
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 23 August 2023
Sec. Ethnopharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1242318
This article is part of the Research TopicGlobal Excellence in Ethnopharmacology: AsiaView all 10 articles
Astragali Radix (Huangqi) is mainly distributed in the Northern Hemisphere, South America, and Africa and rarely in North America and Oceania. It has long been used as an ethnomedicine in the Russian Federation, Mongolia, Korea, Kazakhstan, and China. It was first recorded in the Shennong Ben Cao Jing and includes the effects of reinforcing healthy qi, dispelling pathogenic factors, promoting diuresis, reducing swelling, activating blood circulation, and dredging collaterals. This review systematically summarizes the botanical characteristics, phytochemistry, traditional uses, pharmacology, and toxicology of Astragalus to explore the potential of Huangqi and expand its applications. Data were obtained from databases such as PubMed, CNKI, Wan Fang Data, Baidu Scholar, and Google Scholar. The collected material also includes classic works of Chinese herbal medicine, Chinese Pharmacopoeia, Chinese Medicine Dictionary, and PhD and Master’s theses. The pharmacological effects of the isoflavone fraction in Huangqi have been studied extensively; The pharmacological effects of Huangqi isoflavone are mainly reflected in its anti-inflammatory, anti-tumor, anti-oxidant, anti-allergic, and anti-diabetic properties and its ability to treat several related diseases. Additionally, the medicinal uses, chemical composition, pharmacological activity, toxicology, and quality control of Huangqi require further elucidation. Here, we provide a comprehensive review of the botany, phytochemistry, traditional uses, pharmacology, toxicology, and quality control of Astragalus to assist future innovative research and to identify and develop new drugs involving Huangqi.
Astragalus L. is the largest genus in the family Leguminosae comprising approximately 2,900 species. Astragalus membranaceus (Fisch.) Bunge and Astragalus membranaceus (Fisch.) Bge. Var. mongholicus (Bge) Hsiao are used worldwide because of their high medicinal and nutritional value (Wu et al., 2018). Astragali Radix (Huangqi), the dried roots of A. membranaceus or Astragalus mongholicus, is commonly used as a herbal ethnopharmacological herb in China. Huangqi is mainly distributed in the Russian Federation, Mongolia, and China (Li et al., 2017a). The application of Huangqi can be traced back to the Han Dynasty and was first recorded in Shennong Ben Cao Jing (Han Dynasty, BCE 202–220), where it was categorized as a high-quality product. Li Shizhen’s “Compendium of the Materia Medica” (Ming Dynasty, AD 1552–1578) lists Huangqi as the first tonic herb, which mainly reinforced healthy qi, dispelling pathogenic factors, promoting diuresis, and reducing swelling. Huangqi has been prevalent for more than 2,000 years with over 200 types of herbal decoctions and has experienced extensive clinical application in Chinese medicine (Yuan et al., 2012).
The chemical composition of Astragalus is complex and mainly includes flavonoids, saponins, and polysaccharide compounds, as well as amino acids and trace elements (Wang et al., 2021a). To date, more than 200 compounds have been isolated from Astragalus species, among which isoflavones such as calycosin (CAL), calycosin-7-glucoside (CG), formononetin (FMN), and ononin (ON) have significant value because of their significant antioxidant, anticancer, anti-inflammatory, and neuroprotective pharmacological effects (Jin et al., 2014; Yu et al., 2018b). Modern pharmacological studies have verified that Huangqi has various pharmacological activities, which can improve the body’s immunity; scavenge free radicals; and exert anti-inflammatory, anti-tumor, anti-diabetic, and antioxidant effects (Wu et al., 2016). The aqueous extracts of Huangqi are often used separately or in combination with other drugs to expand the range of its medicinal effects. For example, the combined use of Astragalus and Angelica in Angelica blood tonic soup can improve the deficiency of both qi and blood (Ning et al., 2002). In addition, Huangqi is rich in Astragalus polysaccharides (Shi et al., 2014; Xue et al., 2015), which can treat severe acute respiratory syndrome coronavirus-2 (SARS-CoV2) infection. The combination of Huangqi and Lonicera japonica Thunb. has exhibited significant anti-SARS-CoV2 activity (Yeh et al., 2021). Safety evaluation studies on the toxicological effects of Huangqi have also received extensive attention. The main factors responsible for its pharmacological effects are closely related to its complex chemical composition and chemical-component interactions. Moreover, its wide range of biological activities makes Huangqi an extremely valuable medicinal resource.
As a rare botanical, Huangqi has attracted much attention because of its unique medicinal value and health effects. At present, the research on Huangqi mainly focuses on its chemical composition and pharmacological activity. However, there is a lack of comprehensive and up-to-date information about Huangqi. In this study, the literature on Huangqi since 1983 was collected, and the duplicated and irrelevant literature was removed. This review systematically summarized the literature on the botany, phytochemistry, traditional uses, pharmacology, toxicology, and quality control of Huangqi. This review aims to comprehensively and objectively understand Huangqi, solve the problems in its application, explore its inherent potential, and provide new ideas for future innovative research and the search for new drugs.
Astragalus is widely distributed in the Northern Hemisphere, South America, and Africa; is rare in North America and Oceania; and is used as an ethnomedicine in the Russian Federation, Korea, Mongolia, Kazakhstan, and China (Figure 1). Astragalus membranaceus has the following morphological features: stems 60–150 cm tall, villous; leaves pinnately compound; leaflets 21–31 mm, ovate-lanceolate or elliptic, 7–30 mm long, 4–10 mm wide, white villous on both surfaces; leaf rachis villous; stipules narrowly lanceolate, 4–6 mm white villous; racemes axillary; flowers with striated bracts below; ovary hairy with an ovary stalk; pods membranous, swollen, ovate-tortuous, long-stalked, black pubescent (Figures 2B, D).

FIGURE 2. (A) A. mongholicu above ground parts; (B) A. membranaceus above ground parts; (C) A. mongholicus dry roots; (D) A. membranaceus dry roots.
Astragalus mongholicus is smaller than the original variety, with smaller leaflets (5–10 mm long and 3–5 mm wide) and glabrous pods and grows in an environment similar to that of A. membranaceus, such as sunny grasslands, thickets, and mountain slopes. The roots of these species can be used as a medicine and make a strong tonic that nourishes the kidneys, tonifies the spleen, prevents sweating, expels excess water, and eliminates swelling and pus. A. mongholicus grows on forest edges, thickets, sparse forests, meadows, and mountain slopes and is one of the most commonly used Chinese herbs (Figure 2A, C). Astragalus flowers in June–August and produces fruits in July–September. Good quality Astragalus plants are harvested at 4–5 years of age; transplanted seedlings can be harvested after 3 years. Plants can be harvested in autumn (August–September), after the branches and leaves wither, or in spring (March–April), before the plant sprouts. Plants are dug out after removing the soil and the stems, seedlings, and roots are cut off and dried in the Sun until they have dried by 60% or 70%. Then, they are arranged into small bundles stacked up together, natural pan sugar is added, sundried until soft, rubbed by hand, and then sundried completely (Sun, 2015). Research has revealed that the best harvesting period for Astragalus is from late October to mid-November when it has the highest yield, the best traits, and the best quality. The best time to harvest Huangqi is on a sunny day, and the entire root should be dug deeply to prevent the quality from being reduced through the breakage of the main root. The cleaning method of Huangqi greatly influences the content of the active ingredients, such as astragaloside IV and GC (Tang, 2022).
To date, more than 200 compounds have been isolated from Astragalus species, including flavonoids, triterpenoids, polysaccharides, amino acids, alkaloids, ß-sitosterol, metalloids, and anthraquinones Supplementary Tables S1, S2. Among these, flavonoids and triterpenoids are the most abundant and polysaccharides, isoflavonoids, and triterpenoid saponins are the main active compounds of Huangqi responsible for its various pharmacological properties; these chemical components have been extensively studied (Song et al., 2007).
More than 100 flavonoid compounds have been isolated from A. membranaceus, with isoflavones, flavonoids, isoflavanes, and pterocarpans as the four major groups (Zhang et al., 2021a). Based on their structures, flavonoids can be divided into flavonols, flavones, flavanones, flavanols, anthocyanins, isoflavones, dihydro flavonols, and chalcones. Isoflavones are the most abundant among them, accounting for 80% of the total flavonoid content and are the signature Huangqi flavonoid compound (Huang et al., 2009). FMN and CAL, two important isoflavones in Huangqi, have been widely studied for their multiple pharmacological functions (Gao et al., 2014). CG has been demonstrated to possess various pharmacological activities, including antioxidant, anti-inflammatory, and neuroprotective activities (Choi et al., 2007; Jian et al., 2015). More importantly, CG has been described as a chemical indicator for the quality control of Huangqi in the Chinese Pharmacopoeia (2020).
Astragalus is rich in saponins, with triterpene saponins being the unique bioactive compounds. Saponins such as astragaloside I-VIII, isoastragaloside I-II, acetyl astragaloside, and soy saponin l and more than 100 triterpenoids have been isolated from Astragalus (Kitagawa et al., 1983a; Kitagawa et al., 1983b; Wang et al., 1983; Guo et al., 2019). Further, several cyclohexane-type tetracyclic triterpenes and oleanolane-type pentacyclic triterpenes, which are triterpene glycosides that contain a 30 carbon-atom skeleton, have been isolated from Astragalus. Astragalosides I, II, and IV are the most abundant saponins isolated from Astragalus roots. Astragaloside IV, which has significant pharmacological activities, has been studied extensively and described as one of the important indicators for the quality control of Huangqi in the Chinese Pharmacopoeia (Choi et al., 2007; Qi et al., 2008).
More than 30 types of Astragalus polysaccharide, one of the main components in Huangqi, have been isolated from Astragalus, which are mainly divided into dextrans and heteropolysaccharides (Guo et al., 2019). Additionally, rhamnose, xylose, glucose, galactose, mannose, and alcohol-soluble polysaccharide (ASP) have been isolated from Astragalus. Alcohol-soluble polysaccharide is a neutral polysaccharide composed of mannose, glucose, galactose, and arabinose with pyranose rings and α-glycosidic bonds (Yu et al., 2018a). Recently, a new soluble sugar named APS4 has been isolated from Astragalus. The average molecular weight of APS4, composed of rhamnose, arabinose, xylose, mannose, and galactose, is approximately 1.5 × 103 kDa, as revealed using high-performance gel permeation chromatography. APS4 has been demonstrated to have potential applications in cancer therapy (Yu et al., 2019).
Astragalus species also contain molybdenum, copper, manganese, scandium, rubidium, selenium, chromium, cobalt, cesium, iron, zinc, and more than 20 trace elements; however, the iron, manganese, zinc, and aluminum contents are higher than those of the others (Fu et al., 2014). In addition, Astragalus species contains 20 amino acids, including arginine, aspartic acid, asparagine, proline, and alanine (Shao et al., 2004). Further, other compounds such as folic acid, palmitic acid, bitter elements, coumarin, chloric acid, coumaric acid, choline, linolenic acid, legume sterols, ferulic acid, isoferulic acid, hydroxyphenyl acrylic acid, deerskinol, betaine, caffeic acid, linoleic acid, and β-sitosterol have also been identified from Astragalus species.
Huangqi is named so because of its yellow color and significant tonic potential. Huangqi contains various active ingredients and thus has a wide range of pharmacological effects, playing an important role in traditional Chinese medicine. Huangqi supplements the qi, solidifies the surface, benefits water, supports toxins, and generates muscles. As a traditional Chinese medicine, it is mainly used for treating spleen and stomach weakness, qi deficiency and blood withdrawal, qi deficiency and edema, chronic nephritis, ulcers, or ulcers that remain uncured. In China, it is known as the “Little Ginseng of Northeast China” (Napolitano et al., 2013). The Dictionary of Traditional Chinese Medicine records that Huangqi is taken in a decoction of 9–15 g. Large doses include 30–60 g (Li, 2005). It is suitable for stir-baking with an adjuvant to tonify and replenish the middle qi and used raw to secure the exterior, induce diuresis, and expel toxins. Huangqi has been used clinically in a variety of classical prescriptions. Related formulations of Huangqi with other herbs are shown in Table 1.
Huangqi has a long history and is widely used in classical formulations. Huangqi was first recorded as a high grade herb in the Shennong Ben Cao Jing 《神农本草经》 (Han Dynasty, BCE 202–220), and Li Shizhen’s Compendium of Materia Medica 《本草纲目》 (Ming Dynasty, AD 1552–1578) lists Huangqi as the leading tonic medicine. In Zhang Zhongjing’s the Golden Chamber 《金匮要略》 (Eastern Han Dynasty, AD 200–210), the dosage and preparation of different medicinal formulations are described in detail, and Huangqi is mentioned in eight of these formulations: Huangqi Gui Zhi Wuwu Tang, Huangqi Jianzhong Tang, Fangji Huangqi Tang, Fangji Gui Zhi Tang, Wutou Tang, Gui Zhi Tang, Huangqi Peony Bitter Wine Tang, and Qianjin Sanhuang Tang.However, there are no records of prescribing Huangqi for febrile diseases 《伤寒论》 (Dong Han Dynasty, AD 25–220). In the Secret Record of the Chamber of Orchids 《兰室秘藏》 (Yuan Dynasty, AD 1115–1368), written by Li Dong Yuan, Huangqi is mentioned 19 times as a tonifying agent of the spleen (Liu et al., 2022). In Nei Wai Shang Bian Huo Lun 《内外伤辩惑论》 (Yuan Dynasty, AD 1232–1247), Huangqi is mentioned 11 times in grouping frequency and nine times in combination with ginseng for its effectiveness in benefiting the qi and strengthening the spleen. Huangqi is mentioned 15 times in the Treatise on the Spleen and Stomach 《脾胃论》 (Yuan Dynasty, AD 1249) as an agent widely used to tonify the deficiencies of the spleen and stomach. Wu Jutong wrote Warm Disease Argument 《温病条辩》 (Qing Dynasty, A.D. 1644–1911), where in addition to borrowing Qingshu Yiqi Tang and Buzhong Yiqi Tang and formulating his own formula, he only used the addition and subtraction of Buzhong Yiqi Tang to treat “Qi deficiency and lower trapping, the portal does not hide.” According to Wang Qingren, Huangqi is the preferred qi tonic for treating Yuan Qi deficiency. In 11 of the 33 prescriptions in the Medical Forest Correction 《医林改错》 (Qing Dynasty, AD 1830), Huangqi has been mentioned as the most abundant agent (Sha, 2014). Jing Yue advocated warm tonicity and proposed the idea of “Yang Fei You Yu.” He used qi tonicity in several formulas, including Huangqi in 42 of the 132 formulas (Yi, 2013). In the Orthodox Manual of External Medicine 《外科正宗》 (Ming Dynasty, AD 1617), Huangqi appears 10 times among 32 main treatment formulas for swollen ulcers. Chen Yuren believed that swollen ulcers were caused by “weakness of the spleen and stomach and weakened Yang Qi,” and Huangqi was used in the formulas to nourish qi deficiency and strengthen the spleen (Zhao et al., 2018). Huangqi appears 25 times as a tonic for deficiency, thirst, sores, and fractures in the Prescription of Peaceful Benevolent Dispensary 《太平惠民和剂局方》 (Ming Dynasty, A.D. 1078–1085), written by the Song Dynasty Hodong Bureau (Wang, 2021).
In addition to the studies on Huangqi in classical medicine, it has been extensively studied recently. In Records of Chinese Medicine with Reference to Western Medicine《医学衷中参西录》, Zhang Xichun mentioned Huangqi 35 times for its tonic power that promotes myogenesis, solid qi, diuresis and prevents the collapse of the belt. He also created four formulas of Shengxian Tang, all with Huangqi as the leading substance, that tonify and increase the qi and treat qi trapped in the chest. Zhang Xichun believed that some drugs need to be used raw to obtain the complete benefits of the medicine, and heating weakens concoctions, making them ineffective or may even cause them to have the opposite effect. Thus, we believe that with the increased understanding of the pharmacology and pharmacological effects of Huangqi, its therapeutic effects have also enriched and improved.
The medicinal component of Astragalus is its dried root. Modern pharmacological studies have shown that Huangqi has a wide range of immunological activities and is widely used as an immunostimulant, antioxidant, hepatoprotectant, diuretic, and expectorant. In recent years, astragalus isoflavones have been widely used because of their anti-inflammatory, anti-tumor, treatment of heart diseases, treatment of neurological diseases, anti-diabetic, and Anti-oxidant effects This review discusses the pharmacological effects of the isoflavone compounds in Astragalus Figure 3 and Table 2 to assist further scientific research.
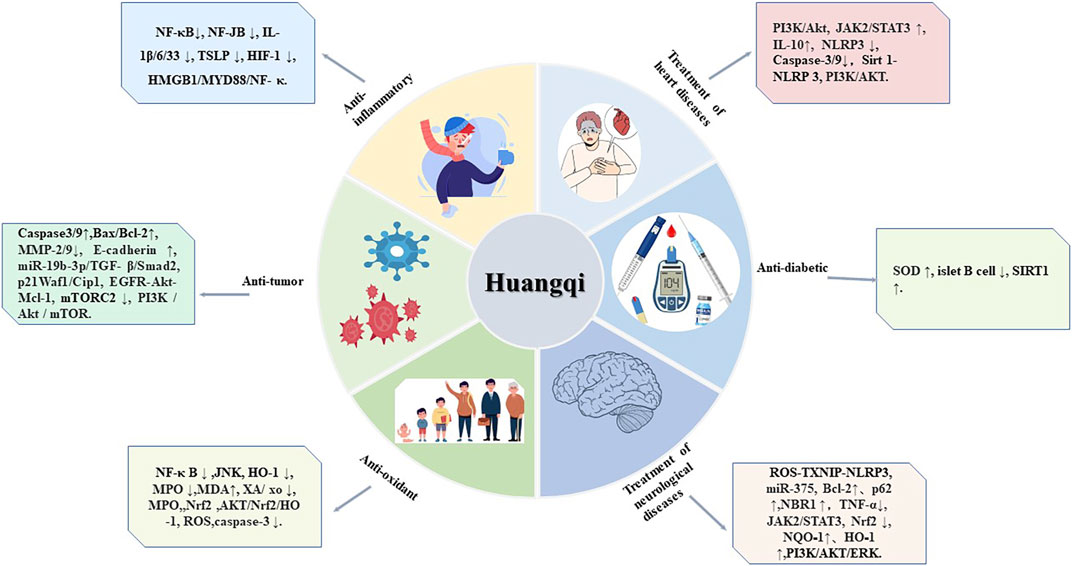
FIGURE 3. Six pharmacological effects of Huangqi. This figure shows the most highlighted six effects in studies on Huangqi.
Inflammation is a defensive response of the body to the external stimuli and is characterized by redness, swelling, fever, pain, and dysfunction. Bacteria such as rickettsiae, mycoplasmas, spirochetes, fungi, and parasites are the most common causes of inflammation (Yang et al., 2021). Modern pharmacology has demonstrated that Astragalus isoflavones have anti-inflammatory effects, the main substances of which are FMN and CAL. Yu Ping Feng San (YPFS) is a traditional Chinese medicinal decoction widely used to treat atopic dermatitis (AD). The active ingredients CAL and FMN extracted from YPFS can reduce epidermal thickening at the initial stage of sensitization alone, and they inhibit thymic stromal lymphopoietin (TSLP) by regulating nuclear factor kappa B (NF-κB) activation and translocation, thereby reducing allergic inflammation. This confirms the anti-inflammatory activity of FMN and CAL(Shen et al., 2014).
Numerous anti-inflammatory studies have demonstrated that drugs usually exert their anti-inflammatory effects by modulating the expression of nuclear factors and κB inhibitors such as NF-κB, Interleukin (IL-1β/6/33), tumor necrosis factor (TNF), Mitogen-activated protein kinase (MAPK), thymic stromal lymphopoietin (TSLP), and hypoxia inducible factor-1 (HIF-1α). A study administered FMN to fluorescein isothiocyanate (FITC)-induced AD mice and FITC-treated HaCaT cells followed by polyinosinic: polycytidylic acid or lipopolysaccharide treatment and reported that TSLP/IL-33 levels were reduced in vitro and in vivo whereas E-calcine mucin levels were increased in vitro (Li et al., 2018). This may be because FMN reduces TSLP/IL-33 production while alleviating the inflammatory response by regulating E-calcine mucin. Moreover, FMN can alleviate AD by promoting the upregulation of tumor necrosis factor alpha-inducible protein 3 (A20) expression by siGPER. FMN significantly increases the expression of A20 protein and mRNA while suppressing the expression of TSLP protein and mRNA (Yuan et al., 2021). FMN inhibits the production of inflammatory mediators and cytokines in osteoarthritis (AO), as well as the expression of cyclooxygenase-2 and nitric oxide synthase, thereby inhibiting the synthesis of matrix metalloproteinases (MMPs) and thrombomodulin. This mechanism involves the activation of phosphatases and the inhibition of IL-1β-induced activation of NF-κB and protein kinase B (AKT) (Jia et al., 2022). CAL has been found to ameliorate lung injury and inflammatory response in mice with pneumonia caused by respiratory syncytial virus infection. The mechanism may be related to the inhibition of NF⁃κB signaling pathway activation. CAL acts on AO by inhibiting IL-1β protein-induced activation of PI3K/AKT/FoxO1 signaling (Guo et al., 2022); it can mitigate sepsis-induced acute lung injury through the HMGB1/MyD88/NF-κB pathway and activation of NLRP3 inflammatory vesicles (Chen et al., 2021). HIF-1α may be a therapeutic target in AD when CAL is used to treat AD. CAL can inhibit HIF-1 α expression both in vivo and in vitro; it downregulates HIF-1 α expression in HaCaT cells to repair tight junctions and reduce allergic inflammation (Jia et al., 2018).
Tumors arise from the proliferation of local tissue cells affected by various tumorigenic factors in the body. Tumors are classified as benign and malignant, and cancer is a type of malignant tumor that originates from epithelial tissue. According to the studies conducted in the last decade, FMN and CAL can treat oncological diseases, including lung (Yang et al., 2014), breast (Yu et al., 2017), colorectal (Hu et al., 2023), ovarian (Yao et al., 2019), and gastric cancers (Zhou et al., 2015), via various molecular pathways. Their mechanism of anti-tumor action includes inhibition of cell proliferation, influence on the cell cycle, and induction of apoptosis.
The four isoflavone extracts of Huangqi, GC, CAL, FMN, and ON, have been found to inhibit the proliferation of SK-BR-3, MCF-7, and MDA-MB-231 cells in a dose-dependent manner, as well as to decrease the levels of p-GS3K β, p-PI3K, p-Akt, and p-mTOR and substantial increase total mTOR levels (Zhou et al., 2018). Additionally, CAL can regulate the circ_0001946/miR-21/GPD1L/HIF-1α signaling axis in a dose-dependent manner. miR-21 is the most recognized and significant miRNA associated with carcinogenesis and is involved in the pathogenesis of many cancers (Huang et al., 2013). CAL downregulates miR-21 at circ_0001946 and GPD1L levels and upregulates HIF-1α levels in lung adenocarcinoma cells, thereby inhibiting cell proliferation, invasion, migration, and epithelial-mesenchymal transitions (EMT) processes (Zhou et al., 2018). Extracellular regulatory protein kinase 1/2 (ERK1/2) can enter the nucleus to promote the transcription and expression of certain genes and is closely related to cell proliferation and differentiation. FMN can act by inhibiting the ERK1/2 pathway and inactivating laminin A/C in nasopharyngeal carcinoma (NPC) cells. Further, B-cell lymphoma-2 (Bcl-2), ERK1/2, laminin A/C, and CK19 expressions have been found to be downregulated in FMN-treated NPC CNE2 cells, whereas intracellular Bax expression is elevated, indicating an inhibition of cell proliferation (Ying et al., 2019).
CAL can inhibit breast cancer cell growth by regulating AKT signaling pathway, inducing the activation of MAPK, STAT3, NF-κB, and related apoptotic proteins and reducing the expression levels of TGF-β1, SMAD2/3, and SLUG to arrest the cell cycle in G0/G1 phase (Zhou et al., 2018). CAL inhibits Bcl-2 expression and promotes Bax, caspase-3, PARP, TGF-β1, SMAD2/3, and SLUG expressions by blocking the growth of hepatocellular carcinoma BEL-7402 cell line in G0/G1 phase. In addition, CAL induces MAPK, STAT3, NF-κB, and related apoptotic proteins in HepG2 hepatocellular carcinoma cells by regulating AKT pathway protein activation to induce G0/G1 phase cell cycle arrest (Liu et al., 2021b). Furthermore, FMN inhibits colon cancer (SW1116 and HCT116) cell growth through miR-149-induced downregulation of EphB3 and inhibition of PI3K/AKT and STAT3 signaling pathways to downregulate cell cycle-associated protein Cyclin D1 expression and block the cell cycle at the G0/G1 point (Wang et al., 2018a). FMN induces G1 phase arrest in MCF-7, SK-BR-3, and MDAMB-231 breast cancer cells by downregulating the expression of Cyclin D1 and Cyclin E and negatively regulating the expression of P21 and P27 (Zhou et al., 2016).
FMN has been shown to alleviate ovarian cancer in SKOV-3 cells by increasing E-cadherin expression and decreasing MMP-9 expression, which inhibits the cancer cell proliferation, migration, and invasion (Gu et al., 2020). Further studies have demonstrated that FMN causes apoptosis in SKOV-3 cells. The anti-tumor effect of FMN is achieved by regulating the miR-19b-3p/TGF-β/Smad2 signaling pathway (Niu et al., 2021). The Bcl-2 protein family significantly inhibits apoptosis, and FMN shows a dose-dependent inhibition of Bax/Bcl-2 and caspase-3/9 protein expressions in ovarian cancer cells, thereby exhibiting anti-proliferative, anti-migratory, and invasive effects. The Bax/Bcl-2 ratio has been found to increase after FMN treatment, whereas caspase-3 and caspase-9 levels are elevated (Lee et al., 2018). CAL induces p21Waf1/Cip1 cycle arrest and promotes caspase apoptosis and MIA PaCa-2 cell migration in macrophages RAW 264.7, which occurs through the induction of the Raf/MEK/ERK pathway and promotion of M2 tumor-associated macrophages acting in the tumor microenvironment (Zhang et al., 2020). CAL can reduce the viability of colorectal cancer (CRC) cells through targeted inhibition of PI3K/Akt signaling pathway and upregulation of Phosphatase gene (PTEN) protein and estrogen receptor ß (Erβ), thereby inducing CRC cell apoptosis. PTEN and ERβ protein expressions are significantly upregulated in CRC cells subjected to CAL, whereas p-AKT/AKT ratio and Bcl-2 levels are downregulated, confirming the anti-tumor effect of CAL(Zhang et al., 2021b). FMN induces apoptosis in OGS cells and inhibits the growth of solid tumors, resulting in an increase in intracellular Apaf-1 positive cells and a decrease in endogenous Ki-67, p-PI3KCATyr317, and p-AKTSer473 immune cells. The mechanism of action is related to the inactivation of miR-375/ERα-PI3K/AKT signaling pathway in cells (Hu et al., 2019).
Cardiovascular diseases account for approximately 17.5 million deaths worldwide annually, it is crucial to screen for effective therapeutic agents against these diseases (Xin et al., 2013). The most common and critical heart diseases include hypertension, coronary artery disease, and arrhythmia, which can occur independently or in combination with other heart diseases. Astragalus isoflavones have anti-apoptotic, autophagy-promoting, anti-inflammatory, and antioxidant roles in heart diseases.
CAL can inhibit cardiomyocyte apoptosis by promoting the activation of the PI3K/AKT signaling pathway, thereby reducing myocardial injury. High-dose CG pretreatment has been shown to significantly improve cardiac function in rats, with the upregulation of superoxide dismutase (SOD), Ejection Fraction (EF), fractional shortening (FS), and left ventricular end-systolic pressure and downregulation of left ventricular end-diastolic pressure and malonaldehyde (MDA). Caspase-3 and caspase-9 activities were also inhibited (Ren et al., 2016). Further studies revealed that CG may mitigate ischemia/reperfusion (I/R) injury by upregulating IL-10 to activate the JAK2/STAT3 signaling pathway (Liu et al., 2020). Using isolated heart tissues from senescent mice and chemically induced senescent H9C2 cells as experimental subjects, a study demonstrated that FMN can attenuate I/R-induced apoptosis in cells or tissues (Huang et al., 2018b). Additionally, FMN can inhibit the activation of nod-like receptor protein 3 (NLRP3) inflammasome in rats and improve IR in rats via the reactive oxygen species (ROS)-TXNIP-NLRP3 signaling pathway (Wang et al., 2020a). CAL protects the heart by eliminating histopathological changes owing to its anti-inflammatory, anti-apoptotic, antioxidant, and anti-lipid peroxidation activities. CAL may exert cardioprotective effects by modulating the Sirt1-NLRP3 pathway, thereby ameliorating adriamycin/adriamycin (DOX)-induced cardiotoxicity, reducing apoptosis and inhibiting oxidative stress. CAL may also be useful in the treatment of myocardial infarction to reduce cardiac dysfunction and its associated complications (Huang et al., 2020). CAL induces apoptosis through the Bcl-2, Bax, and PI3K-Akt signaling pathways and increases H9C2 cell viability. In addition, CAL has been shown to improve Sirt1-NLRP3 levels in cells and mouse hearts. CAL can improve cardiac function in adult zebrafish and restore autophagy through atg7 autophagy-mediated production of protection against DOX-induced cardiotoxicity (Lu et al., 2021). Zhang et al. established a cardiotoxicity model using DOX stimulation in H9C2 cells and C57BL/6J mice. The cardioprotective mechanism was confirmed using in vivo and ex vivo experiments, which showed that CAL alleviated DOX-induced cardiotoxicity by inhibiting the activation of NLRP3 inflammatory vesicles (Zhang et al., 2022).
Neurological diseases are pathological conditions that negatively affect the peripheral nervous system, spinal cord, and/or brain, ultimately leading to functional disorders (Gunata et al., 2020; Cao et al., 2023). Its etiology is complex and includes trauma, infection, genetics, tumors, immunology, and several other factors that can lead to neurological dysfunction, resulting in neurological diseases. Several studies have demonstrated the neuroprotective properties of FMN and CAL against cerebral ischemia, dementia, traumatic brain injury, Alzheimer’s disease, anxiety, and depression. The pathways involved in these neuroprotective mechanisms are ERPI3K-Akt, PI3K/AKT/ERK, and ROS-TXNIP-NLRP3 pathways. The neuroprotective effect of five Astragalus isoflavone compounds on xanthine (XA)/xanthine oxidase (XO)-induced damage in PC12 cells has been investigated. The reduction of SOD, antioxidant glutathione peroxidase, and enzymatic activities is prevented in isoflavone-treated cells; these neuroprotective effects may be produced by increasing endogenous antioxidants (Yu et al., 2009). FMN can promote the expression of NGF, GAP-43, BDNF, p-Trk A, p-Trk B, p-ERK 1/2, and p-AKT by increasing the number of neuronal dendritic spines and ß III-microtubulin, with the best effect at 30 mg/kg (Wu et al., 2020). CAL treatment in stroke patients increases brain BDNF/TrkB expression, ameliorates neurological damage, and transforms microglia from an activated amoeboid state to a resting branching state. BDNF/TrkB -mediated CAL ameliorates ischemic stroke injury in rats by switching microglia from an activated to a resting branching state (Hsu et al., 2020). The pathogenic mechanisms underlying I/R include elevated intracellular Ca2+ levels, excitatory neurotransmitter release, oxidative stress, inflammation, and apoptosis (Durukan and Tatlisumak, 2007). CAL has neuroprotective effects in I/R rats, significantly reducing the brain water content and improving neurological deficits. The mechanism of action may be related to the positive feedback regulation of miR-375 through ER-α (Wang et al., 2014b). The effect of CAL on I/R may be related to its anti-autophagic, anti-apoptotic, and anti-inflammatory activities. A study established an I/R rat model with middle cerebral artery occlusion and reported that CAL pretreatment for 14 days significantly reduced brain edema and improved neurological function in I/R rats, as well as significantly upregulated the expression of Bcl-2, p62, and NBR1 and downregulated the level of tumor necrosis factor alpha (TNF-α) (Wang et al., 2018b). FMN action in I/R rats reduces ASC, p-STAT3, p-JAK2, NLRP3, cl-IL-1β, and cl-caspase-1 protein levels in the brain tissue of rats with infarct volume. The neuroprotective effect of FMN is achieved through the inhibition of the JAK2/STAT3 signaling pathway (Yu et al., 2022). FMN reduces hippocampal neuronal damage and oxidative stress in rats, improves depression-like behavior in rats with mild stress (CUMS)-induced depression, and reverses the CUMS-induced decrease in nuclear factor erythroid-2-related factor 2 (Nrf2) protein and increase in NQO⁃1 and HO⁃1 proteins in the nucleus (Yao et al., 2022). CAL may also be effective against cerebral hemorrhage-induced injury by inhibiting oxidative damage and inflammatory responses, and 50 mg/kg CAL has been shown to significantly inhibit ischemic brain injury. Lesion volume, blood volume, and hemispheric enlargement are significantly reduced after CAL treatment. CAL likely inhibits oxidative stress by enhancing the Nrf2 antioxidant pathway and suppresses the inflammatory response by blocking the activation of NACHT, NALP3 inflammatory vesicles, and NF-κB pathway (Chen et al., 2020). Astragalus isoflavones alleviate I/R by activating the ER-PI3K-Akt pathway, which may be a molecular target for synergistic neuroprotection by Astragalus isoflavones (Gu et al., 2021).
Diabetes is a chronic endocrine disease characterized by glucose, fat, and protein metabolism disorders caused by insulin deficiency, insulin insensitivity, or both, which can lead to the damage and dysfunction of various organs in the body (Krasteva et al., 2014). Type 1 diabetes is characterized by absolute insulin deficiency, whereas type 2 diabetes is characterized by relative insulin deficiency and insulin resistance. CAL ameliorates advanced glycation end products (AGEs)-induced impairment of hepatocyte viability and AGEs-induced dysfunction of hepatocyte glucose uptake in a dose-dependent manner (Xu et al., 2015). The combined application of FMN, CAL, and Tetrandrine has been demonstrated to be effective against hyperglycemia and hypoinsulinemia in streptozotocin (STZ)-induced diabetic mice (Ma et al., 2007); this is because FMN and CAL can enhance the hypoglycemic effect of Tetrandrine. In another study, FMN significantly reduced the fasting blood glucose levels at doses of 5, 10, and 20 mg/kg in alloxan-induced type 1 diabetes mice, indicating that FMN promotes islet B cell regeneration, insulin secretion, and liver glycogen synthesis by inhibiting islet B cell apoptosis (Qiu et al., 2016). FMN can also treat STZ-induced type 2 diabetes. Significant improvement in the fasting blood glucose levels has been observed after 40 mg/kg FMN treatment of rats, and FMN at doses of 10, 20, and 40 mg/kg can significantly reduce serum urea nitrogen, glucose, albumin, and creatinine levels. Further, FMN can significantly increase lipid peroxidation and SOD levels and reduce renal peroxidase activity, cytokine levels, inflammatory changes, and renal cell necrosis, thereby protecting pancreatic ß-cells from necrosis and degeneration (Jain et al., 2020).
Recent studies have reported that Huangqi extract has strong antioxidant activity and may act as a free radical scavenger, thereby alleviating the symptoms of oxidative stress in the early stages of diabetic nephropathy. Some studies have discovered that CAL and GC have significant anti-lipid peroxidation activity (Kim et al., 2003). FMN, CAL, and CA, isolated from Huangqi, have been found to significantly inhibit XA/XO-induced cell damage; they have significant superoxide anion and free radical (DPPH) radical scavenging abilities, which can effectively inhibit cell damage caused by XA and XO. Among these compounds, CAL has the most prominent antioxidant activity (Yu et al., 2005). Studies have reported that Huangqi extract can improve blood lipid levels, inhibit lipid peroxidation, increase the activity of antioxidant enzymes, and reduce the risk of hyperlipidemia and oxidative stress-related coronary heart disease in humans (Ma et al., 2011). In addition, the combination of CAL with gallic acid can significantly inhibit increased myeloperoxidase (MPO) activity due to isoproterenol (ISO) (Cheng et al., 2015). Oxidative stress-induced brain cell damage is an important factor in the pathogenesis of ROS-related nervous system diseases. Astrocytes are important immunocompetent brain cells that play a role in various nervous system diseases. CAL regulates oxidative stress through the AKT/Nrf2/HO-1 pathway, thereby preventing oxidative damage in brain astrocytes. CAL-treated cells exhibit enhanced viability, inhibition of ROS and inflammatory factor production, increased SOD expression, and dose-dependent inhibition of H2O2-induced damage (Lu et al., 2022). CAL was found to exert antioxidant effects by restoring SOD/CAT activity and reducing ROS content and caspase-3 activity in a Parkinson’s disease model, thereby altering α-syn amyloid-induced neurotoxicity (Pan et al., 2021). In an allergic asthma model, treatment with FMN (10, 20, and 40 mg/kg) and the positive control drug dexamethasone (2 mg/kg) decreased ROS activity and increased SOD activity increased. The oxidation-related signaling molecules involved in this action are c-Jun N-terminal kinase (JNK), NF-κB, and the transcription factor Nrf2(Yi et al., 2020).
Astragalus isoflavones also exhibit antiviral, estrogen-like, antibacterial, hepatoprotective, and immune-enhancing effects. Isoflavones have a molecular structure similar to that of estrogen and can therefore bind to estrogen receptors; hence, they are classified as phytoestrogens. CAL has a protective effect on the liver of mice with acute immune liver injury caused by concanavalin A (ConA) (Liang et al., 2018), likely because of its antioxidant effect on free radicals and the enhancement of estrogen-like effects by promoting hepatic ER expression. FMN may enhance estrogen-like effects by promoting estrogen receptor protein expression (El-Bakoush and Olajide, 2018) and exert antimicrobial effects by attenuating the cytotoxic and inflammatory response of Streptococcus suis in vitro; lysozyme could be an ideal target against this pathogen (Wang et al., 2020b). Further, FMN has anti-apoptotic and anti-inflammatory effects on the liver mainly by inhibiting the expression of TNF-α, NF-κB-p65, TLR3, and NLRP3 and upregulating Bcl-2. It also exerts anti-metabolism-related effects on fatty liver disease through lipophagy (Liu et al., 2021a). CAL exhibits hepatoprotective functions mainly by affecting the expression of STAT3, FXR, a-SMA, and ERβ5, which in turn regulates free fatty acid ß-oxidation, gluconeogenesis, triglyceride synthesis, glucose metabolism, collagen deposition, and hydroxyproline content (Duan et al., 2017; Duan et al., 2018).
Although Astragalus has been widely used in clinical practice for several years, comprehensive safety and toxicity assessments have not yet been conducted. Studies on the toxicology of Astragalus have long been of interest to researchers, especially those focusing on the therapeutic toxicity of secondary metabolites of Astragalus. Astragalus species can be classified into three main categories based on their toxic effects on animals: species that can synthesize aliphatic nitro compounds, species that can cause madder poisoning, and species that can accumulate selenium (Rios and Waterman, 1997). Toxicological studies on astragaloside have shown that it is toxic above a dose of 1.0 mL/kg to some embryos as well as mothers. However, no specific toxicities such as acute toxicity, subacute or subchronic toxicity, genotoxicity, or immunotoxicity have been observed (Jiangbo et al., 2009). The extract of Astragalus, historically recognized as a traditional medicine and food, has now been evaluated for its subchronic toxicity and genotoxic safety as a modern dietary ingredient, along with the triterpene glycosidic cyclic element astragalinol (Szabo, 2014). Rats were administered astragalol at 0, 40, 80, and 150 mg/kg/day for 91 consecutive days, but no treatment-related deaths or cardiac effects were observed. In a toxicity study on Huangqi extract, acute and subchronic oral toxicity tests were performed on rats. In acute toxicity studies, a single dose can reach up to 5,000 mg/kg. In a 13-week subchronic toxicity study based on clinical symptoms, body weight, and autopsy results, there were no deaths or toxic reactions (Song et al., 2017). Huangqi can be used for food health and consumed for a long time at standard doses. Because Astragalus species may be contaminated with pesticides or heavy metals during cultivation, leading to increased safety concerns (Jiaojiao et al., 2019) and reducing its value, it is important to control the use of pesticides and conduct soil quality testing.
In addition, the combination of Huangqi can achieve the effect of increasing efficiency and reducing toxicity. Apatinib mesylate combined with astragaloside can significantly inhibit the growth of hepatocellular carcinoma transplantation tumors in nude mice, promote the apoptosis of transplantation tumor cells, and cause inhibitory effects on the proliferation, migration, and invasion of HCC cells (Sun et al., 2023). Astragaloside (Peng et al., 2017), astragaloside (Lina et al., 2022) and doxorubicin can alleviate cardiotoxicity and improve anti-tumor effect. The combination of astragaloside and angiotensin-converting enzyme inhibitors (ACEi) can reduce the degree of proteinuria and delay the progression of diabetic kidney injury in mice (Li et al., 2021). The above experimental results indicate that the combination of Huangqi active ingredients with other drugs has shown certain advantages in basic research, but the mechanism of its efficiency and toxicity reduction needs to continue to be explored. Although the safety of Huangqi has been heavily formalized, independent studies on Huangqi are still lacking, and further in vitro and clinical trials are required for confirmation.
Misidentification and adulteration of varieties are the main problems in the identification of herbal medicines (Zhu et al., 2022). Due to non-standard market systems and market supervision and control, counterfeit and inferior Huangqi products often appear. The quality of Huangqi medicinal herbs is also influenced by different geographical locations, cultivation techniques, and climatic environments (Yang et al., 2020). Therefore, the key to the quality control of Active ingredients in Huangqi lies in the establishment of quality analysis methods.
At present, the 2020 edition of the Chinese Pharmacopoeia controls the quality of Huangqi from three aspects: morphology, microscopy, and thin-layer chromatography. It is required that the moisture content shall not exceed 10.0%, the total ash content shall not exceed 5.0%, and the leaching content shall not be less than 17.0% (Gong et al., 2018). The content of astragaloside A determined by High-performance liquid chromatography shall not be less than 0.080%, and the content of calyx Isoflavone glucoside shall not be less than 0.020%. Traditionally, High-performance liquid chromatography was used to determine the content of Huangqi. However, these methods may not be sufficient to evaluate the quality of Huangqi medicinal herbs. With the improvement of analytical technology, people have adopted other methods to determine the chemical composition of Huangqi and control its quality. For example, chromatography-mass spectrometry (LC-MS), external spectroscopy (IR), and ultraviolet spectroscopy (UV) provide effective means for quantitative analysis of the Active ingredients of Huangqi (Huang et al., 2018a).
Traditional Chinese medicine fingerprint is a comprehensive and quantifiable identification method established based on systematic research on the chemical composition of traditional Chinese medicine, used to evaluate the authenticity, stability, consistency, and effectiveness of traditional Chinese medicine. The fingerprint of traditional Chinese medicine, as a standard for quality control, has also been included in the Chinese Pharmacopoeia. Currently, the Chinese Pharmacopoeia does not include the fingerprint of Huangqi. Scholars (Wang et al., 2023) have established a UPLC fingerprint and content determination method for the stem and leaf of Mongolian Huangqi, and compared and analyzed 15 batches of Mongolian Huangqi stem and leaf samples from different regions. A rapid and effective method for evaluating the quality of Astragalus mongholicu stem and leaf has been established. DNA barcoding technology also shows broad application prospects in the identification of astragalus medicinal materials. The fingerprint determined by LC-MS combined with the ITS interval domain DNA map uses the astragalus plant genome region as the barcode, which can quickly and accurately classify the source plants, and can be used as the barcode mark for quality control of astragalus (Xiao et al., 2011). With the development of technology, quality marker (Q-marker) was proposed in 2016 (Changxiao et al., 2016), and the idea of an “effect component target fingerprint” was discovered (Liao et al., 2018) to predict and identify the quality of Chinese medicine Q-marker through network pharmacology and high-performance liquid chromatography fingerprint. Li et al. (Li et al., 2022) established a reliable analytical method combined with network pharmacology, established fingerprint spectra of 23 batches of Huangqi, successfully isolated and quantified 8 compounds, and is expected to become a new approach for quality control of Huangqi (Huang et al., 2018a). At present, the HPLC-ELSD method is mainly used for the development of fingerprints of saponins and polysaccharides; The HPLC-DAD/HPLC-UV method can be used to establish the fingerprint of flavonoids and polysaccharides.; The PLC-CAD method can be used to develop fingerprints of flavonoids and saponins (Zhen et al., 2023).
With the continuous development of technology, the quality control methods of medicinal materials are also constantly innovating. With the discovery of the pharmacological effects of the Active ingredient of Astragalus, the innovation of quality control methods and technologies of Astragalus is becoming more and more important. These methods and technologies are convenient for people to understand Chinese medicinal materials truly, quickly, and accurately, and provide a reference and basis for the quality control of the Active ingredient of Huangqi.
This review discusses the recent advances in botany, phytochemistry, traditional uses, pharmacology, toxicology, and quality control of Astragalus. Presently, several pharmacological studies have been conducted on Astragalus isoflavones, including FMN and CAL. Therefore, this review mainly focused on the pharmacological effects of isoflavones. Pharmacological studies have shown that isoflavones possess many pharmacological activities, including anti-inflammatory, anti-tumor, anti-diabetic, cardioprotective, neuroprotective, and antioxidant effects. They are also used in many other applications aspects to their diverse activities. However, despite extensive pharmacological research on Astragalus isoflavones, some problems require further discussion.
First, Astragalus has a large demand as a medicine as well food; therefore, its clinical application should be extensively investigated to avoid excessive dosage and incompatibility. Meanwhile, herbs such as Huangqi are highly popular in both China and abroad. Tea, soup, and congee have become important media for healthcare, with the research and production of healthcare products increasing. Additionally, it has been established that Huangqi has a wide range of applications in herbal healthcare, particularly in immunomodulation and regulation of blood glucose. According to the clinical pharmacological effects of Huangqi, the use of Huangqi in combination with other herbal medicines in healthcare products should not be limited to the above functions but can also be studied in the areas of auxiliary improvement of memory, sleep, growth, and development, promotion of digestion, and auxiliary protection against gastric mucosa damage.
Second, more than 200 compounds have been isolated from Astragalus species; although flavonoids and saponins have been comprehensively studied, the study of polysaccharide components of Astragalus remains limited. Some studies show that highly valuable the field of pharmacology is; in fact, pharmacological research and clinical applications are inseparable; therefore, combining pharmacological and clinical studies makes the application of Huangqi possible in many fields.
Third, in terms of pharmacological effects, recent studies on the active ingredients of Astragalus have mainly focused on FMN and CA, and studies on other active compounds and their effects are limited. In addition, most of these studies have focused on the anti-tumor and anti-inflammatory effects of FMN and CAL; however, the mechanism and target of action of the main pharmacological effects, such as anti-tumor and anti-inflammatory effects, are not fully understood. The number of samples was small, the type was single, and the pathological characteristics of different clinical patients were considered and studied. Future pharmacological research should focus on exploring active ingredients and their mechanisms of interaction with specific target ingredients, which can lay the foundation for expanding clinical applications in the future while also providing modern pharmacological interpretations of traditional applications.
Fourth, embryotoxicity and maternal toxicity have been observed above 1.0 mL/kg Astragalus methoside administration. However, the dose–effect relationship between the safety and toxicity of isoflavones, a phytoestrogen of Astragalus, has not been studied. Thus, the mechanism of action and toxicological properties of Astragalus require further investigation. Astragalus may be contaminated by pesticides and heavy metals during cultivation, leading to increased safety problems and reduced value; therefore, there is a need to control the use of pesticides and conduct soil quality tests.
In conclusion, traditional Chinese medicine, Huangqi, has a wide range of medicinal properties. In this review, we discuss the research progress on the botanical features, phytochemistry, traditional applications, pharmacology, toxicology, and quality control of Astragalus. This information can lay a theoretical foundation for the future development and new clinical applications of Huangqi.
PW wrote this paper; ZW checked the chemical structure and formula, and reviewed and edited the manuscript; ZZ, HC, and LK searched for literature, downloaded it, and classified it; WM and WR conceived and designed this review. All authors contributed to the article and approved the submitted version.
This work was supported by National Key Research and Development Program of China (2021YFD1600902), Talent training project supported by the central government for the reform and development of local colleges and Universities (ZYRCB2021008), Heilongjiang Tou yan Innovation Team Program (HLJTYTP2019001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1242318/full#supplementary-material
Aslanipour, B., Gulcemal, D., Nalbantsoy, A., Yusufoglu, H., and Bedir, E. (2017). Secondary metabolites from Astragalus karjaginii BORISS and the evaluation of their effects on cytokine release and hemolysis. Fitoterapia 122, 26–33. doi:10.1016/j.fitote.2017.08.008
Bi, Z., Yu, Q., Li, P., Lin, Y., and Gao, X. (2007). Flavonoids from the aerial parts of Astragalus mongholicus. Chin. J. Biochem. Pharm. 5 (04), 263–265.
Bian, Y., Jia, G., Bi, Z., Yue, S., and Li, P. (2006). Studies on chemical constituents of Astragalus membranaceus (fisch) Bge. Var. mongholicus (Bge) Hsiao. Chin. Pharm. J. 16, 1217–1221.
Bian, Y., and Li, P. (2008). Studies on chemical constituents of Astragalus membranaceus (fisch) Bge. Var. mongholicus (Bge) Hsiao. Chin. Pharm. J. 5 (04), 256–259.
Cao, M., Wu, J., Peng, Y., Dong, B., Jiang, Y., Hu, C., et al. (2023). Ligustri Lucidi Fructus, a traditional Chinese Medicine: comprehensive review of botany, traditional uses, chemical composition, pharmacology, and toxicity. J. Ethnopharmacol. 301, 115789. doi:10.1016/j.jep.2022.115789
Changxiao, L., Shilin, C., Xiaohe, X., Tiejun, Z., Wenbin, H., and Maoliang, L. (2016). A new concept on quality marker of Chinese materia medica: quality control forChinese medicinal products. China Tradit. Herb. Drugs 47 (09), 1443–1457.
Chen, C., Cui, J., Ji, X., and Yao, L. (2020). Neuroprotective functions of calycosin against intracerebral hemorrhage-induced oxidative stress and neuroinflammation. Future Med. Chem. 12 (7), 583–592. doi:10.4155/fmc-2019-0311
Chen, G., Hou, Y., Li, X., Pan, R., and Zhao, D. (2021). Sepsis-induced acute lung injury in young rats is relieved by calycosin through inactivating the HMGB1/MyD88/NF-κB pathway and NLRP3 inflammasome. Int. Immunopharmacol. 96, 107623. doi:10.1016/j.intimp.2021.107623
Cheng, Y., Zhao, J., Tse, H. F., Le, X. C., and Rong, J. (2015). Plant natural products calycosin and gallic acid synergistically attenuate neutrophil infiltration and subsequent injury in isoproterenol-induced myocardial infarction: a possible role for leukotriene B4 12-hydroxydehydrogenase? Oxid. Med. Cell Longev. 2015, 434052. doi:10.1155/2015/434052
Choi, S. I., Heo, T. R., Min, B. H., Cui, J. H., Choi, B. H., and Park, S. R. (2007). Alleviation of osteoarthritis by calycosin-7-O-beta-D-glucopyranoside (CG) isolated from Astragali radix (AR) in rabbit osteoarthritis (OA) model. Osteoarthr. Cartil. 15 (9), 1086–1092. doi:10.1016/j.joca.2007.02.015
Duan, X., Meng, Q., Wang, C., Liu, Z., Liu, Q., Sun, H., et al. (2017). Calycosin attenuates triglyceride accumulation and hepatic fibrosis in murine model of non-alcoholic steatohepatitis via activating farnesoid X receptor. Phytomedicine 25, 83–92. doi:10.1016/j.phymed.2016.12.006
Duan, X., Meng, Q., Wang, C., Liu, Z., Sun, H., Huo, X., et al. (2018). Effects of calycosin against high-fat diet-induced nonalcoholic fatty liver disease in mice. J. Gastroenterol. Hepatol. 33 (2), 533–542. doi:10.1111/jgh.13884
Durukan, A., and Tatlisumak, T. (2007). Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol. Biochem. Be 87 (1), 179–197. doi:10.1016/j.pbb.2007.04.015
El Dib, R. A., Soliman, H. S., Hussein, M. H., and Attia, H. G. (2015). Two new flavonoids and biological activity of Astragalus abyssinicus (hochst) steud. Ex A. Rich. Aerial parts. Drug Res. 65 (5), 259–265. doi:10.1055/s-0034-1377003
El-Bakoush, A., and Olajide, O. A. (2018). Formononetin inhibits neuroinflammation and increases estrogen receptor beta (ERβ) protein expression in BV2 microglia. Int. Immunopharmacol. 61, 325–337. doi:10.1016/j.intimp.2018.06.016
Fu, J., Wang, Z., Huang, L., Zheng, S., Wang, D., Chen, S., et al. (2014). Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 28 (9), 1275–1283. doi:10.1002/ptr.5188
Gao, J., Liu, Z. J., Chen, T., and Zhao, D. (2014). Pharmaceutical properties of calycosin, the major bioactive isoflavonoid in the dry root extract of Radix astragali. Pharm. Biol. 52 (9), 1217–1222. doi:10.3109/13880209.2013.879188
Gong, A., Duan, R., Wang, H., Kong, X., Dong, T., Tsim, K., et al. (2018). Evaluation of the pharmaceutical properties and value of astragali radix. Medicines 5 (2), 46. doi:10.3390/medicines5020046
Gu, C., Yuan, L., and Meng, L. (2020). Effect of formononetin on the viability, migration and invasion of ovarian cancer cells in vitro. Chin. J. Pathophysiol. 36 (08), 1434–1438.
Gu, Y., Chen, X., Fu, S., Liu, W., Wang, Q., Liu, K. J., et al. (2021). Astragali radix isoflavones synergistically alleviate cerebral ischemia and reperfusion injury via activating estrogen receptor-PI3K-akt signaling pathway. Front. Pharmacol. 12, 533028. doi:10.3389/fphar.2021.533028
Gunata, M., Parlakpinar, H., and Acet, H. A. (2020). Melatonin: a review of its potential functions and effects on neurological diseases. Rev. Neurol-France. 176 (3), 148–165. doi:10.1016/j.neurol.2019.07.025
Guo, K., He, X., Zhang, Y., Li, X., Yan, Z., Pan, L., et al. (2016). Flavoniods from aerial parts of Astragalus hoantchy. Fitoterapia 114, 34–39. doi:10.1016/j.fitote.2016.08.009
Guo, X., Pan, X., Wu, J., Li, Y., and Nie, N. (2022). Calycosin prevents IL-1β-induced articular chondrocyte damage in osteoarthritis through regulating the PI3K/AKT/FoxO1 pathway. Vitro Cell Dev-An. 58 (6), 491–502. doi:10.1007/s11626-022-00694-7
Guo, Z., Lou, Y., Kong, M., Luo, Q., Liu, Z., and Wu, J. (2019). A systematic review of phytochemistry, pharmacology and pharmacokinetics on astragali radix: implications for astragali radix as a personalized medicine. Int. J. Mol. Sci. 20 (6), 1463. doi:10.3390/ijms20061463
Hao, J., Li, J., Li, X., Liu, Y., Ruan, J., Dong, Y., et al. (2016). Aromatic constituents from the stems of Astragalus membranaceus (fisch) Bge. Var. Mongholicus (Bge) Hsiao. Molecules 21 (3), 354. doi:10.3390/molecules21030354
He, Z. Q., and Wang, B. Q. (1990). Isolation and identification of chemical constituents of Astragalus root. Acta Pharm. Sin. 25 (9), 694–698.
Hsu, C. C., Kuo, T. W., Liu, W. P., Chang, C. P., and Lin, H. J. (2020). Calycosin preserves BDNF/TrkB signaling and reduces post-stroke neurological injury after cerebral ischemia by reducing accumulation of hypertrophic and TNF-alpha-containing microglia in rats. J. Neuroimmune Pharm. 15 (2), 326–339. doi:10.1007/s11481-019-09903-9
Hu, W., Wu, X., Tang, J., Xiao, N., Zhao, G., Zhang, L., et al. (2019). In vitro and in vivo studies of antiosteosarcoma activities of formononetin. J. Cell Physiol. 234 (10), 17305–17313. doi:10.1002/jcp.28349
Hu, Y., Zhai, W., Tan, D., Chen, H., Zhang, G., Tan, X., et al. (2023). Uncovering the effects and molecular mechanism of Astragalus membranaceus (Fisch) Bunge and its bioactive ingredients formononetin and calycosin against colon cancer: an integrated approach based on network pharmacology analysis coupled with experimental validation and molecular docking. Front. Pharmacol. 14, 1111912. doi:10.3389/fphar.2023.1111912
Hua, T., Yanru, D., Kun, Z., and Huiyuan, C. (2016). Chemical Constituents of Astragalus membranaceus var.mongholicus. Chin. J. Exp. Tradit. Med. Form. 22 (07), 70–73. doi:10.13422/j.cnki.syfjx.2016070070
Huang, J., Liu, Y., and Huang, X. (2018b). Formononetin may protect aged hearts from ischemia/reperfusion damage by enhancing autophagic degradation. Mol. Med. Rep. 18 (6), 4821–4830. doi:10.3892/mmr.2018.9544
Huang, J., Shen, H., Mi Jiang, L. H., Yuan, Y., and Wang, Q. (2020). Calycosin reduces infarct size, oxidative stress and preserve heart function in isoproterenol-induced myocardial infarction model. Pak J. Pharm. Sci. 33 (3), 1341–1347. doi:10.36721/pjps.2020.33.3.Sp.1341-1347.1
Huang, J., Yin, L., Dong, L., Quan, H., Chen, R., Hua, S., et al. (2018a). Quality evaluation for Radix Astragali based on fingerprint, indicative components selection and QAMS. Biomed. Chromatogr. 32 (11), e4343. doi:10.1002/bmc.4343
Huang, X., Liu, Y., Song, F., Liu, Z., and Liu, S. (2009). Studies on principal components and antioxidant activity of different Radix Astragali samples using high-performance liquid chromatography/electrospray ionization multiple-stage tandem mass spectrometry. Talanta 78 (3), 1090–1101. doi:10.1016/j.talanta.2009.01.021
Huang, Y., Yang, Y. B., Zhang, X. H., Yu, X. L., Wang, Z. B., and Cheng, X. C. (2013). MicroRNA-21 gene and cancer. Med. Oncol. 30 (1), 376. doi:10.1007/s12032-012-0376-8
Jain, P. G., Nayse, P. G., Patil, D. J., Shinde, S. D., and Surana, S. J. (2020). The possible antioxidant capabilities of formononetin in guarding against streptozotocin-induced diabetic nephropathy in rats. Futur J. Pharm. Sci. 6 (1), 53. doi:10.1186/s43094-020-00071-9
Janibekov, A. A., Youssef, F. S., Ashour, M. L., and Mamadalieva, N. Z. (2018). New flavonoid glycosides from two Astragalus species (Fabaceae) and validation of their antihyperglycaemic activity using molecular modelling and in vitro studies. Ind. Crop Prod. 118, 142–148. doi:10.1016/j.indcrop.2018.03.034
Jia, C., Hu, F., Lu, D., Jin, H., Lu, H., Xue, E., et al. (2022). Formononetin inhibits IL-1β-induced inflammation in human chondrocytes and slows the progression of osteoarthritis in rat model via the regulation of PTEN/AKT/NF-κB pathway. Int. Immunopharmacol. 113, 109309. doi:10.1016/j.intimp.2022.109309
Jia, Z., Wang, X., Wang, X., Wei, P., Li, L., Wu, P., et al. (2018). Calycosin alleviates allergic contact dermatitis by repairing epithelial tight junctions via down-regulating HIF-1α. J. Cell Mol. Med. 22 (9), 4507–4521. doi:10.1111/jcmm.13763
Jian, L., Xin, L., Yufang, M., and Yifan, H. (2015). Protective effect of calycosin-7-O-β-D-glucopyranoside against oxidative stress of BRL-3A cells induced by thioacetamide. Pharmacogn. Mag. 11 (43), 524–532. doi:10.4103/0973-1296.160461
Jiangbo, Z., Xuying, W., Yuping, Z., Xili, M., Yiwen, Z., and Tianbao, Z. (2009). Effect of astragaloside IV on the embryo-fetal development of Sprague-Dawley rats and New Zealand White rabbits. J. Appl. Toxicol. 29 (5), 381–385. doi:10.1002/jat.1422
Jiaojiao, Y., Dandan, K., Jiaoyang, L., Wenjie, Q., Xuemei, Q., Zhuowen, F., et al. (2019). Analysis of heavy metal pollution in Astragalus membranaceus and its health risk assessment. Chin. J. Chin. Mater Med. 44 (14), 3094–3099. doi:10.19540/j.cnki.cjcmm.20190517.201
Jin, Y. M., Xu, T. M., Zhao, Y. H., Wang, Y. C., and Cui, M. H. (2014). In vitro and in vivo anti-cancer activity of formononetin on human cervical cancer cell line HeLa. Tumour Biol. 35 (3), 2279–2284. doi:10.1007/s13277-013-1302-1
Kavtaradze, N., Alaniya, M., Masullo, M., Cerulli, A., and Piacente, S. (2020). New flavone glycosides from Astragalus tanae endemic to Georgia. Chem. Nat. Compd+ 56 (1), 70–74. doi:10.1007/s10600-020-02946-y
Kim, C., Ha, H., Kim, J. S., Kim, Y. T., Kwon, S. C., and Park, S. W. (2003). Induction of growth hormone by the roots of Astragalus membranaceus in pituitary cell culture. Arch. Pharm. Res. 26 (1), 34–39. doi:10.1007/bf03179928
Kitagawa, I., Saito, M., Takagi, A., and Yoshikawa, M. (1983b). Saponin and sapogenol. XXXVII. Chemical constituents of astragali radix, the root of Astragalus membranaceus Bunge. (4). Astragalosides VII and VIII. Chem. Pharm. Bull. 31 (2), 716–722. doi:10.1248/cpb.31.716
Kitagawa, I., Wang, H., Saito, M., Takagi, A., and Yoshikawa, M. (1983a). Saponin and sapogenol. XXXV. Chemical constituents of Astragali Radix, the root of Astragalus membranaceus Bunge. (2). Astragalosides I,II and IV, acetylastragaloside I and isoastragalosides I and II. Chem. Pharm. Bull. 31 (2), 698–708. doi:10.1248/cpb.31.698
Krasteva, A., Panov, V., Krasteva, A., Kisselova, A., and Krastev, Z. (2014). Oral cavity and systemic diseases—diabetes mellitus. Biotechnol. Biotec Eq 25 (1), 2183–2186. doi:10.5504/bbeq.2011.0022
Krasteva, I., Bratkov, V., Bucar, F., Kunert, O., Kollroser, M., Kondeva-Burdina, M., et al. (2015). Flavoalkaloids and flavonoids from Astragalus monspessulanus. J. Nat. Prod. 78 (11), 2565–2571. doi:10.1021/acs.jnatprod.5b00502
Lee, H., Lee, D., Kang, K. S., Song, J. H., and Choi, Y. K. (2018). Inhibition of intracellular ROS accumulation by formononetin attenuates cisplatin-mediated apoptosis in LLC-PK1 cells. Int. J. Mol. Sci. 19 (3), 813. doi:10.3390/ijms19030813
Li, G., Ai, B., Zhang, W., Feng, X., and Jiang, M. (2021). Efficacy and safety of astragalus injection combined with western medicine in the treatment of early diabetic nephropathy: a protocol for systematic review and meta-analysis. Med. Baltim. 100 (12), e25096. doi:10.1097/md.0000000000025096
Li, L., Li, Z., Yan, S., and Su, Y. (2017b). Chemical constituents in roots of Astragalus membranaceus. China Tradit. Herb. Drugs 48 (13), 2601–2607.
Li, L., Wang, Y., Wang, X., Tao, Y., Bao, K., Hua, Y., et al. (2018). Formononetin attenuated allergic diseases through inhibition of epithelial-derived cytokines by regulating E-cadherin. Clin. Immunol. 195, 67–76. doi:10.1016/j.clim.2018.07.018
Li, L., Zheng, S., Brinckmann, J. A., Fu, J., Zeng, R., Huang, L., et al. (2017a). Chemical and genetic diversity of Astragalus mongholicus grown in different eco-climatic regions. PLoS One 12 (9), e0184791. doi:10.1371/journal.pone.0184791
Li, W. (2005). Dictionary of traditional Chinese medicine. Beijing: The peoples medical publishing house.
Li, X., Guo, X., Sha, M., Gao, W., and Li, X. (2022). Combining network pharmacology with chromatographic fingerprinting and multicomponent quantitative analysis for the quality evaluation of Astragali Radix. Biomed. Chromatogr. 36 (4), e5319. doi:10.1002/bmc.5319
Li, X., Qu, L., Dong, Y., Han, L., Liu, E., Fang, S., et al. (2014). A review of recent research progress on the astragalus genus. Molecules 19 (11), 18850–18880. doi:10.3390/molecules191118850
Liang, C., Zhou, A., Sui, C., and Huang, Z. (2018). The effect of formononetin on the proliferation and migration of human umbilical vein endothelial cells and its mechanism. Biomed. Pharmacother. 111, 86–90. doi:10.1016/j.biopha.2018.12.049
Liao, M., Shang, H., Li, Y., Li, T., Wang, M., Zheng, Y., et al. (2018). An integrated approach to uncover quality marker underlying the effects of Alisma orientale on lipid metabolism, using chemical analysis and network pharmacology. Phytomedicine 45, 93–104. doi:10.1016/j.phymed.2018.04.006
Lina, L., Peiqin, Z., Zhu, H., and Zhang, Y. (2022). Astragaloside - alleviates epirubicin-induced cardiotoxicity in rats. Chin. J. Clin. Med. 29 (6), 1006–1011. doi:10.12025/j.issn.1008-6358.2022.20221746
Liu, G., Piao, X. J., Xu, W. T., Zhang, Y., Zhang, T., Xue, H., et al. (2021b). Calycosin induces mitochondrial-dependent apoptosis and cell cycle arrest, and inhibits cell migration through a ROS-mediated signaling pathway in HepG2 hepatocellular carcinoma cells. Toxicol Vitro 70, 105052. doi:10.1016/j.tiv.2020.105052
Liu, G., Zhao, W., Bai, J., Cui, J., Liang, H., and Lu, B. (2021a). Formononetin protects against concanavalin-A-induced autoimmune hepatitis in mice through its anti-apoptotic and anti-inflammatory properties. Biochem. Cell Biol. 99 (2), 231–240. doi:10.1139/bcb-2020-0197
Liu, W. N., Zhou, S. l., Guan, X., and Li, D. X. (2022). Study on the rules of medication in LAN shi Mi Zang (Lanshi Mi Zang) for the treatment of spleen and stomach diseases. Hunan TCM J. 38 (9), 1–10. doi:10.16808/j.cnki.issn1003-7705.2022.09.008
Liu, Y., Che, G., Di, Z., Sun, W., Tian, J., and Ren, M. (2020). Calycosin-7-O-beta-D-glucoside attenuates myocardial ischemia-reperfusion injury by activating JAK2/STAT3 signaling pathway via the regulation of IL-10 secretion in mice. Mol. Cell Biochem. 463 (1-2), 175–187. doi:10.1007/s11010-019-03639-z
Lu, C. Y., Day, C. H., Kuo, C. H., Wang, T. F., Ho, T. J., Lai, P. F., et al. (2022). Calycosin alleviates H2O2-induced astrocyte injury by restricting oxidative stress through the Akt/Nrf2/HO-1 signaling pathway. Environ. Toxicol. 37 (4), 858–867. doi:10.1002/tox.23449
Lu, X., Lu, L., Gao, L., Wang, Y., and Wang, W. (2021). Calycosin attenuates doxorubicin-induced cardiotoxicity via autophagy regulation in zebrafish models. Biomed. Pharmacother. 137, 111375. doi:10.1016/j.biopha.2021.111375
Ma, J., Qiao, Z., and Xiang, X. (2011). Aqueous extract of Astragalus mongholicus. J. Med. Plants Res. 5 (5), 855–858.
Ma, W., Nomura, M., Takahashi-Nishioka, T., and Kobayashi, S. (2007). Combined effects of fangchinoline from stephania tetrandra radix and formononetin and calycosin from Astragalus membranaceus radix on hyperglycemia and hypoinsulinemia in streptozotocin-diabetic mice. Biol. Pharm. Bull. 30 (11), 2079–2083. doi:10.1248/bpb.30.2079
Maamria, L., Long, C., Haba, H., Lavaud, C., Cannac, A., and Benkhaled, M. (2015). Cycloartane glycosides from Astragalus gombo. Phytochem. Lett. 11, 286–291. doi:10.1016/j.phytol.2015.01.014
Napolitano, A., Akay, S., Mari, A., Bedir, E., Pizza, C., and Piacente, S. (2013). An analytical approach based on ESI-MS, LC-MS and PCA for the quali-quantitative analysis of cycloartane derivatives in Astragalus spp. J. Pharm. Biomed. Anal. 85, 46–54. doi:10.1016/j.jpba.2013.06.021
Ning, L., Chen, C., Jin, R., Wu, Y., Zhang, H., Sun, C., et al. (2002). Effect of components of dang-gui-bu-xue decoction on hematopenia. Zhongguo Zhong Yao Za 27, 50–53.
Niu, Y., Jia, X., and Wang, Y. (2021). Formononetin regulates the proliferation, migration, invasion and apoptosis of ovarian cancer cells by regulating miR-19b-3p/TGF-β/Smad2 signaling pathway. J. Shenyang Pharm. Univ. 38 (10), 1068–1075. doi:10.14066/j.cnki.cn21-1349/r.2020.1202
Oza, M. J., and Kulkarni, Y. A. (2018). Formononetin treatment in type 2 diabetic rats reduces insulin resistance and hyperglycemia. Front. Pharmacol. 9, 739. doi:10.3389/fphar.2018.00739
Pan, Q., Ban, Y., and S, K. (2021). Antioxidant activity of calycosin against α-synuclein amyloid fibrils-induced oxidative stress in neural-like cells as a model of preventive care studies in Parkinson's disease. Int. J. Biol. Macromol. 182, 91–97. doi:10.1016/j.ijbiomac.2021.03.186
Peng, H., Zhang, X., and Li, F. (2017). Mechanisms of Astragalus polysaccharids synergized with doxorubicin against drug resistance in HL-60/A cells. J. Exp. Hemat 25, 743–748. doi:10.7534/j.issn.1009-2137.2017.03.019
Qi, L. W., Yu, Q. T., Yi, L., Ren, M. T., Wen, X. D., Wang, Y. X., et al. (2008). Simultaneous determination of 15 marker constituents in various radix Astragali preparations by solid-phase extraction and high-performance liquid chromatography. J. Sep. Sci. 31 (1), 97–106. doi:10.1002/jssc.200700286
Qiu, G., Tian, W., Huan, M., Chen, J., and Fu, H. (2016). Formononetin exhibits anti-hyperglycemic activity in alloxan-induced type 1 diabetic mice. Exp. Biol. Med. 242 (2), 223–230. doi:10.1177/1535370216657445
Ren, M., Wang, X., Du, G., Tian, J., and Liu, Y. (2016). Calycosin-7-O-beta-D-glucoside attenuates ischemia-reperfusion injury in vivo via activation of the PI3K/Akt pathway. Mol. Med. Rep. 13 (1), 633–640. doi:10.3892/mmr.2015.4611
Rios, J. L., and Waterman, P. G. (1997). A review of the pharmacology and toxicology of Astragalus. Phytother. Res. 11, 411–418. doi:10.1002/(sici)1099-1573(199709)11:6<411::aid-ptr132>3.0.co;2-6
Sha, W. J. (2014). Research on prescription compatibility rules in Wang qing-ren's yi lin qian cuo. China: Hebei Med Univ.
Shao, B. M., Xu, W., Dai, H., Tu, P., Li, Z., and Gao, X. M. (2004). A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem. Bioph Res. Co. 320 (4), 1103–1111. doi:10.1016/j.bbrc.2004.06.065
Shen, D., Xie, X., Zhu, Z., Yu, X., Liu, H., Wang, H., et al. (2014). Screening active components from Yu-ping-feng-san for regulating initiative key factors in allergic sensitization. PLoS One 9 (9), e107279. doi:10.1371/journal.pone.0107279
Shi, L., Yin, F., Xin, X., Mao, S., Hu, P., Zhao, C., et al. (2014). Astragalus polysaccharide protects astrocytes from being infected by HSV-1 through TLR3/NF-κB signaling pathway. Evid-Based Compl Alt. 2014, 285356. doi:10.1155/2014/285356
Song, J., Lee, D., Min, B., Bae, J. S., Chang, G. T., and Kim, H. (2017). Safety evaluation of Astragalus extract mixture HT042 and its constituent herbs in Sprague-Dawley rats. Phytomedicine 32, 59–67. doi:10.1016/j.phymed.2017.03.005
Song, J. Z., Mo, S. F., Yip, Y. K., Qiao, C. F., Han, Q. B., and Xu, H. X. (2007). Development of microwave assisted extraction for the simultaneous determination of isoflavonoids and saponins in radix astragali by high performance liquid chromatography. J. Sep. Sci. 30 (6), 819–824. doi:10.1002/jssc.200600340
Su, H. F., Shaker, S., Kuang, Y., Zhang, M., Ye, M., and Qiao, X. (2021). Phytochemistry and cardiovascular protective effects of huang-qi (astragali radix). Med. Res. Rev. 41 (4), 1999–2038. doi:10.1002/med.21785
Subarnas, A., Sun, O. Y., and Hikino, H. (1991). Isoflavans and a pterocarpan from Astagalus mongholicus. Phytochemistry 30 (8), 2777–2780.
Sun, L. (2015). Investigation on main cultivation of Astragalus membranaceus var. mongholicus and commercial specifications grades. Zhong Yo Cai 38 (12), 2487–2492. doi:10.15979/j.cnki.cn62-1104/f.2015.12.029
Sun, M., Wang, J., and Shi, Z. (2023). Effects of apatinib mesylate combined with astragaloside IV on proliferation, migration and invasion of primary hepatocellular carcinoma cells. Prog. Anat. Sci. 29, 1. doi:10.16695/j.cnki.1006-2947.2023.01.015
Szabo, N. J. (2014). Dietary safety of cycloastragenol from Astragalus spp: subchronic toxicity and genotoxicity studies. Food Chem. Toxicol. 64, 322–334. doi:10.1016/j.fct.2013.11.041
Tang, Z. X. (2022). Effects of different harvesting periods and different cleaning methods on the quality of Astragalus membranaceus. Agric. Tech. Inf. 633 (04), 57–60+67. doi:10.15979/j.cnki.cn62-1057/s.2022.04.026
Un, R., Horo, I., Masullo, M., Falco, A., Senol, S. G., Piacente, S., et al. (2016). Cycloartane and oleanane-type glycosides from Astragalus pennatulus. Fitoterapia 109, 254–260. doi:10.1016/j.fitote.2016.01.015
Vasilev, H., Ross, S., Smejkal, K., Marsik, P., Jankovska, D., Havlik, J., et al. (2019). Flavonoid glycosides from endemic Bulgarian Astragalus aitosensis (ivanisch). Molecules 24 (7), 1419. doi:10.3390/molecules24071419
Wang, Y., Dong, X., Li, Z., Wang, W., Tian, J., and Chen, J. (2014b). Downregulated RASD1 and upregulated miR-375 are involved in protective effects of calycosin on cerebral ischemia/reperfusion rats. J. Neurol. Sci. 339 (1-2), 144–148. doi:10.1016/j.jns.2014.02.002
Wang, A. L., Li, Y., Zhao, Q., and Fan, L. Q. (2018a). Formononetin inhibits colon carcinoma cell growth and invasion by microRNA-149-mediated EphB3 downregulation and inhibition of PI3K/AKT and STAT3 signaling pathways. Mol. Med. Rep. 17 (6), 7721–7729. doi:10.3892/mmr.2018.8857
Wang, A. L., Ren, Q., Zhang, X., Lu, H., and Chen, J. (2018b). Neuroprotective mechanisms of calycosin against focal cerebral ischemia and reperfusion injury in rats. Cell Physiol. Biochem. 45 (2), 537–546. doi:10.1159/000487031
Wang, D. S., Liu, H., Liu, Y., Li, H., Li, Z., Shao, G., et al. (2020b). Formononetin alleviates Streptococcus suis infection by targeting suilysin. Microb. Pathog. 147, 104388. doi:10.1016/j.micpath.2020.104388
Wang, D. S., Yan, L. Y., Yang, D. Z., Lyu, Y., Fang, L. H., Wang, S. B., et al. (2020a). Formononetin ameliorates myocardial ischemia/reperfusion injury in rats by suppressing the ROS-TXNIP-NLRP3 pathway. Biochem. Bioph Res. Co. 525 (3), 759–766. doi:10.1016/j.bbrc.2020.02.147
Wang, D. W. (2021). Research on the characteristics of Taiping Huimin he Yao Fang from the perspective of the theoretical framework of prescription science. China: CDUTCM.
Wang, D. W., Xiao, C. J., Qiu, L., Tian, X. Y., Dong, X., and Jiang, B. (2021a). Two new 8-isopentenyl isoflavane derivatives from Astragalus dolichochaete diels. Nat. Prod. Res. 35 (8), 1323–1330. doi:10.1080/14786419.2019.1647426
Wang, D. W., Xiong, F., Yang, L., Xiao, Y., and Zhou, G. (2021b). A seasonal change of active ingredients and mineral elements in root of Astragalus membranaceus in the qinghai-tibet plateau. Biol. Trace Elem. Res. 199 (10), 3950–3959. doi:10.1007/s12011-020-02486-0
Wang, J. Y., Jiang, M. W., Li, M. Y., Zhang, Z. H., Xing, Y., Ri, M., et al. (2022). Formononetin represses cervical tumorigenesis by interfering with the activation of PD-L1 through MYC and STAT3 downregulation. J. Nutr. Biochem. 100, 108899. doi:10.1016/j.jnutbio.2021.108899
Wang, Q., Guo, S., Li, H.-w., Xie, Y., and Shang, E. (2023). Study on establishment of UPLC fingerprints and chemical pattern recognition of the stems and leaves of Astragalus membranaceus var. mongholicus (Bge) Hsiao from different regions. China Tradit. Herb. Drugs 54 (13), 4312–4320.
Wang, Y., Wang, X., and Ulij, O. (2014a). Chemical constituents of roots of Astragalus membranaceus(fisch) Bge. Var. mongholicus (Bge) Hsiao. Chin. Pharm. J. 49 (05), 357–359.
Wang, Z. B., Zhai, Y. D., Ma, Z. P., Yang, C. J., Pan, R., Yu, J. L., et al. (2015). Triterpenoids and flavonoids from the leaves of Astragalus membranaceus and their inhibitory effects on nitric oxide production. Chem. Biodivers. 12 (10), 1575–1584. doi:10.1002/cbdv.201400371
Wang, Z., Wenbo, Z., Yajun, C., Yu, J., Zhenping, M., Gaosong, W., et al. (2017). Flavonoids from the leaves of Astragalus membranaceus. Chin. Tradit. Pat. Med. 39 (08), 1634–1638.
Wang, Z. X., Cheng, M. Q., He, Q., and Guo, J. S. (1983). Study on chemical composition of astragali radix. Chin. Tradit. Herb. Drugs 1 (8), 1–15.
Wen, Y., Liang, C., Dan, Z., Huang, X., and Li, H. (2010). Studies on chemical constituents of Astragalus mongolicus. Chin. Tradit. Pat. Med. 13 (02), 115–119. doi:10.14053/j.cnki.ppcr.2010.02.007
Wu, B., Guo, Q. S., Shi, H. Z., Shi, G. W., Yan, S. M., Wu, M. J., et al. (2018). Effect of Astragali Radix on growth, immunity and related gene expression of Whitmania pigra. Chin. J. Chin. Mater Med. 43 (18), 3611–3617. doi:10.19540/j.cnki.cjcmm.20180703.006
Wu, J., Ke, X., Ma, N., Wang, W., Fu, W., Zhang, H., et al. (2016). Formononetin, an active compound of Astragalus membranaceus (Fisch) Bunge, inhibits hypoxia-induced retinal neovascularization via the HIF-1α/VEGF signaling pathway. Drug Des. Devel Ther. 10, 3071–3081. doi:10.2147/dddt.S114022
Wu, Q. L., Cheng, Y. Q., Liu, A. J., and Zhang, W. D. (2020). Formononetin recovered injured nerve functions by enhancing synaptic plasticity in ischemic stroke rats. Biochem. Bioph Res. Co. 525 (2), 67–72. doi:10.1016/j.bbrc.2020.02.015
Wu, R., Wang, Q., Wu, X., Tai, W., Dai, N., Wu, J., et al. (2015). Three flavonoids from the leaves of Astragalus membranaceus and their antifungal activity. Monatsh Chem. 146 (10), 1771–1775. doi:10.1007/s00706-015-1473-0
Xiao, C. J., Zhang, Y., Qiu, L., Dong, X., and Jiang, B. (2014). Schistosomicidal and antioxidant flavonoids from Astragalus englerianus. Planta Med. 80 (18), 1727–1731. doi:10.1055/s-0034-1383219
Xiao, W. L., Motley, T. J., Unachukwu, U. J., Lau, C. B., Jiang, B., Hong, F., et al. (2011). Chemical and genetic assessment of variability in commercial Radix Astragali (Astragalus spp) by ion trap LC-MS and nuclear ribosomal DNA barcoding sequence analyses. J. Agric. Food Chem. 59 (5), 1548–1556. doi:10.1021/jf1028174
Xin, M., Olson, E. N., and Bassel-Duby, R. (2013). Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair. Nat. Rev. Mol. Cell Biol. 14 (8), 529–541. doi:10.1038/nrm3619
Xu, Y., Xiong, J., Zhao, Y., He, B., Zheng, Z., Chu, G., et al. (2015). Calycosin rebalances advanced glycation end products-induced glucose uptake dysfunction of hepatocyte in vitro. Am. J. Chin. Med. 43 (6), 1191–1210. doi:10.1142/s0192415x15500688
Xue, H., Gan, F., Zhang, Z., Hu, J., Chen, X., and Huang, K. (2015). Astragalus polysaccharides inhibits PCV2 replication by inhibiting oxidative stress and blocking NF-κB pathway. Int. J. Biol. Macromol. 81, 22–30. doi:10.1016/j.ijbiomac.2015.07.050
Yang, L., Wang, X., Hou, A., Zhang, J., Wang, S., Man, W., et al. (2021). A review of the botany, traditional uses, phytochemistry, and pharmacology of the Flos Inulae. J. Ethnopharmacol. 276, 114125. doi:10.1016/j.jep.2021.114125
Yang, M., Li, Z., Liu, L., Bo, A., Zhang, C., and Li, M. (2020). Ecological niche modeling of Astragalus membranaceus var. mongholicus medicinal plants in Inner Mongolia, China. Sci. Rep. 10 (1), 12482. doi:10.1038/s41598-020-69391-3
Yang, Y., Zhao, Y., Ai, X., Cheng, B., and Lu, S. (2014). Formononetin suppresses the proliferation of human non-small cell lung cancer through induction of cell cycle arrest and apoptosis. Int. J. Clin. Exp. Pathol. 7 (12), 8453–8461. doi:10.1002/3527603344.ch16
Yao, C., Zhao, G., Wang, C., and Qiang, L. (2022). Effect and mechanism of formononetin on depression in rats by activating Nrf2/ARE signaling pathway. J. Trop. Med-Us. 22 (11), 1467–1472+1486+1611. doi:10.3969/j.issn.1672-3619.2022.11.002
Yao, J. N., Zhang, X. X., Zhang, Y. Z., Li, J. H., Zhao, D. Y., Gao, B., et al. (2019). Discovery and anticancer evaluation of a formononetin derivative against gastric cancer SGC7901 cells. Invest. New Drug 37 (6), 1300–1308. doi:10.1007/s10637-019-00767-7
Yazhou, Z., Feng, X., Liang, J., Jingshu, T., Ming-ying, S., Xuan, W., et al. (2012). Isoflavonoids from roots of Astragalus membranaceus var. mongholicus. Zhongguo Zhong yao za zhi 37 (21), 3243–3248.
Yeh, Y. C., Doan, L. H., Huang, Z. Y., Chu, L. W., Shi, T. H., Lee, Y. R., et al. (2021). Honeysuckle (Lonicera japonica) and Huangqi (Astragalus membranaceus) suppress SARS-CoV-2 entry and COVID-19 related cytokine storm in vitro. Front. Pharmacol. 12, 765553. doi:10.3389/fphar.2021.765553
Yi, J. Z. (2013). Study on the compatibility rules of tonic prescriptions in Jing Yue Quan Shu (Jingyue's Complete Works) for treating miscellaneous diseases of internal medicine. Heilongjiang: Heilongjiang Univ TCM.
Yi, L., Cui, J., Wang, W., Tang, W., Teng, F., Zhu, X., et al. (2020). Formononetin attenuates airway inflammation and oxidative stress in murine allergic asthma. Front. Pharmacol. 11, 533841. doi:10.3389/fphar.2020.533841
Yi, L., Lu, Y., Yu, S., Cheng, Q., and Yi, L. (2022). Formononetin inhibits inflammation and promotes gastric mucosal angiogenesis in gastric ulcer rats through regulating NF-κB signaling pathway. J. Recept Signal Transduct. Res. 42 (1), 16–22. doi:10.1080/10799893.2020.1837873
Ying, K., Liu, Y., Zhang, C., and Shangguan, M. (2019). Medical findings of nasopharyngeal carcinoma patients and anti-tumor benefits of formononetin. Eur. J. Pharmacol. 861, 172619. doi:10.1016/j.ejphar.2019.172619
Yu, D.-H., Bao, Y.-M., Wei, C.-L., and An, L.-J. (2005). Studies of chemical constituents and their antioxidant activities from Astragalus mongholicus Bunge. Biomed. Environ. Sci. 18 (5), 297–301.
Yu, D., Bao, Y., An, l. J., and Yang, M. (2009). Protection of PC12 cells against superoxide-induced damage by isoflavonoids from Astragalus mongholicus. Biomed. Environ. Sci. 22 (1), 50–54. doi:10.1016/S0895-3988(09)60022-2
Yu, J., Ji, H., Dong, X., Feng, Y., and Liu, A. (2019). Apoptosis of human gastric carcinoma MGC-803 cells induced by a novel Astragalus membranaceus polysaccharide via intrinsic mitochondrial pathways. Int. J. Biol. Macromol. 126, 811–819. doi:10.1016/j.ijbiomac.2018.12.268
Yu, J., Ji, H. Y., and Liu, A. J. (2018a). Alcohol-soluble polysaccharide from Astragalus membranaceus: preparation, characteristics and antitumor activity. Int. J. Biol. Macromol. 118, 2057–2064. doi:10.1016/j.ijbiomac.2018.07.073
Yu, J., Wang, Z., Wang, Q., and Kuang, H. X. (2018b). Research progress on pharmacological effects of flavonoids in Astragalus membranaceus. Tradit. Chin. Med. Inf. 35 (02), 104–108. doi:10.19656/j.cnki.1002-2406.180065
Yu, J., Zhu, L., Zheng, H., Gong, X., Jiang, H., Chen, J., et al. (2017). Sulfotransferases and breast cancer resistance protein determine the disposition of calycosin in vitro and in vivo. Mol. Pharm. 14 (9), 2917–2929. doi:10.1021/acs.molpharmaceut.7b00042
Yu, L., Zhang, Y., Chen, Q., He, Y., Zhou, H., Wan, H., et al. (2022). Formononetin protects against inflammation associated with cerebral ischemia-reperfusion injury in rats by targeting the JAK2/STAT3 signaling pathway. Biomed. Pharmacother. 149, 112836. doi:10.1016/j.biopha.2022.112836
Yu, X., Gao, F., Li, W., Zhou, L., Liu, W., and Li, M. (2020). Formononetin inhibits tumor growth by suppression of EGFR-Akt-Mcl-1 axis in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 39 (1), 62. doi:10.1186/s13046-020-01566-2
Yuan, W., Chen, Y., Zhou, Y., Bao, K., Yu, X., Xu, Y., et al. (2021). Formononetin attenuates atopic dermatitis by upregulating A20 expression via activation of G protein-coupled estrogen receptor. J. Ethnopharmacol. 266, 113397. doi:10.1016/j.jep.2020.113397
Yuan, Y. M., Gao, J. W., Shi, Z., Huang, P., Lu, Y. S., Yao, M. C., et al. (2012). Herb-drug pharmacokinetic interaction between radix astragali and pioglitazone in rats. J. Ethnopharmacol. 144 (2), 300–304. doi:10.1016/j.jep.2012.09.012
Yujun, W., Hambonny, B., Yanping, S., Hui, J., and Ling, C. (2022). To study the protective effect of calycosin on respiratory syncytial virus (RSV) -induced pneumonia in mice and its mechanism. Zhongguo Zhong yao za zhi 45 (3), 715–719. doi:10.13863/j.issn1001-4454.2022.03.035
Zhai, J., Tao, L., Zhang, S., Gao, H., Zhang, Y., Sun, J., et al. (2020). Calycosin ameliorates doxorubicin-induced cardiotoxicity by suppressing oxidative stress and inflammation via the sirtuin 1-NOD-like receptor protein 3 pathway. Phytother. Res. 34 (3), 649–659. doi:10.1002/ptr.6557
Zhang, C. H., Yang, X., Wei, J. R., Chen, N. M., Xu, J. P., Bi, Y. Q., et al. (2021a). Ethnopharmacology, phytochemistry, pharmacology, toxicology and clinical applications of radix astragali. Chin. J. Integr. Med. 27 (3), 229–240. doi:10.1007/s11655-019-3032-8
Zhang, C. H., Zhang, J. Q., Zhang, T., Xue, H., Zuo, W. B., Li, Y. N., et al. (2021b). Calycosin induces gastric cancer cell apoptosis via the ROS-mediated MAPK/STAT3/NF-κB pathway. OncoTargets Ther. 14, 2505–2517. doi:10.2147/OTT.S292388
Zhang, J., Liu, L., Wang, J., Ren, B., Zhang, L., and Li, W. (2018). Formononetin, an isoflavone from Astragalus membranaceus inhibits proliferation and metastasis of ovarian cancer cells. J. Ethnopharmacol. 221, 91–99. doi:10.1016/j.jep.2018.04.014
Zhang, L., Fan, C., Jiao, H. C., Zhang, Q., Jiang, Y. H., Cui, J., et al. (2022). Calycosin alleviates doxorubicin-induced cardiotoxicity and pyroptosis by inhibiting NLRP3 inflammasome activation. Oxid. Med. Cell Longev. 2022, 1733834. doi:10.1155/2022/1733834
Zhang, L. J., Liu, H. K., Hsiao, P. C., Kuo, L. M., Lee, I. J., Wu, T. S., et al. (2011). New isoflavonoid glycosides and related constituents from astragali radix (Astragalus membranaceus) and their inhibitory activity on nitric oxide production. J. Agric. Food Chem. 59 (4), 1131–1137. doi:10.1021/jf103610j
Zhang, Y., Li, X., Ruan, J., Wang, T., Dong, Y., Hao, J., et al. (2016). Oleanane type saponins from the stems of Astragalus membranaceus (Fisch) Bge. var. mongholicus (Bge) Hsiao. Fitoterapia 109, 99–105. doi:10.1016/j.fitote.2015.12.006
Zhang, Z., Auyeung, K. K., Sze, S. C., Zhang, S., Yung, K. K., and Ko, J. K. (2020). The dual roles of calycosin in growth inhibition and metastatic progression during pancreatic cancer development: a "TGF-β paradox". Phytomedicine 68, 153177. doi:10.1016/j.phymed.2020.153177
Zhao, Z., Mu, C. C., and Zhang, H. (2018). To explore the rules of medication in the book "Zhengzong of Surgery Attending Prescription for Cancer" based on data mining. Chin. J. Basic Med. Tradit. Chin. Med. 35 (6), 832–834+859. doi:10.19945/j.cnki.issn.1006-3250.2018.06.041
Zhen, W., Zhang, J., Jiao, H., Liu, J., and Wang, J. (2023). Research progress on pharmacological action and quality control of effective components of Astragali Radix. Drug Eval. Stud. 46 (04), 917–924.
Zhou, J., Zejing, M., Guoyue, Z., and Gang, R. (2021). Advances in chemical constituents and biological activities of Astragalus SPP. Chin. Tradit. Pat. Med. 43 (07), 1845–1851.
Zhou, Q., Zhang, W., Li, T., Tang, R., Li, C., Yuan, S., et al. (2019). Formononetin enhances the tumoricidal effect of everolimus in breast cancer MDA-MB-468 cells by suppressing the mTOR pathway. Evid. Based Complement. Altern. Med. 2019, 9610629. doi:10.1155/2019/9610629
Zhou, R., Chen, H., Chen, J., Chen, X., Wen, Y., and Xu, L. (2018). Extract from Astragalus membranaceus inhibit breast cancer cells proliferation via PI3K/AKT/mTOR signaling pathway. Bmc Complem Altern. M. 18 (1), 83. doi:10.1186/s12906-018-2148-2
Zhou, R., Chen, H., Ye, M., and Xu, L. (2016). Effect of formononetin on cell cycle gene and protein expression in different subtypes of breast cancer. Drug Eval. Stud. 39 (3), 362–366. doi:10.7501/j.issn.1674-6376.2016.03.006
Zhou, Y., Liu, Q. H., Liu, C. L., and Lin, L. (2015). Calycosin induces apoptosis in human ovarian cancer SKOV3 cells by activating caspases and Bcl-2 family proteins. Tumour Biol. 36 (7), 5333–5339. doi:10.1007/s13277-015-3194-8
Zhu, S., Liu, Q., Qiu, S., Dai, J., and Gao, X. (2022). DNA barcoding: an efficient technology to authenticate plant species of traditional Chinese medicine and recent advances. Chin. Med. 17 (1), 112. doi:10.1186/s13020-022-00655-y
Keywords: Astragalus memeranaceus, ethnopharmacology, botany, traditional uses, phytochemistry, pharmacology, toxicology
Citation: Wang P, Wang Z, Zhang Z, Cao H, Kong L, Ma W and Ren W (2023) A review of the botany, phytochemistry, traditional uses, pharmacology, toxicology, and quality control of the Astragalus memeranaceus. Front. Pharmacol. 14:1242318. doi: 10.3389/fphar.2023.1242318
Received: 19 June 2023; Accepted: 07 August 2023;
Published: 23 August 2023.
Edited by:
Bey Hing Goh, Monash University Malaysia, MalaysiaReviewed by:
Hong-Hua Wu, Tianjin University of Traditional Chinese Medicine, ChinaCopyright © 2023 Wang, Wang, Zhang, Cao, Kong, Ma and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Ma, bWF3ZWlAaGxqdWNtLm5ldA==; Weichao Ren, bHp5cmVud2VpY2hhb0AxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.