- 1Haihe Laboratory of Modern Chinese Medicine, Tianjin, China
- 2National Key Laboratory of Chinese Medicine Modernization, Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 3School of Chinese Materia Medica, Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 4Department of Geriatric, Fourth Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
Arthritis is a common degenerative disease of joints, which has become a public health problem affecting human health, but its pathogenesis is complex and cannot be eradicated. Coptis chinensis (CC) has a variety of active ingredients, is a natural antibacterial and anti-inflammatory drug. In which, berberine is its main effective ingredient, and has good therapeutic effects on rheumatoid arthritis (RA), osteoarthritis (OA), gouty arthritis (GA). RA, OA and GA are the three most common types of arthritis, but the relevant pathogenesis is not clear. Therefore, molecular mechanism and prevention and treatment of arthritis are the key issues to be paid attention to in clinical practice. In general, berberine, palmatine, coptisine, jatrorrhizine, magnoflorine and jatrorrhizine hydrochloride in CC play the role in treating arthritis by regulating Wnt1/β-catenin and PI3K/AKT/mTOR signaling pathways. In this review, active ingredients, targets and mechanism of CC in the treatment of arthritis were expounded, and we have further explained the potential role of AHR, CAV1, CRP, CXCL2, IRF1, SPP1, and IL-17 signaling pathway in the treatment of arthritis, and to provide a new idea for the clinical treatment of arthritis by CC.
1 Introduction
Arthritis is a series of inflammatory diseases occurring in human joints or surrounding tissues, and it can lead to joint disability in serious cases. The incidence of arthritis is increasing year by year. There are more than 355 million arthritis patients worldwide, and the number is still increasing (Jia, 2021). Rheumatoid arthritis (RA) and osteoarthritis (OA) have the highest incidence (Wang, 2020; She, 2020), and the pathogenesis of them is complex. RA is a chronic autoimmune disease caused by synovial joint inflammation, which gradually leads to joint damage, cartilage degradation, disability, with a high disability rate in the later stage of the disease (Bird et al., 2022). OA is the most common type of arthritis associated with age and occurs most often in the elderly (Roškar and Hafner-Bratkovič, 2022). The causes of RA, gouty arthritis (GA) and OA are varied, mainly caused by the combined effects of congenital genetic factors and acquired environmental factors, and the related molecular mechanisms are complicated. The prevention and treatment of these three types of arthritis are the focus of research. Currently, arthritis cannot be cured clinically, and joint function can only be maintained through drug therapy. However, long-term treatment with single drug or combined immunosuppressive drugs have great limitations and cause adverse reactions. Therefore, it has important significance to explore the pathogenesis of arthritis and develop natural drugs to treat arthritis.
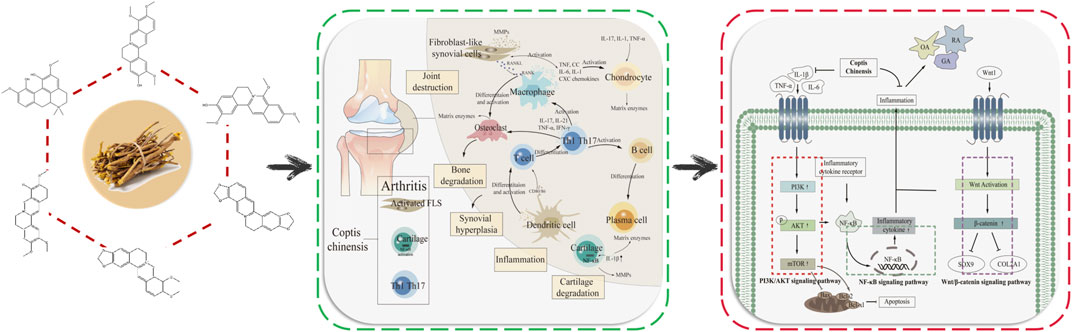
Coptis chinensis (CC) is the dried rhizome of the Ranunculaceae plant Coptis chinensis Franch., Coptis deltoidea C.Y.Cheng et Hsiao, or Coptis teeta Wall (National Pharmacopoeia Commission, 2020), which has antibacterial, anti-inflammatory, antioxidant, anti-tumor, antiarrhythmic and other pharmacological effects (Gai et al., 2018). CC is commonly used in clinical treatment of cardiovascular and cerebrovascular diseases, diabetes, cancer and other diseases (Fu et al., 2021). CC contains more than 130 chemical components, mainly including alkaloids, coumarins, organic acids, and flavonoids (Zhou et al., 2020). CC has good therapeutic effect on RA, OA, GA (Yue et al., 2019; Huang et al., 2021; Zhang et al., 2022; Elkomy et al., 2022). The alkaloid berberine, palmatine, coptisine, jatrorrhizine, magnoflorine and jatrorrhizine hydrochloride show significant antibacterial and anti-inflammatory effects. Studies have shown that berberine can effectively treat RA, OA and GA, mainly by reducing the level of inflammatory factors, regulating intestinal flora, promoting uric acid excretion, and improving the inflammatory response damage of joints and their surrounding tissues (Fan et al., 2021; Xu and Li, 2021). At the molecular level, CC can improve arthritis by regulating Wnt1/β-catenin, PI3K/AKT/mTOR and NF-κB signaling pathways, inhibiting the expression of pro-inflammatory factors such as interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), inducible nitric oxide synthase (iNOS), etc (Zhao et al., 2014; Zhou et al., 2019; Shen et al., 2020; Chen et al., 2023). Berberine, palmatine, coptisine and other components are the main components of CC in the treatment of arthritis. Interleukin-10 (IL-10), IL-1β, mitogen-activated protein kinase (MAPK), IL-6, matrix metalloproteinase-3 (MMP-3), TNF-α and other targets have been confirmed to play important roles in the treatment of arthritis by CC. The regulation of IL-17 signaling pathway in chondrocytes could inhibit the overexpression and activation of key proteins, such as IL-17RA, ACT1 and TRAF6, which could improve the occurrence of cartilage inflammation in OA. Research has shown that aryl hydrocarbon receptor (AHR), caveolin-1 (CAV1), c-reactive protein (CRP), C-X-C motif chemokine 2 (CXCL2), interferon regulatory factor-1 (IRF1), secreted phosphoprotein 1 (SPP1) and other targets are key targets in the pathogenesis of arthritis (Chen et al., 2022; Wang et al., 2022; Sakthiswary et al., 2022; Zhu et al., 2022; Li et al., 2023; Yang et al., 2023), but role of them in the treatment of arthritis by CC has not been verified and further clarification is needed.
Studies have shown that chemical drugs used in the treatment of arthritis have different degrees of toxicity and side effects. CC, as a natural Chinese herbal medicine with low toxicity of antibacterial and anti-inflammatory, is feasible to develop as a drug for treating arthritis. However, there are few studies on the treatment of arthritis by CC. Therefore, it is of great significance to clarify the mechanism of CC in the treatment of arthritis, which can provide new research directions for clinical drug development. We have elaborated the active ingredients, targets and mechanism in the treatment of RA, OA and GA by CC, revealed the potential targets and related pathways of CC in the treatment of arthritis, and provided new insights into the study of the molecular mechanism of CC in treating arthritis. In this review, the chemical components, targets and pathways of CC in the treatment of arthritis were discussed in detail, the molecular mechanism of CC in treating arthritis was elaborated, and the potential therapeutic targets were analyzed, providing new ideas for clinical prevention and treatment of arthritis.
2 The pathogenesis of three types of arthritis
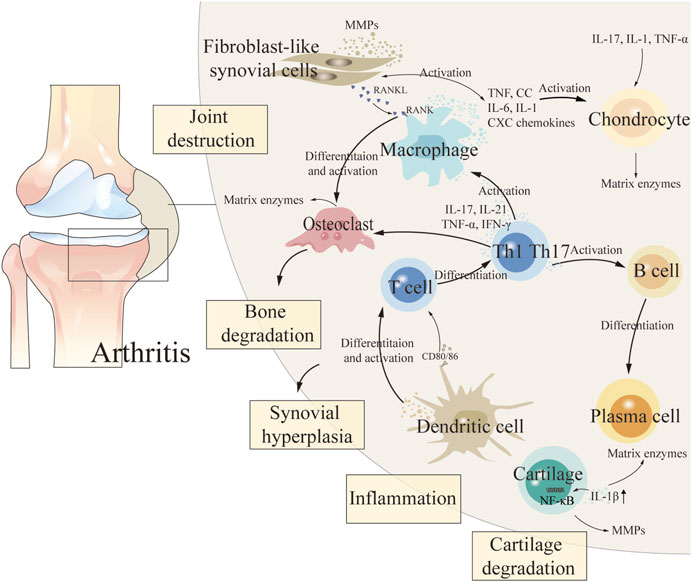
The etiology of arthritis is complex and relevant pathogenesis has not been clarified (Chen et al., 2021). During the pathogenesis of arthritis, fibroblast-like synovial cells (FLS), chondrocytes, intrinsic immune cells (dendritic cells and macrophages), and adaptive immune cells (T and B cells) in synovial tissue release a variety of cytokines that lead to persistent destruction of cartilage and subchondral bone, thereby exacerbating the degree of arthritis (So and Martinon, 2017; Xing, 2021; Xu, 2022) (Figure 1 demonstrated the pathogenesis of arthritis). Non-steroidal anti-inflammatory drugs, anti-rheumatism drugs, traditional Chinese medicine (TCM) compounds and other drugs are commonly used in clinical treatment of arthritis. But chemical therapy cannot cure arthritis, and it can only relieve joint function, and long-term use of these treatments can cause relatively significant toxic and side effects, causing liver, kidney and cardiovascular toxicity (Li, 2022; Yu, 2022). TCM has unique advantages which due to its characteristics of multiple components, low toxicity, few side effects and good curative effects in the treatment of RA and OA (Li et al., 2022; Meng et al., 2022). The effective components of TCM show great potential in the treatment of arthritis by inhibiting inflammatory response, alleviating oxidative stress, regulating chondrocyte metabolism and regulating related signaling pathways (Li et al., 2017).
2.1 Rheumatoid arthritis
RA is an autoimmune inflammatory disease with systemic sequelae (Hyndman, 2017), the main symptoms of which are synovial inflammation, production of rheumatoid factors and antibodies against citrulline proteins, and destruction of cartilage and bone (McInnes and Schett, 2011). The pathogenesis of RA is confused and difficult curative ratio, which is mainly caused by excessive proliferation of synovial cells, increased levels of inflammatory factors and abnormal toll-like receptor signaling pathway (Wen, 2022). Inflammatory response is an important pathological process of RA. Abnormal secretion of proinflammatory cytokines, chemokines and proteases will disturb the balance of the body and lead to cartilage and bone damage (Fang et al., 2020). The pathological feature of RA is the infiltration of synovial inflammatory cells in multiple joints. Nuclear factor кB receptor activating factor (RANKL), prostaglandins and matrix metalloproteinases (MMPs) are induced by pro-inflammatory cytokines, including TNF-α, IL-6 and interleukin-1 (IL-1), causing joint pain and swelling (Wallach, 2016). At the molecular level, MAPK, TLR7/NF-кB and apoptosis signaling pathways are the main signaling pathways involved in regulating the invasion and abnormal behavior of RA-FLS cells (Zheng, 2012; Bottini and Firestein, 2013). Currently, the main treatment is to reduce inflammation and relieve pain (Fan et al., 2020). Modern studies of TCM have shown that a variety of monomer components of TCM have the efficacy of treating RA (Yao et al., 2023), including sinomenine, artemisinin, total glucosides of paeony and berberine (Ou et al., 2010; Wang et al., 2011; Xia and Li, 2017; Sharma et al., 2022).
2.2 Osteoarthritis
OA is the most common type of arthritis that causes joint pain and disability, and it has a high incidence (Li et al., 2022). The main characteristics of OA are cartilage degeneration, synovial hyperplasia, osteophyte formation and subchondral osteosclerosis, but the pathogenesis has not been clearly defined (Chen et al., 2022). The occurrence and development of OA is closely related to degradation of matrix and release of bioactive substances, which promote the release of MMPs and eventually lead to chondrolysis (Uthman et al., 2003). Inflammatory factors and receptors are involved in the occurrence and development of OA, which can cause degenerative changes of chondrocytes through MAPK/ERK, JAK2/STAT3, NF-κB, Wnt/β-catenin and PI3K/AKT signaling pathways (Hwang et al., 2005; Roemer et al., 2011; Min et al., 2017). Chondrocytes are the source and target of pro-inflammatory cytokines in OA. Pro-inflammatory cytokine interleukin-1 is an important inflammatory mediator secreted by early OA and a key inflammatory cytokine involved in the pathogenesis of OA (Li et al., 2019; Yang et al., 2021). IL-1β mainly affects the metabolism of articular cartilage extracellular matrix and chondrocytes, and plays an important role in the pathogenesis of OA by inducing excessive release of inflammatory mediators cycloooxygenase-2 (COX-2) and iNOS, and overexpression of cartilage MMPs. IL-1β, TNF-α and IL-6 are three highly expressed inflammatory cytokines in OA joints, which are actively produced by chondrocytes, synovial cells, macrophages and osteoblasts, and can be used as indicators of the progression of OA (Zhou et al., 2016a).
2.3 Gouty arthritis
GA is an inflammatory reactive disease that causes joint pain due to the dysfunction of purine metabolism and uric acid (UA) excretion in the body (AbdullGaffar et al., 2020). The pathogenesis of GA is related to the inflammatory response caused by the deposition of monosodium urate (MSU) around the joint, which stimulates the synovial membrane to produce pathological reactions such as synovial vasodilation and leukocyte exudation, which mainly involve the mediation of MAPK and NF-κB signaling pathways and the activation of TNF-α, IL-1 and other inflammatory cytokines (Choe et al., 2014; Terkeltaub, 2017; Lv, 2020; Liu et al., 2022).
3 Chemical components and mechanism of Coptis chinensis in the treatment of arthritis
3.1 The chemical components of Coptis chinensis in treating arthritis
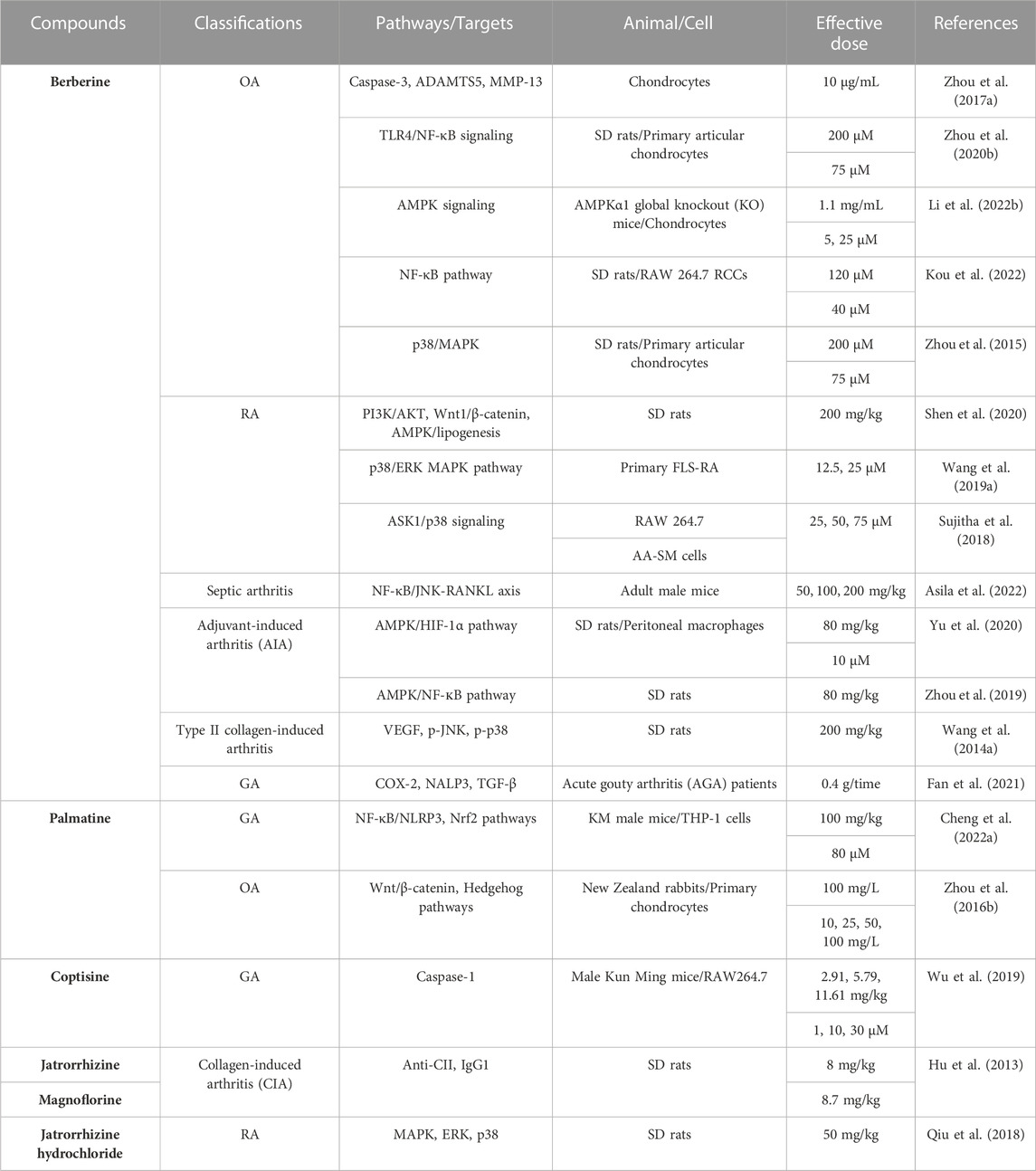
CC has obvious inhibitory effect on acute and chronic inflammatory reactions (Park et al., 2018). CC contains a variety of anti-inflammatory active ingredients, such as berberine, palmatine, coptisine, etc. (Hu et al., 2013; Wang et al., 2014; Zhou et al., 2015; Zhou et al., 2016; Zhou et al., 2017; Qiu et al., 2018; Sujitha et al., 2018; Wang et al., 2019; Wu et al., 2019; Zhou et al., 2019; Zhou et al., 2020; Shen et al., 2020; Yu et al., 2020; Fan et al., 2021; Cheng et al., 2022; Asila et al., 2022; Li et al., 2022; Kou et al., 2022) (Table 1), which can achieve anti-inflammatory effects mainly by inhibiting the activity of key proteins in the inflammatory signaling pathway and blocking the transmission of inflammatory signals (Hu and Mo, 2017; Geng, 2018). Berberine has a significant anti-inflammatory activity and can treat a variety of arthritis, especially RA and OA (Hu et al., 2010; Zhou et al., 2016c).
3.1.1 Berberine
Berberine is the main active ingredient in CC that plays an anti-inflammatory and antibacterial role. It can effectively treat a variety of arthritis by down-regulating the production and expression of various inflammatory mediators and inhibiting the activation of inflammatory pathways (He et al., 2018). Berberine has a strong anti-rheumatoid effect and can slow the progression of RA by targeting mitochondrial oxidative phosphorylation (Fan et al., 2018; Elkomy et al., 2022) confirmed that berberine could effectively inhibit RA inflammation. By inhibiting autophagy of FLS cells, berberine induces RA-FLSs cycle arrest in G0/G1 phase, induces RA-FLSs cell death, inhibits the expression of vascular endothelial growth factor, regulates the level of anti-inflammatory factors, and achieves the purpose of treating RA (Wang et al., 2014; Huang et al., 2021). Wang (2011) verified that berberine induced apoptosis of RA-FLSs mainly through the mechanism of up-regulating the expression of pro-apoptotic protein apoptosis regulator BAX (Bax), inhibiting the expression of anti-apoptotic proteins apoptosis regulator Bcl-2 (Bcl-2) and Bcl-xl, and promoting the activation of caspase-3, caspase-9 and PARP. (Wang et al., 2017) found that berberine could reduce the expression level of interleukin-17 (IL-17) and IL-6, promote the expression of IL-10 and transforming growth factor-β (TGF-β) in serum, and improve the clinical symptoms of RA. Berberine can significantly suppress the activation of p-ERK, p-p38 and p-JNK, reduce the destruction of inflammatory cells on joint tissues, and exert anti-RA activity (Wang et al., 2014). During the treatment of RA, berberine reduced the expression levels of TNF-α, IL-17, interferon-γ (IFN-γ), MMPs and RAR-related orphan receptor γt (RORγt) (Sharma et al., 2022). Studies have shown that berberine treats RA by specifically inhibiting T cells, involving the balance between Treg and Th17 cells, providing a potential target for berberine in the treatment of arthritis (Li et al., 2017; Vita et al., 2021).
(Xie et al., 2018) found that berberine could increase the enzymatic antioxidant levels, such as SOD, glutathione peroxidase, catalase and glutathione-S-transferase in osteoporosis rats, which is helpful to prevent osteoporosis. (Huang et al., 2021) found that berberine promoted the proliferation and activity of IL-1β-induced inflammatory degenerative chondrocytes by inhibiting cell inflammatory response and activating TGF-β/Smad2/3 signaling pathway, and reduced the degree of OA development. Berberine is a potential therapeutic drug for OA. MMPs play a significant role in OA-induced articular cartilage damage (Dean et al., 1989; Hu et al., 2011) showed that berberine could inhibit the expression of matrix metalloproteinase-1 (MMP-1), MMP-3 and matrix metalloproteinase-13 (MMP-13) and effectively treat OA. Connective tissue growth factor (CCN2) is abundantly expressed response. Berberine inhibits CCN2 to produce IL-1β by down-regulating ROS-mediated NF-κB signaling pathway in fibroblast synovial cells, regulates cartilage damage and alleviates OA (Liu et al., 2015). Berberine can inhibit the expression of NO, prostaglandin E2 (PGE2), iNOS, COX-2, MMP-3 and MMP-13 induced by IL-1β, downregulate the expression of inflammatory mediators and reduce the inflammatory response in chondrocytes (Zhou et al., 2016).
By inhibiting the NOD-like receptor thermal protein domain 3 (NLRP3)/Toll-like receptor signaling pathway, berberine downregulated the expression levels of IL-2, IL-6 and TNF-α, and alleviated the degree of ankle swelling in GA mice (Jian et al., 2020). Berberine reduces the expression levels of NLRP3, TNF-α and IL-1β and the level of intracellular reactive oxygen species, thereby reducing MSU crystal-induced inflammation in rats (Liu et al., 2016). Berberine improves the acute symptoms of GA by inhibiting the activity of joint elastase and thereby inhibiting the infiltration of joint synovium neutrophils (Dinesh and Rasool, 2017). The increase of serum UA level is the key to AGA attack. Berberine can dilate blood vessels, improve blood flow and increase the expression of human urate transporter, thus increasing blood UA excretion and reducing UA level in the body. In addition, berberine can also improve insulin resistance and inhibit UA synthesis (Jin et al., 2019; Rondanelli et al., 2020). IL-1β is considered to be the initiating factor of AGA inflammatory response (Li et al., 2019), PGE2 has a strong inflammatory effect and is involved in the whole process of GA inflammatory response, and COX-2 is a key enzyme in the synthesis of PGE2 in the body (Liu et al., 2019). Fan et al. (Fan et al., 2021) showed that assisted treatment of AGA of berberine could significantly inhibit the expressions of inflammatory factors IL-1β, COX-2, nucleotide-binding oligomerization domain-like receptor 3 (NALP3) and TGF-β, reduce the levels of CRP, ESR and UA, and effectively relieve the symptoms of AGA.
3.1.2 Palmatine
Palmatine has been proved to have antipyretic, antibacterial and anti-inflammatory activities, it has been used as an anti-inflammatory agent in clinical practice (Pathan et al., 2015; Zhou et al., 2017). Palmatine has a good effect in the treatment of OA, it can effectively inhibit the expression of MMP-1, MMP-3 and matrix metalloproteinase-9 (MMP-9) induced by IL-1β by blocking Wnt1/β-catenin and Hedgehog signaling pathways, and improve OA (Zhou, 2014). Palmatine can inhibit expression of IL-1β and MMPs, it promotes the expression of cyclopamine which is inhibitor of the Hedgehog signaling pathway and suppress Wnt/β-atenin signaling pathway, to exert protective effect on OA and possess potential antalgic effect (Zhou et al., 2016). Research has shown that palmatine can improve joint swelling and significantly inhibit the expression of IL-1β, IL-6, IL-18, TNF-α in joint tissue, block the infiltration of inflammatory cells into the synovium and joint cavity, to achieve the therapeutic effect of GA (Cheng et al., 2022).
3.1.3 Other ingredients
Alkaloids in CC are the main anti-inflammatory active ingredients. Besides berberine and palmatine, coptisine, jarrorhizine hydrochloride and magnoflorine also play important roles in the anti-inflammatory effect of CC. The expression level of C-X-C motif chemokine 12 (CXCL12) in synovium of patients with OA was significantly increased, and C-X-C chemokine receptor 4 (CXCR4) was its receptor (Wei et al., 2010; Bragg et al., 2019). Coptisine, as a CXCR4 antagonist, inhibits the overexpression of ADAMTS4,5 in chondrocytes induced by CXCL12, improving cartilage degradation and subchondral bone damage (Yang et al., 2023). Coptisine inhibits the activation of NLRP3 inflammatory bodies by blocking caspase-1, which can be used to treat GA associated with NLRP3 inflammatory bodies (Wu et al., 2019). Jarrorhizine hydrochloride can significantly inhibit the expression levels of IL-1β, IL-6, IL-8, matrix metalloproteinase-2 (MMP-2), and MMP-3, suppress the proliferation, migration, and secretion of synovial cells, prevent bone destruction, and thus improve the severity of RA (Qiu et al., 2018). Magnoflorine has significant anti-inflammatory effect, which may improve RA by promoting the synthesis of proteoglycans in chondrocytes (Yue et al., 2013; Gui et al., 2015). Researches have shown that magnoflorine reduces IL-1β-induced inflammatory cytokine levels and inhibits inflammatory responses in AIA rats by regulating the PI3K/AKT/NF-κB signaling pathway (Shen et al., 2022). In a traumatic osteoarthritis model, magnoflorine can promote the proliferation, chondrogenesis and migration of cartilage progenitor cells by activating the chondrogenic signaling pathway, thereby directly reducing articular cartilage degeneration (Cai et al., 2020). By inhibiting the expression of TNF-α, IL-6, IL-1β, MCP-1, iNOS and IFN-β, magnoflorine improved the degree of joint destruction and macrophage infiltration in synovial tissue of CIA mice, and achieved the purpose of treating arthritis by inhibiting the activation of NF-κB and MAPK signaling pathways (Wang et al., 2023a).
3.2 The mechanism of Coptis chinensis in treating arthritis
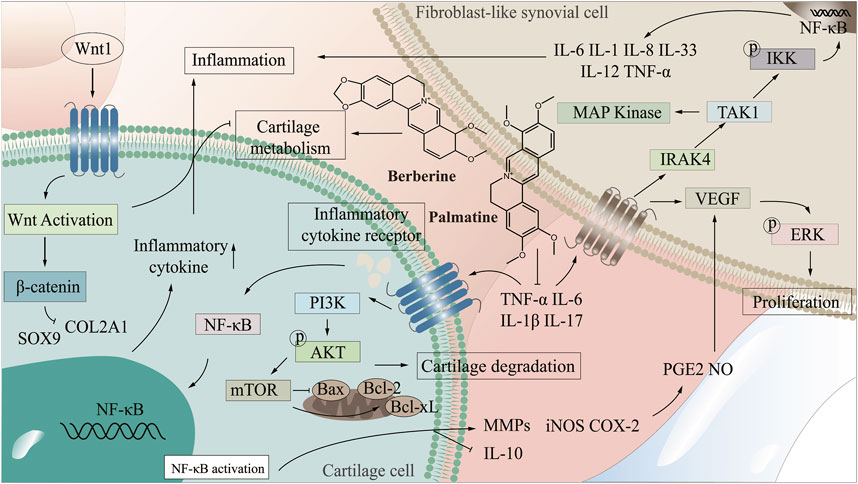
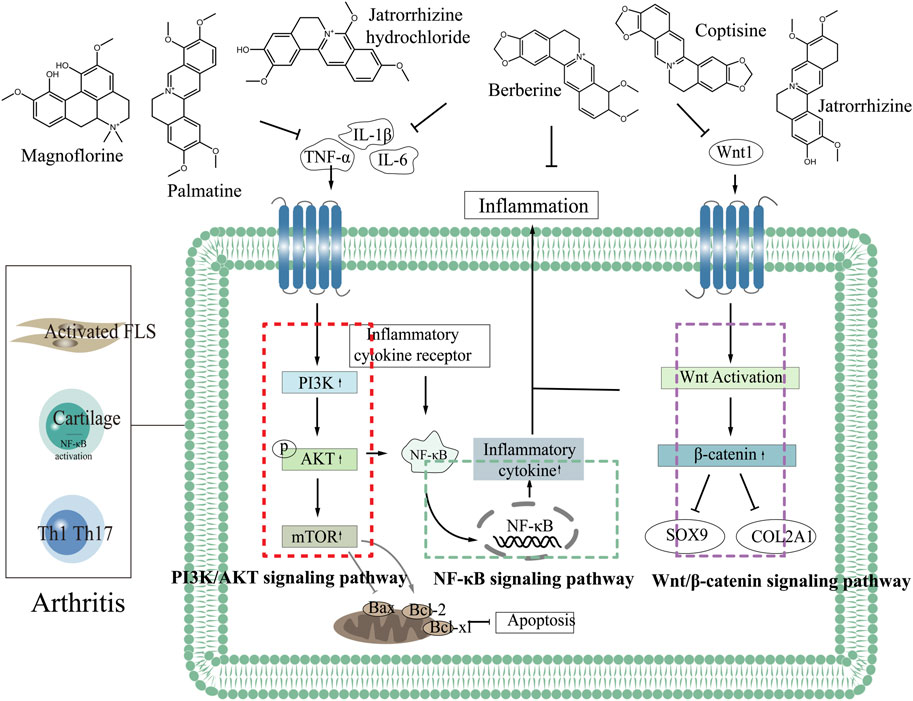
There are many uncomfortable symptoms in the clinical treatment of arthritis, and the development of natural drugs is greatly on demand. CC, as an antibacterial and anti-inflammatory Chinese medicine, presents an excellent potential for treating arthritis. By improving the targeting of CC to arthritic damaged tissues and enhancing the bioavailability of CC in treatment, it is promising to realize the development of novel natural medicines with enhanced curative effect and low side effects. Therefore, it is extremely important to clarify the mechanism of CC in treating arthritis. The NF-κB signaling pathway is one of the crucial pathways in the pathogenesis of arthritis, abnormal activation of which will lead to synovial inflammation, chondrocyte apoptosis and destruction (Qing et al., 2014; Wu, 2021; Zeng, 2022). PI3K/AKT/mTOR signaling pathway is a central regulator of cell growth, proliferation and cell cycle, and plays a significant role in chondrocyte degeneration (Xu et al., 2014). Wnt/β-catenin signaling pathway affects bone modeling and bone remodeling, especially the differentiation of osteoblasts, which may be a potential target for treating bone diseases (Wang et al., 2014). In the treatment of arthritis, CC can reduce the levels of IL-1β, TNF-α, IL-6, COX-2, NALP3 and TGF-β, regulate NF-κB and PI3K/AKT/mTOR signaling pathways, which promote the proliferation of articular chondrocytes, inhibit apoptosis, and enhance cell healing ability, thereby improving bone and joint, inhibiting bone destruction and reducing inflammation in joints and surrounding tissues (Fan et al., 2021; Jie et al., 2022; Liu et al., 2023), (Figure 2 demonstrated the mechanism of CC in the treatment of arthritis).
3.2.1 Wnt1/β-catenin signaling pathway
Wnt1/β-catenin signaling pathway plays a key role in cell proliferation, differentiation and autoimmune regulation (Cici et al., 2019). Wnt1/β-catenin signaling pathway produces a significant role in tissue repair and joint homeostasis by regulating the activity of synovial cells, osteoblasts and chondrocytes in joint tissue, and it can cause a variety of arthritis when abnormal (Mei et al., 2019; Alharbi et al., 2022). FLS are important factors in osteoremodeling in arthritis, and Wnt1/β-catenin signaling pathway exert marked effects in the survival of FLS cells (Dinesh et al., 2020). Studies have shown that abnormal Wnt1/β-catenin signaling pathway is the main mechanism of RA (Miao et al., 2013). In RA, Wnt1/β-catenin pathway signal transduction results in polymorphic changes of osteocytes/chondrocytes, causing bone erosion and cartilage degradation (Sujitha et al., 2020). Wnt1 is mainly expressed in synovial cells, after the activation of Wnt signaling pathway, the expression of β-catenin increases, which promotes the secretion of inflammatory factors. RA-FLS is activated and induces RA, Cai et al. (2022) confirmed that blocking Wnt1/β-catenin signaling pathway and inhibiting TNF-induced migration, invasion and inflammation of RA-FLS cells can effectively alleviate adjuvant arthritis (AA). Some miRNAs can be used as inhibitors of Wnt1/β-catenin signaling pathway to further prevent RA (Miao et al., 2014; Sujitha et al., 2020) showed that berberine could inhibit Wnt1/β-catenin signal transduction through miR-23a activation, thereby improving RA. Wnt1/β-catenin signaling pathway controls bone and joint development and is closely related to the pathogenesis and progression of OA (Ahmad et al., 2020). IL-1β-induced chondrocyte degeneration may be accompanied by activation of Wnt/β-catenin pathway, which exerts an important effect in the degeneration and destruction of OA articular cartilage (Su et al., 2012). Sry-box transcription factor 9 (SOX9) has the function of promoting cartilage anabolism, and abnormal expression may lead to OA (Carmon et al., 2023; Alahdal et al., 2021) showed that activation of Wnt1/β-catenin pathway inhibited the expression of SOX9 and collagen type II, and impaired the cartilage differentiation and regeneration of MSCs in OA patients. (Li et al., 2022) demonstrated that MiR-376c-3p from Adipose mesenchymal stem cell (ADSC) derived exosomes regulated the Wnt1/β-catenin signaling pathway by targeting WNT3 or WNT9a, improving chondrocyte degradation and synovial fibrosis induced by OA. Lietman et al. (2018) found that regulation of Wnt pathway could improve OA symptoms in surgery-induced mouse OA model. Mei et al. (2019) confirmed that inhibition of Wnt1/β-catenin pathway signal transduction and regulation of β-catenin stability in macrophages could effectively improve GA. Berberine can induce Dvl-1 inhibitor-CYLD to inhibit the expression of FZD4, LRP5 and Dvl-1, regulate the Wnt1/β-catenin signaling pathway in adjuvant arthritis FLS cells, and reduce the expression level of intracellular β-catenin, thus improving arthritis (Shen et al., 2020). Palmatine inhibits the progression of OA by regulating the Wnt1/β-catenin signaling pathway (Xuan et al., 2019). Therefore, the Wnt1/β-catenin signaling pathway can be used as a potential target for treating various types of arthritis (Zhou, 2014; Shang et al., 2021).
3.2.2 PI3K/AKT/mTOR signaling pathway
PI3K/AKT/mTOR signaling pathway is mainly mediated by growth factor signal transduction to lipid metabolism, protein synthesis and cell proliferation and survival, and other physiological processes, and it is related to inflammation, autoimmune diseases and hematological malignancies, affecting cell proliferation, differentiation, metastasis and apoptosis (Foster et al., 2012; Abeyrathna and Su, 2015; Liu et al., 2021). The PI3K/AKT/mTOR signaling pathway is crucial for the normal metabolism of joint tissues and is closely related to the occurrence and development of OA and RA (Sun et al., 2020; Zhou et al., 2023; Zhang et al., 2001) found that the spontaneous and induced activation of AKT and the level of pAKT in RA patients were higher than those in OA patients. (Dinesh and Rasool, 2019). demonstrated that PI3K/AKT signaling pathway could be used as a key target for RA treatment, and inhibition of abnormal activation of PI3K/AKT signaling pathway played a key role in the prevention and treatment of RA (Ansari et al., 2022; Hashiramoto et al., 2007) confirmed that abnormal PI3K/AKT signaling pathway can lead to RA synovial overgrowth and joint destruction. Abnormal activation of PI3K/AKT signaling pathway can increase the expression level of anti-apoptotic genes in synovial cells of RA patients, and then leads to the exacerbation of RA disease (Harris et al., 2009; Smith et al., 2010). Wang (Wang, 2020) found that downregulation of PI3K/AKT pathway and inhibition of over-activation of AKT could effectively improve RA. Studies have shown that activation of PI3K/AKT signaling pathway leads to accelerated proliferation of FLS cells in AA and aggravation of the course of arthritis (Dinesh and Rasool, 2019). Abnormal activation of PI3K/AKT/mTOR signaling pathway will destroy the normal function of cartilage and subchondral bone (Sun et al., 2020). As immune cells, synovial macrophages are closely related to the occurrence and development of OA. Activated macrophages are regulated by the PI3K/AKT signaling pathway, and their activation status is highly correlated with the severity of OA (Zhang et al., 2020). Inhibition of PI3K/AKT/mTOR signaling pathway activates autophagy, promotes anabolism and inhibits catabolism of OA chondrocytes, and effectively treats OA (Wang, 2022). Studies have shown that quercetin regulates PI3K/AKT signaling pathway to improve arthritis by binding to and inhibiting PI3K in mouse epidermal cells to inhibit AKT phosphorylation (Khan et al., 2019). Berberine delays the progression of osteoporosis, RA and OA by regulating the PI3K/AKT signaling pathway (Wong et al., 2020). Therefore, the key proteins in PI3K/AKT signaling pathway can be used as potential targets of CC in the treatment of arthritis for in-depth study.
By combining the pathogenesis of three types of arthritis with the related targets and pathways of CC in treating arthritis, we elucidated the mechanism, providing a new idea for the development of CC as a candidate drug for treating arthritis. Most of the components in CC in treating arthritis are alkaloids. Six components including berberine in CC have good effects in the treatment of arthritis, which can inhibit the expression of TNF-α, IL-6, IL-1β through PI3K/AKT, Wnt1/β-catenin and NF-κB signaling pathways, thereby reducing the inflammatory response and achieving the purpose of treating arthritis (Figure 3 demonstrated the chemical constituents and the pathways involved in the treatment of arthritis by CC).
4 Deep exploration based on potential therapeutic targets for arthritis
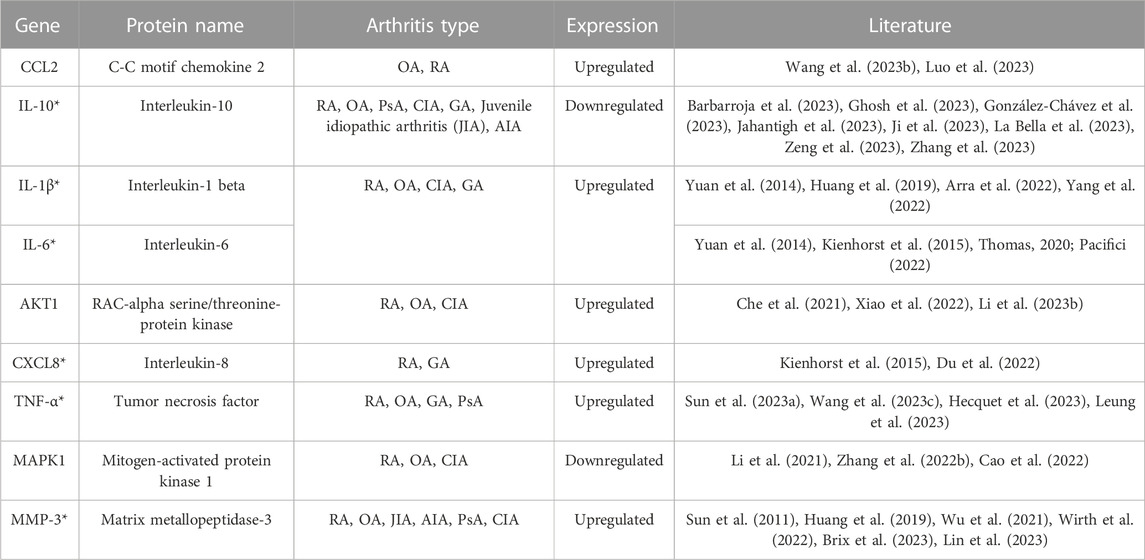
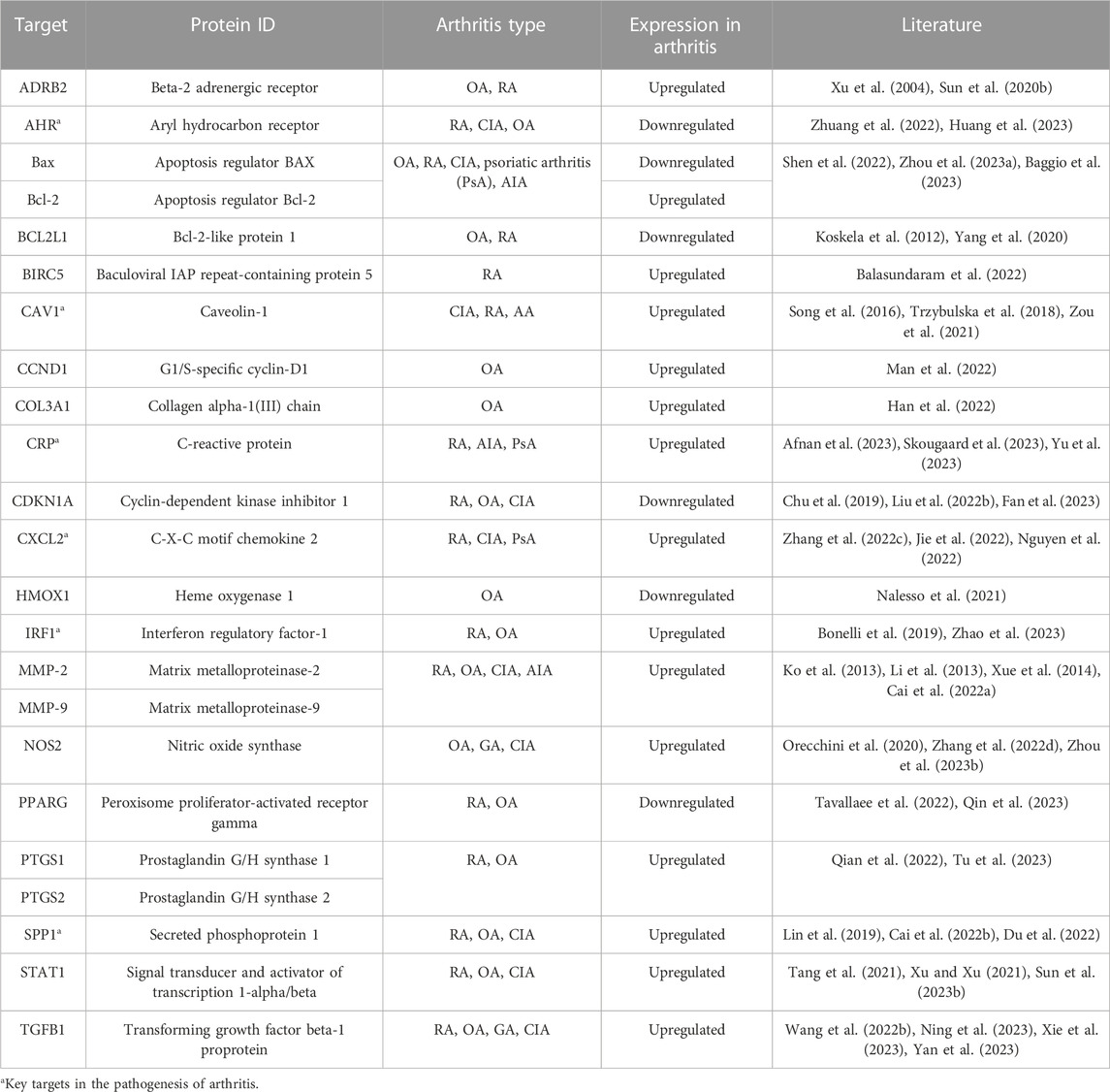
In this review, we have summarized the targets involved in the pathogenesis of arthritis, elaborated potential therapeutic targets, and provided a new idea for the exploration of the mechanism of CC in the treatment of arthritis. Studies have shown that CC can treat a variety of arthritis, and it is better for OA, RA and GA. CC has the characteristics of multiple components, multiple targets, and multiple pathways in the treatment of arthritis. Currently, IL-10, IL-1β, IL-6, MMP-3, TNF-α and other targets have been verified to play a role in treating arthritis by CC (Table 2) (Sun et al., 2011; Yuan et al., 2014; Kienhorst et al., 2015; Huang et al., 2019; Thomas, 2020; Che et al., 2021; Li et al., 2021; Wu et al., 2021; Arra et al., 2022; Zhang et al., 2022; Cao et al., 2022; Du et al., 2022; Pacifici, 2022; Wirth et al., 2022; Xiao et al., 2022; Yang et al., 2022; Sun et al., 2023a; Barbarroja et al., 2023; Li et al., 2023; Brix et al., 2023; Wang et al., 2023b; Wang et al., 2023; Ghosh et al., 2023; González-Chávez et al., 2023; Hecquet et al., 2023; Jahantigh et al., 2023; Ji et al., 2023; La Bella et al., 2023; Leung et al., 2023; Lin et al., 2023; Luo et al., 2023; Zeng et al., 2023; Zhang et al., 2023). However, the types of arthritis treated with these targets are not completely clear. Targets, β2-adrenergic receptor (ADRB2), AHR, CRP, IRF1, prostaglandin G/H synthase 1 (PTGS1), SPP1 and other targets, exert key effects in the pathogenesis of arthritis, but role of them in the treatment of arthritis with CC has not been confirmed (Table 3) (Xu et al., 2004; Koskela et al., 2012; Ko et al., 2013; Li et al., 2013; Xue et al., 2014; Song et al., 2016; Trzybulska et al., 2018; Bonelli et al., 2019; Chu et al., 2019; Lin et al., 2019; Sun et al., 2020; Orecchini et al., 2020; Yang et al., 2020; Nalesso et al., 2021; Tang et al., 2021; Xu and Xu, 2021; Zou et al., 2021; Balasundaram et al., 2022; Cai et al., 2022; Liu et al., 2022; Wang et al., 2022; Zhang et al., 2022; Zhang et al., 2022; Han et al., 2022; Man et al., 2022; Nguyen et al., 2022; Qian et al., 2022; Tavallaee et al., 2022; Zhuang et al., 2022; Afnan et al., 2023; Baggio et al., 2023; Sun et al., 2023b; Zhou et al., 2023; Fan et al., 2023; Huang et al., 2023; Ning et al., 2023; Qin et al., 2023; Skougaard et al., 2023; Tu et al., 2023; Xie et al., 2023; Yan et al., 2023; Yu et al., 2023; Zhao et al., 2023). We have elaborated on the role of targets such as MMP-3, IL-1β, MAPK, IL-6, ADRB2, AHR, CRP, CAV1, CXCL2, SPP1 and other targets in treating arthritis, explored the potential targets and mechanisms of CC in treating arthritis, analyzed the feasibility of CC as an anti-arthritis drug, and provided a theoretical basis for subsequent research.
IL-6 plays an important role in the development of RA (Cheng et al., 2022), and it is associated with inflammatory response and cartilage loss in the pathogenesis of OA (Hou et al., 2020). IL-10 is an important anti-inflammatory and immunosuppressive cytokine that not only prevents the occurrence of arthritis, but also has an inhibitory effect on the development of arthritis (Charbonnier et al., 2010). IL-1β is related to the inflammation of synovium, which can affect the normal metabolism of chondrocytes, change the structure and function of osteocytes, promote the apoptosis of chondrocytes and the decomposition of cartilage matrix, and it plays a key role in the pathogenesis of arthritis (Bai, 2021). TNF-α is a pro-inflammatory cytokine secreted by membrane-forming FLS and mainly distributed in the joint space of RA, anti-TNF therapy is the preferred therapy for severe RA patients (Taylor et al., 2022). Activated ADRB2 in osteoblasts stimulates osteoclastogenesis and upregulates RANKL expression, thereby reducing bone formation and promoting bone resorption, leading to bone loss and osteoarthritis (Ma et al., 2011; Liang et al., 2018). AHR can be used as a key target for the treatment of RA (Xi et al., 2022), it has a variety of potential roles in the immune system. Various natural products can alleviate synovial inflammation and restore immune balance in RA patients by binding to AHR in fibroblast-like synovial cells and T cells (Stockinger et al., 2014; Hui and Dai, 2020). The expression of anti-apoptotic proteins Bcl-xl increased significantly in arthritis patients (Chen et al., 2016). CAV1 is a regulator of various cell signaling pathways. Reducing the expression of CAV1 can inhibit the expression of IL-1β-induced CCL2 mRNA and promote the apoptosis of RA-FLS (Li et al., 2017). CXCL2 promotes osteoclast formation and is associated with bone erosion in RA. Studies have shown that blocking expression of CXCL2 may be a means of treating RA (Wang et al., 2021). IRF1 promotes chondrogenesis of hADSCs by up-regulating HILPDA level, and it provides a new biomarker for the treatment of osteoarthritis (Zhao et al., 2023). As a proteolytic enzyme secreted by synovial fibroblasts, MMPs are involved in the pathogenesis of arthritis and play an important role in inflammatory response and joint destruction (Murphy and Nagase, 2008). The levels of MMP-2 and MMP-9 are elevated in the serum of RA patients, which can reflect the early inflammatory level of RA (Hu et al., 2011). MMP-3 plays a key role in the pathogenesis of RA and is one of the key indicators for the treatment of RA (Lerner et al., 2018) Studies have shown that when PTGS1 is overexpressed, the migration and invasion of OA synovial cells increase, and the apoptosis rate decreases (Wang et al., 2019). In collagen induced arthritis, SPP1 secreted by FLSs promotes the formation of osteoclast through PI3K/AKT signals. Regulating the expression of SPP1 gene in FLSs may be a potential method to treat RA bone injury in the joint microenvironment (Cai et al., 2022). Therefore, ADRB2, AHR, CRP, IRF1, PTGS1, SPP1 and other targets can be used as potential targets for CC in the treatment of arthritis, and it is of great significance to explore its role of CC in the treatment of arthritis.
5 Conclusion
TCM plays an important role in the treatment of arthritis due to its multi-component, multi-efficacy and multi-target characteristics. CC, as an antibacterial and anti-inflammatory Chinese medicine, has a good effect in treating arthritis. In this review, we have summarized the chemical constituents, targets and related pathways of CC in treating arthritis, discussed the mechanism of CC in the treatment of arthritis from the molecular level, clarified the potential targets, and provided reasonable directions for clinical treatment of arthritis. Berberine, palmatine, coptisine, jatrorrhizine, magnoflorine and jatrorrhizine hydrochloride in CC have the effect of treating arthritis, especially berberine can treat a variety of arthritis, such as RA, OA and GA. Berberine improves arthritis by reducing cell inflammation, improving chondrocyte function, promoting cartilage synthesis and repair, and promoting uric acid excretion. Palmatine can significantly block the Wnt1/β-catenin signaling pathway, protect chondrocytes and knee cartilage, and inhibit the progression of OA. At the molecular level, six components including berberine can improve RA, OA, GA and other types of arthritis by regulating PI3K/AKT, Wnt1/β-catenin and NF-кB signaling pathways. IL-10, IL-1β, IL-6, MMP-3, TNF-α, COX-2, TGF-β, Caspase-1, MAPK and other targets have been confirmed to play key roles in the treatment of arthritis by CC, and can be used as targets for clinical treatment of arthritis, providing scientific basis for the development of rational targeted drugs for the treatment of arthritis. AHR, CAV1, CRP, CXCL2, IRF1, SPP1 and other targets play important roles in the pathogenesis of arthritis and can be used as key targets for the treatment of arthritis. However, the role of them of CC in the treatment of arthritis remains to be further verified.
All in all, berberine, palmatine, coptisine, jatrorrhizine, magnoflorine and jatrorrhizine hydrochloride in CC can effectively treat arthritis, and have been proved. At the molecular level, CC plays a critical role in the treatment of arthritis by regulating NF-κB, Wnt1/β-catenin and PI3K/AKT/mTOR signaling pathway, inhibiting the expression of IL-6, IL-1β, MMP-3 and TNF-α. In this review, we have concluded with a summary and our insights on the chemical components, targets and pathways of CC in the treatment of arthritis, and discussed the relevant mechanism and potential targets, providing scientific basis for CC in the clinical treatment of arthritis.
Author contributions
ML: Conceptualization, methodology, writing–original draft, software. FT: Software, writing–review and editing. JG: Formal analysis. XL: Formal analysis. LM: Resources. MJ: Supervision, Project administration. JZ: Supervision, funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant No. 81973699, No. 82274361).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
AbdullGaffar, B., Abdul Hameed, B., Fodeh, S., and Sreeram, R. (2020). Concomitant gouty and tuberculous granulomatous arthritis. Int. J. Surg. Pathol. 28, 288–289. doi:10.1177/1066896919865778
Abeyrathna, P., and Su, Y. (2015). The critical role of Akt in cardiovascular function. Vasc. Pharmacol. 74, 38–48. doi:10.1016/j.vph.2015.05.008
Afnan, A., Saleem, A., and Akhtar, M. F. (2023). Chrysin, a 5,7-dihydroxyflavone restrains inflammatory arthritis in rats via subsiding oxidative stress biomarkers and inflammatory cytokines. Inflammopharmacology 47, 1863–1878. doi:10.1007/s10787-023-01229-6
Ahmad, N., Ansari, M. Y., and Haqqi, T. M. (2020). Role of iNOS in osteoarthritis: pathological and therapeutic aspects. J. Cell. Physiol. 235, 6366–6376. doi:10.1002/jcp.29607
Alahdal, M., Huang, R., Duan, L., Zhiqin, D., Hongwei, O., Li, W., et al. (2021). Indoleamine 2, 3 dioxygenase 1 impairs chondrogenic differentiation of mesenchymal stem cells in the joint of osteoarthritis mice model. Front. Immunol. 12, 781185. doi:10.3389/fimmu.2021.781185
Alharbi, K. S., Afzal, O., Altamimi, A. S. A., Almalki, W. H., Kazmi, I., Al-Abbasi, F. A., et al. (2022). Potential role of nutraceuticals via targeting a Wnt/β-catenin and NF-κB pathway in treatment of osteoarthritis. J. Food Biochem. 46 (2022), e14427. doi:10.1111/jfbc.14427
Ansari, B., Aschner, M., Hussain, Y., Efferth, T., and Khan, H. (2022). Suppression of colorectal carcinogenesis by naringin. Phytomedicine 96 (2022), 153897. doi:10.1016/j.phymed.2021.153897
Arra, M., Swarnkar, G., Alippe, Y., Mbalaviele, G., and Abu-Amer, Y. (2022). IκB-ζ signaling promotes chondrocyte inflammatory phenotype, senescence, and erosive joint pathology. Bone Res. 10, 12. doi:10.1038/s41413-021-00183-9
Asila, A., Liu, J., Liu, J., and Liao, J. (2022). Immunomodulatory effects of berberine on Staphylococcus aureus-induced septic arthritis through down-regulation of Th17 and Treg signaling pathways. Acta. Biochim. Pol. 69, 215–226. doi:10.18388/abp.2020_5948
Baggio, C., Luisetto, R., Boscaro, C., Scanu, A., Ramonda, R., Albiero, M., et al. (2023). Leucocyte abnormalities in synovial fluid of degenerative and inflammatory arthropathies. Int. J. Mol. Sci. 24, 5450. doi:10.3390/ijms24065450
Bai, M. X. (2021). Study on the protective effect of Calcitonin on rat chondrocyte injury induced by IL-1β. Shandong Province: Shandong University.
Balasundaram, A., Udhaya Kumar, S., and George Priya Doss, C. (2022). A computational model revealing the immune-related hub genes and key pathways involved in rheumatoid arthritis (RA). Adv. Protein Chem. Struct. Biol. 129, 247–273. doi:10.1016/bs.apcsb.2021.11.006
Barbarroja, N., López-Montilla, M. D., Cuesta-López, L., Pérez-Sánchez, C., Ruiz-Ponce, M., López-Medina, C., et al. (2023). Characterization of the inflammatory proteome of synovial fluid from patients with psoriatic arthritis: potential treatment targets. Front. Immunol. 14 (2023), 1133435. doi:10.3389/fimmu.2023.1133435
Bird, A., Oakden-Rayner, L., McMaster, C., Smith, L. A., Zeng, M., Wechalekar, M. D., et al. (2022). Artificial intelligence and the future of radiographic scoring in rheumatoid arthritis: a viewpoint. Arthritis Res. Ther. 24, 268. doi:10.1186/s13075-022-02972-x
Bonelli, M., Dalwigk, K., Platzer, A., Olmos Calvo, I., Hayer, S., Niederreiter, B., et al. (2019). IRF1 is critical for the TNF-driven interferon response in rheumatoid fibroblast-like synoviocytes: JAKinibs suppress the interferon response in RA-FLSs. Exp. Mol. Med. 51, 75. doi:10.1038/s12276-019-0267-6
Bottini, N., and Firestein, G. S. (2013). Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat. Rev. Rheumatol. 9, 24–33. doi:10.1038/nrrheum.2012.190
Bragg, R., Gilbert, W., Elmansi, A. M., Isales, C. M., Hamrick, M. W., Hill, W. D., et al. (2019). Stromal cell-derived factor-1 as a potential therapeutic target for osteoarthritis and rheumatoid arthritis. Ther. Adv. Chronic. Dis. 10, 2040622319882531. doi:10.1177/2040622319882531
Brix, N., Glerup, M., Foell, D., Kessel, C., Wittkowski, H., Berntson, L., et al. (2023). Inflammatory biomarkers can differentiate acute lymphoblastic leukemia with arthropathy from juvenile idiopathic arthritis better than standard blood tests. J. Pediatr. 258, 113406. doi:10.1016/j.jpeds.2023.113406
Cai, L., Zhou, M. Y., Hu, S., Liu, F. Y., Wang, M. Q., Wang, X. H., et al. (2022a). Umbelliferone inhibits migration, invasion and inflammation of rheumatoid arthritis fibroblast-like synoviocytes and relieves adjuvant-induced arthritis in rats by blockade of wnt/β-catenin signaling pathway. Am. J. Chin. Med. 50, 1945–1962. doi:10.1142/s0192415x22500835
Cai, X., Zheng, Y., Ren, F., Zhang, S., Wu, L., and Yao, Y. (2022b). Secretory phosphoprotein 1 secreted by fibroblast-like synoviocytes promotes osteoclasts formation via PI3K/AKT signaling in collagen-induced arthritis. Biomed. Pharmacother. 155 (2022), 113687. doi:10.1016/j.biopha.2022.113687
Cai, Z., Hong, M., Xu, L., Yang, K., Li, C., Sun, T., et al. (2020). Prevent action of magnoflorine with hyaluronic acid gel from cartilage degeneration in anterior cruciate ligament transection induced osteoarthritis. Biomed. Pharmacother. 126, 109733. doi:10.1016/j.biopha.2019.109733
Cao, D., Fan, Q., Li, Z., Chen, M., Jiang, Y., Lin, R., et al. (2022). Transcriptomic profiling revealed the role of apigenin-4′-O-α-L-rhamnoside in inhibiting the activation of rheumatoid arthritis fibroblast-like synoviocytes via MAPK signaling pathway. Phytomedicine 102 (2022), 154201. doi:10.1016/j.phymed.2022.154201
Carmon, I., Zecharyahu, L., Elayyan, J., Meka, S. R. K., Reich, E., Kandel, L., et al. (2023). HU308 mitigates osteoarthritis by stimulating sox9-related networks of carbohydrate metabolism. J. Bone Min. Res. 38, 154–170. doi:10.1002/jbmr.4741
Charbonnier, L. M., Han, W. G., Quentin, J., Huizinga, T. W. J., Zwerina, J., Toes, R. E. M., et al. (2010). Adoptive transfer of IL-10-secreting CD4+CD49b+ regulatory T cells suppresses ongoing arthritis. J. Autoimmun. 34, 390–399. doi:10.1016/j.jaut.2009.10.003
Che, N., Sun, X., Gu, L., Wang, X., Shi, J., Sun, Y., et al. (2021). Adiponectin enhances B-cell proliferation and differentiation via activation of akt1/STAT3 and exacerbates collagen-induced arthritis. Front. Immunol. 12, 626310. doi:10.3389/fimmu.2021.626310
Chen, G., Xie, W. G., and Zhou, J. G. (2021). Difference and significance of thymidine kinase 1 expression in gouty arthritis, rheumatoid arthritis and osteoarthritis. J. Chengdu Med. Coll. 16, 730–733. doi:10.3969/j.issn.1674-2257.2021.06.011
Chen, H., Pan, J., Wang, J. D., Liao, Q. M., and Xia, X. R. (2016). Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, induces apoptosis in rheumatoid arthritis fibroblast-like synoviocytes. Inflammation 39, 39–46. doi:10.1007/s10753-015-0220-3
Chen, H., Zhao, J., Hu, J., Xiao, X., Shi, W., Yao, Y., et al. (2022a). Identification of diagnostic biomarkers, immune infiltration characteristics, and potential compounds in rheumatoid arthritis. Biomed. Res. Int. 2022, 1926661. doi:10.1155/2022/1926661
Chen, J., Liu, J., Chen, S., Lai, R., Zheng, C., Lu, J., et al. (2022b). Salinomycin alleviates osteoarthritis progression via inhibiting Wnt/β-catenin signaling. Int. Immunopharmacol. 112, 109225. doi:10.1016/j.intimp.2022.109225
Chen, L., Liu, X., Wang, X., Lu, Z., and Ye, Y. (2023). Berberine alleviates acute lung injury in septic mice by modulating Treg/Th17 homeostasis and downregulating NF-κB signaling. Drug Des. devel. Ther. 17, 1139–1151. doi:10.2147/dddt.s401293
Cheng, J. J., Ma, X. D., Ai, G. X., Yu, Q. X., Chen, X. Y., Yan, F., et al. (2022a). Palmatine protects against MSU-induced gouty arthritis via regulating the NF-κB/NLRP3 and Nrf2 pathways. Drug Des. devel. Ther. 16, 2119–2132. doi:10.2147/dddt.s356307
Cheng, L., Chen, J., and Rong, X. (2022b). Mechanism of emodin in the treatment of rheumatoid arthritis. Evid. Based Complement. Altern. Med. 2022, 9482570. doi:10.1155/2022/9482570
Choe, J. Y., Jung, H. Y., Park, K. Y., and Kim, S. K. (2014). Enhanced p62 expression through impaired proteasomal degradation is involved in caspase-1 activation in monosodium urate crystal-induced interleukin-1b expression. Rheumatol. Oxf. 53, 1043–1053. doi:10.1093/rheumatology/ket474
Chu, Y., Wang, J., and Zhou, X. (2019). Mast cell chymase in synovial fluid of collagen-induced-arthritis rats regulates gelatinase release and promotes synovial fibroblasts proliferation via FAK/p21 signaling pathway. Biochem. Biophys. Res. Commun. 514, 336–343. doi:10.1016/j.bbrc.2019.04.121
Cici, D., Corrado, A., Rotondo, C., and Cantatore, F. P. (2019). Wnt signaling and biological therapy in rheumatoid arthritis and spondyloarthritis. Int. J. Mol. Sci. 20, 5552. doi:10.3390/ijms20225552
Dean, D. D., Martel-Pelletier, J., Pelletier, J. P., Howell, D. S., and Woessner, J. F. (1989). Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J. Clin. Invest. 84, 678–685. doi:10.1172/JCI114215
Dinesh, P., Kalaiselvan, S., Sujitha, S., and Rasool, M. (2020). MiR-145-5p mitigates dysregulated Wnt1/β-catenin signaling pathway in rheumatoid arthritis. Int. Immunopharmacol. 82, 106328. doi:10.1016/j.intimp.2020.106328
Dinesh, P., and Rasool, M. (2019). Berberine mitigates IL-21/IL-21R mediated autophagic influx in fibroblast-like synoviocytes and regulates Th17/Treg imbalance in rheumatoid arthritis. Apoptosis 24, 644–661. doi:10.1007/s10495-019-01548-6
Dinesh, P., and Rasool, M. (2017). Berberine, an isoquinoline alkaloid suppresses TXNIP mediated NLRP3 inflammasome activation in MSU crystal stimulated RAW 264.7 macrophages through the upregulation of Nrf2 transcription factor and alleviates MSU crystal induced inflammation in rats. Int. Immunopharmacol. 44, 26–37. doi:10.1016/j.intimp.2016.12.031
Du, J., Zheng, L., Chen, S., Wang, N., Pu, X., Yu, D., et al. (2022). NFIL3 and its immunoregulatory role in rheumatoid arthritis patients. Front. Immunol. 13 (2022), 950144. doi:10.3389/fimmu.2022.950144
Elkomy, M. H., Alruwaili, N. K., Elmowafy, M., Shalaby, K., Zafar, A., Ahmad, N., et al. (2022). Surface-modified bilosomes nanogel bearing a natural plant alkaloid for safe management of rheumatoid arthritis inflammation. Pharmaceutics 14, 563. doi:10.3390/pharmaceutics14030563
Fan, D. D., Tan, P. Y., Jin, L., Qu, Y., and Yu, Q. H. (2023). Bioinformatic identification and validation of autophagy-related genes in rheumatoid arthritis. Clin. Rheumatol. 42, 741–750. doi:10.1007/s10067-022-06399-2
Fan, X. L., Yang, X. H., and Zhou, X. (2021). Clinical efficacy of berberine in treatment of acute gouty arthritis and its effect on serum levels of inflammation factors. J. Pract. Med. 37, 2413–2417+22. doi:10.3969/j.issn.1006⁃5725.2021.18.021
Fan, X. X., Leung, E. L., Xie, Y., Liu, Z. Q., Zheng, Y. F., Yao, X. J., et al. (2018). Suppression of lipogenesis via reactive oxygen species-AMPK signaling for treating malignant and proliferative diseases. Antioxid. Redox. Signal. 28, 339–357. doi:10.1089/ars.2017.7090
Fan, X. X., Xu, M. Z., Leung, E. L. H., Jun, C., Yuan, Z., and Liu, L. (2020). ROS-responsive berberine polymeric micelles effectively suppressed the inflammation of rheumatoid arthritis by targeting mitochondria. Nano-Micro Lett. 12, 76. doi:10.1007/s40820-020-0410-x
Fang, Q., Zhou, C., and Nandakumar, K. S. (2020). Molecular and cellular pathways contributing to joint damage in rheumatoid arthritis. Mediat. Inflamm. 2020, 3830212. doi:10.1155/2020/3830212
Foster, J. G., Blunt, M. D., Carter, E., and Ward, S. G. (2012). Inhibition of PI3K signaling spurs new therapeutic opportunities in inflammatory/autoimmune diseases and hematological malignancies. Pharmacol. Rev. 64, 1027–1054. doi:10.1124/pr.110.004051
Fu, L., Fu, Q., and Li, J. (2021). Research progress on chemical constituents and pharmacological effects of Coptis chinensis. Acta. Chin. Med. Pharmacol. 49, 87–92. doi:10.19664/j.cnki.1002-2392.210044
Gai, X. H., Liu, S. X., and Ren T, T. (2018). Research progress on chemical constituents and pharmacological effects of Coptis chinensis. Chin. Tradit. Herb. Drugs. 49, 4919–4927. doi:10.7501/j.issn.0253-2670.2018.20.032
Geng, Y. N. (2018). Berberine protects inflammatory injury of pancreatic beta cells induced by cytokines. Tianjin: Tianjin Medical University.
Ghosh, R., Dey, R., Sawoo, R., and Bishayi, B. (2023). Simultaneous neutralization of TGF-β and IL-6 attenuates Staphylococcus aureus-induced arthritic inflammation through differential modulation of splenic and synovial macrophages. Scand. J. Immunol. 97, e13252. doi:10.1111/sji.13252
González-Chávez, S. A., López-Loeza, S. M., Acosta-Jiménez, S., Cuevas-Martínez, R., Pacheco-Silva, C., Chaparro-Barrera, E., et al. (2023). Low-intensity physical exercise decreases inflammation and joint damage in the preclinical phase of a rheumatoid arthritis murine model. Biomolecules 13, 488. doi:10.3390/biom13030488
Gui, Y., Qiu, X., Xu, Y., Li, D., and Wang, L. (2015). Bu-Shen-Ning-Xin decoction suppresses osteoclastogenesis via increasing dehydroepiandrosterone to prevent postmenopausal osteoporosis. Biosci. Trends 9, 169–181. doi:10.5582/bst.2015.01011
Han, Y., Wu, J., Gong, Z., Zhou, Y., Li, H., Chen, Y., et al. (2022). Identification and development of the novel 7-genes diagnostic signature by integrating multi cohorts based on osteoarthritis. Hereditas 159, 10. doi:10.1186/s41065-022-00226-z
Harris, S. J., Foster, J. G., and Ward, S. G. (2009). PI3K isoforms as drug targets in inflammatory diseases: lessons from pharmacological and genetic strategies. Curr. Opin. Investig. Drugs. 10, 1151–1162. doi:10.1016/j.cct.2009.06.009
Hashiramoto, A., Sakai, C., Yoshida, K., Tsumiyama, K., Miura, Y., Shiozawa, K., et al. (2007). Angiopoietin 1 directly induces destruction of the rheumatoid joint by cooperative, but independent, signaling via ERK/MAPK and phosphatidylinositol 3-kinase/Akt. Arthritis Rheum. 56, 2170–2179. doi:10.1002/art.22727
He, X. Y., Shuai, S. Q., and Dang, W. T. (2018). Research progress of berberine in the regulation of inflammation. J. Med. West Chin. 30, 1714–1717. doi:10.3969/j.issn.1672-3511.2018.11.035
Hecquet, S., Combier, A., Steelandt, A., Pons, M., Wendling, D., Molto, A., et al. (2023). Characteristics of patients with difficult-to-treat rheumatoid arthritis in a French single-centre hospital. Rheumatology 2023, kead143. doi:10.1093/rheumatology/kead143
Hou, S. M., Chen, P. C., Lin, C. M., Fang, M. L., Chi, M. C., and Liu, J. F. (2020). CXCL1 contributes to IL-6 expression in osteoarthritis and rheumatoid arthritis synovial fibroblasts by CXCR2, c-Raf, MAPK, and AP-1 pathway. Arthritis Res. Ther. 22, 251. doi:10.1186/s13075-020-02331-8
Hu, C. Y., and Mo, Z. X. (2017). Research progress on pharmacological actions and mechanism of berberine. Chin. J. Exp. Tradit. Med. Formulae. 23, 213–219. doi:10.13422/j.cnki.syfjx.2017200213
Hu, P. F., Chen, W. P., Tang, J. L., Bao, J. p., and Wu, L. d. (2011a). Protective effects of berberine in an experimental rat osteoarthritis model. Phytother. Res. 25, 878–885. doi:10.1002/ptr.3359
Hu, W., Rong, C., and Chen, F. H. (2011b). Research progress on the role of matrix metalloproteinases in the pathogenesis of rheumatoid arthritis. J. Anhui Med. 32, 671–672. doi:10.3969/j.issn.1000-0399.2011.05.046
Hu, Y., Hu, Z., Wang, S., Dong, X., Xiao, C., Jiang, M., et al. (2013). Protective effects of Huang-Lian-Jie-Du-Tang and its component group on collagen-induced arthritis in rats. J. Ethnopharmacol. 150, 1137–1144. doi:10.1016/j.jep.2013.10.038
Hu, Z., Jiao, Q., Ding, J., Liu, F., Liu, R., Shan, L., et al. (2010). Berberine induces dendritic cell apoptosis and has therapeutic potential for rheumatoid arthritis. Arthritis Care Res. 63, 949–959. doi:10.1002/art.30202
Huang, C. C., Chiou, C. H., Liu, S. C., Hu, S. L., Su, C. M., Tsai, C. H., et al. (2019). Melatonin attenuates TNF-α and IL-1β expression in synovial fibroblasts and diminishes cartilage degradation: implications for the treatment of rheumatoid arthritis. J. Pineal. Res. 66, e12560. doi:10.1111/jpi.12560
Huang, D. N., Wu, F. F., Zhang, A. H., Sun, H., and Wang, X. J. (2021a). Efficacy of berberine in treatment of rheumatoid arthritis: from multiple targets to therapeutic potential. Pharmacol. Res. 169, 105667. doi:10.1016/j.phrs.2021.105667
Huang, T., Cheng, L., Jiang, Y., Zhang, L., and Qian, L. (2023). Indole-3-pyruvic acid alleviates rheumatoid arthritis via the aryl hydrocarbon receptor pathway. Ann. Transl. Med. 11, 213. doi:10.21037/atm-23-1074
Huang, W., Ma, S. T., and Yao, J. (2021b). Efficiency limits of concentrating spectral-splitting hybrid photovoltaic-thermal (PV-T) solar collectors and systems. J. Shandong Med. 61, 28–32. doi:10.1038/s41377-021-00465-1
Hui, W., and Dai, Y. (2020). Therapeutic potential of aryl hydrocarbon receptor ligands derived from natural products in rheumatoid arthritis. Basic Clin. Pharmacol. Toxicol. 126, 469–474. doi:10.1111/bcpt.13372
Hwang, S. G., Yu, S. S., Poo, H., and Chun, J. S. (2005). c-Jun/activator protein-1 mediates interleukin-1beta-induced dedifferentiation but not cyclooxygenase-2 expression in articular chondrocytes. J. Biol. Chem. 280, 29780–29787. doi:10.1074/jbc.M411793200
Hyndman, I. J. (2017). Rheumatoid arthritis: past, present and future approaches to treating the disease. Int. J. Rheum. Dis. 20, 417–419. doi:10.1111/1756-185X.12823
Jahantigh, M., Abtahi Froushani, S. M., and Afzale Ahangaran, N. (2023). Benefits of bone marrow-derived mesenchymal stem cells primed with estradiol in alleviating collagen-induced arthritis, Iran. J. Basic. Med. Sci. 26, 400–407. doi:10.22038/IJBMS.2023.68112.14882
Ji, X., Du, W., Che, W., Wang, L., and Zhao, L. (2023). Apigenin inhibits the progression of osteoarthritis by mediating macrophage polarization. Molecules 28, 2915. doi:10.3390/molecules28072915
Jia, J. K. (2021). Effect of macrophage-specific Nfe2l1 deficiency on the occurrence and development of osteoarthritis and its related mechanisms. Liaoning Province: China Medical University.
Jian, R., Yang, M., and Zheng, S. L. (2020). Regulatory effect of berberine on NLRP3/TLRs in mice with gouty arthritis. J. Chongqing Med. Univ. 45, 251–256. doi:10.13406/j.cnki.cyxb.002318
Jie, S. S., Sun, H. J., and Liu, J. X. (2022). Huanglian jiedutang regulates inflammatory immunity to relieve rheumatoid arthritis. Chin. J. Exp. Tradit. Med. Formulae. 28, 28–33. doi:10.13422/j.cnki.syfjx.20221336
Jin, S. J., Wang, G. W., and Chun, L. (2019). Effects of berberine combined with clomiphene on endothelial function, endocrine indexes and clinical outcomes in patients with polycystic ovary syndrome and infertility. J. Pract. Med. 30, 100–103. doi:10.3969/j.issn.1006⁃5725.2019.01.022
Khan, H., Sureda, A., Belwal, T., Çetinkaya, S., Süntar, İ., Tejada, S., et al. (2019). Polyphenols in the treatment of autoimmune diseases. Autoimmun. Rev. 18, 647–657. doi:10.1016/j.autrev.2019.05.001
Kienhorst, L. B., van Lochem, E., Kievit, W., Dalbeth, N., Merriman, M. E., Phipps-Green, A., et al. (2015). Gout is a chronic inflammatory disease in which high levels of interleukin-8 (CXCL8), myeloid-related protein 8/myeloid-related protein 14 complex, and an altered proteome are associated with diabetes mellitus and cardiovascular disease. Arthritis Rheumatol. 67, 3303–3313. doi:10.1002/art.39318
Ko, J. Y., Choi, Y. J., Jeong, G. J., and Im, G. I. (2013). Sulforaphane-PLGA microspheres for the intra-articular treatment of osteoarthritis. Biomaterials 34, 5359–5368. doi:10.1016/j.biomaterials.2013.03.066
Koskela, H. L., Eldfors, S., Ellonen, P., van Adrichem, A. J., Kuusanmäki, H., Andersson, E. I., et al. (2012). Somatic STAT3 mutations in large granular lymphocytic leukemia. N. Engl. J. Med. 366, 1905–1913. doi:10.1056/NEJMoa1114885
Kou, L., Huang, H., Tang, Y., Sun, M., Li, Y., Wu, J., et al. (2022). Opsonized nanoparticles target and regulate macrophage polarization for osteoarthritis therapy: a trapping strategy. J. Control. Release. 347, 237–255. doi:10.1016/j.jconrel.2022.04.037
La Bella, S., Rinaldi, M., Di Ludovico, A., Di Donato, G., Di Donato, G., Salpietro, V., et al. (2023). Genetic background and molecular mechanisms of juvenile idiopathic arthritis. Int. J. Mol. Sci. 24, 1846. doi:10.3390/ijms24031846
Lerner, A., Neidhöfer, S., Reuter, S., and Matthias, T. (2018). MMP3 is a reliable marker for disease activity, radiological monitoring, disease outcome predictability, and therapeutic response in rheumatoid arthritis. Best. Pract. Res. Clin. Rheumatol. 32, 550–562. doi:10.1016/j.berh.2019.01.006
Leung, Y. Y., Kavanaugh, A., and Ritchlin, C. T. (2023). Expert perspective: management of the psoriatic arthritis patient after failure of one tumor necrosis factor inhibitor. Arthritis Rheumatol. 75, 1312–1324. doi:10.1002/art.42498
Li, F., Xu, Z., and Xie, Z. (2022c). Patient-derived functional organoids as a personalized approach for drug screening against hepatobiliary cancers. Apoptosis 17, 319–341. doi:10.1016/bs.acr.2022.01.011
Li, H. M., Dang, W. T., and Yang, X. H. (2017b). Advances in anti-inflammatory mechanism of berberine. Chin. Med. Her. 14, 31–34.
Li, H., Peng, Y., Wang, X., Sun, X., Yang, F., Sun, Y., et al. (2019a). Astragaloside inhibits IL-1β-induced inflammatory response in human osteoarthritis chondrocytes and ameliorates the progression of osteoarthritis in mice. Immunopharmacol. Immunotoxicol. 41, 497–503. doi:10.1080/08923973.2019.1637890
Li, H., Xie, X. W., and Zhao, Y. L. (2022a). Research progress on mechanism of effective components of traditional Chinese medicine in preventing and treating osteoarthritis. Chin. Tradit. Herb. Drugs. 53, 7543–7552. doi:10.7501/j.issn.0253-2670.2022.23.026
Li, J., Wang, Y., Chen, D., and Liu-Bryan, R. (2022b). Oral administration of berberine limits post-traumatic osteoarthritis development and associated pain via AMP-activated protein kinase (AMPK) in mice. Osteoarthr. Cartil. 30, 160–171. doi:10.1016/j.joca.2021.10.004
Li, J., Zhang, X., Guo, D., Shi, Y., Zhang, S., Yang, R., et al. (2023b). The mechanism of action of paeoniae radix rubra-angelicae sinensis radix drug pair in the treatment of rheumatoid arthritis through PI3K/AKT/NF-κB signaling pathway. Front. Pharmacol. 14 (2023), 1113810. doi:10.3389/fphar.2023.1113810
Li, L., Liu, H., Shi, W., Yang, J., and Xu, D. (2017a). Insights into the action mechanisms of traditional Chinese medicine in osteoarthritis. Evid. Based Complement. Altern. Med. 2017, 5190986. doi:10.1155/2017/5190986
Li, Q., Wu, M., Fang, G., Li, K., Cui, W., Li, L., et al. (2021). MicroRNA-186-5p downregulation inhibits osteoarthritis development by targeting MAPK1. Mol. Med. Rep. 23, 253. doi:10.3892/mmr.2021.11892
Li, S., Jin, Z., and Lu, X. (2017c). MicroRNA-192 suppresses cell proliferation and induces apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes by downregulating caveolin 1. Mol. Cell. Biochem. 432, 123–130. doi:10.1007/s11010-017-3003-3
Li, S., Li, L., Yan, H., Jiang, X., Hu, W., Han, N., et al. (2019b). Anti-gouty arthritis and anti-hyperuricemia properties of celery seed extracts in rodent models. Mol. Med. Rep. 20, 4623–4633. doi:10.3892/mmr.2019.10708
Li, X. N. (2022). Preparation of methotrexate-gold nano-targeted formulation and its treatment of rheumatoid arthritis. Jilin Province: Yanbian University.
Li, X., Sun, H., Li, H., Li, D., Cai, Z., Xu, J., et al. (2023a). A single-cell RNA-sequencing analysis of distinct subsets of synovial macrophages in rheumatoid arthritis. DNA Cell. Biol. 42, 212–222. doi:10.1089/dna.2022.0509
Li, Y., Wang, S., Wang, Y., Zhou, C., Chen, G., Shen, W., et al. (2013). Inhibitory effect of the antimalarial agent artesunate on collagen-induced arthritis in rats through nuclear factor kappa B and mitogen-activated protein kinase signaling pathway. Transl. Res. 161, 89–98. doi:10.1016/j.trsl.2012.06.001
Liang, H., Zeng, Y., Feng, Y., Wu, H., Gong, P., and Yao, Q. (2018). Selective β2-adrenoreceptor signaling regulates osteoclastogenesis via modulating RANKL production and neuropeptides expression in osteocytic MLO-Y4 cells. J. Cell. Biochem. 120, 7238–7247. doi:10.1002/jcb.27998
Lietman, C., Wu, B., Lechner, S., Shinar, A., Sehgal, M., Rossomacha, E., et al. (2018). Inhibition of Wnt/β-catenin signaling ameliorates osteoarthritis in a murine model of experimental osteoarthritis. JCI Insight 3, e96308. doi:10.1172/jci.insight.96308
Lin, W., Shen, P., Huang, Y., Han, L., and Ba, X. (2023). Wutou decoction attenuates the synovial inflammation of collagen-induced arthritis rats via regulating macrophage M1/M2 type polarization. J. Ethnopharmacol. 301 (2023), 115802. doi:10.1016/j.jep.2022.115802
Lin, Z., Tian, X. Y., Huang, X. X., He, L. L., and Xu, F. (2019). microRNA-186 inhibition of PI3K-AKT pathway via SPP1 inhibits chondrocyte apoptosis in mice with osteoarthritis. J. Cell. Physiol. 234, 6042–6053. doi:10.1002/jcp.27225
Liu, G., He, G., Zhang, J., Zhang, Z., and Wang, L. (2022b). Identification of SCRG1 as a potential therapeutic target for human synovial inflammation. Front. Immunol. 13 (2022), 893301. doi:10.3389/fimmu.2022.893301
Liu, J. X., Jie, S. S., and Chen, B. (2023). Mechanisms of huanglian jiedu decoction in treating acute gouty arthritis based on NLRP3 inflammasome and TLR4/NF-κB signal pathway. Chin. J. Exp. Tradit. Med. Formulae. doi:10.13422/j.cnki.syfjx.20230802
Liu, L., Wang, D., Liu, M. Y., Yu, H., Chen, Q., Wu, Y., et al. (2022a). The development from hyperuricemia to gout: key mechanisms and natural products for treatment. Acupunct. Herb. Med. 2, 25–32. doi:10.1097/hm9.0000000000000016
Liu, P., Chen, Y., Wang, B., Wang, Z., Li, C., and Wang, Y. (2019). Expression of microRNAs in the plasma of patients with acute gouty arthritis and the effects of colchicine and etoricoxib on the differential expression of microRNAs. Arch. Med. Sci. 159, 1047–1055. doi:10.5114/aoms.2018.75502
Liu, S. C., Lee, H. P., Hung, C. Y., Tsai, C. H., Li, T. M., and Tang, C. H. (2015). Berberine attenuates CCN2-induced IL-1βexpression and prevents cartilage degradation in a rat model of osteoarthritis. Toxico. Appl. Pharmacol. 289, 20–29. doi:10.1016/j.taap.2015.08.020
Liu, S., Ma, H., Zhang, H., Deng, C., and Xin, P. (2021). Recent advances on signaling pathways and their inhibitors in rheumatoid arthritis. Clin. Immunol. 230, 108793. doi:10.1016/j.clim.2021.108793
Liu, Y. F., Wen, C. Y., Chen, Z., Wang, Y., Huang, Y., and Tu, S. H. (2016). Effects of berberine on NLRP3 and IL-1β expressions in monocytic THP-1 cells with monosodium urate crystals-induced inflammation. Biomed. Res. Int. 2016, 2503703. doi:10.1155/2016/2503703
Luo, Y., Lei, Y., Guo, X., Zhu, D., Zhang, H., Guo, Z., et al. (2023). CX-4945 inhibits fibroblast-like synoviocytes functions through the CK2-p53 axis to reduce rheumatoid arthritis disease severity. Int. Immunopharmacol. 119 (2023), 110163. doi:10.1016/j.intimp.2023.110163
Lv, S. (2020). Study on the mechanism of pulchinenoside b4 anti-gouty arthritis (GA) based on metabolomics. Jiangxi Province: Jiangxi University of Traditional Chinese Medicine.
Ma, Y., Nyman, J. S., Tao, H., Moss, H. H., Yang, X., and Elefteriou, F. (2011). β2-adrenergic receptor signaling in osteoblasts contributes to the catabolic effect of glucocorticoids on bone. Endocrinology 152, 1412–1422. doi:10.1210/en.2010-0881
Man, G., Yang, H., Shen, K., Zhang, D., Zhang, J., Wu, H., et al. (2022). Circular RNA RHOT1 regulates miR-142-5p/CCND1 to participate in chondrocyte autophagy and proliferation in osteoarthritis. J. Immunol. Res. 2022, 4370873. doi:10.1155/2022/4370873
McInnes, I. B., and Schett, G. (2011). The pathogenesis of rheumatoid arthritis. New Engl. J. Med. 365, 2205–2219. doi:10.1056/nejmra1004965
Mei, J., Zhou, F., Qiao, H., and Tang, T. (2019). Nerve modulation therapy in gouty arthritis: targeting increased sFRP2 expression in dorsal root ganglion regulates macrophage polarization and alleviates endothelial damage. Theranostics 9, 3707–3722. doi:10.7150/thno.33908
Meng, X. Y., Jiang, L. J., and Qiu, Q. (2022). Research advances on quality markers of Chinese materia medica for prevention and treatment of rheumatoid arthritis. Chin. Arch. Tradit. Chin. Med. 40, 48–53. doi:10.13193/j.issn.1673-7717.2022.04.009
Miao, C. G., Yang, Y. Y., He, X., Huang, C., Huang, Y., Qin, D., et al. (2014). MicroRNA-152 modulates the canonical Wnt pathway activation by targeting DNA methyltransferase 1 in arthritic rat model. Biochimie 106, 149–156. doi:10.1016/j.biochi.2014.08.016
Miao, C. G., Yang, Y. Y., He, X., Li, X. f., Huang, C., Huang, Y., et al. (2013). Wnt signaling pathway in rheumatoid arthritis, with special emphasis on the different roles in synovial inflammation and bone remodeling. Cell. Signal 25, 2069–2078. doi:10.1016/j.cellsig.2013.04.002
Min, S., Wang, C., Lu, W., Xu, Z., Shi, D., Chen, D., et al. (2017). Serum levels of the bone turnover markers dickkopf-1, osteoprotegerin, and TNF-alpha in knee osteoarthritis patients. Clin. Rheumatol. 36, 2351–2358. doi:10.1007/s10067-017-3690-x
Murphy, G., and Nagase, H. (2008). Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair? Rheumatology 4, 128–135. doi:10.1038/ncprheum0727
Nalesso, G., Thorup, A. S., Eldridge, S. E., De Palma, A., Kaur, A., Peddireddi, K., et al. (2021). Calcium calmodulin kinase II activity is required for cartilage homeostasis in osteoarthritis. Sci. Rep. 11, 5682. doi:10.1038/s41598-021-82067-w
National Pharmacopoeia Commission (2020). Pharmacopoeia of the people Republic of China. Beijing: Chemical Industry Press, 316.
Nguyen, C. T., Furuya, H., Das, D., Marusina, A. I., Merleev, A. A., Ravindran, R., et al. (2022). Peripheral γδ T cells regulate neutrophil expansion and recruitment in experimental psoriatic arthritis. Arthritis Rheumatol. 74, 1524–1534. doi:10.1002/art.42124
Ning, X., Ni, Y., Cao, J., and Zhang, H. (2023). Liquiritigenin attenuated collagen-induced arthritis and cardiac complication via inflammation and fibrosis inhibition in mice. Chem. Pharm. Bull. (Tokyo). 71, 269–276. doi:10.1248/cpb.c22-00684
Orecchini, E., Mondanelli, G., Orabona, C., Volpi, C., Adorisio, S., Calvitti, M., et al. (2020). Artocarpus tonkinensis extract inhibits LPS-triggered inflammation markers and suppresses RANKL-induced osteoclastogenesis in RAW264.7. Front. Pharmacol. 11, 593829. doi:10.3389/fphar.2020.593829
Ou, Y., Li, W., Li, X., Lin, Z., and Li, M. (2010). Sinomenine reduces invasion and migration ability in fibroblast-like synoviocytes cells co-cultured with activated human monocytic THP-1 cells by inhibiting the expression of MMP-2, MMP-9, CD147. Rheumatol. Int. 31, 1479–1485. doi:10.1007/s00296-010-1506-2
Pacifici, M. (2022). Osteoarthritis and chronic pain: interleukin-6 as a common denominator and therapeutic target. Sci. Signal. 15, eadd3702. doi:10.1126/scisignal.add3702
Park, S. M., Min, B. G., Jung, J. Y., Jegal, K. H., Lee, C. W., Kim, K. Y., et al. (2018). Combination of Pelargonium sidoides and Coptis chinensis root inhibitsnuclear factor kappa B-mediated inflammatory response in vitro and in vivo. BMC Compl. Altern. Med. 18, 20–32. doi:10.1186/s12906-018-2088-x
Pathan, N. B., Parvez, A., Bader, A., Shaheen, U., and Hadda, T. B. (2015). Synthesis, characterization, crystal structure determination and biological screening of novel N-1 and C5 alkyl substituted scaffolds of pyrimidine. Eur. J. Med. Chem. 103, 594–599. doi:10.1016/j.ejmech.2013.12.036
Qian, Q., Gao, Y., Xun, G., Wang, X., Ge, J., Zhang, H., et al. (2022). Synchronous investigation of the mechanism and substance basis of tripterygium glycosides tablets on anti-rheumatoid arthritis and hepatotoxicity. Appl. Biochem. Biotechnol. 194, 5333–5352. doi:10.1007/s12010-022-04011-6
Qin, C., Diaz-Gallo, L. M., Tang, B., Wang, Y., Nguyen, T. D., Harder, A., et al. (2023). Repurposing antidiabetic drugs for rheumatoid arthritis: results from a two-sample mendelian randomization study. Eur. J. Epidemiol. 38, 809–819. doi:10.1007/s10654-023-01000-9
Qing, Y. F., Zhang, Q. B., Zhou, J. G., and Jiang, L. (2014). Changes in toll-like receptor (TLR)4-NFκB-IL1β signaling in male gout patients might be involved in the pathogenesis of primary gouty arthritis. Rheumatol. Int. 34, 213–220. doi:10.1007/s00296-013-2856-3
Qiu, H., Sun, S., Ma, X., Cui, C., Chen, G., Liu, Z., et al. (2018). Jatrorrhizine hydrochloride suppresses proliferation, migration, and secretion of synoviocytes in vitro and ameliorates rat models of rheumatoid arthritis in vivo. Int. J. Mol. Sci. 19, 1514. doi:10.3390/ijms19051514
Roemer, F. W., Guermazi, A., Felson, D. T., Niu, J., Nevitt, M. C., Crema, M. D., et al. (2011). Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann. Rheum. Dis. 70, 1804–1809. doi:10.1136/ard.2011.150243
Rondanelli, M., Infantino, V., Riva, A., Petrangolini, G., Faliva, M. A., Peroni, G., et al. (2020). Polycystic ovary syndrome management:a review of the possible amazing role of berberine. Arch. Gynecol. Obstet. 301, 53–60. doi:10.1007/s00404-020-05450-4
Roškar, S., and Hafner-Bratkovič, I. (2022). The role of inflammasomes in osteoarthritis and secondary joint degeneration diseases. Life (Basel) 12, 731. doi:10.3390/life12050731
Sakthiswary, R., Uma Veshaaliini, R., Chin, K. Y., Das, S., and Sirasanagandla, S. R. (2022). Pathomechanisms of bone loss in rheumatoid arthritis. Front. Med. (Lausanne). 9, 962969. doi:10.3389/fmed.2022.962969
Shang, X., Böker, K. O., Taheri, S., Hawellek, T., Lehmann, W., and Schilling, A. F. (2021). The interaction between microRNAs and the wnt/β-catenin signaling pathway in osteoarthritis. Int. J. Mol. Sci. 22, 9887. doi:10.3390/ijms22189887
Sharma, A., Tirpude, N. V., Bhardwaj, N., Kumar, D., and Padwad, Y. (2022). Berberis lycium fruit extract and its phytoconstituents berberine and rutin mitigate collagen-CFA-induced arthritis (CIA) via improving GSK3β/STAT/Akt/MAPKs/NF-κB signaling axis mediated oxi-inflammation and joint articulardamage in murine model. Inflammopharmacology 30, 655–666. doi:10.1007/s10787-022-00941-z
She, P. (2020). The application of inflammatory-responsive polymers nanomedicines for treatment of arthritis. Jilin Province: Jilin University.
Shen, P., Jiao, Y., Miao, L., Chen, J. H., and Momtazi-Borojeni, A. A. (2020). Immunomodulatory effects of berberine on the inflamed joint reveal new therapeutic targets for rheumatoid arthritis management. J. Cell. Mol. Med. 24, 12234–12245. doi:10.1111/jcmm.15803
Shen, Y., Fan, X., Qu, Y., Tang, M., Huang, Y., Peng, Y., et al. (2022). Magnoflorine attenuates inflammatory responses in RA by regulating the PI3K/Akt/NF-κB and Keap1-Nrf2/HO-1 signalling pathways in vivo and in vitro. Phytomedicine 104, 154339. doi:10.1016/j.phymed.2022.154339
Skougaard, M., Ditlev, S. B., Søndergaard, M. F., and Kristensen, L. E. (2023). Cytokine signatures in psoriatic arthritis patients indicate different phenotypic traits comparing responders and non-responders of IL-17a and TNFα inhibitors. Int. J. Mol. Sci. 24, 6343. doi:10.3390/ijms24076343
Smith, M. D., Weedon, H., Papangelis, V., Walker, J., Roberts-Thomson, P. J., and Ahern, M. J. (2010). Apoptosis in the rheumatoid arthritis synovial membrane: modulation by disease-modifying anti-rheumatic drug treatment. Rheumatol. Oxf. 49, 862–875. doi:10.1093/rheumatology/kep467
So, A. K., and Martinon, F. (2017). Inflammation in gout: mechanisms and therapeutic targets. Nat. Rev. Rheumatol. 13, 639–647. doi:10.1038/nrrheum.2017.155
Song, L. N., Kong, X. D., Wang, H. J., and Zhan, L. b. (2016). Establishment of a rat adjuvant arthritis-interstitial lung disease model. Biomed. Res. Int. 2016, 2970783. doi:10.1155/2016/2970783
Stockinger, B., Di Meglio, P., Gialitakis, M., and Duarte, J. H. (2014). The aryl hydrocarbon receptor: multitasking in the immune system. Annu. Rev. Immunol. 32, 403–432. doi:10.1146/annurev-immunol-032713-120245
Su, Y. X., Lin, X. Y., and Chen, X. M. (2012). Effect of IL-1β on Wnt/β-catenin pathway in rat articular chondrocytes. Fujian J. Tradit. Chin. Med. 43, 46–48. doi:10.13260/j.cnki.jfjtcm.010478
Sujitha, S., Dinesh, P., and Rasool, M. (2020). Berberine encapsulated PEG-coated liposomes attenuate Wnt1/β-catenin signaling in rheumatoid arthritis via miR-23a activation. Eur. J. Pharm. Biopharm. 149, 170–191. doi:10.1016/j.ejpb.2020.02.007
Sujitha, S., Dinesh, P., and Rasool, M. (2018). Berberine modulates ASK1 signaling mediated through TLR4/TRAF2 via upregulation of miR-23a. Toxicol. Appl. Pharmacol. 359, 34–46. doi:10.1016/j.taap.2018.09.017
Sun, J. L., Yan, J. F., Li, J., Wang, W. R., Yu, S. B., Zhang, H. Y., et al. (2020b). Conditional deletion of Adrb2 in mesenchymal stem cells attenuates osteoarthritis-like defects in temporomandibular joint. Bone 133, 115229. doi:10.1016/j.bone.2020.115229
Sun, K., Luo, J., Guo, J., Yao, X., Jing, X., and Guo, F. (2020a). The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review. Osteoarthr. Cartil. 28, 400–409. doi:10.1016/j.joca.2020.02.027
Sun, L., Wang, X., and Kaplan, D. L. (2011). A 3D cartilage - inflammatory cell culture system for the modeling of human osteoarthritis. Biomaterials 32, 5581–5589. doi:10.1016/j.biomaterials.2011.04.028
Sun, Y., Guo, Y., Chang, L., and Zhang, J. (2023b). Long noncoding RNA H19 synergizes with STAT1 to regulate SNX10 in rheumatoid arthritis. Mol. Immunol. 153, 106–118. doi:10.1016/j.molimm.2022.11.018
Sun, Y., Su, S., Li, M., and Deng, A. (2023a). Inhibition of miR-182-5p targets FGF9 to alleviate osteoarthritis. Anal. Cell. Pathol. (Amst). 2023, 5911546. doi:10.1155/2023/5911546
Tang, K. T., Lin, C. C., Lin, S. C., Wang, J. H., and Tsai, S. W. (2021). Kurarinone attenuates collagen-induced arthritis in mice by inhibiting Th1/Th17 cell responses and oxidative stress. Int. J. Mol. Sci. 22, 4002. doi:10.3390/ijms22084002
Tavallaee, G., Lively, S., Rockel, J. S., Ali, S. A., Im, M., Sarda, C., et al. (2022). Contribution of MicroRNA-27b-3p to synovial fibrotic responses in knee osteoarthritis. Arthritis Rheumatol. 74, 1928–1942. doi:10.1002/art.42285
Taylor, P. C., Matucci Cerinic, M., Alten, R., Avouac, J., and Westhovens, R. (2022). Managing inadequate response to initial anti-TNF therapy in rheumatoid arthritis: optimising treatment outcomes. Ther. Adv. Musculoskelet. Dis. 14, 1759720X221114101. doi:10.1177/1759720X221114101
Terkeltaub, R. (2017). What makes gouty inflammation so variable. BMC Med. 15, 158. doi:10.1186/s12916-017-0922-5
Thomas, D. L. (2020). Caring for patients in a new pandemic: the necessity and challenges of observational research. J. Clin. Invest. 130, 6225–6227. doi:10.1172/JCI143292
Trzybulska, D., Olewicz-Gawlik, A., Sikora, J., Frydrychowicz, M., Kolecka-Bednarczyk, A., Kaczmarek, M., et al. (2018). The effect of caveolin-1 knockdown on interleukin-1β-induced chemokine (C-C motif) ligand 2 expression in synovial fluid-derived fibroblast-like synoviocytes from patients with rheumatoid arthritis. Adv. Clin. Exp. Med. 27, 1491–1497. doi:10.17219/acem/75611
Tu, B., Fang, R., Zhu, Z., Chen, G., Peng, C., and Ning, R. (2023). Comprehensive analysis of arachidonic acid metabolism-related genes in diagnosis and synovial immune in osteoarthritis: based on bulk and single-cell RNA sequencing data. Inflamm. Res. 72, 955–970. doi:10.1007/s00011-023-01720-4
Uthman, I., Raynauld, J. P., and Haraoui, B. (2003). Intra-articular therapy in osteoarthritis. Postgrad. Med. J. 79, 449–453. doi:10.1136/pmj.79.934.449
Vita, A. A., Aljobaily, H., Lyons, D. O., and Pullen, N. A. (2021). Berberine delays onset of Collagen-Induced arthritis through T Cell suppression. Int. J. Mol. Sci. 22, 3522. doi:10.3390/ijms22073522
Wallach, D. (2016). The cybernetics of TNF: old views and newer ones. Semin. Cell. Dev. Biol. 50, 105–114. doi:10.1016/j.semcdb.2015.10.014
Wang, C., Wang, F., Lin, F., Duan, X., and Bi, B. (2019b). Naproxen attenuates osteoarthritis progression through inhibiting the expression of prostaglandinl-endoperoxide synthase 1. J. Cell. Physiol. 234, 12771–12785. doi:10.1002/jcp.27897
Wang, D. D. (2020b). CP-25 down-regulates CXCR4-gβγ-PI3K/AKT mediated migration in fibroblast-like synoviocytes of rheumatoid arthritis by inhibiting GRK2 translocation. Anhui Province: Anhui Medical University.
Wang, H., Tu, S., Yang, S., Shen, P., Huang, Y., Ba, X., et al. (2019a). Berberine modulates LPA function to inhibit the proliferation and inflammation of FLS-RA via p38/ERK MAPK pathway mediated by LPA1. Altern. Med. 2019, 2580207. doi:10.1155/2019/2580207
Wang, J. (2020a). Study on the pharmacodynamics and mechanism of compound Ruteng capsules in treating arthritis based on network pharmacology. Hubei Province: South-central University for Nationalities.
Wang, L., Ishihara, S., Li, J., Miller, R. E., and Malfait, A. M. (2023b). Notch signaling is activated in knee-innervating dorsal root ganglia in experimental models of osteoarthritis joint pain. Arthritis Res. Ther. 25, 63. doi:10.1186/s13075-023-03039-1
Wang, L., Li, P., Zhou, Y., Gu, R., Lu, G., and Zhang, C. (2023a). Magnoflorine ameliorates collagen-induced arthritis by suppressing the inflammation response via the NF-κB/MAPK signaling pathways. J. Inflamm. Res. 16, 2271–2296. doi:10.2147/jir.s406298
Wang, Q. A. (2022). CADM1 regulates chondrocyte anabolism and catabolism in osteoarthritis and its underlying molecular mechanism. Anhui Province: Anhui Medical University.
Wang, Q. T., Zhang, L. L., Wu, H. X., and Wei, W. (2011). The expression change of β-arrestins in fibroblast-like synoviocytes from rats with collagen-induced arthritis and the effect of total glucosides of paeony. J. Ethnopharmacol. 133, 511–516. doi:10.1016/j.jep.2010.10.022
Wang, Q., Yu, X., and Gong, M. (2022a). Single-cell transcriptome analysis reveals the importance of IRF1/FSTL1 in synovial fibroblast subsets for the development of rheumatoid arthritis. Comput. Math. Methods Med. 2022, 1169614. doi:10.1155/2022/1169614
Wang, X., He, X., Zhang, C. F., Guo, C. R., Wang, C. Z., and Yuan, C. S. (2017). Anti-arthritic effect of berberine on adjuvant-induced rheumatoid arthritis in rats. Biomed. Pharmacother. 89, 887–893. doi:10.1016/j.biopha.2017.02.099
Wang, X. H. (2011). Effects of berberine on human rheumatoid arthritis fibroblast-like synoviocytes and its underlying molecular. Shandong Province: Shandong University.
Wang, X., Long, H., Chen, M., Zhou, Z., Wu, Q., Xu, S., et al. (2022b). Modified Baihu decoction therapeutically remodels gut microbiota to inhibit acute gouty arthritis. Front. Physiol. 13 (2022), 1023453. doi:10.3389/fphys.2022.1023453
Wang, X., Sun, L., He, N., An, Z., and Yu, R. (2021). Increased expression of CXCL2 in ACPA-positive rheumatoid arthritis and its role in osteoclastogenesis. Clin. Exp. Immunol. 203, 194–208. doi:10.1111/cei.13527
Wang, Y., Li, Y. P., Paulson, C., Shao, J. Z., Zhang, X., Wu, M., et al. (2014b). Wnt and the Wnt signaling pathway in bone development and disease. Front. Bio (Landmark Ed. 19, 379–407. doi:10.2741/4214
Wang, Y., Zhang, X., Xu, C., Yao, Y., Wu, C., Xu, W., et al. (2023c). κNeoisoastilbin ameliorates acute gouty arthritis via suppression of the NF-B/NLRP3 pathway. Evid. Based Compl. Altern. Med. 2023, 1–8. doi:10.1155/2023/7629066
Wang, Z., Chen, Z., Yang, S., Wang, Y., Huang, Z., Gao, J., et al. (2014a). Berberine ameliorates collagen-induced arthritis in rats associated with anti-inflammatory and anti-angiogenic effects. Inflammation 37, 1789–1798. doi:10.1007/s10753-014-9909-y
Wei, L., Kanbe, K., Lee, M., Wei, X., Pei, M., Sun, X., et al. (2010). Stimulation of chondrocyte hypertrophy by chemokine stromal cell-derived factor 1 in the chondro-osseous junction during endochondral bone formation. Dev. Biol. 341, 236–245. doi:10.1016/j.ydbio.2010.02.033
Wen, Z. H. (2022). Clinical research progress on pathogenesis of rheumatoid arthritis. Clin. Med. 42, 123–125. doi:10.19528/j.issn.1003-3548.2022.07.046
Wirth, T., Balandraud, N., Boyer, L., Lafforgue, P., and Pham, T. (2022). Biomarkers in psoriatic arthritis: a meta-analysis and systematic review. Front. Immunol. 13 (2022), 1054539. doi:10.3389/fimmu.2022.1054539
Wong, S. K., Chin, K. Y., and Ima-Nirwana, S. (2020). Berberine and musculoskeletal disorders: the therapeutic potential and underlying molecular mechanisms. Phytomedicine 73, 152892. doi:10.1016/j.phymed.2019.152892
Wu, B. X. (2021). Experimental study of siRNA interference combined with BMSCs in the treatment of rheumatoid arthritis based on the NF-κB signaling pathway. Jiangsu Province: Yangzhou University.
Wu, J., Luo, Y., Jiang, Q., Li, S., Huang, W., Xiang, L., et al. (2019). Coptisine from Coptis chinensis blocks NLRP3 inflammasome activation by inhibiting caspase-1. Pharmacol. Res. 147, 104348. doi:10.1016/j.phrs.2019.104348
Wu, X., Liu, Y., Jin, S., Wang, M., Jiao, Y., Yang, B., et al. (2021). Single-cell sequencing of immune cells from anticitrullinated peptide antibody positive and negative rheumatoid arthritis. Nat. Commun. 12, 4977. doi:10.1038/s41467-021-25246-7
Xi, X., Ye, Q., Fan, D., Cao, X., Wang, Q., Wang, X., et al. (2022). Polycyclic aromatic hydrocarbons affect rheumatoid arthritis pathogenesis via aryl hydrocarbon receptor. Front. Immunol. 13, 797815. doi:10.3389/fimmu.2022.797815
Xia, T., and Li, J. X. (2017). Effect of artemisia annua combined with methotrexate on levels of IL-1, IL-10, and TNF-α in peripheral blood of patients with rheumatoid arthritis. J. Sichuan Med. 10, 53–55. doi:10.16252/j.cnki.issn1004-0501-2017.10.013
Xiao, J., Zhang, G., Mai, J., He, Q., Chen, W., Li, J., et al. (2022). Bioinformatics analysis combined with experimental validation to explore the mechanism of XianLing GuBao capsule against osteoarthritis. J. Ethnopharmacol. 294 (2022), 115292. doi:10.1016/j.jep.2022.115292
Xie, H., Wang, Q., Zhang, X., Wang, T., Hu, W., Manicum, T., et al. (2018). Possible therapeutic potential of berberine in the treatment of STZ plus HFD-induced diabetic osteoporosis. Biomed. Pharmacother. 108, 280–287. doi:10.1016/j.biopha.2018.08.131
Xie, L., Li, Y., Tang, W., Zhang, Q., Luo, C., and Long, X. (2023). Stattic alleviates pulmonary fibrosis in a mouse model of rheumatoid arthritis-relevant interstitial lung disease. Exp. Biol. Med. (Maywood) 2023, 153537022311579. doi:10.1177/15353702231157934
Xing, J. K. (2021). Polyamidoamine derivative-mediated miR-30a delivery in the treatment of rheumatoid arthritis and the mechanism analysis. Jilin Province: Jilin University.
Xu, B., Arlehag, L., Rantapää-Dahlquist, S. B., and Lefvert, A. K. (2004). beta2-adrenergic receptor gene single-nucleotide polymorphisms are associated with rheumatoid arthritis in northern Sweden. Scand. J. Rheumatol. 33, 395–398. doi:10.1080/03009740410010326
Xu, F., and Li, X. J. (2021). Study on the mechanism of single herb extract in the treatment of gouty arthritis. Rheum. Arthritis. 10, 68–71. doi:10.3969/j.issn.2095-4174.2021.12.019
Xu, H. G., Yu, Y. F., Zheng, Q., Zhang, W., Wang, C. D., Zhao, X. Y., et al. (2014). Autophagy protects end plate chondrocytes from intermittent cyclic mechanical tension induced calcification. Bone 66, 232–239. doi:10.1016/j.bone.2014.06.018
Xu, H., and Xu, B. (2021). Κbmsc-derived exosomes ameliorate osteoarthritis by inhibiting pyroptosis of cartilage via delivering miR-326 targeting HDAC3 and STAT1//NF-B p65 to chondrocytes. Mediat. Inflamm. 2021, 9972805. doi:10.1155/2021/9972805
Xu, Y. (2022). The anti-inflammatory effect and intracellular signling pathway mechanism study of Nootkatone (NTK) in Osteoarthritis. Shandong Province: Shandong University.
Xuan, F., Yano, F., Mori, D., Chijimatsu, R., Maenohara, Y., Nakamoto, H., et al. (2019). Wnt/β-catenin signaling contributes to articular cartilage homeostasis through lubricin induction in the superficial zone. Arthritis Res. Ther. 21, 247. doi:10.1186/s13075-019-2041-5
Xue, M., McKelvey, K., Shen, K., Minhas, N., March, L., Park, S. Y., et al. (2014). Endogenous MMP-9 and not MMP-2 promotes rheumatoid synovial fibroblast survival, inflammation and cartilage degradation. Rheumatol. Oxf. 53, 12270–12279. doi:10.1093/rheumatology/keu254
Yan, Y., Lu, A., Dou, Y., Zhang, Z., Wang, X. Y., Zhai, L., et al. (2023). Nanomedicines reprogram synovial macrophages by scavenging nitric oxide and silencing CA9 in progressive osteoarthritis. Adv. Sci. (Weinh). 10, 2207490. doi:10.1002/advs.202207490
Yang, C., Ni, B., Li, C., Sun, W., Wang, Z., Wang, H., et al. (2023a). circRNA_17725 promotes macrophage polarization towards M2 by targeting FAM46C to alleviate arthritis. Mediat. Inflamm. 2023 (2023), 6818524. doi:10.1155/2023/6818524
Yang, G., Lee, H. E., Moon, S. J., Ko, K. M., Koh, J. H., Seok, J. K., et al. (2022). Direct binding to NLRP3 pyrin domain as a novel strategy to prevent NLRP3-driven inflammation and gouty arthritis. Arthritis Rheumatol. 72, 1192–1202. doi:10.1002/art.41245
Yang, K., Xie, Q., Liao, J., Zhao, N., Liang, J., Liu, B., et al. (2023b). Shang-Ke-Huang-Shui and coptisine alleviate osteoarthritis in the knee of monosodium iodoacetate-induced rats through inhibiting CXCR4 signaling. J. Ethnopharmacol. 311, 116476. doi:10.1016/j.jep.2023.116476
Yang, Y., You, X., Cohen, J. D., Zhou, H., He, W., Li, Z., et al. (2020). Sex differences in osteoarthritis pathogenesis: a comprehensive study based on bioinformatics. Med. Sci. Monit. 26, e923331. doi:10.12659/MSM.923331
Yang, Z., Feng, L., Huang, J., Zhang, X., Lin, W., Wang, B., et al. (2021). Asiatic acid protects articular cartilage through promoting chondrogenesis and inhibiting inflammation and hypertrophy in osteoarthritis. Eur. J. Pharmacol. 907, 174265. doi:10.1016/j.ejphar.2021.174265
Yao, C. J., Li, Y. L., and Xiong, Q. (2023). Bioinformatics prediction of potential traditional Chinese medicines in treatment of rheumatoid arthritis. Acta. Chin. Med. 38, 152–160. doi:10.16368/j.issn.1674-8999.2023.01.027
Yu, J., Hu, C., Dai, Z., Xu, J., Zhang, L., Deng, H., et al. (2023). Dipeptidyl peptidase 4 as a potential serum biomarker for disease activity and treatment response in rheumatoid arthritis. Int. Immunopharmacol. 119 (2023), 110203. doi:10.1016/j.intimp.2023.110203
Yu, Y., Cai, W., Zhou, J., Lu, H., Wang, Y., Song, Y., et al. (2020). Anti-arthritis effect of berberine associated with regulating energy metabolism of macrophages through AMPK/HIF-1α pathway. Int. Immunopharmacol. 87, 106830. doi:10.1016/j.intimp.2020.106830
Yu, Z. L. (2022). Efficacy evaluation, action mechanism and pharmacodynamic material basis of Zhuang medicine Fenghegui against rheumatoid arthritis. Jiangxi Province: Jiangxi University of Traditional Chinese Med.
Yuan, X., Garrett-Sinha, L. A., Sarkar, D., and Yang, S. (2014). Deletion of IFT20 in early stage T lymphocyte differentiation inhibits the development of collagen-induced arthritis. Bone Res. 2, 14038. doi:10.1038/boneres.2014.38
Yue, M., Tao, Y., Fang, Y., Lian, X., and Zhang, Q. (2019). The gut microbiota modulator berberine ameliorates collagen-induced arthritis in rats by facilitating the generation of butyrate and adjusting the intestinal hypoxia and nitrate supply. FASEB J. 33, 12311–12323. doi:10.1096/fj.201900425RR
Yue, R., Zhao, L., Hu, Y., Jiang, P., Wang, S., Xiang, L., et al. (2013). Metabolomic study of collagen-induced arthritis in rats and the interventional effects of Huang-Lian-Jie-Du-Tang, a traditional Chinese medicine. Evid. Based Compl. Altern. Med. 2013, 439690. doi:10.1155/2013/439690
Zeng, W., Shen, C., Mo, S., Ni, C., Lin, Y., Fang, Y., et al. (2023). The effective treatment of purpurin on inflammation and adjuvant-induced arthritis. Molecules 28, 366. doi:10.3390/molecules28010366
Zeng, Z. (2022). The effect and mechanism of pelargonidin on osteoarthritis based on NF-κB signaling pathway. Jiangxi Province: Nanchang University.
Zhang, H., Cai, D., and Bai, X. (2020). Macrophages regulate the progression of osteoarthritis. Osteoarthr. Cartil. 28, 555–561. doi:10.1016/j.joca.2020.01.007
Zhang, H. G., Wang, Y., Xie, J. F., Liang, X., Liu, D., Yang, P., et al. (2001). Regulation of tumor necrosis factor alpha-mediated apoptosis of rheumatoid arthritis synovial fibroblasts by the protein kinase Akt. Arthritis Rheum. 44, 1555–1567. doi:10.1002/1529-0131(200107)44:7<1555::AID-ART279>3.0.CO;2-M
Zhang, J. F., Huang, K., and Cai, H. L. (2022a). Experimental study on the regulation of subchondral bone plate osteoprotegerin/receptor activator of nuclear factor Kappa-B ligand system by berberine to retard the development of osteoarthritis in rabbits, Chin. J. Orthop. Traumatol. 35, 464–469. doi:10.12200/j.issn.1003-0034.2022.05.011
Zhang, N., Zheng, N., Luo, D., Lin, D., Que, W., Wang, H., et al. (2022b). Long non-coding RNA NR-133666 promotes the proliferation and migration of fibroblast-like synoviocytes through regulating the miR-133c/MAPK1 Axis. Front. Pharmacol. 13 (2022), 887330. doi:10.3389/fphar.2022.887330
Zhang, X., Liu, J., Sun, Y., Zhou, Q., Ding, X., and Chen, X. (2023). Chinese herbal compound Huangqin Qingrechubi capsule reduces lipid metabolism disorder and inflammatory response in gouty arthritis via the LncRNA H19/APN/PI3K/AKT cascade. Pharm. Biol. 61, 541–555. doi:10.1080/13880209.2023.2191641
Zhang, X., Zhao, W., Zhao, Y., Zhao, Z., Lv, Z., Zhang, Z., et al. (2022c). Inflammatory macrophages exacerbate neutrophil-driven joint damage through ADP/P2Y1 signaling in rheumatoid arthritis. Sci. Chin. Life Sci. 65, 953–968. doi:10.1007/s11427-020-1957-8
Zhang, Y., Li, S., Jin, P., Shang, T., Sun, R., Lu, L., et al. (2022d). Dual functions of microRNA-17 in maintaining cartilage homeostasis and protection against osteoarthritis. Nat. Commun. 13, 2447. doi:10.1038/s41467-022-30119-8
Zhao, H., Zhang, T., Xia, C., Shi, L., Wang, S., Zheng, X., et al. (2014). Berberine ameliorates cartilage degeneration in interleukin-1β-stimulated rat chondrocytes and in a rat model of osteoarthritis via Akt signalling. J. Cell. Mol. Med. 18, 283–292. doi:10.1111/jcmm.12186
Zhao, Y., Wang, X., and Nie, K. (2023). IRF1 promotes the chondrogenesis of human adipose-derived stem cells through regulating HILPDA. Tissue Cell. 82 (2023), 102046. doi:10.1016/j.tice.2023.102046
Zheng, S. C. (2012). Serum levels and significances of Toll-like receptor 7 in rheumatoid arthritis. Jiangsu Province: Suzhou University.
Zhou, J. T., Li, C. L., Tan, L. H., Xu, Y. F., Liu, Y. H., Mo, Z. Z., et al. (2017b). Inhibition of Helicobacter pylori and its associated urease by palmatine: investigation on the potential mechanism. PLoS One 12, e0168944. doi:10.1371/journal.pone.0168944
Zhou, J., Yu, Y., Yang, X., Wang, Y., Song, Y., Wang, Q., et al. (2019). Berberine attenuates arthritis in adjuvant-induced arthritic rats associated with regulating polarization of macrophages through AMPK/NF-кB pathway. Eur. J. Pharmacol. 852, 179–188. doi:10.1016/j.ejphar.2019.02.036
Zhou, K., Hu, L., Liao, W., Yin, D., and Rui, F. (2016d). Coptisine prevented IL-β-induced expression of inflammatory mediators in chondrocytes. Inflammation 39, 1558–1565. doi:10.1007/s10753-016-0391-6
Zhou, M., Tan, W., Hasimu, H., Liu, J., Gu, Z., and Zhao, J. (2023a). Euphorbium total triterpenes improve Freund's complete adjuvant-induced arthritis through PI3K/AKT/Bax and NF-κB/NLRP3 signaling pathways. J. Ethnopharmacol. 306 (2023), 116146. doi:10.1016/j.jep.2023.116146
Zhou, Q., Sun, H. J., and Liu, S. M. (2023b). Effects of total saponins from Dioscorea nipponica makino on monosodium urate-induced M1-polarized macrophages through arachidonic acid signaling pathway: an in vitro study. Chin. J. Integr. Med. 29, 44–51. doi:10.1007/s11655-022-3721-6
Zhou, R., Xiang, C. P., Zhang, J. J., and Hong-Jun, Y. (2020a). Research progress on chemical compositions of Coptidis Rhizoma and pharmacological effects of berberine. Chin. J. Chin. Mat. Med. 45, 4561–4573. doi:10.19540/j.cnki.cjcmm.20200527.202
Zhou, X. D. (2014). Chondroprotective effects of palmatine on osteoarthritis in vivo and in vitro. Zhejiang Province: Zhejiang University.
Zhou, X., Lin, X., Xiong, Y., Jiang, L., Li, W., Li, J., et al. (2016b). Chondroprotective effects of palmatine on osteoarthritis in vivo and in vitro: a possible mechanism of inhibiting the wnt/β-catenin and Hedgehog signaling pathways. Int. Immunopharmacol. 34, 129–138. doi:10.1016/j.intimp.2016.02.029
Zhou, Y., Deng, M., and He, B. (2016a). Research progress in the construction of rat induced osteoarthritis model. Int. J. Orthop. 37, 304–310. doi:10.3969/j.issn.1673-7083.2016.05.009
Zhou, Y., Liu, S., Ming, J., Li, Y., Deng, M., and He, B. (2017a). Sustained release effects of berberine-loaded chitosan microspheres on in vitro chondrocyte culture. Drug Dev. Ind. Pharm. 403, 1703–1714. doi:10.1080/03639045.2017.1339076
Zhou, Y., Liu, S. Q., Yu, L., He, B., Wu, S. H., Zhao, Q., et al. (2015). Berberine prevents nitric oxide-induced rat chondrocyte apoptosis and cartilage degeneration in a rat osteoarthritis model via AMPK and p38 MAPK signaling. Apoptosis 20, 1187–1199. doi:10.1007/s10495-015-1152-y
Zhou, Y., Ming, J., Deng, M., Li, Y., Li, B., Li, J., et al. (2020b). Berberine-mediated up-regulation of surfactant protein D facilitates cartilage repair by modulating immune responses via the inhibition of TLR4/NF-ĸB signaling. Pharmacol. Res. 155, 104690. doi:10.1016/j.phrs.2020.104690
Zhou, Y., Tao, H., Li, Y., Deng, M., He, B., Xia, S., et al. (2016c). Berberine promotes proliferation of sodium nitroprusside-stimulated rat chondrocytes and osteoarthritic rat cartilage via Wnt/β-catenin pathway. Eur. J. Pharmacol. 789, 109–118. doi:10.1016/j.ejphar.2016.07.027
Zhu, H., Xiong, X. G., Lu, Y., Wu, H. C., Zhang, Z. H., and Sun, M. J. (2022). The mechanism of the anti-inflammatory effect of oldenlandia diffusa on arthritis model rats: a quantitative proteomic and network pharmacologic study. Ann. Transl. Med. 10, 1098. doi:10.21037/atm-22-3678
Zhuang, H., Li, B., Xie, T., Xu, C., Ren, X., Jiang, F., et al. (2022). Indole-3-aldehyde alleviates chondrocytes inflammation through the AhR-NF-κB signalling pathway. Int. Immunopharmacol. 113 (2022), 109314. doi:10.1016/j.intimp.2022.109314
Zou, B., Zheng, J., Deng, W., Tan, Y., Jie, L., Qu, Y., et al. (2021). Kirenol inhibits RANKL-induced osteoclastogenesis and prevents ovariectomized-induced osteoporosis via suppressing the Ca2+-NFATc1 and Cav-1 signaling pathways. Phytomedicine 80, 153377. doi:10.1016/j.phymed.2020.153377
Glossary
Keywords: Coptis chinensis, arthritis, berberine, Wnt1/β-catenin signaling pathway, PI3K/Akt/mTOR signaling pathway
Citation: Li M, Tian F, Guo J, Li X, Ma L, Jiang M and Zhao J (2023) Therapeutic potential of Coptis chinensis for arthritis with underlying mechanisms. Front. Pharmacol. 14:1243820. doi: 10.3389/fphar.2023.1243820
Received: 21 June 2023; Accepted: 01 August 2023;
Published: 11 August 2023.
Edited by:
Sirajudheen Anwar, University of Hail, Saudi ArabiaReviewed by:
Sakeel Ahmed, NIPER-A, IndiaMohd Rabi Bazaz, National Institute of Pharmaceutical Education and Research, India
Ziaur Rahman, Thomas Jefferson University, United States
Copyright © 2023 Li, Tian, Guo, Li, Ma, Jiang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miaomiao Jiang, bWlhb21pYW9qaWFuZ0B0anV0Y20uZWR1LmNu; Jing Zhao, emhhb2ppbmdfdGp1QHRqdXRjbS5lZHUuY24=
†These authors have contributed equally to this work
 Mengyuan Li1†
Mengyuan Li1† Fei Tian
Fei Tian Xiankuan Li
Xiankuan Li Miaomiao Jiang
Miaomiao Jiang Jing Zhao
Jing Zhao