- 1Department of Clinical Pharmacy, Anhui Provincial Children’s Hospital, Hefei, Anhui, China
- 2Department of Respiratory Intensive Care Unit, Anhui Chest Hospital, Hefei, Anhui, China
Chronic Obstructive Pulmonary Disease (COPD) is a chronic respiratory disease characterized by a slow progression and caused by the inhalation of harmful particulate matter. Cigarette smoke and air pollutants are the primary contributing factors. Currently, the pathogenesis of COPD remains incompletely understood. The PI3K/Akt signaling pathway has recently emerged as a critical regulator of inflammation and oxidative stress response in COPD, playing a pivotal role in the disease’s progression and treatment. This paper reviews the association between the PI3K/Akt pathway and COPD, examines effective PI3K/Akt inhibitors and novel anti-COPD agents, aiming to identify new therapeutic targets for clinical intervention in this disease.
1 Introduction
COPD is a heterogeneous lung disease characterized by a variety of chronic respiratory symptoms, such as difficulty breathing, coughing, sputum production, and acute exacerbation. These symptoms arise from abnormal airways (bronchitis) and/or alveolar abnormalities (emphysema), resulting in persistent and frequently progressive airflow obstruction (Venkatesan, 2023). COPD ranks as the third leading cause of death globally, following ischemic heart disease and stroke (Obeidat et al., 2018). COPD arises from the complex interplay of multiple factors. Smoking stands as a significant risk factor for COPD, while environmental exposure and genetic variation can contribute to the development or exacerbation of the disease (Lareau et al., 2019). Presently, COPD is treated with a combination of medication and non-medication strategies, smoking cessation is the primary treatment, bronchodilators and glucocorticoids are the most commonly used drugs (Sandelowsky et al., 2021). Nevertheless, COPD commonly exhibits a progressive nature, and the conventional medications used to manage it entail notable side effects. These challenges emphasize the urgent need for exploring alternative treatment modalities for COPD.
The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB/Akt) signaling pathway is a critical cellular pathway that regulates multiple functions such as cell survival, growth, proliferation, metastasis, and metabolism (Engelman et al., 2006; Abeyrathna and Su, 2015). The abnormal activation of the PI3K/AKT pathway is associated with various human cancers (Noorolyai et al., 2019; Glaviano et al., 2023). Additionally, it is also involved in many chronic diseases such as diabetes (Savova et al., 2023), cardiovascular diseases (Qin et al., 2021), neurological disorders (Wang Q. et al., 2022), autoimmune diseases (Cheng et al., 2022), inflammatory diseases (Xu et al., 2022), and liver diseases (Ye et al., 2023) et al. The PI3K/AKT pathway plays a crucial role in the onset and progression of numerous diseases. Recently, studies have demonstrated that inhibiting the PI3K/Akt signaling pathway reduces inflammation, apoptosis, and oxidative stress in cells, thereby playing a crucial role in COPD treatment (Sun et al., 2019).
This review comprehensively examines the structure and transduction of the PI3K/Akt signaling pathway. Additionally, it highlights the crucial role of PI3K/Akt signaling in COPD and presents a summary of potential drugs that target this pathway. The aim is to expand the therapeutic possibilities for COPD and offer innovative and effective targets for clinical intervention.
2 The PI3K/Akt signaling pathway
The PI3K/Akt signaling pathway plays a crucial role in various cellular regulatory processes such as cell growth, proliferation, migration, metabolism, and secretion. Furthermore, dysregulation of the PI3K/Akt signaling pathway is implicated in a diverse spectrum of human diseases, including cancer (Noorolyai et al., 2019), neurodegenerative disease (Rai et al., 2019), diabetes (Huang et al., 2018), and osteoarthritis (Sun et al., 2020).
2.1 The composition of PI3K
PI3Ks are a class of evolutionarily conserved intracellular lipid kinases called intracellular lipid kinases, classified into classes I, II, and III based on substrate specificity and sequence homology (Cantley, 2002). Type I PI3K can be divided into two subfamilies based on its coupling receptors. Type IA PI3K is a heterogeneous dimer composed of a p85-regulated subgroup and a p110 catalytic subgroup. It is activated by the growth factor receptor tyrosine kinase (RTK) (Katso et al., 2001). Type IB PI3K is a heterogeneous dimer composed of p101 regulatory subunits and p110-γ catalytic subunits. It is activated by G-protein-coupled receptors (GPCRs) (Voigt et al., 2006). Classes II and III PI3K are monomer types. Class II PI3K consists of a catalytic subunit similar to p110, while class III PI3K consists of a single member, Vps34 (Jean and Kiger, 2014). It is generally accepted that class I PI3K is the most widely studied, and class II and III PI3Ks have less knowledge about their specific functions. Here, we will focus on the role of Type I PI3K in COPD.
2.2 The composition of Akt
Akt, a serine/threonine kinase also known as PKB, belongs to the AGC protein kinase family. Three subtypes of Akt exist: Akt1 (PKBa), Akt2 (PKBb), and Akt3 (PKBc) (Risso et al., 2015). The three subtypes of Akt, closely related in mammals, possess three conserved domains: an amino terminal pleckstrin homology (PH) domain, a central kinase domain (excitation domain) highly similar to other AGC protein kinases like PKA and PKC (Peterson and Schreiber, 1999), and a carboxyl terminal regulatory domain that includes HM phosphorylation sites. The three Akt isomers exhibit high sequence similarity and structural resemblance, with Akt1 and Akt2 showing broader expression in mammals (He et al., 2021).
2.3 Mechanisms of PI3K/Akt pathway activation
Common mechanisms of PI3K activation involve the activation of receptor tyrosine kinase under physiological conditions. This leads to the phosphorylation of tyrosine residues and their subsequent binding to one or both SH2 domains of the PI3K splice subunit, resulting in allosteric activation of the PI3K catalytic subunit (Fruman et al., 2017). Additionally, activation of GPCR leads to allosteric activation of PI3K (Rathinaswamy et al., 2021). PI3K activation causes the transformation of PIP2 into PIP3 inside the plasma membrane, which binds specifically to the pleckstrin homlogy (PH) domain of two proteins, PDK-1 and Akt/PKB, to mediate PI3K signaling (Carnero et al., 2008).
The PI3K-dependent activation mechanism of Akt involves the interaction of Akt with PIP3, leading to its translocation to the medial membrane where its Thr308 and Ser473 sites become exposed. PDK1 phosphorylates Akt’s Thr308 site, serving as the initial step in Akt activation. Subsequently, PDK2 phosphorylates the Ser473 sites, located at the hydrophobic carboxyl group’s end, to achieve maximal Akt activation (Cole et al., 2019). Once fully activated, Akt phosphorylates downstream target proteins, thereby regulating various cellular functions such as angiogenesis, metabolism, growth, proliferation, protein synthesis, transcription, and apoptosis (Hemmings and Restuccia, 2015).
2.4 Regulation of the PI3K/Akt pathway
The PI3K/Akt signaling pathway is regulated by various factors, particularly a group of phosphatases that exert negative regulatory effects. These phosphatases include phosphatase and tensin homologues (PTEN), protein phosphatase 2 (PP2A), and protein phosphatase in the PH domain rich in repeated sequences of leucine (PHLPP1/2). PTEN plays a crucial role as an upstream component of the PI3K/Akt signaling pathway. It catalyzes the specific dephosphorylation of PIP3 to produce PIP2, thereby exerting a negative regulatory effect on Akt activation (Viennet et al., 2023). Additionally, Inositol polyphosphate 4-phosphatase type II (INPP4B) inhibits Akt signaling by dephosphorylating PIP2 into PIP (Gewinner et al., 2009). PP2A, a trimeric protein, combats Akt signaling by selectively inhibiting phosphorylation of Akt’s Thr308 site (Zhang Y. et al., 2022) and dephosphorization of Akt’s Ser473 site (Hwang et al., 2013). PHLPP1/2 primarily dephosphorylates Akt at Ser473 sites. Activation of the PI3K pathway leads to stable levels of PHLPP1 and a surge of PHLPP2, both of which attenuate Akt signaling (Chen et al., 2011). Therefore, in the absence of PTEN, PHLPP2 replaces its role in attenuating the output of the PI3K/Akt pathway (Figure 1) (Chen et al., 2014).
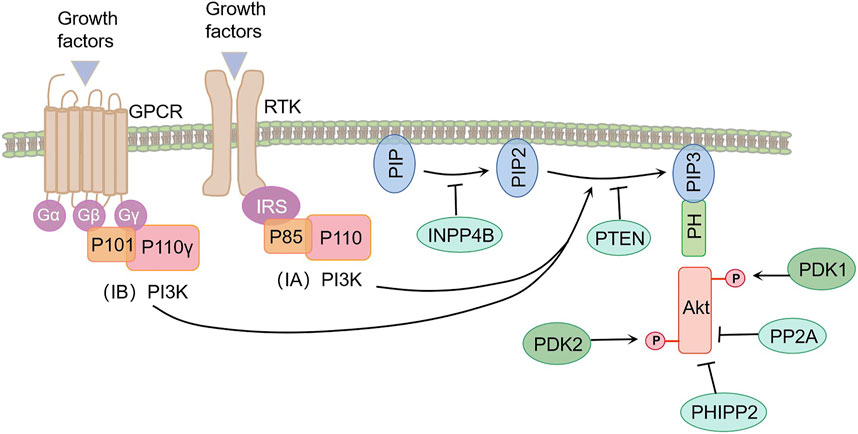
FIGURE 1. Transduction and regulatory pathways of PI3K/Akt pathway. Activation of growth factor receptor tyrosine kinase (RTK) and G-protein-coupled receptors (GPCRs) led to the activation of the PI3K catalytic subunit. Activated PI3K promotes the conversion of PIP2 to PIP3 in the medial membrane, a function that can be reversed by phosphatase and tensin homologues (PTEN). PIP3 activates Akt signaling by specifically binding to the pleckstrin homlogy (PH) domain of both PDK-1 and Akt proteins. Protein phosphatase 2 (PP2A) and the PH domain are rich in leucine repeats of the protein phosphatase (PHLPP1/2), negatively regulating the PI3K/Akt pathway.
3 The PI3K/Akt signaling pathway and its role in the pathogenesis of COPD
3.1 The interplay between inflammation and oxidative stress in the pathogenesis of COPD
Increasing evidence suggests that inflammation and oxidative stress are interconnected pathophysiological processes (Biswas, 2016). In COPD, activated inflammatory cells in the lungs release numerous inflammatory factors that trigger the production of oxygen free radicals, leading to oxidative stress (Guo et al., 2022). Moreover, oxidative stress can amplify lung inflammation by activating multiple signaling pathways within the cells (Barnes, 2022). This closely intertwined process frequently coexists across various chronic diseases, in addition to COPD (Neves et al., 2021), there are inflammatory bowel disease (Tian et al., 2017), alcoholic liver disease (Yang et al., 2022), diabetes (Grabež et al., 2022), neuroinflammatory disease (Xue et al., 2019) and other chronic inflammatory diseases.
3.2 The PI3K/Akt pathway and inflammation in COPD
COPD is a progressive inflammatory lung condition caused by the inhalation of cigarette smoke and other toxic external particulate matter, such as air pollution and biomass fuels. Chronic inflammation of the small airways, known as bronchiolitis, serves as the primary catalyst for COPD development (Brightling and Greening, 2019). Various cytokines secreted by alveolar macrophages, neutrophils, T lymphocytes, B lymphocytes, and structural cells like epithelial, endothelial, and fibroblasts contribute to this inflammatory response (Barnes, 2016). Recent research indicates that smoking acts as an initial trigger for activating innate immune system cells, with tobacco smoke stimulating the PI3K/Akt pathway and exacerbating the inflammatory response in monocytes. Furthermore, elevated PI3K signaling has been linked to sustained inflammation in individuals with COPD, just as shown in Figure 2 (Lee et al., 2018). Furthermore, inflammation underlies significant complications in COPD, including heart and lung diseases, respiratory failure, and cancer (Byrne et al., 2015).
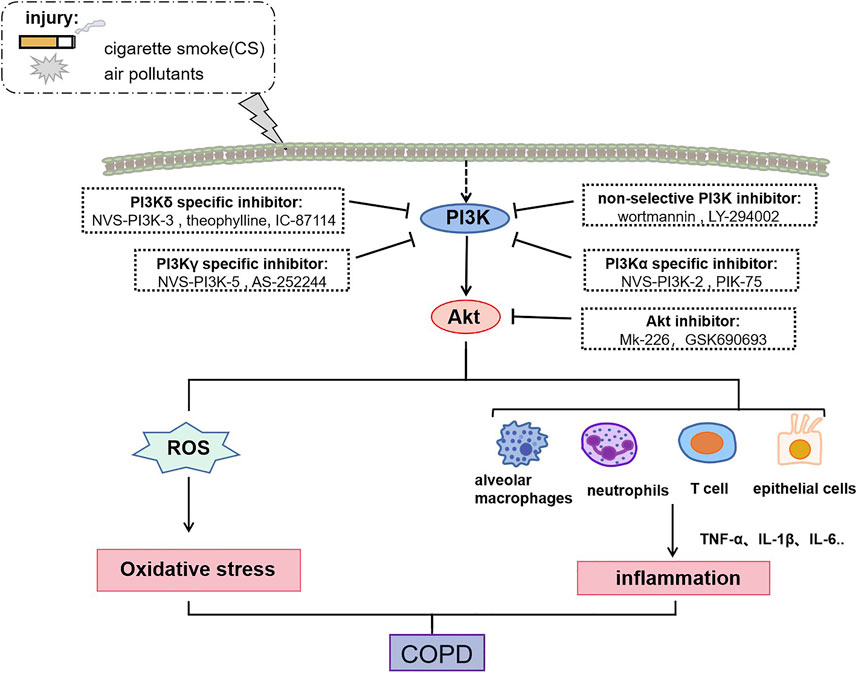
FIGURE 2. Role of PI3K /Akt in COPD regulation. The activation of PI3K/Akt is involved in COPD formation and can exacerbate COPD progression through inflammatory and oxidative stress. Specifically, activated PI3K/Akt promotes the release of ROS, as well as promotes the secretion of multiple pro-inflammatory cytokines by alveolar macrophages, neutrophils, T-lymphocytes, and epithelial cells to participate in COPD pathogenesis. Moreover, small molecule inhibitors targeting the PI3K/Akt signaling pathway can treat COPD through inhibiting oxidative stress and inflammation.
Zhang et al. (2017) discovered that PI3K signaling was activated in alveolar macrophages of COPD mice, leading to a significant increase in pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, thereby enhancing inflammatory responses. Subsequent studies demonstrated that activation of the PI3K/Akt signaling pathway promoted polarization of macrophages in COPD from M1 to M2 phenotypes. The ratio of M1 to M2 macrophages following monocyte polarization has been linked to various inflammatory diseases (Zhang et al., 2023), and an elevated ratio of M2 macrophages has been implicated in lung inflammation (Lu et al., 2017). Neutrophil infiltration in the submucous membrane of the airway is the main driver of airway inflammation in COPD and is regulated by helper T cells 17 (Th17) and macrophages. Macrophages secrete inflammatory mediators that act as chemical inducers, increasing neutrophil infiltration in the airways and promoting lung injury and inflammatory responses in COPD patients (Holl et al., 2013). It is now evident that inflamed airways are exposed to hypoxia, triggering neutrophil degranulation and enhancing their potential for tissue damage. Hoenderdos et al. (2016) discovered that inhibiting the PI3K signaling pathway contributes to the suppression of neutrophil degranulation, suggesting that PI3K plays a crucial role in this process. Additionally, inhibiting Akt phosphorylation had no impact on degranulation regulation, implying that heightened neutrophil reactivity is a result of early PI3K/Akt signaling. Yanagisawa et al. (2017) demonstrated that PI3K signaling is more active in bronchial epithelial cells of COPD patients. In contrast, the negative regulator of PI3K, PTEN, is frequently mutated or absent in the airway epithelial cells of smokers. Knockdown of PTEN leads to significant Akt phosphorylation and increased secretion of pro-inflammatory cytokines (e.g., IL-6, IL-B-induced CXCL8, etc.). Hence, activating PTEN could be an effective approach to impede the progression of COPD.
3.3 The role of the PI3K/Akt pathway in COPD oxidative stress
Oxidative stress arises from an imbalance between free radicals and antioxidants, playing a significant role in inflammatory diseases (Dandekar et al., 2015). Free radicals can originate from activated inflammatory cells, structural cells, cigarette smoke, indoor and outdoor air pollution, among other sources (Valavanidis et al., 2013). COPD is a progressive respiratory disease where inflammatory and structural cells in the lungs release reactive oxygen species (ROS) and reactive nitrogen species (RNS), inducing endogenous oxidative stress during the early stages of the disease. The imbalance between free radicals and antioxidants further exacerbates ROS release (Boukhenouna et al., 2018). Thus, the elevation of oxidative stress persists even after COPD patients stop smoking. This oxidative damage results in endogenous tissue and cellular damage, ultimately leading to chronic inflammation and aging (Barnes et al., 2019). A growing body of research has demonstrated the involvement of the PI3K/Akt/mTOR signaling pathway in promoting lung cell senescence and oxidative stress (Xiaofe et al., 2022), This suggests that blocking the PI3K/Akt pathway as a means to inhibit oxidative stress could hold promise as a therapeutic strategy for COPD patients.
Recent studies have reported a significant reduction of SIRT1 and SIRT6, which are anti-aging molecules, in the lungs of COPD patients (Zhang XY. et al., 2022). Oxidative stress serves as the primary regulator of these proteins’ expression (Lakhdar et al., 2018). Inhibition of the PI3K signaling pathway, as demonstrated by Baker et al. (2016), significantly enhances the expression of SIRT1 and SIRT6 while reversing oxidative stress. Additionally, the knockout of PTEN, which inhibits PI3K signaling, resulted in reduced levels of SIRT1 and SIRT6. Studies by Xie S et al. (Xie and Wang, 2022) have shown that cigarette smoke extract downregulates the expression of CRYAB, a recognized anti-apoptotic protein, in the alveoli of COPD mice. Furthermore, overexpression of CRYAB inhibits oxidative stress, delays the activation of the PI3K/Akt signaling pathway, and reduces apoptosis. Oxidative stress also affects histone deacetylase (HDAC) activity. HDAC, a glucocorticoid functional protein, is frequently associated with glucocorticoid resistance in COPD patients (Rossios et al., 2012), Marwick et al. (2009) found reduced HDAC activity in COPD mice, and knockout of PI3K restored the activity of this enzyme. Consequently, inhibiting the PI3K pathway reinstates histone activity in the presence of oxidative stress-induced glucocorticoid resistance. This restoration, in turn, revives the anti-inflammatory properties of glucocorticoids, leading to a positive inhibition of COPD progression.
4 Potential drug targeting PI3K/Akt for COPD
The pathogenesis of COPD is unclear and is commonly linked to inflammation, oxidative stress and reduced immune function. Current treatments include medication, oxygen therapy and rehabilitation therapy to improve symptoms of airflow restriction caused by reduced lung function (Wang et al., 2020). However, these methods have done little to prevent the progression of COPD disease. Common COPD drugs include beta 2 receptor agonists, anticholinergic drugs, and glucocorticoids. However, chronic inhalation of beta 2 receptor agonists may have adverse cardiovascular and metabolic effects (Vanfleteren et al., 2018). There are a number of side effects associated with anticholinergic drugs, including the dry mouth, blurred vision, and postural hypotension. Glucocorticoids negatively affect the hypothalamic-pituitary-adrenal axis and most COPD patients are insensitive to glucocorticoids. Therefore, there is an urgent need to find novel molecular targeted therapeutics for COPD.
4.1 Application of PI3K inhibitors in COPD treatment
PI3K is a signaling cascade component downstream of multiple cell receptors. Among the three subtypes of PI3K (α, γ and δ), PI3Kα is critical for airway inflammation and angiogenesis (Chen et al., 2021), while Pl3Kγ is pro-inflammatory and involved in inflammatory cell recruitment (Heit et al., 2008) and PI3Kδ contributes to corticosteroid resistance (To et al., 2010).
Wortmannin, a PI3K inhibitor with low substrate specificity. Significantly reduced the activity of neutrophils elastase (NE) and matrix metalloproteinase-9 (MMP-9) released by airway neutrophils in COPD mice and decreased neutrophils inflammation (Vlahos et al., 2012). In addition, Wortmannin induced differentiation of alveolar epithelial stem cells in COPD mice to repair the alveoli and restore respiratory function (Horiguchi et al., 2015). LY 294002 (a non-selective PI3K inhibitor) significantly restored sensitivity to corticosteroids in PBMC cells from COPD patients, but had no effect on the production of the inflammatory factor IL-8. In addition, LY-294002 inhibits the expression of intercellular adhesion molecule −1(ICAM-1) in COPD patients, mediating monocyte/macrophage adhesion and infiltrating inflammatory sites (Liu et al., 2018).
Bewley et al. (2016) found that NVS-PI3K-2 (PI3Kα specific inhibitor), NVS-PI3K-3 (PI3Kδ specific inhibitor) and NVS-PI3K-5 (PI3Kγ specific inhibitor) suppressed lung inflammation and bacterial colonization in COPD patients. Alveolar macrophages play a significant role in clearing bacteria and small apoptotic bodies. However, these inhibitors do not alter the phagocytosis of alveolar macrophages, i.e., do not negatively affect innate immunity of COPD macrophages. Similarly, lower concentrations of theophylline (PI3Kδ specific inhibitors) can target PI3K to reverse oxidative stress-induced corticosteroid resistance and suppress lung inflammation in COPD mice exposed to cigarette smoke (To et al., 2010). In addition, IC-87114, a PI3Kδ specific inhibitor, inhibits neutrophils recruitment and restores corticosteroid sensitivity impaired under oxidative stress by inhibiting PI3K signaling (Rossios et al., 2012). Wang et al. (2019) and others demonstrated that IL-1α and IL-1β expression in human bronchial epithelial cells (HBEC) were significantly upregulated in mucin protein Muc-5ac associated with high mucin secretion, and PIK-75 (PI3Kα specific inhibitor) significantly inhibited PM-induced inflammation and mucin hypersecretion in HBEC, while AS-252244 (PI3Kγ specific inhibitor) and IC-87114 did not. Small molecule inhibitors targeting the PI3K/Akt signaling pathway treat COPD through inhibiting oxidative stress and inflammation, as shown in Figure 2.
4.2 Application of Akt inhibitors in COPD treatment
It is thought that Akt is a central regulator of the molecular pathways involved in smoking-related diseases, particularly COPD. MK-2206 (Akt variant non-ATP competitive inhibitor), an anticancer agent that inhibits all Akt subtypes, is commonly used to synergistically enhance the anti-tumor efficacy of certain molecular targeted drugs (Yap et al., 2011). Jiang et al. (2018) found that MK-2206 reversed changes in markers involved with epithelial mesenchymal transition (EMT) in the lung epithelium of smoking mice. EMT is positively associated with an invasive or metastatic phenotype of COPD (Zhang et al., 2016), so MK-2206 inhibits COPD. In addition, MK-2206 pretreatment inhibited IL-1α and IL-1β and Muc-5ac expression (Wang J. et al., 2019), and also protected the diaphragm in COPD mice induced by hypoxia pretreatment (Chuang et al., 2018). In addition, GSK690693 (ATP-competitive pan-Akt inhibitor) significantly inhibited IL-8 induced apoptosis in inflammatory diaphragm cells (Wang L. et al., 2019). Akt inhibitors are currently understudied and underused in COPD diseases, and more recently, Akt-negative dominant mutant (Akt-DN) transfected cells from Lin CH et al. (Lin et al., 2020) have been shown to inhibit the Akt pathway and IL-8 secretion in human lung epithelial cells.
4.3 Others
In addition to some of these molecular targeted drugs, natural compounds, traditional Chinese medicine formulations, and some anti-inflammatory agents also inhibit the progression of COPD by inhibiting the PI3K/Akt pathway. It has been shown recently that puerarin can relieve over-oxidation in cells (Zhang P. et al., 2022). Wang L. et al. (2022) found that puerarin reverses apoptosis in HBEC stimulated by cigarette smoke extract (CSE) via the PI3K/Akt signaling pathway. Icariin is one of the active components of Bufei Yishen formula, which can inhibit the mucus hypersecretion in COPD rats (Li J. et al., 2020). Icariin in combination with Nobilitin has a positive therapeutic effect on COPD by improving lung inflammation and emphysema and reducing lung pathological damage in COPD rats via the PI3K/Akt pathway (Lu et al., 2022). At the same time, Scutellaria has a reverse effect on lung pathologic injury induced by smoking in COPD rats (Xu et al., 2018). Crocin, the active ingredient in crocus, significantly inhibits the number of neutrophils and macrophages and the concentration of pro-inflammatory cytokines in COPD mice by modulating the PI3K/Akt-mediated inflammatory pathway (Xie et al., 2019).
As a vital component of complementary alternative medicine, TCM is considered to play its pharmacological role via its multi-component, multi-target, and multi-pathway properties (Ren et al., 2019). Notably, Bu-Shen-Fang-Fang-Chuan formula (BSFCF), a commonly used formula for treating COPD in China, attenuates the inflammatory response to COPD by inhibiting PI3K/Akt-Nrf2 and PI3K/Akt-NF-κB (Li Q. et al., 2020). Xuefu Zhuyu Decoction (XFZYD) is also widely used in the treatment of COPD, and (Hu et al., 2022) and others have found that XFZYD is effective in the treatment of COPD by interfering with the PI3K/Akt signaling pathway, improving oxidative stress and inflammatory responses, and relieving airway remodeling and ventilation disorders through web-based pharmacology and molecular docking experiments. In addition, Tiaobu Feishen (TBFS) was observed to reverse lung inflammation and airway remodeling (Li et al., 2012), Zhou et al. (2023) observed that TBFS has more effective in glucocorticoid-resistant COPD patients by modulating PI3K/Akt signaling to improve glucocorticoid resistance.
Anti-inflammatory agents that interfere with the PI3K/Akt pathway also have significant potential to improve COPD steroid resistance. Macrolides may reduce lung inflammation in COPD by modulating the PI3K/Akt pathway, such as erythromycin, which enhances corticosteroid sensitivity by inhibiting the activity of the PI3K/Akt pathway (Miao et al., 2015; Sun et al., 2015). Solithromycin (SOL, CEM-101), a macrolide/fluoronolactone that inhibits airway neutrophils in steroid-insensitive mice, is an effective anti-inflammatory agent for COPD treatment (Kobayashi et al., 2013). The statin simvastatin improves lung remodeling by reversing epithelial mesenchymal transition in alveolar epithelial cells. This effect is mediated by inhibition of the PI3K/Akt pathway (Milara et al., 2015). In addition, the tricyclic antidepressant nortriptyline can also increase corticosteroid sensitivity. Mercado et al. (2011) found that nortriptyline pretreatment inhibited Akt phosphorylation and PI3K activity, restoring oxidative stress-induced corticosteroid sensitivity as a potential treatment for respiratory diseases such as COPD that are corticosteroid insensitive.
5 Conclusion
The morbidity and mortality rates associated with COPD remain substantial, posing numerous challenges for healthcare professionals involved in COPD interventions. There is a growing body of evidence indicating the therapeutic potential of the PI3K/Akt signaling pathway, particularly different PI3K isoforms, in the treatment of COPD. Several non-specific PI3K inhibitors have demonstrated anti-inflammatory and antioxidant effects in COPD models. However, most broad-spectrum PI3K inhibitors exhibit greater genotoxicity, whereas PI3K subtype-specific inhibitors offer the desired therapeutic properties with reduced side effects. This may provide guidance for subsequent drug development targeting PI3K/Akt. In conclusion, there is an urgent need for further insights into the key regulatory mechanisms of the PI3K/Akt pathway in COPD development, as well as the exploration of safer and more effective therapeutic strategies derived from this approach.
Author contributions
YL designed and wrote this manuscript, HK wrote this manuscript, HeC designed this manuscript, GC checked this manuscript, HuC checked this manuscript, WR checked this manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abeyrathna, P., and Su, Y. (2015). The critical role of Akt in cardiovascular function. Vasc. Pharmacol. 74, 38–48. doi:10.1016/j.vph.2015.05.008
Baker, J. R., Vuppusetty, C., Colley, T., Papaioannou, A. I., Fenwick, P., Donnelly, L., et al. (2016). Oxidative stress dependent microRNA-34a activation via PI3Kα reduces the expression of sirtuin-1 and sirtuin-6 in epithelial cells. Sci. Rep. 6, 35871. doi:10.1038/srep35871
Barnes, P. J., Baker, J., and Donnelly, L. E. (2019). Cellular senescence as a mechanism and target in chronic lung diseases. Am. J. Respir. Crit. care Med. 200 (5), 556–564. doi:10.1164/rccm.201810-1975TR
Barnes, P. J. (2016). Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. allergy Clin. Immunol. 138 (1), 16–27. doi:10.1016/j.jaci.2016.05.011
Barnes, P. J. (2022). Oxidative stress in chronic obstructive pulmonary disease. Antioxidants (Basel, Switz. 11 (5), 965. doi:10.3390/antiox11050965
Bewley, M. A., Belchamber, K. B., Chana, K. K., Budd, R. C., Donaldson, G., Wedzicha, J. A., et al. (2016). Differential effects of p38, MAPK, PI3K or rho kinase inhibitors on bacterial phagocytosis and efferocytosis by macrophages in COPD. PloS one 11 (9), e0163139. doi:10.1371/journal.pone.0163139
Biswas, S. K. (2016). Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxidative medicine and cellular longevity. Oxid. Med. Cell Longev. 2016, 5698931. doi:10.1155/2016/5698931
Boukhenouna, S., Wilson, M. A., Bahmed, K., and Kosmider, B. (2018). Reactive oxygen species in chronic obstructive pulmonary disease. Oxidative Med. Cell. Longev. 2018, 5730395. doi:10.1155/2018/5730395
Brightling, C., and Greening, N. (2019). Airway inflammation in COPD: progress to precision medicine. Eur. Respir. J. 54 (2), 1900651. doi:10.1183/13993003.00651-2019
Byrne, A. L., Marais, B. J., Mitnick, C. D., Lecca, L., and Marks, G. B. (2015). Risk factors for and origins of COPD. Lancet (London, Engl. 385 (9979), 1723–1724. doi:10.1016/S0140-6736(15)60884-4
Cantley, L. C. (2002). The phosphoinositide 3-kinase pathway. Sci. (New York, NY) 296 (5573), 1655–1657. doi:10.1126/science.296.5573.1655
Carnero, A., Blanco-Aparicio, C., Renner, O., Link, W., and Leal, J. F. (2008). The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. cancer drug targets 8 (3), 187–198. doi:10.2174/156800908784293659
Chen, M., Nowak, D. G., and Trotman, L. C. (2014). Molecular pathways: PI3K pathway phosphatases as biomarkers for cancer prognosis and therapy. Clin. cancer Res. 20 (12), 3057–3063. doi:10.1158/1078-0432.CCR-12-3680
Chen, M., Pratt, C. P., Zeeman, M. E., Schultz, N., Taylor, B. S., O'Neill, A., et al. (2011). Identification of PHLPP1 as a tumor suppressor reveals the role of feedback activation in PTEN-mutant prostate cancer progression. Cancer Cell 20 (2), 173–186. doi:10.1016/j.ccr.2011.07.013
Chen, X., Zhabyeyev, P., Azad, A. K., Vanhaesebroeck, B., Grueter, C. E., Murray, A. G., et al. (2021). Pharmacological and cell-specific genetic PI3Kα inhibition worsens cardiac remodeling after myocardial infarction. J. Mol. Cell. Cardiol. 157, 17–30. doi:10.1016/j.yjmcc.2021.04.004
Cheng, Q., Chen, M., Liu, M., Chen, X., Zhu, L., Xu, J., et al. (2022). Semaphorin 5A suppresses ferroptosis through activation of PI3K-AKT-mTOR signaling in rheumatoid arthritis. Cell Death Dis. 13 (7), 608. doi:10.1038/s41419-022-05065-4
Chuang, C. C., Zhou, T., Olfert, I. M., and Zuo, L. (2018). Hypoxic preconditioning attenuates reoxygenation-induced skeletal muscle dysfunction in aged pulmonary TNF-α overexpressing mice. Front. physiology 9, 1720. doi:10.3389/fphys.2018.01720
Cole, P. A., Chu, N., Salguero, A. L., and Bae, H. (2019). AKTivation mechanisms. Curr. Opin. Struct. Biol. 59, 47–53. doi:10.1016/j.sbi.2019.02.004
Dandekar, A., Mendez, R., and Zhang, K. (2015). Cross talk between ER stress, oxidative stress, and inflammation in health and disease. Methods Mol. Biol. Clift. NJ) 1292, 205–214. doi:10.1007/978-1-4939-2522-3_15
Engelman, J. A., Luo, J., and Cantley, L. C. (2006). The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 7 (8), 606–619. doi:10.1038/nrg1879
Fruman, D. A., Chiu, H., Hopkins, B. D., Bagrodia, S., Cantley, L. C., and Abraham, R. T. (2017). The PI3K pathway in human disease. Cell 170 (4), 605–635. doi:10.1016/j.cell.2017.07.029
Gewinner, C., Wang, Z. C., Richardson, A., Teruya-Feldstein, J., Etemadmoghadam, D., Bowtell, D., et al. (2009). Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell 16 (2), 115–125. doi:10.1016/j.ccr.2009.06.006
Glaviano, A., Foo, A. S. C., Lam, H. Y., Yap, K. C. H., Jacot, W., Jones, R. H., et al. (2023). PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 22 (1), 138. doi:10.1186/s12943-023-01827-6
Grabež, M., Škrbić, R., Stojiljković, M. P., Vučić, V., Rudić Grujić, V., Jakovljević, V., et al. (2022). A prospective, randomized, double-blind, placebo-controlled trial of polyphenols on the outcomes of inflammatory factors and oxidative stress in patients with type 2 diabetes mellitus. Rev. Cardiovasc. Med. 23 (2), 57. doi:10.31083/j.rcm2302057
Guo, P., Li, R., Piao, T. H., Wang, C. L., Wu, X. L., and Cai, H. Y. (2022). Pathological mechanism and targeted drugs of COPD. Int. J. chronic Obstr. Pulm. Dis. 17, 1565–1575. doi:10.2147/COPD.S366126
He, Y., Sun, M. M., Zhang, G. G., Yang, J., Chen, K. S., Xu, W. W., et al. (2021). Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 6 (1), 425. doi:10.1038/s41392-021-00828-5
Heit, B., Liu, L., Colarusso, P., Puri, K. D., and Kubes, P. (2008). PI3K accelerates, but is not required for, neutrophil chemotaxis to fMLP. J. Cell Sci. 121 (2), 205–214. doi:10.1242/jcs.020412
Hemmings, B. A., and Restuccia, D. F. (2015). The PI3K-PKB/Akt pathway. Cold Spring Harb. Perspect. Biol. 7 (4), a026609. doi:10.1101/cshperspect.a026609
Hoenderdos, K., Lodge, K. M., Hirst, R. A., Chen, C., Palazzo, S. G., Emerenciana, A., et al. (2016). Hypoxia upregulates neutrophil degranulation and potential for tissue injury. Thorax 71 (11), 1030–1038. doi:10.1136/thoraxjnl-2015-207604
Holloway, R. A., and Donnelly, L. E. (2013). Immunopathogenesis of chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 19 (2), 95–102. doi:10.1097/MCP.0b013e32835cfff5
Horiguchi, M., Oiso, Y., Sakai, H., Motomura, T., and Yamashita, C. (2015). Pulmonary administration of phosphoinositide 3-kinase inhibitor is a curative treatment for chronic obstructive pulmonary disease by alveolar regeneration. J. Control. release official J. Control. Release Soc. 213, 112–119. doi:10.1016/j.jconrel.2015.07.004
Hu, Y., Lan, Y., Ran, Q., Gan, Q., and Huang, W. (2022). Analysis of the clinical efficacy and molecular mechanism of xuefu zhuyu decoction in the treatment of COPD based on meta-analysis and network pharmacology. Comput. Math. methods Med. 2022, 2615580. doi:10.1155/2022/2615580
Huang, X., Liu, G., Guo, J., and Su, Z. (2018). The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 14 (11), 1483–1496. doi:10.7150/ijbs.27173
Hwang, J. H., Jiang, T., Kulkarni, S., Faure, N., and Schaffhausen, B. S. (2013). Protein phosphatase 2A isoforms utilizing Aβ scaffolds regulate differentiation through control of Akt protein. J. Biol. Chem. 288 (44), 32064–32073. doi:10.1074/jbc.M113.497644
Jean, S., and Kiger, A. A. (2014). Classes of phosphoinositide 3-kinases at a glance. J. Cell Sci. 127 (5), 923–928. doi:10.1242/jcs.093773
Jiang, B., Guan, Y., Shen, H. J., Zhang, L. H., Jiang, J. X., Dong, X. W., et al. (2018). Akt/PKB signaling regulates cigarette smoke-induced pulmonary epithelial-mesenchymal transition. Lung cancer (Amsterdam, Neth. 122, 44–53. doi:10.1016/j.lungcan.2018.05.019
Katso, R., Okkenhaug, K., Ahmadi, K., White, S., Timms, J., and Waterfield, M. D. (2001). Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 17, 615–675. doi:10.1146/annurev.cellbio.17.1.615
Kobayashi, Y., Wada, H., Rossios, C., Takagi, D., Charron, C., Barnes, P. J., et al. (2013). A novel macrolide/fluoroketolide, solithromycin (CEM-101), reverses corticosteroid insensitivity via phosphoinositide 3-kinase pathway inhibition. Br. J. Pharmacol. 169 (5), 1024–1034. doi:10.1111/bph.12187
Lakhdar, R., McGuinness, D., Drost, E. M., Shiels, P. G., Bastos, R., MacNee, W., et al. (2018). Role of accelerated aging in limb muscle wasting of patients with COPD. Int. J. chronic Obstr. Pulm. Dis. 13, 1987–1998. doi:10.2147/COPD.S155952
Lareau, S. C., Fahy, B., Meek, P., and Wang, A. (2019). Chronic obstructive pulmonary disease (COPD). Am. J. Respir. Crit. care Med. 199 (1), P1–p2. doi:10.1164/rccm.1991P1
Lee, K. H., Lee, C. H., Woo, J., Jeong, J., Jang, A. H., and Yoo, C. G. (2018). Cigarette smoke extract enhances IL-17a-induced IL-8 production via up-regulation of IL-17r in human bronchial epithelial cells. Mol. cells 41 (4), 282–289. doi:10.14348/molcells.2018.2123
Li, J., Ma, J., Tian, Y., Zhao, P., Liu, X., Dong, H., et al. (2020a). Effective-component compatibility of Bufei Yishen formula II inhibits mucus hypersecretion of chronic obstructive pulmonary disease rats by regulating EGFR/PI3K/mTOR signaling. J. Ethnopharmacol. 257, 112796. doi:10.1016/j.jep.2020.112796
Li, J. S., Li, Y., Li, S. Y., Wang, Y. Y., Deng, L., Tian, Y. G., et al. (2012). Long-term effects of Tiaobu Feishen therapies on systemic and local inflammation responses in rats with stable chronic obstructive pulmonary disease. Zhong xi yi jie he xue bao = J. Chin. Integr. Med. 10 (9), 1039–1048. doi:10.3736/jcim20120913
Li, Q., Wang, G., Xiong, S. H., Cao, Y., Liu, B., Sun, J., et al. (2020b). Bu-Shen-Fang-Chuan formula attenuates cigarette smoke-induced inflammation by modulating the PI3K/Akt-Nrf2 and NF-κB signalling pathways. J. Ethnopharmacol. 261, 113095. doi:10.1016/j.jep.2020.113095
Lin, C. H., Shih, C. H., Jiang, C. P., Wen, H. C., Cheng, W. H., and Chen, B. C. (2020). Mammalian target of rapamycin and p70S6K mediate thrombin-induced nuclear factor-κB activation and IL-8/CXCL8 release in human lung epithelial cells. Eur. J. Pharmacol. 868, 172879. doi:10.1016/j.ejphar.2019.172879
Liu, C. W., Lee, T. L., Chen, Y. C., Liang, C. J., Wang, S. H., Lue, J. H., et al. (2018). PM(2.5)-induced oxidative stress increases intercellular adhesion molecule-1 expression in lung epithelial cells through the IL-6/AKT/STAT3/NF-κB-dependent pathway. Part. fibre Toxicol. 15 (1), 4. doi:10.1186/s12989-018-0240-x
Lu, J., Xie, L., Liu, C., Zhang, Q., and Sun, S. (2017). PTEN/PI3k/AKT regulates macrophage polarization in emphysematous mice. Scand. J. Immunol. 85 (6), 395–405. doi:10.1111/sji.12545
Lu, R., Xu, K., Qin, Y., Shao, X., Yan, M., Liao, Y., et al. (2022). Network pharmacology and experimental validation to reveal effects and mechanisms of icariin combined with nobiletin against chronic obstructive pulmonary diseases. Evidence-based complementary Altern. Med. eCAM 2022, 4838650. doi:10.1155/2022/4838650
Marwick, J. A., Caramori, G., Stevenson, C. S., Casolari, P., Jazrawi, E., Barnes, P. J., et al. (2009). Inhibition of PI3Kdelta restores glucocorticoid function in smoking-induced airway inflammation in mice. Am. J. Respir. Crit. care Med. 179 (7), 542–548. doi:10.1164/rccm.200810-1570OC
Mercado, N., To, Y., Ito, K., and Barnes, P. J. (2011). Nortriptyline reverses corticosteroid insensitivity by inhibition of phosphoinositide-3-kinase-δ. J. Pharmacol. Exp. Ther. 337 (2), 465–470. doi:10.1124/jpet.110.175950
Miao, L., Gao, Z., Huang, F., Huang, S., Zhang, R., Ma, D., et al. (2015). Erythromycin enhances the anti-inflammatory activity of budesonide in COPD rat model. Int. J. Clin. Exp. Med. 8 (12), 22217–22226.
Milara, J., Peiró, T., Serrano, A., Artigues, E., Aparicio, J., Tenor, H., et al. (2015). Simvastatin increases the ability of roflumilast N-oxide to inhibit cigarette smoke-induced epithelial to mesenchymal transition in well-differentiated human bronchial epithelial cells in vitro. Copd 12 (3), 320–331. doi:10.3109/15412555.2014.948995
Neves, C. D. C., Lage, V. K. S., Lima, L. P., Matos, M. A., Vieira É, L. M., Teixeira, A. L., et al. (2021). Inflammatory and oxidative biomarkers as determinants of functional capacity in patients with COPD assessed by 6-min walk test-derived outcomes. Exp. Gerontol. 152, 111456. doi:10.1016/j.exger.2021.111456
Noorolyai, S., Shajari, N., Baghbani, E., Sadreddini, S., and Baradaran, B. (2019). The relation between PI3K/AKT signalling pathway and cancer. Gene 698, 120–128. doi:10.1016/j.gene.2019.02.076
Obeidat, M., Zhou, G., Li, X., Hansel, N. N., Rafaels, N., Mathias, R., et al. (2018). The genetics of smoking in individuals with chronic obstructive pulmonary disease. Respir. Res. 19 (1), 59. doi:10.1186/s12931-018-0762-7
Peterson, R. T., and Schreiber, S. L. (1999). Kinase phosphorylation: Keeping it all in the family. Curr. Biol. CB 9 (14), R521–R524. doi:10.1016/s0960-9822(99)80326-1
Qin, W., Cao, L., and Massey, I. Y. (2021). Role of PI3K/Akt signaling pathway in cardiac fibrosis. Mol. Cell Biochem. 476 (11), 4045–4059. doi:10.1007/s11010-021-04219-w
Rai, S. N., Dilnashin, H., Birla, H., Singh, S. S., Zahra, W., Rathore, A. S., et al. (2019). The role of PI3K/Akt and ERK in neurodegenerative disorders. Neurotox. Res. 35 (3), 775–795. doi:10.1007/s12640-019-0003-y
Rathinaswamy, M. K., Dalwadi, U., Fleming, K. D., Adams, C., Stariha, J. T. B., Pardon, E., et al. (2021). Structure of the phosphoinositide 3-kinase (PI3K) p110γ-p101 complex reveals molecular mechanism of GPCR activation. Sci. Adv. 7 (35), eabj4282. doi:10.1126/sciadv.abj4282
Ren, L., Guo, X. Y., Gao, F., Jin, M. L., and Song, X. N. (2019). Identification of the perturbed metabolic pathways associating with renal fibrosis and evaluating metabolome changes of pretreatment with Astragalus polysaccharide through liquid chromatography quadrupole time-of-flight mass spectrometry. Front. Pharmacol. 10, 1623. doi:10.3389/fphar.2019.01623
Risso, G., Blaustein, M., Pozzi, B., Mammi, P., and Srebrow, A. (2015). Akt/PKB: One kinase, many modifications. Biochem. J. 468 (2), 203–214. doi:10.1042/BJ20150041
Rossios, C., To, Y., Osoata, G., Ito, M., Barnes, P. J., and Ito, K. (2012). Corticosteroid insensitivity is reversed by formoterol via phosphoinositide-3-kinase inhibition. Br. J. Pharmacol. 167 (4), 775–786. doi:10.1111/j.1476-5381.2012.01864.x
Sandelowsky, H., Weinreich, U. M., Aarli, B. B., Sundh, J., Høines, K., Stratelis, G., et al. (2021). Copd - do the right thing. BMC Fam. Pract. 22 (1), 244. doi:10.1186/s12875-021-01583-w
Savova, M. S., Mihaylova, L. V., Tews, D., Wabitsch, M., and Georgiev, M. I. (2023). Targeting PI3K/AKT signaling pathway in obesity. Biomed. Pharmacother. 159, 114244. doi:10.1016/j.biopha.2023.114244
Sun, K., Luo, J., Guo, J., Yao, X., Jing, X., and Guo, F. (2020). The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A narrative review. Osteoarthr. Cartil. 28 (4), 400–409. doi:10.1016/j.joca.2020.02.027
Sun, X., Chen, L., and He, Z. (2019). PI3K/Akt-Nrf2 and anti-inflammation effect of macrolides in chronic obstructive pulmonary disease. Curr. drug Metab. 20 (4), 301–304. doi:10.2174/1389200220666190227224748
Sun, X. J., Li, Z. H., Zhang, Y., Zhou, G., Zhang, J. Q., Deng, J. M., et al. (2015). Combination of erythromycin and dexamethasone improves corticosteroid sensitivity induced by CSE through inhibiting PI3K-δ/Akt pathway and increasing GR expression. Am. J. physiology Lung Cell. Mol. physiology 309 (2), L139–L146. doi:10.1152/ajplung.00292.2014
Tian, T., Wang, Z., and Zhang, J. (2017). Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxidative Med. Cell. Longev. 2017, 4535194. doi:10.1155/2017/4535194
To, Y., Ito, K., Kizawa, Y., Failla, M., Ito, M., Kusama, T., et al. (2010). Targeting phosphoinositide-3-kinase-delta with theophylline reverses corticosteroid insensitivity in chronic obstructive pulmonary disease. Am. J. Respir. Crit. care Med. 182 (7), 897–904. doi:10.1164/rccm.200906-0937OC
Valavanidis, A., Vlachogianni, T., Fiotakis, K., and Loridas, S. (2013). Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. public health 10 (9), 3886–3907. doi:10.3390/ijerph10093886
Vanfleteren, L., Fabbri, L. M., Papi, A., Petruzzelli, S., and Celli, B. (2018). Triple therapy (ICS/LABA/LAMA) in COPD: Time for a reappraisal. Int. J. chronic Obstr. Pulm. Dis. 13, 3971–3981. doi:10.2147/COPD.S185975
Venkatesan, P. (2023). GOLD COPD report: 2023 update. Lancet Respir. Med. 11 (1), 18. doi:10.1016/S2213-2600(22)00494-5
Viennet, T., Rodriguez Ospina, S., Lu, Y., Cui, A., Arthanari, H., and Dempsey, D. R. (2023). Chemical and structural approaches to investigate PTEN function and regulation. Methods Enzym. 682, 289–318. doi:10.1016/bs.mie.2022.09.007
Vlahos, R., Wark, P. A., Anderson, G. P., and Bozinovski, S. (2012). Glucocorticosteroids differentially regulate MMP-9 and neutrophil elastase in COPD. PloS one 7 (3), e33277. doi:10.1371/journal.pone.0033277
Voigt, P., Dorner, M. B., and Schaefer, M. (2006). Characterization of p87PIKAP, a novel regulatory subunit of phosphoinositide 3-kinase gamma that is highly expressed in heart and interacts with PDE3B. J. Biol. Chem. 281 (15), 9977–9986. doi:10.1074/jbc.M512502200
Wang, C., Zhou, J., Wang, J., Li, S., Fukunaga, A., Yodoi, J., et al. (2020). Progress in the mechanism and targeted drug therapy for COPD. Signal Transduct. Target. Ther. 5 (1), 248. doi:10.1038/s41392-020-00345-x
Wang, J., Zhu, M., Wang, L., Chen, C., and Song, Y. (2019a). Amphiregulin potentiates airway inflammation and mucus hypersecretion induced by urban particulate matter via the EGFR-PI3Kα-AKT/ERK pathway. Cell. Signal. 53, 122–131. doi:10.1016/j.cellsig.2018.10.002
Wang, L., Gu, W., Shi, Y., Chen, Y., and Tan, Y. (2019b). Protective effects of astragaloside IV on IL-8-treated diaphragmatic muscle cells. Exp. Ther. Med. 17 (1), 519–524. doi:10.3892/etm.2018.6940
Wang, L., Jiang, W., Wang, J., Xie, Y., and Wang, W. (2022b). Puerarin inhibits FUNDC1-mediated mitochondrial autophagy and CSE-induced apoptosis of human bronchial epithelial cells by activating the PI3K/AKT/mTOR signaling pathway. Aging 14 (3), 1253–1264. doi:10.18632/aging.203317
Wang, Q., Shen, Z. N., Zhang, S. J., Sun, Y., Zheng, F. J., and Li, Y. H. (2022a). Protective effects and mechanism of puerarin targeting PI3K/Akt signal pathway on neurological diseases. Front. Pharmacol. 13, 1022053. doi:10.3389/fphar.2022.1022053
Xiaofei, Y., Tingting, L., Xuan, W., and Zhiyi, H. (2022). Erythromycin attenuates oxidative stress-induced cellular senescence via the PI3K-mTOR signaling pathway in chronic obstructive pulmonary disease. Front. Pharmacol. 13, 1043474. doi:10.3389/fphar.2022.1043474
Xie, S., and Wang, X. (2022). CRYAB reduces cigarette smoke-induced inflammation, apoptosis, and oxidative stress by retarding PI3K/Akt and NF-κB signaling pathways in human bronchial epithelial cells. Allergologia Immunopathol. 50 (5), 23–29. doi:10.15586/aei.v50i5.645
Xie, Y., He, Q., Chen, H., Lin, Z., Xu, Y., and Yang, C. (2019). Crocin ameliorates chronic obstructive pulmonary disease-induced depression via PI3K/Akt mediated suppression of inflammation. Eur. J. Pharmacol. 862, 172640. doi:10.1016/j.ejphar.2019.172640
Xu, C., Feng, C., Huang, P., Li, Y., Liu, R., Liu, C., et al. (2022). TNFα and IFNγ rapidly activate PI3K-AKT signaling to drive glycolysis that confers mesenchymal stem cells enhanced anti-inflammatory property. Stem Cell Res. Ther. 13 (1), 491. doi:10.1186/s13287-022-03178-3
Xu, F., Lin, J., Cui, W., Kong, Q., Li, Q., Li, L., et al. (2018). Scutellaria baicalensis attenuates airway remodeling via PI3K/Akt/NF-κB pathway in cigarette smoke mediated-COPD rats model. Evidence-based complementary Altern. Med. eCAM 2018, 1281420. doi:10.1155/2018/1281420
Xue, R., Wan, Y., Sun, X., Zhang, X., Gao, W., and Wu, W. (2019). Nicotinic mitigation of neuroinflammation and oxidative stress after chronic sleep deprivation. Front. Immunol. 10, 2546. doi:10.3389/fimmu.2019.02546
Yanagisawa, S., Baker, J. R., Vuppusetty, C., Fenwick, P., Donnelly, L. E., Ito, K., et al. (2017). Decreased phosphatase PTEN amplifies PI3K signaling and enhances proinflammatory cytokine release in COPD. Am. J. physiology Lung Cell. Mol. physiology 313 (2), L230–l9. doi:10.1152/ajplung.00382.2016
Yang, Y. M., Cho, Y. E., and Hwang, S. (2022). Crosstalk between oxidative stress and inflammatory liver injury in the pathogenesis of alcoholic liver disease. Int. J. Mol. Sci. 23 (2), 774. doi:10.3390/ijms23020774
Yap, T. A., Yan, L., Patnaik, A., Fearen, I., Olmos, D., Papadopoulos, K., et al. (2011). First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J. Clin. Oncol. 29 (35), 4688–4695. doi:10.1200/JCO.2011.35.5263
Ye, Q., Liu, Y., Zhang, G., Deng, H., Wang, X., Tuo, L., et al. (2023). Deficiency of gluconeogenic enzyme PCK1 promotes metabolic-associated fatty liver disease through PI3K/AKT/PDGF axis activation in male mice. Nat. Commun. 14 (1), 1402. doi:10.1038/s41467-023-37142-3
Zhang, A., Qian, F., Li, Y., Li, B., Yang, F., Hu, C., et al. (2023). Research progress of metformin in the treatment of liver fibrosis. Int. Immunopharmacol. 116, 109738. doi:10.1016/j.intimp.2023.109738
Zhang, C., Ding, X. P., Zhao, Q. N., Yang, X. J., An, S. M., Wang, H., et al. (2016). Role of α7-nicotinic acetylcholine receptor in nicotine-induced invasion and epithelial-to-mesenchymal transition in human non-small cell lung cancer cells. Oncotarget 7 (37), 59199–59208. doi:10.18632/oncotarget.10498
Zhang, P., Xin, X., Fang, L., Jiang, H., Xu, X., Su, X., et al. (2017). HMGB1 mediates Aspergillus fumigatus-induced inflammatory response in alveolar macrophages of COPD mice via activating MyD88/NF-κB and syk/PI3K signalings. Int. Immunopharmacol. 53, 125–132. doi:10.1016/j.intimp.2017.10.007
Zhang, P., Zhang, Y., Wang, L., Wang, X., Xu, S., Zhai, Z., et al. (2022c). Reversal of NADPH oxidase-dependent early oxidative and inflammatory responses in chronic obstructive pulmonary disease by puerarin. Oxidative Med. Cell. Longev. 2022, 5595781. doi:10.1155/2022/5595781
Zhang, X. Y., Li, W., Zhang, J. R., Li, C. Y., Zhang, J., and Lv, X. J. (2022b). Roles of sirtuin family members in chronic obstructive pulmonary disease. Respir. Res. 23 (1), 66. doi:10.1186/s12931-022-01986-y
Zhang, Y., Wang, X., Li, A., Guan, Y., Shen, P., Ni, Y., et al. (2022a). PP2A regulates metastasis and vasculogenic mimicry formation via PI3K/AKT/ZEB1 axis in non-small cell lung cancers. J. Pharmacol. Sci. 150 (2), 56–66. doi:10.1016/j.jphs.2022.07.001
Keywords: chronic obstructive pulmonary disease, PI3K/Akt signalling pathway, inhibitor, inflammation, oxidative stress
Citation: Liu Y, Kong H, Cai H, Chen G, Chen H and Ruan W (2023) Progression of the PI3K/Akt signaling pathway in chronic obstructive pulmonary disease. Front. Pharmacol. 14:1238782. doi: 10.3389/fphar.2023.1238782
Received: 12 June 2023; Accepted: 08 September 2023;
Published: 20 September 2023.
Edited by:
Li Wu, Nanjing University of Chinese Medicine, ChinaReviewed by:
Teng He, Zhejiang University, ChinaLan Bai, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, China
Copyright © 2023 Liu, Kong, Cai, Chen, Chen and Ruan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanhui Liu, bGl1eWgxMzA3NTU3OTUxNUAxMjYuY29t
†These authors have contributed equally to this work
 Yanhui Liu
Yanhui Liu Haobo Kong2†
Haobo Kong2† Wenyi Ruan
Wenyi Ruan