- Anhui Provincial Engineering Laboratory for Screening and Re-evaluation of Active Compounds of Herbal Medicines in Southern Anhui, Anhui Provincial Engineering Research Center for Polysaccharide Drugs, Anhui Innovative Center for Drug Basic Research of Metabolic Diseases, School of Pharmacy, Wannan Medical College, Wuhu, China
This manuscript provides an in-depth review of the significance of quality control in herbal medication products, focusing on its role in maintaining efficiency and safety. With a historical foundation in traditional medicine systems, herbal remedies have gained widespread popularity as natural alternatives to conventional treatments. However, the increasing demand for these products necessitates stringent quality control measures to ensure consistency and safety. This comprehensive review explores the importance of quality control methods in monitoring various aspects of herbal product development, manufacturing, and distribution. Emphasizing the need for standardized processes, the manuscript delves into the detection and prevention of contaminants, the authentication of herbal ingredients, and the adherence to regulatory standards. Additionally, it highlights the integration of traditional knowledge and modern scientific approaches in achieving optimal quality control outcomes. By emphasizing the role of quality control in herbal medicine, this manuscript contributes to promoting consumer trust, safeguarding public health, and fostering the responsible use of herbal medication products.
1 Introduction
Herbal medication products have been utilized for centuries as a primary form of healthcare in many cultures worldwide (Motti, 2021). Derived from plants and plant-derived materials, these products offer a rich source of bioactive compounds with potential therapeutic benefits (Chrysant and Chrysant, 2017). The use of herbal medicines spans diverse traditional systems, such as traditional Chinese medicine (TCM), ayurveda, and indigenous healing practices (Liu et al., 2012; Kabak and Dobson, 2017). One of the key reasons for the enduring popularity of herbal medication products is their perceived natural origin and historical use. Many societies have a long-established tradition of utilizing herbal remedies to address a wide range of ailments and promote overall wellbeing (Liu et al., 2006; Alipour et al., 2022). This traditional knowledge has been passed down through generations, offering valuable insights into the healing properties of various plant species (Cho and Kim, 2020). Herbal medication products have demonstrated therapeutic potential across various health conditions, including digestive disorders, respiratory ailments, chronic pain, and immune system support (Ernst, 1998; Kaefer and Milner, 2008; Shaikh et al., 2020). Their bioactive constituents, such as alkaloids, flavonoids, terpenes, and polyphenols, can interact with biological systems, offering potential therapeutic benefits (Wu et al., 2005; Wang and Liu, 2014; Brown, 2017). However, the growing demand for herbal medication products also raises concerns about quality control, safety, and efficacy (Osman et al., 2019; Zhou et al., 2019; Tao et al., 2023). Ensuring the consistent quality and standardization of herbal products is crucial to guarantee their safety, efficacy, and reproducibility (Rahath Kubra et al., 2016). Rigorous quality control measures are essential to protect the health and safety of consumers (Deutch et al., 2019). By implementing stringent testing and quality assurance protocols, potential risks such as contamination, adulteration, and the presence of harmful substances can be minimized, ensuring that the herbal medication products are safe for consumption (Giacometti et al., 2018). Insufficient quality control can pose serious safety risks to consumers. Contaminants, adulterants, or incorrect formulations in herbal medication products may lead to adverse reactions, toxicity, or other health complications (Chen et al., 2018; Cai et al., 2019). Inadequate quality control may result in inconsistent levels of active compounds in herbal medication products (Liu et al., 2020). This inconsistency can lead to variable therapeutic effects, making it challenging for healthcare professionals to prescribe and manage patient treatments effectively (Ma et al., 2022). Quality control measures help maintain consistency in the composition and potency of herbal medication products (Yago and Pla, 2020). Through standardized manufacturing processes and testing methods, the levels of active compounds can be monitored and controlled, ensuring that the products deliver the desired therapeutic effects consistently (Parr et al., 2001; Braga et al., 2020). This commitment helps build trust among consumers, healthcare professionals, and regulatory authorities. The purpose of the review is to provide a comprehensive overview of the importance, methods, and considerations involved in ensuring the quality, efficiency, and safety of herbal medication products.
2 Overview of herbal medication products
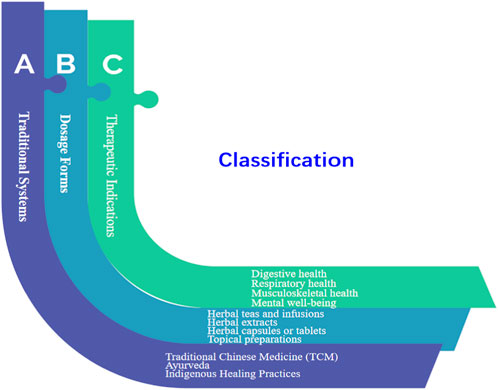
Herbal medication products, also known as herbal medicines or phytotherapeutic products, refer to medicinal products derived from plants or plant materials (Liu et al., 2013). These products utilize the therapeutic properties of various plant species, including their leaves, flowers, roots, stems, or extracts, to promote health and treat or prevent diseases (Sinha et al., 2015). Herbal medication products often contain a combination of active compounds, such as alkaloids, flavonoids, terpenes, and phenolic compounds, which contribute to their pharmacological effects (Chang, 2000; Ranasinghe et al., 2015). Herbal medication products can be classified based on different criteria. Based on different classification methods, we summarize the classification rules of traditional Chinese herbal medicine (Figure 1). Traditional Chinese medicine (TCM) and Ayurvedic herbal medication products have both holistic system of healthcare and healing that has been practiced for over 2,000 years in China and other parts of East Asia (Balachandran and Govindarajan, 2005; Joshi et al., 2017; Prasad et al., 2021). TCM uses a wide range of medicinal herbs, minerals, and animal products to restore balance and treat various health conditions (Wang et al., 2022). These natural substances have been used for centuries for their therapeutic properties and health benefits (Dina et al., 2022). Herbal formulas are often prescribed based on the individual’s unique pattern of disharmony (Zhu et al., 2019). Different herbs have specific chemical compounds that exert various effects on the body. For example, echinacea is used to boost the immune system, ginger for digestive issues, and ginkgo biloba for cognitive function (Karsch-Völk et al., 2014; Sharifi-Rad et al., 2018). Certain minerals and mineral-rich substances are used in traditional medicine for their therapeutic effects. For instance, calcium, magnesium, iron, sulphur, and zinc were supplied for bone health and muscle function, the treatment of anemia and to boost hemoglobin levels, skin conditions like acne and eczema, the immune system, and wound healing (Harper et al., 1987; Song et al., 2021; Xiao et al., 2022). Traditional medicine systems, particularly in East Asia, have used animal products for their medicinal properties (Cheng et al., 2022). Some examples include deer antler velvet which is used to strengthen the body, improve energy, and support joint health, and bear bile used in some traditional Chinese remedies, though the use of bear bile is controversial due to animal cruelty concerns (Wu et al., 2013; Yu et al., 2017). Cordyceps is a fungus that parasitizes insects and is used for various health benefits, including respiratory support and energy enhancement (Chen et al., 2017; Lou et al., 2019; Ashraf et al., 2020; Yang et al., 2020). It is essential to note that while traditional medicine systems have been using these substances for generations, the safety and efficacy of medicinal herbs, minerals, and animal products are not always supported by modern scientific evidence (Yuan et al., 2016). Some of these substances may interact with medications or have potential side effects (Saini et al., 2022). Therefore, it is crucial to consult with qualified healthcare professionals, such as herbalists or traditional medicine practitioners, who have knowledge and experience in the safe use of these natural remedies. In modern times, there is an increasing interest in studying traditional medicinal practices and evaluating the therapeutic potential of these natural substances through rigorous scientific research (Schwabl and Vennos, 2015; Jaiswal et al., 2016). It is precisely because of the diversity of Chinese herbal medicines that the quality control of the herbal medicine industry is particularly important for the efficiency and safety of herbal medication products (Kankanamalage et al., 2014). So, integrative medicine approaches seek to combine the best practices from traditional and modern medicine to provide comprehensive and personalized healthcare solutions.
3 Quality control for herbal medication products
Quality control is a systematic approach that involves monitoring and controlling various aspects of herbal product development, manufacturing, and distribution to guarantee consistent product quality (Ding et al., 2008). Quality control measures are essential in any industry or organization as they play a significant role in ensuring that products or services meet the expected standards and specifications (Osman et al., 2019; Chen et al., 2023). Effective quality control measures involve evaluating processes and identifying areas for improvement (Qin et al., 2012; Zhao et al., 2018; Gong et al., 2023). By streamlining operations, eliminating bottlenecks, and addressing inefficiencies, organizations can enhance productivity and achieve higher output levels with fewer resources (Gong et al., 2023).
3.1 Standardization and identification of herbs
The first step is to built standardization and identification of herbs for effective quality control measures. Standardization and identification of herbs involve establishing consistent and reliable levels of active compounds or markers in herbal medication products (Aleksieva and Yordanov, 2018). It aims to minimize batch-to-batch variability and ensure that each product meets predetermined quality standards (Ketai et al., 2000). Identify the key active compounds or markers in the herb that contribute to its therapeutic properties. Some key aspects of them involve active compound identification, quantitative analysis, and reference standards, which can be done through scientific research, traditional knowledge, or existing literature (Zhao et al., 2022). Develop methods to quantitatively measure the levels of active compounds or markers (An et al., 2022). This can involve techniques such as chromatography (HPLC, GC), spectroscopy (UV-Vis, IR), or specific chemical assays (Pan et al., 2018). Establish reference standards or reference materials that represent the desired levels of active compounds or markers (Xiong et al., 2022). These standards act as benchmarks for comparison during quality control testing and help ensure consistency across batches (Jin et al., 2018). Standardization provides a means to monitor and control the quality and efficacy of herbal medication products, enabling healthcare professionals to prescribe treatments with confidence.
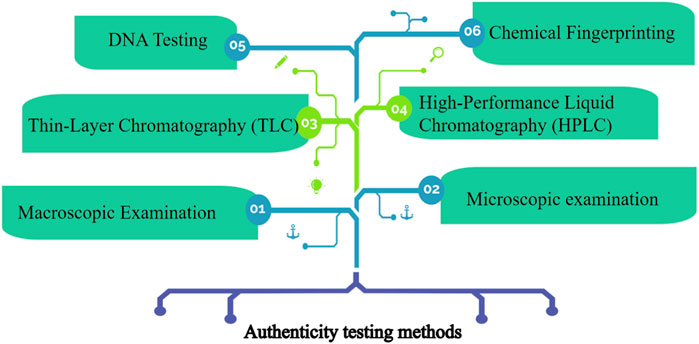
Authenticity testing methods are essential in quality control for herbal medication products to ensure the accurate identification and verification of the herbs used to ensure that the correct herb is being used, as different species or plant parts may have varying therapeutic properties and safety profiles (Zhang et al., 2022). Herb identification is the process of accurately identifying the botanical species or plant material used in herbal medication products (Lo and Shaw, 2019). Some commonly employed authenticity testing methods involve macroscopic examination, microscopic examination, thin-layer chromatography, high-performance liquid chromatography, DNA Barcoding, and chemical profiling (Figure 2).
Macroscopic Examination involves visual inspection and examination of the physical characteristics of the herb, including its color, shape, size, texture, and any unique features (K et al., 2021). Macroscopic examination helps in identifying the herb and distinguishing it from other similar-looking plants. Microscopic examination involves the use of a microscope to examine the cellular structures and characteristic features of the herb (Xiong et al., 2019). This method helps in identifying specific plant parts, such as leaves, stems, or roots, and verifying their authenticity. TLC is a technique used to separate and analyze the chemical constituents of a herb. It involves applying a thin layer of the herb extract onto a solid support, which is then developed using a suitable solvent system (Poole, 2003; Cheng et al., 2011). The resulting chromatogram can be compared to reference standards to identify the herb and detect any adulterants or contaminants (Del Bubba et al., 2013). HPLC is a powerful analytical technique that separates and quantifies the chemical compounds in a herb (Sontag et al., 2019). It can be used to determine the presence and concentration of specific marker compounds or active ingredients, ensuring the consistency and quality of herbal medication products (Malherbe et al., 2012). DNA testing, specifically DNA barcoding, is a molecular technique used to authenticate and identify herbal species (Li et al., 2015). It involves sequencing a specific region of the herb’s DNA, such as the barcode region, which is unique to each species. By comparing the obtained DNA sequence with a reference database, the herb’s genetic identity can be determined, ensuring the use of the correct species and detecting any potential adulteration or substitution (DeSalle et al., 2005; Senapati et al., 2022). DNA testing provides a highly reliable method for herb identification and is particularly useful when the herbs are in processed or powdered forms. Next, chemical fingerprinting involves analyzing the chemical constituents of herbs using analytical techniques such as high-performance liquid chromatography (HPLC), gas chromatography-mass spectrometry (GC-MS), or nuclear magnetic resonance (NMR) (Wibowo et al., 2015; Li et al., 2022). By comparing the chemical profiles obtained from a herb sample with established reference profiles, manufacturers can verify the authenticity, consistency, and quality of the herbs. Chemical fingerprinting helps identify specific marker compounds or active ingredients, ensuring the desired potency and therapeutic efficacy of herbal medication products (Gainza et al., 2020). Chemical fingerprinting and DNA testing provide objective and scientific data for quality control in herbal medication products (Yin et al., 2022). By integrating these techniques into quality control processes, manufacturers can establish robust standards, maintain consistency, meet regulatory requirements, verify the authenticity of herbal ingredients, detect any adulterations or contaminants, and ensure the consistency and safety of herbal medication products. It is worth noting that macroscopic and microscopic examination should be complemented with other testing methods, such as Thin-Layer Chromatography (TLC), High-Performance Liquid Chromatography (HPLC), DNA barcoding, or chemical profiling, to provide a comprehensive quality control approach for herbal medication products (Chen et al., 2006; Hu et al., 2021). These authenticity testing methods help ensure the accurate identification and quality control of herbs used in herbal medication products that may compromise the safety and efficacy of the products.
3.2 Processing and manufacturing controls
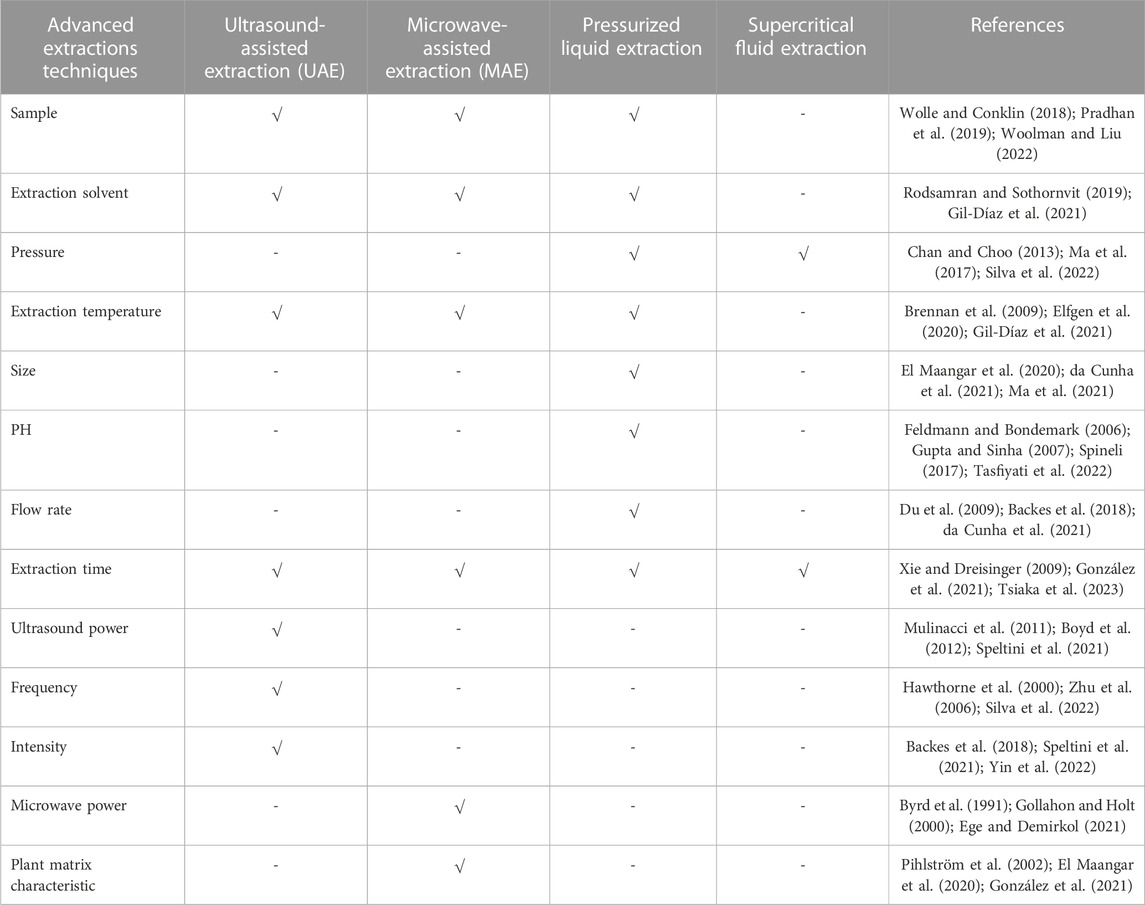
Extraction methods play a crucial role in obtaining the desired active compounds from herbs during the manufacturing process of herbal medication products (Boutron et al., 2007; Abeillé et al., 2020). Optimizing extraction methods is critical to ensure the efficient extraction of bioactive compounds from herbs, which directly impacts the potency and efficacy of herbal medication products (Zhu et al., 2006). Some key points regarding extraction methods and optimization involve selection of solvent, pre-processing of herbal material, ratio of solvent to herbal material, temperature and time, extraction techniques, multiple extractions, pH adjustment, use of co-solvents or enhancers, quality control, safety considerations, sustainability, validation and reproducibility (Hawthorne et al., 2000; Boateng et al., 2023). The selection of the appropriate extraction technique depends on factors such as the nature of the herb, targeted bioactive compounds, desired product characteristics, and manufacturing scale (Gollahon and Holt, 2000). Each technique has its advantages and limitations, and it is important to choose the most suitable method for efficient extraction (Silva et al., 2022). First of all, the choice of solvent is crucial for successful extraction (Brennan et al., 2009). Different solvents have varying polarities and can selectively extract different compounds (Kalkan et al., 2019). Common solvents used include water, ethanol, methanol, and their mixtures. The solvent selection should consider the solubility of target compounds, potential toxicity, environmental impact, and regulatory requirements (Tasfiyati et al., 2022). It is important to optimize the solvent-to-herb ratio to achieve optimal extraction efficiency. Besides, optimization of extraction parameters such as temperature, time, and pressure is essential to maximize the extraction of bioactive compounds while maintaining their stability and minimizing degradation (Ma et al., 2021). These parameters can vary depending on the herb and its targeted compounds. Factors like temperature and extraction duration should be carefully controlled to prevent the loss of heat-sensitive compounds or the degradation of thermally unstable components (Feldmann and Bondemark, 2006). Apparently, pre-treatment techniques, such as size reduction (grinding, milling), drying, or specific treatments like blanching, can influence the efficiency of extraction (Wibowo et al., 2015; An et al., 2022). These techniques help in breaking down the plant matrix, facilitating the release of bioactive compounds and improving their accessibility to the extraction solvent (Dai et al., 2022). Once the extraction method is established, process validation should be performed to ensure consistent and reproducible extraction results. Process validation involves confirming that the extraction process consistently meets predetermined quality standards, including extraction efficiency, yield, and the presence of desired bioactive compounds (González et al., 2021; Kapadia et al., 2022). Validation parameters may include analytical testing, comparison with reference samples, and statistical analysis (Campuzano and González-Martínez, 2017; Onishi et al., 2018). Various extraction techniques and extraction parameters are listed, including maceration, percolation, reflux, Soxhlet extraction, ultrasound-assisted extraction, and supercritical fluid extraction (Table 1).
3.3 Quality assessment during processing
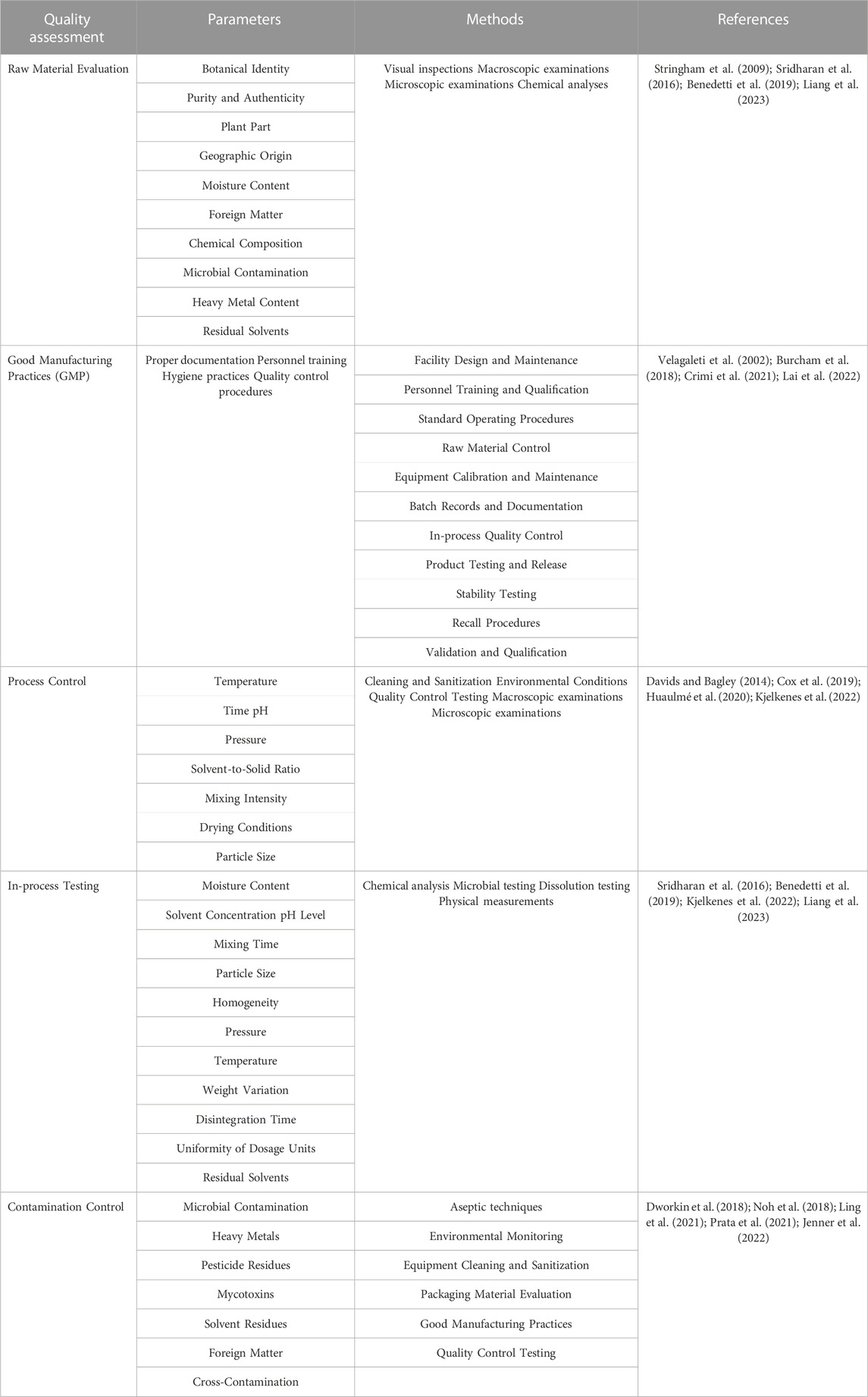
Quality assessment during processing is a crucial aspect of ensuring the safety, efficacy, and consistency of herbal medication products (Fillingim et al., 2016). It involves monitoring and evaluating various parameters and factors throughout the manufacturing process (Sargeant et al., 2022). Here, some key points regarding quality assessment were summarized during processing (Table 2).
3.3.1 Raw material evaluation
The quality assessment starts with a thorough evaluation of the raw materials, which are the herbs used in the product (Liang et al., 2023). Raw material evaluation methods includes checking the identity, authenticity, purity, and quality of the herbs, which involve a combination of techniques and tests to assess the quality, authenticity, and safety of raw materials used in the production of herbal medication products (Sridharan et al., 2016; Benedetti et al., 2019). First of all, it is an organoleptic evaluation that involves the examination of raw materials using the senses, including sight, smell, taste, and touch (Sridharan et al., 2016). It helps identify any abnormalities or inconsistencies in appearance, odor, and taste. Next, a microscopic examination of plant material can help verify its identity and detect any adulteration or foreign substances (Saletnik et al., 2021; Zhang et al., 2021). High-performance thin-layer chromatography (HPTLC) is used to separate and identify different chemical components in a sample. It is often employed to verify the presence of specific markers or active compounds in herbal raw materials (Vestal, 1984; Hussain et al., 2015; Sontag et al., 2019). Fourier transform infrared spectroscopy (FTIR) is used to identify functional groups in raw materials, helping to assess their chemical composition (Agustika et al., 2022; Barnes et al., 2023). Atomic absorption spectroscopy (AAS) is employed to detect and quantify the presence of heavy metals in raw materials, ensuring they are within safe limits (Qu et al., 2016; Ortegón et al., 2022). High-performance liquid chromatography is utilized for quantitative analysis of active compounds in herbal materials, ensuring that they meet specified quality standards (Olsson et al., 2014; Xie et al., 2014). And total organic carbon (TOC) analysis is used to determine the total amount of organic carbon present in a sample, which can indicate the presence of contaminants (Shao et al., 2022; Lee et al., 2023). By using a combination of these raw material evaluation methods, manufacturers can ensure the quality, authenticity, and safety of the herbal ingredients used in their products. These evaluations are crucial for maintaining consistent product quality, meeting regulatory standards, and providing consumers with safe and effective herbal medication products.
3.3.2 Good manufacturing practices for herbal products
Good Manufacturing Practices (GMP) guidelines are essential to the herbal industry to maintain product quality and protect consumer health (Lesch et al., 2021). GMP for herbal products is a set of guidelines and principles that ensure the quality, safety, and consistency of herbal medicines, supplements, and other herbal products (Castiglia et al., 2018). It involves raw material sourcing and identification, facility and equipment, standard operating procedures, batch records and documentation, quality control testing, validation and qualification, stability testing, recalls and complaints, regulatory compliance, and continuous improvement (Boyd, 1994; Jacquemart et al., 2016; Cundell et al., 2023). GMP also involve proper documentation, personnel training, hygiene practices, and quality control procedures to ensure consistent quality throughout the manufacturing process (Castiglia et al., 2018). GMP requires the use of high-quality, authentic, and properly identified herbal raw materials. Suppliers should be carefully selected and qualified to ensure the consistency and purity of the ingredients (Lesch et al., 2021). GMP-compliant facilities should be designed, maintained, and operated in a manner that prevents cross-contamination, ensures cleanliness, and provides a controlled environment for manufacturing (Melethil, 2006; Bai et al., 2022). GMP emphasizes the development and implementation of written standard operating procedures for all critical manufacturing processes (Shukla and Gottschalk, 2013). These procedures help ensure consistent and controlled production. Detailed batch records and documentation should be maintained for each product manufactured (Mager et al., 2007). This includes information about raw materials, manufacturing steps, quality control tests, and any deviations or corrective actions taken during production (Deagle et al., 2017; Smith, 2020). GMP requires routine quality control testing of raw materials, in-process samples, and finished products (Morgan et al., 2015). Testing may include the identification of herbal ingredients, quantitative analysis of active compounds, and evaluation of contaminants. Processes, equipment, and analytical methods used in herbal product manufacturing should be validated to demonstrate their effectiveness and accuracy (Williams et al., 2012). Herbal products should undergo stability testing to determine their shelf life and storage conditions (Cundell et al., 2023). This helps ensure that the product retains its quality and potency throughout its designated shelf life. Regular audits, self-inspections, and reviews of manufacturing processes help identify areas for enhancement and ensure ongoing compliance with GMP principles (Deagle et al., 2017). Finally, adhering to good manufacturing practices is essential for the herbal products industry to maintain product quality, safety, and consistency. GMP guidelines promote the use of standardized procedures, robust quality control, and proper documentation to ensure that herbal products meet the required standards and are safe for consumers (Smith, 2020). By following GMP principles, manufacturers can build trust with consumers, healthcare professionals, and regulatory authorities, contributing to the growth and acceptance of herbal products in the healthcare market.
3.3.3 Process control
Monitoring and controlling critical parameters during processing is essential to maintain product quality (Qu et al., 2021). This includes parameters such as temperature, pressure, pH, mixing time, and drying conditions (Morgan et al., 2015). Regular monitoring and documentation of these parameters help identify any deviations from the desired specifications and allow for necessary adjustments or corrective actions to ensure product consistency (Wasalathanthri et al., 2020). Temperature control is vital in many processes as it directly impacts chemical reactions, phase changes, and microbial growth (Reischauer et al., 2009). Precise temperature control ensures that reactions proceed as intended, preventing unwanted by-products and ensuring the desired product quality. In pharmaceutical manufacturing, maintaining the correct temperature during drug synthesis helps produce stable and effective medications (Pan et al., 2019). Pressure control is particularly important in processes involving gases or liquids (Seo and Shin, 2022). Too much or too little pressure can affect reaction rates, solubility, and the overall efficiency of the process. In applications like chemical reactions, pressure control helps maintain a safe operating environment and prevents equipment failures (Schmidt et al., 2023). pH is a measure of acidity or alkalinity and significantly influences the stability and functionality of many products (Wright, 2021). In industries like food and beverage production, pharmaceuticals, and cosmetics, maintaining the correct pH level is critical for product preservation, taste, and effectiveness (Wiseman et al., 2022). For instance, certain enzymes are only active within specific pH ranges, making pH control essential during enzyme-based processes (Daniel et al., 2022). In processes involving mixing or blending, the duration and intensity of mixing time directly affect product homogeneity and consistency (Hernandez and Perera, 2022). Controlling the mixing time ensures uniform distribution of ingredients, which is crucial in formulations such as pharmaceutical tablets or food products (Adra et al., 2022). Drying is a common step in herbal medication industries, such as product processing, pharmaceuticals, and chemical manufacturing. Controlling drying conditions can change product defects, increase shelf life, or even keep fresh, which involves temperature, humidity, airflow, moisture content, texture, and stability (da Cunha et al., 2021). Quality testing for herbal medication products involves rigorous analysis to ensure the absence of contaminants, heavy metals, pesticide residues, and microbiological safety evaluation (Figure 3). By controlling critical parameters, manufacturers can achieve consistent product quality across different batches, leading to reliable and predictable outcomes, reducing waste and production time, avoiding product defects and minimizing rejections, and increasing its market value and consumer satisfaction.
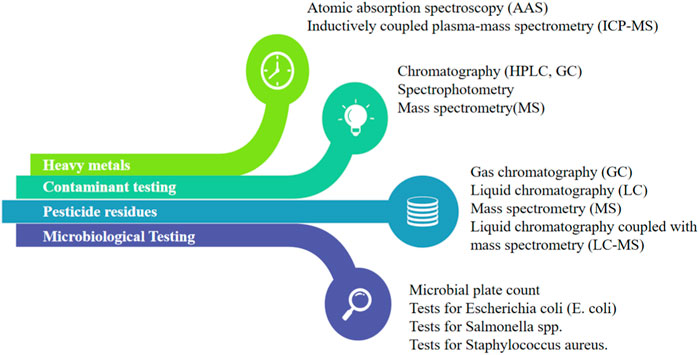
FIGURE 3. The quality control methods for contaminants, heavy metals, pesticide residues, and microbiology.
3.3.4 In-process testing
In-process testing involves performing quality tests at various stages of the manufacturing process to assess the product’s quality and consistency (Silva et al., 2022). These tests may include chemical analysis, microbial testing, dissolution testing, or physical measurements. In-process testing helps identify any variations or issues during processing, allowing for timely corrective actions and ensuring the final product meets the desired quality standards (Chan and Choo, 2013). The presence of contaminants, heavy metals, pesticide residues, and microbiological safety evaluation is a crucial major concern in herbal medication products (Omeje et al., 2021). Therefore, the detection of pollutants, pesticides, microbiological counts, and heavy metals in the environment is key to ensuring the quality of herbal medication products (Varol and Sünbül, 2017). Contaminant testing includes assessing the presence of impurities such as aflatoxins, mycotoxins, residual solvents, and environmental pollutants (Simpson and McKelvie, 2009). Various analytical techniques like chromatography (HPLC, GC), spectrophotometry, and mass spectrometry are employed to detect and quantify these contaminants (Levy et al., 2018). Heavy metals such as lead, mercury, arsenic, and cadmium can pose health risks if present in herbal medication products. Testing methods such as atomic absorption spectroscopy or inductively coupled plasma-mass spectrometry (ICP-MS) are utilized to measure heavy metal content and ensure compliance with permissible limits (Lockwood et al., 2021). Pesticide residues can result from agricultural practices or contamination during cultivation. Analytical techniques like gas chromatography (GC) or liquid chromatography (LC) coupled with mass spectrometry (MS) are employed for pesticide residue analysis, ensuring adherence to regulatory standards (Lockwood et al., 2021). Microbiological testing assesses the presence of harmful microorganisms in herbal medication products (Levy et al., 2018). It includes testing for total microbial counts, specific pathogens, and the absence of certain indicator organisms (Levy et al., 2018). Common methods include microbial plate count, tests for E. coli (Escherichia coli), Salmonella spp., and Staphylococcus aureus (York et al., 2000). The microbial evaluation for total coliform counts (TCC), total viable counts (TVC), and total yeast and mold counts (TYMC) was evaluated using the method described in the compendium of methods for microbiological testing of herb medication products described by Hervert and Alles (2016) with minor modifications. These tests ensure product safety and compliance with microbial limits set by regulatory authorities.
Analytical techniques are employed to determine the presence, identity, and concentration of active constituents in herbal medication products (Seo and Shin, 2022). High-Performance liquid chromatography is widely employed to separate, identify, and quantify individual chemical components, including active compounds, in herbal extracts (Wright, 2021). It provides precise measurements and is useful for standardization and quality control. Gas chromatography-mass spectrometry is used for volatile compounds and essential oils analysis in herbal medication products (Silva et al., 2022). It enables the identification and quantification of specific compounds based on their mass spectra and retention times. Thin-Layer Chromatography is a rapid and cost-effective technique used for qualitative analysis and identification of compounds in herbal medicines (Del Bubba et al., 2013). It involves separating components based on their differential migration on a thin layer of adsorbent material. Spectroscopic Techniques like UV-Vis spectroscopy, infrared (IR) spectroscopy, and nuclear magnetic resonance (NMR) spectroscopy are employed for qualitative and quantitative analysis of herbal constituents. They provide information on chemical structures, functional groups, and concentrations. These quality testing and analytical techniques help ensure the safety, efficacy, and consistency of herbal medication products. By performing these tests, manufacturers can identify potential contaminants, verify active constituent content, and ensure compliance with regulatory requirements, thereby ensuring the quality and safety of the products.
3.3.5 Contamination control
Contamination control is a critical aspect of various herbal medication industries, ensuring safety, product quality, and environmental protection (Raeisossadati et al., 2016). Contamination control methods are techniques and practices employed to prevent, minimize, or eliminate the presence of harmful or unwanted substances in a specific environment, product, or process (Edberg et al., 1989; Simpson and McKelvie, 2009). These methods vary depending on the industry and the nature of the contamination. This includes implementing measures such as cleaning and sanitation, sterilization, disinfection, hand hygiene, cleanroom technology, air filtration, regulatory compliance, and environmental monitoring (Wan et al., 2019). Regular cleaning and sanitation of surfaces, equipment, and facilities are fundamental contamination control methods. Proper cleaning removes dirt, debris, and potential contaminants, reducing the risk of cross-contamination. Sterilization is a critical method used in healthcare settings and the pharmaceutical industry to eliminate all viable microorganisms, including bacteria, viruses, and spores. Techniques like autoclaving, irradiation, and chemical sterilization are commonly used. Disinfection involves the use of chemical agents to reduce the number of microorganisms on surfaces and objects. It is commonly used in healthcare settings and food production to control the spread of pathogens. Clean-rooms are controlled environments designed to minimize airborne particulates and microorganisms (Safra, 2019). They are widely used in industries like semiconductor manufacturing, aerospace, and pharmaceuticals. High-efficiency particulate air (HEPA) filters are used in ventilation systems to remove airborne particles, including microorganisms and other contaminants. In industries like food production and healthcare, pest control measures are essential to prevent contamination from insects and rodents. Many industries have specific regulations and standards related to contamination control (Panteghini et al., 2001). Compliance with these regulations is essential to ensure public safety, product quality, and environmental protection. Regular monitoring of environmental conditions, such as temperature, humidity, and air quality, can help identify potential sources of contamination and ensure appropriate control measures are in place (Omeje et al., 2021). Contamination control methods encompass a range of practices and techniques aimed at preventing or reducing contamination in herbal medication products industries (Levy et al., 2018). These methods are critical to ensure the safety, quality, and efficacy of products, protect public health, and preserve the environment.
4 Discussion
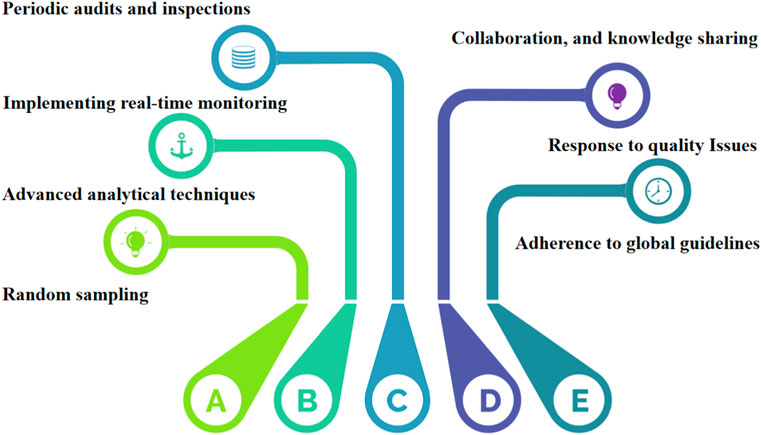
Despite the growing recognition of herbal medicines’ therapeutic potential, the field of quality control in this domain faces several critical gaps and challenges that warrant diligent attention. One notable challenge is the significant variability in product composition and quality due to factors such as geographical variations, cultivation methods, and post-harvest processing. This inherent diversity poses a unique hurdle for establishing consistent quality standards across different herbal products. Rapid advancements in analytical technologies, such as mass spectrometry and DNA barcoding, have transformed the landscape of quality control in other industries, yet their integration into herbal medicine quality assessment has been sporadic and inconsistent. We propose the utilization of molecular fingerprinting techniques, such as DNA bar-coding and metabolic, to authenticate herbal ingredients. These approaches offer accurate species identification and enable the detection of adulterants or contaminants. Secondly, we advocate for the incorporation of real-time monitoring and Internet of Things (IoT) devices along the herbal supply chain. This enables continuous data collection on environmental conditions, ensuring the preservation of botanical integrity and product quality. Besides, Data-driven predictive modeling is also necessary for quality control practices in the herbal industry. Introducing data-driven predictive models, including machine learning and artificial intelligence, can forecast the impact of processing variables on herbal product quality. This empowers manufacturers to optimize production processes for consistent outcomes. Finally, the collaboration between regulatory bodies, herbal practitioners, and manufacturers is proposed to establish comprehensive industry standards. These standards would encompass cultivation, harvesting, processing, and quality control, ensuring adherence to best practices. Moreover, the under-utilization of traditional knowledge is a missed opportunity for elevating the quality control process. Traditional healers and local communities possess invaluable insights into the therapeutic properties and usage of herbs. Integrating this wisdom with modern scientific approaches could enrich the quality control paradigm and enhance product efficacy. In response to these challenges, a holistic approach to quality control is needed—one that bridges the gaps between traditional practices and modern innovations, streamlines regulatory oversight, and nurtures collaborative partnerships among stakeholders. To illustrate the interplay of these elements, a diagram of the road map is presented below in Figure 4. By following this roadmap, herbal medicine manufacturers and regulators can strengthen the safety and efficacy of herbal medication products, thereby instilling confidence in consumers and fostering the responsible growth of the herbal medicine industry. The road map also serves as a foundation for continuous research, improvement, and knowledge-sharing, ultimately contributing to the advancement of quality control practices for herbal medicines. medicines.
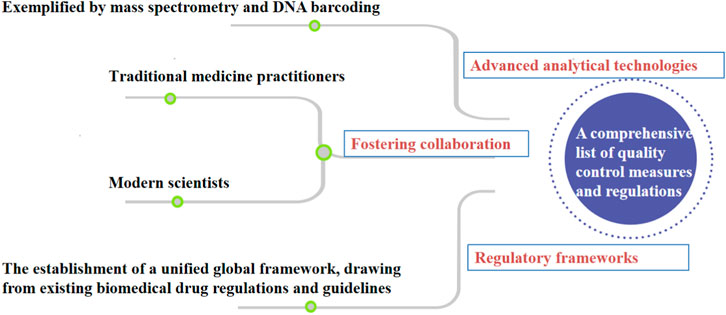
FIGURE 4. A diagram of the road map for the comprehensive list of quality control measures and regulations applied to herbal medicines.
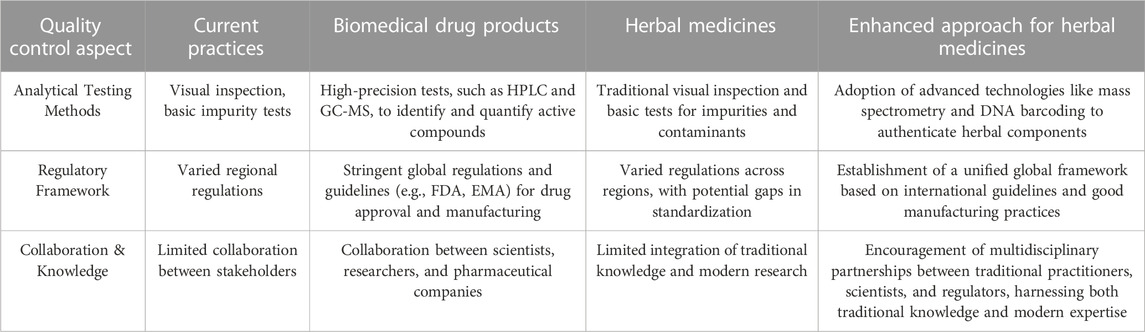
Herbal medicines often draw from centuries of traditional knowledge, which should be thoughtfully integrated into modern quality control practices. It is worth noting that some of the quality control advances suggested for herbal medicines are already well-established practices in the context of biomedical drug products. Building upon the insights highlighted by previous researchers, strategic integration of cutting-edge analytical techniques, harmonized global standards, and collaborative partnerships emerges as the pathway forward. While some aspects of biomedical drug quality control can serve as a model for herbal medicines, there are specific differences between the two domains. Although adopting certain aspects of biomedical drug quality control can be beneficial for the quality control of herbal medicines, the application of such measures to herbal medicines raises distinct considerations. By juxtaposing the existing practices with the proposed enhancements, we underscore the potential evolution of quality control measures for herbs (Table 3). By embracing these enhancements, we hold the promise of transforming the herbal medicine landscape and instilling newfound confidence in consumers seeking natural remedies.
By synthesizing a comprehensive understanding of the challenges and opportunities within the realm of herbal medicine quality control, we equip enterprises with actionable insights to enhance their practices such as a comprehensive review of quality control measures, identification of gaps and challenges, recommendations for enhancements, integration of modern techniques, regulatory harmonization, collaborative approach, continuous improvement, and innovation (Figure 5). Enterprises learn how to maintain uniformity in active ingredients, dosage forms, and potency and improve the core aspects of GMP, including cleanliness, personnel training, documentation, and equipment maintenance. This knowledge empowers enterprises to conduct rigorous quality assessments before products reach the market. By underscoring the role of quality control in ensuring safety, the text assists enterprises in identifying potential risks associated with herbal medicine production. Enterprises are encouraged to regularly review and update their quality control processes in light of emerging research, technological advancements, and changing consumer preferences. By implementing the insights provided in the text, enterprises can confidently navigate the complexities of herbal medicine production and contribute to the availability of safe and consistent products for consumers.
5 Conclusion
Quality control for the efficiency and safety of herbal medication products is of significant importance to safeguard consumer health, establish reliable treatment options, comply with regulations, and foster the growth of the herbal medicine industry. The efficacy of herbal medications depends on the presence and concentration of active compounds. Quality control processes should focus on establishing standardized procedures for sourcing, processing, and formulating herbal ingredients. Besides, regular monitoring of raw materials and finished products, quantitative analysis of these compounds to guarantee consistent therapeutic effects, rigorous testing for potential contaminants, implementing GMP principles in the production process, and post-market surveillance are often attributed to ensure safety and efficacy. With continuous quality control measures in place, any issues related to product quality or safety can be identified and addressed promptly. Advanced techniques such as DNA barcoding can be employed to verify the authenticity of herbs used in the products. This proactive approach minimizes the risk of widespread product recalls or adverse events.
Ensuring the quality, efficiency, and safety of herbal medication products involves standardization, authentication, contaminant testing, quantification of active compounds, adherence to regulations, stability testing, GMP implementation, validated methods, post-market surveillance, and open collaboration among stakeholders. These key findings and takeaways provide a road map for establishing robust quality control processes for herbal medication products.
In conclusion, this comprehensive review underscores the pivotal role of quality control in herbal medication products, emphasizing its significance in ensuring efficiency and safety. The use of robust quality control methods ensures the authenticity and therapeutic value of herbal remedies, fostering consumer trust and promoting the responsible integration of herbal medicine into modern healthcare practices. Continued research and collaboration between traditional knowledge and modern science will undoubtedly enhance the quality and acceptance of herbal medication products, further benefiting public health and wellbeing.
Author contributions
HW: Data curation, Formal Analysis, Funding acquisition, Software, Validation, Writing–original draft, Writing–review and editing. YC: Data curation and editing. LW: Data curation. QL: Investigation and Software. SY:Data curation, Investigation, Software. CW: Formal Analysis, Writing–review and editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Nature Science Research Project of Anhui Province fund (2023AH040268, 2023AH040257).
Acknowledgments
The authors would like to acknowledge the contributions of Anhui Provincial Engineering Research Center for Polysaccharide Drugs that aided the efforts of the authors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AAS, Atomic absorption spectroscopy; E. coli, Escherichia coli; FTIR, Fourier transform infrared spectroscopy; GC, Gas chromatography; GC-MS, Gas Chromatography-Mass Spectrometry; GMP, Good manufacturing practices; HEPA, High-efficiency particulate air; HPLC, High-performance liquid chromatography; HPTLC, High-performance thin-layer chromatography; ICP-MS, Inductively coupled plasma-mass spectrometry; IRS, Infrared spectroscopy; IoT, Internet of things; LC, Liquid chromatography; MS, Mass spectrometry; NMR, Nuclear magnetic resonance; NMRS, Nuclear magnetic resonance spectroscopy; TCM, Traditional chinese medicine; TLC, Thin-Layer Chromatography; TCC, Total coliform counts; TOC, Total organic carbon; TVC, Total viable counts; TYMC, Total yeast and mould counts; UVVS, UV-Vis spectroscopy.
References
Abeillé, A., Hemforth, B., Winckel, E., and Gibson, E. (2020). Extraction from subjects: differences in acceptability depend on the discourse function of the construction. Cognition 204, 104293. doi:10.1016/j.cognition.2020.104293
Adra, H. J., Zhi, J., Luo, K., and Kim, Y. R. (2022). Facile preparation of highly uniform type 3 resistant starch nanoparticles. Carbohydr. Polym. 294, 119842. doi:10.1016/j.carbpol.2022.119842
Agustika, D. K., Mercuriani, I., Purnomo, C. W., Hartono, S., Triyana, K., Iliescu, D. D., et al. (2022). Fourier transform infrared spectrum pre-processing technique selection for detecting PYLCV-infected chilli plants. Spectrochim. Acta A Mol. Biomol. Spectrosc. 278, 121339. doi:10.1016/j.saa.2022.121339
Aleksieva, K. I., and Yordanov, N. D. (2018). Various approaches in EPR identification of gamma-irradiated plant foodstuffs: A review. Food Res. Int. Ott. Ont.) 105, 1019–1028. doi:10.1016/j.foodres.2017.11.072
Alipour, R., Marzabadi, L. R., Arjmand, B., Ayati, M. H., and Namazi, N. (2022). The effects of medicinal herbs on gut microbiota and metabolic factors in obesity models: A systematic review. Diabetes Metab. Syndr. 16, 102586. doi:10.1016/j.dsx.2022.102586
An, Y. L., Wei, W. L., and Guo, D. A. (2022). Application of analytical technologies in the discrimination and authentication of herbs from fritillaria: A review. Crit. Rev. Anal. Chem. 1-22, 1–22. doi:10.1080/10408347.2022.2132374
Ashraf, S. A., Elkhalifa, A., Siddiqui, A. J., Patel, M., Awadelkareem, A. M., Snoussi, M., et al. (2020). Cordycepin for health and wellbeing: A potent bioactive metabolite of an entomopathogenic cordyceps medicinal fungus and its nutraceutical and therapeutic potential. Molecules 25, 2735. doi:10.3390/molecules25122735
Backes, E., Pereira, C., Barros, L., Prieto, M. A., Genena, A. K., Barreiro, M. F., et al. (2018). Recovery of bioactive anthocyanin pigments from Ficus carica L. peel by heat, microwave, and ultrasound based extraction techniques. Food Res. Int. Ott. Ont.) 113, 197–209. doi:10.1016/j.foodres.2018.07.016
Bai, R., Sun, Q., He, Y., Peng, L., Zhang, Y., Zhang, L., et al. (2022). Ceramic toughening strategies for biomedical applications. Front. Bioeng. Biotechnol. 10, 840372. doi:10.3389/fbioe.2022.840372
Balachandran, P., and Govindarajan, R. (2005). Cancer-an ayurvedic perspective. Pharmacol. Res. 51, 19–30. doi:10.1016/j.phrs.2004.04.010
Barnes, M., Sulé-Suso, J., Millett, J., and Roach, P. (2023). Fourier transform infrared spectroscopy as a non-destructive method for analysing herbarium specimens. Biol. Lett. 19, 20220546. doi:10.1098/rsbl.2022.0546
Benedetti, A., Khoo, J., Sharma, S., Facco, P., Barolo, M., and Zomer, S. (2019). Data analytics on raw material properties to accelerate pharmaceutical drug development. Int. J. Pharm. 563, 122–134. doi:10.1016/j.ijpharm.2019.04.002
Boateng, I. D., Kuehnel, L., Daubert, C. R., Agliata, J., Zhang, W., Kumar, R., et al. (2023). Updating the status quo on the extraction of bioactive compounds in agro-products using a two-pot multivariate design. A comprehensive review. Food Funct. 14, 569–601. doi:10.1039/d2fo02520e
Boutron, I., Guittet, L., Estellat, C., Moher, D., Hróbjartsson, A., and Ravaud, P. (2007). Reporting methods of blinding in randomized trials assessing nonpharmacological treatments. PLoS Med. 4, e61. doi:10.1371/journal.pmed.0040061
Boyd, A. R., Champagne, P., McGinn, P. J., MacDougall, K. M., Melanson, J. E., and Jessop, P. G. (2012). Switchable hydrophilicity solvents for lipid extraction from microalgae for biofuel production. Bioresour. Technol. 118, 628–632. doi:10.1016/j.biortech.2012.05.084
Boyd, L. H. (1994). Regulation of drugs and chemicals used by the poultry industry. Good manufacturing practices. Poult. Sci. 73, 1419–1422. doi:10.3382/ps.0731419
Braga, F., Pasqualetti, S., Aloisio, E., and Panteghini, M. (2020). The internal quality control in the traceability era. Clin. Chem. Lab. Med. 59, 291–300. doi:10.1515/cclm-2020-0371
Brennan, A. A., You, J., and Lydy, M. J. (2009). Comparison of cleanup methods for fipronil and its degradation products in sediment extracts. TALANTA 78, 1408–1413. doi:10.1016/j.Talanta.2009.02.034
Brown, A. C. (2017). Liver toxicity related to herbs and dietary supplements: Online table of case reports. Part 2 of 5 series. Food Chem. Toxicol. 107, 472–501. doi:10.1016/j.fct.2016.07.001
Burcham, C. L., Florence, A. J., and Johnson, M. D. (2018). Continuous manufacturing in pharmaceutical process development and manufacturing. Annu. Rev. Chem. Biomol. Eng. 9, 253–281. doi:10.1146/annurev-chembioeng-060817-084355
Byrd, C. L., Schwartz, S. J., Hedin, N., and Beach, M. (1991). Intravascular techniques for extraction of permanent pacemaker leads. J. Thorac. Cardiovasc. Surg. 101, 989–997. doi:10.1016/s0022-5223(19)36615-2
Cai, Z., Wang, C., Zou, L., Liu, X., Chen, J., Tan, M., et al. (2019). Comparison of multiple bioactive constituents in the flower and the caulis of Lonicera japonica based on UFLC-QTRAP-MS/MS combined with multivariate statistical analysis. Molecules 24, 1936. doi:10.3390/molecules24101936
Campuzano, R., and González-Martínez, S. (2017). Influence of process parameters on the extraction of soluble substances from OFMSW and methane production. Waste Manag. (New York, N.Y.) 62, 61–68. doi:10.1016/j.wasman.2017.02.015
Castiglia, S., Adamini, A., Rustichelli, D., Castello, L., Mareschi, K., Pinnetta, G., et al. (2018). Cytokines induced killer cells produced in good manufacturing practices conditions: Identification of the most advantageous and safest expansion method in terms of viability, cellular growth and identity. J. Transl. Med. 16, 237. doi:10.1186/s12967-018-1613-5
Chan, S. Y., and Choo, W. S. (2013). Effect of extraction conditions on the yield and chemical properties of pectin from cocoa husks. Food Chem. 141, 3752–3758. doi:10.1016/j.foodchem.2013.06.097
Chang, J. (2000). Medicinal herbs: Drugs or dietary supplements. Biochem. Pharmacol. 59, 211–219. doi:10.1016/s0006-2952(99)00243-9
Chen, C., Liu, Z., Zou, L., Liu, X., Chai, C., Zhao, H., et al. (2018). Quality evaluation of apocyni veneti folium from different habitats and commercial herbs based on simultaneous determination of multiple bioactive constituents combined with multivariate statistical analysis. Molecules 23, 573. doi:10.3390/molecules23030573
Chen, J., Wang, F., Lee, F. S., Wang, X., and Xie, M. (2006). Separation and identification of water-soluble salvianolic acids from Salvia miltiorrhiza Bunge by high-speed counter-current chromatography and ESI-MS analysis. TALANTA 69, 172–179. doi:10.1016/j.Talanta.2005.09.041
Chen, R., Liu, F., Zhang, C., Wang, W., Yang, R., Zhao, Y., et al. (2023). Trends in digital detection for the quality and safety of herbs using infrared and Raman spectroscopy. Front. Plant Sci. 14, 1128300. doi:10.3389/fpls.2023.1128300
Chen, Y. C., Chen, Y. H., Pan, B. S., Chang, M. M., and Huang, B. M. (2017). Functional study of cordyceps sinensis and cordycepin in male reproduction: A review. J. Food Drug Anal. 25, 197–205. doi:10.1016/j.jfda.2016.10.020
Cheng, S. C., Huang, M. Z., and Shiea, J. (2011). Thin layer chromatography/mass spectrometry. J. Chromatogr. A 1218, 2700–2711. doi:10.1016/j.chroma.2011.01.077
Cheng, W. J., Yang, H. T., Chiang, C. C., Lai, K. H., Chen, Y. L., Shih, H. L., et al. (2022). Deer velvet antler extracts exert anti-inflammatory and anti-arthritic effects on human rheumatoid arthritis fibroblast-like synoviocytes and distinct mouse arthritis. Am. J. Chin. Med. 50, 1617–1643. doi:10.1142/S0192415X22500689
Cho, E. C., and Kim, K. (2020). A comprehensive review of biochemical factors in herbs and their constituent compounds in experimental studies on alopecia. J. Ethnopharmacol. 258, 112907. doi:10.1016/j.jep.2020.112907
Chrysant, S. G., and Chrysant, G. S. (2017). Herbs used for the treatment of hypertension and their mechanism of action. Curr. Hypertens. Rep. 19, 77. doi:10.1007/s11906-017-0775-5
Cox, S., Cleves, A., Clementel, E., Miles, E., Staffurth, J., and Gwynne, S. (2019). Impact of deviations in target volume delineation - time for a new RTQA approach. Radiother. Oncol. 137, 1–8. doi:10.1016/j.radonc.2019.04.012
Crimi, C., Impellizzeri, P., Campisi, R., Nolasco, S., Spanevello, A., and Crimi, N. (2021). Practical considerations for spirometry during the COVID-19 outbreak: Literature review and insights. Pulmonology 27, 438–447. doi:10.1016/j.pulmoe.2020.07.011
Cundell, T., Atkins, J. W., and Lau, A. F. (2023). Sterility testing for hematopoietic stem cells. J. Clin. Microbiol. 61, e0165422. doi:10.1128/jcm.01654-22
da Cunha, R. S., Amorim, K. S., Gercina, A. C., de Oliveira, A., Dos Santos Menezes, L., Groppo, F. C., et al. (2021). Herbal medicines as anxiolytics prior to third molar surgical extraction. A randomized controlled clinical trial. Clin. Oral Investig. 25, 1579–1586. doi:10.1007/s00784-020-03468-1
Dai, Y. L., Li, Y., Wang, Q., Niu, F. J., Li, K. W., Wang, Y. Y., et al. (2022). Chamomile: A review of its traditional uses, chemical constituents, pharmacological activities and quality control studies. Molecules 28, 133. doi:10.3390/molecules28010133
Daniel, S., Kis, Z., Kontoravdi, C., and Shah, N. (2022). Quality by Design for enabling RNA platform production processes. Trends Biotechnol. 40, 1213–1228. doi:10.1016/j.tibtech.2022.03.012
Davids, J. R., and Bagley, A. M. (2014). Identification of common gait disruption patterns in children with cerebral palsy. J. Am. Acad. Orthop. Surg. 22, 782–790. doi:10.5435/JAAOS-22-12-782
Deagle, R. C., Wee, T. E., and Brown, C. M. (2017). Reproducibility in light microscopy: Maintenance, standards and SOPs. Int. J. Biochem. Cell Biol. 89, 120–124. doi:10.1016/j.biocel.2017.06.008
Del Bubba, M., Checchini, L., and Lepri, L. (2013). Thin-layer chromatography enantioseparations on chiral stationary phases: A review. Anal. Bioanal. Chem. 405, 533–554. doi:10.1007/s00216-012-6514-5
DeSalle, R., Egan, M. G., and Siddall, M. (2005). The unholy trinity: Taxonomy, species delimitation and DNA barcoding. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 360, 1905–1916. doi:10.1098/rstb.2005.1722
Deutch, M. R., Grimm, D., Wehland, M., Infanger, M., and Krüger, M. (2019). Bioactive candy: Effects of licorice on the cardiovascular system. Foods 8, 495. doi:10.3390/foods8100495
Dina, E., Vontzalidou, A., Cheilari, A., Bagatzounis, P., Agapidou, E., Giannenas, I., et al. (2022). Sustainable use of Greek herbs by-products, as an alternative source of biologically active ingredients for innovative products. Front. Nutr. 9, 867666. doi:10.3389/fnut.2022.867666
Ding, L., Luo, X. B., Tang, F., Yuan, J. B., Guo, M., and Yao, S. Z. (2008). Quality control of medicinal herbs Fructus gardeniae, Common Andrographis Herb and their preparations for their active constituents by high-performance liquid chromatography-photodiode array detection-electrospray mass spectrometry. TALANTA 74, 1344–1349. doi:10.1016/j.Talanta.2007.09.001
Du, F. Y., Xiao, X. H., Luo, X. J., and Li, G. K. (2009). Application of ionic liquids in the microwave-assisted extraction of polyphenolic compounds from medicinal plants. TALANTA 78, 1177–1184. doi:10.1016/j.Talanta.2009.01.040
Dworkin, J. P., Adelman, L. A., Ajluni, T., Andronikov, A. V., Aponte, J. C., Bartels, A. E., et al. (2018). OSIRIS-REx contamination control strategy and implementation. Space Sci. Rev. 214, 19. [pii]. doi:10.1007/s11214-017-0439-4
Edberg, S. C., Allen, M. J., and Smith, D. B. (1989). National field evaluation of a defined substrate method for the simultaneous detection of total coliforms and Escherichia coli from drinking water: Comparison with presence-absence techniques. Appl. Environ. Microbiol. 55, 1003–1008. doi:10.1128/aem.55.4.1003-1008.1989
Ege, B., and Demirkol, M. (2021). Is the only buccal infiltration anesthesia enough for extraction of mandibular anterior incisors and premolar teeth? A split-mouth randomized clinical trial. Clin. Oral Investig. 25, 3077–3085. doi:10.1007/s00784-020-03628-3
El Maangar, A., Theisen, J., Penisson, C., Zemb, T., and Gabriel, J. P. (2020). A microfluidic study of synergic liquid-liquid extraction of rare earth elements. Phys. Chem. Chem. Phys. 22, 5449–5462. doi:10.1039/c9cp06569e
Elfgen, R., Gehrke, S., and Hollóczki, O. (2020). Ionic liquids as extractants for nanoplastics. ChemSusChem 13, 5449–5459. doi:10.1002/cssc.202001749
Ernst, E. (1998). Harmless herbs? A review of the recent literature. Am. J. Med. 104, 170–178. doi:10.1016/s0002-9343(97)00397-5
Feldmann, I., and Bondemark, L. (2006). Orthodontic anchorage: A systematic review. Angle Orthod. 76, 493–501. doi:10.1043/0003-3219(2006)076[0493:OA]2.0.CO;2
Fillingim, R. B., Loeser, J. D., Baron, R., and Edwards, R. R. (2016). Assessment of chronic pain: Domains, methods, and mechanisms. J. Pain 17, T10–T20. doi:10.1016/j.jpain.2015.08.010
Gainza, P., Sverrisson, F., Monti, F., Rodolà, E., Boscaini, D., Bronstein, M. M., et al. (2020). Deciphering interaction fingerprints from protein molecular surfaces using geometric deep learning. Nat. Methods 17, 184–192. doi:10.1038/s41592-019-0666-6
Giacometti, J., Bursać Kovačević, D., Putnik, P., Gabrić, D., Bilušić, T., Krešić, G., et al. (2018). Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. Ott. Ont.) 113, 245–262. doi:10.1016/j.foodres.2018.06.036
Gil-Díaz, M., Luchsinger-Heitmann, A., García-Gonzalo, P., Alonso, J., and Lobo, M. C. (2021). Selecting efficient methodologies for estimation of as and Hg availability in a brownfield. Environ. Pollut. (Barking, Essex 1987) 270, 116290. doi:10.1016/j.envpol.2020.116290
Gollahon, L. S., and Holt, S. E. (2000). Alternative methods of extracting telomerase activity from human tumor samples. Cancer Lett. 159, 141–149. doi:10.1016/s0304-3835(00)00544-9
Gong, L., Xie, J. B., Luo, Y., Qiu, Z. D., Liu, J. R., Mei, N. J., et al. (2023). Research progress of quality control for the seed of Ziziphus jujuba var. spinosa (Bunge) Hu ex H.F. Chow (Suan-Zao-Ren) and its proprietary Chinese medicines. J. Ethnopharmacol. 307, 116204. doi:10.1016/j.jep.2023.116204
González, F., Quintero, J., Del Río, R., and Mahn, A. (2021). Optimization of an extraction process to obtain a food-grade sulforaphane-rich extract from broccoli (Brassica oleracea var. italica). Molecules 26, 4042. doi:10.3390/molecules26134042
Gupta, A. K., and Sinha, S. (2007). Assessment of single extraction methods for the prediction of bioavailability of metals to Brassica juncea L. Czern. (var. Vaibhav) grown on tannery waste contaminated soil. J. Hazard. Mat. 149, 144–150. doi:10.1016/j.jhazmat.2007.03.062
Harper, D. S., Osborn, J. C., Clayton, R., and Hefferren, J. J. (1987). Modification of food cariogenicity in rats by mineral-rich concentrates from milk. J. Dent. Res. 66, 42–45. doi:10.1177/00220345870660010901
Hawthorne, S. B., Grabanski, C. B., Martin, E., and Miller, D. J. (2000). Comparisons of soxhlet extraction, pressurized liquid extraction, supercritical fluid extraction and subcritical water extraction for environmental solids: Recovery, selectivity and effects on sample matrix. J. Chromatogr. A 892, 421–433. doi:10.1016/s0021-9673(00)00091-1
Hernandez, G. A., and Perera, R. M. (2022). Autophagy in cancer cell remodeling and quality control. Mol. Cell 82, 1514–1527. doi:10.1016/j.molcel.2022.03.023
Hervert, C. J., Alles, A. S., Martin, N. H., Boor, K. J., and Wiedmann, M. (2016). Evaluation of different methods to detect microbial hygiene indicators relevant in the dairy industry. J. Dairy Sci. 99, 7033–7042. doi:10.3168/jds.2016-11074
Hu, G., Li, X., Zhang, J., Zhang, L., Qi, J., and Yu, B. (2021). An integrated strategy for the identification and screening of anti-allergy components from natural products based on calcium fluctuations and cell extraction coupled with HPLC-Q-TOF-MS. Anal. Bioanal. Chem. 413, 6253–6266. doi:10.1007/s00216-021-03580-5
Huaulmé, A., Jannin, P., Reche, F., Faucheron, J. L., Moreau-Gaudry, A., and Voros, S. (2020). Offline identification of surgical deviations in laparoscopic rectopexy. Artif. Intell. Med. Conf. Artif. Intell. Med. 104, 101837. doi:10.1016/j.artmed.2020.101837
Hussain, S. A., Panjagari, N. R., Singh, R. R., and Patil, G. R. (2015). Potential herbs and herbal nutraceuticals: Food applications and their interactions with food components. Crit. Rev. Food Sci. Nutr. 55, 94–122. doi:10.1080/10408398.2011.649148
Jacquemart, R., Vandersluis, M., Zhao, M., Sukhija, K., Sidhu, N., and Stout, J. (2016). A single-use strategy to enable manufacturing of affordable biologics. Comput. Struct. Biotechnol. J. 14, 309–318. doi:10.1016/j.csbj.2016.06.007
Jaiswal, Y., Liang, Z., and Zhao, Z. (2016). Botanical drugs in ayurveda and traditional Chinese medicine. J. Ethnopharmacol. 194, 245–259. doi:10.1016/j.jep.2016.06.052
Jenner, L. C., Rotchell, J. M., Bennett, R. T., Cowen, M., Tentzeris, V., and Sadofsky, L. R. (2022). Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci. Total Environ. 831, 154907. doi:10.1016/j.scitotenv.2022.154907
Jin, Y., Ma, Y., Xie, W., Hou, L., Xu, H., Zhang, K., et al. (2018). UHPLC-Q-TOF-MS/MS-oriented characteristic components dataset and multivariate statistical techniques for the holistic quality control of Usnea. RSC Adv. 8, 15487–15500. doi:10.1039/c8ra00081f
Joshi, V. K., Joshi, A., and Dhiman, K. S. (2017). The Ayurvedic Pharmacopoeia of India, development and perspectives. J. Ethnopharmacol. 197, 32–38. doi:10.1016/j.jep.2016.07.030
Kabak, B., and Dobson, A. D. (2017). Mycotoxins in spices and herbs-An update. Crit. Rev. Food Sci. Nutr. 57, 18–34. doi:10.1080/10408398.2013.772891
Kaefer, C. M., and Milner, J. A. (2008). The role of herbs and spices in cancer prevention. J. Nutr. Biochem. 19, 347–361. doi:10.1016/j.jnutbio.2007.11.003
Kalkan, E., Maness, N. O., and Chrz, D. R. (2019). Partial propane extraction of aromatic compounds from dehydrated basils (Ocimum Lamiaceae). J. Sci. Food Agric. 99, 3776–3784. doi:10.1002/jsfa.9592
Kankanamalage, T. N., Dharmadasa, R. M., Abeysinghe, D. C., and Wijesekara, R. G. (2014). A survey on medicinal materials used in traditional systems of medicine in Sri Lanka. J. Ethnopharmacol. 155, 679–691. doi:10.1016/j.jep.2014.06.016
Kapadia, P., Newell, A. S., Cunningham, J., Roberts, M. R., and Hardy, J. G. (2022). Extraction of high-value chemicals from plants for technical and medical applications. Int. J. Mol. Sci. 23, 10334. doi:10.3390/ijms231810334
Karsch-Völk, M., Barrett, B., Kiefer, D., Bauer, R., Ardjomand-Woelkart, K., and Linde, K. (2014). Echinacea for preventing and treating the common cold. Cochrane database Syst. Rev. 2014, CD000530. doi:10.1002/14651858.CD000530.pub3
Ketai, W., Huitao, L., Yunkun, Z., Xingguo, C., Zhide, H., Yucheng, S., et al. (2000). Separation and determination of alantolactone and isoalantolactone in traditional Chinese herbs by capillary electrophoresis. Talanta 52, 1001–1005. doi:10.1016/s0039-9140(00)00467-7
Kjelkenes, R., Wolfers, T., Alnæs, D., Norbom, L. B., Voldsbekk, I., Holm, M., et al. (2022). Deviations from normative brain white and gray matter structure are associated with psychopathology in youth. Dev. Cogn. Neurosci. 58, 101173. doi:10.1016/j.dcn.2022.101173
Lai, J. J., Chau, Z. L., Chen, S. Y., Hill, J. J., Korpany, K. V., Liang, N. W., et al. (2022). Exosome processing and characterization approaches for research and technology development. Adv. Sci. (Weinheim, Baden-Wurttemberg, Ger. 9, e2103222. doi:10.1002/advs.202103222
Lee, E. J., Lee, S. C., Lee, K., Cha, J. Y., Han, Y. N., Kim, S. G., et al. (2023). Properties of river organic carbon affected by wastewater treatment plants. Sci. Total Environ. 858, 159761. doi:10.1016/j.scitotenv.2022.159761
Lesch, H. P., Valonen, P., and Karhinen, M. (2021). Evaluation of the single-use fixed-bed bioreactors in scalable virus production. Biotechnol. J. 16, e2000020. doi:10.1002/biot.202000020
Levy, J. H., Neal, M. D., and Herman, J. H. (2018). Bacterial contamination of platelets for transfusion: Strategies for prevention. Crit. care (London, Engl. 22, 271. doi:10.1186/s13054-018-2212-9
Li, M., Xu, J., Zheng, Q., Guo, C., and Chen, Y. (2022). Chemical-based surface plasmon resonance imaging of fingerprints. Anal. Chem. 94, 7238–7245. doi:10.1021/acs.analchem.2c00389
Li, X., Yang, Y., Henry, R. J., Rossetto, M., Wang, Y., and Chen, S. (2015). Plant DNA barcoding: From gene to genome. Biol. Rev. Camb Philos. Soc. 90, 157–166. doi:10.1111/brv.12104
Liang, X., Dang, W., Yang, G., and Zhang, Y. (2023). Environmental feasibility evaluation of cement co-production using classified domestic waste as alternative raw material and fuel: A life cycle perspective. J. Environ. Manag. 326, 116726. doi:10.1016/j.jenvman.2022.116726
Ling, J., Xu, Y., Lu, C., Hou, W., Liu, Q., Wang, F., et al. (2021). Microbial contamination control mechanism in lipid production using distillery wastewater and oleaginous yeast - antimicrobial compounds in wastewater as a double-edged sword. J. Environ. Manag. 291, 112672. doi:10.1016/j.jenvman.2021.112672
Liu, H., Lu, X., Hu, Y., and Fan, X. (2020). Chemical constituents of Panax ginseng and Panax notoginseng explain why they differ in therapeutic efficacy. Pharmacol. Res. 161, 105263. doi:10.1016/j.phrs.2020.105263
Liu, X., Zhang, M., He, L., and Li, Y. (2012). Chinese herbs combined with Western medicine for severe acute respiratory syndrome (SARS). Cochrane database Syst. Rev. 10, CD004882. doi:10.1002/14651858.CD004882.pub3
Liu, X., Zhang, M., He, L., Li, Y. P., and Kang, Y. K. (2006). Chinese herbs combined with Western medicine for severe acute respiratory syndrome (SARS). Cochrane database Syst. Rev. 2006, CD004882. doi:10.1002/14651858.CD004882.pub2
Liu, Y., Yin, H., and Chen, K. (2013). Platelet proteomics and its advanced application for research of blood stasis syndrome and activated blood circulation herbs of Chinese medicine. Sci. China Life Sci. 56, 1000–1006. doi:10.1007/s11427-013-4551-8
Lo, Y. T., and Shaw, P. C. (2019). Application of next-generation sequencing for the identification of herbal products. Biotechnol. Adv. 37, 107450. doi:10.1016/j.biotechadv.2019.107450
Lockwood, T. E., Westerhausen, M. T., and Doble, P. A. (2021). Pew(2): Open-Source imaging software for laser ablation-inductively coupled plasma-mass spectrometry. Anal. Chem. 93, 10418–10423. doi:10.1021/acs.analchem.1c02138
Lou, H., Lin, J., Guo, L., Wang, X., Tian, S., Liu, C., et al. (2019). Advances in research on Cordyceps militaris degeneration. Appl. Microbiol. Biotechnol. 103, 7835–7841. doi:10.1007/s00253-019-10074-z
Ma, K., Wu, Z. Z., Wang, G. L., and Yang, X. P. (2021). Separation and purification of pyrroloquinoline quinone from Gluconobacter oxydans fermentation broth using supramolecular solvent complex extraction. Food Chem. 361, 130067. doi:10.1016/j.foodchem.2021.130067
Ma, L., Yang, Z., Kong, Q., and Wang, L. (2017). Extraction and determination of arsenic species in leafy vegetables: Method development and application. Food Chem. 217, 524–530. doi:10.1016/j.foodchem.2016.09.015
Ma, P. Y., Li, X. Y., Wang, Y. L., Lang, D. Q., Liu, L., Yi, Y. K., et al. (2022). Natural bioactive constituents from herbs and nutraceuticals promote browning of white adipose tissue. Pharmacol. Res. 178, 106175. doi:10.1016/j.phrs.2022.106175
Mager, S. R., Oomen, M. H., Morente, M. M., Ratcliffe, C., Knox, K., Kerr, D. J., et al. (2007). Standard operating procedure for the collection of fresh frozen tissue samples. Eur. J. cancer 43, 828–834. doi:10.1016/j.ejca.2007.01.002
Malherbe, C. J., De Beer, D., and Joubert, E. (2012). Development of on-line high performance liquid chromatography (HPLC)-biochemical detection methods as tools in the identification of bioactives. Int. J. Mol. Sci. 13, 3101–3133. doi:10.3390/ijms13033101
Melethil, S. (2006). Proposed rule: Current good manufacturing practice in manufacturing, packing, or holding dietary ingredients and dietary supplements. LIFE Sci. 78, 2049–2053. doi:10.1016/j.lfs.2005.12.020
Morgan, L., New, S., Robertson, E., Collins, G., Rivero-Arias, O., Catchpole, K., et al. (2015). Effectiveness of facilitated introduction of a standard operating procedure into routine processes in the operating theatre: A controlled interrupted time series. BMJ Qual. Saf. 24, 120–127. doi:10.1136/bmjqs-2014-003158
Motti, R. (2021). Wild plants used as herbs and spices in Italy: An ethnobotanical review. Plants (Basel, Switz. 10, 563. doi:10.3390/plants10030563
Mulinacci, N., Innocenti, M., Bellumori, M., Giaccherini, C., Martini, V., and Michelozzi, M. (2011). Storage method, drying processes and extraction procedures strongly affect the phenolic fraction of rosemary leaves: An HPLC/DAD/MS study. TALANTA 85, 167–176. doi:10.1016/j.Talanta.2011.03.050
Noh, W., Kim, J., Lee, S. J., Ryu, B. G., and Kang, C. M. (2018). Harvesting and contamination control of microalgae Chlorella ellipsoidea using the bio-polymeric flocculant α-poly-l-lysine. Bioresour. Technol. 249, 206–211. doi:10.1016/j.biortech.2017.09.157
Olsson, P., Holmbäck, J., and Herslöf, B. (2014). A single step reversed-phase high performance liquid chromatography separation of polar and non-polar lipids. J. Chromatogr. A 1369, 105–115. doi:10.1016/j.chroma.2014.10.010
Omeje, K. O., Ezema, B. O., Okonkwo, F., Onyishi, N. C., Ozioko, J., Rasaq, W. A., et al. (2021). Quantification of heavy metals and pesticide residues in widely consumed Nigerian food crops using atomic absorption spectroscopy (AAS) and gas chromatography (GC). Toxins (Basel) 13, 870. doi:10.3390/toxins13120870
Onishi, T., Kadohira, T., and Watanabe, I. (2018). Relation extraction with weakly supervised learning based on process-structure-property-performance reciprocity. Sci. Technol. Adv. Mater 19, 649–659. doi:10.1080/14686996.2018.1500852
Ortegón, S., Peñaranda, P. A., Rodríguez, C. F., Noguera, M. J., Florez, S. L., Cruz, J. C., et al. (2022). Magnetic torus microreactor as a novel device for sample treatment via solid-phase microextraction coupled to graphite furnace atomic absorption spectroscopy: A route for arsenic pre-concentration. Molecules 27, 6198. doi:10.3390/molecules27196198
Osman, A. G., Haider, S., Chittiboyina, A. G., and Khan, I. A. (2019). Utility of alkaloids as chemical and biomarkers for quality, efficacy, and safety assessment of botanical ingredients. Phytomedicine 54, 347–356. doi:10.1016/j.phymed.2018.03.064
Pan, C., Chen, S., Hao, S., and Yang, X. (2019). Effect of low-temperature preservation on quality changes in pacific white shrimp, Litopenaeus vannamei: A review. J. Sci. Food Agric. 99, 6121–6128. doi:10.1002/jsfa.9905
Pan, M., Pei, W., Yao, Y., Dong, L., and Chen, J. (2018). Rapid and integrated quality assessment of organic-inorganic composite herbs by FTIR spectroscopy-global chemical fingerprints identification and multiple marker components quantification of indigo naturalis (qing dai). Molecules 23, 2743. doi:10.3390/molecules23112743
Panteghini, M., Ceriotti, F., Schumann, G., and Siekmann, L. (2001). Establishing a reference system in clinical enzymology. Clin. Chem. Lab. Med. 39, 795–800. doi:10.1515/CCLM.2001.131
Parr, L. F., Anderson, A. L., Glennon, B. K., and Fetherston, P. (2001). Quality-control issues on high-resolution diagnostic monitors. J. Digit. Imaging 14, 22–26. doi:10.1007/BF03190289
Pihlström, T., Isaac, G., Waldebäck, M., Osterdahl, B. G., and Markides, K. E. (2002). Pressurised fluid extraction (PFE) as an alternative general method for the determination of pesticide residues in rape seed. Analyst 127, 554–559. doi:10.1039/b110814j
Poole, C. F. (2003). Thin-layer chromatography: Challenges and opportunities. J. Chromatogr. A 1000, 963–984. doi:10.1016/s0021-9673(03)00435-7
Pradhan, R., Hoaglin, D. C., Cornell, M., Liu, W., Wang, V., and Yu, H. (2019). Automatic extraction of quantitative data from ClinicalTrials.gov to conduct meta-analyses. J. Clin. Epidemiol. 105, 92–100. doi:10.1016/j.jclinepi.2018.08.023
Prasad, S., Kulshreshtha, A., Lall, R., and Gupta, S. C. (2021). Inflammation and ROS in arthritis: management by ayurvedic medicinal plants. Food Funct. 12, 8227–8247. doi:10.1039/d1fo01078f
Prata, J. C., Reis, V., da Costa, J. P., Mouneyrac, C., Duarte, A. C., and Rocha-Santos, T. (2021). Contamination issues as a challenge in quality control and quality assurance in microplastics analytics. J. Hazard. Mat. 403, 123660. doi:10.1016/j.jhazmat.2020.123660
Qin, Y., Wang, J. B., Zhao, Y. L., Shan, L. M., Li, B. C., Fang, F., et al. (2012). Establishment of a bioassay for the toxicity evaluation and quality control of Aconitum herbs. J. Hazard. Mat. 199-200, 350–357. doi:10.1016/j.jhazmat.2011.11.029
Qu, C. C., Sun, X. Y., Sun, W. X., Cao, L. X., Wang, X. Q., and He, Z. Z. (2021). Flexible wearables for plants. Small 17, e2104482. doi:10.1002/smll.202104482
Qu, Z., Steinvall, E., Ghorbani, R., and Schmidt, F. M. (2016). Tunable diode laser atomic absorption spectroscopy for detection of potassium under optically thick conditions. Anal. Chem. 88, 3754–3760. doi:10.1021/acs.analchem.5b04610
Raeisossadati, M. J., Danesh, N. M., Borna, F., Gholamzad, M., Ramezani, M., Abnous, K., et al. (2016). Lateral flow based immunobiosensors for detection of food contaminants. Biosens. Bioelectron. 86, 235–246. doi:10.1016/j.bios.2016.06.061
Rahath Kubra, I., Kumar, D., and Jagan Mohan Rao, L. (2016). Emerging trends in microwave processing of spices and herbs. Crit. Rev. Food Sci. Nutr. 56, 2160–2173. doi:10.1080/10408398.2013.818933
Ranasinghe, S., Ansumana, R., Lamin, J. M., Bockarie, A. S., Bangura, U., Buanie, J. A., et al. (2015). Herbs and herbal combinations used to treat suspected malaria in Bo, Sierra Leone. J. Ethnopharmacol. 166, 200–204. doi:10.1016/j.jep.2015.03.028
Reischauer, C., Staempfli, P., Jaermann, T., and Boesiger, P. (2009). Construction of a temperature-controlled diffusion phantom for quality control of diffusion measurements. J. Magn. Reson Imaging 29, 692–698. doi:10.1002/jmri.21665
Rodsamran, P., and Sothornvit, R. (2019). Microwave heating extraction of pectin from lime peel: Characterization and properties compared with the conventional heating method. Food Chem. 278, 364–372. doi:10.1016/j.foodchem.2018.11.067
Safra, P. (2019). Importance of photographic standardization. Am. J. Orthod. Dentofac. Orthop. 155, 756–757. doi:10.1016/j.ajodo.2019.03.014
Saini, R., Sharma, N., Oladeji, O. S., Sourirajan, A., Dev, K., Zengin, G., et al. (2022). Traditional uses, bioactive composition, pharmacology, and toxicology of Phyllanthus emblica fruits: A comprehensive review. J. Ethnopharmacol. 282, 114570. doi:10.1016/j.jep.2021.114570
Saletnik, A., Saletnik, B., and Puchalski, C. (2021). Overview of popular techniques of Raman spectroscopy and their potential in the study of plant tissues. Molecules 26, 1537. doi:10.3390/molecules26061537
Sargeant, J. M., Brennan, M. L., and O'Connor, A. M. (2022). Levels of evidence, quality assessment, and risk of bias: Evaluating the internal validity of primary research. Front. Vet. Sci. 9, 960957. doi:10.3389/fvets.2022.960957
Schmidt, R. L., Moore, R. A., Walker, B. S., and Rudolf, J. W. (2023). Precision quality control: A dynamic model for risk-based analysis of analytical quality. Clin. Chem. Lab. Med. 61, 679–687. doi:10.1515/cclm-2022-1094
Schwabl, H., and Vennos, C. (2015). From medical tradition to traditional medicine: A Tibetan formula in the European framework. J. Ethnopharmacol. 167, 108–114. doi:10.1016/j.jep.2014.10.033
Senapati, A., Basak, S., and Rangan, L. (2022). A review on application of DNA barcoding technology for rapid molecular diagnostics of adulterants in herbal medicine. Drug Saf. 45, 193–213. doi:10.1007/s40264-021-01133-4
Seo, C. S., and Shin, H. K. (2022). Simultaneous analysis of 19 marker components for quality control of oncheong-eum using HPLC-DAD. Molecules 27, 2992. doi:10.3390/molecules27092992
Shaikh, A. S., Thomas, A. B., and Chitlange, S. S. (2020). Herb-drug interaction studies of herbs used in treatment of cardiovascular disorders-A narrative review of preclinical and clinical studies. Phytother. Res. 34, 1008–1026. doi:10.1002/ptr.6585
Shao, H., Dong, H., Liu, Y., Zhou, G., and Guan, X. (2022). Chemiluminescence quenching capacity as a surrogate for total organic carbon in wastewater. J. Hazard. Mat. 440, 129765. doi:10.1016/j.jhazmat.2022.129765
Sharifi-Rad, M., Mnayer, D., Morais-Braga, M., Carneiro, J., Bezerra, C. F., Coutinho, H., et al. (2018). Echinacea plants as antioxidant and antibacterial agents: From traditional medicine to biotechnological applications. Phytother. Res. 32, 1653–1663. doi:10.1002/ptr.6101
Shukla, A. A., and Gottschalk, U. (2013). Single-use disposable technologies for biopharmaceutical manufacturing. Trends Biotechnol. 31, 147–154. doi:10.1016/j.tibtech.2012.10.004
Silva, D., Silva, M. S., Coelho, T., Dantas, C., Lopes Júnior, C. A., Caldas, N. M., et al. (2022). Combining high intensity ultrasound and experimental design to improve carotenoid extraction efficiency from Buriti (Mauritia flexuosa). Ultrason. Sonochem 88, 106076. doi:10.1016/j.ultsonch.2022.106076
Simpson, M. J., and McKelvie, J. R. (2009). Environmental metabolomics: new insights into earthworm ecotoxicity and contaminant bioavailability in soil. Anal. Bioanal. Chem. 394, 137–149. doi:10.1007/s00216-009-2612-4
Sinha, R., Abnet, C. C., White, O., Knight, R., and Huttenhower, C. (2015). The microbiome quality control project: baseline study design and future directions. Genome Biol. 16, 276. doi:10.1186/s13059-015-0841-8
Smith, W. S. (2020). Standardizing brain death globally. JAMA Neurol. 77, 1353–1354. doi:10.1001/jamaneurol.2020.1243
Song, P., Zhou, F., Li, F., Han, Z., Wang, L., Xu, J., et al. (2021). Superfine pulverisation pretreatment to enhance crystallinity of cellulose from Lycium barbarum L. leaves. Carbohydr. Polym. 253, 117207. doi:10.1016/j.carbpol.2020.117207
Sontag, G., Pinto, M. I., Noronha, J. P., and Burrows, H. D. (2019). Analysis of food by high performance liquid chromatography coupled with coulometric detection and related techniques: A review. J. Agric. Food Chem. 67, 4113–4144. doi:10.1021/acs.jafc.9b00003
Speltini, A., Merlo, F., Maraschi, F., Villani, L., and Profumo, A. (2021). HA-C@silica sorbent for simultaneous extraction and clean-up of steroids in human plasma followed by HPLC-MS/MS multiclass determination. TALANTA 221, 121496. doi:10.1016/j.Talanta.2020.121496
Spineli, L. M. (2017). Missing binary data extraction challenges from Cochrane reviews in mental health and Campbell reviews with implications for empirical research. Res. Synth. Methods 8, 514–525. doi:10.1002/jrsm.1268
Sridharan, B., Mohan, N., Berkland, C. J., and Detamore, M. S. (2016). Material characterization of microsphere-based scaffolds with encapsulated raw materials. Mater Sci. Eng. C Mater Biol. Appl. 63, 422–428. doi:10.1016/j.msec.2016.02.038
Stringham, R. W., Lynam, K. G., Mrozinski, P., Kilby, G., Pelczer, I., and Kraml, C. (2009). High performance liquid chromatographic evaluation of artemisinin, raw material in the synthesis of artesunate and artemether. J. Chromatogr. A 1216, 8918–8925. doi:10.1016/j.chroma.2009.10.056
Tao, Y., Bao, J., Zhu, F., Pan, M., Liu, Q., and Wang, P. (2023). Ethnopharmacology of rubus idaeus linnaeus: A critical review on ethnobotany, processing methods, phytochemicals, pharmacology and quality control. J. Ethnopharmacol. 302, 115870. doi:10.1016/j.jep.2022.115870
Tasfiyati, A. N., Antika, L. D., Dewi, R. T., Septama, A. W., Sabarudin, A., and Ernawati, T. (2022). An experimental design approach for the optimization of scopoletin extraction from Morinda citrifolia L. using accelerated solvent extraction. TALANTA 238, 123010. doi:10.1016/j.Talanta.2021.123010
Tsiaka, T., Lantzouraki, D. Z., Polychronaki, G., Sotiroudis, G., Kritsi, E., Sinanoglou, V. J., et al. (2023). Optimization of ultrasound- and microwave-assisted extraction for the determination of phenolic compounds in peach byproducts using experimental design and liquid chromatography-tandem mass spectrometry. Molecules 28, 518. doi:10.3390/molecules28020518
Varol, M., and Sünbül, M. R. (2017). Organochlorine pesticide, antibiotic and heavy metal residues in mussel, crayfish and fish species from a reservoir on the Euphrates River, Turkey. Environ. Pollut. (Barking, Essex 1987) 230, 311–319. doi:10.1016/j.envpol.2017.06.066
Velagaleti, R., Burns, P. K., Gill, M., and Prothro, J. (2002). Impact of current good manufacturing practices and emission regulations and guidances on the discharge of pharmaceutical chemicals into the environment from manufacturing, use, and disposal. Environ. Health Perspect. 110, 213–220. doi:10.1289/ehp.02110213
Vestal, M. L. (1984). High-performance liquid chromatography-mass spectrometry. Science 226, 275–281. doi:10.1126/science.6385251
Wan, Q., Yiner-Lee Leemaqz, S., Pederson, S. M., McCullough, D., McAninch, D. C., Jankovic-Karasoulos, T., et al. (2019). Quality control measures for placental sample purity in DNA methylation array analyses. Placenta 88, 8–11. doi:10.1016/j.placenta.2019.09.006
Wang, X., and Liu, Z. (2014). Prevention and treatment of viral respiratory infections by traditional Chinese herbs. Chin. Med. J. 127, 1344–1350. doi:10.3760/cma.j.issn.0366-6999.20132029
Wang, Y. Y., Sun, Y. P., Yang, B. Y., Wang, Q. H., and Kuang, H. X. (2022). Application of metabolomics and network analysis to reveal the ameliorating effect of four typical "hot" property herbs on hypothyroidism rats. Front. Pharmacol. 13, 955905. doi:10.3389/fphar.2022.955905
Wasalathanthri, D. P., Rehmann, M. S., Song, Y., Gu, Y., Mi, L., Shao, C., et al. (2020). Technology outlook for real-time quality attribute and process parameter monitoring in biopharmaceutical development-A review. Biotechnol. Bioeng. 117, 3182–3198. doi:10.1002/bit.27461
Wibowo, S., Grauwet, T., Kebede, B. T., Hendrickx, M., and Van Loey, A. (2015). Study of chemical changes in pasteurised orange juice during shelf-life: A fingerprinting-kinetics evaluation of the volatile fraction. Food Res. Int. Ott. Ont.) 75, 295–304. doi:10.1016/j.foodres.2015.06.020
Williams, D. J., Thomas, R. J., Hourd, P. C., Chandra, A., Ratcliffe, E., Liu, Y., et al. (2012). Precision manufacturing for clinical-quality regenerative medicines. Philos. Trans. A Math. Phys. Eng. Sci. 370, 3924–3949. doi:10.1098/rsta.2011.0049
Wiseman, R. L., Mesgarzadeh, J. S., and Hendershot, L. M. (2022). Reshaping endoplasmic reticulum quality control through the unfolded protein response. Mol. Cell 82, 1477–1491. doi:10.1016/j.molcel.2022.03.025
Wolle, M. M., and Conklin, S. D. (2018). Speciation analysis of arsenic in seafood and seaweed: Part I-evaluation and optimization of methods. Anal. Bioanal. Chem. 410, 5675–5687. doi:10.1007/s00216-018-0906-0
Woolman, M., and Liu, K. (2022). Simplified analysis and expanded profiles of avenanthramides in oat grains. Foods 11, 560. doi:10.3390/foods11040560
Wright, J. F. (2021). Quality control testing, characterization and critical quality attributes of adeno-associated virus vectors used for human gene therapy. Biotechnol. J. 16, e2000022. doi:10.1002/biot.202000022
Wu, F., Li, H., Jin, L., Li, X., Ma, Y., You, J., et al. (2013). Deer antler base as a traditional Chinese medicine: A review of its traditional uses, chemistry and pharmacology. J. Ethnopharmacol. 145, 403–415. doi:10.1016/j.jep.2012.12.008
Wu, T., Chen, X., Duan, X., Juan, N., Liu, G., Qiao, J., et al. (2005). Chinese medicinal herbs for acute bronchitis. Cochrane database Syst. Rev. 2005, CD004560. doi:10.1002/14651858.CD004560.pub2
Xiao, Z., Yang, L., Chen, C., Chen, D., and Zhou, X. (2022). Redox reaction between solid-phase humins and Fe(III) compounds: Toward a further understanding of the redox properties of humin and its possible environmental effects. J. Environ. Manag. 310, 114793. doi:10.1016/j.jenvman.2022.114793
Xie, F., and Dreisinger, D. (2009). Studies on solvent extraction of copper and cyanide from waste cyanide solution. J. Hazard. Mat. 169, 333–338. doi:10.1016/j.jhazmat.2009.03.102
Xie, Y. Y., Xiao, X., Luo, J. M., Fu, C., Wang, Q. W., Wang, Y. M., et al. (2014). Integrating qualitative and quantitative characterization of traditional Chinese medicine injection by high-performance liquid chromatography with diode array detection and tandem mass spectrometry. J. Sep. Sci. 37, 1438–1447. doi:10.1002/jssc.201400129
Xiong, C., Huang, C. H., Wu, L., Xu, R., Xue, J. P., Liu, Z. G., et al. (2022). Identification of Andrographis Herba and its common products using mini-barcode. Chin. J. Nat. Med. 20, 393–400. doi:10.1016/S1875-5364(22)60157-2
Xiong, R., Li, Y., Zheng, K., Zhang, T., Gao, M., Li, Y., et al. (2019). Er Shen Wan extract alleviates polyuria and regulates AQP 2 and AVPR 2 in a rat model of spleen-kidney Yang deficiency-induced diarrhea. Biomed. Pharmacother. 110, 302–311. doi:10.1016/j.biopha.2018.11.147
Yago, M., and Pla, C. (2020). Reference-mean-centered statistical quality control. Clin. Chem. Lab. Med. 58, 1517–1523. doi:10.1515/cclm-2019-1034
Yang, S., Yang, X., and Zhang, H. (2020). Extracellular polysaccharide biosynthesis in Cordyceps. Crit. Rev. Microbiol. 46, 359–380. doi:10.1080/1040841X.2020.1794788
Yin, F., Song, Z., He, Z., Qin, B., John, G. F., Zhang, L., et al. (2022). Chemical fingerprinting and characterization of spilled oils and burnt soot particles - a case study on the Sanchi oil tanker collision in the East China Sea. Sci. Total Environ. 824, 153896. doi:10.1016/j.scitotenv.2022.153896
York, M. K., Baron, E. J., Clarridge, J. E., Thomson, R. B., and Weinstein, M. P. (2000). Multilaboratory validation of rapid spot tests for identification of Escherichia coli. J. Clin. Microbiol. 38, 3394–3398. doi:10.1128/JCM.38.9.3394-3398.2000
Yu, Y., Jin, Y., Wang, F., Yan, J., Qi, Y., and Ye, M. (2017). Protein digestomic analysis reveals the bioactivity of deer antler velvet in simulated gastrointestinal digestion. Food Res. Int. Ott. Ont.) 96, 182–190. doi:10.1016/j.foodres.2017.04.002
Yuan, H., Ma, Q., Ye, L., and Piao, G. (2016). The traditional medicine and modern medicine from natural products. Molecules 21, 559. doi:10.3390/molecules21050559
Zhang, B., Gao, Y., Zhang, L., and Zhou, Y. (2021). The plant cell wall: Biosynthesis, construction, and functions. J. Integr. Plant Biol. 63, 251–272. doi:10.1111/jipb.13055
Zhang, D. Y., Peng, R. Q., Wang, X., Zuo, H. L., Lyu, L. Y., Yang, F. Q., et al. (2022). A network pharmacology-based study on the quality control markers of antithrombotic herbs: Using Salvia miltiorrhiza - ligusticum chuanxiong as an example. J. Ethnopharmacol. 292, 115197. doi:10.1016/j.jep.2022.115197
Zhao, M., Dai, Y., Li, Q., Li, P., Qin, X. M., and Chen, S. (2018). A practical quality control method for saponins without UV absorption by UPLC-QDA. Front. Pharmacol. 9, 1377. doi:10.3389/fphar.2018.01377
Zhao, N., Liu, D., Wang, Y., Zhang, X., and Zhang, L. (2022). Screening and identification of anti-acetylcholinesterase ingredients from Tianzhi granule based on ultrafiltration combined with ultra-performance liquid chromatography-mass spectrometry and in silico analysis. J. Ethnopharmacol. 298, 115641. doi:10.1016/j.jep.2022.115641
Zhou, S. S., Hu, J. W., Kong, M., Xu, J. D., Shen, H., Chen, H. B., et al. (2019). Less SO(2) residue may not indicate higher quality, better efficacy and weaker toxicity of sulfur-fumigated herbs: Ginseng, a pilot study. J. Hazard. Mat. 364, 376–387. doi:10.1016/j.jhazmat.2018.10.038
Zhu, X. R., Wang, H., Sun, J., Yang, B., Duan, X. W., and Jiang, Y. M. (2019). Pericarp and seed of litchi and longan fruits: Constituent, extraction, bioactive activity, and potential utilization. J. Zhejiang Univ. Sci. B 20, 503–512. doi:10.1631/jzus.B1900161
Keywords: herbal medication products, quality control, standardization, quality assurance protocols, quality control methods
Citation: Wang H, Chen Y, Wang L, Liu Q, Yang S and Wang C (2023) Advancing herbal medicine: enhancing product quality and safety through robust quality control practices. Front. Pharmacol. 14:1265178. doi: 10.3389/fphar.2023.1265178
Received: 22 July 2023; Accepted: 15 September 2023;
Published: 25 September 2023.
Edited by:
Jian-Bo Yang, National Institutes for Food and Drug Control, ChinaReviewed by:
Martin Fitzgerald, University of Westminster, United KingdomYongsheng Chen, Jinan University, China
Copyright © 2023 Wang, Chen, Wang, Liu, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongting Wang, bGVvbmFzdXBlckBpY2xvdWQuY29t; Cunqin Wang, d2NxNTE4OGJAMTYzLmNvbQ==
 Hongting Wang
Hongting Wang Ying Chen
Ying Chen Cunqin Wang
Cunqin Wang