
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 09 November 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1255158
Background: Doxorubicin-induced cardiotoxicity represents a prevalent adverse effect encountered in patients undergoing treatment with doxorubicin. To date, there has been no bibliometric study to summarize the field of doxorubicin-induced cardiotoxicity. In our study, we aim to determine the current status and frontiers of doxorubicin-induced cardiotoxicity by bibliometric analysis.
Methods: The documents concerning doxorubicin-induced cardiotoxicity are obtained from the Web of Science Core Collection database (WOSCC), and VOSviewer 1.6.16, CiteSpace 5.1.3 and the WOSCC’s literature analysis wire were used to conduct the bibliometric analysis.
Results: In total, 7,021 publications were encompassed, which are produced by 37,152 authors and 6,659 organizations, 1,323 journals, and 101 countries/regions. The most productive author, institution, country and journal were Bonnie Ky with 35 publications, University of Texas with 190 documents, the United States with 1,912 publications, and PLOS ONE with 120 documents. The first high-cited article was published in the NEJM with 8,134 citations authored by DJ Slamon et al., in 2001. For keyword analysis, there are four clusters depicted in distinct directions. The keywords in the red cluster are oxidative stress, apoptosis, and cardiomyopathy. The keywords in the green cluster are cardiotoxicity, heart failure, and anthracycline. The keywords in the blue cluster are chemotherapy, trastuzumab, and paclitaxel. The keywords in the purple cluster are doxorubicin, adriamycin, and cancer. Most of the documents were derived from the United States, China and Italy (4,080/7,021, 58.1%). The number of studies from other countries should be increased.
Conclusion: In conclusion, the main research hotspots and frontiers in the field of doxorubicin-induced cardiotoxicity include the role of doxorubicin in cardiotoxicity, the mechanisms underlying doxorubicin-induced cardiotoxicity, and the development of treatment strategies for doxorubicin-induced cardiotoxicity. More studies are needed to explore the mechanisms and treatment of doxorubicin-induced cardiotoxicity.
Doxorubicin, an anthracycline antibiotic, has played an indispensable role in the realm of oncology since its discovery. This potent antineoplastic agent has demonstrated significant efficacy against a broad spectrum of malignancies, including hematological malignancies, breast cancer, sarcomas, and solid tumors, among others. Its widespread therapeutic success, However, doxorubicin-induced cardiotoxicity is one of the major side effects which can lead to heart failure and other cardiovascular problems, and limits the clinical use of doxorubicin (Chen et al., 2022; Sangweni et al., 2022; Wu et al., 2022). Clinically, doxorubicin-induced cardiotoxicity manifests across a spectrum (Cheng et al., 2022; Jiang et al., 2022; Jones and Dass, 2022; Liu et al., 2022; Lu et al., 2022; Chen et al., 2023; Ding et al., 2023; Kuno et al., 2023; Luo et al., 2023; Pharoah et al., 2023; Wu et al., 2023; Zhao et al., 2023). Some patients may experience subtle decreases in left ventricular ejection fraction without overt symptoms, while others progress to debilitating heart failure, a consequence that can be fatal and limits the drug’s overall therapeutic index. Patients should be monitored closely during treatment, and their cardiac function should be assessed using tests such as echocardiography or cardiac MRI regularly.
While the advent of new chemotherapeutic agents and targeted therapies has broadened the landscape of cancer treatment options, doxorubicin remains a cornerstone for many protocols. Many studies have explored the mechanisms and treatment of doxorubicin-induced cardiotoxicity. However, these studies are not analyzed and summarized by the method of bibliometric analysis. The bibliometric analysis is a quantitative research method that involves the statistical analysis of patterns in published literature. It is often used to identify trends in a specific field, such as the most cited papers or author, the growth of a particular research area over time, or the impact of the particular publication. The most common bibliometric measures used are citation counts, h-index, and co-citation analysis. It can provide valuable insights into the development of a particular research field, the impact of specific papers or authors, and the relationships between different research areas. It is often used to inform decisions about research funding, hiring and promotion, and the development of research policies and strategies. In our study, we aim to determine the current status and frontiers of doxorubicin-induced cardiotoxicity by bibliometric analysis.
As the most reliable citation-based database that is extensively used for bibliometric analysis, WoSCC was used to download the literature on doxorubicin-induced cardiotoxicity in our study (Lin et al., 2022; Mu et al., 2022). The search term was TS= (“Cardiac” OR “Cardiomyocyte” OR “Cardiac dysfunction” OR “Cardiomyopathy” OR “Cardiopathic” OR “Cardiotoxicity” OR “Myocardial” OR “myocardium” OR “heart”) AND (“Doxorubicin” OR “Adriamycin”). For this study, articles and reviews published in the English language and published between 1 January 2000, and 1 September 2022 were included.
We downloaded the “Plain Text” versions of relevant records from WoSCC. Our analysis utilized WoSCC’s literature analysis wire to identify the top 20 highly cited publications and ten high-yield countries/regions, journals, authors, and institutions. We employed VOSviewer 1.6.16 software to perform a co-occurrence analysis of all keywords and determine the co-authorship of organizations, authors, and countries/regions (van Eck and Waltman, 2010). CiteSpace 5.1.3 is used to perform burst detection of keywords.
According to the search criteria, a total of 7,021 documents were identified in the study. The search flow was displayed in Figure 1. For time periods, the documents can be classified into two phases: the documents published before 2006 were in the first phase, and in this phase, the number of documents was small (no more than 200 publications annually). The second phase was 2015-2022, and in this phase, the overall trend for the number of documents was increased annually, and it is estimated that the publications of Doxorubicin-Induced Cardiotoxicity will continue to increase due the importance of this topic. For types of publications, there are 6,164 articles (87.8%) and 857 reviews (12.2%). For the subject area of documents, the top two subject categories were pharmacology pharmacy (1,732 documents, 24.7%) and oncology (1,584 documents, 22.6%), which was displayed in Figure 2.
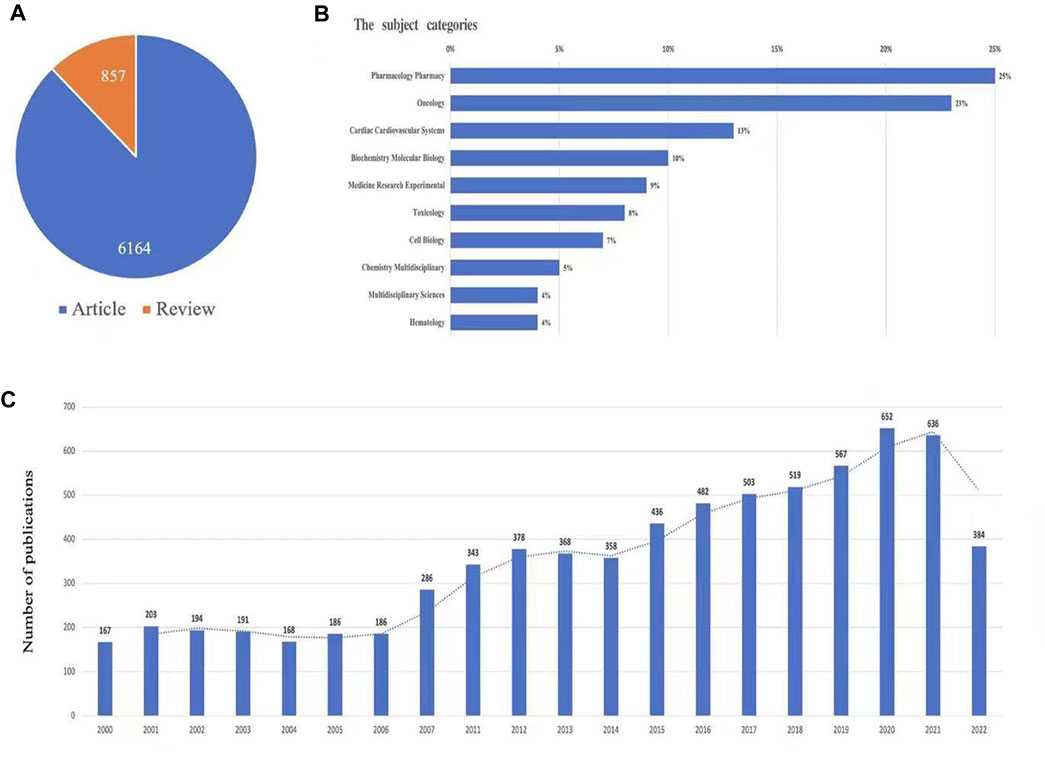
FIGURE 2. The yearly quantity and literature type of publications on TRE from inception to 1 May 2022. The figure includes (A) literature types distribution, (B) subject categories distribution, and (C) annual publications quantitative distribution.
In the field of doxorubicin-induced cardiotoxicity, 37,152 contributors are involved. Bonnie Ky is the most productive author with 35 documents (h-index 23), followed by Paulo J Oliveira with 31 documents (h-index 22), Steven E Lipshultz with 30 publications documents (h-index 20), Leontien M Kremer with documents (h-index 20), and Carlo Gabriele Tocchetti with 23 documents (h-index 19). The most frequently cited authors are Michael S Ewer, Daniela Cardinale, and Bonnie Ky. The co-authorship map is shown in Figure 3A. In general, Bonnie Ky and Steven E Lipshultz are among both the top 10 productive and top 10 most cooperative authors.
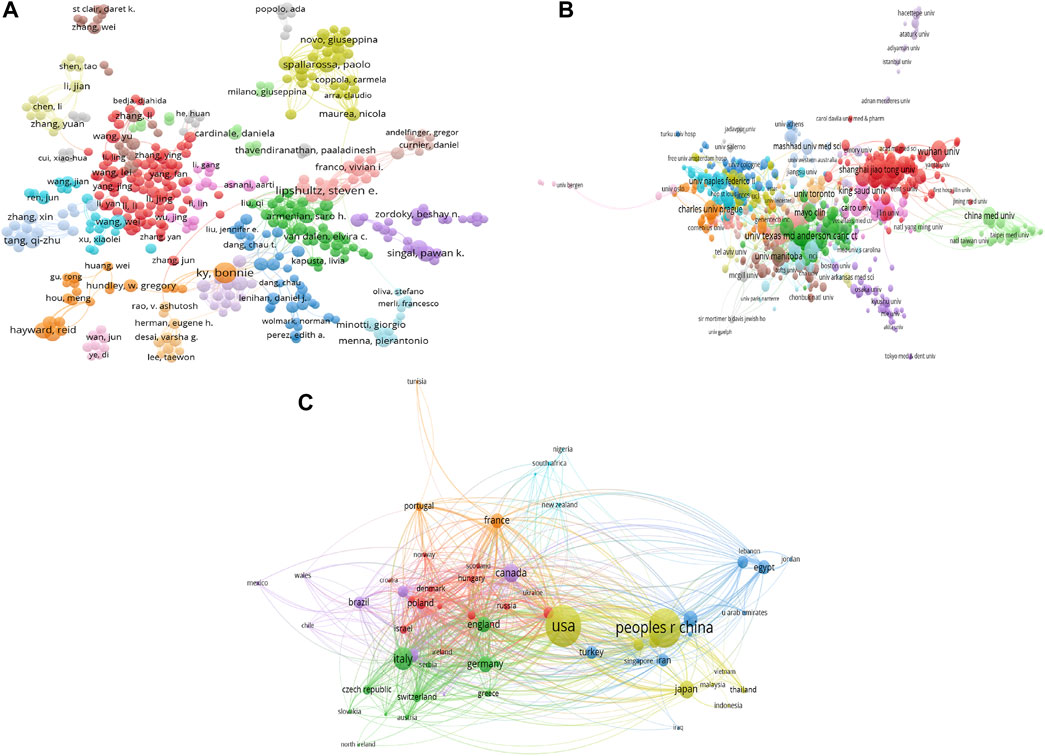
FIGURE 3. The visualization knowledge maps of co-authorship. It includes (A) the co-authorship map of authors that indicates the authors who cooperate in the field of TRE, (B) the co-authorship map of organizations, and (C) the co-authorship map of countries. Different colors indicate different clusters, and the size of nodes indicates the number of publications. The thickness of the lines represents the link strength of the countries.
A total of 6,659 organizations and 101 countries/regions are related to all publications. The most productive institutions are the University of Texas (190 documents/16072 citations), the Harvard University (182 documents/14186 citations), and the University of California System (132 documents/16284 citations). The network visualization map is shown in Figure 3B. For Countries/Regions, the United States has the most publications (1,912 publications), followed by China (1,613 publications), Italy (555 publications), Canada (368 publications), and Japan (331 publications). Figure 3C displays the network visualization map, while Table 1 provides a summary of the top 10 high-yield countries/regions, institutions, and authors. In general, United States, Peoples R China, Italy, Canada, Germany, and England are among both the top 10 productive and top 10 most cooperative countries/regions, and the university of Texas and the Harvard University are among both the top 10 productive and top 10 most cooperative institutions.
All documents are from 1,323 journals, and the highest-cited journal is Journal of Clinical Oncology with 12,428 citations. The top 3 productive journals are PLOS ONE with 120 documents (3,755 citations), Journal of Clinical Oncology with 96 documents (12,428 citations), and Cardiovascular Toxicology with 90 documents (1,364 citations). Figure 4A illustrates the network visualization map of all journals, and Table 2 presents a summary of the top 10 high-yield journals. In general, Journal of Clinical Oncology, PLOS ONE and Annals of Oncology are among the top 10 productive and top 10 most high-cited journals.
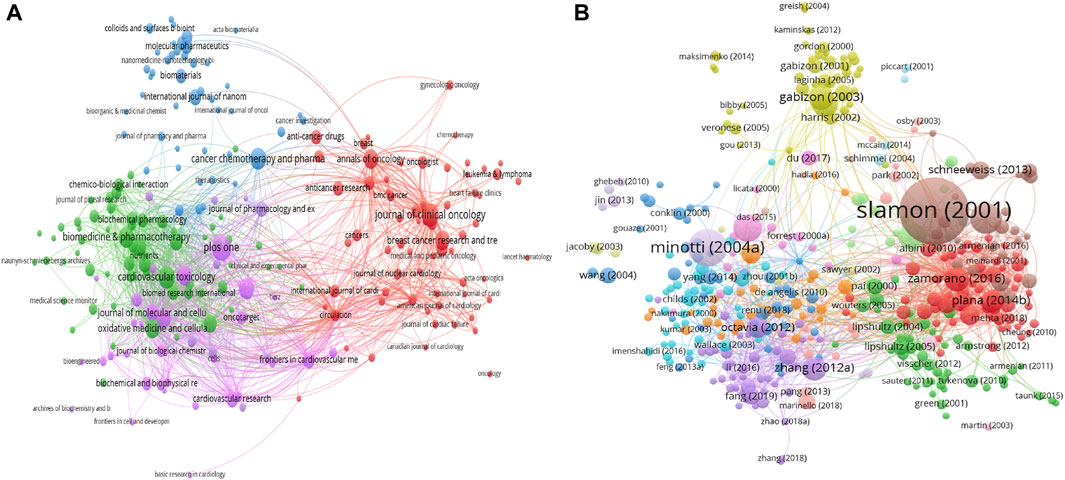
FIGURE 4. The visualization knowledge maps of citation, including (A) citation of Journal and (B) citation of documents. Different colors indicate different clusters, and the size of the nodes represents the counts of citations. The distance between the two nodes indicates their correlation.
Table 3 contains a list of the top 20 highest-cited documents (Armenian et al., 2017; Cardinale et al., 2015; Cardinale et al., 2010; Cardinale et al., 2004; Chatterjee et al., 2010; Demaria et al., 2017; Fang et al., 2019; Gabizon et al., 2003; Minotti et al., 2004; O'Brien et al., 2004; Octavia et al., 2012; Plana et al., 2014; Sawaya et al., 2012; Schneeweiss et al., 2013; Slamon et al., 2011; Slamon et al., 2001; Swain et al., 2003; Tacar et al., 2013; Zamorano et al., 2016; Zhang et al., 2012). Among these publications, 10 studies were clinical trials. The main observations of the clinical trials were summarized in Table 4. Among these clinical trials, nine were completed through collaboration between different institutes. The top 3 high-cited documents are as follows: the first high-cited article was published in NEJM with 8,134 citations authored by Slamon et al., 2001. In this article, they found that concurrent treatment with doxorubicin increased the risk of cardiac dysfunction significantly. The second article was published in Pharmacological Reviews with 2,630 citations authored by Minotti et al. (2004). In this article, they summarized the molecular mechanisms of cardiotoxic synergism between doxorubicin and other anticancer agents, and the clinical recommendations for using cardio-protectants without interfering the tumor response. The third article was published in NEJM with 1,738 citations authored by Slamon et al., 2011. In this study, they randomly assigned 3,222 patients with HER2-positive early-stage breast cancer to receive cyclophosphamide and doxorubicin, and found that the addition of 1 year of adjuvant trastuzumab could lower risks of cardiotoxicity. The network visualization map is displayed in Figure 4B. Figure 5 showed the co-citation reference in the field of Doxorubicin-Induced Cardiotoxicity. Co-cited references are defined as one publication is cited by more than one article of the 7,021 extracted list. The first high-co-cited reference was Minotti et al. (2004), Pharmacological Reviews in 2004 (736 co-citations), which was described above. The second high-co-cited reference was Singal and Iliskovic, 1998, NEJM in 1998 (668 co-citations) (WU et al., 2017). In this review, they discussed the cause, diagnosis, management of doxorubicin-induced cardiomyopathy. The third high-co-cited reference was Swain et al. (2003), Cancer in 2003 (634 co-citations). In this article, they concluded that doxorubicin-related congestive heart failure (CHF) occurred at a lower cumulative dose and with greater frequency than previously reported. In general, there are 11 references among both top 20 high-cited and high-co-cited documents.
Figure 6 displays the network visualization map of keywords, with four clusters (red, green, blue, and purple) depicted in distinct directions. The keywords in red cluster are oxidative stress, apoptosis, cardiomyopathy, and expression. The keywords in green cluster are cardiotoxicity, heart failure, and anthracycline. The keywords in blue cluster are chemotherapy, trastuzumab, and paclitaxel. The keywords in purple cluster are doxorubicin, adriamycin, and cancer. We conducted the burst detection analysis for twenty prominent words from 2010, and displayed in Figure 7. The keywords that begin to burst from 2018 are particularly emphasized, including “inflammation” (burst strength 25.92), “Doxorubicin-Induced Cardiotoxicity” (burst strength 12.55), “autophagy” (burst strength 8.25), suppression (burst strength 7.9), cardiac dysfunction (burst strength 7.46), and nrf2 (burst strength 7.19), which is shown in Figure 7.
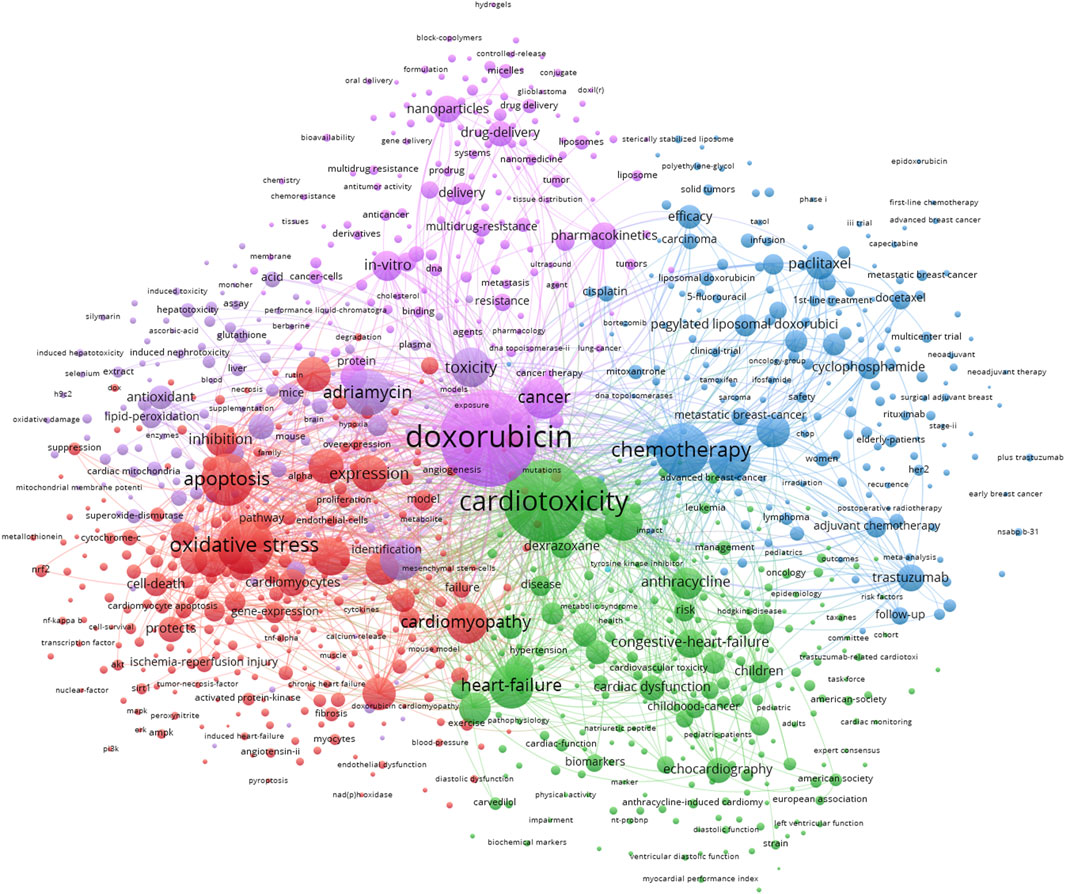
FIGURE 6. The visualization of keyword co-occurrence analysis. The size of nodes indicates the frequency of occurrences of the keywords, and the lines between the nodes represent their co-occurrence in the same publication. The shorter the distance between two nodes, the larger the number of the co-occurrence of the two keywords.
A total of 7,021 publications are included, and 37,152 authors contribute to this field, which are distributed from 6,659 organizations, 1,323 journals, and 101 countries/regions. The most productive author, institution, country and journal were Bonnie Ky with 35 publications, University of Texas with 190 documents, the United States with 1,912 publications, and PLOS ONE with 120 documents. The first high-cited article was published in the NEJM with 8,134 citations authored by Slamon et al., 2001. For keyword analysis, the clusters of red, green, blue and purple, indicate 4 directions. The keywords in red cluster are oxidative stress, apoptosis, cardiomyopathy. The keywords in green cluster are cardiotoxicity, heart failure, and anthracycline. The keywords in blue cluster are chemotherapy, trastuzumab, and paclitaxel. The keywords in purple cluster are doxorubicin, adriamycin, and cancer. Most of the documents were derived from the United States, China and Italy (4,080/7,021, 58.1%). Of the top 10 institutions, five were from the United States. Of the top 10 productive authors, four were from the United States and three were from Italy. Among cooperative relationships of countries/regions institutions and authors, the United States, China and Italy are also prominent. The number of studies from other countries should be increased.
Based on the most highly cited publications and significant keywords, the current frontiers and hotspots in this field can be summarized as follows: 1) The role of doxorubicin in cardiotoxicity. Among the 20 highest-cited references, ten articles explored the role of doxorubicin in cardiotoxicity, (Swain et al., 2003; Cardinale et al., 2010; Chatterjee et al., 2010; Octavia et al., 2012; Tacar et al., 2013; Cardinale et al., 2015; Zamorano et al., 2016; Armenian et al., 2017), and the important keywords of cardiotoxicity, heart failure, and anthracycline were in green cluster, chemotherapy and trastuzumab were in blue cluster, and doxorubicin, adriamycin, and cancer were in purple cluster. The doxorubicin-induced cardiotoxicity is the common adverse effect of cancer treated with doxorubicin, which is usually composed of myocarditis and heart failure/cardiomyopathy, hypertension and vascular toxicity, and QTc prolongation and arrhythmias. 2) The mechanisms of doxorubicin-induced cardiotoxicity. Among the 20 highest-cited references, six articles explored the mechanisms of doxorubicin-induced acute cardiotoxicity (Chatterjee et al., 2010; Octavia et al., 2012; Sawaya et al., 2012; Zhang et al., 2012; Tacar et al., 2013; Cardinale et al., 2015), and the important keywords of oxidative stress, apoptosis, and expression were in red cluster. Oxidative stress and apoptosis play pivotal roles in cardiotoxicity, and several signaling pathways are implicated in mediating these processes (Paradies et al., 2018; Ikeda et al., 2019; Tian et al., 2020; Timm and Tyler, 2020; Wallace et al., 2020; Ahsan et al., 2021; Borisov et al., 2021; Li et al., 2021; Mishra et al., 2021; Rawat et al., 2021; Yang et al., 2021; Chen et al., 2022; Herrmann et al., 2022; Ikeda et al., 2022; Kong et al., 2022; Sangweni et al., 2022; Wu et al., 2022). Understanding these pathways can provide insights into potential therapeutic targets. Here are the major signaling pathways involved in cardiotoxicity under oxidative stress and apoptosis, include:
Oxidative stress can compromise mitochondrial function, heightening the permeability of its membrane. Consequently, pro-apoptotic proteins, notably cytochrome c, are released into the cytosol. This interaction with Apaf-1 and procaspase-9 spawns the apoptosome, activating caspase-9. Subsequent activation of effector caspases, like caspase-3, instigates apoptosis.
The binding of ligands (e.g., FAS and TNF-alpha) to their respective cell surface receptors (FAS, TNF receptor) can set in motion the extrinsic apoptosis pathway. This results in caspase-8 activation, which either directly activates effector caspases or cleaves the Bcl-2 family member Bid, tying back to the intrinsic mitochondrial pathway.
Comprising the ERK, JNK, and p38 MAPK pathways. While ERK typically fosters cell survival, sustained activation under oxidative stress might induce apoptosis. Both JNK and p38 MAPK, triggered by stress, are associated with oxidative stress-induced apoptotic mechanisms in cardiomyocytes.
Fundamentally, the PI3K/Akt pathway enhances cell survival. Akt, upon activation, targets and inhibits numerous pro-apoptotic proteins. However, oxidative stress can disrupt this cardioprotective pathway.
NF-κB wields dual functionality, promoting cell survival and apoptosis. Though it can stimulate anti-apoptotic protein synthesis, extended activation, especially under oxidative duress, can precipitate apoptosis.
Central to apoptosis regulation, this protein family encompasses both pro-apoptotic (e.g., Bax, Bak, Bad) and anti-apoptotic (e.g., Bcl-2, Bcl-xL) constituents. A pro-apoptotic skew can induce mitochondrial dysfunction and, consequently, apoptosis.
The Nrf2 and ARE pathway is vital for cellular defense against oxidative onslaughts. In oxidative scenarios, Nrf2 translocates nucleus-bound, pairing with ARE to stimulate antioxidant gene synthesis. Its dysregulation can accentuate cardiotoxicity.
Sustained oxidative stress can hamper ER efficacy, culminating in ER stress. This elicits the unfolded protein response (UPR), which may lead to apoptosis if the stress remains unchecked. These pathways frequently interconnect, with the cellular outcome (survival or apoptosis) hinging on the equilibrium between pro-survival and pro-apoptotic signals. As oxidative stress and apoptosis are pivotal in cardiac injury, these pathways represent promising therapeutic targets. The main mechanisms of Doxorubicin-induced cardiotoxicity were displayed in Figure 8. 3) The treatment of doxorubicin-induced cardiotoxicity. Among the 20 highest-cited references, ten articles explored the treatment of doxorubicin-induced cardiotoxicity (Armenian et al., 2017; Cardinale et al., 2015; Cardinale et al., 2010; Chatterjee et al., 2010; Minotti et al., 2004; O'Brien et al., 2004; Schneeweiss et al., 2013; Slamon et al., 2011; Slamon et al., 2001; Zamorano et al., 2016), and Dexrazoxane is the first protective agent which is approved for the clinical use of doxorubicin-induced cardiomyopathy by FDA. Dexrazoxane can protect the heart from doxorubicin-induced cardiotoxicity by preventing Top2ß from binding with doxorubicin. More strategies should be developed to treat doxorubicin-induced cardiotoxicity.
It is important to acknowledge certain limitations in this study. Firstly, the data used solely originates from the WOSCC database, excluding other databases such as PubMed, Cochrane Library, and Google Scholar. Secondly, the study exclusively included literature in the English language, potentially introducing bias. Lastly, there may exist inconsistencies in the data, such as variations in institution names over different time periods. These limitations should be taken into consideration when interpreting the findings.
In conclusion, the main research hotspots and frontiers in the field of doxorubicin-induced cardiotoxicity include the role of doxorubicin in cardiotoxicity, the mechanisms underlying doxorubicin-induced cardiotoxicity, and the development of treatment strategies for doxorubicin-induced cardiotoxicity. More studies are needed to explore the mechanisms and treatment of doxorubicin-induced cardiotoxicity.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding authors.
XL: Conceptualization, Data curation, Investigation, Methodology, Software, Writing–original draft. GW: Formal Analysis, Project administration, Validation, Writing–review and editing. SW: Writing–original draft, Writing–review and editing. JH: Supervision, Writing–original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article. This research was supported by the fellowship of the China Postdoctoral Science Foundation (Grant No. 2021M692802) and the Key research and development program of Zhejiang province (2020C03018).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahsan, A., Liu, M. R., Zheng, Y. R., Yan, W., Pan, L., Li, Y., et al. (2021). Natural compounds modulate the autophagy with potential implication of stroke. Acta Pharm. Sin. B 11 (7), 1708–1720. doi:10.1016/j.apsb.2020.10.018
Armenian, S. H., Lacchetti, C., Barac, A., Carver, J., Constine, L. S., Denduluri, N., et al. (2017). Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 35 (8), 893–911. doi:10.1200/JCO.2016.70.5400
Borisov, V. B., Siletsky, S. A., Nastasi, M. R., and Forte, E. (2021). ROS defense systems and terminal oxidases in bacteria. Antioxidants 10 (6), 839. doi:10.3390/antiox10060839
Cardinale, D., Colombo, A., Bacchiani, G., Tedeschi, I., Meroni, C. A., Veglia, F., et al. (2015). Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 131 (22), 1981–1988. doi:10.1161/CIRCULATIONAHA.114.013777
Cardinale, D., Colombo, A., Lamantia, G., Colombo, N., Civelli, M., De Giacomi, G., et al. (2010). Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J. Am. Coll. Cardiol. 55 (3), 213–220. doi:10.1016/j.jacc.2009.03.095
Cardinale, D., Sandri, M. T., Colombo, A., Colombo, N., Boeri, M., Lamantia, G., et al. (2004). Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 109 (22), 2749–2754. doi:10.1161/01.CIR.0000130926.51766.CC
Chatterjee, K., Zhang, J., Honbo, N., and Karliner, J. S. (2010). Doxorubicin cardiomyopathy. Cardiology 115 (2), 155–162. doi:10.1159/000265166
Chen, S., Chen, J., Du, W., Mickelsen, D. M., Shi, H., Yu, H., et al. (2023). PDE10A inactivation prevents doxorubicin-induced cardiotoxicity and tumor growth. Circ. Res. 133 (2), 138–157. doi:10.1161/CIRCRESAHA.122.322264
Chen, Y., Shi, S., and Dai, Y. (2022). Research progress of therapeutic drugs for doxorubicin-induced cardiomyopathy. Biomed. Pharmacother. 156, 113903. doi:10.1016/j.biopha.2022.113903
Cheng, Y., Wu, X., Nie, X., Zhang, C., Lee, S. M. Y., Lv, K., et al. (2022). Natural compound glycyrrhetinic acid protects against doxorubicin-induced cardiotoxicity by activating the Nrf2/HO-1 signaling pathway. Phytomedicine Int. J. phytotherapy Phytopharm. 106, 154407. doi:10.1016/j.phymed.2022.154407
Demaria, M., O'Leary, M. N., Chang, J., Shao, L., Liu, S., Alimirah, F., et al. (2017). Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 7 (2), 165–176. doi:10.1158/2159-8290.CD-16-0241
Ding, M., Shi, R., Fu, F., Li, M., De, D., Du, Y., et al. (2023). Paeonol protects against doxorubicin-induced cardiotoxicity by promoting Mfn2-mediated mitochondrial fusion through activating the PKCε-Stat3 pathway. J. Adv. Res. 47, 151–162. doi:10.1016/j.jare.2022.07.002
Fang, X., Wang, H., Han, D., Xie, E., Yang, X., Wei, J., et al. (2019). Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. U. S. A. 116 (7), 2672–2680. doi:10.1073/pnas.1821022116
Gabizon, A., Shmeeda, H., and Barenholz, Y. (2003). Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin. Pharmacokinet. 42 (5), 419–436. doi:10.2165/00003088-200342050-00002
Herrmann, J., Lenihan, D., Armenian, S., Barac, A., Blaes, A., Cardinale, D., et al. (2022). Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 43 (4), 280–299. doi:10.1093/eurheartj/ehab674
Ikeda, S., Matsushima, S., Okabe, K., Ikeda, M., Ishikita, A., Tadokoro, T., et al. (2019). Blockade of L-type Ca2+ channel attenuates doxorubicin-induced cardiomyopathy via suppression of CaMKII-NF-kappa B pathway. Sci. Rep. 9, 9850. doi:10.1038/s41598-019-46367-6
Ikeda, S., Zablocki, D., and Sadoshima, J. (2022). The role of autophagy in death of cardiomyocytes. J. Mol. Cell Cardiol. 165, 1–8. doi:10.1016/j.yjmcc.2021.12.006
Jiang, Q., Chen, X., Tian, X., Zhang, J., Xue, S., Jiang, Y., et al. (2022). Tanshinone I inhibits doxorubicin-induced cardiotoxicity by regulating Nrf2 signaling pathway. Phytomedicine Int. J. phytotherapy Phytopharm. 106, 154439. doi:10.1016/j.phymed.2022.154439
Jones, I. C., and Dass, C. R. (2022). Doxorubicin-induced cardiotoxicity: causative factors and possible interventions. J. Pharm. Pharmacol. 74 (12), 1677–1688. doi:10.1093/jpp/rgac063
Kong, C. Y., Guo, Z., Song, P., Zhang, X., Yuan, Y. P., Teng, T., et al. (2022). Underlying the mechanisms of doxorubicin-induced acute cardiotoxicity: oxidative stress and cell death. Int. J. Biol. Sci. 18 (2), 760–770. doi:10.7150/ijbs.65258
Kuno, A., Hosoda, R., Tsukamoto, M., Sato, T., Sakuragi, H., Ajima, N., et al. (2023). SIRT1 in the cardiomyocyte counteracts doxorubicin-induced cardiotoxicity via regulating histone H2AX. Cardiovasc Res. 118 (17), 3360–3373. doi:10.1093/cvr/cvac026
Li, D., Yang, Y., Wang, S., He, X., Liu, M., Bai, B., et al. (2021). Role of acetylation in doxorubicin-induced cardiotoxicity. Redox Biol. 46, 102089. doi:10.1016/j.redox.2021.102089
Lin, X., Wu, G., Gao, B., Wang, S., and Huang, J. (2022). Bibliometric and visual analysis of coronary microvascular dysfunction. Front. Cardiovasc. Med. 9, 1021346. doi:10.3389/fcvm.2022.1021346
Liu, X., Li, D., Pi, W., Wang, B., Xu, S., Yu, L., et al. (2022). LCZ696 protects against doxorubicin-induced cardiotoxicity by inhibiting ferroptosis via AKT/SIRT3/SOD2 signaling pathway activation. Int. Immunopharmacol. 113, 109379. doi:10.1016/j.intimp.2022.109379
Lu, D., Chatterjee, S., Xiao, K., Riedel, I., Huang, C. K., Costa, A., et al. (2022). A circular RNA derived from the insulin receptor locus protects against doxorubicin-induced cardiotoxicity. Eur. Heart J. 43 (42), 4496–4511. doi:10.1093/eurheartj/ehac337
Luo, W., Zou, X., Wang, Y., Dong, Z., Weng, X., Pei, Z., et al. (2023). Critical role of the cGAS-STING pathway in doxorubicin-induced cardiotoxicity. Circ. Res. 132 (11), e223–e242. doi:10.1161/CIRCRESAHA.122.321587
Minotti, G., Menna, P., Salvatorelli, E., Cairo, G., and Gianni, L. (2004). Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 56 (2), 185–229. doi:10.1124/pr.56.2.6
Mishra, S. R., Mahapatra, K. K., Behera, B. P., Patra, S., Bhol, C. S., Panigrahi, D. P., et al. (2021). Mitochondrial dysfunction as a driver of NLRP3 inflammasome activation and its modulation through mitophagy for potential therapeutics. Int. J. Biochem. Cell Biol. 136, 106013. doi:10.1016/j.biocel.2021.106013
Mu, F., Tang, M., Guan, Y., Lin, R., Zhao, M., Zhao, J., et al. (2022). Knowledge mapping of the links between the gut microbiota and heart failure: a scientometric investigation (2006-2021). Front. Cardiovasc. Med. 9, 882660. doi:10.3389/fcvm.2022.882660
O'Brien, M. E., Wigler, N., Inbar, M., Rosso, R., Grischke, E., Santoro, A., et al. (2004). Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 15 (3), 440–449. doi:10.1093/annonc/mdh097
Octavia, Y., Tocchetti, C. G., Gabrielson, K. L., Janssens, S., Crijns, H. J., and Moens, A. L. (2012). Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J. Mol. Cell Cardiol. 52 (6), 1213–1225. doi:10.1016/j.yjmcc.2012.03.006
Paradies, G., Paradies, V., Ruggiero, F. M., and Petrosillo, G. (2018). Mitochondrial bioenergetics and cardiolipin alterations in myocardial ischemia-reperfusion injury: implications for pharmacological cardioprotection. Am. J. Physiol-Heart Circ. Physiol. 315 (5), H1341-H1352–H52. doi:10.1152/ajpheart.00028.2018
Pharoah, B. M., Zhang, C., Khodade, V. S., Keceli, G., McGinity, C., Paolocci, N., et al. (2023). Hydropersulfides (RSSH) attenuate doxorubicin-induced cardiotoxicity while boosting its anticancer action. Redox Biol. 60, 102625. doi:10.1016/j.redox.2023.102625
Plana, J. C., Galderisi, M., Barac, A., Ewer, M. S., Ky, B., Scherrer-Crosbie, M., et al. (2014). Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc Imaging 15 (10), 1063–1093. doi:10.1093/ehjci/jeu192
Rawat, P. S., Jaiswal, A., Khurana, A., Bhatti, J. S., and Navik, U. (2021). Doxorubicin-induced cardiotoxicity: an update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 139, 111708. doi:10.1016/j.biopha.2021.111708
Sangweni, N. F., Gabuza, K., Huisamen, B., Mabasa, L., van Vuuren, D., and Johnson, R. (2022). Molecular insights into the pathophysiology of doxorubicin-induced cardiotoxicity: a graphical representation. Arch. Toxicol. 96 (6), 1541–1550. doi:10.1007/s00204-022-03262-w
Sawaya, H., Sebag, I. A., Plana, J. C., Januzzi, J. L., Ky, B., Tan, T. C., et al. (2012). Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ. Cardiovasc Imaging 5 (5), 596–603. doi:10.1161/CIRCIMAGING.112.973321
Schneeweiss, A., Chia, S., Hickish, T., Harvey, V., Eniu, A., Hegg, R., et al. (2013). Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 24 (9), 2278–2284. doi:10.1093/annonc/mdt182
Singal, P. K., and Iliskovic, N. (1998). Doxorubicin-induced cardiomyopathy. N. Engl. J. Med. 339 (13), 900–905. doi:10.1056/NEJM199809243391307
Slamon, D., Eiermann, W., Robert, N., Pienkowski, T., Martin, M., Press, M., et al. (2011). Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 365 (14), 1273–1283. doi:10.1056/NEJMoa0910383
Slamon, D. J., Leyland-Jones, B., Shak, S., Fuchs, H., Paton, V., Bajamonde, A., et al. (2001). Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344 (11), 783–792. doi:10.1056/NEJM200103153441101
Swain, S. M., Whaley, F. S., and Ewer, M. S. (2003). Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 97 (11), 2869–2879. doi:10.1002/cncr.11407
Tacar, O., Sriamornsak, P., and Dass, C. R. (2013). Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 65 (2), 157–170. doi:10.1111/j.2042-7158.2012.01567.x
Tian, W. C., Yang, L., Liu, Y. S., He, J., Zhang, Q., Liu, F., et al. (2020). Resveratrol attenuates doxorubicin-induced cardiotoxicity in rats by up-regulation of vascular endothelial growth factor B. J. Nutr. Biochem. 79, 108132. doi:10.1016/j.jnutbio.2019.01.018
Timm, K. N., and Tyler, D. J. (2020). The role of AMPK activation for cardioprotection in doxorubicin-induced cardiotoxicity. Cardiovasc Drugs Ther. 34 (2), 255–269. doi:10.1007/s10557-020-06941-x
van Eck, N. J., and Waltman, L. (2010). Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84 (2), 523–538. doi:10.1007/s11192-009-0146-3
Wallace, K. B., Sardao, V. A., and Oliveira, P. J. (2020). Mitochondrial determinants of doxorubicin-induced cardiomyopathy. CircRes 126 (7), 926–941. doi:10.1161/CIRCRESAHA.119.314681
Wu, B. B., Leung, K. T., and Poon, E. N. (2022). Mitochondrial-targeted therapy for doxorubicin-induced cardiotoxicity. Int. J. Mol. Sci. 23 (3), 1912. doi:10.3390/ijms23031912
Wu, L., Wang, L., Du, Y., Zhang, Y., and Ren, J. (2023). Mitochondrial quality control mechanisms as therapeutic targets in doxorubicin-induced cardiotoxicity. Trends Pharmacol. Sci. 44 (1), 34–49. doi:10.1016/j.tips.2022.10.003
Wu, S., Seiferheld, W., Lukka, H. R., Major, P. P., Heney, N. M., Grignon, D. J., et al. (2017). Radiation with or without antiandrogen therapy in recurrent prostate cancer. N. Engl. J. Med. 376 (5), 417–428. doi:10.1056/NEJMoa1607529
Yang, G. B., Ji, J. S., and Liu, Z. (2021). Multifunctional MnO2 nanoparticles for tumor microenvironment modulation and cancer therapy. Wiley Interdiscip. Rev-Nanomed Nanobiotechnol 13 (6), e1720. doi:10.1002/wnan.1720
Zamorano, J. L., Lancellotti, P., Rodriguez Munoz, D., Aboyans, V., Asteggiano, R., Galderisi, M., et al. (2016). 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 37 (36), 2768–2801. doi:10.1093/eurheartj/ehw211
Zhang, S., Liu, X., Bawa-Khalfe, T., Lu, L. S., Lyu, Y. L., Liu, L. F., et al. (2012). Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 18 (11), 1639–1642. doi:10.1038/nm.2919
Keywords: doxorubicin-induced cardiotoxicity, mechanisms, treatment, develop, bibliometric analysis
Citation: Lin X, Wu G, Wang S and Huang J (2023) Bibliometric and visual analysis of doxorubicin-induced cardiotoxicity. Front. Pharmacol. 14:1255158. doi: 10.3389/fphar.2023.1255158
Received: 08 July 2023; Accepted: 27 October 2023;
Published: 09 November 2023.
Edited by:
Jan Willem Van Der Laan, Medicinse Evaluation Board, NetherlandsReviewed by:
Priyanka Choudhury, Medical College of Wisconsin, United StatesCopyright © 2023 Lin, Wu, Wang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuai Wang, ZHJ3YW5nc2h1YWlAemp1LmVkdS5jbg==; Jinyu Huang, ZHJodWFuZ2ppbnl1QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.