- 1Department of Oncology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2School of Basic Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3Guang’an Hospital of Traditional Chinese Medicine, Guang’an, China
- 4State Key Laboratory of Southwestern Chinese Medicine Resources, School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 5Department of Gastroenterology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 6TCM Regulating Metabolic Diseases Key Laboratory of Sichuan Province, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
Colorectal cancer (CRC) is the third most common malignant tumor in the world, and it is prone to recurrence and metastasis during treatment. Aerobic glycolysis is one of the main characteristics of tumor cell metabolism in CRC. Tumor cells rely on glycolysis to rapidly consume glucose and to obtain more lactate and intermediate macromolecular products so as to maintain growth and proliferation. The regulation of the CRC glycolysis pathway is closely associated with several signal transduction pathways and transcription factors including phosphatidylinositol 3-kinases/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR), adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK), hypoxia-inducible factor-1 (HIF-1), myc, and p53. Targeting the glycolytic pathway has become one of the key research aspects in CRC therapy. Many phytochemicals were shown to exert anti-CRC activity by targeting the glycolytic pathway. Here, we review the effects and mechanisms of phytochemicals on CRC glycolytic pathways, providing a new method of drug development.
1 Introduction
Colorectal cancer (CRC) ranks third among the globally most prevalent cancers, in terms of incidence and mortality (Sheng et al., 2020). The morbidity and mortality of CRC are consistently increasing, which poses a serious threat to the general population’s health (Jian et al., 2020). After initial CRC diagnosis, 20% of the patients develop metastatic disease, and an additional 25% of the patients with initially localized disease will subsequently develop metastasis (Biller and Schrag, 2021). At present, surgical resection and chemo-radiotherapy are the most common treatment options for CRC patients (Liang et al., 2019). Surgery is the only curative treatment of CRC, however, even after radical surgery, a high recurrence rate remains (van der Stok et al., 2017). Further, there is considerable resistance to CRC to chemotherapy (Van der Jeught et al., 2018). Therefore, the current treatment of CRC is in urgent need of novel and more effective avenues.
The Warburg effect of cancer cells dictates that even under aerobic conditions, cancer cells require glycolysis for energy, which is a hallmark of tumor metabolism (Salimian Rizi et al., 2015). Normal cells generate energy primarily through mitochondrial oxidative phosphorylation, and glycolysis is increased only under hypoxia (Leung et al., 2015). The glucose uptake by cancer cells is markedly higher than that of normal cells, and most of the pyruvate produced is converted to lactic acid through lactate dehydrogenase (LDH). Compared with 36 ATP molecules produced by oxidative metabolism, glycolysis yields only 2 ATP plus 2 lactic acid molecules from 1 molecule of glucose (Gatenby and Gillies, 2004). Although the efficiency of ATP production through glycolysis is lower, the ATP generation rate through glycolysis is nearly 100-fold faster, compared to oxidative phosphorylation (Zhou et al., 2012). Further, rapid aerobic glycolysis produces many intermediates that are conducive to the growth of tumor cells, and these intermediates maintain the proliferation, invasion, and metastasis of tumor cells (Lunt and Vander Heiden, 2011). The metabolic pathway of the Warburg effect has become the focus of tumor treatment in recent years, and its related targeted pathways will be used as an important approach for researching treatment options for colorectal malignancies.
Phytochemicals are an important source of medicines, and plant drugs still play an important role in the treatment of diseases in developing countries (Tamene and Endale, 2019). Despite the rapid development of synthetic drugs, natural compounds remain one of the main sources of drugs (Kazantseva et al., 2022). Approximately 25% of anti-cancer drugs are derived from phytochemicals and contain one or more plant-active ingredients (Kopaskova et al., 2011). At present, many anticancer drugs are derived from phytochemicals, such as vinblastine, paclitaxel, and camptothecin (Wang H. et al., 2018). With the wide application of phytochemicals in cancer treatment and synthetic drug development becoming more difficult, research on anticancer drugs is focused on the development and utilization of phytochemicals and their active substances (Tong et al., 2019), and phytochemicals targeting tumor metabolism have been extensively studied. Moreover, the synergistic effects of phytochemical drugs and anticancer drugs have been attracting attention, as a combination of the two drugs can inhibit the recurrence and metastasis of cancer and reduce the resistance of tumor cells to chemotherapy (Lee et al., 2021). Therefore, it is vital to accelerate the development and utilization of phytochemical drugs for cancer treatment.
Here, we review research on phytochemicals and their active components acting on the glycolysis pathway of CRC cells, and we discuss the molecular mechanisms of phytochemicals targeting the glycolysis pathway to treat CRC. This review will provide a clue for further research on the treatment of CRC targeting the glycolysis pathway.
2 Aerobic glycolysis in CRC
Substantial evidence suggests that metabolic enzymes and transporters associated with the glycolytic pathway are highly expressed in CRC, and these changes may be related to the regulation of several signaling pathways and transcription factors (As shown in Figure 1).

FIGURE 1. Glycolysis in colorectal cancer cells and the related regulatory pathways. Abbreviation: GLUTs, Glucose transporters; HKs, Hexokinases; PFK1, Phosphofructokinase-1; PK, Pyruvate kinase; LDH, Lactate dehydrogenase; PDK, Pyruvate dehydrogenase kinase; PDH, Pyruvate dehydrogenase; AMPK, Adenosine 5′-monophosphate-activated protein kinase; TSC1/2, Tuberous sclerosis complex1/2; AKT, Protein kinase B; mTOR, Mammalian target of rapamycin; HIF-1, Hypoxia-inducible factor-1; PI3K, Phosphatidylinositol 3-kinases; PTEN, Phosphatase and tensin homolog; USP13, Ubiquitin-specific peptidase 13; RPS7, ribosomal protein S7; TCA, Tricarboxylic acid; PPP, Pentose phosphate pathway; HSP90, Heat shock protein 90; MCTs, Monocarboxylic acid transporters; MEG3, lncRNA maternally expressed gene 3; SOD2, Superoxide dismutase 2.
2.1 Upregulation of the glycolytic pathways in CRC
The glycolytic metabolism of CRC cells is closely associated with particular transporters and key enzymes of the glycolytic pathway. Glucose metabolism in cells is mediated by glucose transporters (GLUTs), which occur in the cell membrane. There are 14 subtypes of the GLUT gene (Ancey et al., 2018), of which GLUT1, as one of the most intensively studied membrane transporters, is a key speed-limiting factor of glucose metabolism (Thorens and Mueckler, 2010). This transporter is highly expressed in various cancer tissues, including CRC (Younes et al., 1995; Baer et al., 1997; Mori et al., 2007; Feng et al., 2017), and it has been confirmed to be related to the progress and metastasis of CRC (Younes et al., 1996; Haber et al., 1998). Hexokinase (HK) is a catalytic enzyme that converts glucose into glucose-6-phosphate and is the first rate-limiting enzyme in the glycolysis pathway. There are four types of HK isoenzymes, among which HK2 is abnormally highly expressed in the metabolic pathway of tumor cells, and its expression is closely associated with CRC proliferation and metastasis (Shinohara et al., 1994; Wilson, 2003; Katagiri et al., 2017; Huang et al., 2021). Phosphofructokinase-1, as a catalytic enzyme for the synthesis of 1,6-fructose-diphosphate from fructose-6-phosphate, is one of the key rate-limiting enzymes in the glycolytic pathway. Genes encoding phosphofructokinase-1 activity are highly expressed in CRC (Houddane et al., 2017). The key regulatory enzyme of the pyruvate kinase (PK) glycolysis pathway is responsible for the dephosphorylation of phosphoenolpyruvate to pyruvate and ATP. There are four PK isoenzymes, of which PKM2 is highly expressed in many malignant tumors, including colorectal tumors (Wong et al., 2014; Cui and Shi, 2015; Han et al., 2016). PKM2 maintains the high glycolysis rate of tumor cells and regulates the proliferation of tumor cells (Yu et al., 2015). In the process of tumor glycolysis, most pyruvate is converted into lactic acid by LDH (Li et al., 2020b). LDHA is highly expressed in colorectal and other malignant tumors and is closely related to tumor proliferation and metastasis (Wang et al., 2015; Xian et al., 2015; Cui et al., 2017; Zhao et al., 2017).
Monocarboxylic acid transporters (MCTs) are lactic acid transporters located on the cell membrane, which are responsible for transporting intracellular lactic acid to the exterior of the cell to maintain the pH in tumor cells; further, they are highly expressed in primary CRC and are associated with CRC metastasis and prognosis (Martins et al., 2016). Pyruvate can be converted to acetyl coenzyme A (CoA) under the action of the pyruvate dehydrogenase complex, and enter mitochondria for metabolism through the oxidative phosphorylation pathway (Saunier et al., 2016). Pyruvate dehydrogenase complex acts as the gatekeeper of the oxidative phosphorylation pathway, and the respective enzymes are inhibited in CRC to maintain the high glycolysis rate of CRC and its proliferation and invasion (Hamabe et al., 2014; Ho and Coomber, 2015). Therefore, targeting pyruvate dehydrogenase complex may be a new direction for CRC treatment. The above glycolytic metabolic pathways are abnormally expressed in CRC cells and have exacerbating effects on the occurrence and development of CRC.
2.2 Regulatory mechanisms of glycolytic pathways in CRC
Enzymes and transporters related to the CRC glycolysis pathway are abnormally expressed by different molecular regulatory mechanisms, thus leading to the occurrence and development of CRC.
2.2.1 PI3K/AKT/mTOR
PI3K/AKT/mTOR signaling pathway is a cellular signal transduction pathway, which affects cell growth, proliferation, angiogenesis, and other processes (Coutte et al., 2012; He et al., 2022; Hu et al., 2023). Activation of the PI3K/AKT signal pathway plays an important role in maintaining the glucose metabolism of cells, as it can increase glucose intake by increasing the expression of GLUT and upregulate the expression of glycolysis-related enzymes to promote cell glycolysis (Chang et al., 2015; Prabakaran et al., 2018). AKT is frequently overexpressed in most CRC, whereas phosphatase and tensin homolog (PTEN) expression is lost (Robey and Hay, 2009). As a tumor suppressor gene, PTEN negatively regulates the PI3K/AKT/mTOR pathway (Hollander et al., 2011), and deletion of PTEN can specifically increase the protein level of HIF-1α through PI3K signaling (Jiang et al., 2001), mTOR is closely related to cell growth and mTOR expression is regulated by a variety of factors, including the energy regulator AMPK. mTOR can also regulate the expression of HIF-1α by sensing the intracellular level of hypoxia (Martin and Hall, 2005).
Kallikrein-related peptidase, forkhead box class O6, and S100 calcium-binding protein A2 are highly expressed in CRC and play an activating role in glycolysis by activating PI3K/AKT/mTOR pathway and up-regulating GLUT1 expression (Li et al., 2019; Li C. et al., 2020; Wei et al., 2020). MicroRNAs are non-coding RNA molecules that play a central part in cell differentiation, proliferation, and survival (Rupaimoole and Slack, 2017). MiR-135b is highly expressed in CRC cells, which can activate PI3K pathway and promote glycolysis of CRC cells by down-regulating the expression of ubiquitin-specific peptidase 13 and reducing the stability of PETN (Xiang et al., 2015).
2.2.2 AMPK
AMPK is a conservative Ser/Thr protein kinase, which regulates cell metabolism and maintains the dynamic balance of cell metabolism, thus it is referred to as a regulator of energy homeostasis (Faubert et al., 2013; Narayanankutty, 2019). AMPK is activated by sensing the increase of AMP and ATP (Tokunaga et al., 2019). Once activated, AMPK will immediately block the energy consumption process of cells, and turn to increase the ATP decomposition process (Wang and Guan, 2009). AMPK is an important kinase of cell metabolism and has been widely studied in many metabolic diseases (Towler and Hardie, 2007).
Superoxide dismutase 2 (SOD2) is a key antioxidant enzyme that is highly expressed in CRC. The reduction of SOD2 can significantly downregulate AMPK phosphorylation and the expression of MCT4 and L-lactate and ultimately inhibit the migration and glycolytic metabolism of CRC cells (Zhou C. et al., 2020). Pim1 is an oncogene promoting the growth and metastasis of CRC, which is highly expressed in CRC cells, and it has been found that pim1 can upregulate the expression of HK2 and LDHA in CRC cells, which may be related to the activation of the AMPK pathway under glucose deprivation (Zhang et al., 2018).
2.2.3 HIF-1
Compared with normal cells, the oxygen consumption of tumor cells is markedly increased to maintain cancer cells rapid proliferation, thus leading to relative hypoxia in the local microenvironment (Georges et al., 2018). HIF plays a critical role in driving tumor growth, invasion, and metastasis and is found to be highly expressed in most solid tumors (Kumar and Gabrilovich, 2014; Jiang et al., 2020). HIF - 1 has been demonstrated to participate in regulating key transcription factors of EMT and indirectly promotes EMT by Notch, TGF-β, Wnt, and Hedgehog signal pathways, thereby promoting tumor invasive metastasis (Rankin and Giaccia, 2016). HIF-1 can also induce the expression of the epidermal growth factor receptor (EGFR), transforming growth factor-β, insulin-like growth factor 2 to promote tumor angiogenesis (Bui et al., 2022). HIF has been confirmed to be involved in the glycolytic pathway of tumor cells, and it can directly upregulate the expression of glycolysis-related transporters and key enzymes such as GLUT, PFK, LDH, HK, etc. (Song et al., 2016). At the same time, HIF can also be activated by PI3K/AKT (Yeh et al., 2018), YAP/TAZ (Simula et al., 2022), hippo/YAP1 (Sun et al., 2020) and other signaling pathways to further promote the glycolytic process of tumor cells.
miR-103a-3p is an oncogene that is highly expressed in CRC tissues and cell lines and promotes the glycolysis pathway and proliferation, invasion, and migration of CRC cells by up-regulating the transcription of HK2, LDHA, and PKM1 through the Hippo/YAP1/HIF1α axis (Sun et al., 2020). The ribosomal protein S7 gene (RPS7), as a tumor suppressor gene plays a role in inhibiting glycolysis of CRC cells by inhibiting the expression of HIF-1α, GLUT4, and LDHB (Zhang et al., 2016).
2.2.4 Myc
Myc is highly overexpressed in human cancers, thus providing energy for tumor growth and proliferation and the synthesis of substrates required for the respective metabolism pathways (Dang et al., 2006; Miller et al., 2012). c-myc can directly upregulate the expression of LDHA and promote the conversion of pyruvate to lactic acid, and it can upregulate the expression of enzymes related to glycolytic pathways, such as HK2 and phosphoinositide-dependent protein kinase 1, and promote the glycolytic metabolism of cells (Dang et al., 2006; Miller et al., 2012). Myc is inhibited by HIF-1α, however, the two show synergistic effects in regulating the expression of glycolysis-related enzymes including HK2 and PDK1 (Yeung et al., 2008).
Long non-coding RNAs (lncRNAs) are commonly involved in tumor metabolic rewiring and immune cell infiltration and functioning (Zhang Y. et al., 2021). The lncRNA maternally expressed gene 3 (MEG3) reduces glycolytic levels in CRC cells by degrading the expression of c-myc, including down-regulating the expressions of glycolysis-related enzymes such as LDHA, PKM2, and HK2 (Zuo et al., 2020). Thus, MEG3 expression is inhibited in CRC cells (Zuo et al., 2020). Glycolysis-associated lncRNA of colorectal cancer (lncRNA GLCC1) interacts with heat shock protein90 (HSP90) to stabilize c-myc transcription and thus target c-myc-mediated LDHA expression (Tang et al., 2019).
3 Targeting glycolysis in colorectal cancer therapy
Increasing evidence suggests that metabolic reprogramming is closely related to the occurrence and development of most tumors, including CRC (Nenkov et al., 2021). Cancer cells show a significant increase in aerobic glycolysis in their metabolism, making them potentially more susceptible to inhibition of glycolytic pathways than normal cells. Numerous studies on CRC metabolic pathways attempted to identify new therapeutic directions, including the glycolysis pathway, and the drug-targeting glycolysis pathway is an attractive strategy for CRC therapy.
lncRNAs play an important role in the epigenetic regulation of cancer progression by regulating cell function and development (Zheng et al., 2021). Some lncRNAs are highly expressed in CRC, and they exert anti-tumor effects by regulating the glycolytic pathway (Wang Y. et al., 2019; Liu et al., 2020; Li C. et al., 2021). Chemotherapeutic resistance is a major challenge in CRC treatment, and reprogramming of glucose metabolism is also associated with chemical drug resistance, and high glycolysis levels found in human CRC drug-resistant models (Wang T. et al., 2018). Targeted inhibition of the glycolytic pathway can increase the sensitivity of CRC cells to chemotherapy drugs (Cao et al., 2018; An and Ha, 2022). 5-FU is an important drug in first-line chemotherapy for CRC. In CRC cells resistant to 5-FU, glucose uptake and lactic acid production increase, and glycolysis-related enzymes and GLUT1 and MCT1/4 are significantly upregulated, which may be related to the activation of PI3K/AKT and the Wnt/β-catenin signaling pathway by upregulation of HIF-1α (Dong et al., 2022). The onset of CRC is closely associated with intestinal environment disorders caused by lifestyle and dietary habits, thus probiotics show promising prospects in the treatment of CRC (Donovan et al., 2017; Eslami et al., 2020). Butyrate, a short-chain fatty acid produced by bacterial fermentation of dietary fibers in the colon, was the first probiotic identified as a histone deacetylase inhibitor, which may be related to the inhibition of the Warburg effect in CRC (Hamer et al., 2008; Donohoe et al., 2012; Eslami et al., 2020).
In conclusion, the glycolytic pathway can affect the occurrence and development of CRC through various processes. Therefore, studying the mechanism of CRC and reprogramming glucose metabolism in tumor cells may provide a new research strategy for CRC treatment (Vasaikar et al., 2019).
4 Methods
We conducted a comprehensive literature search using the Web of Science, PubMed, Embase, SpringerLink, ScienceDirect, and EBSCO databases from the beginning of the database to 30 September 2022 and collated and analyzed the literature for this review. The literature search was conducted by two authors independently and separately to minimize potential oversights. This review evaluates and summarizes all previous scientific studies on the effects of phytochemicals on CRC glycolysis pathways. In PubMed, we used MeSH search terms such as “colorectal neoplasm,” “colorectal cancer,” “glycolysis,” “Embden Meyerhof pathway,” “Warburg effect,” “phytochemicals” and “natural product.” We excluded non-experimental articles and repeated articles in the search process.
Through screening and summarizing plant chemicals targeting the CRC glycolytic pathway, we found that numerous in vitro and in vivo studies confirmed that plant chemicals can target the CRC glycolytic pathway and exert anti-cancer effects (As shown in Figures 2, 3; Table 1). Here, the targets affecting the glycolytic pathway are divided into 1) phytochemicals directly targeting the CRC glycolysis pathway; 2) phytochemicals targeting PI3K/AKT/mTOR to affect glycolysis in CRC; 3) phytochemicals targeting AMPK to affect glycolysis in CRC; 4) phytochemicals targeting HIF-1 to affect glycolysis in CRC; 5) phytochemicals targeting c-myc to affect glycolysis in CRC; 6) phytochemicals targeting other pathways that affect CRC glycolysis.
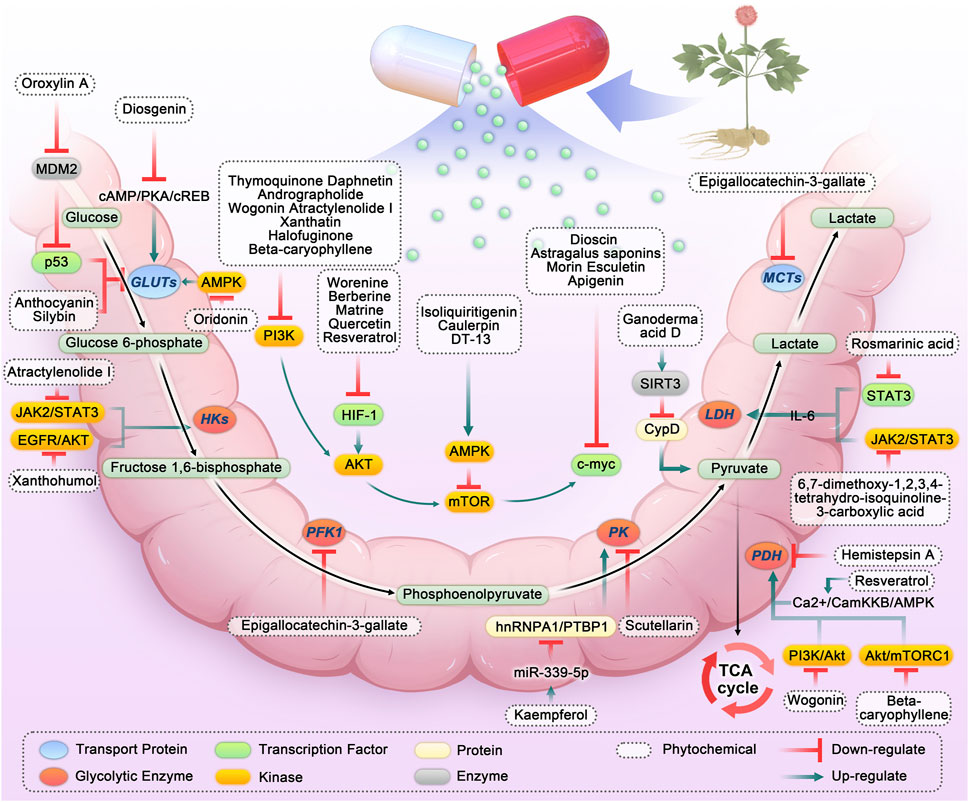
FIGURE 2. Phytochemicals targeting glycolysis in colorectal cancer. Abbreviation: HKs, Hexokinases; PFK1, Phosphofructokinase-1; PK, Pyruvate kinase; LDH, Lactate dehydrogenase; MCTs, Monocarboxylic acid transporters; PDH, Pyruvate dehydrogenase; TCA, Tricarboxylic acid; IL-6, Interleukin 6; SIRT3, Sirtuin-3; JAK2/STAT3, Janus kinase 2/signal transducer and activator of transcription 3; PI3K, Phosphatidylinositol 3-kinases; AKT, Protein kinase B; HIF-1, Hypoxia-inducible factor-1; AMPK, Adenosine 5′-monophosphate-activated protein kinase; hnRNPA1, Heterogeneous nuclear ribonucleoprotein A1; PTBP1, polypyrimidine tract-binding protein 1; PTEN, Phosphatase and tensin homolog.
5 Phytochemicals targeting signal pathways and transcription factors in the CRC glycolysis pathway
5.1 Phytochemicals directly targeting CRC glycolysis pathway
5.1.1 Anthocyanin
Anthocyanin (ANC) is a flavonoid that is common in plants (McGhie and Walton, 2007). And after glycosylation, ANC glycosides can be rapidly absorbed by the human body (McGhie et al., 2003). Cyanidin-3-glucoside, a member of the anthocyanin family, is found in purple or red vegetables and fruits (Shan et al., 2021). A comparative study using cyanidin-3-glucoside and its anthocyanidin aglycone showed that cyanidin-3-glucoside significantly inhibited the expression of GLUT1 in MC38 (mouse colon cancer cells), and it can disturb glucose transport, inhibit energy metabolism, and cause mitochondrial damage and apoptosis in CRC cells (Jing et al., 2020).
5.1.2 Silybin
Silybin is the major component of silymarin, a flavonolignan mixture extracted from the fruits of Silybum marianum (L.) Gaertner displays antioxidant, anti-inflammatory, immunomodulatory and hepatoprotective properties (Chambers et al., 2017). Compared with doxorubicin-sensitive LoVo cells (LoVo WT), doxorubicin-resistant LoVo cells (LoVo DOX) show higher mRNA and protein expression levels of glycolysis-related enzymes, such as GLUT1 and MCT4 (Catanzaro et al., 2018). Further, it also shows a tendency for metabolic transfer from the oxidative phosphorylation pathway to the glycolysis pathway. After treatment of cells with silybin 10–50 μM, glucose uptake and GLUT1 expression is downregulated in sensitive and drug-resistant LoVo cells in silybin 10 μM, and silybin 50 μM resulted effective only in resistant cells (Catanzaro et al., 2018).
5.1.3 Scutellarin
Scutellarin is a flavonoid drug derived from the plant Erigeron breviscapus (Vant.) Hand.-Mazz. (Chen et al., 2006; Dong and Qu, 2022). Erigeron breviscapus (Vant.) Hand.-Mazz. is a Chinese herbal medicine that was first recorded in South Yunnan Materia Medica with multiple pharmacological effects and clinical applications, such as detoxification, inflammation reduction and pain relief (Chen et al., 2006; Dong and Qu, 2022). Studies have found that Oxaliplatin-resistant CRC cells (OR-SW480 and OR-HT29) exhibit higher glycolysis rate and high mRNA and protein expression of PKM2, while scutellarin can inhibit the glucose metabolism rate and ATP production of cells by decreasing the PKM2 expression of OR-SW480 and OR-HT29 cells, leading to mitochondrial dysfunction and finally inducing cell apoptosis (Sun et al., 2021). This previous study established an in vivo model using OR-SW480 cells injected into 4-week-old female immunodeficient nude BALB/c mice, showing that 10 mg/kg scutellarin reversed the drug resistance of oxaliplatin in the OR-SW480 mouse model, increased apoptosis induced by oxaliplatin, and significantly reduced protein expression of PKM2 and ATP production in the tumor (Sun et al., 2021).
5.1.4 Kaempferol
Kaempferol is a flavonoid compound that occurs in many plants such as Acacia nilotica (L.) Delile, Aloe vera (L.) Burm.f., and Crocus sativus L, and has been shown to be cardioprotective, anti-inflammatory, antidiabetic, antioxidant, antitumor, and have anticancer activities (Devi et al., 2015; Imran et al., 2019). Studies have shown that kaempferol can exert anticancer effects by inducing apoptosis of cancer cells, causing cell cycle arrest and autophagy (Wu et al., 2018). Studies have shown that after Kaempferol treatment, the cell cycle of HCT116 and DLD1 cells is delayed, and apoptosis is upregulated (Wu et al., 2021). In addition, glucose consumption, lactic acid production, and ATP level exhibit a downward trend. Kaempferol can also upregulate the expression of miR-339-5p in HCT116 and DLD1 cells, reducing the expression of hnRNPA1 and PTBP1, leading to the downregulation of PKM2 expression, thereby inhibiting glycolysis of CRC (Wu et al., 2021).
5.1.5 Epigallocatechin-3-gallate
Green tea, which originated in China, is one of the most popular drinks in the world and was used in traditional Chinese medicine to treat many illnesses (Akbarialiabad et al., 2021). Epigallocatechin-3-gallate (EGCG) is mainly sourced from green tea (Nagle et al., 2006). EGCG inhibits the proliferation, migration, and metastasis of HCT116 and HT29 cells (Chen et al., 2022). In addition, in cancer-associated fibroblasts (CAFs) co-cultured with HCT116 and HT29 cells, 50 μM EGCG significantly decreased lactate production, PFK, and MCT4 expression (Chen et al., 2022). After further silencing the expression of MCT4, it was found that the above inhibitory effect of EGCG was reversed. Therefore, it is speculated that EGCG may inhibit the glycolysis of CAFs through MCT4.
5.1.6 Hemistepsin A
Hemisteptia lyrata is a kind of Chinese herb, its leaves and flowers are used for the treatment of sore throat and treatment of tumor (Jang et al., 1999). Hemistepsin A (HsA) is a sesquiterpene lactone isolated from Hemistepta lyrata Bunge, which exerts various pharmacological effects including anti-hepatotoxic, anti-inflammatory, and anti-cancer activities (Park et al., 2020). Treatment of various CRC cell lines with HsA showed that HsA had significant cytotoxicity, and significantly inhibited cell growth, but had no significant effect on normal cells (Jin et al., 2020). Further, after treatment with HsA, the extracellular acidification rate (ECAR) of DLD1 and CT26 cells decreased in a dose-dependent manner. Further study of its internal mechanism found that HsA inhibited the activity of PDK1 by interfering with the interaction between PDK1 protein and lipoamide binding domains of PDH-E2 (L1 and L2), reduced the phosphorylation of PDHA1, and thus inhibited the formation of lactic acid. In vivo, experiments showed that HsA (1, 10 mg/kg/day) treated for 10d inhibited the growth of BALB/c mice bearing CT26 cells in a dose-dependent manner, upregulated the expression of apoptosis-related genes, and reduced the phosphorylation of PDHA1, which was consistent with the results of in vitro experiments (Jin et al., 2020).
5.2 Phytochemicals targeting PI3K/AKT/mTOR to affect glycolysis in CRC
5.2.1 Thymoquinone
Nigella sativa is a flowering plant that belongs to the Ranunculaceae family, native to south and southwest Asia, and is used to treat a variety of ailments such as diarrhea, asthma, headaches, cough, eczema, and more (Ciesielska-Figlon et al., 2023). Thymoquinone is a bioactive constituent derived from the seeds of N. sativa and has shown significant anticancer activity in various tumors (Phua et al., 2021). Thymoquinone significantly inhibits the proliferation, invasion, and metastasis of HCT116 and SW480 cells, induces cell apoptosis, and downregulates the lactic acid production, glucose uptake, and ATP levels of tumor cells (Karim et al., 2022). Furthermore, it exerts anti-CRC effects by inhibiting HK2 expression under the PI3K/AKT pathway, thus affecting the glycolysis pathway of CRC cells (Karim et al., 2022).
5.2.2 Daphnetin
Daphnetin, a coumarin derivative, was first isolated from Daphne Korean Nakai, and it exerts various pharmacological activities such as anticoagulation, anti-inflammatory, heart protection, and anti-cancer effects (Hang et al., 2022; Javed et al., 2022). After daphnetin treatment for 24 h, the proliferation and migration of SW480 and HT29 cells are inhibited, apoptosis is increased, glucose uptake and lactic acid production are inhibited, and the protein expression levels of HK2 and GLUT1 are downregulated (He et al., 2018). In addition, the phosphorylated expression of PI3K and AKT is significantly downregulated in SW480 cells treated with daphnetin, LY294002 (a PI3K/AKT inhibitor) could enhance the inhibitory effect of daphnetin on the glycolysis pathway in SW480 cells. Thus daphnetin may exert anti-cancer effects by targeting the PI3K/AKT pathway to affect glycolysis in CRC cells (He et al., 2018).
5.2.3 Andrographolide
Andrographis paniculata belongs to the genus Andrographis, widely distributed in Asian countries such as India, China, and Malaysia, and has been often used to treat inflammatory diseases (Li et al., 2022c). Andrographolide is the principal active ingredient of Andrographis paniculate extract, which has shown great potential in the treatment of a variety of inflammatory diseases as well as tumors (Zhang H. et al., 2021; Qu et al., 2022). Andrographolide not only inhibits glucose uptake and lactic acid production in HCT116 cells, decreases ATP levels, and inhibits the expression of glycolysis proteins and enzymes, such as PFK1, HK2, and GLUT1, but also inhibits the phosphorylation of the PI3K/AKT/mTOR pathway (Li et al., 2020c). Further, IGF-1 (a PI3K activator) can reverse the downregulated expression of PI3K, p-AKT, and p-mTOR by andrographolide, and andrographolide shows similar inhibitory effects on HCT116 as LY294002 (a PI3K inhibitor). Therefore, andrographolide may inhibit CRC by targeting PI3K/AKT signal pathway. Interestingly, andrographolide may also improve the radio sensitivity of HCT116 cells through the PI3K/AKT pathway (Li et al., 2020c).
5.2.4 Wogonin
Scutellaria Baicalensis, belonging to the Lamiaceae family, is a commonly used Chinese herb for clearing heat and detoxification (Yang et al., 2022). Wogonin is a flavonoid from Scutellaria baicalensis, which has anti-tumor, anti-inflammatory, antiviral, and other pharmacological effects (Banik et al., 2022). Wogonin at 20, 40, 60, 80, and 100 mM for 24 h can downregulate the expressions of HK2, PDHK1, and LDHA by inhibiting the transcriptional activity of PI3K/AKT and the expression of HIF-1α, thus affecting the glucose uptake and lactic acid production of HCT116 cells under hypoxia (Wang et al., 2014). Meanwhile, an in vivo study showed that 30 and 60 mg/kg wogonin could significantly reduce the tumor weight of male BALB/c nude mice, and decreased the expression of HIF-1α, glycolysis-related proteins, and PI3K/AKT (Wang et al., 2014). In another study, wogonin inhibited the survival of HCT116 cells and the glycolysis of HCT116 expressing wild type in a dose-dependent manner (Zhao et al., 2018). Wogonin increased the protein level as well as mRNA level of p53 and TIGAR in HCT116 cells, and decreased the protein and mRNA level of PGM, HK2, GLUT1, PDHK1, and LDHA, however, in p53-null and mt-p53 HCT-116 cells the expression and transcription of the glycolysis regulator barely changed. The experiment showed that the inhibitory effect of wogonin on cancer cell glycolysis was dependent on wt-p53. (Zhao et al., 2018).
5.2.5 Atractylenolide I
Atractylodes macrocephala Koidz., which has been used for thousands of years in China, is mainly used to treat gastrointestinal diseases such as loss of appetite, abdominal distension and diarrhea, has the functions of invigorating the spleen for strengthening the stomach, and dispelling dampness for diuresis (Li et al., 2022b). Atractylenolide I (AT-I) is a sesquiterpenoid lactone derivative of Atractylodis macrocephalus (Zhu et al., 2018). AT-I downregulates the phosphorylation of proteins related to the AKT/mTOR pathway and the protein expression of GLUT1, LDH, HK2, and PKM2 in a dose-dependent manner in HCT116 and Colo205 cells, thus disrupting the glucose metabolism of colorectal tumors, followed by induction of apoptosis and reduced cell invasion (Wang K. et al., 2020). In Balb/c nude mice were xenografted with HCT116 cells treated with 25 and 75 mg/kg AT-I, the tumor growth and the expression of glycolysis-related proteins were significantly inhibited, it is consistent with the in vitro experimental results (Wang K. et al., 2020). In addition, AT-I downregulates the expression of HK2 in HCT116 cells by inhibiting the JAK2/STAT3 pathway and reduces glucose consumption and lactate production in HCT116 cells, thus exerting anti-CRC effects (Li Y. et al., 2020). Similar results were also shown in vivo using an HCT116 transplanted mouse model treatment with AT-I at 50 mg/kg/day for 3 weeks. JAK2/STAT3, as a signaling network pathway, is responsible for transducing the signals conveyed by many cytokines from the cell membrane to the nucleus, and extensive studies have confirmed that JAK2/STAT3 plays an important role in the occurrence and development of tumors and drug resistance (Mengie Ayele et al., 2022).
5.2.6 Xanthatin
Xanthium strumarium L (Asteraceae) is a commonly traditional Chinese herb have been applied for treating various diseases, including rhinitis, headache, arthritis, etc. (Fan et al., 2019). Xanthatin is a bioactive sesquiterpene lactone isolated from X. strumarium L. (Fan et al., 2019), which suppresses the growth of many tumors, such as lung cancer (Tao et al., 2016), glioma (Ma et al., 2020), and melanoma (Li et al., 2013). In addition, xanthatin downregulates ATP levels, lactic acid production, and glucose uptake in HCT116 and HT29 cells by inhibiting the mTOR pathway, and the mRNA and protein levels expression of GLUT1, MCT4 and the protein levels expression of HK1 is also inhibited (Li L. et al., 2022). Interestingly, elevated oxygen consumption rates (OCR) and downregulated succinic acid expression were observed in xanthatin-treated HT29 cells, which may be related to xanthatin-induced oxidative phosphorylation (Li L. et al., 2022). These results suggest that xanthatin may inhibit the glycolytic pathway of CRC cells by targeting the mTOR signaling pathway, in compensation for the activation of oxidative phosphorylation metabolism in cells, thereby causing mitochondrial dysfunction and ultimately inhibiting the growth and metastasis of CRC cells.
5.2.7 Halofuginone
Chang Shan (Dichroa febrifuga Lour) is a traditional Chinese medicine as a treatment for malaria, dating back about 2000 years of history (Zhou et al., 2013). Halofuginone is a natural quinazolinone alkaloid extracted from the plant Dichroa febrifuga (Jain et al., 2021). Halofuginone is less toxic to normal cells, but it significantly inhibits the growth of CRC and induces reactive oxygen species (ROS) production and cell apoptosis in a dose-dependent manner (Chen et al., 2015). Moreover, halofuginone downregulates the phosphorylation of AKT, mTORC1, and downstream p70S6K in SW480 and HCT116 cells, while the phosphorylation of 4EBP1 is increased in a dose-dependent manner. P70S6K is one of the effectors of mTORC1, which can activate the pentose phosphate pathway, and 4EBP1 inhibits glucose uptake and glycolysis. Halofuginone also significantly decreases the protein expression of HK2 and GLUT1 and the production of tricarboxylic acid cycle intermediates, indicating that halofuginone inhibits the glycolysis pathway of CRC, which may be mediated by the AKT/mTORC1 signaling pathway. A vivo experiment showed that halofuginone at 0.1 mg/kg for 14 days downregulated the tumor weight and AKT/mTORC1 expression in female BALB/c nude mice xenotransplanted with HCT116 cells, which was consistent with the results of an in vitro experiment (Chen et al., 2015).
5.2.8 Beta-caryophyllene
Beta-caryophyllene (BCP) is a bicyclic sesquiterpene that is a common constituent of essential oils, such as clove oil obtained from the dried flower-buds of Syzygium aromaticum (Machado et al., 2018). Arginine-specific-mono-ADP-ribosyltransfer 1 (ART1) is highly expressed in CRC and inhibits CRC proliferation, invasion, and metastasis (Yang et al., 2016). The glycolytic pathway is upregulated in ART1-expressing CT26 cells, while BCP can inhibit the expression of ART1-influenced glycolysis-related expression, such that of p-AKT, p-mTOR, c-Myc, PDK1 and LDHA, and it reduces lactate concentrations and ATP levels in CT26 cells, as shown using in vivo and in vitro models (Zhou et al., 2018) This suggests that BCP may inhibit ART1-induced glycolysis through the AKT/mTOR pathway, and it may play a role in inhibiting CRC cell proliferation and inducing apoptosis.
5.2.9 Xanthohumol
Xanthohumol is a natural polyphenol chalcone from flowers of the common hop (Humulus lupulus L.) (Vicente de Andrade Silva et al., 2022). Xanthohumol has a variety of pharmacological activities, including antioxidant, anti-inflammatory, and anti-tumor effects (Jiang et al., 2018). The proliferation of HCT116, SW620, and HT29 cells is significantly inhibited and apoptosis is increased after treatment with xanthohumol (Liu et al., 2019); at the same time, glucose consumption, lactic acid production, and HK2 expression in cells are inhibited, and cell pH is decreased. In addition, xanthohumol inhibits the epidermal growth factor receptor (EGFR) and AKT signaling in a dose-dependent manner, and active Akt, Myr-Akt1 can rescued xanthohumol-induced HK2 suppression (Liu et al., 2019). Therefore, xanthohumol may downregulate HK2 expression by inhibiting the activation of the EGFR/AKT pathway, thus affecting the glycolytic pathway and inducing apoptosis in CRC cells. After xenotransplantation of HT29 and HCT116 cells into female athymic nude mice, the tumor weight of 10 mg/kg every 2 days in xanthohumol-treated mice was significantly reduced, and expression of Ki-67, p-AKT, and HK2 was downregulated, which was consistent with the results of in vitro experiment (Liu et al., 2019).
5.3 Phytochemicals targeting AMPK to affect glycolysis in CRC
5.3.1 Isoliquiritigenin
Isoliquiritigenin (ISL) is one of the bioactive ingredients isolated from the roots of plants including Glycyrrhiza uralensis, Mongolian glycyrrhiza, and Glycyrrhiza glabra (Peng et al., 2015). ISL has a variety of biological activities, such as anti-inflammatory, antioxidant, nerve protection, and anti-tumor growth effects (Wang et al., 2021). ISL and ISL-loaded nanoparticles (ISL-NLs) inhibit proliferation, increase apoptosis, inhibit glucose uptake and lactate production, downregulate OCR and ATP levels, and decrease basal ECAR and maximum ECAR in HCT116 cells (Wang G. et al., 2020). Further, the respective study showed that the cell membrane potential was decreased, ROS levels were significantly increased, and the mRNA and protein levels of molecules enolase 1 (ENO1), aldolase, fructose-bisphosphate A (ALDOA), LDHA, MCT4, c-myc, and HIF-1α were also significantly decreased. The authors found that ISL and ISL-NLS could though activate AMPK and downregulate the expression of AKT and mTOR in HCT116 cells, thereby affecting cellular glucose metabolism. Compared with ISL, ISL-NLs have a stronger inhibitory effect on CRC (Wang G. et al., 2020).
5.3.2 Caulerpin
Caulerpin is a bis-indole alkaloid, which has been isolated from Caulerpa racemosa and C. serrulata (Canché Chay et al., 2014). It has been proven to have anti-diabetic, anti-inflammatory, anti-tumor, anti-tuberculosis, and antibacterial effects (Lunagariya et al., 2019). A previous study found that caulerpin regulation of glucose metabolism in CRC cells depended on treatment time (Yu et al., 2017). After 8 h of caulerpin treatment, glucose metabolism in CRC cells was upregulated; GLUT1 was downregulated in LoVo and SW480 cells after 48 h; and p-AMPK increased during the first 30 min and decreased after 60 min. The downstream targets of mTORC1 4E-BP1 and S6 were also found to be inhibited. The above results may be related to the inhibition of ROS by caulerpin, resulting in reduced intracellular ATP levels and activation of the energy sensor AMPK. Once activated, AMPK promotes glycolysis to compensate for the loss of ATP. However, long-term activation of AMPK by caulerpin disrupts glycolysis and glucose metabolism in colorectal cells, ultimately leading to cell death (Yu et al., 2017). In mice transplanted with SW480, caulerpin (30 mg/kg) slowed tumor growth.
5.3.3 Resveratrol
Resveratrol is a natural polyphenol occurring in a wide variety of fruits and vegetables, including peanuts, pistachios, and grapes (Ren et al., 2021), and it has biological activities such as antioxidation and antiproliferation effects (Jang et al., 1997). Studies have shown that in Caco2 and HCT116 cells, resveratrol by targeting the Ca2+/CamKKB/AMPK pathway, activates PDH, increasing the oxidative capacity of colorectal cancer cells, reducing glycolysis, and changing the metabolic pattern of tumor cells (Saunier et al., 2017). Resveratrol-loaded polymeric nanoparticles suppress glucose metabolism and tumor growth of CT26 cells in vitro and in vivo (Jung et al., 2015). In addition, resveratrol can also reduce F-FDG uptake and glycolytic metabolism in HT29 cells by downregulating HIF-1α (Jung et al., 2013).
5.3.4 Oridonin
Rabdosia rubescens is a Chinese medicine to treat sore throat, gingivitis, and rheumatoid arthritis and is widely distributed in China (Abdullah et al., 2021). Oridonin, which is purified from R. rubescens, is an active diterpenoid compound with significant anticancer activity (Fujita et al., 1976; Yang et al., 2017). The inhibition of cell growth and apoptosis induction refers to various cancers, including prostate cancer, non-small cell lung cancer, and glioblastoma (Ikezoe et al., 2003). Oridonin not only showed anti-CRC cell activity in vivo and in vitro but also related to p53. This study further confirmed that oridonin could reduce glucose consumption and extracellular lactate concentration, down-regulating the mRNA and protein expression of GLUT1 and MCT1 in SW480 cells, thereby affecting cancer cell metabolism, and similar results were observed in SW480 xenograft BALB/c nude mice (Yao et al., 2017). However, interestingly, the increased ATP level in the CRC cells treated with oridonin was found, which may be related to the deactivation of AMPK, downregulate the expression of GLUT1 caused by oridonin, metabolic disorder of CRC cells, and thus induced autophagy (Yao et al., 2017).
5.3.5 DT-13
Ophiopogonis Radix (Maidong in Chinese), the root of Ophiopogon japonicus, is widely used as a medicine in East Asia, with functions such as nourishing yin, promoting fluid production, and moistening the lungs (Chen et al., 2016). DT-13 is a saponin monomer of Liriope muscari (Decne.) Bailey has significant anti-tumor, anti-inflammatory, and heart protection effects (Zhang et al., 2015; Khan et al., 2018). DT-13 can inhibit the growth of HT29, HCT116, HCT15, and COLO205 cells (Wei et al., 2019). DT13 significantly inhibited glucose uptake, lactate production, and the expression of GLUT1 in HCT15 and HT29 cells, and also downregulated glycolysis-related mRNA and protein expressions such as HK2, PFK, and LDHA. Blocking GLUT1 attenuated the effects of DT13 on glucose uptake and cell proliferation in CRC cells (Wei et al., 2019). The in vivo study showed that DT-13 at dosages of 0.625, 1.25, and 2.5 mg/kg not only reduced tumor size and weight but also downregulated GLUT1 expression in HCT-15 orthotopic nude mice. Meanwhile, this study also found that DT13 could inhibit the proliferation of CRC cells by activating AMPK and inhibiting mTOR and its downstream phosphorylation. Treatment with an AMPK inhibitor (Compound C) alleviated the proliferation inhibition of DT13 (Wei et al., 2019).
5.4 Phytochemicals targeting HIF-1 to affect glycolysis in CRC
5.4.1 Worenine
Coptis Chinensis (Huanglian in Chinese), a famous traditional herbal medicine used for clearing heat and detoxification, is widely used to treat inflammatory and other diseases (Wang J. et al., 2019; Yang et al., 2021). Worenine is one of the bioactive components in the dried rhizomes of Coptis Chinensis (Meng et al., 2018), and it inhibits cell viability and induces cell cycle arrest of HCT116 and SW620 cells in vitro (Ji et al., 2021). Worenine-treated HCT116 and SW620 cells, the production of lactic acid and the uptake and consumption of glucose is significantly inhibited, the protein and mRNA levels of GLUT3, HK2, PFK-L, PKM2, and LDHA in HCT116 cells are reduced, and PKM activity is also knockdown. The above changes were achieved by reducing the level of HIF-1α, desferrioxamine (stabilize HIF-1α) treatment for HCT116 cells reversed worenine-induced effects on the Warburg effect (Ji et al., 2021).
5.4.2 Berberine
Berberine is a botanical alkaloid from the Ranunculaceae and Papaveraceae plant families (Mao et al., 2018). It is the active component of the Chinese medicine Rhizoma coptidis (Xiong et al., 2022), and it has shown anticancer activity in a variety of cancers, such as breast cancer (Zhong et al., 2022), lung cancer (Achi et al., 2022), gastric cancer (Liu et al., 2022), liver cancer (Wang et al., 2010), CRC (Sun Q. et al., 2022). Berberine significantly inhibits the growth and glucose uptake of HCT116 and KM12C cells in a dose-dependent manner and inhibited the mRNA levels of GLUT1, LDHA, and HK2. Further investigation of the underlying mechanism revealed that berberine inhibited glucose metabolism by suppressing mTOR-dependent HIF-1α protein synthesis in CRC cells (Mao et al., 2018).
5.4.3 Matrine
Sophora flavescens (Fabaceae), which has the effect of killing insects and dispelling dampness, has a long history of use for thousands of years (Sun P. et al., 2022). Matrine is a natural quinoline alkaloid that occurs in the traditional Chinese medicine Sophora flavescens (Lai et al., 2003; Zhang et al., 2020a). A study on the anti-tumor effects of matrine in CRC showed that glucose uptake and lactic acid production in HCT116 and SW620 after matrine treatment decreased, inhibiting the Warburg effect in CRC (Hong et al., 2019). Mechanistically, the transcription and expression of HIF-1α and its downstream related proteins GLUT1, HK2, and LDHA are significantly inhibited by matrine treatment, and knockdown of HIF-1α or overexpression of HIF-1α could reverse matrine’s effect on glucose uptake and lactate production (Hong et al., 2019).
5.4.4 Quercetin
Quercetin is a flavonoid extract that is common in fruits and vegetables and which exerts anticancer effects by inhibiting cell proliferation, inducing cell apoptosis, and delaying the invasion and metastasis of cancer cells (Wang et al., 2022; Xia et al., 2022). Quercetin can induce apoptosis of HCT116 cells by inhibiting the activity of AMPK under hypoxia, down-regulating the expression of HIF-1α and its downstream vascular endothelial growth factor and GLUT1 (Kim et al., 2012). A previous study analyzed the effects of quercetin on the transcriptome and protein change in the discretion-rate colon mucosa of F344 rats and found that quercetin downregulated the expressions of glycolysis-related enzymes such as F-1,6-BP, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), ENO1, PK, and upregulated the expression of genes related to mitochondrial fatty acid degradation, such as 3-hydroxy-3-methylglutaryl-CoA synthase 2, acetyl-CoA acyltransferase 1, and acyl-CoA dehydrogenase short-chain (Dihal et al., 2008). Quercetin may transform the glycolysis of CRC into the degradation of mitochondrial fatty acid in F334 rats, causing mitochondrial dysfunction and inhibiting the development of CRC (Dihal et al., 2008).
5.5 Phytochemicals targeting c-myc to affect glycolysis in CRC
5.5.1 Diosgenin and dioscin
Diosgenin (DSG) is a bioactive steroidal sapogenin, abundant in fenugreek seeds (Raju and Mehta, 2009). DSG not only showed inhibitory effects on the proliferation, invasion, and metastasis of CRC cells but also induced apoptosis (Li S. Y. et al., 2021). In SW1116 cells, DSG showed inhibitory effects on cancer cells glycolysis, including reduction of ECAR, OCR, lactate production, glucose uptake, and expression of GLUT2, GLUT3, and PC (Li S. Y. et al., 2021). Mechanistically, DSG regulated the expression of proteins involved in apoptosis, migration, invasion, and metabolic phenotypes of CRC cells by inhibiting the cAMP/PKA/CREB pathway in SW1116 and RKO cells. On xenograft tumor model of nude mice with SW1116 cells showed that 30 mg/kg DSG can reduce the weight of tumors in vivo, and inhibit the expression of Ki67 and proliferating cell nuclear antigen, Consistent with previous results in cells, DSG also inhibited the cAMP/PKA/CREB signaling pathway in tumor of nude mice (Li S. Y. et al., 2021). The cAMP/PKA/CREB pathway has been confirmed to regulate the growth, migration, invasion, and metabolism of cancer cells, and it is closely related to CRC metastasis (Zhang et al., 2020b; Fujishita et al., 2022).
Dioscin, a structural analog of DSG, is also a steroid saponin isolated from Dioscoreae rhizome and Paridis rhizome, among others. Dioscin downregulates CRC cells (HT-29, HCT-116, and SW480) proliferation and colony formation by promoting FBW-7-mediated c-myc ubiquitination, leading to the downregulation of c-myc and HK2 expression, reduced lactate consumption and glucose absorption, and inhibition of glycolysis. In xenograft models of HCT116 and HT29, tumor growth was inhibited after 5 mg/kg dioscin every 2 days treatment, and expression of c-myc, Ki-67, and HK2 was significantly reduced, and apoptosis was upregulated (Wu et al., 2020). In addition, Dioscin was shown to downregulate S-phase kinase-associated protein 2 (Skp2) protein levels and inhibit the expression of HK2 and aerobic glycolysis in CRC cells in vitro and in vivo by inhibiting Skp2 S72 phosphorylation and enhancing Skp2 ubiquitination and degradation in a cadherin 1(Cdh1) dependent manner (Zhou L. et al., 2020). As an oncogene, the Skp2 gene is closely related to the pathogenesis of CRC and is essential for the growth of CRC cells.
5.5.2 Astragalus saponins
Astragalus membranaceus is a well-known Chinese tonic, that is used as an immune stimulant, antioxidant, diuretic, etc. (Fu et al., 2014). Astragalus saponins (AST), are extracted from the medicinal plant Astragalus membranaceus. Studies have shown that AST downregulates the mRNA expression and enzyme activities of LDHA and HK2 in SW620 cells, inhibits glucose uptake and lactate production, induces apoptosis, and inhibits cell growth and proliferation (Guo et al., 2019). The authors speculated that this may be related to the downregulation of c-myc expression in SW620 cells by AST. In DSS-induced colitis mice, AST attenuates DSS-induced body weight loss and inflammatory response and downregulated the mRNA expression of c-myc and glycolysis-related enzymes, such as LDHA, GLUT, and HK2, which was consistent with the in vitro results (Guo et al., 2019).
5.5.3 Morin and esculetin
Morin is a polyphenol compound originally isolated from members of the Moraceae family such as mulberry figs and fustic (Chung et al., 2016). Esculetin is the principal bioactive ingredient of Fraxinus rhynchophylla Hance (Zhang et al., 2022). In a rat colon cancer model induced by 1,2-dimethylhydrazine (DMH), Morin and esculetin at a dose of 50 mg/kg were shown to reduce the incidence of DMH-induced tumors and down-regulating the expression of related proto-oncogenes. It also affects tumor metabolism through the β-cateinin/c-myc signaling pathway, including inhibiting the protein expression of GLUT, HK2, PKM2, LDHA and other glycolysis-related proteins, and promoting glutaminolysis (Sharma et al., 2017).
Morin also downregulated GLUT1 expression and glucose uptake, inhibited ATP production of SW480 cells, and induced ROS production, affecting mitochondrial function and promoting SW480 cells apoptosis (Sithara et al., 2017).
5.5.4 Apigenin
Apigenin is a natural flavonoid compound, which is common in vegetables and fruits (Sharma et al., 2019) and which significantly restrains cell survival and colony formation of HCT116, HT29, and DLD1 (Shan et al., 2017). Further studies showed that apigenin inhibited the mRNA and protein levels of PKM2 by specifically targeting the allosteric FBP binding site of PKM2 and blocking β-catenin/c-myc/PTBP1 signaling pathway to HCT116 cells, reducing the extracellular acidification rate, glucose consumption, and lactate production, blocking the glycolytic metabolism of HCT116 cells (Shan et al., 2017).
5.6 Phytochemicals targeting other pathways that affect CRC glycolysis
5.6.1 Oroxylin A
Oroxylin A (OA) is a flavonoid isolated from the root of S. baicalensis Georgi., which exerts various functions including cell growth inhibition and apoptosis induction in various cancer cells (Wei et al., 2013). A previous study showed that OA significantly inhibited glucose uptake and lactate production of HCT116 cells, and it significantly increased the protein level of p53 and the expression of TIGAR in cells and inhibited downstream the mRNA and protein levels of PGM and GLUT4; these inhibitory effects were confirmed to be mediated by p53 (Zhao et al., 2015). Mechanism studies showed that OA by promoting the deacetylation of sirtuin-3(SIRT3), increases the lipid phosphatase activity of PTEN and negatively regulates the transcription of MDM2, thus reducing the degradation of P53 (Zhao et al., 2015). An in vivo study showed that 100 mg/kg OA inhibited the growth of nude mice xenograft tumor-inoculated HCT116 cells by down-regulating MDM2 level and glycolytic protein mediated by p53. As one of the widely studied tumor suppressor genes, the p53 gene plays an important role in inhibiting the growth of tumor cells, inducing apoptosis, and regulating metabolism (Komarova and Gudkov, 1998; Vogelstein et al., 2000). SIRT3 is a member of the SIRT family of proteins and has been considered to be related to genomic stability, tumorigenesis, and energy metabolism (Finkel et al., 2009). As a tumor suppressor gene, PTEN has been demonstrated in previous studies to inhibit p53 degradation by controlling MDM2 P1 promoter activity through its lipid phosphatase activity (Freeman et al., 2003).
5.6.2 Ganoderma acid D
Ganoderma lucidum is a type of mushroom that grows on plum trees in Asia and is believed to protect body functions, regulate immunity, and promote health and longevity (Jin et al., 2016). Ganoderma acid D (GAD) is a kind of triterpene compound, which is the main active component of G. lucidum (Yuan et al., 2022). Studies have shown that GAD can reduce glucose uptake, lactic acid production, pyruvate production, and acetyl COA level of HT29 and SW620 cells by upregulating the expression of SIRT3 protein, inactivating acetylation cyclophilin D (CypD), thus playing a role in regulating CRC energy metabolism (Liu et al., 2018). SIRT3 is a mitochondrial deacetylase, and SIRT3 plays a key role in ROS and limiting cell oxidative damage (Torrens-Mas et al., 2017). SIRT3 can cause cell death under stress conditions, thus acting as a tumor suppressor (Torrens-Mas et al., 2017). CypD is one of the SIRT3-modified target proteins in mitochondria.
5.6.3 Rosmarinic acid
Rosmarinus officinalis L. is a plant of the Lamiaceae family native to the Mediterranean region, which has anti-inflammatory, antioxidant, anti-proliferation and other pharmacological effects (de Oliveira et al., 2019). As a water-soluble polyphenol compound, rosmarinic acid (RA) was first isolated from R. officinalis L. (Lamiaceae) (Khojasteh et al., 2020). RA inhibits the inflammatory response to the tumor microenvironment by inhibiting the expression of miR-155-5p in HCT8 and HCT116 cells, down-regulating the levels of transcription factor STAT3 and inflammatory factor Interleukin 6(IL-6), resulting in the inhibition of glucose consumption and lactic acid production in CRC cells, and the down-regulating expression of LDH and HIF-1α, and play the role of anti-Warburg effect (Xu et al., 2016). After further intervention with a miR-155-5p agomir drug, it was found that the above inhibitory effects of RA were reversed (Xu et al., 2016). Recent studies have shown that miR-155 plays an important role in immunity, inflammation, cardiovascular disease, and tumors (Elton et al., 2013). IL-6 is an inflammatory molecule that is highly expressed in tumor tissues and closely related to tumor cell proliferation (Kumari et al., 2016).
5.6.4 6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid
The isoquinoline alkaloids 6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid (M1) are isolated from the seeds of Mucuna pruriens(Kumar et al., 2016). The cytotoxicity of M1 was determined by the cell growth inhibition (MTT) method, which showed its antiproliferative activity against Huh-7 (human hepatoma cell line) (Kumar et al., 2016). M1 also shows anticancer effects in CRC. A study showed that M1 at 10 and 25 mg/kg doses for 15 days inhibited the high expression of inflammatory cells such as IL-6 in DMH-induced CRC cells and inhibited the activation of the JAK2/STAT3 pathway mediated by IL-6 (Mishra et al., 2018); further, it downregulated the expression of enzymes such as LDH in CRC liver metastasis and inhibited the high uptake of lactic acid and glucose in CRC cells (Mishra et al., 2018).
6 Critical considerations
6.1 Potential side-effects of phytochemicals
Although some phytochemicals show specific cytotoxicity to tumor cells, they may elicit adverse effects in humans, depending on the dosage and preparation. Studies have shown that the semi-lethal concentration (LC50) of quercetin is 448.45 ± 0.46 mg/L, and low oral dosages of quercetin (128 mg/kg) do not cause any significant changes in body appearance and general behavior in mice (Pal and Tripathi, 2020). However, higher dosages of quercetin (450 mg/kg) showed mild toxic effects, including weight loss and liver function impairment (Pal and Tripathi, 2020). Oral dosages of quercetin-magnesium were recommended to be restricted to less than 130 mg/kg to avoid possible toxic effects (Ghosh et al., 2017). Andrographolide show toxic side effects in a time-dependent manner, and they can significantly inhibit the proliferation of human renal tubular epithelium cells, induce cell apoptosis and inflammation, and increase nephrotoxicity (Zeng et al., 2022). Meanwhile, it has been reported that with andrographolide (PN355, Paracelsian, Inc.) in a phase-I clinical trial with HIV-positive patients, adverse effects such as anaphylaxis, fatigue, headache, rash, diarrhea, dry mouth, and decreased taste occurred with increasing dosage, and the adverse events disappeared 6 weeks after trial interruption (Calabrese et al., 2000). Determining the safe dosage of phytochemicals in vivo and balancing the relationship between their therapeutic effects and toxic side effects is a prominent difficulty in the development of phytochemical drugs.
6.2 Bioavailability
Some phytochemicals have been demonstrated to inhibit the glycolytic pathway of CRC in vitro and in vivo studies, however, due to their poor water solubility, low bioavailability, low cell uptake, etc., their further application in clinical practice is limited (Mohapatra et al., 2022). How to prepare such drugs so that they can be effectively utilized by humans is one of the bottlenecks of drug development. Therefore, one main objective of drug development is to improve the absorption and pharmacokinetics of drugs in vivo (Estrela et al., 2017). Piperine, a component of black pepper (Piper spp.), can improve the bioavailability of curcumin, the tea polyphenol (-)-Epigallocatechin-3-gallate, and other phytochemicals by inhibiting their glucuronidation (Lambert et al., 2004; Shaikh et al., 2009).
A further prominent avenue to improve the bioavailability of phytochemical drugs is novel drug delivery systems such as nanocarriers, which can alter the pharmacokinetics and improve the stability and half-life of drugs (Aqil et al., 2013). Quercetin has shown promising anticancer activity in current studies, however, its applicability is limited due to its poor water solubility in vivo, poor deliverability, and unstable molecular structure. Nano-conjugated quercetin can overcome the limitations of quercetin and enhance its anticancer effect, thus offering development prospects (Vinayak and Maurya, 2019). Liposome nanocarriers of apigenin can improve its bioavailability, and dual-drug-loaded liposomes with apigenin and 5-FU show higher cytotoxicity, stronger inhibition of angiogenesis and cell proliferation, and increased apoptosis. This delivery mode also showed upregulation of AMPK and downregulation of downstream HIF-1 activity and stronger reversal of the Warburg effect in CRC cells, compared to the single agent (Sen et al., 2019).
6.3 Synergistic interactions of phytochemicals with other treatments
The combination of various phytochemicals can improve the blood concentrations and bioavailability of drugs. Therefore, identifying the synergistic effects of drugs is an important aspect of drug development. The synergistic effect of phytochemical drugs combined with chemoradiotherapy is thus a key direction of treatment research. HF and artemisinin (ATS) show synergistic effects in CRC, manifested as synergistic induction of apoptosis and autophagy by HF-ATS (Gong et al., 2022). Quercetin can be used in combination with 5-FU (Boersma et al., 1994), doxorubicin (Atashpour et al., 2015), and other chemotherapy drugs to enhance its anti-cancer effect and to reduce the cytotoxicity of chemotherapy drugs. Furthermore, quercetin also played a significant role in reducing the mechanism of CRC chemoresistance (Zhou Y. et al., 2020). Oroxylin A and wogonin can synergistically inhibit MCF-7 proliferation and induce cell apoptosis, and baicalein also shows increased gastric cancer AGS cells sensitivity to 5-FU by inhibiting glycolytic flux (Cheng et al., 2018). Currently used phytochemicals have shown great potential in the treatment of cancer, and synergistic effects of phytochemicals and other drugs are a key aspect of drug development.
6.4 Clinical transformation of phytochemicals
Numerous clinical trials have been conducted to develop phytochemical drugs. In a double-blind, randomized, placebo-controlled trial on berberine and patients who had undergone colorectal polypectomy within 6 months before recruitment, the experimental group received berberine (0.3 g, twice per day) and were followed for 2 years; the results showed that the berberine treatment group had a lower incidence of recurrent adenomas (Chen et al., 2020). As a well-known proprietary Chinese medicine, Kangai injection consists of extracts of Astragalus, Ginseng, and Matrine. In a randomized trial, Kangai injection and platinum combination therapy have been shown to have a greater benefit than chemotherapy alone in patients with advanced non-small cell lung cancer, which can enhance the body’s immunity and reduce the toxicity of chemotherapy drugs (Cheng et al., 2018). Aidi injection plays a role of advantages in the treatment of liver cancer (Liu et al., 2021). In CRC patients, the combined use of FOLFOX with Chinese herbal medicines such as Delisheng and Xiaoaiping shows higher safety and improved body functioning than treatment with FOLFOX alone (Zhang et al., 2019). Further evidence also suggests that the use of chemotherapeutic drugs combined with Chinese patent medicine may be more beneficial, and some studies have shown that phytochemicals have significant anticancer properties, however, further research is needed (Thomasset et al., 2007).
7 Summary and prospect
In the past few decades, CRC screening has become increasingly common, and treatment methods have been developed, however, death and incidence rates of CRC are still increasing. At present, there is a lack of effective targeted therapy for CRC. Aerobic glycolysis, as one of the characteristics of tumors, is considered a therapeutic target. It has been found that many malignant tumors, including CRC, have higher glycolysis rates to maintain the proliferation, invasion, and metastasis of cancer cells. In CRC, glycolysis-related enzymes and transporters (e.g., GLUT1, HK2, LDHA, PKM2) are highly expressed, and the upregulation of HIF-1, AMPK, c-Myc, PI3K/Akt/mTOR and other related pathways and transporters also promote the glycolysis process of CRC. This review focuses on phytochemicals that target the glycolytic pathway in CRC. There is in vitro and in vivo evidence that some phytochemicals can affect the growth, invasion, and metastasis of CRC and induce CRC apoptosis by targeting the glycolytic pathway.
The results compiled for this review were classified according to different mechanism pathways targeted, including phytochemicals targeting PI3K/AKT/mTOR pathway (e.g., Thymoquinone and Daphnetin), AMPK pathway (e.g., Isoliquiritigenin and Resveratrol), HIF-1 (e.g., Worenine and Berberine), and c-myc (e.g., Astragalus saponins and Morin), and some phytochemicals directly affecting the glycolytic pathway of CRC without involving the above pathways. Examples include phytochemicals that directly target GLUT1 (e.g., Scutellarin and Kaempferol), PKM2 (e.g., Anthocyanin and Silybin), and MCT4 (e.g., Epigallocatechin-3-gallate). Although the phytochemicals mentioned above affect CRC cells through different pathways, they all suggest bright prospects for targeting CRC glycolytic pathways in the treatment of CRC.
Tumor metabolism is highly complex. Some phytochemicals have complex and diverse molecular targets, and further experiments are needed to verify their anticancer effects. We introduce several phytochemicals targeting CRC glycolysis metabolic pathways, which appears to be a promising direction; however, further research is required, including on how to maintain the stability of plant chemicals in the body, how to prepare plant chemicals that can be transmitted directly to the lesion site, and how to improve blood concentrations of phytochemicals. Therefore, exploiting synergistic effects of phytochemicals with other drugs may provide new ideas for further research and development of anticancer drugs. In addition to the regulation of glucose metabolism, some phytochemicals have shown the therapeutic action of multi-target and multi-pathway, and their anticancer mechanisms need further verification. Although some phytochemicals have shown potential therapeutic effects on colorectal cancer, but long-term evaluation for their possible toxic and side effects is required. In the future, more researches on phytochemicals targeting glycolysis can provide evidence for the drug development in CRC therapy.
Author contributions
LZ: Writing–review and editing, Data curation, Formal Analysis, Writing–original draft. FS: Writing–original draft, Formal Analysis. QL: Writing–original draft, Investigation. YW: Data curation, Methodology, Writing–review and editing. FW: Data curation, Writing–review and editing, Formal Analysis. ZH: Writing–review and editing, Resources, Software. XC: Software, Resources, Writing–review and editing. XY: Writing–review and editing, Formal Analysis, Visualization. JW: Writing–review and editing, Project administration, Resources. YiC: Writing–review and editing, Data curation, Visualization. YG: Visualization, Writing–review and editing. YuC: Writing–review and editing, Funding acquisition, Supervision. XM: Funding acquisition, Supervision, Writing–review and editing, Methodology. JZ: Funding acquisition, Supervision, Writing–review and editing, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Program of Science and Technology Department of Sichuan Province (Grant Nos. 2023YFS0476, 2023YFQ0016, and 2023NSFSC0039), the Project of Traditional Chinese Medicine Administration of Sichuan Province (Grant Nos.MS461), “Hundred Talents Program” of the Hospital of the Chengdu University of Traditional Chinese Medicine (Grant Nos. 20-Q03 and 22-B09), Xinglin Scholar Research Promotion Project of Chengdu University of TCM (Grant Nos. QJJJ2022010 and QJRC2022028).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdullah, N. A., Md Hashim, N. F., Ammar, A., and Muhamad Zakuan, N. (2021). An insight into the anti-angiogenic and anti-metastatic effects of oridonin: current knowledge and future potential. Molecules 26 (4), 775. doi:10.3390/molecules26040775
Achi, I. T., Sarbadhikary, P., George, B. P., and Abrahamse, H. (2022). Multi-target potential of berberine as an antineoplastic and antimetastatic agent: a special focus on lung cancer treatment. Cells 11 (21), 3433. doi:10.3390/cells11213433
Akbarialiabad, H., Dahroud, M. D., Khazaei, M. M., Razmeh, S., and Zarshenas, M. M. (2021). Green tea, A medicinal food with promising neurological benefits. Curr. Neuropharmacol. 19 (3), 349–359. doi:10.2174/1570159x18666200529152625
An, J., and Ha, E. M. (2022). Extracellular vesicles derived from Lactobacillus plantarum restore chemosensitivity through the PDK2-mediated glucose metabolic pathway in 5-FU-resistant colorectal cancer cells. J. Microbiol. 60 (7), 735–745. doi:10.1007/s12275-022-2201-1
Ancey, P. B., Contat, C., and Meylan, E. (2018). Glucose transporters in cancer - from tumor cells to the tumor microenvironment. Febs J. 285 (16), 2926–2943. doi:10.1111/febs.14577
Aqil, F., Munagala, R., Jeyabalan, J., and Vadhanam, M. V. (2013). Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 334 (1), 133–141. doi:10.1016/j.canlet.2013.02.032
Atashpour, S., Fouladdel, S., Movahhed, T. K., Barzegar, E., Ghahremani, M. H., Ostad, S. N., et al. (2015). Quercetin induces cell cycle arrest and apoptosis in CD133(+) cancer stem cells of human colorectal HT29 cancer cell line and enhances anticancer effects of doxorubicin. Iran. J. Basic Med. Sci. 18 (7), 635–643.
Baer, S. C., Casaubon, L., and Younes, M. (1997). Expression of the human erythrocyte glucose transporter Glut1 in cutaneous neoplasia. J. Am. Acad. Dermatol 37 (4), 575–577. doi:10.1016/s0190-9622(97)70174-9
Banik, K., Khatoon, E., Harsha, C., Rana, V., Parama, D., Thakur, K. K., et al. (2022). Wogonin and its analogs for the prevention and treatment of cancer: a systematic review. Phytother. Res. 36 (5), 1854–1883. doi:10.1002/ptr.7386
Biller, L. H., and Schrag, D. (2021). Diagnosis and treatment of metastatic colorectal cancer: a review. Jama 325 (7), 669–685. doi:10.1001/jama.2021.0106
Boersma, H. H., Woerdenbag, H. J., Bauer, J., Scheithauer, W., Kampinga, H. H., and Konings, A. W. (1994). Interaction between the cytostatic effects of quercetin and 5-fluorouracil in two human colorectal cancer cell lines. Phytomedicine 1 (3), 239–244. doi:10.1016/s0944-7113(11)80071-1
Bui, B. P., Nguyen, P. L., Lee, K., and Cho, J. (2022). Hypoxia-inducible factor-1: a novel therapeutic target for the management of cancer, drug resistance, and cancer-related pain. Cancers (Basel) 14 (24), 6054. doi:10.3390/cancers14246054
Calabrese, C., Berman, S. H., Babish, J. G., Ma, X., Shinto, L., Dorr, M., et al. (2000). A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother. Res. 14 (5), 333–338. doi:10.1002/1099-1573(200008)14:5<333::aid-ptr584>3.0.co;2-d
Canché Chay, C. I., Gómez Cansino, R., Espitia Pinzón, C. I., Torres-Ochoa, R. O., and Martínez, R. (2014). Synthesis and anti-tuberculosis activity of the marine natural product caulerpin and its analogues. Mar. Drugs 12 (4), 1757–1772. doi:10.3390/md12041757
Cao, Y., Lin, Y., Wang, D., Pan, D., Zhang, Y., Jin, Y., et al. (2018). Enhancing 5-fluorouracil efficacy through suppression of PKM2 in colorectal cancer cells. Cancer Chemother. Pharmacol. 82 (6), 1081–1086. doi:10.1007/s00280-018-3676-7
Catanzaro, D., Gabbia, D., Cocetta, V., Biagi, M., Ragazzi, E., Montopoli, M., et al. (2018). Silybin counteracts doxorubicin resistance by inhibiting GLUT1 expression. Fitoterapia 124, 42–48. doi:10.1016/j.fitote.2017.10.007
Chambers, C. S., Holečková, V., Petrásková, L., Biedermann, D., Valentová, K., Buchta, M., et al. (2017). The silymarin composition and why does it matter??? Food Res. Int. 100(3), 339–353. doi:10.1016/j.foodres.2017.07.017
Chang, R. C., Shi, L., Huang, C. C., Kim, A. J., Ko, M. L., Zhou, B., et al. (2015). High-fat diet-induced retinal dysfunction. Invest. Ophthalmol. Vis. Sci. 56 (4), 2367–2380. doi:10.1167/iovs.14-16143
Chen, G. Q., Tang, C. F., Shi, X. K., Lin, C. Y., Fatima, S., Pan, X. H., et al. (2015). Halofuginone inhibits colorectal cancer growth through suppression of Akt/mTORC1 signaling and glucose metabolism. Oncotarget 6 (27), 24148–24162. doi:10.18632/oncotarget.4376
Chen, M. H., Chen, X. J., Wang, M., Lin, L. G., and Wang, Y. T. (2016). Ophiopogon japonicus--A phytochemical, ethnomedicinal and pharmacological review. J. Ethnopharmacol. 181, 193–213. doi:10.1016/j.jep.2016.01.037
Chen, S., Nishi, M., Morine, Y., Shimada, M., Tokunaga, T., Kashihara, H., et al. (2022). Epigallocatechin-3-gallate hinders metabolic coupling to suppress colorectal cancer malignancy through targeting aerobic glycolysis in cancer-associated fibroblasts. Int. J. Oncol. 60 (2), 19. doi:10.3892/ijo.2022.5309
Chen, X., Cui, L., Duan, X., Ma, B., and Zhong, D. (2006). Pharmacokinetics and metabolism of the flavonoid scutellarin in humans after a single oral administration. Drug Metab. Dispos. 34 (8), 1345–1352. doi:10.1124/dmd.106.009779
Chen, Y. X., Gao, Q. Y., Zou, T. H., Wang, B. M., Liu, S. D., Sheng, J. Q., et al. (2020). Berberine versus placebo for the prevention of recurrence of colorectal adenoma: a multicentre, double-blinded, randomised controlled study. Lancet Gastroenterol. Hepatol. 5 (3), 267–275. doi:10.1016/s2468-1253(19)30409-1
Cheng, C. S., Chen, J., Tan, H. Y., Wang, N., Chen, Z., and Feng, Y. (2018). Scutellaria baicalensis and cancer treatment: recent progress and perspectives in biomedical and clinical studies. Am. J. Chin. Med. 46 (1), 25–54. doi:10.1142/s0192415x18500027
Chung, S. S., Oliva, B., Dwabe, S., and Vadgama, J. V. (2016). Combination treatment with flavonoid morin and telomerase inhibitor MST-312 reduces cancer stem cell traits by targeting STAT3 and telomerase. Int. J. Oncol. 49 (2), 487–498. doi:10.3892/ijo.2016.3546
Ciesielska-Figlon, K., Wojciechowicz, K., Wardowska, A., and Lisowska, K. A. (2023). The immunomodulatory effect of Nigella sativa. Antioxidants (Basel) 12 (7), 1340. doi:10.3390/antiox12071340
Coutte, L., Dreyer, C., Sablin, M. P., Faivre, S., and Raymond, E. (2012). [PI3K-AKT-mTOR pathway and cancer]. Bull. Cancer 99 (2), 173–180. doi:10.1684/bdc.2011.1384
Cui, R., and Shi, X. Y. (2015). Expression of pyruvate kinase M2 in human colorectal cancer and its prognostic value. Int. J. Clin. Exp. Pathol. 8 (9), 11393–11399.
Cui, X. G., Han, Z. T., He, S. H., Wu, X. D., Chen, T. R., Shao, C. H., et al. (2017). HIF1/2α mediates hypoxia-induced LDHA expression in human pancreatic cancer cells. Oncotarget 8 (15), 24840–24852. doi:10.18632/oncotarget.15266
Dang, C. V., O'Donnell, K. A., Zeller, K. I., Nguyen, T., Osthus, R. C., and Li, F. (2006). The c-Myc target gene network. Semin. Cancer Biol. 16 (4), 253–264. doi:10.1016/j.semcancer.2006.07.014
de Oliveira, J. R., Camargo, S. E. A., and de Oliveira, L. D. (2019). Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J. Biomed. Sci. 26 (1), 5. doi:10.1186/s12929-019-0499-8
Devi, K. P., Malar, D. S., Nabavi, S. F., Sureda, A., Xiao, J., Nabavi, S. M., et al. (2015). Kaempferol and inflammation: from chemistry to medicine. Pharmacol. Res. 99, 1–10. doi:10.1016/j.phrs.2015.05.002
Dihal, A. A., van der Woude, H., Hendriksen, P. J., Charif, H., Dekker, L. J., Ijsselstijn, L., et al. (2008). Transcriptome and proteome profiling of colon mucosa from quercetin fed F344 rats point to tumor preventive mechanisms, increased mitochondrial fatty acid degradation and decreased glycolysis. Proteomics 8 (1), 45–61. doi:10.1002/pmic.200700364
Dong, S., Liang, S., Cheng, Z., Zhang, X., Luo, L., Li, L., et al. (2022). ROS/PI3K/Akt and Wnt/β-catenin signalings activate HIF-1α-induced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer. J. Exp. Clin. Cancer Res. 41 (1), 15. doi:10.1186/s13046-021-02229-6
Dong, X., and Qu, S. (2022). Erigeron breviscapus (vant) hand-mazz: a promising natural neuroprotective agent for alzheimer's disease. Front. Pharmacol. 13, 877872. doi:10.3389/fphar.2022.877872
Donohoe, D. R., Collins, L. B., Wali, A., Bigler, R., Sun, W., and Bultman, S. J. (2012). The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol. Cell 48 (4), 612–626. doi:10.1016/j.molcel.2012.08.033
Donovan, M. G., Selmin, O. I., Doetschman, T. C., and Romagnolo, D. F. (2017). Mediterranean diet: prevention of colorectal cancer. Front. Nutr. 4, 59. doi:10.3389/fnut.2017.00059
Elton, T. S., Selemon, H., Elton, S. M., and Parinandi, N. L. (2013). Regulation of the MIR155 host gene in physiological and pathological processes. Gene 532 (1), 1–12. doi:10.1016/j.gene.2012.12.009
Eslami, M., Sadrifar, S., Karbalaei, M., Keikha, M., Kobyliak, N. M., and Yousefi, B. (2020). Importance of the microbiota inhibitory mechanism on the Warburg effect in colorectal cancer cells. J. Gastrointest. Cancer 51 (3), 738–747. doi:10.1007/s12029-019-00329-3
Estrela, J. M., Mena, S., Obrador, E., Benlloch, M., Castellano, G., Salvador, R., et al. (2017). Polyphenolic phytochemicals in cancer prevention and therapy: bioavailability versus bioefficacy. J. Med. Chem. 60 (23), 9413–9436. doi:10.1021/acs.jmedchem.6b01026
Fan, W., Fan, L., Peng, C., Zhang, Q., Wang, L., Li, L., et al. (2019). Traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics and toxicology of Xanthium strumarium L: a review. Molecules 24 (2), 359. doi:10.3390/molecules24020359
Faubert, B., Boily, G., Izreig, S., Griss, T., Samborska, B., Dong, Z., et al. (2013). AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 17 (1), 113–124. doi:10.1016/j.cmet.2012.12.001
Feng, W., Cui, G., Tang, C. W., Zhang, X. L., Dai, C., Xu, Y. Q., et al. (2017). Role of glucose metabolism related gene GLUT1 in the occurrence and prognosis of colorectal cancer. Oncotarget 8 (34), 56850–56857. doi:10.18632/oncotarget.18090
Finkel, T., Deng, C. X., and Mostoslavsky, R. (2009). Recent progress in the biology and physiology of sirtuins. Nature 460 (7255), 587–591. doi:10.1038/nature08197
Freeman, D. J., Li, A. G., Wei, G., Li, H. H., Kertesz, N., Lesche, R., et al. (2003). PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell 3 (2), 117–130. doi:10.1016/s1535-6108(03)00021-7
Fu, J., Wang, Z., Huang, L., Zheng, S., Wang, D., Chen, S., et al. (2014). Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 28 (9), 1275–1283. doi:10.1002/ptr.5188
Fujishita, T., Kojima, Y., Kajino-Sakamoto, R., Mishiro-Sato, E., Shimizu, Y., Hosoda, W., et al. (2022). The cAMP/PKA/CREB and TGF-β/SMAD4 pathways regulate stemness and metastatic potential in colorectal cancer cells. Cancer Res. 82, 4179–4190. doi:10.1158/0008-5472.Can-22-1369
Fujita, E., Nagao, Y., Node, M., Kaneko, K., Nakazawa, S., and Kuroda, H. (1976). Antitumor activity of the isodon diterpenoids: structural requirements for the activity. Experientia 32 (2), 203–206. doi:10.1007/bf01937766
Gatenby, R. A., and Gillies, R. J. (2004). Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 4 (11), 891–899. doi:10.1038/nrc1478
Georges, L. M., Verset, L., Zlobec, I., Demetter, P., and De Wever, O. (2018). Impact of the microenvironment on tumour budding in colorectal cancer. Adv. Exp. Med. Biol. 1110, 101–111. doi:10.1007/978-3-030-02771-1_7
Ghosh, N., Sandur, R., Ghosh, D., Roy, S., and Janadri, S. (2017). Acute, 28days sub acute and genotoxic profiling of Quercetin-Magnesium complex in Swiss albino mice. Biomed. Pharmacother. 86, 279–291. doi:10.1016/j.biopha.2016.12.015
Gong, R. H., Yang, D. J., Kwan, H. Y., Lyu, A. P., Chen, G. Q., and Bian, Z. X. (2022). Cell death mechanisms induced by synergistic effects of halofuginone and artemisinin in colorectal cancer cells. Int. J. Med. Sci. 19 (1), 175–185. doi:10.7150/ijms.66737
Guo, H., Wan, B., Wang, J., Zhang, J., Yao, W., and Shen, Z. (2019). Astragalus saponins inhibit cell growth, aerobic glycolysis and attenuate the inflammatory response in a DSS-induced colitis model. Int. J. Mol. Med. 43 (2), 1041–1048. doi:10.3892/ijmm.2018.4036
Haber, R. S., Rathan, A., Weiser, K. R., Pritsker, A., Itzkowitz, S. H., Bodian, C., et al. (1998). GLUT1 glucose transporter expression in colorectal carcinoma: a marker for poor prognosis. Cancer 83 (1), 34–40. doi:10.1002/(sici)1097-0142(19980701)83:1<34::aid-cncr5>3.0.co;2-e
Hamabe, A., Yamamoto, H., Konno, M., Uemura, M., Nishimura, J., Hata, T., et al. (2014). Combined evaluation of hexokinase 2 and phosphorylated pyruvate dehydrogenase-E1α in invasive front lesions of colorectal tumors predicts cancer metabolism and patient prognosis. Cancer Sci. 105 (9), 1100–1108. doi:10.1111/cas.12487
Hamer, H. M., Jonkers, D., Venema, K., Vanhoutvin, S., Troost, F. J., and Brummer, R. J. (2008). Review article: the role of butyrate on colonic function. Aliment. Pharmacol. Ther. 27 (2), 104–119. doi:10.1111/j.1365-2036.2007.03562.x
Han, J., Meng, Q., Xi, Q., Zhang, Y., Zhuang, Q., Han, Y., et al. (2016). Interleukin-6 stimulates aerobic glycolysis by regulating PFKFB3 at early stage of colorectal cancer. Int. J. Oncol. 48 (1), 215–224. doi:10.3892/ijo.2015.3225
Hang, S., Wu, W., Wang, Y., Sheng, R., Fang, Y., and Guo, R. (2022). Daphnetin, a coumarin in genus stellera chamaejasme linn: chemistry, bioactivity and therapeutic potential. Chem. Biodivers. 19 (9), e202200261. doi:10.1002/cbdv.202200261
He, L., Lv, S., Ma, X., Jiang, S., Zhou, F., Zhang, Y., et al. (2022). ErbB2 promotes breast cancer metastatic potential via HSF1/LDHA axis-mediated glycolysis. Med. Oncol. 39 (4), 45. doi:10.1007/s12032-021-01641-4
He, Z., Dong, W., Yao, K., Qin, C., and Duan, B. (2018). Retracted article: daphnetin inhibits proliferation and glycolysis in colorectal cancer cells by regulating the PI3K/Akt signaling pathway. RSC Adv. 8 (60), 34483–34490. doi:10.1039/c8ra05583a
Ho, N., and Coomber, B. L. (2015). Pyruvate dehydrogenase kinase expression and metabolic changes following dichloroacetate exposure in anoxic human colorectal cancer cells. Exp. Cell Res. 331 (1), 73–81. doi:10.1016/j.yexcr.2014.12.006
Hollander, M. C., Blumenthal, G. M., and Dennis, P. A. (2011). PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat. Rev. Cancer 11 (4), 289–301. doi:10.1038/nrc3037
Hong, X., Zhong, L., Xie, Y., Zheng, K., Pang, J., Li, Y., et al. (2019). Matrine reverses the Warburg effect and suppresses colon cancer cell growth via negatively regulating HIF-1α. Front. Pharmacol. 10, 1437. doi:10.3389/fphar.2019.01437
Houddane, A., Bultot, L., Novellasdemunt, L., Johanns, M., Gueuning, M. A., Vertommen, D., et al. (2017). Role of Akt/PKB and PFKFB isoenzymes in the control of glycolysis, cell proliferation and protein synthesis in mitogen-stimulated thymocytes. Cell Signal 34, 23–37. doi:10.1016/j.cellsig.2017.02.019
Hu, Q., Chen, Y., Deng, X., Li, Y., Ma, X., Zeng, J., et al. (2023). Diabetic nephropathy: focusing on pathological signals, clinical treatment, and dietary regulation. Biomed. Pharmacother. 159, 114252. doi:10.1016/j.biopha.2023.114252
Huang, C. Y., Weng, Y. T., Li, P. C., Hsieh, N. T., Li, C. I., Liu, H. S., et al. (2021). Calcitriol suppresses Warburg effect and cell growth in human colorectal cancer cells. Life (Basel) 11 (9), 963. doi:10.3390/life11090963
Ikezoe, T., Chen, S. S., Tong, X. J., Heber, D., Taguchi, H., and Koeffler, H. P. (2003). Oridonin induces growth inhibition and apoptosis of a variety of human cancer cells. Int. J. Oncol. 23 (4), 1187–1193. doi:10.3892/ijo.23.4.1187
Imran, M., Salehi, B., Sharifi-Rad, J., Aslam Gondal, T., Saeed, F., Imran, A., et al. (2019). Kaempferol: a key emphasis to its anticancer potential. Molecules 24 (12), 2277. doi:10.3390/molecules24122277
Jain, P. P., Zhao, T., Xiong, M., Song, S., Lai, N., Zheng, Q., et al. (2021). Halofuginone, a promising drug for treatment of pulmonary hypertension. Br. J. Pharmacol. 178 (17), 3373–3394. doi:10.1111/bph.15442
Jang, D. S., Yang, M. S., Ha, T. J., and Park, K. H. (1999). Hemistepsins with cytotoxic activity from Hemisteptia lyrata. Planta Med. 65 (8), 765–766. doi:10.1055/s-2006-960863
Jang, M., Cai, L., Udeani, G. O., Slowing, K. V., Thomas, C. F., Beecher, C. W., et al. (1997). Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275 (5297), 218–220. doi:10.1126/science.275.5297.218
Javed, M., Saleem, A., Xaveria, A., and Akhtar, M. F. (2022). Daphnetin: a bioactive natural coumarin with diverse therapeutic potentials. Front. Pharmacol. 13, 993562. doi:10.3389/fphar.2022.993562
Ji, L., Shen, W., Zhang, F., Qian, J., Jiang, J., Weng, L., et al. (2021). Worenine reverses the Warburg effect and inhibits colon cancer cell growth by negatively regulating HIF-1α. Cell Mol. Biol. Lett. 26 (1), 19. doi:10.1186/s11658-021-00263-y
Jian, X., He, H., Zhu, J., Zhang, Q., Zheng, Z., Liang, X., et al. (2020). Hsa_circ_001680 affects the proliferation and migration of CRC and mediates its chemoresistance by regulating BMI1 through miR-340. Mol. Cancer 19 (1), 20. doi:10.1186/s12943-020-1134-8
Jiang, B. H., Jiang, G., Zheng, J. Z., Lu, Z., Hunter, T., and Vogt, P. K. (2001). Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. 12 (7), 363–369.
Jiang, C. H., Sun, T. L., Xiang, D. X., Wei, S. S., and Li, W. Q. (2018). Anticancer activity and mechanism of xanthohumol: a prenylated flavonoid from hops (humulus lupulus L). Front. Pharmacol. 9, 530. doi:10.3389/fphar.2018.00530
Jiang, X., Wang, J., Deng, X., Xiong, F., Zhang, S., Gong, Z., et al. (2020). The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res. 39 (1), 204. doi:10.1186/s13046-020-01709-5
Jin, L., Kim, E. Y., Chung, T. W., Han, C. W., Park, S. Y., Han, J. H., et al. (2020). Hemistepsin A suppresses colorectal cancer growth through inhibiting pyruvate dehydrogenase kinase activity. Sci. Rep. 10 (1), 21940. doi:10.1038/s41598-020-79019-1
Jin, X., Ruiz Beguerie, J., Sze, D. M., and Chan, G. C. (2016). Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database Syst. Rev. 4 (4), Cd007731. doi:10.1002/14651858.CD007731.pub3
Jing, N., Song, J., Liu, Z., Wang, L., and Jiang, G. (2020). Glycosylation of anthocyanins enhances the apoptosis of colon cancer cells by handicapping energy metabolism. BMC Complement. Med. Ther. 20 (1), 312. doi:10.1186/s12906-020-03096-y
Jung, K. H., Lee, J. H., Park, J. W., Quach, C. H. T., Moon, S. H., Cho, Y. S., et al. (2015). Resveratrol-loaded polymeric nanoparticles suppress glucose metabolism and tumor growth in vitro and in vivo. Int. J. Pharm. 478 (1), 251–257. doi:10.1016/j.ijpharm.2014.11.049
Jung, K. H., Lee, J. H., Thien Quach, C. H., Paik, J. Y., Oh, H., Park, J. W., et al. (2013). Resveratrol suppresses cancer cell glucose uptake by targeting reactive oxygen species-mediated hypoxia-inducible factor-1α activation. J. Nucl. Med. 54 (12), 2161–2167. doi:10.2967/jnumed.112.115436
Karim, S., Burzangi, A. S., Ahmad, A., Siddiqui, N. A., Ibrahim, I. M., Sharma, P., et al. (2022). PI3K-AKT pathway modulation by thymoquinone limits tumor growth and glycolytic metabolism in colorectal cancer. Int. J. Mol. Sci. 23 (4), 2305. doi:10.3390/ijms23042305
Katagiri, M., Karasawa, H., Takagi, K., Nakayama, S., Yabuuchi, S., Fujishima, F., et al. (2017). Hexokinase 2 in colorectal cancer: a potent prognostic factor associated with glycolysis, proliferation and migration. Histol. Histopathol. 32 (4), 351–360. doi:10.14670/hh-11-799
Kazantseva, L., Becerra, J., and Santos-Ruiz, L. (2022). Traditional medicinal plants as a source of inspiration for osteosarcoma therapy. Molecules 27 (15), 5008. doi:10.3390/molecules27155008
Khan, G. J., Rizwan, M., Abbas, M., Naveed, M., Boyang, Y., Naeem, M. A., et al. (2018). Pharmacological effects and potential therapeutic targets of DT-13. Biomed. Pharmacother. 97, 255–263. doi:10.1016/j.biopha.2017.10.101
Khojasteh, A., Mirjalili, M. H., Alcalde, M. A., Cusido, R. M., Eibl, R., and Palazon, J. (2020). Powerful plant antioxidants: a new biosustainable approach to the production of rosmarinic acid. Antioxidants (Basel) 9 (12), 1273. doi:10.3390/antiox9121273
Kim, H. S., Wannatung, T., Lee, S., Yang, W. K., Chung, S. H., Lim, J. S., et al. (2012). Quercetin enhances hypoxia-mediated apoptosis via direct inhibition of AMPK activity in HCT116 colon cancer. Apoptosis 17 (9), 938–949. doi:10.1007/s10495-012-0719-0
Komarova, E. A., and Gudkov, A. V. (1998). Could p53 be a target for therapeutic suppression? Semin. Cancer Biol. 8 (5), 389–400. doi:10.1006/scbi.1998.0101
Kopaskova, M., Hadjo, L., Yankulova, B., Jovtchev, G., Galova, E., Sevcovicova, A., et al. (2011). Extract of Lillium candidum L. can modulate the genotoxicity of the antibiotic zeocin. Molecules 17 (1), 80–97. doi:10.3390/molecules17010080
Kumar, P., Rawat, A., Keshari, A. K., Singh, A. K., Maity, S., De, A., et al. (2016). Antiproliferative effect of isolated isoquinoline alkaloid from Mucuna pruriens seeds in hepatic carcinoma cells. Nat. Prod. Res. 30 (4), 460–463. doi:10.1080/14786419.2015.1020489
Kumar, V., and Gabrilovich, D. I. (2014). Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology 143 (4), 512–519. doi:10.1111/imm.12380
Kumari, N., Dwarakanath, B. S., Das, A., and Bhatt, A. N. (2016). Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 37 (9), 11553–11572. doi:10.1007/s13277-016-5098-7
Lai, J. P., He, X. W., Jiang, Y., and Chen, F. (2003). Preparative separation and determination of matrine from the Chinese medicinal plant Sophora flavescens Ait by molecularly imprinted solid-phase extraction. Anal. Bioanal. Chem. 375 (2), 264–269. doi:10.1007/s00216-002-1675-2
Lambert, J. D., Hong, J., Kim, D. H., Mishin, V. M., and Yang, C. S. (2004). Piperine enhances the bioavailability of the tea polyphenol (-)-epigallocatechin-3-gallate in mice. J. Nutr. 134 (8), 1948–1952. doi:10.1093/jn/134.8.1948
Lee, D. Y., Song, M. Y., and Kim, E. H. (2021). Role of oxidative stress and nrf2/KEAP1 signaling in colorectal cancer: mechanisms and therapeutic perspectives with phytochemicals. Antioxidants (Basel) 10 (5), 743. doi:10.3390/antiox10050743
Leung, C. O., Wong, C. C., Fan, D. N., Kai, A. K., Tung, E. K., Xu, I. M., et al. (2015). PIM1 regulates glycolysis and promotes tumor progression in hepatocellular carcinoma. Oncotarget 6 (13), 10880–10892. doi:10.18632/oncotarget.3534
Li, C., Chen, Q., Zhou, Y., Niu, Y., Wang, X., Li, X., et al. (2020a). S100A2 promotes glycolysis and proliferation via GLUT1 regulation in colorectal cancer. Faseb J. 34 (10), 13333–13344. doi:10.1096/fj.202000555R
Li, C., Wang, P., Du, J., Chen, J., Liu, W., and Ye, K. (2021a). LncRNA RAD51-AS1/miR-29b/c-3p/NDRG2 crosstalk repressed proliferation, invasion and glycolysis of colorectal cancer. IUBMB Life 73 (1), 286–298. doi:10.1002/iub.2427
Li, L., Liu, P., Xie, Y., Liu, Y., Chen, Z., Geng, Y., et al. (2022a). Xanthatin inhibits human colon cancer cells progression via mTOR signaling mediated energy metabolism alteration. Drug Dev. Res. 83 (1), 119–130. doi:10.1002/ddr.21850
Li, Q., Tang, H., Hu, F., and Qin, C. (2019). Silencing of FOXO6 inhibits the proliferation, invasion, and glycolysis in colorectal cancer cells. J. Cell Biochem. 120 (3), 3853–3860. doi:10.1002/jcb.27667
Li, S. Y., Shang, J., Mao, X. M., Fan, R., Li, H. Q., Li, R. H., et al. (2021b). Diosgenin exerts anti-tumor effects through inactivation of cAMP/PKA/CREB signaling pathway in colorectal cancer. Eur. J. Pharmacol. 908, 174370. doi:10.1016/j.ejphar.2021.174370
Li, W. D., Wu, Y., Zhang, L., Yan, L. G., Yin, F. Z., Ruan, J. S., et al. (2013). Characterization of xanthatin: anticancer properties and mechanisms of inhibited murine melanoma in vitro and in vivo. Phytomedicine 20 (10), 865–873. doi:10.1016/j.phymed.2013.03.006
Li, X., Rao, Z., Xie, Z., Qi, H., and Zeng, N. (2022b). Isolation, structure and bioactivity of polysaccharides from atractylodes macrocephala: a review. J. Ethnopharmacol. 296, 115506. doi:10.1016/j.jep.2022.115506
Li, X., Sun, J., Xu, Q., Duan, W., Yang, L., Wu, X., et al. (2020b). Oxymatrine inhibits colorectal cancer metastasis via attenuating PKM2-mediated aerobic glycolysis. Cancer Manag. Res. 12, 9503–9513. doi:10.2147/cmar.S267686
Li, X., Tian, R., Liu, L., Wang, L., He, D., Cao, K., et al. (2020c). Andrographolide enhanced radiosensitivity by downregulating glycolysis via the inhibition of the PI3K-Akt-mTOR signaling pathway in HCT116 colorectal cancer cells. J. Int. Med. Res. 48 (8), 300060520946169. doi:10.1177/0300060520946169
Li, X., Yuan, W., Wu, J., Zhen, J., Sun, Q., and Yu, M. (2022c). Andrographolide, a natural anti-inflammatory agent: an Update. Front. Pharmacol. 13, 920435. doi:10.3389/fphar.2022.920435
Li, Y., Wang, Y., Liu, Z., Guo, X., Miao, Z., and Ma, S. (2020d). Atractylenolide I induces apoptosis and suppresses glycolysis by blocking the JAK2/STAT3 signaling pathway in colorectal cancer cells. Front. Pharmacol. 11, 273. doi:10.3389/fphar.2020.00273
Liang, Z., Li, X., Liu, S., Li, C., Wang, X., and Xing, J. (2019). MiR-141-3p inhibits cell proliferation, migration and invasion by targeting TRAF5 in colorectal cancer. Biochem. Biophys. Res. Commun. 514 (3), 699–705. doi:10.1016/j.bbrc.2019.05.002
Liu, Q., Tang, J., Chen, S., Hu, S., Shen, C., Xiang, J., et al. (2022). Berberine for gastric cancer prevention and treatment: multi-step actions on the Correa's cascade underlie its therapeutic effects. Pharmacol. Res. 184, 106440. doi:10.1016/j.phrs.2022.106440
Liu, R., Zhu, L. L., Yu, C. Y., Shuai, Y. P., Sun, L. L., Bi, K. S., et al. (2021). Quantitative evaluation of the compatibility effects of aidi injection on the treatment of hepatocellular carcinoma using targeted metabolomics: a new strategy on the mechanism study of an anticancer compound in traditional Chinese medicine. World J. Tradit. Chin. Med. 7 (1), 111–119. doi:10.4103/wjtcm.wjtcm_86_20
Liu, S., Huang, F., Ye, Q., Li, Y., Chen, J., and Huang, H. (2020). SPRY4-IT1 promotes survival of colorectal cancer cells through regulating PDK1-mediated glycolysis. Anim. Cells Syst. Seoul. 24 (4), 220–227. doi:10.1080/19768354.2020.1784274
Liu, W., Li, W., Liu, H., and Yu, X. (2019). Xanthohumol inhibits colorectal cancer cells via downregulation of Hexokinases II-mediated glycolysis. Int. J. Biol. Sci. 15 (11), 2497–2508. doi:10.7150/ijbs.37481
Liu, Z., Li, L., and Xue, B. (2018). Effect of ganoderic acid D on colon cancer Warburg effect: role of SIRT3/cyclophilin D. Eur. J. Pharmacol. 824, 72–77. doi:10.1016/j.ejphar.2018.01.026
Lunagariya, J., Bhadja, P., Zhong, S., Vekariya, R., and Xu, S. (2019). Marine natural product bis-indole alkaloid caulerpin: chemistry and biology. Mini Rev. Med. Chem. 19 (9), 751–761. doi:10.2174/1389557517666170927154231
Lunt, S. Y., and Vander Heiden, M. G. (2011). Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 27, 441–464. doi:10.1146/annurev-cellbio-092910-154237
Ma, Y. Y., Di, Z. M., Cao, Q., Xu, W. S., Bi, S. X., Yu, J. S., et al. (2020). Xanthatin induces glioma cell apoptosis and inhibits tumor growth via activating endoplasmic reticulum stress-dependent CHOP pathway. Acta Pharmacol. Sin. 41 (3), 404–414. doi:10.1038/s41401-019-0318-5
Machado, K. D. C., Islam, M. T., Ali, E. S., Rouf, R., Uddin, S. J., Dev, S., et al. (2018). A systematic review on the neuroprotective perspectives of beta-caryophyllene. Phytother. Res. 32 (12), 2376–2388. doi:10.1002/ptr.6199
Mao, L., Chen, Q., Gong, K., Xu, X., Xie, Y., Zhang, W., et al. (2018). Berberine decelerates glucose metabolism via suppression of mTOR‑dependent HIF‑1α protein synthesis in colon cancer cells. Oncol. Rep. 39 (5), 2436–2442. doi:10.3892/or.2018.6318
Martin, D. E., and Hall, M. N. (2005). The expanding TOR signaling network. Curr. Opin. Cell Biol. 17 (2), 158–166. doi:10.1016/j.ceb.2005.02.008
Martins, S. F., Amorim, R., Viana-Pereira, M., Pinheiro, C., Costa, R. F., Silva, P., et al. (2016). Significance of glycolytic metabolism-related protein expression in colorectal cancer, lymph node and hepatic metastasis. BMC Cancer 16, 535. doi:10.1186/s12885-016-2566-9
McGhie, T. K., Ainge, G. D., Barnett, L. E., Cooney, J. M., and Jensen, D. J. (2003). Anthocyanin glycosides from berry fruit are absorbed and excreted unmetabolized by both humans and rats. J. Agric. Food Chem. 51 (16), 4539–4548. doi:10.1021/jf026206w
McGhie, T. K., and Walton, M. C. (2007). The bioavailability and absorption of anthocyanins: towards a better understanding. Mol. Nutr. Food Res. 51 (6), 702–713. doi:10.1002/mnfr.200700092
Meng, F. C., Wu, Z. F., Yin, Z. Q., Lin, L. G., Wang, R., and Zhang, Q. W. (2018). Coptidis rhizoma and its main bioactive components: recent advances in chemical investigation, quality evaluation and pharmacological activity. Chin. Med. 13, 13. doi:10.1186/s13020-018-0171-3
Mengie Ayele, T., Tilahun Muche, Z., Behaile Teklemariam, A., Bogale Kassie, A., and Chekol Abebe, E. (2022). Role of JAK2/STAT3 signaling pathway in the tumorigenesis, chemotherapy resistance, and treatment of solid tumors: a systemic review. J. Inflamm. Res. 15, 1349–1364. doi:10.2147/jir.S353489
Miller, D. M., Thomas, S. D., Islam, A., Muench, D., and Sedoris, K. (2012). c-Myc and cancer metabolism. Clin. Cancer Res. 18 (20), 5546–5553. doi:10.1158/1078-0432.Ccr-12-0977
Mishra, P., Raj, V., Bhadauria, A. S., Singh, A. K., Rai, A., Kumar, P., et al. (2018). 6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid attenuates colon carcinogenesis via blockade of IL-6 mediated signals. Biomed. Pharmacother. 100, 282–295. doi:10.1016/j.biopha.2018.02.009
Mohapatra, P., Singh, P., Singh, D., Sahoo, S., and Sahoo, S. K. (2022). Phytochemical based nanomedicine: a panacea for cancer treatment, present status and future prospective. OpenNano 7, 100055. doi:10.1016/j.onano.2022.100055
Mori, Y., Tsukinoki, K., Yasuda, M., Miyazawa, M., Kaneko, A., and Watanabe, Y. (2007). Glucose transporter type 1 expression are associated with poor prognosis in patients with salivary gland tumors. Oral Oncol. 43 (6), 563–569. doi:10.1016/j.oraloncology.2006.06.006
Nagle, D. G., Ferreira, D., and Zhou, Y. D. (2006). Epigallocatechin-3-gallate (EGCG): chemical and biomedical perspectives. Phytochemistry 67 (17), 1849–1855. doi:10.1016/j.phytochem.2006.06.020
Narayanankutty, A. (2019). PI3K/Akt/mTOR pathway as a therapeutic target for colorectal cancer: a review of preclinical and clinical evidence. Curr. Drug Targets 20 (12), 1217–1226. doi:10.2174/1389450120666190618123846
Nenkov, M., Ma, Y., Gaßler, N., and Chen, Y. (2021). Metabolic reprogramming of colorectal cancer cells and the microenvironment: implication for therapy. Int. J. Mol. Sci. 22 (12), 6262. doi:10.3390/ijms22126262
Pal, A., and Tripathi, A. (2020). Toxicological and behavioral study of two potential antibacterial agents:4-chloromercuribenzoic acid and quercetin on Swiss-albino mice. Drug Chem. Toxicol. 43 (6), 645–655. doi:10.1080/01480545.2018.1517774
Park, C., Lee, H., Noh, J. S., Jin, C. Y., Kim, G. Y., Hyun, J. W., et al. (2020). Hemistepsin A protects human keratinocytes against hydrogen peroxide-induced oxidative stress through activation of the Nrf2/HO-1 signaling pathway. Arch. Biochem. Biophys. 691, 108512. doi:10.1016/j.abb.2020.108512
Peng, F., Du, Q., Peng, C., Wang, N., Tang, H., Xie, X., et al. (2015). A review: the pharmacology of isoliquiritigenin. Phytother. Res. 29 (7), 969–977. doi:10.1002/ptr.5348
Phua, C. Y. H., Teoh, Z. L., Goh, B. H., Yap, W. H., and Tang, Y. Q. (2021). Triangulating the pharmacological properties of thymoquinone in regulating reactive oxygen species, inflammation, and cancer: therapeutic applications and mechanistic pathways. Life Sci. 287, 120120. doi:10.1016/j.lfs.2021.120120
Prabakaran, A. D., Karakkat, J. V., Vijayan, R., Chalissery, J., Ibrahim, M. F., Kaimala, S., et al. (2018). Identification of early indicators of altered metabolism in normal development using a rodent model system. Dis. Model Mech. 11 (3), dmm031815. doi:10.1242/dmm.031815
Qu, J., Liu, Q., You, G., Ye, L., Jin, Y., Kong, L., et al. (2022). Advances in ameliorating inflammatory diseases and cancers by andrographolide: pharmacokinetics, pharmacodynamics, and perspective. Med. Res. Rev. 42 (3), 1147–1178. doi:10.1002/med.21873
Raju, J., and Mehta, R. (2009). Cancer chemopreventive and therapeutic effects of diosgenin, a food saponin. Nutr. Cancer 61 (1), 27–35. doi:10.1080/01635580802357352
Rankin, E. B., and Giaccia, A. J. (2016). Hypoxic control of metastasis. Science 352 (6282), 175–180. doi:10.1126/science.aaf4405
Ren, B., Kwah, M. X., Liu, C., Ma, Z., Shanmugam, M. K., Ding, L., et al. (2021). Resveratrol for cancer therapy: challenges and future perspectives. Cancer Lett. 515, 63–72. doi:10.1016/j.canlet.2021.05.001
Robey, R. B., and Hay, N. (2009). Is Akt the "Warburg kinase"? Akt-energy metabolism interactions and oncogenesis. Semin. Cancer Biol. 19 (1), 25–31. doi:10.1016/j.semcancer.2008.11.010
Rupaimoole, R., and Slack, F. J. (2017). MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 16 (3), 203–222. doi:10.1038/nrd.2016.246
Salimian Rizi, B., Caneba, C., Nowicka, A., Nabiyar, A. W., Liu, X., Chen, K., et al. (2015). Nitric oxide mediates metabolic coupling of omentum-derived adipose stroma to ovarian and endometrial cancer cells. Cancer Res. 75 (2), 456–471. doi:10.1158/0008-5472.Can-14-1337
Saunier, E., Antonio, S., Regazzetti, A., Auzeil, N., Laprévote, O., Shay, J. W., et al. (2017). Resveratrol reverses the Warburg effect by targeting the pyruvate dehydrogenase complex in colon cancer cells. Sci. Rep. 7 (1), 6945. doi:10.1038/s41598-017-07006-0
Saunier, E., Benelli, C., and Bortoli, S. (2016). The pyruvate dehydrogenase complex in cancer: an old metabolic gatekeeper regulated by new pathways and pharmacological agents. Int. J. Cancer 138 (4), 809–817. doi:10.1002/ijc.29564
Sen, K., Banerjee, S., and Mandal, M. (2019). Dual drug loaded liposome bearing apigenin and 5-Fluorouracil for synergistic therapeutic efficacy in colorectal cancer. Colloids Surf. B Biointerfaces 180, 9–22. doi:10.1016/j.colsurfb.2019.04.035
Shaikh, J., Ankola, D. D., Beniwal, V., Singh, D., and Kumar, M. N. (2009). Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 37 (3-4), 223–230. doi:10.1016/j.ejps.2009.02.019
Shan, S., Shi, J., Yang, P., Jia, B., Wu, H., Zhang, X., et al. (2017). Apigenin restrains colon cancer cell proliferation via targeted blocking of pyruvate kinase M2-dependent glycolysis. J. Agric. Food Chem. 65 (37), 8136–8144. doi:10.1021/acs.jafc.7b02757
Shan, X., Lv, Z. Y., Yin, M. J., Chen, J., Wang, J., and Wu, Q. N. (2021). The protective effect of cyanidin-3-glucoside on myocardial ischemia-reperfusion injury through ferroptosis. Oxid. Med. Cell Longev. 2021, 8880141. doi:10.1155/2021/8880141
Sharma, A., Ghani, A., Sak, K., Tuli, H. S., Sharma, A. K., Setzer, W. N., et al. (2019). Probing into therapeutic anti-cancer potential of apigenin: recent trends and future directions. Recent Pat. Inflamm. Allergy Drug Discov. 13 (2), 124–133. doi:10.2174/1872213x13666190816160240
Sharma, S. H., Thulasingam, S., Chellappan, D. R., Chinnaswamy, P., and Nagarajan, S. (2017). Morin and Esculetin supplementation modulates c-myc induced energy metabolism and attenuates neoplastic changes in rats challenged with the procarcinogen 1,2 - dimethylhydrazine. Eur. J. Pharmacol. 796, 20–31. doi:10.1016/j.ejphar.2016.12.019
Sheng, Q. S., He, K. X., Li, J. J., Zhong, Z. F., Wang, F. X., Pan, L. L., et al. (2020). Comparison of gut microbiome in human colorectal cancer in paired tumor and adjacent normal tissues. Onco Targets Ther. 13, 635–646. doi:10.2147/ott.S218004
Shinohara, Y., Yamamoto, K., Kogure, K., Ichihara, J., and Terada, H. (1994). Steady state transcript levels of the type II hexokinase and type 1 glucose transporter in human tumor cell lines. Cancer Lett. 82 (1), 27–32. doi:10.1016/0304-3835(94)90142-2
Simula, L., Alifano, M., and Icard, P. (2022). How phosphofructokinase-1 promotes PI3K and YAP/TAZ in cancer: therapeutic perspectives. Cancers (Basel) 14 (10), 2478. doi:10.3390/cancers14102478
Sithara, T., Arun, K. B., Syama, H. P., Reshmitha, T. R., and Nisha, P. (2017). Morin inhibits proliferation of SW480 colorectal cancer cells by inducing apoptosis mediated by reactive oxygen species formation and uncoupling of Warburg effect. Front. Pharmacol. 8, 640. doi:10.3389/fphar.2017.00640
Song, K., Li, M., Xu, X., Xuan, L. I., Huang, G., and Liu, Q. (2016). Resistance to chemotherapy is associated with altered glucose metabolism in acute myeloid leukemia. Oncol. Lett. 12 (1), 334–342. doi:10.3892/ol.2016.4600
Sun, P., Zhao, W., Wang, Q., Chen, L., Sun, K., Zhan, Z., et al. (2022a). Chemical diversity, biological activities and Traditional uses of and important Chinese herb Sophora. Phytomedicine 100, 154054. doi:10.1016/j.phymed.2022.154054
Sun, Q., Yang, H., Liu, M., Ren, S., Zhao, H., Ming, T., et al. (2022b). Berberine suppresses colorectal cancer by regulation of Hedgehog signaling pathway activity and gut microbiota. Phytomedicine 103, 154227. doi:10.1016/j.phymed.2022.154227
Sun, W., Ge, Y., Cui, J., Yu, Y., and Liu, B. (2021). Scutellarin resensitizes oxaliplatin-resistant colorectal cancer cells to oxaliplatin treatment through inhibition of PKM2. Mol. Ther. Oncolytics 21, 87–97. doi:10.1016/j.omto.2021.03.010
Sun, Z., Zhang, Q., Yuan, W., Li, X., Chen, C., Guo, Y., et al. (2020). MiR-103a-3p promotes tumour glycolysis in colorectal cancer via hippo/YAP1/HIF1A axis. J. Exp. Clin. Cancer Res. 39 (1), 250. doi:10.1186/s13046-020-01705-9
Tamene, D., and Endale, M. (2019). Antibacterial activity of coumarins and carbazole alkaloid from roots of clausena anisata. Adv. Pharmacol. Sci. 2019, 5419854. doi:10.1155/2019/5419854
Tang, J., Yan, T., Bao, Y., Shen, C., Yu, C., Zhu, X., et al. (2019). LncRNA GLCC1 promotes colorectal carcinogenesis and glucose metabolism by stabilizing c-Myc. Nat. Commun. 10 (1), 3499. doi:10.1038/s41467-019-11447-8
Tao, L., Sheng, X., Zhang, L., Li, W., Wei, Z., Zhu, P., et al. (2016). Xanthatin anti-tumor cytotoxicity is mediated via glycogen synthase kinase-3β and β-catenin. Biochem. Pharmacol. 115, 18–27. doi:10.1016/j.bcp.2016.06.009
Thomasset, S. C., Berry, D. P., Garcea, G., Marczylo, T., Steward, W. P., and Gescher, A. J. (2007). Dietary polyphenolic phytochemicals--promising cancer chemopreventive agents in humans? A review of their clinical properties. Int. J. Cancer 120 (3), 451–458. doi:10.1002/ijc.22419
Thorens, B., and Mueckler, M. (2010). Glucose transporters in the 21st century. Am. J. Physiol. Endocrinol. Metab. 298 (2), E141–E145. doi:10.1152/ajpendo.00712.2009
Tokunaga, R., Cao, S., Naseem, M., Battaglin, F., Lo, J. H., Arai, H., et al. (2019). AMPK variant, a candidate of novel predictor for chemotherapy in metastatic colorectal cancer: a meta-analysis using tribe, mavericc and FIRE3. Int. J. Cancer 145 (8), 2082–2090. doi:10.1002/ijc.32261
Tong, J., Shen, Y., Zhang, Z., Hu, Y., Zhang, X., and Han, L. (2019). Apigenin inhibits epithelial-mesenchymal transition of human colon cancer cells through NF-κB/Snail signaling pathway. Biosci. Rep. 39 (5), BSR20190452. doi:10.1042/bsr20190452
Torrens-Mas, M., Oliver, J., Roca, P., and Sastre-Serra, J. (2017). SIRT3: oncogene and tumor suppressor in cancer. Cancers (Basel) 9 (7), 90. doi:10.3390/cancers9070090
Towler, M. C., and Hardie, D. G. (2007). AMP-activated protein kinase in metabolic control and insulin signaling. Circ. Res. 100 (3), 328–341. doi:10.1161/01.Res.0000256090.42690.05
Van der Jeught, K., Xu, H. C., Li, Y. J., Lu, X. B., and Ji, G. (2018). Drug resistance and new therapies in colorectal cancer. World J. Gastroenterol. 24 (34), 3834–3848. doi:10.3748/wjg.v24.i34.3834
van der Stok, E. P., Spaander, M. C. W., Grünhagen, D. J., Verhoef, C., and Kuipers, E. J. (2017). Surveillance after curative treatment for colorectal cancer. Nat. Rev. Clin. Oncol. 14 (5), 297–315. doi:10.1038/nrclinonc.2016.199
Vasaikar, S., Huang, C., Wang, X., Petyuk, V. A., Savage, S. R., Wen, B., et al. (2019). Proteogenomic analysis of human colon cancer reveals new therapeutic opportunities. Cell 177 (4), 1035–1049.e19. doi:10.1016/j.cell.2019.03.030
Vicente de Andrade Silva, G., Demaman Arend, G., Antonio Ferreira Zielinski, A., Di Luccio, M., and Ambrosi, A. (2022). Xanthohumol properties and strategies for extraction from hops and brewery residues: a review. Food Chem. 404, 134629. doi:10.1016/j.foodchem.2022.134629
Vinayak, M., and Maurya, A. K. (2019). Quercetin loaded nanoparticles in targeting cancer: recent development. Anticancer Agents Med. Chem. 19 (13), 1560–1576. doi:10.2174/1871520619666190705150214
Vogelstein, B., Lane, D., and Levine, A. J. (2000). Surfing the p53 network. Nature 408 (6810), 307–310. doi:10.1038/35042675
Wang, G., Yu, Y., Wang, Y. Z., Yin, P. H., Xu, K., and Zhang, H. (2020a). The effects and mechanisms of isoliquiritigenin loaded nanoliposomes regulated AMPK/mTOR mediated glycolysis in colorectal cancer. Artif. Cells Nanomed Biotechnol. 48 (1), 1231–1249. doi:10.1080/21691401.2020.1825092
Wang, H., Zhao, L., Zhu, L. T., Wang, Y., Pan, D., Yao, J., et al. (2014). Wogonin reverses hypoxia resistance of human colon cancer HCT116 cells via downregulation of HIF-1α and glycolysis, by inhibiting PI3K/Akt signaling pathway. Mol. Carcinog. 53 (1), E107–E118. doi:10.1002/mc.22052
Wang, H., Zhao, W., Zhou, L., Wang, J., Liu, L., Wang, S., et al. (2018a). Soft particles of gemini surfactant/conjugated polymer for enhanced anticancer activity of chemotherapeutics. ACS Appl. Mater Interfaces 10 (1), 37–41. doi:10.1021/acsami.7b16396
Wang, J., Wang, H., Liu, A., Fang, C., Hao, J., and Wang, Z. (2015). Lactate dehydrogenase A negatively regulated by miRNAs promotes aerobic glycolysis and is increased in colorectal cancer. Oncotarget 6 (23), 19456–19468. doi:10.18632/oncotarget.3318
Wang, J., Wang, L., Lou, G. H., Zeng, H. R., Hu, J., Huang, Q. W., et al. (2019a). Coptidis rhizoma: a comprehensive review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. Pharm. Biol. 57 (1), 193–225. doi:10.1080/13880209.2019.1577466
Wang, K., Huang, W., Sang, X., Wu, X., Shan, Q., Tang, D., et al. (2020b). Atractylenolide I inhibits colorectal cancer cell proliferation by affecting metabolism and stemness via AKT/mTOR signaling. Phytomedicine 68, 153191. doi:10.1016/j.phymed.2020.153191
Wang, K. L., Yu, Y. C., and Hsia, S. M. (2021). Perspectives on the role of isoliquiritigenin in cancer. Cancers (Basel) 13 (1), 115. doi:10.3390/cancers13010115
Wang, M., Chen, X., Yu, F., Zhang, L., Zhang, Y., and Chang, W. (2022). The targeting of noncoding RNAs by quercetin in cancer prevention and therapy. Oxid. Med. Cell Longev. 2022, 4330681. doi:10.1155/2022/4330681
Wang, N., Feng, Y., Zhu, M., Tsang, C. M., Man, K., Tong, Y., et al. (2010). Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: the cellular mechanism. J. Cell Biochem. 111 (6), 1426–1436. doi:10.1002/jcb.22869
Wang, T., Ning, K., Sun, X., Zhang, C., Jin, L. F., and Hua, D. (2018b). Glycolysis is essential for chemoresistance induced by transient receptor potential channel C5 in colorectal cancer. BMC Cancer 18 (1), 207. doi:10.1186/s12885-018-4123-1
Wang, W., and Guan, K. L. (2009). AMP-activated protein kinase and cancer. Acta Physiol. (Oxf) 196 (1), 55–63. doi:10.1111/j.1748-1716.2009.01980.x
Wang, Y., Lu, J. H., Wu, Q. N., Jin, Y., Wang, D. S., Chen, Y. X., et al. (2019b). LncRNA LINRIS stabilizes IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer. Mol. Cancer 18 (1), 174. doi:10.1186/s12943-019-1105-0
Wei, H., Dong, C., and Shen, Z. (2020). Kallikrein-related peptidase (KLK10) cessation blunts colorectal cancer cell growth and glucose metabolism by regulating the PI3K/Akt/mTOR pathway. Neoplasma 67 (4), 889–897. doi:10.4149/neo_2020_190814N758
Wei, L., Zhou, Y., Dai, Q., Qiao, C., Zhao, L., Hui, H., et al. (2013). Oroxylin A induces dissociation of hexokinase II from the mitochondria and inhibits glycolysis by SIRT3-mediated deacetylation of cyclophilin D in breast carcinoma. Cell Death Dis. 4 (4), e601. doi:10.1038/cddis.2013.131
Wei, X., Mao, T., Li, S., He, J., Hou, X., Li, H., et al. (2019). DT-13 inhibited the proliferation of colorectal cancer via glycolytic metabolism and AMPK/mTOR signaling pathway. Phytomedicine 54, 120–131. doi:10.1016/j.phymed.2018.09.003
Wilson, J. E. (2003). Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J. Exp. Biol. 206 (12), 2049–2057. doi:10.1242/jeb.00241
Wong, N., Yan, J., Ojo, D., De Melo, J., Cutz, J. C., and Tang, D. (2014). Changes in PKM2 associate with prostate cancer progression. Cancer Invest. 32 (7), 330–338. doi:10.3109/07357907.2014.919306
Wu, H., Cui, M., Li, C., Li, H., Dai, Y., Cui, K., et al. (2021). Kaempferol reverses aerobic glycolysis via miR-339-5p-mediated PKM alternative splicing in colon cancer cells. J. Agric. Food Chem. 69 (10), 3060–3068. doi:10.1021/acs.jafc.0c07640
Wu, P., Meng, X., Zheng, H., Zeng, Q., Chen, T., Wang, W., et al. (2018). Kaempferol attenuates ROS-induced hemolysis and the molecular mechanism of its induction of apoptosis on bladder cancer. Molecules 23 (10), 2592. doi:10.3390/molecules23102592
Wu, Z., Han, X., Tan, G., Zhu, Q., Chen, H., Xia, Y., et al. (2020). Dioscin inhibited glycolysis and induced cell apoptosis in colorectal cancer via promoting c-myc ubiquitination and subsequent hexokinase-2 suppression. Onco Targets Ther. 13, 31–44. doi:10.2147/ott.S224062
Xia, L. M., Zhang, A. P., Zheng, Q., Ding, J., Jin, Z., Yu, H., et al. (2022). Quercetin-3-O-β-D-glucuronide inhibits mitochondria pathway-mediated platelet apoptosis via the phosphatidylinositol-3-kinase/AKT pathway in immunological bone marrow failure. World J. Tradit. Chin. Med. 8 (1), 115–122. doi:10.4103/wjtcm.wjtcm_44_21
Xian, Z. Y., Liu, J. M., Chen, Q. K., Chen, H. Z., Ye, C. J., Xue, J., et al. (2015). Inhibition of LDHA suppresses tumor progression in prostate cancer. Tumour Biol. 36 (10), 8093–8100. doi:10.1007/s13277-015-3540-x
Xiang, S., Fang, J., Wang, S., Deng, B., and Zhu, L. (2015). MicroRNA-135b regulates the stability of PTEN and promotes glycolysis by targeting USP13 in human colorectal cancers. Oncol. Rep. 33 (3), 1342–1348. doi:10.3892/or.2014.3694
Xiong, R. G., Huang, S. Y., Wu, S. X., Zhou, D. D., Yang, Z. J., Saimaiti, A., et al. (2022). Anticancer effects and mechanisms of berberine from medicinal herbs: an update review. Molecules 27 (14), 4523. doi:10.3390/molecules27144523
Xu, Y., Han, S., Lei, K., Chang, X., Wang, K., Li, Z., et al. (2016). Anti-Warburg effect of rosmarinic acid via miR-155 in colorectal carcinoma cells. Eur. J. Cancer Prev. 25 (6), 481–489. doi:10.1097/cej.0000000000000205
Yang, I. H., Shin, J. A., Lee, K. E., Kim, J., Cho, N. P., and Cho, S. D. (2017). Oridonin induces apoptosis in human oral cancer cells via phosphorylation of histone H2AX. Eur. J. Oral Sci. 125 (6), 438–443. doi:10.1111/eos.12387
Yang, L., Xiao, M., Li, X., Tang, Y., and Wang, Y. L. (2016). Arginine ADP-ribosyltransferase 1 promotes angiogenesis in colorectal cancer via the PI3K/Akt pathway. Int. J. Mol. Med. 37 (3), 734–742. doi:10.3892/ijmm.2016.2473
Yang, X., Zheng, S., Wang, X., Wang, J., Ali Shah, S. B., Wang, Y., et al. (2022). Advances in pharmacology, biosynthesis, and metabolic engineering of Scutellaria-specialized metabolites. Crit. Rev. Biotechnol. 29, 1–17. doi:10.1080/07388551.2022.2149386
Yang, Y., Vong, C. T., Zeng, S., Gao, C., Chen, Z., Fu, C., et al. (2021). Tracking evidences of Coptis chinensis for the treatment of inflammatory bowel disease from pharmacological, pharmacokinetic to clinical studies. J. Ethnopharmacol. 268, 113573. doi:10.1016/j.jep.2020.113573
Yao, Z., Xie, F., Li, M., Liang, Z., Xu, W., Yang, J., et al. (2017). Oridonin induces autophagy via inhibition of glucose metabolism in p53-mutated colorectal cancer cells. Cell Death Dis. 8 (2), e2633. doi:10.1038/cddis.2017.35
Yeh, Y. H., Hsiao, H. F., Yeh, Y. C., Chen, T. W., and Li, T. K. (2018). Inflammatory interferon activates HIF-1α-mediated epithelial-to-mesenchymal transition via PI3K/AKT/mTOR pathway. J. Exp. Clin. Cancer Res. 37 (1), 70. doi:10.1186/s13046-018-0730-6
Yeung, S. J., Pan, J., and Lee, M. H. (2008). Roles of p53, MYC and HIF-1 in regulating glycolysis - the seventh hallmark of cancer. Cell Mol. Life Sci. 65 (24), 3981–3999. doi:10.1007/s00018-008-8224-x
Younes, M., Brown, R. W., Mody, D. R., Fernandez, L., and Laucirica, R. (1995). GLUT1 expression in human breast carcinoma: correlation with known prognostic markers. Anticancer Res. 15 (6), 2895–2898.
Younes, M., Lechago, L. V., and Lechago, J. (1996). Overexpression of the human erythrocyte glucose transporter occurs as a late event in human colorectal carcinogenesis and is associated with an increased incidence of lymph node metastases. Clin. Cancer Res. 2 (7), 1151–1154.
Yu, G., Yu, W., Jin, G., Xu, D., Chen, Y., Xia, T., et al. (2015). PKM2 regulates neural invasion of and predicts poor prognosis for human hilar cholangiocarcinoma. Mol. Cancer 14, 193. doi:10.1186/s12943-015-0462-6
Yu, H., Zhang, H., Dong, M., Wu, Z., Shen, Z., Xie, Y., et al. (2017). Metabolic reprogramming and AMPKα1 pathway activation by caulerpin in colorectal cancer cells. Int. J. Oncol. 50 (1), 161–172. doi:10.3892/ijo.2016.3794
Yuan, H., Xu, Y., Luo, Y., Zhang, J. R., Zhu, X. X., and Xiao, J. H. (2022). Ganoderic acid D prevents oxidative stress-induced senescence by targeting 14-3-3ε to activate CaM/CaMKII/NRF2 signaling pathway in mesenchymal stem cells. Aging Cell 21 (9), e13686. doi:10.1111/acel.13686
Zeng, B., Wei, A., Zhou, Q., Yuan, M., Lei, K., Liu, Y., et al. (2022). Andrographolide: a review of its pharmacology, pharmacokinetics, toxicity and clinical trials and pharmaceutical researches. Phytother. Res. 36 (1), 336–364. doi:10.1002/ptr.7324
Zhang, D., Wu, J., Duan, X., Wang, K., Ni, M., Liu, S., et al. (2019). Network meta-analysis of Chinese herbal injections plus the FOLFOX regimen for the treatment of colorectal cancer in China. Integr. Cancer Ther. 18, 1534735419827098. doi:10.1177/1534735419827098
Zhang, H., Chen, L., Sun, X., Yang, Q., Wan, L., and Guo, C. (2020a). Matrine: a promising natural product with various pharmacological activities. Front. Pharmacol. 11, 588. doi:10.3389/fphar.2020.00588
Zhang, H., Kong, Q., Wang, J., Jiang, Y., and Hua, H. (2020b). Complex roles of cAMP-PKA-CREB signaling in cancer. Exp. Hematol. Oncol. 9 (1), 32. doi:10.1186/s40164-020-00191-1
Zhang, H., Li, S., Si, Y., and Xu, H. (2021a). Andrographolide and its derivatives: current achievements and future perspectives. Eur. J. Med. Chem. 224, 113710. doi:10.1016/j.ejmech.2021.113710
Zhang, L., Xie, Q., and Li, X. (2022). Esculetin: a review of its pharmacology and pharmacokinetics. Phytother. Res. 36 (1), 279–298. doi:10.1002/ptr.7311
Zhang, M., Liu, T., Sun, H., Weng, W., Zhang, Q., Liu, C., et al. (2018). Pim1 supports human colorectal cancer growth during glucose deprivation by enhancing the Warburg effect. Cancer Sci. 109 (5), 1468–1479. doi:10.1111/cas.13562
Zhang, W., Tong, D., Liu, F., Li, D., Li, J., Cheng, X., et al. (2016). RPS7 inhibits colorectal cancer growth via decreasing HIF-1α-mediated glycolysis. Oncotarget 7 (5), 5800–5814. doi:10.18632/oncotarget.6807
Zhang, Y., Mao, Q., Xia, Q., Cheng, J., Huang, Z., Li, Y., et al. (2021b). Noncoding RNAs link metabolic reprogramming to immune microenvironment in cancers. J. Hematol. Oncol. 14 (1), 169. doi:10.1186/s13045-021-01179-y
Zhang, Y., Sun, M., Han, Y., Zhai, K., Tang, Y., Qin, X., et al. (2015). The saponin DT-13 attenuates tumor necrosis factor-α-induced vascular inflammation associated with Src/NF-кB/MAPK pathway modulation. Int. J. Biol. Sci. 11 (8), 970–981. doi:10.7150/ijbs.11635
Zhao, J., Huang, X., Xu, Z., Dai, J., He, H., Zhu, Y., et al. (2017). LDHA promotes tumor metastasis by facilitating epithelial-mesenchymal transition in renal cell carcinoma. Mol. Med. Rep. 16 (6), 8335–8344. doi:10.3892/mmr.2017.7637
Zhao, K., Zhou, Y., Qiao, C., Ni, T., Li, Z., Wang, X., et al. (2015). Oroxylin A promotes PTEN-mediated negative regulation of MDM2 transcription via SIRT3-mediated deacetylation to stabilize p53 and inhibit glycolysis in wt-p53 cancer cells. J. Hematol. Oncol. 8, 41. doi:10.1186/s13045-015-0137-1
Zhao, Y., Zhang, L., Wu, Y., Dai, Q., Zhou, Y., Li, Z., et al. (2018). Selective anti-tumor activity of wogonin targeting the Warburg effect through stablizing p53. Pharmacol. Res. 135, 49–59. doi:10.1016/j.phrs.2018.07.011
Zheng, Y., Wang, Y., Liu, Y., Xie, L., Ge, J., Yu, G., et al. (2021). N6-Methyladenosine modification of PTTG3P contributes to colorectal cancer proliferation via YAP1. Front. Oncol. 11, 669731. doi:10.3389/fonc.2021.669731
Zhong, X. D., Chen, L. J., Xu, X. Y., Liu, Y. J., Tao, F., Zhu, M. H., et al. (2022). Berberine as a potential agent for breast cancer therapy. Front. Oncol. 12, 993775. doi:10.3389/fonc.2022.993775
Zhou, C., Lyu, L. H., Miao, H. K., Bahr, T., Zhang, Q. Y., Liang, T., et al. (2020a). Redox regulation by SOD2 modulates colorectal cancer tumorigenesis through AMPK-mediated energy metabolism. Mol. Carcinog. 59 (5), 545–556. doi:10.1002/mc.23178
Zhou, H., Sun, L., Yang, X. L., and Schimmel, P. (2013). ATP-directed capture of bioactive herbal-based medicine on human tRNA synthetase. Nature 494 (7435), 121–124. doi:10.1038/nature11774
Zhou, L., Yu, X., Li, M., Gong, G., Liu, W., Li, T., et al. (2020b). Cdh1-mediated Skp2 degradation by dioscin reprogrammes aerobic glycolysis and inhibits colorectal cancer cells growth. EBioMedicine 51, 102570. doi:10.1016/j.ebiom.2019.11.031
Zhou, L., Zhan, M. L., Tang, Y., Xiao, M., Li, M., Li, Q. S., et al. (2018). Effects of β-caryophyllene on arginine ADP-ribosyltransferase 1-mediated regulation of glycolysis in colorectal cancer under high-glucose conditions. Int. J. Oncol. 53 (4), 1613–1624. doi:10.3892/ijo.2018.4506
Zhou, Y., Tozzi, F., Chen, J., Fan, F., Xia, L., Wang, J., et al. (2012). Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res. 72 (1), 304–314. doi:10.1158/0008-5472.Can-11-1674
Zhou, Y., Zhang, J., Wang, K., Han, W., Wang, X., Gao, M., et al. (2020c). Quercetin overcomes colon cancer cells resistance to chemotherapy by inhibiting solute carrier family 1, member 5 transporter. Eur. J. Pharmacol. 881, 173185. doi:10.1016/j.ejphar.2020.173185
Zhu, B., Zhang, Q. L., Hua, J. W., Cheng, W. L., and Qin, L. P. (2018). The traditional uses, phytochemistry, and pharmacology of atractylodes macrocephala Koidz.: a review. J. Ethnopharmacol. 226, 143–167. doi:10.1016/j.jep.2018.08.023
Zuo, S., Wu, L., Wang, Y., and Yuan, X. (2020). Long non-coding RNA MEG3 activated by vitamin D suppresses glycolysis in colorectal cancer via promoting c-myc degradation. Front. Oncol. 10, 274. doi:10.3389/fonc.2020.00274
Glossary
Keywords: colorectal cancer, warburg effect, glycolysis, phytochemicals, molecular pathways
Citation: Zhan L, Su F, Li Q, Wen Y, Wei F, He Z, Chen X, Yin X, Wang J, Cai Y, Gong Y, Chen Y, Ma X and Zeng J (2023) Phytochemicals targeting glycolysis in colorectal cancer therapy: effects and mechanisms of action. Front. Pharmacol. 14:1257450. doi: 10.3389/fphar.2023.1257450
Received: 12 July 2023; Accepted: 10 August 2023;
Published: 24 August 2023.
Edited by:
Pranav Kumar Prabhakar, Lovely Professional University, IndiaReviewed by:
Jinjun Wu, Guangzhou University of Chinese Medicine, ChinaAli AbdelWahab, Cairo University, Egypt
Copyright © 2023 Zhan, Su, Li, Wen, Wei, He, Chen, Yin, Wang, Cai, Gong, Chen, Ma and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Chen, NzM1NDA1NjYxQHFxLmNvbQ==; Xiao Ma, dG9ieW1heGlhb0BjZHV0Y20uZWR1LmNu; Jinhao Zeng, emVuZ2ppbmhhb0BjZHV0Y20uZWR1LmNu
†These authors have contributed equally to this work
 Lu Zhan
Lu Zhan Fangting Su1†
Fangting Su1† Feng Wei
Feng Wei Jinhao Zeng
Jinhao Zeng