- 1The Second Clinical Medical College of Beijing University of Chinese Medicine, Dongfang Hospital of Beijing University of Chinese Medicine, Beijing, China
- 2The First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, China
- 3Dongfang Hospital of Beijing University of Chinese Medicine, Beijing, China
Background: Allergic rhinitis is prevalent among children and can cause nasal itching, fatigue, and even hinder growth and development. The main discomfort symptom of allergic rhinitis is nasal itching. Clinical reports suggest that Chinese herbal medicine (CHM) is effective in allergy rhinitis treatment. Therefore, we evaluate the clinical efficacy of Chinese herbal medicine in treating nasal itching caused by allergic rhinitis in children.
Methods: Nine databases, including PubMed, Embase, The Cochrane Library, Web of Science, China National Knowledge Infrastructure, Wan Fang Data, CQVIP, Chinese Biological Medicine, and ClinicalTrials.gov, were systematically searched from their inception until March 2023. Randomized controlled trials (RCTs) comparing the efficacy of Chinese herbal medicine, either alone or in combination with Western medicine, to Western medicine treatment or placebo intervention for treating allergic rhinitis in children were eligible for inclusion. The effectiveness of Chinese herbal medicines for nasal itching was mainly evaluated. The Risk of Bias tool 2.0 assessed the risk of bias. Statistical analysis using RevMan 5.3 and Stata/SE 12. The quality of evidence was evaluated by GRADEpro 3.6. Risk ratios (RR) with corresponding 95% confidence intervals (CI) were utilized to evaluate and present dichotomous data, while mean difference (MD) and standardized mean difference (SMD) were employed for continuous data. A fixed-effects model was applied in cases where the data exhibited homogeneity (p > 0.1, I2 < 50%), whereas a random-effects model was utilized for heterogeneous data. Statistical significance was determined by a p-value <0.05. This study was conducted by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and its review protocol was registered on the International Platform for Registered Systematic Reviews and Meta-Analysis Programs (INPLASY202340076).
Results: The review incorporated 23 studies. The meta-analysis indicated that herbal medicine was significantly related to the reduction of nasal itching (MD = −0.59, 95%CI: −0.94–0.24) and the increase of interleukin 10 level (SMD = 1.47, 95% CI: 0.90–2.05). Compared to Western medicine, the combining herbs and Western medicine showed better efficacy in relieving nasal itching, inhibiting immunoglobulin E, interleukin 4 and 33, enhancing interleukin 10, improving therapeutic efficiency, and reducing recurrent. Oral herbal medicine was more effective in treating nasal itching (MD = −0.45, 95% CI: −0.62–0.29). Combining oral and external herbal medicines was more efficient in treating nasal itching (MD = −0.44, 95% CI: −0.54–0.33), inhibiting immunoglobulin E, interleukin 4 (SMD = −0.87, 95% CI: −1.24–0.50) and 33 (SMD = −1.16, 95% CI: −1.54–0.77), and improving therapeutic efficiency. External herbal medicine did not show differences compared to Western medicines. Regarding safety, herbal medicine alone exhibited fewer adverse events than Western medicine; combining herbal and Western medicine showed no significant variation in adverse event incidence.
Conclusion: Chinese herbal medicine (CHM) holds great potential in alleviating symptoms, modulating immune factors levels, and reducing relapse in pediatric rhinitis. Meanwhile, CHM is relatively safe. However, the efficacy and safety of CHM in treating pediatric rhinitis still need to be confirmed due to the inclusion of studies with low methodological quality, small sample sizes, and potential heterogeneity. More high-quality research is necessary to provide reliable evidence for the clinical application of CHM.
Systematic Review Registration: INPLASY.com, identifier INPLASY202340076
1 Introduction
Allergic rhinitis is a prevalent condition in children, mediated by immunoglobulin E. Its prevalence is approximately 40% and gradually rising (Zhang and Zhang, 2019; Hox et al., 2020). In China, the current prevalence of allergic rhinitis among children is about 18.61% (Ruikun et al., 2022). The main symptoms include nasal itching, congestion, runny nose, and sneezing. Since there is a correlation between allergic diseases, allergic rhinitis is closely linked to other conditions such as asthma, upper airway cough syndrome, and cough variant asthma (Marple, 2010; Zvezdin et al., 2015; Donaldson, 2023). Additionally, allergic rhinitis can affect children’s nervous system, including attention deficit hyperactivity disorder and Tourette syndrome (Zhou et al., 2017; Xu et al., 2020; Liu X. et al., 2021). As a result, allergic rhinitis has become one of the research priorities in pediatric studies.
The pharmacological treatment of allergic rhinitis involves glucocorticoids, leukotriene receptor antagonists, antihistamines, and immunotherapy (Cobanoğlu et al., 2013; Klimek et al., 2019). These medications can have side effects such as impaired height growth, rhinorrhea, mental arousal, and drowsiness (Wolthers and Pedersen, 1993; Sastre et al., 2012; Mener et al., 2015; Marques et al., 2022), interfering with the standardized treatment of allergic rhinitis in children. Since allergic rhinitis can impede physical and intellectual development in children (He et al., 2017; Morais-Almeida et al., 2019; Sirufo et al., 2020), better management of its symptoms is required for optimal growth and development. Therefore, exploring alternative pharmacological therapies is necessary.
Chinese herbal medicine is a cornerstone of complementary alternative medicine, used in China for thousands of years. Herbal medicine can relieve allergic rhinitis nasal symptoms (Chan and Ng, 2020) by regulating inflammatory factors and immune function in affected children (Liu et al., 2022; Dou et al., 2023). Although some studies show the therapeutic effectiveness of herbal treatment in children with allergic rhinitis, meta-analyses examining the control of nasal symptoms from herbal medicine on allergic rhinitis in children are inconclusive. This review and meta-analysis aim to gather the appropriate evidence to comprehensively assess the overall therapeutic efficacy of herbal medicine on allergic rhinitis in children.
2 Materials and methods
This study adheres to the Preferred Reporting Items for Systematic Evaluations and Meta-Analyses (PRISMA) guidelines, and its synthesis protocol is registered on International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY202340076).
2.1 Search strategies
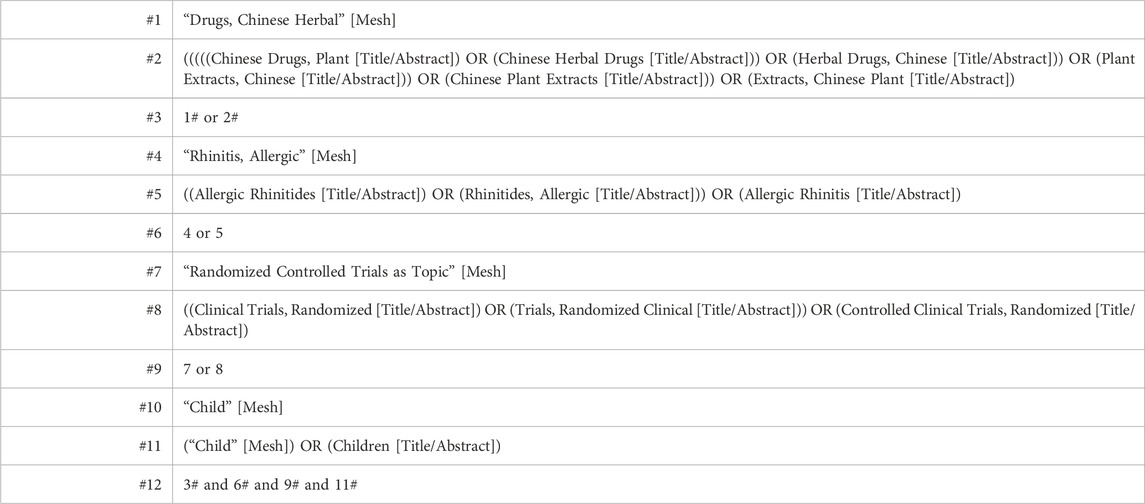
Nine databases were searched from their creation to March 2023 included PubMed, Embase, The Cochrane Library, Web of Science, China National Knowledge Infrastructure, Wan Fang Data, CQVIP, Chinese Biological Medicine, and ClinicalTrials.gov. No language or country restrictions were applied. Medical subject terms combined with free terms enhanced our search parameters. Primary search terms were “herbal medicine,” “traditional Chinese medicine,” “allergic rhinitis,” “pediatric,” and “randomized.” Table 1 displays the search strategy utilized in PubMed.
2.2 Inclusion and exclusion criteria
Inclusion criteria: 1) children with allergic rhinitis diagnosed using clear diagnostic criteria (Nasal Group and Pediatrics Group, 2022), between the ages of 3–18 years; 2) randomized controlled trials; 3) compared Chinese herbal medicine (Including alone or in combination with western medicine) to Western medicine or placebo. No restrictions on the type, use, or duration of Chinese herbal medicine; 4) nasal itching score was reported in study.
Exclusion criteria were: 1) use acupuncture, massage, or any non-Chinese herbal treatments or control group treatment with Chinese medicine; 2) Children with other co-morbidities. Two independent reviewers (CYH and WJ) screened the studies based on the selection criteria. Any discrepancies between the assessments of these reviewers were resolved by a third reviewer (WLQ).
2.3 Types of outcome measures
The primary outcomes were nasal itching score (Gu and Dong, 2005), scored from 0–3 (The scoring system for itchy nose symptoms is as follows: a score of 0 indicates an absence of itchy nose symptoms, a score of 1 signifies occasional and intermittent itchy nose, a score of 2 represents a tolerable creeper sensation, and a score of 3 indicates the most severe level, characterized by an intolerable creeper sensation.). Secondary outcomes were efficiency, serum IgE levels, serum IL-4, IL-10, and IL-33 levels, recurrent rate, and adverse events.
2.4 Data extraction and bias assessment
Upon completing the literature search, we employed Endnote 20.0 software to manage the collected literature. Two reviewers (CYH and WJ) independently screened the identified studies’ titles, abstracts, and full texts using the predetermined inclusion and exclusion criteria. Essential information from the included studies, such as authors’ names, publication year, sample size, participant demographics (gender and age), intervention methods, outcome measures, and adverse effects, was extracted by the same two reviewers (CYH and WJ) utilizing a pre-established data collection form. Subsequently, this information was cross-validated by another two reviewers (CH and ZZW). In cases requiring additional details, one reviewer (ZY) proactively contacted the authors of specific studies via phone or email. Any reviewer disagreements will be resolved through discussion with another reviewer (WLQ). The Risk of Bias in included literature was evaluated by two independent reviewers (CYH and WJ) using the Risk of Bias 2 tool, which assessed six specific areas: randomization process, deviations from intended interventions, missing data, outcome measurement, selection of the reported result, and overall bias. After the data was extracted, we transformed raw continuous variable data into post-treatment minus pre-treatment delta values following guidelines proposed by Cochrane (JPT et al., 2022).
2.5 Evidence synthesis and statistical analysis
The statistical analysis of this study was conducted using Review Manager 5.3 software and STATA/SE 12.0. The quality of evidence was evaluated using GRADE profiler 3.6. Effect sizes for dichotomous data were analyzed using risk ratio (RR) and their 95% confidence interval (CI). Mean difference (MD) effect sizes with 95% confidence intervals were used to analyze nasal itching data. Standardized mean difference (SMD) with 95% confidence interval was used for continuous variables represented in different units as reported in the original studies. A fixed effects model was utilized to analyze data with good homogeneity (I2 < 50%, p > 0.1), while a random effects model was used for data with poor homogeneity. p < 0.05 were considered to be statistically significant. Heterogeneity sources were elucidated by subgroup analysis when appropriate. Begg’s analysis was employed for studies with literature sizes equal to or greater than ten to ascertain publication bias. The stability of the study’s findings was determined using sensitivity analysis.
3 Results
3.1 Literature search results
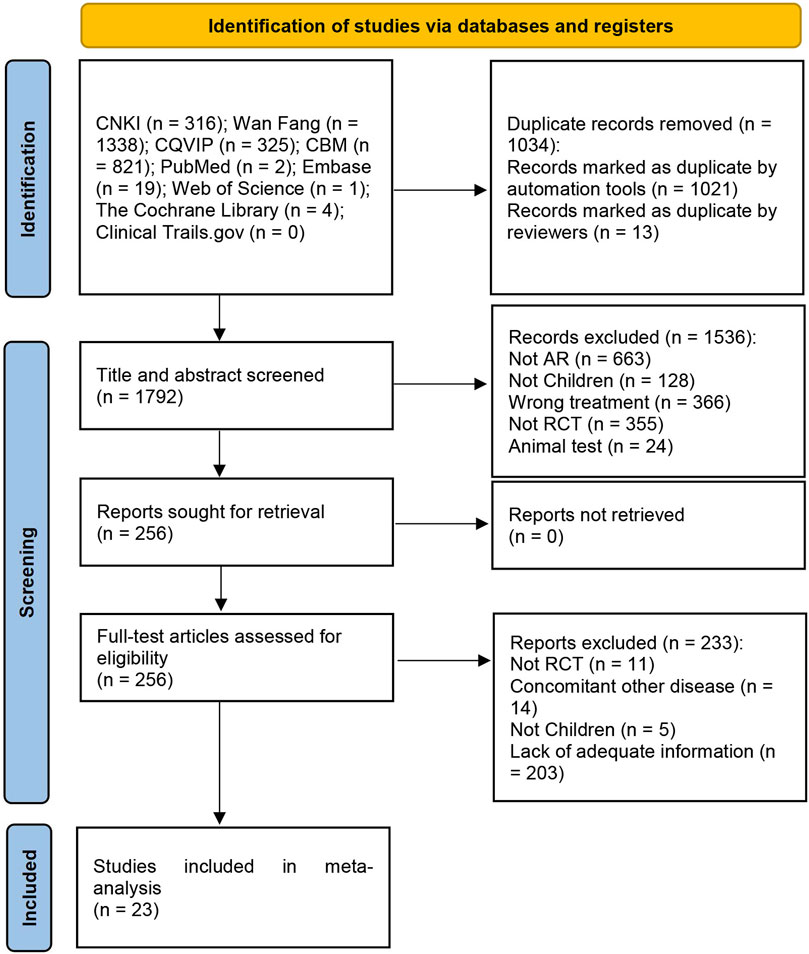
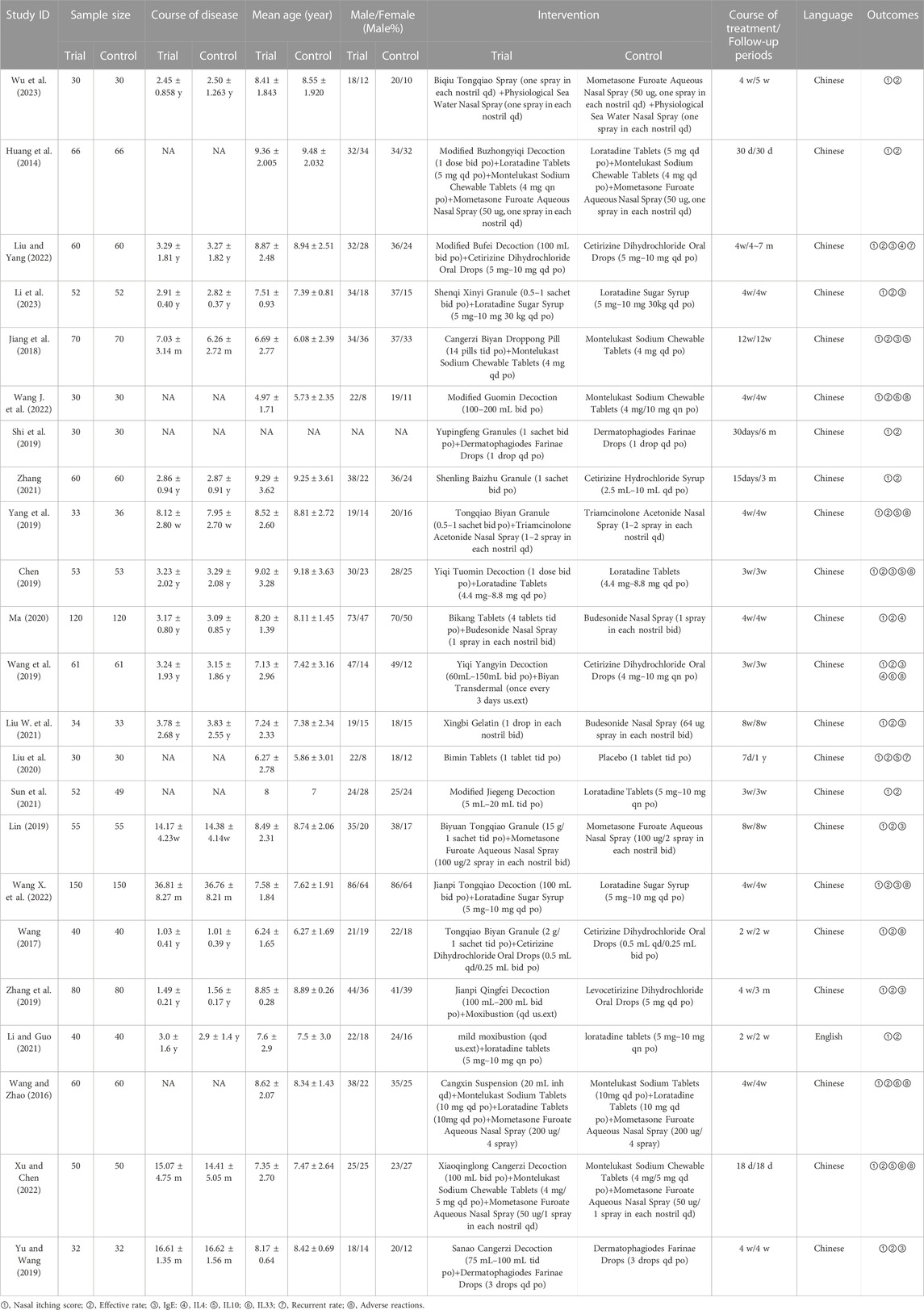
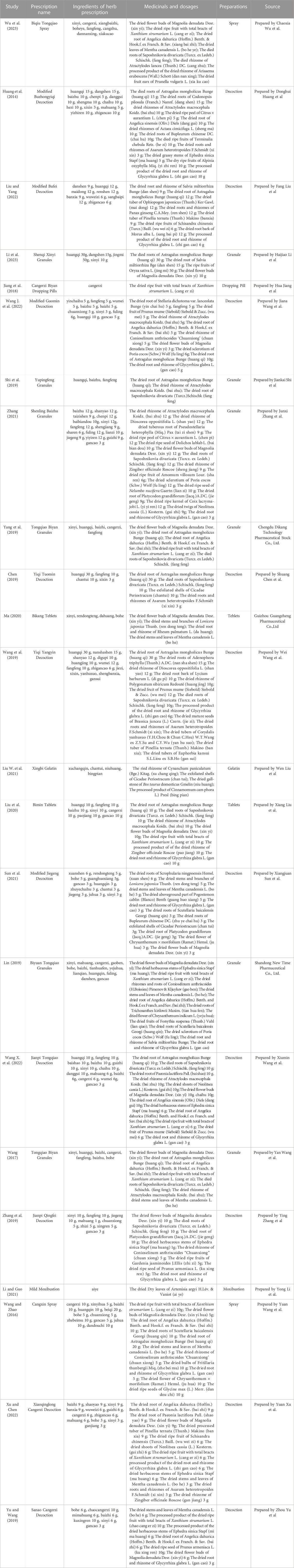
The initial search yielded 2,826 articles, from which 1,034 duplicates were identified and removed. A total of 1,536 articles were subsequently excluded based on their titles/abstracts according to the inclusion and exclusion criteria. After a full-text reading of the remaining 256 articles, 233 studies were excluded, leaving 23 RCTs (Huang et al., 2014; Wang and Zhao, 2016; Wang, 2017; Jiang et al., 2018; Chen, 2019; Lin, 2019; Shi et al., 2019; Wang et al., 2019; Yang et al., 2019; Yu and Wang, 2019; Zhang et al., 2019; Liu et al., 2020; Ma, 2020; Liu W. et al., 2021; Li and Guo, 2021; Sun et al., 2021; Zhang, 2021; Wang J. et al., 2022; Wang X. et al., 2022; Liu and Yang, 2022; Xu and Chen, 2022; Li et al., 2023; Wu et al., 2023) suitable for inclusion in this meta-analysis. Figure 1 illustrates the specific screening process, while Table 2 presents the distinctive characteristics of the analyzed studies. The drug utilization details, including dosage form, dose, and frequency, for each study and the duration of follow-up are presented in Table 2. Supplementary Table S1 provides additional information on the included studies’ patient sources, TCM syndromes, and funding sources. The characteristics of the included CHM are presented in Table 3.
Nine of the 23 randomized controlled trials (RCTs) explicitly stated that the patients were sourced from the outpatient clinic. One RCT included patients from the outpatient clinic and inpatient wards, while the remaining 13 did not provide explicit information regarding the patient source (Supplementary Table S1). In the 23 RCTs, one RCT (Liu et al., 2020) compared oral Chinese herbal medicine with placebo. Seven RCTs compared Chinese herbal medicine versus Western medicine, including three (Sun et al., 2021; Zhang, 2021; Wang J. et al., 2022) studies using oral CHM, two (Liu W. et al., 2021; Wu et al., 2023) using external CHM, and two studies (Wang et al., 2019; Zhang et al., 2019) using a combination of oral and external CHM. Fifteen RCTs used CHM in combination with WM compared with WM, of which thirteen RCTs (Huang et al., 2014; Wang and Zhao, 2016; Wang, 2017; Jiang et al., 2018; Chen, 2019; Lin, 2019; Shi et al., 2019; Yang et al., 2019; Yu and Wang, 2019; Ma, 2020; Wang X. et al., 2022; Liu and Yang, 2022; Li et al., 2023) used oral CHM and two RCTs (Wang and Zhao, 2016; Li and Guo, 2021) used external CHM. All 23 RCTs reported nasal itching score and efficiency rate. With serum IgE levels mentioned in ten (Jiang et al., 2018; Chen, 2019; Lin, 2019; Wang et al., 2019; Yu and Wang, 2019; Zhang et al., 2019; Liu W. et al., 2021; Wang X. et al., 2022; Liu and Yang, 2022; Li et al., 2023), IL-4 in two (Wang et al., 2019; Liu and Yang, 2022), IL-10 in five (Jiang et al., 2018; Chen, 2019; Yang et al., 2019; Liu et al., 2020; Xu and Chen, 2022) and IL33 in two (Wang et al., 2019; Xu and Chen, 2022). Recurrent rates were noted in two RCTs (Liu et al., 2020; Liu and Yang, 2022), while adverse events were reported in eight RCTs (Wang and Zhao, 2016; Wang, 2017; Chen, 2019; Wang et al., 2019; Yang et al., 2019; Wang J. et al., 2022; Wang X. et al., 2022; Xu and Chen, 2022). Of the 23 RCTs, 12 RCTs (Chen, 2019; Shi et al., 2019; Wang et al., 2019; Zhang et al., 2019; Liu W. et al., 2021; Li and Guo, 2021; Sun et al., 2021; Zhang, 2021; Wang J. et al., 2022; Wang X. et al., 2022; Li et al., 2023; Wu et al., 2023) indicated TCM syndromes, of which 2 RCTs (Sun et al., 2021; Wu et al., 2023) had a TCM syndrome of Lung meridian latent heat syndrome, 1 RCT (Li et al., 2023) was Spleen qi deficiency syndrome, 3 RCTs (Chen, 2019; Li and Guo, 2021; Wang J. et al., 2022) had a Lung-qi deficiency cold pattern, 4 RCTs (Shi et al., 2019; Zhang et al., 2019; Zhang, 2021; Wang X. et al., 2022) were Pulmonosplenic asthenia, 1 RCT (Wang et al., 2019) was Deficiency of both vital energy and yin, and 1 RCT (Liu W. et al., 2021) was Syndrome of wind invading the lung (Supplementary Table S1).
3.2 Risk of bias assessment
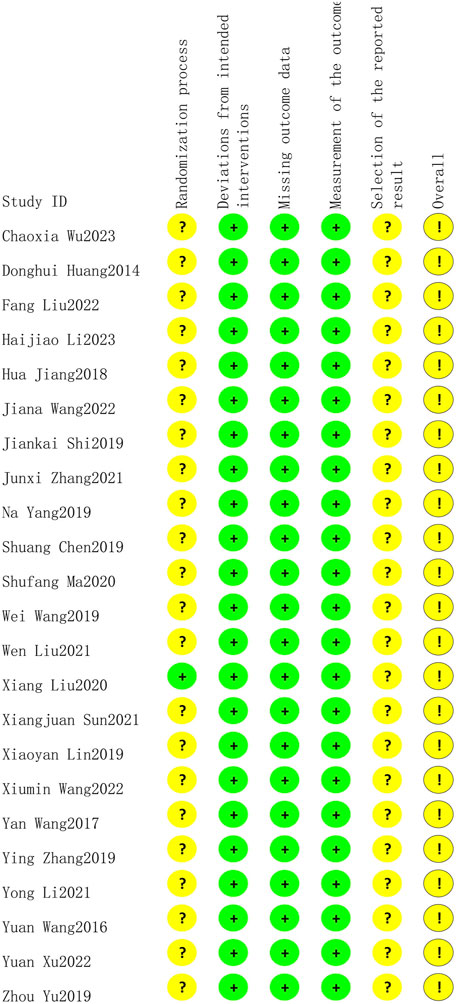
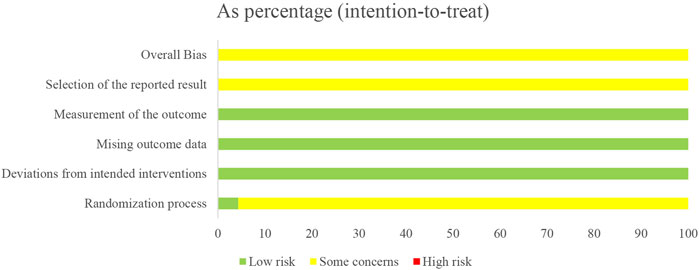
Only one study (Liu et al., 2020) reported low risk of randomization as the utilized of blinding, other studies did not utilize blinding so reported moderate risk of bias. All twenty-three studies lacked specification on study enrollment information, resulting in a moderate risk of selection of reported bias. All twenty-three studies reported low risk of deviations from intended intervention, missing outcome date, and measurement of outcome. The overall risk of bias for all twenty-three studies was moderate. The risk of article bias is presented in Figures 2, 3.
3.3 Primary outcomes
3.3.1 Nasal itching
3.3.1.1 Chinese herbal medicine versus placebo
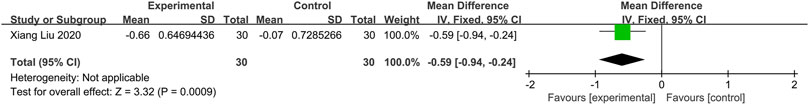
One study (Liu et al., 2020) compared herbal medicine to placebo. The fixed-effects model analysis showed that Chinese herbal medicine is significantly related to an alleviation of nasal itching (n = 60; MD = −0.59, 95%CI: −0.94 to −0.24, p = 0.0009; Figure 4).
3.3.1.2 Chinese herbal medicine versus western medicine
3.3.1.2.1 Oral CHM
Three RCTs (Sun et al., 2021; Zhang, 2021; Wang J. et al., 2022) compared the effects of oral Chinese herbal medicine to Western medicine, all of which had no pharmacological intervention lasting longer than 4 weeks. Fixed-effects model (p = 0.20, I2 = 38%), CHM demonstrated a statistically significant advantage in the relief of nasal itching (n = 281, MD = −0.45, 95% CI: −0.62 to −0.29, p < 0.00001; Figure 5A).
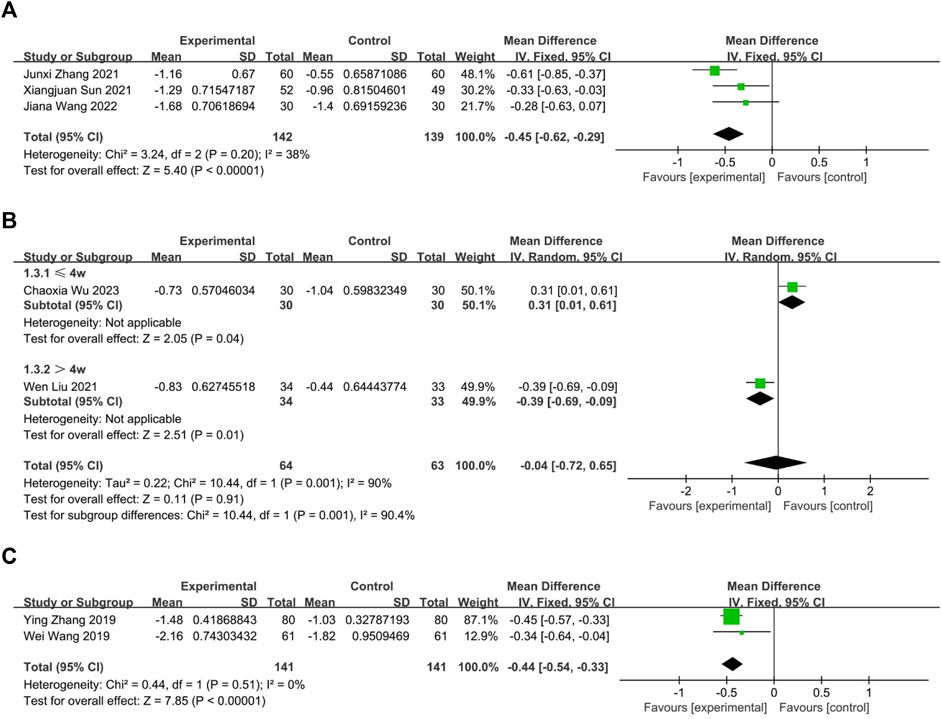
FIGURE 5. The result of meta-analysis of CHM versus WM on nasal itching. (A) oral CHM; (B) external CHM; (C) combination of oral and external CHM.
3.3.1.2.2 External CHM
Two RCTs (Liu W. et al., 2021; Wu et al., 2023) compared the effects of external Chinese herbal medicine to Western medicine. A random-effects model analysis (p = 0.001, I2 = 90%) revealed no significant difference between the two treatments (n = 127, MD = −0.04, 95% CI: −0.72 to 0.65, p = 0.91; Figure 5B). However, subgroup analysis based on intervention duration showed that one RCT with an intervention lasting more than 4 weeks favored external CHM over WM (n = 67, MD = −0.39, 95% CI: −0.69 to −0.09, p = 0.01; Figure 5B), while another RCT with an intervention lasting no more than 4 weeks reported better efficacy for WM than CHM (n = 60, MD = 0.31, 95% CI: 0.01 to 0.61, p = 0.04; Figure 5B).
3.3.1.2.3 Oral and external CHM
Two trials (Wang et al., 2019; Zhang et al., 2019) compared the combination of oral and external CHM to Western medicine. None of the interventions lasted longer than 4 weeks. Based on a fixed-effects model analysis (p = 0.51, I2 = 0%), Chinese herbal medicine demonstrated a statistically significant advantage over WM in treating nasal itching (n = 282, MD = −0.44, 95% CI: −0.54 to −0.33, p < 0.00001; Figure 5C).
3.3.1.3 Combination of CHM and WM versus WM alone
3.3.1.3.1 Oral CHM treatment
Thirteen trials compared the effectiveness of combining oral CHM with WM to that of WM alone. Based on a random-effects model analysis (p < 0.00001, I2 = 84%), the CHM group demonstrated a statistically significant advantage over the WM group (n = 1,625, MD = −0.37, 95% CI: −0.47 to −0.27, p < 0.00001; Figure 6A). Of the thirteen RCTs, nine (Wang, 2017; Chen, 2019; Yang et al., 2019; Yu and Wang, 2019; Ma, 2020; Wang X. et al., 2022; Liu and Yang, 2022; Xu and Chen, 2022; Li et al., 2023) had interventions lasting no more than 4 weeks (p < 0.00001, I2 = 84%), while four (Huang et al., 2014; Jiang et al., 2018; Lin, 2019; Shi et al., 2019) had interventions lasting more than 4 weeks (p = 0.16, I2 = 42%). The subgroup analysis results were consistent with the overall findings (n = 1,183; MD = −0.35; 95% CI: −0.48 to −0.23; p < 0.00001; n = 442; MD = −0.39; 95% CI: −0.53 to −0.25; p < 0.00001; Figure 6A). No publication bias was found by Begg’s test (p = 0.30; Supplementary Figure S1). Sensitivity analysis was performed with one-by-one exclusion and the results of the meta-analysis were found to be stable (Supplementary Figure S2).
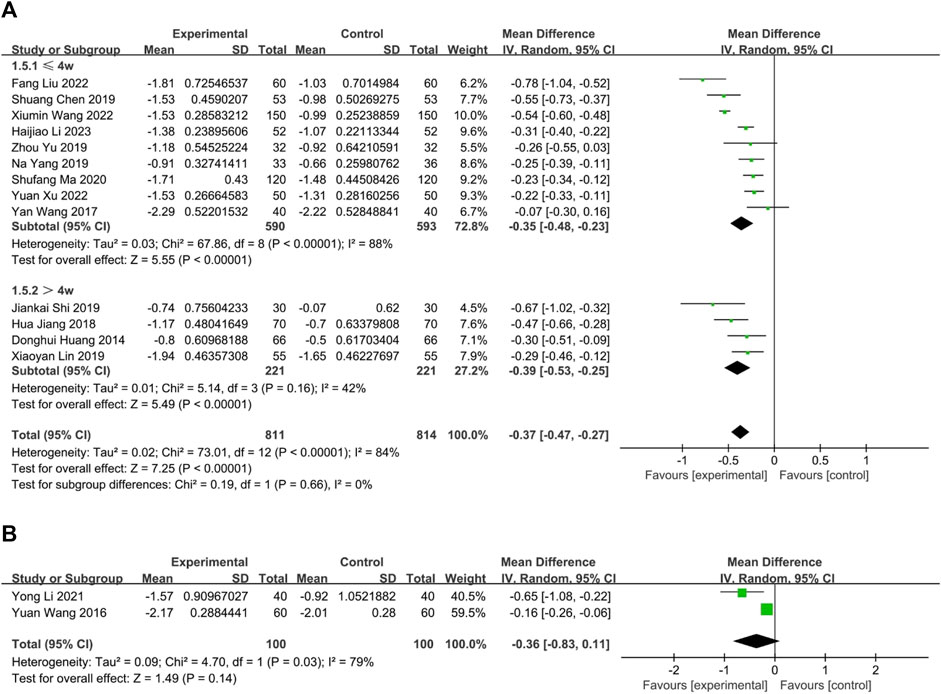
FIGURE 6. The result of meta-analysis of Combination of CHM and WM versus WM alone. (A) oral CHM; (B) external CHM.
3.3.1.3.2 External treatment with CHM
In two RCTs (Wang and Zhao, 2016; Li and Guo, 2021), external CHM combined with WM was compared to WM alone, with an intervention period lasting up to 4 weeks. Although the results of both RCTs indicated better efficacy of CHM in relieving nasal itching than WM, a random-effects model analysis did not show any significant difference between the two treatments (p = 0.03, I2 = 79%, n = 200, MD = −0.36, 95% CI: −0.83 to 0.11, p = 0.14; Figure 6B).
3.4 Secondary outcomes
3.4.1 Effective rate
3.4.1.1 Chinese herbal medicine versus placebo
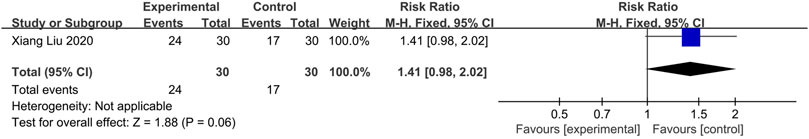
One study (Liu et al., 2020) compared CHM with Placebo. Fixed-effects models showed no statistical difference between the two groups (n = 60, RR = 1.41, 95% CI: 0.98 to 2.02, p = 0.06; Figure 7).
3.4.1.2 CHM versus WM
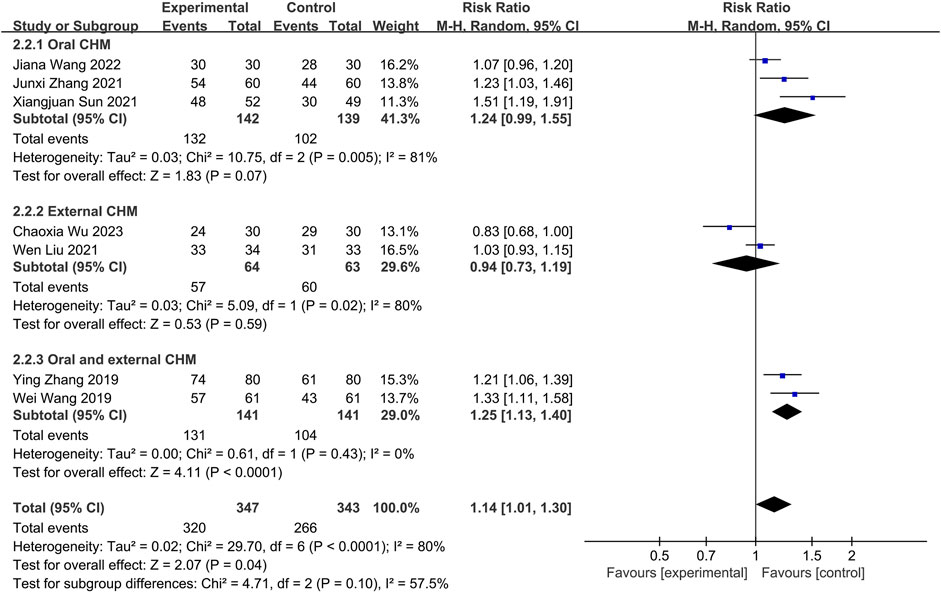
Seven studies compared CHM to WM. Of these, three (Sun et al., 2021; Zhang, 2021; Wang J. et al., 2022) investigated oral CHM, two (Liu W. et al., 2021; Wu et al., 2023) evaluated external CHM, and two (Wang et al., 2019; Zhang et al., 2019) assessed oral combined with external CHM. Based on a random-effects model analysis (p < 0.0001, I2 = 80%), there was no significant difference between oral CHM (n = 281, RR = 1.24, 95% CI: 0.99 to 1.55, p = 0.07; Figure 8) and external CHM (n = 127, RR = 0.94, 95% CI: 0.73 to 1.19, p = 0.59; Figure 8) and WM. Oral combined with external CHM (n = 282, RR = 1.25; 95% CI: 1.13 to 1.40; p < 0.0001; Figure 8) demonstrated a statistically significant advantage over WM.
3.4.1.3 Combination of CHM and WM versus WM
Fifteen studies were conducted to compare the effectiveness of combining CHM with WM to WM alone. Thirteen (Huang et al., 2014; Wang, 2017; Jiang et al., 2018; Chen, 2019; Lin, 2019; Shi et al., 2019; Yang et al., 2019; Yu and Wang, 2019; Ma, 2020; Wang X. et al., 2022; Liu and Yang, 2022; Xu and Chen, 2022; Li et al., 2023) utilizing oral CHM and two (Wang and Zhao, 2016; Li and Guo, 2021) using external CHM were included in this comparison. A fixed-effects model (p = 0.11, I2 = 34%) demonstrated that both oral and external CHM combined with WM had a significant statistical advantage over WM alone (n = 1,625, RR = 1.18, 95% CI: 1.13 to 1.22, p < 0.00001; n = 200, RR = 1.21, 95% CI: 1.07 to 1.35, p = 0.002; Figure 9). Publication bias was found by Begg’s test (p = 0.006; Supplementary Figure S3).
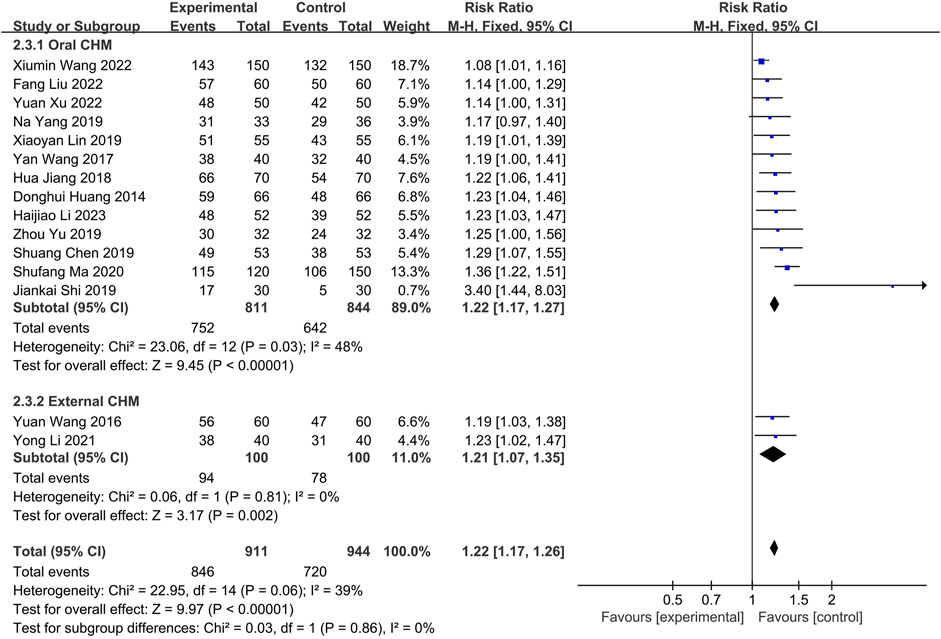
FIGURE 9. The result of meta-analysis of the combination of CHM and WM versus WM alone on effective rate.
3.4.2 Serum IgE level
3.4.2.1 CHM versus WM*
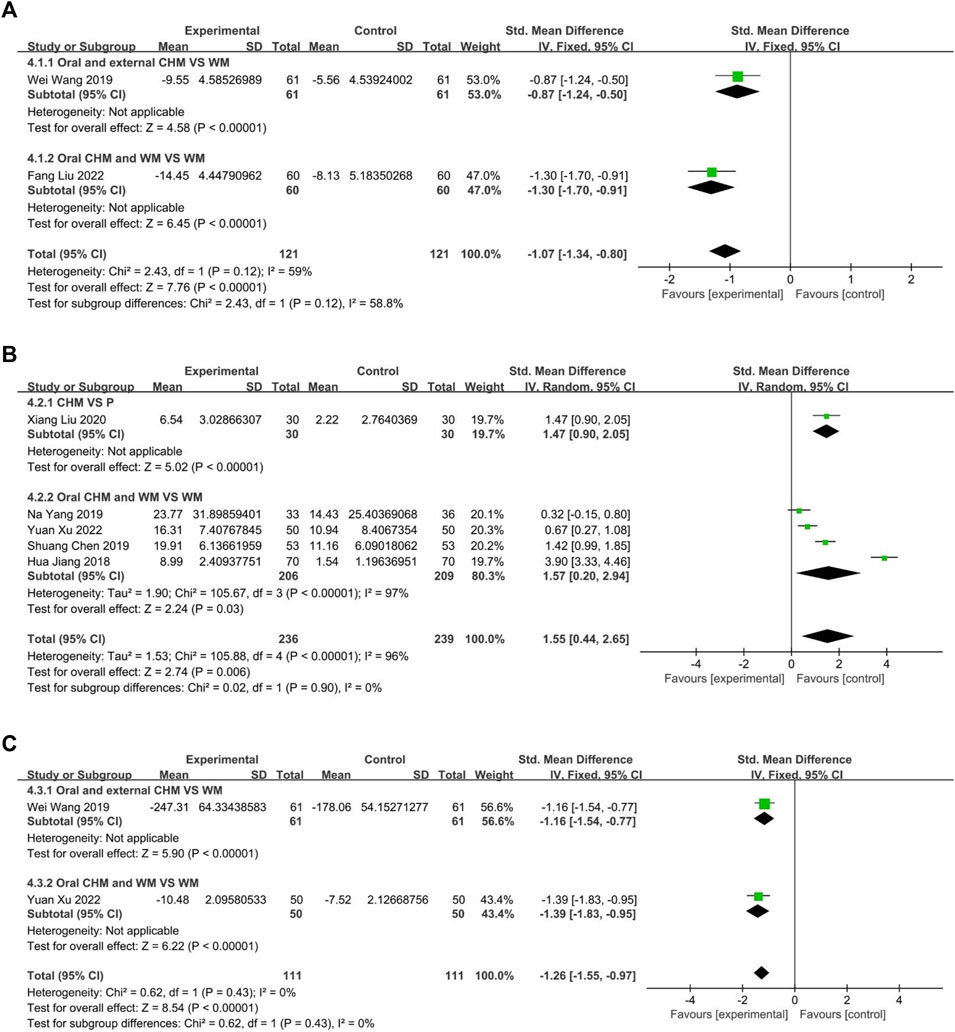
Three studies compared CHM to WM, with one (Liu W. et al., 2021) evaluating external CHM, while two (Wang et al., 2019; Zhang et al., 2019) assessing oral combined with external CHM. Based on a random-effects model analysis (p < 0.00001, I2 = 98%), there was insufficient evidence to suggest that external CHM was significantly different from WM in relieving nasal itching (n = 67, SMD = −0.04, 95% CI: −0.52 to 0.44, p = 0.87; Figure 10A). Oral combined with external CHM did not significantly differ from WM (n = 282, SMD = −1.77, 95% CI: −3.69 to 0.15, p = 0.07; Figure 10A).
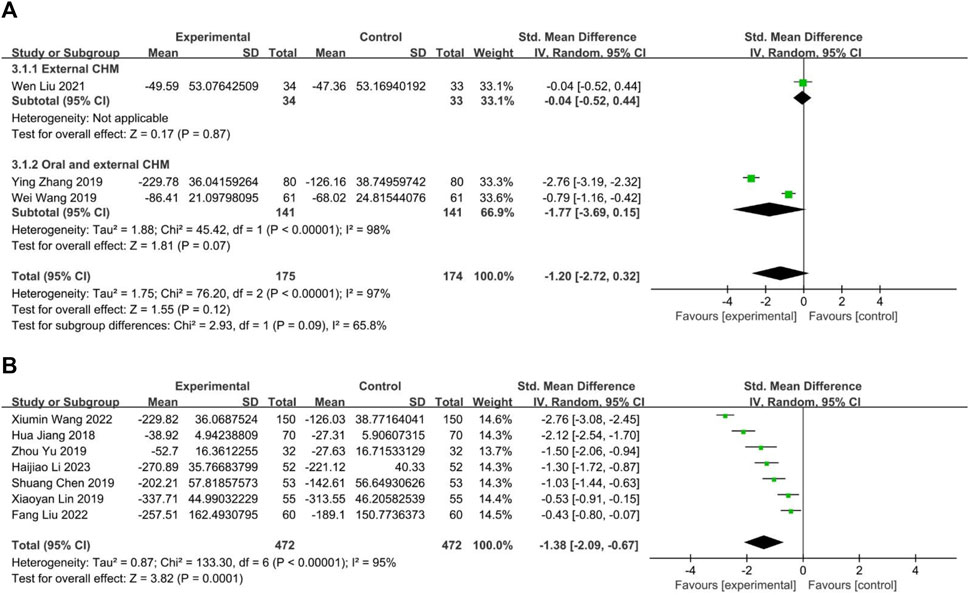
FIGURE 10. The result of meta-analysis of IgE. (A) CHM versus WM; (B) Combination of CHM and WM versus WM alone.
3.4.2.2 Combination of CHM and WM versus WM
Seven studies (Jiang et al., 2018; Chen, 2019; Lin, 2019; Yu and Wang, 2019; Wang X. et al., 2022; Liu and Yang, 2022; Li et al., 2023) compared combining oral CHM with WM to WM alone. The random-effects models (p < 0.00001, I2 = 95%) demonstrated a statistically significant advantage of CHM combined with WM over WM alone (n = 944, SMD = −1.38, 95% CI: −2.09 to −0.67, p = 0.0001; Figure 10B).
3.4.3 Serum IL-4 level
Two studies measured the level of IL4, with one (Wang et al., 2019) comparing CHM to WM, and one (Liu and Yang, 2022) evaluated the efficacy of CHM combined with WM versus WM alone. The fixed-effect model (p = 0.12, I2 = 59%) demonstrated that both CHM alone and CHM combined with WM were significantly superior to WM in reducing IL4 levels (n = 122, SMD = −0.87, 95% CI: −1.24 to −0.50, p < 0.00001; n = 120, SMD = −1.30, 95% CI: −1.70 to −0.91, p < 0.00001; Figure 11A).
3.4.4 Serum IL-10 level
Five trials reported IL10 levels, one (Liu et al., 2020) assessed CHM against Placebo, the remaining four studies (Jiang et al., 2018; Chen, 2019; Yang et al., 2019; Xu and Chen, 2022) evaluated the efficacy of combining CHM with WM versus WM alone. The result indicated that CHM is significantly related to decreased IL10 levels (n = 60, SMD = 1.47, 95% CI: 0.90 to 2.05, p < 0.00001; Figure 11B). A random-effect model (p < 0.00001, I2 = 96%) revealed a statistically significant advantage of combining CHM with WM over WM alone (p < 0.00001, I2 = 96%, n = 415, SMD = 1.57, 95% CI: 0.20 to 2.94, p < 0.00001; Figure 11B).
3.4.5 Serum IL-33 level
This study analyzed two trials, one (Wang et al., 2019) comparing CHM to WM and the other (Xu and Chen, 2022) investigating the efficacy of combining CHM and WM versus WM alone. The fixed-effect model (p = 0.43, I2 = 0%) demonstrated that both CHM alone and in combination with WM resulted in significantly better outcomes than WM (n = 122, SMD = −1.16, 95% CI: −1.54 to −0.77, p < 0.00001; n = 100, SMD = −1.39, 95% CI: −1.83 to −0.95, p < 0.00001; Figure 11C).
3.4.6 Recurrence rate
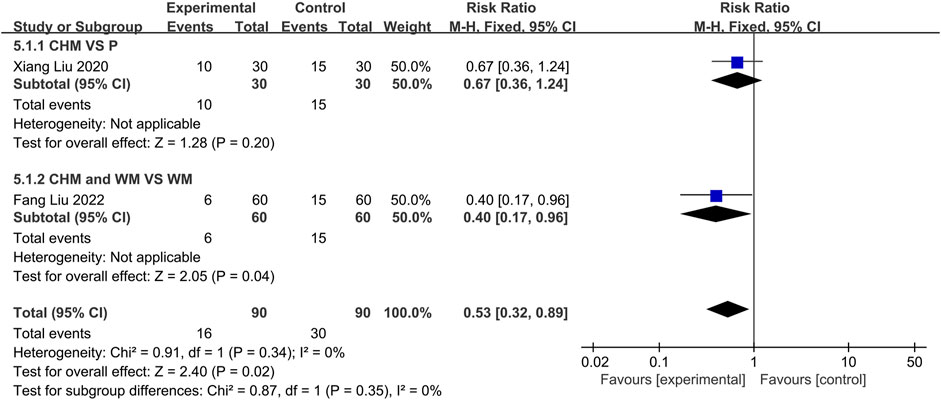
Two trials reported the recurrence rates. One(Liu et al., 2020) compared CHM to Placebo, and the other (Liu and Yang, 2022) compared the combination of CHM with WM to using WM alone. The fixed-effect (p = 0.34, I2 = 0%) models demonstrated no statistically significant difference between CHM and placebo groups (n = 60, RR = 0.67, 95% CI: 0.36 to 1.24, p = 0.20; Figure 12). However, a significant difference was observed between CHM with WM compared to WM alone, indicating lower recurrence rates among the former (n = 120, RR = 0.40, 95% CI: 0.17 to 0.96, p = 0.04; Figure 12).
3.4.7 Safety
The safety profiles of CHM and WM were evaluated in eight trials. Two (Wang et al., 2019; Wang J. et al., 2022) compared CHM to WM, and six (Wang and Zhao, 2016; Wang, 2017; Chen, 2019; Yang et al., 2019; Wang X. et al., 2022; Xu and Chen, 2022) compared combined CHM with WM to WM alone. The fixed-effect model (p = 0.68, I2 = 0%) indicated that CHM had a lower incidence of adverse drug reactions compared to WM (p = 0.68, I2 = 0%; n = 182, RR = 0.19, 95% CI: 0.06 to 0.57, p = 0.003; Figure 13A) and no significant difference between the combination of CHM with WM and WM alone (p = 0.55, I2 = 0%; n = 775, RR = 0.79, 95% CI: 0.55 to 1.14, p = 0.21; Figure 13B), according to the fixed-effect model (p = 0.68, I2 = 0%).
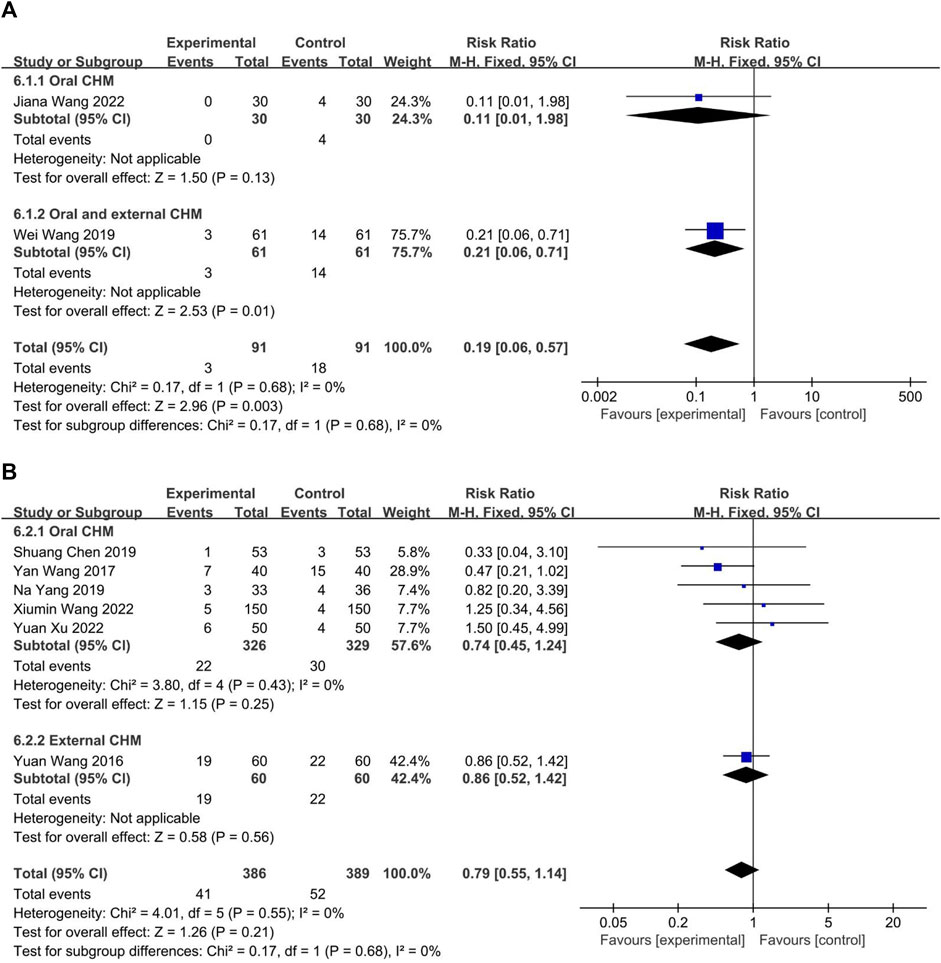
FIGURE 13. The result of meta-analysis of adverse reaction. (A) CHM versus WM; (B) Combination of CHM and WM versus WM alone.
3.5 GRADE for the main comparisons
The GRADE quality of evidence for all outcomes was evaluated. The quality of Nasal itch, effective rate, IgE, IL10, IL33, and recurrent rate were low. The quality of IL4 was very low. The quality of adverse reaction was moderate (Supplementary Table S2).
3.6 Description of CHM
A total of 68 different herbal medicines were used in 23 RCTs. Eight herbals were used more than seven times (10%), includes Biond Magnolia Immature Flower, Liquorice Root, Membranous Milkvetch Root, Divaricate Saposhnikovia Root, Siberian Cocklebur Fruit, Dahurian Angelica Root, Peppermint Rhizome, and Largehead Atractylodes Rhizome (Supplementary Table S3).
4 Discussion
4.1 Summary of evidence
Chinese herbal medicine is a widely used treatment for allergic rhinitis in children in China. Although previous Chinese studies have demonstrated the therapeutic effects of CHM on allergic rhinitis in children, their level of evidence was low. Therefore, we conducted a meta-analysis of 23 randomized controlled trials (RCTs) with 2,605 children to pool the information on CHM’s efficacy for nasal itching symptom relief, modulation of immune imbalance (IgE, IL-4, IL-10, and IL-33), relapse reduction, and safety.
The risk of bias assessment indicates the need for attention to the risk of bias in the randomization process and selective reporting of endpoints in the included studies. This concern primarily arises from the absence of blinding, lack of reporting on specific methods of random allocation concealment, and failure to report study protocols.
Our primary findings indicate that Chinese herbal medicine (CHM) is related to reducing nasal itching and raising interleukin-10 (IL-10) levels compared to placebo. When administered orally, herbal medicines alone alleviate nasal itching and suppress levels of immunoglobulin E (IgE), interleukin-4 (IL-4), and interleukin-33 (IL-33), while simultaneously increasing IL-10 levels in contrast to Western medicines. Additionally, the combination of oral and topical CHM substantially alleviates nasal itching. External CHM was overall similar to WM in relieving nasal itching, efficiency, and lowering IgE levels. The combined external application of CHM and WM did not differ statistically from using WM alone for relieving nasal itching. Both studies within the subgroup indicated that external CHM combined with WM was superior in relieving nasal itching, but the meta-analysis indicated the opposite result. This was due to the use of a randomized effect model to account for the high heterogeneity. When using a fixed-effects model can obtain a more significant result. Therefore, we believe that topical CHM combined with WM is beneficial in the treatment of nasal itching. Statistically significant advantages were also observed when CHM was used with WM in other indicators. For the same reason, we believe that oral combined with external CHM is effective in reducing IgE. Eight randomized controlled trials (RCTs) reported adverse effects such as headache, dizziness, malaise, and dry mouth; however, all self-heal and no serious adverse events were reported. Meta-analysis results suggest that CHM alone or combined with WM has a favorable safety profile.
We found that the results of using external CHM alone varied widely across studies. The effectiveness of external CHM may depend on the duration of intervention and the use of different WM controls. External CHM was more effective when the duration of treatment was longer than 4 weeks, with the control group using antihistamines. Conversely, when the duration of treatment was shorter, and the control group received glucocorticoids, the opposite result was observed. Nasal hormones act on glucocorticoid receptors in the nasal mucosa by reducing inflammatory factors and inhibiting inflammation cells, thus decreasing inflammatory factor-mediated hyperirritability of the nasal mucosa. These two explanations may account for the lack of efficacy of external CHM.
4.2 Implications for practice
We have compiled comprehensive information regarding the herbal medicines utilized in this study, encompassing their types, frequency of use, and the outcomes associated with CHM prescriptions incorporated within commonly used herbal medicines. The presented results are detailed below: Across the 23 studies analyzed, the detailed composition of the CHM varied widely and included over 60 different herbs. The most frequently used CHMs were Magnolia denudate Desr, Glycyrrhiza glabra L, Astragalus mongholicus Bunge, Saposhnikovia divaricate (Turcz. ex Ledeb.) Schischk, Xanthium strumarium L, Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav, Mentha canadensis L, Atractylodes macrocephala Koidz. Cang Erzi San (Xanthium strumarium L, Magnolia denudate Desr, Angelica dahurica (Hoffm) Benth. & Hook. f. ex Franch. & Sav, Mentha canadensis L) and Yu Pingfeng San (Astragalus mongholicus Bunge, Atractylodes macrocephala Koidz., Saposhnikovia divaricate (Turcz. ex Ledeb.) Schischk) are most commonly used CHM redescription for treating allergic rhinitis. The present findings may have important implications for the TCM management of rhinitis in children, informing the development of relevant guidelines.
4.3 Limitations of the study
This study conducted a thorough and comprehensive literature search to examine the efficacy and safety of Chinese herbal medicine (CHM) therapy for children with allergic rhinitis. The findings indicate that CHM holds significant potential in alleviating symptoms of nasal itchiness, modulating inflammatory responses, and reducing recurrence.
However, the study still has several limitations. Firstly, the methodological quality of the included studies was low. Only one study implemented blinding, and no study reported trial protocol, making determining adherence to the prescribed protocols challenging. Most studies did not explicitly report the concealment of random assignment. Secondly, the assessment of nasal itchiness relied on subjective measures, while the blood test results were objective. However, different studies used varying units for measurement, making it challenging to fully mitigate heterogeneity, despite using Delta values and standardized mean differences (SMDs). Thirdly, substantial variability in the composition of Chinese herbal medicine (CHM) prescriptions and administration methods contributed to heterogeneity across the studies. Fourthly, most of the included studies did not evaluate the long-term efficacy of CHM. Given the association between allergic rhinitis and other conditions such as asthma and ADHD, it is essential to consider the co-occurrence of these diseases when assessing the long-term efficacy of CHM. Lastly, it is essential to note that all the included studies were conducted in single-centre settings in China, potentially introducing publication bias.
4.4 Implications for research
Based on the findings and limitations, we propose the following key considerations for future research: Firstly, it is crucial to enhance the methodological quality of studies. This can be achieved by pre-registering the study protocol, ensuring data transparency, and rigorously implementing randomization, allocation concealment, and blinding throughout the study process to uphold the integrity of the research. Secondly, it is crucial to improve the design of clinical studies by selecting more objective outcome measures, authenticating the data, and minimizing individual differences. Additionally, incorporating appropriate follow-up periods that align with the characteristics of the disease, such as monitoring recurrence rates and associated comorbidities in the case of rhinitis, will provide valuable clinical insights. Thirdly, for randomized controlled trials involving Chinese herbal medicine (CHM), adherence to the CONSORT Extension for Chinese Herbal Medicine Formulas 2017 (Cheng et al., 2017) is recommended to ensure standardization and authenticity of the trials. Fourthly, implementing multicenter, large-sample, and high-quality clinical studies is warranted to enhance the generalizability of research findings. Fifth, in view of the research potential of Chinese herbal medicines in allergic diseases, clinical and animal experimental studies focusing on the active ingredients of Chinese herbal medicines may elucidate the specific effects and intrinsic mechanisms of different Chinese herbal medicinal ingredients and Chinese herbal prescriptions, to better guide the use of CHM in pediatric rhinitis. For example, studies (Liu et al., 2023) have confirmed that the CHM prescription YPF and its main active compound wogonin may alleviate airway inflammation in asthma by inhibiting PI3K/AKT, IL-17 and TNF-α signaling pathways.
Given the distinctive prescriptions and dosage forms of CHM, achieving blinding in clinical research involving CHM presents a challenging task. This study identified two potential common CHM prescriptions and eight herbal medicines for childhood rhinitis. It is worth considering future investigations to explore the feasibility of utilizing these prescriptions and drugs to develop new CHM dosage forms. This would facilitate blinding in clinical studies of childhood rhinitis and enhance the completeness of research protocols for RCTs. This study included 12 RCTs, which reported similar TCM evidence. Subgroup analysis of TCM evidence demonstrated an association between CHM use and a reduction in itch (Supplementary Figure S4). However, due to the overall low methodological quality of the original studies included in this systematic review, we believe that additional high-quality basic and clinical studies are necessary to further validate the role of herbal and CHM prescriptions, as well as traditional Chinese medicine (TCM) evidence, in clinical practice.
5 Conclusion
Chinese herbal medicine (CHM) holds great potential in alleviating symptoms, modulating immune factors levels, and reducing relapse in pediatric rhinitis. Meanwhile, CHM is relatively safe. However, the efficacy and safety of CHM in treating pediatric rhinitis still need to be confirmed due to the inclusion of studies with low methodological quality, small sample sizes, and potential heterogeneity. More large-sample, high-quality RCTs are necessary to provide reliable evidence for the clinical application of CHM.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
YC designed the study. YC, JW, YZ, HC, ZZ, and LW collaborated to conduct the literature search, collect and process data, assess quality, and perform statistical analysis. YC completed the final discussion and summary section with guidance from LW. All authors contributed to the article and approved the submitted version.
Funding
The authors of this review were supported by the National Natural Science Foundation of China (81874487).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1240917/full#supplementary-material
References
Chan, H. H. L., and Ng, T. (2020). Traditional Chinese medicine (TCM) and allergic diseases. Curr. Allergy Asthma Rep. 20 (11), 67. doi:10.1007/s11882-020-00959-9
Chen, S. (2019). Clinical observation on the treatment of 53 cases of children’s allergic rhinitis by self-made Yiqi Tuomin decoction combined with western medicine. J. Pediatr. Traditional Chin. Med. 15 (02), 38–45. doi:10.16840/j.issn1673-4297.2019.02.12
Cheng, C. W., Wu, T. X., Shang, H. C., Li, Y. P., Altman, D. G., Moher, D., et al. (2017). CONSORT extension for Chinese herbal medicine Formulas 2017: recommendations, explanation, and elaboration (traditional Chinese version). Ann. Intern Med. 167 (2), W7–w20. doi:10.7326/IsTranslatedFrom_M17-2977_1
Cobanoğlu, B., Toskala, E., Ural, A., and Cingi, C. (2013). Role of leukotriene antagonists and antihistamines in the treatment of allergic rhinitis. Curr. Allergy Asthma Rep. 13 (2), 203–208. doi:10.1007/s11882-013-0341-4
Donaldson, A. M. (2023). Upper airway cough syndrome. Otolaryngol. Clin. North Am. 56 (1), 147–155. doi:10.1016/j.otc.2022.09.011
Dou, Y., Zhu, Z., and Di, G. (2023). Evaluation of curative effect of acupoint application in treatment of allergic rhinitis and asthma and the effect of serum IL - 33. Liaoning J. Traditional Chin. Med. 50 (03), 177–180. doi:10.13192/j.issn.1000-1719.2023.03.050
Gu, Z., and Dong, Z. (2005). The diagnosis and treatment principle and recommended scheme of allergic rhinitis (Lanzhou, China, 2004). Chin. J. Otorhinolaryngology Head Neck Surg. (03), 8–9.
He, X., Feng, Y., Dong, Y., and Wang, B. (2017). Allergic rhinitis and psychological state assessment. J. Clin. Otorhinolaryngology Head Neck Surg. 31 (05), 400–403. doi:10.13201/j.issn.1001-1781.2017.05.019
Higgins, J. P. T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., et al. (2022). Cochrane handbook for systematic reviews of interventions . Available at: www.training.cochrane.org/handbook.
Hox, V., Lourijsen, E., Jordens, A., Aasbjerg, K., Agache, I., Alobid, I., et al. (2020). Benefits and harm of systemic steroids for short- and long-term use in rhinitis and rhinosinusitis: an EAACI position paper. Clin. Transl. Allergy 10, 1. doi:10.1186/s13601-019-0303-6
Huang, D., Chen, J., Ji, S., and Zhong, Y. (2014). Clinical observation on treating allergic rhinitis in children with buzhong yiqi decoction. Clin. J. Chin. Med. (11), 3–5. doi:10.3969/j.issn.1674-7860.2014.11.002
Jiang, H., Zhao, J., Zhang, Q., and Yang, J. (2018). Efficacy of Xanthium rhinitis dripping pills combined with montelukast in the treatment of allergic rhinitis in children. Shaanxi J. Traditional Chin. Med. 39 (7), 851–853. doi:10.3969/j.issn.1000-7369.2018.07.011
Klimek, L., Sperl, A., Becker, S., Mösges, R., and Tomazic, P. V. (2019). Current therapeutical strategies for allergic rhinitis. Expert Opin. Pharmacother. 20 (1), 83–89. doi:10.1080/14656566.2018.1543401
Li, H., Chen, X., and Chen, Q. (2023). Efficacy observation of Shenqi Xinyi granules on allergic rhinitis with spleen-qi weakness type in children. J. Mod. Med. Health 39 (4), 595–598. doi:10.3969/j.issn.1009-5519.2023.04.013
Li, Y., Guo, X., Zhao, D., Liu, J., Wang, L., Li, M., et al. (2021). Mild moxibustion plus loratadine tablets for children with allergic rhinitis: A randomized controlled trial. J. Acupunct. Tuina Sci. 19 (6), 419–424. doi:10.1038/s41392-021-00843-6
Lin, X. (2019). Clinical study on biyuan tongqiao granules combined with mometasone furfurate nasal spray in treatment of allergic rhinitis in children. Drugs and Clin. 34 (6), 1744–1747. doi:10.7501/j.issn.1674-5515.2019.06.030
Liu, F., and Yang, C. (2022). Clinical observation of modified bufei decoction combined with cetirizine in the treatment of children's allergic rhitinis. J. Pract. TRADITIONAL Chin. Med. 38 (8), 1382–1384. doi:10.3969/j.issn.1004-2814.2022.8.syzyyzz202208053
Liu, W., Wu, Y., Yang, W., Shen, F., and Qiu, C. (2021). Clinical study on Xingbi gel nasal drops in the treatment of allergic rhinitis in children. Chin. Foreign Med. Res. 19 (21), 40–43. doi:10.14033/j.cnki.cfmr.2021.21.014
Liu, X., Tang, H., Peng, W., and Chen, S. (2020). The effect of BI MIN PIAN on eosinophil level in nasal secretion of children with allergic rhinitis. Chin. J. Otorhinolaryngology Integr. Med. 28(4), 251–254. doi:10.16542/j.cnki.issn.1007-4856.2020.04.003
Liu, X., Wang, X., Zhang, X., and Cao, A. H. (2021). Allergic diseases influence symptom severity and T lymphocyte subgroups of children with tic disorders. J. Investig. Med. 69 (8), 1453–1457. doi:10.1136/jim-2021-001788
Liu, X., Yu, Y., Wu, Y., Luo, A., Yang, M., Li, T., et al. (2023). A systematic pharmacology-based in vivo study to reveal the effective mechanism of Yupingfeng in asthma treatment. Phytomedicine 114, 154783. doi:10.1016/j.phymed.2023.154783
Liu, X., Zhang, L., Hu, Y., Yang, X., Di, Z., Wang, Y., et al. (2022). Effects of Tiaoxi and Tongqiao Regimen on nasal morphology and total serum IgE and other immune indexes in patients with allergic rhinitis. Hebei Med. J. 44 (16), 2461–2465. doi:10.3969/j.issn.1002-7386.2022.16.014
Ma, S. (2020). Clinical study on the treatment of allergic rhinitis in children with bikang tablets combined with budesonide nasal spray. Maternal Child Health Care China 35 (19), 3610–3613. doi:10.19829/j.zgfybj.issn.1001-4411.2020.19.028
Marple, B. F. (2010). Allergic rhinitis and inflammatory airway disease: interactions within the unified airspace. Am. J. Rhinol. Allergy 24 (4), 249–254. doi:10.2500/ajra.2010.24.3499
Marques, C. F., Marques, M. M., and Justino, G. C. (2022). Leukotrienes vs. Montelukast-activity, metabolism, and toxicity hints for repurposing. Pharm. (Basel) 15 (9), 1039. doi:10.3390/ph15091039
Mener, D. J., Shargorodsky, J., Varadhan, R., and Lin, S. Y. (2015). Topical intranasal corticosteroids and growth velocity in children: A meta-analysis. Int. Forum Allergy Rhinol. 5 (2), 95–103. doi:10.1002/alr.21430
Morais-Almeida, M., Wandalsen, G. F., and Solé, D. (2019). Growth and mouth breathers. J. Pediatr. (Rio J. 95 (Suppl. 1), 66–71. doi:10.1016/j.jped.2018.11.005
Nasal Group and Pediatrics Group, (2022). Guidelines for the diagnosis and treatment of allergic rhinitis in children (2022, revised edition). Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 57(04), 392–404. doi:10.3760/cma.j.cn115330-20220303-00092
Ruikun, W., Jieqiong, L., Wei, H., Wenpeng, W., Yingxia, L., and Qinglong, G. (2022). Prevalence of allergic rhinitis in Chinese children from 2001 to 2021: meta analysis. Chin. J. Prev. Med. (06), 784–793. doi:10.3760/cma.j.cn112150-20220315-00242
Sastre, J., Mullol, J., Valero, A., and Valiente, R.Bilastine Study Group (2012). Efficacy and safety of bilastine 20 mg compared with cetirizine 10 mg and placebo in the treatment of perennial allergic rhinitis. Curr. Med. Res. Opin. 28 (1), 121–130. doi:10.1185/03007995.2011.640667
Shi, J., Liu, G., and Wu, F. (2019). Clinical observation of 90 cases of children with allergic rhinitis treated by sublingual immunotherapy with dust mile combined yupingfeng granule. Chin. J. Otorhinolaryngology Integr. Med. 27 (01), 41–44. doi:10.16542/j.cnki.issn.1007-4856.2019.01.008
Sirufo, M. M., Suppa, M., Ginaldi, L., and De Martinis, M. (2020). Does allergy break bones? Osteoporosis and its connection to allergy. Int. J. Mol. Sci. 21 (3), 712. doi:10.3390/ijms21030712
Sun, X., Liu, L., Weng, D., Yu, Q., and Zhang, F. (2021). Effects of modified jiegeng decoction on children with allergic rhinitis with underlying heat in lung meridian. Chin. Pediatr. Integr. Traditional West. Med. 13 (4), 350–353. doi:10.3969/j.issn.16743865.2021.04.018
Wang, J., Qiu, Z., and Xie, J. (2022). Clinical observation on treating allergic rhinitis of lung Qi deficiency and cold syndrome with the Guomin decoction. Clin. J. Chin. Med. 14 (30), 22–25. doi:10.3969/j.issn.1674-7860.2022.30.008
Wang, W., Xing, X., Liu, W., Sun, Y., and Du, M. (2019). Effect of yiqi yangyin recipe combined with acupoint application on patients with allergic rhinitis. Hebei J. Traditional Chin. Med. 41(12), 1795–1798. doi:10.3969/j.issn.1002-2619.2019.12.008
Wang, X., Yuan, S., Jiang, R., and Gu, Y. (2022). Efficacy of bufei jianpi recipe combined with loratadine syrup in the treatment of allergic rhinitis in children with deficiency of lung and spleen. Eval. analysis drug-use Hosp. China 22 (7), 847–851. doi:10.3760/cma.j.cn112150-20211214-01155
Wang, Y. (2017). The clinical efficacy analysis of tongqiao rhinitis particles combined with cetirizine drops treatment of children with allergic rhinitis. J. Pediatr. Pharm. 23 (03), 30–32. doi:10.13407/j.cnki.jpp.1672-108X.2017.03.010
Wang, Y., and Zhao, S. (2016). Efficacy observation of cangxin liquid aerosol combined with normal saline nasal irrigation in the treatment of children's allergic rhinitis. Guizhou Med. J. 40 (3), 263–264. doi:10.3969/j.issn.1000-744X.2016.03.016
Wolthers, O. D., and Pedersen, S. (1993). Short-term growth in children with allergic rhinitis treated with oral antihistamine, depot and intranasal glucocorticosteroids. Acta Paediatr. 82 (8), 635–640. doi:10.1111/j.1651-2227.1993.tb18030.x
Wu, C., Xue, B., Li, J., and Tian, J. (2023). Clinical observation of biqiu tongqiao nasal spray in the treatment of allergic rhinitis with underlying heat in lung meridian. J. Extern. Ther. Traditional Chin. Med. 32 (01), 43–45. doi:10.3969/j.issn.1006-978X.2023.01.018
Xu, Y., and Chen, F. (2022). Clinical effect and safety of xiaoqinglong decoction combined with Xanthium powder in the treatment of allergic rhinitis in children. Chin. J. Clin. Ration. Drug Use 15 (25), 42–45. doi:10.15887/j.cnki.13-1389/r.2022.25.011
Xu, Y. C., Wang, J. P., Zhu, W. J., and Li, P. (2020). Childhood atopic dermatitis as a precursor for developing attention deficit/hyperactivity disorder. Int. J. Immunopathol. Pharmacol. 34, 2058738420962902. doi:10.1177/2058738420962902
Yang, N., Liu, Y., and Cheng, C. (2019). Clinical effect of Tongqiao Biyan granule on the children with allergic rhinitis. WORLD Clin. DRUGS 40 (12), 862–866. doi:10.13683/j.wph.2019.12.008
Yu, Z., and Wang, L. (2019). Effect of sanao decoction and Xanthium powder on children’s allergic rhinitis and its effect on serum IgE level,EOS level and Th1/Th2 cytokine level. Med. Innovation China 16 (22), 84–88. doi:10.3969/j.issn.1674-4985.2019.22.022
Zhang, J. (2021). Study on the clinical value of Shenlingbaizhu powder in treating children with allergic rhinitis. Chin. Med. Digest:Otorhinolaryngology 36 (02), 99–100. doi:10.19617/j.issn1001-1307.2021.02.99
Zhang, Y., Zhang, J., Zhao, J., Zhang, L., Liu, H., and Wang, J. (2019). Clinical study on jianpi qingfei decoction combined with shenque moxibustion in the treatment of children's allergic rhinitis and its effect on the expression of IgE and EOS. J. Hebei TCM Pharmacol. 34 (02), 30–32. doi:10.16370/j.cnki.13-1214/r.2019.02.009
Zhang, Y., and Zhang, L. (2019). Increasing prevalence of allergic rhinitis in China. Allergy Asthma Immunol. Res. 11 (2), 156–169. doi:10.4168/aair.2019.11.2.156
Zhou, R. Y., Wang, J. J., Sun, J. C., You, Y., Ying, J. N., and Han, X. M. (2017). Attention deficit hyperactivity disorder may be a highly inflammation and immune-associated disease (Review). Mol. Med. Rep. 16 (4), 5071–5077. doi:10.3892/mmr.2017.7228
Keywords: Chinese herbal medicine (CHM), nasal itching, allergic rhinitis (AR), children, systematic review, meta-analysis
Citation: Chen Y, Wang J, Wu L, Zhang Y, Chen H and Zhang Z (2023) Efficacy of Chinese herbal medicine on nasal itching in children with allergic rhinitis: a systematic review and meta-analysis. Front. Pharmacol. 14:1240917. doi: 10.3389/fphar.2023.1240917
Received: 15 June 2023; Accepted: 08 August 2023;
Published: 23 August 2023.
Edited by:
Adolfo Andrade-Cetto, National Autonomous University of Mexico, MexicoReviewed by:
Nuray Bayar Muluk, Kırıkkale University, TürkiyeHong Li, Southern Medical University, China
Copyright © 2023 Chen, Wang, Wu, Zhang, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liqun Wu, V3VscTEyMTFAMTYzLmNvbQ==
 Yuhang Chen
Yuhang Chen Jie Wang
Jie Wang Liqun Wu3*
Liqun Wu3*