- 1Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Pharmacy, Peking Union Medical College Hospital, Beijing, China
- 3Department of Pharmacy, Hospital of Honghe State Affiliated to Kunming Medical University, Southern Central Hospital of Yunnan Province, Mengzi, China
- 4Institute of Medicinal Biotechnology, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
Background: The sedative role of dexmedetomidine (DEX) in gastrointestinal endoscopic procedures is unclear. We performed this systematic review and meta-analysis to assess the efficacy and safety of sedation with DEX during gastrointestinal endoscopic procedures with a view to providing evidence-based references for clinical decision-making.
Methods: The PubMed, Embase, Cochrane Library, Web of Science, and ClinicalTrials.gov databases were searched for randomized controlled trials (RCTs) that compared DEX with different sedatives comparators (such as propofol, midazolam, and ketamine) for sedation in a variety of adult gastrointestinal endoscopic procedures from inception to 1 July 2022. Standardized mean difference (SMD) and weighted mean difference (WMD) with 95% confidence interval (CI) or pooled risk ratios (RR) with 95% CI were used for continuous outcomes or dichotomous outcomes, respectively, and a random-effect model was selected regardless of the significance of the heterogeneity.
Results: Forty studies with 2,955 patients were assessed, of which 1,333 patients were in the DEX group and 1,622 patients were in the control (without DEX) group. The results suggested that the primary outcomes of sedation level of DEX are comparable to other sedatives, with similar RSS score and patient satisfaction level, and better in some clinical outcomes, with a reduced risk of body movements or gagging (RR: 0.60; 95% CI: 0.37 to 0.97; p = 0.04; I2 = 68%), and a reduced additional requirement for other sedatives, and increased endoscopist satisfaction level (SMD: 0.41; 95% CI: 0.05 to 0.77; p = 0.03; I2 = 86%). In terms of secondary outcomes of adverse events, DEX may benefit patients in some clinical outcomes, with a reduced risk of hypoxia (RR:0.34; 95% CI: 0.20 to 0.55; p < 0.0001; I2 = 52%) and cough (RR: 0.25; 95% CI: 0.12 to 0.54; p = 0.0004; I2 = 0%), no significant difference in the risk of hypotension, while an increased risk of bradycardia (RR: 3.08; 95% CI: 2.12 to 4.48; p < 0.00001; I2 = 6%).
Conclusion: This meta-analysis indicates that DEX is a safe and effective sedative agent for gastrointestinal endoscopy because of its benefits for patients in some clinical outcomes. Remarkably, DEX is comparable to midazolam and propofol in terms of sedation level. In conclusion, DEX provides an additional option in sedation for gastrointestinal endoscopic procedures.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/#searchadvanced
Introduction
Conscious sedation is a common strategy to increase patient comfort during gastrointestinal endoscopy, as it is an uncomfortable and stressful procedure for most patients. It improves clinical outcomes by decreasing procedural pain, increasing patient satisfaction, relieving patient anxiety and discomfort, and minimizes the risk of adverse effects by avoiding involuntary and untimely patient movements that might interfere with endoscopic procedures (Early et al., 2018; Kamal et al., 2021; Mason et al., 2021). A number of agents are available for conscious sedation during endoscopic procedures, including benzodiazepines (midazolam), opioids (fentanyl and meperidine), propofol, and dexmedetomidine (DEX) (Waring et al., 2003; Vargo et al., 2012).
Dexmedetomidine (DEX) is a potent, highly selective α2-adrenergic receptor agonist with the properties of sedation, analgesia, anxiolysis, and sympathetic tone inhibition (Aantaa and Scheinin, 1993; Kamibayashi and Maze, 2000). DEX was first approved for sedation in intensive care units (ICU) in 1999, and its use has been rapidly extended to patients sedation in a variety of clinical situations (Takrouri et al., 2002). DEX has recently gained popularity in gastrointestinal endoscopic procedures due to its superiority over conventional sedatives, including cooperative or semi-rousable sedation, minimal respiratory depression at high doses, and fewer cardiopulmonary complications (Ebert et al., 2000; Venn et al., 2000; Liu X et al., 2021). However, associated adverse effects of DEX such as hypotension, the biphasic dose-response relationship of mean arterial pressure, and bradycardia have been reported (Ebert et al., 2000; Talke et al., 2003; Bharati et al., 2011). Despite the widespread use of DEX, significant concerns remain about its safety and efficacy. Several meta-analyses of randomized controlled trials (RCTs) and many clinical studies have compared DEX with other conventional sedatives (Nishizawa et al., 2015; Zhang et al., 2016; Nishizawa et al., 2017; Liu W et al., 2021), due to the limited number of studies and the single perspective (simply comparing DEX with a particular sedative), no clear conclusions have been drawn about the role of dexmedetomidine for sedation in gastrointestinal endoscopic procedures. There are even some conflicting conclusions, for instance, a previous study reported that DEX is associated with better sedation than midazolam in gastrointestinal endoscopic procedures, however, another study showed that DEX may be a possible alternative to midazolam in sedation (Nishizawa et al., 2015; Zhang et al., 2016).
The many favorable physiological effects of DEX have made it increasingly popular and applied in gastrointestinal endoscopic procedures, however, no current literature reviews are providing a definite conclusion about the role of DEX in gastrointestinal endoscopic procedures sedation. We, therefore, performed an updated, systematic, and pooled meta-analysis of the currently associated RCTs to evaluate the efficacy and safety of DEX compared with multiple conventional sedatives in various gastrointestinal endoscopic procedures.
Methods
Data sources and literature search strategy
We conducted this meta-analysis in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guideline (Higgins et al., 2011). It was prospectively registered on PROSPERO (CRD42022345358). We performed a comprehensive search of the MEDLINE (via PubMed), Embase (via Ovid), Cochrane Central Register of Controlled Trials (CENTRAL), Institute for Scientific Information (ISI) Web of Science, and the ClinicalTrials.gov database from inception to 1 July 2022. The search comprised free-text terms and database-specific subject headings for DEX in combination with endoscopic procedures. The full search strategies for all databases are provided in Supplementary Material SA. The PRISMA checklist is provided in SI B.
Two authors (RT and YH) independently screened the titles and abstracts of all studies retrieved by the search strategy. We excluded obviously irrelevant studies and documented the reason for exclusion of studies when the reason for exclusion is not explicit. Eligibility for the remaining studies then be identified by reading the full text and according to predefined inclusion and exclusion criteria, besides, we reviewed the reference lists of all eligible studies and previously published systematic reviews and meta-analyses to identify additional relevant randomized controlled trials. We attempted to contact the first author of the relevant trial when further information was required or any queries arose. We resolved disagreements between the two authors (RT and YH) by discussion until consensus is reached or by consulting with a third author (YZ).
Inclusion and exclusion criteria
We included studies meeting the following inclusion criteria: 1) study type: RCT; 2) population: adult patients (16 years of age or older) undergoing all types of diagnostic and therapeutic gastrointestinal endoscopic procedures; 3) intervention: perioperative administration of DEX alone or in combination, irrespective of the route of administration, dosage, frequency, and duration; 4) comparator: any other pharmacological interventions, including other sedative agents such as propofol, midazolam, and ketamine, or 0.9% sodium chloride, or placebo; 5) outcomes: eligible studies had to report at least one of the predetermined outcomes listed in the following: a) primary outcomes: sedation level (Ramsay sedation scale (RSS) score, body movements or gagging, endoscopist and patient satisfaction level, and reduction in other sedative requirements); b) secondary outcomes: adverse events (hypoxia, hypotension, bradycardia, and cough). Only full articles published in English were considered.
Duplicate publications, reviews, prospective cohort studies, cross-over trials, quasi-randomized trials, and all nonrandomized trials were excluded.
Data extraction
Four authors (RT and YH; XM and HY; in pairs) independently performed data extraction using predesigned data extraction forms. We extracted the following characteristics from each included study: the first author, year of publication, country or location where the study was conducted, study quality, sample size, details of participants (such as age and sex), inclusion criteria, exclusion criteria, type of endoscopic procedure, details of intervention (such as route of administration, dosage, and duration of DEX, and comparator medication), outcomes (as listed in above), adverse events, and risk of bias. When studies reported multiple treatment arms using additional sedatives, only data from the groups utilizing DEX were extracted. Data reported in graph form were extracted by the software GetData Graph Digitizer (v2.25, Canopus, Japan).
Any discrepancies in extracted data were resolved by a repeat review of the original text and discussion with a third author (KS and LR).
Risk of bias assessment
Two authors (YZ and KS) independently assessed risk of bias for each included study using the Cochrane risk of bias tool described in the Cochrane Collaboration Handbook for Systematic Reviews of Interventions (Higgins et al., 2011). Included studies were assessed at low, high, or unclear risk of bias across seven domains applicable to RCTs: random sequence generation, allocation concealment, blinding methods, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias. Any disagreements were resolved by a repeat review of the data and consensus through discussion, or arbitration by a third author if necessary.
Data synthesis and statistical analysis
We calculated the standardized mean difference (SMD) and weighted mean difference (WMD) with 95% confidence interval (CI) or pooled risk ratios (RRs) with 95% CI for continuous outcomes or dichotomous outcomes, respectively; for other outcomes, we performed a qualitative analysis. Measurement data presented as mean (SE) or mean will be excluded. For the purposes of this review, we included the studies reporting the range or inter-quartile range (IQR), and standard deviation (SD) was estimated with the formulas:
The heterogeneity across studies was assessed with Cochran’s Q test (p < 0.10 for statistical significance) and the I2 statistic (I2 > 50% for significant heterogeneity). We always used a random-effect model, regardless of the significance of the heterogeneity. Regardless of the level of heterogeneity (significant or not), we performed subgroup analyses to explore possible sources of clinical heterogeneity or to assess the effect of grouping factors on outcomes: 1) different comparators (saline or other sedative agents such as propofol, midazolam, and ketamine, or opioids (including fentanyl, sufentanil, remifentanil, and meperidine)); 2) surgery type that is divided into two groups based on the procedure length (the non-advanced endoscopic procedures consisting of gastroscopy, colonoscopy, gastrointestinal endoscopy, diagnostic esophagogastroduodenal endoscopy, and esophagogastroduodenoscopy, and the advanced endoscopic procedures including endoscopic retrograde cholangiopancreatography (ERCP), and endoscopic submucosal dissection (ESD) ESD; 3) different scoring systems or definitions for some outcomes (a. different scoring systems for endoscopist satisfaction level (the numeric rating scores (1–4), or the visual analogue scale (VAS) scores (0–10 or 0–100)); b. different scoring systems for patient satisfaction level (the seven-step numeric range Likert scale (1–7), or the VAS scores (0–10 or 0–100)); c. different definitions for hypoxia (SpO2 < 90% or <94%)). Subgroup analysis was performed only if there were at least two studies in each subgroup, and the data were analysed by χ2 test.
Additionally, sensitivity analyses were performed to assess the effect of individual studies with a high risk of bias on the stability of pooled data. Finally, publication bias was detected by funnel plot asymmetry with Egger’s regression tests. All statistical analyses will be performed using Review Manager 5.4. (RevMan, v5.4, The Cochrane Collaboration, Oxford, UK) and Stata/SE 17.0 (Stata Corp., TX, United States).
Results
Literature search results and study characteristics
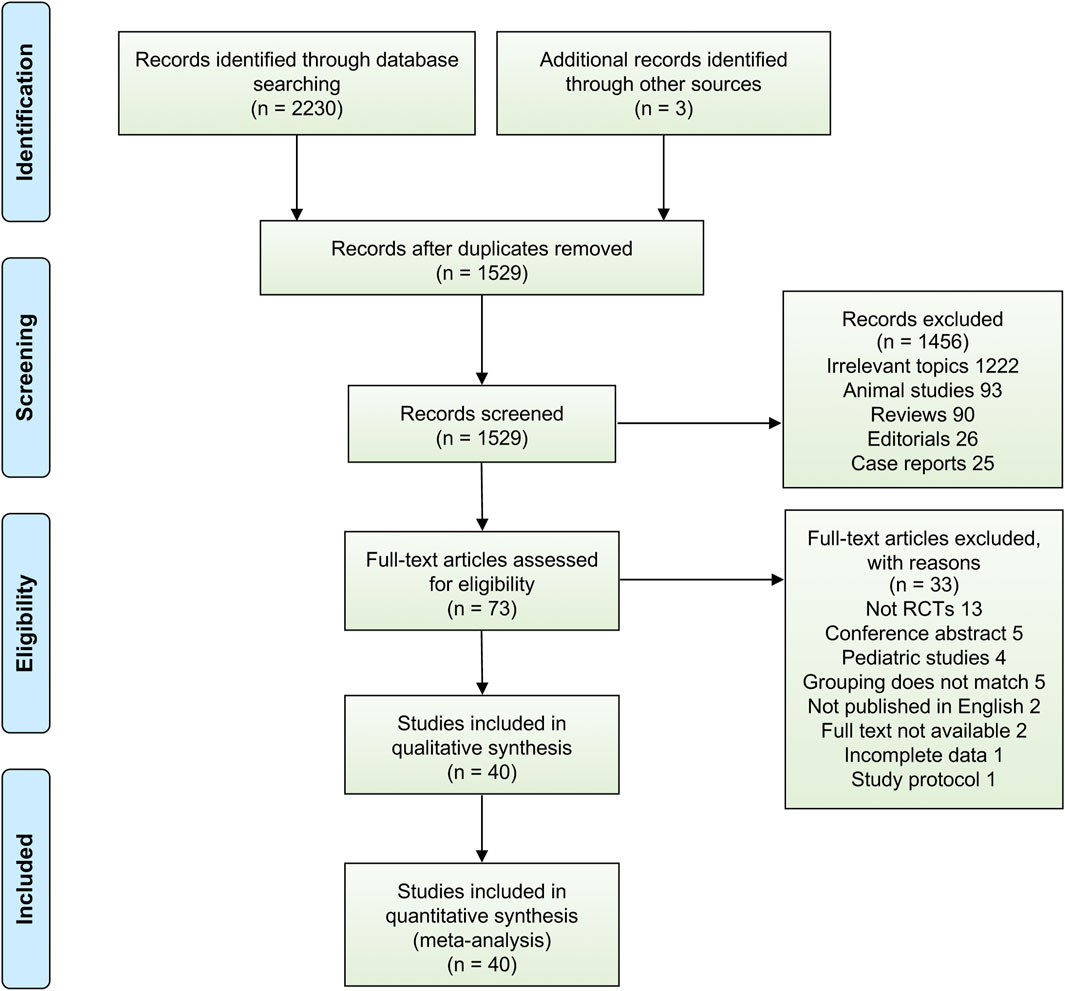
Using the aforementioned literature search strategy, 2,233 potentially relevant citations were found through a systematic search, and 1,529 articles remained after exclusion of duplicates. Of those, 1,456 citations were removed after title and abstract screening, 73 studies underwent a full-text review, and 33 studies were subsequently excluded after the full-text review. Finally, we included 40 RCTs that fulfilled the eligibility criteria for analysis, and a detailed overview of PRISMA flowchart of database search and study identification is shown in Figure 1, reflecting the search process and the reasons for exclusion.
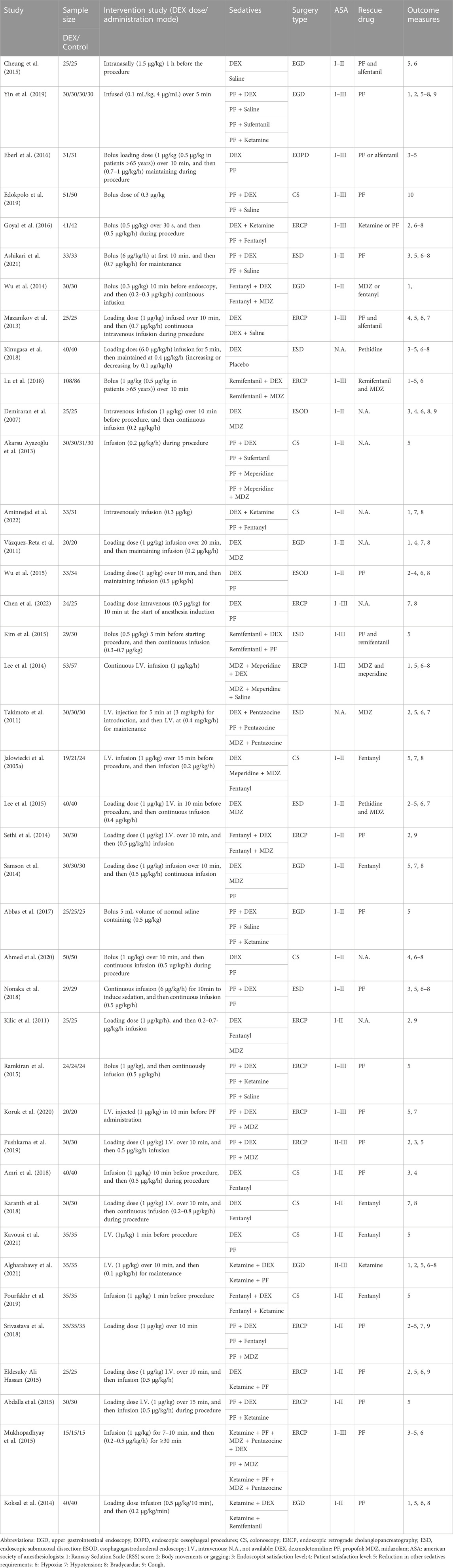
The characteristics of included trials are summarized in Table 1. 40 RCTs enrolling a total of 2,955 patients who underwent gastrointestinal endoscopic procedures were included in this meta-analysis. Of those, 1,333 patients were in the DEX group (alone or in combination) and 1,622 patients were in the control (without DEX) group. We pooled saline and other sedatives as a collective control group. Of these included trials, 18 studies used DEX alone, whilst 22 studies used DEX in combination with other sedatives; 31 studies involved one DEX intervention group and one control group, whilst 9 studies set ≥2 control groups; 8 studies compared DEX with saline, whilst 32 studies included other comparators (propofol, midazolam, ketamine, fentanyl, remifentanil, sufentanil, and meperidine). Nineteen studies were non-advanced endoscopic procedures, and the other 21 studies were advanced endoscopic procedures.
Risk-of-bias assessment
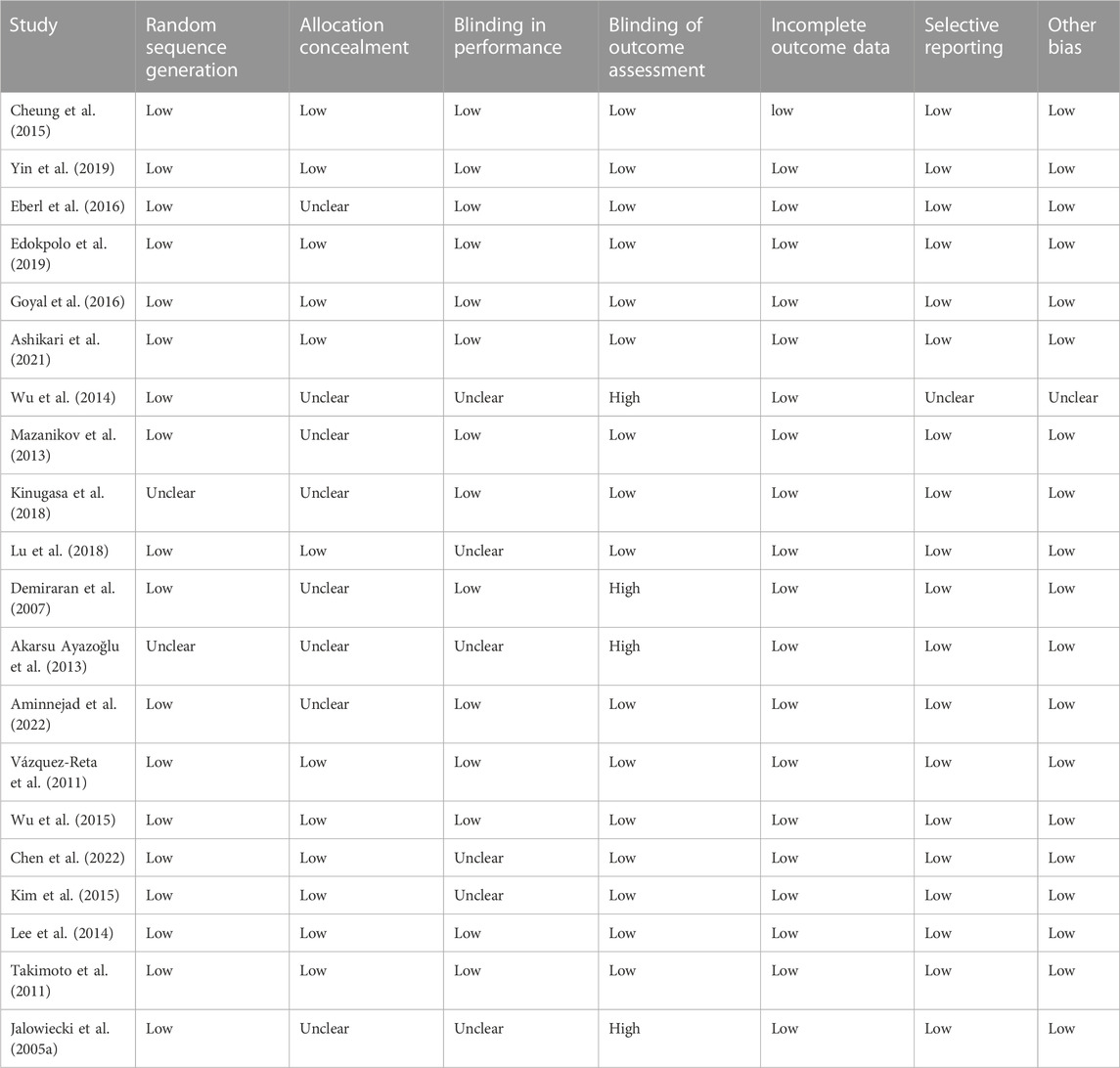
The risk of bias assessment for included RCTs is summarized in Table 2 and Supplementary Figure S1. In general, the included trials had a low risk of bias, apart from several studies. Four RCTs did not describe the specific methods used for random sequence generation. Allocation concealment was unclear in 16 RCTs. Blinding of participants was unclear in 2 RCTs, and 7 RCTs were inadequate blinding of participants (single-blind). Six RCTs were deemed to be of high risk of bias due to blinding of outcomes assessment was not carried out. Adequate assessment of incomplete outcomes was reported in all 40 RCTs. The study by Wu et al. was a retrospective randomized study and therefore had a potential bias in selective reporting and other biases. All other RCTs avoided selective outcome reporting and were free from other biases.
Meta-analysis results
Primary outcomes
Ramsay sedation scale (RSS) score
Seven studies (Algharabawy et al., 2021; Aminnejad et al., 2022; Koksal et al., 2014; B. S. Lee et al., 2014; Lu et al., 2018; Wu et al., 2014; Yin et al., 2019) reported the RSS score of patients, with 369 patients in the control group vs. 389 patients in the DEX group. There was no significant difference between the DEX group and the control group in RSS score of patients (WMD: 0.34; 95% CI: −0.04 to 0.72; p = 0.08; I2 = 96%) (Figure 2A).
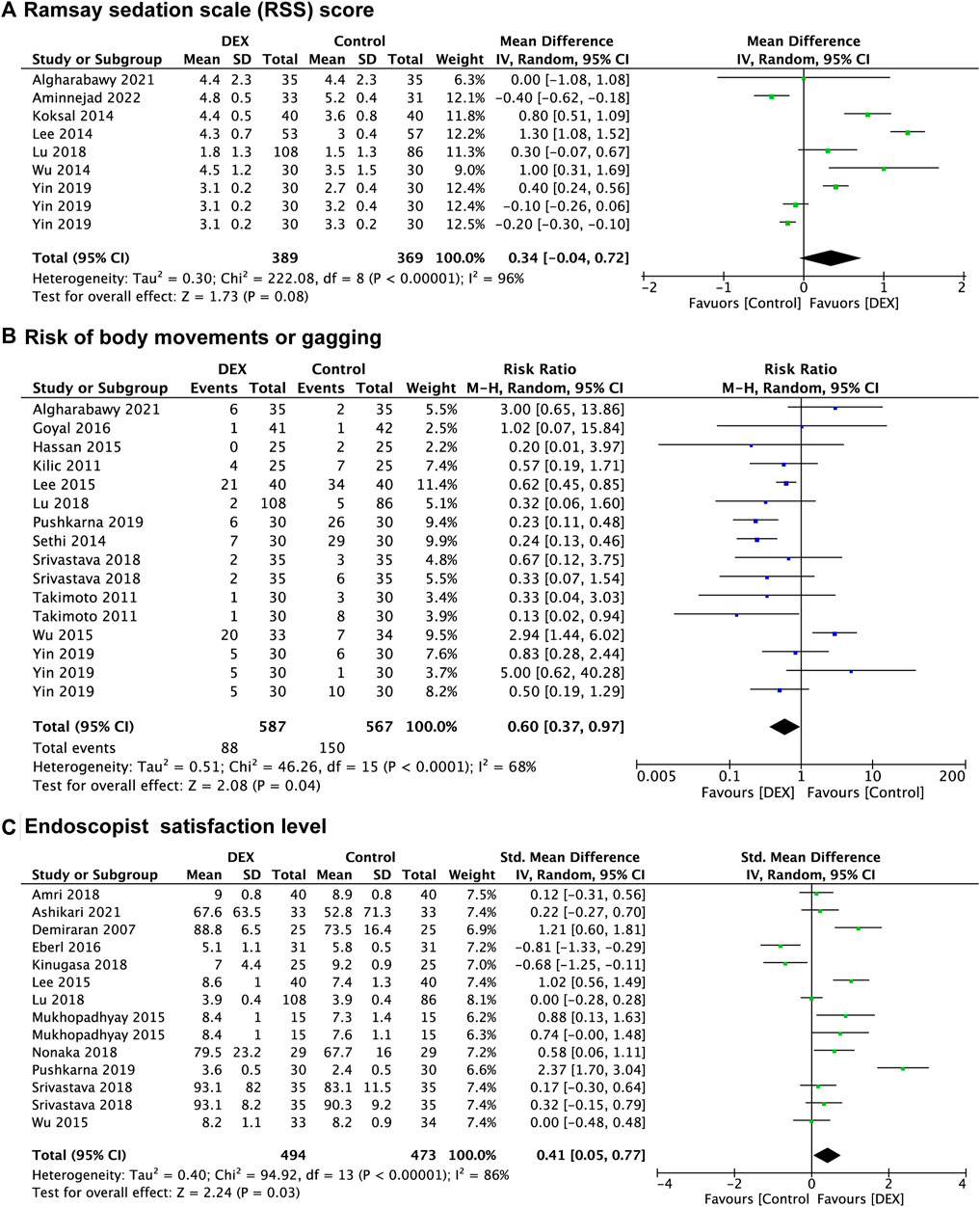
FIGURE 2. Forest plot depicting Ramsay sedation scale (RSS) score (A), risk of body movements or gagging (B), and endoscopist satisfaction level (C). Boxes represent mean differences, and the line across each box represents respective 95% CI. CI, confidence interval; DEX, dexmedetomidine; SD, standard deviation.
In the subgroup analysis (Supplementary Figure S2), there was no significant difference in RSS score of patients between the DEX group and the saline group (p = 0.06), the midazolam group (p = 0.09), and the opioids group (p = 0.45). Subgroup analysis also indicated that there was no significant difference in RSS score of patients between the DEX group and the control group in both the non-advanced endoscopic procedures (p = 0.29) and the advanced endoscopic procedures (p = 0.10).
Body movements or gagging
Twelve studies (Kilic et al., 2011; Takimoto et al., 2011; Eldesuky Ali Hassan, 2015; Lee et al., 2015; Wu et al., 2015; Goyal et al., 2016; Lu et al., 2018; Srivastava et al., 2018; Pushkarna et al., 2019; Yin et al., 2019; Algharabawy et al., 2021) recorded the prevalence of body movements or gagging, with 567 patients in the control group vs. 587 patients in the DEX group. The prevalence of body movements or gagging was significantly decreased in the DEX group (RR: 0.60; 95% CI: 0.37 to 0.97; p = 0.04; I2 = 68%) compared with the control group (Figure 2B).
In the subgroup analysis (Supplementary Figure S3), the prevalence of body movements or gagging was significantly decreased in the DEX group compared with the midazolam group (RR: 0.35; 95% CI: 0.20 to 0.59; p < 0.0001; I2 = 59%), but not in the propofol group (p = 0.18) and the opioids group (p = 0.60). There was a significant decrease in the prevalence of body movements or gagging between the DEX group and the control group in the advanced endoscopic procedures (RR: 0.37; 95% CI: 0.25 to 0.57; p < 0.00001; I2 = 35%), but not in the non-advanced endoscopic procedures (p = 0.32).
Endoscopist satisfaction level
Twelve studies (Demiraran et al., 2007; Abdalla et al., 2015; Lee et al., 2015; Wu et al., 2015; Eberl et al., 2016; Amri et al., 2018; Kinugasa et al., 2018; Lu et al., 2018; Nonaka et al., 2018; Srivastava et al., 2018; Pushkarna et al., 2019; Ashikari et al., 2021) evaluated endoscopist satisfaction level, and data from 967 patients were recorded, of which 473 were in the control group and 494 were in the DEX group. There was a significant increase in endoscopist satisfaction level in the DEX group (SMD: 0.41; 95% CI: 0.05 to 0.77; p = 0.03; I2 = 86%) compared with the control group (Figure 2C).
In the subgroup analysis (Supplementary Figure S4), endoscopist satisfaction level was significantly increased in the DEX group (SMD: 0.92; 95% CI: 0.15 to 1.69; p = 0.02; I2 = 93%) compared with the midazolam group, but no significant differences were found in the propofol group (p = 0.33) and the opioids group (p = 0.19). There was no significant difference between groups in the non-advanced endoscopic procedures (p = 0.23), and the advanced endoscopic procedures (p = 0.07). For the subgroup analysis of different scoring systems, the pooled results suggested that there was no significant difference between the DEX group and the control group in both the Numeric rating scores (p = 0.32) and the VAS scores (0–10) (p = 0.19), however, endoscopist satisfaction level of DEX group was higher than the control group in the VAS scores (0–100) (SMD: 9.63; 95% CI: 2.15 to 17.12; p = 0.01; I2 = 63%).
Patient satisfaction level
Twelve studies (Demiraran et al., 2007; Vázquez-Reta et al., 2011; Mazanikov et al., 2013; Lee et al., 2015; Mukhopadhyay et al., 2015; Wu et al., 2015; Eberl et al., 2016; Amri et al., 2018; Kinugasa et al., 2018; Lu et al., 2018; Srivastava et al., 2018; Ahmed et al., 2020) evaluated patient satisfaction level, and data from 973 patients were recorded, of which 476 were in the control group and 497 were in the DEX group. Compared with the control group, the pooled SMD of patient satisfaction level in the DEX group was 0.09 (95% CI: −0.13 to 0.30; p = 0.44; I2 = 63%), indicating no significant difference between the two groups (Figure 3A).
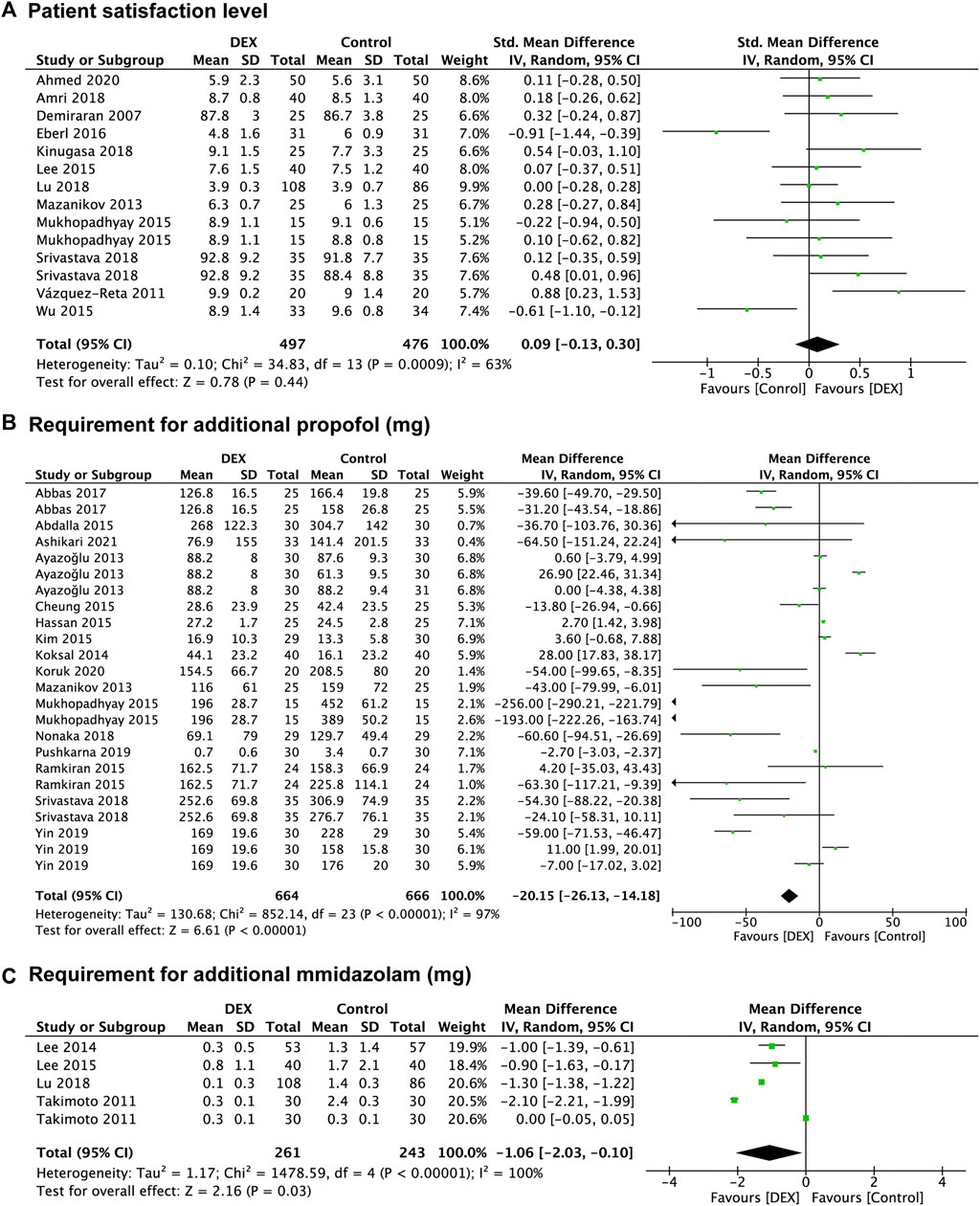
FIGURE 3. Forest plot depicting patient satisfaction level (A), the requirement for additional propofol (B) and midazolam (C). Boxes represent mean differences, and the line across each box represents respective 95% CI. CI, confidence interval; DEX, dexmedetomidine; SD, standard deviation.
In the subgroup analysis (Supplementary Figure S5), no significant difference was found in terms of patient satisfaction level between the DEX group and the propofol group (p = 0.16), midazolam group (p = 0.06), and opioids group (p = 0.35). Both the non-advanced endoscopic procedures (p = 0.49) and the advanced endoscopic procedures (p = 0.68) had no significant difference between the DEX group and the control group. For the subgroup analysis of different scoring systems, the pooled results indicated that there was no significant difference in patient satisfaction level between the DEX group and the control group in the Seven-step numeric range Likert scale (p = 0.68) or the VAS scores (0–10) (p = 0.50) or the VAS scores (0–100) (p = 0.06).
Reduction in other sedatives requirements
Twenty-seven studies provided data regarding the reduction in other sedatives requirements, which was measured in 1,256 patients in the control group and 1,287 patients in the DEX group. The requirement for additional propofol was significantly reduced in the DEX group (WMD: −20.15; 95% CI: −26.13 to −14.18; p < 0.00001; I2 = 97%) compared with the control group (Figure 3B), and data from 16 studies (Akarsu Ayazoğlu et al., 2013; Mazanikov et al., 2013; Koksal et al., 2014; Abdalla et al., 2015; Cheung et al., 2015; Eldesuky Ali Hassan, 2015; Kim et al., 2015; Mukhopadhyay et al., 2015; Ramkiran et al., 2015; Abbas et al., 2017; Nonaka et al., 2018; Srivastava et al., 2018; Pushkarna et al., 2019; Yin et al., 2019; Koruk et al., 2020; Ashikari et al., 2021) with 24 comparison groups. There was also a significant reduction of the requirement for additional midazolam in the DEX group (WMD: −1.06; 95% CI: −2.03 to −0.10; p = 0.03; I2 = 100%) compared with the control group (Figure 3C), and data from 3 studies (Takimoto et al., 2011; Lee et al., 2014; Lee et al., 2015) with 5 comparison groups. We found no significant difference in the requirement for additional opioids between the two groups (p = 0.20) (Figure 4A), and data from 12 studies (Jalowiecki et al., 2005a; Mazanikov et al., 2013; Lee et al., 2014; Samson et al., 2014; Sethi et al., 2014; Cheung et al., 2015; Lee et al., 2015; Eberl et al., 2016; Kinugasa et al., 2018; Lu et al., 2018; Pourfakhr et al., 2019; Kavousi et al., 2021) with 14 comparison groups.
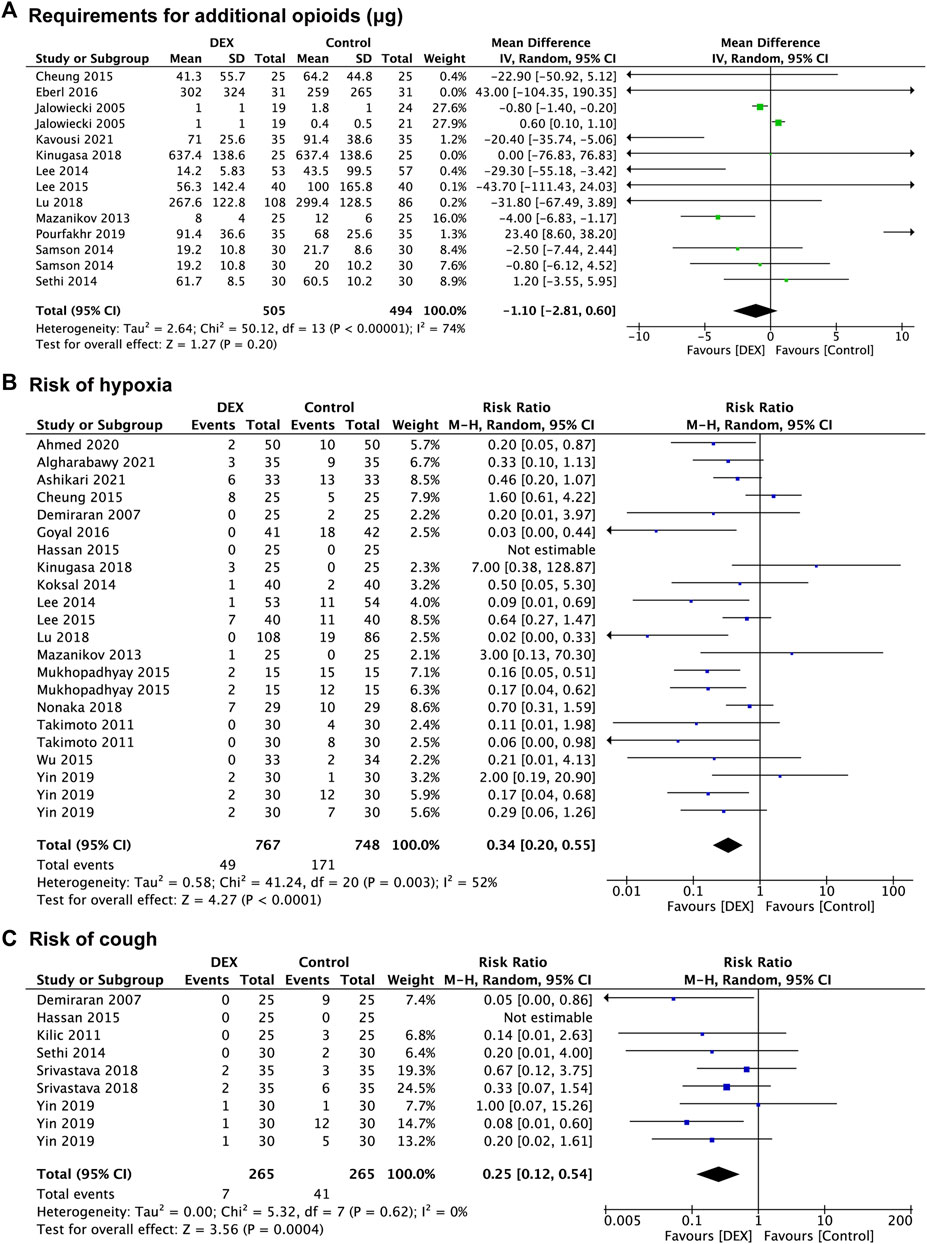
FIGURE 4. Forest plot depicting the requirement for additional opioids (A), risk of hypoxia (B), and risk of cough (C). Boxes represent mean differences, and the line across each box represents respective 95% CI. CI, confidence interval; DEX, dexmedetomidine; SD, standard deviation.
In the subgroup analysis of opioids consumption, the requirement for pethidine was significantly decreased in the DEX group (WMD: −28.33; 95% CI: −51.39 to −5.27; p = 0.02; I2 = 0%) compared with the control group, however, no significant difference was found between the two groups in the requirement for fentanyl (p = 0.83) and alfentanil (p = 0.17). The subgroup analysis of the requirement for opioids between the two groups identified no significant difference in both the non-advanced endoscopic procedures (p = 0.64) and the advanced endoscopic procedures (p = 0.19) (Supplementary Figure S6). The subgroup analysis of the requirement for propofol showed that the DEX group had significantly lower requirements than the control group in the advanced endoscopic procedures (WMD: −36.10; 95% CI: −44.74 to −27.45; p < 0.00001; I2 = 97%), but not in the non-advanced endoscopic procedures (p = 0.27). There was no significant difference in the requirement for midazolam between the DEX group and the control group in the advanced endoscopic procedures (p = 0.16) (Supplementary Figure S7).
Secondary outcomes
Adverse events
Hypoxia
Eighteen studies (Demiraran et al., 2007; Takimoto et al., 2011; Mazanikov et al., 2013; Koksal et al., 2014; Lee et al., 2014; Cheung et al., 2015; Eldesuky Ali Hassan, 2015; Lee et al., 2015; Mukhopadhyay et al., 2015; Wu et al., 2015; Goyal et al., 2016; Kinugasa et al., 2018; Lu et al., 2018; Nonaka et al., 2018; Yin et al., 2019; Ahmed et al., 2020; Algharabawy et al., 2021; Ashikari et al., 2021) reported data concerning the risk of hypoxia, which was observed in 748 patients in the control group and 767 patients in the DEX group. The risk of hypoxia was significantly decreased in the DEX group (RR:0.34; 95% CI: 0.20 to 0.55; p < 0.0001; I2 = 52%) compared with the control group (Figure 4B).
In the subgroup analysis, the DEX group carried a lower risk of hypoxia (RR: 0.23; 95% CI: 0.10 to 0.54; p = 0.0007; I2 = 0%) than the propofol group, however, the results indicated no significant difference between the DEX group and saline group (p = 0.18), and the midazolam group (p = 0.07), and the opioids group (p = 0.09). Compared with the control group, the pooled RR of hypoxia when using DEX was 0.42 (95% CI: 0.21 to 0.85; p = 0.01; I2 = 36%) in the non-advanced endoscopic procedures and 0.28 (95% CI: 0.13 to 0.58; p = 0.0006; I2 = 61%) in the non-advanced endoscopic procedures, showing a significantly lower risk of hypoxia in the DEX group (Supplementary Figure S8). When the definition of hypoxia was SpO2 < 90%, the risk of hypoxia was decreased in the DEX group (RR: 0.28; 95% CI: 0.15 to 0.53; p < 0.0001; I2 = 40%) compared with the control group, but no significant difference was found when the definition of hypoxia was SpO2 < 94% (p = 0.06) (Supplementary Figure S8).
Hypotension
Nineteen studies (Jalowiecki et al., 2005a; Takimoto et al., 2011; Vázquez-Reta et al., 2011; Mazanikov et al., 2013; Lee et al., 2014; Samson et al., 2014; Lee et al., 2015; Goyal et al., 2016; Karanth et al., 2018; Kinugasa et al., 2018; Nonaka et al., 2018; Srivastava et al., 2018; Yin et al., 2019; Ahmed et al., 2020; Koruk et al., 2020; Algharabawy et al., 2021; Ashikari et al., 2021; Aminnejad et al., 2022; Chen et al., 2022) recorded the risk of hypotension, with 787 patients in the control group vs. 776 patients in the DEX group. There was no significant difference in risk of hypotension between the DEX group (RR: 1.31; 95% CI: 0.85 to 2.02; p = 0.22; I2 = 61%) and the control group (Figure 5A).
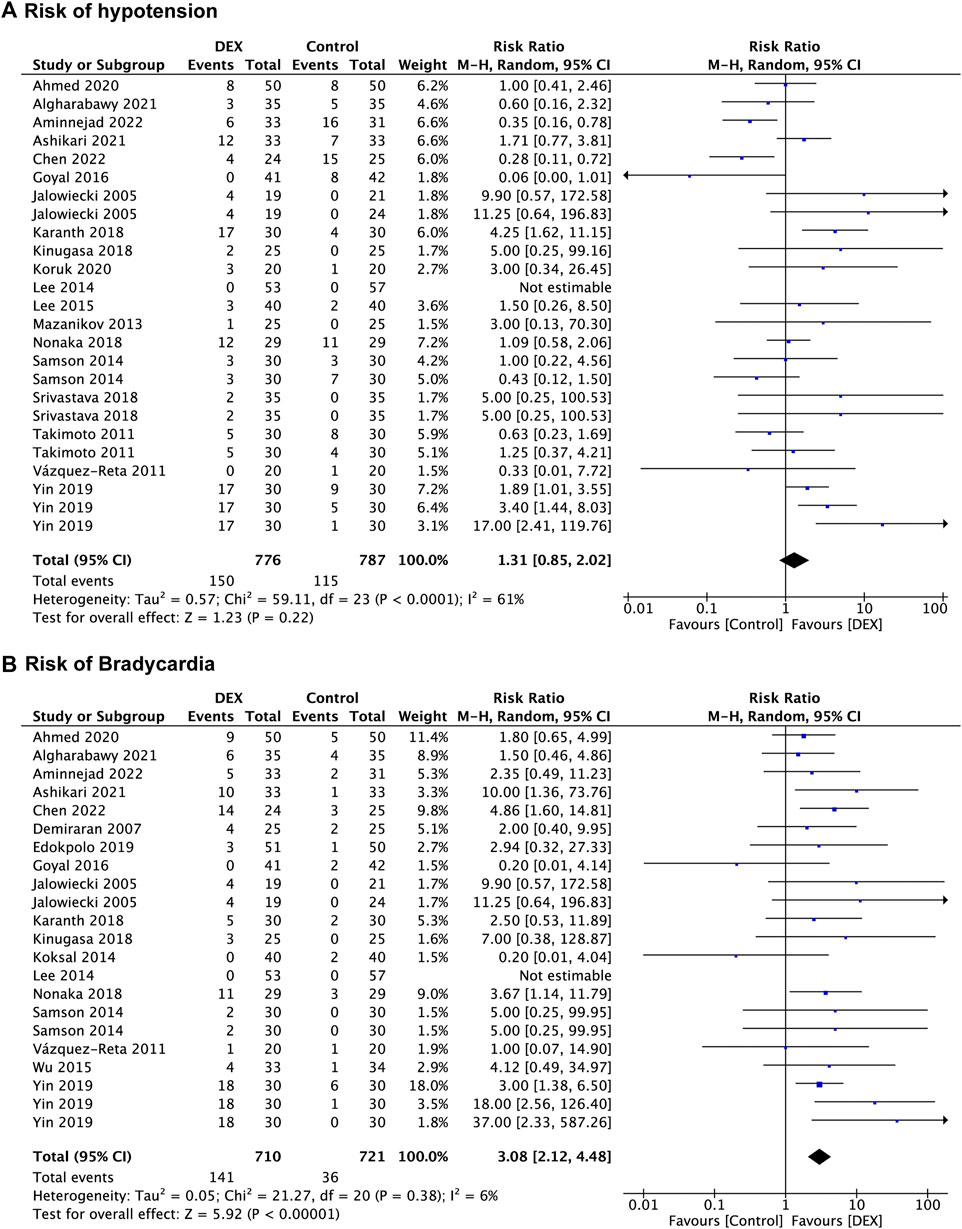
FIGURE 5. Forest plot depicting risk of hypotension (A), and risk of bradycardia (B). Boxes represent mean differences, and the line across each box represents respective 95% CI. CI, confidence interval; DEX, dexmedetomidine; SD, standard deviation.
In the subgroup analysis, the risk of hypotension in the DEX group was higher than saline group (RR: 1.84; 95% CI: 1.13 to 3.00; p = 0.01; I2 = 0%) and the opioids group (RR: 3.99; 95% CI: 2.16 to 7.37; p < 0.00001; I2 = 0%), whereas the DEX group had a lower risk of hypotension than the propofol group (RR: 0.55; 95% CI: 0.34 to 0.88; p = 0.01; I2 = 0%), and no significant difference was found between the DEX group and the midazolam group (p = 0.40). Pooled results revealed that both the non-advanced endoscopic procedures (p = 0.19) and the advanced endoscopic procedures (p = 0.87) had no significant difference in hypotension risk between the control group and the DEX group (Supplementary Figure S9).
Bradycardia
Eighteen studies (Jalowiecki et al., 2005a; Demiraran et al., 2007; Vázquez-Reta et al., 2011; Koksal et al., 2014; Lee et al., 2014; Samson et al., 2014; Wu et al., 2015; Goyal et al., 2016; Karanth et al., 2018; Kinugasa et al., 2018; Nonaka et al., 2018; Edokpolo et al., 2019; Yin et al., 2019; Ahmed et al., 2020; Algharabawy et al., 2021; Ashikari et al., 2021; Aminnejad et al., 2022; Chen et al., 2022) reported data concerning the risk of bradycardia, which was observed in 721 patients in the control group and 710 patients in the DEX group. There was a significant increase in bradycardia risk in the DEX group (RR: 3.08; 95% CI: 2.12 to 4.48; p < 0.00001; I2 = 6%) compared with the control group (Figure 5B).
In the subgroup analysis (Supplementary Figure S10), compared with the saline group (RR: 3.45; 95% CI: 1.74 to 6.85; p = 0.0004; I2 = 0%) and the propofol group (RR: 2.53; 95% CI: 1.40 to 4.59; p = 0.002; I2 = 0%), the DEX group had a higher risk of bradycardia, but no significant difference was found between the midazolam group (p = 0.27), the opioids group (p = 0.14) and the DEX group. The results of subgroup analysis displayed a higher risk of bradycardia in the DEX group than in the control group in both non-advanced endoscopic procedures (RR: 2.80; 95% CI: 1.82 to 4.32; p < 0.00001; I2 = 6%) and advanced endoscopic procedures (RR: 4.04; 95% CI: 1.77 to 9.19; p = 0.0009; I2 = 17%).
Cough
Six studies (Demiraran et al., 2007; Kilic et al., 2011; Sethi et al., 2014; Eldesuky Ali Hassan, 2015; Srivastava et al., 2018; Yin et al., 2019) recorded the risk of cough, with 265 patients in the control group vs. 265 patients in the DEX group. The risk of cough was significantly decreased in the DEX group (RR: 0.25; 95% CI: 0.12 to 0.54; p = 0.0004; I2 = 0%) compared with the control group (Figure 4C).
In the subgroup analysis (Supplementary Figure S11), the risk of cough was lower in the DEX group (RR: 0.20; 95% CI: 0.07 to 0.62; p = 0.005; I2 = 0%) than in the midazolam group, but no difference in the opioids group (p = 0.19). The risk of cough in the DEX group was significantly decreased in the DEX group compared with the control group in both the non-advanced endoscopic procedures (RR: 0.16; 95% CI: 0.05 to 0.50; p = 0.002; I2 = 0%) and the advanced endoscopic procedures (RR: 0.36; 95% CI: 0.13 to 0.98; p = 0.05; I2 = 0%).
Sensitivity analysis and publication bias
A sensitivity analysis was performed to reduce the potential bias (Supplementary Figures S1–S11), and no single trial significantly affected the overall results of most outcomes in this meta-analysis, except for the requirement for additional propofol in which three studies (Eldesuky Ali Hassan, 2015; Mukhopadhyay et al., 2015; Pushkarna et al., 2019) with a high risk of bias but did not alter the findings. Potential publication bias was evaluated graphically using funnel plot asymmetry, and funnel plot asymmetry was measured by Egger’s regression test. No asymmetry was demonstrated by a visual indication of the funnel plots, and Egger’s regression test suggested no significant asymmetry of the funnel plots in all outcomes (p > 0.05) (Supplementary Table S1), indicating no evidence of significant publication bias in this meta-analysis.
Discussion
These data suggest that the primary outcomes of sedation level of DEX are comparable to other sedatives, with similar RSS score and patient satisfaction level, and even better in some clinical outcomes, with decreased risk of body movements or gagging and reduced additional requirement for other sedatives and increased endoscopist satisfaction level. In terms of secondary outcomes of adverse events, DEX may benefit patients in some clinical outcomes (reduced risk of hypoxia and cough), with no significant difference in the risk of hypotension, while there may be potential drawbacks in other outcomes (increased risk of bradycardia) (Figure 6B).
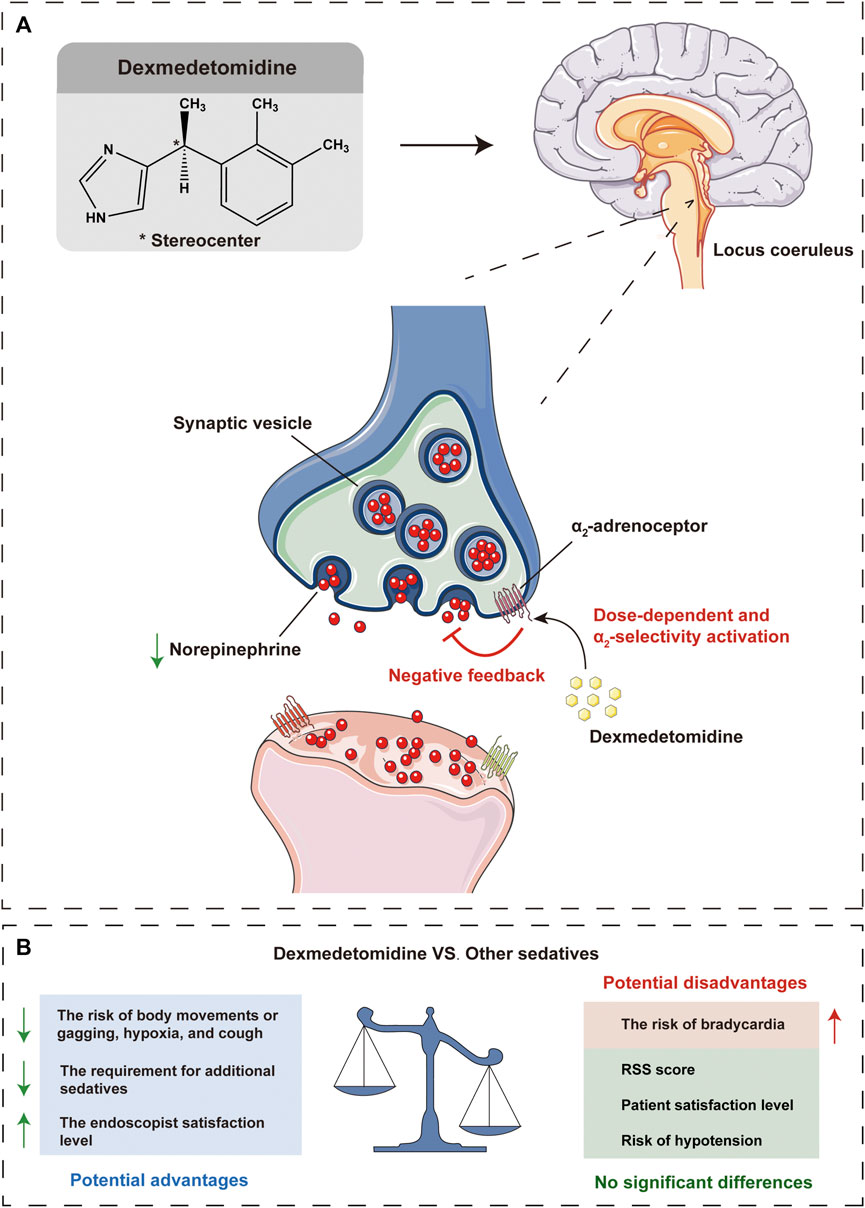
FIGURE 6. Schematic illustration that dexmedetomidine inhibits norepinephrine release, resulting in a reduction of excitation, especially in the locus coeruleus, which mediates the sedative and antinociceptive effects (A). Potential advantages and disadvantages of dexmedetomidine compared with other sedatives in gastrointestinal endoscopic procedures (B). VS, versus; RSS, Ramsay sedation scale.
In terms of sedation level, our results indicated that DEX is comparable to other sedatives with similar RSS score and patient satisfaction level, but a higher endoscopist satisfaction level. An admirable property of DEX is arousable sedation mimicking natural sleep, which is indicative of the potential of DEX for an easy transition from sleep to wakefulness potential of DEX, thus allowing patients to be cooperative and communicative when stimulated which may be the reason for the higher satisfaction level of endoscopists and also make the endoscopy a more smooth procedure (Wijeysundera et al., 2009; Liu X et al., 2021). The results of sedation level in the present analysis are conflicted with several previous studies comparing DEX with propofol or midazolam (Nishizawa et al., 2015; Zhang et al., 2016; Nishizawa et al., 2017), which may be related to the inclusion of multiple different comparators, and we will discuss these outcomes in subgroup analysis for different comparators. Another desirable property of DEX is the additional opioids and sedatives sparing effect (Akarsu Ayazoğlu et al., 2013; Wang and Shi, 2017; Nonaka et al., 2018), which may be associated with the synergistic interactions of DEX with other sedatives due to its different sedative mechanism than conventional sedatives. In our meta-analysis, additional consumption of propofol and midazolam was significantly reduced with DEX sedatives, and there was a trend toward a reduction in opioids consumption although no statistical difference was found, however, a subgroup analysis confined to the 3 studies showed a lower consumption of pethidine when using DEX sedative. This founding revealed that DEX can provide adequate conscious sedation during gastrointestinal endoscopic procedures while reducing the requirement for other additional sedatives, with significant clinical implications for decreasing the dose of each drug and minimizing individual adverse side effects.
As a highly selective α2-adrenoceptor agonist, DEX acts primarily on the central pre-and postsynaptic α2-receptors in the locus coeruleus which gives it a unique sedative activity that differs from conventional sedatives (Figure 6A) (Hoy and Keating, 2011; Liu X et al., 2021). For example, DEX exhibits minimal respiratory depressive effects and lower physiologic stress response to surgical stimulation than GABA receptor agonists such as propofol and the benzodiazepines (Riker et al., 2009; Candiotti et al., 2010; Akarsu Ayazoğlu et al., 2013). The results of this current meta-analysis suggested that DEX sedation had statistically lower hypoxia risk and body movements or gagging rate, which was consistent with the conclusions of the previous studies (Nishizawa et al., 2015; Liu W et al., 2021), and also with the pharmacological characteristics of DEX, but contradicted by others (no significant difference was found) (Nishizawa et al., 2015; Nishizawa et al., 2017). This is a potentially critical finding, as involuntary patient body movements or gagging can severely interfere with endoscopic interventions and may increase risk of adverse events. Furthermore, DEX sedation with a lower risk of hypoxia may result in a more stable respiratory system, which may be beneficial in patients with a history of respiratory disease.
Bradycardia and hypotension are two other common cardiorespiratory complications of DEX besides hypoxia, both usually resolve without intervention (Hoy and Keating, 2011). As the mainly hemodynamic effects and known side effects of DEX, dose-dependent bradycardia and hypotension are caused by its endogenous catecholamine reduction, peripheral vasoconstrictive, sympatholytic, and baroreflex-mediated parasympathetic activation properties (Jalowiecki et al., 2005b; Carollo et al., 2008; Weerink et al., 2017), which are associated with the activation of α2-adrenoceptor agonist and imidazoline-preferring receptors in the ventrolateral medulla and solitarius nucleus tract (Hayashi et al., 1995). This current meta-analysis found that DEX significantly increased the risk of bradycardia, although we pooled the data with different definitions of bradycardia, the heterogeneity was as low as 6%. Although previous studies have not reached consistent conclusions about risk of bradycardia associated with DEX (Nishizawa et al., 2015; Zhang et al., 2016; Nishizawa et al., 2017), we suggest that DEX should be used with caution in patients diagnosed with severe sinus bradycardia or heart block. During gastrointestinal endoscopic procedures, bradycardia could be managed with atropine or butylscopolamine bromide. However, no statistically significant difference was found in the risk of hypotension. It is important to note that we appear to have identified a source of heterogeneity in the risk of hypotension, which was reduced to 0% for all subgroups by subgroup analysis with different comparators, but there were considerable variations in results between subgroups which would be discussed below. Therefore, a complicated method of DEX administration in somewhat, including a loading dose that should be given over no less than 10 min and a maintenance dose with appropriate infusion velocity, may achieve favorable cardiovascular stability (Schaffrath et al., 2004; Nishizawa et al., 2015).
Perioperative DEX administration has been suggested to reduce the occurrence of cough in many surgeries since its property of mitigating airway reflexes (Kim et al., 2013; Aouad et al., 2019). Perioperative coughing is highly undesirable for patients as it may prolong extubation and delay postoperative recovery, which may lead to unfavorable postoperative complications. The results and several subgroup analyses in this present meta-analysis showed that DEX significantly reduced the risk of cough, with 0% heterogeneity in all comparisons. Although no previous meta-analysis has pooled outcomes of cough risk in gastrointestinal endoscopic procedures, our findings support that DEX administration may be effective for perioperative cough prevention.
In the main analysis, we included all comparators and procedure types, most analysis results were limited by significant heterogeneity that may have influenced the validity of our results. Therefore, we performed subgroup analyses to address this issue, among which the subgroup analysis of different comparators had a more obvious effect of reducing heterogeneity. We discuss the advantages and disadvantages of DEX over other sedatives and present the results of the subgroup analysis in Table 3.
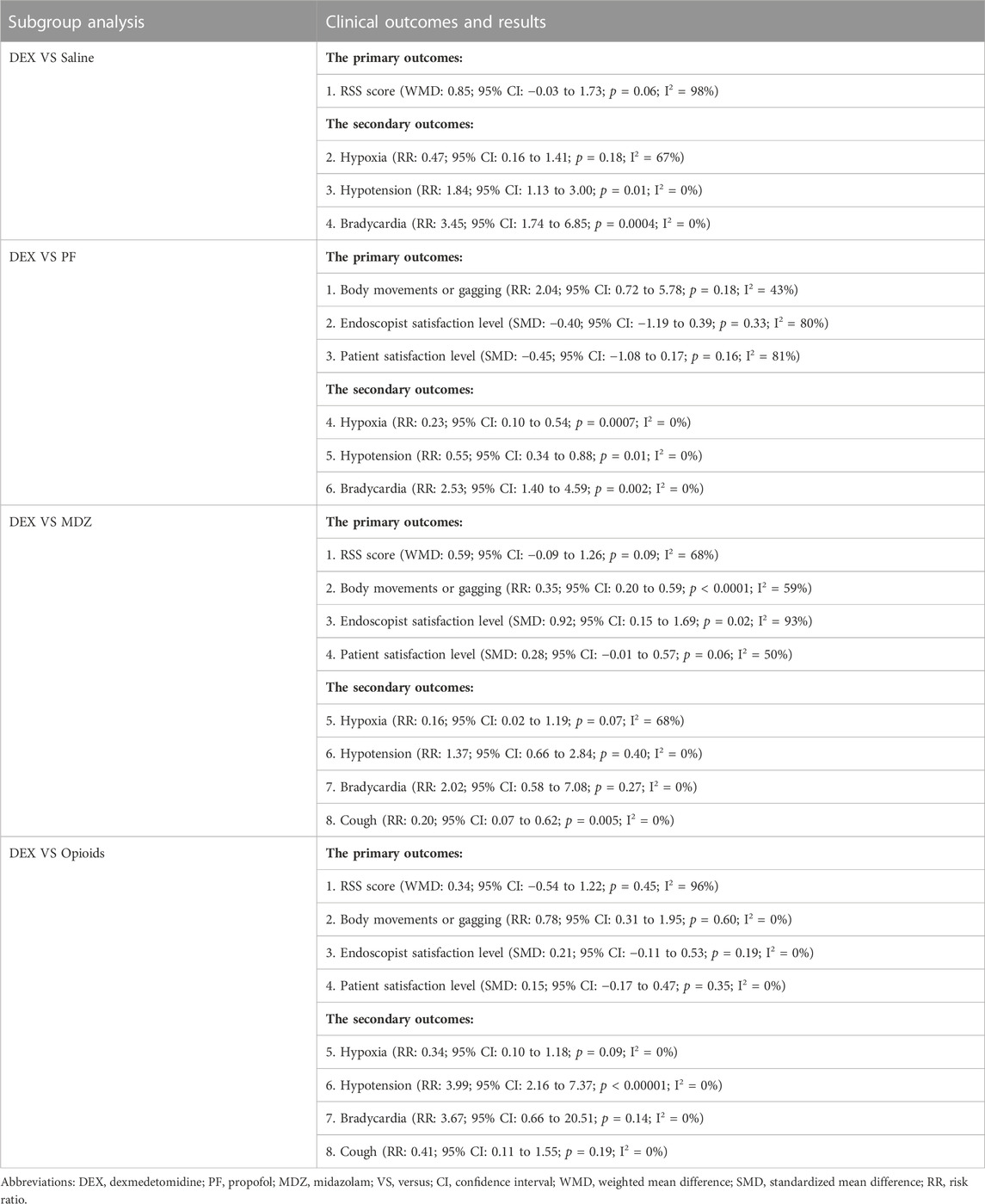
TABLE 3. Subgroup analysis of DEX versus different sedation comparators in the primary and secondary outcomes.
Limited by the number of included studies, too few outcomes can be analysed in studies comparing DEX with saline or ketamine, and we may not be able to draw firm conclusions from these data. What we do know, however, is that DEX has both pharmacodynamic advantages of a significantly greater α 2:α 1-adrenoceptor affinity ratio and a pharmacokinetic advantage over similar α 2-adrenoceptor agonists such as ketamine and a better sedation level over saline (Liu X et al., 2021).
An interesting discovery is that although previous researchers have suggested that pooling data on outcomes of different definitions or evaluation systems may be one source of heterogeneity, however, subgroup analyses were not performed due to the small number of included studies in previous meta-analyses. In this present meta-analysis, we performed a subgroup analysis in terms of different definitions or evaluation systems of certain outcomes, and the results showed that they had little effect on heterogeneity, which deviates from the conjecture of the previous researchers.
Limitations
Confinement to RCTs representing the highest level of evidence is a major strength of our work, however, there are also several limitations in our meta-analysis. First, the number of studies in our meta-analysis was insufficient, especially for several outcomes such as midazolam consumption and risk of cough, which may increase Type 1 error and publication bias. Of the 40 trials enrolled in our meta-analysis, only three and one trial originated from Europe and the United States, respectively, which may be another source of publication bias. Second, most of the analyses results were limited by substantial heterogeneity, however, no exact reason for the observed heterogeneity was determined although several subgroup analyses were performed, among which the subgroup analysis of different comparators had a more obvious effect of decreasing heterogeneity. It is more important to note that the association between the dose use/the mode of DEX administration and cardiac side effects such as bradycardia and hypotension was not available yet. Remarkably, synergistic sedation regimens containing DEX based on different sedatives may benefit patients more because of the admirable potential to reduce side effects, improve tolerability, and reduce the requirement for additional sedatives while providing a safe and effective sedation level. Therefore, further trials focusing on specific surgery-type-related, various dose use/the mode of DEX administration, and different synergistic sedation regimens based on DEX are warranted. Third, the extensive exclusion criteria in most trials, including American Society of Anesthesiologists (ASA) class IV or more, pregnancy, cardiovascular disease, renal or hepatic or pulmonary insufficiency, limited the applicability of the results to the general critically ill patient population, moreover, the paediatric population was excluded in this study due to the limited number of studies, however, the role of DEX as a potential sedative in the paediatric population warrants further investigation (Mason et al., 2021). Fourth, subgroup analyses were conducted in an attempt to reduce heterogeneity, but many subgroup analyses contained small studies and sample sizes and were therefore of limited value. Finally, other outcomes (such as economic cost) should be evaluated in the subgroup analysis of different sedatives to better understand their role in gastrointestinal endoscopic procedures.
Conclusion
In summary, we did find evidence of certain advantages of DEX in gastrointestinal endoscopic procedures, whilst some potential disadvantages also exist. DEX is comparable to midazolam and propofol in maintaining light to moderate sedation and even better in some clinical outcomes during gastrointestinal endoscopic procedures, and all these indicate that DEX is a safe and effective sedative agent for gastrointestinal endoscopy and provides a more sedative option for patients undergoing gastrointestinal endoscopic procedures. However, definitive conclusions on the clinical practice of DEX in gastrointestinal endoscopic procedures may not be given due to issues of limited sample size and heterogeneity. What we did is a systematic and pooled meta-analysis of the current literature regarding DEX use in gastrointestinal endoscopic procedures, which could stimulate new research that may potentially guide future clinical sedation practices in this field. Further large, multicenter RCTs with multiple sedation protocols are warranted to enhance understanding of its pharmacological properties, patient selection, dosage, and adverse effects. Therefore, we will continue to pay attention to updating our conclusions in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
RT: This author helped with study design, literature search, data extraction, data analysis, drafting the manuscript, and revising the manuscript. YH: This author helped with study design, literature search, data extraction, data analysis, drafting the manuscript, and revising the manuscript. YZ: This author helped assess bias and draft the manuscript. XM: This author helped with the data extraction of the manuscript. HY: This author helped with the data extraction of the manuscript. KS: This author helped assess bias and conduct data analysis of the manuscript. BZ: This author helped revise the manuscript. LW: This author helped revise the manuscript. WZ: This author helped revise the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by CAMS Innovation Fund for Medical Sciences (No. 2021-I2M-1-070, 2021-I2M-1-026, 2022-I-2M-002) and National Key Research and Development Project of China (No.2019YFC1708901).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1241714/full#supplementary-material
Abbreviations
ASA, American Society of Anesthesiologists; CI, Confidence interval; CS, colonoscopy; DEX, Dexmedetomidine; EGD, upper gastrointestinal endoscopy; ERCP, Endoscopic retrograde cholangiopancreatography; ESD, Endoscopic submucosal dissection; EOPD, endoscopic oesophageal procedures; ESOD, esophagogastroduodenal endoscopy; IQR, Inter-quartile range; I.V., intravenous; SE, Mean; N.A., not available; PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis; RSS, Ramsay sedation scale; RCT, Randomized controlled trial; RR, Risk ratios; SD, Standard deviation; SMD, Standardized mean difference; VAS, Visual analogue scale; N.A., not available; WMD, Weighted mean difference.
References
Aantaa, R., and Scheinin, M. (1993). Alpha2-adrenergic agents in anaesthesia. Acta Anaesthesiol. Scand. 37 (5), 433–448. doi:10.1111/j.1399-6576.1993.tb03743.x
Abbas, I., Hassanein, A., and Mokhtar, M. (2017). Effect of low dose ketamine versus dexmedetomidine on gag reflex during propofol based sedation during upper gastrointestinal endoscopy. A randomized controlled study. Egypt. J. Anaesth. 33 (2), 165–170. doi:10.1016/j.egja.2017.01.003
Abdalla, M. W., El Shal, S. M., El Sombaty, A. I., Abdalla, N. M., and Zeedan, R. B. (2015). Propofol dexmedetomidine versus propofol ketamine for anesthesia of endoscopic retrograde cholangiopancreatography (ERCP) (A randomized comparative study). Egypt. J. Anaesth. 31 (2), 97–105. doi:10.1016/j.egja.2014.12.008
Ahmed, S. A., Hawash, N., Rizk, F. H., Elkadeem, M., Elbahnasawy, M., and Abd-Elsalam, S. (2020). Randomised study comparing the use of propofol versus dexmedetomidine as a sedative agent for patients presenting for lower gastrointestinal endoscopy. Curr. Drug Ther. 15 (1), 61–66. doi:10.2174/1574885514666190904161705
Akarsu Ayazoğlu, T., Polat, E., Bolat, C., Yasar, N. F., Duman, U., Akbulut, S., et al. (2013). Comparison of propofol-based sedation regimens administered during colonoscopy. Rev. Medica Chile 141 (4), 477–485. doi:10.4067/S0034-98872013000400009
Algharabawy, W. S., Abusinna, R. G., and AbdElrahman, T. N. (2021). Dexmedetomidine-ketamine versus propofol-ketamine for sedation during upper gastrointestinal endoscopy in hepatic patients (a comparative randomized study). Egypt. J. Anaesth. 37 (1), 364–372. doi:10.1080/11101849.2021.1961428
Aminnejad, R., Hormati, A., Shafiee, H., Alemi, F., Hormati, M., Saeidi, M., et al. (2022). Comparing the efficacy and safety of dexmedetomidine/ketamine with propofol/fentanyl for sedation in colonoscopy patients: a doubleblinded randomized clinical trial. CNS Neurological Disord. Drug Targets 21 (8), 724–731. doi:10.2174/1871527320666211006141406
Amri, P., Nahrini, S., Hajian-Tilaki, K., Hamidian, M., Alipour, S. F., Hamidi, S. H., et al. (2018). Analgesic effect and hemodynamic changes due to dexmedetomidine versus fentanyl during elective colonoscopy: a double-blind randomized clinical trial. Anesthesiol. Pain Med. 8 (6), e81077. doi:10.5812/aapm.81077
Aouad, M. T., Zeeni, C., Al Nawwar, R., Siddik-Sayyid, S. M., Barakat, H. B., Elias, S., et al. (2019). Dexmedetomidine for improved quality of emergence from general anesthesia: a dose-finding study. Anesth. Analgesia 129 (6), 1504–1511. doi:10.1213/ane.0000000000002763
Ashikari, K., Nonaka, T., Higurashi, T., Takatsu, T., Yoshihara, T., Misawa, N., et al. (2021). Efficacy of sedation with dexmedetomidine plus propofol during esophageal endoscopic submucosal dissection. J. Gastroenterology Hepatology 36 (7), 1920–1926. doi:10.1111/jgh.15417
Bharati, S., Pal, A., Biswas, C., and Biswas, R. (2011). Incidence of cardiac arrest increases with the indiscriminate use of dexmedetomidine: a case series and review of published case reports. Acta Anaesthesiol. Taiwanica Official J. Taiwan Soc. Anesthesiol. 49 (4), 165–167. doi:10.1016/j.aat.2011.11.010
Candiotti, K. A., Bergese, S. D., Bokesch, P. M., Feldman, M. A., Wisemandle, W., Bekker, A. Y., et al. (2010). Monitored anesthesia care with dexmedetomidine: a prospective, randomized, double-blind, multicenter trial. Anesth. Analgesia 110 (1), 47–56. doi:10.1213/ane.0b013e3181ae0856
Carollo, D. S., Nossaman, B. D., and Ramadhyani, U. (2008). Dexmedetomidine: a review of clinical applications. Curr. Opin. Anesthesiol. 21 (4), 457–461. doi:10.1097/aco.0b013e328305e3ef
Chen, M., Sun, Y., Li, X., Zhang, C., Huang, X., Xu, Y., et al. (2022). Effectiveness of single loading dose of dexmedetomidine combined with propofol for deep sedation of endoscopic retrograde cholangiopancreatography (ERCP) in elderly patients: a prospective randomized study. BMC Anesthesiol. 22 (1), 85. doi:10.1186/s12871-022-01630-8
Cheung, C. W., Qiu, Q., Liu, J., Chu, K. M., and Irwin, M. G. (2015). Intranasal dexmedetomidine in combination with patient-controlled sedation during upper gastrointestinal endoscopy: a randomised trial. Acta Anaesthesiol. Scand. 59 (2), 215–223. doi:10.1111/aas.12445
Demiraran, Y., Korkut, E., Tamer, A., Yorulmaz, I., Kocaman, B., Sezen, G., et al. (2007). The comparison of dexmedetomidine and midazolam used for sedation of patients during upper endoscopy: a prospective, randomized study. Can. J. Gastroenterology 21 (1), 25–29. doi:10.1155/2007/350279
Early, D. S., Lightdale, J. R., Vargo, J. J., Acosta, R. D., Chandrasekhara, V., Chathadi, K. V., et al. (2018). Guidelines for sedation and anesthesia in GI endoscopy. Gastrointest. Endosc. 87 (2), 327–337. doi:10.1016/j.gie.2017.07.018
Eberl, S., Preckel, B., Bergman, J. J., van Dieren, S., and Hollmann, M. W. (2016). Satisfaction and safety using dexmedetomidine or propofol sedation during endoscopic oesophageal procedures: a randomised controlled trial. Eur. J. Anaesthesiol. 33 (9), 631–637. doi:10.1097/eja.0000000000000438
Ebert, T. J., Hall, J. E., Barney, J. A., Uhrich, T. D., and Colinco, M. D. (2000). The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology 93 (2), 382–394. doi:10.1097/00000542-200008000-00016
Edokpolo, L. U., Mastriano, D. J., Serafin, J., Weedon, J. C., Siddiqui, M. T., and Dimaculangan, D. P. (2019). Discharge readiness after propofol with or without dexmedetomidine for colonoscopy: a randomized controlled trial. Anesthesiology 131 (2), 279–286. doi:10.1097/ALN.0000000000002809
Eldesuky Ali Hassan, H. I. (2015). Dexmedetomidine versus ketofol for moderate sedation in Endoscopic Retrograde Cholangiopancreatography (ERCP) comparative study. Egypt. J. Anaesth. 31 (1), 15–21. doi:10.1016/j.egja.2014.11.002
Goyal, R., Hasnain, S., Mittal, S., and Shreevastava, S. (2016). A randomized, controlled trial to compare the efficacy and safety profile of a dexmedetomidine-ketamine combination with a propofol-fentanyl combination for ERCP. Gastrointest. Endosc. 83 (5), 928–933. doi:10.1016/j.gie.2015.08.077
Hayashi, Y., Guo, T.-Z., and Maze, M. (1995). Desensitization to the behavioral effects of alpha 2-adrenergic agonists in rats. J. Am. Soc. Anesthesiol. 82 (4), 954–962. doi:10.1097/00000542-199504000-00019
Higgins, J. P. T., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ Clin. Res. ed.) 343, d5928. doi:10.1136/bmj.d5928
Hoy, S. M., and Keating, G. M. (2011). Dexmedetomidine: a review of its use for sedation in mechanically ventilated patients in an intensive care setting and for procedural sedation. Drugs 71 (11), 1481–1501. doi:10.2165/11207190-000000000-00000
Jalowiecki, P., Rudner, R., Gonciarz, M., Kawecki, P., Petelenz, M., and Dziurdzik, P. (2005a). Sole use of dexmedetomidine has limited utility for conscious sedation during outpatient colonoscopy. Anesthesiology 103 (2), 269–273. doi:10.1097/00000542-200508000-00009
Jalowiecki, P., Rudner, R., Gonciarz, M., Kawecki, P., Petelenz, M., and Dziurdzik, P. (2005b). Sole use of dexmedetomidine has limited utility for conscious sedation during outpatient colonoscopy. J. Am. Soc. Anesthesiol. 103 (2), 269–273. doi:10.1097/00000542-200508000-00009
Kamal, F., Khan, M. A., Lee-Smith, W., Sharma, S., Imam, Z., Jowhar, D., et al. (2021). Efficacy and safety of supplemental intravenous lidocaine for sedation in gastrointestinal endoscopic procedures: systematic review and meta-analysis of randomized controlled trials. Gastrointest. Endosc. 93 (6), 1241–1249.e6. doi:10.1016/j.gie.2021.01.008
Kamibayashi, T., and Maze, M. (2000). Clinical uses of alpha2 -adrenergic agonists. Anesthesiology 93 (5), 1345–1349. doi:10.1097/00000542-200011000-00030
Karanth, H., Murali, S., Koteshwar, R., Shetty, V., and Adappa, K. (2018). Comparative study between propofol and dexmedetomidine for conscious sedation in patients undergoing outpatient colonoscopy. Anesth. Essays Res. 12 (1), 98–102. doi:10.4103/aer.AER_206_17
Kavousi, E., Shariefnia, H. R., Pourfakhr, P., Khajavi, M., and Behseresht, A. (2021). Dexmedetomidine versus propofol in combination with fentanyl for sedation-analgesia in colonoscopy procedures: a randomized prospective study. Middle East J. Dig. Dis. 13 (4), 328–332. doi:10.34172/mejdd.2021.242
Kilic, N., Sahin, S., Aksu, H., Yavascaoglu, B., Gurbet, A., Turker, G., et al. (2011). Conscious sedation for endoscopic retrograde cholangiopancreatography: dexmedetomidine versus midazolam. Eurasian J. Med. 43 (1), 13–17. doi:10.5152/eajm.2011.03
Kim, N., Yoo, Y.-C., Lee, S. K., Kim, H., Ju, H. M., and Min, K. T. (2015). Comparison of the efficacy and safety of sedation between dexmedetomidine-remifentanil and propofol-remifentanil during endoscopic submucosal dissection. World J. Gastroenterology 21 (12), 3671–3678. doi:10.3748/wjg.v21.i12.3671
Kim, S., Kim, J., Lee, J., Song, B., and Koo, B. (2013). Efficacy of intraoperative dexmedetomidine infusion on emergence agitation and quality of recovery after nasal surgery. Br. J. Anaesth. 111 (2), 222–228. doi:10.1093/bja/aet056
Kinugasa, H., Higashi, R., Miyahara, K., Moritou, Y., Hirao, K., Ogawa, T., et al. (2018). Dexmedetomidine for conscious sedation with colorectal endoscopic submucosal dissection: a prospective double-blind randomized controlled study. Clin. Transl. Gastroenterology 9 (7), 167. doi:10.1038/s41424-018-0032-5
Koksal, E., Ustun, Y. B., Kaya, C., Torun, A. C., Yilmaz, M. Z., Atalay, Y. O., et al. (2014). Use of remifentanil or dexmedetomidine with ketamine for upper gastrointestinal endoscopy. J. Exp. Clin. Med. Turk. 31 (4), 221–224. doi:10.5835/jecm.omu.31.04.004
Koruk, S., Koruk, I., Arslan, A. M., Bilgi, M., Gul, R., and Bozgeyik, S. (2020). Dexmedetomidine or midazolam in combination with propofol for sedation in endoscopic retrograde cholangiopancreatography: a randomized double blind prospective study. Wideochirurgia I Inne Tech. Maloinwazyjne = Videosurgery Other Miniinvasive Tech. 15 (3), 526–532. doi:10.5114/wiitm.2020.95066
Lee, B. S., Ryu, J., Lee, S. H., Lee, M. G., Jang, S. E., Hwang, J.-H., et al. (2014). Midazolam with meperidine and dexmedetomidine vs. midazolam with meperidine for sedation during ERCP: prospective, randomized, double-blinded trial. Endoscopy 46 (4), 291–298. doi:10.1055/s-0033-1358909
Lee, S. P., Sung, I.-K., Kim, J. H., Lee, S.-Y., Park, H. S., Shim, C. S., et al. (2015). Comparison of dexmedetomidine with on-demand midazolam versus midazolam alone for procedural sedation during endoscopic submucosal dissection of gastric tumor. J. Dig. Dis. 16 (7), 377–384. doi:10.1111/1751-2980.12254
Liu, W., Yu, W., Yu, H., and Sheng, M. (2021). Comparison of clinical efficacy and safety between dexmedetomidine and propofol among patients undergoing gastrointestinal endoscopy: a meta-analysis. J. Int. Med. Res. 49 (7), 3000605211032786. doi:10.1177/03000605211032786
Liu, X., Li, Y., Kang, L., and Wang, Q. (2021). Recent advances in the clinical value and potential of dexmedetomidine. J. Inflamm. Res. 14, 7507–7527. doi:10.2147/JIR.S346089
Lu, Z., Li, W., Chen, H., and Qian, Y. (2018). Efficacy of a dexmedetomidine-remifentanil combination compared with a midazolam-remifentanil combination for conscious sedation during therapeutic endoscopic retrograde cholangio-pancreatography: a prospective, randomized, single-blinded preliminary trial. Dig. Dis. Sci. 63 (6), 1633–1640. doi:10.1007/s10620-018-5034-3
Luo, D., Wan, X., Liu, J., and Tong, T. (2018). Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 27 (6), 1785–1805. doi:10.1177/0962280216669183
Mason, K. P., Park, R. S., Sullivan, C. A., Lukovits, K., Halpin, E. M., Imbrescia, S. T., et al. (2021). The synergistic effect of dexmedetomidine on propofol for paediatric deep sedation: a randomised trial. Eur. J. Anaesthesiol. 38 (5), 541–547. doi:10.1097/eja.0000000000001350
Mazanikov, M., Udd, M., Kylänpää, L., Mustonen, H., Lindström, O., Halttunen, J., et al. (2013). Dexmedetomidine impairs success of patient-controlled sedation in alcoholics during ERCP: a randomized, double-blind, placebo-controlled study. Surg. Endosc. 27 (6), 2163–2168. doi:10.1007/s00464-012-2734-1
Mukhopadhyay, S., Niyogi, M., Sarkar, J., Mukhopadhyay, B. S., and Halder, S. K. (2015). The dexmedetomidine "augmented" sedato analgesic cocktail: an effective approach for sedation in prolonged endoscopic retrograde cholangio-pancreatography. J. Anaesthesiol. Clin. Pharmacol. 31 (2), 201–206. doi:10.4103/0970-9185.155149
Nishizawa, T., Suzuki, H., Hosoe, N., Ogata, H., Kanai, T., and Yahagi, N. (2017). Dexmedetomidine vs propofol for gastrointestinal endoscopy: a meta-analysis. United Eur. Gastroenterology J. 5 (7), 1037–1045. doi:10.1177/2050640616688140
Nishizawa, T., Suzuki, H., Sagara, S., Kanai, T., and Yahagi, N. (2015). Dexmedetomidine versus midazolam for gastrointestinal endoscopy: a meta-analysis. Dig. Endosc. 27 (1), 8–15. doi:10.1111/den.12399
Nonaka, T., Inamori, M., Miyashita, T., Inoh, Y., Kanoshima, K., Higurashi, T., et al. (2018). Can sedation using a combination of propofol and dexmedetomidine enhance the satisfaction of the endoscopist in endoscopic submucosal dissection? Endosc. Int. Open 6 (1), E3-E10. doi:10.1055/s-0043-122228
Pourfakhr, P., Nouri, K., Shariefnia, H. R., Moharari, R. S., Khajavi, M. R., and Pourfakhr, P. (2019). Dexmedetomidine versus ketamine combined with fentanyl for sedation-analgesia in colonoscopy procedures: a randomized prospective study. Acta Medica Iran. 57 (6), 355–358. doi:10.18502/acta.v57i6.1878
Pushkarna, G., Sarangal, P., Pushkarna, V., and Gupta, R. (2019). Comparative evaluation of dexmedetomidine versus midazolam as premedication to propofol anesthesia in endoscopic retrograde cholangiopancreatography. Anesth. Essays Res. 13 (2), 297–302. doi:10.4103/aer.AER_62_19
Ramkiran, S., Iyer, S. S., Dharmavaram, S., Mohan, C. V. R., Balekudru, A., and Kunnavil, R. (2015). BIS targeted propofol sparing effects of dexmedetomidine versus ketamine in outpatient ERCP: a prospective randomised controlled trial. J. Clin. Diagnostic Res. JCDR 9 (5), UC07–UC12. doi:10.7860/JCDR/2015/12435.5991
Riker, R. R., Shehabi, Y., Bokesch, P. M., Ceraso, D., Wisemandle, W., Koura, F., et al. (2009). Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. Jama 301 (5), 489–499. doi:10.1001/jama.2009.56
Samson, S., George, S. K., Vinoth, B., Khan, M. S., and Akila, B. (2014). Comparison of dexmedetomidine, midazolam, and propofol as an optimal sedative for upper gastrointestinal endoscopy: a randomized controlled trial. J. Dig. Endosc. 05 (02), 051–057. doi:10.4103/0976-5042.144826
Schaffrath, E., Kuhlen, R., and Tonner, P. (2004). Analgesia and sedation in intensive care medicine. Der Anaesthesist 53 (11), 1111–1130. doi:10.1007/s00101-004-0773-2
Sethi, P., Mohammed, S., Bhatia, P. K., and Gupta, N. (2014). Dexmedetomidine versus midazolam for conscious sedation in endoscopic retrograde cholangiopancreatography: an open-label randomised controlled trial. Indian J. Anaesth. 58 (1), 18–24. doi:10.4103/0019-5049.126782
Srivastava, V. K., Singh, D., Agrawal, S., Khan, S., Gupta, A., and Miree, R. D. (2018). Comparative evaluation of propofol fentanyl, propofol-midazolam and propofol-dexmedetomidine on haemodynamic and postoperative recovery for endoscopic retrograde cholangiopancreatography. J. Clin. Diagnostic Res. 12 (7), UC01–UC05. doi:10.7860/JCDR/2018/32201.11730
Takimoto, K., Ueda, T., Shimamoto, F., Kojima, Y., Fujinaga, Y., Kashiwa, A., et al. (2011). Sedation with dexmedetomidine hydrochloride during endoscopic submucosal dissection of gastric cancer. Dig. Endosc. Official J. Jpn. Gastroenterological Endosc. Soc. 23 (2), 176–181. doi:10.1111/j.1443-1661.2010.01080.x
Takrouri, M. S., Seraj, M. A., Channa, A. B., el-Dawlatly, A. A., Thallage, A., Riad, W., et al. (2002). Dexmedetomidine in intensive care unit: a study of hemodynamic changes. Middle East J. Anaesthesiol. 16 (6), 587–595.
Talke, P., Lobo, E., and Brown, R. (2003). Systemically administered alpha2-agonist-induced peripheral vasoconstriction in humans. Anesthesiology 99 (1), 65–70. doi:10.1097/00000542-200307000-00014
Vargo, J. J., DeLegge, M. H., Feld, A. D., Gerstenberger, P. D., Kwo, P. Y., Lightdale, J. R., et al. (2012). Multisociety sedation curriculum for gastrointestinal endoscopy. Gastroenterology 143 (1), e1–e25. doi:10.1016/j.gie.2012.03.001
Vázquez-Reta, J. A., Jiménez Ferrer, M. C., Colunga-Sánchez, A., Pizarro-Chávez, S., Vázquez-Guerrero, A. L., and Vázquez-Guerrero, A. R. (2011). Midazolam versus dexmedetomidine for sedation for upper gastrointestinal endoscopy. Rev. Gastroenterol. Mex. 76 (1), 13–18.
Venn, R. M., Hell, J., and Grounds, R. M. (2000). Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit. Care (London, Engl. 4 (5), 302–308. doi:10.1186/cc712
Wan, X., Wang, W., Liu, J., and Tong, T. (2014). Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14 (1), 135–213. doi:10.1186/1471-2288-14-135
Wang, H.-M., Shi, X.-Y., Qin, X. R., Zhou, J. L., and Xia, Y. F. (2017). Comparison of dexmedetomidine and propofol for conscious sedation in inguinal hernia repair: a prospective, randomized, controlled trial. J. Int. Med. Res. 45 (2), 533–539. doi:10.1177/0300060516688408
Waring, J. P., Baron, T. H., Hirota, W. K., Goldstein, J. L., Jacobson, B. C., Leighton, J. A., et al. (2003). Guidelines for conscious sedation and monitoring during gastrointestinal endoscopy. Gastrointest. Endosc. 58 (3), 317–322. doi:10.1067/s0016-5107(03)00001-4
Weerink, M. A. S., Struys, M. M. R. F., Hannivoort, L. N., Barends, C. R. M., Absalom, A. R., and Colin, P. (2017). Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin. Pharmacokinet. 56 (8), 893–913. doi:10.1007/s40262-017-0507-7
Wijeysundera, D. N., Bender, J. S., and Beattie, W. S. (2009). Alpha-2 adrenergic agonists for the prevention of cardiac complications among patients undergoing surgery. Cochrane Database Syst. Rev. 2009 (4), CD004126. doi:10.1002/14651858.cd004126.pub2
Wu, W., Chen, Q., Zhang, L.-C., and Chen, W.-H. (2014). Dexmedetomidine versus midazolam for sedation in upper gastrointestinal endoscopy. J. Int. Med. Res. 42 (2), 516–522. doi:10.1177/0300060513515437
Wu, Y., Zhang, Y., Hu, X., Qian, C., Zhou, Y., and Xie, J. (2015). A comparison of propofol vs. dexmedetomidine for sedation, haemodynamic control and satisfaction, during esophagogastroduodenoscopy under conscious sedation. J. Clin. Pharm. Ther. 40 (4), 419–425. doi:10.1111/jcpt.12282
Yin, S., Hong, J., Sha, T., Chen, Z., Guo, Y., Li, C., et al. (2019). Efficacy and tolerability of sufentanil, dexmedetomidine, or ketamine added to propofol-based sedation for gastrointestinal endoscopy in elderly patients: a prospective, randomized, controlled trial. Clin. Ther. 41 (9), 1864–1877. doi:10.1016/j.clinthera.2019.06.011
Keywords: dexmedetomidine, gastrointestinal endoscopic procedures, sedative, metaanalysis, randomized controlled trial
Citation: Tang R, Huang Y, Zhang Y, Ma X, Yu H, Song K, Ren L, Zhao B, Wang L and Zheng W (2023) Efficacy and safety of sedation with dexmedetomidine in adults undergoing gastrointestinal endoscopic procedures: systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 14:1241714. doi: 10.3389/fphar.2023.1241714
Received: 17 June 2023; Accepted: 02 November 2023;
Published: 15 November 2023.
Edited by:
Liqun Yang, Shanghai Jiao Tong University, ChinaReviewed by:
Habib Md Reazaul Karim, All India Institute of Medical Sciences, Deoghar (AIIMS Deoghar), IndiaQazi Zeeshan, Mayo Clinic Arizona, United States
Copyright © 2023 Tang, Huang, Zhang, Ma, Yu, Song, Ren, Zhao, Wang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Zhao, Wmhhb2JpbkBwdW1jaC5jbg==; Lulu Wang, d2FuZ2x1bHVAaW1iLmNhbXMuY24=; Wensheng Zheng, emhlbmd3ZW5zaGVuZ0BpbW0uYWMuY24=
 Rou Tang
Rou Tang Yaqun Huang3
Yaqun Huang3