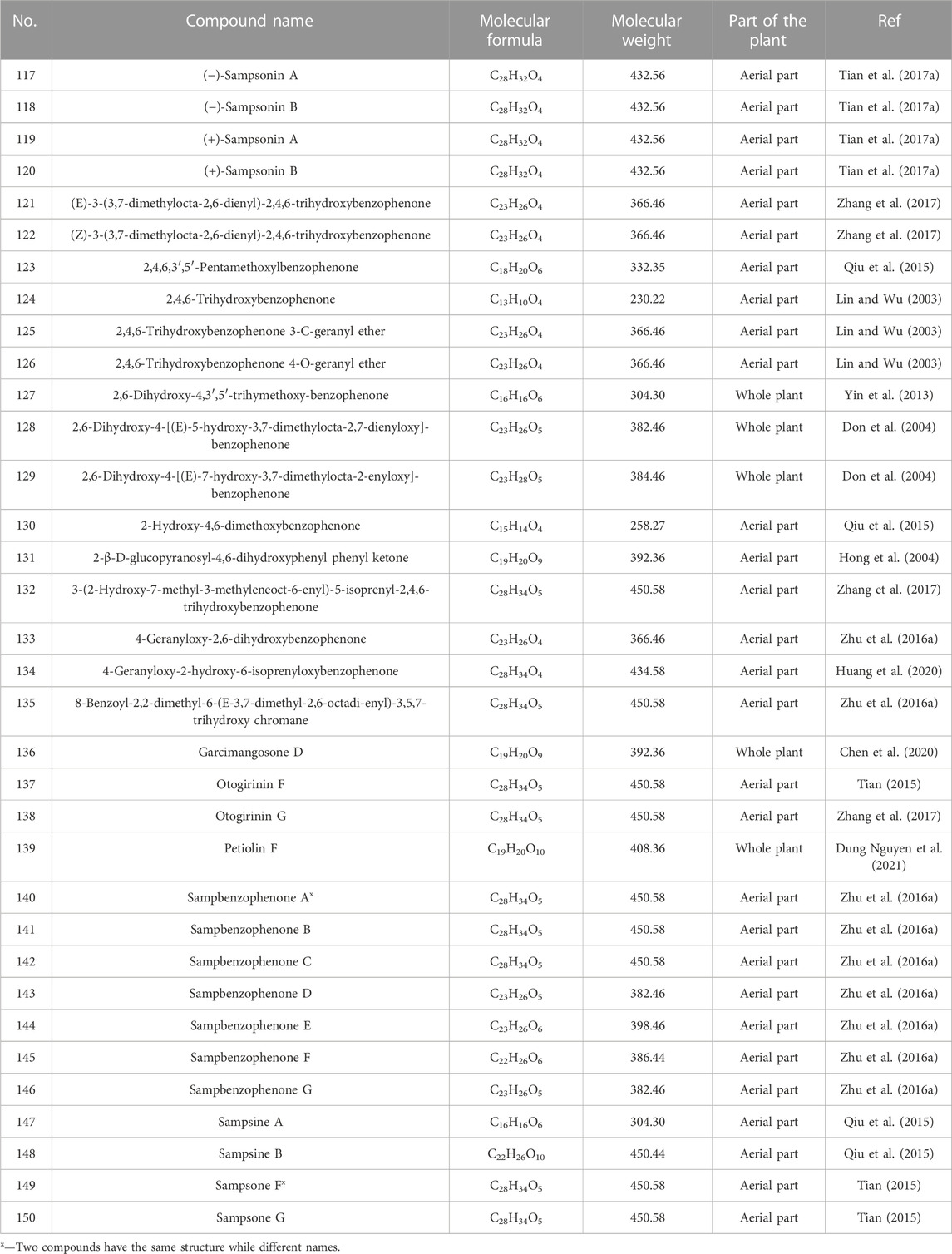
- 1Guangdong Provincial Key Laboratory of Utilization and Conservation of Food and Medicinal Resources in Northern Region, Shaoguan University, Shaoguan, China
- 2College of Food Science and Technology, Shaoguan University, Shaoguan, China
- 3School of Pharmaceutical Sciences, Guangzhou University of Chinese Medicine, Guangzhou, China
Ethnopharmacological relevance: Hypericum sampsonii Hance, also known as Yuanbao Cao in Chinese, is a traditional medicinal herb from the Guttiferae family and has been widely used in China to treat various conditions, including dysentery, enteritis, mastitis, scrofula, and contusion.
Aim of the review: This review aims to provide a comprehensive overview of the botany, traditional uses, phytochemistry, biological activity and safety of H. sampsonii and to highlight its potential for medical application and drug development.
Materials and methods: We searched several databases, i.e., Web of Science, SciFinder, PubMed, CBM, CNKI, Google Scholar, etc., for relevant information on H. sampsonii. Additionally, we also consulted some books on Chinese medicine.
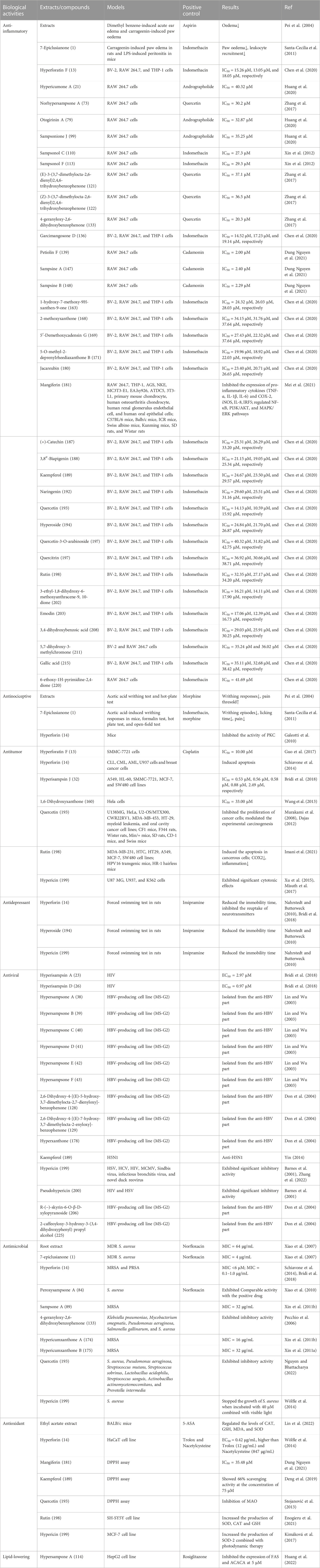
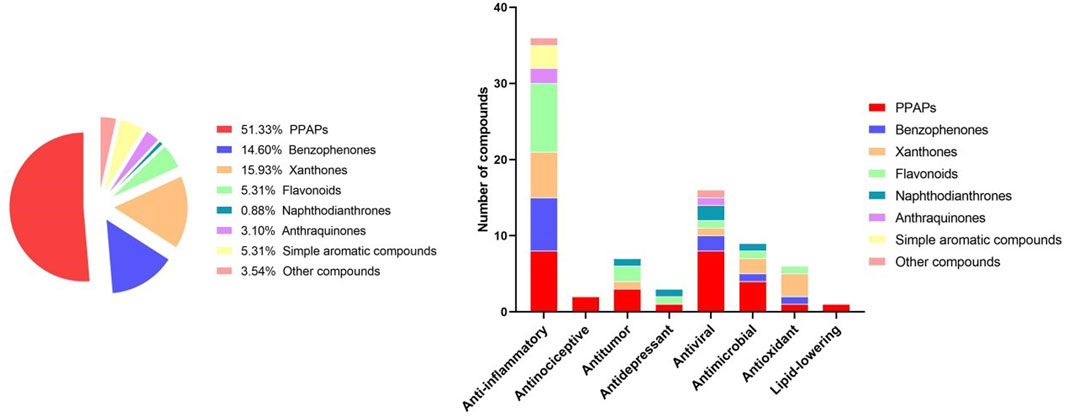
Results: To date, 227 secondary metabolites have been isolated from H. sampsonii, including polycyclic polyprenylated acylphloroglucinols (PPAPs), benzophenones, xanthones, flavonoids, naphthodianthrones, anthraquinones and aromatic compounds. These metabolites exhibit various biological activities such as anti-inflammatory, anti-tumor, anti-depressant, anti-oxidant, anti-viral and anti-bacterial effects. PPAPs are considered the main active metabolites with rich biological activities. Despite being known as rich source of PPAPs, the full extent of H. sampsonii biological activities, including their potential as PDE4 inhibitors, remained unclear. Since, previous studies have mainly been based on structural identification of metabolites in H. sampsonii, and efficacy evaluations of these metabolites based on clinical applications of H. sampsonii lack sufficient data. However, current evidence suggest that PPAPs are the most likely material basis for efficacy. From the limited information available so far, there is no evidence of potential safety issues and the safety data are limited.
Conclusion: Collectively, this review provides a comprehensive overview of the botany, traditional uses, phytochemistry, pharmacology, and safety of H. sampsonii, a valuable medicinal plant in China with various pharmacological activities. Based on pharmacological studies, H. sampsonii shows potential for treating gastrointestinal and gynecological disorders as well as traumatic injuries, which aligns with traditional medicinal use due to the presence of PPAPs, benzophenones, xanthones, and flavonoids. Therefore, further studies are needed to evaluate the pharmacological effects and elucidate the pharmacological mechanisms. In addition, pharmacological mechanisms and safety evaluation of PPAPs on animal models need to be clarified. Yet, further comprehensive studies are required to elucidate the phytochemical constituents, pharmacological mechanisms, structure-activity relationships, safety evaluation, and quality standards of this plant. Takentogether, this review highlights the potential of H. sampsonii for medical application and drug development.
1 Introduction
Plants have been used in traditional medicine for centuries to prevent and treat various diseases. Ethnomedicinal plants, which have clinical evidence of efficacy and safety, play an important role in drug discovery and development (Cragg and Pezzuto, 2016; Anand et al., 2019; Choudhari et al., 2020). The Hypericum genus (Guttiferae) boasts over 460 species that are distributed worldwide, with the exception of arctic and desert regions and most tropical lowlands. (Committee, 2007). Some species are widely used in official medicine throughout the world, such as Hypericum perforatum L. (St. John’s wort) (Stojanovic et al., 2013). However, some endemic species of Hypericum have traditionally been used as folk medicine or ethnomedicine in East Asia, particularly in China (Zhang et al., 2020). Hypericum sampsonii Hance (known as “Yuanbao Cao” in Chinese), which is used as a traditional medicine in south of Changjiang River, has been commonly used as a folk medicine with functions of traumatic bleeding, enteritis, dysentery, and acute mastitis (Gong, 2014; Xie et al., 2021).
Recent years have shown, growing interest of researchers in the chemical constituents and pharmacological effects of H. sampsonii. Previous chemical investigation of H. sampsonii reports a series of metabolites including polycyclic polyprenylated acylphloroglucinols (PPAPs), benzophenones, flavonoids, xanthones, naphthodianthrones, anthraquinones, and aromatic compounds (Xie et al., 2021). The previously reported literatures have revealed that H. sampsonii possesses multiple biological properties, including anti-inflammatory, antinociceptive, antitumor, antidepressant, antimicrobial, antiviral, and antioxidant activities (Tian, 2015; Xie et al., 2021). Accordingly, in the present srudy, we have attempted a pharmacological analysis of the whole plant H. sampsonii to understand the primary target of inflammation and to validate the ethnomedicine reports due to its numerous pharmacological activities and traditional claims of anti-inflammatory properties in the intestinal tract (Lin et al., 2022). However, biological activities and molecular mechanisms of constituents in H. sampsonii have not been fully explored, and a comprehensive and systematic review of this plant is lacking. The present study will not only provide motivation to the growing interest in recent years for a better understanding of the indication-discovery strategies but also assist the concept of drug repurposing in the treatment of many other related clinical conditions that may direct guide towords future research plan.
In this review, the botany, traditional uses, phytochemistry, pharmacological action, and safety of H. sampsonii have been summarized along with discussion over future direction and focus of H. sampsonii in the field of pharmacology.
2 Materials and methods
The relevant information was collected from various search engines: Web of Science, SciFinder, PubMed, CBM, CNKI, Google Scholar, etc. Other literature sources, i.e., classic books of Chinese herbal medicine were also screened to get the maximal information on this plant. The keywords used included H. sampsonii Hance, botany, phytochemistry, pharmacological activity, traditional uses, safety, and other related words. The plant name was also checked with World Flora Online (WFO (2023): Hypericum sampsonii Hance. Published on the Internet; http://www.worldfloraonline.org/taxon/wfo-0000728267. Accessed on: 04 February 2023).
3 Botany
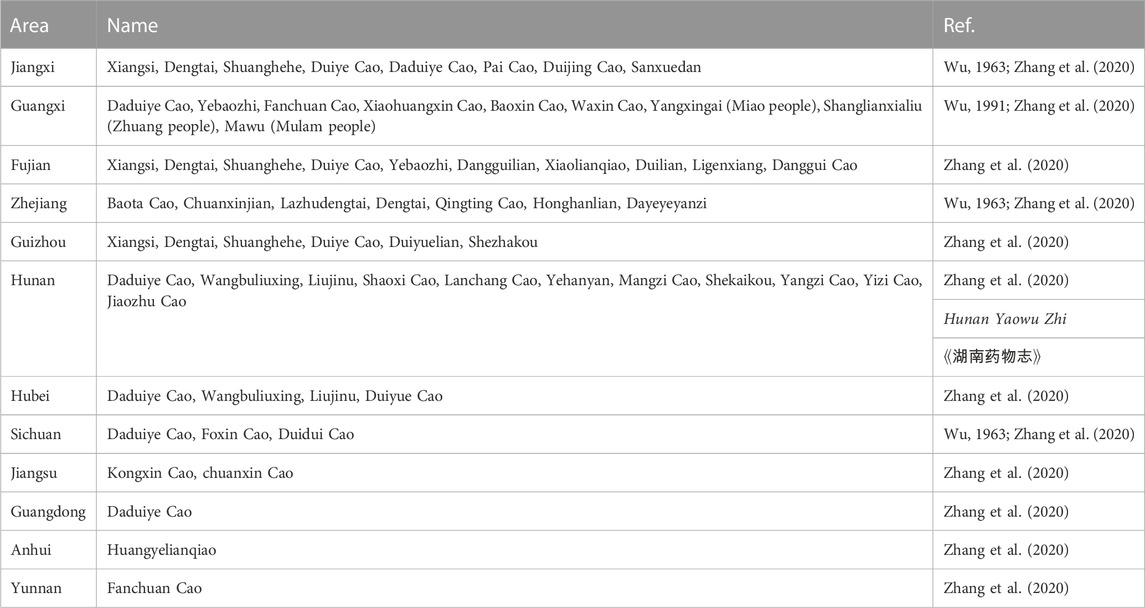
According to World Flora Online, this name of H. sampsonii Hance (Figure 1) of Hypericaceae family has been accepted, with other four synonyms including Hypericum electrocarpum Maxim, H. electrocarpum f. parvifolium R. Keller, Hypericum esquirolii H. Lév, and Hypericum oshimaense R. Keller, in the genus Hypericum (family Hypericaceae). As a folk medicine, various vernacular names of H. sampsonii have been known in China, such as Hezhang Cao, Shangtianti, Dahuanhun, etc (Table 1).
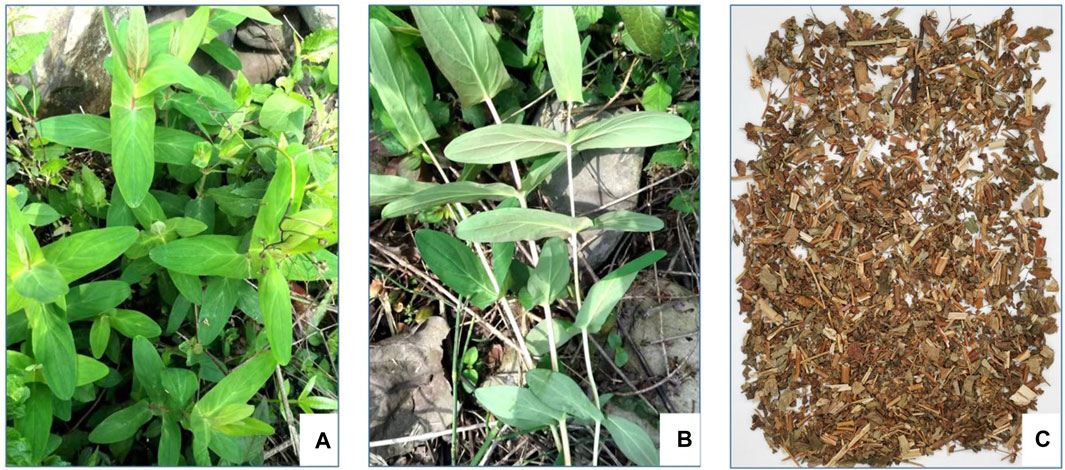
FIGURE 1. Photographs of H. sampsonii: the whole plant (A), the lateral view of connate perfoliate leaves (B), and medicinal material (C).
H. sampsonii, which look like gold ingot (Yuanbao in Chinese) by the connate perfoliate leaves, is a perennial herb with a height of approximately 0.2–0.8 m. The stem is erect and glabrous, with slender and short fibrous roots. The upright stem is cylindroid, and the upper part is branched. The shape of leaves is oblong to lanceolate or oblanceolate, (2–) 2.5–7 (8) cm long, (0.7–) 1–3.5 cm wide, and the apex is obtuse or rounded, sessile, with entire margins. Leaves are arranged in opposite and basally completely connate, green above and light green below with dense marginal black glands. The leaf midrib passes through the leaf apex, and both sides have four lateral veins oblique upward; the vein network is fine and sparse. Its inflorescences like corymbose, terminal, which form into the cylindrical panicle; the bracts and bractlets are linear-lanceolate or linear, 4 mm long, with an acuminate apex. The flowers are 6–10 (−15) mm in diameter, nearly oblate, and cup-shaped at the base; the buds are ovate with an obtuse apex. The pedicel is slender and 2–3 mm long; the sepals are oblong to oblong-spatulate or oblong-linear with 3–7 (–10) mm in length and 1–3 mm in width; the petals are light yellow, ovate, persistent, about 4–8 (−13) mm long and 1.5–4 (−7) mm wide, with marginal sessile or nearly sessile black glands. There are three stamens, each containing 10–14 stamens; the anther is light yellow with black glands. The ovary is ovoid to narrowly conical, three-celled, and about 3 mm long; the style is 3, about 2 mm long, separated from the base. The capsule is ovate with a length of 6–9 mm and covered with yellowish-brown glands; seeds are small long ovate, about 1 mm long with yellowish-brown. The fluorescence duration is from May to June and the fruiting period is from July to August. The whole plant is collected in summer or autumn, dried, and used for medicinal purposes (Editorial Committee of Flora of China, 1990; Xie, 2014; Li et al., 2019).
H. sampsonii not only favors a warm and humid environment but also tolerant to cold and drought. This plant is usually living on hillsides, roadsides, scrub, grassland, fields, and ditches, with an altitude of 0–1,200 m. It is commonly distributed in the south of the Yangtze River and Taiwan in China, and is also found in Japan, Northern Viet Nam, Eastern Myanmar, and Northeast India. Guangxi, Jiangsu, Zhejiang, and Sichuan are major provinces producing this plant (Editorial Committee of Flora of China, 1990).
4 Traditional uses
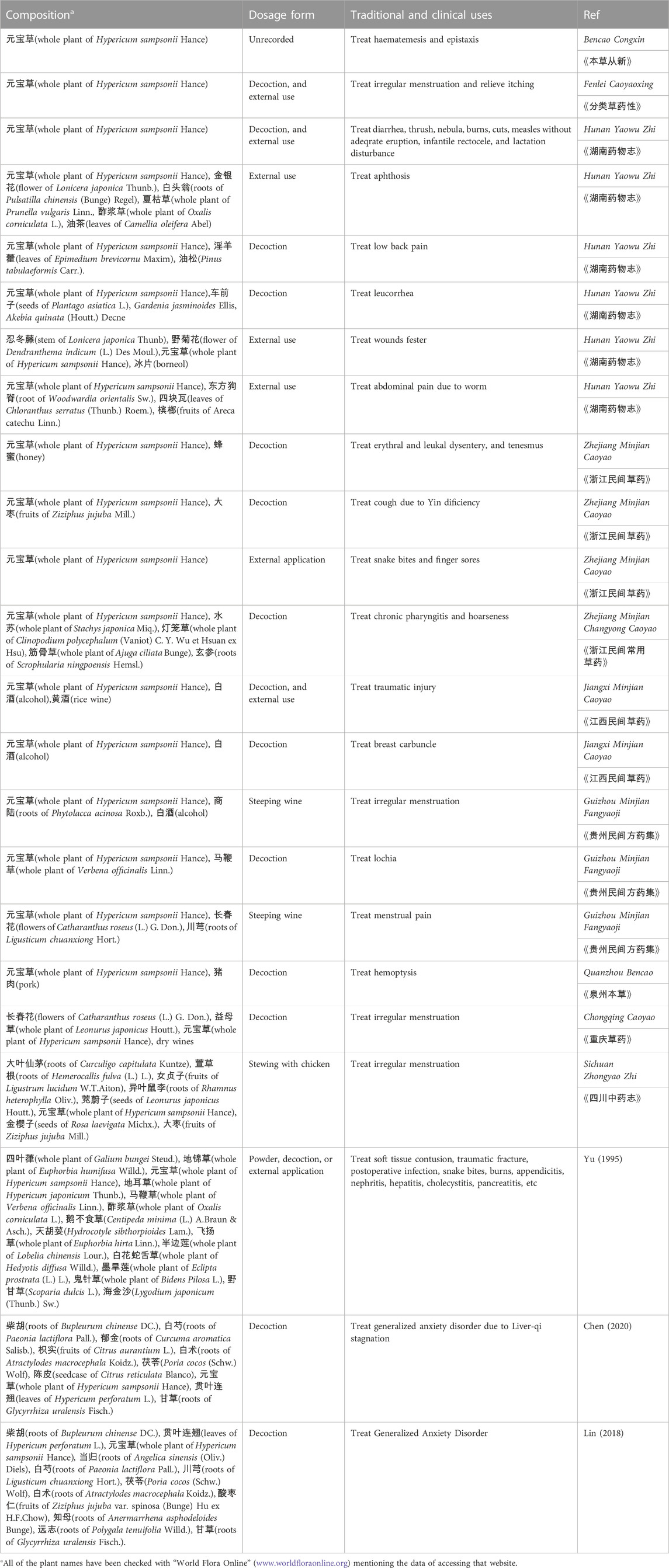
H. sampsonii has been traditionally used as a folk medicine for the treatment of gastrointestinal diseases and traumatic bleeding in China. The medicinal use of this plant was first recorded in the book of Ben Cao Cong Xin (本草从新) in Qing Dynasty, which proposed that it tastes acrid, is cold in nature, functions as nourishing Yin, and can be used to treat haematemesis and epistaxis (Wu, 1957). Textual Research on other monographs of Materia Medica, such as Bai Cao Jing and An Illustrated Book on Plants, records that the plant can be used for treating carbuncle due to toxins, traumatic injury, and deep-rooted breast carbuncles. It has the functions of clearing heat and detoxifying, relaxing tendons and activating collaterals, cooling blood and stopping bleeding. Clinically, H. sampsonii has been used to treat a variety of diseases, such as dysentery, enteritis, infantile fever, infantile convulsion, haematemesis, epistaxis, irregular menstruation, leucorrhea, traumatic bleeding, wounds, mastitis, burns, bedsores, and snakebites, etc (Editorial Board of Chinese Materia Medica, 1999; Xie, 2014; Xu, 2016; Vincent et al., 2021). According to the Gu Shang Zhong Cao Yao Shi Yong Tu Ce (Li et al., 2019), the fresh herb is often processed by pounding or grinding and used to treat traumatic injuries, gouty arthritis, and finger sores. In addition to external application, the whole plant is generally made into a decoction and taken orally for the treatment of rheumatic arthralgia, hemoptysis due to pulmonary trauma, stranguria due to hematuria, dysmenorrhea, and aphthous ulcers, etc. As a common folk medicine, the medicinal uses of H. sampsonii are documented in many local medicinal classics (Table 2). For example, Hunan Yaowu Zhi recorded that the whole plant of H. sampsonii can be used to treat diarrhea.
H. sampsonii was also widely utilized as ethnomedicine by national minorities in China. In Sandu Shui Autonomous County located in the south of Guizhou Province of China, the botanical drug was commonly used as the liquor fermentation starter by the Shui people. Besides the edible value, this wild plant also possesses a wide range of medicinal values and can be used to treat irregular menstruation, leucorrhea, dysentery, and fever (Hong et al., 2015b). Furthermore, it was often used as an herbal tea to treat gynaecopathia by the Yao minority (Jin et al., 2018). H. sampsonii was also the medicinal plant traditionally used by Mulam people in Guangxi Province. It was mainly used to treat internal hemorrhage, abnormal menstruation, dysmenorrhea, and bleeding wound (Hu et al., 2020). According to the ethnobotanical data collected from the Maonan minority, H. sampsonii was used for the treatment of traumatic injury, pain, indigestion, chest congestion, and acute icteric hepatitis (Hong et al., 2015a; Xiang et al., 2018).
Additionally, the whole plant of H. sampsonii is most frequently reported as a traditional treatment for various diseases. Commonly, two processing methods (internal use and external application) are used before clinical use or self-medication. Firstly, it is processed by removing impurities and non-medicinal parts together with auxiliary materials such as honey and alcohol. Subsequently, the dried or fresh herb is often made as a decoction for oral administration to treat various diseases. Furthermore, in the second step, the dried herb can be ground or freshly pounded, and applied to the affected area for external use.
5 Phytochemistry
Chemical investigation of the Hypericum species include a series of phloroglucinol derivatives, naphthodianthrones, xanthones, flavonoids, and other phenols and terpenoids (Zhang et al., 2020). Of these, phloroglucinol derivatives are the main secondary metabolites. H. sampsonii is a rich source of natural products with diverse chemical structures. To date, a total of 223 metabolites including polycyclic polyprenylated acylphloroglucinols (PPAPs), benzophenones, xanthones, flavonoids, bisanthraquinones, and anthraquinones have been separated and identified from H. sampsonii (Supplementary Figure S10).
5.1 Polycyclic polyprenylated acylphloroglucinols (PPAPs)
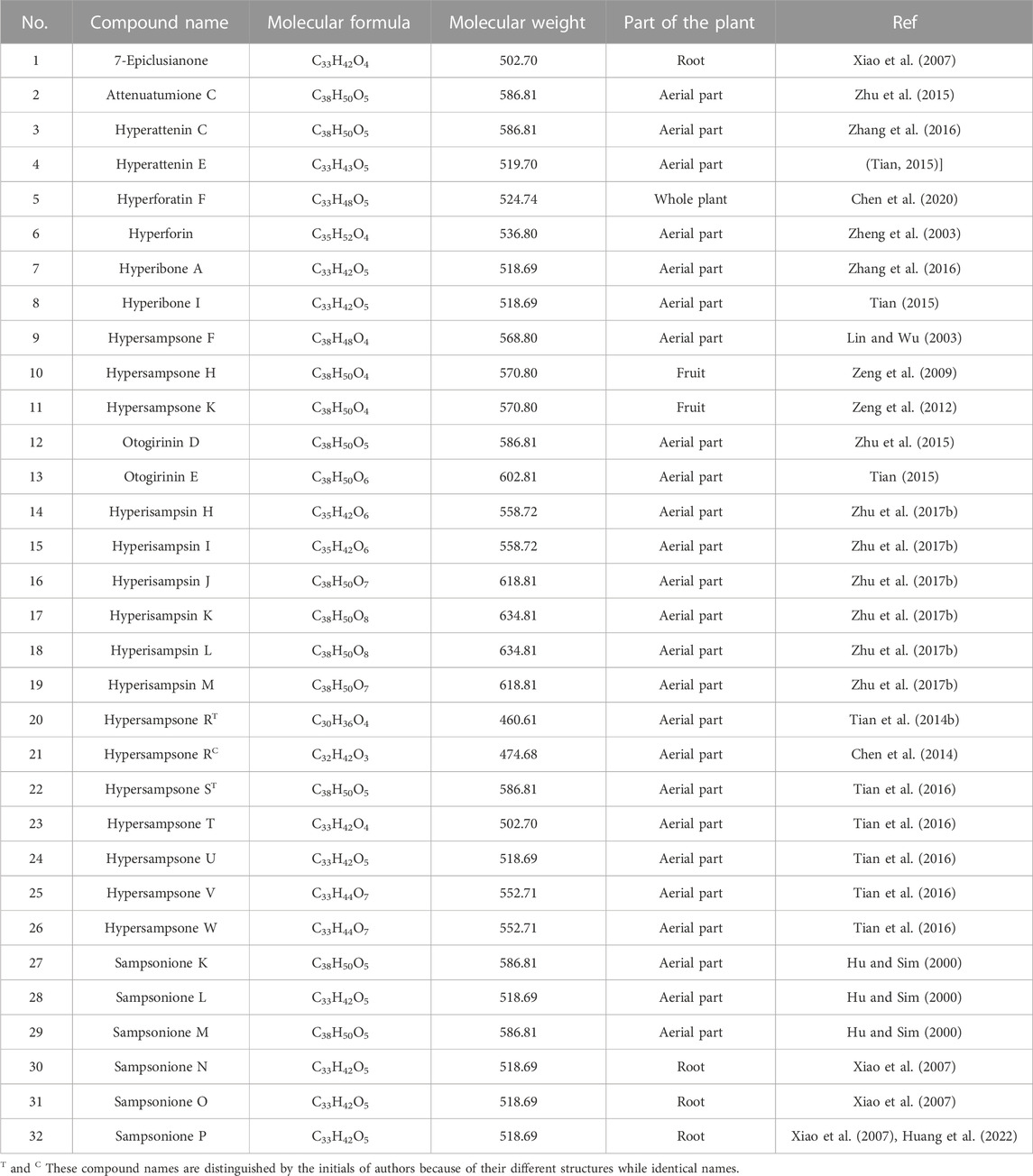
Phloroglucinols, a type of natural products showing strong oxidizing properties, variable stereochemical structures, and a wide range of pharmacological activities, are decorated with isoprenyl and hydroxyl groups which are substituted at multiple positions on the benzene ring or fused together to form a ring (Xiao and Mu, 2007). PPAPs were highly oxygenated acylphloroglucinol derivatives which decorated with complicated side chains. In the past decades, PPAPs have received extensive attention due to their considerable structural diversity and remarkable biological activities (Yang et al., 2018). Biogenetically, all PPAPs which are derived from a common biosynthetic pathway via different cyclizations of the less complex monocyclic polyprenylated acylphloroglucinols, are generated via three main biosynthetic pathways (Yang et al., 2018). Interestingly, this special class of phloroglucinols has been exclusively isolated from the plants of family Guttiferae (Clusiaceae) and mainly from the genera Hypericum and Garcinia. Up to December 2022, 116 PPAPs comprise the major family of metabolites identified from H. sampsonii (Tables 3, 4, 5). All of the PPAP profiles are generated via three major biosynthetic pathways and may be divided into three groups according to their different scaffolds. Group I are the bicyclic polyprenylated acylphloroglucinols (BPAPs) with major bicyclo [3.3.1]nonane-2,4,9-trione core and related seco-BPAPs. Group II include the caged PPAPs with adamantane (tricyclo [3.3.1.1]decane) and homoadamantane (tricyclo [4.3.1.1]undecane) skeletons. Group III contain other biosynthetically related derivatives which derived from direct cyclizations of monocyclic polyprenylated acylphloroglucinols (MPAPs).
5.1.1 Bicyclic polyprenylated acylphloroglucinols (BPAPs)
The bicyclic polyprenylated acylphloroglucinols (BPAPs) with major bicyclo [3.3.1] nonane-2,4,9-trione core and related seco-BPAPs (1–32, Table 3) include 32 BPAPs in which the acyl group is located at the C-1 or C-3 position. In 2000, BPAPs (Sampsoniones K-M, 27-29) were first discovered in the ethanolic extract of the aerial parts of H. sampsonii (Hu and Sim, 2000). Since then, BPAPs have been increasingly explored from the roots, fruits, and aerial part of H. sampsonii.
5.1.2 Caged PPAPs with adamantane or homoadamantane skeletons
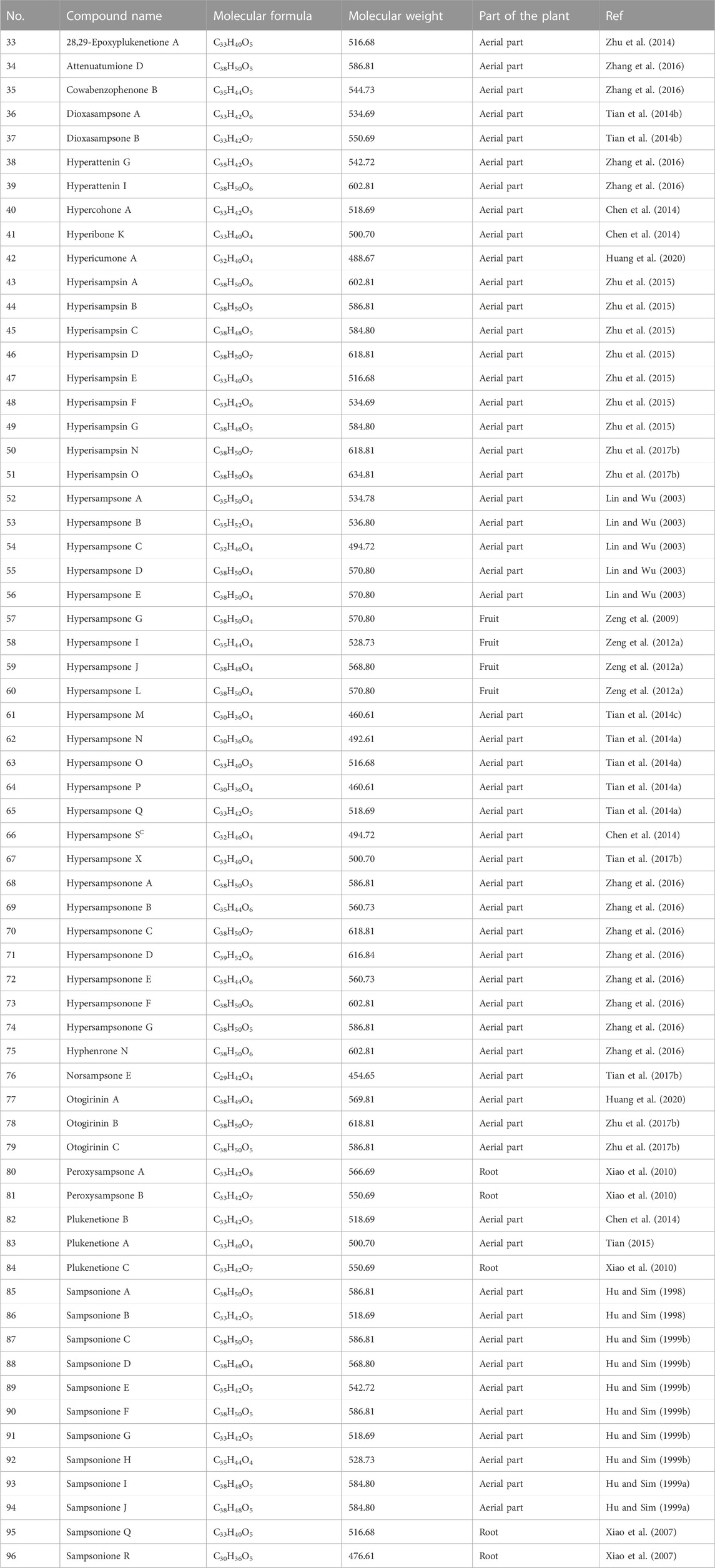
It is noteworthy that H. sampsonii is a rich source of caged PPAPs, and about 64 adamantane- and homoadamantane-type derivatives with adamantane (tricyclo [3.3.1.1]decane) and homoadamantane (tricyclo [4.3.1.1]undecane) (33–96, Table 4) have also been isolated from this plant. As early as 1998, Hu and Sim isolated two caged PPAPs (sampsoniones A and B, 85–86) from the aerial parts of H. sampsonii (Hu and Sim, 1998). Subsequently, they discovered sampsoniones C-J (87–94) (Hu and Sim, 1999a; b, 2000). A few years later, sampsoniones Q-R (95–96) were isolated from the root of H. sampsonii (Xiao et al., 2007). Since then, a large number of studies of caged PPAPs which were isolated from H. sampsonii have been reported, primarily focusing on its structure diversity with an unprecedented carbon skeleton. The tetracyclo [6.3.1.1(3,10).0(3,7)]tridecane skeletons and biogenetically related congeners, such as 28,29-Epoxyplukenetione A (33) (Zhu et al., 2014), hyperisampsins A-G (43–49) (Zhu et al., 2014), hyperisampsins N (50), and hyperisampsins O (51) (Zhu et al., 2017a), hypersampsones A-E (52–56) (Lin and Wu, 2003), hypersampsones L-S (60–66) (Zeng et al., 2012b), and hypersampsonones A-G (68–74) (Zhang et al., 2016).
5.1.3 Other PPAPs
A total of 20 other PPAPs such as spirocyclic PPAPs with octahydrospiro-[cyclohexan-1,5′-indene] core and complicated PPAPs via intramolecular [4 + 2] cycloadditions from MPAPs (97-116, Table 5) have been isolated from H. sampsonii. In 2011, a novel prenylated aromatic lactone (sampsone A, 107) was isolated from the aerial parts of H. sampsonii (Xin et al., 2011). Soon afterwards, six new acylphloroglucinol derivatives, (sampsonols A-F, 108–113), were discovered from the aerial parts of H. sampsonii (Xin et al., 2012). In 2014, four new decarbonyl PPAPs, (norsampsones A-D, 102-105), were isolated from the 60% EtOH extract of the aerial parts of H. sampsonii (Tian et al., 2014c). Recently, three nor-polycyclic polyprenylated acylphloroglucinols with a tetracyclic 6/5/5/6 ring system, (Hypersampones A-C, 114-116), which showed a lipid-lowering activity, were isolated from H. sampsonii. (Huang et al., 2022).
5.2 Benzophenones
Natural benzophenone derivatives have attracted extensive attention due to their unique structures and extensive biological activities. In accordance with the literature, benzophenones, mainly including simple benzophenone derivatives (SBDS) and polyprenylated benzophenones (PPBS), may be the precursors of some xanthones (Kitanov and Nedialkov, 2001). Currently, there are 33 benzophenones isolated from H. sampsonii (Supplementary Figure S4; Table 6). Among them, two pairs of racemic PPBS, (±)-sampsonin A-B (117–120) were chirally separated from H. sampsonii (Tian et al., 2017a). In addition, seven benzophenone derivatives sampbenzophenones A-G (140–146) were isolated from the aerial parts of H. sampsonii (Zhu et al., 2016a).
5.3 Xanthones
Xanthones, a class of iso-tricyclic compounds mainly divided into simple xanthones, glycosylated xanthones, prenylated xanthones, and sulfonated xanthones, are known to possess a variety of biological activities, such as antihypertensive, antiviral, and antitumor activities. In addition, the discrepancy in xanthones activity depends on the substituents on the aromatic rings.
In 1985, Chen MT and Chen CM isolated hyperxanthone (178) from the whole plant of H. sampsonii, and firstly discovered 2-hydroxy-3.4-dimethoxyxanthone (164) and isomangiferin (179) in the genus Hypericum (Chen and Chen, 1985). Further study on the constituents in the whole plant of H. sampsonii, Hong et al. also isolated two xanthone sulfonates, 1,3-dihydroxy-5-methoxyxanthone-4-sulfonate (158) and 1,3-dihydroxy-5-O-β-D-glucopyranosylxanthone-4-sulfonate (159) (Hong et al., 2004). The reported metabolites and structures of xanthones are shown in Supplementary Figure S5; Table 7.
5.4 Flavonoids
Flavonoids, including flavanols, biflavonoids, and common flavonoids, constitute an important class of metabolites in H. sampsonii. To date, twelve flavonoids have been isolated and identified from H. sampsonii (Supplementary Figure S6; Table 8). According to the documents, we have found that structures of these metabolites are generally based on the structure quercetin (193), in which the groups usually substitute at the 3- and 3′- positions, while all of saccharide groups located at C-3 in flavonoid glycosides.
5.5 Naphthodianthrones
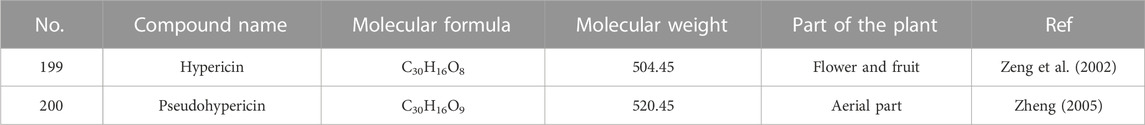
Naphthodianthrones, one out of the most biologically active substances in H. sampsonii, are mainly represented by hypericin and pseudohypericin (Supplementary Figure S7; Table 9). Hypericin (199), the active metabolite isolated from the flowers and fruits of H. sampsonii, is considered the characteristic constituent for the identification of this plant (Zeng et al., 2002). Subsequently, pseudohypericin (200) was isolated from the aerial parts of H. sampsonii (Zheng, 2005).
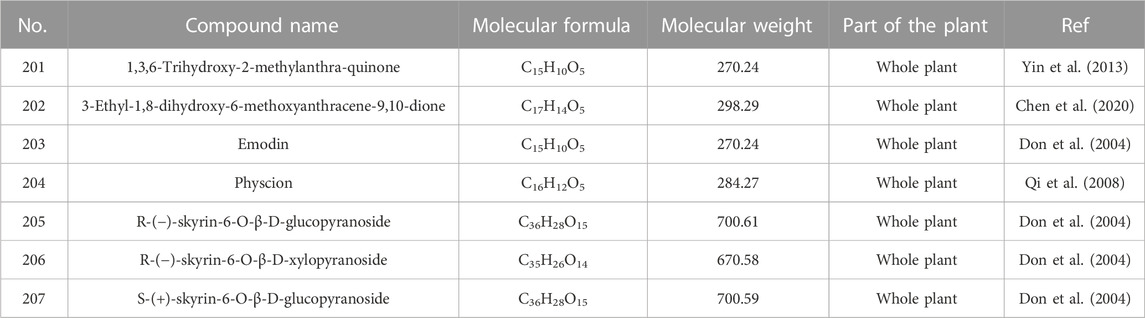
5.6 Anthraquinones
Anthraquinones found in H. sampsonii generally include two types of single anthraquinones and bisanthraquinones. As shown in Supplementary Figure S8, compounds 201–204 are single anthraquinones, while compounds 205–207 are bisanthraquinones. The chemical structures of anthraquinones are listed in Table 10.
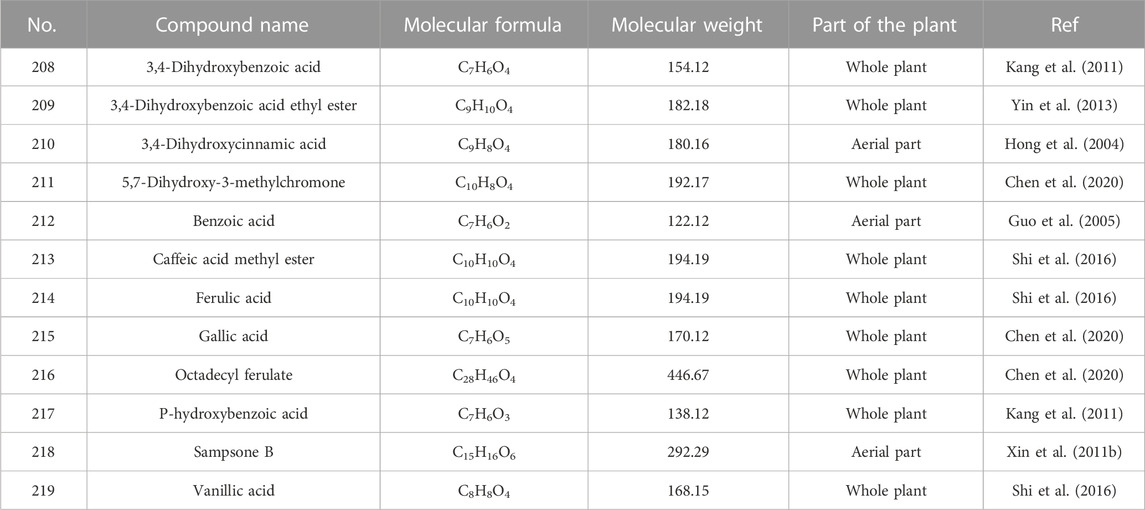
5.7 Simple aromatic compounds
Simple aromatic compounds in the extracts of H. sampsonii refer to the compounds with a benzene ring, which have simple structure and small relative molecular weight. The main compounds are presented in Supplementary Figure S9; Table 11. Xin WB and his co-works found a rare chemical structure sampsone B (218) in the aerial parts of H. sampsonii (Xin et al., 2011).
5.8 Other secondary metabolites
In addition to the aforementioned compounds, other compounds including alkaloids, porphyrins, steroids, pentacyclic triterpenoids and so on have been found in H. sampsonii. Chen Q isolated 6-ethoxy-1H-pyrimidine-2,4-dione (220) from the whole plant of H. sampsonii (Chen et al., 2020). Qi JB and his colleagues found chlorophyll A (221) from the extract of H. sampsonii (Qi et al., 2008). Additionally, Chen Q also discovered β-sitosterol (222) from this plant (Chen et al., 2020). And Guo et al. isolated stigmasteol (223) from the aerial parts of H. sampsonii (Guo et al., 2005). Betulinic acid (224), a pentacyclic triterpenoid compound, was also discovered in this botanical drug (Don et al., 2004). Furthermore, this plant was also demonstrated to contain 2-caffeoyloxy-3-hydroxy-3-(3,4-dihydroxyphenyl) propyl alcohol (225) (Don et al., 2004), octacosanol (226) (Guo et al., 2005), and triacontanoic acid (227) (Guo et al., 2005). The variety and structure of other compounds are displayed in Supplementary Figure S10; Table 12.
6 Biological activities
Recent studies have revealed that several biological activities including anti-inflammatory, anti-tumor, anti-depressant, antiviral, antimicrobial, and antioxidant activities have been documented for extracts and secondary metabolites of H. sampsonii (Tian, 2015). These pharmacological effects have been summarized in Table 13; Figure 2.
6.1 Anti-inflammatory activity
In vitro studies have suggested that extracts of H. sampsonii showed anti-inflammatory activity in lipopolysaccharide (LPS)-treated BV-2, RAW 264.7, and THP-1 cells (Chen et al., 2020). On the other hand, in vivo studies have demonstrated that the alcohol extracts of H. sampsonii had antinociceptive and anti-inflammatory properties. The antinociceptive potential carried out using acetic acid-induced writhing responses in mice and hot-plate test suggested that extracts of H. sampsonii effectively suppressed the writhing symptom and increased the pain threshold of mice. Also, the anti-inflammatory effect was investigated using dimethyl benzene-induced acute ear edema and carrageenin-induced paw edema in rats. Besides, the anti-inflammatory activity have been demonstrated by the reduction of acute ear edema induced by dimethyl benzene and carrageenin-induced paw oedema (Pei et al., 2004). A study of our group to investigate the therapeutic effects and molecular mechanisms of H. sampsonii (HS) in a dextran sulfate sodium (DSS)-induced ulcerative colitis (UC) mice model (Lin et al., 2022). These results indicate that HS distinctly alleviated DSS-stimulated UC-like lesions symptoms as evidenced by a significant recovery from body weight, colon lengths, and histological injuries of colons. HS reduced the accumulation of pro-inflammatory cytokines and improved the mRNA level of IL-10. Simultaneously, the colonic mRNA expression levels of IL- 1β, IL-17, iNOS and COX-2 were all significantly suppressed by HS in a dose-dependent manner. Furthermore, HS restored the protein expression of tight junction-associated protein (ZO-1 and occluding). Further studies have also reported that HS can significantly inhibit the protein level of PDE4 and reduced the expressions of PKA and phosphorylated CREB.
Experimental evidence has emerged to indicate that PPAPs are one of the major constituents required for anti-inflammatory effects. Since, it has been reported that a series of compounds isolated from H. sampsonii, including hyperattenin C (3), otogirinin D (12), hyperisampsin I (15), hyperisampsin J (16), sampsonione L (28), hyperattenin G (38), hypersampsone O (63), hypersampsonone A (68), sampsonione A (85), and sampsonione B (86), were found to have significant PDE4D2 inhibitory activity (Zhang et al., 2016). PDE4D2 is one of the subtypes of phosphodiesterase-4 (PDE4), which can specifically hydrolyze cAMP and participate in various physiological responses, and is a promising drug target for inflammatory diseases such as psoriasis and ulcerative colitis. Moreover, the antinociceptive and anti-inflammatory properties have been reported for 7-epiclusianone (1) using animal models (Santa-Cecilia et al., 2011). In addition to PPAPs, other compounds such as benzophenone, xanthones, flavonoids, anthraquinones, and phenols, are also stated to possess anti-inflammatory properties (Chen et al., 2020).
Besides, benzophenone derivatives have also been shown to be important in the anti-inflammatory effects of H. sampsonii. Recently, our group investigated the therapeutic effect and potential mechanisms of 4-geranyloxy-2,6-dihydroxybenzophenonel (4-GDB, 133) on DSS-induced ulcerative colitis in mice (Wang et al., 2023). This study showed that intragastric administration of 4-GDB (20 mg/kg/day) for 8 days significantly attenuated colonic injury, reduced the expression of inflammatory mediators, and improved colonic barrier function in mice with colitis. Furthermore, in vivo and in vitro experiments indicated that 4-GDB could activate cAMP/PKA/CREB and inhibit the NF-κB pathway. Collectively, 4-GDB may be a potential agent for treating UC by regulating the cAMP/PKA/CREB and NF-κB pathways.
6.2 Antitumor activity
The antitumor activity of H. sampsonii has been evaluated in various cancer cell lines in vitro including A375, MDA-MB-231, SHSY-5Y, and SiHa cell lines (Chen et al., 2020). Studies have suggested that regulation of subcellular localization of retinoid X receptor-alpha (RXR-α) is a potential method to induce tumor cell apoptosis. Zeng et al. have found that H. sampsonii extracts can induce the translocation of RXR-α from the nucleus to the cytoplasm, and promote the apoptosis of NIH-H460, MGC-803, and SMMC-7721 (Jin-Zhang et al., 2006). Besides, the ethanol extract and the chloroform fraction especially were demonstrated for apoptosis-inducing and antitumor properties via inhibiting RXR-α transcription (Han et al., 2007).
Moreover, the antitumor effect has also been documented for a panel of natural products in H. sampsonii, such as 7-epiclusianone (1) (Sales et al., 2015), sampsonione A (85) (Hu and Sim, 1999a), sampsonione I (93) (Hu and Sim, 1999a), mangiferin (181) (Mei et al., 2021), naringenin (192) (Memariani et al., 2021), quercetin (193) (Murakami et al., 2008; Dajas, 2012) and rutin (198) (Imani et al., 2021).
6.3 Antidepressant activity
Depression is a common mental disorder characterized by syndromes like depressed mood, hopelessness, and even thoughts of suicide. Clinical studies have demonstrated that H. perforatum L. (St. John’s Wort), a member of the Hypericum genus, has significant antidepressant impacts. The extract of this plant was introduced into the market as an antidepressant in Germany in 1988, and became the preferred phytomedicine for the treatment of depressive disorder in European and American regions. Intriguingly, previously reported studies have also isloated an antidepressant active metabolite hyperforin rom H. perforatum and its also present in H. sampsonii (Zheng et al., 2003).
In 2003, the ethanol extracts of H. sampsonii were demonstrated for a significant antidepressant effect on the behavior despair animal models (Wan et al., 2003). It was believed that the total flavonoids of H. sampsonii showed antidepressant activity in the hypothermia experiments induced by reserpine and the forced swimming test. In studies utilizing the forced swimming test, tail suspension test, and open-field test, H. sampsonii extracts, HTX fraction, and mangiferin (181) induced a significant reduction in immobility, and the antidepressant mechanism of HTX might be related to neurotransmitters (Gong, 2014). The antidepressant properties of H. sampsonii have been attributed to various phytochemical constituents, such as hyperforin (6), hyperoside (194), and hypericin (199) (Nahrstedt and Butterweck, 2010; Bridi et al., 2018). However, the precise mechanism of action for the antidepressant capacity of this plant remains indistinct.
6.4 Antiviral activity
Previous studies have suggested that the extracts and several compounds of H. sampsonii have antiviral activity. For instance, the chloroform and n-butyl alcohol fractions as well as kaempferol (189) were shown to possess antiviral activity against avian influenza virus H5N1 in the Madin Darby Canine Kidney (MDCK) screening experiment (Yin, 2014). Besides, Lin and Wu found that hypersampsone A-F (52–56, 9) isolated from H. sampsonii exhibited anti-HBV activity on the MS-G2 cell line (Lin and Wu, 2003). In addition, the antiviral activity has been reported for hypericin (199) and pseudohypericin (200) against herpes simplex virus types 1 and 2 and HIV-1 in vitro. Hypericin (199) has also exhibited activity against HCV, murine cytomegalovirus (MCMV), Sindbis virus, infectious bronchitis virus, and novel duck reovirus (Barnes et al., 2001; Zhang et al., 2022).
6.5 Antimicrobial activity
Plants belonging to the Hypericum genus are a crucial source of antimicrobial compounds (Marrelli et al., 2016). Previous evidence indicated that the antibacterial activity has been demonstrated for hyperforin (6) and quercetin (193) against Staphylococcus aureus, Streptococcus mutans, Streptococcus pyogenes, and Corynebacterium diphtheria, etc (Barnes et al., 2001; Nguyen and Bhattacharya, 2022). In studies using MDR S. aureus strain SA-1199B to determine the antibacterial effect of H. sampsonii, the MIC of the petroleum ether extract of the root was up to 64 μg/mL (Xiao et al., 2007). Moreover, 7-epiclusianone (1) induced potent antibacterial activity against SA-1199B with a MIC of 4 μg/mL, while MIC of the positive control (norfloxacin) was 32 μg/mL (Xiao et al., 2007). In other antibacterial experiments, some PPAPs including sampsone A (107) and hypericumxanthone A (174) were shown to exhibit good antibacterial activity on Methicillin-resistant S. aureus (MRSA), with MIC values of 32 μg/mL and 16 μg/mL respectively (Xin et al., 2011).
6.6 Antioxidant activity
Reactive oxygen species (ROS), the important substances released from neutrophils, play a part in cell signaling and homeostasis. It is, however, important to note, that the overproduction of ROS can initiate the inflammatory cascade and subsequent cell damage as well as tissue dysfunction under oxidative stress (Chen et al., 2009). Research revealed that H. sampsonii showed antioxidant capacity by regulating the content of oxidase (GSH and SOD) (Chen et al., 2009). It has also been reported that the ethyl acetate extract of H. sampsonii could alleviate oxidative stress as indicated by reversing the abnormal levels of CAT, GSH, MDA, and SOD in mice with colitis (Lin et al., 2022). Additionally, the antioxidant activity of mangiferin (181) from H. sampsonii was assessed by means of the DPPH radical scavenging assay with an IC50 value of 35.48 μM (Dung Nguyen et al., 2021).
7 Safety
Many ancient classics and medicinal books have recorded that the clinical administration dosage of H. sampsonii should be 9–15 g for dried herb or 30–60 g for fresh herb. To further determine the safety of therapeutic doses of H. sampsonii, a previous study by Lin et al. (2022), fed mice with the ethyl acetate extract at a dose of 2000 mg/kg. After 14 days of observation, there was no morphological abnormality in major organs, indicating that the ethyl acetate extract of H. sampsonii showed no toxicity. Although the toxicity studies and the wide range of edible and medicinal values of H. sampsonii may provide a preliminary reference for its high safety in clinical application; however, the potential toxicity cannot be completely excluded.
According to the Chinese Materia Medica, morphological and microscopic examinations as well as physicochemical identification should be used to control the quality of H. sampsonii. Meanwhile, for its medicinal application, it is should also contain hypericin (199) and flavonoids (Editorial Board of Chinese Materia Medica, 1999). Yet, thin-layer chromatography (TLC) identification and content determination as well as other analytical methods have not been employed to control the quality of this plant, indicating a lack of quality standard despite its extensive folk utilization. Besides, there is no indication of potential safety issues. Therefore, further research work is essentially required to meet these standards.
8 Conclusion and perspectives
As a common botanical drug for the treatment of dysentery, enteritis, and irregular menstruation in folk, H. sampsonii is safe and effective. It is a versatile plant with a complexity of phytochemicals and remarkable pharmacological actions. In this paper, we reviewed the botany, traditional uses, phytochemistry, pharmacological activities, and safety of this species for the first time. It was found that more than 220 chemicals have been isolated and identified from this plant, including PPAPs, benzophenones, xanthones, flavonoids, naphthodianthrones, anthraquinones, and aromatic compounds, among others. Among the identified compounds, PPAPs are the most abundant compounds with novel structures as well as up-and-coming biological characteristics, such as PDE4 inhibitory activity. Although, accumulating studies have shown the progress in the understanding of its anti-inflammatory, anti-tumor, antidepressant, antiviral, antibacterial, and antioxidant properties. However, further studies should focused on the isolation of new compounds and biological screening tests in vitro from H. sampsonii. Yet, it is also important to mention that empirical pharmacologic studies are insufficient to validate the claimed healing properties.
It is noteworthy that H. perforatum L., the most familiar species of the Hypericum genus, has been extensively investigated due to its medicinal values and was listed in the Chinese Pharmacopoeia in 2015. Nevertheless, H. sampsonii has not yet been listed in the Chinese Pharmacopoeia, which potentially prevents in-depth research to a large extent. Taken together, the current pharmacological research on H. sampsonii remains in infancy, and other aspects such as safety evaluation and quality control standards are scanty. This review provided a systematic overview of this plant based on the available research while not comprehensive. Therefore, further studies including pharmacological mechanisms in vitro and in vivo, structure-activity relationship appraisal, safety evaluation, and quality standards should be done. More emerging studies may reveal the scientific connotation of the traditional application and lay the foundation for the development and utilization of H. sampsonii.
Author contributions
ZS: methodology, writing—original draft, writing—review and editing, funding acquisition, supervision. YL and RL: data curation. RZ: conceptualization, writing—original draft, funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Science and Technology Program of Guangdong Province of China (2022B1212010014) and the Guangdong Province’s Projects of High-Level University Construction Funds (No. A1-2601-21-414-001Z06).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1247675/full#supplementary-material
Abbreviations
CNKI, China National Knowledge Infrastructure; IC50, 50% Inhibitory concentration; uM, Micromolar; Pre, isoprenyl; Ger, geranyl; Bz, benzoyl.
References
Anand, U., Jacobo-Herrera, N., Altemimi, A., and Lakhssassi, N. (2019). A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 9, 258. doi:10.3390/metabo9110258
Barnes, J., Anderson, L. A., and Phillipson, J. D. (2001). St john's wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 53, 583–600. doi:10.1211/0022357011775910
Bridi, H., Meirelles, G. d. C., and von Poser, G. L. (2018). Structural diversity and biological activities of phloroglucinol derivatives from Hypericum species. Phytochemistry 155, 203–232. doi:10.1016/j.phytochem.2018.08.002
Chen, C. L., Huang, C. H., and Sung, J. M. (2009). Antioxidants in aerial parts of Hypericum sampsonii, Hypericum japonicum and Hypericum perforatum. Int. J. Food Sci. Technol. 44, 2249–2255. doi:10.1111/j.1365-2621.2009.02066.x
Chen, D. F. (2020). The clinical efficacy observation of huayu changshen decoction in theTreatment of generalized anxiety disorder for liver-qi stagnation type. Fuzhou, China: Fujian University of Traditional Chinese Medicine.
Chen, J. J., Chen, H. J., and Lin, Y. L. (2014). Novel polyprenylated phloroglucinols from Hypericum sampsonii. Molecules 19, 19836–19844. doi:10.3390/molecules191219836
Chen, M. T., and Chen, C. M. (1985). Xanthones from Hypericum sampsonii. Heterocycles 23, 2543–2548. doi:10.3987/r-1985-10-2543
Chen, Q., Di, L., Zhang, Y., and Li, N. (2020). Chemical constituents with cytotoxic and anti-inflammatory activity in Hypericum sampsonii and the antitumor potential under the view of cancer-related inflammation. J. Ethnopharmacol. 259, 112948. doi:10.1016/j.jep.2020.112948
Choudhari, A. S., Mandave, P. C., Deshpande, M., Ranjekar, P., and Prakash, O. (2020). Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front. Pharmacol. 10, 1614. doi:10.3389/fphar.2019.01614
Cragg, G. M., and Pezzuto, J. M. (2016). Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Princ. Pract. 25, 41–59. doi:10.1159/000443404
Dajas, F. (2012). Life or death: Neuroprotective and anticancer effects of quercetin. J. Ethnopharmacol. 143, 383–396. doi:10.1016/j.jep.2012.07.005
Deng, S. P., Yang, Y. L., Cheng, X. X., Li, W. R., and Cai, J. Y. (2019). Synthesis, spectroscopic study and radical scavenging activity of kaempferol derivatives: Enhanced water solubility and antioxidant activity. Int. J. Mol. Sci. 20, 975. doi:10.3390/ijms20040975
Don, M. J., Huang, Y. J., Huang, R. L., and Lin, Y. L. (2004). New phenolic principles from Hypericum sampsonii. Chem. Pharm. Bull. 52, 866–869. doi:10.1248/cpb.52.866
Dong, H. X., Xia, C., Liu, Y., Fan, M., Wu, T., and Shi, J. C. (2015). Study on chemical constituents in Hypericum sampsonii. J. Plant Resour. Environ. 24, 110–112.
Dung Nguyen, V., Vinh Le, B., Thuan Nguyen, D., Van Anh Pham, T., Han Tran, T., Viet Cuong Le, C., et al. (2021). Bioactive compounds from the aerial parts of Hypericum sampsonii. Nat. Prod. Res. 35, 646–648. doi:10.1080/14786419.2019.1586690
Editorial Board of Chinese Materia Medica (1999). Chinese Materia Medica. Shanghai: Science and Technology Publishing House.
Enogieru, A. B., Haylett, W., Hiss, D., and Ekpo, O. (2021). Inhibition of γH2AX, COX-2 and regulation of antioxidant enzymes in MPP+-exposed SH-SY5Y cells pre-treated with rutin. Metab. Brain. Dis. 36, 2119–2130. doi:10.1007/s11011-021-00746-z
Galeotti, N., Vivoli, E., Bilia, A. R., Bergonzi, M. C., Bartolini, A., and Ghelardini, C. (2010). A prolonged protein kinase C-mediated, opioid-related antinociceptive effect of st john's wort in mice. J. Pain 11, 149–159. doi:10.1016/j.jpain.2009.06.013
Gong, Y. Z. (2014). Study of antidepressive effect of HTX from Hypericum sampsonii hance. Fuzhou, China: Fujian University of Traditional Chinese Medicine.
Guo, C., Zheng, Q. M., and Zheng, H. C. (2005). Study of the chemical constituents of Hypericum sampsonii. Pharm. Care Res. 5, 341–344.
Guo, C., Zheng, Q. M., and Zheng, H. C. (2007). Study on xanthones from Hypericum samponii hance. Chin. Pharm. J., 418–419+436.
Guo, Y., Zhang, N., Chen, C. M., Huang, J. F., Li, X. N., Liu, J. J., et al. (2017). Tricyclic polyprenylated acylphloroglucinols from st john's wort, Hypericum perforatum. J. Nat. Prod. 80, 1493–1504. doi:10.1021/acs.jnatprod.6b01178
Han, C. L., Sun, D. F., Wu, D. J., Guo, Y. Q., Wang, L., Qi, J. B., et al. (2007). Hypericum sampsonii exerts its apoptotic activity on lung cancer cells through binding and inducing RXRa translocation. Med. J. Chin. People's Armed Police Forces, 729–732.
Hong, D., Yin, F., Hu, L. H., and Lu, P. (2004). Sulfonated xanthones from Hypericum sampsonii. Phytochemistry 65, 2595–2598. doi:10.1016/j.phytochem.2004.08.014
Hong, L., Guo, Z., Huang, K., Wei, S., Liu, B., Meng, S., et al. (2015a). Ethnobotanical study on medicinal plants used by Maonan people in China. J. Ethnobiol. Ethnomed. 11, 32. doi:10.1186/s13002-015-0019-1
Hong, L., Zhuo, J., Lei, Q., Zhou, J., Ahmed, S., Wang, C., et al. (2015b). Ethnobotany of wild plants used for starting fermented beverages in Shui communities of southwest China. J. Ethnobiol. Ethnomed. 11, 42. doi:10.1186/s13002-015-0028-0
Hu, L. H., and Sim, K. Y. (1998). Complex caged polyisoprenylated benzophenone derivatives, sampsoniones A and B, from Hypericum sampsonii. Tetrahedron Lett. 39, 7999–8002. doi:10.1016/s0040-4039(98)01741-9
Hu, L. H., and Sim, K. Y. (1999a). Cytotoxic polyprenylated benzoylphloroglucinol derivatives with an unusual adamantyl skeleton from Hypericum sampsonii (Guttiferae). Org. Lett. 1, 879–882. doi:10.1021/ol9907825
Hu, L. H., and Sim, K. Y. (2000). Sampsoniones A-M, a unique family of caged polyprenylated benzoylphloroglucinol derivatives, from Hypericum sampsonii. Tetrahedron 56, 1379–1386. doi:10.1016/s0040-4020(00)00010-7
Hu, L. H., and Sim, K. Y. (1999b). Sampsoniones C-H, a unique family of polyprenylated benzophenone derivatives with the novel tetracyclo 7.3.1.1(3,11).0(3,7) tetradecane-2,12,14-trione skeleton, from Hypericum sampsonii (Guttiferae). Tetrahedron Lett. 40, 759–762. doi:10.1016/s0040-4039(98)02366-1
Hu, R., Lin, C., Xu, W., Liu, Y., and Long, C. (2020). Ethnobotanical study on medicinal plants used by Mulam people in Guangxi, China. J. Ethnobiol. Ethnomed. 16, 40. doi:10.1186/s13002-020-00387-z
Huang, C. Y., Chang, T. C., Wu, Y. J., Chen, Y., and Chen, J. J. (2020). Benzophenone and benzoylphloroglucinol derivatives from Hypericum sampsonii with anti-inflammatory mechanism of otogirinin A. Molecules 25, 4463. doi:10.3390/molecules25194463
Huang, L., Zhang, Z.-Z., Li, Y.-N., Yi, P., Gu, W., Yang, J., et al. (2022). Hypersampones A-C, three nor-polycyclic polyprenylated acylphloroglucinols with lipid-lowering activity from Hypericum sampsonii. Org. Lett. 24, 5967–5971. doi:10.1021/acs.orglett.2c02240
Imani, A., Maleki, N., Bohlouli, S., Kouhsoltani, M., Sharifi, S., and Dizaj, S. M. (2021). Molecular mechanisms of anticancer effect of rutin. Phytother. Res. 35, 2500–2513. doi:10.1002/ptr.6977
Jin, B., Liu, Y., Xie, J., Luo, B., and Long, C. (2018). Ethnobotanical survey of plant species for herbal tea in a Yao autonomous county (jianghua, China): Results of a 2-year study of traditional medicinal markets on the dragon boat festival. J. Ethnobiol. Ethnomed. 14, 58. doi:10.1186/s13002-018-0257-0
Jin-Zhang, Z., De-Fu, S., Li, W., Xihua, C., Jian-Bin, Q., Ting, Y., et al. (2006). Hypericum sampsonii induces apoptosis and nuclear export of retinoid X receptor-alpha. Carcinogenesis 27, 1991–2000. doi:10.1093/carcin/bgl046
Kang, J. M., Ouyang, S., Xiao, B. K., Yang, J. Y., and Huang, R. Q. (2011). Isolation and identification of the chemical constituents from the Hypericum sampsonii hance. Lishizhen Med. Mat. Med. Res. 22, 2641–2642.
Kimáková, P., Solár, P., Fecková, B., Sačková, V., Solárová, Z., Ilkovičová, L., et al. (2017). Photoactivated hypericin increases the expression of SOD-2 and makes MCF-7 cells resistant to photodynamic therapy. Biomed. Pharmacother. 85, 749–755. doi:10.1016/j.biopha.2016.11.093
Kitanov, G. M., and Nedialkov, P. T. (2001). Benzophenone O-glucoside, a biogenic precursor of 1,3,7-trioxygenated xanthones in Hypericum annulatum. Phytochemistry 57, 1237–1243. doi:10.1016/s0031-9422(01)00194-7
Li, S. G., Dai, Y. L., Li, C. R., and Xu, S. Z. (2019). Gu Shang Zhong Cao Yao Shi Yong Tu Ce. Fuzhou, China: Fujian Science and Technology Publishing House.
Li, Z. Q., Luo, L., Ma, G. Y., Huang, R., and Hu, Z. H. (2004). Sampsoniones and xanthones of Hypericum sampsonii from yunnan province. Chin. Tradit. Herb. Drugs 35, 17–20.
Lin, Q. F. (2018). The clinical efficacy observation of wangyou ningshen decoction to patients with generalized anxiety Disorder. Fuzhou, China: Fujian University of Traditional Chinese Medicine.
Lin, Y. L., and Wu, Y. S. (2003). Polyprenylated phloroglucinol derivatives from Hypericum sampsonii. Helv. Chim. Acta 86, 2156–2163. doi:10.1002/hlca.200390173
Lin, Y., Su, J., Wang, M., Li, Y., Zhao, Z., and Sun, Z. (2022). Hypericumsampsonii attenuates inflammation in mice with ulcerative colitis via regulation of PDE4/PKA/CREB signaling pathway. J. Ethnopharmacol. 296, 115447. doi:10.1016/j.jep.2022.115447
Marrelli, M., Statti, G., Conforti, F., and Menichini, F. (2016). New potential pharmaceutical applications of Hypericum species. Mini-Rev. Med. Chem. 16, 710–720. doi:10.2174/1389557515666150709105844
Mei, S. H., Ma, H. L., and Chen, X. M. (2021). Anticancer and anti-inflammatory properties of mangiferin: A review of its molecular mechanisms. Food Chem. Toxicol. 149, 111997. doi:10.1016/j.fct.2021.111997
Memariani, Z., Abbas, S. Q., Ul Hassan, S. S., Ahmadi, A., and Chabra, A. (2021). Naringin and naringenin as anticancer agents and adjuvants in cancer combination therapy: Efficacy and molecular mechanisms of action, a comprehensive narrative review. Pharmacol. Res. 171, 105264. doi:10.1016/j.phrs.2020.105264
Misuth, M., Horvath, D., Miskovsky, P., and Huntosova, V. (2017). Synergism between PKC delta regulators hypericin and rottlerin enhances apoptosis in U87 MG glioma cells after light stimulation. Photodiagn. Photodyn. Ther. 18, 267–274. doi:10.1016/j.pdpdt.2017.03.018
Murakami, A., Ashida, H., and Terao, J. (2008). Multitargeted cancer prevention by quercetin. Cancer Lett. 269, 315–325. doi:10.1016/j.canlet.2008.03.046
Nahrstedt, A., and Butterweck, V. (2010). Lessons learned from herbal medicinal products: The example of st. John's wort (perpendicular). J. Nat. Prod. 73, 1015–1021. doi:10.1021/np1000329
Nguyen, T. L. A., and Bhattacharya, D. (2022). Antimicrobial activity of quercetin: An approach to its mechanistic principle. Molecules 27, 2494. doi:10.3390/molecules27082494
Pecchio, M., Solis, P. N., Lopez-Perez, J. L., Vasquez, Y., Rodriguez, N., Olmedo, D., et al. (2006). Cytotoxic and antimicrobial benzophenones from the leaves of Tovomita longifolia. J. Nat. Prod. 69, 410–413. doi:10.1021/np050338c
Pei, J., Yang, L., Huang, L. F., and Wang, G. Z. (2004). “Experimental study on antinociceptive and anti-inflammatory effects of three species of sect. Hypericum medicinal plants,” in The fourth national symposium on medicinal botany and botanical medicine (Nanjing, China: China Academic Journal Electronic Publishing House), 201–204.
Qi, J. B., Wang, L., Chen, C., Zeng, J. Z., and Hu, C. Q. (2008). Anti-tumor components targeting to RXRα from Hypericum sampsonii. Nat. Prod. Res. Dev., 129–130+169.
Qiu, Y. Q., Tian, W. J., Li, C., Dai, Y., and Yao, X. S. (2015). Two new compounds from Hypericum sampsonii. Chin. Tradit. Herb. Drugs 46, 625–628.
Sales, L., Pezuk, J. A., Borges, K. S., Brassesco, M. S., Scrideli, C. A., Tone, L. G., et al. (2015). Anticancer activity of 7-epiclusianone, a benzophenone from Garcinia brasiliensis, in glioblastoma. BMC Complement. Altern. Med. 15, 393. doi:10.1186/s12906-015-0911-1
Santa-Cecilia, F. V., Freitas, L. A. S., Vilela, F. C., Veloso, C. D., da Rocha, C. Q., Moreira, M. E. C., et al. (2011). Antinociceptive and anti-inflammatory properties of 7-epiclusianone, a prenylated benzophenone from Garcinia brasiliensis. Eur. J. Pharmacol. 670, 280–285. doi:10.1016/j.ejphar.2011.08.032
Schiavone, B. I. P., Verotta, L., Rosato, A., Marilena, M., Gibbons, S., Bombardelli, E., et al. (2014). Anticancer and antibacterial activity of hyperforin and its derivatives. Anti-Cancer Agents Med. Chem. 14, 1397–1401. doi:10.2174/1871520614999140829122803
Shi, L. L., Xu, M. P., Tan, H. B., and Qiu, S. X. (2016). Chemical constituents from Hypericum sampsonii. J. Shanxi Univ. 39, 264–268.
Stojanovic, G., Dordevic, A., and Smelcerovic, A. (2013). Do other Hypericum species have medical potential as st. John's wort (Hypericum perforatum)? Curr. Med. Chem. 20, 2273–2295. doi:10.2174/0929867311320180001
Stojanović, G., Ðorđević, A., and Šmelcerović, A. (2013). Do other Hypericum species have medical potential as St. John's wort (Hypericum perforatum)? Curr. Med. Chem. 20, 2273–2295. doi:10.2174/0929867311320180001
Tian, W. J., Qiu, Y. Q., Chen, H. F., Jin, X. J., Yao, X. J., Dai, Y., et al. (2017a). Chiral separation and absolute configurations of two pairs of racemic polyprenylated benzophenones from Hypericum sampsonii. Fitoterapia 116, 39–44. doi:10.1016/j.fitote.2016.10.014
Tian, W. J., Qiu, Y. Q., Chen, J. J., Yao, X. J., Wang, G. H., Dai, Y., et al. (2017b). Norsampsone E, an unprecedented decarbonyl polycyclic polyprenylated acylphloroglucinol with a homoadamantyl core from Hypericum sampsonii. RSC Adv. 7, 33113–33119. doi:10.1039/c7ra05947g
Tian, W. J., Qiu, Y. Q., Jin, X. J., Chen, H. F., Yao, X. J., Dai, Y., et al. (2016). Hypersampsones S-W, new polycyclic polyprenylated acylphloroglucinols from Hypericum sampsonii. RSC Adv. 6, 50887–50894. doi:10.1039/c5ra26332h
Tian, W. J., Qiu, Y. Q., Jin, X. J., Chen, H. F., Yao, X. J., Dai, Y., et al. (2014a). Novel polycyclic polyprenylated acylphloroglucinols from Hypericum sampsonii. Tetrahedron 70, 7912–7916. doi:10.1016/j.tet.2014.08.062
Tian, W. J., Qiu, Y. Q., Yao, X. J., Chen, H. F., Dai, Y., Zhang, X. K., et al. (2014b). Dioxasampsones A and B, two polycyclic polyprenylated acylphloroglucinols with unusual epoxy-ring-fused skeleton from Hypericum sampsonii. Org. Lett. 16, 6346–6349. doi:10.1021/ol503122m
Tian, W. J. (2015). Study on the chemical constituents of the aerial parts of Hypericum sampsonii. Shenyang, China: Shenyang Pharmaceutical University.
Tian, W. J., Yu, Y., Yao, X. J., Chen, H. F., Dai, Y., Zhang, X. K., et al. (2014c). Norsampsones A-D, four new decarbonyl polycyclic polyprenylated acylphloroglucinols from Hypericum sampsonii. Org. Lett. 16, 3448–3451. doi:10.1021/ol501333k
Vincent, O. M., Nguta, J. M., Mitema, E. S., Musila, F. M., Nyak, D. M., Mohammed, A. H., et al. (2021). Ethnopharmacology, pharmacological activities, and chemistry of the Hypericum genus. J. Phytopharm. 10, 105–113. doi:10.31254/phyto.2021.10206
Wan, D., Pei, J., Zhou, M., and Wang, G. (2003). Antidepressant effect of ethanol extracts from three species of sect. Hypericum medicinal plants in mice. J. Chin. Med. Mat. 26, 187–189.
Wang, H. F., Wei, L. Q., Yan, H., Gao, X. H., Xu, B. S., and Tang, N. (2013). Antitumor activity and DNA-binding investigations of isoeuxanthone and its piperidinyl derivative. Chem. Pharm. Bull. 61, 599–603. doi:10.1248/cpb.c12-00500
Wang, M. Q., Li, Y. Z., Su, J. H., Bai, J. Y., Zhao, Z. X., and Sun, Z. H. (2023). Protective effects of 4-geranyloxy-2,6-dihydroxybenzophenonel on DSS-induced ulcerative colitis in mice via regulation of cAMP/PKA/CREB and NF-kappa B signaling pathways. Phytotherapy Res. 37, 1330–1345. doi:10.1002/ptr.7689
Wölfle, U., Seelinger, G., and Schempp, C. M. (2014). Topical application of St. John's wort (Hypericum perforatum). Planta Med. 80, 109–120. doi:10.1055/s-0033-1351019
Xiang, S., Zhang, L., and Zou, K. (2018). A kind of maonan formula for treating acute icteric hepatitis and preparation method thereof. Peop. Rep. China.
Xiao, Z. Y., and Mu, Q. (2007). Advance on chemical investigation of Hypericum. Nat. Prod. Res. Dev., 344–355.
Xiao, Z. Y., Mu, Q., Shiu, W. K. P., Zeng, Y. H., and Gibbons, S. (2007). Polyisoprenylated benzoylphloroglucinol derivatives from Hypericum sampsonii. J. Nat. Prod. 70, 1779–1782. doi:10.1021/np0704147
Xiao, Z. Y., Shiu, W. K. P., Zeng, Y. H., Mu, Q., and Gibbons, S. (2008). A naturally occurring inhibitory agent from Hypericum sampsonii with activity against multidrug-resistant staphylococcus aureus. Pharm. Biol. 46, 250–253. doi:10.1080/13880200701739405
Xiao, Z. Y., Zeng, Y. H., Mu, Q., Shiu, W. K. P., and Gibbons, S. (2010). Prenylated benzophenone peroxide derivatives from Hypericum sampsonii. Chem. Biodivers. 7, 953–958. doi:10.1002/cbdv.200900247
Xie, M. J., Guo, Y. X., Li, F. C., Chen, H. Y., Lai, X. H., Sui, J. N., et al. (2021). Research progress on chemical components and pharmacological effects of Hypericum sampsonii and analysis and prediction of its quality markers. Acta Chin. Med. Pharmacol. 49, 40–44.
Xin, W.-b., Man, X.-h., Zheng, C.-j., Jia, M., Jiang, Y.-p., Zhao, X.-x., et al. (2012). Prenylated phloroglucinol derivatives from Hypericum sampsonii. Fitoterapia 83, 1540–1547. doi:10.1016/j.fitote.2012.08.022
Xin, W.-B., Mao, Z.-J., Jin, G.-L., and Qin, L.-P. (2011a). Two new xanthones from Hypericum sampsonii and biological activity of the isolated compounds. Phytother. Res. 25, 536–539. doi:10.1002/ptr.3291
Xin, W. B., Jin, G. L., Mao, Z. J., and Qin, L. P. (2011b). Two unusual phenolic substances and one new xanthone from Hypericum sampsonii. Helv. Chim. Acta 94, 686–692. doi:10.1002/hlca.201000281
Xu, C. (2016). A traditional Chinese medicine preparation for treating infantile convulsion and its preparation method. Peop. Rep. China.
Xu, Y. X., Wang, D. X., Zhuang, Z. Z., Jin, K. K., Zheng, L. Z., Yang, Q., et al. (2015). Hypericin-mediated photodynamic therapy induces apoptosis in K562 human leukemia cells through JNK pathway modulation. Mol. Med. Rep. 12, 6475–6482. doi:10.3892/mmr.2015.4258
Yang, X.-W., Grossman, R. B., and Xu, G. (2018). Research progress of polycyclic polyprenylated acylphloroglucinols. Chem. Rev. 118, 3508–3558. doi:10.1021/acs.chemrev.7b00551
Yin, H. J., Li, B., Zhou, Q., and Dong, J. X. (2013). A new benzophenone derivatives from Hypericum sampsonii. Nat. Prod. Res. Dev. 25, 875–877.
Yin, H. J. (2014). Study on the anti-H5N1 constituents of Hypericum sampsonii. Hefei: Anhui Medical University.
Yu, Y. P., and Diamandis, E. P. (1995). Prostate-specific antigen in milk of lactating women. Fujian J. TCM 26, 54–58. doi:10.1093/clinchem/41.1.54
Zeng, H. Y., Zhou, P. H., and Gang, P. (2002). Studies on chemical constituents of Hypericum sampsonii. Nat. Prod. Res. Dev. 14, 50–53.
Zeng, Y.-H., Osman, K., Xiao, Z.-Y., Gibbons, S., and Mu, Q. (2012). Four geranyl-bearing polyisoprenylated benzoylphloroglucinol derivatives from Hypericum sampsonii. Phytochem. Lett. 5, 200–205. doi:10.1016/j.phytol.2011.09.009
Zeng, Y. H., Mu, Q., Xiao, Z. Y., Xu, Y., Rahman, M. M., and Gibbons, S. (2009). Geranyl bearing polyisoprenylated benzoylphloroglucinol derivatives from Hypericum sampsonii. Chem. Lett. 38, 440–441. doi:10.1246/cl.2009.440
Zhang, J.-S., Zou, Y.-H., Guo, Y.-Q., Li, Z.-Z., Tang, G.-H., and Yin, S. (2016). Polycyclic polyprenylated acylphloroglucinols: Natural phosphodiesterase-4 inhibitors from Hypericum sampsonii. RSC Adv. 6, 53469–53476. doi:10.1039/c6ra08805h
Zhang, J., Gao, L., Hu, J., Wang, C. J., Hagedoorn, P. L., Li, N., et al. (2022). Hypericin: Source, determination, separation, and properties. Sep. Purif. Rev. 51, 1–10. doi:10.1080/15422119.2020.1797792
Zhang, J. S., Huang, J. L., Zou, Y. H., Liu, X., Ahmed, A., Tang, G. H., et al. (2017). Novel degraded polycyclic polyprenylated acylphloroglucinol and new polyprenylated benzophenone from Hypericum sampsonii. Phytochem. Lett. 21, 190–193. doi:10.1016/j.phytol.2017.06.023
Zhang, R., Ji, Y., Zhang, X., Kennelly, E. J., and Long, C. (2020). Ethnopharmacology of Hypericum species in China: A comprehensive review on ethnobotany, phytochemistry and pharmacology. J. Ethnopharmacol. 254, 112686. doi:10.1016/j.jep.2020.112686
Zhang, Z. Z., Zeng, Y. R., Li, Y. N., Hu, Z. X., Huang, L. J., Gu, W., et al. (2021). Two new seco-polycyclic polyprenylated acylphloroglucinol from Hypericum sampsonii. Org. Biomol. Chem. 19, 216–219. doi:10.1039/d0ob02072a
Zheng, Q. M. (2005). Pharmacognostical studies on Hypericum sampsonii and other species of Hypericum. Shanghai , China: Second Military Medical University.
Zheng, Q. M., Qin, L. P., Zheng, H. C., Chen, Y., Zhang, C., Zhang, Q. Y., et al. (2003). Quantitative phytochemical analysis of 11 Hypericum species growing in China. Acad. J. Second Mil. Med. Univ. 24, 457–459.
Zhu, H. C., Chen, C. M., Zhang, J. W., Guo, Y., Tan, D. D., Wei, G. Z., et al. (2017). Hyperisampsins N and O, two new benzoylated phloroglucinol derivatives from Hypericum sampsonii. Chin. Chem. Lett. 28, 986–990. doi:10.1016/j.cclet.2016.11.014
Zhu, H., Chen, C., Tan, D., Li, D., Guo, Y., Wei, G., et al. (2016a). Sampbenzophenones A-G, prenylated benzoylphloroglucinol derivatives from Hypericum sampsonii. RSC Adv. 6, 86710–86716. doi:10.1039/c6ra17885e
Zhu, H., Chen, C., Tong, Q., Chen, X., Yang, J., Liu, J., et al. (2015). Hyperisampsins H-M, cytotoxic polycyclic polyprenylated acylphloroglucinols from Hypericum sampsonii. Sci. Rep. 5, 14772. doi:10.1038/srep14772
Zhu, H., Chen, C., Yang, J., Li, D., Zhang, J., Guo, Y., et al. (2016b). Hyperhexanone A, a crucial intermediate from bicyclo 3.3.1 - to cyclohexanone monocyclic-polycyclic polyprenylated acylphloroglucinols. Tetrahedron 72, 4655–4659. doi:10.1016/j.tet.2016.06.035
Keywords: Hypericum sampsonii Hance, botany, traditional uses, phytochemistry, biological activities, safety
Citation: Sun Z, Li Y, Zhong R and Li R (2023) Hypericum sampsonii Hance: a review of its botany, traditional uses, phytochemistry, biological activity, and safety. Front. Pharmacol. 14:1247675. doi: 10.3389/fphar.2023.1247675
Received: 26 June 2023; Accepted: 31 August 2023;
Published: 19 September 2023.
Edited by:
Daqian Wan, Tongji Hospital Affiliated to Tongji University, ChinaReviewed by:
Changling Hu, North Carolina Agricultural and Technical State University, United StatesHong-Hua Wu, Tianjin University of Traditional Chinese Medicine, China
Copyright © 2023 Sun, Li, Zhong and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhanghua Sun, c3lzdXN6aEAxMjYuY29t; Ruimin Zhong, c2d1X3pybUBzZ3UuZWR1LmNu
†These authors contributed equally to this work
 Zhanghua Sun
Zhanghua Sun Yanzhen Li3†
Yanzhen Li3† Ran Li
Ran Li