Revealing the Mechanism of Huazhi Rougan Granule in the Treatment of Nonalcoholic Fatty Liver Through Intestinal Flora Based on 16S rRNA, Metagenomic Sequencing and Network Pharmacology
- 1Department of Clinical Chinese Pharmacy, School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, China
- 2Institute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China, Chengdu, China
- 3State Key Laboratory of Generic Manufacture Technology of Chinese Traditional Medicine, Linyi, China
by Liu Y, Tan Y, Huang J, Wu C, Fan X, Stalin A, Lu S, Wang H, Zhang J, Zhang F, Wu Z, Li B, Huang Z, Chen M, Cheng G, Mou Y and Wu J (2022). 13:875700. doi: 10.3389/fphar.2022.875700
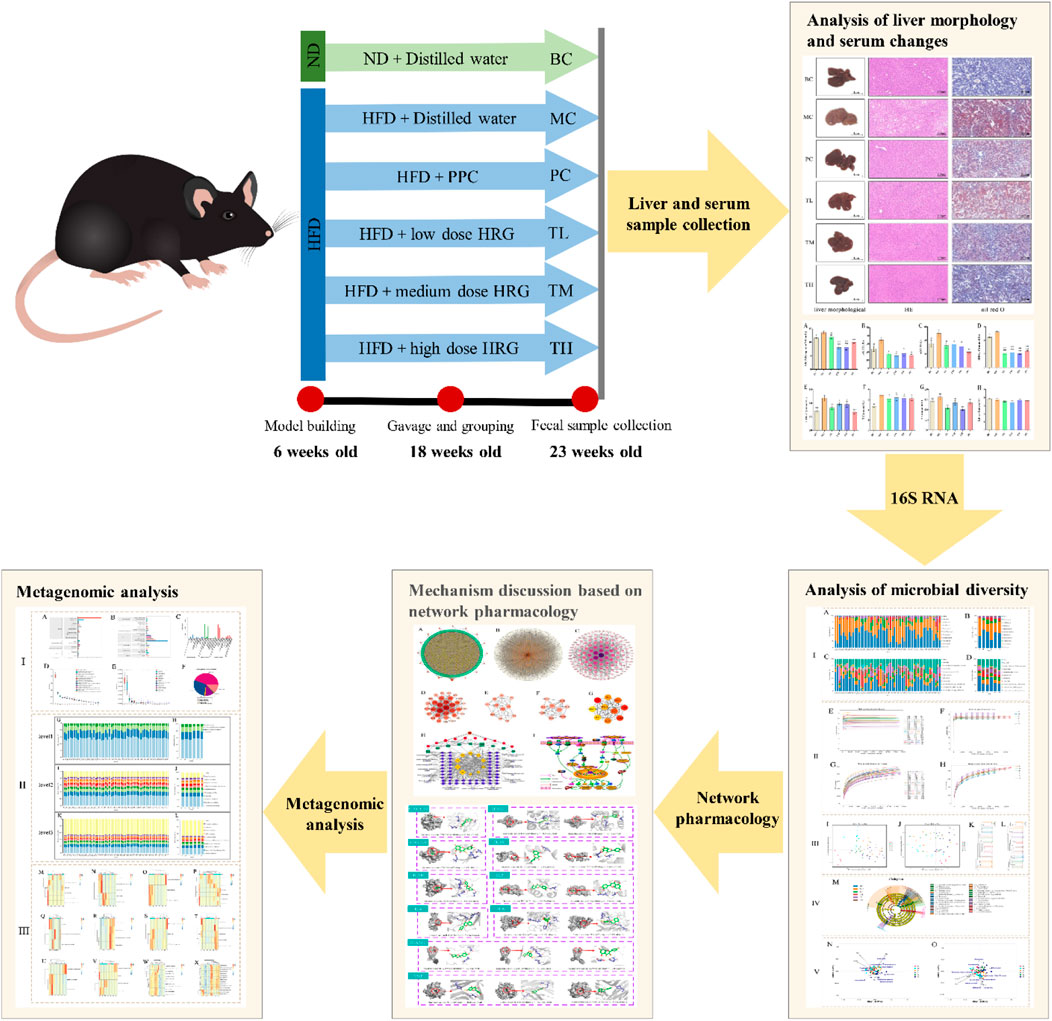
In the published article, there was an error in the Graphical Abstract as published. The number of weeks in the picture are incorrect. The Graphical Abstract has been corrected.
Additionally, a correction has been made to the section Abstract, subsection Methods, 2nd sentence. This sentence previously stated:
“In this study, C57BL/6J mice were fed a high-fat diet for 8 weeks, and the high-fat diet plus HRG or polyene phosphatidylcholine capsules were each administered by gavage for 4 weeks.”
The corrected sentence appears below:
“In this study, C57BL/6J mice were fed a high-fat diet for 10 weeks, and the high-fat diet plus HRG or polyene phosphatidylcholine capsules were each administered by gavage for 5 weeks.”
Lastly, a correction has been made to section Materials and Methods, subsection Animal Grouping and Model Establishment, 2nd and 3rd sentences. These sentences previously stated:
“After 12 consecutive weeks, relevant indicators were evaluated. Mice that passed the evaluation could be considered successful modeling, gavage at week 13, and sampled at week 17.”
The corrected sentences appear below:
“After 10 consecutive weeks, relevant indicators were evaluated. Mice that passed the evaluation could be considered successful modeling, gavage at week 12, and sampled at week 17.”
The authors apologize for these errors and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: nonalcoholic simple fatty liver, high-fat diet, intestinal flora disorder, 16S sequencing, network pharmacology
Citation: Liu Y, Tan Y, Huang J, Wu C, Fan X, Stalin A, Lu S, Wang H, Zhang J, Zhang F, Wu Z, Li B, Huang Z, Chen M, Cheng G, Mou Y and Wu J (2023) Corrigendum: Revealing the mechanism of Huazhi Rougan granule in the treatment of nonalcoholic fatty liver through intestinal flora based on 16S rRNA, metagenomic sequencing and network pharmacology. Front. Pharmacol. 14:1243304. doi: 10.3389/fphar.2023.1243304
Received: 20 June 2023; Accepted: 20 July 2023;
Published: 26 July 2023.
Edited and reviewed by:
Ana Blas-García, University of Valencia, SpainCopyright © 2023 Liu, Tan, Huang, Wu, Fan, Stalin, Lu, Wang, Zhang, Zhang, Wu, Li, Huang, Chen, Cheng, Mou and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoliang Cheng, Y2dsLnliQDE2My5jb20=; Yanfang Mou, bG5jYXR0bGV5YUAxNjMuY29t; Jiarui Wu, ZXhvZ2FteUAxNjMuY29t
 Yingying Liu
Yingying Liu Yingying Tan1
Yingying Tan1 Xiaotian Fan
Xiaotian Fan Antony Stalin
Antony Stalin Fanqin Zhang
Fanqin Zhang Jiarui Wu
Jiarui Wu