- 1Beijing Huilongguan Hospital, Peking University Huilongguan Clinical Medical School, Beijing, China
- 2Qingdao Mental Health Center, Qingdao, China
- 3North University of China, Taiyuan, China
- 4Department of Nutritional and Metabolic Psychiatry, The Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou, China
- 5Guangdong Engineering Technology Research Center for Translational Medicine of Mental Disorders, Guangzhou, China
- 6Key Laboratory of Neurogenetics and Channelopathies of Guangdong Province and the Ministry of Education of China, Guangzhou Medical University, Guangzhou, China
Background: There is sufficient evidence of the high prevalence of obesity in schizophrenia (SZ) compared to the general population. Previous studies have reported that weight gain correlated with the response to antipsychotics in patients with SZ. Nonetheless, the relationship between body mass index (BMI) and therapeutic benefits remains unclear. This study was designed to investigate the association between baseline BMI and improvements in clinical symptoms after treatment with antipsychotics in first-episode and medication-naïve SZ (FEMNS).
Methods: A total of 241 FEMNS patients were enrolled and received risperidone over 12 weeks. The severity of symptoms was assessed by the Positive and Negative Syndrome Scale (PANSS) and BMI was measured at baseline and 12-week follow-up.
Results: We found that risperidone treatment raised the body weight of FEMNS patients and baseline BMI was negatively correlated with the improvement in negative symptoms (r = −0.14, p = 0.03) after 12-week treatment. Linear regression analysis indicated that baseline BMI was an independent predictor of response to risperidone in the early stage of SZ.
Conclusion: The current study suggests a close relationship between baseline BMI and improvement in negative symptoms in SZ.
1 Introduction
Atypical antipsychotics are reported to be associated with severe side effects like weight gain, obesity, and metabolic dysfunction (Li et al., 2018; Luckhoff et al., 2019; Li et al., 2021). It is reported that the prevalence of obesity ranges from 10% to 60% in SZ patients (Naslund et al., 2016; Annamalai et al., 2017). Weight gain/obesity impacts the quality of life and adherence to antipsychotic drugs in SZ (Dibonaventura et al., 2012; Pillinger et al., 2020).
A large of evidence has identified weight gain as a prognosis biomarker of the response to antipsychotic drugs (Ascher-Svanum et al., 2005; Bai et al., 2006; Sharma et al., 2014), underscoring the need for early monitoring of weight in SZ patients. Even in adolescents with SZ, substantial decreases in global psychopathology have been reported to be associated with weight gain after taking antipsychotic medications (Sharma et al., 2014). Studies have demonstrated that risperidone was associated with intermediate weight gain (1.76 kg), compared with olanzapine with the highest (3.45 kg) (De Hert et al., 2011). It is reported that individuals who were younger, female and first-episode and were less exposed to antipsychotics previously were more likely to increase the risk of obesity.
Antipsychotics have different treatment outcomes for patients with SZ (Zhu et al., 2022). Some studies have revealed the prognostic role of weight gain after treatment with antipsychotics in favorable treatment outcomes (Chen et al., 2021). However, considering the independence from confounding factors, such as treatment adherence and duration, the clinical significance of such a relationship has been questioned (Correll et al., 2011; De Hert et al., 2011; Hermes et al., 2011). Notably, a previous study has reported that baseline BMI correlated with antipsychotic-induced weight gain, and it was a predictive biomarker in the therapeutic response to antipsychotics (Verma et al., 2009). Another study in adolescents with SZ revealed that olanzapine-associated weight gain was not independently correlated with therapeutic response to olanzapine, but baseline obesity was related to more olanzapine-associated weight gain and symptomatic outcome (Kemp et al., 2013).
Studies have shown that the first-episode and medication-naive SZ (FEMNS) patients display the advantage of reducing the possible impacts of the use of antipsychotics, the duration of illness, and the impact of comorbidities associated with chronic illness (Xiu et al., 2020). In exploring the link between BMI and response to antipsychotics, thus, we recruited FEMNS patients in the current study. This study was designed to determine the predictive role of baseline BMI in the symptom improvements after 12-week treatment with risperidone in SZ.
2 Materials and methods
2.1 Patients
FEMNS patients were recruited from Beijing Hui-long-guan Hospital and Zhu-ma-dian Hospital. Patients were diagnosed with SZ as the Structured Clinical Interview for DSM-IV (SCID). The inclusion criteria were: 1) male and female inpatients; 2) first onset of psychosis; 3) between the ages of 18 and 45; 4) duration of illness less than 5 years; 5) previous antipsychotics exposure less than 14 days; 6) without substance abuse except for tobacco; 7) without major medical illness, such as diabetes, metabolic syndrome, hypertension, and cardiovascular disease; and 8) without taking weight-loss drug.
FEMNS patients received a flexible dose of risperidone for 12 weeks. During this 12-week study, all participants were hospitalized and nurses monitored compliance with risperidone. The protocol was approved by the ethical committee of Beijing Hui-long-guan Hospital (Ethic No.: 2011-4). Written informed consent was obtained from each patient.
2.2 BMI measurement
Height was determined by a metric stadiometer after removing the shoes. Weight was determined following overnight fasting at baseline and 12-week follow-up. Weight was measured in a hospital uniform with the pocket empty and without shoes. BMI was calculated using the weight divided by the height. All measurements were taken twice for each patient, and the mean was recorded.
In the present study, patients were classified into a high-BMI group when their BMI was 24 kg/m2 or higher and a low BMI group when their BMI was lower than 24 kg/m2, as in previous studies (An et al., 2018; Chen et al., 2023).
2.3 Clinical symptoms
The severity of symptoms was evaluated using the Positive and Negative Syndrome Scale (PANSS). The interviewers were trained before the assessment. After training, the inter-observer correlation coefficient for the PANSS total score was maintained at >0.8 during repeated assessments. PANSS scales were assessed at baseline and the end of 12 weeks. Improvement in clinical symptoms was calculated as the changes in PANSS score between baseline and 12-week follow-up after treatment with risperidone.
2.4 Statistical analysis
All statistical analyses were conducted using SPSS version 20.0. Statistical significance was defined as p < 0.05.
As described previously, the Last-observation-carried-forward (LOCF) was used for the data of the last time point of patients who dropped out (Wang et al., 2014; Raven et al., 2020; Wimms et al., 2020). ANOVA and X2 test were used to investigate whether there was a difference in demographic and clinical characteristics, body weight and BMI between the groups at baseline. If there were differences between the two groups, then the analysis of covariance (ANCOVA) was performed after controlling for the confounding variables. Then, Pearson correlation analysis was used to explore the relationship between BMI and PANSS scores at baseline, and further the relationship between baseline BMI and reductions in PANSS scores after treatment. Further regression analysis was performed to assess the association of BMI at baseline with the decrease in PANSS scores after 12 weeks of treatment after adjusting for various confounding factors.
3 Results
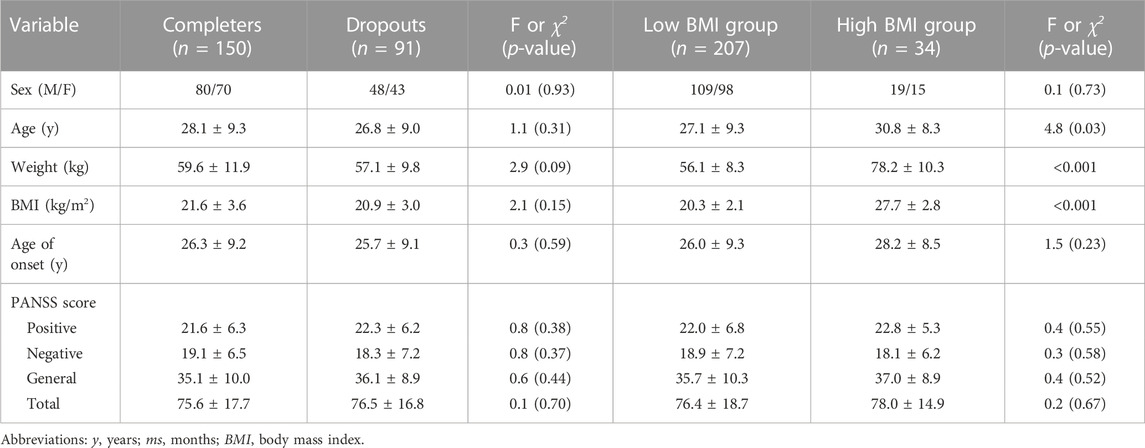
The sample included 241 FEMNS patients (128 men, 113 women). Thirty-eight participants were lost before the 2-month follow-up and 53 patients were lost after 2 months of treatment with risperidone. Finally, a total of 91 patients were lost. Table 1 shows the differences between completers and those who dropped out in the follow-up. There were no significant differences in the demographic characteristics and clinical data between completers and drop-outs (all p > 0.05).
After treatment, the mean changes in weight were 2.7 kg (SD = 3.8). According to the criteria of obesity, we identified 34 patients in the high BMI group and 207 patients in the low BMI group. Comparisons of demographic characteristics and clinical data between the high BMI and low BMI groups are shown in Table 1. The mean changes in weight were 0.4 (95% CI: −0.7–1.5) in the high BMI subgroup and 3.1 (95% CI: 2.5–3.6) in the low BMI subgroup. Significant differences were observed in weight gain between the low BMI and high BMI subgroups (p < 0.01).
After treatment with risperidone, clinical symptoms were significantly improved (Bonferroni corrected all p < 0.05) (Table 2). Pearson correlation analysis revealed that baseline BMI was negatively correlated with the improvement in negative symptoms (r = −0.14, p = 0.03). After subgroup analysis, we found that the negative association was only present in the high BMI group (r = −0.43, p = 0.01), but not in the low BMI group (Figure 1). Moreover, baseline BMI was not associated with the improvement in positive symptoms, general psychopathology, and PANSS total score (all p > 0.05). Further linear regression analysis confirmed that baseline BMI (β = −0.15, t = −2.2, p = 0.027) was a predictive factor for negative symptom improvements, while sex, age and years of education were not associated with negative symptom improvement (all p > 0.05).
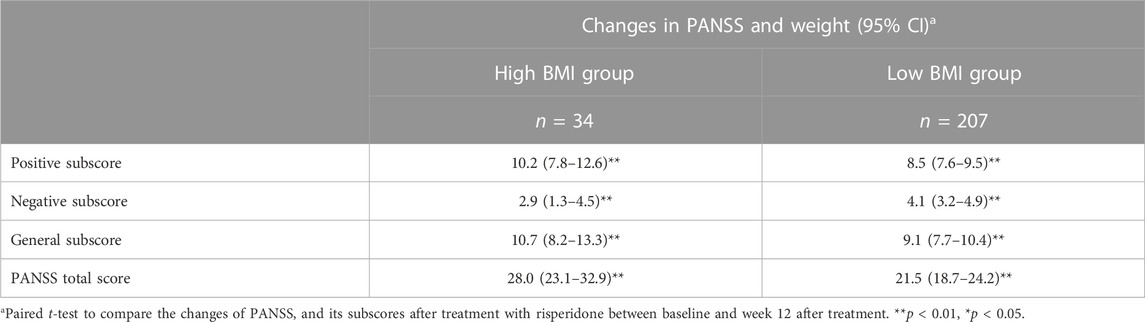
TABLE 2. Reduction of symptoms after 12-week treatment with risperidone in the low BMI and high BMI groups.
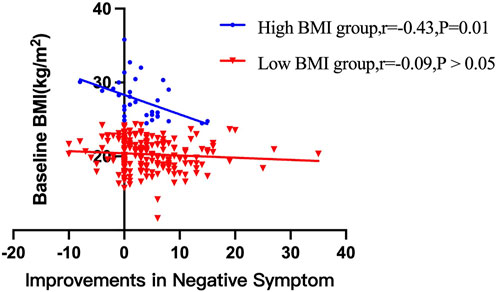
FIGURE 1. There were significant associations between baseline BMI and improvements in negative symptom improvements after 12 weeks of treatment with risperidone in high BMI group (p < 0.05), but not in low BMI group.
Further, among those with low BMI, we found 28 patients were underweight or with undernutrition. To rule out their influence on the results, we reanalyzed the data without these 28 patients and there was no difference in the result (Supplementary Tables S1).
4 Discussion
We here found that baseline BMI was associated with weight gain and negative symptom improvements in this relatively large sample of FEMNS patients on 12-week treatment with risperidone. However, we did not find an association between female sex, younger age education levels, and symptom improvements.
This study found that baseline BMI was significantly associated with weight gain after 3 months of risperidone monotherapy. Patients with a lower baseline BMI gained more weight after risperidone medication than those with a higher baseline BMI. Our findings are in line with most previous studies (Jones et al., 2001; Leucht et al., 2013; Huhn et al., 2019) and the recent longitudinal cohort study in FEMN patients with SCZ (Vázquez-Bourgon et al., 2022), providing further evidence for a relationship between baseline BMI/bodyweight and antipsychotic-induced weight gain in SCZ (Gentile, 2009; Verma et al., 2009). Notably, previous evidence-based data reveal that a variety of other risk factors, including ethnicity, young age, recent onset of psychotic symptoms, unemployment, unhealthy lifestyle and low income, may contribute to the rate or magnitude of antipsychotic-induced weight gain during long-term treatments (Gentile, 2009). However, in this study, we did not find an association between weight gain and education years, which was well-known to be strongly associated with unemployment, unhealthy diet and low income. This may be due to the differences between the patients with SCZ we recruited in this study and those in previous studies, considering that a majority of previous studies have investigated weight gain in outpatients on long-term antipsychotic medication.
We further found that baseline BMI was an independent predictor for the improvement of negative symptoms after treatment, after controlling for age, sex, and education years. Indeed, there is evidence to show a close relationship between negative symptoms in SZ patients and BMI at baseline (Jones et al., 2001; Leucht et al., 2013; Sharma et al., 2014; Raben et al., 2017; Huhn et al., 2019), which is consistent with our findings. The findings in the current study were also in line with the studies on FEMNS patients (Zipursky et al., 2005; Venkatasubramanian et al., 2010; Kemp et al., 2013). Importantly, the abovementioned studies have all controlled for confounding factors, such as sex, age, antipsychotic medications, and duration of illness.
However, the exact mechanism remains unclear. It may be due to the shared mechanistic pathway between body weight regulation and negative symptom improvements after antipsychotics. Risperidone has been reported to have a relatively high affinity for D2, 5-HT2, histamine H1 receptors, and NE alpha-2 receptors (Nasrallah, 2008). Animal studies also revealed that obesity induced by a high-fat diet and increased food intake correlated with 5-HT2 deficiency (Mercer et al., 1994). In particular, the use of antipsychotics may lead to the preferential metabolism of carbohydrates over fats and further lead to increased fat storage (Tiwari et al., 2018; Kaar et al., 2019). Leptin, a key signal for determining the size of fat depots in the brain, was found to be increased following atypical antipsychotic medication (Brömel et al., 1998; Kraus et al., 1999). On the other hand, dopamine D2 receptors play an important role in the reward circuit and in mediating both obesity and therapeutic response to antipsychotics, which may explain the association between BMI and therapeutic benefits in SZ.
Higher BMI was associated with fewer negative symptom changes in this study. It is reported that in patients with higher BMI in the early phase of SZ, brain functional connectivity associated with food cravings and weight control was decreased (Homan et al., 2019). Additionally, the prefrontal cortex of obese patients exhibited altered insulin and DA gene expression, resulting in relatively poorer outcomes than those with lower BMI (Mansur et al., 2018). Altogether, this study suggests that BMI at baseline may be a more sensitive indicator of the therapeutic benefits in the early stage of SZ, as most of the reported correlations between weight gain and therapeutic responses to antipsychotics are mixed.
Some strengths should be mentioned in this study. This is a prospective and longitudinal study examining a well-characterized group of FEMNS patients. Standardized treatment with a single antipsychotic excluded the different impacts of antipsychotic drugs. However, this study has several limitations. First, in this study, obesity-related biomarkers such as glucose, cholesterol, insulin resistance, and lipids were not recorded. Additionally, other metabolic parameters, such as food preference, dietary record, and caloric intake were also not collected. Second, only risperidone was assessed in our study. Therefore, the results of this study may not be generalized to other types of antipsychotics that may be different in metabolic disturbance. Third, the criteria for obesity in our study are only for Chinese, thus the conclusions in this study cannot be generalized to other populations. Fourth, the regulation of body weight may overlap with the pharmacological mechanisms of antipsychotic drugs. Genetic factors are known to be associated with the pharmacodynamics, pharmacokinetics, and adverse effects of antipsychotics. Additional pharmacogenetic analyses may provide new insights into the current findings. Fifth, considering the role of lifestyle, diet, and exercise in explaining the contradictory findings across studies, we did not collect the detailed diet and exercise in the present study.
In conclusion, the current study found that BMI was negatively correlated with the improvements in negative symptoms after treatment with risperidone for 12 weeks in FEMNS patients. In addition, we identified baseline BMI as an independent predictor for negative symptom improvements in SZ. Our study underscores the key role of BMI in the clinical management of patients in the early stage of SZ. These findings provide further evidence that greater efforts should be made to prevent obesity in clinical practice from the early phase of SZ.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethics committee of Beijing Huilongguan Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YF: Conceptualization, Writing–original draft, Investigation. XG: Conceptualization, Investigation, Writing–original draft. MX: Conceptualization, Writing–original draft. SL: Data curation, Writing–original draft. XC: Writing-original draft, review and editing. MS: Data curation. WR: Investigation, review and editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Guangzhou Municipal Health Commission (2023C-TS26), Opening Foundation of Jiangsu Key Laboratory of Neurodegeneration, Nanjing Medical University (KF202202), Open Project Program of State Key Laboratory of Virtual Reality Technology and Systems, Beihang University (VRLAB2022 B02), and Shanghai Key Laboratory of Psychotic Disorders Open Grant (21-K03), Guangzhou High-level Clinical Key Specialty, and Guangzhou Research-oriented Hospital.
Acknowledgments
We would like to thank the participants in the study and their families.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1264591/full#supplementary-material
References
An, H., Du, X., Huang, X., Qi, L., Jia, Q., and Yin, G., (2018). Obesity, altered oxidative stress, and clinical correlates in chronic schizophrenia patients. Transl. Psychiatry 8 (1), 258. doi:10.1038/s41398-018-0303-7
Annamalai, A., Kosir, U., and Tek, C. (2017). Prevalence of obesity and diabetes in patients with schizophrenia. World J. Diabetes 8 (8), 390–396. doi:10.4239/wjd.v8.i8.390
Ascher-Svanum, H., Stensland, M. D., Kinon, B. J., and Tollefson, G. D. (2005). Weight gain as a prognostic indicator of therapeutic improvement during acute treatment of schizophrenia with placebo or active antipsychotic. J. Psychopharmacol. 19 (6), 110–117. doi:10.1177/0269881105058978
Bai, Y. M., Lin, C. C., Chen, J. Y., Lin, C. Y., Su, T. P., and Chou, P. (2006). Association of initial antipsychotic response to clozapine and long-term weight gain. Am. J. Psychiatry 163 (7), 1276–1279. doi:10.1176/appi.ajp.163.7.1276
Brömel, T., Blum, W. F., Ziegler, A., Schulz, E., Bender, M., and Fleischhaker, C., (1998). Serum leptin levels increase rapidly after initiation of clozapine therapy. Mol. Psychiatry 3 (1), 76–80. doi:10.1038/sj.mp.4000352
Chen, K., Shen, Z., Gu, W., Lyu, Z., Qi, X., and Mu, Y., (2023). Prevalence of obesity and associated complications in China: a cross-sectional, real-world study in 15.8 million adults. Diabetes Obes. Metab. 25, 3390–3399. doi:10.1111/dom.15238
Chen, Y. Q., Li, X. R., Zhang, L., Zhu, W. B., Wu, Y. Q., and Guan, X. N., (2021). Therapeutic response is associated with antipsychotic-induced weight gain in drug-naive first-episode patients with schizophrenia: an 8-week prospective study. J. Clin. Psychiatry 82 (3), 20m13469. doi:10.4088/JCP.20m13469
Correll, C. U., Lencz, T., and Malhotra, A. K. (2011). Antipsychotic drugs and obesity. Trends Mol. Med. 17 (2), 97–107. doi:10.1016/j.molmed.2010.10.010
De Hert, M., Detraux, J., van Winkel, R., Yu, W., and Correll, C. U. (2011). Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat. Rev. Endocrinol. 8 (2), 114–126. doi:10.1038/nrendo.2011.156
De Hert, M., Dobbelaere, M., Sheridan, E. M., Cohen, D., and Correll, C. U. (2011). Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: a systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Eur. Psychiatry 26 (3), 144–158. doi:10.1016/j.eurpsy.2010.09.011
Dibonaventura, M., Gabriel, S., Dupclay, L., Gupta, S., and Kim, E. (2012). A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry 12, 20. doi:10.1186/1471-244X-12-20
Gentile, S. (2009). Contributing factors to weight gain during long-term treatment with second-generation antipsychotics. A systematic appraisal and clinical implications. Obes. Rev. 10 (5), 527–542. doi:10.1111/j.1467-789X.2009.00589.x
Hermes, E., Nasrallah, H., Davis, V., Meyer, J., McEvoy, J., and Goff, D., (2011). The association between weight change and symptom reduction in the CATIE schizophrenia trial. Schizophr. Res. 128 (1-3), 166–170. doi:10.1016/j.schres.2011.01.022
Homan, P., Argyelan, M., Fales, C. L., Barber, A. D., DeRosse, P., and Szeszko, P. R., (2019). Striatal volume and functional connectivity correlate with weight gain in early-phase psychosis. Neuropsychopharmacology 44 (11), 1948–1954. doi:10.1038/s41386-019-0464-y
Huhn, M., Nikolakopoulou, A., Schneider-Thoma, J., Krause, M., Samara, M., and Peter, N., (2019). Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet 394 (10202), 939–951. doi:10.1016/S0140-6736(19)31135-3
Jones, B., Basson, B. R., Walker, D. J., Crawford, A. M., and Kinon, B. J. (2001). Weight change and atypical antipsychotic treatment in patients with schizophrenia. J. Clin. Psychiatry 62 (2), 41–44.
Kaar, S. J., Natesan, S., McCutcheon, R., and Howes, O. D. (2019). Antipsychotics: mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology 172, 107704. doi:10.1016/j.neuropharm.2019.107704
Kemp, D. E., Correll, C. U., Tohen, M., Delbello, M. P., Ganocy, S. J., and Findling, R. L., (2013). Associations among obesity, acute weight gain, and response to treatment with olanzapine in adolescent schizophrenia. J. Child. Adolesc. Psychopharmacol. 23 (8), 522–530. doi:10.1089/cap.2012.0099
Kraus, T., Haack, M., Schuld, A., Hinze-Selch, D., Kühn, M., and Uhr, M., (1999). Body weight and leptin plasma levels during treatment with antipsychotic drugs. Am. J. Psychiatry 156 (2), 312–314. doi:10.1176/ajp.156.2.312
Leucht, S., Cipriani, A., Spineli, L., Mavridis, D., Orey, D., and Richter, F., (2013). Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382 (9896), 951–962. doi:10.1016/S0140-6736(13)60733-3
Li, S., Chen, D., Xiu, M., Li, J., and Zhang, X. Y. (2021). Diabetes mellitus, cognitive deficits and serum BDNF levels in chronic patients with schizophrenia: a case-control study. J. Psychiatr. Res. 134, 39–47. doi:10.1016/j.jpsychires.2020.12.035
Li, S., Gao, Y., Lv, H., Zhang, M., Wang, L., and Jiang, R., (2018). T(4) and waist:hip ratio as biomarkers of antipsychotic-induced weight gain in Han Chinese inpatients with schizophrenia. Psychoneuroendocrinology 88, 54–60. doi:10.1016/j.psyneuen.2017.11.010
Luckhoff, H., Phahladira, L., Scheffler, F., Asmal, L., Du Plessis, S., and Chiliza, B., (2019). Weight gain and metabolic change as predictors of symptom improvement in first-episode schizophrenia spectrum disorder patients treated over 12 months. Schizophr. Res. 206, 171–176. doi:10.1016/j.schres.2018.11.031
Mansur, R. B., Fries, G. R., Subramaniapillai, M., Frangou, S., De Felice, F. G., and Rasgon, N., (2018). Expression of dopamine signaling genes in the post-mortem brain of individuals with mental illnesses is moderated by body mass index and mediated by insulin signaling genes. J. Psychiatr. Res. 107, 128–135. doi:10.1016/j.jpsychires.2018.10.020
Mercer, L. P., Kelley, D. S., Humphries, L. L., and Dunn, J. D. (1994). Manipulation of central nervous system histamine or histaminergic receptors (H1) affects food intake in rats. J. Nutr. 124 (7), 1029–1036. doi:10.1093/jn/124.7.1029
Naslund, J. A., Aschbrenner, K. A., Scherer, E. A., Pratt, S. I., Wolfe, R. S., and Bartels, S. J. (2016). Lifestyle intervention for people with severe obesity and serious mental illness. Am. J. Prev. Med. 50 (2), 145–153. doi:10.1016/j.amepre.2015.07.012
Nasrallah, H. A. (2008). Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol. Psychiatry 13 (1), 27–35. doi:10.1038/sj.mp.4002066
Pillinger, T., McCutcheon, R. A., Vano, L., Mizuno, Y., Arumuham, A., and Hindley, G., (2020). Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry 7 (1), 64–77. doi:10.1016/S2215-0366(19)30416-X
Raben, A. T., Marshe, V. S., Chintoh, A., Gorbovskaya, I., Müller, D. J., and Hahn, M. K. (2017). The complex relationship between antipsychotic-induced weight gain and therapeutic benefits: a systematic review and implications for treatment. Front. Neurosci. 11, 741. doi:10.3389/fnins.2017.00741
Raven, S. F. H., Hoebe, C., Vossen, A., Visser, L. G., Hautvast, J. L. A., and Roukens, A. H. E., (2020). Serological response to three alternative series of hepatitis B revaccination (Fendrix, Twinrix, and HBVaxPro-40) in healthy non-responders: a multicentre, open-label, randomised, controlled, superiority trial. Lancet Infect. Dis. 20 (1), 92–101. doi:10.1016/S1473-3099(19)30417-7
Sharma, E., Rao, N. P., and Venkatasubramanian, G. (2014). Association between antipsychotic-induced metabolic side-effects and clinical improvement: a review on the Evidence for "metabolic threshold. Asian J. Psychiatr. 8, 12–21. doi:10.1016/j.ajp.2013.11.017
Tiwari, A. K., Zhang, D., Pouget, J. G., Zai, C. C., Chowdhury, N. I., and Brandl, E. J., (2018). Impact of histamine receptors H1 and H3 polymorphisms on antipsychotic-induced weight gain. World J. Biol. Psychiatry 19 (3), S97-S105–S105. doi:10.1080/15622975.2016.1262061
Vázquez-Bourgon, J., Ortiz-García de la Foz, V., Gómez-Revuelta, M., Mayoral-van Son, J., Juncal-Ruiz, M., and Garrido-Torres, N., (2022). Aripiprazole and risperidone present comparable long-term metabolic profile; data from a pragmatic randomized controlled trial in drug-naïve first episode psychosis. Int. J. Neuropsychopharmacol. doi:10.1093/ijnp/pyac033
Venkatasubramanian, G., Chittiprol, S., Neelakantachar, N., Shetty, T., and Gangadhar, B. N. (2010). Effect of antipsychotic treatment on Insulin-like Growth Factor-1 and cortisol in schizophrenia: a longitudinal study. Schizophr. Res. 119 (1-3), 131–137. doi:10.1016/j.schres.2010.01.033
Verma, S., Liew, A., Subramaniam, M., and Poon, L. Y. (2009). Effect of treatment on weight gain and metabolic abnormalities in patients with first-episode psychosis. Aust. N. Z. J. Psychiatry 43 (9), 812–817. doi:10.1080/00048670903107609
Wang, K., Birring, S. S., Taylor, K., Fry, N. K., Hay, A. D., and Moore, M., (2014). Montelukast for postinfectious cough in adults: a double-blind randomised placebo-controlled trial. Lancet Respir. Med. 2 (1), 35–43. doi:10.1016/S2213-2600(13)70245-5
Wimms, A. J., Kelly, J. L., Turnbull, C. D., McMillan, A., Craig, S. E., and O'Reilly, J. F., (2020). Continuous positive airway pressure versus standard care for the treatment of people with mild obstructive sleep apnoea (MERGE): a multicentre, randomised controlled trial. Lancet Respir. Med. 8 (4), 349–358. doi:10.1016/S2213-2600(19)30402-3
Xiu, M. H., Li, Z., Chen, D. C., Chen, S., Curbo, M. E., and Wu, H. E., (2020). Interrelationships between BDNF, superoxide dismutase, and cognitive impairment in drug-naive first-episode patients with schizophrenia. Schizophr. Bull. 46, 1498–1510. doi:10.1093/schbul/sbaa062
Zhu, M. H., Liu, Z. J., Hu, Q. Y., Yang, J. Y., Jin, Y., and Zhu, N., (2022). Amisulpride augmentation therapy improves cognitive performance and psychopathology in clozapine-resistant treatment-refractory schizophrenia: a 12-week randomized, double-blind, placebo-controlled trial. Mil. Med. Res. 9 (1), 59. doi:10.1186/s40779-022-00420-0
Keywords: schizophrenia, weight, improvements, negative symptoms, risperidone
Citation: Chen X, Fan Y, Ren W, Sun M, Guan X, Xiu M and Li S (2023) Baseline BMI is associated with clinical symptom improvements in first-episode schizophrenia: a longitudinal study. Front. Pharmacol. 14:1264591. doi: 10.3389/fphar.2023.1264591
Received: 21 July 2023; Accepted: 20 October 2023;
Published: 09 November 2023.
Edited by:
Song Zhang, Shanghai Jiao Tong University, ChinaCopyright © 2023 Chen, Fan, Ren, Sun, Guan, Xiu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuyun Li, aml1amlhbmcxOTk2QDE2My5jb20=
 Xiaofang Chen1
Xiaofang Chen1 Meihong Xiu
Meihong Xiu