
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 08 January 2024
Sec. Pharmacology of Anti-Cancer Drugs
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1242548
This article is part of the Research Topic Synthetic and non-synthetic anti-cancer drugs in gastric cancer: emphasis on the RAS/RAF/MAPK and PI3K/AKT/mTOR signaling pathways View all 6 articles
Objective: To systematically evaluate the safety and efficacy of docetaxel plus S-1-based therapy in gastric cancer treatment.
Methods: PubMed, Embase, The Cochrane Library, and Web of Science electronic databases were searched for randomized controlled trials on docetaxel plus S-1-based therapy in the treatment of gastric cancer from the establishment of the database to 1 September 2022. Relevant studies were included per pre-defined eligibility criteria, and two researchers independently screened and assessed the included literature using Review Manager v5. Outcome measures and statistics related with efficacy and safety profiles were extracted from the included studies, and Stata v15.1 was used for pooled analysis.
Results: Objective response rate (odds ratio = 2.34, 95% CI = [1.32, 4.13], p = 0.003), relapse-free survival (HR = 0.68, 95% CI = [0.58, 0.79], p < 0.001), progression-free survival (HR = 0.81, 95% CI = [0.68, 0.96], p = 0.016), and overall survival (HR = 0.86, 95% CI = [0.79, 0.95], p = 0.002) of docetaxel plus S-1-based therapy (DS-based therapy) in gastric cancer treatment were better than those of the non-DS-based therapy. However, DS-based therapy was associated with increased risk of certain adverse drug effects, such as alopecia, leukopenia, and oral mucositis. Further studies are warranted to validate the efficacy superiority of DS-based versus non-DS-based regimens as per our trial sequential analysis findings.
Conclusion: DS-based therapy significantly improves patients’ clinical outcomes in gastric cancer, albeit at the cost of increased toxicity. Further RCTs are needed to confirm the efficacy superiority of DS-based regimens.
Gastric cancer (GC) is one of the most common malignancies, especially in Asian countries including China. GC ranks fifth and fourth in terms of incidence and mortality among all malignant cancer types (H Sung, et al., 2021). Surgical resection in combination with neoadjuvant/adjuvant chemotherapy, namely, multimodal therapy (Y Kakeji, et al., 2022), represents the mainstay of care for treatment of GC; however, tumor recurrence rates can reach 25%–40% in GC patients classified as stages II–IV (American Joint Committee on Cancers; AJCC2) (Bang, et al., 2012; Cristescu, et al., 2015; Kim, et al., 2005; Lee, et al., 2012; Macdonald, et al., 2001). Given the poor prognosis of advanced gastric cancer (AGC) patients, development of more effective chemotherapy regimens remains an unmet clinical need (Y Kurokawa, et al., 2021), which is also the current research hotspot (Zhu, et al., 2020). To date, fluoropyrimidine plus platinum compounds are the most widely used chemotherapeutic agents against GC worldwide (Cunningham, et al., 2008; Kang, et al., 2009; Koizumi, et al., 2008; Kurokawa, et al., 2021). S-1 monotherapy, which leverages the compounds tegafur, gimeracil, and oteracil potassium, is one of the most common treatments for stage II/III gastric cancer in Asia (Kakeji, et al., 2022; Sakuramoto, et al., 2007; Sasako, et al., 2011). However, as the cancer progresses to more advanced stages, S-1 monotherapy lacks efficacy and fails to reduce the incidence of hematogenous recurrence (Sakuramoto, et al., 2007; Sasako, et al., 2011). Recently, a long-term follow-up study of an international Phase III (START) trial showed that the docetaxel plus S-1 (DS) regimen significantly improved patients’ prognosis compared with S-1 monotherapy, although the initial analysis failed to show a significant difference in overall survival (OS) (Koizumi, et al., 2014). Although DS-based regimens may cause mildly increased toxicity compared with their non-DS-based counterparts, significantly improved efficacy outcomes were reported in multiple studies (Koizumi, et al., 2014; Kurokawa, et al., 2021). However, inconsistencies and discrepancies were observed among studies comparing DS-based therapy vs non-DS-based regimens in terms of efficacy and safety profiles (Kakeji, et al., 2022; Kang, et al., 2021; Kurokawa, et al., 2021; Lee, et al., 2019; Yamada, et al., 2019).
To the best of our knowledge, there is no systematic review/meta-analysis addressing this topic. Therefore, we by literature search identified randomized controlled trials (RCTs) on DS-based vs non-DS-based treatment regimens in gastric cancer and systematically assessed and quantitatively synthesized the relevant literature.
The literature about the safety and efficacy of DS-based treatment for gastric cancer was searched in the database of PubMed, The Cochrane Library, Embase, and Web of Science from the establishment of the database to 1 September 2022. All RCTs comparing docetaxel plus S-1-based therapy with other treatments were considered. The language was limited to English. The following keywords were used to create the search strategy: “stomach neoplasms,” “docetaxel,” “S-1,” and “TS-1,” and all relevant MeSH terms of those words were considered as well. Those keywords were used in combination with logic “and” and “or” to ensure complete retrieval.
The inclusion criteria are as follows: 1) subjects: patients aged ≥18 years with pathologically diagnosed gastric cancer; 2) interventions: DS-based regimens vs. non-DS-based controls; 3) efficacy outcomes: objective response rate (ORR), relapse-free survival (RFS), progression-free survival (PFS), and overall survival (OS); safety outcomes: adverse event rates, including hematologic toxicity and non-hematologic toxicity; 4) research type: RCTs; 5) language: English.
The exclusion criteria are as follows 1) animal experiments; 2) the full text cannot be obtained; 3) data cannot be extracted; 4) meta-analysis, review, conference abstracts, and letters.
Risk of bias assessment for the included RCTs was performed as per standards recommended in Cochrane handbook (version 6.3). Evaluation criteria included randomization methods, concealed assignments, blinded participants, outcome evaluators, incomplete/missing outcome data, selective reporting, and other potential threats to validity. Quality assessment was conducted independently by two investigators (Rui-Zhi RAN and Xue-Ping JIANG) using the software RevMan 5, and discrepancies were resolved through discussion with a third reviewer.
Data were extracted independently by two reviewers (Hui-Fen LV and Li-Feng QIN). If there were any disagreements, a third reviewer was consulted, and differences were resolved through discussion. The extracted data included the following: baseline demographic characteristics, treatment regimens, efficacy outcome measurements, and detailed data on adverse events.
Stata v15.1 software was used for statistical analysis. Cochrane’s Q test p-value and I2 statistic were used to analyze the heterogeneity of the included literature. In case that significant between-study heterogeneity was detected, as indicated by an I2 ≥ 50% and/or Cochrane’s Q test p-value <0.1, the random-effects inverse-variance weighted meta-analysis model was used; otherwise, the fixed-effects model was used. Leave-one-out sensitivity analyses were performed to identify studies that contribute most to the heterogeneity. For dichotomous data, odds ratio (OR) and 95% confidence intervals (95% CI) were calculated based on event counts, as outcome measurements for meta-analyses. For time-to-event data, natural logarithmic hazard ratios and corresponding standard errors were extracted/calculated and pooled. For the efficacy outcomes, trial sequential analysis was performed using the metacumbounds command add-on to Stata, which was developed by Dr. Miladinovic and colleagues, to determine whether adequate information was accumulated for a definitive conclusion (Miladinovic, et al., 2013). The a priori diversity-adjusted information size (APDIS) was used as the information measurement. The pre-specified type I error was set as two-sided α = 0.05 and type II error as β = 20% (1-β = 80% power). A conservative relative risk reduction (RRR) of 15% was used as described previously (B Miladinovic, et al., 2013). O’Brian–Fleming boundaries were used (DeMets and Lan, 1994). Egger’s test was used to evaluate publication bias among the included studies, and a p > 0.05 of Egger’s test indicated no publication bias. A 95% CI not covering 1 or a p < 0.05 suggests statistical significance, unless otherwise specified.
The present study was reported in line with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) reporting guidelines.
A total of 2,264 references were retrieved by literature search in electronic databases, and 10 RCTs were finally included for quantitative synthesis, after removing irrelevant literature according to inclusion and exclusion criteria. The flowchart of literature screening is shown in Figure 1.
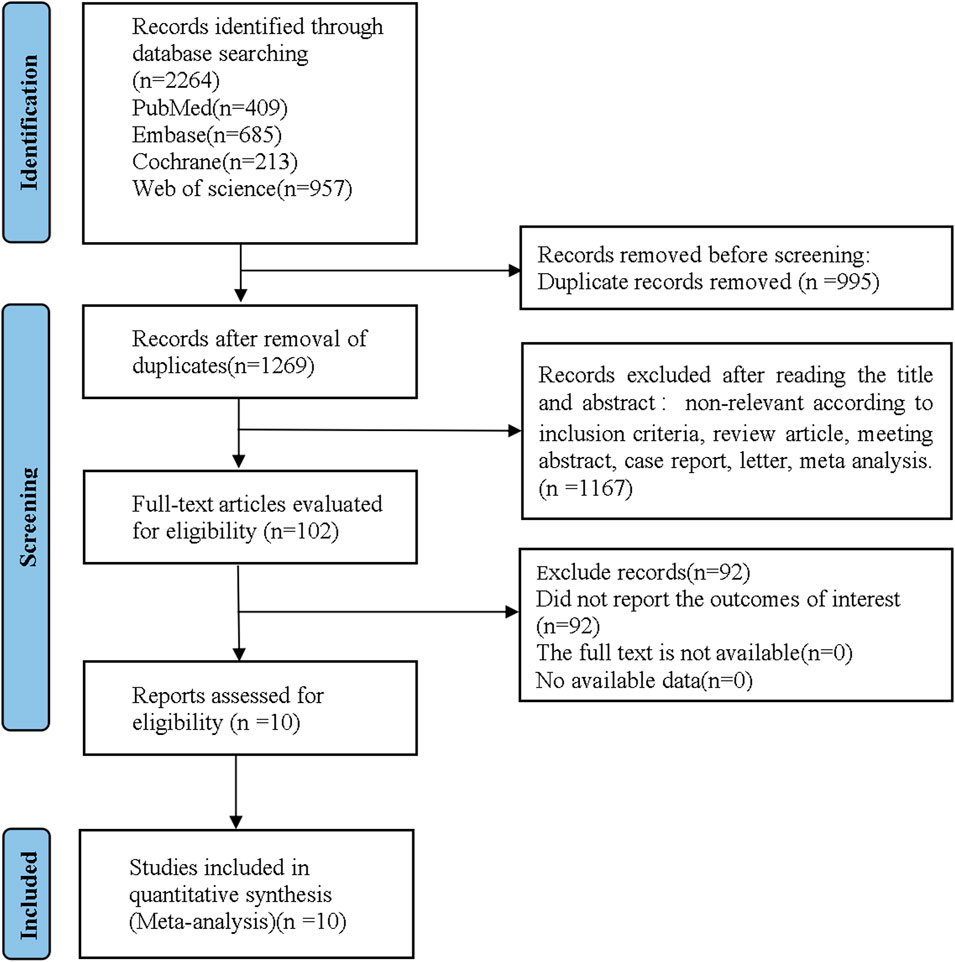
FIGURE 1. Flow diagram of the study as per Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) style.
A total of 10 trials were included in this meta-analysis, containing 4,145 patients. The details of baseline characteristics and types of participants are shown in Table 1 (Jeung et al., 2011; Koizumi, et al., 2014; Lee, et al., 2017; Lee, et al., 2019; Yamada, et al., 2019; Yoshida, et al., 2019; Zhu, et al., 2020; Kang, et al., 2021; Kurokawa, et al., 2021; Kakeji, et al., 2022).
The included literatures were basically of high quality. There are uncertain risks in the generation of random sequences in five literatures and uncertain risks in the distribution in seven papers. The experimental results were objective data and were not affected by whether the distribution scheme was hidden or not. The details are listed in Figures 2, 3.
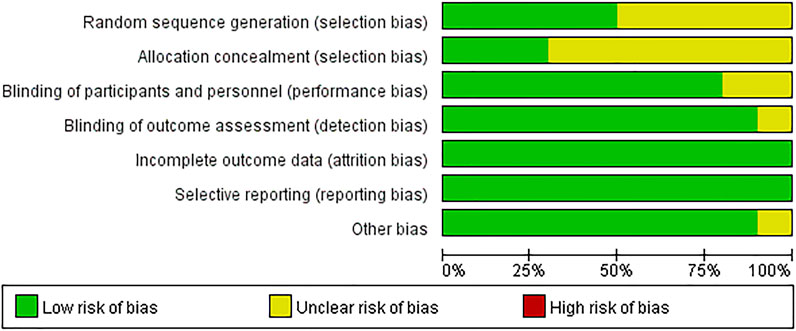
FIGURE 2. Risk of bias. Revman 5 software was used to evaluate the risk of bias. There is no high risk in assessment.
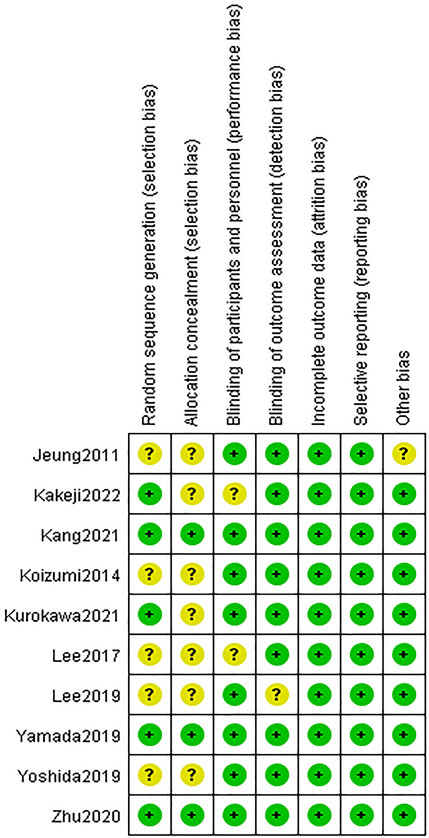
FIGURE 3. Risk of bias summary. Green indicates high quality. Yellow indicates that the article is unclear in this respect.
A total of three studies with available data on the ORR were included in the meta-analysis of the ORR (Jeung, et al., 2011; Lee, et al., 2017; Zhu, et al., 2020). The heterogeneity of the ORR among included studies was minimal (I2 = 0.0%, p = 0.928), and the fixed-effects model was used for meta-analysis. The results of the analysis showed that the ORR of the DS-based therapy was better than that of the non-DS-based therapy (OR = 2.34, 95% CI = [1.32, 4.13], p = 0.003), and the difference was statistically significant. The meta-analysis forest plot is shown in Figure 4. Egger’s test suggested no publication bias (p = 0.942).
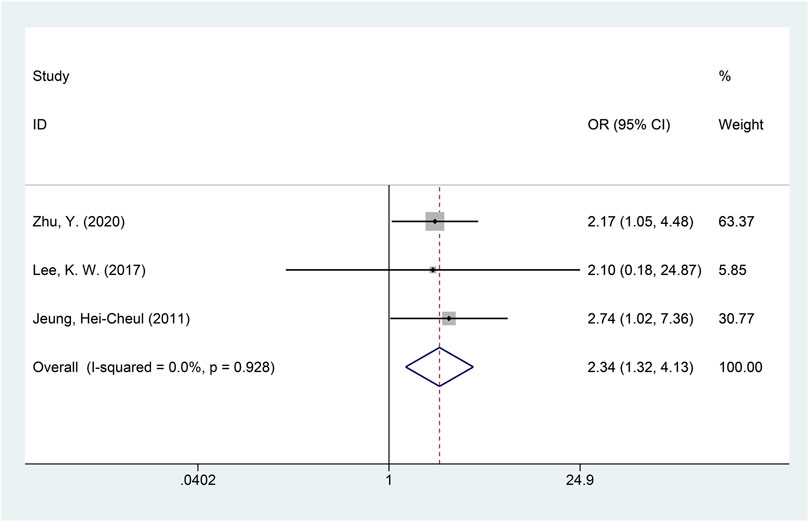
FIGURE 4. Forest plot of the objective response rate (ORR). The forest plot depicts the individual and pooled ORs with 95% CIs.
Only one RCT, the JACCRO GC-07, addressed the RFS (Kakeji, et al., 2022; Yoshida, et al., 2019), which reported superior RFS in the DS-based vs. the non-DS-based group (HR = 0.715; 95% CI = [0.59, 0.87]; p = 0.0008).
A total of five studies with available data on PFS were included in the meta-analysis of PFS (Kang, et al., 2021; Koizumi, et al., 2014; Kurokawa, et al., 2021; Lee, et al., 2017; Yamada, et al., 2019). Significant heterogeneity was detected (I2 = 52.0%, p = 0.080), and the random-effects model was used for the meta-analysis of PFS. The results showed that, compared with the non-DS-based group, the DS-based group demonstrated significantly improved PFS (HR = 0.81, 95% CI = [0.68, 0.96], p = 0.016). The forest plot of meta-analysis is shown in Figure 5. Egger’s test suggested no publication bias (p = 0.335).
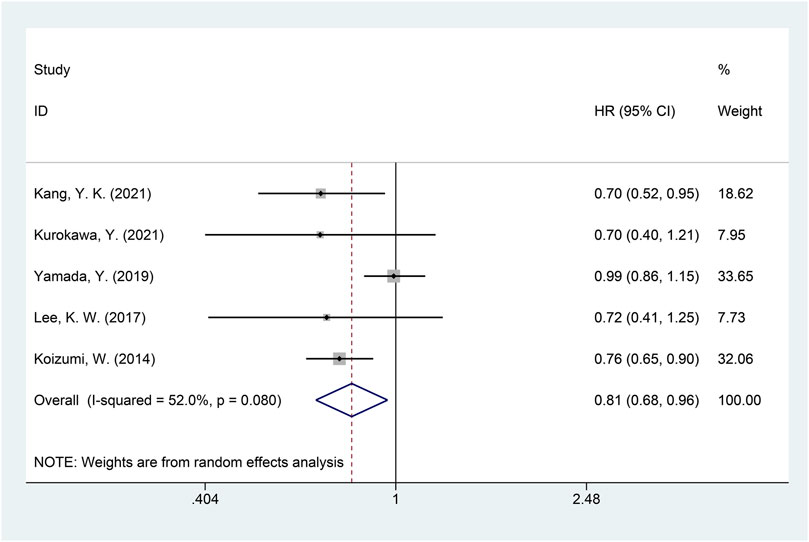
FIGURE 5. Forest plot of progression-free survival (PFS). The forest plot depicts the individual and pooled ORs with 95% CIs.
A total of seven studies were included in the meta-analysis of OS (Kakeji, et al., 2022; Kang, et al., 2021; Koizumi, et al., 2014; Kurokawa, et al., 2021; Lee, et al., 2019; Lee, et al., 2017; Yamada, et al., 2019). The heterogeneity of OS among included studies was minimal (I2 = 28.8%, p = 0.208), and the fixed-effects model was used for the meta-analysis of OS. The results showed that, compared with the non-DS-based group, the DS-based group demonstrated significantly improved OS (HR = 0.86, 95% CI = [0.79, 0.95], p = 0.002). The forest plot of OS is shown in Figure 6. Egger’s test suggested no publication bias (p = 0.313).
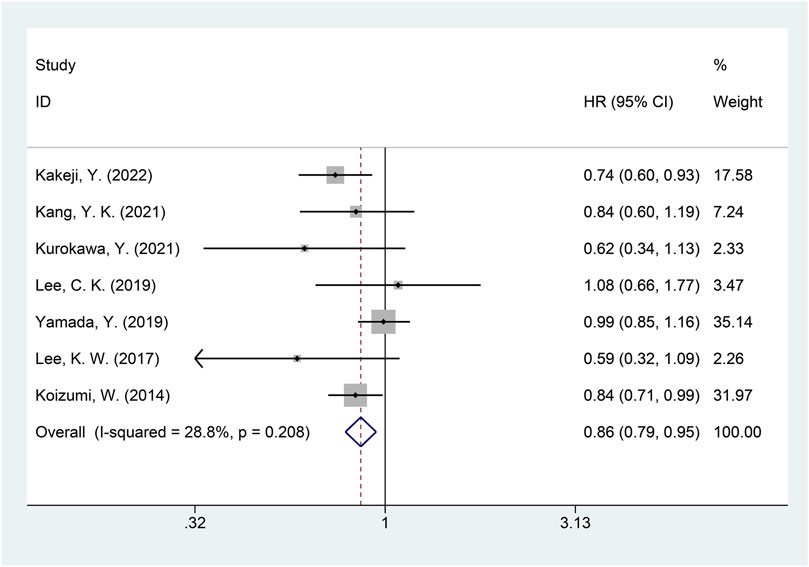
FIGURE 6. Forest plot of overall survival (OS). The forest plot depicts the individual and pooled ORs with 95% CIs.
The results of all-grade drug-induced adverse events suggested increased risk of developing alopecia (OR = 9.35, 95% CI = [1.94, 45.18], p = 0.005), oral mucositis (OR = 1.78, 95% CI = [1.47, 2.17], p < 0.001), and neutropenia (OR = 1.72, 95% CI = [1.21, 2.45], p = 0.002) in patients treated with DS-based regimens than in those treated with non-DS-based regimens. The heterogeneity of alopecia (I2 = 96.3%, p < 0.001) and neutropenia (I2 = 56.8%, p = 0.041) was high among studies, and the random-effects model was used for analysis. No single study with significant contribution to heterogeneity was found for alopecia and neutropenia. The studies on oral mucositis (I2 = 49.0%, p = 0.098) had little heterogeneity and were analyzed by the fixed-effects model.
Except for the above adverse reactions, no statistically significant difference was observed between the DS-based and the non-DS-based groups in other all-grade adverse events. Detailed data of all grades of adverse events are listed in Table 2.
The analysis of ≥ grade 3 drug-induced adverse events showed that leukopenia (OR = 3.04, 95% CI = [1.04, 8.94], p = 0.043), neutropenia (OR = 2.28, 95% CI = [1.36, 3.83], p = 0.002), and oral mucositis (OR = 2.07, 95% CI = [1.25, 3.44], p = 0.005) in the DS-based group were more frequent than in the non-DS-based group. The heterogeneity of leukopenia (I2 = 89.2%, p < 0.001) and neutropenia (I2 = 81.9%, p < 0.001) was significant, and the random-effects model was adopted for analysis. No single study with significant contribution to heterogeneity was found for leukopenia and neutropenia. The studies on oral mucositis had little heterogeneity (I2 = 0.0%, p = 0.592), and the fixed-effects model was adopted for analysis.
Diarrhea (OR = 0.63, 95% CI = [0.45, 0.88], p = 0.007) was less common in the DS-based therapy than in the non-DS-based therapy. There was little heterogeneity among various studies on diarrhea (I2 = 24.1%, p = 0.245), and the fixed-effects model was used for analysis.
There was no statistically significant difference between the DS-based therapy and the non-DS-based therapy in terms of other adverse events of ≥ grade 3. Detailed data are listed in Table 3.
Egger’s test was conducted for assessing the publication bias of included studies. The results showed no bias in the ORR (p = 0.942), PFS (p = 0.335), and OS (p = 0.313). Bilirubin increase had a publication bias in all levels of adverse events. In ≥ grade 3 adverse events, anorexia and bilirubin increase were associated with publication bias. Among the adverse events, no bias was found in the analysis of other results, and the detailed p values are shown in Table 2 and Table 3. No studies that significantly influenced the results were found in the leave-one-out sensitivity analysis.
The results of TSAs are shown in Figure 7. For the ORR, PFS, and OS efficacy outcome measures, although the cumulative Z-curves passed the pre-specified α-threshold, none of them crossed the Lan–DeMets trial sequential monitoring boundaries, suggesting the possibility of false positivity. For RFS with only one trial available, we instead calculated the APDIS as per the formula (Zα/2+ Zβ)2/[(1 − w) (1 − S)]×[(HR0 + 1)/(HR0–1)]2, as previously described, using the statistics based on a 3-year follow-up by Kakeji et al. (HR0 = 0.715, the censoring rate w was estimated as 0.5, and the average survival rate during the follow-up period was estimated as 0.5, as per the RFS Kaplan–Meier curve given in the original study) (Kakeji, et al., 2022; Miladinovic, et al., 2013). The estimated APDIS was 1,137, larger than the actually enrolled sample size, which was 912. Therefore, further studies plus updated meta-analysis are warranted to validate and confirm the currently observed efficacy effects of DS-based regimens.
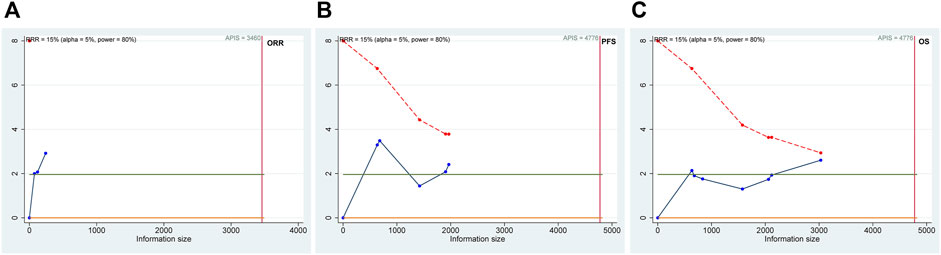
FIGURE 7. Trial sequential analysis (TSA) of the objective response rate (ORR; (A)), progression-free survival (PFS; (B)), and overall survival (OS; (C)) efficacy outcome measurements. The possibility of false positivity might exist in the ORR, PFS, and OS according to the results of TSA.
Our systematic review and meta-analysis on DS-based therapy vs. non-DS-based therapy shows the following: ORR, RFS (HR = 0.68, 95% CI = [0.58, 0.79], p < 0.001), PFS (HR = 0.81, 95% CI = [0.68, 0.96], p = 0.016), and OS (HR = 0.86, 95% CI = [0.79, 0.95], p = 0.002) in gastric cancer treatment, suggesting better efficacy profiles of DS-based therapy. However, DS-based therapy was associated with increased risk of certain adverse drug effects, such as alopecia, leukopenia, and oral mucositis.
Docetaxel is a paclitaxel drug, which can inhibit microtubule depolymerization. When used in combination with chemotherapy drugs (Van Cutsem, et al., 2006), docetaxel has been shown to increase the response rate in AGC patients (Zhu, et al., 2020). The anti-tumor mechanism of docetaxel is different from that of fluoropyrimidine and platinum, and there is no cross-resistance with these drugs. Docetaxel is often seen as a second-line regimen in the case of failed first-line therapy based on fluorouracil and/or platinum (Lee, et al., 2017). S-1 is a compound oral drug composed of tegafur, gimeracil, and oteracil potassium, which has a significant curative effect on advanced gastric cancer (Gyurkovska and Ivanovska, 2016). Fluorouracil phosphorylation could be blocked by oteracil potassium, so that the toxic effects of fluorouracil in the gastrointestinal tract could be reduced. Decomposition of fluorouracil could be inhibited by gimeracil, thus extending the action time of fluorouracil in vivo, maintaining blood drug concentration of fluorouracil, and ultimately preventing tumor growth (F Pietrantonio, et al., 2016). Docetaxel regulates the expression of enzymes involved in fluorouracil metabolism, which are thymidine synthase, dihydropyrimidine dehydrogenase, and rotary phosphotransferase, showing synergistic anti-tumor effects with fluorouracil, especially S-1 (Maeda, et al., 2004; Takahashi, et al., 2005; Wada, et al., 2006). Considering that the two drugs have a good synergistic effect in the anti-tumor mechanism, the survival rate of the DS-based therapy based on docetaxel plus S-1 is higher than that of the non-DS-based therapy.
We also analyzed adverse drug events, and the results showed that alopecia, oral mucositis, and neutropenia were more frequent in the DS-based therapy than in the non-DS-based therapy in all grades of adverse reactions. In grade 3 and higher adverse events, leukopenia, neutropenia, and oral mucositis were more common in the DS-based therapy than in the non-DS-based therapy. Diarrhea was less frequently observed in the DS-based therapy than in the non-DS-based therapy. Differences of adverse drug events between all above the DS-based therapy and the non-DS-based therapy were statistically significant.
In this analysis, neutropenia was more frequent for patients in the DS-based therapy group than those in the non-DS-based therapy in all grades of adverse events and ≥3 grade, which may be related to the fact that the main adverse event of docetaxel is myelosuppression. Despite the toxicity of docetaxel which may cause fatigue, anorexia, alopecia, leukopenia, and neutropenia (Koizumi, et al., 2014; Yoshida, et al., 2004; Yoshida, et al., 2006), all adverse events including febrile neutropenia (2%) were within the tolerable range in patients in our included studies, and no treatment-related deaths were reported (Fujitani, et al., 2014; Yoshida, et al., 2019). In the V325 study, for AGC patients approved to be treated with docetaxel plus cisplatin/5-fluorouracil in Western countries, the incidence rate of severe neutropenia was 82% (Van Cutsem, et al., 2006). Many modifications of docetaxel plus cisplatin/5-fluorouracil can be used with superiority in safety (Inal, et al., 2012; Koizumi, et al., 2014; Lorenzen, et al., 2007; Roth, et al., 2007; Tebbutt, et al., 2010). For management of grade 3 and higher hematologic adverse events, such as leukopenia and neutropenia, recombinant-human granulocyte colony-stimulating factor could be applied; therefore, these severe hematologic adverse events could be controlled and handled (Bian, et al., 2019).
Although meta-analysis found positive results of efficacy, for all efficacy outcome measurements considered in this work, the findings of TSA analysis suggest that the false positivity cannot be ruled out; thus, more original studies and updated meta-analysis are needed for further validation.
This study has certain limitations. First, the tumor stage was not analyzed as a potential confounding factor, and the efficacy and adverse effects were not analyzed by different stages of gastric cancer. This should be further explored in stage-stratified subgroup analyses when more data from additional RCTs become available. Second, the chemotherapy regimens used in the control groups of the included studies were heterogeneous, and different DS-based regimens were considered a common strategy while the possible differences were left unaddressed. Further head-to-head comparisons with increased number of trials and sample size, as well as network meta-analysis differentiating various treatment options, are warranted to validate our findings. Nevertheless, our study provides new insights for clinical management of gastric cancer.
DS-based therapy significantly improves the patients’ clinical outcome in gastric cancer, albeit at the cost of increased toxicity. Further RCTs are needed to confirm the efficacy superiority of DS-based regimens.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Study design: LB; data collection/analysis: H-FL and L-FQ; data interpretation: R-ZR and X-PJ; manuscript preparation: H-FL; manuscript revision: F-YZ; approval of the final version. All authors contributed to the article and approved the submitted version.
This study was supported by the following funding sources: the Open fund project of Hubei Provincial Key Laboratory of Occurrence and Intervention of Rheumatic Diseases (Hubei Minzu University) (PT022216), the Health Commission of Hubei Province scientific research project (WJ 2021M259), and the National Natural Science Foundation of Hubei Province (2022CFB808).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bang, Y. J., Kim, Y. W., Yang, H. K., Chung, H. C., Park, Y. K., Lee, K. H., et al. (2012). Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet (London, Engl. 379 (9813), 315–321. doi:10.1016/S0140-6736(11)61873-4
Bian, N. N., Wang, Y. H., and Min, G. T. (2019). S-1 combined with paclitaxel may benefit advanced gastric cancer: evidence from a systematic review and meta-analysis. Int. J. Surg. Lond. Engl. 62, 34–43. doi:10.1016/j.ijsu.2018.11.010
Cristescu, R., Lee, J., Nebozhyn, M., Kim, K. M., Ting, J. C., Wong, S. S., et al. (2015). Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 21 (5), 449–456. doi:10.1038/nm.3850
Cunningham, D., Starling, N., Rao, S., Iveson, T., Nicolson, M., Coxon, F., et al. (2008). Capecitabine and oxaliplatin for advanced esophagogastric cancer. N. Engl. J. Med. 358 (1), 36–46. doi:10.1056/NEJMoa073149
DeMets, D. L., and Lan, K. K. (1994). Interim analysis: the alpha spending function approach. Statistics Med. 13 (13-14), 1341–1352. doi:10.1002/sim.4780131308
Fujitani, K., Tamura, S., Kimura, Y., Tsuji, T., Matsuyama, J., Iijima, S., et al. (2014). Three-year outcomes of a phase II study of adjuvant chemotherapy with S-1 plus docetaxel for stage III gastric cancer after curative D2 gastrectomy. Gastric cancer official J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 17 (2), 348–353. doi:10.1007/s10120-013-0273-7
Gyurkovska, V., and Ivanovska, N. (2016). Distinct roles of TNF-related apoptosis-inducing ligand (TRAIL) in viral and bacterial infections: from pathogenesis to pathogen clearance. Inflamm. Res. official J. Eur. Histamine Res. Soc. [et al] 65 (6), 427–437. doi:10.1007/s00011-016-0934-1
Inal, A., Kaplan, M. A., Kucukoner, M., and Isikdogan, A. (2012). Docetaxel and Cisplatin Plus Fluorouracil compared with Modified Docetaxel, Cisplatin, and 5-Fluorouracil as first-line therapy for advanced gastric cancer: a retrospective analysis of single institution. Neoplasma 59 (2), 233–236. doi:10.4149/neo_2012_030
Jeung, H. C., Rha, S. Y., Im, C. K., Shin, S. J., Ahn, J. B., Yang, W. I., et al. (2011). A randomized phase 2 study of docetaxel and S-1 versus docetaxel and cisplatin in advanced gastric cancer with an evaluation of SPARC expression for personalized therapy. Cancer 117 (10), 2050–2057. doi:10.1002/cncr.25729
Kakeji, Y., Yoshida, K., Kodera, Y., Kochi, M., Sano, T., Ichikawa, W., et al. (2022). Three-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 plus docetaxel versus S-1 alone in stage III gastric cancer: JACCRO GC-07. Gastric cancer official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 25 (1), 188–196. doi:10.1007/s10120-021-01224-2
Kang, Y. K., Kang, W. K., Shin, D. B., Chen, J., Xiong, J., Wang, J., et al. (2009). Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Annals of oncology official journal of the European Society for Medical Oncology 20 (4), 666–673. doi:10.1093/annonc/mdn717
Kang, Y. K., Yook, J. H., Park, Y. K., Lee, J. S., Kim, Y. W., Kim, J. Y., et al. (2021). PRODIGY: a phase III study of neoadjuvant docetaxel, oxaliplatin, and S-1 plus surgery and adjuvant S-1 versus surgery and adjuvant S-1 for resectable advanced gastric cancer. Journal of clinical oncology official journal of the American Society of Clinical Oncology 39 (26), 2903–2913. doi:10.1200/JCO.20.02914
Kim, S., Lim, D. H., Lee, J., Kang, W. K., MacDonald, J. S., Park, C. H., et al. (2005). An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. International journal of radiation oncology, biology, physics 63 (5), 1279–1285. doi:10.1016/j.ijrobp.2005.05.005
Koizumi, W., Kim, Y. H., Fujii, M., Kim, H. K., Imamura, H., Lee, K. H., et al. (2014). Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). Journal of cancer research and clinical oncology 140 (2), 319–328. doi:10.1007/s00432-013-1563-5
Koizumi, W., Narahara, H., Hara, T., Takagane, A., Akiya, T., Takagi, M., et al. (2008). S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. The Lancet Oncology 9 (3), 215–221. doi:10.1016/S1470-2045(08)70035-4
Kurokawa, Y., Matsuyama, J., Nishikawa, K., Takeno, A., Kimura, Y., Fujitani, K., et al. (2021). Docetaxel plus S-1 versus cisplatin plus S-1 in unresectable gastric cancer without measurable lesions: a randomized phase II trial (HERBIS-3). Gastric cancer official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 24 (2), 428–434. doi:10.1007/s10120-020-01112-1
Lee, C. K., Jung, M., Kim, H. S., Jung, I., Shin, D. B., Kang, S. Y., et al. (2019). S-1 based doublet as an adjuvant chemotherapy for curatively resected stage III gastric cancer: results from the randomized phase III POST trial. Cancer research and treatment 51 (1), 1–11. doi:10.4143/crt.2018.028
Lee, J., Lim, D. H., Kim, S., Park, S. H., Park, J. O., Park, Y. S., et al. (2012). Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. Journal of clinical oncology official journal of the American Society of Clinical Oncology 30 (3), 268–273. doi:10.1200/JCO.2011.39.1953
Lee, K. W., Kim, B. J., Kim, M. J., Han, H. S., Kim, J. W., Park, Y. I., et al. (2017). A multicenter randomized phase II study of docetaxel vs. Docetaxel plus cisplatin vs. Docetaxel plus S-1 as second-line chemotherapy in metastatic gastric cancer patients who had progressed after cisplatin plus either S-1 or capecitabine. Cancer research and treatment 49 (3), 706–716. doi:10.4143/crt.2016.216
Lorenzen, S., Hentrich, M., Haberl, C., Heinemann, V., Schuster, T., Seroneit, T., et al. (2007). Split-dose docetaxel, cisplatin and leucovorin/fluorouracil as first-line therapy in advanced gastric cancer and adenocarcinoma of the gastroesophageal junction: results of a phase II trial. Annals of oncology official journal of the European Society for Medical Oncology 18 (10), 1673–1679. doi:10.1093/annonc/mdm269
Macdonald, J. S., Smalley, S. R., Benedetti, J., Hundahl, S. A., Estes, N. C., Stemmermann, G. N., et al. (2001). Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. The N. Engl. journal of medicine 345 (10), 725–730. doi:10.1056/NEJMoa010187
Maeda, S., Sugiura, T., Saikawa, Y., Kubota, T., Otani, Y., Kumai, K., et al. (2004). Docetaxel enhances the cytotoxicity of cisplatin to gastric cancer cells by modification of intracellular platinum metabolism. Cancer science 95 (8), 679–684. doi:10.1111/j.1349-7006.2004.tb03329.x
Miladinovic, B., Hozo, I., and Djulbegovic, B. (2013). Trial sequential boundaries for cumulative meta-analyses. The Stata Journal. 13 (1), 77–91. doi:10.1177/1536867x1301300106
Pietrantonio, F., Caporale, M., Morano, F., Scartozzi, M., Gloghini, A., De Vita, F., et al. (2016). HER2 loss in HER2-positive gastric or gastroesophageal cancer after trastuzumab therapy: implication for further clinical research. International journal of cancer 139 (12), 2859–2864. doi:10.1002/ijc.30408
Roth, A. D., Fazio, N., Stupp, R., Falk, S., Bernhard, J., Saletti, P., et al. (2007). Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic treatment for advanced gastric carcinoma: a randomized phase II trial of the Swiss Group for Clinical Cancer Research. Journal of clinical oncology official journal of the American Society of Clinical Oncology 25 (22), 3217–3223. doi:10.1200/JCO.2006.08.0135
Sakuramoto, S., Sasako, M., Yamaguchi, T., Kinoshita, T., Fujii, M., Nashimoto, A., et al. (2007). Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. The N. Engl. journal of medicine 357 (18), 1810–1820. doi:10.1056/NEJMoa072252
Sasako, M., Sakuramoto, S., Katai, H., Kinoshita, T., Furukawa, H., Yamaguchi, T., et al. (2011). Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. Journal of clinical oncology official journal of the American Society of Clinical Oncology 29 (33), 4387–4393. doi:10.1200/JCO.2011.36.5908
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA a cancer journal for clinicians 71 (3), 209–249. doi:10.3322/caac.21660
Takahashi, I., Emi, Y., Kakeji, Y., Uchida, J., Fukushima, M., and Maehara, Y. (2005). Increased antitumor activity in combined treatment TS-1 and docetaxel. A preclinical study using gastric cancer xenografts. Oncology 68 (2-3), 130–137. doi:10.1159/000086767
Tebbutt, N. C., Cummins, M. M., Sourjina, T., Strickland, A., Van Hazel, G., Ganju, V., et al. (2010). Randomised, non-comparative phase II study of weekly docetaxel with cisplatin and 5-fluorouracil or with capecitabine in oesophagogastric cancer: the AGITG ATTAX trial. British journal of cancer 102 (3), 475–481. doi:10.1038/sj.bjc.6605522
Van Cutsem, E., Moiseyenko, V. M., Tjulandin, S., Majlis, A., Constenla, M., Boni, C., et al. (2006). Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. Journal of clinical oncology official journal of the American Society of Clinical Oncology 24 (31), 4991–4997. doi:10.1200/JCO.2006.06.8429
Wada, Y., Yoshida, K., Suzuki, T., Mizuiri, H., Konishi, K., Ukon, K., et al. (2006). Synergistic effects of docetaxel and S-1 by modulating the expression of metabolic enzymes of 5-fluorouracil in human gastric cancer cell lines. International journal of cancer 119 (4), 783–791. doi:10.1002/ijc.21879
Yamada, Y., Boku, N., Mizusawa, J., Iwasa, S., Kadowaki, S., Nakayama, N., et al. (2019). Docetaxel plus cisplatin and S-1 versus cisplatin and S-1 in patients with advanced gastric cancer (JCOG1013): an open-label, phase 3, randomised controlled trial. The lancet Gastroenterology and hepatology 4 (7), 501–510. doi:10.1016/S2468-1253(19)30083-4
Yoshida, K., Hirabayashi, N., Takiyama, W., Ninomiya, M., Takakura, N., Sakamoto, J., et al. (2004). Phase I study of combination therapy with S-1 and docetaxel (TXT) for advanced or recurrent gastric cancer. Anticancer research 24 (3b), 1843–1851.
Yoshida, K., Kodera, Y., Kochi, M., Ichikawa, W., Kakeji, Y., Sano, T., et al. (2019). Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: interim analysis of JACCRO GC-07, a randomized controlled trial. Journal of clinical oncology official journal of the American Society of Clinical Oncology 37 (15), 1296–1304. doi:10.1200/JCO.18.01138
Yoshida, K., Ninomiya, M., Takakura, N., Hirabayashi, N., Takiyama, W., Sato, Y., et al. (2006). Phase II study of docetaxel and S-1 combination therapy for advanced or recurrent gastric cancer. Clinical cancer research an official journal of the American Association for Cancer Research 12 (11 Pt 1), 3402–3407. doi:10.1158/1078-0432.CCR-05-2425
Zhu, Y., Zhang, Z., and Zou, X. (2020). Comparative study of tegafur gimeracil and oteracil potassium (TS-1) plus docetaxel and oxaliplatin versus TS-1 plus oxaliplatin in postoperative adjuvant chemotherapy for gastric cancer. International Journal of Clinical and Experimental Medicine 13 (4), 2527–2533.
Keywords: chemotherapy, DS-based regimen, meta-analysis, clinical outcome, adverse event
Citation: Lv H-F, Qin L-F, Ran R-Z, Jiang X-P, Zhao F-Y and Li B (2024) Efficacy and safety of docetaxel plus S-1-based therapy in gastric cancer: a quantitative evidence synthesis of randomized controlled trials. Front. Pharmacol. 14:1242548. doi: 10.3389/fphar.2023.1242548
Received: 19 June 2023; Accepted: 13 December 2023;
Published: 08 January 2024.
Edited by:
Sukkum Chang, Tulane University, United StatesReviewed by:
Asit Kumar Manna, The University of Utah, United StatesCopyright © 2024 Lv, Qin, Ran, Jiang, Zhao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Li, bGlib29wcGFAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.