- 1Key Lab, Ningbo Kangning Hospital, Ningbo, Zhejiang, China
- 2Department of Geriatric, Ningbo Kangning Hospital, Ningbo, Zhejiang, China
- 3Medical College, Ningbo University, Ningbo, Zhejiang, China
Background: As a non-pharmacologic treatment, bright light therapy (BLT) is often used to improve affective disorders and memory function. In this study, we aimed to determine the effect of BLT on depression and electrophysiological features of the brain in patients with Alzheimer’s disease (AD) and their caregivers using a light-emitting diode device of 14000 lux.
Methods: A 4-week case-control trial was conducted. Neuropsychiatric and electroencephalogram (EEG) examination were evaluated at baseline and after 4 weeks. EEG power in delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), and beta (12–30 Hz) bands was calculated for our main analysis. Demographic and clinical variables were analyzed using Student’s t test and the chi-square test. Pearson’s correlation was used to determine the correlation between electrophysiological features, blood biochemical indicators, and cognitive assessment scale scores.
Results: In this study, 22 in-patients with AD and 23 caregivers were recruited. After BLT, the Hamilton depression scale score decreased in the fourth week. Compared with the age-matched controls of their caregivers, a higher spectral power at the lower delta and theta frequencies was observed in the AD group. After BLT, the EEG power of the delta and theta frequencies in the AD group decreased. No change was observed in blood amyloid concentrations before and after BLT.
Conclusion: In conclusion, a 4-week course of BLT significantly suppressed depression in patients with AD and their caregivers. Moreover, changes in EEG power were also significant in both groups.
Introduction
Alzheimer’s disease (AD) is a progressive degenerative disease affecting cognitive functions and mental health. Its clinical manifestations include memory impairment and additional cognitive domain impairments in executive functions, attention, language, social cognition and judgment, psychomotor speed, and visuoperceptual or visuospatial abilities (Masters et al., 2015; Scheltens et al., 2016; Scheltens et al., 2021). Cognitive impairment is considered a transitional stage between normal aging and AD. China has a high prevalence of dementia and cognitive impairment (Jia et al., 2020).
AD may be accompanied by mental and behavioral symptoms, such as depressed mood and apathy in the initial stages, and also by psychotic symptoms, irritability, aggression, confusion, gait and mobility abnormalities, and seizures in the later stages (Altomari et al., 2022). In the long-term care process, caregivers of patients with AD may also suffer from mental health problems (Corrêa et al., 2019; Carbone et al., 2021). In previous studies, depression occurred in 50% of patients with AD, thereby increasing the caregivers’ burden (Chi et al., 2014). Symptoms of depression can precede a clinical diagnosis of AD for years or occur around the onset of AD (Diniz et al., 2013). Caring for a loved one with AD can increase the risk of depression in caregivers, and symptoms of depression typically persist over time (Liu et al., 2017; Chai et al., 2018).
Aside from medication, a number of non-pharmacological treatments can be used to improve a patient’s health condition. According to previous studies, exposure to bright light may improve sleep and ease depression and agitation in people with AD (Peter-Derex et al., 2015; van Maanen et al., 2016; Roccaro et al., 2020). Persons living with AD or vascular dementia who were exposed to bright light therapy (BLT) demonstrated significantly improved scores on the Mini-Mental State Examination (MMSE) scale, compared to exposure to dim light therapy (Lu et al., 2023). BLT has also been used for decades to treat nonseasonal depression and other mood disorders (Al-Karawi and Jubair, 2016; Wang et al., 2020).
Regardless of clinical manifestations, AD biomarkers, including amyloidosis, tauopathy, and neurodegeneration, are important for diagnosis and to evaluate the effectiveness of therapy in this disease (Jack et al., 2018). Electroencephalogram (EEG) biomarkers can be used to reflect the effects of AD neuropathology on functional brain networks (Pfurtscheller and Lopes da Silva, 1999). Because of its high temporal resolution, we can investigate EEG rhythms at different frequency bands during a resting-state condition in patients with cognitive decline (Caravaglios et al., 2023). Although changes in electrophysiological features are not specific for patients with AD, compared to older adults without cognitive impairment, patients with AD or dementia with mild cognitive impairment were characterized by changes in EEG rhythms during resting-state condition (Babiloni et al., 2020).
This study aimed to determine the effect of BLT on depression and electrophysiological features of the brain in patients with AD and their caregivers using a light-emitting diode device with an intensity of 14000 lux.
Methods
Participants
We recruited inpatients and their caregivers form Geriatric Center of Ningbo Kangning Hospital between September 2022 and March 2023. Potential participants were recommended to our experienced research psychiatrists for further study. Ultimately, 22 patients with AD and 23 caregivers were recruited in this study. The patients with AD were diagnosed using the Diagnostic and Statistical Manual of Mental Disorders, fifth edition criteria (First, 2013). All patients met the following inclusion and exclusion criteria: 1) diagnosed with AD by two research psychiatrists; 2) provision of informed consent; 3) disease course >3 months; 4) cholinesterase inhibitor (donepezil) and non-competitive N-methyl-d-aspartate receptor antagonist (memantine) use; 5) no history of other mental illnesses, including schizophrenia and delirium; and 6) no physical diseases.
Neuropsychiatric evaluation
The neuropsychological evaluation of cognition was confirmed by MMSE scores <17, 20, and 24 in people with no, primary school, and junior high school education, respectively (Li et al., 2016). The AD assessment scale—cognitive subscale (ADAScog) was used to evaluate patients’ memory, language, and other cognitive impairments (Rosen et al., 1984). The Hamilton depression scale (HAMD) was adopted to evaluate depression in patients and their caregivers (Zimmerman et al., 2013).
Experimental protocol
The light therapy equipment used for treatment was designed by the Geriatric Center of Ningbo Kangning Hospital as described in our previous study (Zou et al., 2022). Light therapy with peak strength of 14000 lux was conducted from 9:00 a.m. to 9:30 a.m. each day for 4 weeks. The patients and their caregivers sat in front of the light therapy device with their eyes open to allow the light to reach the retinas for BLT. The distance between the participants and light source was 50 cm.
EEG examination and analysis
The participants were seated in a comfortable chair. A 128-channel EEG (EGI System 300; Electrical Geodesic Inc., Eugene, OR, United States) configured in the standard 10–20 montage was recorded with reference to linked mastoids. The sampling rate was 500 Hz using an amplifier with low and high cutoff frequencies of 0.1 and 100 Hz, respectively.
EEG recordings were compiled during a resting state using a structured testing and acquisition software platform (5 min with the eyes opened and 5 min with the eyes closed). During the open-eye task, participants were instructed to stare directly at a black fixation cross located in the center of a gray background. During the closed-eye task, they were instructed to close their eyes while maintaining wakefulness. NetStation software (Electrical Geodesic Inc., OR, United States) was used for recording. The impedance for all electrodes was kept below 50 kΩ. Offline data analysis was conducted with the open-source EEGLAB toolbox.
Blood enzyme-linked immunosorbent assay
Blood samples were collected before breakfast using a winged blood collection set. Approximately 5 mL of whole blood was collected in a procoagulant tube. To allow the measurement of plasma peptides, blood was immediately centrifuged at 1,400 rpm using a BY-600A type medical centrifuge (Beijing Baiyang Medical Devices Co., Beijing, China) for 10 min. All blood samples were processed within 30 min of collection and immediately frozen at −80°C. Blood samples were thawed immediately before analysis.
Serum amyloid-β (Aβ), Aβ40, Aβ42, interleukin (IL)-1β, and melatonin (MT) levels were estimated using enzyme-linked immunosorbent assay kits (Shanghai Yuanye Bio-Technology Co., Shanghai, China). All procedures were performed according to the manufacturer’s instructions. Absorbance was measured at 450 nm using a Sunrise-basic enzyme labeling instrument (Tecan Group Ltd., Mannedorf, Switzerland) with a reference wavelength of 690 nm. These measurements were transformed into concentrations by comparing the optical densities of the samples with the standard curve values.
Statistical analysis
Data are presented as the mean ± standard deviation (SD). Demographic and clinical variables were compared and analyzed between the different groups using Student’s t test for continuous variables and the chi-square test for categorical variables. Pearson’s correlation was used to determine the correlation among electrophysiological features, blood indicators, and cognitive assessment scale scores. Statistical significance was set at p < 0.05. Statistical Package for the Social Sciences (SPSS version 19.0, IBM Corp., Armonk, NY, United States) was used for all analyses.
Results
Clinical assessment
The protocol for BLT in patients with AD and their caregivers is shown in Figure 1. First, we recruited participants based on the inclusion and exclusion criteria. At baseline, all the participants were asked to complete assessments including neurophysiological scales (MMSE, ADAScog, and HAMD), EEG examination, and blood tests (i.e., Aβ, IL-1β, and MT levels). Subsequently, they were exposed to bright light at an illumination intensity of 14000 lux twice daily. After 4 weeks of therapy, the same assessments were repeated.
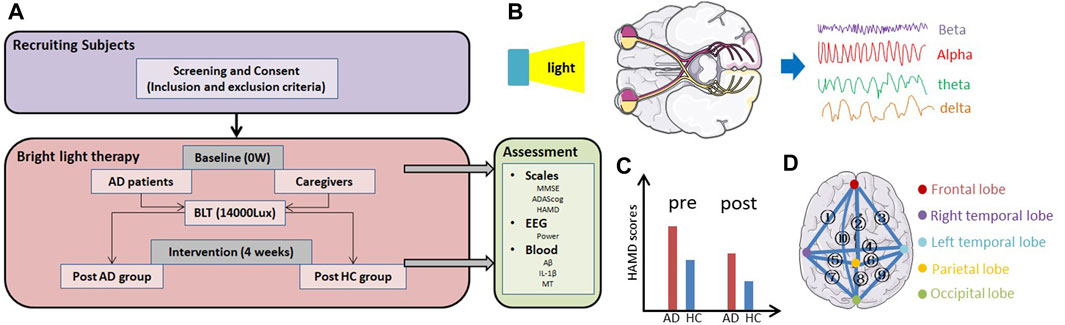
FIGURE 1. Overview of the study. (A) Protocol for bright light therapy in patients with AD and their caregivers (HC). (B) Light therapy modulated the EEG signal through the retina to the cortex. (C) HAMD scores, which reflect the level of depression in participants, may be change before and after therapy. (D) ① to ⑩ represent the relationship between the frontal and right temporal lobes, frontal and parietal lobes, frontal and left temporal lobes, right temporal and left temporal lobes, right temporal and parietal lobes, left temporal and parietal lobes, right temporal and occipital lobes, parietal and occipital lobes, left temporal and occipital lobes, frontal and occipital lobes.
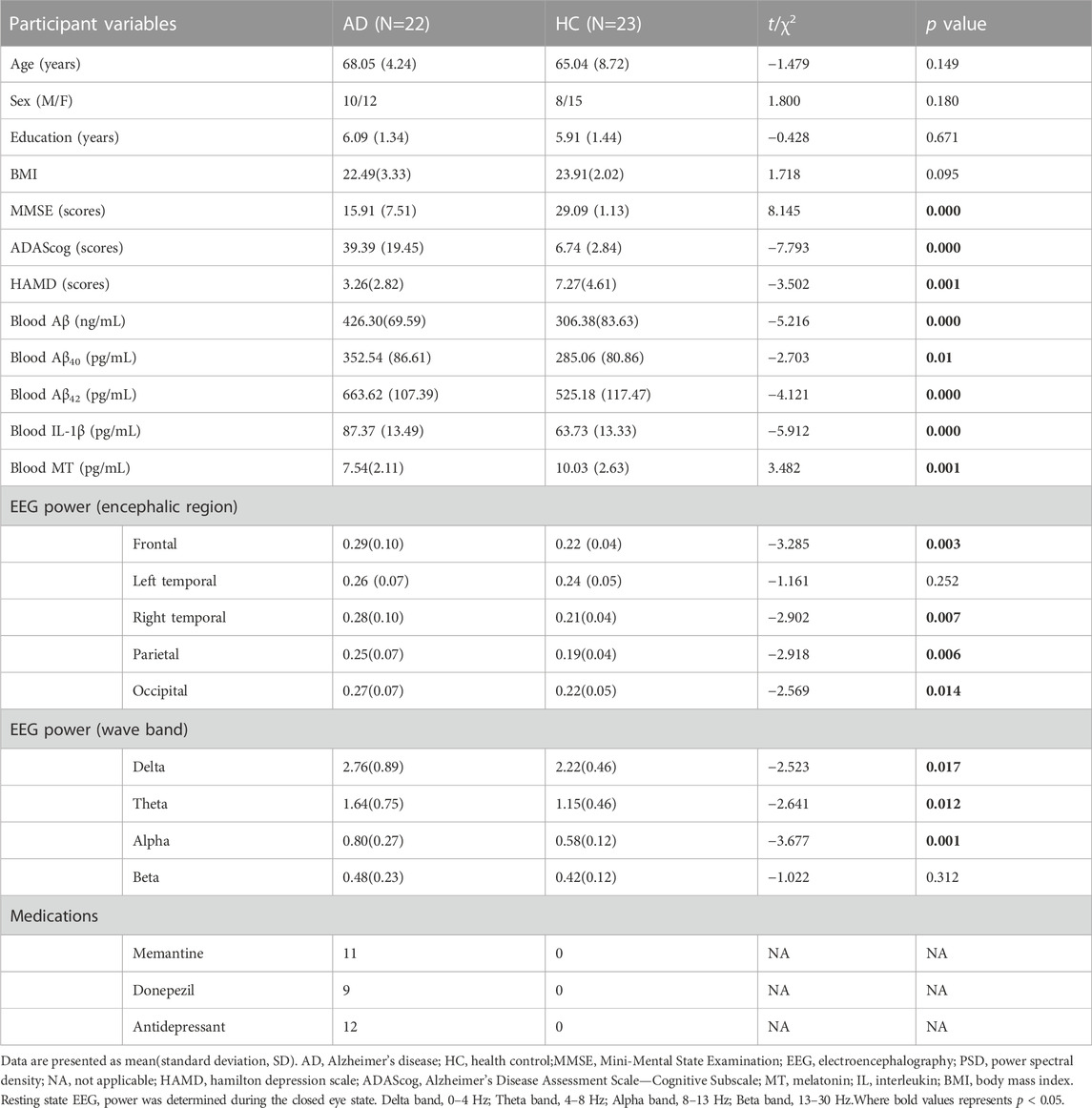
The characteristics of the patients included in this study are summarized in Table 1. In total, 22 patients with AD were included, with a mean age of 68.05 years, 10 men and 12 women. The caregiver group consisted of 23 individuals (8 men and 15 women) with a mean age of 65.04 years. Eleven patients with AD were taking memantine and nine were taking donepezil.
Effect of BLT on patients with AD and their caregivers
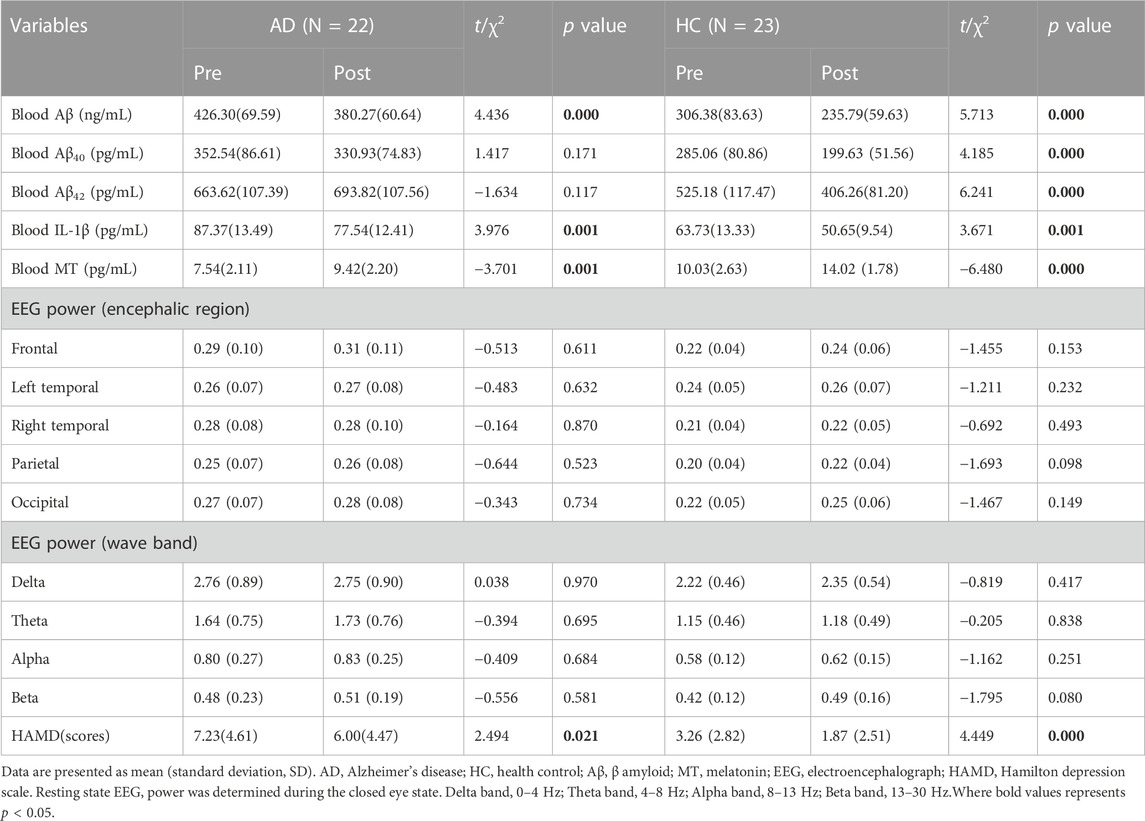
Blood test results and EEG parameters of patients with AD and their caregivers were evaluated. The results are presented in Table 2. After BLT, blood Aβ and IL-1β levels decreased significantly in both the AD and HC groups, whereas MT levels increased significantly in both groups. The HAMD score also decreased. Blood Aβ40 and Aβ42 levels were only significantly decreased in the HC group.
Changes in EEG power after BLT
Resting state EEG was performed in patients with AD and their caregivers with normal cognitive levels, as shown in Figures 2A–D. Compared to people with normal cognition, patients with AD had higher resting state EEG power at baseline. In the open-eye state (Figure 2A), the power of the HC group decreased, whereas that of the AD group increased, although no significant change in the EEG power after BLT was observed in both groups compared with that at baseline,. BLT suppressed the resting state power in the occipital lobe of patients with AD and in the frontal and right temporal lobes of caregivers in the HC group; however, these results were not significant. Regarding the differences in EEG power between the AD and HC groups in the closed-eye state (Figure 2B), the differences after BLT decreased in frontal, parietal and occipital lobes compared with that before BLT.
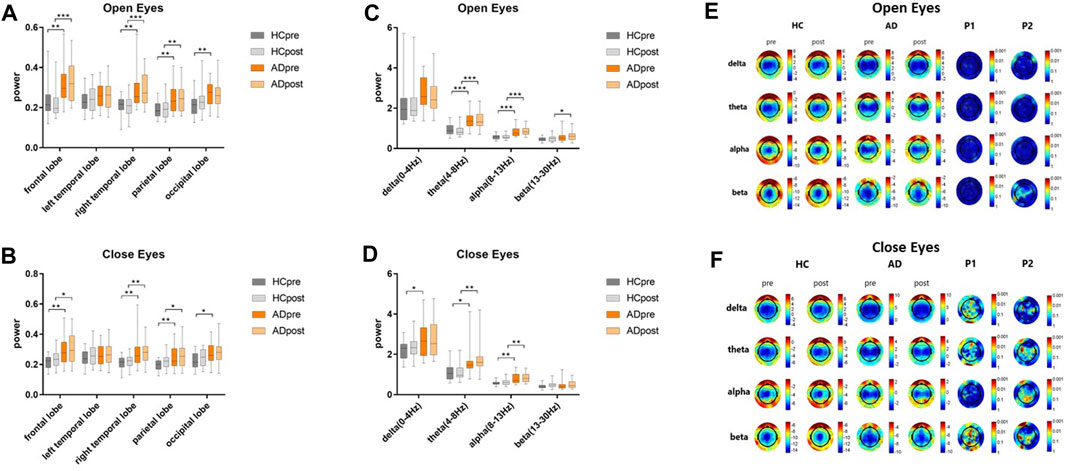
FIGURE 2. Resting state EEG power of patients with AD and their caregivers with normal cognition (CN group) in the open-eye (A) and closed-eye (B) states at the frontal, left temporal, right temporal, parietal, and occipital lobes, and in the open-eye (C) and closed-eye (D) states in the delta (0–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), and beta (13–30 Hz) bands. The gray and orange bars represent the HC and AD groups, respectively before (dark color) and after (light color) bright light therapy. Resting state EEG topographic maps of patients with AD and their care givers with normal cognition (HC group) in the open-eye (E) and closed-eyes (F) states. P1 and P2 represent different p values before and after therapy in the HC and AD groups, respectively.
Regarding the EEG power band, differences were observed at baseline between patients with AD and their caregivers in the theta, alpha, and beta bands in the open-eye state and in the delta, theta, and alpha bands in the closed-eye state. Although no significant changes were observed in the power bands of both the AD and CG groups after BLT, differences were observed between the beta band in the open-eye state and that of the theta band in the closed-eye state, whereas the differences between the delta bands in the closed-eye state changed from significant to not significant.
Regarding the EEG topographic map (Figures 2E, F) the difference between pre- and post-therapy in the HC and AD groups was not significant in the open-eye state. In the closed-eye state, the differences between the delta, theta, and beta bands of the occipital lobe in the HC group were significant (P1 < 0.001); the differences between the theta and beta bands of the left temporal parietal lobe in the AD group were also significant (P2 < 0.001).
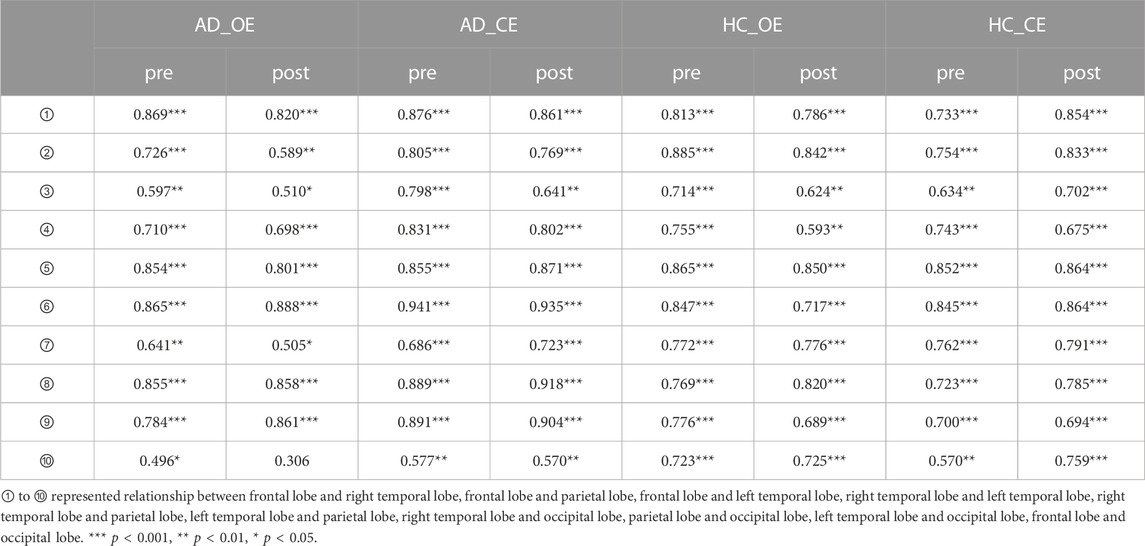
In Figure 3, the effects of BLT on the correlations between different regions of the cortex were investigated using the EEG power network in patients with AD and cognitive normal controls. The Pearson coefficient (r) was used to represent the connection strength between regions of the cortex as shown in Table 3. Compared with the pre therapy condition, significant improvements were observed in the numbers and strengths of connections after BLT.
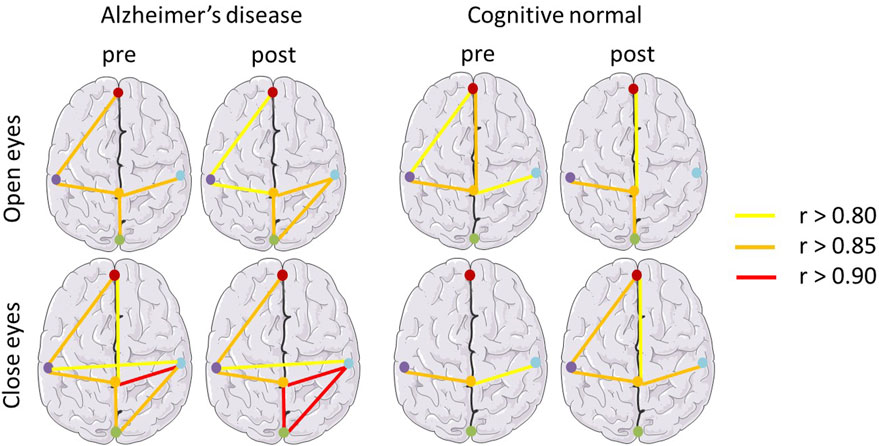
FIGURE 3. EEG power network of the brain in patients with Alzheimer’s disease and cognitively normal controls. Pearson coefficient (r) was used to represent the connection strength between regions of the cortex. r values greater than 0.80, 0.85, 0.90 are represented by yellow, orange and red bars respectively.
Pearson correlation between cognitive, blood, and EEG parameters
As shown in Figure 4, MMSE and ADAScog scores were both significantly correlated with the blood parameters of Aβ (r = −0.587, p < 0.001; r = 0.555, p < 0.001), Aβ40 (r = −0.410, p = 0.005; r = 0.319, p = 0.033), Aβ42 (r = −0.519, p < 0.001; r = 0.437, p = 0.003), IL-1β (r = −0.682, p < 0.001; r = 0.649, p < 0.001), and MT (r = 0.503, p < 0.001; r = −0.471, p = 0.001) respectively. The EEG parameter of the delta power band in the closed- (p = 0.054) and open-eye (p = 0.187) states showed no significant correlation with MMSE scores. The theta and delta power bands in the closed- (p = 0.096) and open-eye (p = 0.127) states showed no significant correlation with ADAScog scores. The EEG power of the left temporal lobe showed no significant correlation with both MMSE (p = 0.072) and ADAScog (p = 0.069) scores.
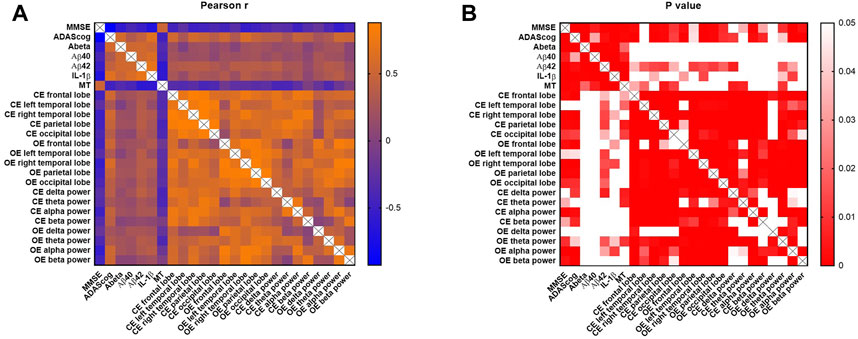
FIGURE 4. Pearson correlation analysis of cognitive, blood, and EEG parameters. MMSE and ADAScog scores represent the cognitive levels of participants. The blood parameters included Aβ, Aβ40, Aβ42, IL-1β and MT. EEG parameters included the power of five lobes (frontal, left temporal, right temporal, parietal, and occipital lobes) and four bands (delta [0–4 Hz], theta [4–8 Hz], alpha [8–13 Hz] and beta [13–30 Hz]). The scale bar represents the Pearson coefficient, r (A) and p values (B). CE, closed-eye state; OE, open-eye state.
Discussion
BLT is a medical treatment that utilizes natural or artificial light to improve a health condition. Our study showed that BLT may affect the EEG power and blood indicators, and may suppress depression, potentially owing to its effects on the circadian system via brain regions that respond preferentially to light. Patients with AD have high EEG power and strong electrical activity and more wastage in the resting state (Hsiao et al., 2013). Although the EEG power may be high and electrical activity of nerve cells may be strong in patients with AD, the synaptic function is incomplete and the signal conduction between neurons is abnormal; therefore, the brain cannot perform the functions of execution, memory, and learning (Babiloni et al., 2023).
To the best of our knowledge, EEG has been used as a non-invasive tool to study AD for many years. In recent years, modern methods of EEG analysis have been used to investigate physiological brain aging and AD (Cassani et al., 2018). Many recent studies focused on EEG signals for AD diagnosis, identifying and comparing key steps of EEG-based AD detection (Poil et al., 2013; Jesus et al., 2021). Resting state EEG was used as an effective tool to detect and assess cognitive impairment in patients with early AD (Meghdadi et al., 2021; Özbek et al., 2021). Compared to age-matched controls, an increase in spectral power was observed at the lower delta and theta frequencies in the AD group (Meghdadi et al., 2021), which was also observed in our study. The power of the delta and theta bands in the AD group decreased after BLT. These indicators may be potentially used as therapy efficacy assessment indicators.
In this study, the control group included cognitively normal individuals, rather than patients with AD who were not exposed to BLT. To some extent, this method avoided the placebo effect in participants with or without BLT. Moreover, the baseline values of the AD group were considered the self-control condition (positive control). The caregiver group was considered the normal condition (negative control). In previous studies, low intensity light was used as a sham stimulation (Hirakawa et al., 2020), which may be an ideal sham procedure if the lighting device is adjustable.
According to a previous study, non-invasive phototherapy is emerging as a strategy for suppressing Aβ self-assembly against AD (Ma et al., 2023). However, developing efficient photosensitizers for Aβ oxygenation that are active in deep brain tissues through the scalp and skull while reducing side effects remains a daunting challenge. Although light treatment improved cognitive function in AD mice and cleared amyloid levels in the brain (Yao et al., 2020; Kim et al., 2022), no changes in blood amyloid concentrations before and after BLT were observed. Another study found that BLT resulted in less daytime sleeping and increased night-time sleeping in people with dementia (Tan et al., 2022). Aβ levels can be cleared during sleep although accumulate during sleep deprivation (Shokri-Kojori et al., 2018; Wang and Holtzman, 2020).
Phototherapy is an effective method of treatment for patients with seasonal affective disorders (Knapen et al., 2014; Meyerhoff et al., 2018). Research confirms that phototherapy markedly improved mood and sleep quality in older adults (Zhang et al., 2023). Light signals are projected through retinal ganglion cells to depressed brain areas to participate in non-visual imaging functions, thereby activating nerve cell activity, secreting neurotransmitters to induce physiological changes in neural pathways, and regulating circadian rhythms, mood, and sleep in the biological organism to improve depressive symptoms (Fernandez et al., 2018). Moreover, the circadian system is composed of the central autonomous clock, suprachiasmatic nucleus, and body systems that follow the signals of the suprachiasmatic nucleus which mediate the effects of light on cognition and mood (Fernandez et al., 2018). It continuously changes the homeostatic set points of the body over the day-night cycle. The circadian rhythm is a natural, internal process that regulates the sleep-wake cycle that repeats approximately every 24 h.
Several treatments for mood disorders are available, including medication (anticonvulsants, antipsychotics, lithium, antidepressants, and benzodiazepines) and psychotherapy (Otte et al., 2016). Anticonvulsants, antipsychotics, and lithium are used to stabilize mood whereas antidepressants are used to treat depressive and anxiety disorders. As a non-pharmacological treatment, BLT can avoid the side effects and drug interactions associated with drug therapy, and also have the advantages of good compliance and ease of operation, making it suitable for use in patients with AD and their caregivers to treat depression.
This study has the following limitations and strengths. First, the sample size was relatively small. Regarding the long therapy duration and advanced age of participants, clinical samples were prone to shedding, and the dropout rate was high. The strength of this study is that we explored the non-pharmacological treatment BLT in patients with AD accompanied by depression, and revealed significant effects and useful clinical information regarding EEG power. Future studies will include a prolonged study duration and larger sample size to further investigate the association between changes in depression and EEG power.
Conclusion
BLT can suppress depression in patients with AD and their caregivers. Meanwhile, BLT significantly affected the EEG power and blood indicators. Non-pharmacological antidepressant therapy may be an effective and safe method for improving the emotional states of patients with AD and their caregivers.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ningbo Kangning Hospital ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’; legal guardians/next of kin.
Author contributions
XM, CZo, and ZS contributed to the original draft of the manuscript. TX and JH conducted the experiments. XW and CZh proofread the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The study was funded by Ningbo City Key R&D plan “Jie Bang Gua Shuai” (2023Z170), Ningbo City Public welfare Science and technology Plan project (2022S025), Zhejiang Medical and Health Science and Technology Project (2022KY1174), Ningbo Medical and Health Leading Academic Discipline Project (2022-F28), and the Major Fund Project of Ningbo Science and Technology Bureau (2019B10034). The sponsor had no role in the design or conduct of this research.
Acknowledgments
We acknowledge the Ningbo Kangning Hospital and all hospital staff that were involved in patient treatment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Karawi, D., and Jubair, L. (2016). Bright light therapy for nonseasonal depression: meta-analysis of clinical trials. J. Affect. Disord. 198, 64–71. doi:10.1016/j.jad.2016.03.016
Altomari, N., Bruno, F., Laganà, V., Smirne, N., Colao, R., Curcio, S., et al. (2022). A comparison of behavioral and psychological symptoms of dementia (bpsd) and bpsd sub-syndromes in early-onset and late-onset alzheimer's disease. J. Alzheimer's Dis. JAD 85, 691–699. doi:10.3233/JAD-215061
Babiloni, C., Blinowska, K., Bonanni, L., Cichocki, A., De Haan, W., Del Percio, C., et al. (2020). What electrophysiology tells us about alzheimer's disease: a window into the synchronization and connectivity of brain neurons. Neurobiol. aging 85, 58–73. doi:10.1016/j.neurobiolaging.2019.09.008
Babiloni, C., Lopez, S., Noce, G., Ferri, R., Panerai, S., Catania, V., et al. (2023). Relationship between default mode network and resting-state electroencephalographic alpha rhythms in cognitively unimpaired seniors and patients with dementia due to alzheimer's disease. Cereb. Cortex 33, 10514–10527. doi:10.1093/cercor/bhad300
Caravaglios, G., Muscoso, E. G., Blandino, V., Di Maria, G., Gangitano, M., Graziano, F., et al. (2023). Eeg resting-state functional networks in amnestic mild cognitive impairment. Clin. EEG Neurosci. 54, 36–50. doi:10.1177/15500594221110036
Carbone, E. A., de Filippis, R., Roberti, R., Rania, M., Destefano, L., Russo, E., et al. (2021). The mental health of caregivers and their patients with dementia during the covid-19 pandemic: a systematic review. Front. Psychol. 12, 782833. doi:10.3389/fpsyg.2021.782833
Cassani, R., Estarellas, M., San-Martin, R., Fraga, F. J., and Falk, T. H. (2018). Systematic review on resting-state eeg for alzheimer's disease diagnosis and progression assessment. Dis. Markers 2018, 5174815. doi:10.1155/2018/5174815
Chai, Y. C., Mahadevan, R., Ng, C. G., Chan, L. F., and Md Dai, F. (2018). Caregiver depression: the contributing role of depression in patients, stigma, social support and religiosity. Int. J. Soc. psychiatry 64, 578–588. doi:10.1177/0020764018792585
Chi, S., Yu, J. T., Tan, M. S., and Tan, L. (2014). Depression in alzheimer's disease: epidemiology, mechanisms, and management. J. Alzheimer's Dis. JAD 42, 739–755. doi:10.3233/JAD-140324
Corrêa, M. S., de Lima, D. B., Giacobbo, B. L., Vedovelli, K., Argimon, I. I. L., and Bromberg, E. (2019). Mental health in familial caregivers of alzheimer's disease patients: are the effects of chronic stress on cognition inevitable? Stress Amsterdam, Neth. 22, 83–92. doi:10.1080/10253890.2018.1510485
Diniz, B. S., Butters, M. A., Albert, S. M., Dew, M. A., and Reynolds, C. F. (2013). Late-life depression and risk of vascular dementia and alzheimer's disease: systematic review and meta-analysis of community-based cohort studies. Br. J. psychiatry J. Ment. Sci. 202, 329–335. doi:10.1192/bjp.bp.112.118307
Fernandez, D. C., Fogerson, P. M., Lazzerini Ospri, L., Thomsen, M. B., Layne, R. M., Severin, D., et al. (2018). Light affects mood and learning through distinct retina-brain pathways. Cell 175, 71–84. doi:10.1016/j.cell.2018.08.004
First, M. B. (2013). Diagnostic and statistical manual of mental disorders, 5th edition, and clinical utility. J. Nerv. Ment. Dis. 201, 727–729. doi:10.1097/NMD.0b013e3182a2168a
Hirakawa, H., Terao, T., Muronaga, M., and Ishii, N. (2020). Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 10, e01876. doi:10.1002/brb3.1876
Hsiao, F. J., Wang, Y. J., Yan, S. H., Chen, W. T., and Lin, Y. Y. (2013). Altered oscillation and synchronization of default-mode network activity in mild alzheimer's disease compared to mild cognitive impairment: an electrophysiological study. PloS one 8, e68792. doi:10.1371/journal.pone.0068792
Jack, C. R., Bennett, D. A., Blennow, K., Carrillo, M. C., Dunn, B., Haeberlein, S. B., et al. (2018). Nia-aa research framework: toward a biological definition of alzheimer's disease. Alzheimer's dementia 14, 535–562. doi:10.1016/j.jalz.2018.02.018
Jesus, B., Cassani, R., McGeown, W. J., Cecchi, M., Fadem, K. C., and Falk, T. H. (2021). Multimodal prediction of alzheimer's disease severity level based on resting-state eeg and structural mri. Front. Hum. Neurosci. 15, 700627. doi:10.3389/fnhum.2021.700627
Jia, L., Du, Y., Chu, L., Zhang, Z., Li, F., Lyu, D., et al. (2020). Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public health 5, e661–e671. doi:10.1016/S2468-2667(20)30185-7
Kim, S. H., Park, S. S., Kim, C. J., and Kim, T. W. (2022). Exercise with 40-hz light flicker improves hippocampal insulin signaling in alzheimer disease mice. J. Exerc. Rehabil. 18, 20–27. doi:10.12965/jer.2244042.021
Knapen, S. E., van de Werken, M., Gordijn, M. C., and Meesters, Y. (2014). The duration of light treatment and therapy outcome in seasonal affective disorder. J. Affect. Disord. 166, 343–346. doi:10.1016/j.jad.2014.05.034
Li, H., Jia, J., and Yang, Z. (2016). Mini-mental state examination in elderly Chinese: a population-based normative study. J. Alzheimer's Dis. JAD 53, 487–496. doi:10.3233/JAD-160119
Liu, S., Li, C., Shi, Z., Wang, X., Zhou, Y., Liu, S., et al. (2017). Caregiver burden and prevalence of depression, anxiety and sleep disturbances in alzheimer's disease caregivers in China. J. Clin. Nurs. 26, 1291–1300. doi:10.1111/jocn.13601
Lu, X., Liu, C., and Shao, F. (2023). Phototherapy improves cognitive function in dementia: a systematic review and meta-analysis. Brain Behav. 2023, e2952. doi:10.1002/brb3.2952
Ma, M., Wang, J., Jiang, H., Chen, Q., Xiao, Y., Yang, H., et al. (2023). Transcranial deep-tissue phototherapy for alzheimer's disease using low-dose x-ray-activated long-afterglow scintillators. Acta biomater. 155, 635–643. doi:10.1016/j.actbio.2022.10.049
Masters, C. L., Bateman, R., Blennow, K., Rowe, C. C., Sperling, R. A., and Cummings, J. L. (2015). Alzheimer's disease. Nat. Rev. Dis. Prim. 1, 15056. doi:10.1038/nrdp.2015.56
Meghdadi, A. H., Stevanović Karić, M., McConnell, M., Rupp, G., Richard, C., Hamilton, J., et al. (2021). Resting state eeg biomarkers of cognitive decline associated with alzheimer's disease and mild cognitive impairment. PloS one 16, e0244180. doi:10.1371/journal.pone.0244180
Meyerhoff, J., Young, M. A., and Rohan, K. J. (2018). Patterns of depressive symptom remission during the treatment of seasonal affective disorder with cognitive-behavioral therapy or light therapy. Depress. anxiety 35, 457–467. doi:10.1002/da.22739
Otte, C., Gold, S. M., Penninx, B. W., Pariante, C. M., Etkin, A., Fava, M., et al. (2016). Major depressive disorder. Nat. Rev. Dis. Prim. 2, 16065. doi:10.1038/nrdp.2016.65
Özbek, Y., Fide, E., and Yener, G. G. (2021). Resting-state eeg alpha/theta power ratio discriminates early-onset alzheimer's disease from healthy controls. Clin. neurophysiology official J. Int. Fed. Clin. Neurophysiology 132, 2019–2031. doi:10.1016/j.clinph.2021.05.012
Peter-Derex, L., Yammine, P., Bastuji, H., and Croisile, B. (2015). Sleep and alzheimer's disease. Sleep. Med. Rev. 19, 29–38. doi:10.1016/j.smrv.2014.03.007
Pfurtscheller, G., and Lopes da Silva, F. H. (1999). Event-related eeg/meg synchronization and desynchronization: basic principles. Clin. Neurophysiol. 110, 1842–1857. doi:10.1016/s1388-2457(99)00141-8
Poil, S. S., de Haan, W., van der Flier, W. M., Mansvelder, H. D., Scheltens, P., and Linkenkaer-Hansen, K. (2013). Integrative eeg biomarkers predict progression to alzheimer's disease at the mci stage. Front. aging Neurosci. 5, 58. doi:10.3389/fnagi.2013.00058
Roccaro, I., Smirni, D., and lux, F. (2020). Fiat lux: the light became therapy. An overview on the bright light therapy in alzheimer's disease sleep disorders. J. Alzheimer's Dis. JAD 77, 113–125. doi:10.3233/JAD-200478
Rosen, W. G., Mohs, R. C., and Davis, K. L. (1984). A new rating scale for alzheimer's disease. Am. J. psychiatry 141, 1356–1364. doi:10.1176/ajp.141.11.1356
Scheltens, P., Blennow, K., Breteler, M. M., de Strooper, B., Frisoni, G. B., Salloway, S., et al. (2016). Alzheimer's disease. Lancet London, Engl. 388, 505–517. doi:10.1016/S0140-6736(15)01124-1
Scheltens, P., De Strooper, B., Kivipelto, M., Holstege, H., Chételat, G., Teunissen, C. E., et al. (2021). Alzheimer's disease. Lancet (London, Engl. 397, 1577–1590. doi:10.1016/S0140-6736(20)32205-4
Shokri-Kojori, E., Wang, G. J., Wiers, C. E., Demiral, S. B., Guo, M., Kim, S. W., et al. (2018). Β-amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci. U. S. A. 115, 4483–4488. doi:10.1073/pnas.1721694115
Tan, J. S. I., Cheng, L. J., Chan, E. Y., Lau, Y., and Lau, S. T. (2022). Light therapy for sleep disturbances in older adults with dementia: a systematic review, meta-analysis and meta-regression. Sleep. Med. 90, 153–166. doi:10.1016/j.sleep.2022.01.013
van Maanen, A., Meijer, A. M., van der Heijden, K. B., and Oort, F. J. (2016). The effects of light therapy on sleep problems: a systematic review and meta-analysis. Sleep. Med. Rev. 29, 52–62. doi:10.1016/j.smrv.2015.08.009
Wang, C., and Holtzman, D. M. (2020). Bidirectional relationship between sleep and alzheimer's disease: role of amyloid, tau, and other factors. Neuropsychopharmacology 45, 104–120. doi:10.1038/s41386-019-0478-5
Wang, S., Zhang, Z., Yao, L., Ding, N., Jiang, L., and Wu, Y. (2020). Bright light therapy in the treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS One 15, e0232798. doi:10.1371/journal.pone.0232798
Yao, Y., Ying, Y., Deng, Q., Zhang, W., Zhu, H., Lin, Z., et al. (2020). Non-invasive 40-hz light flicker ameliorates alzheimer's-associated rhythm disorder via regulating central circadian clock in mice. Front. physiology 11, 294. doi:10.3389/fphys.2020.00294
Zhang, M., Wang, Q., Pu, L., Tang, H., Chen, M., Wang, X., et al. (2023). Light therapy to improve sleep quality in older adults living in residential long-term care: a systematic review. J. Am. Med. Dir. Assoc. 24, 65–74.e1. doi:10.1016/j.jamda.2022.10.008
Zimmerman, M., Martinez, J. H., Young, D., Chelminski, I., and Dalrymple, K. (2013). Severity classification on the Hamilton depression rating scale. J. Affect. Disord. 150, 384–388. doi:10.1016/j.jad.2013.04.028
Keywords: bright light therapy, Alzheimer’s disease, depression, EEG, biomarkers
Citation: Mei X, Zou C, Si Z, Xu T, Hu J, Wu X and Zheng C (2023) Antidepressant effect of bright light therapy on patients with Alzheimer’s disease and their caregivers. Front. Pharmacol. 14:1235406. doi: 10.3389/fphar.2023.1235406
Received: 06 June 2023; Accepted: 06 November 2023;
Published: 15 November 2023.
Edited by:
Jiehui Jiang, Shanghai University, ChinaReviewed by:
Ioannis Zaganas, University of Crete, GreeceCan Sheng, Affiliated Hospital of Jining Medical University, China
Copyright © 2023 Mei, Zou, Si, Xu, Hu, Wu and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengying Zheng, emhlbmdjaGVuZ3lpbmdAMTI2LmNvbQ==
 Xi Mei
Xi Mei Chenjun Zou2
Chenjun Zou2 Xiangping Wu
Xiangping Wu