
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 09 January 2024
Sec. Pharmacology of Anti-Cancer Drugs
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1238579
This article is part of the Research Topic Advances in the use of EGFR TKIs in the Treatment of NSCLC View all 21 articles
Background: The synergistic effects of antiangiogenic inhibitor bevacizumab and epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKI) therapy were encouraging in patients with EGFR-mutant advanced NSCLC, though some controversy remains. The specific subgroup of patients who might benefit most from the EGFR-TKI and bevacizumab combination therapy is yet to be determined.
Methods: Randomized clinical trials (RCTs) that had compared the clinical efficacy of EGFR-TKI and bevacizumab combination therapy with EGFR-TKI monotherapy in treating EGFR-mutant advanced NSCLC patients published before 23 December 2022 were searched in the Cochrane, PubMed and Embase. We performed a meta-analysis for the overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and treatment-related adverse events with a grade equal or more than 3 (grade≥3 TRAEs). Subgroup analyses of PFS and OS stratified by clinical characteristics and treatment were conducted.
Results: We included 10 RCTs involving 1520 patients. Compared with EGFR-TKI monotherapy, addition of bevacizumab to EGFR-TKI resulted in a significantly higher PFS (hazard ratio (HR) = 0.74, 95% confidence interval (95% CI): 0.62–0.87)) and ORR (risk ratio (RR) = 1.07, 95% CI: 1.01–1.13). However, no significant difference in OS (HR = 0.96, 95% CI: 0.83–1.12) was noticed. Patients with EGFR-mutant advanced NSCLC receiving combination therapy showed PFS improvement regardless of gender (male or female), Eastern Cooperative Oncology Group performance status (0 or 1), baseline central nervous system (CNS) metastasis (presence or absence) and EGFR mutation type (19del or 21L858R). Subgroup analyses showed that, with the treatment of bevacizumab and EGFR-TKI, patients who ever smoked achieved significantly better OS and PFS benefits (HR = 0.68, 95% CI: 0.48–0.95; HR = 0.59, 95% CI: 0.46–0.74, respectively), and those aged <75 years and the Asian population had significantly prolonged PFS (HR = 0.69, 95% CI: 0.52–0.91; HR = 0.71, 95% CI: 0.58–0.87; respectively). The superiority of EGFR-TKI and bevacizumab combination therapy against EGFR-TKI monotherapy in improving PFS was more significant in the erlotinib regimen subgroup. The risk of grade≥3 TRAEs was remarkably higher in the combination therapy group (HR = 1.73, 95% CI: 1.39–2.16).
Conclusion: Addition of bevacizumab to EGFR-TKI therapy provided significantly better PFS and ORR for EGFR-mutant advanced NSCLC patients, though with higher risk of grade≥3 TRAEs. Patients who ever smoked, aged <75 years, and Asian population might benefit more from the combination regimen.
Systematic Review Registration: This systematic review and meta-analysis was registered in the PROSPERO database (CRD42023401926)
Lung cancer is one of the most common leading causes of death worldwide (Miller and Hanna, 2021). Non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) account for nearly 85% and 15% of all lung cancers, respectively (Molina et al., 2008; Wang et al., 2021). Epidermal growth factor receptor (EGFR), a transmembrane receptor tyrosine kinase in the ERBB family, plays fundamental role in cell proliferation and survival (Jorissen et al., 2003). The overall EGFR mutation frequency was about 50% in Asia-Pacific patients and 15%–20% in western NSCLC patients, with higher frequency in women compared with men, as well as in non-smokers compared with ever-smokers (Midha et al., 2015). Moreover, exon 19 (19del) deletion and L858R point mutation are most prevalent (Lee, 2017). The mutation and overexpression of EGFR was the pharmaceutical basis for the development and employment of EGFR-tyrosine kinase inhibitors (EGFR-TKI), and it has been widely adopted in front-line treatment for NSCLC patients with EGFR mutation (Lau et al., 2019; Li et al., 2019; Ito et al., 2020). Nevertheless, most patients inevitably develop resistance to these TKIs within 9–13 months (Lee, 2017), which has been found to be associated with increased vascular endothelial growth factor (VEGF) levels (Hung et al., 2016). It is reported that inhibition of angiogenesis could effectively enhance the anti-tumor activity of EGFR-TKI by targeting both the EGFR and VEGF pathways (Zhang et al., 2020; Watanabe et al., 2021). Therefore, addition of antiangiogenic agents might be able to prevent EGFR-TKI resistance and exert synergistic anti-tumor effects.
Bevacizumab is a kind of recombinant, anti-VEGF monoclonal antibody, which targets vascular endothelial growth factor-A (Goyal et al., 2022). The addition of bevacizumab to chemotherapy or immune check point inhibitors in the treatment of advanced NSCLC was demonstrated to be favorable (Systematic review and meta, 2013; Socinski et al., 2021; Sugawara et al., 2021), whereas its role in EGFR-TKI combination therapy remains controversy. The combination of erlotinib and bevacizumab was shown to be encouraging and has been accepted as an alternative choice of front-line therapy (Hsu et al., 2018). However, in the trials that mostly included non-Asian patients, no superiority of the combination regimen was found in terms of the anti-tumor effect, as compared with EGFR-TKI alone (Stinchcombe et al., 2019; Soo et al., 2021).
As more clinical trials had reported the outcome of combination therapy involving bevacizumab and different EGFR-TKIs, the present study aimed to clarify the clinical value of bevacizumab and EGFR-TKI combination therapy in EGFR-mutant advanced NSCLC patients, and further explore its role in predefined subgroups, in an attempt to provide evidence for selection of NSCLC individuals who might benefit most by adding bevacizumab to EGFR-TKI.
This systematic review and meta-analysis was registered in the International prospective register of systematic reviews (PROSPERO) database (CRD42023401926). We conducted a thorough search to identify relevant RCTs that had compared the clinical efficacy of combination EGFR-TKI and bevacizumab therapy with EGFR-TKI monotherapy in the treatment of advanced NSCLC using the following databases: PUBMED, EMBASE, and Cochrane. The last retrieval was performed on 23 December 2022. The keywords used were as follows: all terms related to “NSCLC,” “bevacizumab,” “erlotinib,” “gefitinib,” “icotinib,” “afatinib,” “Osimertinib,” and other EGFR-TKIs, “epidermal growth factor receptor,” “EGFR,” all terms related to clinical trial. The retrieval strategy for the PubMed database is listed in Supplementary Table S1.
Studies fulfilling all the following criteria were included (Miller and Hanna, 2021) RCTs; (Molina et al., 2008) studies that had compared combination EGFR-TKI and bevacizumab therapy with EGFR-TKI monotherapy in treating advanced NSCLC (Wang et al., 2021); studies included patients with EGFR mutations (Jorissen et al., 2003); with at least one of the following reported outcomes: overall survival (OS), progression-free survival (PFS), objective response rate (ORR) and treatment-related adverse events with a grade equal or more than 3 (grade≥3 TRAEs) (Midha et al., 2015); studies with a sample size of at least 40 patients. For the overlapping reports obtained from the same group of patients, the latest and most complete reports were included. Duplicate publications, review articles, meta-analyses, editorials, case reports, letters, animal or cellular experiments and studies with incomplete data were excluded.
Data extraction was performed independently by two investigators according to the predefined criteria. The information extracted from each study was as follows: the name of study, year of publication, trial number and design, ethnicity involved, sample size (female%), treatment regimens, follow-up time, age (median, range, years), Eastern Cooperative Oncology Group performance status (ECOG PS), smoking status, baseline central nervous system (CNS) metastasis condition, pathological features, EGFR mutation status, outcomes including PFS, OS, ORR and grade≥3 TRAEs. A third investigator was consulted when there were any disagreements during the process, and the discrepancies were resolved by discussion.
The quality assessment of included trials was conducted independently by two investigators. The quality of RCT was evaluated according to the Cochrane Collaboration tool, with a total of 6 items included: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias (Supplementary Figure S1). There are three levels for each item, that is, a high, low or unclear risk of bias. A third investigator was consulted when there were any disagreements during the process, and the discrepancies were resolved by discussion.
R software (version 4.1.0) with package meta was adopted to perform meta-analysis. The primary outcomes were OS and PFS, and the secondary outcomes were ORR and grade≥3 TRAEs. Hazard ratios (HR) with 95% CIs for OS and PFS, odds ratios (OR) with 95% CIs for ORR and grade≥3 TRAEs were extracted from the original report.
For each outcome, statistical heterogeneity was evaluated using the Cochran’s Q test and the I2 measure. An I2 value greater than 50% or p-value equal or less than 0.1 is generally considered to indicate a substantial level of heterogeneity, which requires a random effects model for pooled analysis and initiates subsequent sensitivity analysis to identify the source. Otherwise, a fixed effects model was adopted. The Egger regression test with a funnel plot was used to evaluate the publication bias, and a p-value of less than 0.10 was considered to indicate significant asymmetry and publication bias. When there was publication bias, trim-and-fill method was used for data correction. Subgroup analyses were conducted with the following stratifications: gender, age, baseline CNS metastasis, EGFR mutation type, smoking status, different type of EGFR-TKI, treatment line, ethnicity, and ECOG PS.
We identified 797 records from the databases. After excluding 153 duplicates and 604 reports for irrelevant titles and abstracts, a total of 40 studies were reviewed for full-text assessment. Finally, 14 studies from 10 trials were included in our work (Seto et al., 2014; Kato et al., 2018; Saito et al., 2019; Stinchcombe et al., 2019; Akamatsu et al., 2021; Soo et al., 2021; Yamamoto et al., 2021; Zhou et al., 2021; Ishikawa et al., 2022; Kawashima et al., 2022; Kenmotsu et al., 2022; Lee et al., 2022; Nakamura et al., 2022; Piccirillo et al., 2022), with 1 trial only reported in conference abstract (Ishikawa et al., 2022) (Figure 1).
The detailed information of the 14 studies were shown in Table 1 and Table 2. A total of 1520 patients were included in our work, with 760 in the combination therapy group and 760 in the monotherapy group. One out of 10 trials had evaluated the efficacy of afatinib plus bevacizumab as compared with afatinib alone (Ishikawa et al., 2022), 6 had compared erlotinib plus bevacizumab with erlotinib alone (Seto et al., 2014; Kato et al., 2018; Saito et al., 2019; Stinchcombe et al., 2019; Yamamoto et al., 2021; Zhou et al., 2021; Kawashima et al., 2022; Lee et al., 2022; Piccirillo et al., 2022), and 3 had compared osimertinib plus bevacizumab with osimertinib monotherapy (Akamatsu et al., 2021; Kenmotsu et al., 2022; Nakamura et al., 2022). There were 3 phase III RCTs and 7 phase II RCTs. The majority of the included patient population was Asian. There were 8 RCTs adopted the EGFR-TKI regimen as first-line treatment (Seto et al., 2014; Saito et al., 2019; Stinchcombe et al., 2019; Zhou et al., 2021; Ishikawa et al., 2022; Kenmotsu et al., 2022; Lee et al., 2022; Piccirillo et al., 2022). Most patients were ECOG PS 0-1.
There were 10 studies involving 1520 patients with EGFR-mutant advanced NSCLC eligible for the pooling analysis of PFS. The pooled PFS result derived from a random-effect model showed that the combination therapy group had a significantly longer PFS as compared with the EGFR-TKI monotherapy group (HR = 0.74, 95% CI: 0.62–0.87, Cochran’s Q p = 0.06, I2 = 44%; Figure 2A). The funnel plot and Egger’s test both demonstrated publication bias (Supplementary Figure S2A, p = 0.0227). Thus, trim-and-fill method was adopted. The data after correction also suggested significant PFS benefit in the combination therapy group (HR = 0.655, 95% CI: 0.5439–0.7889; Supplementary Figure S2B). Sensitivity analysis showed that removal of any study did not affect the pooled HR, which indicates stability of the result (Supplementary Figure S3A).
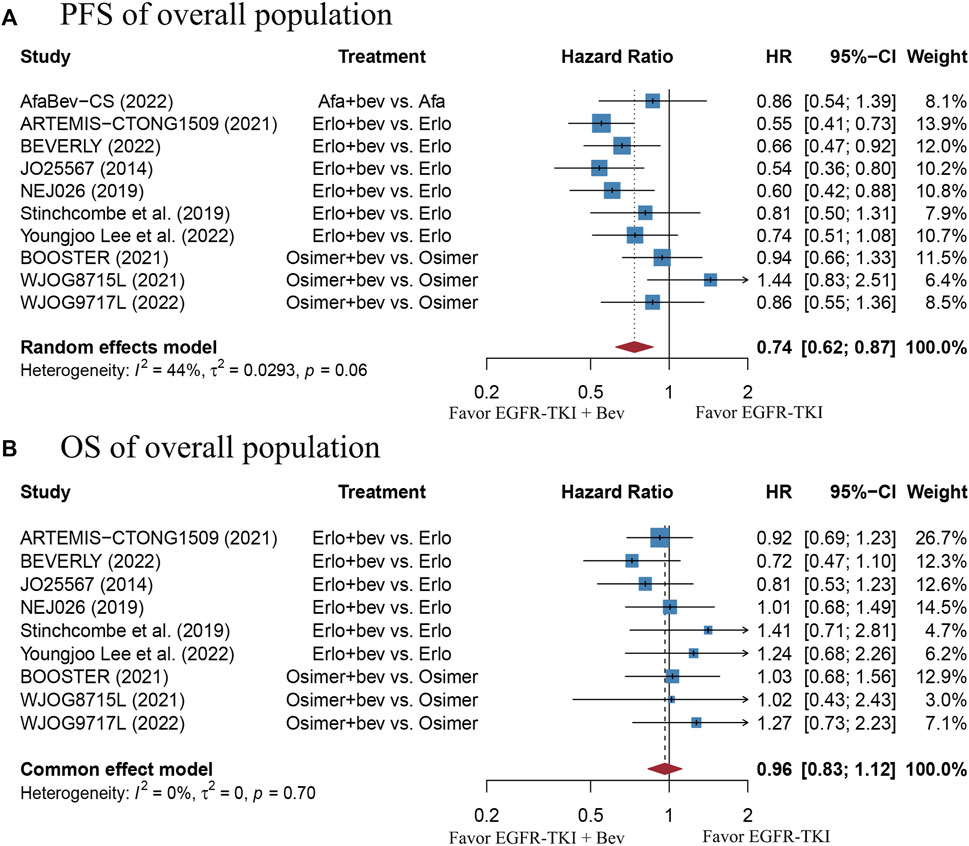
FIGURE 2. (A) Forest plot of HRs for PFS in the overall population. (B) Forest plot of HRs for OS in the overall population. Afa, Afatinib; Bev, Bevacizumab; Erlo, Erlotinib; Osimer, Osimertinib; CI, confidence interval.
A total of 9 studies including 1420 patients with EGFR-mutant advanced NSCLC were enrolled for the pooling analysis of OS. The pooled HR was 0.96 (95% CI: 0.83–1.12), with no heterogeneity (Cochran’s Q p = 0.7, I2 = 0%; Figure 2B), suggesting that there was no significant difference in OS between the combination therapy group and EGFR-TKI monotherapy group. The funnel plot and Egger’s test showed no publication bias (Supplementary Figure S2C, p = 0.1486). Sensitivity analysis showed that removal of any study did not affect the pooled HR, which indicates stability of the result (Supplementary Figure S3B).
There were 10 studies with 1520 EGFR-mutant advanced NSCLC patients provided the ORR outcome. The pooled RR was 1.07 (95% CI: 1.01–1.13), with no heterogeneity (Cochran’s Q p = 0.45, I2 = 0%; Figure 3A), indicating a slightly better response in the combination therapy group, as compared with the EGFR-TKI monotherapy group. The funnel plot and Egger’s test showed no publication bias (Supplementary Figure S2D, p = 0.1524). Nevertheless, sensitivity analysis showed that removal of the BEVERLY research would affect the pooled RR, which indicates instability of the result (Supplementary Figure S3C). This data should be interpreted with caution.
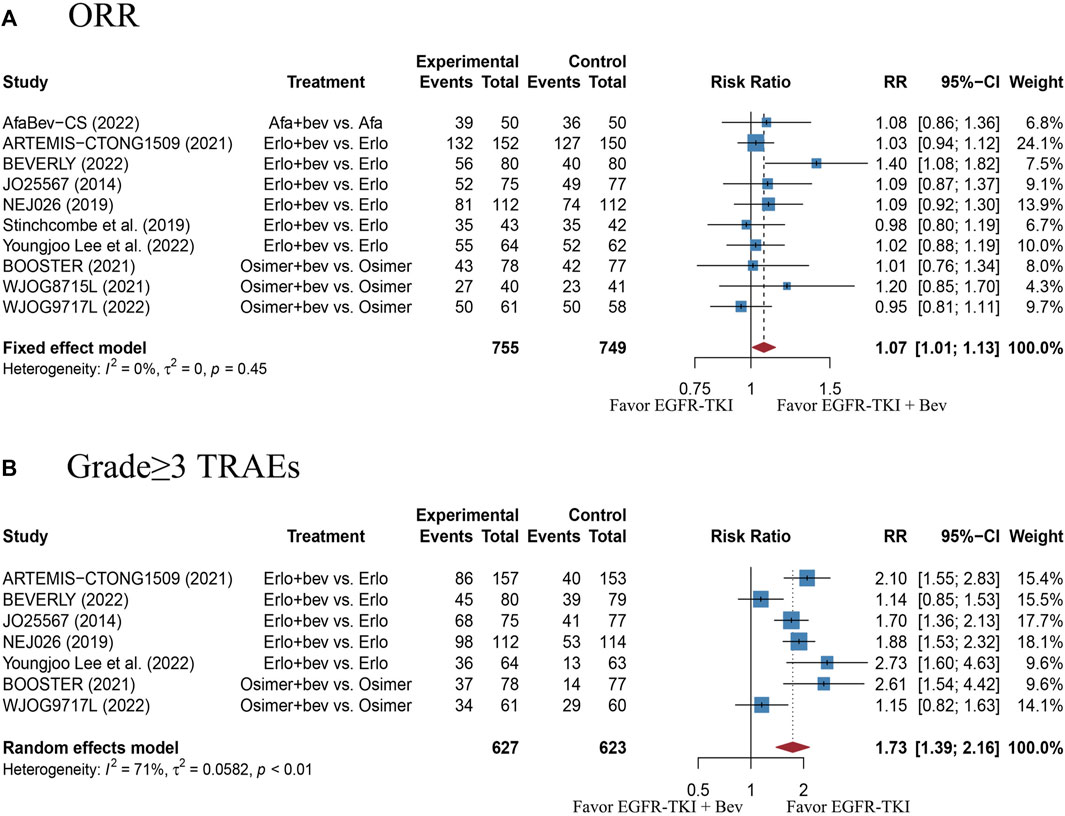
FIGURE 3. (A) Forest plot of RRs for ORR in the overall population. (B) Forest plot of RRs for grade≥3 TRAEs in the overall population. Afa, Afatinib; Bev, Bevacizumab; Erlo, Erlotinib; Osimer, Osimertinib; CI, confidence interval.
There were 7 studies with 1250 EGFR-mutant advanced NSCLC patients reported data on grade≥3 TRAEs. The pooled RR was 1.73 (95% CI: 1.39–2.16), with high heterogeneity (Cochran’s Q p < 0.01, I2 = 71%; Figure 3B), which suggests a significantly higher risk of grade≥3 TRAEs with combination therapy, as compared with the EGFR-TKI monotherapy. The funnel plot and Egger’s test showed no publication bias (Supplementary Figure S2E, p = 0.6441). Sensitivity analysis showed that removal of any study did not affect the pooled RR, indicating stability of the result (Supplementary Figure S3D). Moreover, the most reported grade≥3 TRAEs were listed in Supplementary Table S2. Of those, the increased risks of hypertension, proteinuria and rash in the combination therapy group were statistically significant, as compared with monotherapy group.
Subgroup analyses of PFS and OS were conducted with the following stratifications: gender, age, baseline CNS metastasis condition, EGFR mutation type, smoking status, different type of EGFR-TKI, treatment line, ethnicity, and ECOG PS (Table 3).
The stratified analysis showed that addition of bevacizumab to EGFR-TKI therapy could significantly improve the PFS for all EGFR-mutant advanced NSCLC patients irrespective of the differences in gender, EGFR mutation type, ECOG PS, and baseline CNS metastasis (Table 3). However, significant PFS benefit of combination therapy was noticed in patients with age below 75 years (HR = 0.69, 95% CI: 0.52–0.91, Cochran’s Q p = 0.09, I2 = 50%; Figures 4A,B), in the smoker population (HR = 0.59, 95% CI: 0.46–0.74, Cochran’s Q p = 0.43, I2 = 0%; Figures 5A,B), and in the Asian population (HR = 0.71, 95% CI: 0.58–0.87, Cochran’s Q p = 0.07, I2 = 46%; Figures 6A,B). Moreover, patients treated with erlotinib and bevacizumab combination therapy yielded remarkably better PFS (HR = 0.63, 95% CI: 0.54–0.73, Cochran’s Q p = 0.65, I2 = 0%; Figure 7), whereas those treated with osimertinib or afatinib and bevacizumab had comparable efficacy with those treated with EGFR-TKI monotherapy (For osimertinib, HR = 1, 95% CI: 0.78–1.28, Cochran’s Q p = 0.34, I2 = 8%; Figure 7). Further analyses revealed that EGFR-TKI and bevacizumab had significantly better PFS outcome when adopted as first-line treatment (HR = 0.66, 95% CI: 0.58–0.76, Cochran’s Q p = 0.49, I2 = 0%; Figure 8).
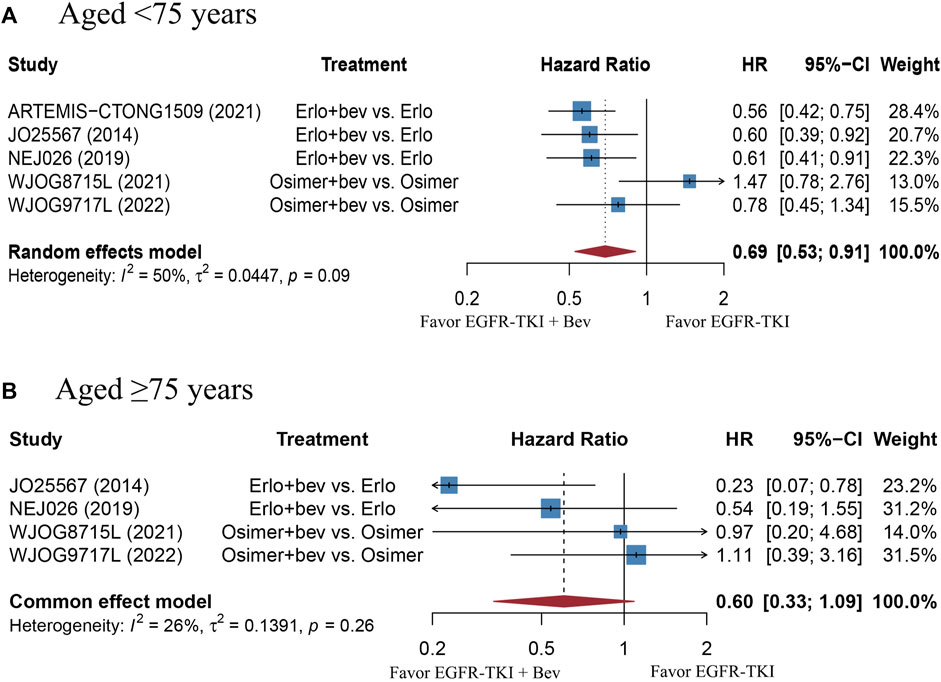
FIGURE 4. (A) Forest plot of HRs for PFS in patients aged less than 75 years old. (B) Forest plot of HRs for OS in patients aged equal or more than 75 years old. Afa, Afatinib; Bev, Bevacizumab; Erlo, Erlotinib; Osimer, Osimertinib; CI, confidence interval.
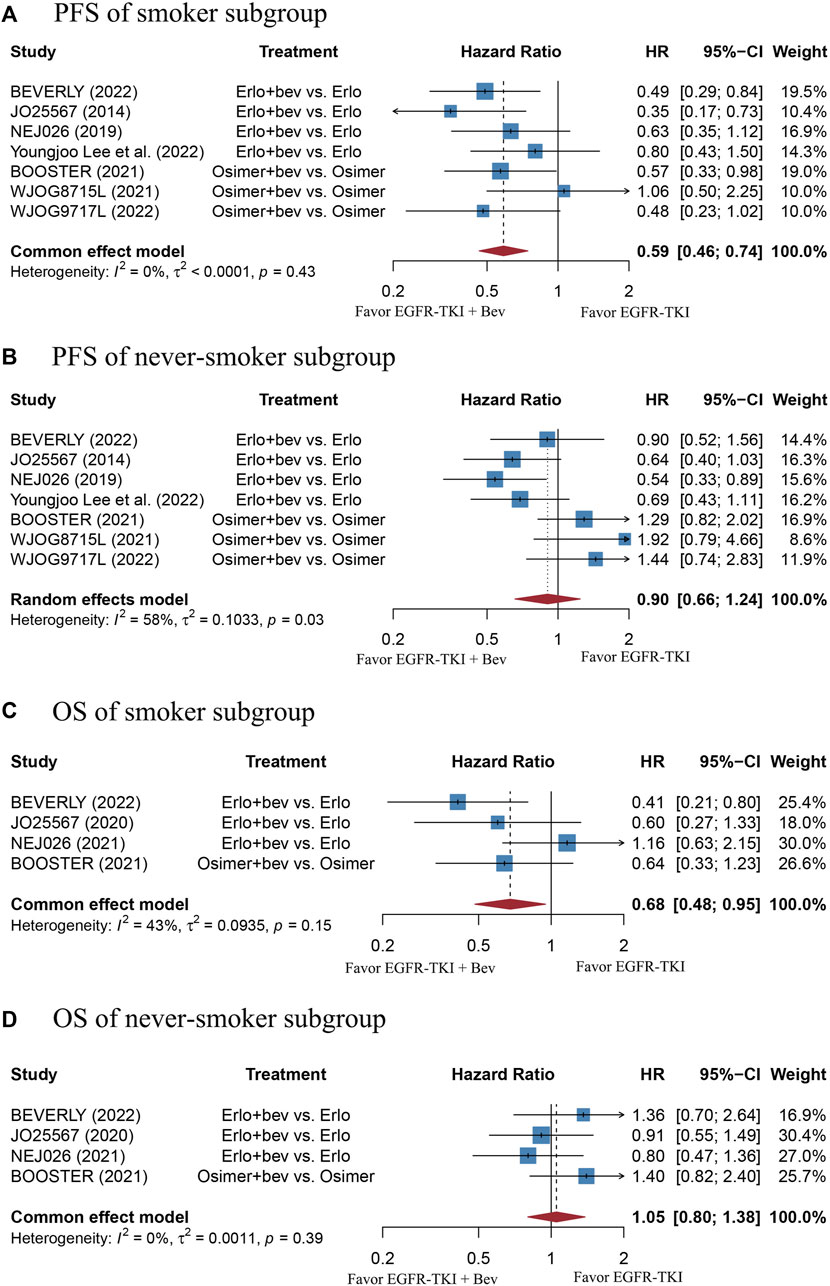
FIGURE 5. (A) Forest plot of HRs for PFS in smoker subgroup. (B) Forest plot of HRs for PFS in never-smoker subgroup. (C) Forest plot of HRs for OS in smoker subgroup. (D) Forest plot of HRs for OS in never-smoker subgroup. Note: There were 13 former light smokers from the NEJ026 study excluded from the analysis. The 15 former light smokers from the JO25567 study were included in the never-smoker subgroup. Afa, Afatinib; Bev, Bevacizumab; Erlo, Erlotinib; Osimer, Osimertinib; CI, confidence interval.
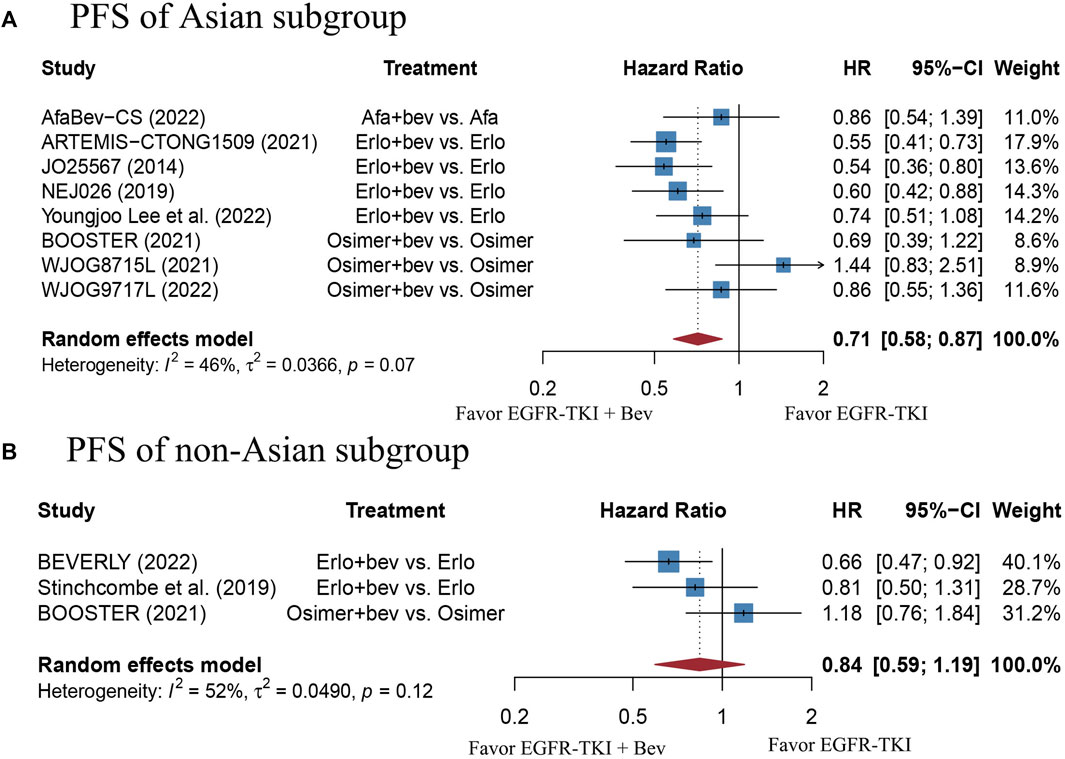
FIGURE 6. (A) Forest plot of HRs for PFS in Asian subgroup. (B) Forest plot of HRs for PFS in non-Asian subgroup. Note: The BEVERLY study conducted in Italian centers and the work proposed by Stinchcombe et al. including mostly non-Asian people were both categorized as non-Asian group. Afa, Afatinib; Bev, Bevacizumab; Erlo, Erlotinib; Osimer, Osimertinib; CI, confidence interval.
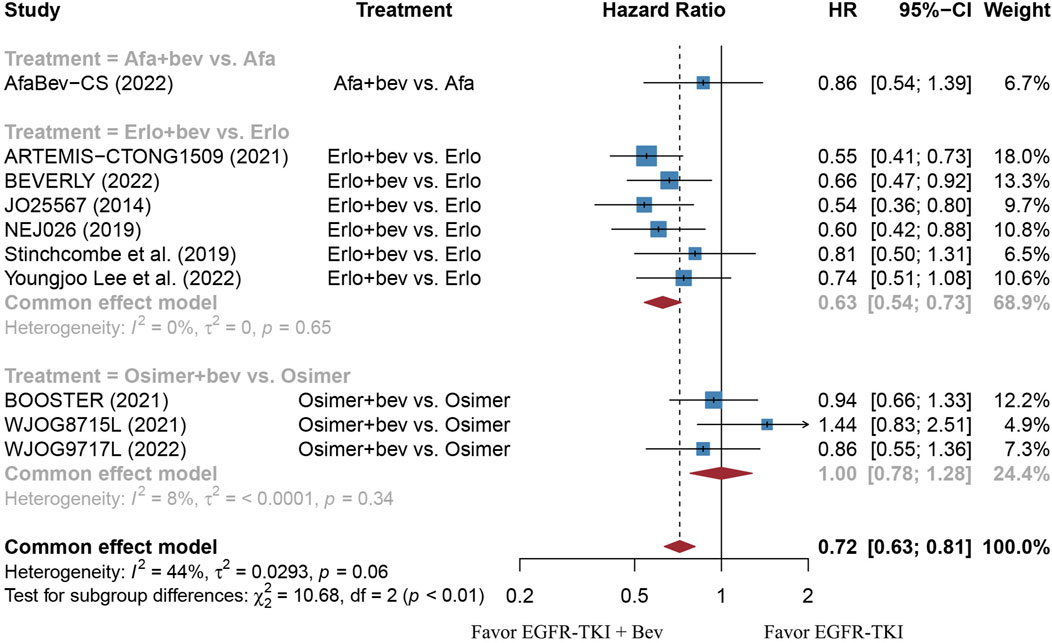
FIGURE 7. Forest plot of HRs for PFS based on different types of EGFR-TKI. Afa, Afatinib; Bev, Bevacizumab; Erlo, Erlotinib; Osimer, Osimertinib; CI, confidence interval.
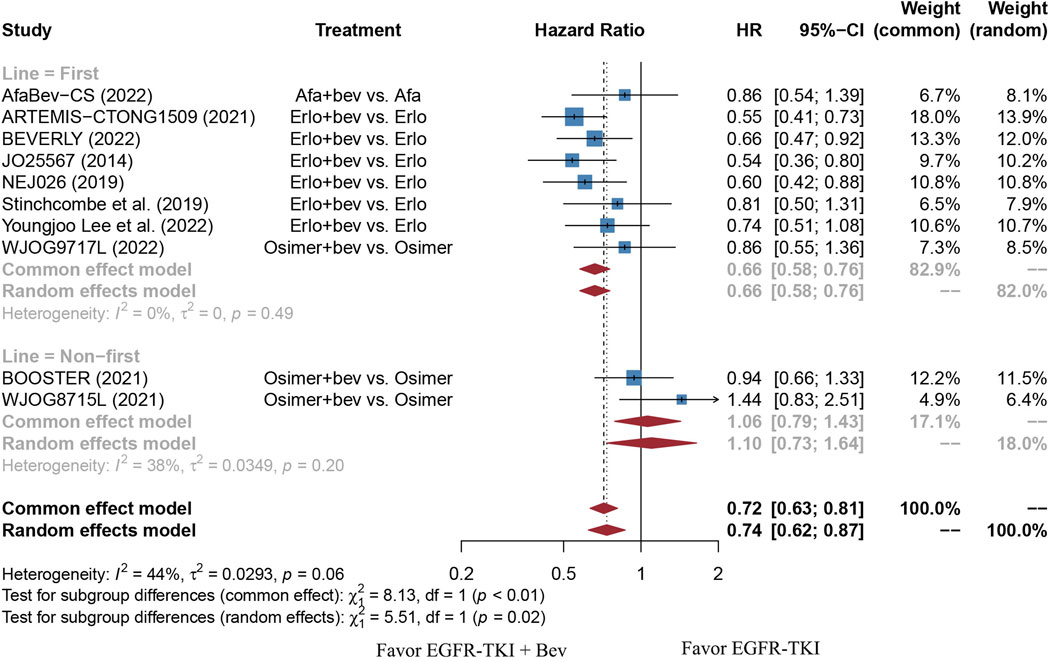
FIGURE 8. Forest plot of HRs for PFS based on different treatment lines. Afa, Afatinib; Bev, Bevacizumab; Erlo, Erlotinib; Osimer, Osimertinib; CI, confidence interval.
Adding bevacizumab to EGFR-TKI therapy did not affect the OS for all EGFR-mutant advanced NSCLC patients, regardless of their gender, EGFR mutation type, different type of EGFR-TKI, treatment line, and ECOG PS (Table 3). Interestingly, significant OS benefit of combination therapy was observed in the smoker subgroup, with no heterogeneity (HR = 0.68, 95%CI: 0.48–0.95, Cochran’s Q p = 0.15, I2 = 43%; Figures 5C,D).
The results of this meta-analysis showed that adding bevacizumab to EGFR-TKI therapy provided significantly better PFS and ORR results for NSCLC patients harboring EGFR mutations, though this benefit failed to translate into prolonging OS. The subgroup analyses stratified by patients’ clinical features also proved that EGFR-TKI and bevacizumab combination therapy consistently resulted in longer PFS regardless of the gender, ECOG PS, baseline CNS metastasis and EGFR mutation type. Interestingly, in the smoker subgroup (former or current smoker), addition of bevacizumab to EGFR-TKI could significantly prolong the PFS and OS. Moreover, as compared with those aged equal or more than 75 years, combination therapy provided with significantly favorable PFS results for EGFR-mutant advanced NSCLC patients who aged less than 75 years.
VEGF, a family of polypeptide growth factors, mainly included VEGF-A, -B, -C and -D (32). Of those, VEGF-A is the most investigated variant, which primarily binds to VEGF receptor 1 and 2, thus inducing angiogenesis (Ferrara and Adamis, 2016). Bevacizumab, a humanized monoclonal antibody directed against VEGF-A, has been approved for the treatment of NSCLC globally. Given that the VEGF and EGFR pathways share common downstream signaling pathway that regulate cellular proliferation, it is suggested that EGFR-mutant tumors are more VEGF-dependent, thus dual inhibition of EGFR and VEGF might yield better antitumor effects (Abid et al., 2004; Le et al., 2021). In addition, it has been found that VEGF contributes to the acquired EGFR-TKI resistance, which supports the hypothesis that dual inhibition of EGFR and VEGF could delay resistance to EGFR-TKI, thus prolonging antitumor activity (Byers and Heymach, 2007; Le et al., 2021).
Subgroup analyses showed that the PFS benefit was consistently observed in EGFR-mutant advanced NSCLC patients of different gender (male or female), patients with different ECOG PS (0 or 1), baseline CNS metastasis (presence or absence) and EGFR mutation type (19del or 21L858R). The finding is echoed with the same subgroup analyses in the study of Deng et al., 2021. In addition, we found that combination bevacizumab and EGFR-TKI therapy significantly improved the PFS and OS result in smokers rather than those who never smoked, which is in line with the findings of Dafni et al., 2022. One possible explanation of this phenomenon is that TP53 mutation triggered by cigarette exposure would lead to increased sensitivity to anti-VEGF therapy (Schwaederlé et al., 2015). Moreover, we also noticed a significantly improved PFS in patients younger than 75 years old, as compared with those aged equal or more than 75 years. This finding is contradictory to that of Deng et al. (Deng et al., 2021). Nevertheless, it should be noted that the sample size of patients who aged equal or more than 75 years were too small in the ARTEMIS-CTONG1509 study and the PFS of the population could not be calculated, the number of patients aged equal or more than 75 years included was much less than those aged less than 75 years. In terms of different types of EGFR-TKI, our work included trials using all three generations of EGFR-TKI. Our data found that patients treated with erlotinib and bevacizumab combination therapy resulted in significantly better PFS than monotherapy, whereas the regimen involving osimertinib did not. The result may be partially explained by the fact that osimertinib and bevacizumab combination therapy adopted in both BOOSTER and WJOG8715L trials were used as non-first-line treatment. In the WJOG9717L trial, in which osimertinib and bevacizumab combination therapy was used in un-treated EGFR-mutant advanced NSCLC patients, bevacizumab was administered with a median duration of 33.4 weeks, which is shorter than that used with erlotinib (11–12 months) (Kenmotsu et al., 2022). There is another clinical trial (NCT04181060) currently evaluating the efficacy of osimertinib and bevacizumab combination therapy in un-treated EGFR-mutant advanced NSCLC patients, and the results are anticipated. Currently, most published work had focused on the Asian population. Our data showed that Asian population experienced significantly prolonged PFS than the non-Asian group. However, it should be noted that the sample size of non-Asian population is limited. There are several ongoing RCTs of EGFR-TKI with or without bevacizumab in EGFR-mutant advanced NSCLC that had primarily included non-Asian population (NCT04181060, NCT02971501), and the results are anticipated.
Noteworthy, several studies aimed to investigate the clinical value of multi-drugs therapy in treatment-naïve EGFR-mutant advanced NSCLC patients. The recently published FLAURA2 study confirmed significantly prolonged PFS in EGFR-mutant advanced NSCLC patients treated with osimertinib and chemotherapy, as compared with osimertinib alone (median PFS 25.5 months vs. 16.7 months, HR = 0.62, p < 0.001) (Planchard et al., 2023). The MARIPOSA study proved the superiority of amivantamab (with dual activity against EGFR and MET) and Lazertinib (a third-generation EGFR-TKI with CNS permeability) combination therapy in un-treated EGFR-mutant advanced NSCLC patients, as compared with osimertinib alone (median PFS 23.7 months vs. 16.6 months, HR = 0.7, p < 0.001) (Soria et al., 2013). The updated median PFS of the osimertinib monotherapy arm in the WJO9717L study was 20.2 months, which is longer than that reported in the FLAURA2 and MARIPOSA study. The reason may be that both FLAURA2 and MARIPOSA study had included more patients with CNS metastasis at baseline. With the emerging evidence of various combination therapy, the optimal choice for EGFR-mutant advanced NSCLC patients awaits further exploration.
However, the increased risk of combination therapy is non-neglectable. The most frequently observed grade≥3 TRAEs were hypertension, proteinuria, thrombotic events, rash, diarrhea and increased aminotransferase, which were similar to the established profiles of bevacizumab and EGFR-TKI, with no new safety concerns. Though it had been reported that the adverse effects of combination therapy were manageable (Kato et al., 2018), combination therapy of bevacizumab and EGFR-TKI should be applied with caution, and the occurrence of adverse events should be monitored carefully.
To the best of our knowledge, this is the meta-analysis that had included the most recently published RCTs comparing the clinical efficacy of combination therapy of bevacizumab and EGFR-TKI with EGFR-TKI monotherapy, and it is also the first meta-analysis that had performed subgroup analyses for both PFS and OS outcomes. However, some limitations should be taken under consideration. First, the majority of included trials had only involved Asian patients, and the non-Asian population is limited, which may affect the subgroup comparison between Asian group and non-Asian group. Second, the OS data of the AfaBev-CS study is immature and the subgroup analyses result are not reported, thus we failed to include the information in our work.
Addition of bevacizumab to EGFR-TKI therapy provided significantly better PFS and ORR results for NSCLC patients harboring EGFR mutations, but no obvious OS benefit was observed and the risk of grade≥3 AEs was higher. Patients who ever smoked, aged <75 years old, and the Asian population might benefit more from the combination regimen, whereas gender, ECOG PS, baseline CNS metastasis and EGFR mutation type did not lead to significant differences.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
HZ, XQ, and HL contributed to the study conceptualization and design. HZ, YZ, and XY contributed to data acquisition and formal analysis. XQ, JT, and WC contributed to manuscript writing. HL and SH contributed to manuscript revision and study supervision. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1238579/full#supplementary-material
2013). Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann. Oncol. official J. Eur. Soc. Med. Oncol. 24 (4), 1133. doi:10.1093/annonc/mdt075
Abid, M., Guo, S., Minami, T., Spokes, K., Ueki, K., Skurk, C., et al. (2004). Vascular endothelial growth factor activates PI3K/Akt/forkhead signaling in endothelial cells. Arteriosclerosis, thrombosis, Vasc. Biol. 24 (2), 294–300. doi:10.1161/01.ATV.0000110502.10593.06
Akamatsu, H., Toi, Y., Hayashi, H., Fujimoto, D., Tachihara, M., Furuya, N., et al. (2021). Efficacy of osimertinib plus bevacizumab vs osimertinib in patients with EGFR t790m-mutated non-small cell lung cancer previously treated with epidermal growth factor receptor-tyrosine kinase inhibitor: west Japan Oncology group 8715L phase 2 randomized clinical trial. JAMA Oncol. 7 (3), 386–394. doi:10.1001/jamaoncol.2020.6758
Byers, L., and Heymach, J. (2007). Dual targeting of the vascular endothelial growth factor and epidermal growth factor receptor pathways: rationale and clinical applications for non-small-cell lung cancer. Clin. lung cancer 8 Suppl 2, S79–S85. doi:10.3816/clc.2007.s.006
Dafni, U., Soo, R., Peters, S., Tsourti, Z., Zygoura, P., Vervita, K., et al. (2022). Impact of smoking status on the relative efficacy of the EGFR TKI/angiogenesis inhibitor combination therapy in advanced NSCLC-a systematic review and meta-analysis. ESMO open 7 (3), 100507. doi:10.1016/j.esmoop.2022.100507
Deng, Z., Qin, Y., Liu, Y., Zhang, Y., and Lu, Y. (2021). Role of antiangiogenic agents combined with EGFR tyrosine kinase inhibitors in treatment-naive lung cancer: a meta-analysis. Clin. lung cancer 22 (1), e70–e83. doi:10.1016/j.cllc.2020.08.005
Ferrara, N., and Adamis, A. (2016). Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 15 (6), 385–403. doi:10.1038/nrd.2015.17
Goyal, P., Vats, B., Subbarao, M., Honnappa, C., Kabadi, P., Rohil, S., et al. (2022). Analytical similarity assessment of MYL-1402O to reference Bevacizumab. Expert Opin. Biol. Ther. 22 (2), 271–298. doi:10.1080/14712598.2021.1973426
Hsu, W., Yang, J., Mok, T., and Loong, H. (2018). Overview of current systemic management of EGFR-mutant NSCLC. Ann. Oncol. official J. Eur. Soc. Med. Oncol. 29, i3–i9. doi:10.1093/annonc/mdx702
Hung, M., Chen, I., Lin, P., Lung, J., Li, Y., Lin, Y., et al. (2016). Epidermal growth factor receptor mutation enhances expression of vascular endothelial growth factor in lung cancer. Oncol. Lett. 12 (6), 4598–4604. doi:10.3892/ol.2016.5287
Ishikawa, N., Ninomiya, T., Kozuki, T., Kuyama, S., Inoue, K., Yokoyama, T., et al. (2022). Afatinib (Afa) + bevacizumab (Bev) versus afatinib alone as first-line treatment of patients with EGFR-mutated advanced non-squamous NSCLC: primary analysis of the multicenter, randomized, phase II study?AfaBev-CS study. J. Clin. Oncol. 40 (16), 9112. doi:10.1200/jco.2022.40.16_suppl.9112
Ito, K., Murotani, K., Kubo, A., Kunii, E., Taniguchi, H., Shindoh, J., et al. (2020). Propensity score analysis of overall survival between first- and second-generation EGFR-TKIs using real-world data. Cancer Sci. 111 (10), 3705–3713. doi:10.1111/cas.14560
Jorissen, R., Walker, F., Pouliot, N., Garrett, T., Ward, C., and Burgess, A. (2003). Epidermal growth factor receptor: mechanisms of activation and signalling. Exp. Cell Res. 284 (1), 31–53. doi:10.1016/s0014-4827(02)00098-8
Kato, T., Seto, T., Nishio, M., Goto, K., Yamamoto, N., Okamoto, I., et al. (2018). Erlotinib plus bevacizumab phase ll study in patients with advanced non-small-cell lung cancer (JO25567): updated safety results. Drug Saf. 41 (2), 229–237. doi:10.1007/s40264-017-0596-0
Kawashima, Y., Fukuhara, T., Saito, H., Furuya, N., Watanabe, K., Sugawara, S., et al. (2022). Bevacizumab plus erlotinib versus erlotinib alone in Japanese patients with advanced, metastatic, EGFR-mutant non-small-cell lung cancer (NEJ026): overall survival analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Respir. Med. 10 (1), 72–82. doi:10.1016/S2213-2600(21)00166-1
Kenmotsu, H., Wakuda, K., Mori, K., Kato, T., Sugawara, S., Kirita, K., et al. (2022). Randomized phase 2 study of osimertinib plus bevacizumab versus osimertinib for untreated patients with nonsquamous NSCLC harboring EGFR mutations: WJOG9717L study. J. Thorac. Oncol. 17 (9), 1098–1108. doi:10.1016/j.jtho.2022.05.006
Lau, S., Chooback, N., Ho, C., and Melosky, B. (2019). Outcome differences between first- and second-generation EGFR inhibitors in advanced EGFR mutated NSCLC in a large population-based cohort. Clin. lung cancer 20 (5), e576–e583. doi:10.1016/j.cllc.2019.05.003
Le, X., Nilsson, M., Goldman, J., Reck, M., Nakagawa, K., Kato, T., et al. (2021). Dual EGFR-VEGF pathway inhibition: a promising strategy for patients with EGFR-mutant NSCLC. J. Thorac. Oncol. official Publ. Int. Assoc. Study Lung Cancer 16 (2), 205–215. doi:10.1016/j.jtho.2020.10.006
Lee, D. (2017). Treatments for EGFR-mutant non-small cell lung cancer (NSCLC): the road to a success, paved with failures. Pharmacol. Ther. 174, 1–21. doi:10.1016/j.pharmthera.2017.02.001
Lee, Y., Kim, H. R., Hong, M. H., Lee, K. H., Park, K. U., Lee, G. K., et al. (2022). A randomized Phase 2 study to compare erlotinib with or without bevacizumab in previously untreated patients with advanced non-small cell lung cancer with EGFR mutation. Cancer 129, 405–414. doi:10.1002/cncr.34553
Li, H., Wang, C., Wang, Z., Hu, Y., Zhang, G., Zhang, M., et al. (2019). Efficacy and long-term survival of advanced lung adenocarcinoma patients with uncommon EGFR mutations treated with 1st generation EGFR-TKIs compared with chemotherapy as first-line therapy. Lung cancer (Amsterdam, Neth. 130, 42–49. doi:10.1016/j.lungcan.2019.02.001
Midha, A., Dearden, S., and McCormack, R. (2015). EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am. J. cancer Res. 5 (9), 2892–2911.
Miller, M., and Hanna, N. (2021). Advances in systemic therapy for non-small cell lung cancer. BMJ Clin. Res. ed) 375, n2363. doi:10.1136/bmj.n2363
Molina, J., Yang, P., Cassivi, S., Schild, S., and Adjei, A. (2008). Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 83 (5), 584–594. doi:10.4065/83.5.584
Nakamura, A., Kenmotsu, H., Sakai, K., Mori, K., Kato, T., Kirita, K., et al. (2022). Final results and biomarker analysis of a randomized phase II study of osimertinib plus bevacizumab versus osimertinib monotherapy for untreated patients with non-squamous non-small cell lung cancer harboring EGFR mutations: WJOG9717L study. Ann. Oncol. 33, S1000–S1. doi:10.1016/j.annonc.2022.07.1110
Piccirillo, M. C., Bonanno, L., Garassino, M. C., Esposito, G., Dazzi, C., Cavanna, L., et al. (2022). Addition of bevacizumab to erlotinib as first-line treatment of patients with EGFR-mutated advanced nonsquamous NSCLC: the BEVERLY multicenter randomized phase 3 trial. J. Thorac. Oncol. 17, 1086–1097. doi:10.1016/j.jtho.2022.05.008
Planchard, D., Jänne, P., Cheng, Y., Yang, J., Yanagitani, N., Kim, S., et al. (2023). Osimertinib with or without chemotherapy in EGFR-mutated advanced NSCLC. N. Engl. J. Med. 389 (21), 1935–1948. doi:10.1056/NEJMoa2306434
Saito, H., Fukuhara, T., Furuya, N., Watanabe, K., Sugawara, S., Iwasawa, S., et al. (2019). Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 20 (5), 625–635. doi:10.1016/S1470-2045(19)30035-X
Schwaederlé, M., Lazar, V., Validire, P., Hansson, J., Lacroix, L., Soria, J., et al. (2015). VEGF-A expression correlates with TP53 mutations in non-small cell lung cancer: implications for antiangiogenesis therapy. Cancer Res. 75 (7), 1187–1190. doi:10.1158/0008-5472.CAN-14-2305
Seto, T., Kato, T., Nishio, M., Goto, K., Atagi, S., Hosomi, Y., et al. (2014). Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. lancet Oncol. 15 (11), 1236–1244. doi:10.1016/S1470-2045(14)70381-X
Socinski, M., Nishio, M., Jotte, R., Cappuzzo, F., Orlandi, F., Stroyakovskiy, D., et al. (2021). IMpower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first-line metastatic nonsquamous NSCLC. J. Thorac. Oncol. official Publ. Int. Assoc. Study Lung Cancer 16 (11), 1909–1924. doi:10.1016/j.jtho.2021.07.009
Soo, R. A., Han, J. Y., Dafni, U., Cho, B. C., Yeo, C. M., Nadal, E., et al. (2021). A randomised phase II study of osimertinib and bevacizumab versus osimertinib alone as second-line targeted treatment in advanced NSCLC with confirmed EGFR and acquired T790M mutations: the European Thoracic Oncology Platform (ETOP 10-16) BOOSTER trial. Ann. Oncol. 33, 181–192. doi:10.1016/j.annonc.2021.11.010
Soria, J., Mauguen, A., Reck, M., Sandler, A., Saijo, N., Johnson, D., et al. (2013). Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann. Oncol. official J. Eur. Soc. Med. Oncol. 24 (1), 20–30.
Stinchcombe, T. E., Jänne, P. A., Wang, X., Bertino, E. M., Weiss, J., Bazhenova, L., et al. (2019). Effect of erlotinib plus bevacizumab vs erlotinib alone on progression-free survival in patients with advanced EGFR-mutant non-small cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 5 (10), 1448–1455. doi:10.1001/jamaoncol.2019.1847
Sugawara, S., Lee, J., Kang, J., Kim, H., Inui, N., Hida, T., et al. (2021). Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann. Oncol. official J. Eur. Soc. Med. Oncol. 32 (9), 1137–1147. doi:10.1016/j.annonc.2021.06.004
Wang, M., Herbst, R., and Boshoff, C. (2021). Toward personalized treatment approaches for non-small-cell lung cancer. Nat. Med. 27 (8), 1345–1356. doi:10.1038/s41591-021-01450-2
Watanabe, H., Ichihara, E., Kayatani, H., Makimoto, G., Ninomiya, K., Nishii, K., et al. (2021). VEGFR2 blockade augments the effects of tyrosine kinase inhibitors by inhibiting angiogenesis and oncogenic signaling in oncogene-driven non-small-cell lung cancers. Cancer Sci. 112 (5), 1853–1864. doi:10.1111/cas.14801
Yamamoto, N., Seto, T., Nishio, M., Goto, K., Yamamoto, N., Okamoto, I., et al. (2021). Erlotinib plus bevacizumab vs erlotinib monotherapy as first-line treatment for advanced EGFR mutation-positive non-squamous non-small-cell lung cancer: survival follow-up results of the randomized JO25567 study. Lung cancer (Amsterdam, Neth. 151, 20–24. doi:10.1016/j.lungcan.2020.11.020
Zhang, K., Wang, L., Wei, A., Jia, X., and Liu, X. (2020). CM082, a novel angiogenesis inhibitor, enhances the antitumor activity of gefitinib on epidermal growth factor receptor mutant non-small cell lung cancer in vitro and in vivo. Thorac. cancer 11 (6), 1566–1577. doi:10.1111/1759-7714.13430
Keywords: EGFR, NSCLC, bevacizumab, EGFR-TKI, combination therapy
Citation: Zheng H, Qin X, Zheng Y, Yang X, Tan J, Cai W, He S and Liao H (2024) Addition of bevacizumab to EGFR tyrosine kinase inhibitors in advanced NSCLC: an updated systematic review and meta-analysis. Front. Pharmacol. 14:1238579. doi: 10.3389/fphar.2023.1238579
Received: 12 June 2023; Accepted: 21 December 2023;
Published: 09 January 2024.
Edited by:
Yusuke Okuma, National Cancer Center Hospital, JapanReviewed by:
Horace C. W. Choi, The University of Hong Kong, Hong Kong SAR, ChinaCopyright © 2024 Zheng, Qin, Zheng, Yang, Tan, Cai, He and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongying Liao, bGlhb2h5MkBtYWlsLnN5c3UuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.