- Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
1 Introduction
Alzheimer’s disease (AD) is a predominant form of dementia characterized by progressive cognitive impairments including behavioral changes, language disorder, and mental symptoms, ultimately leading to death (Wu et al., 2022). Since first described in 1907 by Alois Alzheimer, awareness of the pathological characteristics and clinical manifestations of AD has improved. However, the etiology of AD remains uncertain and is considered the result of interactions among biological, genetic, and environmental factors. A wide range of pathological hypotheses have been proposed, mainly centered on the deposition of amyloid-β (Aβ), neurofibrillary tangles induced by hyperphosphorylated tau, synaptic dysfunction, neuroinflammation, and neurotransmitter imbalance (Decourt et al., 2022). However, at present, only symptomatic treatments exist for AD.
MicroRNAs (miRNAs) are a type of regulatory non-coding RNAs (ncRNAs) important in modulating gene expression (Ren et al., 2022). miRNAs are 19–24 nt length single-stranded RNAs with a role in post-transcriptional gene silencing. Over 38,000 miRNAs have been identified to date (https://www.mirbase.org/), approximately 68 miRNAs of which have been comprehensively studied and associated with the onset and development of AD (Supplementary Table S1). Among these, miR-23b-3p has been broadly investigated in peripheral circulatory systems and various AD models. In this opinion article, informed by published data and our own findings, we outline the role of miR-23b-3p in AD pathology and its biomarker potential and target properties for future miRNA-based applications in AD.
2 MiR-23b-3p plays a key role in AD
MiR-23b-3p is a member of the miR-23b family. Initially described in 2012, miR-23b-3p plays important roles in carcinogenesis (Grossi et al., 2018) and non-cancerous diseases, especially nervous system diseases like ischemic stroke (Wu et al., 2017), seizure (Zhan et al., 2016), Parkinson’s disease (Geng et al., 2023), peripheral nerve regeneration (Xia et al., 2020), and neuroinflammation-related diseases (Burrows et al., 2023). The first reported relationship between miR-23b-3p and AD was altered expression of miR-23b-3p in AD patients (Lugli et al., 2015). The role and underlying mechanism of miR-23b-3p, as well its potential applications in AD, have also been investigated by our group.
Our recent research indicates that miR-23b-3p plays a neuroprotective role in AD. Low expression of miR-23b-3p was revealed to correlate with AD progression (Liu et al., 2021). Downregulation of miR-23b-3p has been reported with disease development in various AD models, including in the hippocampus and cortex of amyloid precursor protein (APP)/presenilin 1 (PS1) mice and senescence-accelerated mouse prone 8 (SAMP8) mice at different ages (Liu et al., 2020). Decrease of miR-23b-3p in neuronal cells with Aβ toxicity also has a time-effect relationship (Jiang et al., 2022). Furthermore, the correlation analysis of plasma miR-23b-3p levels and mini-mental state examination scores in AD patients has a good clinical diagnostic value with 81.8% sensitivity and 92.9% specificity (Jiang et al., 2022).
Importantly, functional evidence of the beneficial roles of miR-23b-3p suggests that modifying its expression could counteract AD pathology. Intracerebral ventricular infusion of adeno-associated virus (AAV) constructs expressing miR-23b-3p in APP/PS1 mice rescued learning and memory deficits in the Morris water maze test (Jiang et al., 2020). The neurotic plaques forming Aβ and neurofibrillary composed of hyperphosphorylated tau are indicators of AD. It has been found that restoring miR-23b-3p levels via direct delivery of AAV-loaded miR-23b-3p into the brain or miR-23b-3p synthetic mimics transfected into neuronal cells both suppressed Aβ1-42 levels and tau phosphorylation against AD-related deficits, thereby inhibiting cell apoptosis (Jiang et al., 2022; Liu et al., 2022). In contrast, use of a miR-23b-3p inhibitor led to the opposite effects in vitro. These findings are in line with related reports for other neurological diseases that miR-23b alleviates hypoxia-induced neuronal apoptosis (Chen et al., 2014) and that upregulation of miR-23b-3p has positive effects on neuronal activity, plasticity, and cognitive functions like long-term potentiation and memory in response to music-listening (Nair et al., 2021). Together, these findings support that increasing miR-23b-3p level in the brain of AD models is potentially therapeutic, as it could concomitantly modify various aspects of AD’s hallmark pathology and potentially lead to amelioration of cognitive deficits.
3 MiR-23b-3p participates in multi-pathway crosstalk for AD therapy
Our findings suggest that miR-23b-3p is downregulated in AD, causing a loss of the effective inhibitory effect on the expression of its target gene glycogen synthase kinase 3 beta (GSK-3β). Activation of GSK-3β/tau hyperphosphorylation signaling is crucial in causing cognitive impairment (Lauretti et al., 2020). Increased GSK-3β activity is known to contribute to Aβ production by inducing the beta-site APP cleaving enzyme 1-mediated amyloidogenic pathway and downregulating disintegrin and metalloprotease 10 (ADAM10) in the nonamyloidogenic pathway (Liu et al., 2018; Ly et al., 2013). miR-23b-3p has been shown to directly suppress tau hyperphosphorylation by targeting GSK-3β and to indirectly reduce Aβ1-42 production by increasing the ADAM10-mediated nonamyloidogenic pathway that GSK-3β is involved in. GSK-3β is intimately associated with apoptosis. In line with this relationship, the GSK-3β/Bax/caspase-3 axis was shown to be inhibited by administering miR-23b-3p to both in vitro and in vivo AD models (Jiang et al., 2022). These findings suggest that miR-23b-3p multimodally inhibits Aβ generation, tau phosphorylation, and neuronal apoptosis by regulating one target, namely, GSK-3β, acting upstream of several signaling pathways (Figure 1).
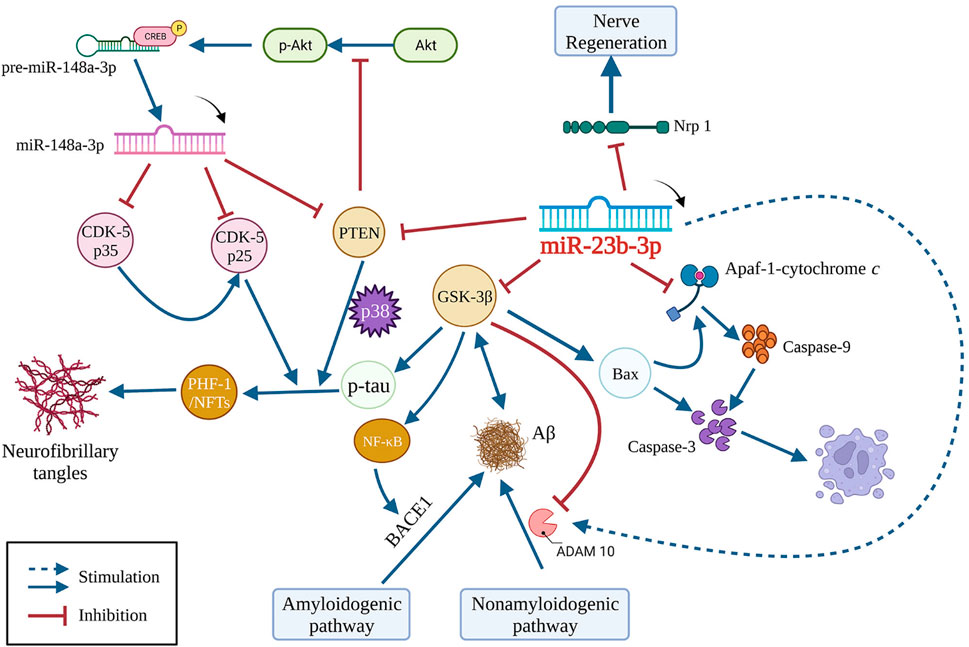
FIGURE 1. Multi-Pathway Crosstalk of miR-23b-3p in AD. Bax, Bcl-2-associated X protein; GSK-3β, glycogen synthase kinase-3β; Aβ, amyloid-β; BACE1, beta-site APP cleaving enzyme 1; ADAM10, disintegrin and metalloprotease 10; PTEN, phosphatase and tensin homolog; CDK-5, cyclin-dependent kinase-5; PHF-1, paired helical filament-1; NFTs, non-fungible tokens; Nrp 1, neuropilin 1; Apaf-1, apoptotic protease activating factor-1; CREB, cyclic adenosine monophosphate response element-binding protein; NF-κB, nuclear factor-κB.
Emerging evidence suggests additional targets for miR-23b-3p in non-cancerous diseases, such as neuropilin 1 (Xia et al., 2020), apoptotic protease activating factor-1 (Apaf-1) (Chen et al., 2014), α-synuclein (Geng et al., 2023), and phosphatase and tensin homolog (PTEN) (Guo et al., 2022). Among these, Apaf-1 mediates apoptosis via recruitment and activation of caspase-9 by binding with cytochrome c. Notably, PTEN is also a regulator of tau phosphorylation. As a negative regulator of PI3 kinase signaling, downregulation of PTEN-mediated Akt pathway induces activation of p38 MAPK, leading to tau phosphorylation (Zeng et al., 2021). The effect of PTEN on tau phosphorylation in AD is independent of GSK-3β and the tau phosphatases PP1 and PP2A (Kerr et al., 2006). PTEN also plays a role in a negative feedforward loop involving the miR-148a-3p-associated Akt/CREB pathway to recover cognitive impairment in AD (Zeng et al., 2021). In summary, various direct or indirect targets involved in AD pathogenesis have been shown to be regulated by miR-23b-3p, supporting the putatively central role of miR-23b-3p in AD.
4 Transformational potential of MiR-23b-3p from bench to bedside
Using artificial miR-23b-3p duplexes similar to its specific miRNA precursors, it is possible to affect a number of molecules in response to miR-23b-3p-mediated reduction of AD hallmarks, such as tau phosphorylation and Aβ production. GSK-3β is thought to be a key player in AD since its dysregulation is associated with several significant hallmarks of the disease, including tau hyperphosphorylation, Aβ accumulation, synaptic dysfunction, and microglia-mediated neuroinflammation (Lauretti et al., 2020). GSK-3β thus represents a promising disease-modifying target against AD, and several inhibitors are currently being used in pre-clinical and clinical studies. miR-23b-3p inhibits GSK-3β overexpression by specifically binding to GSK3β mRNA, upregulates inhibitory GSK-3β phosphorylation at the Ser9 site, and decreases GSK-3β overactivity in the brain (Jiang et al., 2022). A negative correlation has been observed between GSK-3β and miR-23b-3p in the cortex and hippocampus during AD progression, suggesting miR-23b-3p as a promising drug target. Specifically, we have started developing anti-AD candidate drugs to inhibit the GSK-3β/p-tau/Bax/caspase-3 pathways dependent on interference with miR-23b-3p (Li et al., 2023). However, clinical trials have not identified an effective and safe inhibitor of GSK-3β for AD due to its ubiquitous expression and multiple regulatory functions. Precise brain delivery of miR-23b-3p and specific regulation of GSK-3β may provide new prospects for the treatment of AD.
5 Final considerations
In this opinion article, we discuss the role and potential of targeting miR-23b-3p signaling in AD. To our knowledge, our research group was the first to report and thoroughly investigate the role and underlying mechanisms of miR-23b-3p in AD. Collectively, our studies support the cognitive enhancement effects of miR-23b-3p, identify the miR-23b-3p-elicited GSK-3β/p-tau/Bax/caspase-3 pathway as a promising network-based target, and investigate novel compounds based on miR-23b-3p interference for AD. Although specific miRNAs have potential as clinical biomarkers and drug targets, much work is still needed before miRNA-based applications are available. The combination of increased t-tau and p-tau levels and decreased Aβ levels in blood or cerebrospinal fluid is a sensitive and specific measure for AD diagnosis and monitoring. Before miR-23b-3p-associated candidate biomarkers can achieve routine clinical applications, they require critical evaluation, including clinical analysis, validity, and utility. miRNA-based therapeutics are currently in the development pipeline, along with other oligonucleotide-based approaches. However, there are still many future challenges for characterizing miR-23b-3p-mediated optimal target pathways and systematically mapping on- and off-target toxic effects. Despite these issues, we will continue to pursue this line of research and strive to incorporate interventions targeting miR-23b-3p into clinical diagnosis and treatment of AD.
Author contributions
CW and ZC drafted the manuscript; RL and ZL supervised and revised the draft. All authors have read and approved the final manuscript.
Funding
The paper was supported by the National Natural Science Foundation of China (No. 82173806).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1245352/full#supplementary-material
References
Burrows, K., Figueroa-Hall, L. K., Alarbi, A. M., Stewart, J. L., Kuplicki, R., Tan, C., et al. (2023). Association between inflammation, reward processing, and ibuprofen-induced increases of miR-23b in astrocyte-enriched extracellular vesicles: A randomized, placebo-controlled, double-blind, exploratory trial in healthy individuals. Brain Behav. Immun. Health 27, 100582. doi:10.1016/j.bbih.2022.100582
Chen, Q., Xu, J., Li, L., Li, H., Mao, S., Zhang, F., et al. (2014). MicroRNA-23a/b and microRNA-27a/b suppress Apaf-1 protein and alleviate hypoxia-induced neuronal apoptosis. Cell Death Dis. 5 (3), e1132. doi:10.1038/cddis.2014.92
Decourt, B., D’Souza, G. X., Shi, J., Ritter, A., Suazo, J., and Sabbagh, M. N. (2022). The cause of alzheimer’s disease: The theory of multipathology convergence to chronic neuronal stress. Aging Dis. 13 (1), 37–60. doi:10.14336/ad.2021.0529
Geng, X., Zou, Y., Li, S., Qi, R., Yu, H., and Li, J. (2023). MALAT1 mediates α-synuclein expression through miR-23b-3p to induce autophagic impairment and the inflammatory response in microglia to promote apoptosis in dopaminergic neuronal cells. Mediators Inflamm., 4477492. doi:10.1155/2023/4477492
Grossi, I., Salvi, A., Baiocchi, G., Portolani, N., and De Petro, G. (2018). Functional role of microRNA-23b-3p in cancer biology. Microrna 7 (3), 156–166. doi:10.2174/2211536607666180629155025
Guo, Y., Yuan, X., Hong, L., Wang, Q., Liu, S., Li, Z., et al. (2021). Promotor hypomethylation mediated upregulation of miR-23b-3p targets PTEN to promote bronchial epithelial-mesenchymal transition in chronic asthma. Front. Immunol. 12, 771216. doi:10.3389/fimmu.2021.771216
Jiang, H., Li, Z., Liu, R., Zeng, L., and Zhang, J. (2020). miRNA marker for diagnosis and treatment of ALZHEIMER’S DISEASE. A.U. Patent No 2,020,103,707. Available at: https://patentscope2.wipo.int/search/zh/detail.jsf?docId=AU313300333&_cid=JP2-LJ3LIO-07852-1.
Jiang, H., Liu, J., Guo, S., Zeng, L., Cai, Z., Zhang, J., et al. (2022). miR-23b-3p rescues cognition in Alzheimer’s disease by reducing tau phosphorylation and apoptosis via GSK-3β signaling pathways. Mol. Ther. Nucleic Acids 28, 539–557. doi:10.1016/j.omtn.2022.04.008
Kerr, F., Rickle, A., Nayeem, N., Brandner, S., Cowburn, R. F., and Lovestone, S. (2006). PTEN, a negative regulator of PI3 kinase signalling, alters tau phosphorylation in cells by mechanisms independent of GSK-3. FEBS Lett. 580 (13), 3121–3128. doi:10.1016/j.febslet.2006.04.064
Lauretti, E., Dincer, O., and Praticò, D. (2020). Glycogen synthase kinase-3 signaling in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Cell Res. 1867 (5), 118664. doi:10.1016/j.bbamcr.2020.118664
Li, Z., Liu, R., Xue, S., Jiang, H., Zhang, L., Tan, L., et al. (2023). Application, structure and preparation method of polysubstituted benzene compound in preparation of medicine for treating cognitive impairment diseases. C.N. Patent No 113,521,048. Available at: https://patentscope2.wipo.int/search/zh/detail.jsf?docId=CN340389254&_cid=JP2-LJ3RZG-39401-1.
Liu, Q. S., Jiang, H. L., Wang, Y., Wang, L. L., Zhang, J. X., He, C. H., et al. (2018). Total flavonoid extract from Dracoephalum moldavica L. attenuates β-amyloid-induced toxicity through anti-amyloidogenesic and neurotrophic pathways. Life Sci. 193, 214–225. doi:10.1016/j.lfs.2017.10.041
Liu, R., Li, Z., Jiang, H., Zeng, L., and Wang, Q. (2020). MiRNA marker for diagnosis and/or treatment of Alzheimer’s disease (AD). C.N. Patent No 110,791,560. Available at: https://patentscope2.wipo.int/search/zh/detail.jsf?docId=CN289085025&_cid=JP2-LJ3LHZ-07419-1.
Liu, R., Li, Z., Jiang, H., Zeng, L., and Zhang, J. (2021). Mirna marker for diagnosis and/or treatment of ALZHEIMER’S disease. W.O. Patent No 2,021,088,317. Available at: https://patentscope2.wipo.int/search/zh/detail.jsf?docId=WO2021088317&_cid=JP2-LJ3LGY-06244-1.
Liu, R., Li, Z., Zeng, L., Jiang, H., Sun, T., Liu, M., et al. (2022). miRNA marker for diagnosis AND/OR treatment of ALZHEIMER’S DISEASE. U.S. Patent No 20,220,259,658. Available at: https://patentscope2.wipo.int/search/zh/detail.jsf?docId=US371833316&_cid=JP2-LJ3LMP-11357-1.
Lugli, G., Cohen, A. M., Bennett, D. A., Shah, R. C., Fields, C. J., Hernandez, A. G., et al. (2015). Plasma exosomal miRNAs in persons with and without Alzheimer disease: altered expression and prospects for biomarkers. PLoS One 10 (10), e0139233. doi:10.1371/journal.pone.0139233
Ly, P. T., Wu, Y., Zou, H., Wang, R., Zhou, W., Kinoshita, A., et al. (2013). Inhibition of GSK3β-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J. Clin. Invest. 123 (1), 224–235. doi:10.1172/JCI64516
Nair, P. S., Raijas, P., Ahvenainen, M., Philips, A. K., Ukkola-Vuoti, L., and Järvelä, I. (2021). Music-listening regulates human microRNA expression. Epigenetics 16 (5), 554–566. doi:10.1080/15592294.2020.1809853
Ren, H., Guo, Z., Liu, Y., and Song, C. (2022). Stem cell-derived exosomal MicroRNA as therapy for vascular age-related diseases. Aging Dis. 13 (3), 852–867. doi:10.14336/ad.2021.1110
Wu, J., Fan, C. L., Ma, L. J., Liu, T., Wang, C., Song, J. X., et al. (2017). Distinctive expression signatures of serum microRNAs in ischaemic stroke and transient ischaemic attack patients. Thromb. Haemost. 117 (5), 992–1001. doi:10.1160/th16-08-0606
Wu, T., Lin, D., Cheng, Y., Jiang, S., Riaz, M. W., Fu, N., et al. (2022). Amyloid cascade hypothesis for the treatment of alzheimer’s disease: Progress and challenges. Aging Dis. 13 (6), 1745–1758. doi:10.14336/ad.2022.0412
Xia, B., Gao, J., Li, S., Huang, L., Zhu, L., Ma, T., et al. (2020). Mechanical stimulation of Schwann cells promote peripheral nerve regeneration via extracellular vesicle-mediated transfer of microRNA 23b-3p. Theranostics 10 (20), 8974–8995. doi:10.7150/thno.44912
Zeng, L., Jiang, H., Ashraf, G. M., Liu, J., Wang, L., Zhao, K., et al. (2021). Implications of miR-148a-3p/p35/PTEN signaling in tau hyperphosphorylation and autoregulatory feedforward of Akt/CREB in Alzheimer’s disease. Mol. Ther. Nucleic Acids 27, 256–275. doi:10.1016/j.omtn.2021.11.019
Keywords: Alzheimer’s disease, microRNA-23b-3p, microRNA therapy, glycogen synthase kinase-3β, tau protein
Citation: Wang C, Cai Z, Li Z and Liu R (2023) The potential of targeting microRNA-23b-3p signaling in Alzheimer’s disease: opinions on recent findings. Front. Pharmacol. 14:1245352. doi: 10.3389/fphar.2023.1245352
Received: 23 June 2023; Accepted: 03 July 2023;
Published: 12 July 2023.
Edited by:
Yunfeng Li, Academy of Military Medical Sciences (AMMS), ChinaReviewed by:
Jun Zhao, Xinjiang Institute of Materia Medica, ChinaCopyright © 2023 Wang, Cai, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Liu, bGl1cnVpQGltYi5wdW1jLmVkdS5jbg==
†These authors have contributed equally to this work
 Chenyu Wang
Chenyu Wang Zhongdi Cai
Zhongdi Cai Zhuorong Li
Zhuorong Li Rui Liu
Rui Liu