
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 25 September 2023
Sec. Pharmacoepidemiology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1244486
This article is part of the Research Topic Biologic Drugs in Immune-Mediated Inflammatory Diseases: Validation, Drug-Utilization, Effectiveness, Regulation, Costs, and Safety in the Real World View all 10 articles
 Irma Convertino1†
Irma Convertino1† Massimiliano Cazzato2†
Massimiliano Cazzato2† Silvia Tillati3
Silvia Tillati3 Sabrina Giometto3
Sabrina Giometto3 Rosa Gini4
Rosa Gini4 Giulia Valdiserra1
Giulia Valdiserra1 Emiliano Cappello1
Emiliano Cappello1 Sara Ferraro1
Sara Ferraro1 Marco Bonaso1
Marco Bonaso1 Claudia Bartolini4
Claudia Bartolini4 Olga Paoletti4
Olga Paoletti4 Valentina Lorenzoni5
Valentina Lorenzoni5 Leopoldo Trieste5
Leopoldo Trieste5 Matteo Filippi6
Matteo Filippi6 Giuseppe Turchetti5
Giuseppe Turchetti5 Michele Cristofano6
Michele Cristofano6 Corrado Blandizzi1,7
Corrado Blandizzi1,7 Marta Mosca2
Marta Mosca2 Ersilia Lucenteforte3*
Ersilia Lucenteforte3* Marco Tuccori1,7
Marco Tuccori1,7Introduction: The disease activity associated with the drug-utilization patterns of biologic Disease Modifying Anti-Rheumatic Drugs (DMARDs) is poorly investigated in real-world studies on rheumatoid arthritis (RA) patients. To investigate the relationship between biologic DMARD initiation/discontinuations in RA patients identified in the healthcare administrative databases of Tuscany and the Disease Activity Score 28 (DAS28) reported in the medical charts.
Methods: This retrospective population-based study included RA’s first-ever biologic DMARD users of the Pisa University Hospital from 2014 to 2016. Patients were followed up until 31 December 2019. We evaluated the DAS28 recorded before (T0) and after (T1) the biologic DMARD initiation and before (TD0) and after (TD1) discontinuations. Patients were classified as “off-target” (DAS28 > 3.2) or “in-target” (DAS28 ≤ 3.2). We described the disease activity trends at initiation and discontinuation.
Results: Ninety-five users were included (73 women, mean age 59.6). Among 70 patients (74%) with at least three DAS28 measures, 28 (40.0%) were off-target at T0 and 38 (54.3%) in-target at T1. Thirty-three (47%) patients had at least one discontinuation, among those with at least three DAS28 assessments. In the disease activity trend, disease stability or improvement was observed in 28 out of 37 (75.7%) patients at initiation and in 24 out of 37 (64.9%) at discontinuation.
Discussion: Biologic DMARD discontinuations identified in the healthcare administrative databasese of Tuscany are frequently observed in situations of controlled RA disease. Further studies are warranted to confirm that these events can be used in studies using healthcare administrative databases as proxies of treatment effectiveness.
Healthcare administrative databases (HADs) are demonstrated to be reliable data sources for drug utilization studies (Gini et al., 2014; Trifirò et al., 2021). However, investigating clinical outcomes associated with drug use in these databases is conditioned by the aims these data are collected for, particularly the management of healthcare costs. Therefore, proxies must often define clinical outcomes (Convertino et al., 2021a). For example, the treatment initiation identified in these databases can be interpreted as a clinical need (i.e., an uncontrolled disease) and a change in therapy (switching) or interruption (discontinuation) as efficacy loss or as a consequence of tolerability problems.
The Pathfinder project (EUPAS29263) (Convertino et al., 2021d) was developed to describe the use of biological drugs in rheumatoid arthritis (RA) patients and the related clinical outcomes by combining data from the HAD of the Tuscan region with the information contained in the individual medical charts. The extraction algorithm of subjects with RA demonstrated high values of sensitivity (0.93; 95% confidence interval, CI, 0.86–0.97), specificity (0.84; 95% CI 0.78–0.90), and positive predictive value (0.78; 95% CI 0.70–0.85) (Convertino et al., 2021b). The project characterized the use of biological drugs in these patients, classifying them based on adherence trajectories. This evaluation observed that about 88% of the subjects fall into the adherence category of continuous users characterized by alternation of phases of treatment coverage with phases not covered by treatment (Convertino et al., 2021c). In accordance with the guidelines that provide for the biologic prescription in subjects with uncontrolled disease, and the treatment tapering in subjects showing disease remission (Smolen et al., 2010; Smolen et al., 2014; Smolen et al., 2017), we hypothesized that the disease activity is high in correspondence with events of biologic initiation and improves or remains stable in subsequent assessments. Disease activity is also expected to be reduced before discontinuations and to remain almost stable thereafter.
To test these hypotheses, we described the disease activity reported in the medical charts of an RA population in correspondence with the events of initiation and discontinuations of biologic disease-modifying antirheumatic drugs (DMARDs), identified from the HAD of the Tuscany Region (Italy).
This investigation is part of the Pathfinder (Convertino et al., 2021d), a retrospective population-based cohort study on RA first ever biologic DMARD users extracted from the HAD of Tuscany. The regional healthcare system, comprising the national, universal, single-payer, and public health system, provides healthcare assistance to over 3 million Tuscan residents. All data about services supplied have been recorded electronically in the HAD since 2004. Data are periodically analyzed by the Agenzia Regionale di Sanità Toscana (ARST), and these have been used to conduct pharmacoepidemiological studies (Gini et al., 2014; Trifirò et al., 2021). The study was conducted from 1 January 2004 to 31 December 2019. We extracted data on 29 April 2020 from the HAD. We also collected information from the corresponding medical charts of the Rheumatology Unit of Pisa University Hospital. We used the following repositories encompassed in the Tuscan HAD: drug supply to inpatient and outpatient databases (Anatomical Therapeutic Chemical, ATC, Classification codes), exemptions from the co-payment database (exemption from co-payment codes), hospital discharge records, Emergency Department (ED) admission records (International Classification of Diseases Ninth Revision, ICD-9, codes), and outpatient services for specialist visits. The pseudo-anonymized information of Tuscan RA first ever biologic DMARD users identified by a validated algorithm (Convertino et al., 2021b) from the HAD was linked to the corresponding medical chart data in the Rheumatology Ward of Pisa University Hospital. According to the ethical and data protection requirements, data were managed by the Hospital Healthcare Office and through a unique identification number (Supplementary Material S1, Supplementary Figure S1). The informed consent for participating in the study was obtained from patients during the scheduled visits or by phone. From the medical charts, the following information was collected retrospectively: RA diagnosis date, Disease Activity Score 28 (DAS28), adverse events, adverse event dates, and dates of the DAS28 assessments. Information extracted from the HAD involved the biologic DMARD supplies, RA visits, RA diagnosis, RA exemption from co-payment, the first biologic DMARD supply date, the index date (ID), and the dates of the subsequent biologic DMARD dispensations.
The Pathfinder study received consent from the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) (EUPAS29263) (Convertino et al., 2021d) and was approved by the Ethical Committee of Pisa University Hospital (Protocol number 18724). This article was written in accordance with the guidelines on conducting and reporting drug utilization studies (Vrijens et al., 2012; De Geest et al., 2018; Dima et al., 2021).
During the validation analysis (Convertino et al., 2021b), we identified RA first ever biologic DMARD (infliximab, adalimumab, certolizumab pegol, etanercept, golimumab, abatacept, tocilizumab, sarilumab, and rituximab) users in the period between 1 January 2014 and 31 December 2016 (inclusion period), accessing healthcare facilities at the Rheumatology Unit of Pisa University Hospital in the year preceding the ID. First ever users were defined by no biologic DMARD dispensation recorded in the period ranging from the first record available in the Tuscan HAD to the ID (look-back period). We decided to exclude patients with rituximab as an index drug since the timing of administration is different from that of other biologic DMARDs, and this could have affected the frequency of DAS28 assessments. We followed up patients from the ID until the occurrence of the following events: disenrollment from the healthcare coverage plan, death, or end of the study period (31 December 2019), whichever came first.
For each patient, we identified the ID and the date of each discontinuation event, defined as the first day not covered with biologic DMARDs. The coverage was calculated based on the defined daily dose (DDD) (WHO, 2021) and the number of doses supplied plus a grace period of 60 days not covered by treatment (i.e., without any other biologic DMARD supply). Disease activity was measured using the DAS28. The disease activity recorded during RA visits was classified according to EULAR guidelines into two clinical categories based on DAS28 values (Fransen and van Riel, 2005): i) in-target disease with DAS28 ≤ 3.2 and ii) off-target disease with DAS28 > 3.2.
We defined the DAS28T0 as the DAS28 value recorded in the closest date before the ID (T0) (including the ID), the DAS28T1 as the DAS28 recorded in the closest date after the ID (T1), the DAS28TD0 as the DAS28 recorded in the closest date before the date of any discontinuation event (TD0) (including the date of the discontinuation event), and the DAS28TD1 as the DAS28 recorded in the closest date after the date of any discontinuation event (TD1) (Figure 1). We measured improvements in DAS28 by classifying the changes according to the EULAR response criteria (Fransen and van Riel, 2005): i) good, when a change >1.2 of the DAS28 from DAS28T0 to DAS28T1 (or from DAS28TD0 to DAS28TD1) was recorded; ii) moderate, when a change > 0.6 of the DAS28 from DAS28T0 to DAS28T1 (or from DAS28TD0 to DAS28TD1) was registered; and iii) no improvement, when the change of DAS28 from T0 to T1 or from TD0 to TD1 ranged between −0.5 and 0.
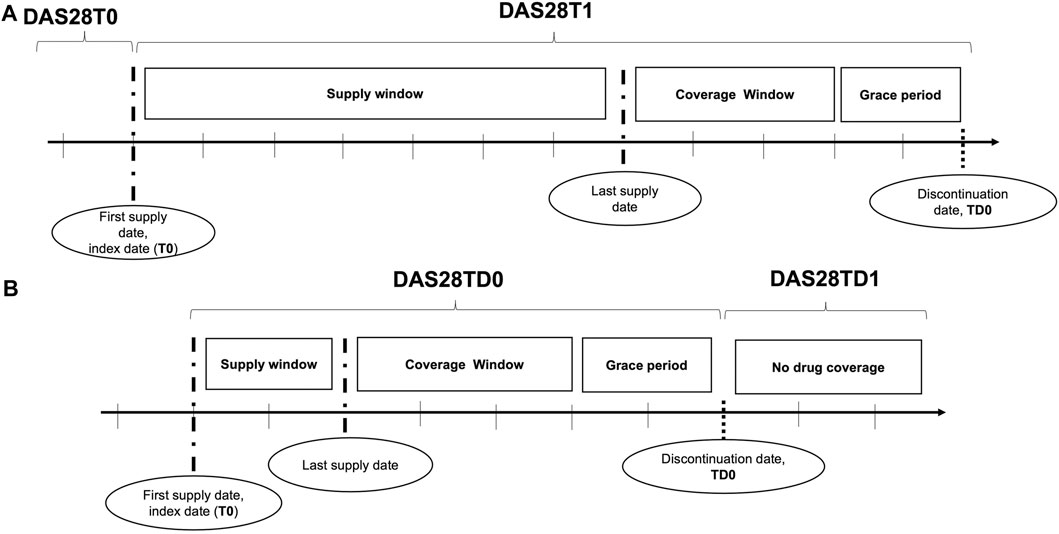
FIGURE 1. Disease activity assessments: (A) at the initiation of biologic DMARDs and (B) at the discontinuations. The disease activity was assessed through the DAS28 at initiation of biologic DMARDs (A) and at discontinuation (B). Section A displayed how DAS28 was detected at initiation: the DAS28 recorded at the visit before (T0) the first biologic DMARD supply, corresponding to the index date, and that after the index date (T1) were used. Section B showed the DAS28 detection at discontinuations: the DAS28 reported in medical charts at the visit before (TD0) the discontinuation date, defined by the end of the drug coverage plus a grace period, and that after the discontinuation date (TD1) were adopted for the investigation.
We considered the following variables as baseline characteristics: time invariant (age at ID and gender), single event (index biologic DMARDs, conventional synthetic DMARDs, azathioprine, cyclophosphamide, ciclosporin, hydroxychloroquine sulfate, leflunomide, methotrexate, mycophenolate mofetil, and sulfasalazine supplied in 3 months prior to ID), and time variant (RA disease duration, as the time from the date of diagnosis in the medical chart to the ID recorded in the HAD, days).
We performed a step-by-step descriptive analysis by progressively classifying the population based on DAS28 measure availability and occurrence of discontinuation events (Figure 2). The results were reported as the numbers and percentages for categorical variables and as the mean and standard deviation (SD) or median and interquartile range [IQR] for continuous variables. In the first step, we described the baseline characteristics of all RA patients. In the second step, we classified patients based on the presence of at least three available DAS28 assessments, and we tested these two groups for differences at the baseline by using the t-test and chi-square test, as appropriate. In the group with at least three DAS28 assessments, we described available DAS28T0 and DAS28T1. Discontinuation events were identified, and DAS28TD0 and DAS28TD1 were described. We computed discontinuations as dichotomous variables and categorical variables: no discontinuation, 1 discontinuation, 2–3 discontinuations, and ≥4 discontinuations. In the third step, among patients with at least three DAS28 available, we identified those with at least one discontinuation. We tested the baseline differences between continuers and discontinuers, and we described the related DAS28 observed at T0, T1, TD0, and TD1 and the corresponding time elapsed (days). In the fourth step, we separately analyzed the disease activity trends at the ID and at discontinuations. We restricted the evaluation to patients with records of both DAS28T0 and DAS28T1, and with records of both DAS28TD0 and DAS28TD1 within the group of patients with at least three DAS28 available. We used Sankey plots to illustrate the variation in disease activity with respect to the index date and discontinuation date, where the width of the flows represents the proportion of subjects. To check for discontinuations, possibly due to safety reasons, we retrieved adverse events recorded in patients from medical charts included in the analysis for disease activity trends, and we estimated the time between the date of the event and that of the discontinuation.
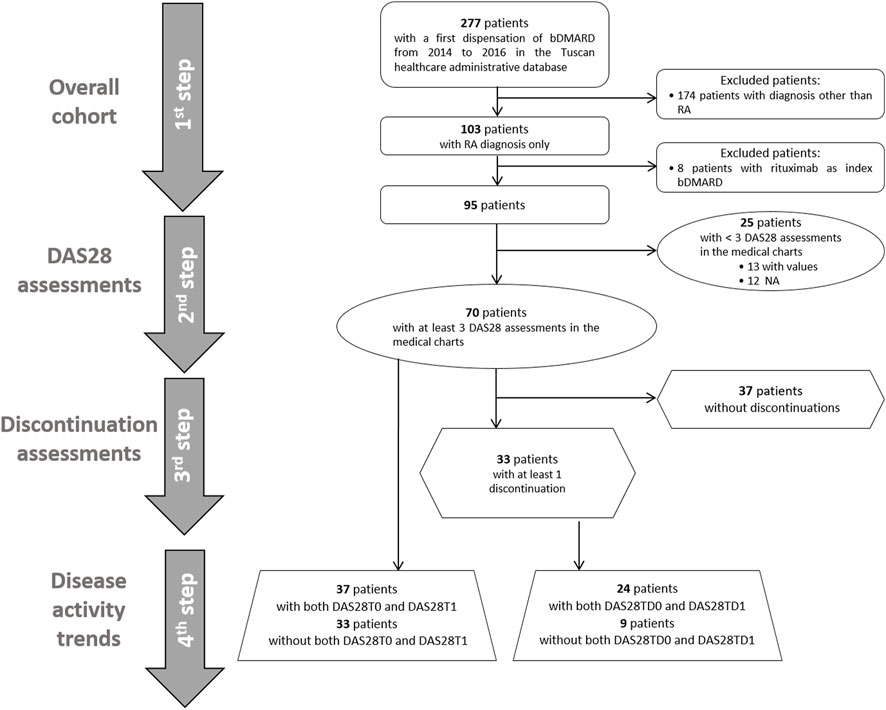
FIGURE 2. Flow chart of selection of the cohorts for the study analyses. First ever biologic DMARD users from 2014 to 2016 with rheumatoid arthritis were selected, and we included 95 patients in the first-step analysis. In the second step, patients were classified based on the presence of at least three DAS28 assessments reported in the medical charts or not. In the third step, patients were distinguished between those with at least one discontinuation and those without any discontinuation. In the fourth step, the disease activity trend was evaluated at initiation and at discontinuations in available patients with both DAS28T0 and DAS28T1 and DAS28TD0 and DAS28TD1, respectively.
We performed a sensitivity analysis by varying the grace period for estimating discontinuations to 30 days. All these analyses were performed on anonymized data using R, version 3.6.3.
Overall, 95 patients with RA first ever biologic DMARD use had the inclusion criteria (Figure 2). The majority of biologic DMARD first ever users were women (76.8%), and the mean age was 59.6 (SD 12.1). The index biologic DMARDs most frequently supplied were as follows: abatacept (33.7%), etanercept (29.5%), and adalimumab (15.8%). At baseline, 66 patients had at least one supply of csDMARDs, with hydroxychloroquine sulfate (33.7%) and methotrexate (30.5%) as the most frequently observed. The median time elapsed from the RA diagnosis date, and the first biologic DMARD supply was 2,633 [IQR = 966.5–5,518.5] days, i.e., 7.2 years, and most part of the biologic DMARD first ever users belonged to the categories exceeding the 5 years from RA diagnosis (Table 1 and Supplementary Figure S2).
The median age observed at RA diagnosis was 54 [IQR = 41–60]. Overall, 91 discontinuations were identified (Supplementary Table S1), 67.0% had a DAS28TD0 and 59.3% DAS28TD1 (Supplementary Table S2). At TD0, 41.8% of discontinuations were associated with and in-target disease, while at TD1, the majority of discontinuations (40.7%) had no DAS28 recorded, and 35.2% reported an in-target RA (Supplementary Table S2).
Seventy patients (74.0%) had at least three DAS28 assessments recorded in the medical charts (Figure 2; Table 1). No statistical differences at the baseline were observed between these patients and those with less than three DAS28 values registered (Table 1). We identified 60 discontinuation events. Out of 70 patients with at least three DAS28 assessments available, 33 (47.1%) had at least one discontinuation; this distribution is similar to that observed in the general study population (47/95 patients, 49.5%) (Supplementary Table S1). Only six patients moved away from Tuscany, as reported in the medical charts, and no discontinuations of biologic treatment were detected before their moving. No significant differences were observed in the baseline characteristics between the population with at least one discontinuation and those without discontinuation (Supplementary Table S3). The DAS28T0 was available in 41 patients (58.6%) and the DAS28T1 in 66 (94.3%). At T0, 40% of patients were off-target and 41.4% had no DAS28 recorded, while at T1, 54.3% were in-target. At T1, patients with at least one discontinuation had a significant distribution of DAS28 associated with an off-target disease, while those continuing treatment displayed, most frequently, an in-target condition (Supplementary Table S4). Among the available 37 patients with both the DAS28T0 and DAS28T1 (Supplementary Table S5 and Table 2), 13 (35%) displayed a good improvement and six (16.2%) a moderate one (Table 2; Figure 3).
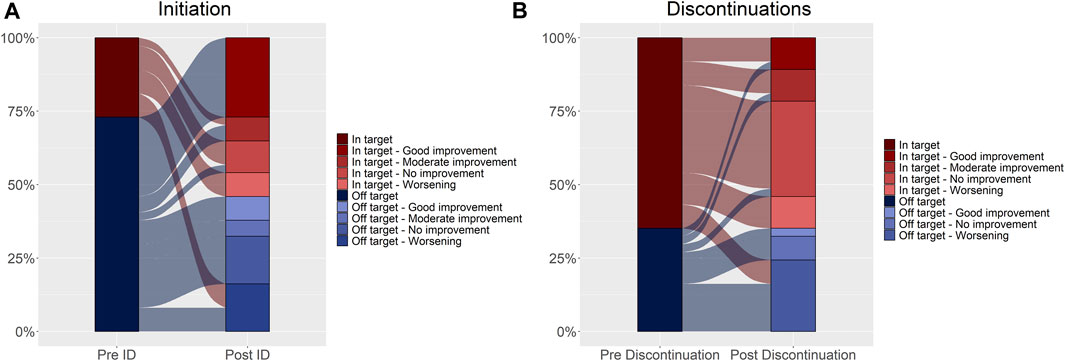
FIGURE 3. Disease activity trend assessed at initiation (A) and discontinuations (B). Changes in DAS28 relative to the index date and discontinuations are shown in Figure 3 (Panel A, B, respectively). The reference unit is the subject in Panel A, and the discontinuation in Panel (B). In the left column of both panels, disease activity expressed as DAS28 is reported and categorized as in-target (dark red) and off-target (dark blue) (pre-index date, Panel A and pre-discontinuation date, Panel (B). In the right column, disease activity was further categorized based on the movement over time (post-index date, Panel A and post-discontinuation date, Panel (B) as good improvement, moderate improvement, no improvement, and worsening. Shades of red represent in-target conditions, and shades of blue represent off-target conditions. The flows connecting the different layers of the two bars represent the movement over time, relative to the index date (Panel A) and the discontinuation date (Panel B). The width of the flow reflects the proportion of subjects (Panel A) or discontinuations (Panel B).
The median time elapsed between the DAS28T0 assessment and index date was 36 days (IQR 0–132), while the median time between DAS28T1 assessment and index date was 93 days (IQR 31–252). The subjects with both pre- and post-index DAS28 measurements within 180 days of it were 22 (59.5%), and 20/22 had off-target disease (91%), while 2/22 (9%) had an in-target RA. Of these, five (22.7%) experienced a deterioration, with one (20.0%) moving from in-target to off-target, three (60.0%) remaining off-target, and one (20.0%) remaining in-target.
Most of the patients showed disease stability or improvement after initiating biologic DMARDs (75.7%) (Table 2; Figure 3), as well as after the discontinuation events (64.9%) (Table 3; Figure 3).
Out of 33 patients with at least three DAS28 and a discontinuation, 24 had 37 events with both DAS28TD0 and DAS28TD1 (Supplementary Table S6). The reasons for discontinuations were deterioration of disease (13), adverse drug events (7), surgery/hospitalizations (5), pregnancy (4), and no information about were reported (8). Among these 37 discontinuation events (Supplementary Table S5 and Table 3), the disease activity showed a good or moderate improvement in 10 events (27.0%) (Table 3; Figure 3). The median time elapsed between the DAS28TD0 assessment and discontinuation date was 113 days (IQR 51–168), while the median time between the DAS28TD1 assessment and discontinuation date was 117 days (IQR 49–221). The subjects with both pre- and post-discontinuation DAS28 measurements within 180 days of it were 18 (48.6%), and 10/18 patients were in target and 8/10 off-target at TD0. Of these, nine (50.0%) experienced a deterioration, with one (11.1%) moving from in-target to off-target, six (66.7%) remaining off-target, and two (22.2%) remaining in-target.
Out of 24 patients with both DAS28TD0 and DAS28TD1, 11 had adverse events recorded. In particular, among the 37 discontinuations, we retrieved 15 adverse events, of which 9/15 occurred ±1 year at the discontinuation date and 3/9 showed off-target RA and disease worsening. These included neutropenia, bile acid increase, and pneumonia. Out of the six remaining adverse events reported within 1 year of the discontinuation, three (ovarian cancer, hypersensibility, and cough/sinusitis) occurred in a condition of the in-target disease and stability, one (intolerance) in an in-target and improvement disease, and one (hypersensibility) in an off-target and improvement condition. The drugs discontinued were etanercept, adalimumab, certolizumab, and abatacept. No further information was reported in the medical charts (Supplementary Table S7).
In sensitivity analysis, we almost confirmed the main analysis observations (Supplementary Table S8–S13).
This study describing the relationship between drug utilization patterns assessed using HAD information and the disease activity reported in the medical charts of RA patients showed that over half of the discontinuation events had an in-target disease before and after the biologic interruption.
In line with the clinical recommendations (Smolen et al., 2014; Smolen et al., 2017) and literature evidence (Silvagni et al., 2018), in our study, etanercept and adalimumab were among the most frequently supplied index drugs. We found a high percentage of abatacept supplies in both the overall population and in the subgroup of patients with three available DAS28 assessments. A high number of DAS28 assessments could reflect closer monitoring of patients by rheumatologists, probably in relationship with the disease burden. At the time of observation, abatacept was recommended as a first-line biologic DMARD in subjects with co-morbidities (Smolen et al., 2010; Monti et al., 2017), and therefore its higher use in subjects with more assessments (i.e., those with more complicated disease) seems to be plausible. However, statistical significance was not confirmed; therefore, these results should be considered with caution.
When investigating the initiation of the first ever biologic drugs in subjects with available DAS28 measure before and after the ID, we observed that 73% of patients (27/37) started with an off-target disease that provides the rationale for prescribing a biologic drug in accordance with the clinical guidelines. However, (10/37) 27% of patients started biologic treatment with an in-target disease. It is important to remark that the causal relationship between disease activity and the initiation of biologics is conditioned by the temporal distance between the available DAS28 assessments and the initiation event. It is unlikely that a DAS28 measure recorded several years before and after the ID can provide a reliable disease activity measure at the time of biologic DMARD initiation. In this regard, it is important to note that patients with DAS28 assessed within 6 months of the ID (i.e., those with the most reliable disease activity assessment) showed off-target disease in the majority of cases (20/22 patients, 91%). In 13/27 patients starting with an off-target disease (48%), the disease control was achieved at the subsequent assessment, while in six (22.2%) users, the improvements were without achieving the disease control. These results are in line with those of other real-world studies. For instance, a study using the Corrona registry data and evaluating biologic naïve patients with moderate and severe RA pointed out that among 817 patients with severe RA and 779 with moderate disease, 41.2% and 60.1% achieved a controlled disease after 1 year, respectively (Kavanaugh et al., 2017).
When disease activity was measured in relationship with discontinuations, 24 (64.9%) events with both DAS28TD0 and DAS28TD1 measures available presented an in-target disease before discontinuing the treatment and 17/24 (70.8%) displayed disease improvement or stability after the discontinuation. Adverse events recorded in the medical charts rarely occurred in plausible temporal relationships to suggest a causal role for discontinuation events. These results suggest that, according to our discontinuation definition, disease control could often drive the clinical decision of tapering biologic DMARDs in accordance with the clinical guidelines (Smolen et al., 2014; Smolen et al., 2017) and disease remains controlled after tapering. The robustness of these findings is confirmed by the sensitivity analysis, performed to evaluate whether the discontinuation definition could have affected our observations. Even in this case, the time distance between available DAS28 assessments and the date of the discontinuation event could affect the reliability of the results (18/24 patients, 75%, had DAS28 assessments within 6 months before and after the discontinuation date). Overall, 10/18 patients were in target and 8/18 were off-target. Nine discontinuation events showed a disease worsening, six starting from an off-target condition, and four from an in-target disease. Out of these, only one patient with an off-target disease switched to a JAK inhibitor. In these cases, the decision to discontinue the treatment should have been driven by reasons other than the achievement of disease control [i.e., patient deterioration due to co-morbidity-related events (Listing et al., 2015), lack of biologic DMARD response (Olsen et al., 2019), safety issues (Capogrosso Sansone et al., 2015; Codreanu and Damjanov, 2015; Antonazzo et al., 2022), or non-clinical events] that deserve further investigations.
Our results about the in-target disease observed before discontinuations and disease improvement or stability after the interruption are in line with those in the medical literature. A prospective observational study on 43 first ever biologic DMARD users interrupting treatment showed that 58.1% of patients maintained discontinuation along with the in-target condition for up to 1 year. In these patients, the disease activity recorded after the biologic DMARD initiation was significantly lower than in patients restarting biologic DMARD, who relapsed to off-target within 1 year (Ochiai et al., 2021). Another multicenter observational study performed in Japan using data from medical records of 102 RA infliximab users having DAS28 < 3.2 for at least 24 weeks displayed that 55% of patients maintained disease stability and 43% achieved RA remission after infliximab discontinuation (Tanaka et al., 2010). In the HOPEFUL-3 study, a follow-up to the HOPEFUL-1 and HOPEFUL-2 studies evaluating adalimumab users in Japanese patients with early RA, out of 74 patients discontinuing adalimumab for low disease activity, 59 (79.7%) retained the status of low RA activity for about 4 years (Tanaka et al., 2017). Schlager et al. (2020), in a recent systematic literature review and meta-analysis of clinical trials and observational studies, highlighted that the low disease activity at the time of discontinuation should be explored as a predictor of interruption. However, this study displayed that among patients discontinuing biologic DMARDs owing to the low disease activity, the probability for RA relapsing was high at 13 months of observation (overall Odds Ratio, OR 3.87; 95% CI 2.31–6.49).
Our study has some elements of strength. First, we used ecological data from Tuscan HAD that have been consolidated from previous population-based studies (Gini et al., 2014; Convertino et al., 2021a; Convertino et al., 2021b; Convertino et al., 2021c; Trifirò et al., 2021; Convertino et al., 2023). Second, while disease activity is usually measured by proxies in HAD studies, in our investigation, instead, we used the medical charts that represent a more reliable source of this information. Third, since therapeutic indications of drugs are not recorded in the HAD in Italy, by linking information from medical charts, we could have the certainty that all biologic users included in our study had RA.
Some limitations have to be considered. First, the small number of patients included in the study could limit the extension of the results to the general population of RA patients. However, the majority of studies investigating similar topics in the literature are not more than ours (Tanaka et al., 2010; Tanaka et al., 2017; Schlager et al., 2020; Ochiai et al., 2021). Second, we have a limited number of disease activity assessments recorded in the medical charts, and this could affect our findings. Nevertheless, a UK study (Choy et al., 2012) showed that the baseline recording of DAS28 is available in about 45% starting a biologic DMARD therapy, which is lower than the frequency observed in our study (59%). The authors of this study hypothesized that disease activity is likely measured at any visit but recorded in medical charts more probably when a relevant improvement or worsening occurs. Therefore, the probability of not recording important disease activity modification in two consecutive measures, even distant in time, is unlikely. Third, the assessment of two consecutive DAS28 without time restriction could have influenced our results. However, the disease activity monitoring should be scheduled at least every 180 days (Smolen et al., 2010; Smolen et al., 2014; Smolen et al., 2017), which is not far from the 113 and 117 days in median observed before and after the discontinuation events in our study. Fourth, the quality of available data could have affected the results. However, we have carefully measured the characteristics of patients, and discontinuation events progressively dropped out from the analysis due to the lack of records of disease activities, and by comparing these with those remaining in the cohort, we controlled for the possible selection bias. Fifth, we cannot exclude that information bias (Grimes and Schulz, 2002) could have occurred. Sixth, the discontinuation measurement was based on drug coverage estimated through the DDD and not by the prescribed daily dose (PDD). Since the use of DDD could overestimate the persistence of patients and inversely underestimate the discontinuation assessment, we cannot exclude that a definition based on the actually prescribed dose could have changed by increasing the number and the timing of discontinuation events. However, we performed the sensitivity analysis by varying the discontinuation definition, which confirmed the robustness of the main analysis results.
In conclusion, half of the RA patients achieve an in-target disease after starting biologic DMARDs, and the disease activity trend mainly reveals RA stability or improvement. As far as discontinuations are concerned, the majority of discontinuation events reported an in-target condition before the interruption date, and disease control is also confirmed after the discontinuation. Future studies on a larger RA population are needed to confirm our findings and support the use of this discontinuation definition in Tuscan HAD as effectiveness proxy.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by this retrospective chart review study involving human participants which was in accordance with the ENCePP Code of Conduct, the ethical standards of the Institutional and National Research Committee, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Ethical Committee of the Pisa University Hospital approved this study (Protocol number 18724). Informed consent was obtained from all individual participants included in the study. All patients were required to give their consent to the publication of study results. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
IC, MaC, SG, RG, ST, VL, LT, GT, CoB, MM, EL, and MT: drafting the article, analysis, and interpretation of data. IC, MC, SG, RG, GV, EC, SF, ST, MB, CB, OP, VL, LT, MF, GT, MC, CoB, MM, EL, and MT: conception and design of the study, acquisition of data, analysis, and interpretation of data. IC, MaC, SG, RG, ST, VL, LT, GT, CoB, MM, EL, and MT: revising the manuscript critically for important intellectual content. All authors contributed to the article and approved the submitted version.
This work is dedicated in memory of CoB who spent his life in the passion for experimental research and in teaching the values of science to his students.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1244486/full#supplementary-material
Antonazzo, I. C., Lombardi, N., Fusaroli, M., Battini, V., Convertino, I., and Crisafulli, S. (2022). Real-world evidence and pharmacovigilance: Workshop of the Italian epidemiological association and of the Italian society of Pharmacology; [Real-world evidence e farmacovigilanza: Workshop della Associazione italiana di epidemiologia e della Società itali. Epidemiol. Prev. 46, 217–218. doi:10.19191/EP22.3.A452.047
Capogrosso Sansone, A., Mantarro, S., Tuccori, M., Ruggiero, E., Montagnani, S., Convertino, I., et al. (2015). Safety profile of certolizumab pegol in patients with immune-mediated inflammatory diseases: A systematic review and meta-analysis. Drug Saf. 38, 869–888. doi:10.1007/s40264-015-0336-2
Choy, E., Taylor, P., McAuliffe, S., Roberts, K., and Sargeant, I. (2012). Variation in the use of biologics in the management of rheumatoid arthritis across the UK. Curr. Med. Res. Opin. 28, 1733–1741. doi:10.1185/03007995.2012.731388
Codreanu, C., and Damjanov, N. (2015). Safety of biologics in rheumatoid arthritis: data from randomized controlled trials and registries. Biologics 9, 1–6. doi:10.2147/BTT.S68949
Convertino, I., Cazzato, M., Giometto, S., Gini, R., Valdiserra, G., Cappello, E., et al. (2021b). Validation of algorithms for selecting rheumatoid arthritis patients in the Tuscan healthcare administrative databases. Sci. Rep. 11, 20314. doi:10.1038/S41598-021-98321-0
Convertino, I., Giometto, S., Gini, R., Cazzato, M., Fornili, M., Valdiserra, G., et al. (2021c). Trajectories of adherence to biologic disease-modifying anti-rheumatic drugs in tuscan administrative databases: The pathfinder study. J. Clin. Med. 10, 5743. doi:10.3390/JCM10245743
Convertino, I., Lorenzoni, V., Gini, R., Turchetti, G., Fini, E., Giometto, S., et al. (2023). Drug-utilization, healthcare facilities accesses and costs of the first generation of JAK inhibitors in rheumatoid arthritis. Pharmaceuticals 16, 465. doi:10.3390/ph16030465
Convertino, I., Lucenteforte, E., Gini, R., Lorenzoni, V., Cazzato, M., Turchetti, G., et al. (2021a). Utilisation patterns and clinical impact of the introduction of infliximab-biosimilar in Tuscany, Italy: Real world evidence following the recommendation of switching for non-medical reasons. Clin. Exp. Rheumatol. 39, 753–762. doi:10.55563/clinexprheumatol/pa6lap
Convertino, I., Lucenteforte, E., Gini, R., and Tuccori, M. (2021d). ExPloring efficAcy safeTy and adHerence oF dIseasemodifyiNg antirheumatic drugs through trajEctoRy model: the PATHFINDER study. ENCEPP 2019 EU PASS Register #29263. Available at: http://www.encepp.eu/encepp/viewResource.htm?id=37113.
De Geest, S., Zullig, L. L., Dunbar-Jacob, J., Helmy, R., Hughes, D. A., Wilson, I. B., et al. (2018). ESPACOMP medication adherence reporting guideline (EMERGE). Ann. Intern Med. 169, 30–35. doi:10.7326/M18-0543
Dima, A. L., Allemann, S. S., Dunbar-Jacob, J., Hughes, D. A., Vrijens, B., and Wilson, I. B. (2021). Teos: A framework for constructing operational definitions of medication adherence based on timelines-events-objectives-sources. Br. J. Clin. Pharmacol. 87, 2521–2533. doi:10.1111/BCP.14659
Fransen, J., and van Riel, P. L. C. M. (2005). The disease activity score and the EULAR response criteria. Clin. Exp. Rheumatol. 23, S93–S99.
Gini, R., Schuemie, M. J., Francesconi, P., Lapi, F., Cricelli, I., Pasqua, A., et al. (2014). Can Italian healthcare administrative databases Be used to compare regions with respect to compliance with standards of care for chronic diseases? PLoS One 9, e95419. doi:10.1371/JOURNAL.PONE.0095419
Grimes, D. A., and Schulz, K. F. (2002). Bias and causal associations in observational research. Lancet (London, Engl. 359, 248–252. doi:10.1016/S0140-6736(02)07451-2
Kavanaugh, A., Keystone, E., Greenberg, J. D., Reed, G. W., Griffith, J. M., Friedman, A. W., et al. (2017). Benefit of biologics initiation in moderate versus severe rheumatoid arthritis: Evidence from a United States registry. Rheumatology 56, 1095–1101. doi:10.1093/rheumatology/kex042
Listing, J., Kekow, J., Manger, B., Burmester, G-R., Pattloch, D., Zink, A., et al. (2015). Mortality in rheumatoid arthritis: the impact of disease activity, treatment with glucocorticoids, TNFα inhibitors and rituximab. Ann. Rheum. Dis. 74, 415–421. doi:10.1136/ANNRHEUMDIS-2013-204021
Monti, S., Klersy, C., Gorla, R., Sarzi-Puttini, P., Atzeni, F., Pellerito, R., et al. (2017). Factors influencing the choice of first- and second-line biologic therapy for the treatment of rheumatoid arthritis: real-life data from the Italian LORHEN registry. Clin. Rheumatol. 36, 753–761. doi:10.1007/S10067-016-3528-Y
Ochiai, M., Tanaka, E., Sato, E., Inoue, E., Abe, M., Saka, K., et al. (2021). Successful discontinuation of biological disease-modifying antirheumatic drugs in patients with rheumatoid arthritis in real-world settings. Mod. Rheumatol. 31, 790–795. doi:10.1080/14397595.2021.1883252
Olsen, I. C., Lie, E., Vasilescu, R., Wallenstein, G., Strengholt, S., and Kvien, T. K. (2019). Assessments of the unmet need in the management of patients with rheumatoid arthritis: analyses from the NOR-DMARD registry. Rheumatol. Oxf. 58, 481–491. doi:10.1093/RHEUMATOLOGY/KEY338
Schlager, L., Loiskandl, M., Aletaha, D., and Radner, H. (2020). Predictors of successful discontinuation of biologic and targeted synthetic DMARDs in patients with rheumatoid arthritis in remission or low disease activity: a systematic literature review. Rheumatol. Oxf. 59, 324–334. doi:10.1093/rheumatology/kez278
Silvagni, E., Bortoluzzi, A., Carrara, G., Zanetti, A., Govoni, M., and Scirè, C. (2018). Comparative effectiveness of first-line biological monotherapy use in rheumatoid arthritis: a retrospective analysis of the RECord-linkage on rheumatic diseases study on health care administrative databases. BMJ Open 8, e021447. doi:10.1136/BMJOPEN-2017-021447
Smolen, J. S., Landewé, R., Bijlsma, J., Burmester, G., Chatzidionysiou, K., Dougados, M., et al. (2017). EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann. Rheum. Dis. 76, 960–977. doi:10.1136/annrheumdis-2016-210715
Smolen, J. S., Landewé, R., Breedveld, F. C., Buch, M., Burmester, G., Dougados, M., et al. (2014). EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann. Rheum. Dis. 73, 492–509. doi:10.1136/annrheumdis-2013-204573
Smolen, J. S., Landewé, R., Breedveld, F. C., Dougados, M., Emery, P., Gaujoux-Viala, C., et al. (2010). EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann. Rheum. Dis. 69, 964–975. doi:10.1136/ARD.2009.126532
Tanaka, Y., Takeuchi, T., Mimori, T., Saito, K., Nawata, M., Kameda, H., et al. (2010). Discontinuation of infliximab after attaining low disease activity in patients with rheumatoid arthritis: RRR (remission induction by remicade in RA) study. Ann. Rheum. Dis. 69, 1286–1291. doi:10.1136/ard.2009.121491
Tanaka, Y., Yamanaka, H., Ishiguro, N., Miyasaka, N., Kawana, K., Kimura, J., et al. (2017). Low disease activity for up to 3 years after adalimumab discontinuation in patients with early rheumatoid arthritis: 2-year results of the HOPEFUL-3 study. Arthritis Res. Ther. 19, 56. doi:10.1186/s13075-017-1264-6
Trifirò, G., Isgrò, V., Ingrasciotta, Y., Ientile, V., L’Abbate, L., Foti, S. S., et al. (2021). Large-scale postmarketing surveillance of biological drugs for immune-mediated inflammatory diseases through an Italian distributed multi-database healthcare Network: The VALORE project. BioDrugs 35, 749–764. doi:10.1007/S40259-021-00498-3
Vrijens, B., De Geest, S., Hughes, D. A., Przemyslaw, K., Demonceau, J., Ruppar, T., et al. (2012). A new taxonomy for describing and defining adherence to medications. Br. J. Clin. Pharmacol. 73, 691–705. doi:10.1111/j.1365-2125.2012.04167.x
WHO (2021). WHOCC - ATC/DDD Index n.d. Available at: https://www.whocc.no/atc_ddd_index/(Accessed August 30, 2021).
Keywords: DAS28, initiation, discontinuation, biologic, DMARD, real-world, drug-utilization
Citation: Convertino I, Cazzato M, Tillati S, Giometto S, Gini R, Valdiserra G, Cappello E, Ferraro S, Bonaso M, Bartolini C, Paoletti O, Lorenzoni V, Trieste L, Filippi M, Turchetti G, Cristofano M, Blandizzi C, Mosca M, Lucenteforte E and Tuccori M (2023) Assessing disease activity of rheumatoid arthritis patients and drug-utilization patterns of biologic disease-modifying antirheumatic drugs in the Tuscany region, Italy. Front. Pharmacol. 14:1244486. doi: 10.3389/fphar.2023.1244486
Received: 22 June 2023; Accepted: 04 September 2023;
Published: 25 September 2023.
Edited by:
Carlos Alves, University of Coimbra, PortugalReviewed by:
Maria Giner-Soriano, Fundació Institut Universitari per a la Recerca a l'Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol), SpainCopyright © 2023 Convertino, Cazzato, Tillati, Giometto, Gini, Valdiserra, Cappello, Ferraro, Bonaso, Bartolini, Paoletti, Lorenzoni, Trieste, Filippi, Turchetti, Cristofano, Blandizzi, Mosca, Lucenteforte and Tuccori. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ersilia Lucenteforte, ZXJzaWxpYS5sdWNlbnRlZm9ydGVAdW5pcGkuaXQ=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.