Background: The temporomandibular joint is often afflicted by osteoarthritis (TMJOA), causing pain and dysfunction, which is particularly prevalent in the elderly population. IL-37 is effective in avoiding excessive inflammatory damage to the organism. This article investigates the role and mechanism of intracellular IL-37 in TMJOA.
Methods: Enzyme-linked immunosorbent assay, quantitative real-time polymerase chain reaction, Western blotting, Senescence-associated β-galactosidase staining, immunofluorescence, and lentivirus were performed to elucidate the underlying mechanism.
Results: The results confirmed that IL-37 in synovial cells decreased with aging. Inflammatory stimulus elevated intracellular IL-37 in synoviocytes, while lentiviral knockdown of IL-37 resulted in more inflammatory factor production. Dynamic changes of IL-37 were observed in the nucleus and supernatant. In addition, Caspease-1 inhibitor hindered intracellular IL-37 maturation, and Smad3 inhibitor caused the loss of nuclear translocation of mature IL-37. Transfection of synovial cells with IL-37-expressing lentivirus resulted in relief not only of synovitis but also of the cartilage damage and inflammation caused by synovitis.
Conclusion: This study provides new insights into the intracellular anti-inflammatory mechanism of IL-37. It also confirms that IL-37 decreases with cellular senescence and that increasing intracellular IL-37 can effectively treat synovitis and synovitis-induced inflammatory damage to cartilage.
1 Introduction
The temporomandibular joint (TMJ) is crucial in the maxillofacial region because it is involved in complex movements such as chewing, pronunciation and facial expression (Schellhas, 1989). However, TMJ is often affected by osteoarthritis, which causes pain and dysfunction in patients. TMJ Osteoarthritis (TMJOA) is a chronic painful disease whose pathological features mainly include degenerative cartilage changes, subchondral bone remodeling and synovitis (Cardoneanu et al., 2022; Wang et al., 2023). Although the etiology of TMJOA is multi-factorial and complex, continuous inflammatory stimulation is still one of the main reasons for TMJOA (Zhang et al., 2023; Zhong et al., 2023). TMJOA is age-related, and much research has confirmed that its incidence and severity increase with the increase of age (Bernhardt et al., 2007; Schmitter et al., 2010; Alzahrani et al., 2020). However, further studies on the high incidence of TMJOA in the aging population are still lacking.
The synovial membrane can produce and secrete a variety of nutrients to maintain the viscosity of the synovial fluid, nourish cartilage and protect the bone (Ulmner et al., 2022). However, activated synoviocytes in inflammatory synovitis produce and release a large number of pro-inflammatory factors when synovitis occurs, such as IL-1β, IL-6, MMPs and TNF-α, which mediate cartilage destruction and TMJ inflammation through various ways (Yuan et al., 2004; Sellam and Berenbaum, 2010; Jiang et al., 2016; Ulmner et al., 2022). Although synovitis is not a prerequisite for the occurrence of TMJOA, more and more studies have indicated that the pro-inflammatory mediators released by synovitis are critical for the occurrence and development of osteoarthritis and the destruction of articular cartilage. On the other hand, disrupted and decomposed cartilage generally leads to excessive production of proteolytic enzymes, which in turn aggravates the inflammation of synovial tissue, forming a positive feedback loop (Sellam and Berenbaum, 2010; Felson et al., 2016; Mathiessen and Conaghan, 2017; Liao et al., 2020).
As a natural inhibitor of immune response and inflammation, IL-37 is upregulated to avoid further development of inflammation when the body is stimulated. It has been demonstrated in various tissues, such as the myocardial, periodontal tissue, and respiratory tract (Feng et al., 2022; Sun et al., 2022; Li et al., 2023). In these inflammatory tissues, IL-37 effectively reduces the expression of inflammatory factors such as IL-1β, IL-6, TNF-α and IL-18, and the inflammatory damage of tissues. IL-37 is a dual function cytokine with both intracellular and extracellular anti-inflammatory properties. Extracellular IL-37 binds to IL-18Rα on the cell surface of chondrocytes and macrophages to exert its anti-inflammatory effect in TMJOA (Luo et al., 2019; Luo et al., 2020). However, the role and mechanism of intracellular IL-37 in the arthritis of TMJ are unclear.
In previous studies, we observed that the expression of IL-37 was most abundant in the synovial tissue of TMJ and concentrated in the nucleus through immunohistochemical staining (Luo et al., 2019). These findings suggest that the intracellular pathway of IL-37 may play an essential anti-inflammatory role in TMJ inflammation. Our study found that elderly TMJOA patients expressed less IL-37, which is present in synoviocytes as an inflammatory antagonist, compared to young adults. We also confirmed the therapeutic effect of intracellular IL-37 on TMJ inflammation and explored its anti-inflammatory pathway and mechanism. This study may provide a therapeutic target for the prevention and treatment of the inflammation associated with TMJ.
2 Materials and methods
2.1 Sample collection and synovial cell culture
1 mL of synovial fluid diluted with normal saline was extracted during intra-articular injections in 58 TMJOA patients (17–80 years old, mean age 44 years old). The specific articular synovial fluid extraction method was described previously. The synovial fluid was centrifuged at 2000 r/min for 10 min and then stored at −80°C.
Synovial tissue was obtained from patients undergoing TMJ condylectomy and arthroplasty. Synovialis collected after surgery was washed with PBS and immersed in DMEM/F-12 medium (Gibco, Carlsbad, CA, United States). The synovial was cut to approximately 1 mm in size using ophthalmic scissors. The scissored synovial blocks were evenly distributed at the bottom of the cell culture bottle, and then DMEM/F-12 complete medium containing 10% fetal bovine serum (Gibco) and 1% penicillin/streptococcus was added. The extracted synovial cells were cultured in a 37°C incubator containing 5% CO2. Chondrocytes were extracted and cultured the same way as before.
The above experiments were performed with patient consent and approved by the Ethics Committee of Chongqing Medical University. Samples were collected in compliance with the Declaration of Helsinki.
2.2 Enzyme-linked immunosorbent assay (ELISA)
Cells were treated accordingly and the supernatant was retained and then centrifuged at 4,000 rpm for 20 min The supernatant obtained was used for experiments immediately or stored at −80°C. The supernatant was used to measure the expression of IL-37 in the cells. The ELISA kit for measuring IL-37, IL-6, MMP9, and MMP13 in cell supernatants was purchased from Jianglai Biotech (Shanghai, China). All experiments were performed according to the manufacturer’s instructions. The website is http://www.laibio.com/. The absorbance of the supernatants was evaluated at 450 nm in an enzyme labeling instrument (Molecular Devices, Shanghai, China).
2.3 Senescence-associated β-galactosidase (SA-β-gal) staining
Synovial cells were inoculated in 6-well plates when grown to the first or seventh generation, respectively. After 12 h, the plates were washed once with PBS, and 1 mL of β-galactosidase staining fixative (Beyotime, Shanghai, China) was added to each well to fix the cells for 15 min 1mL of staining working solution was added to each well and then incubated at 37°C for 12 h. Finally, the staining results were observed and recorded under a microscope.
2.4 Quantitative real-time polymerase chain reaction (qRT-PCR)
For gene expression analysis, total RNA was extracted from synovial cells using RNAiso plus (Takara, Nogihigashi, Japan). RNA was reverse transcribed using PrimeScript™ RT Reagent Kit with gDNA Eraser (Takara) to obtain cDNA. real-time PCR was carried out using Power TB Green PCR Master Mix (Takara). The expression of data was evaluated by the 2-△△Ct method. Primer sequences used in the article are displayed in Table 1.
2.5 Western blotting (WB)
Total intracellular proteins were extracted using RIPA, and nuclear or cytoplasmic proteins were obtained using a Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime). For Western blot analysis, 30 μg of cell lysates were loaded for each sample. Then, the proteins were separated in SDS-PAGE gels and transferred to PVDF membranes (Millipore). After blocking with 5% skimmed milk in TBST, the proteins were blotted with antibodies for IL-37 (1:1,000; Abcam, ab153889) and Proteintech, 11863-1-AP), p-Smad3 (1:1,000; CST, 9520S), IL-6 (1:1,000; CST, 12153S), TNF-α (1:1,000; Proteintech, 17590-1-AP), MMP9 (1:1,000; Bioss, bs-22502R), MMP13 (1:1,000; Abcam, ab39012), GAPDH (1:1,000; HUABIO, ET1601-4), Histone-H3 (1:1,000; Proteintech, 17168-1-AP) at 4°C overnight, followed by incubating with HRP- labeled secondary antibodies (1:5,000; HUABIO, HA1001) for 2 h. Images were obtained and analyzed by a computer program (Bio-Rad, United States).
2.6 Transfection of lentivirus
Synovial cells were transfected with lentiviruses (Tsingke Biotech, Beijing, China) expressing or targeting IL-37 with the help of Polybrene (Beyotime) according to the manufacturer’s instructions (https://www.tsingke.com.cn/). The multiplicity of infection (MOI) of synoviocytes is 20; Virus concentration of 2×106 transducing units/mL. At 48 h after lentivirus transfection, synoviocytes were treated with Puromycin Dihydrochloride (1 μg/mL, Beyotime) for 1 week to remove transfection failures. Subsequent treatments were started after 48 h of ordinary culture.
2.7 Immunofluorescence
Synovial cells were treated with IL-1β (10 ng/mL) for 12 h to induce intracellular expression of p-Smad3 and IL-37. After washing the cells with PBS, the cells were fixed with 4% paraformaldehyde and closed with goat serum for 1 h. Next, cells were incubated overnight with p-Smad3 primary antibody (1:200; CST, 9520S) and the following day with Alexa Fluor 594-coupled goat anti-rabbit antibody (1:500; Abcam, ab150080) for 1 h. Cell nuclei were stained with DAPI. Images were observed under a fluorescent microscope.
2.8 Statistical analysis
The data were statistically analyzed by GraphPad Prism 8 (GraphPad Software Inc., La Jolla, CA). Statistical differences were analyzed by t-test or one-way ANOVA. For condylar chondrocytes, experiments were conducted in triplicatewith cells from three donors, and data were statistically significant if p < 0.05.
3 Results
3.1 Reduced IL-37 expression in senescent sy novial cells
At first, IL-37 was analyzed by ELISA in the synovial fluid of 58 TMJOA patients. The results revealed that IL-37 decreased with age in TMJOA patients, indicating that older patients have a reduced ability to produce IL-37 (Figure 1B). Further studies extracted primary synovial cells by tissue-sticking method. The results showed short spindle-shaped synovial cells crawling out from within the synovial tissue (Figure 1C). Then, the synovial cells were passaged to the first and seventh generations (P1 and P7) to simulate synovial tissue in the young and aging states. Microscopically, it was observed that P7 became larger and showed more bifurcation compared to P1 (Figure 1D). Also, β-galactosidase staining confirmed that P7 had darker blue staining and a greater proportion of stained cells than P1 (Figure 1E). When P1 and P7 were stimulated with IL-1β to simulate the inflammatory state of young and old synovium, the expression of IL-37 precursor (Pro-IL-37) and mature IL-37 (Mat-IL-37) in P7 was significantly reduced both at rest and when stimulated with IL-1β (Figures 1F–H).
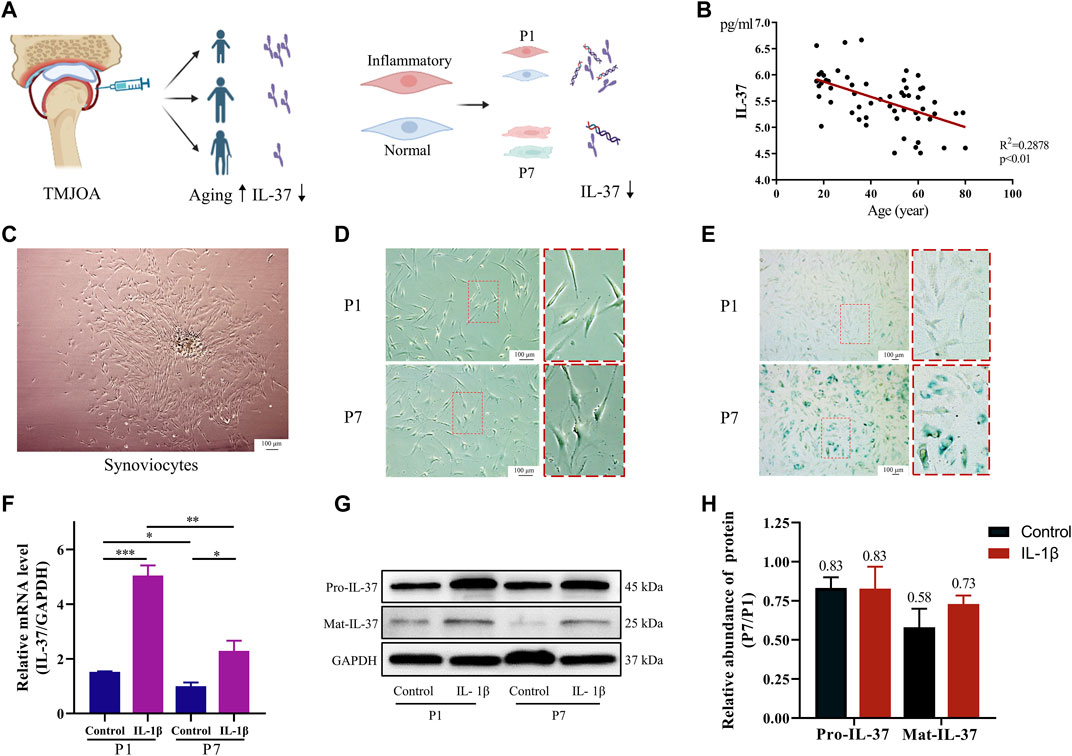
FIGURE 1. Reduced IL-37 expression in senescent sy novial cells. (A) Diagram of synovial fluid and intracellular IL-37 detection. (B) IL-37 in synovial fluid of 58 TMJOA patients was detected by ELISA. (C) Primary synovial cells were extracted by explant cultures. (D) Observation of the morphology of P1 and P7 under electron microscope. (E) SA-β-gal staining of P1 and P7 synovial cells, and aging synovial cells appear dark blue. Synovial cells were stimulated with IL-1β (10 ng/mL) for 24 h. PCR (F) and Western blot (G) were performed to detect intracellular IL-37. (H) Quantitative analysis of Western blot. The numbers above the bars indicate fold changes. Results are expressed as the mean ± S.E.M (n = 3). *p < 0.05; **p < 0.01; ***p < 0.001.
3.2 IL-37 exerts anti-inflammatory effects
Synovial cells were stimulated with inflammatory factors (IL-1β, HMGB1, LPS) to mimic the state of synovial tissue during inflammatory stimulation, which resulted in a significant increase of IL-37 in synovial cells at the protein and gene levels (Figures 2B–D). To verify the anti-inflammatory effect of IL-37 in synoviocytes, we knocked down IL-37 with lentivirus, which resulted in a Remarkable reduction of IL-37 (Figure 2E). In response to IL-1β stimulation, IL-6 and TNF-α were elevated in synovial cells. However, silencing of intracellular IL-37 using lentivirus resulted in an additional elevation of IL-6 and TNF-α (Figure 2F).
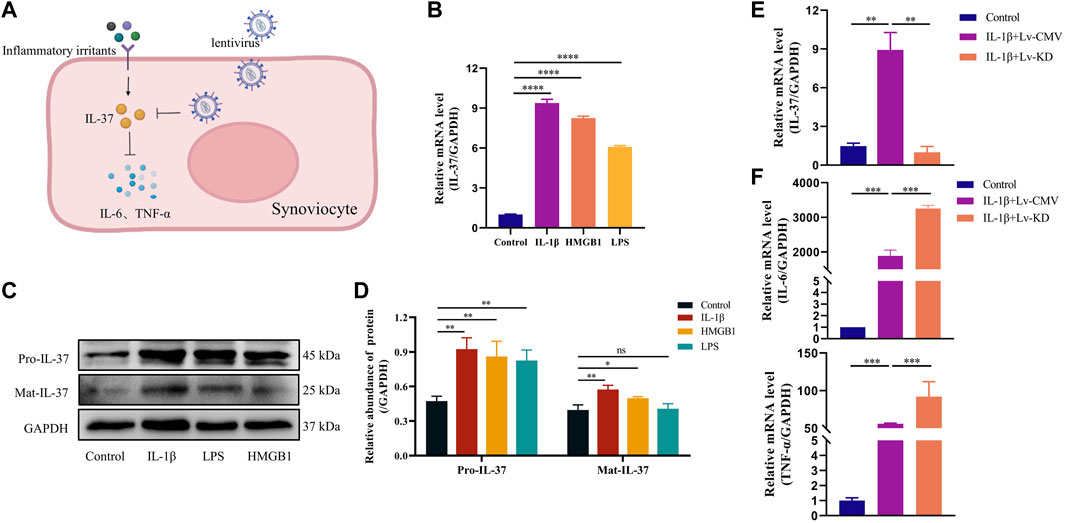
FIGURE 2. IL-37 exerts anti-inflammatory effects. (A) Diagram of il-37 against inflammation. IL-1β (10 ng/mL), HMGB1 (10 ng/mL) and LPS (1 μg/mL) stimulated synovial cells for 24 h. The levels of protein (B) and RNA (C) were detected. (D) Quantitative analysis of Western blot. IL-1β was stimulated for 24 h after transfection of synovial cells with IL-37 knockdown lentivirus (Lv-KD) and control lentivirus (Lv-CMV). PCR was used to detect the RNA levels of IL-37 (E), IL-6 and TNF-α (F) in Synovial cells. Results are expressed as the mean ± S.E.M (n = 3). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns, not significant.
3.3 Dynamic changes of IL-37 inside and outside the cell
IL-37 is a bifunctional anti-inflammatory cytokine, so we examined the dynamic changes of IL-37 inside and outside the cells after 0–48 h stimulation of synovial cells using IL-1β. Within the cytoplasm, Pro-IL-37 exhibited a consistently elevated trend. Although Mat-IL-37 also showed an elevated trend in the cytoplasm, the most significant elevation occurred at 6–12 h and started to decrease after 12 h (Figure 3A). In the nucleus, No visible expression of Pro-IL-37 can be observed. On the other hand, Mat-IL-37 showed a tendency of elevation followed by a decrease, and the most pronounced upward shift occurs at 9 and 12 h (Figures 3B, C). ELISA detected a slow release of extracellular IL-37 into the extracellular compartment starting at 6 h, reaching a maximum at 24 h, and following a decline (Figure 3D).
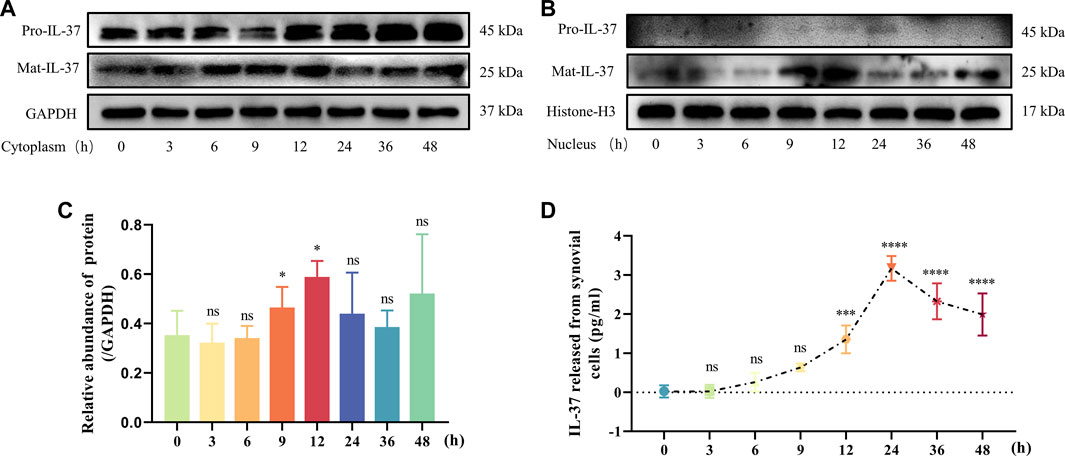
FIGURE 3. Dynamic changes of IL-37 inside and outside the cell. Samples were collected at 3, 6, 9, 12, 24, 36, and 48 h after treatment with IL-1β. IL-37 in cytoplasm (A) and nucleus (B) were detected by Western blot. (C) Quantitative analysis of Mat-IL-37 in the nucleus. (D) Cell supernatants were collected after treatments. ELISA was used to detect the content of IL-37. Results are expressed as the mean ± S.E.M (n = 3). Ns, not significant; ***p < 0.001; ****p < 0.0001 for treatment group compared to control group.
3.4 Mechanism of intracellular IL-37 maturation and nuclear translocation in synoviocytes
Upon exposure to stimuli, IL-37 is produced intracellularly as Pro-IL-37. The Pro-IL-37 becomes Mat-IL-37 after cleavage by Caspase-1, as pretreatment with Caspase-1 inhibitor (VX-765) almost completely inhibited the IL-1β-triggered elevation of Mat-IL-37. In contrast, the Pro-IL-37 exhibited elevated (Figures 4B, C). On the other hand, analysis of IL-37 levels in the nucleus revealed that VX-765 curtailed the increased nuclear translocation of Mat-IL-37 caused by IL-1β stimulation (Figures 4D, E).
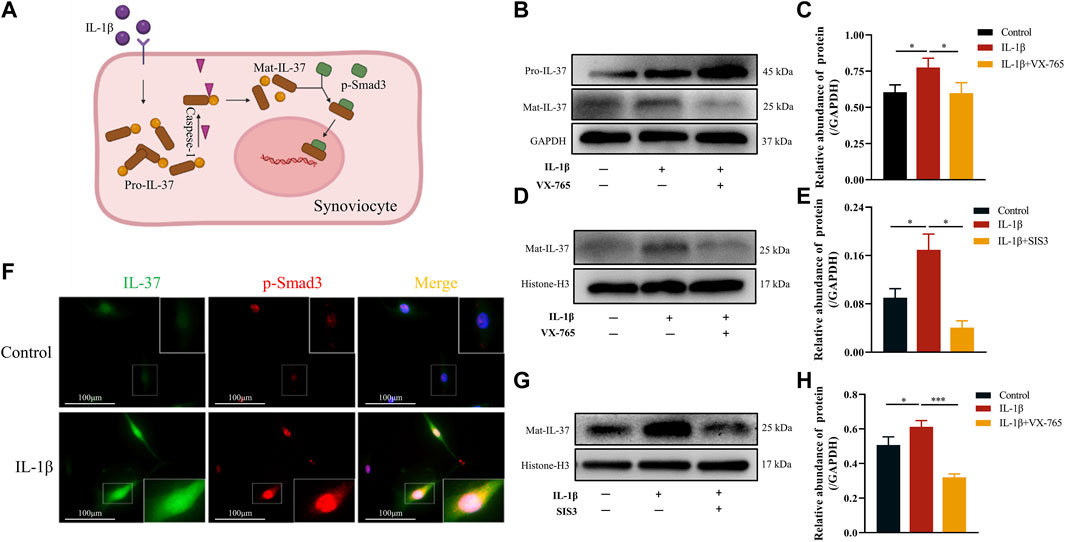
FIGURE 4. Mechanism of intracellular IL-37 maturation and nuclear translocation in synoviocytes. (A) Schematic diagram of Mat IL-37 translocation into the nucleus. Synovial cells were pretreated with Caspase-1 (VX-765, 10 μM) and p-Smad3 (SIS3, 10 μM) inhibitors for 2 h prior to IL-1β treatment for 12 h. Then Western blot was used to detect IL-37 protein levels in whole cell lysates (B,C) and nuclear lysates (D,E) (G,H). (F) The synovial cells were transfected with lentivirus to express IL-37 with green fluorescent label. Then, the intracellular distribution of IL-37 and p-Smad3 (red) was observed by immunofluorescence after 12 h treatment with IL-1β. The nuclei are stained by DAPI. Results are expressed as the mean ± S.E.M (n = 3). *p < 0.05; ***p < 0.001.
Regarding the mechanism of nuclear translocation of IL-37, synoviocytes transfected with lentivirus expressing IL-37 labeled by green fluorescent. Upon stimulation with IL-1β, intracellular expression of both IL-37 and p-Smad3 upregulated. At the same time, the intracellularly abundantly expressed IL-37 co-localized with p-Smad3 in the nucleus and perinucleus, especially in the nucleus (Figure 4F). In contrast, pretreatment of synoviocytes with SIS3 attenuated or even eliminated IL-37 nuclear translocation induced by IL-1β stimulation (Figures 4G, H).
3.5 Intracellular IL-37 inhibits synovial inflammation
To verify if intracellular IL-37 can exert anti-inflammatory effects, lentivirus transfected synovial cells to obtain intracellular overexpression of IL-37. Increased production of IL-37 in lentivirus-transfected synoviocytes at the protein level was observed (Figure 5B). Although IL-1β stimulation caused an increase of IL-6, TNF-α, MMP9, and MMP13, the production of the above-mentioned inflammatory factors received significant inhibition when intracellular IL-37 was abundantly expressed (Figures 5C–E). Smad3 is a protein necessary for IL-37 nuclear translocation to exert anti-inflammatory effects. When Sma d3 was inhibited by SIS3, intracellular IL-37 resistance to inflammatory factors is lost.
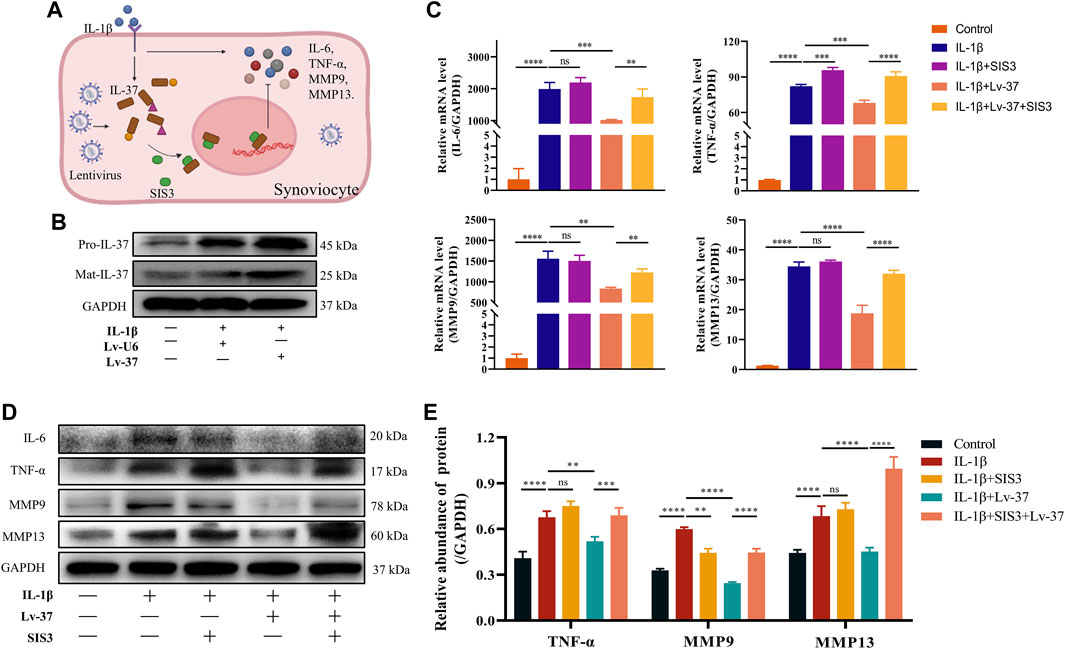
FIGURE 5. Intracellular IL-37 inhibits synovial inflammation. (A) Schematic illustration of Anti-inflammatory mechanism of intracellular IL-37. IL-37 was overexpressed in synovial cells with NC (Lv-U6) or IL-37 (Lv-37) lentivirus. Then SIS3 was pretreated for 2 h before IL-1β (12 h) treatment. (B) Western blot observed intracellular IL-37 levels. The protein and RNA levels of IL-6, TNF-α, MMP9, and MMP13 were analysed by PCR (C) and Western blot (D). (E) Quantitative analysis of Western blot. Results are expressed as the mean ± S.E.M (n = 3). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns, not significant.
3.6 Synoviocyte conditioned medium regulates the inflammatory response in chondrocytes
During the development of TMJOA, inflamed synovium tends to release multiple inflammatory factors leading to the progression of osteoarthritis. We, therefore, collected conditioned medium (CM) from synovial cells (Figure 6B). ELISA was performed to detect the water of inflammatory factors in the supernatant. The results showed that in response to IL-1β stimulation, synovial cells released more IL-6, TNF-α and MMP13 (Figure 6C). At the same time, intracellular IL-37 significantly inhibited the release of these inflammatory factors. When chondrocytes were cultured using CM with synovial cells, we found that the supernatant of inflamed synovial cells also triggered chondrocyte inflammation, resulting in higher levels of IL-6, IL-8, IL-1β, TNF-α, MMP1, MMP9, MMP13, and ADAMTS4. However, when the intracellular IL-37 in synovial cells was increased, CM from inflammatory synoviocytes showed remarkably reduced inflammation induction in chondrocytes.
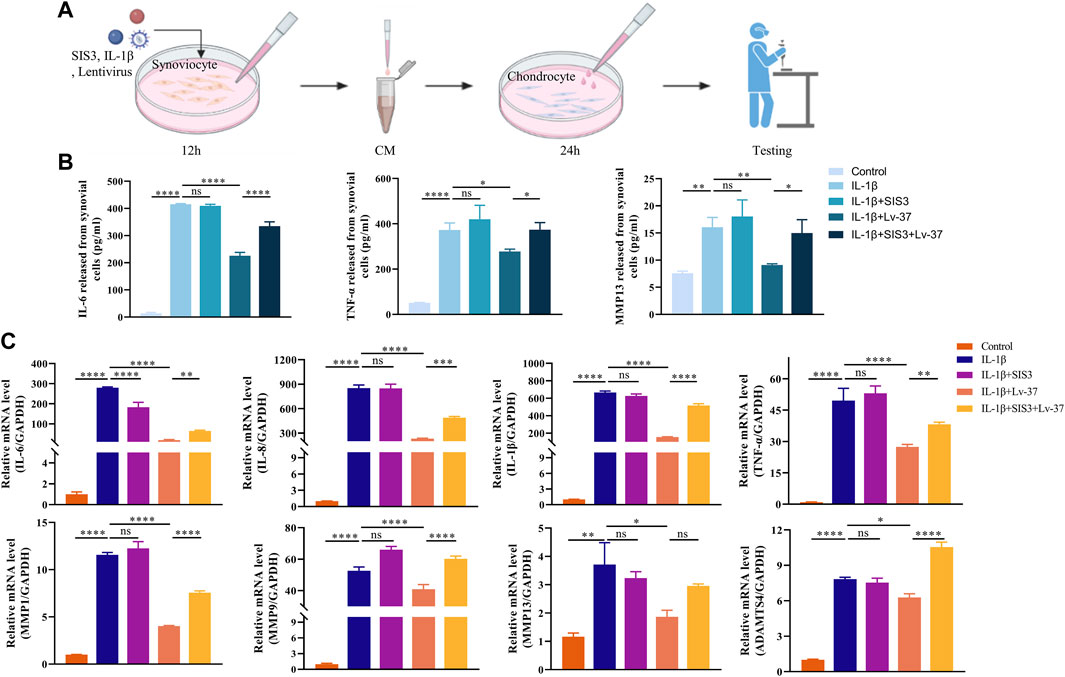
FIGURE 6. Synoviocyte conditioned medium regulates the inflammatory response in chondrocytes. (A) Conditioned media (CM) of synovial cells were collected for cartilage culture. The synovial cells were transfected with lentivirus expressing IL-37, After pretreated with SIS3 for 2h, synoviocytes stimulated by IL-1β for 12 h. Chondrocytes were cultured in the CM form synovial cells for 24 h. (B) The levels of sIL-6, TNF-α, MMP9, and MMP13 in supernatant were sdetected by ELISA. (C) PCR was conducted to measure IL-6, IL-8, IL-1β, TNF-α, MMP1, MMP9, MMP13, and ADAMTS4 in chondrocytes. Results are expressed as the mean ± S.E.M (n = 3). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns, not significant.
4 Discussion
The incidence and severity of TMJOA increase significantly with aging, which is confirmed in a large body of literature (Bernhardt et al., 2007; Schmitter et al., 2010; Alzahrani et al., 2020). Although the etiology of TMJOA is complex and multifactorial, inflammatory stimuli still play an essential role in its development, such as IL-6, TNF-α, IL-1β, and MMP13 (Yuan et al., 2004; Sellam and Berenbaum, 2010; Jiang et al., 2016; Ulmner et al., 2022). Given the strong anti-inflammatory effect of IL-37, we speculated whether the high prevalence of TMJOA in the elderly population was related to the reduced expression of IL-37. We, therefore, examined the level of IL-37 in the synovial fluid of patients with TMJOA and found that it decreased with aging. This initially verified our speculation.
The components of synovial fluid are mainly derived from synovial tissue, which includes various inflammatory irritants that contribute to cartilage destruction (Wang et al., 2015; Qi et al., 2020). In recent years, attention has begun to be paid to the importance of synovium in the etiology of OA, and some studies have even proposed synovitis as an independent risk factor for OA (Felson et al., 2016; MacFarlane et al., 2019; Wang et al., 2022). And we found much higher levels of IL-37 in the synovial membrane than in the articular disc, cartilage and condyle (Luo et al., 2019). This evidence led us to select synovium for further study. First- and seventh-generation synovial cells were then cultured to mimic the inflammation of young and old synovium. The results confirmed that P7 exhibited significant senescence and a reduced ability to produce IL-37 compared to P1. This further confirms our speculation that low intracellular of IL-37 may be responsible for the development of synovitis in senescent synoviocytes, which is detrimental to both the development and progression of TMJOA.
IL-37 counteracts inflammatory progression in the heart, periodontal and respiratory tracts (Feng et al., 2022; Sun et al., 2022; Li et al., 2023). In a synovitis cell model constructed using IL-1β, HMGB1, and LPS, intracellular IL-37 was responsively upregulated (more pronounced with stimulation of IL-1β). However, more pronounced synovitis was observed when IL-37 was knocked down using lentivirus. The above experiments confirm that IL-37 is generated as an inflammatory stimulus counteractant when the synovium is subjected to inflammatory stimuli. However, the knockdown of IL-37 using lentivirus decreases both intracellular and extracellular IL-37, which results in the inhibition of the anti-inflammatory effects of both intracellular and extracellular il-37. Our previous study found that IL-37 in inflamed joints is predominantly concentrated intracellularly, suggesting an important role for the intracellular anti-inflammatory pathway of IL-37 (Luo et al., 2019). Therefore, whether there is an anti-inflammatory effect of intracellular IL-37 still needs further confirmation.
The intracellular anti-inflammatory effect of IL-37 requires entry into the nucleus (Sharma et al., 2008; Bulau et al., 2014). When looking at IL-37 in and out of cells under inflammatory stimuli, we found that IL-37 was the first to translocate to the nucleus and then released into the extracellular space. This suggests that intracellular IL-37s are the first to exert anti-inflammatory effects in response to inflammatory stimuli. Furthermore, the intracellular anti-inflammatory mechanism of IL-37 appears to be achieved through its mature form, as no significant pro-IL-37 was observed in the nucleus.
Further, we explored the mechanism of IL-37 maturation and nuclear translocation in synovial cells. Consistent with previous studies (Kumar et al., 2002; Bulau et al., 2014), Casepase-1 was involved in the maturation process of IL-37, as its inhibitor significantly decreased the expression of Mat-IL-37. Furthermore, we observed that VX-765 impeded the movement of Mat-IL-37 toward the nucleus. We speculate that this may be because VX-765 reduces the total intracellular level of Mat-IL-37. The presence of Smad3 is necessary for the intracellular anti-inflammatory process of IL-37 (Nold et al., 2010; Luo et al., 2017), but the exact mechanism is still unknown. Our study also observed fluorescent co-localization of p-Smad3 with IL-37 in the nucleus and perinucleus. Importantly, we confirmed that SIS3 reversed the IL-1β-induced nuclear translocation of Mat-IL-37. This suggests that the role of p-Smad3 is to assist in the nuclear translocation of IL-37 during its intracellular anti-inflammatory process.
After elaborating on the mechanism of intracellular IL-37 maturation and nuclear translocation, we explore whether intracellular IL-37 is effective against synovitis. Synovial cells transfected with IL-37-expressing lentivirus significantly inhibited IL-6, TNFα, MMP9, and MMP13 upregulation caused by IL-1β. Notably, this resulted in both increased IL-37 secretion as well as increased extracellular anti-inflammatory effects. Therefore, we blocked the intracellular anti-inflammatory pathway of IL-37 using SIS3, which reversed the anti-inflammatory effect of intracellular IL-37. It confirms that intracellular IL-37 can effectively counteract inflammation and alleviate tissue damage caused by excessive inflammation.
Numerous studies have shown that synovitis occurs well before the onset of OA and releases various inflammatory factors promoting OA procession (Loeuille et al., 2005; Scanzello and Goldring, 2012; Hügle and Geurts, 2017; Liao et al., 2020). To further confirm the role of synovitis in promoting osteoarthrosis, we collected conditioned media from inflamed synovial cells. ELISA showed that inflamed synovium released more IL-6, TNF-α, and MMP13. These are all undesirable stimuli leading to cartilage breakage and the progression of osteoarthritis (Wang and He, 2018; Wang et al., 2020). Meanwhile, factors such as IL-6, IL-8, IL-1β, TNF-α, MMP1, MMP9, MMP13, and ADAMTS4 were elevated when chondrocytes were stimulated by CM from inflammatory synovium. These factors play a role in promoting cartilage destruction and osteoarthritis progression (Burrage et al., 2006; Latourte et al., 2017; Wang and He, 2018; Fei et al., 2019; Li et al., 2022). In contrast, when IL-37 levels are elevated in synovial cells, inflamed synovium will release fewer inflammatory factors and have significantly less damage to chondrocytes and induction of inflammation.
5 Conclusion
In summary, our study confirm that the high prevalence of TMJOA in the elderly population is associated with reduced IL-37 levels. Restoring intracellular IL-37 levels effectively reduces synovial tissue inflammation and alleviates the destruction of cartilage and progression of inflammation caused by synovitis (Figure 7). This study provides new ideas on the anti-inflammatory mechanism of intracellular IL-37 and new insights into the etiology and treatment of TMJOA.

FIGURE 7. An illustration of intracellular IL-37 against arthritis in TMJ. In response to the stimulation of inflammatory factors, IL-37 is upregulated responsively in synovial cells in the form of precursors. Pro-IL-37 matures after cleavage by caspase-1 and translocates to the nucleus mediated by Smad3. This avoids excessive inflammatory responses in synovial cells and attenuates the release of IL-6, TNF-α, MMP9, and MMP13 and the resulting osteoarthritis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Chongqing Medical University [CQHS-REC-2021 (LSNo27)]. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JL; Methodology; visualization; writing–original draft; investigation. SP: Methodology; writing–original draft; formal analysis. YY: Data curation; methodology; writing–review and editing. SY: Data curation; methodology. XC: Conceptualization; methodology. LZ: Data curation; investigation; methodology. YL: Supervision ; writing–review and editing. JX: Conceptualization; resources; writing–review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81800999 to JX), the Chongqing Health Commission and Science and Technology Bureau Foundation (2023ZDXM019 to JX).
Acknowledgments
Schematics in the figures were created with BioRender.com (https://www.biorender.com/).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1250216/full#supplementary-material
References
Alzahrani, A., Yadav, S., Gandhi, V., Lurie, A. G., and Tadinada, A. (2020). Incidental findings of temporomandibular joint osteoarthritis and its variability based on age and sex. Imaging Sci. Dent. 50, 245–253. doi:10.5624/isd.2020.50.3.245
Bernhardt, O., Biffar, R., Kocher, T., and Meyer, G. (2007). Prevalence and clinical signs of degenerative temporomandibular joint changes validated by magnetic resonance imaging in a non-patient group. Ann. Anat. 189, 342–346. doi:10.1016/j.aanat.2007.02.008
Bulau, A. M., Nold, M. F., Li, S., Nold-Petry, C. A., Fink, M., Mansell, A., et al. (2014). Role of caspase-1 in nuclear translocation of IL-37, release of the cytokine, and IL-37 inhibition of innate immune responses. Proc. Natl. Acad. Sci. U. S. A. 111, 2650–2655. doi:10.1073/pnas.1324140111
Burrage, P. S., Mix, K. S., and Brinckerhoff, C. E. (2006). Matrix metalloproteinases: role in arthritis. Front. Biosci. 11, 529–543. doi:10.2741/1817
Cardoneanu, A., Macovei, L. A., Burlui, A. M., Mihai, I. R., Bratoiu, I., Rezus, I. I., et al. (2022). Temporomandibular joint osteoarthritis: pathogenic mechanisms involving the cartilage and subchondral bone, and potential therapeutic strategies for joint regeneration. Int. J. Mol. Sci. 24, 171. doi:10.3390/ijms24010171
Fei, J., Liang, B., Jiang, C., Ni, H., and Wang, L. (2019). Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed. Pharmacother. 109, 1586–1592. doi:10.1016/j.biopha.2018.09.161
Felson, D. T., Niu, J., Neogi, T., Goggins, J., Nevitt, M. C., Roemer, F., et al. (2016). Synovitis and the risk of knee osteoarthritis: the MOST Study. Osteoarthr. Cartil. 24, 458–464. doi:10.1016/j.joca.2015.09.013
Feng, K. N., Meng, P., Zhang, M., Zou, X. L., Li, S., Huang, C. Q., et al. (2022). IL-24 contributes to neutrophilic asthma in an IL-17a-dependent manner and is suppressed by IL-37. Allergy Asthma Immunol. Res. 14, 505–527. doi:10.4168/aair.2022.14.5.505
HüGLE, T., and Geurts, J. (2017). What drives osteoarthritis? synovial versus subchondral bone pathology. Rheumatol. Oxf. 56, 1461–1471. doi:10.1093/rheumatology/kew389
Jiang, L., Lin, X., and Ji, P. (2016). Effect of p38 mitogen activated protein kinase inhibitor on temporomandibular joint synovitis induced by occlusal alteration. J. Oral Maxillofac. Surg. 74, 1131–1139. doi:10.1016/j.joms.2015.12.020
Kumar, S., Hanning, C. R., Brigham-Burke, M. R., Rieman, D. J., Lehr, R., Khandekar, S., et al. (2002). Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine 18, 61–71. doi:10.1006/cyto.2002.0873
Latourte, A., Cherifi, C., Maillet, J., Ea, H. K., Bouaziz, W., Funck-Brentano, T., et al. (2017). Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann. Rheum. Dis. 76, 748–755. doi:10.1136/annrheumdis-2016-209757
Liao, L., Zhang, S., Zhao, L., Chang, X., and Huang, J., (2020). Acute synovitis after trauma precedes and is associated with osteoarthritis onset and progression. Int. J. Biol. Sci. 16, 970–980. doi:10.7150/ijbs.39015
Li, L., Li, J., Li, S., Chen, H., Wu, Y., and Qiu, Y. (2023). IL-37 alleviates alveolar bone resorption and inflammatory response through the NF-κB/NLRP3 signaling pathway in male mice with periodontitis. Arch. Oral Biol. 147, 105629. doi:10.1016/j.archoralbio.2023.105629
Li, T., Peng, J., Li, Q., Shu, Y., Zhu, P., and Hao, L. (2022). The mechanism and role of ADAMTS protein family in osteoarthritis. Biomolecules 12, 959. doi:10.3390/biom12070959
Loeuille, D., Chary-Valckenaere, I., Champigneulle, J., Rat, A. C., Toussaint, F., Pinzano-Watrin, A., et al. (2005). Macroscopic and microscopic features of synovial membrane inflammation in the osteoarthritic knee: correlating magnetic resonance imaging findings with disease severity. Arthritis Rheum. 52, 3492–3501. doi:10.1002/art.21373
Luo, C., Shu, Y., Luo, J., Liu, D., Huang, D. S., Han, Y., et al. (2017). Intracellular IL-37b interacts with Smad3 to suppress multiple signaling pathways and the metastatic phenotype of tumor cells. Oncogene 36, 2889–2899. doi:10.1038/onc.2016.444
Luo, P., Feng, C., Jiang, C., Ren, X., Gou, L., Ji, P., et al. (2019). IL-37b alleviates inflammation in the temporomandibular joint cartilage via IL-1R8 pathway. Cell Prolif. 52, e12692. doi:10.1111/cpr.12692
Luo, P., Peng, S., Yan, Y., Ji, P., and Xu, J. (2020). IL-37 inhibits M1-like macrophage activation to ameliorate temporomandibular joint inflammation through the NLRP3 pathway. Rheumatol. Oxf. 59, 3070–3080. doi:10.1093/rheumatology/keaa192
Macfarlane, L. A., Yang, H., Collins, J. E., Jarraya, M., Guermazi, A., Mandl, L. A., et al. (2019). Association of changes in effusion-synovitis with progression of cartilage damage over eighteen months in patients with osteoarthritis and meniscal tear. Arthritis Rheumatol. 71, 73–81. doi:10.1002/art.40660
Mathiessen, A., and Conaghan, P. G. (2017). Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res. Ther. 19, 18. doi:10.1186/s13075-017-1229-9
Nold, M. F., Nold-Petry, C. A., Zepp, J. A., Palmer, B. E., Bufler, P., and Dinarello, C. A. (2010). IL-37 is a fundamental inhibitor of innate immunity. Nat. Immunol. 11, 1014–1022. doi:10.1038/ni.1944
Qi, D., Sun, S., Han, L., Wang, Q., Kong, J., Zhang, Y., et al. (2020). Stromal cell-derived factor-1 regulates the secretion of interleukin-1β in the temporomandibular joint of rats with synovial inflammation. J. Oral Pathol. Med. 49, 933–939. doi:10.1111/jop.13040
Scanzello, C. R., and Goldring, S. R. (2012). The role of synovitis in osteoarthritis pathogenesis. Bone 51, 249–257. doi:10.1016/j.bone.2012.02.012
Schellhas, K. P. (1989). Temporomandibular joint injuries. Radiology 173, 211–216. doi:10.1148/radiology.173.1.2781010
Schmitter, M., Essig, M., Seneadza, V., Balke, Z., Schröder, J., and Rammelsberg, P. (2010). Prevalence of clinical and radiographic signs of osteoarthrosis of the temporomandibular joint in an older persons community. Dentomaxillofac Radiol. 39, 231–234. doi:10.1259/dmfr/16270943
Sellam, J., and Berenbaum, F. (2010). The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 6, 625–635. doi:10.1038/nrrheum.2010.159
Sharma, S., Kulk, N., Nold, M. F., Gräf, R., Kim, S. H., Reinhardt, D., et al. (2008). The IL-1 family member 7b translocates to the nucleus and down-regulates proinflammatory cytokines. J. Immunol. 180, 5477–5482. doi:10.4049/jimmunol.180.8.5477
Sun, L., Yuan, H., and Zhao, G. (2022). IL-37 alleviates Coxsackievirus B3-induced viral myocarditis via inhibiting NLRP3 inflammasome-mediated pyroptosis. Sci. Rep. 12, 20077. doi:10.1038/s41598-022-22617-y
Ulmner, M., Sugars, R., Naimi-Akbar, A., Alstergren, P., and Lund, B. (2022). Cytokines in temporomandibular joint synovial fluid and tissue in relation to inflammation. J. Oral Rehabil. 49, 599–607. doi:10.1111/joor.13321
Wang, X. D., Zhang, J. N., Gan, Y. H., and Zhou, Y. H. (2015). Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J. Dent. Res. 94, 666–673. doi:10.1177/0022034515574770
Wang, C., Gao, Y., Zhang, Z., Chi, Q., Liu, Y., Yang, L., et al. (2020). Safflower yellow alleviates osteoarthritis and prevents inflammation by inhibiting PGE2 release and regulating NF-κB/SIRT1/AMPK signaling pathways. Phytomedicine 78, 153305. doi:10.1016/j.phymed.2020.153305
Wang, D., Qi, Y., Wang, Z., Guo, A., Xu, Y., and Zhang, Y. (2023). Recent advances in animal models, diagnosis, and treatment of temporomandibular joint osteoarthritis. Tissue Eng. Part B Rev. 29, 62–77. doi:10.1089/ten.TEB.2022.0065
Wang, T., and He, C. (2018). Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 44, 38–50. doi:10.1016/j.cytogfr.2018.10.002
Wang, X., Chen, T., Liang, W., Fan, T., Zhu, Z., Cao, P., et al. (2022). Synovitis mediates the association between bone marrow lesions and knee pain in osteoarthritis: data from the Foundation for the National Institute of Health (FNIH) Osteoarthritis Biomarkers Consortium. Osteoarthr. Cartil. 30, 1270–1277. doi:10.1016/j.joca.2022.06.004
Yuan, G. H., Tanaka, M., Masuko-Hongo, K., Shibakawa, A., Kato, T., Nishioka, K., et al. (2004). Characterization of cells from pannus-like tissue over articular cartilage of advanced osteoarthritis. Osteoarthr. Cartil. 12, 38–45. doi:10.1016/j.joca.2003.08.004
Zhang, Z., Yuan, L., Liu, Y., Wang, R., Zhang, Y., Yang, Y., et al. (2023). Integrated cascade nanozyme remodels chondrocyte inflammatory microenvironment in temporomandibular joint osteoarthritis via inhibiting ROS-NF-κb and MAPK pathways. Adv. Healthc. Mater 12, e2203195. doi:10.1002/adhm.202203195
Zhong, Y., Zhou, Y., Ding, R., Zou, L., Zhang, H., Wei, X., et al. (2023). Intra-articular treatment of temporomandibular joint osteoarthritis by injecting actively-loaded meloxicam liposomes with dual-functions of anti-inflammation and lubrication. Mater Today Bio 19, 100573. doi:10.1016/j.mtbio.2023.100573
Keywords: intracellular IL-37, inflammation, temporomandibular joint, synovitis, osteoarthritis
Citation: Li J, Peng S, Yan Y, Yan S, Cao X, Li Y, Zhu L and Xu J (2023) IL-37 counteracts inflammatory injury in the temporomandibular joint via the intracellular pathway. Front. Pharmacol. 14:1250216. doi: 10.3389/fphar.2023.1250216
Received: 29 June 2023; Accepted: 02 November 2023;
Published: 20 November 2023.
Edited by:
Yubiao Zhang, Renmin Hospital of Wuhan University, ChinaReviewed by:
Eduardo Dominguez, University of Santiago de Compostela, SpainKang Xu, Hubei University of Chinese Medicine, China
Copyright © 2023 Li, Peng, Yan, Yan, Cao, Li, Zhu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luying Zhu, NTAwMjY2QGhvc3BpdGFsLmNxbXUuZWR1LmNu; Jie Xu, eHVqaWVAaG9zcGl0YWwuY3FtdS5lZHUuY24=
†These authors have contributed equally to this work
 Jun Li
Jun Li Sisi Peng1,2,3†
Sisi Peng1,2,3† Jie Xu
Jie Xu