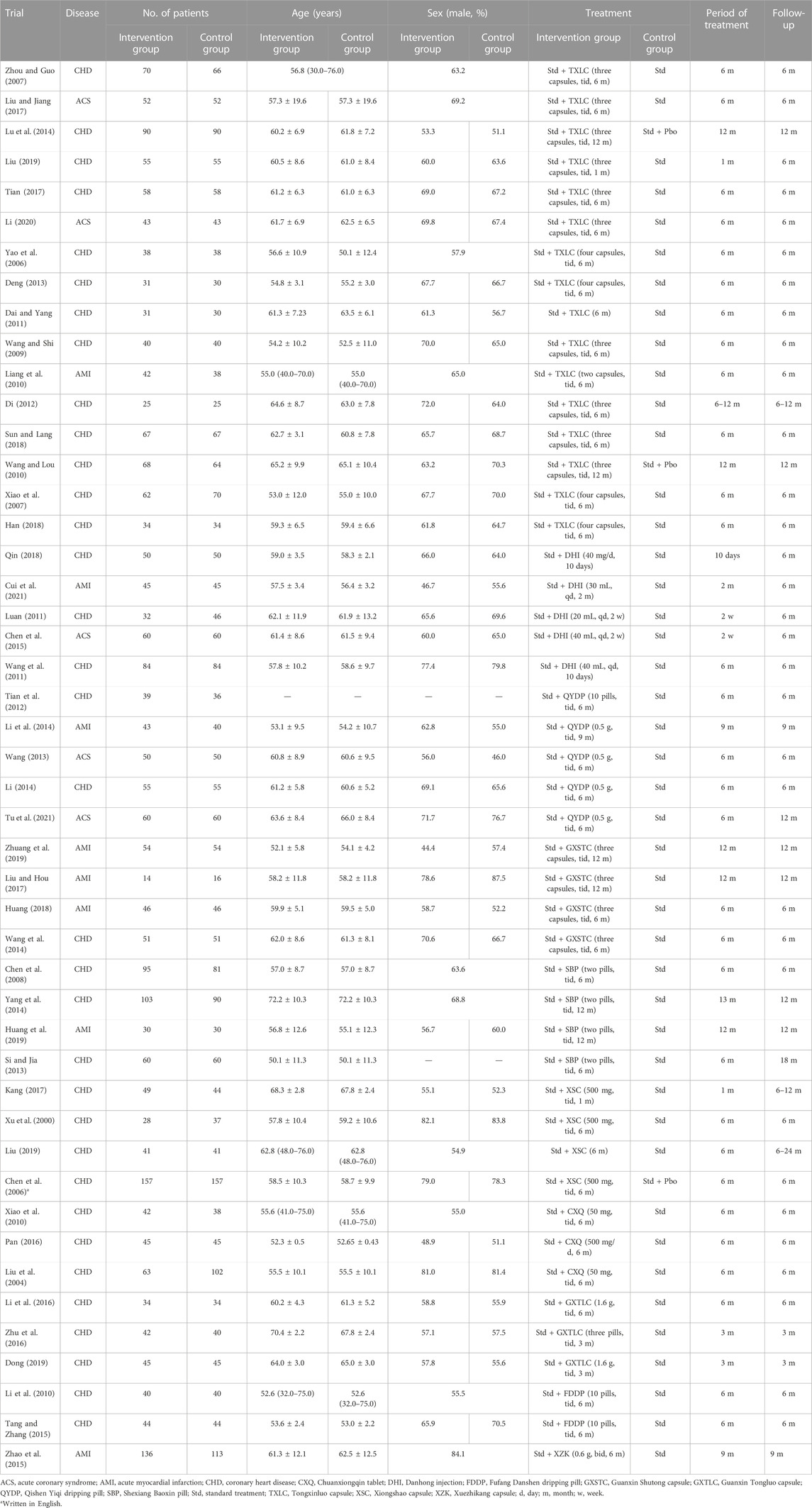
The Bayesian NMA was conducted including 47 studies that enrolled a total of 5,077 patients. All trials were conducted in China. One of them was written in English, and 46 were written in Chinese. NMA evaluated 11 different treatments in preventing restenosis: Std, Tongxinluo capsule plus Std (represented as TXLC), Danhong injection plus Std (represented as DHI), Qishen Yiqi dripping pill plus Std (represented as QYDP), Guanxin Shutong capsule plus Std (represented as GXSTC), Shexiang Baoxin pill plus Std (represented as SBP), Xiongshao capsule plus Std (represented as XSC), Chuanxiongqin tablet plus Std (represented as CXQ), Guanxin Tongluo capsule plus Std (represented as GXTLC), Fufang Danshen dripping pill plus Std (represented as FDDP), and Xuezhikang capsule plus Std (represented as XZK). The mean (or median) age of the patients ranged from 50 to 72 years, and the follow-up time varied from 3 to 24 months (Table 1).
3.3 Risk of bias assessment
In terms of the randomization process, 11 studies had clear random sequence generation, 34 studies mentioned “random” only and did not describe the random method, and two studies enrolled patients according to their order of the first visit. Only three studies were double-blind trials. Six studies failed to follow up with all patients and did not mention appropriate analysis to estimate the effect of assignment to intervention. Five studies missed complete outcome data. All studies had an appropriate method of measuring the outcomes and did not have the risk of bias in selection of the reported result. The details of bias risk assessment are shown in Figure 2 and Supplementary Excel.
3.4 Assessment of heterogeneity and model fitting
For the outcomes of angiographic restenosis and TLR, there was heterogeneity in comparison pairs between XSC and Std (I2 = 69.8%) and DHI and Std (I2 = 50.6%). No heterogeneity was observed for the outcomes of recurrence angina and AMI after PCI.
The trace and density and Brooks–Gelman–Rubin diagnostic plots are shown in Supplementary Figures S1, S2. In the trace and density plot, the Markov chain Monte Carlo chain fluctuated steadily with good overlap, and the curve was smooth; in addition, the potential scale reduction factor value was close to 1. The above results showed that the model had a strong degree of convergence.
3.5 Primary outcome: angiographic restenosis
3.5.1 Traditional pairwise meta-analysis
A traditional pairwise meta-analysis of the included study data was performed. There were 11 different interventions and 10 pairwise comparisons generated. All CPMs combined with Std except XZK were superior to Std alone (Supplementary Figure S3).
3.5.2 Evidence network
In total, 47 studies including 11 medication strategies involving 5,077 patients reported angiographic restenosis. The studies provided 10 direct comparisons (Figure 3A). Line thickness matches the number of included trials, and the circle size is related to the number of included patients. The number of studies that compared TXLC with Std and the number of patients who received the treatment were 16 and 1,606, respectively, and these were the largest numbers compared with other direct comparisons.
FIGURE 3. Network of treatment comparisons for Bayesian network meta-analysis. (A) Outcome of angiographic restenosis; (B) outcome of recurrence angina; (C) outcome of acute myocardial infarction; and (D) outcome of target lesion revascularization. CXQ, Chuanxiongqin tablet; DHI, Danhong injection; FDDP, Fufang Danshen dripping pill; GXSTC, Guanxin Shutong capsule; GXTLC, Guanxin Tongluo capsule; QYDP, Qishen Yiqi dripping pill; SBP, Shexiang Baoxin pill; Std, standard treatment; TXLC, Tongxinluo capsule; XSC, Xiongshao capsule; XZK, Xuezhikang capsule.
3.5.3 NMA and SUCRA
A total of 55 pairwise comparisons of angiographic restenosis were generated by NMA (Figure 4). In addition to nine pairwise direct comparisons, two pairwise indirect comparisons were also statistically significant. Compared with XSC and XZK, GXST reduced the risk of angiographic restenosis by 53% (RR 0.47, 95% CI 0.21–0.99) and 75% (RR 0.25, 95% CI 0.06–0.95), respectively. As shown in Figure 5 and Supplementary Table S3 for SUCRA of angiographic restenosis, GXST was most likely the best intervention. Certainty of evidence for all comparisons was very low to moderate. The details of evidence evaluation are available in Supplementary Table S4.

FIGURE 4. Summary of results of angiographic restenosis from network meta-analysis. CXQ, Chuanxiongqin tablet; DHI, Danhong injection; FDDP, Fufang Danshen dripping pill; GXSTC, Guanxin Shutong capsule; GXTLC, Guanxin Tongluo capsule; QYDP, Qishen Yiqi dripping pill; SBP, Shexiang Baoxin pill; Std, standard treatment; TXLC, Tongxinluo capsule; XSC, Xiongshao capsule; XZK, Xuezhikang capsule.
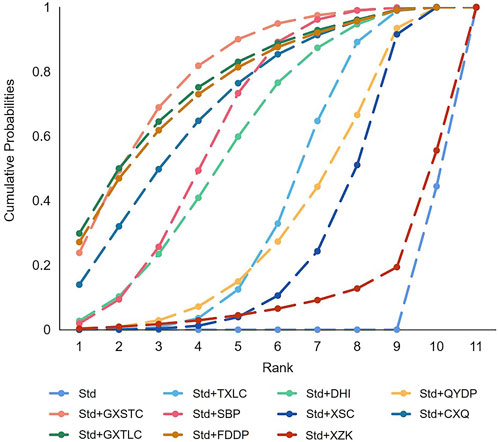
FIGURE 5. SUCRA curve with regard to reducing angiographic restenosis. CXQ, Chuanxiongqin tablet; DHI, Danhong injection; FDDP, Fufang Danshen dripping pill; GXSTC, Guanxin Shutong capsule; GXTLC, Guanxin Tongluo capsule; QYDP, Qishen Yiqi dripping pill; SBP, Shexiang Baoxin pill; Std, standard treatment; TXLC, Tongxinluo capsule; XSC, Xiongshao capsule; XZK, Xuezhikang capsule; SUCRA, surface under the cumulative ranking.
3.6 Secondary outcome: recurrence angina
3.6.1 Traditional pairwise meta-analysis
A traditional pairwise meta-analysis of the included study data was performed. There were nine different interventions and eight pairwise comparisons generated. All CPMs combined with Std were superior to Std alone (Supplementary Figure S4).
3.6.2 Evidence network
In total, 33 studies including nine medication strategies involving 3,749 patients reported recurrence angina. The studies provided eight direct comparisons (Figure 3B). The number of studies that compared TXLC with Std and the number of patients who received the treatment were 11 and 1,214, respectively.
3.6.3 NMA and SUCRA
A total of 36 pairwise comparisons of recurrence angina were generated by NMA (Figure 6). In addition to eight pairwise direct comparisons, four pairwise indirect comparisons were also statistically significant. Compared with GXSTC, FDDP, and TXLC, CXQ reduced the risk of recurrence angina by 72% (RR 0.28, 95% CI 0.06–0.89), 54% (RR 0.46, 95% CI 0.29–0.76), and 49% (RR 0.51, 95% CI 0.30–0.86), respectively. As shown in Figure 7 and Supplementary Table S3 for the SUCRA of recurrence angina, FDDP was most likely the best intervention. Certainty of evidence for all comparisons was very low to moderate. The details of evidence evaluation are available in Supplementary Table S5.
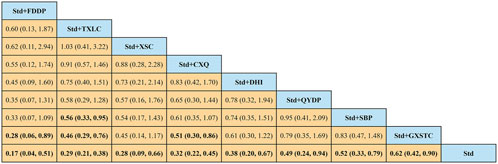
FIGURE 6. Summary of results of recurrence angina from network meta-analysis. CXQ, Chuanxiongqin tablet; DHI, Danhong injection; FDDP, Fufang Danshen dripping pill; GXSTC, Guanxin Shutong capsule; QYDP, Qishen Yiqi dripping pill; SBP, Shexiang Baoxin pill; Std, standard treatment; TXLC, Tongxinluo capsule; XSC, Xiongshao capsule.
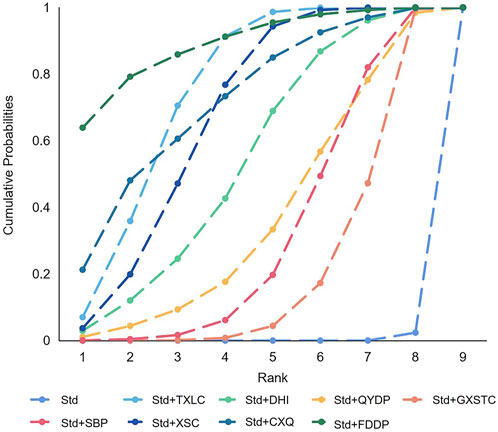
FIGURE 7. SUCRA curve with regard to reducing recurrence angina. CXQ, Chuanxiongqin tablet; DHI, Danhong injection; FDDP, Fufang Danshen dripping pill; GXSTC, Guanxin Shutong capsule; QYDP, Qishen Yiqi dripping pill; SBP, Shexiang Baoxin pill; Std, standard treatment; TXLC, Tongxinluo capsule; XSC, Xiongshao capsule; SUCRA, surface under the cumulative ranking.
3.7 Secondary outcome: AMI
3.7.1 Traditional pairwise meta-analysis
A traditional pairwise meta-analysis of the included study data was performed. There were nine different interventions and eight pairwise comparisons generated. Only DHI or TXLC was superior to Std (Supplementary Figure S5).
3.7.2 Evidence network
In total, 17 studies including nine medication strategies involving 2,143 patients reported AMI. The studies provided eight direct comparisons (Figure 3C). The number of studies that compared TXLC with Std and the number patients who received the treatment were 7 and 844, respectively, and these are the largest numbers compared with other direct comparisons.
3.7.3 NMA and SUCRA
A total of 36 pairwise comparisons of AMI were generated by NMA (Figure 8). Only two pairwise direct comparisons were statistically significant. Compared with Std, DHI or TXLC reduced the risk of AMI by 89% (RR 0.11, 95% CI 0.00–0.69) and 73% (RR 0.27, 95% CI 0.10–0.64), respectively. As shown in Figure 9 and Supplementary Table S3 for the SUCRA of recurrence angina, DHI was most likely the best intervention. Certainty of evidence for all comparisons was very low to moderate. The details of evidence evaluation are available in Supplementary Table S6.
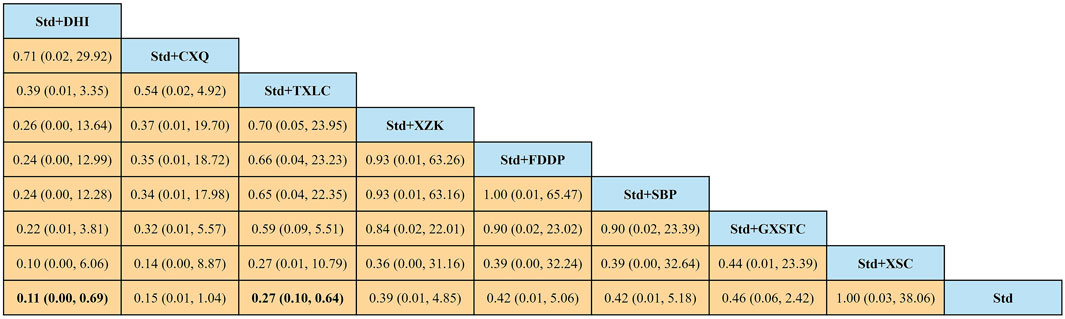
FIGURE 8. Summary of results of acute myocardial infarction from network meta-analysis. CXQ, Chuanxiongqin tablet; DHI, Danhong injection; FDDP, Fufang Danshen dripping pill; GXSTC, Guanxin Shutong capsule; SBP, Shexiang Baoxin pill; Std, standard treatment; TXLC, Tongxinluo capsule; XSC, Xiongshao capsule; XZK, Xuezhikang capsule.
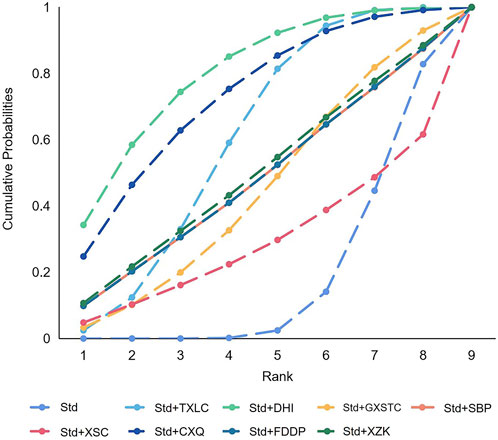
FIGURE 9. SUCRA curve with regard to reducing acute myocardial infarction after PCI. CXQ, Chuanxiongqin tablet; DHI, Danhong injection; FDDP, Fufang Danshen dripping pill; GXSTC, Guanxin Shutong capsule; QYDP, Qishen Yiqi dripping pill; SBP, Shexiang Baoxin pill; Std, standard treatment; TXLC, Tongxinluo capsule; XSC, Xiongshao capsule; SUCRA, surface under the cumulative ranking.
3.8 Secondary outcome: TLR
3.8.1 Traditional pairwise meta-analysis
A traditional pairwise meta-analysis of the included study data was performed. There were 10 different interventions and nine pairwise comparisons generated. CXQ, FDDP, TXLC, or XSC was superior to Std (Supplementary Figure S6).
3.8.2 Evidence network
In total, 15 studies including 10 medication strategies involving 1,839 patients reported TLR. The studies provided nine direct comparisons (Figure 3D). The number of studies that compared DHI with Std and the number of patients who received the treatment were 4 and 477, respectively, and these were the largest numbers compared with other direct comparisons.
3.8.3 NMA and SUCRA
In total, 45 pairwise comparisons of TLR were generated by NMA (Figure 10). In addition to four pairwise direct comparisons, two pairwise indirect comparisons were also statistically significant. Compared with DHI, CXQ reduced the risk of TLR by 92% (RR 0.08, 95% CI 0.00–0.78). Compared with XZK, FDDP reduced the risk of TLR by 91% (RR 0.09, 95% CI 0.00–0.84). As shown in Figure 11 and Supplementary Table S3 for SUCRA, CXQ was most likely the best intervention. Certainty of evidence for all comparisons was very low to moderate. The details of evidence evaluation are available in Supplementary Table S7.
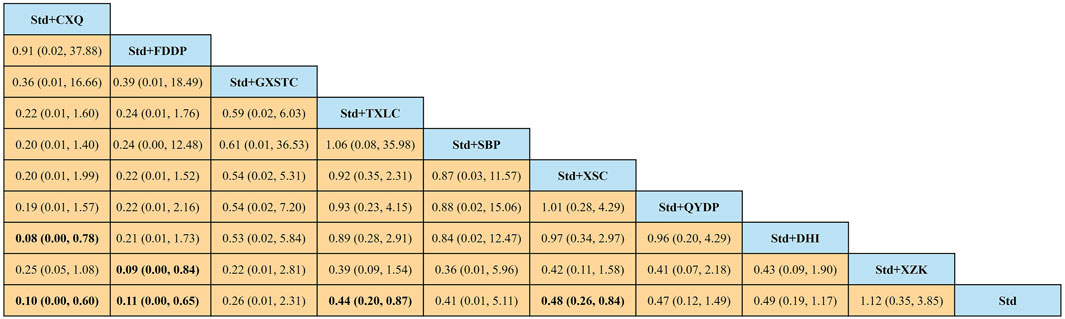
FIGURE 10. Summary of results of target lesion revascularization from network meta-analysis. CXQ, Chuanxiongqin tablet; DHI, Danhong injection; FDDP, Fufang Danshen dripping pill; GXSTC, Guanxin Shutong capsule; QYDP, Qishen Yiqi dripping pill; SBP, Shexiang Baoxin pill; Std, standard treatment; TXLC, Tongxinluo capsule; XSC, Xiongshao capsule; XZK, Xuezhikang capsule.
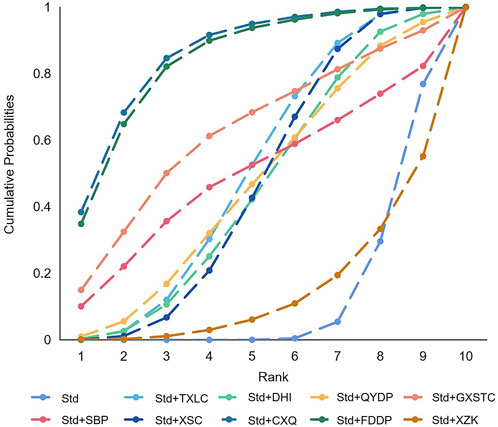
FIGURE 11. SUCRA curve with regard to reducing target lesion revascularization. CXQ, Chuanxiongqin tablet; DHI, Danhong injection; FDDP, Fufang Danshen dripping pill; GXSTC, Guanxin Shutong capsule; QYDP, Qishen Yiqi dripping pill; SBP, Shexiang Baoxin pill; Std, standard treatment; TXLC, Tongxinluo capsule; XSC, Xiongshao capsule; XZK, Xuezhikang capsule; SUCRA, surface under the cumulative ranking.
3.9 Subgroup and sensitivity analysis
The subgroup network meta-analysis for angiographic restenosis showed that QYDP performed better in studies involving ≥100 patients, while CXQ performed better in studies involving <100 patients (Supplementary Figures S7, S8). In sensitivity analysis, the results revealed that removing these studies with a high risk of bias had no discernible impact on the overall analysis (Supplementary Figures S9–S12).
3.10 Publication bias assessment
An assessment was conducted to determine the bias of different drugs regarding angiographic restenosis, recurrence angina, AMI after PCI, and TLR (Supplementary Figure S13). The included studies were represented by dots, with different interventions indicated by varying colors. The symmetry was acceptable.
4 Discussion
In this NMA, we reviewed, compared, and ranked the effectiveness of CPMs combined with Std in preventing restenosis after PCI. A total of 47 randomized controlled studies that enrolled a total of 5,077 patients assigned to 11 interventions were included in this study. Except XZK, CPMs including GXSTC, GXTLC, FDDP, CXQ, SBP, DHI, TXLC, QYDP, and XSC reduced the risk of angiographic restenosis. Among them, GXSTC showed the highest probability of being superior. For recurrent angina, FDDP was demonstrated to be the most effective intervention. In addition, DHI was more effective than other CPMs in preventing AMI after PCI. The network rank of the cumulative probability indicated that CXQ was the optimal treatment for TLR.
In fact, the previous meta-analyses have explored the effectiveness of TCM in preventing restenosis. Mao et al. (2015) conducted a systematic review of 16 RCTs involving 1,063 patients to evaluate the effectiveness of TXLC on patients with coronary heart disease after PCI. Similar to our findings, the results showed that TXLC reduced the risk of angiographic restenosis, recurrent angina, AMI, and revascularization by 84%, 76%, 68%, and 74%, respectively. Furthermore, TXLC exhibited a specific advantage in reducing the occurrence of adverse cardiovascular events without compromising safety on the 6-month course rather than the 3-month course, indicating that the effectiveness of TXLC might be influenced by different time courses (Hui et al., 2022). Our research results are also consistent with the meta-analysis study by Zheng et al. (2013) who reported that XSC has been beneficial in preventing restenosis after PCI in patients with coronary heart disease in certain aspects. Some other systematic reviews and meta-analyses reported that the combination treatment of TCM and Std showed promising results regarding preventing restenosis (Ren et al., 2008; Zheng et al., 2012; Chen et al., 2018; Wu et al., 2019). Compared with these studies, we not only analyzed the effectiveness of each CPM individually in preventing restenosis but also compared the effectiveness of different CPMs using a Bayesian NMA approach.
CPMs prevent restenosis through multiple targets and multiple ways (Shen, 2006). Clinical studies have demonstrated that FDDP (Tang and Zhang, 2015), DHI (Chen et al., 2015), XZK (Zhao et al., 2015), and TXLC (Sun and Lang, 2018) reduced the levels of blood lipids including cholesterol, low-density lipoprotein cholesterol, and triglyceride and suppressed inflammation by lowering hs-CRP, IL-6, TNF-α, and NF-κB. Furthermore, studies with animal models of arterial balloon injury were conducted to reveal the underlying mechanism. Paeonia lactiflora Pall. (Chi shao), one of the main components of TXLC and XSC, can reduce oxidative stress by targeting NADPH oxidase (Zhu and Zhu, 2004), inhibiting the expression of monocyte chemoattractant protein-1 mRNA (Zhu and Mou, 2008), and blocking type I collagen synthesis to prevent restenosis (Zhu and Zhu, 2004). FDDP also inhibits vascular smooth muscle cell proliferation, migration, and extracellular matrix synthesis and promotes the repair of damaged endothelial cells (Ma et al., 2006; Song, 2013), and TXLC can inhibit platelet aggregation (Huang et al., 2001), which are both the vital cause of restenosis.
Restenosis after PCI has remained an important clinical problem due to the higher mortality and poor prognosis once it occurs. Modern medicine generally focuses on the drug-release coatings of stents and balloons such as paclitaxel, sirolimus, everolimus, and rapamycin (Dibra et al., 2005; Stettler et al., 2008; Palmerini et al., 2015; Ahmad et al., 2022), which inhibit vascular smooth muscle cell proliferation and migration and prevent coronary artery restenosis by interrupting the cell cycle (Melnik et al., 2022). However, these agents also suppress the multiplication of vascular endothelial cells, resulting in incomplete endothelization and late thrombosis (Torii et al., 2020). Furthermore, a meta-analysis (Katsanos et al., 2018) reported that paclitaxel-coated balloons and stents increased the risk of death following application in the femoropopliteal artery of the lower limbs. Subsequently, the Federal Drug Administration alerted healthcare providers to continue closely monitoring this problem.
Another way to prevent restenosis is that modern medicine concerned with is the materials of opening the stenosed or occluded coronary arteries. Trials that compared permanent drug-eluting stent implantation with drug-coated balloon dilation (Cortese et al., 2023; 2020; Jeger et al., 2020) or bioresorbable drug-eluting stent implantation (Saito et al., 2014; Zong et al., 2022) have yielded neutral or somewhat exciting results. Nevertheless, drug-coated balloons are only suitable for a limited population and bioresorbable drug-eluting stents face technical challenges, leading to limited clinical application. To the best of our knowledge, there have been few studies on oral contemporary Western medicine to prevent restenosis. The randomized controlled trial named “OPTION” (indobufen or aspirin on top of clopidogrel after coronary drug-eluting stent implantation) (Wu et al., 2023) still failed to elucidate the antiplatelet mechanism. Therefore, considering new therapeutic strategies such as TCM may be feasible based on the complex mechanism involved in restenosis.
Some reasons may affect the extrapolation of our results. Clinical predictors of coronary restenosis include older age, diabetes mellitus, female sex, and higher body mass index (Giustino et al., 2022). However, more than 95% of the patients included in this study were ≤65 years of age, and individual-level data on gender, body mass index, and history of diabetes are not available. The efficacy of Chinese patent medicine in preventing restenosis in these populations is unclear. Future additional randomized controlled trials of different populations are needed.
In summary, this NMA provided a comprehensive picture of the likelihood of a range of CPMs to prevent coronary restenosis. We also reported the rank probability for all 11 treatment strategies including standard treatment using the Bayesian approach. The Bayesian approach has significant advantages over classical frequentist statistical approach for presenting evidence to decision-makers. Therefore, this study provided a reference recommendation for clinicians on further reducing the risk of restenosis for patients receiving PCI and which CAP should be chosen.
Our study also had several limitations. First, included studies did not clearly describe randomization procedures or whether allocation concealment and blinding occurred, leading to “some concerns” about quality of evidence of most studies, which may affect the analysis results. The treatment effects of CPMs must be evaluated by rigorous RCT design in the future. Second, lacking direct head-to-head trials led to our finding of low or very low certainty of evidence. Third, the most effective drugs for different outcomes were inconsistent, confusing clinicians when prescribing them. Based on the above limitation, treatment rank may have a substantial degree of imprecision, and the results in terms of treatment rank should be interpreted with caution although we performed the Bayesian approach.
5 Conclusion
This NMA indicated that addition of CPMs to standard treatment may prevent restenosis in patients with coronary heart disease (CHD) after PCI. GXSTC, FDDP, DHI, and CXQ combined with Std were more effective for angiographic restenosis, recurrence angina, AMI after PCI, and TLR, respectively. However, interpreting these results should be cautious due to the poor quality of original research and low grade of evidence with this study.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author contributions
JF: methodology, writing–original draft, and writing–review and editing. TL: conceptualization and writing–review and editing. FP: methodology and writing–review and editing. NG: data curation, investigation, and writing–review and editing. JW: data curation, investigation, and writing–review and editing. YG: data curation, software, and writing–review and editing. HZ: data curation and writing–review and editing. HZ: supervision, validation, and writing–review and editing. XW: funding acquisition, supervision, and writing–review and editing.
Funding
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (grant number 82274326), National Natural Science Foundation of China (grant number 82074263), and Research Platform Construction Project of the Beijing University of Chinese Medicine (grant number 90070161220034).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1265766/full#supplementary-material
Abbreviations
ACS, acute coronary syndrome; AMI, acute myocardial infarction; CI, confidence interval; CHD, coronary heart disease; CPMs, Chinese patent medicines; CXQ, Chuanxiongqin tablet; DHI, Danhong injection; FDDP, Fufang Danshen dripping pill; GXSTC, Guanxin Shutong capsule; GXTLC, Guanxin Tongluo capsule; NMA, network meta-analysis; PCI, percutaneous coronary intervention; RCT, randomized controlled trial; RR, relative risk; SBP, Shexiang Baoxin pill; SUCRA, surface under the cumulative ranking; TCM, traditional Chinese medicine; TLR, target lesion revascularization; Std, standard treatment; TXLC, Tongxinluo capsule; XSC, Xiongshao capsule; QYDP, Qishen Yiqi dripping pill; XZK, Xuezhikang capsule.
References
Ahmad, W. A. W., Nuruddin, A. A., Abdul Kader, M. A. S. K., Ong, T. K., Liew, H. B., Ali, R. M., et al. (2022). Treatment of coronary de novo lesions by a sirolimus- or paclitaxel-coated balloon. JACC Cardiovasc Interv. 15 (7), 770–779. doi:10.1016/j.jcin.2022.01.012
Brignardello-Petersen, R., Florez, I. D., Izcovich, A., Santesso, N., Hazlewood, G., Alhazanni, W., et al. (2020). GRADE approach to drawing conclusions from a network meta-analysis using a minimally contextualised framework. BMJ 371, m3900. doi:10.1136/bmj.m3900
Chen, X. D., Qian, L. Y., and Gao, R. L. (2008). Clinical efficacy of SXBXW to prevent the restenosis after PCI in patient with coronary artery disease. Chinese Archives of Traditional Chinese Medicine 26 (4), 765–766. doi:10.13193/j.archtcm.2008.04.94.chexd.025
Chen, K., Shi, D., Xu, H., Lü, S., Li, T., Ke, Y., et al. (2006). XS0601 reduces the incidence of restenosis: a prospective study of 335 patients undergoing percutaneous coronary intervention in China. Chin. Med. J. (Engl). 119, 6–13.
Chen, J., Xu, H., Zhou, D. P., Dong, M., and Du, H. L. (2015). The curative effectiveness of Danhong injection in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Liaoning J. Traditional Chin. Med. 42 (02), 341–344. doi:10.13192/j.issn.1000-1719.2015.02.048
Chen, R., Xiao, Y., Chen, M., He, J., Huang, M., Hong, X., et al. (2018). A traditional Chinese medicine therapy for coronary heart disease after percutaneous coronary intervention: a meta-analysis of randomized, double-blind, placebo-controlled trials. Biosci. Rep. 38 (5), BSR20180973. doi:10.1042/BSR20180973
Chinese Cardiovascular Health and Disease report writing group (2023). Report on cardiovascular health and diseases in China 2022: an updated summary. Chin. Circulation J. 38 (6). Serial No.300. doi:10.3969/j.issn.1000-3614.2023.06.001
Cortese, B., Di Palma, G., Guimaraes, M. G., Piraino, D., Orrego, P. S., Buccheri, D., et al. (2020). Drug-coated balloon versus drug-eluting stent for small coronary vessel disease: PICCOLETO II randomized clinical trial. JACC Cardiovasc Interv. 13 (24), 2840–2849. doi:10.1016/j.jcin.2020.08.035
Cortese, B., Testa, G., Rivero, F., Erriquez, A., and Alfonso, F. (2023). Long-Term outcome of drug-coated balloon vs drug-eluting stent for small coronary vessels: PICCOLETO-II 3-year follow-up. JACC Cardiovasc Interv. 16 (9), 1054–1061. doi:10.1016/j.jcin.2023.02.011
Cui, X. P., Yan, W., and He, J. H. (2021). Clinical effect of tigrillo combined with Danhong injection on postoperative PCI of acute myocardial infarction. Journal of Guangxi Medical University 38 (8), 1609–1614. doi:10.16190/j.cnki.45-1211/r.2021.08.026
Dai, G. F., and Yang, S. J. (2011). Clinical observation of Tongxinluo capsule in intervention of restenosis in patients with coronary heart disease after PCI. Guangming Journal of Chinese Medicine 26 (09), 1823–1824. doi:10.3969/j.issn.1003-8914.2011.09.043
Deng, X. D. (2013). Effect of Tongxinluo capsule on restenosis after PCI in patients with CHD. China Medicine and Pharmacy 3 (12), 67–68.
Di, J. (2012). Research on the effect of Tongxinluo capsule on the in-stent restenosis of small vessels and long affection in coronary artery. Master's thesis. China: Nanjing University of Chinese Medicine.
Dibra, A., Kastrati, A., Mehilli, J., Pache, J., Schühlen, H., von Beckerath, N., et al. (2005). Paclitaxel-eluting or sirolimus-eluting stents to prevent restenosis in diabetic patients. N. Engl. J. Med. 353 (7), 663–670. doi:10.1056/NEJMoa044372
Dong, F. X. (2019). Analysis of clinical effect of Guanxin Tongluo capsule on preventing restenosis after stent implantation in coronary heart disease. Journal of Practical Medical Techniques 26 (7), 897–899. doi:10.19522/j.cnki.1671-5098.2019.07.038
Giustino, G., Colombo, A., Camaj, A., Yasumura, K., Mehran, R., Stone, G. W., et al. (2022). Coronary in-stent restenosis: JACC state-of-the-art review. J. Am. Coll. Cardiol. 80 (4), 348–372. doi:10.1016/j.jacc.2022.05.017
Grines, C. L., Harjai, K. J., and Schreiber, T. L. (2016). Percutaneous coronary intervention: 2015 in review. J. Interv. Cardiol. 29 (1), 11–26. doi:10.1111/joic.12272
Han, Z. X. (2018). Effect of Tongxinluo capsule on prevention of restenosis in patients with coronary heart disease after PCI. Modern Medical Imageology 27 (2), 651–652.
Heinrich, M., Appendino, G., Efferth, T., Fürst, R., Izzo, A. A., Kayser, O., et al. (2020). Best practice in research – overcoming common challenges in phytopharmacological research. J. Ethnopharmacol. 246, 112230. doi:10.1016/j.jep.2019.112230
Huang, J., Li, C. J., Yang, G. P., and Yu, G. P. (2001). Efficacy of herbal Tongxinluo on thrombus formation and neointimal hyperplasia of the balloon injured rabbit peripheral arteries. Chin. J. Clin. Pharmacol. Ther. (01), 35–37+98.
Huang, Y. P. (2018). The effect of Guanxin Shutong capsules on inflammatory response and restenosis of patients with acute myocardial infarction undergoing interventional therapy. International Medicine and Health Guidance News 24 (11), 1677–1679. doi:10.3760/cma.j.issn.1007-1245.2018.11.024
Huang, Y. X., Fang, K. F., Zhang, Y. H., Liu, Y. Y., Liu, W. B., Lin, P. H., et al. (2019). Effects of shexiang baoxin pills on TCM symptoms and in-stent restenosis in the patients with acute myocardial infarction after PCI. World Journal of Integrated Traditional and Western Medicine 14 (2), 227–231. doi:10.13935/j.cnki.sjzx.190220
Hui, J., Yuan, R., Li, P., Xin, Q., Miao, Y., Shen, X., et al. (2022). Efficacy and safety of different courses of Tongxinluo capsule as adjuvant therapy for coronary heart disease after percutaneous coronary intervention: a systematic review and meta-analysis of randomized controlled trials. J. Clin. Med. 11 (11), 2991. doi:10.3390/jcm11112991
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of Health care interventions: checklist and explanations. Ann. Intern Med. 162, 777–784. doi:10.7326/M14-2385
Jeger, R. V., Farah, A., Ohlow, M.-A., Mangner, N., Möbius-Winkler, S., Weilenmann, D., et al. (2020). Long-term efficacy and safety of drug-coated balloons versus drug-eluting stents for small coronary artery disease (BASKET-SMALL 2): 3-year follow-up of a randomised, non-inferiority trial. Lancet 396 (10261), 1504–1510. doi:10.1016/S0140-6736(20)32173-5
Jukema, J. W., Verschuren, J. J. W., Ahmed, T. A. N., and Quax, P. H. A. (2012). Restenosis after PCI. Part 1: pathophysiology and risk factors. Nat. Rev. Cardiol. 9 (1), 53–62. doi:10.1038/nrcardio.2011.132
Kang, W. (2017). Clinical effect of Xiongshao Capsule on restenosis in patients with coronary heart disease after PCI. Shenzhen Journal of Integrated Traditional Chinese and Western Medicine 27 (8), 47–48. doi:10.16458/j.cnki.1007-0893.2017.08.023
Katsanos, K., Spiliopoulos, S., Kitrou, P., Krokidis, M., and Karnabatidis, D. (2018). Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 7 (24), e011245. doi:10.1161/JAHA.118.011245
Lawton, J. S., Tamis-Holland, J. E., Bangalore, S., Bates, E. R., Beckie, T. M., Bischoff, J. M., et al. (2022). 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of cardiology/American heart association joint committee on clinical practice guidelines. Circulation 145 (3), e18–e114. doi:10.1161/CIR.0000000000001038
Li, A., Gong, K., Yan, J., Sun, X., Feng, Y., and Zhang, Z. (2004). Effect of Shuxuetong in preventing restenosis after intracoronary stenting. Zhongguo Zhong Xi Yi Jie He Za Zhi 24 (10), 879–881.
Li, B. (2014). Effect of Qishenyiqi Dripping pills on restenosis in stent after coronary artery disease stenting. China Health Care and nutrition 5, 2877–2878.
Li, C. W., Yuan, F., and Li, X. J. (2014). Effect of Qishen Yiqi drop pill on coronary blood flow and left ventricular function in patients with anterior wall AMI after PCI. Chinese Journal of Integrative Medicine on Cardio-Cerebrovascular Disease 12, 1441–1443.
Li, D. J. (2020). Effect of Tongxinluo capsule combined with atorvastatin on restenosis and inflammatory factors in stents after PCI. Journal of Aerospace Medicine 31 (03), 325–327.
Li, G. H., Fu, G. S., and Zhou, X. Q. (2010). Clinical observation of compound Danshen drop pill preventing restenosis of patients with coronary heart disease after stenting operation. Modern Journal of Integrated Traditional Chinese and Western Medicine 19 (8), 920–921.
Li, Q. Z., Hu, G., and Wang, Y. L. (2016). Clinical observation of 34 cases of stent restenosis after PCI treated with Guanxin Tongluo Capsule combined with dual antiplatelet drugs. Guiding Journal of Traditional Chinese Medicine and Pharmacy 22 (3), 80–82. doi:10.13862/j.cnki.cn43-1446/r.2016.03.029
Liang, Y. M., Wang, Z. J., and Su, X. Y. (2010). The effect of Tongxinluo capsules on coronary restennosis of patients with acute myocardial infarction after percutaneoas coronary intervention. Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care 3, 175–176. doi:10.3969/j.issn.1008-9691.2010.03.017
Liu, L., and Jiang, T. (2017). The influence of Tongxinluo capsule and atorvastatin on in-stent restenosis and inflammatory factors after percutaneous coronary intervention. Chinese Journal of Integrative Medicine on Cardio-Cerebrovascular Disease 15 (7), 769–771. doi:10.3969/j.issn.1672-1349.2017.07.001
Liu, S. H. (2019). Application effect of Tongxinluo capsule combined with clopidogrel and aspirin in patients with coronary heart disease after PCI. Clinical Research and Practice 4 (19), 127–128+131. doi:10.19347/j.cnki.2096-1413.201919054
Liu, S. N., and Hou, Y. (2017). Study on the effect of Mongolian medicine Guanxintong Capsule on restenosis after stenting in acute myocardial infarction. Journal of Medicine and Pharmacy of Chinese Minorities 23 (3), 12–13. doi:10.16041/j.cnki.cn15-1175.2017.03.009
Liu, Y., Li, W., and Qi, W. J. (2012). XSC on restenosis after percutaneous coronary intervention effects observed. China Health Industry 9 (1), 17–18. doi:10.16659/j.cnki.1672-5654.2012.02.024
Liu, Y. J., Sun, G. Y., Liu, Z. Y., Zhang, S. S., and Liang, S. L. (2004). Clinical effects of tetramethyl pyrazine on preventing restenosis after percutaneous coronary intervention. Chinese Journal of Cardiovascular Medicine 9 (2), 90–91+95.
Lu, H. W., Chen, J. X., and Zheng, C. (2014). Clinical observation on Tongxinluo capsule combined with routine Western medicine in the prevention of restenosis after percutaneous coronary intervention in 90 cases. J. Tradit. Chin. Med. 55 (24), 2117–2120. doi:10.13288/j.11-2166/r.2014.24.013
Luan, B. (2011). Observation on Danhong injection for prevention of coronary in-stent restenosis. Journal of Jinzhou Medical University 32 (3), 237–239.
Ma, X. J., Zhang, X. H., Ma, H. J., Shao, J. H., and Zhao, T. (2006). Effect of compound Danshen dripping pills on the repair of intimal hyperplasia in balloon injured vessels. J. Clin. Cardiol. 22 (7), 437–438.
Mao, C., Fu, X.-H., Yuan, J. Q., Yang, Z. Y., Chung, V. C. H., Qin, Y., et al. (2015). Tong-xin-Luo capsule for patients with coronary heart disease after percutaneous coronary intervention. Cochrane Database Syst. Rev. 5, CD010237. doi:10.1002/14651858.CD010237.pub2
Melnik, T., Jordan, O., Corpataux, J.-M., Delie, F., and Saucy, F. (2022). Pharmacological prevention of intimal hyperplasia: a state-of-the-art review. Pharmacol. Ther. 235, 108157. doi:10.1016/j.pharmthera.2022.108157
Moussa, I. D., Mohananey, D., Saucedo, J., Stone, G. W., Yeh, R. W., Kennedy, K. F., et al. (2020). Trends and outcomes of restenosis after coronary stent implantation in the United States. J. Am. Coll. Cardiol. 76 (13), 1521–1531. doi:10.1016/j.jacc.2020.08.002
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, 89. doi:10.1186/s13643-021-01626-4
Palmerini, T., Benedetto, U., Biondi-Zoccai, G., Della Riva, D., Bacchi-Reggiani, L., Smits, P. C., et al. (2015). Long-Term safety of drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J. Am. Coll. Cardiol. 65 (23), 2496–2507. doi:10.1016/j.jacc.2015.04.017
Pan, Z. H. (2016). Clinical analysis of Chuanxiongqin in the prevention and treatment of restenosis after percutaneous coronary intervention. Asia-Pacific Traditional Medicine 12 (18), 143–144. doi:10.11954/ytctyy.201618070
Puhan, M. A., Schünemann, H. J., Murad, M. H., Li, T., Brignardello-Petersen, R., Singh, J. A., et al. (2014). A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 349, g5630. doi:10.1136/bmj.g5630
Qin, W. (2018). To study the effect of Danhong injection on restenosis and thrombosis in coronary stent. Journal of Aerospace Medicine 29 (10), 1241–1242.
Ren, Y., Chen, K.-J., and Ruan, X.-M. (2008). Systematic review of randomized controlled trials on preventing and treating restenosis after percutaneous coronary intervention with Chinese medicine. Zhongguo Zhong Xi Yi Jie He Za Zhi 28 (7), 597–601.
Rivera, D., Allkin, R., Obón, C., Alcaraz, F., Verpoorte, R., and Heinrich, M. (2014). What is in a name? The need for accurate scientific nomenclature for plants. J. Ethnopharmacol. 152 (3), 393–402. doi:10.1016/j.jep.2013.12.022
Saito, S., Valdes-Chavarri, M., Richardt, G., Moreno, R., Iniguez Romo, A., Barbato, E., et al. (2014). A randomized, prospective, intercontinental evaluation of a bioresorbable polymer sirolimus-eluting coronary stent system: the CENTURY II (clinical evaluation of new terumo drug-eluting coronary stent system in the treatment of patients with coronary artery disease) trial. Eur. Heart J. 35 (30), 2021–2031. doi:10.1093/eurheartj/ehu210
Shen, W. F., and Liu, H. (2006). Potential role of Chinese medicinal herbs in the prevention of coronary artery restenosis. Chin. Med. J. Engl. 119 (1), 3–5. doi:10.1097/00029330-200601010-00001
Si, X. C., and Jia, Y. P. (2013). Effect of Shexiang Baoxin pellet on long-term prognosis in patients with coronary disease after PCI. J. Chin. Clin. Med. 5 (1), 5–6. doi:10.3969/j.issn.1674-7860.2013.01.002
Song, J. L. (2013). Study on the effect and molecular mechanism of Fufang danshen dropping pills in restenosis after angioplasty. China Pract. Med. 8 (05), 259–260. doi:10.14163/j.cnki.11-5547/r.2013.05.012
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Stettler, C., Allemann, S., Wandel, S., Kastrati, A., Morice, M. C., Schömig, A., et al. (2008). Drug eluting and bare metal stents in people with and without diabetes: collaborative network meta-analysis. BMJ 337, a1331. doi:10.1136/bmj.a1331
Sun, Z. R., and Lang, Z. L. (2018). Effect of Tongxinluo capsule on NF-κB, IL-6 and TNF-α expression after coronary stenting in patients with coronary artery heart disease. Chin. J. Front. Med. Sci. Electron. Version) 10 (08), 43–45. doi:10.12037/YXQY.2018.08-10
Sutton, A. J., and Abrams, K. R. (2001). Bayesian methods in meta-analysis and evidence synthesis. Stat. Methods Med. Res. 10 (4), 277–303. doi:10.1177/096228020101000404
Tang, Y., and Zhang, H. (2015). Clinical research of compound Danshen dripping pills in preventing and curing coronary heart disease with restenosis after percutaneous coronary intervention. J. Community Med. 13 (21), 16–19.
Tian, F. (2017). Effect of Tongxinluo capsule combined with atorvastatin on stent restenosis and inflammatory factors in patients after PCI. Journal of Huaihai Medicine 35 (06), 644–646. doi:10.14126/j.cnki.1008-7044.2017.06.006
Tian, F. L., Wei, W. L., Zhang, E. J., Zhang, B., Li, X. Y., Zhu, J. Y., et al. (2012). Intervention of Qishen Yiqi Diwan combining atorvastatin to coronary in-stent restenosis. Chinese Journal of Evidence-Based Cardiovascular Medicine 4 (3), 224–227.
Torii, S., Jinnouchi, H., Sakamoto, A., Kutyna, M., Cornelissen, A., Kuntz, S., et al. (2020). Drug-eluting coronary stents: insights from preclinical and pathology studies. Nat. Rev. Cardiol. 17 (1), 37–51. doi:10.1038/s41569-019-0234-x
Tsao, C. W., Aday, A. W., Almarzooq, Z. I., Alonso, A., Beaton, A. Z., Bittencourt, M. S., et al. (2022). Heart disease and stroke statistics-2022 update: a report from the American heart association. Circulation 145 (8), e153–e639. doi:10.1161/CIR.0000000000001052
Tu, W. L., Huang, Q., Chen, Z. Q., Huang, X., Hu, J., and Li, L. F. (2021). Qishen-Yiqi dripping pills on effect of in-stent restenosis after PCI for type 2 diabetes mellitus patients with ACS. Jiangxi Medical Journal 56 (4), 422–423+427. doi:10.3969/j.issn.1006-2238.2021.04.004
Wang, H. (2013). The curative effect of Qishen Yiqi drop pills in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Master's thesis. China: Tianjin Medical University.
Wang, Q. S., Liu, D. L., Lv, Y. X., and Dong, Y. C. (2014). The influence of Guanxinshutong capsules on in-stent restenosis after coronary stent implantation. Chinese Journal of Cardiovascular Research 12 (8), 757–761.
Wang, X. D., and Lou, B. (2010). Effect of Tongxinluo capsule on restenosis after stenting of small vessels and long affection in type 2 diabetes mellitus. Journal of Nanjing University of Traditional Chinese Medicine 26 (06), 464–467. doi:10.14148/j.issn.1672-0482.2010.06.010
Wang, Y. H., and Shi, H. N. (2009). Curative effect of Tongxinluo capsule on restenosis after coronary stenting. Modern Journal of Integrated Traditional Chinese and Western Medicine 18 (01), 39.
Wang, Z. H., Xu, Y., Ma, L. H., Zhao, Z., Yue, M., and Yan, Z. H. (2011). Effect of Danhong Injection on restenosis and thrombosis in coronary stent. Chinese Journal of Gerontology 31 (16), 3151–3152.
Wu, H., Xu, L., Zhao, X., Zhang, H., Cheng, K., Wang, X., et al. (2023). Indobufen or aspirin on top of clopidogrel after coronary drug-eluting stent implantation (option): a randomized, open-label, end point-blinded, noninferiority trial. Circulation 147 (3), 212–222. doi:10.1161/CIRCULATIONAHA.122.062762
Wu, J., Zhao, L., Lin, K., Lu, L., and Luo, C. (2019). Chinese herbal medicines for restenosis after percutaneous coronary intervention: a meta-analysis of randomized controlled trials. J. Altern. Complement. Med. 25 (10), 983–992. doi:10.1089/acm.2018.0516
Xiao, H. B., Zhang, D. D., and Gu, J. (2007). Effects of Tongxinluo on C-reactive protein and clinical prognosis in patients after coronary stenting. Journal of Interventional Radiology 8, 520–522.
Xiao, Y., Gu, W. X., and Gao, Y. (2010). Clinical study on prevention of restenosis after stenting in patients with coronary heart disease by Chuanxiongqin. Shaanxi Medical Journal 39 (8), 1070–1071.
Xu, H., Shi, D., and Chen, K. (2000). Clinical effect of Xiongshao capsule on preventing restenosis post-PTCA or/and stenting. Zhongguo Zhong Xi Yi Jie He Za Zhi 20 (7), 494–497.
Yang, J. H., Yang, X. L., Sun, Z. H., Zong, T. Y., Yao, L., and Wang, S. J. (2014). Effect of Shexiang Baoxin Pills combined with trimetazidine on restenosis after coronary stenting in elderly patients with angina pectoris. Chinese Journal of Clinical Rational Drug Use (7), 325.
Yao, F. M., Liu, N., and Ge, G. Y. (2006). Clinical study of Tongxinluo capsule intervention on restenosis after PCI in patients with coronary heart disease. Chinese Journal of Difficult and Complicated 3, 191–192.
Zhao, G. Y., Geng, X. B., Li, L., Zhao, B. Q., and Tian, M. R. (2015). Evaluation of efficacy and safety of coronary artery restenosis after coronary stenting in patients with acute myocardial infarction. J. North China Univ. Sci. Technol. Heal. Sci. Ed. 17 (04), 22–24. doi:10.19539/j.cnki.2095-2694.2015.04.007
Zheng, G. H., Liu, J. P., Chu, J. F., Mei, L., and Chen, H. Y. (2013). Xiongshao for restenosis after percutaneous coronary intervention in patients with coronary heart disease. Cochrane Database Syst. Rev. 5, CD009581. doi:10.1002/14651858.CD009581.pub2
Zheng, G. H., Liu, J. P., Wang, N. S., Chen, H. Y., and Chu, J. F. (2012). Systematic review of Chinese herbal medicines for preventing in-stent coronary restenosis after percutaneous coronary intervention. Evid. Based Complement. Altern. Med. 2012, 253409. doi:10.1155/2012/253409
Zhou, J., and Guo, J. T. (2007). Clinical study of Tongxinluo on preventing restenosis of stents in CHD. Hebei Medicine (10), 1188–1191.
Zhou, Y. S., Mao, S., Guo, L. H., Gao, X. Y., Zou, X., and Zhang, M. Z. (2021). Effect of tongguan capsules on restenosis after coronary stent implantation: study protocol for A randomized controlled trial. Chin. J. Integr. Med. 27 (1), 16–23. doi:10.1007/s11655-020-2722-6
Zhu, H. B., Zhang, C. Q., Zhang, W. N., and Wang, F. L. (2016). Prevention and treatment of in-stent restenosis after PCI by Guanxintongluo capsule combined dual antiplatelet drugs. Chinese J. Rehabilitation Med. 25 (6), 629–632. doi:10.3969/j.issn.1008-0074.2016.06.20
Zhu, H. M., and Mou, H. M. (2008). Effectiveness of Chishao on monocyte chemotactic protein-1 Mrna expression of vascular intima after balloon injury in cholesterol-fed rabbits. Chin. J. Integr. Traditional West. Med. Intensive Crit. Care (03), 138–141+193.
Zhu, H. M., and Zhu, P. D. (2004). Experimental study on preventive effect of Radix Paeoniae Rubra to restenosis after carotid balloon injury in high fat-diet rabbits. Chin. J. Integr. Traditional West. Med. 24 (6), 538–540.
Zhuang, H., Zhang, S. C., Yin, J., Jiang, W., Yao, F., Liu, B., et al. (2019). Therapeutic effect of Mongolian medicine Guanxinshutong capsule on restenosis after stenting in AMI patients. Chinese J. Rehabilitation Med. 28 (2), 237–240. doi:10.3969/j.issn.1008-0074.2019.02.27
Keywords: Chinese patent medicines, restenosis, percutaneous coronary intervention, efficacy, Bayesian network meta-analysis
Citation: Fan J, Li T, Pu F, Guo N, Wang J, Gao Y, Zhao H, Wang X and Zhu H (2024) Comparative efficacy of different Chinese patent medicines in preventing restenosis after percutaneous coronary intervention: a systematic review and Bayesian network meta-analysis of randomized clinical trials. Front. Pharmacol. 14:1265766. doi: 10.3389/fphar.2023.1265766
Received: 24 July 2023; Accepted: 21 November 2023;
Published: 05 January 2024.
Edited by:
Jeff Guo, University of Cincinnati, United StatesReviewed by:
Zhikang Ye, McMaster University, CanadaXiaomo Xiong, University of Cincinnati, United States
Copyright © 2024 Fan, Li, Pu, Guo, Wang, Gao, Zhao, Wang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyan Zhu, emh1aGFpeWFuZ3lAMTYzLmNvbQ==; Xian Wang, d3g2NTA1MTVAMTYzLmNvbQ==
†These authors share first authorship
 Jiasai Fan
Jiasai Fan Tianli Li
Tianli Li Fenglan Pu
Fenglan Pu Nan Guo4†
Nan Guo4†