
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 08 August 2023
Sec. Inflammation Pharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1241954
This article is part of the Research TopicGlobal Excellence in Inflammation PharmacologyView all 5 articles
Objective: Janus kinase (JAK) inhibitors are a novel class of drugs that have shown efficacy in treating immune-mediated inflammatory diseases (IMIDs). However, their safety profile in terms of herpes zoster infection remains unclear. We aimed to evaluate the risk of herpes zoster associated with JAK inhibitors in patients with IMIDs.
Methods: A systematic search of electronic databases was conducted to identify randomized controlled trials (RCTs) that evaluated the safety of JAK inhibitors in patients with IMIDs including inflammatory bowel disease (IBD), rheumatoid arthritis (RA), spondyloarthritis (SpA), psoriasis (PsO), and psoriatic arthritis (PsA). The primary outcome of interest was the incidence of herpes zoster infection. Network meta-analysis was performed to compare the risk of herpes zoster among different JAK inhibitors and placebo.
Results: A network meta-analysis was conducted using data from 47 RCTs including 24,142 patients. In patients with IMIDs, peficitinib 100 mg QD was associated with the highest risk of herpes zoster infection in patients with IMIDs, followed by baricitinib 4 mg QD and upadacitinib 30 mg QD. No difference in herpes zoster risk was found for other JAK inhibitors compared with placebo. Subgroup analysis indicated that higher incidence of herpes zoster was found in patients treated by baricitinib 4 mg QD, peficitinib 100 mg QD, and upadacitinib 30 mg QD only in patients with RA.
Conclusion: Our study suggests that some JAK inhibitors, particularly peficitinib, baricitinib, and tofacitinib, are associated with a higher risk of herpes zoster infection in patients with IMIDs.
Immune-mediated inflammatory diseases (IMIDs) are a group of diverse conditions without known cures (McInnes and Gravallese, 2021a). Although these diseases have unique characteristics such as clinical phenotype, tissue localization, and therapeutic response profile, they also share common underlying pathogenic features such as Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway, provide an exceptional opportunity for the application of modern molecular and computational techniques in the discovery of immunological targets and development of effective therapies (McInnes and Gravallese, 2021b; Schett et al., 2021; Rusiñol and Puig, 2023). Examples of IMIDs include broad and refractory disease spectrum including inflammatory bowel disease (IBD), rheumatoid arthritis (RA), spondyloarthritis (SpA) [including both ankylosing spondylitis (AS) and non-radiographic axial spondyloarthritis (nr-axSpA)], psoriasis (PsO), psoriatic arthritis (PsA), autoimmune thyroiditis (Hashimoto’s thyroiditis), asthma, and other immune-mediated diseases (Ortega et al., 2022; Gialouri et al., 2023). Therefore, they present significant systemic medical difficulties.
Herpes zoster, commonly known as shingles, is a viral infection caused by the reactivation of the varicella-zoster virus in individuals who have previously been infected with chickenpox (Sampathkumar et al., 2009). Herpes zoster typically presents as a painful rash, usually in a unilateral dermatome, and can result in severe complications including postherpetic neuralgia, ophthalmic involvement, and dissemination (Valladales-Restrepo et al., 2023). The incidence of herpes zoster increases with age and is further elevated in individuals with IMIDs (Harbecke et al., 2021).
JAK inhibitors are a relatively new class of immunosuppressive drugs that target intracellular signaling pathways involved in the pathogenesis of IMIDs (McInnes and Gravallese, 2021b). JAK inhibitors have demonstrated efficacy in managing IMIDs. On the other hands, their usage is linked with a higher risk of severe infections, particularly herpes zoster (Benucci et al., 2023; Yamaoka and Oku, 2023). However, the comparative risk of herpes zoster associated with different JAK inhibitors in patients with IMIDs is not well established. Some studies have suggested that certain JAK inhibitors may have a higher risk of herpes zoster than others, but these findings have not been consistently replicated across studies, and no comparison of incidence of herpes zoster were conducted in patients with IMIDs as an entity (Campanaro et al., 2021; Harkins et al., 2023; Yang et al., 2023).
The aim of this study is to systematically review and synthesize the available evidence on the risk of herpes zoster associated with JAK inhibitor therapy in patients with IMIDs, including IBD, RA, SpA, PsO, and PsA. We will also perform a network meta-analysis to compare the risk of herpes zoster across different JAK inhibitors and IMIDs.
This study was designed and conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for network meta-analyses for healthcare interventions (Hutton et al., 2015), utilizing its methods and recommendations. The study protocol was created in advance and registered in PROSPERO (CRD42023423787). All data included in the study can be found in the article and Supplementary Material and have been made openly available.
A thorough search of eligible studies was conducted by utilizing MEDLINE through PubMed, Embase, Web of science, and Cochrane Library. The search strategy incorporated medical subject heading (MeSH) terms or Emtree terms and followed the PICOS format: Population (P)—Patients with IMIDs including IBD, RA, SpA, PsO and PsA. Intervention (I)—JAK inhibitors. Comparison (C)—Placebo, and/or conventional disease-modifying agents (csDMARDs). Outcomes (O)—Incidence of herpes zoster infection. Study design (S)—Randomized placebo- or active-controlled clinical trials.
We established a timeframe from the inception of each database up to 1 May 2023, and only studies published in English were included. The search terms included “herpes zoster”, “JAK inhibitor”, “immune-mediated inflammatory diseases”, “inflammatory bowel disease”, “rheumatoid arthritis”, “spondyloarthritis”, “psoriasis”, and “psoriatic arthritis”. A detailed description of the search strategy is outlined in Supplementary Table S1. We also screened the references of the eligible studies to identify any additional studies meeting our inclusion criteria.
We included phase II or III randomized controlled trials (RCTs) that met the following criteria: (1) Population: adults (age≥18 years) patients with IMIDs, including IBD, RA, SpA, PsO, and PsA; (2) Intervention: patients received treatment with a JAK inhibitor (baricitinib, decernotinib, filgotinib, ivarmacitinib, peficitinib, tofacitinib, upadacitinib), either alone or in combination with immunosuppressants; It is noteworthy that this analysis specifically focused on approved JAK inhibitors with available RCT evidence related to herpes zoster risk. Upstream kinase inhibitors could be considered in an updated analysis in the future as more data accumulates; (3) Comparator: comparisons were made between JAK inhibitor treatments and placebo or another JAK inhibitor; (4) Outcome: the incidence of herpes zoster infection associated with JAK inhibitor treatment. (5) Studies published in English.
Studies were excluded if: (1) They were pediatric trials, or included pregnant patients, patients hypersensitive to JAK inhibitors, patients with systemic disease, or those previously treated with JAK inhibitors; (2) They did not provide sufficient data to estimate odds ratios (ORs) and 95% confidence intervals (95%CIs); (3) They were reviews, lectures, comments, letter or research unable to be extracted for statistical analysis.
Two reviewers (QX and LH) independently screened the titles and abstracts of the identified papers to determine their eligibility. Full texts of the potentially eligible papers were then reviewed for inclusion. Data were extracted using a standardized data extraction form that included study characteristics, patient demographics, intervention and comparison groups, and follow-up period. Any discrepancies were resolved through discussion and consensus.
Two reviewers (QX and LH) independently evaluated the risk of bias of each study and any disagreements were resolved through consensus. The risk of bias for each included study was assessed using the Revised Cochrane Risk-of-Bias Tool (RoB 2) (Sterne et al., 2019). The following aspects were evaluated to determine the bias risk: randomization process, deviations from the intended interventions, missing outcome data, outcome measurement, and selection of the reported result. The certainty of evidence was classified into three levels: low risk of bias, some concerns, and high risk of bias.
The quantitative analysis for this network meta-analysis involved a multivariate frequentist framework which allows combining direct and indirect evidence while accounting for the correlations between the multiple treatments (Chaimani et al., 2013; Shim et al., 2017). Evidence network diagrams were constructed to clearly visualize the available direct comparisons between treatments as well as the indirect comparisons enabled through the network meta-analysis (Chaimani et al., 2013; Shim et al., 2017). The results were reported as ORs with 95%CIs. Summary ORs with 95%CIs were calculated and presented using league matrix. To predict the potential effectiveness of future trials, 95% predictive intervals (95%PrIs) of ORs were also calculated and presented using forest plots alongside the meta-analysis estimates. Surface under cumulative ranking (SUCRA) curves were used to identify the JAK inhibitors with the greatest association with herpes zoster infection. SUCRA value is expressed as a percentage ranging from 0% to 100%, with the higher the SUCRA value, the more probability achievement of endpoint. Consistency tests were conducted using Wald test (Hoaglin et al., 2011; van Valkenhoef et al., 2016). Funnel plots were drawn to evaluate any small sample effects and the publication bias of the final screening. Statistical analyses were conducted using Stata/SE (version 17.0) and R software package (version 4.2.2) with the “netmeta” and “gemtc” packages. A significance level of p < 0.05 was used for all statistical tests.
The flowchart of the literature selection is shown in Figure 1. Of 3,428 studies initially identified, a total of 44 citations including 47 distinct RCTs (Fleischmann et al., 2012;van Vollenhoven et al., 2012;Kremer et al., 2013;van der Heijde et al., 2013;Lee et al., 2014;Bachelez et al., 2015;Fleischmann et al., 2015;Keystone et al., 2015;Papp et al., 2015;Tanaka et al., 2015;Genovese et al., 2016a;Genovese et al., 2016b;Takeuchi et al., 2016;Abe et al., 2017;Dougados et al., 2017;Fleischmann et al., 2017;Gladman et al., 2017;Kavanaugh et al., 2017;Kivitz et al., 2017;Mease et al., 2017;Panés et al., 2017;Sandborn et al., 2017;Taylor et al., 2017;van der Heijde et al., 2017;Vermeire et al., 2017;Westhovens et al., 2017;Zhang et al., 2017;Burmester et al., 2018;Genovese et al., 2018;Mease et al., 2018;Genovese et al., 2019;Smolen et al., 2019;Takeuchi et al., 2019;van der Heijde et al., 2019;Deodhar et al., 2021;Feagan et al., 2021;McInnes et al., 2021;Mease et al., 2021;Chen et al., 2022;Danese et al., 2022;Deodhar et al., 2022;Loftus et al., 2022;van der Heijde et al., 2022;Leng et al., 2023) were included in this network meta-analysis based on the selection criteria. The evidence network comprising 7 JAK inhibitor with 13 different dosages therapies and placebo in 4 kinds of IMIDs is shown in Figure 2; Supplementary Figure S1.
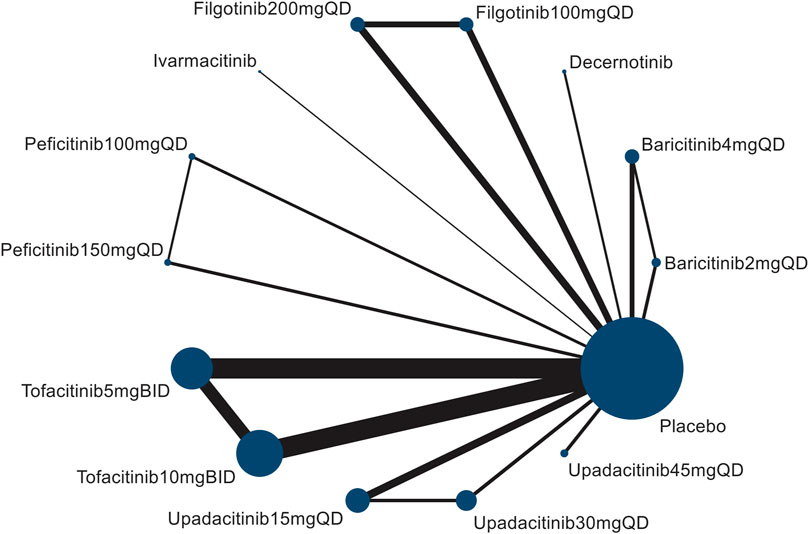
FIGURE 2. Network diagram of herpes zoster risk formed by interventions and both direct and indirect comparisons. The node size (the size of the circle) reflects the number of patients allocated to each intervention, whereas connection size (line thickness) is in proportion to each direct comparison.
The characteristics of the studies included are presented in Table 1. The studies were conducted in various countries and published between 2012 and 2022. The sample sizes of the included studies ranged from 58 to 1348, with a total of 24,142 patients. The patients had a diagnosis of IMIDs, including IBD, RA, SpA (including AS and nr-axSpA), PsO, and PsA. According to the types of diseases, there were 7 studies in IBD, 25 studies in RA, 5 studies in axSpA and 4 studies in PsO, and 6 studies in PsA. The JAK inhibitors evaluated in the studies included baricitinib, decernotinib, filgotinib, ivarmacitinib, peficitinib, tofacitinib, and upadacitinib, with various dosages administered. The risk of herpes zoster was reported as ORs with 95%CIs or as incidence rates.
The quality of all eligible studies included in this network meta-analysis was evaluated using the Jaded scale (Jadad et al., 1996). The Jaded scale ranged from 0 to 5, with 0 representing the lowest quality and 5 indicating the highest quality. Studies with a score ≥4 were considered as high quality. Common reasons for downgrading the quality of studies were: 1) not mention the method of randomization; 2) not describe the concealment of treatment allocation; 3) not blind the participant and assessor; 4) not give the reason for loss of follow up. As shown in Supplementary Table S2, the quality scores of the most included studies have very low to moderate risk of bias.
A total of 47 RCTs were incorporated into the network meta-analysis. In patients with IMIDs, baricitinib 4 mg QD (OR = 3.46, 95%CI 1.38, 8.67), peficitinib 100 mg QD (OR = 6.06, 95%CI 1.76, 20.82), tofacitinib 10 mg BID (OR = 1.96, 95%CI 1.01, 3.78), and upadacitinib 30 mg QD (OR = 3.25, 95%CI 1.50, 7.02) were associated with a higher incidence of herpes zoster infection compared with placebo. No difference in herpes zoster risk was found for other JAK inhibitors (baricitinib 2 mg QD, decernotinib, filgotinib, peficitinib 150 mg QD, tofacitinib 5 mg BID, upadacitinib 15 mg QD and upadacitinib 45 mg QD) compared with placebo. Besides, upadacitinib 30 mg QD (OR = 2.00, 95%CI 1.01, 3.96) had higher risk of herpes zoster compared to lower dosage of upadacitinib 15 mg QD (Figure 3).
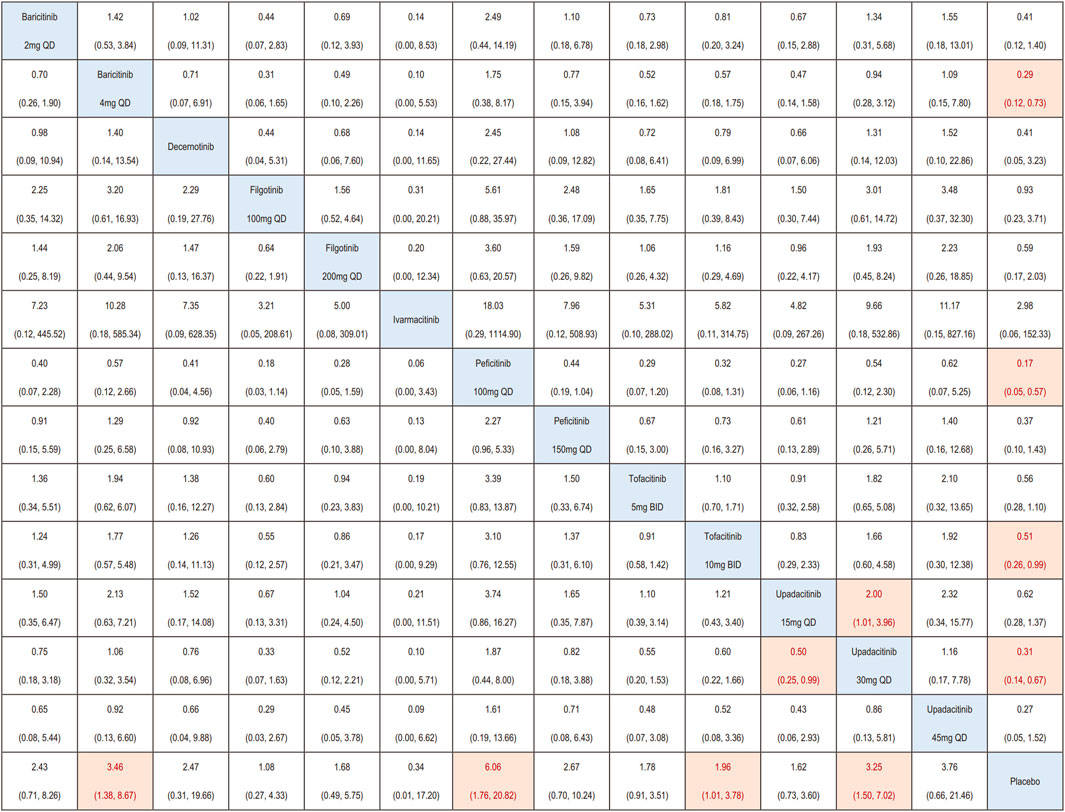
FIGURE 3. League matrix showing the comparative risk of herpes zoster included in this network meta-analysis. For interpreting the odds ratios (ORs), comparisons should be read from left to right, with reciprocals taken to obtain ORs from right to left. The ORs (95% CIs) for each comparison is shown in the cell intersecting the column-defining and row-defining treatments. Orange boxes represent statistically significant comparisons, while white boxes indicate non-statistically significant comparisons.
Subgroup analysis based on different types of IMIDs indicated that, higher incidence of Herpes zoster was found in patients treated by baricitinib 4 mg QD (OR = 3.46, 95%CI 1.38, 8.67), peficitinib 100 mg QD (OR = 6.06, 95%CI 1.76, 20.82), and upadacitinib 30 mg QD (OR = 3.87, 95%CI 1.07,13.98) only in patients with RA (Supplementary Figure S2).
To provide a hierarchy of the assessed interventions, SUCRA ranking plots were generated based on the results of network meta-analysis. SUCRA values range from 0% to 100%, with higher values indicating higher hierarchy, while 50% indicates moderate risk. SUCRA plots were depicted in Supplementary Figure S3.
The SUCRA ranking plot showed that among the assessed JAK inhibitors, peficitinib 100 mg QD (SUCRA = 88.1%), were associated with the highest risk of herpes zoster infection in patients with IMIDs, followed by baricitinib 4 mg QD (SUCRA = 72.2%), upadacitinib 30 mg QD (SUCRA = 71.2%), upadacitinib 45 mg QD (SUCRA = 70.6%), peficitinib 100 mg QD (SUCRA = 58.8%), baricitinib2mgqd (SUCRA = 55.5%), and decernotinib (SUCRA = 54.8%), other JAK inhibitors dosages showed a SUCRA value less than 50%, indicating a low risk of herpes zoster infection relative to other interventions (Figure 4).
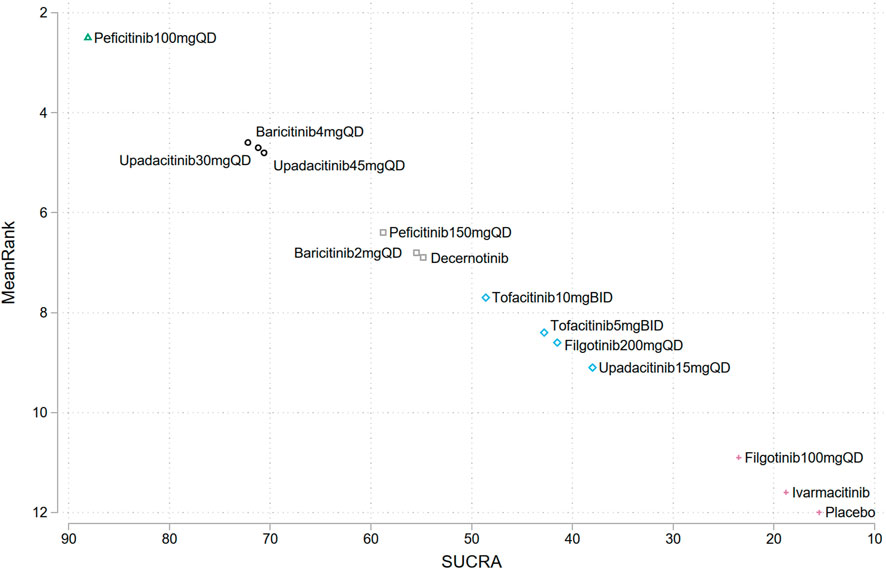
FIGURE 4. Surface under the cumulative ranking curves ranking (SUCRA) plots for the risk of herpes zoster in different JAK inhibitors. Treatments have been ranked (vertical axis) according to the SUCRA value (horizontal axis). Treatments positioned in the upper left corner of the plot have a higher risk of herpes zoster compared to the other treatments.
Subgroup analysis suggested a highest herpes zoster risk in patients with IBD, RA, axSpA and PsO or PsA treated with upadacitinib 45 mg QD (SUCRA = 81.2%), peficitinib100mgqd (SUCRA = 88.4%), tofacitinib10 mg BID (SUCRA = 69.6%), and tofacitinib 5 mg BID (SUCRA = 54.8%), respectively (Supplementary Figure S4).
The forest plots of the relative mean effects of treatments, along with 95%CIs and respective 95%PrI, were showed in (Supplementary Figure S5). The parameters of 95%PrI are crucial in effectively appraising heterogeneity among the included studies and interpreting results of the future trials by giving the range within which the results of a future study might lie (IntHout et al., 2016; Lin, 2019). Wider predictive intervals indicate more heterogeneity and uncertainty. Compared with placebo in patients with IMIDs, the 95%PrI of baricitinib 4 mg QD (0.11, 0.76) and peficitinib 100 mg QD (0.05, 0.60), and upadacitinib 30 mg QD (0.14, 0.69) exclude the null value (OR = 1), providing more confidence that these interventions are associated with higher herpes zoster risk.
Compared with placebo in patients with RA, the 95% predictive intervals of baricitinib 4 mg QD (0.09, 0.97) and peficitinib 100 mg QD (0.03, 0.83) exclude the null value, providing more confidence that these interventions are associated with higher herpes zoster risk. For other IMIDs, the 95%PrI for all comparisons crossing the null value reflect the uncertainty and inconsistency in study findings.
An inconsistency test was conducted to assess whether there were any significant differences between direct and indirect treatment effects, which would suggest that the studies were not consistent (Supplementary Table S3). The results of the inconsistency test showed no evidence of inconsistency in any of the analyses, indicating that the direct and indirect treatment effects were in agreement and that the studies were consistent with each other. This supports the validity of our findings and highlights the strength of our study.
Publication bias was assessed using funnel plots (Supplementary Figure S6). Based on the funnel plots generated in our network meta-analysis, the studies are expected to be symmetrically distributed at the top of the plot, which indicated the absence of publication bias. This suggests that the studies included in our analysis were not selected or published based on their results, and our findings are therefore less likely to be biased.
The current study aimed to evaluate the risk of herpes zoster associated with JAK inhibitor therapy in patients with IMIDs including IBD, RA, SpA, PsO and PsA. The findings of this network meta-analysis suggest that there is a higher risk of herpes zoster infection in patients with RA who are treated with baricitinib 4 mg QD, peficitinib 100 mg QD, and upadacitinib 30 mg QD compared to placebo. There was no difference in herpes zoster infection risk observed in patients with RA treated with other JAK inhibitors and placebo. Besides, in patients with IBD, SpA, PsO, and PsA, no significant difference in herpes zoster infection was found with any type of JAK inhibitor or dosage compared to placebo.
The current study findings support the results of previous studies that have also reported an increased risk of herpes zoster infection with JAK inhibitors in patients with RA. A previous meta-analysis by Bechman et al. (2019) reported a significantly increased risk of herpes zoster infection with the incidence rate ratios (IRRs) of 2.86 (95% CI: 1.26, 6.50) in RA patients treated by baricitinib 4 mg QD comparing with placebo, while non-significant IRRs were seen with other JAK inhibitors such as tofacitinib and upadacitinib. A network meta-analysis also indicated that, the baricitinib 4 mg and upadacitinib 15 mg showed the highest ACR response rates; but otherwise, these two JAK inhibitors ranked higher probability in herpes zoster infection, followed by tofacitinib, adalimumab, filgotinib100 mg, and filgotinib 200 mg (Lee and Song, 2020). A meta-analysis conducted by Olivera et al. (2020) revealed an elevated risk of herpes zoster infection in patients with IMIDs (including RA, PsO, IBD and AS) who were treated with various JAK inhibitors. However, upon conducting a subgroup analysis, no significant differences were observed among the specific IMIDs. In a recent meta-analysis by Wang et al. (2022), a total of 37 RCTs with 15,174 participants treated with six different JAK inhibitors (tofacitinib, baricitinib, upadacitinib, decernotinib, peficitinib, and filgotinib) were included. The analysis revealed that only baricitinib was associated with a higher risk of herpes zoster (RR = 3.15; 95% CI: 1.19, 8.33). Our findings add to this literature by evaluating a wider range of JAK inhibitors and additional IMIDs in a network meta-analysis, allowing for more indirect comparisons between treatments.
In general populations, various risk factors such as increasing age, female gender, ethnicity of Asia and Oceania, substance abuse such as smoking or alcohol, genetic predisposition, psychological stress, and exposure to immunotoxins can collectively contribute to the elevated susceptibility to herpes zoster (Thomas and Hall, 2004; van Oorschot et al., 2021; Curran et al., 2022; Kawahira et al., 2023). The pre-existing IMIDs themselves are also associated with an increased risk of herpes zoster (Leung et al., 2022), and the use of JAK inhibitors can further increase the risk of herpes zoster in these patients (Clarke et al., 2021). We have reason to believe that the combination of these factors may lead to an even higher incidence of herpes zoster infection. However, there have been no detailed reports on the effect of the above-mentioned risk factors (age, gender, ethnicity, substance abuse et al.) on the development of herpes zoster associated with JAK inhibitors in patients with IMIDs. Besides, several phase II and III studies investigating the incidence of herpes zoster infection treated by JAK inhibitors in other IMIDs (such as atopic dermatitis, juvenile idiopathic arthritis, and non-infectious uveitis) are also in progress (Harigai and Honda, 2020). The emergence of more studies will provide more data to compare the effectiveness and safety of JAK inhibitors in different IMIDs.
The mechanism underlying the association between JAK inhibitor therapy and increased risk of herpes zoster infection is not entirely clear. It has been suggested that the activation of JAK-STAT signaling pathways is important in host defense against viral infections (Hu et al., 2021). Inhibition of JAK signaling may impair the immune system’s ability to produce antiviral cytokines such as interferons and tumor necrosis factor α, which may be a contributing factor to the increased risk of viral infections and reactivation of latent viral infections such as herpes zoster (Sunzini et al., 2020). Interestingly, even JAK inhibitors designed to target specific JAKs with high precision seem to affect the immunogenic network in overlapping ways (Moodley et al., 2016). Consequently, the precise mechanism through which certain JAK inhibitors heighten the risk of herpes zoster infection remains elusive and necessitates further investigation.
Our study found that different JAK inhibitors may be associated with different risks of herpes zoster infection in patients with RA. However, in patients with IMIDs other than RA, our study found no significant differences in the risk of herpes zoster infection among the different JAK inhibitors. In the individual studies included in our meta-analysis, the higher incidences of herpes zoster infection were found in patients with CD treated by upadacitinib 45 mg (2.9%) (Loftus et al., 2022) and PsA treated by upadacitinib 30 mg (3.7%) (Mease et al., 2021) compared with placebo. This suggests that the increased risk observed in RA patients may be specific to this population, and that caution may be warranted when prescribing certain JAK inhibitors to patients with RA. However, these results should be interpreted cautiously due to the potential limitations, including the relatively small number of studies available for analysis in some of the IMID subgroups, and the potential for heterogeneity in study design and patient populations.
It is important to note that the results of this study should be interpreted with caution due to some limitations. Firstly, the number of studies and patients included in the analysis were limited, and some JAK inhibitors were only studied in a small number of trials. Secondly, the duration of the included studies varied, which may have affected the incidence of herpes zoster infection observed. Thirdly, the current study only included studies published in English, which may have resulted in publication bias. Finally, there were some variations in the dosages of JAK inhibitors used in the included studies, which could have influenced the results.
Despite these limitations, the findings of this study have important clinical implications. Gastroenterologist, rheumatologists, and other healthcare professionals should be aware of the increased risk of herpes zoster infection associated with certain JAK inhibitors in patients with IMIDs. Patients should be informed of this risk and monitored closely for the related signs and symptoms during treatment with JAK inhibitors. It may also be necessary to consider vaccination against herpes zoster infection in these patients.
In conclusion, the results of this network meta-analysis suggest that there is a higher risk of herpes zoster infection in patients with RA who are treated with baricitinib 4 mg QD, peficitinib 100 mg QD, and upadacitinib 30 mg QD compared to placebo. No higher risk of herpes zoster infection was observed in patients with other IMIDs (including IBD, SpA, PsO, and PsA) treated with JAK inhibitors. Further studies conducted in real-world settings and direct head-to-head comparisons will be required to completely understand the safety profile of the various JAK inhibitors.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
QX and YY contributed to the conception and design. QX, LH, and YY contributed to the collection and assembly of the data. QX and YY contributed to the analysis and interpretation of the data. QX, LH, and YY contributed to drafting and revision of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1241954/full#supplementary-material
Abe, M., Nishigori, C., Torii, H., Ihn, H., Ito, K., Nagaoka, M., et al. (2017). Tofacitinib for the treatment of moderate to severe chronic plaque psoriasis in Japanese patients: Subgroup analyses from a randomized, placebo-controlled phase 3 trial. J. Dermatol 44, 1228–1237. doi:10.1111/1346-8138.13956
Bachelez, H., Van De Kerkhof, P. C., Strohal, R., Kubanov, A., Valenzuela, F., Lee, J. H., et al. (2015). Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: A phase 3 randomised non-inferiority trial. Lancet 386, 552–561. doi:10.1016/S0140-6736(14)62113-9
Bechman, K., Subesinghe, S., Norton, S., Atzeni, F., Galli, M., Cope, A. P., et al. (2019). A systematic review and meta-analysis of infection risk with small molecule JAK inhibitors in rheumatoid arthritis. Rheumatol. Oxf. 58, 1755–1766. doi:10.1093/rheumatology/kez087
Benucci, M., Bernardini, P., Coccia, C., De Luca, R., Levani, J., Economou, A., et al. (2023). JAK inhibitors and autoimmune rheumatic diseases. Autoimmun. Rev. 22, 103276. doi:10.1016/j.autrev.2023.103276
Burmester, G. R., Kremer, J. M., Van Den Bosch, F., Kivitz, A., Bessette, L., Li, Y., et al. (2018). Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 391, 2503–2512. doi:10.1016/S0140-6736(18)31115-2
Campanaro, F., Batticciotto, A., Zaffaroni, A., Cappelli, A., Donadini, M. P., and Squizzato, A. (2021). JAK inhibitors and psoriatic arthritis: A systematic review and meta-analysis. Autoimmun. Rev. 20, 102902. doi:10.1016/j.autrev.2021.102902
Chaimani, A., Higgins, J. P., Mavridis, D., Spyridonos, P., and Salanti, G. (2013). Graphical tools for network meta-analysis in STATA. PloS one 8, e76654. doi:10.1371/journal.pone.0076654
Chen, B., Zhong, J., Li, X., Pan, F., Ding, Y., Zhang, Y., et al. (2022). Efficacy and safety of ivarmacitinib in patients with moderate-to-severe, active, ulcerative colitis: A phase II study. Gastroenterology 163, 1555–1568. doi:10.1053/j.gastro.2022.08.007
Clarke, B., Yates, M., Adas, M., Bechman, K., and Galloway, J. (2021). The safety of JAK-1 inhibitors. Rheumatol. Oxf. 60, ii24–ii30. doi:10.1093/rheumatology/keaa895
Curran, D., Callegaro, A., Fahrbach, K., Neupane, B., Vroling, H., Van Oorschot, D., et al. (2022). Meta-regression of herpes zoster incidence worldwide. Infect. Dis. Ther. 11, 389–403. doi:10.1007/s40121-021-00567-8
Danese, S., Vermeire, S., Zhou, W., Pangan, A. L., Siffledeen, J., Greenbloom, S., et al. (2022). Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: Results from three phase 3, multicentre, double-blind, randomised trials. Lancet 399, 2113–2128. doi:10.1016/S0140-6736(22)00581-5
Deodhar, A., Sliwinska-Stanczyk, P., Xu, H., Baraliakos, X., Gensler, L. S., Fleishaker, D., et al. (2021). Tofacitinib for the treatment of ankylosing spondylitis: A phase III, randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 80, 1004–1013. doi:10.1136/annrheumdis-2020-219601
Deodhar, A., Van Den Bosch, F., Poddubnyy, D., Maksymowych, W. P., Van Der Heijde, D., Kim, T. H., et al. (2022). Upadacitinib for the treatment of active non-radiographic axial spondyloarthritis (SELECT-AXIS 2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 400, 369–379. doi:10.1016/S0140-6736(22)01212-0
Dougados, M., Van Der Heijde, D., Chen, Y. C., Greenwald, M., Drescher, E., Liu, J., et al. (2017). Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: Results from the RA-BUILD study. Ann. Rheum. Dis. 76, 88–95. doi:10.1136/annrheumdis-2016-210094
Feagan, B. G., Danese, S., Loftus, E. V., Vermeire, S., Schreiber, S., Ritter, T., et al. (2021). Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): A phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet 397, 2372–2384. doi:10.1016/S0140-6736(21)00666-8
Fleischmann, R., Kremer, J., Cush, J., Schulze-Koops, H., Connell, C. A., Bradley, J. D., et al. (2012). Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N. Engl. J. Med. 367, 495–507. doi:10.1056/NEJMoa1109071
Fleischmann, R. M., Damjanov, N. S., Kivitz, A. J., Legedza, A., Hoock, T., and Kinnman, N. (2015). A randomized, double-blind, placebo-controlled, twelve-week, dose-ranging study of decernotinib, an oral selective JAK-3 inhibitor, as monotherapy in patients with active rheumatoid arthritis. Arthritis Rheumatol. 67, 334–343. doi:10.1002/art.38949
Fleischmann, R., Schiff, M., Van Der Heijde, D., Ramos-Remus, C., Spindler, A., Stanislav, M., et al. (2017). Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and No or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol. 69, 506–517. doi:10.1002/art.39953
Genovese, M. C., Fleischmann, R., Combe, B., Hall, S., Rubbert-Roth, A., Zhang, Y., et al. (2018). Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): A double-blind, randomised controlled phase 3 trial. Lancet 391, 2513–2524. doi:10.1016/S0140-6736(18)31116-4
Genovese, M. C., Kalunian, K., Gottenberg, J. E., Mozaffarian, N., Bartok, B., Matzkies, F., et al. (2019). Effect of filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: The FINCH 2 randomized clinical trial. Jama 322, 315–325. doi:10.1001/jama.2019.9055
Genovese, M. C., Kremer, J., Zamani, O., Ludivico, C., Krogulec, M., Xie, L., et al. (2016a). Baricitinib in patients with refractory rheumatoid arthritis. N. Engl. J. Med. 374, 1243–1252. doi:10.1056/NEJMoa1507247
Genovese, M. C., Van Vollenhoven, R. F., Pacheco-Tena, C., Zhang, Y., and Kinnman, N. (2016b). VX-509 (decernotinib), an oral selective JAK-3 inhibitor, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheumatol. 68, 46–55. doi:10.1002/art.39473
Gialouri, C. G., Moustafa, S., Thomas, K., Hadziyannis, E., and Vassilopoulos, D. (2023). Herpes zoster in patients with inflammatory arthritides or ulcerative colitis treated with tofacitinib, baricitinib or upadacitinib: A systematic review of clinical trials and real-world studies. Rheumatol. Int. 43, 421–435. doi:10.1007/s00296-022-05270-6
Gladman, D., Rigby, W., Azevedo, V. F., Behrens, F., Blanco, R., Kaszuba, A., et al. (2017). Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N. Engl. J. Med. 377, 1525–1536. doi:10.1056/NEJMoa1615977
Harbecke, R., Cohen, J. I., and Oxman, M. N. (2021). Herpes zoster vaccines. J. Infect. Dis. 224, S429–s442. doi:10.1093/infdis/jiab387
Harigai, M., and Honda, S. (2020). Selectivity of Janus kinase inhibitors in rheumatoid arthritis and other immune-mediated inflammatory diseases: Is expectation the root of all headache? Drugs 80, 1183–1201. doi:10.1007/s40265-020-01349-1
Harkins, P., Burke, E., Swales, C., Silman, A., and Conway, R. (2023). Are Janus kinase inhibitors safe and effective in treating the key clinical domains of psoriatic arthritis? A systematic review and meta-analysis. Int. J. Rheum. Dis. 26, 31–42. doi:10.1111/1756-185X.14447
Hoaglin, D. C., Hawkins, N., Jansen, J. P., Scott, D. A., Itzler, R., Cappelleri, J. C., et al. (2011). Conducting indirect-treatment-comparison and network-meta-analysis studies: Report of the ISPOR task force on indirect treatment comparisons good research practices: Part 2. Value Health 14, 429–437. doi:10.1016/j.jval.2011.01.011
Hu, X., Li, J., Fu, M., Zhao, X., and Wang, W. (2021). The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target Ther. 6, 402. doi:10.1038/s41392-021-00791-1
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern Med. 162, 777–784. doi:10.7326/M14-2385
Inthout, J., Ioannidis, J. P. A., Rovers, M. M., and Goeman, J. J. (2016). Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 6, e010247. doi:10.1136/bmjopen-2015-010247
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J., Gavaghan, D. J., et al. (1996). Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin. Trials 17, 1–12. doi:10.1016/0197-2456(95)00134-4
Kavanaugh, A., Kremer, J., Ponce, L., Cseuz, R., Reshetko, O. V., Stanislavchuk, M., et al. (2017). Filgotinib (GLPG0634/GS-6034), an oral selective JAK1 inhibitor, is effective as monotherapy in patients with active rheumatoid arthritis: Results from a randomised, dose-finding study (Darwin 2). Ann. Rheum. Dis. 76, 1009–1019. doi:10.1136/annrheumdis-2016-210105
Kawahira, K., Imano, H., Yamada, K., Mori, Y., Asada, H., Okuno, Y., et al. (2023). Risk of herpes zoster according to past history in the general population: The Japanese Shozu herpes zoster study. J. Dermatol. doi:10.1111/1346-8138.16828
Keystone, E. C., Taylor, P. C., Drescher, E., Schlichting, D. E., Beattie, S. D., Berclaz, P. Y., et al. (2015). Safety and efficacy of baricitinib at 24 weeks in patients with rheumatoid arthritis who have had an inadequate response to methotrexate. Ann. Rheum. Dis. 74, 333–340. doi:10.1136/annrheumdis-2014-206478
Kivitz, A. J., Gutierrez-Urena, S. R., Poiley, J., Genovese, M. C., Kristy, R., Shay, K., et al. (2017). Peficitinib, a JAK inhibitor, in the treatment of moderate-to-severe rheumatoid arthritis in patients with an inadequate response to methotrexate. Arthritis Rheumatol. 69, 709–719. doi:10.1002/art.39955
Kremer, J., Li, Z. G., Hall, S., Fleischmann, R., Genovese, M., Martin-Mola, E., et al. (2013). Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: A randomized trial. Ann. Intern Med. 159, 253–261. doi:10.7326/0003-4819-159-4-201308200-00006
Lee, E. B., Fleischmann, R., Hall, S., Wilkinson, B., Bradley, J. D., Gruben, D., et al. (2014). Tofacitinib versus methotrexate in rheumatoid arthritis. N. Engl. J. Med. 370, 2377–2386. doi:10.1056/NEJMoa1310476
Lee, Y. H., and Song, G. G. (2020). Relative efficacy and safety of tofacitinib, baricitinib, upadacitinib, and filgotinib in comparison to adalimumab in patients with active rheumatoid arthritis. Z Rheumatol. 79, 785–796. doi:10.1007/s00393-020-00750-1
Leng, X., Lin, W., Liu, S., Kanik, K., Wang, C., Wan, W., et al. (2023). Efficacy and safety of tofacitinib in Chinese patients with active psoriatic arthritis: A phase 3, randomised, double-blind, placebo-controlled study. RMD Open 9, e002559. doi:10.1136/rmdopen-2022-002559
Leung, J., Anderson, T. C., Dooling, K., Xie, F., and Curtis, J. R. (2022). Recombinant zoster vaccine uptake and risk of flares among older adults with immune-mediated inflammatory diseases in the US. Arthritis and Rheumatology 74, 1833–1841. doi:10.1002/art.42261
Lin, L. (2019). Use of prediction intervals in network meta-analysis. JAMA Netw. Open 2, e199735. doi:10.1001/jamanetworkopen.2019.9735
Loftus, E., Colombel, J. F., Lacerda, A. P., Peyrin-Biroulet, L., D'haens, G., Panaccione, R., et al. (2022). Efficacy and safety of upadactinib induction therapy in patients with moderately to severely active Crohn's disease: Results from a randomized phase 3 U-EXCEL study. United Eur. Gastroenterol. J. 10 (Suppl. 8), 103–104. doi:10.14309/01.ajg.0000859584.83560.ef
Mcinnes, I. B., Anderson, J. K., Magrey, M., Merola, J. F., Liu, Y., Kishimoto, M., et al. (2021). Trial of upadacitinib and adalimumab for psoriatic arthritis. N. Engl. J. Med. 384, 1227–1239. doi:10.1056/NEJMoa2022516
Mcinnes, I. B., and Gravallese, E. M. (2021a). Immune-mediated inflammatory disease therapeutics: Past, present and future. Nat. Rev. Immunol. 21, 680–686. doi:10.1038/s41577-021-00603-1
Mcinnes, I. B., and Gravallese, E. M. (2021b). Immune-mediated inflammatory disease therapeutics: Past, present and future. Nat. Rev. Immunol. 21, 680–686. doi:10.1038/s41577-021-00603-1
Mease, P., Coates, L. C., Helliwell, P. S., Stanislavchuk, M., Rychlewska-Hanczewska, A., Dudek, A., et al. (2018). Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active psoriatic arthritis (EQUATOR): Results from a randomised, placebo-controlled, phase 2 trial. Lancet 392, 2367–2377. doi:10.1016/S0140-6736(18)32483-8
Mease, P., Hall, S., Fitzgerald, O., Van Der Heijde, D., Merola, J. F., Avila-Zapata, F., et al. (2017). Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N. Engl. J. Med. 377, 1537–1550. doi:10.1056/NEJMoa1615975
Mease, P. J., Lertratanakul, A., Anderson, J. K., Papp, K., Van Den Bosch, F., Tsuji, S., et al. (2021). Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann. Rheum. Dis. 80, 312–320. doi:10.1136/annrheumdis-2020-218870
Moodley, D., Yoshida, H., Mostafavi, S., Asinovski, N., Ortiz-Lopez, A., Symanowicz, P., et al. (2016). Network pharmacology of JAK inhibitors. Proc. Natl. Acad. Sci. U. S. A. 113, 9852–9857. doi:10.1073/pnas.1610253113
Olivera, P. A., Lasa, J. S., Bonovas, S., Danese, S., and Peyrin-Biroulet, L. (2020). Safety of Janus kinase inhibitors in patients with inflammatory bowel diseases or other immune-mediated diseases: A systematic review and meta-analysis. Gastroenterology 158, 1554–1573. doi:10.1053/j.gastro.2020.01.001
Ortega, M. A., García-Montero, C., Fraile-Martinez, O., Alvarez-Mon, M. A., Gómez-Lahoz, A. M., Lahera, G., et al. (2022). Immune-mediated diseases from the point of view of psychoneuroimmunoendocrinology. Biol. (Basel) 11, 973. doi:10.3390/biology11070973
Panés, J., Sandborn, W. J., Schreiber, S., Sands, B. E., Vermeire, S., D'haens, G., et al. (2017). Tofacitinib for induction and maintenance therapy of crohn's disease: Results of two phase IIb randomised placebo-controlled trials. Gut 66, 1049–1059. doi:10.1136/gutjnl-2016-312735
Papp, K. A., Menter, M. A., Abe, M., Elewski, B., Feldman, S. R., Gottlieb, A. B., et al. (2015). Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: Results from two randomized, placebo-controlled, phase III trials. Br. J. Dermatol 173, 949–961. doi:10.1111/bjd.14018
Rusiñol, L., and Puig, L. (2023). Tyk2 targeting in immune-mediated inflammatory diseases. Int. J. Mol. Sci. 24, 3391. doi:10.3390/ijms24043391
Sampathkumar, P., Drage, L. A., and Martin, D. P. (2009). Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin. Proc. 84, 274–280. doi:10.1016/S0025-6196(11)61146-4
Sandborn, W. J., Su, C., Sands, B. E., D'haens, G. R., Vermeire, S., Schreiber, S., et al. (2017). Tofacitinib as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 376, 1723–1736. doi:10.1056/NEJMoa1606910
Schett, G., Mcinnes, I. B., and Neurath, M. F. (2021). Reframing immune-mediated inflammatory diseases through signature cytokine hubs. N. Engl. J. Med. 385, 628–639. doi:10.1056/NEJMra1909094
Shim, S., Yoon, B. H., Shin, I. S., and Bae, J. M. (2017). Network meta-analysis: Application and practice using Stata. Epidemiol. Health 39, e2017047. doi:10.4178/epih.e2017047
Smolen, J. S., Pangan, A. L., Emery, P., Rigby, W., Tanaka, Y., Vargas, J. I., et al. (2019). Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): A randomised, placebo-controlled, double-blind phase 3 study. Lancet 393, 2303–2311. doi:10.1016/S0140-6736(19)30419-2
Sterne, J. a. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. Bmj 366, l4898. doi:10.1136/bmj.l4898
Sunzini, F., Mcinnes, I., and Siebert, S. (2020). JAK inhibitors and infections risk: Focus on herpes zoster. Ther. Adv. Musculoskelet. Dis. 12, 1759720X20936059. 1759720x20936059. doi:10.1177/1759720X20936059
Takeuchi, T., Tanaka, Y., Iwasaki, M., Ishikura, H., Saeki, S., and Kaneko, Y. (2016). Efficacy and safety of the oral Janus kinase inhibitor peficitinib (ASP015K) monotherapy in patients with moderate to severe rheumatoid arthritis in Japan: A 12-week, randomised, double-blind, placebo-controlled phase IIb study. Ann. Rheum. Dis. 75, 1057–1064. doi:10.1136/annrheumdis-2015-208279
Takeuchi, T., Tanaka, Y., Tanaka, S., Kawakami, A., Iwasaki, M., Katayama, K., et al. (2019). Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to methotrexate: Results of a phase III randomised, double-blind, placebo-controlled trial (RAJ4) in Japan. Ann. Rheum. Dis. 78, 1305–1319. doi:10.1136/annrheumdis-2019-215164
Tanaka, Y., Takeuchi, T., Yamanaka, H., Nakamura, H., Toyoizumi, S., and Zwillich, S. (2015). Efficacy and safety of tofacitinib as monotherapy in Japanese patients with active rheumatoid arthritis: A 12-week, randomized, phase 2 study. Mod. Rheumatol. 25, 514–521. doi:10.3109/14397595.2014.995875
Taylor, P. C., Keystone, E. C., Van Der Heijde, D., Weinblatt, M. E., Del Carmen Morales, L., Reyes Gonzaga, J., et al. (2017). Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N. Engl. J. Med. 376, 652–662. doi:10.1056/NEJMoa1608345
Thomas, S. L., and Hall, A. J. (2004). What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect. Dis. 4, 26–33. doi:10.1016/s1473-3099(03)00857-0
Valladales-Restrepo, L. F., Velasquez-Quimara, S., and Machado-Alba, J. E. (2023). Pharmacological treatment of herpes zoster and factors associated with its recurrence. Antibiot. (Basel) 12, 757. doi:10.3390/antibiotics12040757
Van Der Heijde, D., Baraliakos, X., Sieper, J., Deodhar, A., Inman, R. D., Kameda, H., et al. (2022). Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: A double-blind, randomised, placebo-controlled phase 3 trial. Ann. Rheum. Dis. 81, 1515–1523. doi:10.1136/ard-2022-222608
Van Der Heijde, D., Deodhar, A., Wei, J. C., Drescher, E., Fleishaker, D., Hendrikx, T., et al. (2017). Tofacitinib in patients with ankylosing spondylitis: A phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann. Rheum. Dis. 76, 1340–1347. doi:10.1136/annrheumdis-2016-210322
Van Der Heijde, D., Song, I. H., Pangan, A. L., Deodhar, A., Van Den Bosch, F., Maksymowych, W. P., et al. (2019). Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): A multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial. Lancet 394, 2108–2117. doi:10.1016/S0140-6736(19)32534-6
Van Der Heijde, D., Tanaka, Y., Fleischmann, R., Keystone, E., Kremer, J., Zerbini, C., et al. (2013). Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: Twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 65, 559–570. doi:10.1002/art.37816
Van Oorschot, D., Vroling, H., Bunge, E., Diaz-Decaro, J., Curran, D., and Yawn, B. (2021). A systematic literature review of herpes zoster incidence worldwide. Hum. Vaccin Immunother. 17, 1714–1732. doi:10.1080/21645515.2020.1847582
Van Valkenhoef, G., Dias, S., Ades, A. E., and Welton, N. J. (2016). Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res. Synth. Methods 7, 80–93. doi:10.1002/jrsm.1167
Van Vollenhoven, R. F., Fleischmann, R., Cohen, S., Lee, E. B., Garcia Meijide, J. A., Wagner, S., et al. (2012). Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N. Engl. J. Med. 367, 508–519. doi:10.1056/NEJMoa1112072
Vermeire, S., Schreiber, S., Petryka, R., Kuehbacher, T., Hebuterne, X., Roblin, X., et al. (2017). Clinical remission in patients with moderate-to-severe crohn's disease treated with filgotinib (the FITZROY study): Results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 389, 266–275. doi:10.1016/S0140-6736(16)32537-5
Wang, F., Tang, X., Zhu, M., Mao, H., Wan, H., and Luo, F. (2022). Efficacy and safety of JAK inhibitors for rheumatoid arthritis: A meta-analysis. J. Clin. Med. 11, 4459. doi:10.3390/jcm11154459
Westhovens, R., Taylor, P. C., Alten, R., Pavlova, D., Enriquez-Sosa, F., Mazur, M., et al. (2017). Filgotinib (GLPG0634/GS-6034), an oral JAK1 selective inhibitor, is effective in combination with methotrexate (MTX) in patients with active rheumatoid arthritis and insufficient response to MTX: Results from a randomised, dose-finding study (Darwin 1). Ann. Rheum. Dis. 76, 998–1008. doi:10.1136/annrheumdis-2016-210104
Yamaoka, K., and Oku, K. (2023). JAK inhibitors in rheumatology. Immunol. Med., 1–10. doi:10.1080/25785826.2023.2172808
Yang, F., Lu, C., Wang, Y., Liu, H., Leng, X., and Zeng, X. (2023). Efficacy and safety of Janus kinase inhibitors in patients with psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Clin. Rheumatol. 42, 1593–1605. doi:10.1007/s10067-023-06529-4
Zhang, J., Tsai, T. F., Lee, M. G., Zheng, M., Wang, G., Jin, H., et al. (2017). The efficacy and safety of tofacitinib in asian patients with moderate to severe chronic plaque psoriasis: A phase 3, randomized, double-blind, placebo-controlled study. J. Dermatol Sci. 88, 36–45. doi:10.1016/j.jdermsci.2017.05.004
Keywords: herpes zoster, JAK inhibitors, immune-mediated inflammatory diseases, systematic review, meta-analysis
Citation: Xu Q, He L and Yin Y (2023) Risk of herpes zoster associated with JAK inhibitors in immune-mediated inflammatory diseases: a systematic review and network meta-analysis. Front. Pharmacol. 14:1241954. doi: 10.3389/fphar.2023.1241954
Received: 17 June 2023; Accepted: 28 July 2023;
Published: 08 August 2023.
Edited by:
Sukanya Saha, National Institute of Environmental Health Sciences (NIH), United StatesReviewed by:
Tarunendu Mapder, Bristol Myers Squibb, United StatesCopyright © 2023 Xu, He and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yufeng Yin, eWlueXVmZW5nQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.