- Department of Rheumatology, The Affiliated Hospital of Qingdao University, Shandong, China
Systemic Lupus Erythematosus (SLE) is a chronic autoimmune systemic disease with a wide range of clinical symptoms, complex development processes, and uncertain prognosis. The clinical treatment of SLE is mainly based on hormones and immunosuppressants. Research on novel therapy strategies for SLE has flourished in recent years, especially the emergence of new targeted drugs and natural products that can modulate related symptoms. This review discusses the current experience including B-cell targeted drugs (belimumab, tabalumab, blisibimod, atacicept, rituximab, ofatumumab, ocrelizumab, obexelimab, and epratuzumab), T-cell targeted drugs (abatacept, dapirolizumab, and inhibitor of syk and CaMKIV), cytokines targeted drugs (anifrolumab and sifalimumab), and natural products (curcumin, oleuropein, punicalagin, sulforaphane, icariin, apigenin, and resveratrol). The aim of this paper is to combine the existing in vitro and in vivo models and clinical research results to summarize the efficacy and mechanism of natural drugs and targeted drugs in SLE for the reference and consideration of researchers.
1 Introduction
SLE is a complex autoimmune inflammatory disease with a diverse course and prognosis that can involve multiple organs throughout the body. It mostly affects women, and its clinical symptoms range from mild to life-threatening. This clinical heterogeneity is most likely caused by intricate immunological dysregulation, like the loss of immunological tolerance to autoantigens and the development of multiple autoantibodies (Bentham et al., 2015). Immune complexes formed by autoantibodies binding to intracellular autoantigens are deposited in the skin, blood vessels, kidneys, and liver. Over time, these immune complexes will cause tissue damage and the emergence of a variety of diseases or manifestations, such as zygomatic rash, arthralgia, fever, renal failure, and cardiovascular disease (Fava and Petri, 2019; Herrada et al., 2019). Lupus nephritis (LN) is the primary clinical symptom of SLE. It may have a negative impact on the life quality and long-term prognosis of SLE patients (Davidson, 2016). Roughly 50% of SLE patients will eventually acquire renal dysfunction (Almaani et al., 2017).
The disease cannot be cured for now, but its progression can be controlled by early diagnosis and medications. Currently, the drugs used to treat SLE include antimalarial therapy, glucocorticoids (GC), non-steroidal anti-inflammatory drugs (NSAIDs), and immunosuppressants including azathioprine (AZA), cyclophosphamide (CYC), tacrolimus (TAC), and mycophenolate mofetil (MMF). However, conventional treatments are accompanied by significant side effects and ideal treatment options are rare. The anti-inflammatory effects of these medicines are accompanied by adverse effects caused by the toxic effects of the drug, including cataracts, osteoporotic fractures, cardiovascular injury, severe infections, malignancies, teratogenicity, and infertility, potentially leading to additional organ damage and mortality (Curtis et al., 2006; Apostolopoulos and Morand, 2017; Ponticelli and Moroni, 2017; Deng et al., 2019; Felten et al., 2019). Therefore, The medical community is eager for safer treatment profiles and more targeted therapies.
Fortunately, an increasing number of studies have focused on exploring new drugs and therapies and found that targeted drugs and natural products offer significant therapeutic promise in the treatment of SLE.
2 Targeted agents
Due to the low specificity and adverse effects of traditional therapeutic agents, there is still some unmet need for more targeted agents with better safety profiles in SLE treatment. Based on recent advances in understanding the complex pathogenesis of SLE, several targeted therapies are presently being evaluated in clinical trials.
2.1 Targeted drugs against B lymphocytes
A wealth of evidence points to B cells as key players in the pathogenesis of SLE. B cells initiate self-reactive T cells, such as antigen-presenting cells, to release pro-inflammatory cytokines and chemokines, promote the generation of autoimmune responses in target organs, and are considered as potential targets for SLE therapy (Table 1) (Sanz and Lee, 2010; Tsokos et al., 2016).
2.1.1 Belimumab
B lymphocyte stimulator (BLyS), often referred to as B-cell-activation factor (BAFF), is a member of the tumor necrosis factor (TNF) cytokine superfamily and is essential for B-cell survival and development (Figure 1) (Blair and Duggan, 2018). SLE is associated with B-cell hyperactivity, autoantibodies, and increased concentrations of BLyS (Zhang et al., 2001).
Belimumab, a fully humanized anti-BAFF monoclonal antibody, has been the first biologic drug to receive a license for the treatment of SLE to date. This medication binds to BAFF and prevents soluble BAFF from attaching to B cell receptors, thereby preventing B cell survival, differentiation, and activation (Blair and Duggan, 2018). Belimumab was formally approved for the treatment of SLE through a phase III randomized controlled trial (RCT) in 2011. And its efficacy is demonstrated by four large double-blinded phase Ⅲ RCT (Furie et al., 2011; Navarra et al., 2011; Stohl et al., 2017; Zhang et al., 2018). In the RCT, this medicine was shown to be effective in reducing SLE disease activity and severe flares and delaying the onset of lupus in addition to standard therapy (Furie et al., 2011; Navarra et al., 2011). And according to the findings of a prospective cohort trial, belimumab may have clinical benefits for people with acute and subacute cutaneous lupus erythematosus by decreasing the frequency of flares and slowing the progression of skin lesions in patients with active SLE (Iaccarino et al., 2017). In a 104-week trial conducted at 107 sites in 21 countries, the probability of kidney-related events, morbidity, and mortality was all lower in the trial group compared to the control group, which demonstrates the utility of belimumab with standard therapy (Furie et al., 2020). The effectiveness, safety, and pharmacokinetics of intravenous belimumab were assessed in a Phase-2, randomized, placebo-controlled, double-blind research for children with systemic lupus erythematosus (cSLE). The results show that belimumab was well tolerated by paediatric patients, and the pharmacokinetics, pharmacodynamics and safety profiles were similar to those of adults with SLE (Brunner et al., 2020). A 28-week open-label study found that increases occurred in the proportions of patients achieving primary efficacy renal response and complete renal response from open-label baseline to week 28. Additionally, no new safety signals were identified, and efficacy was generally maintained throughout the open-label phase (Furie et al., 2022).
2.1.2 Tabalumab
Similar to belimumab, tabalumab is a fully human IgG4 monoclonal antibody against soluble and membrane BAFF. Two phase Ⅲ randomized, multicenter, double-blinded, placebo-controlled trials (ILLUMINATE-1 and ILLUMINATE-2) were taken to assess efficacy and safety of subcutaneous tabalumab in patients with SLE. The results of the study demonstrated that the key clinical efficacy endpoints of ILLUMINATE-1 failed to achieve statistical significance, and the safety profile of tabalumab was similar to placebo (Isenberg et al., 2016). However, ILLUMINATE-2 met the primary endpoint of SLE response index 5 in the cohort receiving tabalumab 120 mg every 2 weeks (Merrill et al., 2016).
Although both studies noted that there were no serious safety signals, LUMMINATE-2 revealed that more patients (8.5%) receiving tabalumab than placebo reported depression and 3 patients in the tabalumab cohort attempted suicide while on treatment (Merrill et al., 2016). Although the results of the two phase Ⅲ trials of tabalumab were unsatisfactory, they did not indicate little or no efficacy. That may be a result of tabalumab’s researchers skipping a phase Ⅱ clinical trial and going directly to a phase Ⅲ trial.
2.1.3 Blisibimod
Blisibimod is a potent and selective inhibitor of BAFF and has a unique tetravalent, ‘peptibody’ structure features. The results of randomized, double-blind phase 1a and phase 1b trials demonstrated that the safety and tolerability profile of blisibimod in SLE was comparable with that of placebo (Stohl et al., 2015). Subsequently, a phase 2 RCT in patients with moderate-to-severe SLE was taken. In this RCT, SLE responder index 5 response rates were not significantly improved in the pooled blisibimod groups compared with placebo; however, reductions in proteinuria, changes in anti-double standard DNA antibody, complement C3 and C4, and reductions in B cells were observed significantly with blisibimod (Furie et al., 2015). Therefore, the phase Ⅲ RCT of blisibimod enrolled patients with high disease activity. Although the SLE responder index 6 was not met in the study, blisibimod was well-tolerated and was associated with steroid reduction, decreased proteinuria and biomarker responses (Merrill et al., 2018a).
2.1.4 Atacicept
Atacicept, a fusion protein of the transmembrane activator calcium moderator and cyclophilin lingand interactor (TACI) receptor and IgG, inhibit both BAFF and a proliferation-inducing ligand (APRIL). APRIL is another cytokine that has been identified as an important B-cell regulator. BAFF and APRIL levels are increased in patients with SLE, which indicates that dual blockade by atacicept may be more effective than blockading BAFF alone (Bag-Ozbek and Hui-Yuen, 2021).
A phase Ⅱ b RCT of 24-week, multicenter, randomized, double-blind, placebo-controlled, parallelism was taken to evaluate the safety and efficacy of atacicept in patients with SLE. The results of the study showed that there was a trend toward an improved SLE responder index 4 response rate with atacicept 75 mg and 150 mg as compared with placebo. Additionally, the risk of serious adverse events and serious or severe infection was not increased with atacicept as compared with placebo (Merrill et al., 2018b). The post hoc analyses of phase Ⅱ b RCT demonstrated that low disease activity (LDA) and lupus low disease activity state (LLDAS) were attainable at week 24 in the patients with high disease activity (HDA) receiving atacicept 150 mg compared to placebo (Morand et al., 2020b). Additionally, patients on continuous 150 mg atacicept had a reduced risk of first severe flare and a longer time to first severe flare. The study has drawn the conclusion that long-term treatment with atacicept 150 mg in SLE patients had an acceptable safety profile and durable efficacy (Wallace et al., 2021).
2.1.5 Rituximab
Rituximab (RTX) is a chimeric monoclonal antibody that targets the B-cell CD20 (Figure 1) (Durcan et al., 2019). CD20 is a cell-surface antigen expressed on most B cells, except plasma cells, the majority of plasmablasts, and lymphoid stem cells (Gelfand et al., 2017; Payandeh et al., 2019). RTX was initially developed and approved by the Food and Drug Administration (FDA) for the treatment of non-Hodgkin’s lymphoma and has successfully entered rheumatology with benefits in the treatment of rheumatoid arthritis (RA) and antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (Stone et al., 2010; van Vollenhoven et al., 2015). RTX was first used in SLE in 2002, and 5/6 patients with refractory lupus erythematosus had a clinical response to the combination of RTX, CYC, and high-dose corticosteroids (Leandro et al., 2002). Subsequently, RTX is successful in treating refractory SLE symptoms such as nephritis and neuropsychiatric illness (Gottenberg et al., 2005; Leandro et al., 2005; Roccatello et al., 2011).
For the treatment of SLE, the combination of RTX and belimumab has undergone substantial research and appears to be a valuable potion for several clinical situations. Although RTX has a greater capacity to deplete B cells compared to belimumab, RTX’s SLE RCT has not achieved its primary clinical endpoint (Wise and Stohl, 2020). A randomized controlled trial was taken to evaluate the effectiveness of belimumab after RTX in SLE patients. In this study, patients were treated with RTX and then 4–8 weeks later were randomized (1:1) to receive belimumab or placebo for 52 weeks. The result of this study showed that belimumab after rituximab significantly reduced serum IgG anti-dsDNA antibody levels and reduced the risk of severe flare in SLE patients who are refractory to conventional therapy at 52 weeks (Shipa et al., 2021). The efficacy of the combination of RTX and belimumab is theoretically plausible but requires more clinical studies and case reports to confirm it.
2.1.6 Ofatumumab
The main side event of RTX is allergic response because it is a chimeric anti-CD20 antibody. Therefore, other anti-CD20 antibodies, like ofatumumab, are investigated in SLE. Ofatumumab is a fully humanized anti-CD20 monoclonal antibody and is a safe, well-tolerated and effective alternative for B cell depletion (Cinar et al., 2021). Ofatumumab has recently received permission for treatment in individuals with chronic lymphocytic leukemia (Sandhu and Mulligan, 2015) and multiple sclerosis (Hauser et al., 2020). In a single-center retrospective case series of 16 patients treated with ofatumumab, 14/16 patients were well-tolerated. In this study, B cell depletion was achieved in 12 patients and was associated with improvement in serological markers of disease activity including ANA, anti-dsDNA antibody, and complement levels. Additionally, half of the patients with LN achieved renal remission by 6 months (Masoud et al., 2018). In an endogamous Pakistani kindred with monogenic SLE, 2/3 of the siblings had severe infusion-related reactions to RTX. Therefore, ofatumumab has been used and resulted in marked clinical improvement in both patients (Lei et al., 2018). A study of single-center retrospective case series for juvenile SLE found that significant clinical improvement was observed in all cases after using ofatumumab, mirrored by improved laboratory markers of disease activity including anti-dsDNA antibody, complement levels, and proteinuria (Cinar et al., 2021).
2.1.7 Ocrelizumab
Ocrelizumab is another fully humanized anti-CD20 antibody. The CD20 epitopes binding ocrelizumab and RTX are overlapping. The result of a phase Ⅲ study showed that overall renal response rates with ocrelizumab were numerically superior to those with placebo. However, ocrelizumab in SLE patients with class III/IV LN was terminated prematurely due to a high rate of serious infections in the cohort treated with ocrelizumab (Mysler et al., 2013). Anyhow, initial results suggested ocrelizumab may have some efficacy in treating LN. And ocrelizumab was shown to have great efficacy in treating multiple sclerosis in several phase Ⅲ trials (Lamb, 2022).
2.1.8 Obexelimab
CD19 is a transmembrane protein on the surface of B lymphocytes and is an important co-receptor for B cell antigen receptor (BCR) signaling. It is closely related to the activation, signal transduction, and growth of B cells. CD19 co-linked with BCR synergistically enhances calcium release, mitogen-activated protein kinase activity and cell proliferation (Li et al., 2017). CD19 has been widely used in the diagnosis and prognosis of leukemia, lymphoma, and immune system diseases, and is an important target for immunotherapy. Fcγ receptor (FcγR) IIB is a low-affinity IgG receptor expressed on the surface of B cells, which, when combined with the Fc segment of IgG, crosslinks with the BCR, raises the threshold of B cell activation, reduces antibody production, and thus plays a negative feedback regulatory function in humoral immunity (Espéli et al., 2016).
XmAb5871 (also known as obexelimab), a monoclonal antibody targeting CD19 and FcγRIIb, was found to inhibit calcium transport, proliferation, and co-stimulatory molecule expression in B cells in SLE patients and healthy volunteers, thereby suppressing humoral immunity and reducing IgM, IgG and IgE levels (Horton et al., 2011). The result of a phase Ⅱ study showed that XmAb5871 was well-tolerated, the infection rate was low, and flares were similar to other trials, which supports further evaluation of XmAb5871 in SLE (Merrill et al., 2018c).
2.1.9 Epratuzumab
CD22 is expressed in mature B cells and modulates activation and migration of B cells by acting as an inhibitory co-receptor of the B-cell receptor. Epratuzumab is a humanized antibody binding to the glycoprotein CD22 of the cell surface of mature B cells (Clowse et al., 2017).
Initially, two phase Ⅲ study (ALLEVIATE-1 and -2) was taken to evaluate the efficacy and safety of epratuzumab in patients with moderate/severe flaring SLE. However, both initial ALLEVIATE studies were discontinued prematurely because of the drug supply (Wallace et al., 2013). Subsequently, phase Ⅲ study (EMBLEM) with 227 patients showed that proportion of responders was higher in all epratuzumab groups than with placebo (Wallace et al., 2014). An open-label extension study indicated that open-label epratuzumab treatment was well tolerated for up to 3.2 years, and associated with sustained improvements in disease activity along with a reduction of corticosteroid dose (Wallace et al., 2016). This result has been confirmed in several other studies (Strand et al., 2014; Tsuru et al., 2016). On the contrary, results from two phase Ⅲ trials showed that patients treated with epratuzumab + standard therapy did not result improvements in response rates over that observed in the placebo + standard therapy group (Clowse et al., 2017).
2.2 Targeted drugs against T lymphocytes
Studies have shown that the production of anti-DNA antibodies and other pathogenic autoantibodies in SLE patients or mice is T-cell dependent (Comte et al., 2015; Shan et al., 2020). SLE has multiple T-cell abnormalities, and the activation of auto-reactive T lymphocytes and the resulting autoimmune responses are central to the occurrence of the disease (Yasutomo, 2003).
2.2.1 Abatacept
Abatacept is a fusion protein composed of cytotoxic T-associated protein 4 (CTLA-4) (a leukocyte differentiation antigen) and the Fc region of IgG1 (an immunoglobulin fused to extracellular structures). This medicine could regulate T cell costimulatory molecules by interrupting the interaction between CD80/CD86 and CD28 (Figure 1) (Pimentel-Quiroz et al., 2016). Two signals are required for T cells to activate and attack antigens to produce an immune response. For the first signal, antigen-presenting cells (APCs) bind to major histocompatibility complex (MHC) molecules and be presentd to T cell receptors on the T cell surface; for the second signal, APCs present B7 protein on their cell surface to CD28 protein on the T cell surface. Abatacept binds to B7-1 and B7-2 and blocks the signaling 2 pathway, preventing T cells from being activated.
However, a study showed that the phase II b clinical study of abatacept failed to meet the primary/secondary endpoints measured by the British Isles Lupus Assessment Group (BILAG) (Merrill et al., 2010a). In another clinical trial, a total of 298 patients were treated for 52 weeks to compare the efficacy and safety of intravenous (IV) abatacept. The result showed that although the primary endpoint was not met, abatacept showed evidence of biological activity (greater improvements in anti-double-stranded DNA antibody, C3, and C4 levels and a 20%–30% greater reduction in mean urinary protein-to-creatinine ratio compared with placebo) and was well tolerated in patients with active class III or IV lupus nephritis (Furie et al., 2014).
These unsatisfactory results may be related to a flawed study design that requires continuous optimization at a later stage. The safety profile of abatacept in patients with SLE suggests that abatacept may be considered as an alternative in refractory cases.
2.2.2 anti-CD40 antibody
Interaction between the CD40 ligand (CD40L, CD154; mainly expressed on activated T cells and platelets) and the CD40 receptor (expressed on a variety of cells, including antigen-presenting cells and B cells) is essential for the activation of the adaptive immune system and drive the pathological process in SLE, including B cell differentiation and proliferation (Furie et al., 2021).
In human SLE, CD40L has long been a desirable therapeutic target. Dapirolizumab is a polyethylene glycol-conjugated Fab’ fragment that targets CD40 ligand (Narain et al., 2020). Since then, two phase Ⅰ clinical studies have investigated dapirolizumab. The first study showed predictable pharmacokinetics in both healthy individuals and patients with SLE and was well tolerated, with no safety signals of concern (Tocoian et al., 2015). The second study showed that multiple doses of dapirolizumab were well-tolerated, and there were no thromboembolic events during the study (Chamberlain et al., 2017). A phase Ⅱ study was taken to investigate the dose response, efficacy, and safety of dapirolizumab. The result of the study showed that although the primary objective was not met, dapirolizumab appeared to be well tolerated, and patients exhibited improvements across multiple clinical and immunological measures of disease activity after 24 weeks relative to placebo (Furie et al., 2021). Further research is necessary to determine the clinical benefit of dapirolizumab.
2.2.3 Inhibition of syk and CaMKIV
Spleen tyrosine kinase (Syk) and calcium/calmodulin kinase IV (CaMKIV), small molecule inhibitors of kinases, are aberrantly expressed in immune cells of SLE patients and may present novel treatment prospects.
Syk is implicated in membrane-mediated signal transduction in a variety of cells and overexpressed in T cells of patients with SLE (Krishnan et al., 2008; Grammatikos et al., 2013). In the MRL/lpr mouse model, the Syk inhibitor, R788, avoided the onset of skin disease and dramatically reduced established skin disease, with clinical effects lasting at least 1 month after discontinuation of the drug. Syk inhibitor also decreased the growth of spleen and lymph nodes and inhibited kidney disease progression (Deng et al., 2010).
When the combination of T cell receptor and CD3 is activated, CaMKIV, which is expressed at high levels in SLE T cells, translocates to the nucleus and results in aberrant T cell function. A study using the CaMKIV inhibitor KN-93 to treat MRL/lpr mice found that CaMKIV inhibitor in MRL/lpr mice significantly suppressed nephritis and dermatosis, reduced the expression of co-stimulatory molecules CD86 and CD80 on B cells, and prevented the generation of inhibition of interferon (IFN)-γ and TNF-α. In human SLE T cells, the silence of CaMKIV results in the inhibition of IFN-γ production, which confirms the rationality of developing small molecule CaMKIV inhibitors for the treatment of SLE patients (Ichinose et al., 2011).
2.3 Targeted drugs against cytokines
Patients with SLE have significantly elevated genetic markers of type I IFN, a cytokine involved in the inflammatory response. Type 1 IFN has long been implicated in the pathogenesis of lupus (Rönnblom et al., 2011). IFN-α is associated with SLE and its disease activity (Nikpour et al., 2008; Bauer et al., 2009), which can promote the development of various immune cells, lead to the expression of BAFF, upregulation of T cells, and inactivation of T regulatory cells.
2.3.1 Anifrolumab
Anifrolumab, a monoclonal antibody that targets the type I IFN receptor, successfully treated individuals with SLE in a phase 3 randomized trial in 2020 (Morand et al., 2020a). The results showed that adverse events in patients in the anifrolumab group included respiratory infections, nasopharyngitis, and infusion-related reactions. The number of adverse outcomes was lower than that in the placebo group, as well as better GC tapering and severity of skin disease than with the placebo (Morand et al., 2020a). A phase Ⅱ study was taken to assess the efficacy and safety of anifrolumab. Although the result showed that the primary endpoint was not met, anifrolumab intensified regimen was associated with numerical improvements over the placebo acrossing endpoint, including complete renal response (CRR), urine protein-creatinine ratio, CRR with inactive urinary sediment, and sustained glucocorticoid reductions (Jayne et al., 2022). Still, more studies are required to confirm the effectiveness and safety of this drug.
2.3.2 Sifalimumab
Sifalimumab is a human-derived anti-IFN-α antibody. A recent phase II b clinical trial demonstrated that Sifalimumab has an inhibitory effect on IFN gene expression in patients with moderately to severely active SLE, resulting in remission of disease activity and joint injury (Takeuchi et al., 2020). Therefore, it is important to further explore the role of the type I IFN signaling pathway in autoimmune diseases to develop more effective and safe therapies approaches.
Cytokines play a role in the pathogenesis of SLE, for example, IL-4, IL-5, IL-6, IL-10, and IL-15 are increased in the serum of SLE patients. The pathogenesis of SLE is still unclear, and multi-level analysis of relevant cytokines is beneficial to provide a basis for new target therapy for SLE in the future.
3 Natural products
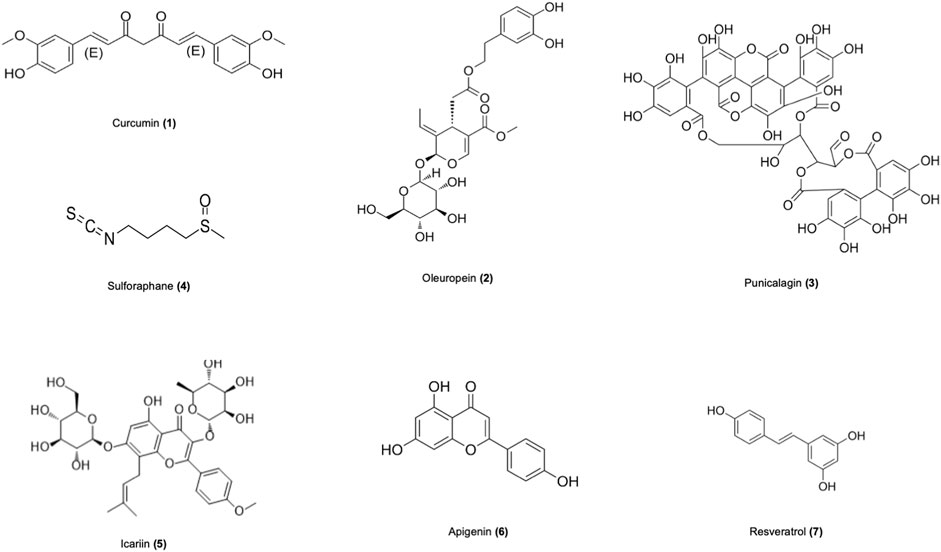
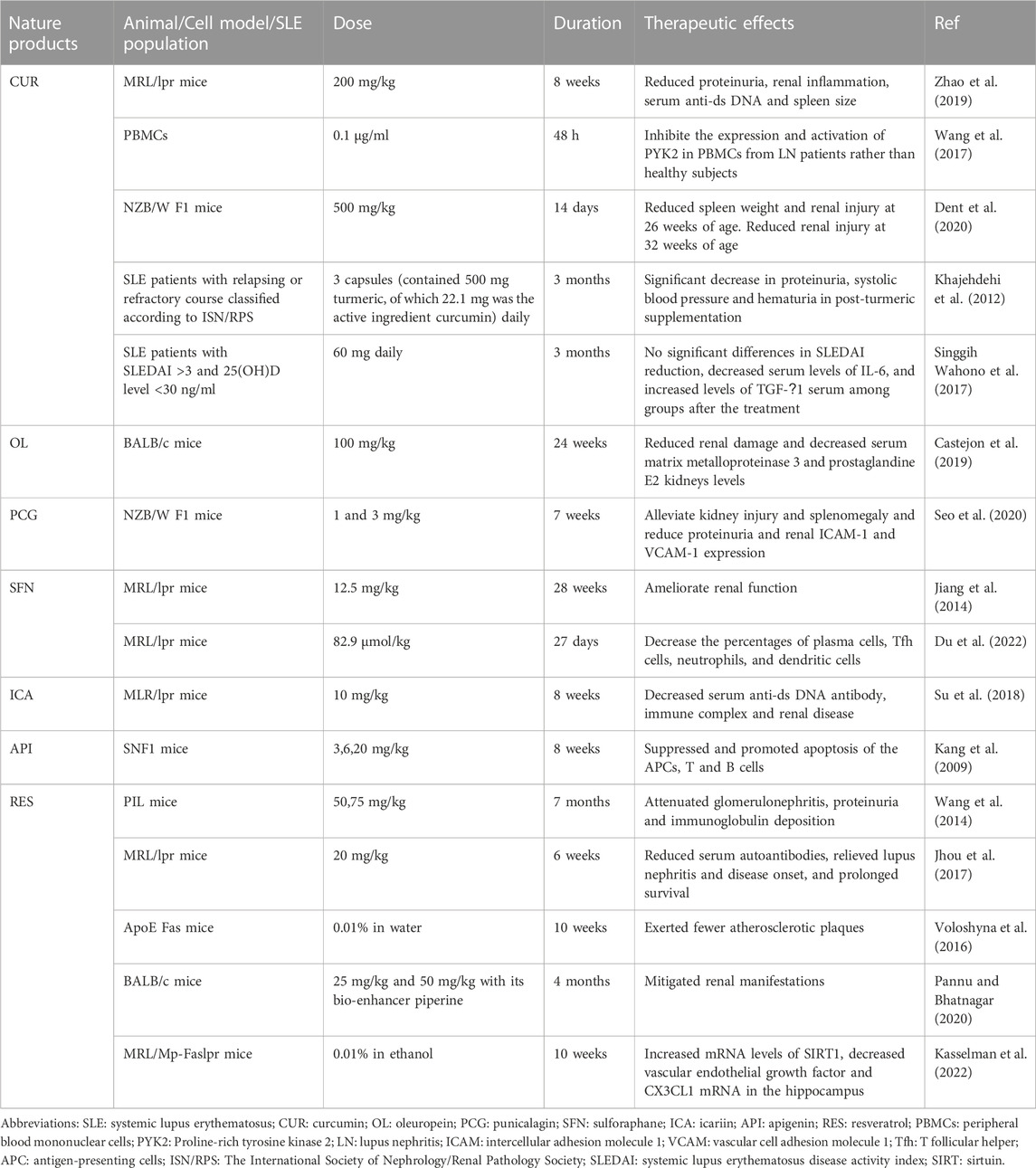
Numerous studies in recent years have revealed that natural products derived from animals and plants are effective in healing human diseases. Nature products showed promising further therapeutic effects in vitro and in vivo models of SLE through various mechanisms (Figure 2), as shown in Table 2. The mechanisms of nature products in the treatment of SLE are diverse and complex, which urgently need to be summarized and analyzed.
3.1 Curcumin
Ginger is thought to have anti-inflammatory and antioxidant effects, and the health-promoting properties of ginger have been attributed to its rich phenolic phytochemicals, such as curcumin (CUR) (Jolad et al., 2004; Grzanna et al., 2005; Jolad et al., 2005). CUR, a phenolic component extracted from turmeric, belongs to the ginger family and has been shown to have immunomodulatory and anti-inflammatory effects in various diseases (Chamani et al., 2022). According to animal reseatch, CUR has the potential to cure a wide range of inflammatory diseases (Aggarwal and Harikumar, 2009).
LN is one of the most severe SLE side effects and a significant contributor to morbidity and mortality in SLE patients (Anders et al., 2020). Although immunotherapy considerably improves the prognosis of patients with SLE and nephropathy, several patients with LN eventually get end-stage renal disease (Faurschou et al., 2010). In a mouse model prone to lupus erythematosus, CUR-treated MLR/lpr mice displayed a significant decrease in proteinuria and renal inflammatory response, lower serum anti-ds DNA levels, and smaller spleens, as well as downregulation of nucleotide-binding domain (NOD) like receptor protein 3 (NLRP3) inflammasome (Zhao et al., 2019). Proline-rich tyrosine kinase 2 (PYK2) is a non-receptor protein tyrosine kinase belonging to the superfamily of adhesion kinases. It plays a significant role in the development of autoimmune disorders and delivers critical signals during lymphocyte activation (Sasaki et al., 1995). According to in vitro research, CUR can inhibit the proliferation of peripheral blood mononuclear cells in LN patients by suppressing the expression and activation of PYK2. Additionally, CUR inhibition showed a negative correlation with complement levels in the serum and positive correlation with proteinuria levels measured after 24 h, without affecting normal subjects (Wang et al., 2017). A RCT clinical study used CUR 60 mg combined with cholecalciferol as the treatment regimen for the trial group. The result of the study found that decreased levels of serum IL-6 have a positive correlation with Safety of Estrogens in Lupus Erythematosus National Assessment–Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) reduction, and there were no significant differences in SLEDAI reduction, decreased serum level of IL-6, and increased levels of TGF-β1 serum among group after treatment (Singgih Wahono et al., 2017). A study in an experimental model of SLE found that CUR-treated SLE mice had lower spleen weight and renal injury compared to vehicle-treated SLE mice when treatment started at 26 weeks of age. When CUR treatment started at 32 weeks of age, renal injury was reduced in SLE mice (Dent et al., 2020).
Although CUR has potential therapeutic value for SLE, its poor bioavailability limits its therapeutic effect due to poor absorption, rapid metabolism, and rapid systemic elimination (Anand et al., 2007). One study found that reconstituting CUR with a non-curcumin-like component of turmeric can greatly improve the bioavailability of turmeric (Antony et al., 2008). An RCT study was taken to evaluate the effect of turmeric and the result showed that proteinuria, systolic blood pressure and hematuria decreased significantly in SLE patients after received capsules that contained turmeric for 3 months (Khajehdehi et al., 2012).
3.2 Oleuropein
Oleuropein (OL) is key to the main anti-inflammatory action of olive leaf extract and has a pharmacologically important antioxidant, anti-inflammatory, and immunomodulatory properties (Ryu et al., 2015; Qabaha et al., 2018). Peracetylated oleuropein (Per-OL) are acyl derivatives of OL and have the potential to protect membrane components due to their lipophilic nature, which enables them to cross cytoplasmic cell membranes and be taken up by cells. This results in better absorption through intestinal epithelial cell monolayers than OL (Stamatopoulos et al., 2013).
OL and Per-OL can significantly reduce renal injury and serum matrix metalloproteinase-3 and prostaglandin E2 levels. Studies have shown that in mouse models of SLE, the expression of nuclear factor E2-related factor 2 (Nrf2) and heme oxygenase (HO-1) antioxidant protein was upregulated in mice fed with OL and Per-OL diets. However, there was a significant improvement in the stimulation of Janus kinase/signal transducer and activator of transcription (JAK/STAT), mitogen-activated protein kinases (MAPK), nuclear transcription factor-kappa B (NF-κB) and inflammasome nucleotide-binding domain, leucine-rich repeats-containing family, pyrin domain-containing-3 inflammatory vesicle pathway (Castejon et al., 2019). STAT3 signaling pathway is essential for the development of the Th17, and STAT3/IL-17 expression was upregulated in SLE patients (Chen et al., 2019). In peritoneal macrophages of SLE mice, it was discovered that OL and Per-OL diets decreased T helper (Th)1, Th2, and Th17 cytokines, as well as the protein expressions of p-STAT3, COX-2, and inducible nitric oxide synthase (iNOS). At the same time, NF-κB activation was inhibited (Castejón et al., 2020).
3.3 Punicalagin
Punicalagin (PCG), a major polyphenol-rich in pomegranate, has antioxidant, anti-inflammatory, and immunosuppressive effects (Venusova et al., 2021).
Studies have shown that PCG can effectively inhibit protease-activated receptor-2 (PAR2), which is abundantly expressed in human tissues, particularly in renal cells, such as podocytes, thylakoid cells, tubular epithelial cells and infiltrating immune cells (Vesey et al., 2007; D'Andrea et al., 1998). In the NZB/W F1 mouse model and human podocyte cell lines, PCG significantly reduced PAR2-mediated activation of extracellular signal-regulated kinases 1 and 2, NF-κB signaling pathways, intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1) as well as expression of IL-8, IFN-γ and TNF-α. Meanwhile, injection via PCG dramatically reduced renal injury and splenomegaly, decreased proteinuria, and decreased the expression of renal ICAM-1 and VCAM-1 expression in NZB/W F1 mice (Seo et al., 2020).
3.4 Sulforaphane
Sulforaphane (SFN), found in cruciferous plants, is an activator of NRF2 and has several biological properties that may lower the risk of developing chronic diseases, such as anti-inflammatory, antioxidant, and anti-tumor activities (Raiola et al., 2017).
SFN is an NRF2 agonist with positive effects in ameliorating oxidative stress. Previous research suggests that Nrf2 deficiency increases the development of LN, revealing a preventative or protective role for SFN. It found that Nrf2−/− mice spontaneously develop lupus-like autoimmune nephritis at 60 weeks (Yoh et al., 2001). Experimental data have demonstrated the significance of anti-oxidative stress and the NF-kB pathway in lupus in a mouse model of LN (Jiang et al., 2014). SFN can act as an NRF2 activator to effectively ameliorate oxidative stress and inflammatory disease and suppress LN by inhibiting oxidative stress and NF-κB signaling pathway (Jiang et al., 2014).
Another study on the disease found that SFN improved the autoimmune response in the kidney of lupus-prone MRL/lpr mice. Th1 and Th17 cells expressed more and lived longer after receiving SFN therapy. The proportion of plasma cells, T follicular helper (Tfh) cells, neutrophils, dendritic cells, and quantity of malondialdehyde all decreased. Further studies revealed that the antioxidant activity of SFN was dependent on the activation of the peroxiredoxin 1 (PRDX 1) gene, which was increased in SFN-treated MRL/lpr mice (Du et al., 2022). The above studies also suggest that antioxidant therapy may be a potential approach to treating SLE.
3.5 Icariin
Icariin (ICA) is recognized as a traditional herb in eastern Asia, which benefits osteoporosis, chronic nephritis, asthma, hepatitis, and cardiovascular disease. ICA is the most abundant flavonol glycoside in Epimedium. It has a range of therapeutic effects, including anti-inflammatory, antioxidant, anti-cancer, and anti-aging properties (Li et al., 2015).
NF-κB is a crucial regulator of the production of several proteins linked to inflammation (Li and Verma, 2002). The severity of LN is directly correlated with NF-B activation, which is crucial for LN pathogenesis (Li et al., 2015). It was discovered that ICA treatment dramatically decreased serum urea nitrogen and creatine levels in MRL/lpr mice, and improved renal pathologies such as glomerular hyperplasia, glomerulosclerosis, and periglomerular inflammation. Further experiments revealed that ICA inhibited renal NF-κb activation, NLRP3 inflammatory vesicle activation, and TNF-α expression in MRL/lpr mice (Su et al., 2018). As a diagnostic biomarker for active LN, C-C motif chemokine ligand (CCL)2, also known as monocyte chemoattractant protein 1 (MCP-1), is employed (Gupta et al., 2016). CCL2 levels were significantly elevated in MRL/lpr mice, while CCL2 overexpression was reduced by ICA therapy (Su et al., 2018). These results suggest a protective effect of ICA on SLE.
3.6 Apigenin
Flavonoid subclass Apigenin (API) is chemically known as 4′,5,7 trihydroxyflavone. Flavonoids have various biological benefits include free radical scavenging, antioxidant, anti-inflammatory, and anti-cancer. Based on in vivo, and clinical trial studies, API is a potential therapeutic agent. This compound has the ability to reduce PG synthesis, nitric oxide generation, and the activity of numerous enzymes that control cell growth (Kasiri et al., 2018).
20 mg/kg of apigenin was daily administered into SNF1 mice with lupus-like lesions to watch the progression of severe nephritis. The results showed that API inhibited the response of Th1, Th17 cells, and B cells to major lupus autoantigens by suppressing autoantigen presentation and the value-added function of APCs. Additionally, API induced apoptosis in lupus T cells, B cells, and APCs via downregulating COX-2 expression (Kang et al., 2009).
3.7 Resveratrol
Resveratrol (RES) is a molecule known chemically as 35,4-trihydroxystilbene. It is a natural plant antitoxin that can be synthesized from various plants, including grapes, wine, soy, nuts, and chocolate, and is produced in pathogenic and stressful environments (Oliveira et al., 2017). The molecule can be distributed into tissues by reversible interaction with serum albumin by its cis-trans isomeric (Tang et al., 2017).
Treatment of pristane-induced lupus mice with different doses of RES has been shown to reduce proteinuria, renal immunoglobulin deposition, glomerulonephritis, and serum IgG1 and IgG2a levels. Additionally, CD4+ T cell proliferation and CD4+ T cell death were both decreased by RES, as well as the expression of CD69 and CD71 in CD4+ T cells. It caused CD4+ T cells to undergo apoptosis. CD4 IFN γ+ Th1 cells and Th1/Th2 cell ratio were decreased. In in vitro, antibody production and B-cell proliferation were also inhibited. The treatment of pristane-induced lupus mice with RES may inhibit CD4+ T cells by triggering the silent mating type information regulator 2 homolog 1 (SIRT1) (Wang et al., 2014). Further studies revealed that RES increased the expression of FcγRIIB in B cells of MRL/lpr mice, which led to a considerable decrease of plasma cells in the spleen and bone marrow, thereby reducing serum autoantibody titers. This may be due to the upregulation of FcγRIIB by affecting the increase of SIRT1 protein and p65 NF-κB deacetylation (Jhou et al., 2017). In the ApoE−/−Fas−/− double knockout mouse model, RES attenuated atherosclerosis in the mouse model, with the RES-treated group generating fewer atherosclerotic plaques than the untreated group. Also, the data showed that RES inhibited cholesterol efflux in macrophages (Voloshyna et al., 2016). A study was taken to evaluate the combinatorial effect of RES and piperine on the murine model. Renal manifestations (proteinuria and decreased creatinine in urine) were successfully mitigated by the combination of RES and piperine (Pannu and Bhatnagar, 2020). In a RES-treated atherosclerosis-prone lupus mouse model, RES tends to increase mRNA levels of sirtuin 1, and decrease vascular endothelial growth factor and CX3CL1 (neurotactin ligand) mRNA in the hippocampus. The study demonstrated that RES could potentially be a therapeutic candidate in the modulation of cognitive dysfunction in neuropsychiatric lupus, especially motor incoordination. (Kasselman et al., 2022).
RES also has some limitations. Its metabolism in in vivo is rapid and its bioavailability is limited (Walle, 2011; Pujara et al., 2017). It also shows some mild toxic effects at high doses of treatment, such as headache, lower extremity myalgia, drowsiness, blood electrolyte changes, and rash (Cottart et al., 2010). Therefore, its bioavailability and toxicity should be taken into account when considering its possible therapeutic effects.
4 Conclusion
In this review, we enumerate the great potential of antibody drugs and natural medicines in the treatment of SLE. Despite the great therapeutic value of targeted and natural drugs, there are still some limitations in the treatment of autoimmune diseases. Therefore, every effort should be made to develop safer and more effective therapies, develop treatment regimens that rapidly control lupus activity, prevent future relapses, and prevent additional short-term and long-term complications.
Author contributions
YH: writing–original draft, data charting, and data collation. LL: data collation. BZ: data collation. RL: data collation. XZ: data collation. BL: revising-orginal draft. All authors contributed to the article and approved the submitted version.
Funding
This work is supported by the National Natural Science Foundation of China (81671600); the Natural Science Foundation of Shandong Province, China (ZR2016HM13).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aggarwal, B. B., and Harikumar, K. B. (2009). Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell. Biol. 41, 40–59. doi:10.1016/j.biocel.2008.06.010
Almaani, S., Meara, A., and Rovin, B. H. (2017). Update on lupus nephritis. Clin. J. Am. Soc. Nephrol. 12, 825–835. doi:10.2215/cjn.05780616
Anand, P., Kunnumakkara, A. B., Newman, R. A., and Aggarwal, B. B. (2007). Bioavailability of curcumin: Problems and promises. Mol. Pharm. 4, 807–818. doi:10.1021/mp700113r
Anders, H. J., Saxena, R., Zhao, M. H., Parodis, I., Salmon, J. E., and Mohan, C. (2020). Lupus nephritis. Nat. Rev. Dis. Prim. 6, 7. doi:10.1038/s41572-019-0141-9
Antony, B., Merina, B., Iyer, V. S., Judy, N., Lennertz, K., and Joyal, S. (2008). A pilot cross-over study to evaluate human oral bioavailability of BCM-95cg (biocurcumax), A novel bioenhanced preparation of curcumin. Indian J. Pharm. Sci. 70, 445–449. doi:10.4103/0250-474x.44591
Apostolopoulos, D., and Morand, E. F. (2017). It hasn't gone away: The problem of glucocorticoid use in lupus remains. Rheumatol. Oxf. 56, i114–i122. doi:10.1093/rheumatology/kew406
Bag-Ozbek, A., and Hui-Yuen, J. S. (2021). Emerging B-cell therapies in systemic lupus erythematosus. Ther. Clin. Risk Manag. 17, 39–54. doi:10.2147/tcrm.S252592
Bauer, J. W., Petri, M., Batliwalla, F. M., Koeuth, T., Wilson, J., Slattery, C., et al. (2009). Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: A validation study. Arthritis Rheum. 60, 3098–3107. doi:10.1002/art.24803
Bentham, J., Morris, D. L., Graham, D. S. C., Pinder, C. L., Tombleson, P., Behrens, T. W., et al. (2015). Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat. Genet. 47, 1457–1464. doi:10.1038/ng.3434
Blair, H. A., and Duggan, S. T. (2018). Belimumab: A review in systemic lupus erythematosus. Drugs 78, 355–366. doi:10.1007/s40265-018-0872-z
Brunner, H. I., Abud-Mendoza, C., Viola, D. O., Calvo Penades, I., Levy, D., Anton, J., et al. (2020). Safety and efficacy of intravenous belimumab in children with systemic lupus erythematosus: Results from a randomised, placebo-controlled trial. Ann. Rheum. Dis. 79, 1340–1348. doi:10.1136/annrheumdis-2020-217101
Castejón, M. L., Montoya, T., Alarcón-De-La-Lastra, C., González-Benjumea, A., Vázquez-Román, M. V., and Sánchez-Hidalgo, M. (2020). Dietary oleuropein and its acyl derivative ameliorate inflammatory response in peritoneal macrophages from pristane-induced SLE mice via canonical and noncanonical NLRP3 inflammasomes pathway. Food Funct. 11, 6622–6631. doi:10.1039/d0fo00235f
Castejon, M. L., Sánchez-Hidalgo, M., Aparicio-Soto, M., Montoya, T., Martín-Lacave, I., Fernández-Bolaños, J. G., et al. (2019). Dietary oleuropein and its new acyl-derivate attenuate murine lupus nephritis through HO-1/Nrf2 activation and suppressing JAK/STAT, NF-κB, MAPK and NLRP3 inflammasome signaling pathways. J. Nutr. Biochem. 74, 108229. doi:10.1016/j.jnutbio.2019.108229
Chamani, S., Moossavi, M., Naghizadeh, A., Abbasifard, M., Majeed, M., Johnston, T. P., et al. (2022). Immunomodulatory effects of curcumin in systemic autoimmune diseases. Phytother. Res. 36, 1616–1632. doi:10.1002/ptr.7417
Chamberlain, C., Colman, P. J., Ranger, A. M., Burkly, L. C., Johnston, G. I., Otoul, C., et al. (2017). Repeated administration of dapirolizumab pegol in a randomised phase I study is well tolerated and accompanied by improvements in several composite measures of systemic lupus erythematosus disease activity and changes in whole blood transcriptomic profiles. Ann. Rheum. Dis. 76, 1837–1844. doi:10.1136/annrheumdis-2017-211388
Chen, S. Y., Liu, M. F., Kuo, P. Y., and Wang, C. R. (2019). Upregulated expression of STAT3/IL-17 in patients with systemic lupus erythematosus. Clin. Rheumatol. 38, 1361–1366. doi:10.1007/s10067-019-04467-8
Cinar, O. K., Marlais, M., Al Obaidi, M., Cheng, I. L., Tullus, K., Brogan, P., et al. (2021). Ofatumumab use in juvenile systemic lupus erythematosus: A single centre experience. Lupus 30, 527–530. doi:10.1177/0961203320981137
Clowse, M. E., Wallace, D. J., Furie, R. A., Petri, M. A., Pike, M. C., Leszczyński, P., et al. (2017). Efficacy and safety of epratuzumab in moderately to severely active systemic lupus erythematosus: Results from two phase III randomized, double-blind, placebo-controlled trials. Arthritis Rheumatol. 69, 362–375. doi:10.1002/art.39856
Comte, D., Karampetsou, M. P., and Tsokos, G. C. (2015). T cells as a therapeutic target in SLE. Lupus 24, 351–363. doi:10.1177/0961203314556139
Cottart, C. H., Nivet-Antoine, V., Laguillier-Morizot, C., and Beaudeux, J. L. (2010). Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 54, 7–16. doi:10.1002/mnfr.200900437
Curtis, J. R., Westfall, A. O., Allison, J., Bijlsma, J. W., Freeman, A., George, V., et al. (2006). Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 55, 420–426. doi:10.1002/art.21984
D'andrea, M. R., Derian, C. K., Leturcq, D., Baker, S. M., Brunmark, A., Ling, P., et al. (1998). Characterization of protease-activated receptor-2 immunoreactivity in normal human tissues. J. Histochem Cytochem 46, 157–164. doi:10.1177/002215549804600204
Davidson, A. (2016). What is damaging the kidney in lupus nephritis? Nat. Rev. Rheumatol. 12, 143–153. doi:10.1038/nrrheum.2015.159
Deng, G. M., Liu, L., Bahjat, F. R., Pine, P. R., and Tsokos, G. C. (2010). Suppression of skin and kidney disease by inhibition of spleen tyrosine kinase in lupus-prone mice. Arthritis Rheum. 62, 2086–2092. doi:10.1002/art.27452
Deng, J., Chalhoub, N. E., Sherwin, C. M., Li, C., and Brunner, H. I. (2019). Glucocorticoids pharmacology and their application in the treatment of childhood-onset systemic lupus erythematosus. Semin. Arthritis Rheum. 49, 251–259. doi:10.1016/j.semarthrit.2019.03.010
Dent, E. L., Taylor, E. B., Turbeville, H. R., and Ryan, M. J. (2020). Curcumin attenuates autoimmunity and renal injury in an experimental model of systemic lupus erythematosus. Physiol. Rep. 8, 14501. doi:10.14814/phy2.14501
Du, P., Zhang, W., Cui, H., He, W., Lu, S., Jia, S., et al. (2022). Sulforaphane ameliorates the severity of psoriasis and SLE by modulating effector cells and reducing oxidative stress. Front. Pharmacol. 13, 805508. doi:10.3389/fphar.2022.805508
Durcan, L., O'dwyer, T., and Petri, M. (2019). Management strategies and future directions for systemic lupus erythematosus in adults. Lancet 393, 2332–2343. doi:10.1016/s0140-6736(19)30237-5
Espéli, M., Smith, K. G., and Clatworthy, M. R. (2016). FcγRIIB and autoimmunity. Immunol. Rev. 269, 194–211. doi:10.1111/imr.12368
Faurschou, M., Dreyer, L., Kamper, A. L., Starklint, H., and Jacobsen, S. (2010). Long-term mortality and renal outcome in a cohort of 100 patients with lupus nephritis. Arthritis Care Res. Hob. 62, 873–880. doi:10.1002/acr.20116
Fava, A., and Petri, M. (2019). Systemic lupus erythematosus: Diagnosis and clinical management. J. Autoimmun. 96, 1–13. doi:10.1016/j.jaut.2018.11.001
Felten, R., Scher, F., Sibilia, J., Chasset, F., and Arnaud, L. (2019). Advances in the treatment of systemic lupus erythematosus: From back to the future, to the future and beyond. Jt. Bone Spine 86, 429–436. doi:10.1016/j.jbspin.2018.09.004
Furie, R. A., Bruce, I. N., Dörner, T., Leon, M. G., Leszczyński, P., Urowitz, M., et al. (2021). Phase 2, randomized, placebo-controlled trial of dapirolizumab pegol in patients with moderate-to-severe active systemic lupus erythematosus. Rheumatol. Oxf. 60, 5397–5407. doi:10.1093/rheumatology/keab381
Furie, R. A., Leon, G., Thomas, M., Petri, M. A., Chu, A. D., Hislop, C., et al. (2015). A phase 2, randomised, placebo-controlled clinical trial of blisibimod, an inhibitor of B cell activating factor, in patients with moderate-to-severe systemic lupus erythematosus, the PEARL-SC study. Ann. Rheum. Dis. 74, 1667–1675. doi:10.1136/annrheumdis-2013-205144
Furie, R., Nicholls, K., Cheng, T. T., Houssiau, F., Burgos-Vargas, R., Chen, S. L., et al. (2014). Efficacy and safety of abatacept in lupus nephritis: A twelve-month, randomized, double-blind study. Arthritis Rheumatol. 66, 379–389. doi:10.1002/art.38260
Furie, R., Petri, M., Zamani, O., Cervera, R., Wallace, D. J., Tegzová, D., et al. (2011). A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 63, 3918–3930. doi:10.1002/art.30613
Furie, R., Rovin, B. H., Houssiau, F., Contreras, G., Teng, Y. K. O., Curtis, P., et al. (2022). Safety and efficacy of belimumab in patients with lupus nephritis: Open-label extension of BLISS-LN study. Clin. J. Am. Soc. Nephrol. 17, 1620–1630. doi:10.2215/cjn.02520322
Furie, R., Rovin, B. H., Houssiau, F., Malvar, A., Teng, Y. K. O., Contreras, G., et al. (2020). Two-year, randomized, controlled trial of belimumab in lupus nephritis. N. Engl. J. Med. 383, 1117–1128. doi:10.1056/NEJMoa2001180
Gelfand, J. M., Cree, B. a. C., and Hauser, S. L. (2017). Ocrelizumab and other CD20(+) B-Cell-Depleting therapies in multiple sclerosis. Neurotherapeutics 14, 835–841. doi:10.1007/s13311-017-0557-4
Gottenberg, J. E., Guillevin, L., Lambotte, O., Combe, B., Allanore, Y., Cantagrel, A., et al. (2005). Tolerance and short term efficacy of rituximab in 43 patients with systemic autoimmune diseases. Ann. Rheum. Dis. 64, 913–920. doi:10.1136/ard.2004.029694
Grammatikos, A. P., Ghosh, D., Devlin, A., Kyttaris, V. C., and Tsokos, G. C. (2013). Spleen tyrosine kinase (Syk) regulates systemic lupus erythematosus (SLE) T cell signaling. PLoS One 8, e74550. doi:10.1371/journal.pone.0074550
Grzanna, R., Lindmark, L., and Frondoza, C. G. (2005). Ginger-an herbal medicinal product with broad anti-inflammatory actions. J. Med. Food 8, 125–132. doi:10.1089/jmf.2005.8.125
Gupta, R., Yadav, A., and Aggarwal, A. (2016). Longitudinal assessment of monocyte chemoattractant protein-1 in lupus nephritis as a biomarker of disease activity. Clin. Rheumatol. 35, 2707–2714. doi:10.1007/s10067-016-3404-9
Hauser, S. L., Bar-Or, A., Cohen, J. A., Comi, G., Correale, J., Coyle, P. K., et al. (2020). Ofatumumab versus teriflunomide in multiple sclerosis. N. Engl. J. Med. 383, 546–557. doi:10.1056/NEJMoa1917246
Herrada, A. A., Escobedo, N., Iruretagoyena, M., Valenzuela, R. A., Burgos, P. I., Cuitino, L., et al. (2019). Innate immune cells' contribution to systemic lupus erythematosus. Front. Immunol. 10, 772. doi:10.3389/fimmu.2019.00772
Horton, H. M., Chu, S. Y., Ortiz, E. C., Pong, E., Cemerski, S., Leung, I. W., et al. (2011). Antibody-mediated coengagement of FcγRIIb and B cell receptor complex suppresses humoral immunity in systemic lupus erythematosus. J. Immunol. 186, 4223–4233. doi:10.4049/jimmunol.1003412
Iaccarino, L., Bettio, S., Reggia, R., Zen, M., Frassi, M., Andreoli, L., et al. (2017). Effects of belimumab on flare rate and expected damage progression in patients with active systemic lupus erythematosus. Arthritis Care Res. Hob. 69, 115–123. doi:10.1002/acr.22971
Ichinose, K., Juang, Y. T., Crispín, J. C., Kis-Toth, K., and Tsokos, G. C. (2011). Suppression of autoimmunity and organ pathology in lupus-prone mice upon inhibition of calcium/calmodulin-dependent protein kinase type IV. Arthritis Rheum. 63, 523–529. doi:10.1002/art.30085
Isenberg, D. A., Petri, M., Kalunian, K., Tanaka, Y., Urowitz, M. B., Hoffman, R. W., et al. (2016). Efficacy and safety of subcutaneous tabalumab in patients with systemic lupus erythematosus: Results from ILLUMINATE-1, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 75, 323–331. doi:10.1136/annrheumdis-2015-207653
Jayne, D., Rovin, B., Mysler, E. F., Furie, R. A., Houssiau, F. A., Trasieva, T., et al. (2022). Phase II randomised trial of type I interferon inhibitor anifrolumab in patients with active lupus nephritis. Ann. Rheum. Dis. 81, 496–506. doi:10.1136/annrheumdis-2021-221478
Jhou, J. P., Chen, S. J., Huang, H. Y., Lin, W. W., Huang, D. Y., and Tzeng, S. J. (2017). Upregulation of FcγRIIB by resveratrol via NF-κB activation reduces B-cell numbers and ameliorates lupus. Exp. Mol. Med. 49, e381. doi:10.1038/emm.2017.144
Jiang, T., Tian, F., Zheng, H., Whitman, S. A., Lin, Y., Zhang, Z., et al. (2014). Nrf2 suppresses lupus nephritis through inhibition of oxidative injury and the NF-κB-mediated inflammatory response. Kidney Int. 85, 333–343. doi:10.1038/ki.2013.343
Jolad, S. D., Lantz, R. C., Chen, G. J., Bates, R. B., and Timmermann, B. N. (2005). Commercially processed dry ginger (zingiber officinale): Composition and effects on LPS-stimulated PGE2 production. Phytochemistry 66, 1614–1635. doi:10.1016/j.phytochem.2005.05.007
Jolad, S. D., Lantz, R. C., Solyom, A. M., Chen, G. J., Bates, R. B., and Timmermann, B. N. (2004). Fresh organically grown ginger (zingiber officinale): Composition and effects on LPS-induced PGE2 production. Phytochemistry 65, 1937–1954. doi:10.1016/j.phytochem.2004.06.008
Kang, H. K., Ecklund, D., Liu, M., and Datta, S. K. (2009). Apigenin, a non-mutagenic dietary flavonoid, suppresses lupus by inhibiting autoantigen presentation for expansion of autoreactive Th1 and Th17 cells. Arthritis Res. Ther. 11, R59. doi:10.1186/ar2682
Kasiri, N., Rahmati, M., Ahmadi, L., and Eskandari, N. (2018). The significant impact of apigenin on different aspects of autoimmune disease. Inflammopharmacology 26, 1359–1373. doi:10.1007/s10787-018-0531-8
Kasselman, L. J., Renna, H. A., Voloshyna, I., Pinkhasov, A., Gomolin, I. H., Teboul, I., et al. (2022). Cognitive changes mediated by adenosine receptor blockade in a resveratrol-treated atherosclerosis-prone lupus mouse model. J. Tradit. Complement. Med. 12, 447–454. doi:10.1016/j.jtcme.2022.01.006
Khajehdehi, P., Zanjaninejad, B., Aflaki, E., Nazarinia, M., Azad, F., Malekmakan, L., et al. (2012). Oral supplementation of turmeric decreases proteinuria, hematuria, and systolic blood pressure in patients suffering from relapsing or refractory lupus nephritis: A randomized and placebo-controlled study. J. Ren. Nutr. 22, 50–57. doi:10.1053/j.jrn.2011.03.002
Krishnan, S., Juang, Y. T., Chowdhury, B., Magilavy, A., Fisher, C. U., Nguyen, H., et al. (2008). Differential expression and molecular associations of Syk in systemic lupus erythematosus T cells. J. Immunol. 181, 8145–8152. doi:10.4049/jimmunol.181.11.8145
Lamb, Y. N. (2022). Ocrelizumab: A review in multiple sclerosis. Drugs 82, 323–334. doi:10.1007/s40265-022-01672-9
Leandro, M. J., Cambridge, G., Edwards, J. C., Ehrenstein, M. R., and Isenberg, D. A. (2005). B-Cell depletion in the treatment of patients with systemic lupus erythematosus: A longitudinal analysis of 24 patients. Rheumatol. Oxf. 44, 1542–1545. doi:10.1093/rheumatology/kei080
Leandro, M. J., Edwards, J. C., Cambridge, G., Ehrenstein, M. R., and Isenberg, D. A. (2002). An open study of B lymphocyte depletion in systemic lupus erythematosus. Arthritis Rheum. 46, 2673–2677. doi:10.1002/art.10541
Lei, L., Muhammad, S., Al-Obaidi, M., Sebire, N., Cheng, I. L., Eleftheriou, D., et al. (2018). Successful use of ofatumumab in two cases of early-onset juvenile SLE with thrombocytopenia caused by a mutation in protein kinase C δ. Pediatr. Rheumatol. Online J. 16, 61. doi:10.1186/s12969-018-0278-1
Li, C., Li, Q., Mei, Q., and Lu, T. (2015). Pharmacological effects and pharmacokinetic properties of icariin, the major bioactive component in Herba Epimedii. Life Sci. 126, 57–68. doi:10.1016/j.lfs.2015.01.006
Li, Q., and Verma, I. M. (2002). NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2, 725–734. doi:10.1038/nri910
Li, X., Ding, Y., Zi, M., Sun, L., Zhang, W., Chen, S., et al. (2017). CD19, from bench to bedside. Immunol. Lett. 183, 86–95. doi:10.1016/j.imlet.2017.01.010
Masoud, S., Mcadoo, S. P., Bedi, R., Cairns, T. D., and Lightstone, L. (2018). Ofatumumab for B cell depletion in patients with systemic lupus erythematosus who are allergic to rituximab. Rheumatol. Oxf. 57, 1156–1161. doi:10.1093/rheumatology/key042
Merrill, J. T., Burgos-Vargas, R., Westhovens, R., Chalmers, A., D'cruz, D., Wallace, D. J., et al. (2010a). The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: Results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 62, 3077–3087. doi:10.1002/art.27601
Merrill, J. T., June, J., Koumpouras, F., Machua, W., Khan, M. F., Askanase, A., et al. (2018c). Efficacy and safety of atacicept in patients with systemic lupus erythematosus: Results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled, parallel-arm, phase IIb study. Arthritis Rheum. 70, 266–276. doi:10.1002/art.4036
Merrill, J. T., Neuwelt, C. M., Wallace, D. J., Shanahan, J. C., Latinis, K. M., Oates, J. C., et al. (2010b). Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: The randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 62, 222–233. doi:10.1002/art.27233
Merrill, J. T., Shanahan, W. R., Scheinberg, M., Kalunian, K. C., Wofsy, D., and Martin, R. S. (2018a). Phase III trial results with blisibimod, a selective inhibitor of B-cell activating factor, in subjects with systemic lupus erythematosus (SLE): Results from a randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 77, 883–889. doi:10.1136/annrheumdis-2018-213032
Merrill, J. T., Van Vollenhoven, R. F., Buyon, J. P., Furie, R. A., Stohl, W., Morgan-Cox, M., et al. (2016). Efficacy and safety of subcutaneous tabalumab, a monoclonal antibody to B-cell activating factor, in patients with systemic lupus erythematosus: Results from ILLUMINATE-2, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 75, 332–340. doi:10.1136/annrheumdis-2015-207654
Merrill, J. T., Wallace, D. J., Wax, S., Kao, A., Fraser, P. A., Chang, P., et al. (2018b). Efficacy and safety of atacicept in patients with systemic lupus erythematosus: Results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled, parallel-arm, phase IIb study. Arthritis Rheumatol. 70, 266–276. doi:10.1002/art.40360
Morand, E. F., Furie, R., Tanaka, Y., Bruce, I. N., Askanase, A. D., Richez, C., et al. (2020a). Trial of anifrolumab in active systemic lupus erythematosus. N. Engl. J. Med. 382, 211–221. doi:10.1056/NEJMoa1912196
Morand, E. F., Isenberg, D. A., Wallace, D. J., Kao, A. H., Vazquez-Mateo, C., Chang, P., et al. (2020b). Attainment of treat-to-target endpoints in SLE patients with high disease activity in the atacicept phase 2b ADDRESS II study. Rheumatol. Oxf. 59, 2930–2938. doi:10.1093/rheumatology/keaa029
Mysler, E. F., Spindler, A. J., Guzman, R., Bijl, M., Jayne, D., Furie, R. A., et al. (2013). Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: Results from a randomized, double-blind, phase III study. Arthritis Rheum. 65, 2368–2379. doi:10.1002/art.38037
Narain, S., Berman, N., and Furie, R. (2020). Biologics in the treatment of Sjogren's syndrome, systemic lupus erythematosus, and lupus nephritis. Curr. Opin. Rheumatol. 32, 609–616. doi:10.1097/bor.0000000000000754
Navarra, S. V., Guzmán, R. M., Gallacher, A. E., Hall, S., Levy, R. A., Jimenez, R. E., et al. (2011). Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: A randomised, placebo-controlled, phase 3 trial. Lancet 377, 721–731. doi:10.1016/s0140-6736(10)61354-2
Nikpour, M., Dempsey, A. A., Urowitz, M. B., Gladman, D. D., and Barnes, D. A. (2008). Association of a gene expression profile from whole blood with disease activity in systemic lupus erythaematosus. Ann. Rheum. Dis. 67, 1069–1075. doi:10.1136/ard.2007.074765
Oliveira, A. L. B., Monteiro, V. V. S., Navegantes-Lima, K. C., Reis, J. F., Gomes, R. S., Rodrigues, D. V. S., et al. (2017). Resveratrol role in autoimmune disease-A mini-review. Nutrients 9, 1306. doi:10.3390/nu9121306
Pannu, N., and Bhatnagar, A. (2020). Combinatorial therapeutic effect of resveratrol and piperine on murine model of systemic lupus erythematosus. Inflammopharmacology 28, 401–424. doi:10.1007/s10787-019-00662-w
Payandeh, Z., Bahrami, A. A., Hoseinpoor, R., Mortazavi, Y., Rajabibazl, M., Rahimpour, A., et al. (2019). The applications of anti-CD20 antibodies to treat various B cells disorders. Biomed. Pharmacother. 109, 2415–2426. doi:10.1016/j.biopha.2018.11.121
Pimentel-Quiroz, V. R., Ugarte-Gil, M. F., and Alarcón, G. S. (2016). Abatacept for the treatment of systemic lupus erythematosus. Expert Opin. Investig. Drugs 25, 493–499. doi:10.1517/13543784.2016.1154943
Ponticelli, C., and Moroni, G. (2017). Hydroxychloroquine in systemic lupus erythematosus (SLE). Expert Opin. Drug Saf. 16, 411–419. doi:10.1080/14740338.2017.1269168
Pujara, N., Jambhrunkar, S., Wong, K. Y., Mcguckin, M., and Popat, A. (2017). Enhanced colloidal stability, solubility and rapid dissolution of resveratrol by nanocomplexation with soy protein isolate. J. Colloid Interface Sci. 488, 303–308. doi:10.1016/j.jcis.2016.11.015
Qabaha, K., Al-Rimawi, F., Qasem, A., and Naser, S. A. (2018). Oleuropein is responsible for the major anti-inflammatory effects of olive leaf extract. J. Med. Food 21, 302–305. doi:10.1089/jmf.2017.0070
Raiola, A., Errico, A., Petruk, G., Monti, D. M., Barone, A., and Rigano, M. M. (2017). Bioactive compounds in brassicaceae vegetables with a role in the prevention of chronic diseases. Molecules 23, 15. doi:10.3390/molecules23010015
Roccatello, D., Sciascia, S., Rossi, D., Alpa, M., Naretto, C., Baldovino, S., et al. (2011). Intensive short-term treatment with rituximab, cyclophosphamide and methylprednisolone pulses induces remission in severe cases of SLE with nephritis and avoids further immunosuppressive maintenance therapy. Nephrol. Dial. Transpl. 26, 3987–3992. doi:10.1093/ndt/gfr109
Rönnblom, L., Alm, G. V., and Eloranta, M. L. (2011). The type I interferon system in the development of lupus. Semin. Immunol. 23, 113–121. doi:10.1016/j.smim.2011.01.009
Rovin, B. H., Furie, R., Latinis, K., Looney, R. J., Fervenza, F. C., Sanchez-Guerrero, J., et al. (2012). Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: The lupus nephritis assessment with rituximab study. Arthritis Rheum. 64, 1215–1226. doi:10.1002/art.34359
Ryu, S. J., Choi, H. S., Yoon, K. Y., Lee, O. H., Kim, K. J., and Lee, B. Y. (2015). Oleuropein suppresses LPS-induced inflammatory responses in RAW 264.7 cell and zebrafish. J. Agric. Food Chem. 63, 2098–2105. doi:10.1021/jf505894b
Sandhu, S., and Mulligan, S. P. (2015). Ofatumumab and its role as immunotherapy in chronic lymphocytic leukemia. Haematologica 100, 411–414. doi:10.3324/haematol.2015.124107
Sanz, I., and Lee, F. E. (2010). B cells as therapeutic targets in SLE. Nat. Rev. Rheumatol. 6, 326–337. doi:10.1038/nrrheum.2010.68
Sasaki, H., Nagura, K., Ishino, M., Tobioka, H., Kotani, K., and Sasaki, T. (1995). Cloning and characterization of cell adhesion kinase beta, a novel protein-tyrosine kinase of the focal adhesion kinase subfamily. J. Biol. Chem. 270, 21206–21219. doi:10.1074/jbc.270.36.21206
Seo, Y., Mun, C. H., Park, S. H., Jeon, D., Kim, S. J., Yoon, T., et al. (2020). Punicalagin ameliorates lupus nephritis via inhibition of PAR2. Int. J. Mol. Sci. 21, 4975. doi:10.3390/ijms21144975
Shan, J., Jin, H., and Xu, Y. (2020). T cell metabolism: A new perspective on Th17/treg cell imbalance in systemic lupus erythematosus. Front. Immunol. 11, 1027. doi:10.3389/fimmu.2020.01027
Shipa, M., Embleton-Thirsk, A., Parvaz, M., Santos, L. R., Muller, P., Chowdhury, K., et al. (2021). Effectiveness of belimumab after rituximab in systemic lupus erythematosus: A randomized controlled trial. Ann. Intern Med. 174, 1647–1657. doi:10.7326/m21-2078
Singgih Wahono, C., Diah Setyorini, C., Kalim, H., Nurdiana, N., and Handono, K. (2017). Effect of curcuma xanthorrhiza supplementation on systemic lupus erythematosus patients with hypovitamin D which were given vitamin D(3) towards disease activity (SLEDAI), IL-6, and TGF-β1 serum. Int. J. Rheumatol. 2017, 7687053. doi:10.1155/2017/7687053
Stamatopoulos, K., Chatzilazarou, A., and Katsoyannos, E. (2013). Optimization of multistage extraction of olive leaves for recovery of phenolic compounds at moderated temperatures and short extraction times. Foods 3, 66–81. doi:10.3390/foods3010066
Stohl, W., Merrill, J. T., Looney, R. J., Buyon, J., Wallace, D. J., Weisman, M. H., et al. (2015). Treatment of systemic lupus erythematosus patients with the BAFF antagonist "peptibody" blisibimod (AMG 623/A-623): Results from randomized, double-blind phase 1a and phase 1b trials. Arthritis Res. Ther. 17, 215. doi:10.1186/s13075-015-0741-z
Stohl, W., Schwarting, A., Okada, M., Scheinberg, M., Doria, A., Hammer, A. E., et al. (2017). Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: A fifty-two-week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. 69, 1016–1027. doi:10.1002/art.40049
Stone, J. H., Merkel, P. A., Spiera, R., Seo, P., Langford, C. A., Hoffman, G. S., et al. (2010). Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N. Engl. J. Med. 363, 221–232. doi:10.1056/NEJMoa0909905
Strand, V., Petri, M., Kalunian, K., Gordon, C., Wallace, D. J., Hobbs, K., et al. (2014). Epratuzumab for patients with moderate to severe flaring SLE: Health-related quality of life outcomes and corticosteroid use in the randomized controlled ALLEVIATE trials and extension study SL0006. Rheumatol. Oxf. 53, 502–511. doi:10.1093/rheumatology/ket378
Su, B., Ye, H., You, X., Ni, H., Chen, X., and Li, L. (2018). Icariin alleviates murine lupus nephritis via inhibiting NF-κB activation pathway and NLRP3 inflammasome. Life Sci. 208, 26–32. doi:10.1016/j.lfs.2018.07.009
Takeuchi, T., Tanaka, Y., Matsumura, R., Saito, K., Yoshimura, M., Amano, K., et al. (2020). Safety and tolerability of sifalimumab, an anti-interferon-α monoclonal antibody, in Japanese patients with systemic lupus erythematosus: A multicenter, phase 2, open-label study. Mod. Rheumatol. 30, 93–100. doi:10.1080/14397595.2019.1583832
Tang, F., Xie, Y., Cao, H., Yang, H., Chen, X., and Xiao, J. (2017). Fetal bovine serum influences the stability and bioactivity of resveratrol analogues: A polyphenol-protein interaction approach. Food Chem. 219, 321–328. doi:10.1016/j.foodchem.2016.09.154
Tocoian, A., Buchan, P., Kirby, H., Soranson, J., Zamacona, M., Walley, R., et al. (2015). First-in-human trial of the safety, pharmacokinetics and immunogenicity of a PEGylated anti-CD40L antibody fragment (CDP7657) in healthy individuals and patients with systemic lupus erythematosus. Lupus 24, 1045–1056. doi:10.1177/0961203315574558
Tsokos, G. C., Lo, M. S., Costa Reis, P., and Sullivan, K. E. (2016). New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 12, 716–730. doi:10.1038/nrrheum.2016.186
Tsuru, T., Tanaka, Y., Kishimoto, M., Saito, K., Yoshizawa, S., Takasaki, Y., et al. (2016). Safety, pharmacokinetics, and pharmacodynamics of epratuzumab in Japanese patients with moderate-to-severe systemic lupus erythematosus: Results from a phase 1/2 randomized study. Mod. Rheumatol. 26, 87–93. doi:10.3109/14397595.2015.1079292
Van Vollenhoven, R. F., Fleischmann, R. M., Furst, D. E., Lacey, S., and Lehane, P. B. (2015). Longterm safety of rituximab: Final report of the rheumatoid arthritis global clinical trial program over 11 years. J. Rheumatol. 42, 1761–1766. doi:10.3899/jrheum.150051
Venusova, E., Kolesarova, A., Horky, P., and Slama, P. (2021). Physiological and immune functions of punicalagin. Nutrients 13, 2150. doi:10.3390/nu13072150
Vesey, D. A., Hooper, J. D., Gobe, G. C., and Johnson, D. W. (2007). Potential physiological and pathophysiological roles for protease-activated receptor-2 in the kidney. Nephrol. Carlt. 12, 36–43. doi:10.1111/j.1440-1797.2006.00746.x
Voloshyna, I., Teboul, I., Littlefield, M. J., Siegart, N. M., Turi, G. K., Fazzari, M. J., et al. (2016). Resveratrol counters systemic lupus erythematosus-associated atherogenicity by normalizing cholesterol efflux. Exp. Biol. Med. (Maywood) 241, 1611–1619. doi:10.1177/1535370216647181
Wallace, D. J., Gordon, C., Strand, V., Hobbs, K., Petri, M., Kalunian, K., et al. (2013). Efficacy and safety of epratuzumab in patients with moderate/severe flaring systemic lupus erythematosus: Results from two randomized, double-blind, placebo-controlled, multicentre studies (ALLEVIATE) and follow-up. Rheumatol. Oxf. 52, 1313–1322. doi:10.1093/rheumatology/ket129
Wallace, D. J., Hobbs, K., Clowse, M. E., Petri, M., Strand, V., Pike, M., et al. (2016). Long-term safety and efficacy of epratuzumab in the treatment of moderate-to- severe systemic lupus erythematosus: Results from an open-label extension study. Arthritis Care Res. Hob. 68, 534–543. doi:10.1002/acr.22694
Wallace, D. J., Isenberg, D. A., Morand, E. F., Vazquez-Mateo, C., Kao, A. H., Aydemir, A., et al. (2021). Safety and clinical activity of atacicept in the long-term extension of the phase 2b ADDRESS II study in systemic lupus erythematosus. Rheumatol. Oxf. 60, 5379–5389. doi:10.1093/rheumatology/keab115
Wallace, D. J., Kalunian, K., Petri, M. A., Strand, V., Houssiau, F. A., Pike, M., et al. (2014). Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: Results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Ann. Rheum. Dis. 73, 183–190. doi:10.1136/annrheumdis-2012-202760
Walle, T. (2011). Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 1215, 9–15. doi:10.1111/j.1749-6632.2010.05842.x
Wang, M., Zhou, G., Lv, J., Zeng, P., Guo, C., and Wang, Q. (2017). Curcumin modulation of the activation of PYK2 in peripheral blood mononuclear cells from patients with lupus nephritis. Reumatologia 55, 269–275. doi:10.5114/reum.2017.72623
Wang, Z. L., Luo, X. F., Li, M. T., Xu, D., Zhou, S., Chen, H. Z., et al. (2014). Resveratrol possesses protective effects in a pristane-induced lupus mouse model. PLoS One 9, e114792. doi:10.1371/journal.pone.0114792
Wise, L. M., and Stohl, W. (2020). Belimumab and rituximab in systemic lupus erythematosus: A tale of two B cell-targeting agents. Front. Med. (Lausanne) 7, 303. doi:10.3389/fmed.2020.00303
Yasutomo, K. (2003). Pathological lymphocyte activation by defective clearance of self-ligands in systemic lupus erythematosus. Rheumatol. Oxf. 42, 214–222. doi:10.1093/rheumatology/keg081
Yoh, K., Itoh, K., Enomoto, A., Hirayama, A., Yamaguchi, N., Kobayashi, M., et al. (2001). Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 60, 1343–1353. doi:10.1046/j.1523-1755.2001.00939.x
Zhang, F., Bae, S. C., Bass, D., Chu, M., Egginton, S., Gordon, D., et al. (2018). A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Ann. Rheum. Dis. 77, 355–363. doi:10.1136/annrheumdis-2017-211631
Zhang, J., Roschke, V., Baker, K. P., Wang, Z., Alarcón, G. S., Fessler, B. J., et al. (2001). Cutting edge: A role for B lymphocyte stimulator in systemic lupus erythematosus. J. Immunol. 166, 6–10. doi:10.4049/jimmunol.166.1.6
Keywords: systemic lupus erythematosus, antibody drug, natural products, treatment, immune system
Citation: Han Y, Liu L, Zang B, Liang R, Zhao X and Liu B (2023) Advances in natural products and antibody drugs for SLE: new therapeutic ideas. Front. Pharmacol. 14:1235440. doi: 10.3389/fphar.2023.1235440
Received: 07 June 2023; Accepted: 03 July 2023;
Published: 10 July 2023.
Edited by:
Mariateresa Cristani, University of Messina, ItalyReviewed by:
Rahul Kakalij, University of Nebraska Medical Center, United StatesQingjun Pan, Affiliated Hospital of Guangdong Medical University, China
Copyright © 2023 Han, Liu, Zang, Liang, Zhao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Liu, YmlubGl1NzIzMTRAMTYzLmNvbQ==
 Yibing Han
Yibing Han Lingwei Liu
Lingwei Liu Bo Zang
Bo Zang Ruiwen Liang
Ruiwen Liang Bin Liu
Bin Liu