
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 31 October 2023
Sec. Pharmacoepidemiology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1253799
This article is part of the Research TopicPharmacoepidemiology and pharmacovigilance post-marketing drug safety studiesView all 17 articles
Molnupiravir, an urgently approved drug during the Coronavirus Disease 2019 (COVID-19) pandemic, serves as the basis for our study, which relies on the Food and Drug Administration Adverse Event Reporting System (FAERS). The objective is to extract adverse event (AE) signals associated with molnupiravir from the FAERS database, thereby providing a reference for post-marketing monitoring of adverse events. Specifically, we extracted individual case safety reports (ICSRs) from the database, focusing on cases with COVID-19 indications and molnupiravir identified as the primary suspect drug. Descriptive analysis of the extracted data was performed, followed by four disproportionality analyses using the reporting odds ratio (ROR) method. These analyses were conducted across four levels, encompassing overall data, reports by health professionals, as well as age and gender differentiations, ensuring the robustness of the analysis results. In total, 116,576 ICSRs with COVID-19 indications and 2,285 ICSRs with molnupiravir as the primary suspect were extracted. Notably, after excluding cases with unknown age or gender, a higher proportion of molnupiravir-related ICSRs were observed among individuals aged 65 years and older (70.07%) and women (54.06%). The most frequently reported adverse events and AE signals were associated with gastrointestinal disorders, as well as skin and subcutaneous tissue disorders. Moreover, individuals aged 65 years and older exhibited a higher risk of cardiac disorders, hepatobiliary disorders, renal and urinary disorders, and vascular disorders. In conclusion, this study found molnupiravir demonstrated a lower risk of serious adverse events compared to other RNA antiviral drugs like remdesivir in patients under 65 years old. However, close monitoring of its safety is still necessary for elderly patients aged 65 years and above. Further studies are warranted to continuously assess the safety profile of molnupiravir as its usage increases, especially in high risk populations.
The Coronavirus Disease 2019 (COVID-19) pandemic caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is a highly infectious disease, with a rapid person-to-person transmission rate. According to the WHO Coronavirus (COVID-19) Dashboard, as of 10 February 2023, there have been 760 million confirmed cases of COVID-19 worldwide, resulting in 6.86 million deaths (World Health Organization, 2023). COVID-19 can range from asymptomatic to severe respiratory failure and multi-organ involvement (Huang et al., 2020; Wang et al., 2020). The major symptoms of COVID-19 include fever, cough, and dyspnea (De Vito et al., 2021; Cascella et al., 2023). The minor symptoms are less specific and require comprehensive evaluation. They include anosmia/dysgeusia, headache, diarrhea, vomiting, nausea, sore throat, fatigue, malaise, myalgia, etc (Cheung et al., 2020; Grant et al., 2020; Mao et al., 2020; Vaira et al., 2020). Skin lesions such as vasculitis-like skin eruption can also be seen in COVID-19 patients (Geremia et al., 2020). Although vaccination has significantly reduced the risk of morbidity and mortality (Polack et al., 2020), breakthrough infections remain a concern. Before molnupiravir, remdesivir was the first antiviral against SARS-CoV-2, sharing the same target as molnupiravir. Initially, remdesivir was administered to patients with severe pneumonia and illness (De Vito et al., 2022), but following the PINE-TREE trial, it was also recommended for mild cases to prevent progression (Gottlieb et al., 2022). However, remdesivir requires intravenous infusion, making an oral alternative highly desirable.
Targeting the RNA-dependent RNA polymerase (RdRp), which is a crucial enzyme for SARS-CoV-2 replication, has been proven to be an effective strategy to combat COVID-19, regardless of the variant type. This is because RdRps are widely conserved among all SARS-CoV-2 strains (Rabie, 2022). Molnupiravir is a biologically active prodrug of b-D-N4-hydroxycytidine (NHC, EIDD-1931) that targets RdRp. Upon oral administration, molnupiravir is quickly converted into active NHC in plasma and distributed to various organs. Host kinases then convert it into NHC 5′-triphosphate. This NHC 5′-triphosphate can serve as a competitive alternative substrate for viral RdRp, which integrates into viral RNA and causes the accumulation of mutations in the viral genome, ultimately leading to lethal mutations (Tian et al., 2022). In the MOVE-OUT trial, a phase 3, double-blind, randomized, placebo-controlled trial, molnupiravir demonstrated a 31% relative risk reduction in all-cause mortality compared to placebo. Additionally, the proportion of participants experiencing at least one adverse event was similar in both the molnupiravir and placebo groups, indicating its safety (Jayk Bernal et al., 2022). Being a potential therapeutic option, molnupiravir received emergency use authorization from the Food and Drug Administration (FDA) on 23 December 2021, for the management of mild-to-moderate COVID-19 in adults who are at a heightened risk of developing severe illness (CDER Division of Drug Information, 2023). In addition to the United States, molnupiravir was granted approval in the United Kingdom in November 2021. Furthermore, it received special emergency approval in Japan on 24 December 2021 (Medicines and Healthcare products Regulatory Agency, 2021; Merck & Co, I, 2021). However, the PANORAMIC trial showed no benefits of molnupiravir over placebo in reducing mortality or hospitalization duration for hospitalized COVID-19 patients (Butler et al., 2023). Additionally, the European Medicines Agency’s controversial withdrawal of molnupiravir’s regulatory application (EMA, 2023) has raised doubts about its efficacy. While the efficacy of molnupiravir remains questionable given these latest trial results and regulatory decisions, some advantages of molnupiravir’s safety profile should be considered. Specifically, unlike other antiviral options like remdesivir and nirmatrelvir, molnupiravir has demonstrated a lower potential for clinically significant drug-drug interactions (DDIs) so far (Wanounou et al., 2022; Akhvlediani et al., 2023). The low DDI risk makes molnupiravir an easier oral therapy to administer alongside other medications patients may be taking. Therefore, if the efficacy concerns can be adequately addressed with additional studies, molnupiravir’s favorable DDI profile could position it as an alternative oral antiviral option, especially for patients on polypharmacy regimens.
Molnupiravir has not only demonstrated a comparable incidence of adverse reactions in both the molnupiravir and placebo groups during the MOVE-OUT trial but also consistently across all current clinical trials at doses of 200 mg, 400 mg, and 800 mg (Caraco et al., 2022; Fischer et al., 2022; Sinha et al., 2022; Tippabhotla et al., 2022; Zou et al., 2022; Butler et al., 2023; Khoo et al., 2023). Moreover, systematic reviews and meta-analyses suggest that molnupiravir may represent a safe and effective treatment option for patients with COVID-19 (Joseph Mari and Erwin, 2022; Mali et al., 2022; Pitre et al., 2022). Although current clinical trials and systematic reviews indicate that molnupiravir appears to have a favorable safety profile, it is important to note that the safety outcomes in these trials are typically assessed over a limited period of 28–29 days. Therefore, there is still a lack of comprehensive real-world research and post-marketing monitoring for this drug. To address this critical gap, our study aims to gather individual case safety reports associated with molnupiravir from the FDA Adverse Event Reporting System (FAERS) database. By analyzing these reports, we intend to identify potential adverse drug reactions associated with molnupiravir and compare them with other SARS-CoV-2 RNA drugs such as remdesivir, ribavirin, favipiravir, and azvudine. This study aims to contribute to the post-marketing monitoring of molnupiravir and provide valuable insights for its clinical use.
FAERS is a computerized database specifically designed for the spontaneous reporting of adverse events and medication errors involving human drugs and therapeutic biological products. The data structure of FAERS adheres to the International Council for Harmonisation (ICH) guidelines for international safety reporting. Adverse events and therapeutic indications are coded at the “preferred term” (PT) level using the Medical Dictionary for Regulatory Activities (MedDRA).
Access to FAERS data is provided through quarterly data files, which can be obtained from the following link: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html. These files are available in two distinct formats: ASCII files and XML files. For our study, we downloaded FAERS data in ASCII format, covering the period from January 2020 to December 2022. We managed and analyzed the data using Microsoft SQL Server 2019 software. Since the study was an analysis of the third party anonymized publicly available database with pre-existing institutional review board (IRB) approval, IRB approval was exempted by the institutional ethics board of The first Affiliated Hospital Of Jinan University.
To prevent the duplication of multiple report versions, we conducted a deduplication process on the DEMO table. Firstly, we removed identical records, keeping only one instance. Next, if the “CASEID” column was the same, we deleted the duplicate “PRIMARYID” column with the lower value. Additionally, if multiple rows had the same “PRIMARYID” values, we eliminated the earliest “FDA_DT” column to ensure data consistency (Silva et al., 2021).
In order to investigate the adverse event (AE) signals of molnupiravir in COVID-19 prevention and treatment, we utilized the following narrow Standardized MedDRA Query (SMQ) within the “INDI” table: COVID-19, COVID-19 pneumonia, COVID-19 immunization, COVID-19 prophylaxis, COVID-19 treatment, Suspected COVID-19, Asymptomatic COVID-19, SARS-CoV-2 test positive, SARS-CoV-2 carrier, SARS-CoV-2 test false negative, SARS-CoV-2 antibody test positive, SARS-CoV-2 sepsis, SARS-CoV-2 viremia, Occupational exposure to SARS-CoV-2, Exposure to SARS-CoV-2, Coronavirus infection, Coronavirus test positive, Multisystem inflammatory syndrome in children. These queries were employed to extract reports involving COVID-19 from the FAERS database (Wu et al., 2022).
We proceeded to identify cases in the “DRUG” table where the “drugname” and “prod_ai” columns aligned with the regular expression "%MOLNUPIRAVIR%" or "% LAGEVIRIO%", and the “role_cod” column matched the regular expression “PS”. These cases represented instances where molnupiravir was administered.
Initially, we examined the attributes of the included Individual Case Safety Reports (ISCRs), which encompassed age, gender, reporter type, reporting country, serious outcomes and dosage. The serious outcomes evaluated encompassed death, life-threatening conditions, interventions, disabilities, congenital anomalies, hospitalizations, and other significant events.
We proceeded with a detailed analysis of the attributes of the ISCRs. Furthermore, a disproportionality analysis was conducted to detect any potential AE signals. To ensure the reliability of the findings, separate disproportionality analyses were performed based on patient age and sex. Additionally, a sensitivity analysis was conducted by restricting the analysis to reports from healthcare professionals as the reporters. In our study, adverse event signals such as “product use issue,” “no adverse event,” “wrong technique in product usage process,” “COVID-19”and similar cases were excluded to enhance the reliability of the findings.
The descriptive statistical analysis encompassed patient age, gender, reporter type, reporting country, dosage and the outcomes of adverse events. The variables are presented as frequencies and percentages.
For the disproportionality analysis, we employed the reporting odds ratio (ROR) method and conducted calculations using a 2-by-2 contingency table (Table 1). In this analysis, reports where molnupiravir was suspected as the causative drug were classified in the target drug group, while reports without molnupiravir as the suspected drug were included in the other drug group. A risk signal was deemed significant if the total number of drugs and adverse events was three or more, and the lower limit of the 95% confidence interval (CI) for the ROR exceeded 1 (Jung et al., 2021; Kim et al., 2021; Rocca et al., 2021).
A comprehensive analysis was conducted on a dataset of 4,747,645 individual case safety reports (ICSRs) from the FAERS database, spanning January 2020 to December 2022, after removing duplicates. Among them, 116,576 ICSRs indicated COVID-19. For the analysis, 2,285 ICSRs were included in the molnupiravir group, while 114,291 ICSRs were assigned to the other drugs group. More information on ICSR identification can be found in Figure 1.
The characteristics of the 116,576 ICSRs are presented in Table 2. Notably, the molnupiravir group exhibited a higher proportion (60.96%) of individuals aged 65 years and older, compared to the other drugs group (27.42%). The male-to-female ratio was 0.85 in the molnupiravir group and 0.75 in the other drugs group. Assessing outcomes, the other drugs group reported a higher incidence of life-threatening events (3.40% vs. 2.19%), interventions (0.94% vs. 0.70%), and hospitalizations (20.14% vs. 19.65%) compared to the molnupiravir group. Conversely, the molnupiravir group reported a higher rate of deaths (12.12% vs. 6.99%) compared to the other drugs group. The top 20 drugs involved in adverse events in the other drugs group are summarized in Figure 2.
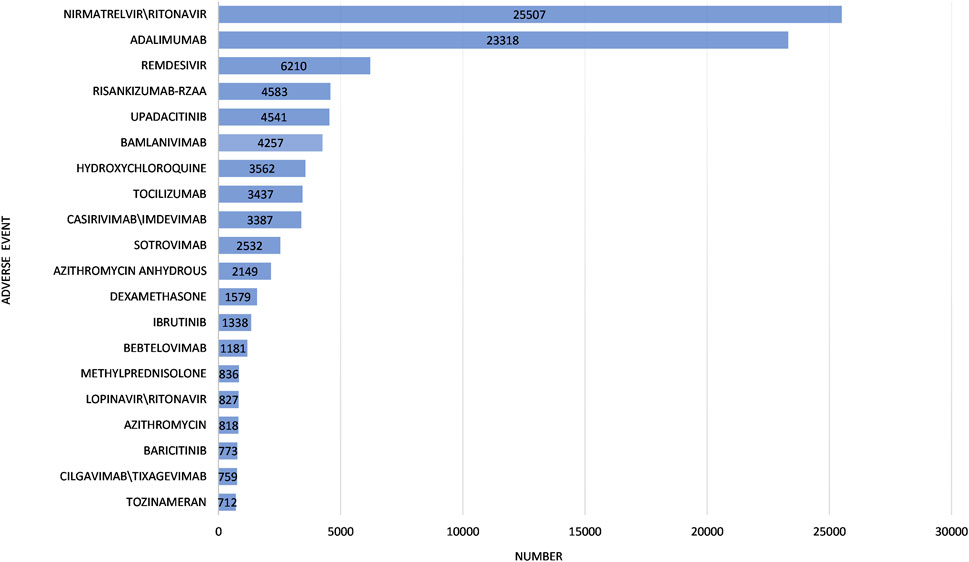
FIGURE 2. Top 20 drugs involved in adverse events in the other Coronavirus Disease 2019 drugs group.
The molnupiravir group included 2,285 individual safety case reports (ISCRs) encompassing a total of 4,888 adverse events, while the non-molnupiravir group comprised 114291 ISCRs involving 374575 adverse events. We identified the top 15 AEs based on occurrence and deaths. The most common AEs were Diarrhoea (142, 2.91%), Rash (125, 2.56%), Nausea (97, 1.98%), Dizziness (83, 1.70%) and Vomiting (80, 1.64%); AEs leading to death included, Pneumonia aspiration (23, 0.47%), Respiratory failure (21, 0.43%), Pneumonia (16, 0.33%), Diarrhoea (13, 0.27%) and Dysphagia (11, 0.23%) (Figure 3).
The molnupiravir group comprised 2,285 ICSRs. Using the ROR method, 74 AE signals were identified and classified into 18 System Organ Classes (SOCs). The top 20 AE signals at the preferred term PT level are shown in Table 3. They cover 9 SOCs, with skin/subcutaneous tissue disorders and gastrointestinal disorders (GI) being the most common.
According to the drug labels, the most common adverse events associated with molnupiravir use are diarrhea, nausea, and dizziness, which align with the findings from our analysis (MSD LLC, 2023). These adverse events have been included in our monitoring list. In addition to the adverse events mentioned in the drug labels, we have detected 71 AE signals that are not specifically mentioned.
To ensure reliability, we extracted ICSRs reported by health professionals (physicians, pharmacists, and other healthcare professionals) from the initial pool of 2,285 ICSRs associated with molnupiravir. The health professional group consisted of 1,830 ICSRs related to molnupiravir. Using the ROR method, we identified 52 AE signals in the health professional group. The top 20 AE signals at the PT level are presented in Table 4.
Of the 2,285 ICSRs associated with molnupiravir, 2,042 reported known age and were divided into <65 years and ≥65 years groups. The remaining 243 ICSRs had unknown age. The <65 years group had 595 ICSRs, while the ≥65 years group had 1,393 ICSRs. Using the ROR method, we identified 30 AE signals in the <65 years group and 56 AE signals in the ≥65 years group. It is worth noting that only five signals were common among the top 20 signals in the two age groups. According to Common Terminology Criteria for Adverse Events (CTCAE), in <65 years group, 5 PTs were Grade 3 and 2 PTs were Grade 5 among top 20 adverse events at PT level. In ≥65 years group, 1 PT was Grade 3 and 5 PTs were Grade 5, excluding those not covered by CTCAE (U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES, 2017). Overall, adverse events appeared to be more severe in the <65 years group (Figure 4).
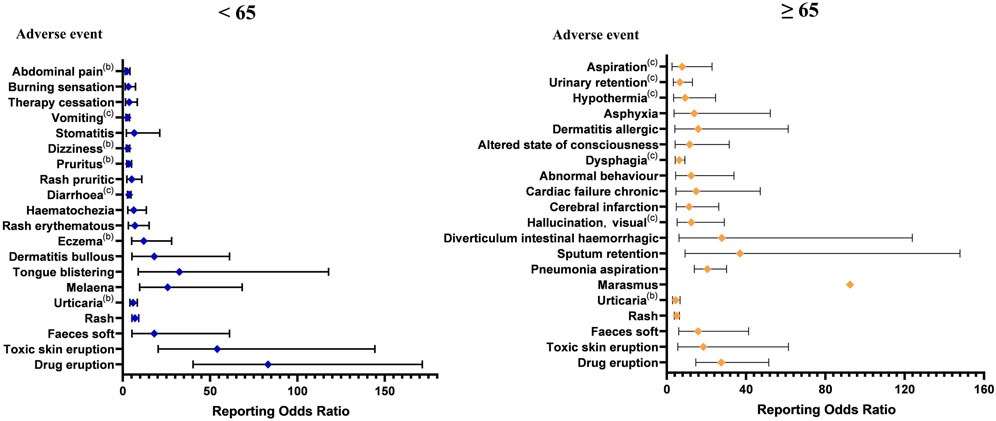
FIGURE 4. The top 20 adverse event signals at the preferred term level base on age. (a The adverse event is up to level 2 in Common Terminology Criteria for Adverse Events. b The adverse event is up to level 3 in Common Terminology Criteria for Adverse Events. c The adverse event is up to level 5 in Common Terminology Criteria for Adverse Events.).
In addition to age sensitivity analysis, we also conducted a sex sensitivity analysis. Of the 2,285 ICSRs associated with molnupiravir, 2,107 reported known gender and were divided into female (1,139 ICSRs) and male (968 ICSRs) groups. The remaining 178 ICSRs had unknown gender. Using ROR, we found 54 AE signals in the female group and 43 AE signals in the male group. RORs with 95% CIs were calculated for each group, and the top 20 adverse drug event signals at the PT level are listed in Figure 5.
It is noteworthy that marasmus (ROR: 408.07, 95% CI: 47.66–3494.22) emerged as the strongest adverse drug event signal in the female group, but it did not appear among the top 20 adverse drug event signals in the male group. The mean time to marasmus onset was 1.75 ± 1.71 days in the molnupiravir group and 16.50 ± 10.60 days in the other COVID-19 drugs group, p = 0.001.
Molnupiravir, an RNA drug targeting SARS-CoV-2, is being utilized on an emergency basis in numerous countries for the treatment of COVID-19. Various studies have suggested that molnupiravir could be a secure therapeutic option to lower hospitalizations and/or mortality rates among nonhospitalized individuals with COVID-19 (Gao et al., 2023). However, the presence of certain unknown concerns, including teratogenicity, carcinogenicity, mutation, and potential bone and cartilage toxicity, cannot be overlooked (Focosi, 2022). Currently, there is a scarcity of studies investigating the long-term toxicity of molnupiravir. Most of these studies are prospective in nature and have small sample sizes, while there is a lack of retrospective studies with large sample cohorts. In our research, we conducted the first largescale retrospective study utilizing the FAERS database to examine adverse events associated with molnupiravir. Our findings indicate that molnupiravir is associated with an elevated likelihood of gastrointestinal disorders and skin and subcutaneous tissue disorders as adverse events. Moreover, we observed a significant increase in the risk of AEs related to cardiac disorders, hepatobiliary disorders, renal and urinary disorders, and vascular disorders among individuals aged 65 years and older. However, there was no statistically significant difference observed between males and females.
The characteristics of individual safety reports linked to molnupiravir highlight that individuals aged 65 years and older account for over 60% (60.96%) of the reported cases. This observation may be attributed to the approved usage scope of molnupiravir, such as in the United States where LAGEVRIO™ (molnupiravir) is an investigational medicine for treating mild to moderate COVID-19 in adults who have a positive result from a direct-to-consumer SARS-CoV-2 virus test and are at risk of progressing to severe COVID-19. These individuals are typically unable to access or receive clinically suitable FDA-approved or licensed COVID-19 treatment options. Adolescents, on the other hand, tend to possess robust immune systems and generally experience milder illness following severe acute respiratory syndrome coronavirus 2 infection. Additionally, elderly individuals and those at higher risk often receive chronic treatments that may interact with Nirmatrelvir/ritonavir, making molnupiravir a more suitable option for them. The substantial number of elderly individuals using molnupiravir has contributed to the multitude of individual case safety reports, which is one of the factors we suspect. Diarrhea, nausea, and dizziness are the most frequently reported adverse drug events associated with molnupiravir treatment in most clinical trials, aligning closely with the outcomes of our analysis on the common adverse events linked to molnupiravir usage (Sinha et al., 2022). This study validates the effectiveness of utilizing a data mining method for detecting AE signals, making it a valuable reference for clinical applications. However, it should be noted that diarrhea and rash can also manifest as common symptoms of COVID-19 itself, making it difficult to ascertain whether they are truly drug-related adverse events or merely symptoms of the viral infection.
The results of the disproportionality analysis for the molnupiravir group and health professional group showed that adverse events mainly linked to molnupiravir were related to GI disorders and skin/subcutaneous tissue disorders. We found 71 adverse event signals not mentioned in the drug label, with the majority involving skin/subcutaneous tissue disorders. In a phase I, randomized, placebo-controlled study with healthy Japanese participants, toxic skin eruption was the most frequently reported adverse event associated with molnupiravir use (Nakamura et al., 2022). In another trial, a similar observation was made where discontinuation of the treatment occurred due to a rash in one participant (Painter et al., 2020). Skin and subcutaneous tissue disorder adverse events were infrequent in some COVID-19 patient studies. However, the ethnic sensitivity of these findings remains unclear due to the limited scale of our study. Continuous surveillance is crucial to further investigate and monitor these aspects effectively.
Close monitoring of individuals aged ≥65 years is recommended to mitigate serious adverse reactions with molnupiravir. Among the AE signals, 30 were observed in the <65 years group, while 56 were reported in the ≥65 years group, involving 9 SOCs and 14 SOCs, respectively. Notably, the four SOCs related to cardiac disorders, hepatobiliary disorders, renal and urinary disorders, and vascular disorders were exclusively present in the ≥65 years group. These SOCs are associated with more severe PTs not mentioned in the drug labels, such as chronic cardiac failure, abnormal hepatic function, urinary retention, and hypertensive crisis. Additionally, certain clinical trials have reported serious adverse events like cardiac chest pain, haematuria, hypertension, and increased transaminase levels, which are not included in the molnupiravir drug labels (Painter et al., 2020; Fischer et al., 2022). Therefore, increased surveillance of cardiotoxicity, nephrotoxicity, hepatotoxicity, and vascular disease is warranted in elderly patients. Additionally, notable differences exist between the two age groups in the respiratory, thoracic, and mediastinal disorders system. To enhance clarity, we created a heat map (Figure 6). The ≥65 years group showed more signals in respiratory, thoracic, and mediastinal disorders, with the strongest signal being sputum retention (ROR: 36.97, 95% CI: 9.24–147.88). Hence, middle-aged and elderly individuals may require heightened monitoring of adverse events, particularly for cardiac disorders, hepatobiliary disorders, renal and urinary disorders, vascular disorders, and the respiratory, thoracic, and mediastinal disorders system. We also found females ≥65 years old had a higher risk of developing marasmus. Possible mechanisms include appetite and metabolic changes, and direct drug toxicity. Further studies on pathophysiology are warranted. In the interim, clinicians should closely monitor nutritional status and wasting in molnupiravir patients, especially elderly females in the first week. Prompt nutrition and physical therapy support may mitigate progression. The risk of rapid severe marasmus onset should be considered when evaluating molnupiravir’s risk-benefit profile.
Molnupiravir has demonstrated excellent safety as an SARS-CoV-2 RNA drug. In comparison, other SARS-CoV-2 RNA drugs have exhibited distinct adverse events in pharmacovigilance studies based on real FAERS database. Remdesivir has shown a significant association with acute kidney injury, with the top three adverse events being elevated liver function test, acute kidney injury, and death. The ribavirin-interferon combination has been linked to an increased risk of anemia, vomiting, neutropenia, diarrhea, and insomnia. Favipiravir has shown side effects such as QTC prolongation, hyperuricemia, abnormalities in liver enzymes, elevation of uric acid, total bilirubin, and liver enzymes, along with gastrointestinal disorders in clinical trials. Conversely, no adverse events were reported in the azvudine group in a clinical trial (Ren et al., 2020; Shan et al., 2020; Wu et al., 2022; Alsuhaibani et al., 2023; Batool et al., 2023). In addition, clinical trials have indicated that Molnupiravir is associated with fewer side effects compared to nirmatrelvir/ritonavir (Mazzitelli et al., 2023). Although GI disorders and skin/subcutaneous tissue disorders are the most common AEs related to molnupiravir, it is essential to enhance safety monitoring in older and medically vulnerable individuals.
Our study has limitations. Firstly, as a spontaneous reporting system, the FAERS database lacks information on patients’ baseline characteristics (e.g., age, gender, BMI), onset timing AE severity and so on. These factors limit the predictive ability and detail of our analysis. Secondly, the substantially larger sample size of the non-molnupiravir group compared to the molnupiravir group may lead to baseline imbalance that cannot be readily addressed due to the inherent data source discrepancy. We mention that this could be a limitation in result interpretation. Furthermore, FAERS does not contain information on patient ethnicity, making it impossible to analyze potential differences in adverse events between populations such as Japanese versus Caucasians. The lack of ethnicity data is an important limitation, as previous studies have shown pharmacokinetic differences between ethnic groups (Shimada et al., 1994). In addition, this study did not evaluate the impacts of COVID-19 co-infections with other viruses and medication interactions on adverse events due to data limitations. The lack of COVID-19 co-infection and drug interaction data represents another constraint of this analysis. Lastly, the calculation of the reporting odds ratio (ROR) is sensitive to individual values, and it may be unreliable when one of the theoretical frequencies in the 2 × 2 contingency table is small or the denominator is 0.
This pharmacovigilance study used the real FAERS database to assess adverse event risks associated with molnupiravir therapy in COVID-19 patients. However, further clinical studies are needed for confirmation.
Overall, besides common gastrointestinal disorders, skin and subcutaneous tissue disorders were also prevalent. The study found that compared to other RNA antiviral drugs such as remdesivir, molnupiravir demonstrated a lower risk of serious adverse events in individuals under 65 years old. Nonetheless, closer monitoring of drug safety is necessary for individuals ≥65 years old. The adverse events reported in FAERS data align with most clinical trial findings. Ongoing monitoring is vital as the use of molnupiravir increases, enabling a more comprehensive understanding of its safety profile. These findings provided invaluable real-world data for post-marketing surveillance of molnupiravir and impor€tant guidance for its future clinical use.
Publicly available datasets were analyzed in this study. This data can be found here: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
YL: Methodology, Data curation, Formal Analysis, Investigation, Software, Writing–original draft. LM: Writing–original draft, Conceptualization, Validation. YW: Formal Analysis, Software, Writing–review and editing. JZ: Formal Analysis, Software, Writing–review and editing. LS: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Writing–review and editing. JL: Formal Analysis, Funding acquisition, Methodology, Resources, Validation, Writing–review and editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Akhvlediani, T., Bernard-Valnet, R., Dias, S. P., Eikeland, R., Pfausler, B., Sellner, J., et al. (2023). Neurological side effects and drug interactions of antiviral compounds against SARS-CoV-2. Eur. J. Neurol. doi:10.1111/ene.16017
Alsuhaibani, D. S., Edrees, H. H., and Alshammari, T. M. (2023). The use and safety risk of repurposed drugs for COVID-19 patients: lessons learned utilizing the Food and drug administration’s adverse event reporting system. Saudi Pharm. J. 31, 1360–1366. doi:10.1016/j.jsps.2023.05.023
Batool, S., Vuthaluru, K., Hassan, A., Bseiso, O., Tehseen, Z., Pizzorno, G., et al. (2023). Efficacy and safety of favipiravir in treating COVID-19 patients: a meta-analysis of randomized control trials. Cureus 15 (1), e33676. doi:10.7759/cureus.33676
Butler, C. C., Hobbs, F. D. R., Gbinigie, O. A., Rahman, N. M., Hayward, G., Richards, D. B., et al. (2023). Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet 401 (10373), 281–293. doi:10.1016/s0140-6736(22)02597-1
Caraco, Y., Crofoot, G. E., Moncada, P. A., Galustyan, A. N., Musungaie, D. B., Payne, B., et al. (2022). Phase 2/3 trial of molnupiravir for treatment of covid-19 in nonhospitalized adults. NEJM Evid. 1 (2). doi:10.1056/EVIDoa2100043
Cascella, M., Rajnik, M., Aleem, A., Dulebohn, S. C., and Di Napoli, R. (2023). “Features, evaluation, and treatment of coronavirus (COVID-19),” in StatPearls (Treasure Island (FL): StatPearls Publishing). Copyright 2023.
CDER Division of Drug Information (2023). Coronavirus (COVID-19) | drugs. Available: https://www.fda.gov/drugs/emergency-preparedness-drugs/coronavirus-covid-19-drugs (Accessed March 9, 2023).
Cheung, K. S., Hung, I. F. N., Chan, P. P. Y., Lung, K. C., Tso, E., Liu, R., et al. (2020). Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology 159 (1), 81–95. doi:10.1053/j.gastro.2020.03.065
De Vito, A., Fiore, V., Princic, E., Geremia, N., Panu Napodano, C. M., Muredda, A. A., et al. (2021). Predictors of infection, symptoms development, and mortality in people with SARS-CoV-2 living in retirement nursing homes. PLoS One 16 (3), e0248009. doi:10.1371/journal.pone.0248009
De Vito, A., Poliseno, M., Colpani, A., Zauli, B., Puci, M. V., Santantonio, T., et al. (2022). Reduced risk of death in people with SARS-CoV-2 infection treated with remdesivir: a nested case-control study. Curr. Med. Res. Opin. 38 (12), 2029–2033. doi:10.1080/03007995.2022.2129801
EMA (2023). Lagevrio: withdrawal of the marketing authorisation application. Available: https://www.ema.europa.eu/en/medicines/human/withdrawn-applications/lagevrio (Accessed September 10, 2023).
Fischer, W. A., Eron, J. J., Holman, W., Cohen, M. S., Fang, L., Szewczyk, L. J., et al. (2022). A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci. Transl. Med. 14(628), eabl7430. doi:10.1126/scitranslmed.abl7430
Focosi, D. (2022). Molnupiravir: from hope to epic fail? Viruses 14 (11), 2560. doi:10.3390/v14112560
Gao, Y., Liu, M., Li, Z., Xu, J., Zhang, J., and Tian, J. (2023). Molnupiravir for treatment of adults with mild or moderate COVID-19: a systematic review and meta-analysis of randomized controlled trials. Clin. Microbiol. Infect. 29, 979–999. doi:10.1016/j.cmi.2023.04.014
Geremia, N., De Vito, A., Gunnella, S., Fiore, V., Princic, E., Panu Napodano, C., et al. (2020). A case of vasculitis-like skin eruption associated with COVID-19. Infect. Dis. Clin. Pract. 28 (6), e30–e31. doi:10.1097/ipc.0000000000000952
Gottlieb, R. L., Vaca, C. E., Paredes, R., Mera, J., Webb, B. J., Perez, G., et al. (2022). Early remdesivir to prevent progression to severe covid-19 in outpatients. N. Engl. J. Med. 386 (4), 305–315. doi:10.1056/NEJMoa2116846
Grant, M. C., Geoghegan, L., Arbyn, M., Mohammed, Z., McGuinness, L., Clarke, E. L., et al. (2020). The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): a systematic review and meta-analysis of 148 studies from 9 countries. PLoS One 15 (6), e0234765. doi:10.1371/journal.pone.0234765
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395 (10223), 497–506. doi:10.1016/s0140-6736(20)30183-5
Jayk Bernal, A., Gomes da Silva, M. M., Musungaie, D. B., Kovalchuk, E., Gonzalez, A., Delos Reyes, V., et al. (2022). Molnupiravir for oral treatment of covid-19 in nonhospitalized patients. N. Engl. J. Med. 386 (6), 509–520. doi:10.1056/NEJMoa2116044
Joseph Mari, B. Q., and Erwin, M. F. (2022). A mini-review on clinical studies of Molnupiravir for COVID-19 conducted in selected Asian countries. GSC Biol. Pharm. Sci. 18 (3), 126–129. doi:10.30574/gscbps.2022.18.3.0105
Jung, S. Y., Kim, M. S., Li, H., Lee, K. H., Koyanagi, A., Solmi, M., et al. (2021). Cardiovascular events and safety outcomes associated with remdesivir using a World Health Organization international pharmacovigilance database. Clin. Transl. Sci. 15 (2), 501–513. doi:10.1111/cts.13168
Khoo, S. H., FitzGerald, R., Saunders, G., Middleton, C., Ahmad, S., Edwards, C. J., et al. (2023). Molnupiravir versus placebo in unvaccinated and vaccinated patients with early SARS-CoV-2 infection in the UK (AGILE CST-2): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Infect. Dis. 23 (2), 183–195. doi:10.1016/S1473-3099(22)00644-2
Kim, M. S., Jung, S. Y., Lee, S. W., Li, H., Koyanagi, A., Kronbichler, A., et al. (2021). Hepatobiliary adverse drug reactions associated with remdesivir: the WHO international pharmacovigilance study. Clin. Gastroenterology Hepatology 19 (9), 1970–1972.e3. doi:10.1016/j.cgh.2021.04.039
Mali, K. R., Eerike, M., Raj, G. M., Bisoi, D., Priyadarshini, R., Ravi, G., et al. (2022). Efficacy and safety of Molnupiravir in COVID-19 patients: a systematic review. Ir. J. Med. Sci. 192, 1665–1678. doi:10.1007/s11845-022-03139-y
Mao, L., Jin, H., Wang, M., Hu, Y., Chen, S., He, Q., et al. (2020). Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, China. JAMA Neurol. 77 (6), 683–690. doi:10.1001/jamaneurol.2020.1127
Mazzitelli, M., Mengato, D., Sasset, L., Ferrari, A., Gardin, S., Scaglione, V., et al. (2023). Molnupiravir and nirmatrelvir/ritonavir: tolerability, safety, and adherence in a retrospective cohort study. Viruses 15 (2), 384. doi:10.3390/v15020384
Medicines and Healthcare products Regulatory Agency (2021). First oral antiviral for COVID-19, Lagevrio (molnupiravir), approved by MHRA. Available: https://www.gov.uk/government/news/first-oral-antiviral-for-covid-19-lagevrio-molnupiravir-approved-by-mhra (Accessed March 9, 2023).
Merck and Co, I (2021). Merck and ridgeback’s molnupiravir, an investigational oral antiviral COVID-19 treatment, receives special approval for emergency in Japan - Merck.com. Available: https://www.merck.com/news/merck-and-ridgebacks-molnupiravir-an-investigational-oral-antiviral-covid-19-treatment-receives-special-approval-for-emergency-in-japan/(Accessed March 9, 2023).
MSD LLC (2023). FACT SHEET FOR HEALTHCARE PROVIDERS: EMERGENCY USE AUTHORIZATION FOR LAGEVRIO™ (molnupiravir) CAPSULES. Available: https://www.accessdata.fda.gov/spl/data/14c88fa1-7205-49d6-9ccb-0800dd2978fd/14c88fa1-7205-49d6-9ccb-0800dd2978fd.xml (Accessed March 9, 2023).
Nakamura, K., Fujimoto, K., Hasegawa, C., Aoki, I., Yoshitsugu, H., Ugai, H., et al. (2022). A phase I, randomized, placebo-controlled study of molnupiravir in healthy Japanese to support special approval in Japan to treat COVID-19. Clin. Transl. Sci. 15 (11), 2697–2708. doi:10.1111/cts.13395
Pitre, T., Van Alstine, R., Chick, G., Leung, G., Mikhail, D., Cusano, E., et al. (2022). Antiviral drug treatment for nonsevere COVID-19: a systematic review and network meta-analysis. CMAJ 194 (28), E969–E980. doi:10.1503/cmaj.220471
Polack, F. P., Thomas, S. J., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., et al. (2020). Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 383 (27), 2603–2615. doi:10.1056/NEJMoa2034577
Rabie, A. M. (2022). Efficacious preclinical repurposing of the nucleoside analogue didanosine against COVID-19 polymerase and exonuclease. ACS Omega 7 (25), 21385–21396. doi:10.1021/acsomega.1c07095
Ren, Z., Luo, H., Yu, Z., Song, J., Liang, L., Wang, L., et al. (2020). A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv. Sci. (Weinh) 7 (19), e2001435. doi:10.1002/advs.202001435
Rocca, E., Gauffin, O., Savage, R., Vidlin, S. H., and Grundmark, B. (2021). Remdesivir in the COVID-19 pandemic: an analysis of spontaneous reports in VigiBase during 2020. Drug Saf. 44 (9), 987–998. doi:10.1007/s40264-021-01091-x
Shan, W., Hong, D., Zhu, J., and Zhao, Q. (2020). Assessment of the potential adverse events related to ribavirin-interferon combination for novel coronavirus therapy. Comput. Math. Methods Med. 2020, 1391583. doi:10.1155/2020/1391583
Shimada, T., Yamazaki, H., Mimura, M., Inui, Y., and Guengerich, F. P. (1994). Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J. Pharmacol. Exp. Ther. 270 (1), 414–423.
Silva, N. A. O., Zara, A., Figueras, A., and Melo, D. O. (2021). Potential kidney damage associated with the use of remdesivir for COVID-19: analysis of a pharmacovigilance database. Cad. Saude Publica 37 (10), e00077721. doi:10.1590/0102-311X00077721
Sinha, S., N, K., Suram, V. K., Chary, S. S., Naik, S., Singh, V. B., et al. (2022). Efficacy and safety of molnupiravir in mild COVID-19 patients in India. Cureus 14 (11), e31508. doi:10.7759/cureus.31508
Tian, L., Pang, Z., Li, M., Lou, F., An, X., Zhu, S., et al. (2022). Molnupiravir and its antiviral activity against COVID-19. Front. Immunol. 13, 855496. doi:10.3389/fimmu.2022.855496
Tippabhotla, S. K., Lahiri, D. S., and Kandi, C. (2022). Efficacy and safety of molnupiravir for the treatment of non-hospitalized adults with mild COVID-19: a randomized, open-label, parallel-group phase 3 trial. Available at https://ssrn.com/abstract=4042673.
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES (2017). Common Terminology Criteria for adverse events (CTCAE). National Cancer Institute.
Vaira, L. A., Hopkins, C., Salzano, G., Petrocelli, M., Melis, A., Cucurullo, M., et al. (2020). Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head. Neck 42 (7), 1560–1569. doi:10.1002/hed.26269
Wang, D., Hu, B., Hu, C., Zhu, F., Liu, X., Zhang, J., et al. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. Jama 323 (11), 1061–1069. doi:10.1001/jama.2020.1585
Wanounou, M., Caraco, Y., Levy, R. H., Bialer, M., and Perucca, E. (2022). Clinically relevant interactions between ritonavir-boosted nirmatrelvir and concomitant antiseizure medications: implications for the management of COVID-19 in patients with epilepsy. Clin. Pharmacokinet. 61 (9), 1219–1236. doi:10.1007/s40262-022-01152-z
World Health Organization (2023). WHO coronavirus (COVID-19) dashboard. Available: https://covid19.who.int (Accessed March 2, 2023).
Wu, B., Luo, M., Wu, F., He, Z., Li, Y., and Xu, T. (2022). Acute kidney injury associated with remdesivir: a comprehensive pharmacovigilance analysis of COVID-19 reports in FAERS. Front. Pharmacol. 13, 692828. doi:10.3389/fphar.2022.692828
Zou, R., Peng, L., Shu, D., Zhao, L., Lan, J., Tan, G., et al. (2022). Antiviral efficacy and safety of molnupiravir against omicron variant infection: a randomized controlled clinical trial. Front. Pharmacol. 13, 939573. doi:10.3389/fphar.2022.939573
Keywords: molnupiravir, pharmacovigilance, coronavirus disease 2019, food and drug administration adverse event reporting system, adverse events, safety
Citation: Liang Y, Ma L, Wang Y, Zheng J, Su L and Lyu J (2023) Adverse events associated with molnupiravir: a real-world disproportionality analysis in food and drug administration adverse event reporting system. Front. Pharmacol. 14:1253799. doi: 10.3389/fphar.2023.1253799
Received: 06 July 2023; Accepted: 17 October 2023;
Published: 31 October 2023.
Edited by:
Yusuf Karatas, Çukurova University, TürkiyeReviewed by:
Sandhiya Selvarajan, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), IndiaCopyright © 2023 Liang, Ma, Wang, Zheng, Su and Lyu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Lyu, bHl1anVuMjAyMEBqbnUuZWR1LmNu; Ling Su, MzgxMDU1OTZAMTYzLmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.